Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.109687
Revised: June 21, 2025
Accepted: August 15, 2025
Published online: September 21, 2025
Processing time: 118 Days and 6.5 Hours
Predicting early recurrence (ER), (≤ 12 months) after pancreatic ductal adenocarcinoma (PDAC) resection remains challenging. Preoperative biomarkers such as carbohydrate antigen 19-9 (CA19-9) and computed tomography (CT) lack optimal specificity and reproducibility. Extracellular volume (ECV), measured on equi
To investigate the utility of CT-ECV for preoperative prediction of ER in PDAC patients after R0 resection.
This retrospective study included 93 PDAC patients undergoing R0 resection and preoperative pancreatic CT from January 2020 to November 2023. Clinical and CT features were analyzed. ECV was calculated using unenhanced and equilibrium-phase CT. Univariable and multivariable Cox regression identified ER predictors, followed by receiver operating characteristic analysis. Recurrence-free survival (RFS) was assessed by the Kaplan-Meier method.
Multivariable analysis identified elevated CT-ECV [hazard ratio (HR) = 1.05; 95% confidence interval (CI): 1.02-1.09; P = 0.003], high preoperative CA19-9 (HR = 1.00; 95%CI: 1.00-1.00; P = 0.002), and poor tumor grade (HR = 2.51; 95%CI: 1.20-5.22; P = 0.014) as independent ER predictors. CT-ECV demonstrated comparable predictive accuracy to tumor grade [areas under the curve (AUC): 0.736 vs 0.650; P = 0.202]. Combining CT-ECV and CA19-9 achieved a higher AUC than tumor grade alone (0.759 vs 0.650; P < 0.05). Kaplan-Meier analysis revealed sig
CT-derived ECV is a promising non-invasive biomarker for preoperative ER prediction in PDAC. Combined with CA19-9, it outperforms tumor grade in stratifying recurrence risk, offering a clinically actionable tool for opti
Core Tip: This study demonstrates that preoperative computed tomography (CT)-derived extracellular volume (CT-ECV) is an independent predictor of early recurrence in pancreatic ductal adenocarcinoma after R0 resection. Elevated CT-ECV, high carbohydrate antigen 19-9 (CA19-9), and poor tumor grade were significantly associated with shorter recurrence-free survival. Combining CT-ECV and CA19-9 improved predictive accuracy over tumor grade alone, offering a non-invasive tool for preoperative risk stratification and personalized postoperative management.
- Citation: Zhang ZW, Liu HT, Zhou ZH, Liao HF, Zhang LL, Li YM, Liang HW. Preoperative risk stratification of early recurrence in resected pancreatic ductal adenocarcinoma: Novel equilibrium-phase-computed tomography biomarker of extracellular volume. World J Gastroenterol 2025; 31(35): 109687
- URL: https://www.wjgnet.com/1007-9327/full/v31/i35/109687.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i35.109687
Pancreatic ductal adenocarcinoma (PDAC) is the most fatal pancreatic malignancy and the seventh leading cause of global cancer-related deaths, with an estimated 660000 deaths in 2024[1]. The 5-year survival rate has been stagnant at 7%-12% for a long time despite advancements in diagnostics and multimodal therapies[2]. In 2024, the mortality/incidence ratio is close to 0.99 (i.e., nearly one death for every new case) and the annual incidence has continually increased at a rate of 1%[1]. While radical resection remains the sole curative option[3], only 15%-20% of patients qualify for surgery, and over half experience early recurrence (ER) within a median recurrence-free survival (RFS) of 12 months post-resection[4-6]. This aggressive recurrence pattern underscores the critical need for preoperative risk stratification to optimize treatment strategies. Although neoadjuvant therapy shows promise in mitigating micrometastases and improving surgical margins[7,8], concerns regarding tumor progression during treatment and heterogeneous patient responses highlight the urgency for reliable biomarkers to guide therapeutic decisions[9].
Current ER predictors, including tumor grade, tumor node metastasis (TNM) stage, and lymphovascular invasion, are exclusively postoperative, limiting preoperative risk assessment[10]. Carbohydrate antigen 19-9 (CA19-9), the most widely used serum biomarker, correlates with recurrence risk but suffers from suboptimal specificity[11,12]. Computed tomography (CT), pivotal for PDAC diagnosis and staging, provides prognostic insights through features such as perivascular infiltration, peripancreatic tumor infiltration, and peripheral enhancement[7,12,13]; however, its reliance on subjective interpretation restricts reproducibility.
PDAC is pathologically characterized by extracellular matrix expansion driven by collagen deposition, quantified as extracellular volume (ECV)[14,15]. The iodinated contrast agent is uniformly spread across both intravascular and extravascular spaces in the CT equilibrium-phase. CT-derived ECV, calculated by comparing tissue and blood pool (aortic) enhancement corrected for hematocrit, provides an objective measure of fibrotic burden. Previous studies have established CT-ECV as an imaging biomarker for cardiac and hepatic fibrosis. In pancreatic imaging, CT-ECV has also emerged as a noninvasive quantitative biomarker for evaluating pancreatic fibrosis and pancreas-related clinical events. Specifically, Fukukura et al[15] and Wang et al[16] demonstrated that CT-ECV predicts survival outcomes and che
This study investigates the utility of preoperative CT-ECV as a novel imaging biomarker for predicting ER in PDAC patients undergoing R0 resection, aiming to bridge the gap in preoperative risk stratification and inform personalized therapeutic strategies.
This retrospective study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University (No. K2023-356), which waived the requirement for informed consent.
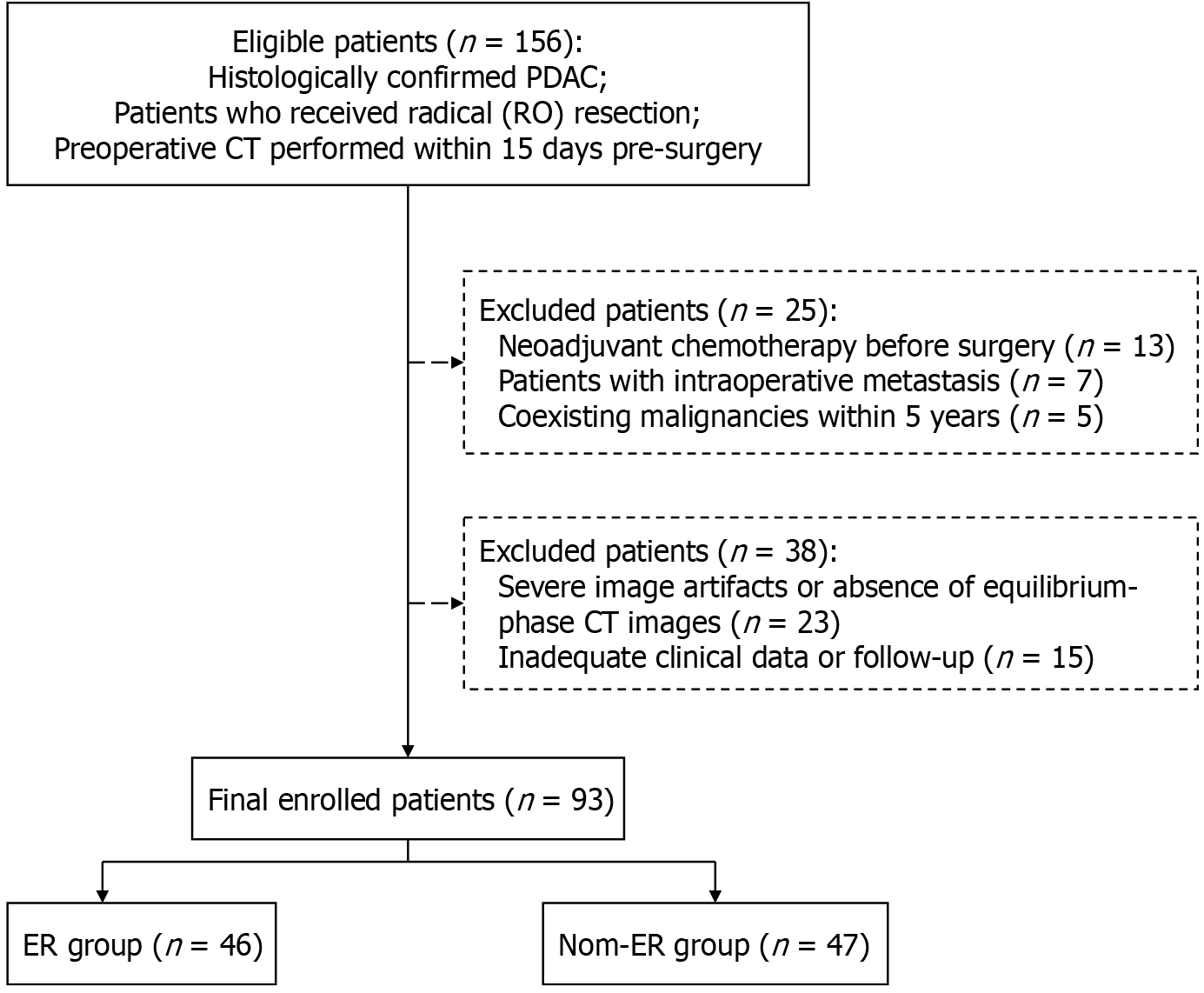
We consecutively reviewed resectable PDAC patients who underwent preoperative pancreatic CT from January 2020 to November 2023. Inclusion criteria included the following: (1) Histologically confirmed PDAC; (2) Patients who received radical (R0) resection; and (3) Preoperative CT examination performed within 15 days pre-surgery. Exclusion criteria included the following: (1) Neoadjuvant chemotherapy before surgery; (2) Intraoperative metastasis (excluding lymph node metastases); (3) Coexisting other malignancies within 5 years other than PDAC; (4) Severe image artifacts or absence of equilibrium-phase CT images; and (5) Inadequate clinical data or follow-up information. Resectable disease was defined as tumors with no major arterial involvement and no contact or ≤ 180° contact without contour irregularity with the portal vein or superior mesenteric vein, according to the National Comprehensive Cancer Network criteria[19]. Patients with PDAC underwent initial eligibility screening based on imaging and medical records, followed by retrospective confirmation of CT scans reviewed by an experienced radiologist (Li YM, 20 years’ expertise in pancreatic imaging). Finally, 93 patients were enrolled in this study (Figure 1). The sample size is determined by the number of patients who meet the criteria and have complete data during the study period.
Multiphasic contrast-enhanced CT was performed for all patients using two 128-channel multidetector row CT scanners (SOMATOM Definition Flash and SOMATOM Force, Siemens Healthineers). After the non-enhanced scan was performed, a standard bolus dosage of 1.2 mL/kg of nonionic contrast agent (Iopamiro 370 from Bracco Healthcare; or Ultravist 370 from Bayer Healthcare) was injected at a rate of 3.0-5.0 mL/second. The scans for the arterial and parenchymal phases were postponed by 8 seconds and 35 seconds, respectively, after the descending aorta reached 100 Hounsfield units (HU). Portal venous and equilibrium phases were performed with a fixed scan delay of 72 seconds and 3-5 minutes.
The detailed scan parameters were as follows: Tube voltages of 120 kVp, and 200-440 mA with use of automatic exposure control (Care dose 4D; Siemens), collimation of 128 mm × 0.6 mm, gantry rotation time of 0.5 seconds, and spiral pitch of 1.0 (for SOMATOM Definition Flash) or 0.7 (for SOMATOM Force). For image reconstruction, iterative reconstruction algorithms were employed (SAFIRE for SOMATOM Definition Flash; ADMIRE for SOMATOM Force). Images were reconstructed with a thickness of 1 mm and increment of 0.8 mm.
The following clinicopathologic and biochemical data were collected from the electronic medical records and pathological reports: Age, sex, body mass index, bilirubin, albumin, lymphocytes, hematocrit (Hct), alkaline phosphatase and serum CA 19-9 Levels, surgery type, tumor location, tumor diameter, tumor grade, and tumor stage of PDAC according to the American Joint Committee on Cancer (AJCC) 8th edition. All biochemical variables were routinely measured within 15 days before surgery. According to the international study group for pancreatic surgery definition[20], the type of pancreatic surgery was categorized as standard pancreaticoduodenectomy/distal pancreatectomy or extended pancreaticoduodenectomy/distal pancreatectomy involving concomitant veins or additional organ resection. Radical (R0) resection was defined as the complete removal of all tumors with a histologically negative margin.
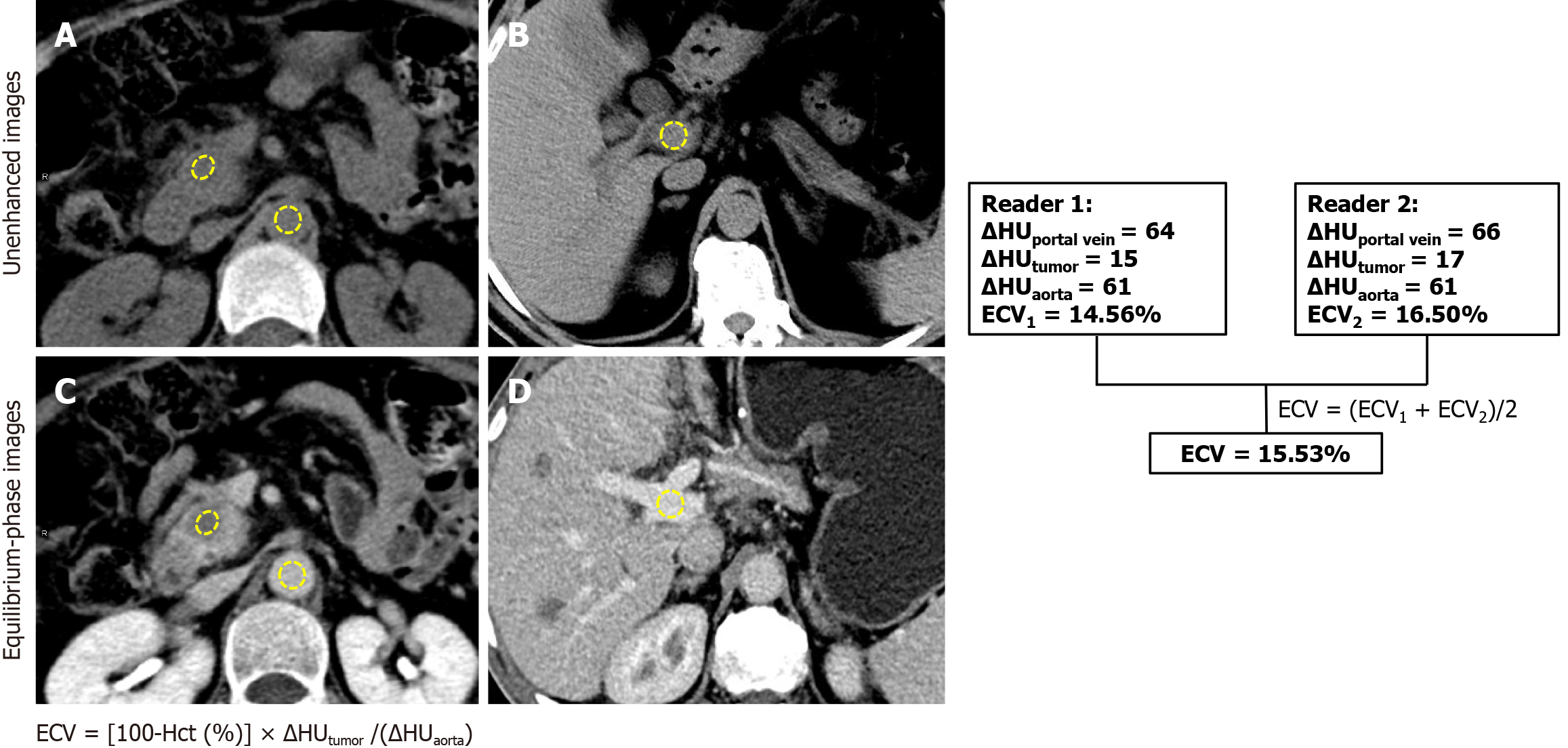
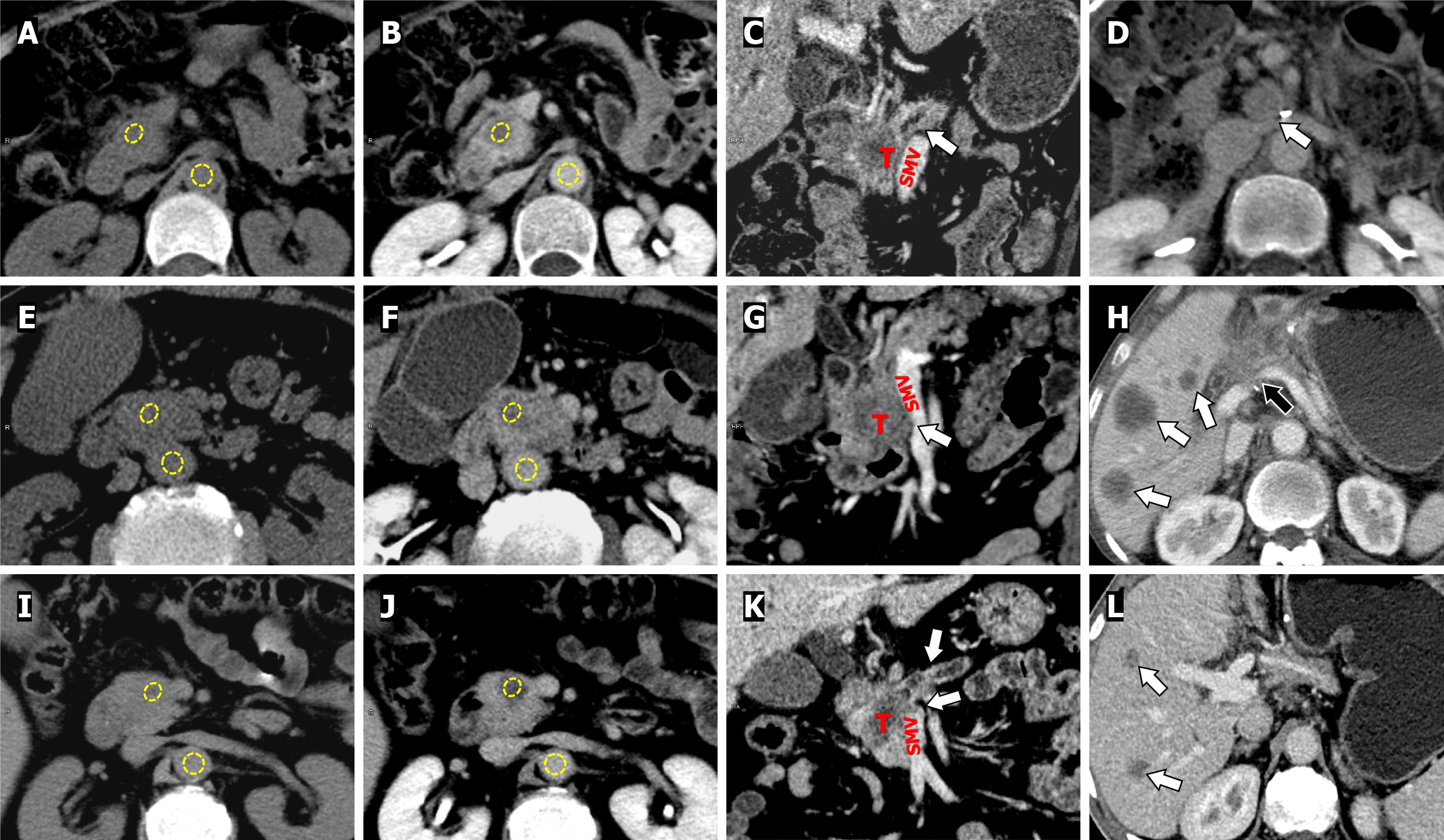
Two radiologists with 4 and 6 years of experience in abdominal imaging who were blinded to the clinical data and patient outcomes reviewed CT images at a picture archive and communication system (PACS) workstation (Communication System Carestream Vue PACS, version 12.1). Regions of interest (ROIs) were used to measure the CT values (HU) of the primary PDAC, portal vein and abdominal aorta on the same plane on unenhanced and equilibrium-phase images. For the tumor ROIs, areas of visible vessels, calcifications, and necrotic collections were avoided. For the aorta and portal vein, the ROIs were as large as possible but carefully avoided the aortic wall and calcification. ROIs placements were kept identical and visually co-localized on the CT images during unenhanced and equilibrium phases. The CT-ECV of the tumor were calculated using the following formula:
ECV = [100-Hct (%)] × ΔHUtumor/(ΔHUaorta)
Where ΔHU represents the change in HU between unenhanced and equilibrium-phase CT images. To confirm that the equilibrium phase was adequately obtained, ΔHUaorta and ΔHUportal vein for each patient were compared. Any significant differences between ΔHUaorta and ΔHUportal vein exceeding ± 10 HU were deemed outliers and removed. All measurements were taken twice by two radiologists and then averaged for analysis. The delineation of ROI is shown in Figure 2.
The same two observers also assessed the following CT variables based on previous imaging studies[7,21]: (1) Margins (well circumscribed vs ill defined); (2) Main pancreatic duct dilation (present vs absent); (3) Parenchymal atrophy (present vs absent, based on the ratio of the main pancreatic duct’s diameter to the pancreatic parenchyma’s width at the same level >0.5); (4) Vein abutment (present vs absent, defined as ≤ 180° of circumferential contact of the tumor with the portal vein, superior mesenteric vein and splenic vein); and (5) Peripancreatic tumor infiltration (tumor with expansive growth beyond the pancreas). Any ambiguous CT findings were addressed by consultation with the senior radiologist, who has 28 years of experience in abdominal imaging.
Every patient in our institution underwent a contrast-enhanced CT scan every three months during the first year after surgery. Magnetic resonance imaging, positron emission tomography/CT or biopsy was performed if necessary to clarify tumor recurrence. By comparing these results with preoperative data, ER was assessed in each patient after R0 resection. RFS was defined as the time from surgery to tumor recurrence, death, or last follow-up examination (censored). Each patient was followed up for at least 1 year and the ER status was reviewed up to November 2024. Recurrence within 1 year after R0 resection was defined as ER.
Continuous variables are presented as mean ± SD or medians and ranges conforming to a normal distribution, and compared using the t-test or Mann-Whitney U test. Categorical variables are expressed as numbers and percentages, and compared using the χ2 test or Fisher’s exact test. Patients were divided into ER and non-ER (NER) groups according to whether they had ER.
Univariable and multivariable Cox proportional-hazards models were used to identify independent predictors of ER. Variables with a P value of < 0.10 in the univariable analysis were then included for multivariable analysis. The results are presented as odds ratio with 95% confidence interval (CI). To evaluate the predictive performance of the predictor for ER, receiving operating characteristic curve analysis was used, and areas under the curve (AUC) were calculated. The optimal cutoff value was established by the Youden index, defined as the value maximizing the mean of sensitivity and specificity. Comparisons of AUC values between parameters were performed using the DeLong method. Kaplan-Meier survival curves for RFS were generated and analyzed using the log-rank test.
Intraclass correlation coefficient (ICC) was calculated to evaluate inter-observer agreement for attenuations and ECV measurements (ICC: 0.00-0.20, poor correlation; ICC: 0.21-0.40, fair correlation; ICC: 0.41-0.60, moderate correlation; ICC: 0.61-0.80, good correlation; and ICC: 0.81-1.00, excellent correlation). All statistical analyses were performed by SPSS software (version 26.0; IBM Corporation, 2013) and MedCalc Statistical Software (version 20.0.14), with significance at P < 0.05.
A total of 156 PDAC patients underwent preoperative pancreatic CT and R0 resection during the study period. After excluding 25 patients who either underwent neoadjuvant chemotherapy before surgery (n = 13), had intraoperative metastasis (n = 7) and coexisting malignancies within 5 years (n = 5), we also excluded 38 patients due to severe image artifacts or absence of equilibrium-phase CT images (n = 23) and inadequate clinical data or follow-up (n = 15). Finally, 93 patients (61.0 ± 9.1 years; 58 males) were included.
Tumors were found in the head (n = 68), body or tail (n = 25) of the pancreas, with an average primary tumor size of 2.8 cm. The AJCC classification identified 62 patients as stage IA or IB, 29 as stage IIA or IIB, and 2 as stage III. Out of the total, 79 patients received standard pancreatic resection, while 14 had an extended version of the surgery. Postoperative adjuvant therapy was administered to 53 patients.
Patients were categorized into ER (n = 46) and NER (n = 47) groups according to whether the tumor recurred within one year. The median RFS of ER patients was 5 months (interquartile range: 3.25-8.45).
Our results revealed no significant differences in demographic data, surgery type, biochemical results except CA19-9, tumor location, AJCC tumor stage, postoperative adjuvant therapy and CT findings of the pancreas between the two groups (P > 0.05), whereas significant differences were noted in the CA19-9, maximum tumor diameter, tumor grade, ΔHUtumor, and CT-derived ECV (P < 0.05). The main characteristics of the patients are summarized in Table 1.
| Characteristic | Non-ER (n = 47) | ER (n = 46) | P value |
| Age (years) | 60.76 ± 1.50 | 61.27 ± 1.33 | 0.802 |
| Male sex | 29 (61.7) | 29 (63.0) | 0.867 |
| Female sex | 18 (38.3) | 17 (37.0) | 0.894 |
| BMI (kg/m2), IQR | 21.85 (20.50-23.93) | 21.78 ± 4.70 | 0.895 |
| Type of pancreatic surgery | 0.165 | ||
| Standard pancreatic surgery | 42 (89.4) | 37 (80.4) | |
| Extended pancreatic surgery | 5 (10.6) | 9 (19.6) | |
| Laboratory results | |||
| CA19-9 (U/mL), IQR | 54.37 (15.48-268.88) | 328.00 (90.45-500.00) | 0.006 |
| Haematocrit level (%), IQR | 38.80 ± 7.86 | 38.00 (34.55-41.45) | 0.339 |
| Bilirubin (μmol/L), IQR | 47.05 (12.98-159.25) | 64.00 (10.20-201.55) | 0.809 |
| Albumin (g/L), IQR | 40.50 (37.00-44.75) | 38.97 ± 1.14 | 0.161 |
| Lymphocytes (109/L), IQR | 24.41 ± 1.38 | 19.50 (14.75-30.75) | 0.207 |
| Alkaline phosphatase (μkat/L), IQR | 188.0 (88.0-428.0) | 154.00 (87.00-461.00) | 0.913 |
| Tumor location | 0.077 | ||
| Head | 31 (66.0) | 37 (80.4) | |
| Body/tail | 16 (34.0) | 9 (19.6) | |
| Tumor size (cm), IQR | 2.0 (1.50-3.50) | 2.8 (2.25-3.90) | 0.024 |
| AJCC tumor stage | 0.867 | ||
| I | 31 (66.0) | 31 (67.4) | |
| II/III | 16 (34.0) | 15 (32.6) | |
| Tumor grade | 0.006 | ||
| G1/2 | 32 (68.1) | 19 (41.3) | |
| G3 | 15 (31.9) | 27 (58.7) | |
| Postoperative adjuvant therapy | 26 (49.1) | 27 (50.9) | 0.430 |
| ΔHUtumor, IQR | 53.05 ± 3.43 | 37.00 (32.66-45.00) | 0.003 |
| CT-derived ECV (%) | 39.78 ± 1.95 | 30.01 ± 1.90 | 0.001 |
| CT findings of the pancreas | |||
| Ill-defined margins | 13 (27.7) | 13 (28.3) | 0.948 |
| Main pancreatic duct dilation | 21 (44.7) | 20 (43.5) | 0.979 |
| Parenchymal atrophy | 7 (14.9) | 11 (23.9) | 0.271 |
| Vein abutment | 9 (19.1) | 17 (37.0) | 0.056 |
| Peripancreatic tumor infiltration | 11 (23.4) | 18 (39.1) | 0.102 |
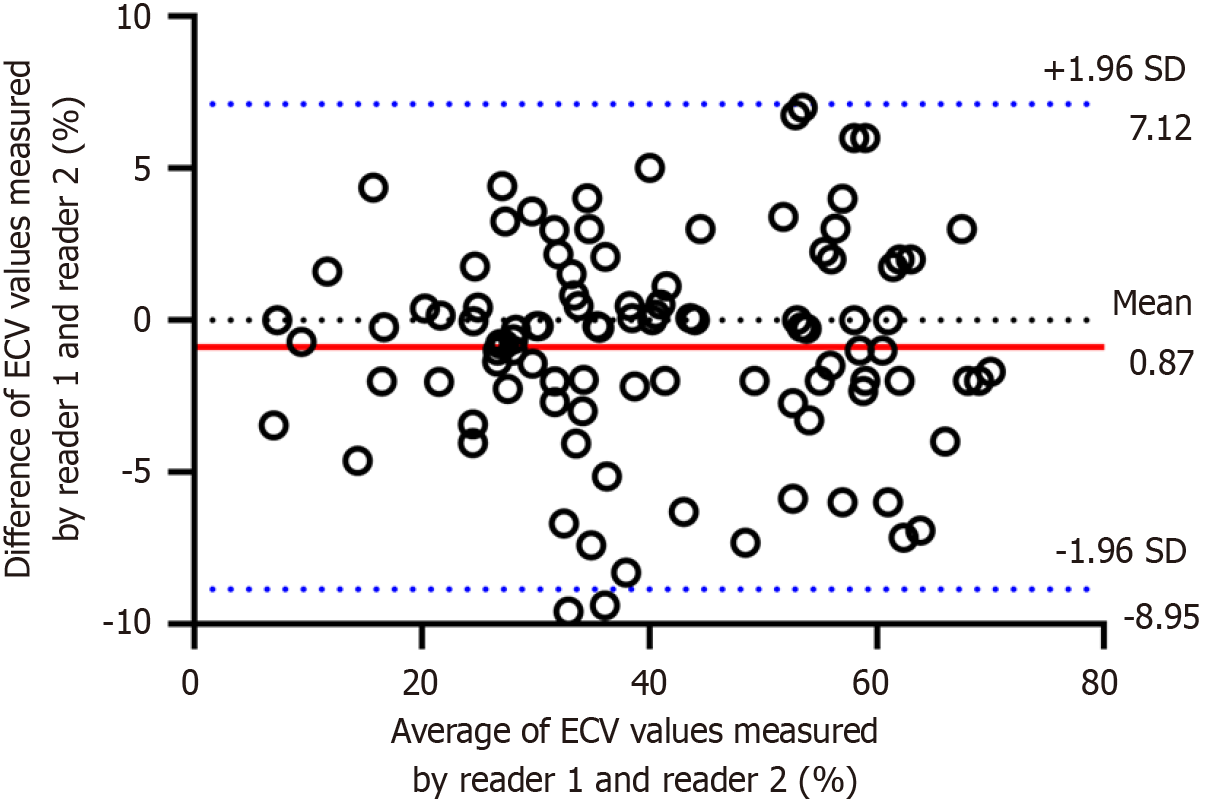
The interobserver reproducibility of CT-derived ECV was excellent (ICC = 0.926, 95%CI: 0.882-0.953). The Bland-Altman plots showed good interobserver agreement, with the mean bias of agreement in CT-ECV values between reader 1 and reader 2 of 0.87% and the upper and lower limits were -8.95% and 7.12%. Neither fixed bias nor proportional bias was observed. Therefore, the average value of CT-ECV measured by the two radiologists was used for further analysis. The Bland-Altman plot is shown in Figure 3.
The results of univariate and multivariate analyses for ER are shown in Table 2. Univariate analysis revealed that CA 19-9 [hazard ratio (HR) = 1.001; 95%CI: 1.000-1.001; P = 0.050], T stage (HR = 3.256; 95%CI: 1.499-7.071; P = 0.003), poorly differentiated PDAC (HR = 2.542; 95%CI: 1.352-4.780; P = 0.004), vein abutment (HR = 1.778; 95%CI: 0.954-3.313; P = 0.070), ΔHUtumor (HR = 0.974; 95%CI: 0.958-1.090; P = 0.002) and CT-derived ECV (HR = 1.057; 95%CI: 1.025-1.090; P = 0.001) were significantly associated with ER. In the multivariate analysis, CA 19-9 (HR = 1.000; 95%CI: 1.000-1.001; P = 0.002), poorly differentiated PDAC (HR = 2.506; 95%CI: 1.204-5.215; P = 0.014) and CT-derived ECV (HR = 1.050; 95%CI: 1.017-1.085; P = 0.003) were risk predictors of ER.
| Variable | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Age (years) | 1.008 (0.974-1.042) | 0.660 | ||
| Male sex | 0.842 (0.429-1.650) | 0.616 | ||
| Postoperative adjuvant therapy | 0.734 (0.423-1.274) | 0.272 | ||
| CA19-9 (U/mL) | 1.001 (1.000-1.001) | 0.050 | 1.000 (1.000-1.001) | 0.002a |
| Tumor location | 0.131 | |||
| Head | 1 (Reference) | |||
| Body to tail | 0.404 (0.125-1.310) | |||
| Tumor size (cm) | 1.196 (0.959-1.492) | 0.112 | ||
| T stage | 0.003 | 0.319 | ||
| T1 | 1 (Reference) | 1 (Reference) | ||
| T2 + T3 | 3.256 (1.499-7.071) | 1.556 (0.652-3.712) | ||
| N, yes | 1.211 (0.578-2.539) | 0.612 | ||
| Tumor grade, G3 | 2.542 (1.352-4.780) | 0.004 | 2.506 (1.204-5.215) | 0.014a |
| AJCC tumor stage | 0.942 | |||
| I | 1 (Reference) | |||
| II/III | 0.975 (0.498-1.912) | |||
| CT findings of the pancreas | ||||
| Ill-defined margins | 0.987 (0.511-1.905) | 0.968 | ||
| Main pancreatic duct dilation | 0.895 (0.485-1.652) | 0.723 | ||
| Parenchymal atrophy | 1.306 (0.654-2.609) | 0.449 | ||
| Vein abutment | 1.778 (0.954-3.313) | 0.070 | 2.099 (0.977-4.510) | 0.057 |
| Peripancreatic tumor infiltration | 0.762 (0.441-1.315) | 0.329 | 0.879 (0.530-1.457) | 0.616 |
| ΔHUtumor | 0.974 (0.958-0.990) | 0.002 | 0.988 (0.965-1.013) | 0.340 |
| CT-derived ECV (%) | 1.057 (1.025-1.090) | 0.001 | 1.050 (1.017-1.085) | 0.003a |
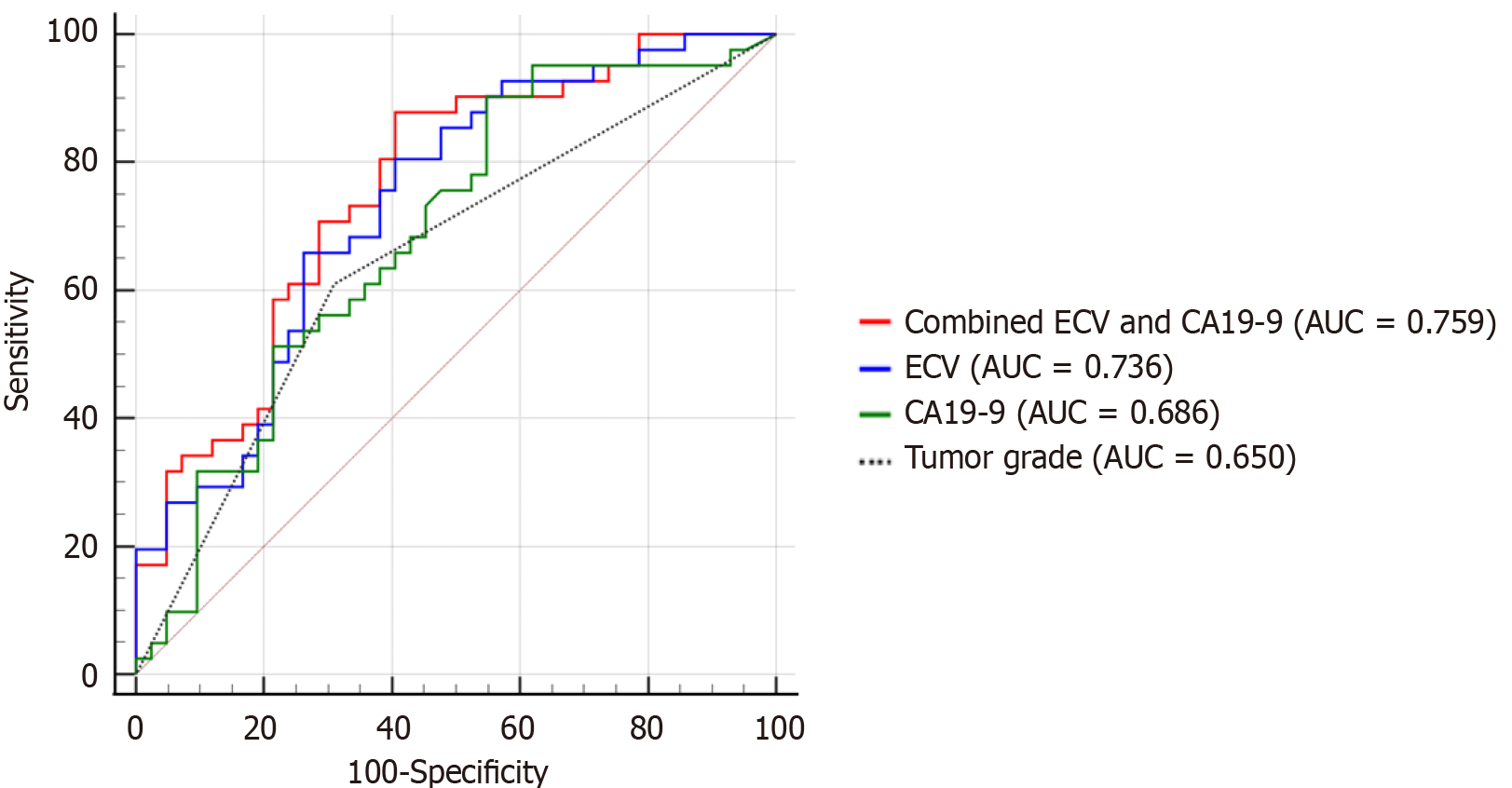
The AUC for predicting ER using the three identified independent predictors for CT-derived ECV was 0.736 (95%CI: 0.628-0.827), for CA19-9 was 0.686 (95%CI: 0.575-0.784), and for tumor grade was 0.650 (95%CI: 0.538-0.752). The higher AUC of CT-ECV and CA19-9 compared with tumor grade was not statistically significant (P = 0.202 and P = 0.653, respectively). However, the combination of ECV and CA19-9 yielded a significantly higher AUC [0.759 (95%CI: 0.653-0.846)] compared with tumor grade (P = 0.044) (Figure 4).
As shown in Table 3, using the Youden index, CT-derived ECV with the cutoff value of ≤ 35.37% was associated with a sensitivity of 80.49% and specificity of 59.42%, CA19-9 with the cutoff value of > 55 was associated with a sensitivity of 90.24% and specificity of 45.24%, tumor grade had a sensitivity of 60.98% and specificity of 69.05%. The combination of ECV and CA19-9 (threshold predicted probability of 0.45) achieved a sensitivity of 87.80% and specificity of 69.52%.
| Characteristic | Combined ECV and CA19-9 | CT-derived ECV | CA19-9 | Tumor grade |
| Cutoff value | 0.45 | 35.37 | 55.00 | Ref. |
| AUC (95%CI) | 0759 (0.653-0.846) | 0.736 (0.628-0.827) | 0.686 (0.575-0.784) | 0.650 (0.538-0.752) |
| Sensitivity | 87.80 | 80.49 | 90.24 | 60.98 |
| Specificity | 69.52 | 59.42 | 45.24 | 69.05 |
| P value | < 0.001 | < 0.001 | 0.002 | 0.005 |
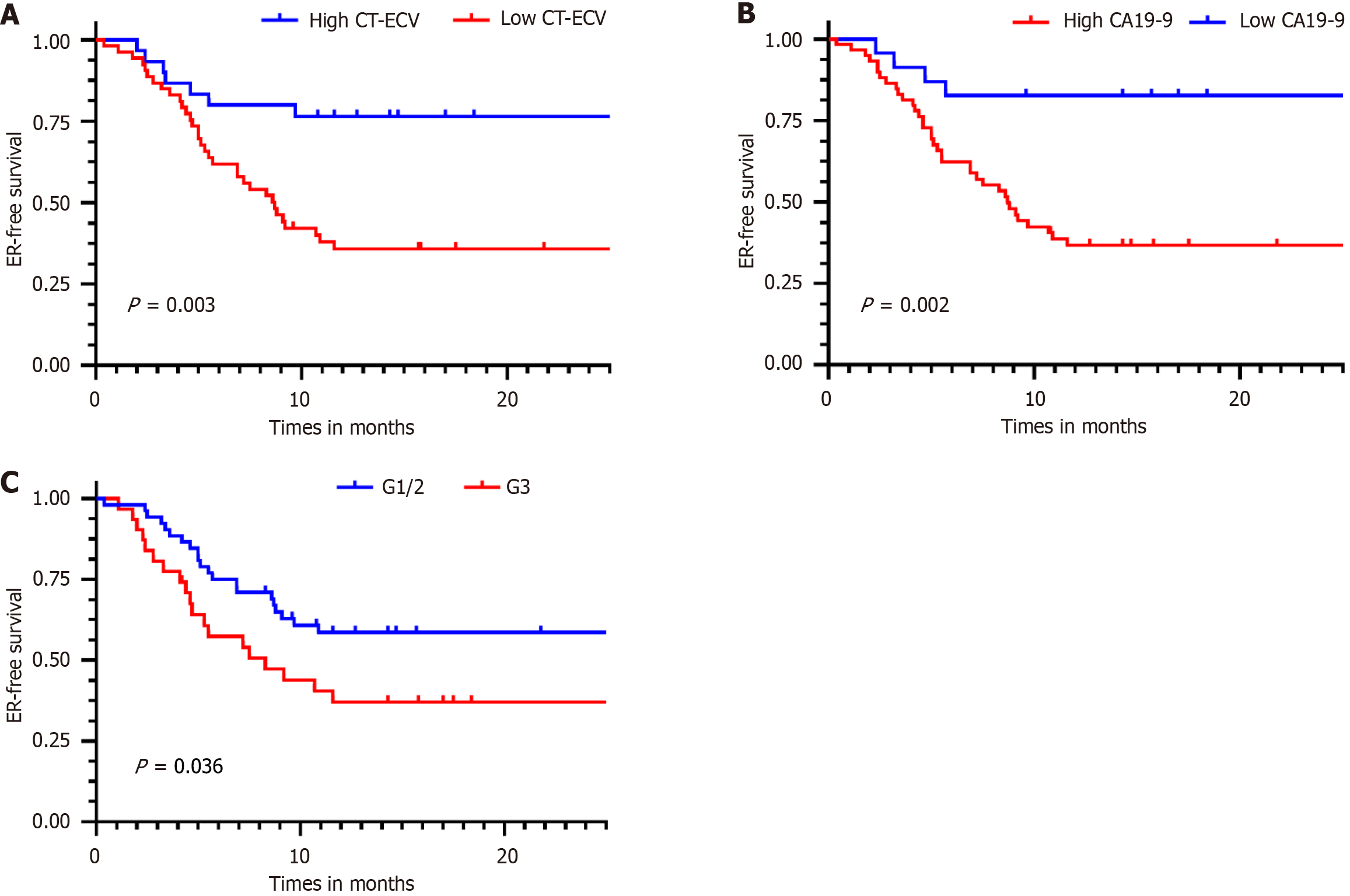
When survival analysis was stratified according to the optimal threshold, the Kaplan-Meier curves showed a higher ER rate in patients with low CT-ECV than in patients with high CT-ECV (66.0% vs 24.24%, log-rank test: P = 0.003). The results also showed that patients with high CA19-9 Levels had a higher ER rate than those with low CA19-9 Levels (61.67% vs 17.39%, log-rank test: P = 0.002). Tumor grade was stratified by high/moderate differentiation and poor differentiation, and patients with poorly differentiated PDAC had a higher ER rate than those with highly/moderately differentiated PDAC (65.79% vs 35.56%, log-rank test: P = 0.036) (Figure 5). Representative cases are shown in Figure 6.
Our study establishes CT-derived ECV as a novel preoperative predictor of ER in resectable PDAC, demonstrating comparable efficacy to tumor grade (AUC: 0.736 vs 0.650) and showing synergistic value when combined with CA19-9 (AUC: 0.759). These findings address a critical gap in preoperative risk stratification, as traditional predictors such as TNM stage or vascular invasion require postoperative histopathology. The prognostic superiority of CT-ECV over subjective CT features (e.g., vein abutment) and its technical advantages of rapid acquisition (< 3 minutes), minimal radiation, and reproducibility, position it as a practical biomarker for clinical integration.
Notably, low CT-ECV (≤ 35.37%) was correlated with a twofold increased ER risk, aligning with its pathophysiological basis: ECV quantifies fibrotic extracellular matrix expansion, a hallmark of PDAC aggression[15]. Our results are consistent with those of previous studies showing that CT-ECV was useful in estimating the risk of pancreas-related events, such as pancreas fibrosis, postoperative acute pancreatitis and chemotherapy response[15-17]. While fluorodeoxyglucose positron emission tomography/CT (AUC: 0.748) and radiomics (AUC: 0.701) show similar predictive potential[4,22], CT-ECV avoids their limitations of high cost, complex feature extraction, or protocol variability. Although magnetic resonance imaging offers superior soft-tissue contrast for ECV calculation, its longer scan time and contraindications limit practicality compared to equilibrium-phase CT[23].
Several well-known clinical and pathological variables, including T stage, lymph node status, post-surgical adjuvant chemotherapy and tumor differentiation influence PDAC outcomes[12,24]. Poor tumor differentiation, in particular, has been linked to greater heterogeneity, invasiveness, and a higher likelihood of ER. Consistent with previous reports[24,25], our results showed that poorly differentiated PDAC was associated with a twofold increased risk of ER compared to moderately or well-differentiated tumors. Notably, tumor grade exhibited marginally lower predictive accuracy AUC compared to CT-ECV, underscoring the utility of CT-ECV in preoperative evaluation. We found that T stage and lymph node metastasis were not identified as independent predictors of ER, which may be due to the relatively small sample size or the superior discriminatory power of tumor grade in reflecting tumor heterogeneity.
Serum CA19-9 is a well-established prognostic factor in PDAC, with elevated concentrations correlating with higher tumor burden and poorer survival[10,26]. Multiple studies have demonstrated the association between preoperative CA19-9 Levels and ER following resection of PDAC, with threshold values ranging from 100 to 529 U/mL[12,27,28]. In our study, patients experiencing ER exhibited significantly elevated CA19-9 Levels (optimal threshold: 55 U/mL; AUC: 0.686, sensitivity: 90.24%, specificity: 45.24%). While our threshold yielded lower specificity than previous studies, the high sensitivity (90.24%) supports its utility in high-risk screening, particularly given potential confounding effects from biliary obstruction in some patients.
Pancreatic CT remains the preferred imaging modality of choice for pancreatic tumor evaluation. Beyond its diagnostic and staging roles, tumor enhancement features on CT may hold prognostic value[7]. Recent studies have linked morphological and enhancement characteristics, such as tumor diameter, peripancreatic infiltration, and suspicious lymph node metastasis, to tumor recurrence and poor survival after R0 resection[7,12]. In our study, vein abutment and tumor enhancement were significant predictors of ER in univariate analysis but did not emerge as independent predictors in multivariate analysis. This may be attributed to the strong discriminatory power of CT-ECV, CA19-9, and tumor grade, which diminished the contribution of these CT characteristics. The prognostic role of vascular invasion in PDAC has been explored, with some studies indicating that portal or splenic vein invasion is associated with worse outcomes[13,29]. We speculate that tumor is more likely to be systemic when it has invaded the portal vein or its branches. Indeed, recent findings have confirmed the prognostic significance of portal vein circulation tumor cells in PDAC patients[30,31], supporting the hypothesis that vascular invasion reflects systemic disease.
This study has some limitations. First, single-center relatively small R0-resected cohorts may limit generalizability; thus, validation in a larger population of PDAC individuals is warranted. Second, this study used retrospective observational data and selective bias is unavoidable. Prospective studies will be needed to further validate the findings. Third, the small sample size leads to insufficient adjustment efficacy for key confounding variables, such as age and surgery types. Future studies should expand the sample size through multicenter cohorts to more comprehensively control for confounding factors. Fourth, ECV was calculated using a single CT approach, and the prognostic value of ECV from dual-energy CT in post-surgery PDAC needs more investigation. Finally, 3-minute equilibrium-phase timing, although validated for hepatic fibrosis[32], requires optimization for pancreatic applications.
Combined CT-derived ECV and CA19-9 provide superior ER prediction compared to tumor grade alone in PDAC patients. As a noninvasive, widely accessible imaging biomarker, CT-ECV offers critical insights for risk stratification and personalized therapeutic strategies in PDAC management.
All authors would like to thank all the subjects who participated in this study.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6057] [Article Influence: 3028.5] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56666] [Article Influence: 7083.3] [Reference Citation Analysis (135)] |
| 3. | Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Wargo JA, Lillemoe KD, Ferrone CR. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? Ann Surg. 2013;257:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Xie T, Wang X, Li M, Tong T, Yu X, Zhou Z. Pancreatic ductal adenocarcinoma: a radiomics nomogram outperforms clinical model and TNM staging for survival estimation after curative resection. Eur Radiol. 2020;30:2513-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, Urba S, Zeh HJ, Katz MH. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 6. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 547] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 7. | Kim DW, Lee SS, Kim SO, Kim JH, Kim HJ, Byun JH, Yoo C, Kim KP, Song KB, Kim SC. Estimating Recurrence after Upfront Surgery in Patients with Resectable Pancreatic Ductal Adenocarcinoma by Using Pancreatic CT: Development and Validation of a Risk Score. Radiology. 2020;296:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 316] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 9. | van Dam JL, Janssen QP, Besselink MG, Homs MYV, van Santvoort HC, van Tienhoven G, de Wilde RF, Wilmink JW, van Eijck CHJ, Groot Koerkamp B; Dutch Pancreatic Cancer Group. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 10. | Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med. 2013;13:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, Büchler MW, Werner J. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20:2188-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Li D, Peng Q, Wang L, Cai W, Liang M, Liu S, Ma X, Zhao X. Preoperative prediction of disease-free survival in pancreatic ductal adenocarcinoma patients after R0 resection using contrast-enhanced CT and CA19-9. Eur Radiol. 2024;34:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Xie T, Xie X, Liu W, Chen L, Liu K, Zhou Z. Prediction of postoperative recurrence in resectable pancreatic body/tail adenocarcinoma: a novel risk stratification approach using a CT-based nomogram. Eur Radiol. 2023;33:7782-7793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Fukui H, Onishi H, Nakamoto A, Tsuboyama T, Ota T, Yano K, Enchi Y, Yamada D, Takeda Y, Kobayashi S, Fukuda Y, Eguchi H, Matsui T, Tatsumi M, Tomiyama N. Pancreatic fibrosis by extracellular volume fraction using Contrast-enhanced computed tomography and relationship with pancreatic cancer. Eur J Radiol. 2022;156:110522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Fukukura Y, Kumagae Y, Higashi R, Hakamada H, Takumi K, Maemura K, Higashi M, Kamimura K, Nakajo M, Yoshiura T. Extracellular volume fraction determined by equilibrium contrast-enhanced multidetector computed tomography as a prognostic factor in unresectable pancreatic adenocarcinoma treated with chemotherapy. Eur Radiol. 2019;29:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Wang ZJ, Zhang TT, An C, Ko AH, Tempero M, Collisson E, Yeh BM. Estimation of Fractional Extracellular Space at CT for Predicting Chemotherapy Response and Survival in Pancreatic Ductal Adenocarcinoma. AJR Am J Roentgenol. 2020;215:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Bai XH, Yin J, Yu SY, Shu YP, Lu ZP, Jiang KR, Xu Q. Extracellular volume fraction derived from dual-energy CT: a potential predictor for acute pancreatitis after pancreatoduodenectomy. Eur Radiol. 2024;34:6957-6966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Kameda F, Tanabe M, Higashi M, Ariyoshi S, Ihara K, Iida E, Furukawa M, Okada M, Ito K. The extracellular volume fraction of the pancreas measured by dual-energy computed tomography: The association with impaired glucose tolerance. Eur J Radiol. 2021;141:109775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Hong SB, Lee SS, Kim JH, Kim HJ, Byun JH, Hong SM, Song KB, Kim SC. Pancreatic Cancer CT: Prediction of Resectability according to NCCN Criteria. Radiology. 2018;289:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Marqués Gubern A. [The publications of Cirugía Pediátrica]. Cir Pediatr. 1989;2:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Wang Y, Hu X, Shi S, Song C, Wang L, Yuan J, Lin Z, Cai H, Feng ST, Luo Y. Utility of Quantitative Metrics From Dual-Layer Spectral-Detector CT for Differentiation of Pancreatic Neuroendocrine Tumor and Neuroendocrine Carcinoma. AJR Am J Roentgenol. 2022;218:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Yamamoto T, Sugiura T, Mizuno T, Okamura Y, Aramaki T, Endo M, Uesaka K. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Suzuki M, Toba T, Izawa Y, Fujita H, Miwa K, Takahashi Y, Toh H, Kawamori H, Otake H, Tanaka H, Fujiwara S, Watanabe Y, Kono AK, Okada K, Hirata KI. Prognostic Impact of Myocardial Extracellular Volume Fraction Assessment Using Dual-Energy Computed Tomography in Patients Treated With Aortic Valve Replacement for Severe Aortic Stenosis. J Am Heart Assoc. 2021;10:e020655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, Burkhart RA, Rinkes IHMB, Molenaar IQ, Cameron JL, Weiss MJ, Wolfgang CL, He J. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 25. | Hong S, Song KB, Hwang DW, Lee JH, Lee W, Jun E, Kwon J, Park Y, Park SY, Kim N, Shin D, Kim H, Sung M, Ryu Y, Kim SC. Preoperative serum carbohydrate antigen 19-9 levels predict early recurrence after the resection of early-stage pancreatic ductal adenocarcinoma. World J Gastrointest Surg. 2021;13:1423-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Jones WE 3rd, Suh WW, Abdel-Wahab M, Abrams RA, Azad N, Das P, Dragovic J, Goodman KA, Jabbour SK, Konski AA, Koong AC, Kumar R, Lee P, Pawlik TM, Small W Jr, Herman JM; Expert Panel on Radiation OncologyGastrointestinal. ACR Appropriateness Criteria® Resectable Pancreatic Cancer. Am J Clin Oncol. 2017;40:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 2103] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 28. | Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, Tomlinson JS. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312-2320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Mizumoto T, Toyama H, Asari S, Terai S, Mukubo H, Yamashita H, Shirakawa S, Nanno Y, Ueda Y, Sofue K, Tanaka M, Kido M, Ajiki T, Fukumoto T. Pathological and Radiological Splenic Vein Involvement are Predictors of Poor Prognosis and Early Liver Metastasis After Surgery in Patients with Pancreatic Adenocarcinoma of the Body and Tail. Ann Surg Oncol. 2018;25:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Choi YH, Hong TH, Yoon SB, Lee IS, Lee MA, Choi HJ, Choi MH, Jung ES. Prognostic Implications of Portal Venous Circulating Tumor Cells in Resectable Pancreatic Cancer. Biomedicines. 2022;10:1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 31. | Morimoto D, Yamada S, Murotani K, Sonohara F, Takami H, Suenaga M, Hayashi M, Niwa Y, Tashiro M, Hattori N, Iwata N, Kanda M, Tanaka C, Kobayashi D, Nakayama G, Koike M, Fujiwara M, Fujii T, Kodera Y. Prognostic Impact of Portal System Invasion in Pancreatic Cancer Based on Image Classification. Pancreas. 2018;47:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Cicero G, Mazziotti S, Silipigni S, Blandino A, Cantisani V, Pergolizzi S, D'Angelo T, Stagno A, Maimone S, Squadrito G, Ascenti G. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol Med. 2021;126:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/