Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.108990
Revised: May 22, 2025
Accepted: June 23, 2025
Published online: July 28, 2025
Processing time: 87 Days and 23 Hours
Gastric adenocarcinoma with primitive phenotypes has recently attracted increa
To investigate the clinicopathological and molecular characteristics of GAPEP and establish an integrative diagnostic strategy to guide therapeutic decision-making.
Based on the expression profile and morphology, patients were divided into three groups: GAPEP (including GAED and HAC), conventional gastric cancer (CGC), and CGC expressing primitive phenotypic markers. We analyzed clinicopathological features and overall survival. Data from The Cancer Genome Atlas were also ana
GAPEP showed diverse morphology, and immunohistochemical staining alone was not adequate for accurate diagnosis. Histologically, GAPEP was characterized by large, polygonal tumor cells with supranuclear or sub
The diversity and aggressiveness of GAPEP are driven by deregulated methylation, necessitating the integration of morphological and immunohistochemical diagnosis. Targeting methylation can provide new therapeutic oppor
Core Tip: Gastric adenocarcinoma with primitive enterocyte phenotype (GAPEP) exhibits aggressive behavior and diverse morphologies, including tubular-papillary and solid types, which necessitate integrated histopathological, immunohistochemical, and molecular experiments for accurate identification. This study highlighted GAPEP’s methylation-driven pathogenesis, with methylation dysregulation (e.g., DNMT3A/B/L, TET1/3 and EZH2 upregulation). Gastric adenocarcinoma with enteroblastic differentiation and hepatoid adenocarcinoma shared similar clinicopathological and genetic features, suggesting a common origin. Targeting methylation pathways (e.g., DNMT inhibitors) offers potential therapeutic avenues for this lethal cancer, emphasizing the critical role of epigenetic mechanisms in the pathogenesis of GAPEP.
- Citation: Li HQ, Zheng LQ, Huang WT, Yu XB, Zhang X, Lin L, Lv SS, Yan XY, Chen XY. Clinicopathological significance of histological diversity in gastric adenocarcinoma with primitive enterocyte phenotype: A methylation-driven aggressive entity. World J Gastroenterol 2025; 31(28): 108990
- URL: https://www.wjgnet.com/1007-9327/full/v31/i28/108990.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i28.108990
Gastric cancer (GC) is among the most common malignant tumors of the digestive system, ranking third in terms of cancer-related deaths in China[1]. A major contributor to the development of GC is chronic Helicobacter pylori infection, which remodels the environment of the stomach in atrophic gastritis and induces the phenotypic transformation of gastric stem cells[2-4]. Gastric mucosal stem cells differ in origin, proliferation, differentiation, and expression profiles across different anatomical sites, contributing to GC’s heterogeneity[5]. Advances in high-throughput sequencing have shifted the focus of pathology from morphology-based to molecular-based classifications. However, the heterogeneity of GC continues to complicate precise diagnosis and treatment strategies, even with The Cancer Genome Atlas (TCGA) mo
GC’s striking histological heterogeneity features have led to the development of various classification systems[7-9]. Alpha-fetoprotein (AFP)-producing GC is an example of GC heterogeneity. Regardless of morphology, cases with posi
Although GAPEP subtypes share similar immunophenotypes, their histomorphology may overlap, making diagnosis challenging. Previous studies demonstrated that the coexistence of GAED and HAC can be frequently observed[15]. The relationship between GAED and HAC has been challenging for researchers. Histologically, GAED has a low-grade tubule-papillary architecture and a distinctly clear cytoplasm that resembles the primitive gut. In contrast, HAC consists of large polygonal eosinophilic cells resembling hepatocellular carcinoma. These cells are arranged in various patterns, including pseudo-glandular, cord-like, or solid patterns[15]. IHC for primitive markers, including AFP, GPC3, and SALL4, is crucial to distinguish GAPEP from other types of GC, though these markers can also be seen in more conventional types. This overlap complicates accurate diagnosis and highlights the phenotypic plasticity of GAPEP. GAPEP is known for its poor prognosis, and its morphological heterogeneity makes diagnosis difficult. The accurate diagnosis of GAPEP directly affects therapeutic decision-making.
Currently, there is limited information regarding the morphological and molecular features of GAPEP, underscoring the importance of further research. In this paper, we present a series of typical cases of GAPEP to delineate the morphological and molecular characteristics of this aggressive tumor. Our study systematically identified that the histological heterogeneity of GAPEP is intrinsically linked with methylation aberrations, providing critical molecular evidence for stratified therapeutic strategies. Additionally, we conducted a comparative analysis of clinicopathological and molecular characteristics between GAED and HAC to ascertain their relationship.
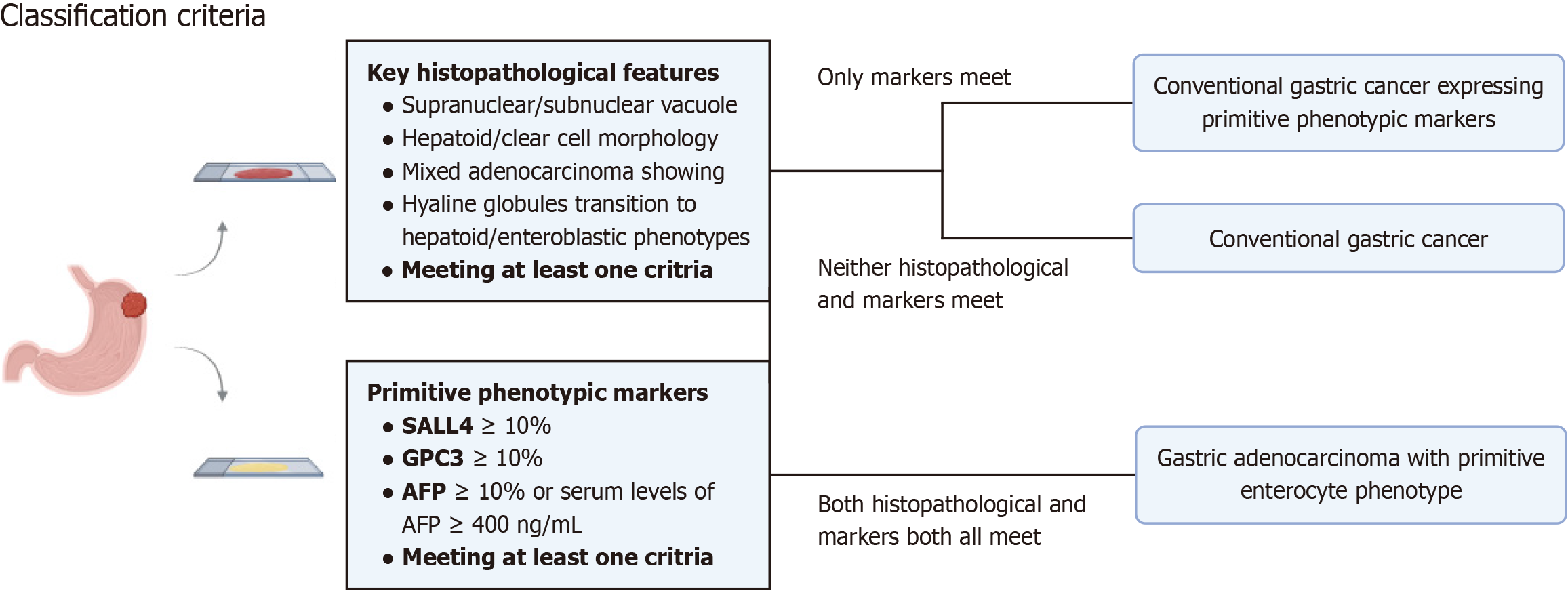
Inclusion and exclusion criteria: Patients with progressive GC from the Fujian Provincial Hospital (January 2014 to December 2020) who were pathologically confirmed were included in this study. Patients were included in two cohorts. 407 consecutive patients who underwent surgical resection of GC from January 2014 to December 2015 were included in cohort I, and 114 patients with GC expressing at least one of three primitive phenotypic markers (AFP, GPC3, and SALL4) were randomly included in cohort II. The classification criteria of various histological subtypes are defined as follows (Figure 1): (1) GAPEP: GC expressing at least one of the primitive phenotypic markers and histologically exhibiting features of GAED or HAC; (2) Conventional GC (CGC) primitive phenotypic markers (CGC-P): GC lacking the histolo
Clinicopathological characteristics and follow-up: Four pathologists (Li HQ, Zhang X, Lin L and Chen XY) reviewed all original hematoxylin and eosin slides and recorded clinicopathological parameters from the hospital medical records. The following clinical pathological parameters were recorded: Age, sex, tumor location, histologic type, lymphovascular invasion, perineural invasion, tumor budding, tumor size, depth of tumor invasion, and lymph node metastasis. All cases with complete follow-up data and a follow-up period of more than 5 years were included in the survival analysis. The tumor-node-metastasis staging was conducted following the eighth edition of the American Joint Committee on Cancer staging manual[17]. The detailed histological classification was determined according to the criteria of the sixth edition of the Japanese GC Association classification[7].
Immunohistochemical staining: The 4-μm-thick unstained sections of the representative regions of samples were pre
Fluorescence in situ hybridization: The fluorescence in situ hybridization assay was conducted using the HER-2 DNA Probe Kit II (PathVysion Kit II, Abbott, United States) on the paraffin-embedded tissue blocks. The fluorescence in situ hybridization assay was conducted following the instructions, and the results were analyzed using the methods described by the American Society of Clinical Oncology guidelines[19]. The total numbers of HER2 and CEP17 signals were counted in 20 adjacent interphase tumor cell nuclei, using fluorescent microscopes and appropriate filters. The ratios of HER2 signals to CEP17 signals were calculated regardless of IHC status as follows: When the ratio was < 1.8, the gene was considered non-amplified, and when it was > 2.2, the gene was considered to be amplified. If the ratio was within the range of 1.8 to 2.2 at the initial count, an additional 20 tumor cells were counted. If the final ratio for 40 nuclei was 2.0 or higher, the case was deemed to have HER2 amplification.
Data from the Stomach Adenocarcinoma (TCGA, Nature 2014) dataset were obtained using the public tool: CBioPortal (http://www.cbioportal.org). The clinicopathological information and molecular data, including the gene expression profile and mutated genes, were obtained from the TCGA database. We analyzed the expression of primitive phenotypic genes (SALL4, GPC3, and AFP) at the mRNA level. Clinical parameters, including clinicopathological features and patient prognosis, were extracted. The whole slide images of tumors were reviewed following the Genomic Data Commons portal (https://portal.gdc.cancer.gov/). In cBioPortal, Onco Query Language was used to identify samples with specific expression values. For mRNA (RNA Seq V2 RSEM) expression, z-score thresholds were set to ± 1. We analyzed the correlation between GAPEP, CGC-P, and CGC and clinicopathological features, including patient prognosis and somatic genomic alterations, including somatic mutations, DNA copy-number alterations, mRNA expression, and DNA methyla
GraphPad Prism 9.0 (La Jolla, CA, United States) was used to draw graphs and conduct statistical analyses. Descriptive variables are presented as percentages. Continuous data were compared using the Mann-Whitney U test. Categorical variables were compared using the χ2 test and Fisher’s exact test. A log-rank test was performed for survival analysis, and R studio was used to generate Kaplan-Meier curves. For molecular genetics data, continuous data were used to analyze copy-number alterations. For all other parameters (mRNA, methylation, and protein expression), normalized continuous values were used for statistical analysis. The Kruskal-Wallis test was used to assess differences in DNA copy-number alterations, mRNA, and methylation between various types of GC. The Mann-Whitney U test was employed to compare individual groups. Log2 ratio test/control thresholds of 0.25 and -0.25 were defined as copy number gains and losses, respectively. Statistical significance was defined as a two-tailed P < 0.05.
The results of single-center dataset analysis: The results of IHC for cohort I showed that 133 of 407 (32.7%) tumors had at least one of the three primitive phenotypes, including SALL4 (63/407, 15.5%), GPC3 (85/407, 20.9%), and AFP (6/407, 1.5%). In the cohort I, 63 cases (15.5%) were GAPEP, 70 cases (17.2%) were CGC-P. Meanwhile, 71 cases of cohort II were GAPEP. In total, 134 cases of GAPEP (including 63 GAED, 49 HAC and 22 cases of a mixture of GAED and HAC) were included in subsequent analyses of clinicopathological data. This cohort of 134 GAPEP cases represents the largest single-center cohort reported to date. Additionally, 92 cases of CGC-P and 274 cases of CGC were included.
Results of TCGA datasets: After excluding cases with no mRNA data for any of the three genes and cases with preope
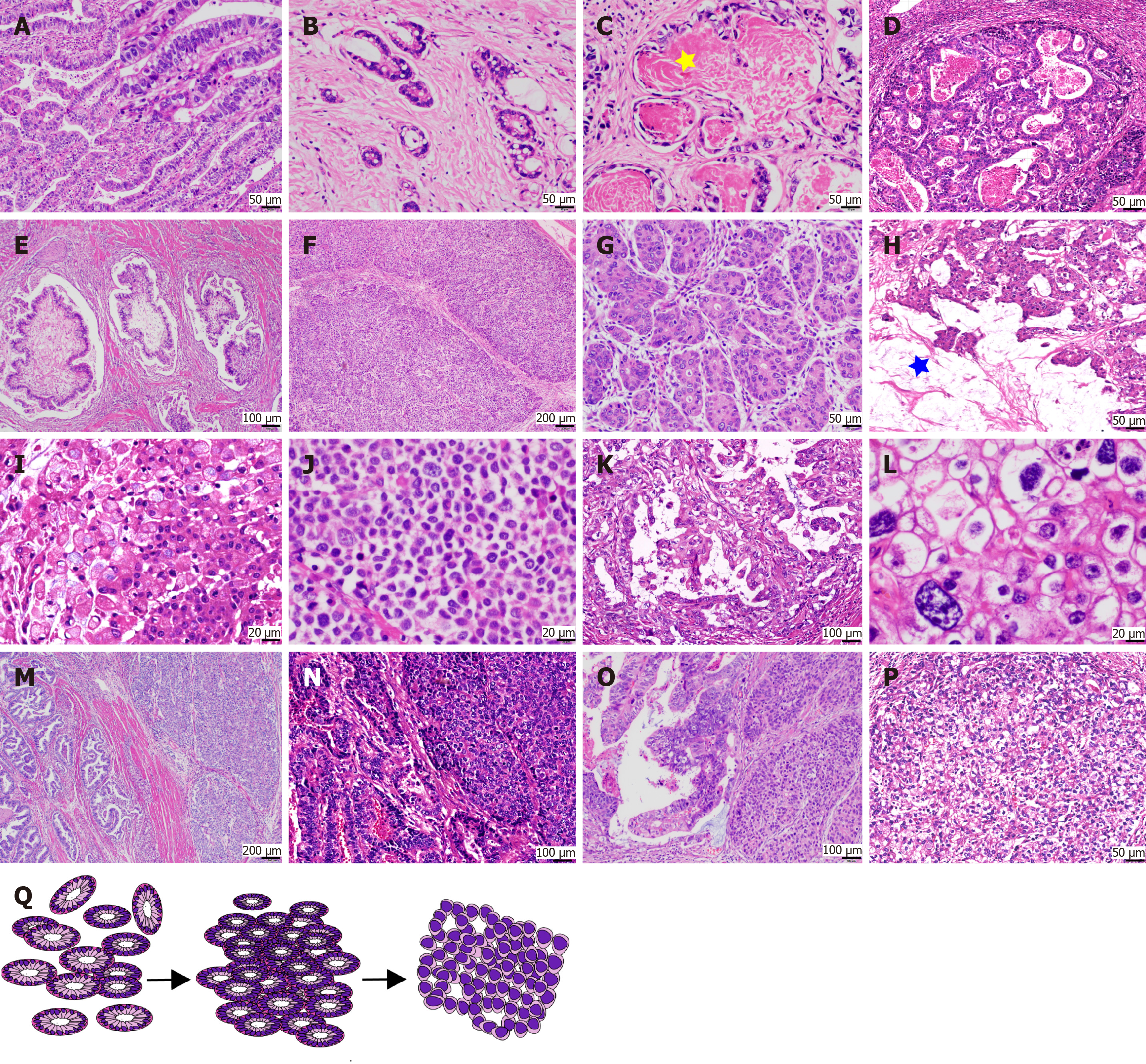
The morphology of GAPEP was diverse, with classical features visible in all cases. In the local dataset, the morphology of GAPEP was classified into two main types. The “tubular-papillary type” was characterized by prominent tubular and papillary structures with columnar and cuboidal cells containing hyaline cytoplasm similar to the intestinal epithelium of a newly formed fetus (Figure 2A and B). The tubular and papillary structures in GAPEP presented with supranuclear and subnuclear vacuoles, showing a “piano keyboard-like” appearance (Figure 2A). Most glandular lumens contained a large amount of eosinophilic secretion, which may be a feature of this type of GAPEP (Figure 2C). In some cases, the tumor exhibited sieve-like and sac-like structures (Figure 2D), tumor cells had a flat shape, and the tumor had a villi-like structure (Figure 2E). The “solid type” possessed a morphology similar to hepatocellular carcinoma (Figure 2F and G). It had large polygonal cells with eosinophilic or clear cytoplasm and round to oval nuclei in nested mass structure. The “solid type” contained eosinophilic intracellular and extracellular hyaline microspheres. Extracellular and intracellular mucus formation was seen in a subset of tumors (Figure 2H and I). In rare instances, tumor cells did not adhere to each other, similar to the poorly cohesive gastric carcinoma (Figure 2J). A combination of tubule-papillary and solid compo
Results of the local dataset: The mean age of patients with GAPEP was 67 years in the local dataset (range: 44-90), with a male predominance (3:1). Most tumors were located in the fundus, body, and cardia (62.22%), with a median tumor size of 5 cm (range: 1.5-15 cm). Differentiated subtypes were the main histological types of GAPEP (73.9%), and most cases showed well-differentiated tumors (69.17%). However, GAPEP showed aggressiveness, and most cases were in stage T4 (73.13%). Compared with CGC, GAPEP was significantly and positively correlated with the age of onset (P < 0.001), tumor size (P < 0.0001), T stage (P < 0.0001), tumor differentiation (P = 0.0001), differentiated subtypes (P = 0.0001), perineural invasion (P < 0.001), lymph node metastasis (P < 0.05), MMR (P < 0.05), p53 expression (P = 0.001), and HER2/ERBB2 amplification (P < 0.001) (Table 1). In contrast, no differences were found in gender, tumor location, lymphovascu
| Parament | GAPEP (n = 134) | CGC-P (n = 92) | CGC (n = 274) | HAC (n = 49) | GAED (n = 63) | P value | |||
| GAPEP vs CGC | GAPEP vs CGC-P | CGC-P vs CGC | HAC vs GAED | ||||||
| Age (years) | < 0.01 | < 0.05 | 0.4103 | < 0.05 | |||||
| > 65 | 74 | 38 | 100 | 21 | 39 | ||||
| ≤ 65 | 60 | 54 | 174 | 28 | 24 | ||||
| Gender | 0.407 | 0.4426 | 0.1206 | 0.568 | |||||
| Male | 102 | 74 | 198 | 35 | 48 | ||||
| Female | 32 | 18 | 76 | 14 | 15 | ||||
| Location | 0.749 | 0.6455 | 0.4288 | 0.4778 | |||||
| Fundus-body and cardia | 84 | 60 | 166 | 32 | 37 | ||||
| Antrum | 51 | 32 | 108 | 17 | 26 | ||||
| Size (cm) | < 0.0001 | < 0.0001 | 0.5841 | 0.2630 | |||||
| > 5 | 65 | 21 | 219 | 27 | 28 | ||||
| ≤ 5 | 69 | 71 | 55 | 22 | 35 | ||||
| Histological grade | < 0.0001 | < 0.01 | 0.4677 | < 0.001 | |||||
| G1-2 | 92 | 47 | 128 | 25 | 53 | ||||
| G3 | 41 | 45 | 146 | 24 | 10 | ||||
| JGCA type | 0.0001 | 0.0822 | 0.1309 | < 0.001 | |||||
| Differentiated | 99 | 58 | 148 | 26 | 54 | ||||
| Undifferentiated | 35 | 34 | 126 | 23 | 9 | ||||
| Vascular invasion | 0.846 | 0.6119 | 0.6883 | 0.1278 | |||||
| Positive | 105 | 70 | 214 | 41 | 45 | ||||
| Negative | 28 | 22 | 60 | 8 | 18 | ||||
| Perineural invasion | < 0.0001 | < 0.05 | 0.1168 | 0.7850 | |||||
| Positive | 98 | 58 | 158 | 37 | 46 | ||||
| Negative | 31 | 34 | 136 | 12 | 17 | ||||
| T-staging | < 0.001 | 0.4323 | < 0.0001 | 0.8249 | |||||
| T2-3 | 9 | 20 | 53 | 11 | 16 | ||||
| T4 | 124 | 72 | 221 | 38 | 47 | ||||
| AJCC-staging | 0.6875 | 0.0967 | 0.127 | 0.5434 | |||||
| I-II | 42 | 39 | 92 | 14 | 22 | ||||
| III-IV | 91 | 53 | 182 | 35 | 41 | ||||
| Tumor budding | 0.0656 | 0.6422 | 0.2247 | NA | |||||
| Bd1 | 16 | 16 | 47 | NA | NA | ||||
| Bd 2-3 | 43 | 52 | 227 | NA | NA | ||||
| MMR | |||||||||
| pMMR | 86 | 209 | 71 | 30 | 40 | < 0.05 | 0.9554 | 0.0571 | 0.7564 |
| dMMR | 13 | 63 | 11 | 6 | 6 | ||||
| p53 | 0.001 | 0.5465 | < 0.05 | 0.7880 | |||||
| Mutant type | 81 | 59 | 128 | 28 | 42 | ||||
| Wild type | 21 | 19 | 83 | 6 | 11 | ||||
| HER2 | < 0.01 | 0.3301 | 0.1179 | 0.6284 | |||||
| Amplification | 29 | 16 | 30 | 8 | 14 | ||||
| Not amplification | 93 | 72 | 228 | 36 | 45 | ||||
TCGA dataset results: Data mining from TCGA databases demonstrated that the mean age of GAPEP was 71.86 years (range: 51-90), with a male predominance (3:2). Most tumors were located in the fundus, body, and cardia (62.1%). Intestinal subtypes were the main histological types (87.1%), and most cases were in the T3-T4 stage (77.8%). In terms of molecular subtypes defined by the TCGA project, the majority of GAPEP cases in TCGA datasets were chromosome instability (83.9%), and the remaining subtypes, microsatellite stability (MSI), genomic stability and tumors positive for Epstein-Barr virus, constituted a small proportion of GAPEP (16.1%). TP53 mutation was frequently observed in GAPEP (67.7%), and ERBB2 was amplified in seven cases (22.6%). Compared with CGC, GAPEP was significantly and positively correlated with the age of onset (P < 0.05), intestinal type (P < 0.05), World Health Organization classification (P < 0.05), TP53 mutation (P < 0.01), HER2/ERBB2 amplification (P = 0.0183), ARID1A mutation (P < 0.05), MSI status (P < 0.05), CpG island methylator phenotype (CIMP) category (P < 0.05), and molecular subtypes (P < 0.001). Moreover, comparing other molecular genetic characteristics between GAPEP and CGC showed significant differences in the fraction genome alteration, copy number cluster, methylation cluster, gene expression cluster, and hyper-mutation. Meanwhile, there were significant differences between GAPEP and CGC-P in clinicopathological and molecular genetic characteristics. Subgroup analysis revealed that HAC and GAED shared similar clinicopathological and molecular genetic characteristics. Signi
| Parament | Statisticians | P value | ||
| GAPEP (n = 31) vs CGC (n = 40) | GAPEP (n = 31) vs CGC-P (n = 157) | HAC (n = 12) vs GAED (n = 19) | ||
| Sex | χ2 test | 0.652 | 0.893 | 0.518 |
| Diagnosis age | Kruskal-Wallis test | < 0.05 | 0.0346 | 0.525 |
| Lauren class | χ2 test | < 0.05 | < 0.01 | 0.366 |
| WHO class | χ2 test | < 0.05 | < 0.05 | 0.216 |
| TNM stage | χ2 test | 0.572 | 0.129 | 0.435 |
| AJCC stage | χ2 test | 0.319 | 0.0878 | 0.778 |
| T stage | χ2 test | 0.071 | - | - |
| NDS-AJCC | χ2 test | 0.443 | 0.277 | 0.299 |
| EBV present | χ2 test | 0.48 | 0.132 | 0.814 |
| Molecular-subtypes | χ2 test | < 0.0001 | < 0.05 | 0.183 |
| Fraction genome altered | Kruskal-Wallis test | < 0.0001 | < 0.01 | 0.598 |
| Copy number cluster | χ2 test | < 0.0001 | < 0.05 | 0.295 |
| Molecular subtype | χ2 test | < 0.001 | < 0.05 | 0.136 |
| Absolute extract ploidy | Kruskal-Wallis test | < 0.001 | 0.126 | 0.653 |
| Methylation cluster | χ2 test | < 0.001 | 0.0688 | 0.0625 |
| Gene expression cluster | χ2 test | < 0.05 | < 0.05 | < 0.05 |
| CIMP category | χ2 test | < 0.05 | 0.167 | < 0.05 |
| Hyper-mutated | χ2 test | < 0.05 | 0.738 | 0.244 |
| TP53 mutation | χ2 test | < 0.01 | < 0.05 | 0.869 |
| CDKN2A silencing | χ2 test | 0.0622 | 0.26 | 0.0894 |
| ARID1A mutation | χ2 test | < 0.05 | 0.175 | 0.249 |
| MSI status | χ2 test | < 0.05 | 0.85 | 0.145 |
| MLH1 silencing | χ2 test | 0.0655 | 0.648 | 0.809 |
| ERBB2 amplificated | χ2 test | < 0.05 | 0.2799 | 0.363 |
| Percent tumor nuclei | Kruskal-Wallis test | 0.285 | < 0.05 | 0.0418 |
| MET skipped exons 18 and 19 | χ2 test | 0.129 | 0.682 | 0.571 |
| Percent lymphocyte infiltration | Kruskal-Wallis test | 0.444 | 0.902 | 0.0833 |
| ARHGAP26-ARHGAP6-CLDN18 rearrangement | χ2 test | 0.86 | 0.821 | 0.814 |
| KRAS mutation | χ2 test | 0.156 | 0.123 | - |
| PIK3CA mutation | χ2 test | 0.15 | 0.42 | 0.613 |
| MicroRNA expression cluster | χ2 test | 0.377 | 0.0688 | - |
| Mutation count | Kruskal-Wallis test | 0.208 | 0.262 | 0.389 |
| Mutation rate | Kruskal-Wallis test | 0.19 | 0.285 | 0.378 |
| MET skipped exon 2 | χ2 test | 0.957 | 0.105 | 0.237 |
| TMB | Kruskal-Wallis test | 0.18 | 0.378 | 0.655 |
| RHOA mutation | χ2 test | 0.239 | - | - |
| Percent tumor cells | Kruskal-Wallis test | 0.277 | < 0.01 | 0.178 |
| Estimated leukocyte percentage | Kruskal-Wallis test | < 0.05 | 0.101 | 0.372 |
| Intestinal type subclass | χ2 test | 0.133 | 0.257 | 0.527 |
| Anatomic region | χ2 test | 0.732 | 0.72 | 0.0754 |
| NDS-AJCC | χ2 test | 0.443 | 0.277 | 0.299 |
| EBV present | χ2 test | 0.48 | 0.132 | 0.814 |
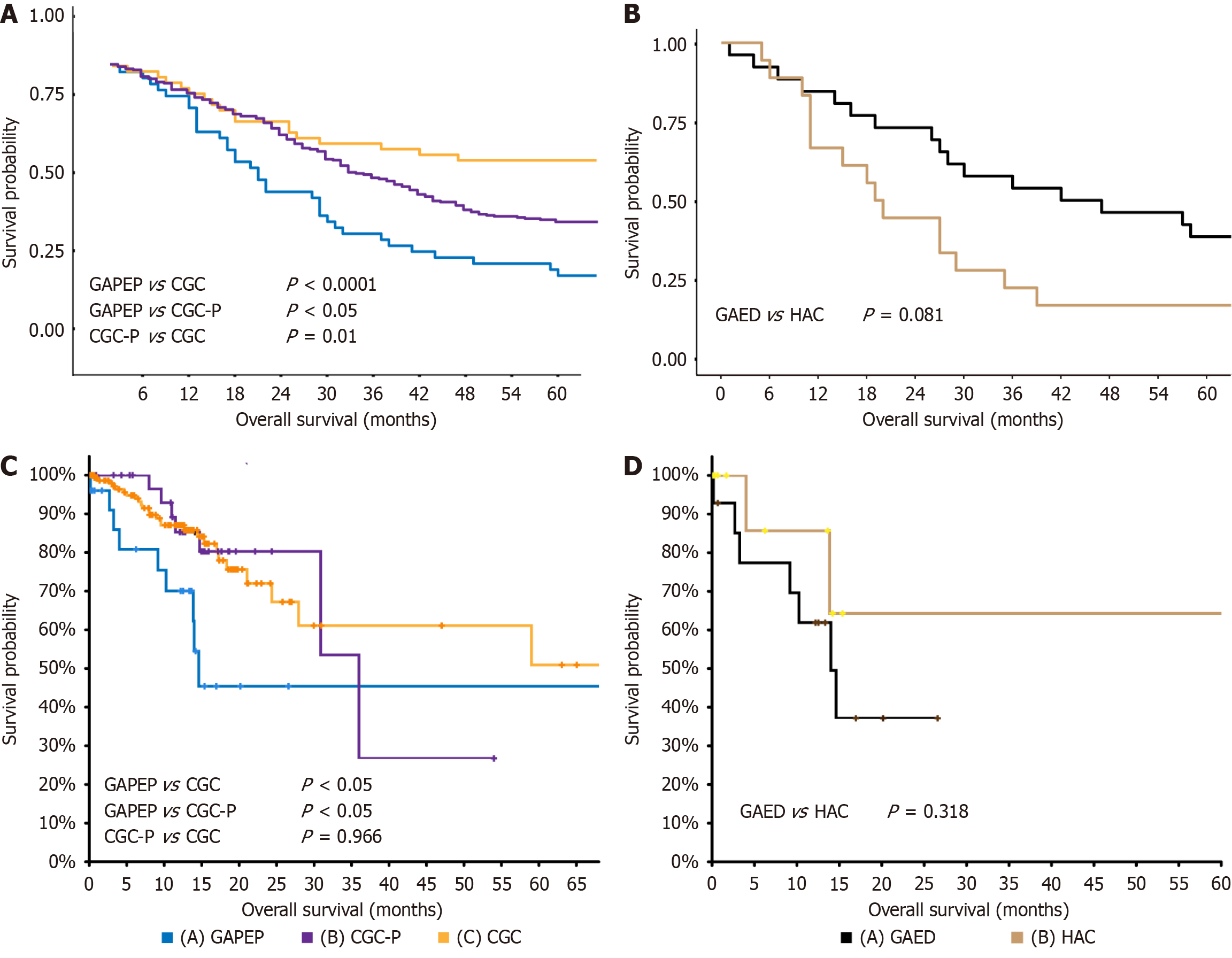
GAPEP was markedly associated with poor prognosis: Prognosis analysis of our data indicated that compared with CGC-P and CGC, GAPEP was associated with poorer overall survival (P < 0.01 and P < 0.0001, respectively) (Figure 3A). Stratified analysis revealed no differences in prognosis between GAED and HAC (P = 0.0810) (Figure 3B). Moreover, the TCGA dataset was analyzed to better clarify the differences in prognosis between GAPEP, CGC-P, and CGC. The results showed that GAPEP had worse overall survival compared to CGC-P (P < 0.05) and CGC (P < 0.05). There was no difference in prognosis between CGC-P and CGC (P = 0.9960) (Figure 3C). The results of stratified analysis of data from the TCGA dataset also exhibited no differences in prognosis between GAED and HAC (P = 0.0810) (Figure 3D).
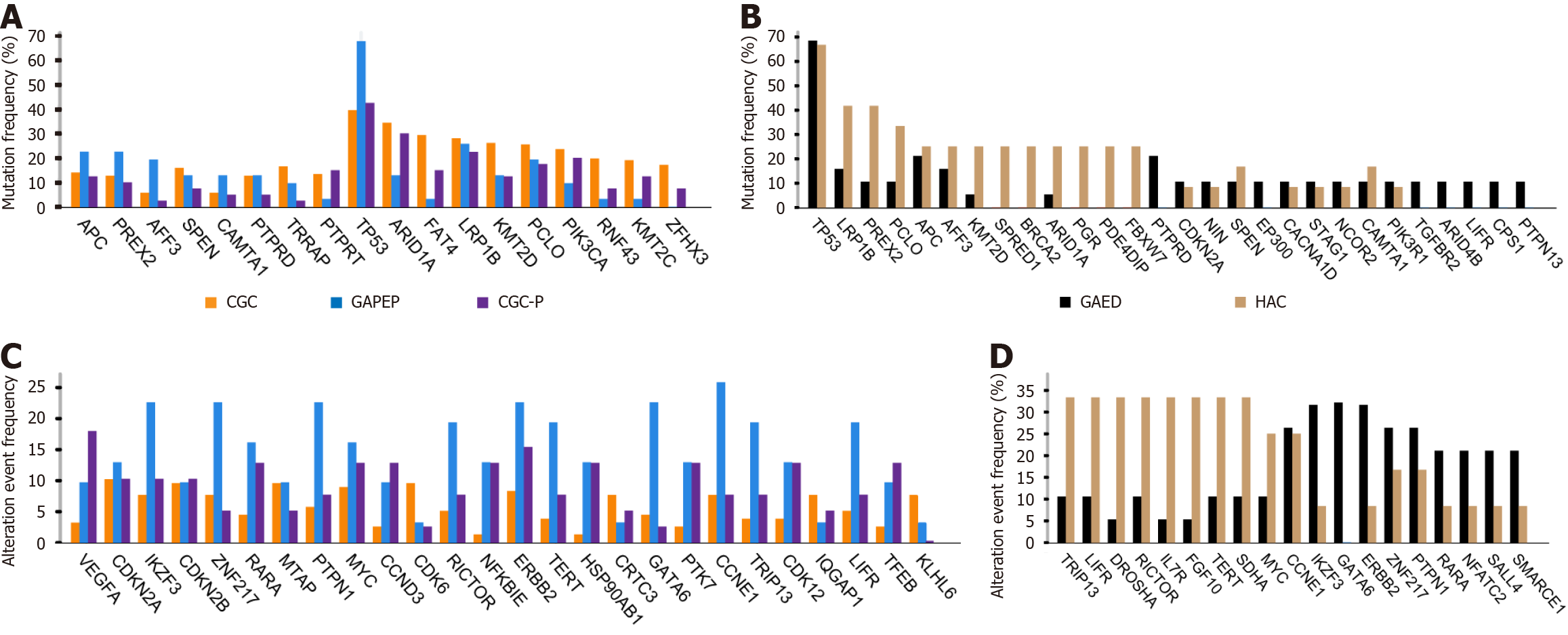
Mutation analysis: After applying our filtering strategy, 361, 525, and 984 known cancer-associated genes were detected among the mutated genes in GAPEP, CGC-P, and CGC, respectively. The TP53 was the most frequently mutated gene in GAPEP with a mutation frequency of 67.7%, followed by LRP1B (25.8%), APC (22.6%), PREX2 (22.6%), PCLO (19.4%), AFF3 (19.4%), SPEN (12.9%), CAMTA1 (12.9%), PTPRD (12.9%), and ARID1A (12.9%). In CGC-P, TP53 exhibited the highest mutation frequency (42.5%), followed by ARID1A (30%), LRP1B (22.5%), RNF43 (20.5%), PIK3CA (20%), PCLO (17.5%), TRRAP (16.8%), FAT4 (15%), PTPRT (15%), KMT2C (12.5%), RELN (12.5%), and KMT2D (12.5%). Moreover, CGC harbored the highest number of mutated cancer genes, including TP53 (40.5%), ARID1A (36.2%), FAT4 (30.3%), LRP1B (28.1%), KMT2D (27%), PCLO (25.4%), PIK3CA (23.2%), RNF43 (20.5%), KMT2C (19.5%), TRRAP (16.8%), ZFHX3 (16.8%), and RELN (16.8%) (Figure 4A). TP53 mutations were frequently observed in GAPEP, CGC-P, and CGC. TP53, APC, and AFF3 were more frequently mutated in GAPEP than in CGC-P and CGC. However, CGC-P and CGC had a higher frequency of ARID1A, PIK3CA, KMT2C, and FAT4 mutations. No significant differences were found in gene mutation between GAPEP, CGC-P, and CGC. CGC and CGC-P exhibited similar profiles of gene mutation.
In GAPEP, the HAC group harbored the highest number of mutated genes, including TP53 (66.7%), LRP1B (41.7%), PREX2 (41.7%), PCLO (33.3%), APC (25%), AFF3 (25%), KMT2D (25%), BRCA2 (25%), ARID1A (25%), and PDE4DIP (25%). On the other hand, the GAED group had the highest number of mutated genes, including TP53 (68.4%), APC (21.1%), PTPRD (21.1%), LRP1B (15.8%), AFF3 (15.8%), CDKN2A (10.5%), NIN (10.5%), SPEN (10.5%), PCLO (10.5%), and ARID4B (10.5%) (Figure 4B). Both HAC and GAED showed extremely high frequencies of TP53 mutation. There was no significant genetic difference between HAC and GAED.
Copy number alteration analysis: After applying the filtering strategy, we detected 672, 371, and 1295 known cancer-associated genes with DNA copy number alteration in GAPEP, CGC-P, and CGC, respectively. CCNE1 most frequently showed copy number alteration in GAPEP with an alteration frequency of 25.8%, followed by IKZF3 (22.60%), ZNF217 (22.60%), PTPN1 (22.60%), ERBB2 (22.6%), RICTOR (19.40%), TERT (19.40%), GATA6 (19.40%), TRIP13 (19.40%), and LIFR (19.40%). The most frequently altered gene in CGC-P was VEGFA (17.9%), followed by ERBB2 (15.4%), RARA (12.8%), MYC (12.8%), CCND3 (12.8%), NFKBIE (12.8%), HSP90AB1 (12.8%), PTK7 (12.8%), CDK12 (12.8%), and TFEB (12.8%). Moreover, MYC (10.30%), CDKN2A (9.80%), CDK6 (9.2%), ERBB2 (9.2%), CDKN2B (9.2%), MTAP (9.2%), GATA4 (8.7%), IKZF3 (8.2%), TRRAP (7.6%), and ZNF217 (7.6%) exhibited the highest number of DNA copy-number alterations in CGC. The amplified genes, such as CCNE1, IKZF3, ZNF217, PTPN1, ERBB2, RICTOR, TERT, GATA6, TRIP13, and LIFR, all had higher frequencies in GAPEP than in CGC and CGC-P; however, no significant differences were found (Figure 4C). Further analysis showed that DNA copy number alteration in CIC (P < 0.0001), DUSP22 (P < 0.0001), IRF4 (P < 0.0001), BRCA1 (P < 0.001), and ETV4 (P < 0.001) was significantly more prevalent in GAPEP compared to CGC-P and CGC.
Moreover, stratified analyses showed that in GAPEP, the HAC group harbored more genes with copy number alterations, such as TRIP13 (33.3%), LIFR (33.3%), DROSHA (33.3%), RICTOR (33.3%), IL7R (33.3%), FGF10 (33.3%), TERT (33.3%), SDHA (33.3%), MYC (25%), and CCNE1 (25%). On the other hand, the GAED group had the highest copy number alterations in ERBB2 (31.6%), GATA6 (31.6%), IKZF3 (31.6%), CCNE1 (26.3%), ZNF217 (26.3%), PTPN1 (26.3%), RARA (21.1%), NFATC2 (21.1%), SALL4 (21.1%), and SMARCE1 (21.1%) (Figure 4D); however, no significant differences were found between HAC and GAED.
mRNA expression level and key signaling pathways: According to the standard deviation of log2 expression, the following genes showed the highest levels of mRNA expression in GAPEP: GAPDH, HSP90AB1, CALR, P4HB, HSP90B1, ATP5F1B, TUBB, MDH2, HSPA5, and NCL. In CGC-P, the following genes showed the highest levels of mRNA expre
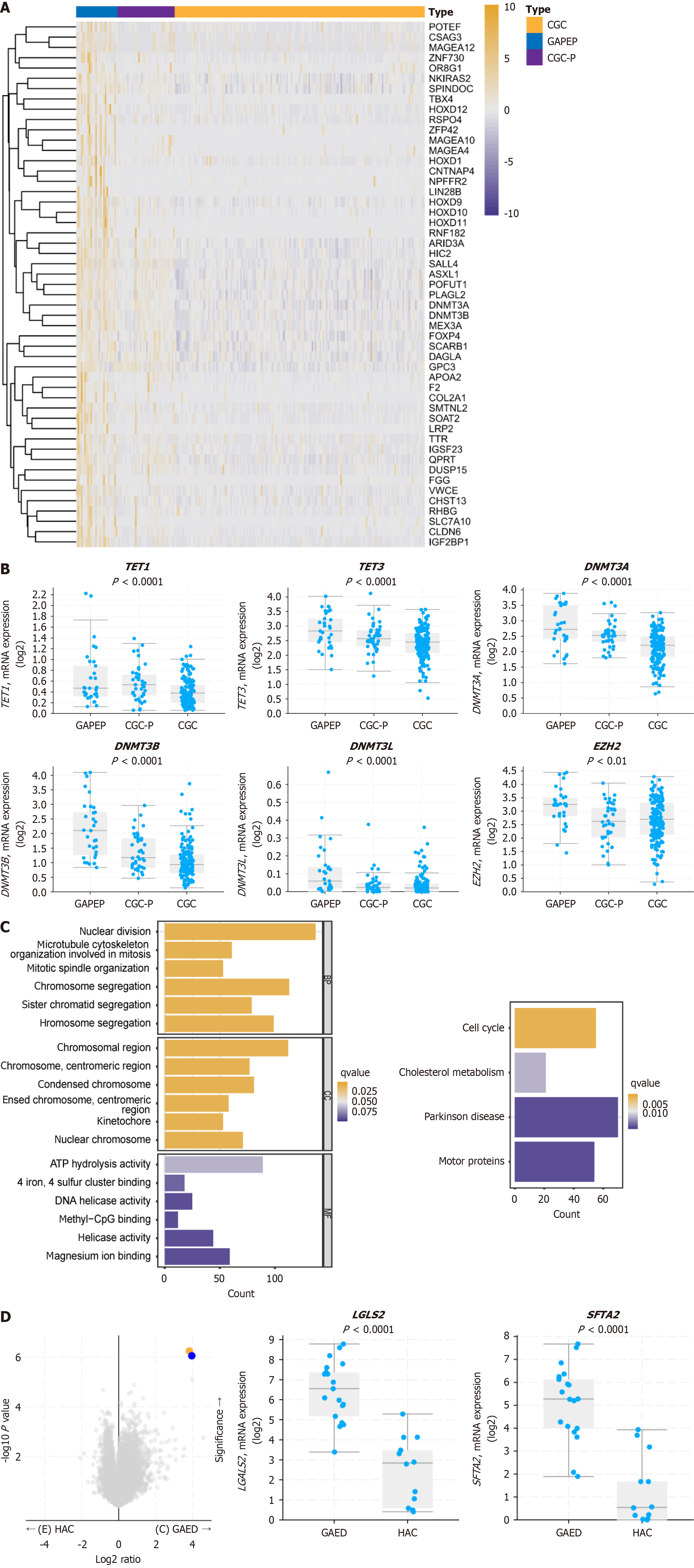
In total, 3541 differentially expressed genes (DEGs) were identified between GAPEP, CGC-P, and CGC. The heatmap showed the top 50 DEGs of GAPEP, CGC-P, and CGC (Figure 5A). Further analysis revealed that the DNMT, TET, TDG, IDH, and EZH2 genes, which are involved in methylation, were upregulated in GAPEP. Specifically, TET1 (P < 0.0001), TET3 (P < 0.0001), DNMT3A (P < 0.0001), DNMT3B (P < 0.0001), DNMT3 L (P < 0.0001), EZH2 (P < 0.01), IDH1 (P < 0.01), IDH3B (P < 0.01), and TDG (P < 0.0001) were upregulated in GAPEP compared with CGC-P and CGC (Figure 5B). These findings indicated that methylation-related genes play a crucial role in the development of GAPEP.
GO and KEGG pathway analyses were performed to unravel the biological function and molecular mechanism of the DEGs in GAPEP, CGC-P, and CGC. The top 1000 positively and negatively correlated DEGs were selected for analysis. The GO analysis revealed that biological process terms were implicated in nuclear division and chromosome segregation. The cellular component terms were related to chromosomal regions, condensed chromosomes, and centromeric regions. The molecular function terms were associated with ATP hydrolysis, methyl-CpG binding, and helicase activity (Figure 5C). The KEGG pathway analysis indicated that the GAPEP-related signaling pathways were enriched in cell cycle signaling, cholesterol metabolism, Parkinson’s disease, and motor proteins (Figure 5C).
Stratified analyses showed that the GAED group harbored the highest expression of mRNA in the following genes: ACTB, GAPDH, FTL, MT-ND5, ACTG1, TMSB10, RPLP1, TPT1, RPS11, and RPL27. The HAC group harbored the highest mRNA expression of the following genes: GAPDH, ACTB, ACTG1, RPS11, RPS6, RPLP1, FTL, TPT1, TMSB10, and B2M. Both the GAED and HAC groups had extremely high expression of GAPDH and ACTB. SFTA2 and LGALS2 were the only genes that showed significantly different expression between HAC and GAED (P < 0.001 and P < 0.001, respectively) (Figure 5D).
According to the research results of Yamazawa et al[10], GAPEP is a subtype of GC that expresses at least one primitive phenotypic marker. However, this study has certain limitations, as it primarily focuses on the value of immunophenotype in diagnosing GAPEP and does not delve into the morphological significance. Our study indicated that some cases of conventional tubular or papillary adenocarcinomas, mucinous adenocarcinomas, and indolent cell carcinomas can also express SALL4, GPC3, and AFP. These types of adenocarcinomas were classified as CGC-P in this article, as they do not have the morphological characteristics of GAPEP (including HAC or GAED). Further analyses also showed that CGC-P did not exhibit aggressive clinicopathological characteristics and unfavorable prognosis compared to GAPEP, and it was comparable to CGC. Additionally, molecular studies demonstrated that GAPEP and CGC-P exhibited significantly diffe
Compared to CGC-P and CGC, GAPEP was associated with aggressive clinicopathological features, in terms of tumor size, tumor differentiation, differentiated subtypes, perineural invasion, lymph node metastasis, and p53 overexpression. Bioinformatic analysis revealed that GAPEP, CGC-P, and CGC were significantly different in terms of molecular subtype, TP53 mutation, ERBB2 amplification, ARID1A mutation, MSI status, fraction genome altered, copy number cluster, CIMP category, and hyper-mutated. Subgroup analysis unveiled that HAC and GAED shared similar clinicopathological and molecular genetic characteristics. There were no significant differences in prognosis between GAED and HAC. Geneti
We also analyzed data from the TCGA to clarify the molecular aspects of GAPEP and found that methylation modi
The pathogenesis of GAPEP remains to be found in future studies. Between embryonic days 17-23 of human gestation, the caudal and terminal ventral walls of the foregut develop into the stomach and liver, respectively[30]. Therefore, the histological features and immunophenotypes of GAPEP are similar to those of the early fetal gut and embryonic liver, both of which express SALL4, GPC3, and AFP. The development of GAPEP may be a reenactment of foregut differentiation during embryonic development[10]. SALL4 is a critical marker of stem cells and plays a key role in the self-renewal of embryonic stem cells[31]. Overexpression of SALL4 contributes to the proliferation, development, invasion, and mi
Our study has several limitations. First, although our single-center cohort represents the largest GAPEP cohort, the sample size remains modest due to the low incidence of this subtype, potentially limiting the generalizability of our conclusions. To enhance the robustness of our findings, we validated key methylation differences between GAPEP and CGC/CGC-P using the TCGA-Stomach Adenocarcinoma cohort and confirmed their consistency. Future multi-center collaborations should expand the cohort size and assess the stability of molecular features across diverse populations. Second, although the TCGA database provides an authoritative resource, we could not access all whole-slide imaging sections for all cases. Therefore, we could not comprehensively assess the histologic pattern of the disease. The retro
The aggressiveness of GAPEP is closely associated with methylation dysregulation, and achieving a precise diagnosis necessitates a combined approach integrating morphological analysis with molecular biomarkers. Accurate diagnosis of GAPEP is of great importance. The morphology of GAPEP was diverse, suggesting a multilineage differentiation. We also emphasized that HAC and GAED have a monoclonal origin. Targeting methylation could provide new therapeutic opportunities for treating this aggressive cancer.
We would like to sincerely thank Professor Giulia De Falco from the School of Biological and Chemical Sciences, Queen Mary University of London, and Professor Jiang Huang from Guangzhou Huayin Healthcare Group.
| 1. | Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 1238] [Article Influence: 619.0] [Reference Citation Analysis (0)] |
| 2. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 3. | Sigal M, Rothenberg ME, Logan CY, Lee JY, Honaker RW, Cooper RL, Passarelli B, Camorlinga M, Bouley DM, Alvarez G, Nusse R, Torres J, Amieva MR. Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology. 2015;148:1392-404.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S, Renz BW, Ariyama H, Dubeykovskaya ZA, Takemoto Y, Lee Y, Muley A, Tailor Y, Chen D, Muthupalani S, Fox JG, Shulkes A, Worthley DL, Takaishi S, Wang TC. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Kim TH, Shivdasani RA. Stomach development, stem cells and disease. Development. 2016;143:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 5100] [Article Influence: 425.0] [Reference Citation Analysis (4)] |
| 7. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 851] [Article Influence: 283.7] [Reference Citation Analysis (2)] |
| 8. | World Health Organization. Publication of WHO Classification of Tumours, 5th Edition, Volume 1: Digestive System Tumours. Jul 11, 2019. [cited 27 April 2025]. Available from: https://www.iarc.who.int/news-events/publication-of-who-classification-of-tumours-5th-edition-volume-1-digestive-system-tumours/. |
| 9. | Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4391] [Article Influence: 146.4] [Reference Citation Analysis (1)] |
| 10. | Yamazawa S, Ushiku T, Shinozaki-Ushiku A, Hayashi A, Iwasaki A, Abe H, Tagashira A, Yamashita H, Seto Y, Aburatani H, Fukayama M. Gastric Cancer With Primitive Enterocyte Phenotype: An Aggressive Subgroup of Intestinal-type Adenocarcinoma. Am J Surg Pathol. 2017;41:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 11. | Matsunou H, Konishi F, Jalal RE, Yamamichi N, Mukawa A. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer. 1994;73:534-540. [PubMed] [DOI] [Full Text] |
| 12. | Wang Y, Wei X, Ke B, Liu J, Guo Y, Liu Y, Chen Y, Ding T, Wang Y, Meng B, Sun B, Zang F. Exploring the molecular characteristics of the malignant potential of gastric adenocarcinoma with enteroblastic differentiation. Histopathology. 2023;83:631-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Ushiku T, Uozaki H, Shinozaki A, Ota S, Matsuzaka K, Nomura S, Kaminishi M, Aburatani H, Kodama T, Fukayama M. Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomas. Cancer Sci. 2009;100:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Ushiku T, Shinozaki A, Shibahara J, Iwasaki Y, Tateishi Y, Funata N, Fukayama M. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol. 2010;34:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Akazawa Y, Saito T, Hayashi T, Yanai Y, Tsuyama S, Akaike K, Suehara Y, Takahashi F, Takamochi K, Ueyama H, Murakami T, Watanabe S, Nagahara A, Yao T. Next-generation sequencing analysis for gastric adenocarcinoma with enteroblastic differentiation: emphasis on the relationship with hepatoid adenocarcinoma. Hum Pathol. 2018;78:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, Yuan J, Wang X, Li J, Lu Z, Gong J, Lu M, Zhou J, Peng Z, Shen L, Zhang X. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Amin MB, Gress DM, Meyer Vega LR, Edge SB. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2018. |
| 18. | Li H, Zheng L, Zhang X, Yu X, Zhong G, Chen X, Chen X, Chen L. SH3 domainbinding glutamic acidrich proteinlike 3 is associated with hyperglycemia and a poor outcome in EpsteinBarr virusnegative gastric carcinoma. Oncol Lett. 2025;29:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142:1364-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 738] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 20. | Wang HL, Kim CJ, Koo J, Zhou W, Choi EK, Arcega R, Chen ZE, Wang H, Zhang L, Lin F. Practical Immunohistochemistry in Neoplastic Pathology of the Gastrointestinal Tract, Liver, Biliary Tract, and Pancreas. Arch Pathol Lab Med. 2017;141:1155-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Kishimoto T, Nagai Y, Kato K, Ozaki D, Ishikura H. Hepatoid adenocarcinoma: a new clinicopathological entity and the hypotheses on carcinogenesis. Med Electron Microsc. 2000;33:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Akiyama S, Tamura G, Endoh Y, Fukushima N, Ichihara Y, Aizawa K, Kawata S, Motoyama T. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer. 2003;106:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kumashiro Y, Yao T, Aishima S, Hirahashi M, Nishiyama K, Yamada T, Takayanagi R, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol. 2007;38:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | He F, Fu Y, Sun Q, Geng P, Zheng Z, Pu X, Shi J, Fan X. Integrated clinicopathological and immunohistochemical analysis of gastric adenocarcinoma with hepatoid differentiation: an exploration of histogenesis, molecular characteristics, and prognostic markers. Hum Pathol. 2021;115:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 1029] [Article Influence: 114.3] [Reference Citation Analysis (1)] |
| 26. | Zhang X, Su J, Jeong M, Ko M, Huang Y, Park HJ, Guzman A, Lei Y, Huang YH, Rao A, Li W, Goodell MA. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet. 2016;48:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 27. | He LJ, Cai MY, Xu GL, Li JJ, Weng ZJ, Xu DZ, Luo GY, Zhu SL, Xie D. Prognostic significance of overexpression of EZH2 and H3k27me3 proteins in gastric cancer. Asian Pac J Cancer Prev. 2012;13:3173-3178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | He ZC, Yang F, Guo LL, Wei Z, Dong X. LncRNA TP73-AS1 promotes the development of Epstein-Barr virus associated gastric cancer by recruiting PRC2 complex to regulate WIF1 methylation. Cell Signal. 2021;110094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Wang P, Zhao L, Rui Y, Ding Y. SMYD3 regulates gastric cancer progression and macrophage polarization through EZH2 methylation. Cancer Gene Ther. 2023;30:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Han L, Chaturvedi P, Kishimoto K, Koike H, Nasr T, Iwasawa K, Giesbrecht K, Witcher PC, Eicher A, Haines L, Lee Y, Shannon JM, Morimoto M, Wells JM, Takebe T, Zorn AM. Single cell transcriptomics identifies a signaling network coordinating endoderm and mesoderm diversification during foregut organogenesis. Nat Commun. 2020;11:4158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, Lasota J, Lee YS. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Yang Y, Wang X, Liu Y, Hu Y, Li Z, Li Z, Bu Z, Wu X, Zhang L, Ji J. Up-Regulation of SALL4 Is Associated With Survival and Progression via Putative WNT Pathway in Gastric Cancer. Front Cell Dev Biol. 2021;9:600344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 33. | Wang J, Huang J, Ma Q, Liu G. Association between quantitative parameters of CEUS and Sall4/Wnt/β-catenin signaling in patients with hepatocellular carcinoma. Cancer Manag Res. 2019;11:3339-3347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Wang M, Qiu R, Gong Z, Zhao X, Wang T, Zhou L, Lu W, Shen B, Zhu W, Xu W. miR-188-5p emerges as an oncomiRNA to promote gastric cancer cell proliferation and migration via upregulation of SALL4. J Cell Biochem. 2019;120:15027-15037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Zhang X, Zhang P, Shao M, Zang X, Zhang J, Mao F, Qian H, Xu W. SALL4 activates TGF-β/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res. 2018;10:4459-4470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Zhao B, Wang Y, Tan X, Ke K, Zheng X, Wang F, Lan S, Liao N, Cai Z, Shi Y, Zheng Y, Lai Y, Wang L, Li Q, Liu J, Huang A, Liu X. Inflammatory Micro-environment Contributes to Stemness Properties and Metastatic Potential of HCC via the NF-κB/miR-497/SALL4 Axis. Mol Ther Oncolytics. 2019;15:79-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Diener J, Baggiolini A, Pernebrink M, Dalcher D, Lerra L, Cheng PF, Varum S, Häusel J, Stierli S, Treier M, Studer L, Basler K, Levesque MP, Dummer R, Santoro R, Cantù C, Sommer L. Epigenetic control of melanoma cell invasiveness by the stem cell factor SALL4. Nat Commun. 2021;12:5056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Misawa K, Misawa Y, Mima M, Yamada S, Imai A, Mochizuki D, Nakagawa T, Kurokawa T, Endo S, Kawasaki H, Brenner JC, Mineta H. Overexpression of Sal-like protein 4 in head and neck cancer: epigenetic effects and clinical correlations. Cell Oncol (Dordr). 2020;43:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Xia Z, Qiu D, Deng J, Jiao X, Yang R, Sun Z, Wan X, Li J. Methylation-induced downregulation and tumor-suppressive role of microRNA-98 in glioma through targeting Sal-like protein 4. Int J Mol Med. 2018;41:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Peng Z, Zhang Y, Shi D, Jia Y, Shi H, Liu H. miR-497-5p/SALL4 axis promotes stemness phenotype of choriocarcinoma and forms a feedback loop with DNMT-mediated epigenetic regulation. Cell Death Dis. 2021;12:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Sun B, Xu L, Bi W, Ou WB. SALL4 Oncogenic Function in Cancers: Mechanisms and Therapeutic Relevance. Int J Mol Sci. 2022;23:2053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/