Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.111541
Revised: August 1, 2025
Accepted: August 14, 2025
Published online: September 14, 2025
Processing time: 63 Days and 18.8 Hours
Deep learning-based super-resolution (SR) reconstruction can obtain high-quality images with more detailed information.
To compare multiparametric normal-resolution (NR) and SR magnetic resonance imaging (MRI) in predicting the histopathologic grade in hepatocellular carcinoma.
We retrospectively analyzed a total of 826 patients from two medical centers (training 459; validation 196; test 171). T2-weighted imaging, diffusion-weighted imaging, and portal venous phases were collected. Tumor segmentations were conducted automatically by 3D U-Net. Based on generative adversarial network, we utilized 3D SR reconstruction to produce SR MRI. Radiomics models were developed and validated by XGBoost and Catboost. The predictive efficiency was demonstrated by calibration curves, decision curve analysis, area under the curve (AUC) and net reclassification index (NRI).
We extracted 3045 radiomic features from both NR and SR MRI, retaining 29 and 28 features, respectively. For XGBoost models, SR MRI yielded higher AUC value than NR MRI in the validation and test cohorts (0.83 vs 0.79; 0.80 vs 0.78), respectively. Consistent trends were seen in CatBoost models: SR MRI achieved AUCs of 0.89 and 0.80 compared to NR MRI’s 0.81 and 0.76. NRI indicated that the SR MRI models could improve the prediction accuracy by -1.6% to 20.9% compared to the NR MRI models.
Deep learning-based SR MRI could improve the predictive performance of histopathologic grade in HCC. It may be a powerful tool for better stratification management for patients with operable HCC.
Core Tip: In this study, multiparametric magnetic resonance imaging radiomics could non-invasively classify histopathologic grade in hepatocellular carcinoma. The image quality is crucial for radiomics feature extraction and model development. Deep learning-based super-resolution (SR) reconstruction further improved the prediction by optimizing radiomics features. Deep learning-based SR reconstruction may provide deeper insights for precision medicine and disease management.
- Citation: Wang ZZ, Song SM, Zhang G, Chen RQ, Zhang ZC, Liu R. Multiparametric magnetic resonance imaging of deep learning-based super-resolution reconstruction for predicting histopathologic grade in hepatocellular carcinoma. World J Gastroenterol 2025; 31(34): 111541
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/111541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.111541
Hepatocellular carcinoma (HCC) is the sixth most frequent malignant tumor and the fourth leading cause of cancer-related deaths worldwide[1,2]. Despite widespread adoption of radical resection, the 5-year post-operative recurrence rate of HCC remains as high as 70%[3,4]. Poorly differentiated (PD) tumors are a major contributor to both high recur
Most current radiomics studies assessing preoperative tumor status in HCC focused on microvascular invasion, while relatively few studies have investigated the grade of tumor differentiation[6,7]. PD HCC is typically highly aggressive and prone to metastasis or multifocal disease[8,9]. Accurate preoperative assessment of tumor differentiation could guide the development of personalized treatment strategies. For instance, preoperative adjuvant anti-tumor therapies may increase radical resection rates and reduce postoperative recurrence[10,11]. In addition, considering the high aggressiveness of PD HCC, which render it more susceptible to vascular invasion, a wide margin (≥ 1 cm) hepatectomy may further improve postoperative survival in patients with HCC[5,12,13].
Radiomics has significantly improved the accuracy and efficiency of diagnosis and prognosis, yet its performance in HCC is often constrained by image quality. For patients with HCC, especially those with early-stage lesions or multifocal tumors, the subtlety of pathological features, such as indistinct tumor boundaries or microvascular invasion, makes reliable detection and characterization highly dependent on high-resolution imaging. However, in clinical practice, factors like limited scanner field strength, motion artifacts, or the need for rapid acquisition to reduce patient burden often result in low-quality images with blurred details, directly hindering the effectiveness of radiomics analysis.
Super-resolution (SR) reconstruction, a deep learning-based technique that enhances image resolution while preserving the critical anatomical information, offers a potential solution to this bottleneck[14,15]. By refining normal-resolution (NR) images into high-resolution counterparts with richer details, SR could help visualize subtle HCC lesions and improve the robustness of radiomic features derived from these images. While SR has shown promise in enhancing the image quality for other modalities [e.g., coronary computed tomography (CT) angiography] and disease types (e.g., rectal cancer staging), its application in HCC-related radiomics remains underexplored[16-18], thereby leaving a critical gap in addressing the unmet need for reliable imaging-based HCC assessment. Thus, this study aimed to investigate the value of multiparametric SR magnetic resonance imaging (MRI) for the preoperative prediction of histopathologic grade in HCC.
The study was approved by the institutional review board of Chinese PLA General Hospital and the requirement for informed consent was waived. From January 2018 to July 2022, consecutive HCC patients who underwent preoperative MRI and subsequently received hepatectomy were retrospectively collected in The First Medical Center of Chinese PLA General Hospital.
Inclusion criteria: (1) Patients who underwent radical surgical resection of HCC; (2) Patients who completed multiparametric MRI examinations within 4 weeks before surgery; and (3) Postoperative pathological examination confirmed the diagnosis of HCC.
Exclusion criteria: (1) History of previous liver surgery; (2) Received anti-tumor therapies before surgery; (3) Incomplete medical records; (4) Presence of portal vein invasion or distant metastasis confirmed by preoperative imaging or postoperative pathology; and (5) MRI data with poor quality.
Patient grouping: A total of 655 patients from this hospital were included in this study. These patients were randomly divided into the training (n = 459) and validation (n = 196) cohorts. Additionally, to better validate the performance of the model, data from the Sixth Medical Center of Chinese PLA General Hospital, public data from The Cancer Imaging Archive, and data from the First Medical Center of Chinese PLA General Hospital, between August 2022 and June 2023, were incorporated as the test cohort, which included 171 patients.
Information on tumor differentiation was extracted from routine pathological reports as the reference standard. The tumors were categorized as PD, moderately-differentiated (MD), and well-differentiated (WD), of which MD and WD were classified into none-PD (nPD) HCC. When HCC tumors exhibited various differentiation results, the predominant differentiation determined the final diagnosis.
All patients underwent MRI on 1.5 T or 3.0 T MRI scanner. The following sequences were acquired: T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and contrast-enhanced T1-weighted imaging [T1WI; portal venous phase (PVP)]. For T2WI, the repetition time (TR) and echo time (TE) were set within commonly used ranges for abdominal imaging, with slice thickness in the typical range for liver examinations. DWI sequences employed standard b-values for assessing diffusion in hepatic tissues, with TR and TE adjusted appropriately, and slice thickness similar to that of T2WI. Contrast-enhanced T1WI in the PVP was obtained at the usual time point after contrast agent administration, with TR and TE set to optimize T1 weighting, and slice thickness suitable for detailed visualization of liver structures. The detailed parameters of MRI sequences are described in Supplementary Table 1.
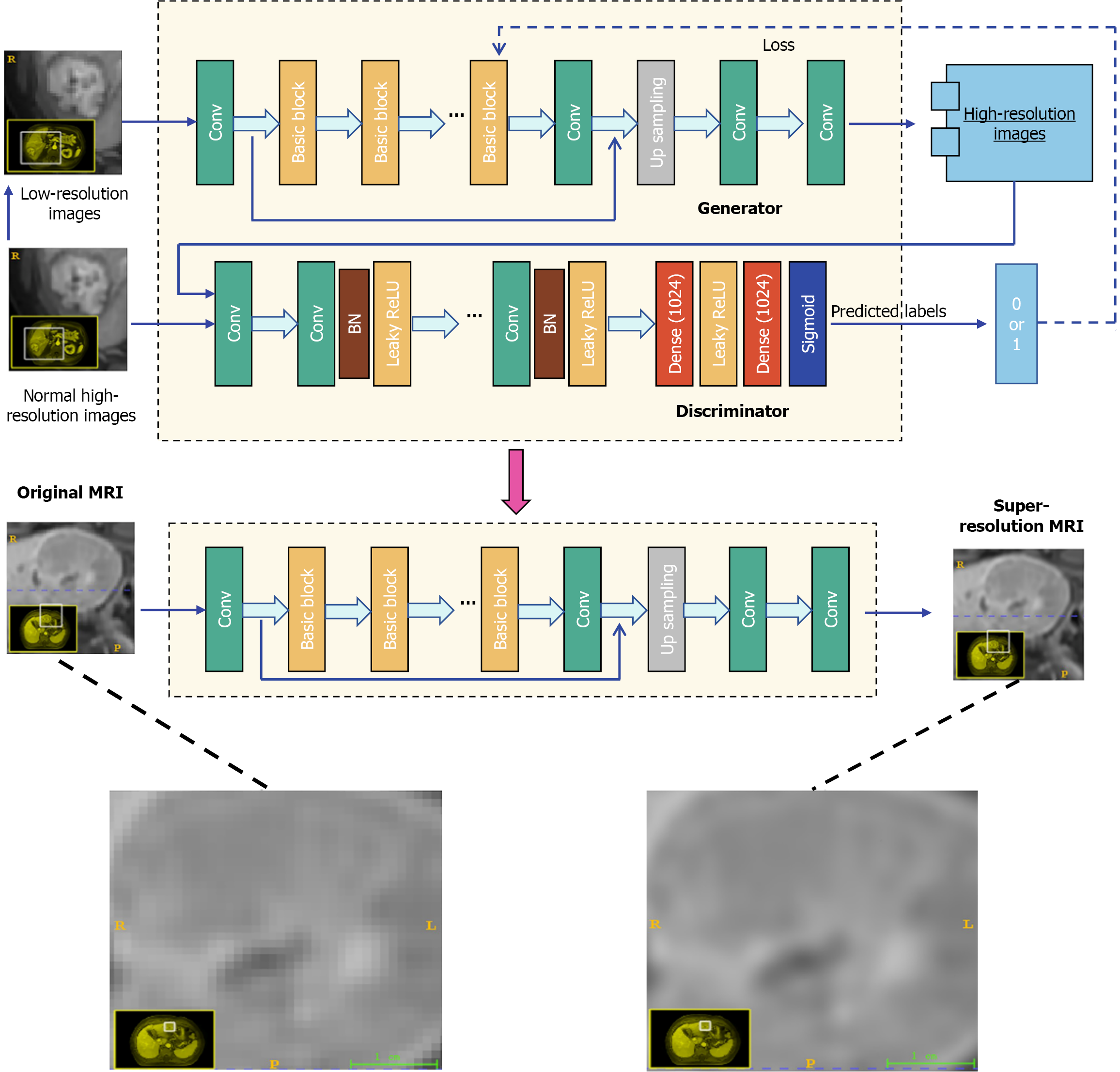
We used a 3D SR reconstruction technique based on generative adversarial networks (GANs; Figure 1). In simple terms, GANs work through a "cooperative competition" between two core parts: One-part (generator) focuses on improving the resolution of MRI images, and the other (discriminator) checks whether the improved images look real and natural. Through this process, the generated high-resolution images can better match the characteristics of real high-resolution MRI scans. In our design, we optimized this GAN framework to ensure that the enhanced images not only look visually realistic but also accurately preserve the key anatomical details in the original NR MRI scans. By clearly restoring the fine anatomical structures, such as small lesions or subtle tissue boundaries, the reconstructed images can provide more reliable imaging basis for doctors' diagnosis and treatment decision-making, thereby helping to improve the accuracy of disease detection and evaluation. Thus, this is crucial for clinical practice. For more detailed information on the technical implementation of this SR reconstruction method, please refer to Supplementary material 1.
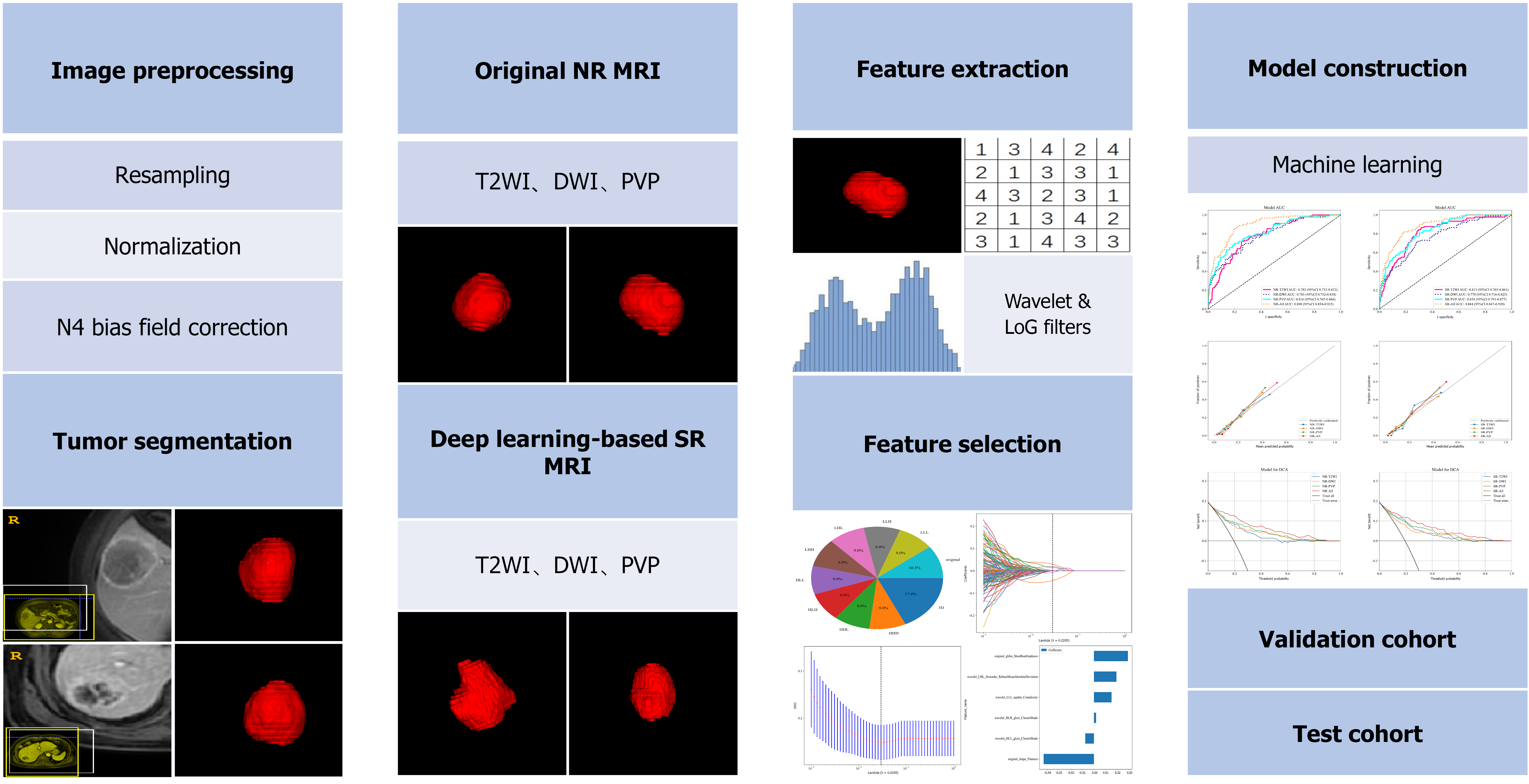
Image preprocessing was performed, including resampling, normalization, and N4 bias field correction. Algorithm engineers utilized the 3D U-Net to automatically detect and segment tumors in the liver region. Our implementation of the 3D U-Net model featured an encoder-decoder structure with dense skip pathways that facilitated the precise localization of tumor margins. The deep supervision mechanism embedded within the network enhanced the learning process by providing additional training signals at various stages of the decoding process. The automated segmentations produced by the 3D U-Net were rigorously reviewed by two experienced radiologists. They manually checked each segmentation for accuracy and made refinements where necessary. The details of tumor segmentation are provided in Supplementary material 2.
The Pyradiomics package (Version 3.6) was utilized to extract radiomics features from the tumor regions. The radiomics feature selection was performed according to the following procedures. First, all radiomic features underwent Z-score standardization to ensure uniform scaling [0, 1]. The Pearson correlation coefficient was then calculated to assess the association between highly redundant features. Subsequently, we applied the Least Absolute Shrinkage and Selection Operator to adjust the regularization parameter (λ) through a minimization criterion.
The radiomics models were constructed using the XGBoost and CatBoost (Figure 2). Hyperparameter optimization for the model using the Grid search was conducted after analyzing the outcomes of five-fold cross-validation on the primary training set[19]. After fixing the optimal hyperparameters, the models were finally trained using the training cohort, and evaluated in the validation and test cohorts. We applied the synthetic minority oversampling technique when the datasets were unbalanced[20].
The predictive efficiency was demonstrated qualitatively by receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA), and quantitatively by the area under the curve (AUC), net reclassification index (NRI), and Delong test.
Continuous variables were presented as mean ± SD and analyzed by t-test or Mann-Whitney U test. Fisher’s exact test or χ2 tests were used to analyze the categorical variables. Survival analyses were assessed using the Kaplan-Meier method and log-rank test. Cox proportional-hazards models were used to analyze the potential prognostic variables. A two-sided P value of < 0.05 was considered as the criterion to indicate a statistically significant difference. Statistical analysis was performed using the Python (version 3.11) and SPSS (version 26.0) software.
A total of 826 patients were included in this study, of whom 155 patients had PD HCC. The patient characteristics are summarized in Table 1. The PD HCC group had more patients with cirrhosis than that of nPD HCC group in the validation cohort (P = 0.03). In addition, variables of α-fetoprotein (AFP) < 20 and AFP > 400 were found to be significantly different between PD HCC and nPD HCC patients in all the three cohorts. No significant difference was observed in age, sex, body mass index, hepatitis B virus, hepatitis C virus, alanine aminotransferase, aspartate aminotransferase, total bilirubin, thromboplastin time, albumin, tumor size, and number of tumors (all P > 0.05) between the groups.
| Training cohort (n = 459) | Validation cohort (n = 196) | Test cohort (n = 171) | |||||||
| PD HCC (n = 89) | nPD HCC (n = 370) | P value | PD HCC (n = 30) | nPD HCC (n = 166) | P value | PD HCC (n = 36) | nPD HCC (n = 135) | P value | |
| Age (years) | 55.67 ± 10.56 | 57.24 ± 9.69 | 0.18 | 58.07 ± 9.12 | 58.95 ± 9.96 | 0.65 | 57.58 ± 8.75 | 59.67 ± 10.41 | 0.16 |
| Sex (male), n (%) | 73 (82.02) | 312 (84.32) | 0.71 | 25 (83.33) | 141 (84.94) | 1.00 | 27 (75.00) | 112 (82.96) | 0.15 |
| BMI (kg/m2) | 25.39 ± 3.39 | 25.14 ± 3.58 | 0.60 | 24.69 ± 3.68 | 24.95 ± 3.28 | 0.61 | 24.55 ± 3.23 | 25.07 ± 3.08 | 0.40 |
| HBV, n (%) | 61 (68.54) | 253 (68.38) | 1.00 | 23 (76.67) | 125 (75.30) | 1.00 | 26 (72.22) | 92 (68.15) | 0.79 |
| HCV, n (%) | 6 (6.74) | 23 (6.22) | 1.00 | 0 (0) | 5 (3.01) | 0.74 | 1 (2.78) | 7 (5.19) | 0.87 |
| Cirrhosis, n (%) | 64 (71.91) | 236 (63.78) | 0.19 | 25 (83.33) | 100 (60.24) | 0.03 | 24 (66.67) | 76 (56.30) | 0.35 |
| AFP (ng/mL), n (%) | |||||||||
| < 20 | 21 (23.60) | 215 (58.11) | < 0.001 | 6 (20.00) | 89 (53.61) | 0.001 | 11 (30.56) | 88 (65.19) | < 0.001 |
| 20-400 | 29 (32.58) | 83 (22.43) | 0.06 | 11 (36.67) | 49 (29.52) | 0.57 | 12 (33.33) | 27 (20.00) | 0.14 |
| > 400 | 39 (43.82) | 72 (19.46) | < 0.001 | 13 (43.33) | 28 (16.87) | 0.002 | 13 (36.11) | 20 (14.81) | 0.01 |
| ALT (U/L) | 39.43 ± 31.08 | 33.74 ± 27.29 | 0.06 | 30.81 ± 12.57 | 33.48 ± 26.51 | 0.47 | 37.92 ± 34.03 | 31.51 ± 25.20 | 0.17 |
| AST (U/L) | 32.54 ± 23.91 | 30.03 ± 22.88 | 0.13 | 29.26 ± 9.25 | 28.70 ± 18.83 | 0.09 | 32.58 ± 22.14 | 27.73 ± 14.45 | 0.11 |
| TB (umol/L) | 18.91 ± 27.90 | 13.55 ± 5.85 | 0.31 | 14.38 ± 5.32 | 13.78 ± 6.48 | 0.45 | 13.76 ± 9.14 | 12.16 ± 5.22 | 0.35 |
| Albumin (g/L) | 41.65 ± 4.19 | 41.53 ± 3.95 | 0.79 | 41.64 ± 4.63 | 41.14 ± 3.90 | 0.53 | 40.58 ± 4.12 | 41.78 ± 3.86 | 0.08 |
| PT (S) | 13.45 ± 0.97 | 13.51 ± 1.07 | 0.56 | 13.63 ± 0.85 | 13.39 ± 1.01 | 0.34 | 13.56 ± 1.14 | 13.60 ± 0.84 | 0.77 |
| Tumors size (mm) | 47.57 ± 22.08 | 47.91 ± 21.1 | 0.73 | 48.10 ± 25.78 | 49.83 ± 23.06 | 0.52 | 45.64 ± 19.51 | 50.90 ± 21.53 | 0.19 |
| Number of tumors, n (%) | |||||||||
| Solitary | 80 (89.89) | 330 (89.19) | 1.00 | 26 (86.67) | 153 (92.17) | 0.53 | 36 (100.00) | 126 (93.33) | 0.24 |
| Multiple | 9 (10.11) | 40 (10.81) | 4 (13.33) | 13 (7.83) | 0 (0) | 9 (6.67) | |||
A total of 3045 features were extracted from the three sequences (T2WI, DWI, PVP) in both NR and SR MRI. Among the extracted features, 13, 8, 23, and 29 features were retained on T2WI, DWI, PVP, and on All-sequence (T2WI + DWI + PVP) in NR MRI, respectively. Regarding SR MRI, 14, 6, 19, and 28 features were retained on T2WI, DWI, PVP, and on all-sequence, respectively, for further analyses. These selected features are provided in Supplementary Figure 1.
Among the three single-sequence radiomics models, the PVP model showed better results, achieving the highest AUC of 0.73 (95%CI: 0.61-0.84) and the highest accuracy of 0.80 on the validation cohort (Table 2). Even in the test cohort, the PVP model maintained an AUC of 0.71 (95%CI: 0.62-0.81).
| Models | Cohorts | Original NR MRI | Deep learning-based SR MRI | ||||||
| AUC (95%CI) | Accuracy | Sensitivity | Specificity | AUC (95%CI) | Accuracy | Sensitivity | Specificity | ||
| T2WI | Training | 0.782 (0.732-0.832) | 0.741 | 0.708 | 0.749 | 0.813 (0.765-0.861) | 0.717 | 0.854 | 0.684 |
| Validation | 0.721 (0.613-0.828) | 0.745 | 0.600 | 0.771 | 0.738 (0.636-0.840) | 0.755 | 0.633 | 0.777 | |
| Test | 0.685 (0.585-0.785) | 0.637 | 0.722 | 0.615 | 0.721 (0.620-0.820) | 0.637 | 0.833 | 0.585 | |
| DWI | Training | 0.785 (0.732-0.834) | 0.678 | 0.742 | 0.662 | 0.770 (0.716-0.825) | 0.715 | 0.708 | 0.716 |
| Validation | 0.697 (0.595-0.800) | 0.653 | 0.733 | 0.639 | 0.721 (0.614-0.827) | 0.801 | 0.500 | 0.855 | |
| Test | 0.695 (0.595-0.795) | 0.550 | 0.861 | 0.467 | 0.694 (0.586-0.802) | 0.696 | 0.639 | 0.711 | |
| PVP | Training | 0.816 (0.765-0.866) | 0.778 | 0.685 | 0.800 | 0.834 (0.791-0.877) | 0.741 | 0.764 | 0.735 |
| Validation | 0.727 (0.610-0.844) | 0.801 | 0.567 | 0.843 | 0.762 (0.664-0.859) | 0.816 | 0.567 | 0.861 | |
| Test | 0.713 (0.620-0.805) | 0.678 | 0.611 | 0.696 | 0.752 (0.659-0.845) | 0.743 | 0.667 | 0.763 | |
| All-sequences1 | Training | 0.890 (0.854-0.925) | 0.793 | 0.876 | 0.773 | 0.884 (0.847-0.920) | 0.815 | 0.809 | 0.816 |
| Validation | 0.792 (0.700-0.883) | 0.842 | 0.533 | 0.898 | 0.832 (0.748-0.915) | 0.735 | 0.800 | 0.723 | |
| Test | 0.779 (0.695-0.862) | 0.667 | 0.778 | 0.637 | 0.798 (0.720-0.875) | 0.766 | 0.695 | 0.785 | |
In addition, multi-sequence MRI radiomics models showed better performance than single-sequence radiomics models (Table 2). The all-sequence model had superior AUCs for the training (0.89, 95%CI: 0.85-0.93), validation (0.79, 95%CI: 0.70-0.88), and test (0.78, 95%CI: 0.70-0.86) cohorts. The ROC of different models are shown in Supplementary Figure 2.
As for deep learning-based SR MRI, the PVP model also had better preoperative prediction of HCC tumor differentiation in the validation and test cohorts, with AUCs of 0.76 (95%CI: 0.66-0.86) and 0.75 (95%CI: 0.66-0.85).
Similarly, the all-sequence model had the superior performance in predicting the grade of tumor differentiation in HCC, with AUCs of 0.80-0.88 (Table 2). In the validation cohort, the all-sequence model showed an accuracy, sensitivity, and specificity of 74%, 80%, and 72%, respectively. In the test cohort, the accuracy, sensitivity, and specificity were 77%, 70%, and 79%, respectively. The ROC curves of these models are demonstrated in Supplementary Figure 2.
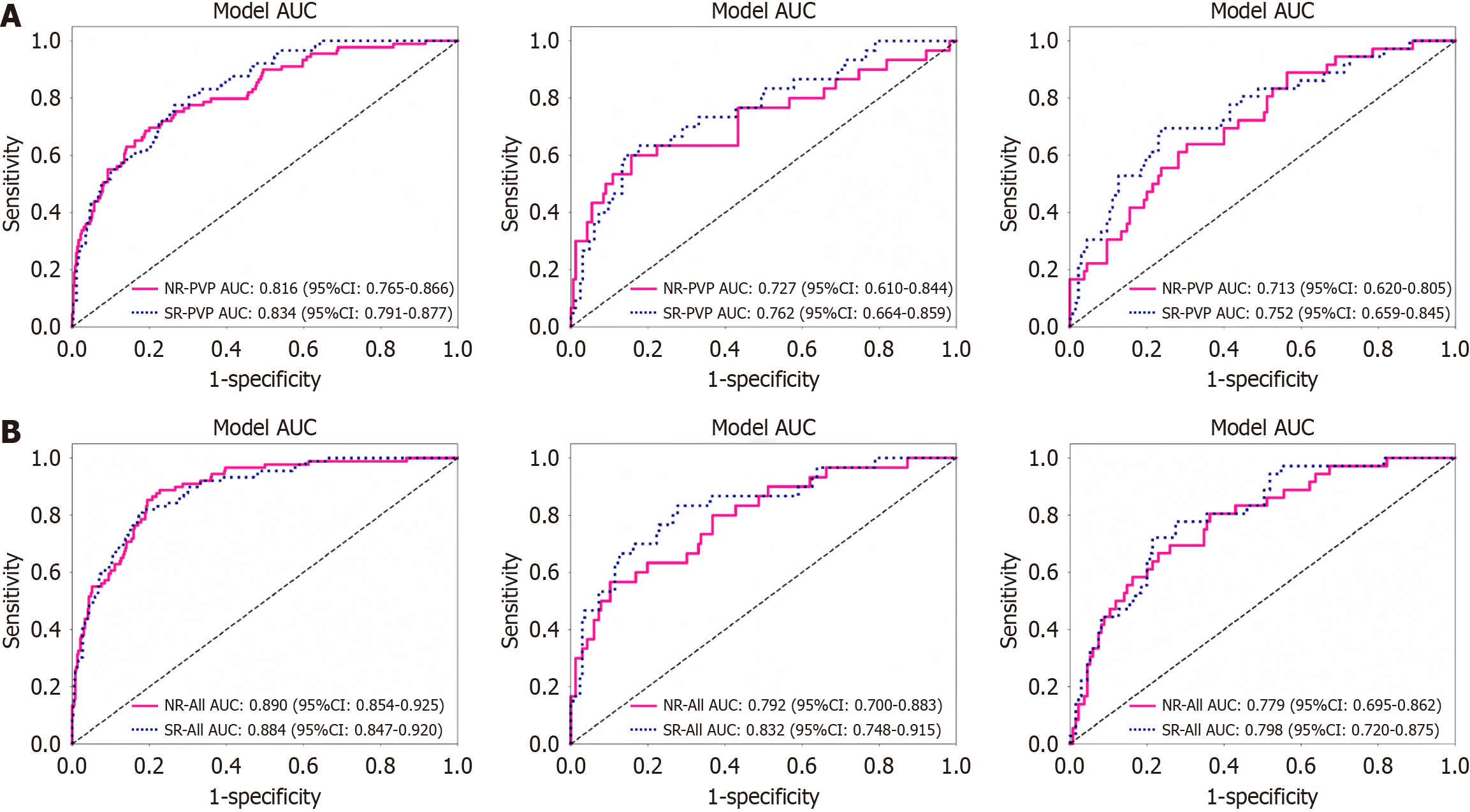
Figure 3 shows the ROCs based on NR and SR MRI. Regarding the PVP models, the AUCs were 0.82, 0.73, and 0.71 in the three cohorts based on NR MRI, and the AUCs of the model based on SR MRI were 0.83, 0.76, and 0.75, respectively. Meanwhile, the All-sequence model based on SR MRI showed an enhanced performance (AUCs of 0.88, 0.83, and 0.80) than that based on NR MRI (AUCs of 0.89, 0.79, and 0.78) in the three cohorts, respectively.
The calibration curves suggested a slightly better agreement in the all-sequence model based on SR MRI compared with that of NR MRI. The DCA of these models also showed that the SR MRI model had a slightly higher net benefit than the NR MRI models in distinguishing between PD and nPD HCC over most of the threshold probability ranges. The NRIs indicated that the SR MRI models could improve the prediction accuracy by -1.6% to 14% compared to the NR MRI models. However, the Delong test indicated that this difference did not reach a statistical significance (P > 0.05; Supplementary Table 2).
To further verify the consistency of SR’s benefits, we supplemented analyses with Catboost machine learning algorithm (Supplementary Figure 3). Across all models, SR consistently improved the predictive performance: AUC increased from 0.80 (NR) to 0.89 (SR), NRI = 20.9% in the validation cohort, and AUC increased from 0.76 (NR) to 0.81 (SR), NRI = 8% in the test cohort. The DCA showed that the SR model consistently yielded higher net benefit across a range of threshold probabilities compared to the NR model based on XGBoost (0.1 to 0.7) and Catboost (0.2 to 0.48) machine learning algorithms. This indicated that integrating SR reconstruction improved the model’s clinical utility in guiding decision-making. The calibration curve for the NR and SR models are close to the ideal reference line, confirming that SR-enhanced predictions are clinically trustworthy in their probability estimates.
In addition, we supplemented subgroup analyses stratified by both field strength (1.5 T) and key scanner models (Supplementary Figure 4). Among 1.5 T scanners, United imaging 560 and 570 were the most frequently used in our cohort (n = 449). Focusing on these two models, we observed consistent trends: SR reconstruction showed numerical improvements in radiomic model performance compared to NR MRI, with AUCs increasing from 0.792 to 0.852 (XGBoost) and from 0.807 to 0.878 (Catboost) for predicting tumor differentiation. These results reinforced that SR maintains relative stability in enhancing image utility for radiomic analysis. For other scanner models, due to smaller sample sizes, we did not perform separate subgroup analyses.
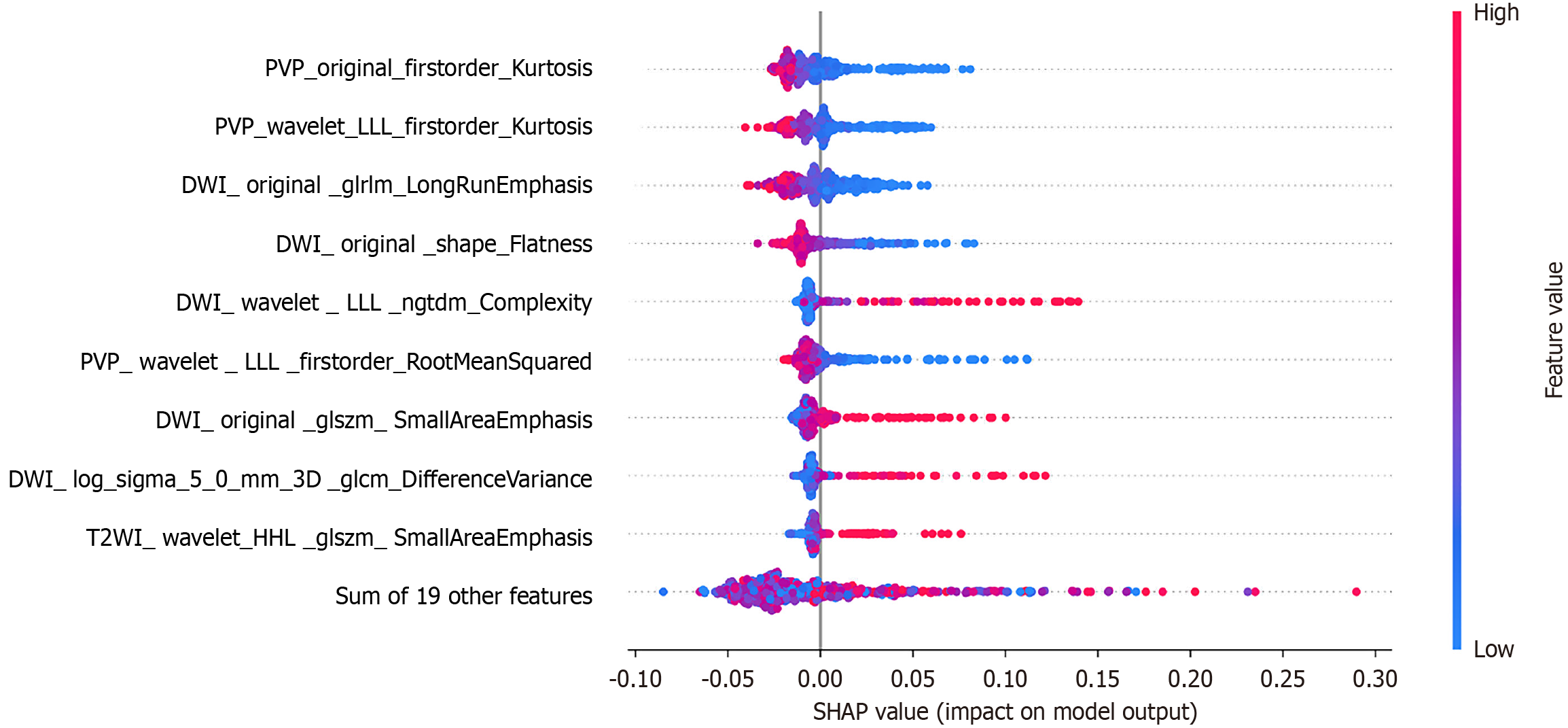
SHapley Additive exPlanations (SHAP) connects the feature’s values with their impact on the model predictions and ranks these features according to their global importance. Instead of investigating all the selected features between NR and SR MRI, concentrating on their differential features seems more insightful (Table 3). We found that six out of the 12 differential features selected after SR reconstruction were among the most prominent features, whereas only four out of the 13 differential features from conventional MRI were included in this top list (Figure 4 and Table 3).
| Original NR MRI | Deep learning-based SR MRI | |
| 1 | DWI_original_glrlm_RunVariance | DWI_original_glrlm_LongRunEmphasis |
| 2 | DWI_original_glszm_ZonePercentage | DWI_original_glszm_SmallAreaEmphasis |
| 3 | DWI_original_shape_Elongation | DWI_wavelet_HLL_glcm_ClusterShade |
| 4 | DWI_wavelet_LHH_firstorder_Skewness | DWI_wavelet_LLL_ngtdm_Complexity |
| 5 | DWI_wavelet_LHL_firstorder_RobustMeanAbsoluteDeviation | DWI_log_sigma_5_0_mm_3D_glcm_DifferenceVariance |
| 6 | DWI_wavelet_LLL_glszm_GrayLevelVariance | PVP_original_firstorder_Kurtosis |
| 7 | PVP_log_sigma_5_0_mm_3D_glrlm_ShortRunEmphasis | PVP_wavelet_HLL_firstorder_Kurtosis |
| 8 | PVP_wavelet_LHH_glcm_ClusterShade | PVP_wavelet_LHL_firstorder_Median |
| 9 | PVP_wavelet_LHH_glcm_Correlation | PVP_wavelet_LLL_glszm_ZonePercentage |
| 10 | PVP_wavelet_LHL_firstorder_Median | T2WI_wavelet_HHL_glszm_SmallAreaEmphasis |
| 11 | T2WI_wavelet_HHL_firstorder_Kurtosis | T2WI_wavelet_HLH_firstorder_Median |
| 12 | T2WI_wavelet_HHL_firstorder_Median | T2WI_wavelet_HLH_glszm_SmallAreaEmphasis |
| 13 | T2WI_wavelet_LLL_firstorder_RootMeanSquared |
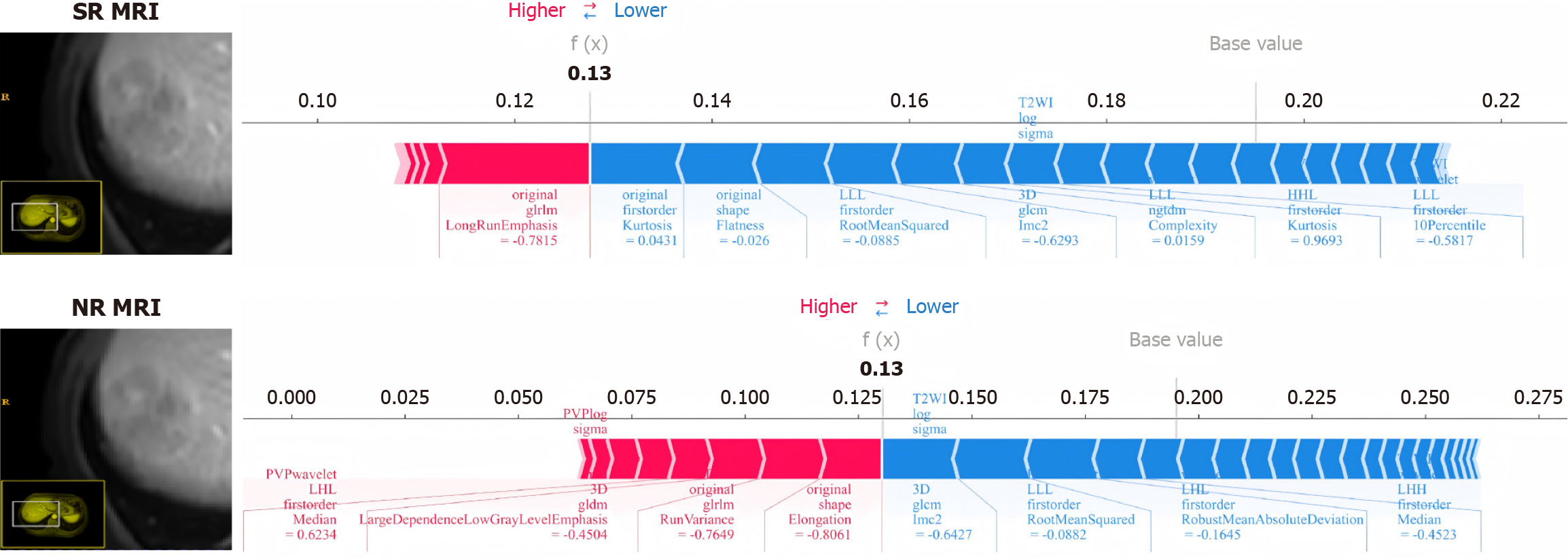
Moreover, force plots were utilized to interpret the evaluations for individual patient. The length and color of the arrows represent the contribution of specific features to the SHAP values. As shown in Figure 5, the patient’s SHAP value was 0.13, which was below the baseline, suggesting that this patient could be classified into the nPD group. Specifically, in the force plot of SR MRI, eight important features evaluated the differentiation degree as nPD, while only one important feature assessed it as PD. The force plot derived from NR MRI indicated that four important features evaluate the differentiation degree as nPD, while four important features assess it as PD. Despite the final assessment being nPD, the model’s prediction showed less satisfactory consistency. Due to space constraints, we could not detail and introduce the contrast force plots for each patient, but we did find better consistency in the SR MRI model.
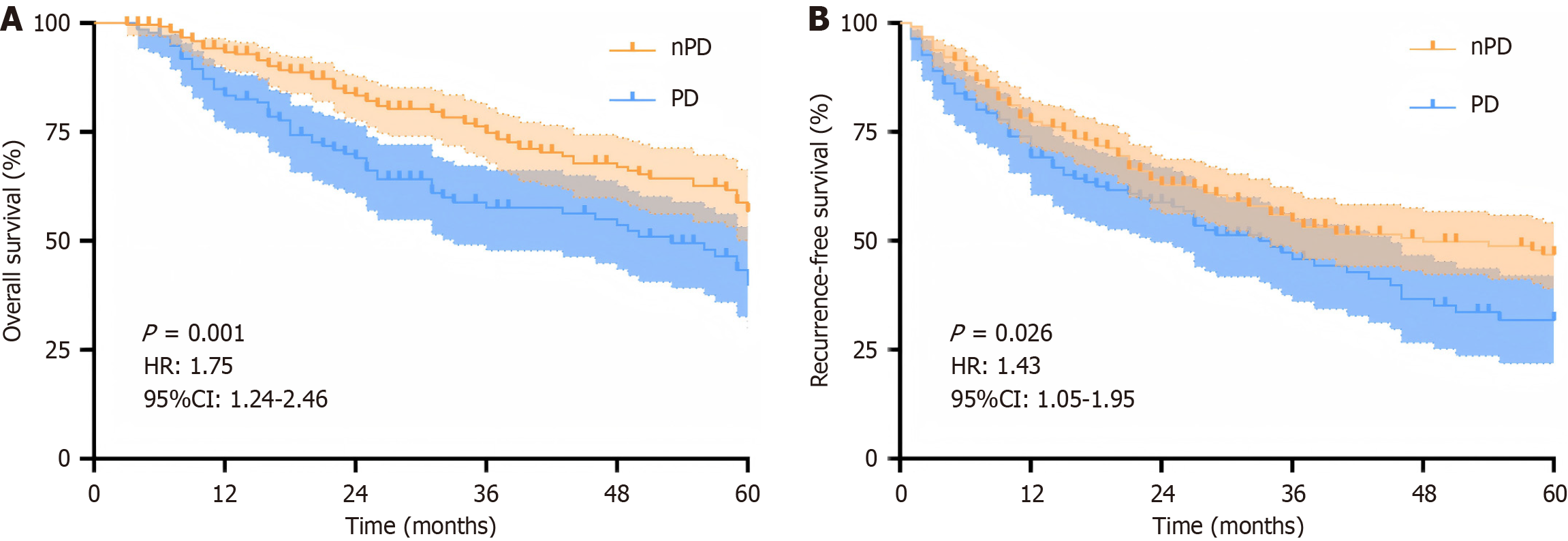
To investigate the clinical utility of the signature from the SR MRI model in prognostic stratification, we divided the patients (n = 395) from the primary cohort into two different risk groups based on thresholds. As shown in Figure 6, the overall survival and recurrence-free survival in these patients could be significantly stratified by the signature (P < 0.05). In addition, the results of multivariable Cox regressions indicated that the signature from SR MRI were identified as risk predictors of poor overall survival (HR: 1.81, 95%CI: 1.29-2.55, P < 0.001) and recurrence-free survival (HR: 1.36, 95%CI: 1.02-1.85, P = 0.042; Table 4).
| Variable | Overall survival | Recurrence-free survival | ||||||
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| Signature from SR MRI | 1.67 (1.21, 2.30) | 0.002 | 1.81 (1.29, 2.55) | 0.001 | 1.39 (1.04, 1.88) | 0.028 | 1.36 (1.02, 1.85) | 0.042 |
| Age (> 65 years) | 1.20 (0.80, 1.79) | 0.381 | 1.12 (0.79, 1.59) | 0.533 | ||||
| Gender (male) | 1.08 (0.70, 1.67) | 0.723 | 1.13 (0.75, 1.71) | 0.559 | ||||
| HBV | 0.97 (0.67, 1.39) | 0.855 | 0.93 (0.67, 1.29) | 0.677 | ||||
| Cirrhosis | 1.30 (0.94, 1.80) | 0.118 | 1.15 (0.86, 1.54) | 0.341 | ||||
| MVI | 1.78 (1.28, 2.47) | < 0.001 | 1.48 (1.04, 2.09) | 0.029 | 1.80 (1.34, 2.42) | 0.001 | 1.67 (1.23, 2.28) | 0.001 |
| INR (> 1.5 ratio) | 2.31 (0.32, 16.64) | 0.405 | 2.61 (0.64, 10.54) | 0.179 | ||||
| AFP | 1.48 (1.02, 2.14) | 0.04 | 1.53 (1.06, 2.19) | 0.219 | 1.44 (1.03, 2.02) | 0.031 | ||
| Multiple | 1.07 (0.67, 1.72) | 0.766 | 1.59 (1.07, 2.37) | 0.021 | 1.53 (1.03, 2.28) | 0.036 | ||
| Pseudocapsule | 0.74 (0.52, 1.04) | 0.083 | 0.88 (0.65, 1.20) | 0.426 | ||||
| Tumor size | 1.38 (1.01, 1.90) | 0.049 | 1.53 (1.06, 2.19) | 0.022 | 1.38 (1.01, 1.91) | 0.048 | 1.38 (0.96, 1.98) | 0.08 |
Over the past few decades, the mortality rate of HCC has remained high, with low differentiation serving as a predictor of poor prognosis. This study developed and validated radiomics models based on single and multiple sequences. The deep learning-based SR reconstruction further improved the predictive performance for preoperative histopathologic grade in HCC. These multiparametric MRI radiomics models have the potential to non-invasively classify tumor status and may contribute to clinical decision-making.
Currently, besides MRI, various medical imaging approaches have also been applied to preoperatively predict the histopathologic grade of HCC, such as CT, contrast-enhanced ultrasound (CEUS), and positron emission tomography (PET)-MRI[21-23]. According to the clinical practice guideline of HCC in China, CT, MRI and CEUS are the most commonly used approaches[24]. Similar to MRI, CT provides whole-liver coverage and reproducible arterial/portal enhancement patterns; however, it suffers from lower soft-tissue contrast and a higher miss-rate for small HCC lesions. This results in lower diagnostic sensitivity and inferior grading accuracy. Additionally, the cumulative ionizing radiation further restricts its applicability for repeated assessment.
CEUS offers bedside, real-time, and repeated evaluation of HCC; however, it has lower spatial resolution and operator-dependent patterns, leading to reduced sensitivity for small lesions and poor reproducibility in the evaluation of HCC. Furthermore, its inability to provide multiparametric intra-tumor information limits quantitative assessment of HCC differentiation. PET-MRI, by integrating metabolic information from tracers such as 18F-FDG or 11C-choline with high-field MRI, addresses some limitations inherent to purely morphological imaging. Nevertheless, PET-MRI requires on-site cyclotron for radionuclide tracers and has significantly higher ionizing radiation than CT and higher examination costs than MRI. It only serves as a complementary tool in equivocal cases and is not recommended to be regularly used for HCC in clinical practice.
Clarifying the degree of tumor differentiation before surgery is of great significance for the treatment and prognostic assessment of HCC[10,11,25]. Mao et al[26] developed a predictive model of radiomic features based on the arterial phase and hepatobiliary phase images in 122 patients with HCC, and the results showed that the logistic regression-based model [AUC: 0.792 (0.681–0.904)] had favorable correction and classification accuracy for predicting the degree of differentiation of HCC. In addition, Liu et al[7] reported a radiomic model with an AUC of 0.82 for distinguishing PD HCC based on the arterial, portal venous, and delayed phases in 265 patients with HCC, which further highlighted the potential of the radiomic model based on multiparametric MRI[7]. Similar to previous studies, this study demonstrated that the multiparametric radiomics model exhibited a high discriminatory power when predicting the grade of tumor differentiation in HCC[7,26-29].
The image quality is crucial for extracting radiomics features and constructing the radiomics model[30-32]. In this study, we transformed these original NR MRI into SR images based on the deep learning model utilizing GAN as the basic architecture. Subsequently, we developed and validated different radiomics models to investigate the feasibility and efficacy of deep learning-based SR MRI for the preoperative prediction of tumor differentiation in HCC. The results indicated that the radiomics models based on SR MRI had higher AUC and accuracy than those of the original NR MRI in most single-sequence. In addition, the multi-sequence model combined with SR reconstruction further demonstrated this high and stable predictive performance in the validation and test cohorts. In addition, 1.5 T MRI, due to its lower magnetic field strength, typically has characteristics such as relatively low signal-to-noise ratio, limited spatial resolution, and insufficiently clear tissue contrast in its original images, all of which are common manifestations of "low quality" in clinical practice. Theoretically, this provides a greater room for improvement for the SR technology. The results showed that in the 1.5T subgroup, the SR technology did lead to a certain improvement in the AUC value (0.825 vs 0.792; 0.878 vs 0.807).
The advantage of SR reconstruction is its ability to eliminate blurriness and largely enhance the image details[31]. We summarized several features from SR MRI that are closely related to the microstructure and heterogeneity of the tumor, which may be valuable in predicting the degree of differentiation of HCC. To begin with, the SR model included three new SmallAreaEmphasis features that may be more sensitive to small heterogeneous areas within the tumor that are associated with aggressive margins or microvascular changes, which are important indicators of tumor differentiation. Complexity can well quantify the inhomogeneity of signal intensity within a tumor, and this inhomogeneity is also an important indicator of tumor heterogeneity, which is closely related to the aggressiveness and differentiation of the tumor. We speculated that the SR reconstruction technique enhanced the identification of tumor cell density, alignment, and tumor microenvironment, which could provide a more detailed texture and accurately reflect tumor heterogeneity and microenvironmental characteristics[33,34].
In addition to increased AUCs, the NRIs indicated that compared with NR MRI, the radiomics models by SR MRI showed superior performance in most cases. However, there was no significant difference in the Delong test, and the DCA and calibration curves also demonstrated a slight improvement. One possible explanation for this is that problems such as the image being of low quality itself, having low contrast, or containing noise may affect the accuracy of SR reconstruction in improving the specific information of tumor regions. Six MRI scanners were included in this study, which can show the generalizability of the model. The choice of parameters during MRI acquisition, such as slice thickness scan time, resolution, etc., may affect the quality of original images, resulting in SR reconstructions still not being informative enough to significantly improve the performance of radiomics models in this study. Moreover, the multiple of SR reconstruction significantly reflects the ability of algorithms to convert original images into higher-resolution ones. The upscaling factor for SR reconstruction was 2x in this study. A higher reconstruction magnification means that the resulting image has a higher spatial resolution and may contain more detailed information, which may help improve the predictive accuracy of radiomics models.
The SR reconstruction helps in accurately assessing the shape, size, borders, and internal structure of tumors. With the development of deep learning, we believe the SR reconstruction has a broader prospect in the field of medical image processing[35-37].
There were several limitations in this study. First, although this study included patients from two medical centers, given the small sample size in center B, further large-scale, multi-center studies are needed to validate these results. Second, PD HCC has a high aggressiveness; therefore, some of these patients were treated with anti-tumor therapy before surgery. These patients were excluded to ensure that the tumor region in preoperative MRI was consistent with postoperative pathologic tissue; however, this may have led to selection bias. Finally, multi-omics research, including clinical characterization, genomics, and proteomics, may yield better indicators; however, the focus of this study was to investigate the efficacy and feasibility of SR reconstruction in HCC.
In conclusion, this study developed and validated classification models for HCC differentiation grade using multiparametric MRI, showing commendable predictive accuracy and stability. Notably, deep learning-based 3D SR reconstruction could further improve this predictive efficacy. As technology advances, we anticipate that 3D SR reconstruction would enhance the predictive precision for HCC preoperative tumor status and provide deeper insights for precision medicine and disease management.
| 1. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1498] [Article Influence: 299.6] [Reference Citation Analysis (2)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68549] [Article Influence: 13709.8] [Reference Citation Analysis (201)] |
| 3. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4359] [Article Influence: 544.9] [Reference Citation Analysis (6)] |
| 4. | Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 5. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 437] [Article Influence: 62.4] [Reference Citation Analysis (1)] |
| 6. | Mo ZY, Chen PY, Lin J, Liao JY. Pre-operative MRI features predict early post-operative recurrence of hepatocellular carcinoma with different degrees of pathological differentiation. Radiol Med. 2023;128:261-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Liu HF, Wang M, Wang Q, Lu Y, Lu YJ, Sheng Y, Xing F, Zhang JL, Yu SN, Xing W. Multiparametric MRI-based intratumoral and peritumoral radiomics for predicting the pathological differentiation of hepatocellular carcinoma. Insights Imaging. 2024;15:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Moazzam Z, Alaimo L, Endo Y, Lima HA, Woldesenbet S, Rueda BO, Yang J, Ratti F, Marques HP, Cauchy F, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Guglielmi A, Hugh T, Aldrighetti L, Shen F, Endo I, Pawlik TM. A Prognostic Model To Predict Survival After Recurrence Among Patients With Recurrent Hepatocellular Carcinoma. Ann Surg. 2024;279:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Tran BV, Moris D, Markovic D, Zaribafzadeh H, Henao R, Lai Q, Florman SS, Tabrizian P, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, Taner CB, Hoteit M, Levine MH, Cillo U, Vitale A, Verna EC, Halazun KJ, Tevar AD, Humar A, Chapman WC, Vachharajani N, Aucejo F, Lerut J, Ciccarelli O, Nguyen MH, Melcher ML, Viveiros A, Schaefer B, Hoppe-Lotichius M, Mittler J, Nydam TL, Markmann JF, Rossi M, Mobley C, Ghobrial M, Langnas AN, Carney CA, Berumen J, Schnickel GT, Sudan DL, Hong JC, Rana A, Jones CM, Fishbein TM, Busuttil RW, Barbas AS, Agopian VG. Development and validation of a REcurrent Liver cAncer Prediction ScorE (RELAPSE) following liver transplantation in patients with hepatocellular carcinoma: Analysis of the US Multicenter HCC Transplant Consortium. Liver Transpl. 2023;29:683-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Alliance of Chinese Expert Consensus on Neoadjuvant Therapy for Hepatocellular Carcinoma; Committee of Digestive Surgery of Chinese Research Hospital Association; Committee of Liver Cancer, Chinese Anti-Cancer Association. [Chinese expert consensus on neoadjuvant therapy for hepatocellular carcinoma (2023 edition)]. Zhonghua Wai Ke Za Zhi. 2023;61:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Pinyol R, Yarchoan M, Singal AG, Marron TU, Schwartz M, Pikarsky E, Kudo M, Finn RS. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol. 2024;21:294-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 215] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 12. | Xu XF, Wu H, Li JD, Yao LQ, Huang B, Diao YK, Chen TH, Gu WM, Chen Z, Li J, Zhang YM, Wang H, Liang YJ, Zhou YH, Li C, Wang MD, Zhang CW, Pawlik TM, Lau WY, Shen F, Yang T. Association of tumor morphology with long-term prognosis after liver resection for patients with a solitary huge hepatocellular carcinoma-a multicenter propensity score matching analysis. Hepatobiliary Surg Nutr. 2023;12:314-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Zhang XP, Xu S, Lin ZY, Gao QL, Wang K, Chen ZL, Yan ML, Zhang F, Tang YF, Zhao ZM, Li CG, Lau WY, Cheng SQ, Hu MG, Liu R. Significance of anatomical resection and resection margin status in patients with HBV-related hepatocellular carcinoma and microvascular invasion: a multicenter propensity score-matched study. Int J Surg. 2023;109:679-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Klintström E, Klintström B, Ferguson SJ, Helgason B, Persson C. A convolutional neural network-based method for the generation of super-resolution 3D models from clinical CT images. Comput Methods Programs Biomed. 2024;245:108009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Kim DK, Lee SY, Lee J, Huh YJ, Lee S, Lee S, Jung JY, Lee HS, Benkert T, Park SH. Deep learning-based k-space-to-image reconstruction and super resolution for diffusion-weighted imaging in whole-spine MRI. Magn Reson Imaging. 2024;105:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Nagayama Y, Emoto T, Kato Y, Kidoh M, Oda S, Sakabe D, Funama Y, Nakaura T, Hayashi H, Takada S, Uchimura R, Hatemura M, Tsujita K, Hirai T. Improving image quality with super-resolution deep-learning-based reconstruction in coronary CT angiography. Eur Radiol. 2023;33:8488-8500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Sood RR, Shao W, Kunder C, Teslovich NC, Wang JB, Soerensen SJC, Madhuripan N, Jawahar A, Brooks JD, Ghanouni P, Fan RE, Sonn GA, Rusu M. 3D Registration of pre-surgical prostate MRI and histopathology images via super-resolution volume reconstruction. Med Image Anal. 2021;69:101957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Hou M, Zhou L, Sun J. Deep-learning-based 3D super-resolution MRI radiomics model: superior predictive performance in preoperative T-staging of rectal cancer. Eur Radiol. 2023;33:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 19. | Wang W, Liang H, Zhang Z, Xu C, Wei D, Li W, Qian Y, Zhang L, Liu J, Lei D. Comparing three-dimensional and two-dimensional deep-learning, radiomics, and fusion models for predicting occult lymph node metastasis in laryngeal squamous cell carcinoma based on CT imaging: a multicentre, retrospective, diagnostic study. EClinicalMedicine. 2024;67:102385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 20. | Nakamura M, Kajiwara Y, Otsuka A, Kimura H. LVQ-SMOTE - Learning Vector Quantization based Synthetic Minority Over-sampling Technique for biomedical data. BioData Min. 2013;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Sokmen BK, Inan N. 18 F-FDG PET/MRI of Primary Hepatic Malignancies: Differential Diagnosis and Histologic Grading. Curr Med Imaging. 2024;20:e080523216636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Zhang R, Li D, Chen Y, Xu W, Zhou W, Lin M, Xie X, Xu M. Development and Comparison of Prediction Models Based on Sonovue- and Sonazoid-Enhanced Ultrasound for Pathologic Grade and Microvascular Invasion in Hepatocellular Carcinoma. Ultrasound Med Biol. 2024;50:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Wu M, Yu H, Pang S, Liu A, Liu J. Application of CT-based radiomics combined with laboratory tests such as AFP and PIVKA-II in preoperative prediction of pathologic grade of hepatocellular carcinoma. BMC Med Imaging. 2025;25:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Su H, Wei Y, Liao X, Zhu G, Peng M, Fan F, Peng T. Interpretation of the updates of the chinese guidelines for the diagnosis and treatment of primary liver cancer (CNLC-2024 Edition). Hepatoma Res. 2024;10:30. [DOI] [Full Text] |
| 25. | Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 26. | Mao Y, Wang J, Zhu Y, Chen J, Mao L, Kong W, Qiu Y, Wu X, Guan Y, He J. Gd-EOB-DTPA-enhanced MRI radiomic features for predicting histological grade of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Han YE, Cho Y, Kim MJ, Park BJ, Sung DJ, Han NY, Sim KC, Park YS, Park BN. Hepatocellular carcinoma pathologic grade prediction using radiomics and machine learning models of gadoxetic acid-enhanced MRI: a two-center study. Abdom Radiol (NY). 2023;48:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Tomino T, Itoh S, Fujita N, Okamoto D, Nakayama Y, Toshida K, Tomiyama T, Tsutsui Y, Kosai Y, Kurihara T, Nagao Y, Morita K, Harada N, Ushijima Y, Kohashi K, Ishigami K, Oda Y, Yoshizumi T. Clinical association between intraoperative indocyanine green fluorescence imaging pattern, preoperative Gd-EOB-DTPA-enhanced magnetic resonance imaging findings, and histological differentiation in hepatocellular carcinoma. Hepatol Res. 2023;53:723-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Weng S, Xu X, Li Y, Yan C, Chen J, Ye R, Zhu Y, Wen L, Hong J. Quantitative analysis of multiphase magnetic resonance images may assist prediction of histopathological grade of small hepatocellular carcinoma. Ann Transl Med. 2020;8:1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Beirinckx Q, Jeurissen B, Nicastro M, Poot DHJ, Verhoye M, Dekker AJD, Sijbers J. Model-based super-resolution reconstruction with joint motion estimation for improved quantitative MRI parameter mapping. Comput Med Imaging Graph. 2022;100:102071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Kang L, Tang B, Huang J, Li J. 3D-MRI super-resolution reconstruction using multi-modality based on multi-resolution CNN. Comput Methods Programs Biomed. 2024;248:108110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 32. | Wang L, Guo T, Wang L, Yang W, Wang J, Nie J, Cui J, Jiang P, Li J, Zhang H. Improving radiomic modeling for the identification of symptomatic carotid atherosclerotic plaques using deep learning-based 3D super-resolution CT angiography. Heliyon. 2024;10:e29331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Altmann S, Grauhan NF, Mercado MAA, Steinmetz S, Kronfeld A, Paul R, Benkert T, Uphaus T, Groppa S, Winter Y, Brockmann MA, Othman AE. Deep Learning Accelerated Brain Diffusion-Weighted MRI with Super Resolution Processing. Acad Radiol. 2024;31:4171-4182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Lyu J, Li G, Wang C, Cai Q, Dou Q, Zhang D, Qin J. Multicontrast MRI Super-Resolution via Transformer-Empowered Multiscale Contextual Matching and Aggregation. IEEE Trans Neural Netw Learn Syst. 2024;35:12004-12014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Arora V, Ng EY, Leekha RS, Darshan M, Singh A. Transfer learning-based approach for detecting COVID-19 ailment in lung CT scan. Comput Biol Med. 2021;135:104575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Chen Z, Niu C, Gao Q, Wang G, Shan H. LIT-Former: Linking In-Plane and Through-Plane Transformers for Simultaneous CT Image Denoising and Deblurring. IEEE Trans Med Imaging. 2024;43:1880-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Marin Z, Graff M, Barentine AES, Soeller C, Chung KKH, Fuentes LA, Baddeley D. PYMEVisualize: an open-source tool for exploring 3D super-resolution data. Nat Methods. 2021;18:582-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/