Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.108297
Revised: May 18, 2025
Accepted: July 2, 2025
Published online: July 28, 2025
Processing time: 105 Days and 13.3 Hours
The gastrointestinal (GI) tract is essential for digestion, absorption, excretion, and protection, supported by a diverse microbial ecosystem. Traditional in-vitro mo
Core Tip: This review highlights the need for advanced in-vitro models to better replicate the gastrointestinal (GI) tract’s complexity for translational research. It compares the evolution from conventional 2-dimensional cultures toward 3-dimensional and microfluidic systems. Key advancements include the use of patient-derived cells, engineered microenvironments, and bioprinting techniques like micro-extrusion and laser-assisted printing. These technologies enable modeling of essential processes such as peristalsis, molecular transport, and liver coupling. By enhancing physiological relevance, these models support personalized medicine and improve the predictive power of preclinical GI research.
- Citation: Skok K, Vihar B, Maver U, Gradišnik L, Bräutigam K, Trapecar M, Skok P. Gastrointestinal tract, its pathophysiology and in-vitro models: A “quick” reference guide to translational studies. World J Gastroenterol 2025; 31(28): 108297
- URL: https://www.wjgnet.com/1007-9327/full/v31/i28/108297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i28.108297
Despite significant advances in gastrointestinal (GI) research, existing in-vitro models often fail to recapitulate the full complexity of the GI tract. Functions of the digestive system include digestion, absorption, excretion, and protection. In addition to these functions, which are maintained by the respective organs individually, as well as in concert, there is a growing body of evidence that supports the importance of the microbiome, composed of a collection of bacteria, fungi, viruses and archaea. These produce a diverse ecosystem of about 1014 microorganisms[1]. Studying such a diverse and complicated system requires multidisciplinary approaches. A potential approach to studying these systems in a controlled environment are in-vitro as well as in vivo models[2]. The benefit of these models is the fact that they adhere to the “3Rs” principles, defined by Russell and Burch in 1959[3]. These 3Rs are: “Replace” animals used in experiments with non-sentient alternatives; “Reduce” the number of animals employed; and “Refine” animal experiments so that they cause minimum pain and distress[3,4]. This review aims to provide a comprehensive reference guide that bridges the gap between simple cell cultures and complex, dynamic systems used in translational studies[1,5-7]. It systematically details the anatomy and pathophysiology of specific digestive tract diseases, especially those with an inflammatory etiology, providing clear rationale for the necessity of complex model systems. Furthermore, it highlights the wide range of applications for these models and explores emerging trends in culturing techniques that better replicate the human digestive tract environment.
Following this introduction, we detail the GI tract and its pathologies. This will serve as a discussion basis. Following that we continue with in-vitro models, compare their performance, and discuss their translational relevance.
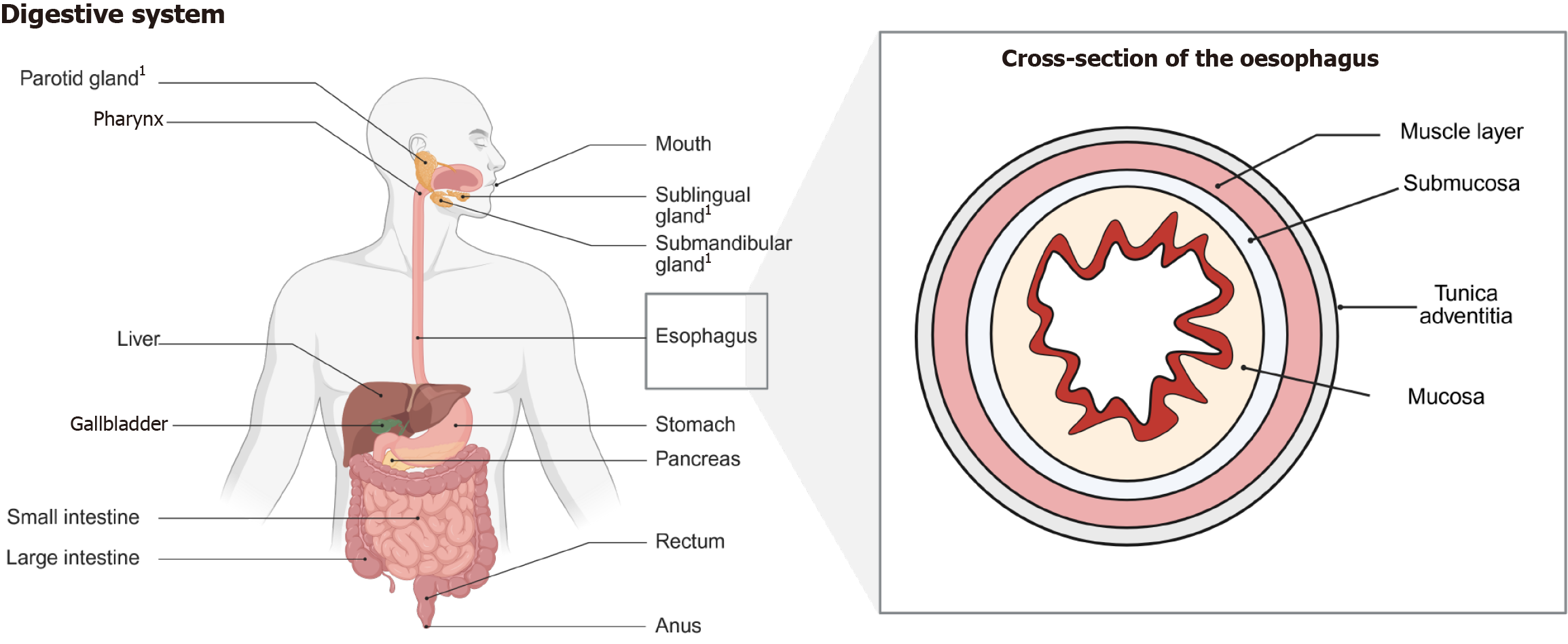
The digestive tract is estimated to be 8 m-9 m long and is composed of different interconnected organs (Figure 1). Malig
The adult oesophagus is an 18- to 25-cm-long muscular tube (Figure 1). In contrast to other portions of the GI tract the oesophagus does not have a serosal covering. The most common types of cancers of the oesophagus are SCCs and adeno
The stomach has been formerly only known as a hollow muscular structure, today, it is regarded as one of the most com
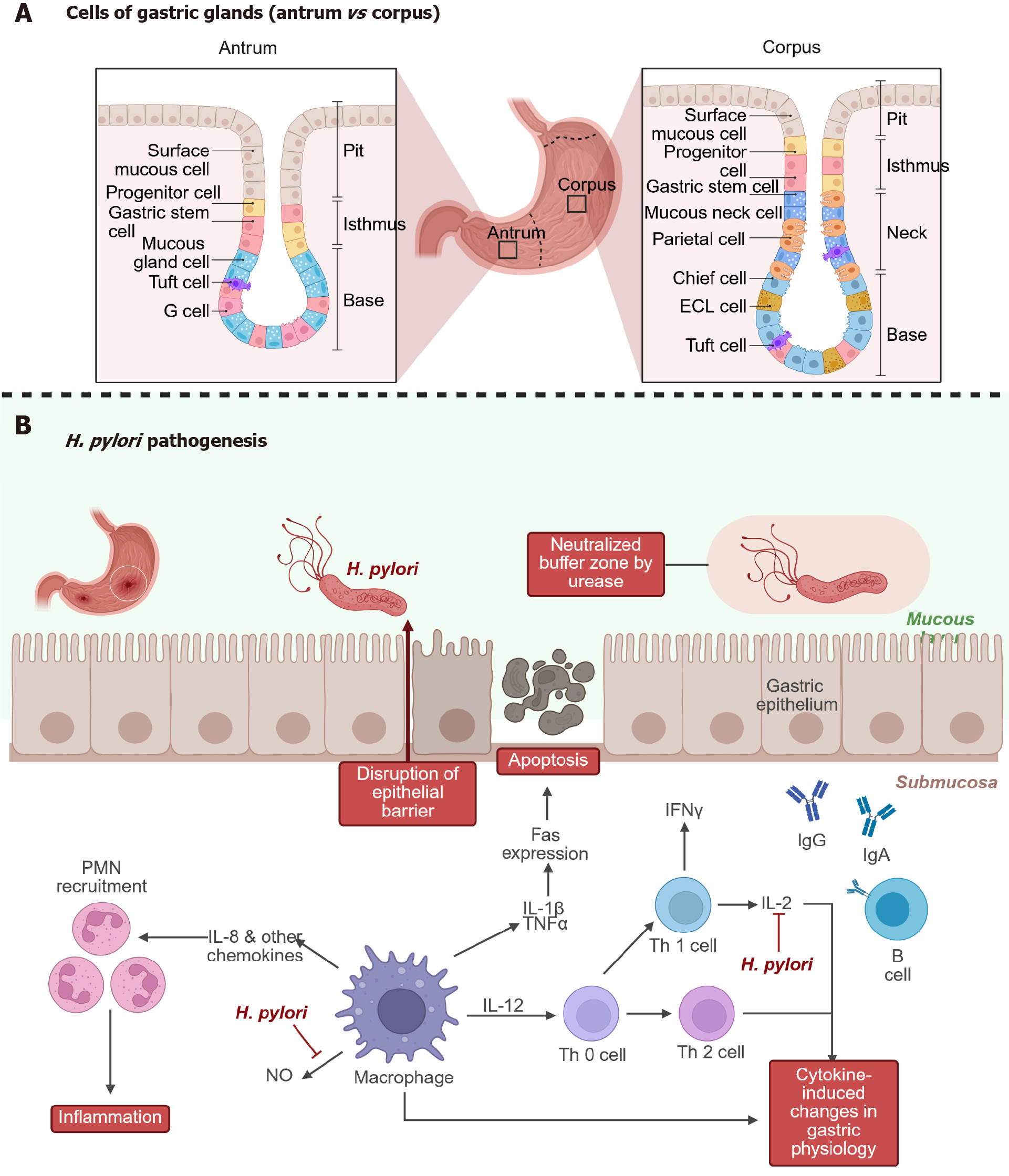
Chronic infection (Figure 2B) with Helicobacter pylori (H. pylori) is recognized as a key factor in the development of several gastric disorders, including gastric cancer, and remains a significant global health issue due to its widespread occurrence[18,19]. After entering the stomach, H. pylori adheres to the gastric epithelium, particularly at cell junctions, establishing a protected niche that allows it to survive in the otherwise hostile acidic environment[20,21]. Once estab
Understanding the underlying mechanisms remains challenging, largely because commonly used model systems do not accurately mimic the complex environment of the human stomach[23]. Standard in-vitro approaches often lack key physiological features such as dynamic potential of hydrogen conditions, mucus secretion, and the full spectrum of diffe
Hofer et al[27] just recently presented and patterned a homeostatic human gastric organ-on-a-chip (OoC) system with bilateral access. They claim that is capable of modeling H. pylori niche establishment and persistent colonization of the gastric epithelium[27]. The authors stated that under physiologically relevant acidic conditions at the apical surface, the OoC system supported the development of more mature gastric pit cells compared to conventional organoid cultures[27]. The differentiated pit cells displayed, after exposure to H. pylori, a distinct response that sets them apart from other epithelial cell types an aspect that had not been previously described. The authors concluded that the model could prove to be powerful tool for broader investigations into gastric epithelial dynamics, mucosal immune responses, and host-microbe interactions[27].
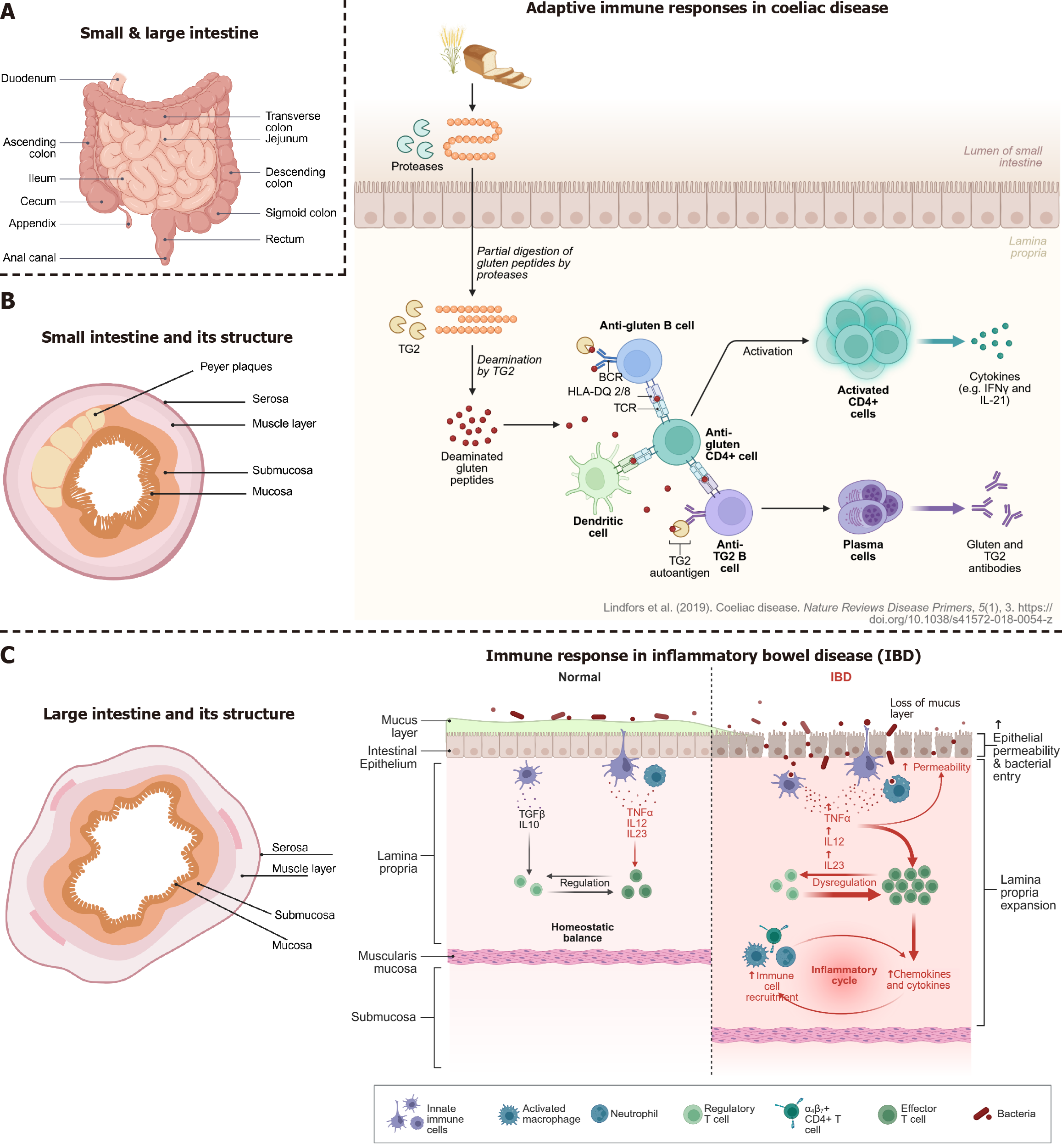
Diseases of the small intestine (Figure 3A and B) that are noteworthy in the context of in-vitro cell culturing are speci
CeD is an autoimmune enteropathy, 1%-2% of the general population, triggered by gluten in genetically susceptible individuals risk haplotypes [human leucocyte antigen (HLA)-DQ2.5, -DQ2.2, -DQ8, and -DQ8.5][32,33], with HLA-DQ2.5 carrying the strongest risk association[34]. The immune pathophysiology is complex. It involves innate and adaptive immune responses as well as the intestinal epithelium, which may interact with environmental risk factors (e.g., gut microbiome, luminal antigens, viral infections etc.)[34]. The disease leads to atrophy of the small intestinal mucosa and consequently to malabsorption[32]. The main histological features include villous atrophy, crypt hyperplasia and intraepithelial lymphocytosis. These findings are summarized in the modified MARSH criteria, which are used for histological diagnosis[35]. Ingested gluten is partially digested into peptides, including immunogenic fragments like gliadin [parti
To date, there is no model system that fully recapitulates the complexity of CeD[40]. Current in-vitro models include immortalized cell lines (CLs) and mucosal biopsies. The immune system has been investigated using CLs of monocytes, such as THP-1, or intestinally derived T cells[41,42]. Existing data on epithelial barrier function are largely based on the CLs Caco2, T84, and HT-29[43-45]. Immortalized CLs do not represent the genetics of CeD and have poor genomic integrity[40]. Patient-derived intestinal biopsy material does contain the CeD-associated genetic background and directly reflects the disease phenotype, but is scarce because of its invasive nature. Another option are murine models. In terms of completeness they present a living model with working inter-organ communication[46]. However, the model requires thorough understanding of induction of disease and is due to interspecies differences in physiology, pharmacology and cellular processes difficult to extrapolate to humans.
OoC technology may solve many of these drawbacks[47]. A recent paper[48] showcased the potential of an induced pluripotent stem cells (iPSCs)-derived small intestine-on-chip with a self-organised tight epithelial layer, including villus-like structures and a cell type composition that resembles the human small intestine. Just recently, an in-depth review on human organoids and OoC in CeD has been published[39].
Although significant progress has been made in managing inflammatory bowel disease (IBD)[49], a definitive cure remains out of reach. This is largely due to an incomplete understanding of the disease’s complex origins and biological mechanisms[31,50]. Over recent decades, a range of experimental models spanning in-vitro, in vivo, and ex vivo systems has been introduced to help close these knowledge gaps[31,40,46,51].
Crohn’s disease and ulcerative colitis (UC) are two main forms of IBD, but they differ significantly[52]. Crohn’s can impact any segment of the GI tract in a patchy, discontinuous pattern, often involving all layers of the gut wall[53]. UC, in contrast, is confined to the colon and rectum and affects only the inner lining in a continuous manner[54].
Current thinking emphasizes an abnormal immune response to intestinal microbes[55], likely triggered by environmental factors in genetically susceptible individuals[56]. In IBD, several key defense mechanisms in the gut are impaired. These include weakened tight junctions, changes in the mucus layer, and microbial imbalances[52]. Dysfunctional goblet and Paneth cells contribute to this, as they produce less protective mucus and antimicrobial substances, respectively. Defects in autophagy-related genes such as NOD2 and ATG16 L1 are also associated with increased disease risk[52].
The weakened barrier allows gut microbes to invade the intestinal wall, prompting immune cells like macrophages and dendritic cells to release pro-inflammatory cytokines [e.g., tumor necrosis factor (TNF)-α, IL-6, IL-23], which attract more immune cells and fuel ongoing inflammation[57-59]. This results in increased gut permeability and a self-sustaining inflammatory cycle[59]. A key feature is the imbalance between regulatory T cells (Tregs) and inflammatory Th17 cells, which contributes to immune overactivation[52].
Furthermore, mitochondrial dysfunction has emerged as a factor in IBD pathology[60]. Genes linked to mitochondrial stability (e.g., MDR1, HNF4A) are disrupted, leading to oxidative stress and impaired energy production in intestinal cells[52,61]. This mitochondrial damage affects cell renewal and further weakens the gut barrier. Such findings suggest that targeting mitochondrial health could be a promising strategy for restoring intestinal function and controlling inflammation in IBD[62].
There are various three-dimensional (3D) intestinal inflammation models that can at least partially, recapitulate IBD features. These include models based on scaffolds or hydrogels and those based on decellularized tissue models, as well as more complex intestine-on-a-chip systems and organoids.
Some of these include: Leonard et al[63] developed a 3D co-culture model by embedding human blood monocyte-derived macrophages and DCs in a collagen matrix on a semi-permeable Transwell® filter insert, with Caco-2 cells seeded on top. This model was subjected to different types of proinflammatory stimuli [lipopolysaccharides (LPS) from Escherichia coli and Salmonella typhimurium, IL-1β, IFN-γ], IL-1β presented the strongest induction of inflammation[63].
Later on, the model was further improved by replacing the primary immune cells with a macrophage-derived CLs and dendritic-like cells (MUTZ-3), in an effort to enhance reproducibility and facilitate a more comprehensive assessment of cytotoxicity[64].
Another study described a triple co-culture intestinal model consisting of an intestinal epithelial layer (Caco-2/HT29-MTX cells) and immunocompetent cells. The main goal was to represent a healthy intestine characterized by a stable intestinal barrier and to evaluate the efficacy of anti-inflammatory drugs[65].
Le et al[66] described a complex in-vitro triple-culture model aiming to develop an inflammation-triggered in-vitro leaky gut model using Caco-2/HT29-MTX-E12 combined with macrophage-like THP-1 cells or primary human-derived macrophages.
The large intestine is approximately 1.5 m long and its primary functions include desiccation, compaction of waste and storage in the sigmoid colon and rectum (Figure 3A). The colon is inhabited by a multitude of different bacteria, which produce vitamins (vitamin K and B), other metabolic by-products [e.g., short-chain fatty acids (SCFAs)] and help in regulating other important organic systems (e.g., gut-brain axis).
The most common disorders in the colon are different types of inflammations (“colitis”), ischaemic changes, distur
What is more, the role of the microbiota has gained in importance even in oncological therapy[81-83]. Emerging evi
Following this chapter, which was dedicated to the structural properties of the GI tract and their malignancies, we now explore the inhabitants, their impact on the physiology, individual cells and their translational value in modelling.
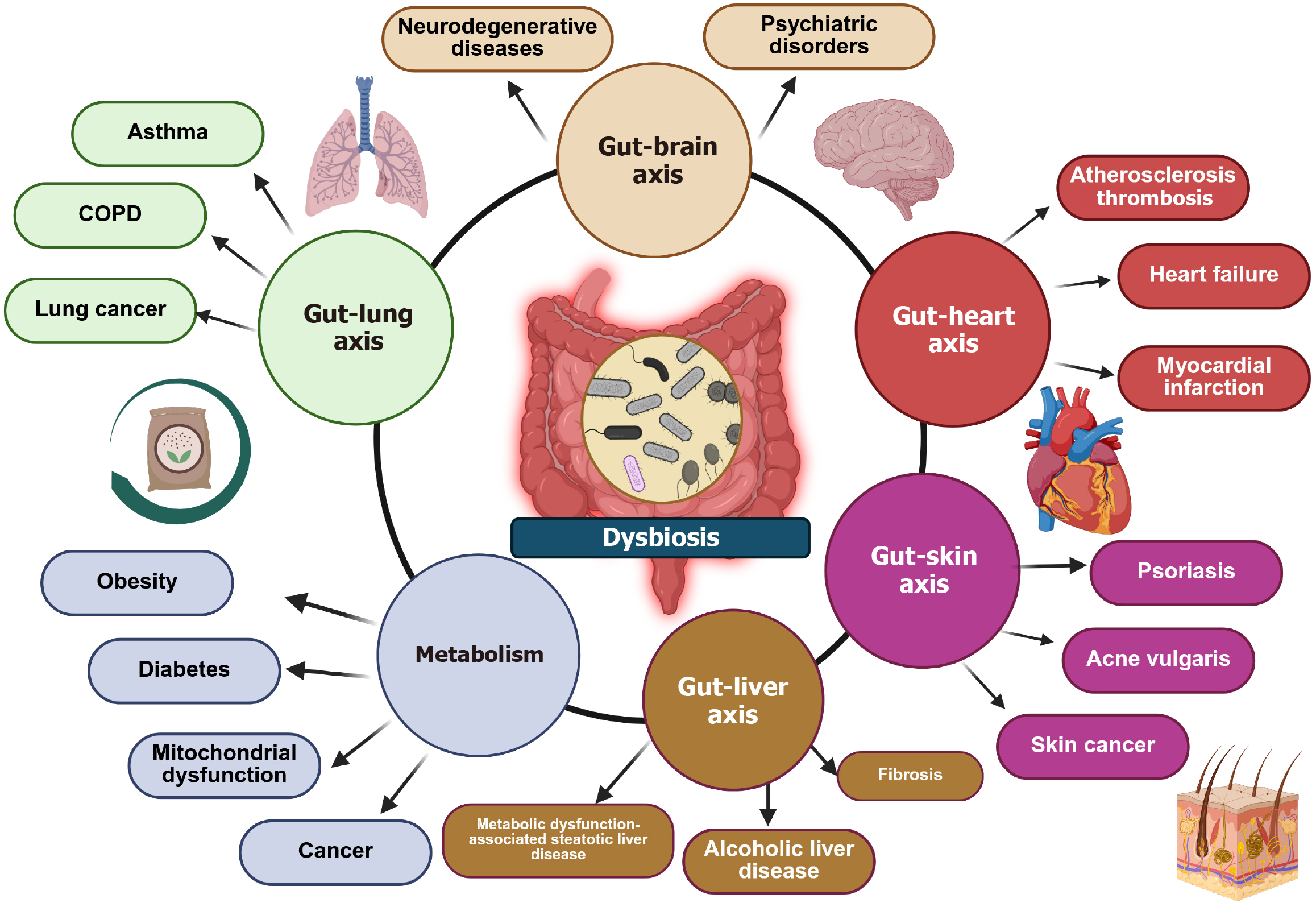
The GI tract gives home to a microbiome, composed of a collection of bacteria, fungi, viruses and archaea. These produce a diverse ecosystem of about 1014 microorganisms[1]. The term dysbiosis presents a change in the composition of the gut microbiota[1,85-87]. Over the last decade, knowledge about the relationship between dysbiosis and the pathogenesis of various diseases (especially cardiovascular disease) has rapidly accumulated[88-90]. It comes as no surprise that some of these potential diseases include cardiovascular disease, chronic kidney disease, type 2 diabetes mellitus, non-alcoholic fatty liver disease, and even certain types of cancer[85,91-94] (Figure 4). On the pathophysiological level it has been pro
A reduced expression of tight junction proteins (e.g., zonula occludens-1, claudin-1 and occluding) and an imbalance between epithelial cell death and regeneration can lead to a leaky-gut[85,95,96]. What follows is the translocation of bacteria, which stimulate, via the recognition of their pathogen associated molecular patterns, an immune response and general inflammatory reaction [secretion of pro-inflammatory cytokines (like IL-18, IL-1, IL-6, and TNF-α)]. This affects the whole organism (e.g., damaging the integrity of the blood brain barrier).
Gut dysbiosis increases systemic inflammation (via LPS, IL-6, TNF-α), contributing to endothelial dysfunction, hyper
Microbial metabolites (e.g., SCFAs, tryptophan metabolites) and neuroactive compounds (e.g., gamma-aminobutyric acid, serotonin, dopamine) modulate brain function and behavior. Dysbiosis can also lead to an altered synthesis of neu
The portal vein transports gut-derived bacterial metabolites and endotoxins directly to the liver. Dysbiosis and leaky gut lead to endotoxemia and hepatic inflammation via activation of Kupffer cells, which in turn release pro-inflammatory mediators, such as TNF-α, ILs (IL-1 and IL-10), lysosomal enzymes (protease and phosphatase)[100,101]. It has been shown that there are distinctive gut-liver axis disruption patterns in the prevalent chronic liver diseases, adrenoleukodystrophy and metabolic dysfunction-associated steatotic liver disease[102].
Gut microbiota shape systemic and pulmonary immunity through SCFAs and Treg induction. Gut dysbiosis increases pro-inflammatory cytokines and compromises lung mucosal immunity[103].
Microbiota regulate systemic and cutaneous inflammation, influence Treg/Th17 balance, and affect skin barrier integrity. SCFAs and tryptophan-derived metabolites promote anti-inflammatory pathways. Dysbiosis leads to skin flares via immune dysregulation and altered lipid metabolism. This has been linked to diseases such as psoriasis, atopic der
As an illustration, it has been shown that there is a potential fourfold increase in obesity risk within 15 years of emigrating to the United States, compared to populations remaining in their birth country. This fact is accompanied with a decrease in their gut microbial diversity and function[86,105].
Furthermore, environmental factors (e.g. diet, household cohabitation) greatly outweigh heritable genetic contributions to the composition and function of gut microbiota[106]. Rothschild et al[107] showed with their microbiome-association index, mimicking heritability statistics, that most significant associations were between the gut microbiome and host phenotypes for body mass index, waist-to-hip ratio, fasting glucose levels, glycemic status, high-density lipoprotein cholesterol levels, and monthly lactose consumption[106,107].
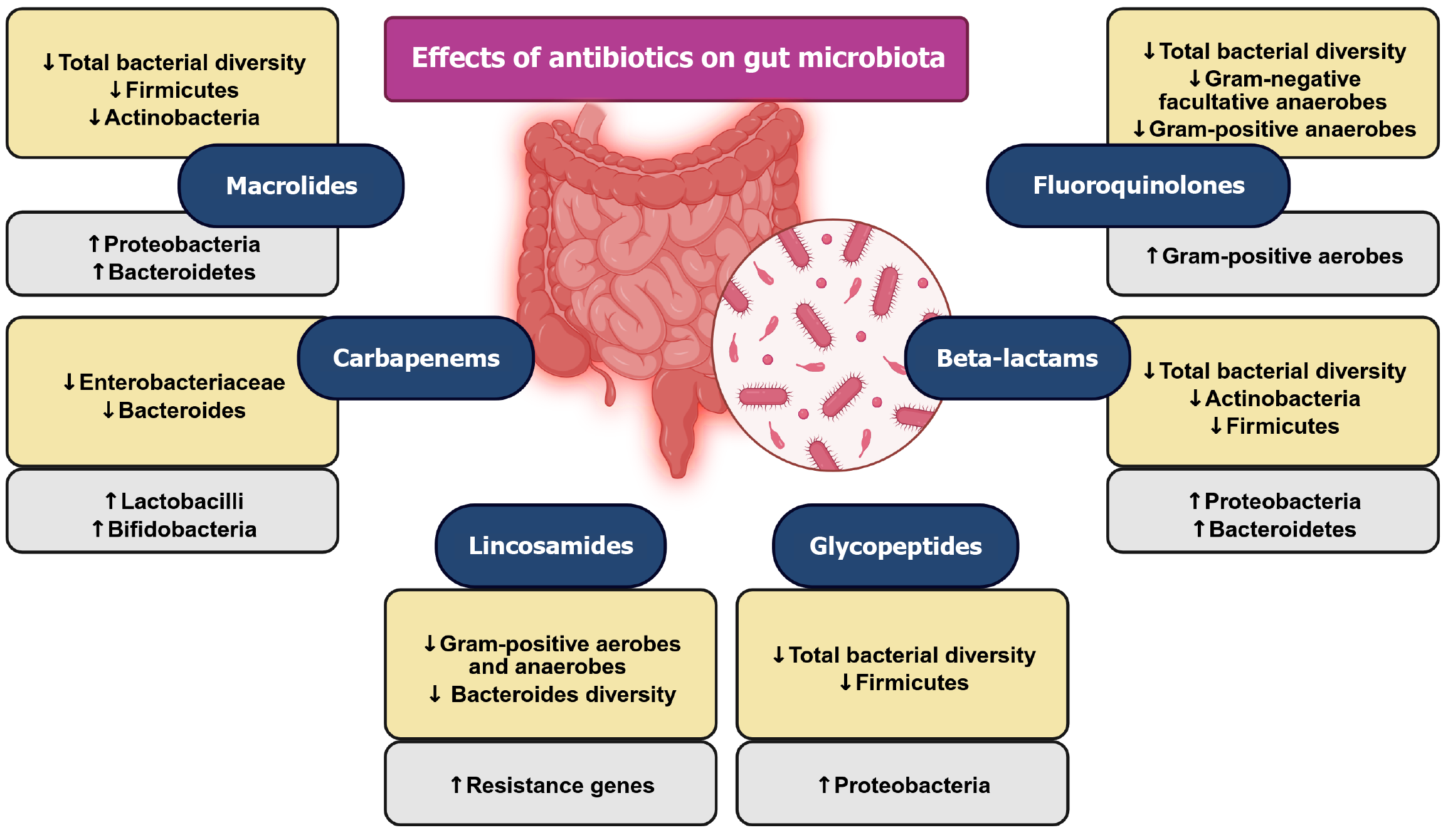
Antibiotic use for microbiota manipulation remains debatable due to potential side-effects (Figure 5). It represents an aggressive approach with drawbacks such as reduced bacterial diversity, altered gene expression, and selection for resistant bacteria[108,109], earning antibiotics the label of “deep modulators” of gut microbiota[109,110]. Studies link antibiotics to various outcomes, including effects on obesity, insulin resistance, diabetes, and myocardial infarction[111-113]. Faecal microbiota transplantation has already therapeutically confirmed the importance of a healthy gut microbiota in certain patients. This form of treatment is several decades old and still presents an important intervention[114].
Regarding the potential for modelling some of the gut axes, significant advances have been made. Trapecar et al[115] utilized interconnected human microphysiological systems of the gut, liver, and circulating Treg and Th17 cells to model UC ex vivo. The research revealed that microbiome-derived SCFAs can either alleviate or exacerbate UC severity, depending on the involvement of effector CD4 T cells. Their findings highlight the complex role of SCFAs in modulating inflammation within the gut-liver axis[115].
Also, in another study Trapecar et al[116] developed a human multi-organ OoC microphysiological system integrating the gut, liver, and brain to investigate how microbial metabolites influence neurodegenerative diseases. The study demonstrated that microbial metabolites, particularly SCFAs, can modulate neuroinflammation and neuronal health. The platform provides insights into the gut-liver-brain axis and its role in the pathogenesis of neurodegenerative conditions, offering a novel approach to study complex inter-organ interactions.
Furthermore, Zhang et al[117] presented a protocol for co-culturing primary human colon epithelial cells with human gut bacteria under controlled oxygen conditions, including anaerobic environments. The method enables studying host-microbe interactions in a physiologically relevant setting, facilitating research into how gut bacteria influence colon health and disease. Finally, Zhang et al[118] developed in 2024 an immune-competent human gut microphysiological system to examine the anti-inflammatory effects of the commensal bacterium Faecalibacterium prausnitzii. The findings indicate that Faecalibacterium prausnitzii can modulate inflammation within the human gut environment, highlighting its potential therapeutic role in treating IBD[118].
Collectively, these studies utilize advanced human microphysiological systems to explore complex interactions between the gut microbiota, host tissues, and the immune system, providing valuable insights into inflammatory and neurodegenerative diseases and show the great potential of these methods. These studies directly link us to our next section on the specifics of cell culturing.
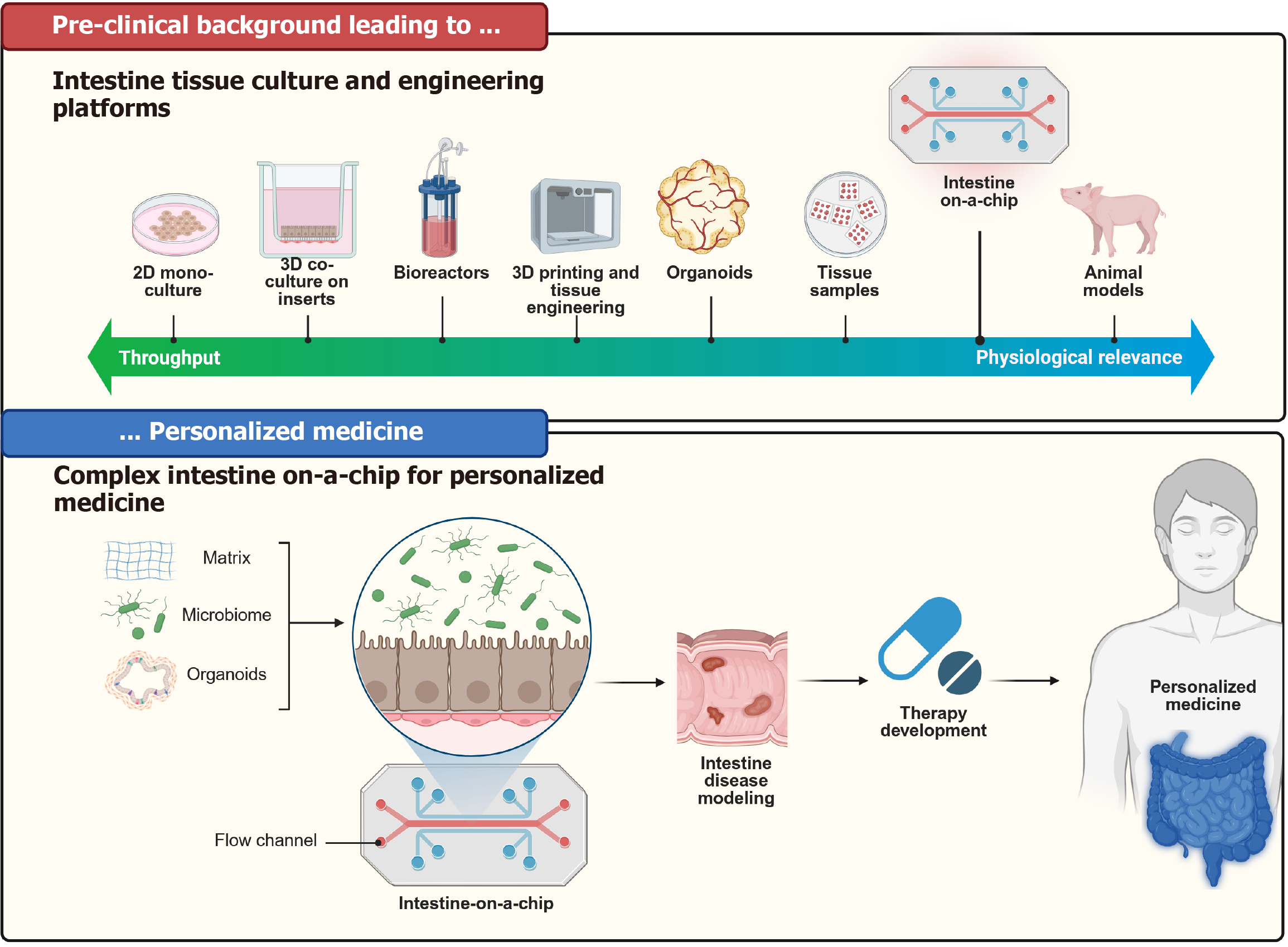
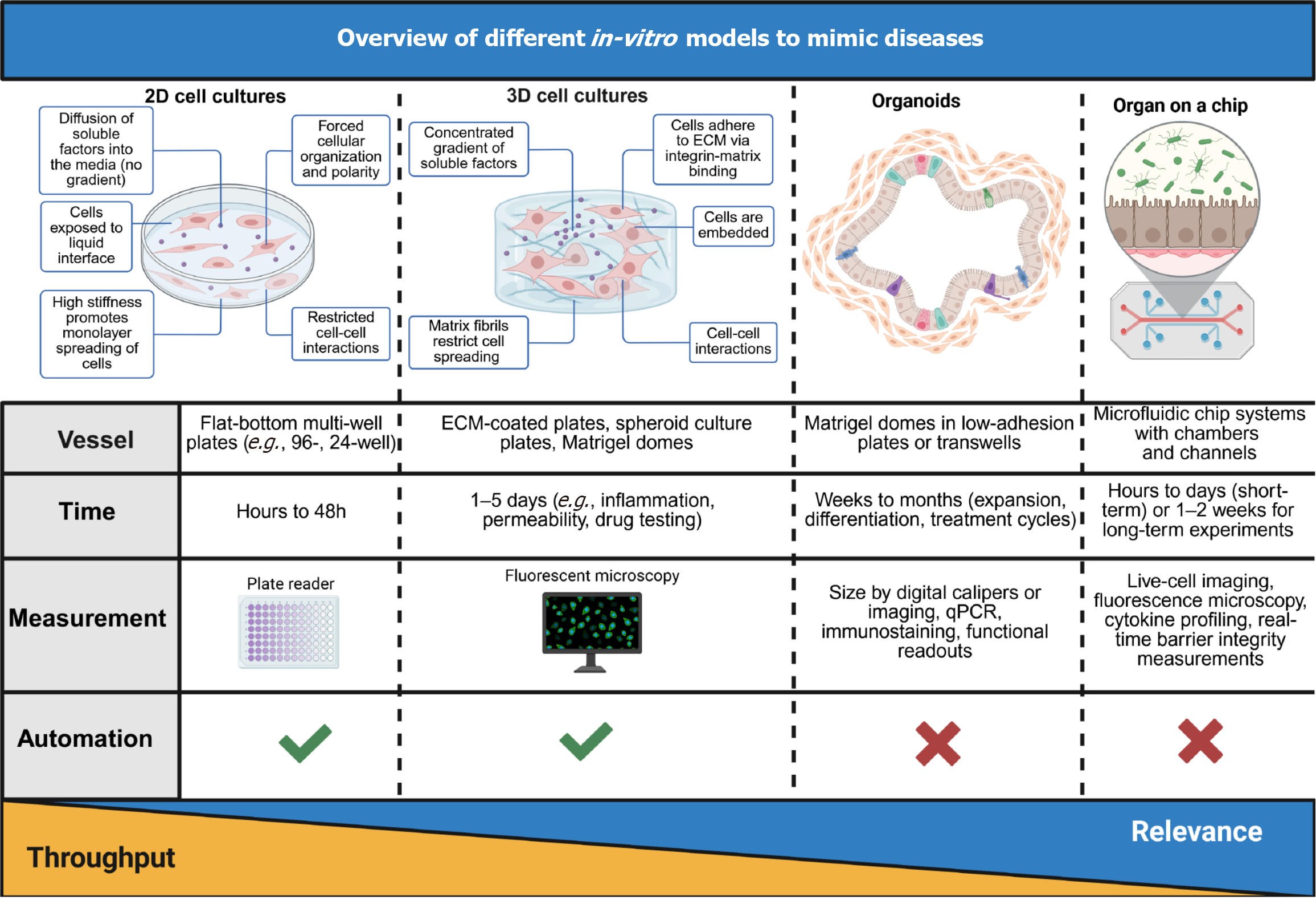
Key elements of the intestinal microenvironment are the biochemical interactions, the cells, which constitute the 3D architecture, flow dynamics and motility. Traditionally used two-dimensional (2D) immortalised (tumour) CLs survive long-term but are not genetically stable nor represent any human in particular. In contrast, primary cultures are patient-unique, but short-lived. Some other approaches, which have shown promise, include organoids[119-122] (spheroids and tumoroids)[123], multi-well systems[119,124], as well as microphysiological OoC models[125]. Their hierarchy, regarding complexity and usage applicability, can be seen in Figure 6. 3D organoid cultures resemble the crypt-villus domain and contain all cell lineages, are long-lived and genetically stable. Unfortunately, manipulation of the 3D organoid system is more challenging[126-128]. The applications of these models are manifold (e.g., functional test with drug and toxicity testing, tissue engineering and simulation of absorption, metabolism etc.)[2]. A comparison of the individual methods in relation to modelling human disease can be seen in Figure 7.
Many in-vitro intestinal models exist in the form of simple 2D systems, which rely on culturing an intestinal epithelial cell monolayer (e.g., Caco-2 cells) or co-cultured mixtures of intestinal cells on static micro-porous Transwell supports[129-131]. For modelling different disease states, immune cells and microbes can be added to the apical and basolateral side of the culture well, respectively[132].
However, to date, 2D in-vitro models have not readily provided an intestinal model that accurately recapitulates the architecture, segment specificity, paracrine and autocrine molecular signalling of healthy tissue. Additionally, various drug transporters are either mis-localized or have inaccurate expression in transformed cells when compared to the human intestine[133].
The biggest drawback, however, is the lack of cellular diversity in a single cell type system. Normal intestinal epithe
Overall, despite the drawbacks of 2D culturing, this method has significantly impacted our understanding of spatially organized structures, with cell identities resembling those found in tissues in vivo[135].
Caco-2 was developed in the 1970’s[136]. It has been widely studied in various domains (e.g., pharmacological, nutri
The HT-29 CL was isolated from a 44-year-old Caucasian female with colorectal adenocarcinoma in the 1970s’ by Fogh and Trempe[142]. The CL has a doubling time from 24 hours to 60 hours, is MSS and has 69 derived CLs[143]. It was initially used for cancer biology research but later transitioned to other studies due to its versatile phenotype. HT-29 cells can be cultured in an undifferentiated state or differentiated to form a polarized membrane, providing flexibility for various research objectives. It has been shown that these cells produce cytokines, including ILs and TNF-α, making them valuable for nutrition and host-microbiome interaction investigations[144,145]. Using a Transwell system with apical and basolateral polarity, HT-29 cells are suitable for studying bacterial adhesion and transport. What sets them apart from Caco-2 is the presence of mucus-producing goblet cells, making them particularly useful for immune function and bacterial-host interaction studies[146,147]. According to literature, mucins expressed in this CL include both secretory (MUC2, MUC5AC) as well as membrane bound (MUC1, MUC3, MUC4) types[148].
The T84 CL was established from a xenograft produced by subcutaneous injection of tumour cells into BALB/c nude mice[149]. The tumour cells were derived from a lung metastasis of a colorectal adenocarcinoma in a 72-year-old Cauca
The HuTu 80 CL was isolated from a 53-year-old male of allegedly, Caucasian ethnicity, but exome analysis finds it to be mostly of African lineage, with a duodenal adenocarcinoma. It has a doubling time of approximately 26 hours, is MSS and has one subline. The literature on its origin is conflicting, since it is listed as colon or small intestine[138,150]. Th CL was included in multiple studies trying to understand the molecular landscape of intestinal cancers[150] and the land
Some non-neoplastic CLs that are worth mentioning are JFCF-6 (derived from the small intestine, jejunum; also called jejunal fibroblast cystic fibrosis-6; 14 derived CLs), H-4 (enterocytes from a fetal small intestine; spontaneously immorta
An intestinal organ culture was first described in 1969[154]. This method utilized biopsy tissue and a traditional culture-dish system but was limited in the amount of time tissue could be cultured[140]. In 2009, a significant breakthrough occurred with the development of intestinal organoids[155]. Furthermore, in 2014 a reproducible method to direct the differentiation of LGR5 + stem cells to become a specific cell type was published (e.g., enterocytes, goblet cells, stem cells, enteroendocrine cells)[156]. GI organoids can be derived from various portions of the GI tract, including the oesophagus[157], stomach[23], pancreas[158], small intestine[159,160], and colon[161,162].
3D organotypic cultures, derived from primary tissue, embryonic stem cells, or iPSCs[163], exhibit self-renewal, self-organization, and mimic the functionality of the original tissue[164]. They hold great promise for various applications, from basic research to translational uses like disease modelling, drug testing, and host-microbe interactions[165]. Moreover, induced Human Intestinal Organoids can be created from iPSCs. Organoids are mostly grown in 3D, however, they can also be seeded and grown as monolayers that allow easier access to the apical side and studies towards the intestinal barrier function[166].
Noel et al[167] used a macrophage-enteroid co-culture model to investigate mucosal gut physiology. They found out that macrophages enhanced barrier function and maturity of enteroid monolayers by measuring an increased transe
To confront the above-mentioned limitations of traditional 2D and 3D in-vitro models, biomimetic systems (OoC or microfluidic devices), which are based on recent advances in microfabrication, microchip technology, microfluidics, and tissue engineering approaches, have emerged.
It is well established that the 3D physical environment plays an important role in the morphology development, biochemistry, and metabolism of cells[174,175] and has been shown specifically for the small intestine as well[176,177]. Existing microfluidic platforms are focusing on introducing physical cues such as fluid sheer stress, flow rate and direction, shear stress and chemical gradients that are important at both the tissue and organ level[178]. Often, well-characterized intestinal transformed CLs are used to examine changes in cellular microenvironment, polarization, spatial organization, and/or differentiation of the cultures better correlate with those found in vivo.
There are examples of 3D models of the intestine: IPSC-derived intestinal organoid cultures[177,179,180] and ex vivo intestinal enteroid cultures derived from either a single LGR5 + stem cell/Paneth cell unit or multi-cell human intestinal crypts[155] obtained from human intestinal tissue. The iPSC-derived cultures have a more immature, fetal tissue phenotype making them valuable to study developmental biology. These ex vivo organoids self-organize into a 3D “mini-intestine” and contain all intestinal epithelial cell types that are found in vivo, and can be expanded for years without genetic instabilities[155]. Epithelial culture systems in the form of organoids and enteroids recapitulate the central features of normal intestinal epithelial architecture and function including cell polarization, the presence of the brush border with microvilli and appropriate physiologic responsiveness in-vitro. They are often cultured in Matrigel because it resembles the extracellular matrix composition of the native basement membrane. The extracellular matrix has a crucial role for epithelial cells as it helps them to maintain their apical to basal cell polarity when cultured both in Matrigel or on Transwell inserts[162]. As mentioned, organoids produce higher levels of intestinal differentiation; however, the cells in organoids do not experience physiological peristalsis-like motions and cannot be cultured with a living microbiome under such static conditions because bacterial overgrowth will result in death of the epithelium[181]. This is a critical limitation because mechanical deformations resulting from peristalsis influence both normal epithelial cell differentiation[182] and restrain microbial overgrowth in vivo and in-vitro[183].
Creating effective OoC models poses the challenge of reproducing physiologically relevant biology akin to complex in vivo tissues. OoC is a device where biology is coupled with microtechnology. The chip takes the form of a microfluidic device containing networks of hair-fine microchannels for guiding and manipulating minute volumes (picolitres up to millilitres) of solution[125]. The so-called organs present miniaturized tissues in microfluidic chips, which can replicate specific tissue functions, proving to be simplified yet effective models for human physiology and disease. As of the hierarchy of cultures, 2D cell cultures are regarding the simulation of complex physiological processes the least relevant, followed by 3D cell cultures, organoids, and OoCs in increasing order.
The typical workflow of such a system is as follows. Intestinal epithelial cells are introduced into the device and allowed to adhere briefly[153]. Subsequently, a media solution is pumped through channels, either using syringes or peristaltic pumps to establish fluid flow[184-186]. Alternatively, gravity can drive flow in platforms like multi-well plates, which require incubation on a rocking platform[187,188]. This flow induces the spontaneous differentiation of intestinal cells, leading to the formation of 3D villi-like structures[189]. These villi structures are covered with brush borders and mucus, connected by tight junctions, closely mimicking the structure and function of human intestinal villi. In some gut-on-chip models, peristaltic motions can be generated by applying a vacuum to hollow side chambers[184,190]. Addi
Interestingly, when exposing Caco-2 cells embedded in a gut-on-a-chip microfluidic device to cyclic mechanical distor
These systems often use transformed CLs raising concerns as to whether the findings have biological relevance. The co-development of microfluidic devices with integrated stem cell-derived organoids and enteroids will increase the com
In 2017 Alimperti et al[205] developed a biomimetic vascular model on a chip, creating a perfusable channel with an endothelial lining in a collagen matrix to evaluate mural cell-endothelial cell regulated barrier function and the impact of inflammatory factors, N-cadherin expression etc. They were able to provide a model for drug testing and diseases.
Zhang et al[206], 2016 developed a biodegradable scaffold with build in vasculature for OoC research and surgical anastomosis. Vascular networks can be built from natural hydrogels, which are, however, soft and only provide tempo
OoC devices can also include microbes and mimic the gut microbiome, as shown is chapter 3, Trapecar et al[115,116], Zhang et al[117,118] and host interactions[207,208]. Some examples are a co-culture of Caco-2 cells with Lactobacillus rhamnosus[184], enterohemorrhagic Escherichia coli[209], or obligate anaerobes[210]. What is more, when looking at disease models, there are a number of articles on studying IBD[211-214], virus pathogenesis and infections[215-217], metabolic diseases[218] etc., Yoon et al[213] cultured IBD patient cells to validate the synergistic actions of peptides and hydrogels used to treat IBD, Khan et al[214] propose synthetic- and engineered community-based microbiota transplants as a potential therapy approach.
However, as depicted in Figure 7, some of the major challenges in the development and application of advanced in-vitro models, such as organoids and OoC platforms, compared to simpler in-vitro culture methods include scaling up pro
To address the number, or rather the lack of, CLs. A very useful resource in the field of CL research is the Cellosaurus database[143]. The authors have, to gain an overview of the topic at hand, looked through the database by searching for “small intestine”. In November 2023 there were 124 hits for this search term. Now, in January 2025 there are 135 hits. From the old record 2 CL were removed (HT-29 since it is a colon adenocarcinoma; and CVCL A5HM since it is also of colonic origin) and 13 new ones were included (6 of human origin from the same patient-iPSCs; the remaining 7 were of animal origin). These consist of 63 human (46.7% of 135) and 7 animal CLs (53.3% of 135). From these 63 human CLs: 3 CLs are Hutu-80 derivatives, one CL (JFCF-6) is the parent of 14 CLs, 1 CL has been discontinued, 3 CLs are reported to be contaminated, 4 CLs possibly misidentified. Furthermore, from these 63 CLs, 17 CLs are either metastases or do not belong in the category “small intestine”. By excluding metastases, derived/children CLs, induced pluripotent CLs this leaves us with merely 18 CLs of which 6 are benign and 12 malignant. As a comparison, a Cellosaurus search for “Triple-negative breast cancer CL” on 20 September 2021 resulted in 144 entries[220]. On November 2023 the search yielded 163 hits with only 2 being of animal origin (November 2023) and now (January 2025) the search yields 204 hits with only 4 being of animal origin. This shows that the number and variety of available CLs in this field is profoundly lacking. Regarding the fabrication methods, it is highly desirable these can use a broad spectrum of available materials that facilitate fast printing of several mm large structures while keeping sub-micron resolution to create nano-patterns and localised mechanical improvements. Joining different bioprinting techniques, such as micro-extrusion and laser-assisted lithography for simultaneous use, could be a major stepping stone in this direction and enabling the fabrication of complex tissue models such as a vascularised interstitium underlying a parenchymal epithelium. The ultimate goal of these techniques will therefore be the construction of advanced tissue models for simulations of molecular transport and other physiological phenomena occurring on epithelia lined on vascularised connective tissues, focusing on the gut. To successfully fabricate tissues of such complexity, micro-extrusion bioprinting for constructing several mm large scaffolds will be combined with laser-assisted bioprinting for local nano-patterning and structural modification. This will allow the fabrication of a branched microfluidic system in a biopolymeric matrix (vascularised connective tissue), which will induce polarisation on an attached epithelial layer of enterocytes. The intestinal epithelium boasts a wide range of gradients, offering significant potential when combining primary intestinal culture systems with chemical and matrix gradients. These gradients can be integrated into surfaces, solutions, scaffolds, or generated through co-cultured cells, including bacteria or stroma. In addition to the 3D environment, it is important to consider several other critical features when developing a complex physiologically relevant in-vitro model. The epithelium must be supported by matrices that mimic the lamina propria and the muscular layers that form a concentric tube along with the epithelium. Further, it has to contain a heterogeneous population of cells that is spatially organized to mimic the crypt-villus axis. The luminal unidirectional shear stress on epithelium’s apical and blood flow on the basolateral surface has to be simulated. The intestine also periodically squeezes by the muscular layers (peristalsis). More complex models need to consider the unique coupling of the intestine to the liver. Specifically, there is basolateral outflow from the intestinal epithelium to the liver through the portal system, whereas liver products are secreted into the lumen of the duodenum acting on the apical side of the epithelium. Some key challenges and dilemmas that remain are ethical considerations surrounding the sourcing and further use of these models. Namely, older informed consents do not sufficiently address many of the issues that include chimeric research, gene-editing technologies etc.[221]. Furthermore, there is the urgent need for improved standardization and rigorous validation of in-vitro models to ensure reproducibility and reliability[221-223]. Establishing standardized protocols for organoid culture, differentiation, and functional assessment will facilitate broader adoption and regulatory acceptance. Moreover, the integration of more sophisticated co-culture systems that incorporate immune cells, enteric neurons, and other relevant cell types is crucial to better recapitulate tissue complexity and physiological interactions[224]. Such multi-cellular models can provide deeper insights into tissue homeostasis, inflammation, and disease pathogenesis. A next-generation approach would integrate OoC platforms with embedded biosensors, such as impedance spectroscopy or metabolite-sensitive fluorescent probes, to enable real-time monitoring of both microbial metabolite dynamics and epithelial barrier function. This type of dynamic model could offer valuable insights into the bidirectional communication between host tissues and the microbiome. For example, it could reveal how SCFAs influence tight junction regulation and, conversely, how epithelial responses shape microbial composition. In parallel, advance
| 1. | Skok P, Skok K. Gut microbiota and the pathophysiology of cardiovascular disease. Arch Med Sci. 2021. [DOI] [Full Text] |
| 2. | Cencic A, Langerholc T. Functional cell models of the gut and their applications in food microbiology--a review. Int J Food Microbiol. 2010;141 Suppl 1:S4-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Tannenbaum J, Bennett BT. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci. 2015;54:120-132. [PubMed] |
| 4. | Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav. 1997;53:229-234. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kiela PR, Ghishan FK. Physiology of Intestinal Absorption and Secretion. Best Pract Res Clin Gastroenterol. 2016;30:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 461] [Article Influence: 46.1] [Reference Citation Analysis (2)] |
| 6. | Skok P, Skok K. Prebavna cev in srčno-žilne bolezni – ali imajo kaj skupnega? ZdravVestn. 2020;89:528-538. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2747] [Article Influence: 457.8] [Reference Citation Analysis (3)] |
| 8. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12617] [Article Influence: 6308.5] [Reference Citation Analysis (6)] |
| 9. | Kang H, Kiess A, Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat Rev Clin Oncol. 2015;12:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Tian S, Switchenko JM, Jhaveri J, Cassidy RJ, Ferris MJ, Press RH, Pfister NT, Patel MR, Saba NF, McDonald MW, Higgins KA, Yu DS, Curran WJ, Gillespie TW, Beitler JJ. Survival outcomes by high-risk human papillomavirus status in nonoropharyngeal head and neck squamous cell carcinomas: A propensity-scored analysis of the National Cancer Data Base. Cancer. 2019;125:2782-2793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 12. | Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Egashira A, Morita M, Kumagai R, Taguchi KI, Ueda M, Yamaguchi S, Yamamoto M, Minami K, Ikeda Y, Toh Y. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12:e0173501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Singhi AD, Seethala RR, Nason K, Foxwell TJ, Roche RL, McGrath KM, Levy RM, Luketich JD, Davison JM. Undifferentiated carcinoma of the esophagus: a clinicopathological study of 16 cases. Hum Pathol. 2015;46:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 16. | Hunt RH, Camilleri M, Crowe SE, El-Omar EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM, Rugge M, Sonnenberg A, Sugano K, Tack J. The stomach in health and disease. Gut. 2015;64:1650-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 17. | Sharkey KA, Beck PL, McKay DM. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium. Nat Rev Gastroenterol Hepatol. 2018;15:765-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2208] [Article Influence: 245.3] [Reference Citation Analysis (3)] |
| 19. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 567] [Article Influence: 189.0] [Reference Citation Analysis (1)] |
| 20. | Noach LA, Rolf TM, Tytgat GN. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 502] [Article Influence: 38.6] [Reference Citation Analysis (2)] |
| 22. | Carrasco G, Corvalan AH. Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol Res Pract. 2013;2013:393015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Pompaiah M, Bartfeld S. Gastric Organoids: An Emerging Model System to Study Helicobacter pylori Pathogenesis. Curr Top Microbiol Immunol. 2017;400:149-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Choi E, Roland JT, Barlow BJ, O'Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126-136.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 625] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 26. | Boccellato F, Woelffling S, Imai-Matsushima A, Sanchez G, Goosmann C, Schmid M, Berger H, Morey P, Denecke C, Ordemann J, Meyer TF. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut. 2019;68:400-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Hofer M, Kim Y, Broguiere N, Gorostidi F, Klein JA, Amieva MR, Lutolf MP. Accessible homeostatic gastric organoids reveal secondary cell type-specific host-pathogen interactions in Helicobacter pylori infections. Nat Commun. 2025;16:2767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Gagliardi M, Clemente N, Monzani R, Fusaro L, Ferrari E, Saverio V, Grieco G, Pańczyszyn E, Carton F, Santoro C, Del Mare-Roumani S, Amidror S, Yissachar N, Boccafoschi F, Zucchelli S, Corazzari M. Gut-Ex-Vivo system as a model to study gluten response in celiac disease. Cell Death Discov. 2021;7:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Joshi A, Soni A, Acharya S. In vitro models and ex vivo systems used in inflammatory bowel disease. In Vitro Model. 2022;1:213-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Zhao Q, Shao M, Ma L, Zhou R. Insights into Modeling Inflammatory Bowel Disease from Stem Cell Derived Intestinal Organoids. Stem Cell Rev Rep. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Ferreira B, Barros AS, Leite-Pereira C, Viegas J, das Neves J, Nunes R, Sarmento B. Trends in 3D models of inflammatory bowel disease. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 32. | Lindfors K, Ciacci C, Kurppa K, Lundin KEA, Makharia GK, Mearin ML, Murray JA, Verdu EF, Kaukinen K. Coeliac disease. Nat Rev Dis Primers. 2019;5:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 33. | Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823-836.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1025] [Article Influence: 128.1] [Reference Citation Analysis (1)] |
| 34. | Iversen R, Sollid LM. The Immunobiology and Pathogenesis of Celiac Disease. Annu Rev Pathol. 2023;18:47-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 35. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1223] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 36. | Meresse B, Malamut G, Cerf-Bensussan N. Celiac disease: an immunological jigsaw. Immunity. 2012;36:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 629] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 38. | Barone MV, Zanzi D, Maglio M, Nanayakkara M, Santagata S, Lania G, Miele E, Ribecco MT, Maurano F, Auricchio R, Gianfrani C, Ferrini S, Troncone R, Auricchio S. Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS One. 2011;6:e17039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Simpson HL, Smits E, Moerkens R, Wijmenga C, Mooiweer J, Jonkers IH, Withoff S. Human organoids and organ-on-chips in coeliac disease research. Trends Mol Med. 2025;31:117-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Moerkens R, Mooiweer J, Withoff S, Wijmenga C. Celiac disease-on-chip: Modeling a multifactorial disease in vitro. United European Gastroenterol J. 2019;7:467-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Stoven S, Murray JA, Marietta EV. Latest in vitro and in vivo models of celiac disease. Expert Opin Drug Discov. 2013;8:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Marietta EV, Schuppan D, Murray JA. In vitro and in vivo models of celiac disease. Expert Opin Drug Discov. 2009;4:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851-4855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Vincentini O, Maialetti F, Gonnelli E, Silano M. Gliadin-dependent cytokine production in a bidimensional cellular model of celiac intestinal mucosa. Clin Exp Med. 2015;15:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Villella VR, Venerando A, Cozza G, Esposito S, Ferrari E, Monzani R, Spinella MC, Oikonomou V, Renga G, Tosco A, Rossin F, Guido S, Silano M, Garaci E, Chao YK, Grimm C, Luciani A, Romani L, Piacentini M, Raia V, Kroemer G, Maiuri L. A pathogenic role for cystic fibrosis transmembrane conductance regulator in celiac disease. EMBO J. 2019;38:e100101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Abadie V, Khosla C, Jabri B. A Mouse Model of Celiac Disease. Curr Protoc. 2022;2:e515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Alonso-Roman R, Mosig AS, Figge MT, Papenfort K, Eggeling C, Schacher FH, Hube B, Gresnigt MS. Organ-on-chip models for infectious disease research. Nat Microbiol. 2024;9:891-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 48. | Moerkens R, Mooiweer J, Ramírez-Sánchez AD, Oelen R, Franke L, Wijmenga C, Barrett RJ, Jonkers IH, Withoff S. An iPSC-derived small intestine-on-chip with self-organizing epithelial, mesenchymal, and neural cells. Cell Rep. 2024;43:114247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 49. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 187] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 50. | Singh N, Bernstein CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 51. | Santos AJM, van Unen V, Lin Z, Chirieleison SM, Ha N, Batish A, Chan JE, Cedano J, Zhang ET, Mu Q, Guh-Siesel A, Tomaske M, Colburg D, Varma S, Choi SS, Christophersen A, Baghdasaryan A, Yost KE, Karlsson K, Ha A, Li J, Dai H, Sellers ZM, Chang HY, Dunn JCY, Zhang BM, Mellins ED, Sollid LM, Fernandez-Becker NQ, Davis MM, Kuo CJ. A human autoimmune organoid model reveals IL-7 function in coeliac disease. Nature. 2024;632:401-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 52. | Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int J Mol Sci. 2023;24:1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 326] [Reference Citation Analysis (0)] |
| 53. | Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med. 2020;383:2652-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 892] [Article Influence: 148.7] [Reference Citation Analysis (0)] |
| 54. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2254] [Article Influence: 132.6] [Reference Citation Analysis (10)] |
| 55. | Iliev ID, Ananthakrishnan AN, Guo CJ. Microbiota in inflammatory bowel disease: mechanisms of disease and therapeutic opportunities. Nat Rev Microbiol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 67] [Article Influence: 67.0] [Reference Citation Analysis (2)] |
| 56. | Ananthakrishnan AN, Gerasimidis K, Ho SM, Mayer E, Pollock J, Soni S, Wu GD, Benyacoub J, Ali B, Favreau A, Smith DE, Oh JE, Heller C, Hurtado-Lorenzo A, Moss A, Croitoru K. Challenges in IBD Research 2024: Environmental Triggers. Inflamm Bowel Dis. 2024;30:S19-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1086] [Cited by in RCA: 1445] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 58. | Chassaing B, Compher C, Bonhomme B, Liu Q, Tian Y, Walters W, Nessel L, Delaroque C, Hao F, Gershuni V, Chau L, Ni J, Bewtra M, Albenberg L, Bretin A, McKeever L, Ley RE, Patterson AD, Wu GD, Gewirtz AT, Lewis JD. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology. 2022;162:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 59. | Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed). 2009;14:2765-2778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 483] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 60. | Mancini NL, Goudie L, Xu W, Sabouny R, Rajeev S, Wang A, Esquerre N, Al Rajabi A, Jayme TS, van Tilburg Bernandes E, Nasser Y, Ferraz JGP, Shutt T, Shearer J, McKay DM. Perturbed Mitochondrial Dynamics Is a Novel Feature of Colitis That Can Be Targeted to Lessen Disease. Cell Mol Gastroenterol Hepatol. 2020;10:287-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Ho GT, Aird RE, Liu B, Boyapati RK, Kennedy NA, Dorward DA, Noble CL, Shimizu T, Carter RN, Chew ETS, Morton NM, Rossi AG, Sartor RB, Iredale JP, Satsangi J. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 2018;11:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | Jackson DN, Theiss AL. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes. 2020;11:285-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 63. | Leonard F, Collnot EM, Lehr CM. A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in vitro. Mol Pharm. 2010;7:2103-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Susewind J, de Souza Carvalho-Wodarz C, Repnik U, Collnot EM, Schneider-Daum N, Griffiths GW, Lehr CM. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology. 2016;10:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Marescotti D, Lo Sasso G, Guerrera D, Renggli K, Ruiz Castro PA, Piault R, Jaquet V, Moine F, Luettich K, Frentzel S, Peitsch MC, Hoeng J. Development of an Advanced Multicellular Intestinal Model for Assessing Immunomodulatory Properties of Anti-Inflammatory Compounds. Front Pharmacol. 2021;12:639716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Le NPK, Altenburger MJ, Lamy E. Development of an Inflammation-Triggered In Vitro "Leaky Gut" Model Using Caco-2/HT29-MTX-E12 Combined with Macrophage-like THP-1 Cells or Primary Human-Derived Macrophages. Int J Mol Sci. 2023;24:7427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Holtmann G, Shah A, Morrison M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig Dis. 2017;35 Suppl 1:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 68. | Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13:120-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 109] [Reference Citation Analysis (0)] |
| 69. | Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 285] [Reference Citation Analysis (0)] |
| 70. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1772] [Article Influence: 253.1] [Reference Citation Analysis (2)] |
| 71. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3822] [Article Influence: 347.5] [Reference Citation Analysis (8)] |
| 72. | Schürch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, Zlobec I, Chu P, Black S, Demeter J, McIlwain DR, Kinoshita S, Samusik N, Goltsev Y, Nolan GP. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell. 2020;182:1341-1359.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 73. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4493] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 74. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8096] [Article Influence: 224.9] [Reference Citation Analysis (1)] |
| 75. | Patai AV, Molnár B, Tulassay Z, Sipos F. Serrated pathway: alternative route to colorectal cancer. World J Gastroenterol. 2013;19:607-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 76. | Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 740] [Article Influence: 46.3] [Reference Citation Analysis (2)] |
| 77. | Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D, Curtis C. A Big Bang model of human colorectal tumor growth. Nat Genet. 2015;47:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 711] [Cited by in RCA: 787] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 78. | Cornish AJ, Gruber AJ, Kinnersley B, Chubb D, Frangou A, Caravagna G, Noyvert B, Lakatos E, Wood HM, Thorn S, Culliford R, Arnedo-Pac C, Househam J, Cross W, Sud A, Law P, Leathlobhair MN, Hawari A, Woolley C, Sherwood K, Feeley N, Gül G, Fernandez-Tajes J, Zapata L, Alexandrov LB, Murugaesu N, Sosinsky A, Mitchell J, Lopez-Bigas N, Quirke P, Church DN, Tomlinson IPM, Sottoriva A, Graham TA, Wedge DC, Houlston RS. The genomic landscape of 2,023 colorectal cancers. Nature. 2024;633:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 79. | Househam J, Heide T, Cresswell GD, Spiteri I, Kimberley C, Zapata L, Lynn C, James C, Mossner M, Fernandez-Mateos J, Vinceti A, Baker AM, Gabbutt C, Berner A, Schmidt M, Chen B, Lakatos E, Gunasri V, Nichol D, Costa H, Mitchinson M, Ramazzotti D, Werner B, Iorio F, Jansen M, Caravagna G, Barnes CP, Shibata D, Bridgewater J, Rodriguez-Justo M, Magnani L, Sottoriva A, Graham TA. Phenotypic plasticity and genetic control in colorectal cancer evolution. Nature. 2022;611:744-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 80. | Heide T, Househam J, Cresswell GD, Spiteri I, Lynn C, Mossner M, Kimberley C, Fernandez-Mateos J, Chen B, Zapata L, James C, Barozzi I, Chkhaidze K, Nichol D, Gunasri V, Berner A, Schmidt M, Lakatos E, Baker AM, Costa H, Mitchinson M, Piazza R, Jansen M, Caravagna G, Ramazzotti D, Shibata D, Bridgewater J, Rodriguez-Justo M, Magnani L, Graham TA, Sottoriva A. The co-evolution of the genome and epigenome in colorectal cancer. Nature. 2022;611:733-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 81. | Ramesh A, Srinivasan D, Subbarayan R, Chauhan A, Krishnamoorthy L, Kumar J, Krishnan M, Shrestha R. Enhancing Colorectal Cancer Treatment: The Role of Bifidobacterium in Modulating Gut Immunity and Mitigating Capecitabine-Induced Toxicity. Mol Nutr Food Res. 2025;69:e70023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 82. | Deng Y, Hou X, Wang H, Du H, Liu Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals (Basel). 2024;17:604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 83. | Yixia Y, Sripetchwandee J, Chattipakorn N, Chattipakorn SC. The alterations of microbiota and pathological conditions in the gut of patients with colorectal cancer undergoing chemotherapy. Anaerobe. 2021;68:102361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Kim SH, Lim YJ. The role of microbiome in colorectal carcinogenesis and its clinical potential as a target for cancer treatment. Intest Res. 2022;20:31-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Lau K, Srivatsav V, Rizwan A, Nashed A, Liu R, Shen R, Akhtar M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients. 2017;9:859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 86. | Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, Lucas SK, Beura LK, Thompson EA, Till LM, Batres R, Paw B, Pergament SL, Saenyakul P, Xiong M, Kim AD, Kim G, Masopust D, Martens EC, Angkurawaranon C, McGready R, Kashyap PC, Culhane-Pera KA, Knights D. US Immigration Westernizes the Human Gut Microbiome. Cell. 2018;175:962-972.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 557] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 87. | Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1185] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 88. | Zhu Q, Gao R, Zhang Y, Pan D, Zhu Y, Zhang X, Yang R, Jiang R, Xu Y, Qin H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol Genomics. 2018;50:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 89. | Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA; LifeLines cohort study, Weersma RK, Feskens EJ, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1380] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 90. | Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. 2018;44:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 91. | Nallu A, Sharma S, Ramezani A, Muralidharan J, Raj D. Gut microbiome in chronic kidney disease: challenges and opportunities. Transl Res. 2017;179:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 92. | Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 2517] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 93. | Abdallah Ismail N, Ragab SH, Abd Elbaky A, Shoeib AR, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. 2011;7:501-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 94. | Xu H, Wang X, Feng W, Liu Q, Zhou S, Liu Q, Cai L. The gut microbiota and its interactions with cardiovascular disease. Microb Biotechnol. 2020;13:637-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 95. | Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M; SPRING Trial Group. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension. 2016;68:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 96. | Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 630] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 97. | Ahmadmehrabi S, Tang WHW. Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol. 2017;32:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 98. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 99. | Foster JA, Lyte M, Meyer E, Cryan JF. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience. Int J Neuropsychopharmacol. 2016;19:pyv114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 100. | Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1055] [Article Influence: 131.9] [Reference Citation Analysis (1)] |
| 101. | Zheng Z, Wang B. The Gut-Liver Axis in Health and Disease: The Role of Gut Microbiota-Derived Signals in Liver Injury and Regeneration. Front Immunol. 2021;12:775526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 102. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1456] [Article Influence: 242.7] [Reference Citation Analysis (1)] |
| 103. | Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 703] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 104. | Salem I, Ramser A, Isham N, Ghannoum MA. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front Microbiol. 2018;9:1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 407] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 105. | Lauderdale DS, Rathouz PJ. Body mass index in a US national sample of Asian Americans: effects of nativity, years since immigration and socioeconomic status. Int J Obes Relat Metab Disord. 2000;24:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 106. | Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11:1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 722] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 107. | Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1974] [Article Influence: 246.8] [Reference Citation Analysis (0)] |
| 108. | Francino MP. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front Microbiol. 2015;6:1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 543] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 109. | Bhalodi AA, van Engelen TSR, Virk HS, Wiersinga WJ. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother. 2019;74:i6-i15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 110. | Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 459] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 111. | Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 388] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 112. | Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1234] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 113. | Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS One. 2016;11:e0160840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 114. | Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854-859. [PubMed] |
| 115. | Trapecar M, Communal C, Velazquez J, Maass CA, Huang YJ, Schneider K, Wright CW, Butty V, Eng G, Yilmaz O, Trumper D, Griffith LG. Gut-Liver Physiomimetics Reveal Paradoxical Modulation of IBD-Related Inflammation by Short-Chain Fatty Acids. Cell Syst. 2020;10:223-239.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 116. | Trapecar M, Wogram E, Svoboda D, Communal C, Omer A, Lungjangwa T, Sphabmixay P, Velazquez J, Schneider K, Wright CW, Mildrum S, Hendricks A, Levine S, Muffat J, Lee MJ, Lauffenburger DA, Trumper D, Jaenisch R, Griffith LG. Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases. Sci Adv. 2021;7:eabd1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 117. | Zhang J, Hernandez-Gordillo V, Trapecar M, Wright C, Taketani M, Schneider K, Chen WLK, Stas E, Breault DT, Carrier RL, Voigt CA, Griffith LG. Coculture of primary human colon monolayer with human gut bacteria. Nat Protoc. 2021;16:3874-3900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 118. | Zhang J, Huang YJ, Trapecar M, Wright C, Schneider K, Kemmitt J, Hernandez-Gordillo V, Yoon JY, Poyet M, Alm EJ, Breault DT, Trumper DL, Griffith LG. An immune-competent human gut microphysiological system enables inflammation-modulation by Faecalibacterium prausnitzii. NPJ Biofilms Microbiomes. 2024;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 119. | Dutta D, Heo I, Clevers H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med. 2017;23:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 608] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 120. | Lehmann R, Lee CM, Shugart EC, Benedetti M, Charo RA, Gartner Z, Hogan B, Knoblich J, Nelson CM, Wilson KM. Human organoids: a new dimension in cell biology. Mol Biol Cell. 2019;30:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 121. | Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 1385] [Article Influence: 230.8] [Reference Citation Analysis (0)] |
| 122. | Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A, Tesei A. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016;6:19103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 825] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 123. | Xu H, Jiao D, Liu A, Wu K. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol. 2022;15:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 183] [Reference Citation Analysis (0)] |
| 124. | Wasson EM, He W, Ahlquist J, Hynes WF, Triplett MG, Hinckley A, Karelehto E, Gray-Sherr DR, Friedman CF, Robertson C, Shusteff M, Warren R, Coleman MA, Moya ML, Wheeler EK. A perfused multi-well bioreactor platform to assess tumor organoid response to a chemotherapeutic gradient. Front Bioeng Biotechnol. 2023;11:1193430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 125. | Leung CM, de Haan P, Ronaldson-bouchard K, Kim G, Ko J, Rho HS, Chen Z, Habibovic P, Jeon NL, Takayama S, Shuler ML, Vunjak-novakovic G, Frey O, Verpoorte E, Toh Y. A guide to the organ-on-a-chip. Nat Rev Methods Primers. 2022;2:33. [DOI] [Full Text] |
| 126. | Noben M, Vanhove W, Arnauts K, Santo Ramalho A, Van Assche G, Vermeire S, Verfaillie C, Ferrante M. Human intestinal epithelium in a dish: Current models for research into gastrointestinal pathophysiology. United European Gastroenterol J. 2017;5:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 127. | Rahman S, Ghiboub M, Donkers JM, van de Steeg E, van Tol EAF, Hakvoort TBM, de Jonge WJ. The Progress of Intestinal Epithelial Models from Cell Lines to Gut-On-Chip. Int J Mol Sci. 2021;22:13472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 128. | Costa J, Ahluwalia A. Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest. Front Bioeng Biotechnol. 2019;7:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 129. | Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736-749. [PubMed] |
| 130. | Hilgers AR, Conradi RA, Burton PS. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990;7:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 467] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 131. | Parlesak A, Haller D, Brinz S, Baeuerlein A, Bode C. Modulation of cytokine release by differentiated CACO-2 cells in a compartmentalized coculture model with mononuclear leucocytes and nonpathogenic bacteria. Scand J Immunol. 2004;60:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 132. | Bernardo D, Sánchez B, Al-Hassi HO, Mann ER, Urdaci MC, Knight SC, Margolles A. Microbiota/host crosstalk biomarkers: regulatory response of human intestinal dendritic cells exposed to Lactobacillus extracellular encrypted peptide. PLoS One. 2012;7:e36262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 133. | Larregieu CA, Benet LZ. Drug discovery and regulatory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 2013;15:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 134. | Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang KJ, Karalis K, Kim HJ, MacQueen L, Mahmoodian R, Musah S, Torisawa YS, van der Meer AD, Villenave R, Yadid M, Parker KK, Ingber DE. Engineered in vitro disease models. Annu Rev Pathol. 2015;10:195-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 395] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 135. | Ballard DH, Boyer CJ, Alexander JS. Organoids - Preclinical Models of Human Disease. N Engl J Med. 2019;380:1981-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 137. | Simon-Assmann P, Turck N, Sidhoum-Jenny M, Gradwohl G, Kedinger M. In vitro models of intestinal epithelial cell differentiation. Cell Biol Toxicol. 2007;23:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 138. | Bazzocco S, Dopeso H, Carton-Garcia F, Macaya I, Andretta E, Chionh F, Rodrigues P, Garrido M, Alazzouzi H, Nieto R, Sanchez A, Schwartz S Jr, Bilic J, Mariadason JM, Arango D. Highly Expressed Genes in Rapidly Proliferating Tumor Cells as New Targets for Colorectal Cancer Treatment. Clin Cancer Res. 2015;21:3695-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 139. | Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 719] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 140. | Pearce SC, Coia HG, Karl JP, Pantoja-Feliciano IG, Zachos NC, Racicot K. Intestinal in vitro and ex vivo Models to Study Host-Microbiome Interactions and Acute Stressors. Front Physiol. 2018;9:1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 141. | Pereira C, Araújo F, Barrias CC, Granja PL, Sarmento B. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials. 2015;56:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 142. | Fogh J, Trempe G. New Human Tumor Cell Lines. In: Fogh J. Human Tumor Cells in Vitro. Springer, Boston, MA. 1975: 115-159. [DOI] [Full Text] |
| 143. | Bairoch A. The Cellosaurus, a Cell-Line Knowledge Resource. J Biomol Tech. 2018;29:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 527] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 144. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 145. | Khan Mirzaei M, Haileselassie Y, Navis M, Cooper C, Sverremark-Ekström E, Nilsson AS. Morphologically Distinct Escherichia coli Bacteriophages Differ in Their Efficacy and Ability to Stimulate Cytokine Release In Vitro. Front Microbiol. 2016;7:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 146. | Altamimi M, Abdelhay O, Rastall RA. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe. 2016;39:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 147. | Raja SB, Murali MR, Devaraj H, Devaraj SN. Differential expression of gastric MUC5AC in colonic epithelial cells: TFF3-wired IL1 β/Akt crosstalk-induced mucosal immune response against Shigella dysenteriae infection. J Cell Sci. 2012;125:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 148. | Huet G, Kim I, de Bolos C, Lo-Guidice JM, Moreau O, Hemon B, Richet C, Delannoy P, Real FX, Degand P. Characterization of mucins and proteoglycans synthesized by a mucin-secreting HT-29 cell subpopulation. J Cell Sci. 1995;108 ( Pt 3):1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 149. | Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204-G208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 158] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 150. | Medico E, Russo M, Picco G, Cancelliere C, Valtorta E, Corti G, Buscarino M, Isella C, Lamba S, Martinoglio B, Veronese S, Siena S, Sartore-Bianchi A, Beccuti M, Mottolese M, Linnebacher M, Cordero F, Di Nicolantonio F, Bardelli A. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat Commun. 2015;6:7002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 151. | Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, Aben N, Gonçalves E, Barthorpe S, Lightfoot H, Cokelaer T, Greninger P, van Dyk E, Chang H, de Silva H, Heyn H, Deng X, Egan RK, Liu Q, Mironenko T, Mitropoulos X, Richardson L, Wang J, Zhang T, Moran S, Sayols S, Soleimani M, Tamborero D, Lopez-Bigas N, Ross-Macdonald P, Esteller M, Gray NS, Haber DA, Stratton MR, Benes CH, Wessels LFA, Saez-Rodriguez J, McDermott U, Garnett MJ. A Landscape of Pharmacogenomic Interactions in Cancer. Cell. 2016;166:740-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1398] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 152. | Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci U S A. 1996;93:7717-7722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 153. | Gradisnik L, Trapecar M, Rupnik MS, Velnar T. HUIEC, Human intestinal epithelial cell line with differentiated properties: process of isolation and characterisation. Wien Klin Wochenschr. 2015;127 Suppl 5:S204-S209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Browning TH, Trier JS. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969;48:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 155. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5463] [Article Influence: 321.4] [Reference Citation Analysis (0)] |
| 156. | Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 458] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 157. | DeWard AD, Cramer J, Lagasse E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014;9:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 158. | April-Monn SL, Kirchner P, Detjen K, Bräutigam K, Trippel MA, Grob T, Statzer C, Maire RS, Kollàr A, Chouchane A, Kunze CA, Horst D, Sadowski MC, Schrader J, Marinoni I, Wiedenmann B, Perren A. Patient derived tumoroids of high grade neuroendocrine neoplasms for more personalized therapies. NPJ Precis Oncol. 2024;8:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 159. | Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc. 2013;8:2471-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 608] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 160. | Moore SR, Pruszka J, Vallance J, Aihara E, Matsuura T, Montrose MH, Shroyer NF, Hong CI. Robust circadian rhythms in organoid cultures from PERIOD2::LUCIFERASE mouse small intestine. Dis Model Mech. 2014;7:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 161. | Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HG, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 2016;8:344ra84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 430] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 162. | Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2253] [Cited by in RCA: 2866] [Article Influence: 191.1] [Reference Citation Analysis (0)] |
| 163. | Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 814] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 164. | Creff J, Malaquin L, Besson A. In vitro models of intestinal epithelium: Toward bioengineered systems. J Tissue Eng. 2021;12:2041731420985202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 165. | Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 2039] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 166. | Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. Human Enteroids as a Model of Upper Small Intestinal Ion Transport Physiology and Pathophysiology. Gastroenterology. 2016;150:638-649.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 167. | Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep. 2017;7:45270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 168. | Bardenbacher M, Ruder B, Britzen-Laurent N, Naschberger E, Becker C, Palmisano R, Stürzl M, Tripal P. Investigating Intestinal Barrier Breakdown in Living Organoids. J Vis Exp. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 169. | Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 783] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 170. | Okamoto R, Shimizu H, Suzuki K, Kawamoto A, Takahashi J, Kawai M, Nagata S, Hiraguri Y, Takeoka S, Sugihara HY, Yui S, Watanabe M. Organoid-based regenerative medicine for inflammatory bowel disease. Regen Ther. 2020;13:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 171. | Engel RM, Chan WH, Nickless D, Hlavca S, Richards E, Kerr G, Oliva K, McMurrick PJ, Jardé T, Abud HE. Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment. J Clin Med. 2020;9:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 172. | Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, Date S, Nishikori S, Nakazato Y, Nakamura T, Kanai T, Sato T. Reconstruction of the Human Colon Epithelium In Vivo. Cell Stem Cell. 2018;22:171-176.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 173. | Sun J. Intestinal organoid as an in vitro model in studying host-microbial interactions. Front Biol (Beijing). 2017;12:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 174. | Anselme K, Bigerelle M. Role of materials surface topography on mammalian cell response. Int Mater Rev. 2011;56:243-266. [DOI] [Full Text] |
| 175. | Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48:5406-5415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1104] [Cited by in RCA: 875] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 176. | Creff J, Courson R, Mangeat T, Foncy J, Souleille S, Thibault C, Besson A, Malaquin L. Fabrication of 3D scaffolds reproducing intestinal epithelium topography by high-resolution 3D stereolithography. Biomaterials. 2019;221:119404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 177. | Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 906] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 178. | Mehling M, Tay S. Microfluidic cell culture. Curr Opin Biotechnol. 2014;25:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 179. | Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1668] [Cited by in RCA: 1484] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 180. | McCracken KW, Howell JC, Wells JM, Spence JR. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 181. | van Rijn JM, Schneeberger K, Wiegerinck CL, Nieuwenhuis EE, Middendorp S. Novel approaches: Tissue engineering and stem cells--In vitro modelling of the gut. Best Pract Res Clin Gastroenterol. 2016;30:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 182. | Basson MD. Paradigms for mechanical signal transduction in the intestinal epithelium. Category: molecular, cell, and developmental biology. Digestion. 2003;68:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 183. | Quigley EM. Microflora modulation of motility. J Neurogastroenterol Motil. 2011;17:140-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 184. | Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1209] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 185. | Ashammakhi N, Nasiri R, Barros NR, Tebon P, Thakor J, Goudie M, Shamloo A, Martin MG, Khademhosseini A. Gut-on-a-chip: Current progress and future opportunities. Biomaterials. 2020;255:120196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 186. | Xiang Y, Wen H, Yu Y, Li M, Fu X, Huang S. Gut-on-chip: Recreating human intestine in vitro. J Tissue Eng. 2020;11:2041731420965318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 187. | Trietsch SJ, Naumovska E, Kurek D, Setyawati MC, Vormann MK, Wilschut KJ, Lanz HL, Nicolas A, Ng CP, Joore J, Kustermann S, Roth A, Hankemeier T, Moisan A, Vulto P. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat Commun. 2017;8:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 188. | Naumovska E, Aalderink G, Wong Valencia C, Kosim K, Nicolas A, Brown S, Vulto P, Erdmann KS, Kurek D. Direct On-Chip Differentiation of Intestinal Tubules from Induced Pluripotent Stem Cells. Int J Mol Sci. 2020;21:4964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 189. | Kim HJ, Ingber DE. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb). 2013;5:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 190. | Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 2814] [Article Influence: 175.9] [Reference Citation Analysis (0)] |
| 191. | Jalili-Firoozinezhad S, Prantil-Baun R, Jiang A, Potla R, Mammoto T, Weaver JC, Ferrante TC, Kim HJ, Cabral JMS, Levy O, Ingber DE. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018;9:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 192. | Gijzen L, Marescotti D, Raineri E, Nicolas A, Lanz HL, Guerrera D, van Vught R, Joore J, Vulto P, Peitsch MC, Hoeng J, Lo Sasso G, Kurek D. An Intestine-on-a-Chip Model of Plug-and-Play Modularity to Study Inflammatory Processes. SLAS Technol. 2020;25:585-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 193. | Costello CM, Hongpeng J, Shaffiey S, Yu J, Jain NK, Hackam D, March JC. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng. 2014;111:1222-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 194. | Nematollahi M, Hamilton DW, Jaeger NJ, Brunette DM. Hexagonal micron scale pillars influence epithelial cell adhesion, morphology, proliferation, migration, and cytoskeletal arrangement. J Biomed Mater Res A. 2009;91:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 195. | Wang L, Murthy SK, Fowle WH, Barabino GA, Carrier RL. Influence of micro-well biomimetic topography on intestinal epithelial Caco-2 cell phenotype. Biomaterials. 2009;30:6825-6834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 196. | Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Cell guidance by ultrafine topography in vitro. J Cell Sci. 1991;99 ( Pt 1):73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 293] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 197. | Barrila J, Radtke AL, Crabbé A, Sarker SF, Herbst-Kralovetz MM, Ott CM, Nickerson CA. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol. 2010;8:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 198. | Goodwin TJ, Schroeder WF, Wolf DA, Moyer MP. Rotating-wall vessel coculture of small intestine as a prelude to tissue modeling: aspects of simulated microgravity. Proc Soc Exp Biol Med. 1993;202:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 199. | Skardal A, Sarker SF, Crabbé A, Nickerson CA, Prestwich GD. The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials. 2010;31:8426-8435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 200. | Höner zu Bentrup K, Ramamurthy R, Ott CM, Emami K, Nelman-Gonzalez M, Wilson JW, Richter EG, Goodwin TJ, Alexander JS, Pierson DL, Pellis N, Buchanan KL, Nickerson CA. Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes Infect. 2006;8:1813-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 201. | Chen X, Zhao J, Gregersen H. The villi contribute to the mechanics in the guinea pig small intestine. J Biomech. 2008;41:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 202. | Yu J, Peng S, Luo D, March JC. In vitro 3D human small intestinal villous model for drug permeability determination. Biotechnol Bioeng. 2012;109:2173-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 203. | VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 411] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 204. | Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, Dziki J, Badylak S, Sodhi CP, March JC, Hackam DJ. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11:45-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 205. | Alimperti S, Mirabella T, Bajaj V, Polacheck W, Pirone DM, Duffield J, Eyckmans J, Assoian RK, Chen CS. Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell-endothelial cell-regulated barrier function. Proc Natl Acad Sci U S A. 2017;114:8758-8763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 206. | Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 2016;15:669-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 432] [Article Influence: 43.2] [Reference Citation Analysis (7)] |
| 207. | Baddal B, Marrazzo P. Refining Host-Pathogen Interactions: Organ-on-Chip Side of the Coin. Pathogens. 2021;10:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 208. | Puschhof J, Pleguezuelos-Manzano C, Clevers H. Organoids and organs-on-chips: Insights into human gut-microbe interactions. Cell Host Microbe. 2021;29:867-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 209. | Tovaglieri A, Sontheimer-Phelps A, Geirnaert A, Prantil-Baun R, Camacho DM, Chou DB, Jalili-Firoozinezhad S, de Wouters T, Kasendra M, Super M, Cartwright MJ, Richmond CA, Breault DT, Lacroix C, Ingber DE. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome. 2019;7:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 210. | Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, Bein A, Swenor B, Nestor B, Cronce MJ, Tovaglieri A, Levy O, Gregory KE, Breault DT, Cabral JMS, Kasper DL, Novak R, Ingber DE. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019;3:520-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 580] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 211. | Beaurivage C, Naumovska E, Chang YX, Elstak ED, Nicolas A, Wouters H, van Moolenbroek G, Lanz HL, Trietsch SJ, Joore J, Vulto P, Janssen RAJ, Erdmann KS, Stallen J, Kurek D. Development of a Gut-On-A-Chip Model for High Throughput Disease Modeling and Drug Discovery. Int J Mol Sci. 2019;20:5661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 212. | Beaurivage C, Kanapeckaite A, Loomans C, Erdmann KS, Stallen J, Janssen RAJ. Development of a human primary gut-on-a-chip to model inflammatory processes. Sci Rep. 2020;10:21475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 213. | Yoon HJ, Lee S, Kim TY, Yu SE, Kim HS, Chung YS, Chung S, Park S, Shin YC, Wang EK, Noh J, Kim HJ, Ku CR, Koh H, Kim CS, Park JS, Shin YM, Sung HJ. Sprayable nanomicelle hydrogels and inflammatory bowel disease patient cell chips for development of intestinal lesion-specific therapy. Bioact Mater. 2022;18:433-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 214. | Khan R, Roy N, Ali H, Naeem M. Fecal Microbiota Transplants for Inflammatory Bowel Disease Treatment: Synthetic- and Engineered Communities-Based Microbiota Transplants Are the Future. Gastroenterol Res Pract. 2022;2022:9999925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 215. | Villenave R, Wales SQ, Hamkins-Indik T, Papafragkou E, Weaver JC, Ferrante TC, Bahinski A, Elkins CA, Kulka M, Ingber DE. Human Gut-On-A-Chip Supports Polarized Infection of Coxsackie B1 Virus In Vitro. PLoS One. 2017;12:e0169412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 216. | Zhang M, Wang P, Luo R, Wang Y, Li Z, Guo Y, Yao Y, Li M, Tao T, Chen W, Han J, Liu H, Cui K, Zhang X, Zheng Y, Qin J. Biomimetic Human Disease Model of SARS-CoV-2-Induced Lung Injury and Immune Responses on Organ Chip System. Adv Sci (Weinh). 2021;8:2002928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 217. | Bein A, Kim S, Goyal G, Cao W, Fadel C, Naziripour A, Sharma S, Swenor B, LoGrande N, Nurani A, Miao VN, Navia AW, Ziegler CGK, Montañes JO, Prabhala P, Kim MS, Prantil-Baun R, Rodas M, Jiang A, O'Sullivan L, Tillya G, Shalek AK, Ingber DE. Enteric Coronavirus Infection and Treatment Modeled With an Immunocompetent Human Intestine-On-A-Chip. Front Pharmacol. 2021;12:718484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 218. | Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA, Anderson CL, Li N, Fisher AB, West KA, Reeder PJ, Momin MM, Bergeron CG, Guilmain SE, Miller PF, Kurtz CB, Falb D. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol. 2018;36:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 219. | Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nat Rev Mater. 2018;3:257-278. [DOI] [Full Text] |
| 220. | Skok K, Gradišnik L, Čelešnik H, Milojević M, Potočnik U, Jezernik G, Gorenjak M, Sobočan M, Takač I, Kavalar R, Maver U. MFUM-BrTNBC-1, a Newly Established Patient-Derived Triple-Negative Breast Cancer Cell Line: Molecular Characterisation, Genetic Stability, and Comprehensive Comparison with Commercial Breast Cancer Cell Lines. Cells. 2021;11:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 221. | Munsie M, Hyun I, Sugarman J. Ethical issues in human organoid and gastruloid research. Development. 2017;144:942-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 222. | Wang J, Chen X, Li R, Wang S, Geng Z, Shi Z, Jing Y, Xu K, Wei Y, Wang G, He C, Dong S, Liu G, Hou Z, Xia Z, Wang X, Ye Z, Zhou F, Bai L, Tan H, Su J. Standardization and consensus in the development and application of bone organoids. Theranostics. 2025;15:682-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 223. | Zhou C, Wu Y, Wang Z, Liu Y, Yu J, Wang W, Chen S, Wu W, Wang J, Qian G, He A. Standardization of organoid culture in cancer research. Cancer Med. 2023;12:14375-14386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 224. | Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 225. | Fei K, Zhang J, Yuan J, Xiao P. Present Application and Perspectives of Organoid Imaging Technology. Bioengineering (Basel). 2022;9:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 226. | Dekkers JF, Alieva M, Wellens LM, Ariese HCR, Jamieson PR, Vonk AM, Amatngalim GD, Hu H, Oost KC, Snippert HJG, Beekman JM, Wehrens EJ, Visvader JE, Clevers H, Rios AC. High-resolution 3D imaging of fixed and cleared organoids. Nat Protoc. 2019;14:1756-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 227. | Lee MJ, Lee J, Ha J, Kim G, Kim HJ, Lee S, Koo BK, Park Y. Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography. Exp Mol Med. 2024;56:2162-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/