Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.108977
Revised: July 1, 2025
Accepted: July 24, 2025
Published online: August 21, 2025
Processing time: 113 Days and 18.8 Hours
Pepsinogen (PG) and the PG I/II ratio (PGR) are critical indicators for diagnosing Helicobacter pylori infection and chronic atrophic gastritis, and assessing gastric cancer risk. Existing reference intervals (RIs) often overlook age, sex, and demographic variations. Partitioned RIs, while considering these factors, fail to capture the gradual age-related physiological changes. Next-generation RIs offer a solution to this limitation.
To investigate age- and sex-specific dynamics of PG and establish next-generation RIs for adults and the elderly in northern China.
After screening, 708 healthy individuals were included in this observational study. Serum PG was measured using chemiluminescence immunoassay. Age- and sex-related effects on PG were analyzed with a two-way analysis of variance. RI partitioning was determined by the standard deviation ratio (SDR). Traditional RIs were established using a non-parametric approach. Generalized Additive Models for Location, Scale, and Shape (GAMLSS) modeled age-related trends and continuous reference percentiles for PG I and PG II. Reference limit flagging rates for both RI types were compared.
PG I and PG II levels were influenced by age (P < 0.001) and sex (P < 0.001), while PGR remained stable. Age-specific RIs were required for PG I (SDR = 0.366) and PG II (SDR = 0.424). Partitioned RIs were established for PG I and PG II, with a single RI for PGR. GAMLSS modeling revealed distinct age-dependent trajectories: PG I increased from a median of 39.75 μg/L at age 20 years to 49.75 μg/L at age 60 years, a 25.16% increase, after which it plateaued through age 80 years. In contrast, PG II showed a continuous rise throughout the age range, with the median value increasing from 5.07 μg/L at age 20 years to 8.36 μg/L at age 80 years, corresponding to a 64.89% increase. Continuous reference percentiles intuitively reflected these trends and were detailed in this study. Next-generation RIs demonstrated superior accuracy compared to partitioned RIs when applied to specific age subgroups.
This study elucidates the age- and sex-specific dynamics of PG and, to our knowledge, is the first to establish next-generation RIs for PG, supporting more individualized interpretation in laboratory medicine.
Core Tip: This study pioneers the establishment of next-generation reference intervals (RIs) for pepsinogen (PG) in Chinese adults and elderly populations using Generalized Additive Models for Location, Scale, and Shape. We identified age- and sex-specific dynamics: PG I and PG II levels increased with age (PG I plateaued post-60 years; PG II rose continuously), with males exhibiting higher values. Partitioned RIs based on age were established, but next-generation RIs demonstrated superior accuracy. By capturing continuous physiological trends, next-generation RIs could potentially reduce diagnostic misinterpretation.
- Citation: Zhang MM, Zhu D, Zhao HB, Zhao XY. Age- and gender-specific dynamics and next-generation reference intervals for pepsinogen in northern China. World J Gastroenterol 2025; 31(31): 108977
- URL: https://www.wjgnet.com/1007-9327/full/v31/i31/108977.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i31.108977
Pepsinogen (PG), the inactive precursor of pepsin, comprises two primary subtypes: PG I and PG II[1]. These biomarkers reflect the functional and structural integrity of the gastric mucosa and are widely utilized to evaluate gastric acid secretion, diagnose Helicobacter pylori infection and chronic atrophic gastritis, and assess gastric cancer risk[2-6]. The PG I/II ratio (PGR) also serves as a critical diagnostic indicator for related conditions[4,7,8].
Reference intervals (RIs) are important for proper interpretation of laboratory results and disease diagnosis. Inappropriate RIs may delay early disease detection or misinterpret physiological variations as pathological, potentially leading to unnecessary anxiety or waste of medical resources, particularly in the context of tumor markers[9,10].
Studies have suggested that PG levels could be influenced by age, sex, and geography, indicating that these factors should be considered when using PG for health assessments and disease diagnoses[11,12]. However, most clinical laboratories currently rely on single, generalized RIs or clinical decision limits for PG I, PG II, and PGR, raising concerns about their clinical applicability[13]. Partitioned RIs based on these factors can partially address this limitation, but for biomarkers notably influenced by age, they cannot capture gradual physiological changes. Abrupt shifts at RI par
In recent years, the concept of “next-generation RIs”, also known as “continuous RIs”, has emerged alongside advancements in data analytics and computational modeling. These RIs rely on advanced statistical techniques that generate continuous percentile curves to replace static reference limits[15-19]. Unlike traditional RIs, next-generation RIs continuously reflect age-related physiological changes in biomarkers, thereby reducing the risk of false positives and negatives and potentially enhancing clinical decision-making[16,20].
This study aimed to analyze age- and sex-specific dynamics and innovatively establish next-generation RIs for PG in Chinese adult and elderly populations.
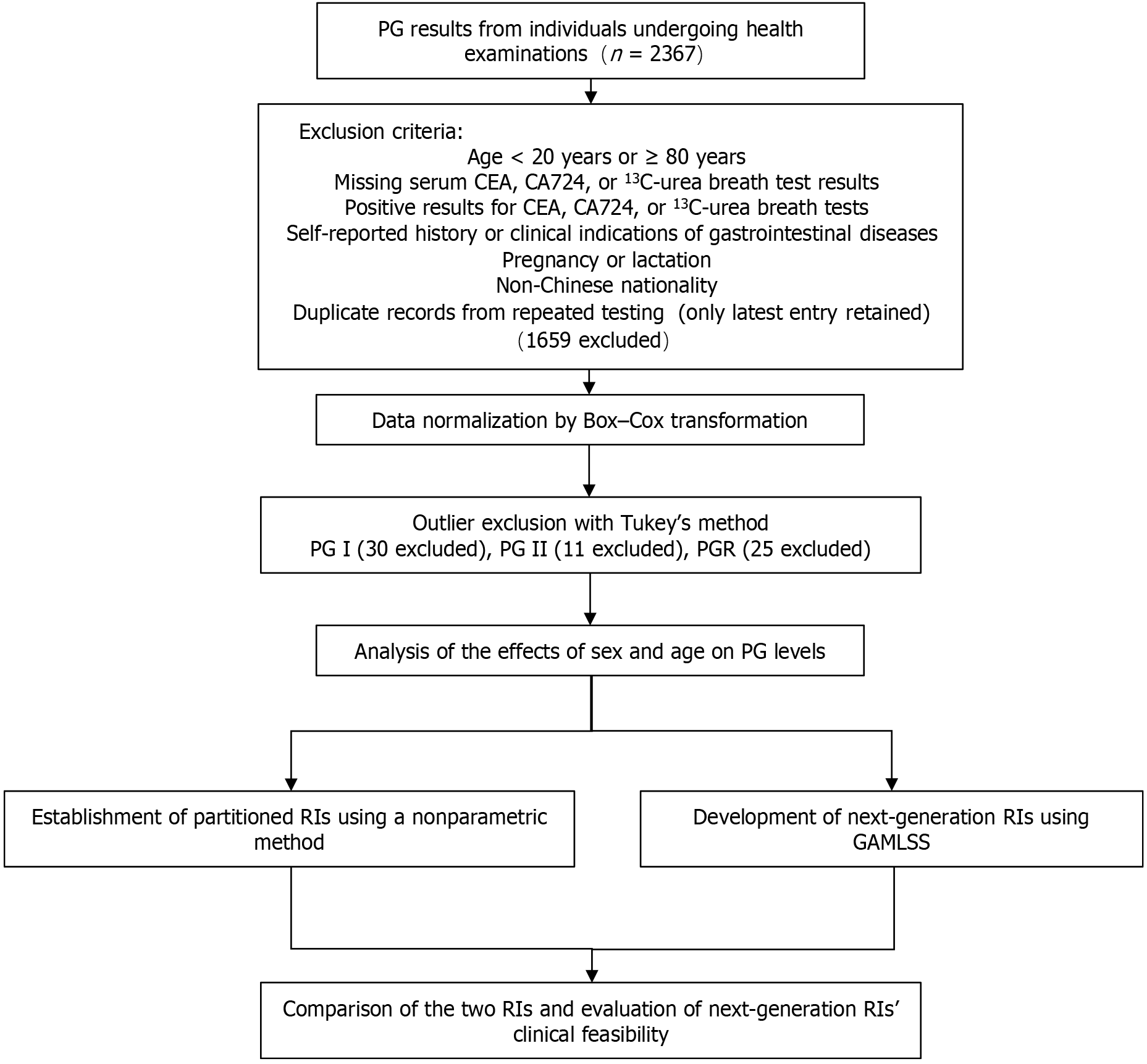
A total of 2367 individuals who underwent routine health examinations and were tested for PG I and PG II at Beijing Tsinghua Changgung Hospital (February 2023 to July 2024) were initially included retrospectively. The setting of Beijing, a major northern Chinese hub, ensured demographic diversity through its mix of long-term residents and migrants, reflecting northern Chinese populations[21].
Participants were excluded based on the following criteria: (1) Age < 20 years or ≥ 80 years; (2) Absence of serum carcinoembryonic antigen (CEA), carbohydrate antigen 724 (CA724), or 13C-urea breath test results; (3) Positive results for CEA, CA724, or 13C-urea breath test; (4) Self-reported history or indications of gastrointestinal diseases; (5) Pregnant or lactating women; (6) Non-Chinese individuals; and (7) Duplicate records from repeat testing.
After rigorous screening, 708 reference (healthy) individuals were included in the study. Of these, 698 had complete laboratory results for liver function, renal function, and hematological parameters. The participant screening process and study flow are illustrated in Figure 1. This study was approved by the Ethics Committee of Beijing Tsinghua Changgung Hospital (Approval No. 24791-6-01).
All biomarker tests were performed in the clinical laboratory of Beijing Tsinghua Changgung Hospital, which is accredited under International Organization for Standardization (ISO) 15189 standards. PG I, PG II, and CEA were analyzed using the Alinity i immunoassay analyzer (Abbott, Abbott Park, IL, United States). CA724 was measured using the Cobas e801 electrochemiluminescence immunoassay analyzer (Roche Diagnostics, Switzerland). Liver and renal function markers, including alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, and creatinine, were quantified using the Cobas c702 clinical chemistry analyzer (Roche Diagnostics, Switzerland). Hematological parameters, including white blood cell count and hemoglobin concentration, were assessed with the XN-Series automated hematology analyzer (Sysmex Corporation, Kobe, Japan). All assays were conducted using reagents and calibrators provided by the manufacturers, and internal quality control procedures were implemented using at least two levels of quality control materials for each testing batch.
Data preprocessing: The screened PG I, PG II, and PGR results were assessed for distribution characteristics using the Kolmogorov-Smirnov test. Non-normally distributed data were transformed into a normal or approximately normal distribution using the Box-Cox method. Outliers were identified and excluded using Tukey’s method.
Gender- and age-related effects analysis: PG I, PG II, and PGR values were stratified by sex and age group (20-29, 30-39, 40-49, 50-59, and ≥ 60 years). A two-way ANOVA was performed to assess the effects of sex and age on biomarker levels.
Partitioned RI establishment: The necessity of establishing RIs based on sex or age was evaluated by the standard deviation ratio (SDR) derived from two-way ANOVA[22,23]. The formulas can be concisely defined as follows:
SDR (age) = SD (between-age groups)/SD (residual); SDR (sex) = SD (between-sex groups)/SD (residual).
A higher SDR suggests that the mean biomarker levels differ substantially between sex groups or across age groups. When SDR was ≥ 0.3, a threshold suggesting clinically relevant heterogeneity, age- or sex-specific RIs are warranted. For age-based stratification, the Kruskal-Wallis test, followed by Dunn’s post hoc pairwise comparisons, was used to refine partition boundaries. Each partitioned subgroup included no fewer than 120 reference individuals. Using a non-parametric approach, RIs for PG I, PG II, and PGR were established by defining the 2.5th and 97.5th percentiles as lower reference limits (LRLs) and upper reference limits (URLs), respectively[24].
Development of next-generation RI models: Generalized Additive Models for Location, Scale, and Shape (GAMLSS) were employed to flexibly model age-related dynamics in PG I and PG II and construct continuous reference percentiles. The models were fitted using the Box-Cox power exponential distribution to account for skewness and kurtosis in the data. Cubic splines were applied to smooth the relationship of PG I and PG II levels with age. The optimal degrees of freedom were determined to be 2, balancing model fit and generalizability. The 2.5th, 50th- (median), and 97.5th-percentile curves derived from the models were used to define the LRLs, central tendency, and URLs, respectively, thereby capturing the age-related variation.
Comparison of flagging rates between partitioned and next-generation RIs: We compared the reference limit flagging rates between partitioned and next-generation RIs in the overall reference individuals and across stratified age groups (20-29, 30-39, 40-49, 50-59, and ≥ 60 years). The reference limit flagging rate is defined as the percentage of test results exceeding the URLs or LRLs. Theoretically, a flagging rate of URL or LRL closer to the ideal value of 2.5% indicates the RIs more accurately represent the central 95% of the distribution of a healthy reference population.
Software: All statistical analyses were conducted using R (version 4.3.0; R Development Core Team, Vienna, Austria). The significance level for statistical tests was set at 0.05. The GAMLSS models were fitted using the “gamlss” package in R.
A total of 708 participants were enrolled after rigorous screening. The detailed baseline characteristics are summarized in Table 1. After data normalization and outlier exclusion, 678 (PG I), 697 (PG II), and 683 (PGR) valid cases were retained for analysis. Distribution changes before and after normalization are shown in Supplementary Figure 1.
| Variable | Value |
| Age, years | 45 (35-54) |
| Age group, years | |
| 20-29 | 73 (10.3) |
| 30-39 | 173 (24.4) |
| 40-49 | 207 (29.2) |
| 50-59 | 142 (20.1) |
| ≥ 60 | 113 (16.0) |
| Sex (male/female) | 417 (58.9)/291 (41.1) |
| PG I (μg/L) | 44.0 (36.7-54.0) |
| PG II (μg/L) | 5.9 (4.8-7.3) |
| CEA (ng/mL) | 2.41 (2.05-2.98) |
| CA724 (KU/L) | 1.87 (1.22-3.21) |
| ALT (U/L) | 18.5 (13.3-27.9) |
| AST (U/L) | 19.2 (16.4-23.2) |
| GGT (U/L) | 21.0 (14.0-34.0) |
| CREA (µmol/L) | 76.0 (67.0-88.0) |
| WBC (× 109/L) | 5.5 (4.7-6.5) |
| Hb (g/L) | 147.0 (134.0-157.0) |
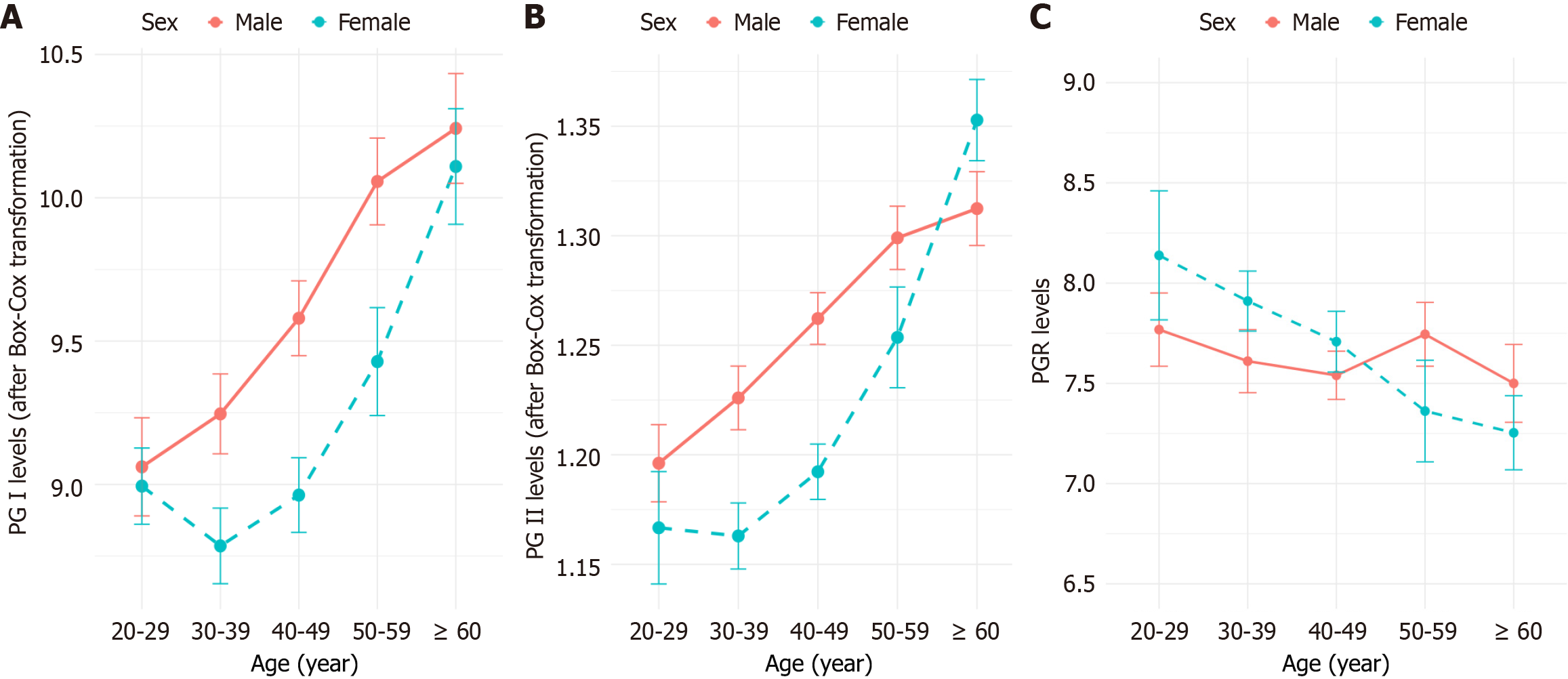
A two-way ANOVA was performed to evaluate the effects of sex and age on serum PG I, PG II, and PGR levels. Both PG I and PG II showed statistically significant differences across age groups (P < 0.001) and between sexes (P < 0.001), indicating clear variation related to both factors. In contrast, PGR did not show significant differences between sexes (P = 0.261) or across age groups (P = 0.163; Figure 2).
The necessity for stratifying RIs by age or sex was evaluated based on the SDR, with a critical threshold of 0.3[22]. The SDR values for sex were 0.215 for PG I and 0.177 for PG II, both below the threshold, indicating that sex-specific RIs are not required in clinical practice. In contrast, age-specific SDR values for PG I (0.366) and PG II (0.424) exceeded the threshold, necessitating age-specific RIs for both biomarkers. Since PGR show no significant age or sex differences, only a single, unified RI is needed.
To refine age-partitioning boundaries, age subgroups without statistically significant differences for PG I and PG II were merged to determine the optimal partitioning pattern of reference values (Table 2). PG I was ultimately stratified into two age groups: 20-49 years (n = 447) and ≥ 50 years (n = 231). Similarly, PG II was partitioned into 20-49 years (n = 447) and ≥ 50 years (n = 250). PGR showed no significant variation by age or sex, and a single unified RI was established (n = 684). Final age-partitioned RIs for PG I and PG II, along with a unified RI for PGR, were established as follows: PG I: 20-49 years, 23.55-69.06 μg/L; ≥ 50 years, 30.58-78.85 μg/L. PG II: 20-49 years, 3.30-10.06 μg/L; ≥ 50 years, 3.60-12.38 μg/L. PGR: 5.03-10.79 μg/L.
| Comparison groups (age, years) | P value | |
| PG I | PG II | |
| 20-29 vs 30-39 | 1 | 1 |
| 20-29 vs 40-49 | 0.82 | 0.266 |
| 20-29 vs 50-59 | 0.001 | < 0.001 |
| 20-29 vs 60+ | < 0.001 | < 0.001 |
| 30-39 vs 40-49 | 0.296 | 0.144 |
| 30-39 vs 50-59 | < 0.001 | < 0.001 |
| 30-39 vs 60+ | < 0.001 | < 0.001 |
| 40-49 vs 50-59 | 0.001 | < 0.001 |
| 40-49 vs 60+ | < 0.001 | < 0.001 |
| 50-59 vs 60+ | 0.325 | 0.057 |
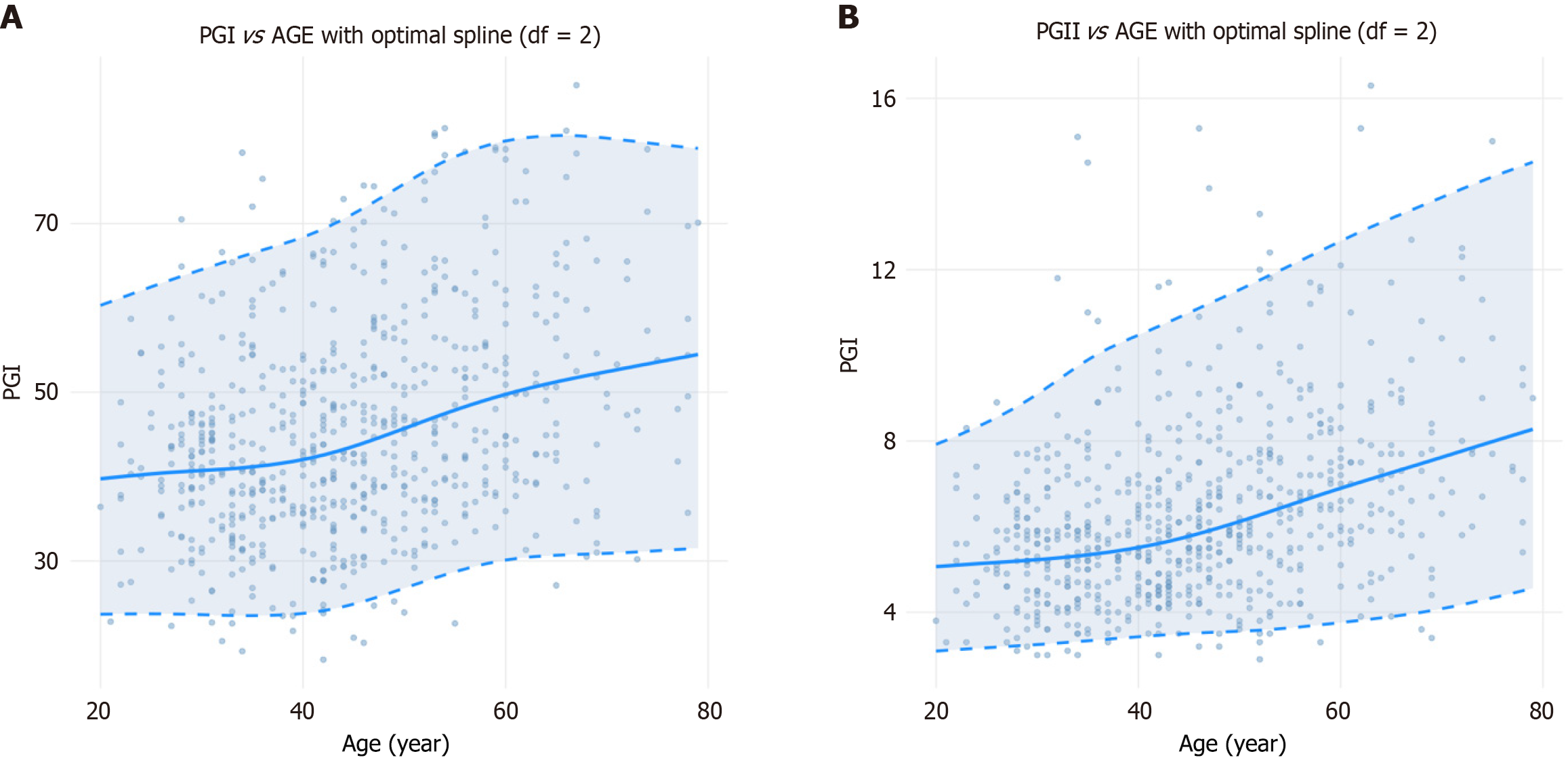
The continuous reference percentiles generated using the GAMLSS revealed clear age-related trends for both PG I and PG II. Overall, both biomarkers increased with age, with a more pronounced rise observed after 40 years. However, PG I levels plateaued after the age of 60 years, whereas PG II continued to increase steadily across all ages.
Specifically, for PG I, the modeled median concentration increased from 39.75 μg/L at age 20 years to 49.75 μg/L at age 60 years (+25.16%). Correspondingly, the LRL rose from 23.08 μg/L to 30.10 μg/L (+30.42%), and the URL from 60.30 μg/L to 79.89 μg/L (+32.49%). After age 60 years, all percentiles plateaued, showing minimal age-related variation beyond this point. For PG II, the median concentration increased continuously from 5.07 μg/L at age 20 years to 8.36 μg/L at age 80 years (+64.89%), accompanied by an LRL increase from 3.09 μg/L to 4.61 μg/L (+49.19%) and an URL increase from 7.92 μg/L to 14.60 μg/L (+84.34%). These trajectories, and the corresponding continuous reference percentiles for PG I and PG II, are visualized in Figure 3 and detailed in Table 3.
| Age (years) | PG I (μg/L) | PG II (μg/L) | ||||
| 2.5th percentile | Median | 97.5th percentile | 2.5th percentile | Median | 97.5th percentile | |
| 20 | 23.08 | 39.75 | 60.3 | 3.09 | 5.07 | 7.92 |
| 21 | 23.7 | 39.87 | 60.73 | 3.11 | 5.08 | 8.02 |
| 22 | 23.71 | 39.98 | 61.17 | 3.13 | 5.1 | 8.13 |
| 23 | 23.71 | 40.1 | 61.62 | 3.14 | 5.12 | 8.23 |
| 24 | 23.72 | 40.21 | 62.06 | 3.16 | 5.13 | 8.32 |
| 25 | 23.74 | 40.32 | 62.49 | 3.17 | 5.15 | 8.43 |
| 26 | 23.75 | 40.41 | 62.92 | 3.19 | 5.16 | 8.53 |
| 27 | 23.77 | 40.5 | 63.34 | 3.2 | 5.18 | 8.65 |
| 28 | 23.79 | 40.58 | 63.76 | 3.21 | 5.19 | 8.8 |
| 29 | 23.66 | 40.65 | 64.15 | 3.23 | 5.21 | 8.94 |
| 30 | 23.63 | 40.72 | 64.55 | 3.25 | 5.23 | 9.09 |
| 31 | 23.59 | 40.79 | 64.95 | 3.26 | 5.25 | 9.23 |
| 32 | 23.56 | 40.86 | 65.33 | 3.28 | 5.27 | 9.42 |
| 33 | 23.53 | 40.95 | 65.73 | 3.3 | 5.29 | 9.58 |
| 34 | 23.52 | 41.04 | 66.11 | 3.32 | 5.31 | 9.74 |
| 35 | 23.52 | 41.16 | 66.48 | 3.33 | 5.33 | 9.89 |
| 36 | 23.53 | 41.29 | 66.85 | 3.35 | 5.37 | 10.03 |
| 37 | 23.56 | 41.43 | 67.21 | 3.37 | 5.4 | 10.15 |
| 38 | 23.61 | 41.6 | 67.58 | 3.39 | 5.43 | 10.27 |
| 39 | 23.68 | 41.8 | 67.95 | 3.41 | 5.47 | 10.37 |
| 40 | 23.79 | 42.02 | 68.39 | 3.43 | 5.51 | 10.47 |
| 41 | 23.93 | 42.27 | 68.86 | 3.44 | 5.56 | 10.57 |
| 42 | 24.11 | 42.56 | 69.37 | 3.46 | 5.6 | 10.68 |
| 43 | 24.33 | 42.87 | 69.89 | 3.48 | 5.66 | 10.79 |
| 44 | 24.59 | 43.22 | 70.42 | 3.49 | 5.71 | 10.89 |
| 45 | 24.89 | 43.59 | 71.18 | 3.51 | 5.77 | 10.99 |
| 46 | 25.22 | 43.96 | 71.85 | 3.52 | 5.84 | 11.1 |
| 47 | 25.59 | 44.4 | 72.54 | 3.53 | 5.9 | 11.21 |
| 48 | 25.97 | 44.83 | 73.29 | 3.54 | 5.97 | 11.31 |
| 49 | 26.37 | 45.26 | 73.98 | 3.55 | 6.05 | 11.42 |
| 50 | 26.77 | 45.7 | 74.71 | 3.56 | 6.12 | 11.53 |
| 51 | 27.18 | 46.15 | 75.43 | 3.57 | 6.19 | 11.64 |
| 52 | 27.59 | 46.62 | 76.17 | 3.58 | 6.27 | 11.76 |
| 53 | 27.98 | 47.08 | 76.78 | 3.6 | 6.36 | 11.87 |
| 54 | 28.35 | 47.46 | 77.38 | 3.62 | 6.43 | 11.98 |
| 55 | 28.71 | 47.85 | 77.92 | 3.64 | 6.52 | 12.09 |
| 56 | 29.07 | 48.26 | 78.48 | 3.66 | 6.59 | 12.21 |
| 57 | 29.36 | 48.67 | 78.82 | 3.68 | 6.67 | 12.32 |
| 58 | 29.65 | 49.05 | 79.19 | 3.71 | 6.74 | 12.44 |
| 59 | 29.89 | 49.41 | 79.52 | 3.73 | 6.82 | 12.55 |
| 60 | 30.1 | 49.75 | 79.89 | 3.76 | 6.9 | 12.67 |
| 61 | 30.28 | 50.07 | 80 | 3.79 | 6.97 | 12.78 |
| 62 | 30.42 | 50.38 | 80.17 | 3.81 | 7.04 | 12.89 |
| 63 | 30.53 | 50.67 | 80.29 | 3.84 | 7.11 | 13 |
| 64 | 30.61 | 50.95 | 80.38 | 3.87 | 7.18 | 13.1 |
| 65 | 30.67 | 51.22 | 80.44 | 3.9 | 7.26 | 13.2 |
| 66 | 30.73 | 51.48 | 80.44 | 3.93 | 7.33 | 13.3 |
| 67 | 30.77 | 51.73 | 80.41 | 3.96 | 7.4 | 13.39 |
| 68 | 30.81 | 51.97 | 80.34 | 4 | 7.47 | 13.49 |
| 69 | 30.85 | 52.2 | 80.21 | 4.03 | 7.55 | 13.59 |
| 70 | 30.9 | 52.44 | 80.01 | 4.08 | 7.62 | 13.69 |
| 71 | 30.95 | 52.67 | 79.98 | 4.13 | 7.69 | 13.79 |
| 72 | 31.01 | 52.9 | 79.84 | 4.18 | 7.77 | 13.88 |
| 73 | 31.07 | 53.18 | 79.7 | 4.23 | 7.84 | 13.98 |
| 74 | 31.14 | 53.35 | 79.56 | 4.28 | 7.91 | 14.08 |
| 75 | 31.21 | 53.58 | 79.42 | 4.34 | 7.98 | 14.17 |
| 76 | 31.28 | 53.8 | 79.28 | 4.39 | 8.06 | 14.26 |
| 77 | 31.36 | 54.02 | 79.14 | 4.45 | 8.13 | 14.34 |
| 78 | 31.43 | 54.25 | 79.02 | 4.5 | 8.2 | 14.43 |
| 79 | 31.51 | 54.47 | 78.91 | 4.56 | 8.27 | 14.51 |
| 80 | 31.58 | 54.7 | 78.81 | 4.61 | 8.36 | 14.6 |
Reference limit flagging rates were compared between partitioned and next-generation RIs for PG I and PG II (Table 4). In the overall population, the flagging rates for both RI types were satisfactory and comparable, ranging from 2.0% to 3.0% for both biomarkers. However, age-stratified analyses highlighted superior accuracy of next-generation RIs. These RIs achieved flagging rates closer to the optimal 2.5% in a greater number of age groups, particularly for URL flagging. Notably, all age-specific flagging rates for next-generation RIs fell within the acceptable range of 1%-4%. Conversely, partitioned RIs exhibited flagging rates exceeding this range in one age group for PG I and two age groups for PG II.
| Age group, years | PG I | PG II | ||||||
| URL flagging rate (%) | LRL flagging rate (%) | URL flagging rate (%) | LRL flagging rate (%) | |||||
| Partitioned | Continuous | Partitioned | Continuous | Partitioned | Continuous | Partitioned | Continuous | |
| 20-29 | 1.37 | 2.74 | 2.74 | 2.74 | 0 | 2.78 | 2.78 | 2.78 |
| 30-39 | 1.75 | 2.34 | 4.09 | 3.51 | 2.96 | 2.96 | 2.37 | 2.96 |
| 40-49 | 3.94 | 1.97 | 1.48 | 2.96 | 3.4 | 1.94 | 1.46 | 1.94 |
| 50-59 | 3.01 | 3.76 | 2.26 | 1.5 | 1.43 | 2.14 | 3.57 | 3.57 |
| ≥ 60 | 2.04 | 2.04 | 3.06 | 3.06 | 4.55 | 2.73 | 0.91 | 1.82 |
| Total | 2.65 | 2.51 | 2.65 | 2.95 | 2.73 | 2.44 | 2.15 | 2.58 |
In this study, we revealed age- and sex-related variations in PG and PGR. Both PG I and PG II levels showed evident increases, but while PG I plateaued after age 60 years, PG II showed a continuous rise. We visualized and quantified the complex dynamics with GAMLSS, and for the first time, to our knowledge, established next-generation RIs for PG I and PG II. Comparative analysis based on reference limit flagging rates demonstrated the next-generation RIs provided greater accuracy than traditional partitioned RIs.
RIs are essential for proper health assessments and disease diagnosis, typically defined as the range between the 2.5th and 97.5th percentiles of test values from a healthy reference population[24]. RIs can be established using either the direct method, namely strict recruitment of reference individuals, or the indirect method, which utilizes stored laboratory data, often from individuals undergoing health checkups or outpatient care. While the direct method is considered the preferred approach, its complexity, high cost, and challenges in recruiting reference individuals limit its feasibility[24,25]. As data analytics and information technologies advance, the indirect method is increasingly recognized and applied to establish RIs[26,27].
The validity of RIs derived from the indirect method depends largely on data quality, sample size, and the appropriateness of the statistical methods. Previous studies have shown that the inclusion of a certain proportion of “abnormal” data (e.g., from outpatient populations) does not compromise validity[28,29]. However, to further ensure study reliability, we applied comprehensive and strict screening criteria (Figure 1) to ensure the dataset accurately reflected a reference population. Additionally, as an ISO 15189-accredited laboratory, we followed rigorous quality control protocols to ensure the analytical accuracy of test results. According to the Clinical and Laboratory Standards Institute’s EP28-A3c document, at least 120 reference individuals are required to establish traditional RIs[24]. For next-generation RIs, a minimum of 400 individuals were suggested in a study[30]. Our dataset exceeded these thresholds, contributing to the robustness of both RI types. Moreover, we used well-validated statistical methods and selected GAMLSS based on its superior flexibility and stability for next-generation RIs modeling[19,23,24,31].
Serum PG I and PG II levels were influenced by both age and sex, consistent with previous studies[11,32,33]. PG I and PG II levels were significantly higher in males compared to females, likely due to the inhibitory effect of estradiol on gastric acid secretion, as males generally exhibit higher levels of gastric acid secretion than females[34,35]. Both PG I and PG II levels increased with age, and the GAMLSS models provided a more intuitive visualization of these age-related dynamics. However, the rise in PG II was more pronounced, exhibiting a stronger age-dependent trend. In contrast, PG I showed a different pattern, with the upward trend plateauing after the age of 60 years. For PGR, no significant sex differences were observed, which is consistent with most previous studies[32,33]. However, one study reported significantly lower PGR levels in males compared to females, possibly due to differences in population composition between studies, such as the inclusion of a large proportion of unhealthy individuals in that study[36]. Regarding age, although a slight downward trend in PGR values with increasing age was noted in our study (Figure 2), the difference did not reach statistical significance. Previous studies have reported inconsistent results, with some suggesting a correlation[11,36] and others not[32,33]. These inconsistencies may stem from variations in sample size or population characteristics.
Although age and sex were shown to influence PG I and PG II levels in our study, the decision to stratify RIs by these factors should not solely rely on statistical significance but also consider the actual contribution of these factors to the overall variability and clinically relevant heterogeneity. In this study, the SDR was used to assess the necessity of establishing age- or sex-specific RIs, and only age-based RIs were deemed necessary for both biomarkers[23].
A major contribution of this study is the development of next-generation RIs for PG I and PG II, along with a comparative evaluation against partitioned RIs. While age-partitioned RIs offer an improvement over single RIs as biomarkers, they inherently introduce abrupt shifts at defined boundaries (e.g., age 50 years in our study), which fail to capture the gradual nature of physiological changes and could result in misinterpretation of laboratory results[37]. The flagging rates comparison between two RI types supports this concern. While the overall flagging rates were comparable, partitioned RIs demonstrated reduced accuracy in discrete age groups. Notably, in three age groups, the flagging rates exceeded the acceptable range of 1%-4% (Table 4). In contrast, the next-generation RIs more accurately reflect the distribution of the age-based population, potentially enhancing clinical utility and reliability.
In this study, we established a single RI for PGR, along with age-partitioned and next-generation RIs for PG I and PG II. Given the current limitations in implementing next-generation RIs in most clinical laboratories, the RIs from this study can also be adopted. Compared to the single RIs commonly provided by manufacturers, partitioned RIs offer clear advantages[38]. However, it is important to note that laboratories must ensure method comparability and population applicability, and to verify the RIs before RI implementation[24]. Although next-generation RIs offer significant advantages, their implementation in clinical laboratories remains limited due to methodological complexity and technological constraints. Integrating next-generation RI models into laboratory instrument middleware or laboratory information systems represents a promising path for practical application. As advancements in both methodology and technology continue, and as the clinical utility of next-generation RIs becomes more evident, their broader imple
This study has several limitations. First, as a real-world study, its reliability may be lower than that of a clinical study. However, we applied comprehensive and strict screening criteria to exclude “unhealthy” cases and employed robust statistical methods to strengthen validity. Second, since this study was based on a single testing platform, the generalizability of its RIs should be further validated across different testing systems. Laboratories should ensure method comparability and population applicability before implementation. Finally, RIs for individuals over 80 years of age were not established due to the limited number of participants, which warrants further investigation in future studies.
We revealed age- and gender-specific dynamics and established next-generation RIs for PG in adult and elderly populations in China. Our results demonstrated the superiority of next-generation RIs derived from GAMLSS over partitioned RIs. With the further application of next-generation RIs, these advancements could propel laboratory medicine toward better individualization.
| 1. | Herriott RM. Pepsinogen and pepsin. J Gen Physiol. 1962;45(4)Pt 2:57-76. [PubMed] |
| 2. | Gritti I, Banfi G, Roi GS. Pepsinogens: physiology, pharmacology pathophysiology and exercise. Pharmacol Res. 2000;41:265-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lee SY. Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection. Korean J Intern Med. 2016;31:835-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Mansour-Ghanaei F, Joukar F, Baghaee M, Sepehrimanesh M, Hojati A. Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer. Biomol Concepts. 2019;10:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | De Re V, Realdon S, Vettori R, Zaramella A, Maiero S, Repetto O, Canzonieri V, Steffan A, Cannizzaro R. A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area. Int J Mol Sci. 2023;24:3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Matsukura N, Onda M, Tokunaga A, Fujita I, Okuda T, Mizutani T, Kyono S, Yamashita K. Significance of serum markers pepsinogen I and II for chronic atrophic gastritis, peptic ulcer, and gastric cancer. J Clin Gastroenterol. 1993;17 Suppl 1:S146-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Yamada S, Matsuhisa T, Makonkawkeyoon L, Chaidatch S, Kato S, Matsukura N. Helicobacter pylori infection in combination with the serum pepsinogen I/II ratio and interleukin-1beta-511 polymorphisms are independent risk factors for gastric cancer in Thais. J Gastroenterol. 2006;41:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Takahashi S, Igarashi H, Masubuchi N, Ishiyama N, Saito S, Aoyagi T, Itoh T, Hirata I. [Helicobacter pylori and the development of atrophic gastritis]. Nihon Rinsho. 1993;51:3231-3235. [PubMed] |
| 9. | Tahmasebi H, Higgins V, Fung AWS, Truong D, White-Al Habeeb NMA, Adeli K. Pediatric Reference Intervals for Biochemical Markers: Gaps and Challenges, Recent National Initiatives and Future Perspectives. EJIFCC. 2017;28:43-63. [PubMed] |
| 10. | Stollberg SM, Näpflin M, Nagler M, Huber CA. Are Tumor Marker Tests Applied Appropriately in Clinical Practice? A Healthcare Claims Data Analysis. Diagnostics (Basel). 2023;13:3379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Huang RG, Xiao HL, Zhou B, Song XH, Zhang J, Wang CM, Jiang YH, Chen DZ, Huang B. Serum Pepsinogen Levels Are Correlated With Age, Sex and the Level of Helicobacter pylori Infection in Healthy Individuals. Am J Med Sci. 2016;352:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Tong Y, Wang H, Zhao Y, He X, Xu H, Li H, Shuai P, Gong L, Wu H, Xu H, Luo Y, Wang D, Liu S, Song Z. Serum pepsinogen levels in different regions of China and its influencing factors: a multicenter cross-sectional study. BMC Gastroenterol. 2021;21:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ichihara K, Itoh Y, Lam CW, Poon PM, Kim JH, Kyono H, Chandrawening N, Muliaty D; Science Committee for the Asian-Pacific Federation of Clinical Biochemistry. Sources of variation of commonly measured serum analytes in 6 Asian cities and consideration of common reference intervals. Clin Chem. 2008;54:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Doyle K, Bunch DR. Reference intervals: past, present, and future. Crit Rev Clin Lab Sci. 2023;60:466-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 15. | Klawitter S, Kacprowski T. A visualization tool for continuous reference intervals based on GAMLSS. J Lab Med. 2023;47:165-170. [DOI] [Full Text] |
| 16. | Fang H, Li J, Wen X, Ren L, Liu E. Next-generation reference interval for total IgE in the United States: A retrospective real-world analysis. Clin Chim Acta. 2024;563:119895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Ma C, Yu Z, Qiu L. Development of next-generation reference interval models to establish reference intervals based on medical data: current status, algorithms and future consideration. Crit Rev Clin Lab Sci. 2024;61:298-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Yang Q, Lew HY, Peh RH, Metz MP, Loh TP. An automated and objective method for age partitioning of reference intervals based on continuous centile curves. Pathology. 2016;48:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Zhao H, Zhu D, Zhang M, Wang T, Han N, Ge T, Ma X, Wu A, Li R, Zhao X. Establishing Neuron-Specific Enolase Reference Intervals: A Comparative Analysis of Partitioned Approach- and Gender-Based Continuous Age- and Season-Related Models. Diagnostics (Basel). 2024;14:2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Ammer T, Schützenmeister A, Prokosch HU, Rauh M, Rank CM, Zierk J. A pipeline for the fully automated estimation of continuous reference intervals using real-world data. Sci Rep. 2023;13:13440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Lu Q, Wu PL, Lu LX, Wang GX. [The Relation between the Characteristics of the Migrants and the Economic Development in Beijing and the Regional Differentiation of Their Distribution]. Dili Xuebao. 2005;60:851-862. [DOI] [Full Text] |
| 22. | Ichihara K, Ozarda Y, Barth JH, Klee G, Qiu L, Erasmus R, Borai A, Evgina S, Ashavaid T, Khan D, Schreier L, Rolle R, Shimizu Y, Kimura S, Kawano R, Armbruster D, Mori K, Yadav BK; Committee on Reference Intervals and Decision Limits, International Federation of Clinical Chemistry and Laboratory Medicine. A global multicenter study on reference values: 1. Assessment of methods for derivation and comparison of reference intervals. Clin Chim Acta. 2017;467:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Ichihara K, Boyd JC; IFCC Committee on Reference Intervals and Decision Limits (C-RIDL). An appraisal of statistical procedures used in derivation of reference intervals. Clin Chem Lab Med. 2010;48:1537-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. CLSI document EP28-A3c. 3rd ed. Wayne, PA, United States: Clinical and Laboratory Standards Institute, 2008. |
| 25. | Yan R, Li K, Lv Y, Peng Y, Van Halm-Lutterodt N, Song W, Peng X, Ni X. Comparison of reference distributions acquired by direct and indirect sampling techniques: exemplified with the Pediatric Reference Interval in China (PRINCE) study. BMC Med Res Methodol. 2022;22:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Farrell CL, Nguyen L. Indirect Reference Intervals: Harnessing the Power of Stored Laboratory Data. Clin Biochem Rev. 2019;40:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Obstfeld AE, Patel K, Boyd JC, Drees J, Holmes DT, Ioannidis JPA, Manrai AK. Data Mining Approaches to Reference Interval Studies. Clin Chem. 2021;67:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Katayev A, Balciza C, Seccombe DW. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Liu J, Zhan S, Jia Y, Li Y, Liu Y, Dong Y, Tang G, Li L, Zhai Y, Cao Z. Retinol and α-tocopherol in pregnancy: Establishment of reference intervals and associations with CBC. Matern Child Nutr. 2020;16:e12975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Bellera CA, Hanley JA. A method is presented to plan the required sample size when estimating regression-based reference limits. J Clin Epidemiol. 2007;60:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Li K, Hu L, Peng Y, Yan R, Li Q, Peng X, Song W, Ni X. Comparison of four algorithms on establishing continuous reference intervals for pediatric analytes with age-dependent trend. BMC Med Res Methodol. 2020;20:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Huang M, Tang AG, Mu S, Yang JJ, Xiang ZY, Liu B, Yang JJ. Serum pepsinogen reference intervals in apparently healthy Chinese population with latex enhanced turbidimetric immunoassay. J Clin Pathol. 2014;67:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Zhang L, Niu Y, Lv YJ, Wu LF, Hu QL, Huang R, Xu RJ. Preliminary Study on Reference Interval of Serum Pepsinogen in Healthy Subjects. Patient Prefer Adherence. 2021;15:2725-2730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Iijima K, Ohara S, Koike T, Sekine H, Shimosegawa T. Gastric acid secretion of normal Japanese subjects in relation to Helicobacter pylori infection, aging, and gender. Scand J Gastroenterol. 2004;39:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Adeniyi KO. Gastric acid secretion and parietal cell mass: effect of sex hormones. Gastroenterology. 1991;101:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Sun LP, Gong YH, Wang L, Yuan Y. Serum pepsinogen levels and their influencing factors: a population-based study in 6990 Chinese from North China. World J Gastroenterol. 2007;13:6562-6567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Hall A, Bohn MK, Wilson S, Higgins V, Adeli K. Continuous reference intervals for 19 endocrine, fertility, and immunochemical markers in the CALIPER cohort of healthy children and adolescents. Clin Biochem. 2021;94:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Lahti A. Partitioning biochemical reference data into subgroups: comparison of existing methods. Clin Chem Lab Med. 2004;42:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/