Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.109459
Revised: May 31, 2025
Accepted: July 1, 2025
Published online: July 21, 2025
Processing time: 70 Days and 19.7 Hours
Crohn's disease (CD) is a type of inflammatory bowel disease, with chronic and progressive characteristics. Infliximab (IFX) can rapidly relieve CD-related sym
To develop a radiomics-based model via integrative analysis of intestinal wall and creeping fat to predict SLOR in CD.
We retrospectively analyzed clinical and imaging data from 220 CD patients in two centers. Univariate and multivariate analyses were used to screen out clini
White blood cell count, disease duration and Harvey-Bradshaw Index were identified as clinically independent predictors of SLOR to develop the clinical model. Fifteen most valuable radiomics features were selected to develop the radiomics model. Compared with the clinical and radiomics models, the combined model achieved the best prediction performance, with AUCs were 0.871 (95%CI: 0.814-0.929) in the training cohort and 0.854 (95%CI: 0.759-0.949) in the validation cohort.
The combined model that integrates intestinal wall and creeping fat analysis is valuable for predicting the SLOR of IFX in CD.
Core Tip: This study is the first to present the integrative analysis of intestinal wall and creeping fat in the prediction of secondary loss of response (SLOR) to infliximab in Crohn's disease (CD). This model can accurately identify patients with high risk of SLOR in CD, providing reliable pre-treatment references for clinicians and thereby assisting in treatment decisions.
- Citation: Li S, Zhu C, Tong L, Zheng XM, Rong C, Gao YK, Yuan DC, Wu XW. Correlation between radiomic features of Crohn's disease and secondary loss of response to infliximab. World J Gastroenterol 2025; 31(27): 109459
- URL: https://www.wjgnet.com/1007-9327/full/v31/i27/109459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i27.109459
Crohn's disease (CD) is a chronic, nonspecific granulomatous inflammation that affects the entire gastrointestinal tract. It is characterized by frequent recurrences, is challenging to cure, and typically necessitates lifelong treatment[1]. Study have proved that tumor necrosis factor-α (TNF-α) plays an important role in the pathogenesis of CD[2]. Infliximab (IFX) is a human-mouse chimeric IgG1 monoclonal antibody. By binding to both transmembrane and soluble TNF-α, it prevents the interaction between TNF-α and its receptor, thereby inhibiting its biological activity and exerting anti-inflammatory effect[3]. However, a noteworthy problem was that 23%-46% of CD patients who achieved clinical remission via induction therapy experienced secondary loss of response (SLOR) over time[4], which is primarily because low serum drug concentrations of IFX, IFX-related antibodies and the disease itself (such as the size and location of the lesion)[5-7].
As a chronic transmural inflammatory bowel disease, CD often has characteristic mesenteric fat hyperplasia, which wraps around the intestinal tract and forms creeping fat[8]. As one of the main differences between CD and other gastrointestinal diseases, it was reported that there is a close correlation between the creeping fat and the development of intestinal inflammation, stenosis, and fibrosis in CD patients[9-12].
Radiomics transforms medical images into deep spatial features via high-throughput data analysis. Hence, it is capable of extracting more detailed features than traditional visual interpretation and providing additional information for the treatment strategies[13]. It has been increasingly used in CD research in recent years. Previous study have developed radiomics model to predict SLOR based on computed tomography enterography (CTE)[14], while Yueying et al[15] predicted the IFX response in bio-naïve CD patients through magnetic resonance enterography (MRE). Nevertheless, previous loss of response related studies have just focused on the intestinal wall, and no study has explored the relevance between creeping fat and SLOR. Therefore, we hypothesize that the integrated analysis of radiomics features extracted from the intestinal wall and creeping fat may provide a novel approach to better predict the SLOR of IFX in CD patients.
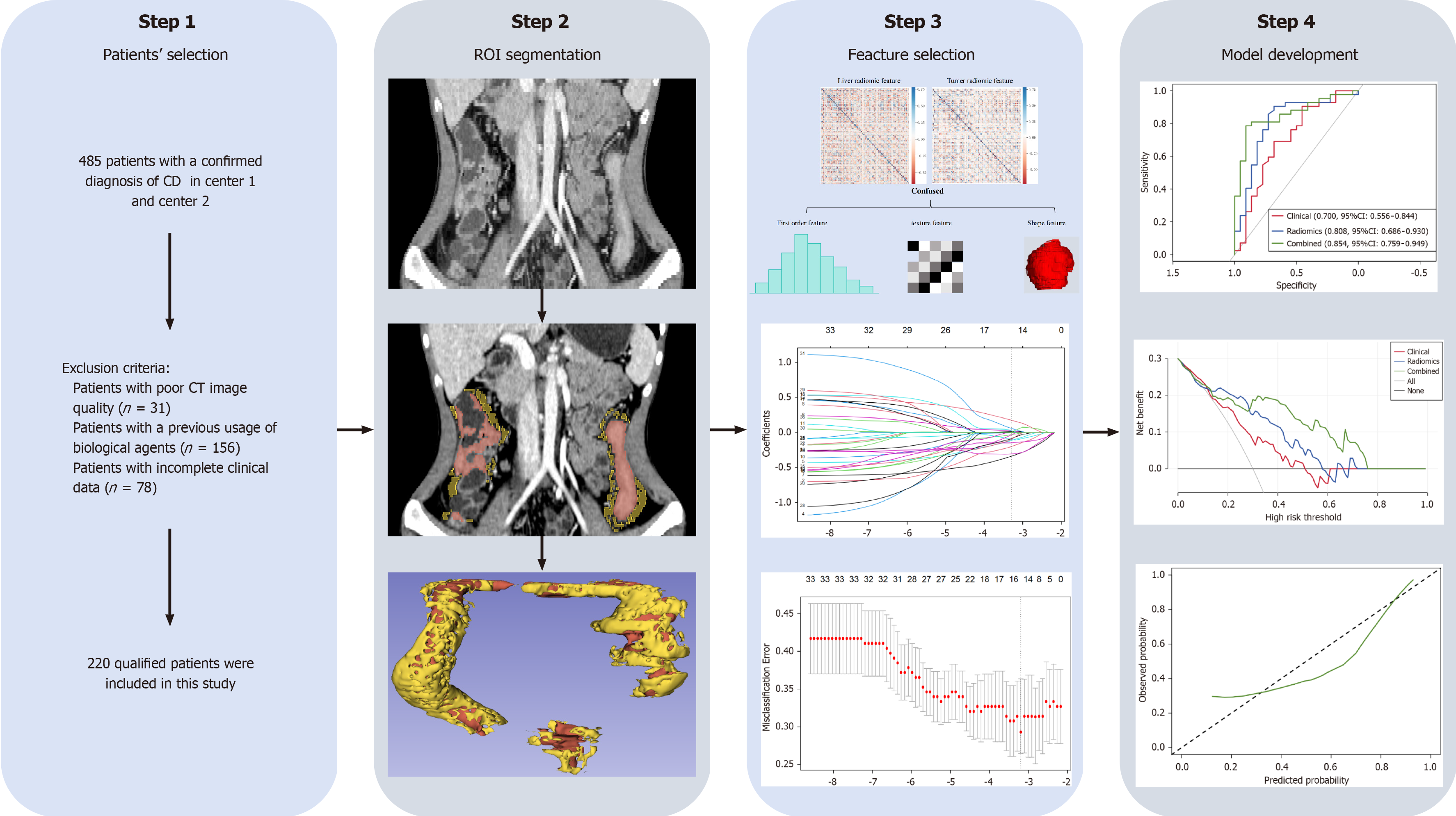
This was a retrospective study approved by the corresponding institutional review board. The flowchart of this study is showing in Figure 1. The 485 patients with confirmed diagnosis of CD from March 2017 to February 2023 in center 1 and center 2 were retrospectively enrolled according to the European Crohn’s and Colitis Organization guideline[16]. The inclusion criteria were as follows: (1) Patients with a confirmed diagnosis of CD; (2) Patients who underwent CTE within one month before IFX treatment; and (3) Patients with initial response to standardized IFX treatment. The exclusion criteria were as follows: (1) Patients with poor CT image quality (n = 31); (2) Patients with a previous usage of biological agents (n = 156); and (3) Patients with incomplete clinical data (n = 78). After screening, a total of 220 CD patients were included in this study, of whom 164 (74.5%) were males (with an average age of 27.12 ± 8.91 years old), and 56 (25.5%) were females (with an average age of 27.79 ± 12.34 years old). Patients in center 1 (n = 156) served as training cohort, and patients in center 2 (n = 64) served as validation cohort. This study strictly adhered to the Declaration of Helsinki, and the informed consent requirement was waived.
Demographic and laboratory data at baseline were obtained from medical record system. A standardized treatment strategy[16] was used for all of the CD patients, which involved intravenous administration of IFX at 5-10 mg/kg for 0, 2, and 6 weeks as the induction therapy. The same dose was subsequently given every 8 weeks for the maintenance therapy. Initial response was defined as a decrease in the Harvey-Bradshaw Index (HBI) score of more than 2 points at week 12, whereas clinical remission was defined as a decrease in the HBI score of more than 5 points at week 54[17]. SLOR was defined as an initial response followed by a relapse or progression of CD. The therapeutic effect of IFX was evaluated by two gastroenterologists using an independent blind method based on the patients' HBI score, radiological and endoscopic examination results. If there was a discrepancy in opinions, the final decision was made by another senior physician. The endpoint of follow-up was the time of SLOR onset in patients with SLOR or the 54th week after the start of IFX treatment in patients without SLOR. The follow-up duration from baseline to endpoint was defined as progression-free remission (PRF).
All of the CD patients underwent CTE examination within one month before IFX treatment at both centers with the same scanning protocol: Patients were given a light diet and intestinal preparation the day before the CTE examination. About 45 minutes before CT examination, each patient drank 1500-2000 mL 2.5% isotonic mannitol solution to fill the intestinal tract. Scan range is from diaphragm to perineum. Scanning parameters included a rotation time 0.5 second, tube voltage 120 kV, tube current 150-300 mA, detector collimation 64 mm × 0.625 mm, pitch 1.375:1, Matrix 512 × 512, layer thickness 5 mm, layer spacing 5 mm, contrast agent (iodohexanol, 320 mgI/mL, 1.5 mL/kg) was injected intravenously (3.0 mL/s). Enteric phase (45 seconds after injection of contrast agent) CTE images were obtained for radiomics analysis. The segmentation of the intestinal wall [volume of interest (VOI)-intestine] was conducted by a deep learning automatic segmentation model. This model, built upon the nn-Unet algorithm and achieved the best segmentation result at 91.61%, 91.60%, and 90.69% for dice similarity coefficient, precision, and recall, respectively. Creeping fat (VOI-fat) was then obtained via an expansion algorithm of Python, which automatically extends the fat density (-150 HU to -30HU) within 10 mm outward along the margin of the VOI-intestine (Supplementary Figure 1).
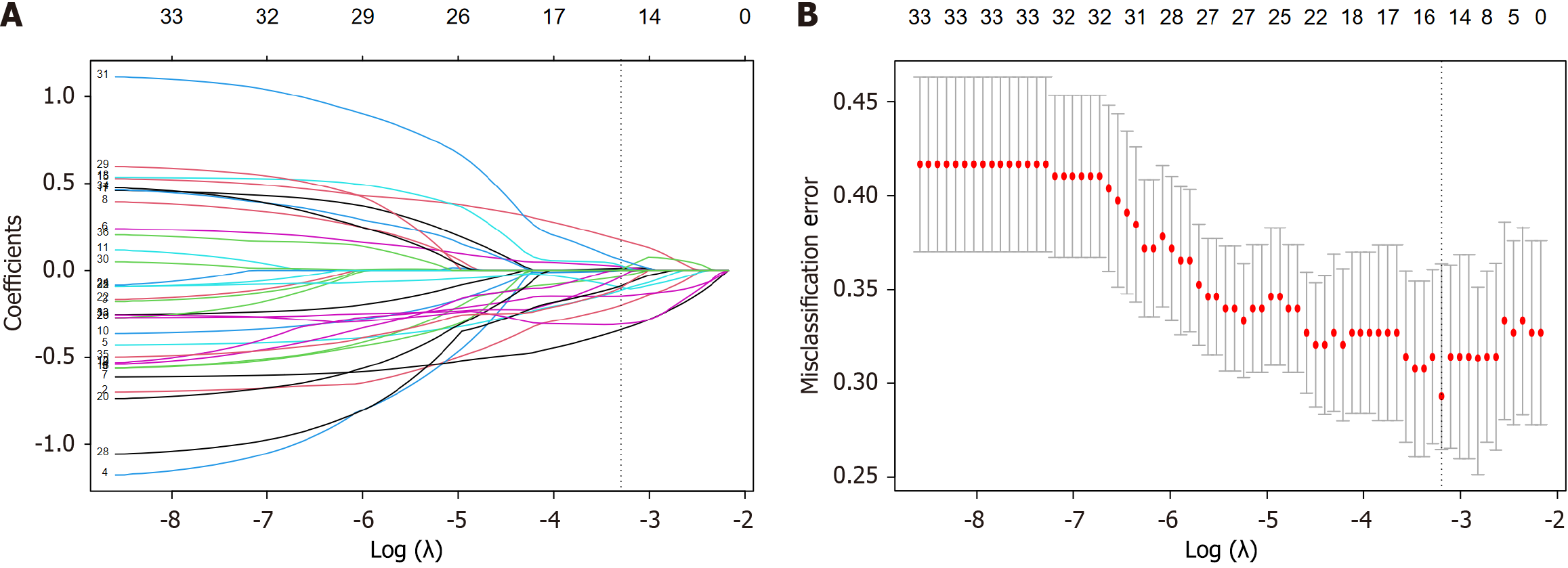
Radiomics features of intestinal wall and creeping fat were separately extracted via the PyRadiomics package in Python and then fused together for radiomics analysis. All of the extracted features were Z-score normalized before analysis. Spearman’s test and univariate analysis were applied to downscale the radiomics features, and the most valuable features were selected using least absolute shrinkage and selection operator (LASSO) to calculate Radscore and develop the radiomics predictive model. As for clinical characteristics, univariate and multivariate analyses were applied to screen out the clinically independent predictors of SLOR and develop the clinical predictive model. Finally, a combined predictive model was developed by combining Radscore and clinically independent predictors via multivariate logistic regression analysis. The predictive performance of different models were assessed via the area under the receiver operating characteristic curve (AUC), calibration curve and decision curve analysis (DCA).
All of the statistical analyses were performed via SPSS 26.0 and R software (version 3.6.3). The normality of the measurements was examined via the Kolmogorov-Smirnov test. The normally distributed characteristics were compared between groups via the t test, and the non-normally distributed characteristics were compared between groups via the Mann-Whitney U test. Count data between-group comparisons were conducted via the χ2 test. And the AUCs comparison between different models were conducted via the Delong test. The X-tile program (version 3.6.1, Rimm Laboratory, Yale University School of Medicine) was applied to calculate the optimal cutoff value to classify CD patients into high and low risk groups for SLOR. PRF probabilities were estimated using the Kaplan-Meier method and compared with the log-rank test. A two-sided P < 0.05 was considered to be statistically significant.
A total of 69 CD patients developed SLOR in this study, with 47 in the training cohort and 22 in the validation cohort. The clinical characteristics of all of the patients are showing in Table 1. There was no statistically significant between the training and validation cohorts (both P > 0.05). Univariate analysis based on the training cohort revealed that SLOR patients has longer disease duration (24 months vs 12 months), higher HBI score (7 vs 6), white blood cell (WBC; 7.07 ± 2.21 × 109/L vs 6.24 ± 2.14 × 109/L) and lower albumin level (35.70 g/L vs 37.80 g/L) than patients without SLOR (both
| Clinical characteristics | Training cohort (n = 156) | Validation cohort (n = 64) | P value |
| Age (years) | 25.00 (20.00, 31.00) | 24.00 (19.50, 29.00) | 0.351 |
| Gender (male/female) | 111/45 | 53/11 | 0.071 |
| Disease duration (months) | 14.50 (7.00, 36.00) | 12.00 (5.00, 46.00) | 0.706 |
| Montreal location, n (%) | |||
| Ileal (L1) | 21 (13.5) | 15 (23.5) | 0.346 |
| Colonic (L2) | 43 (27.6) | 18 (28.1) | 0.268 |
| Ileocolonic (L3) | 92 (58.9) | 31 (48.4) | 0.725 |
| Montreal behavior, n (%) | |||
| Nonstricturing, nonpenetrating (B1) | 105 (67.3) | 46 (71.9) | 0.356 |
| Stricturing (B2) | 39 (25.0) | 14 (21.9) | 0.623 |
| Penetrating (B3) | 22 (14.1) | 11 (17.2) | 0.561 |
| Perianal disease modifier (P) | 82 (52.6) | 33 (51.6) | 0.893 |
| Smoking (yes) | 8 (5.1) | 4 (6.3) | 0.739 |
| CRP (mg/L) | 12.95 (4.05, 30.16) | 14.60 (2.17, 27.06) | 0.438 |
| ESR (mm/h) | 26.50 (13.00, 36.00) | 22.00 (12.00, 31.00) | 0.252 |
| ALB (g/L) | 37.00 (33.90, 40.30) | 39.40 (34.70, 42.80) | 0.104 |
| WBC (× 109/L) | 6.49 ± 2.19 | 7.93 ± 1.56 | 0.105 |
| HBI | 6.00 (5.00, 8.00) | 6.50 (5.00, 7.00) | 0.388 |
| Previous abdominal surgery (yes), n (%) | 18 (11.5) | 9 (14.1) | 0.604 |
| Previous steroids or immunomodulators use (yes), n (%) | 69 (44.2) | 26 (40.6) | 0.624 |
| Clinical factors | Univariate analysis | Multivariate analysis | ||||
| Loss of response (n = 47) | Response (n = 109) | P value | β | P value | OR (95%CI) | |
| Age (years) | 25 (18, 34) | 25 (20, 30.5) | 0.902 | |||
| Gender (male/female) | 12/35 | 33/76 | 0.549 | |||
| Disease duration (months) | 24 (12, 48) | 12 (4, 36) | 0.011 | -0.012 | 0.041 | 0.988 (0.976, 0.999) |
| Montreal location, n (%) | ||||||
| Ileal (L1) | 9 (19.1) | 12 (11.0) | 0.168 | |||
| Colonic (L2) | 13 (27.7) | 30 (27.5) | 0.575 | |||
| Ileocolonic (L3) | 25 (53.2) | 67 (61.5) | 0.319 | |||
| Montreal behavior, n (%) | ||||||
| Nonstricturing, nonpenetrating (B1) | 38 (81.0) | 67 (61.5) | 0.687 | |||
| Stricturing (B2) | 7 (14.9) | 32 (29.4) | 0.056 | |||
| Penetrating (B3) | 3 (6.4) | 19 (17.4) | 0.069 | |||
| Perianal disease modifier (P) | 24 (51.1) | 58 (53.2) | 0.805 | |||
| Smoking (yes) | 3 (6.4) | 5 (4.6) | 0.641 | |||
| CRP (mg/L) | 17.70 (8.85, 40.10) | 11.10 (3.55, 25.79) | 0.058 | |||
| ESR (mm/h) | 31.00 (15.00, 39.00) | 23.00 (13.00, 34.01) | 0.105 | |||
| ALB (g/L) | 35.70 (32.70, 39.80) | 37.80 (34.73, 40.50) | 0.049 | 0.613 | ||
| WBC (× 109/L) | 7.07 ± 2.21 | 6.24 ± 2.14 | 0.028 | -0.248 | 0.008 | 0.780 (0.649, 0.939) |
| HBI | 7 (6, 9) | 6 (5, 8) | 0.001 | -0.029 | 0.018 | 0.812 (0.683, 0.965) |
| Previous abdominal surgery (yes), n (%) | 4 (8.5) | 14 (12.8) | 0.437 | |||
| Previous steroids or immunomodulators use (yes), n (%) | 18 (38.3) | 51 (46.8) | 0.327 | |||
We separately extracted 1874 radiomics features from the VOI-intestine and VOI-fat of each patient. All of the radiomics features were fused together and Z-score normalized for further radiomics analysis. After the spearman’s test, those features with a correlation coefficient > 0.8 were eliminated, and 738 out of 3748 radiomics features were retained. Following the univariate and LASSO analyses (Figure 2), 15 radiomics features were selected as the most valuable features to calculate Radscore (Supplementary material) and develop radiomics predictive model, including 9 from VOI-intestine and 6 from VOI-fat. The AUCs of radiomics predictive were 0.818 (95%CI: 0.752-0.884) for the training cohort and 0.808 (95%CI: 0.686-0.930) for the validation cohort.
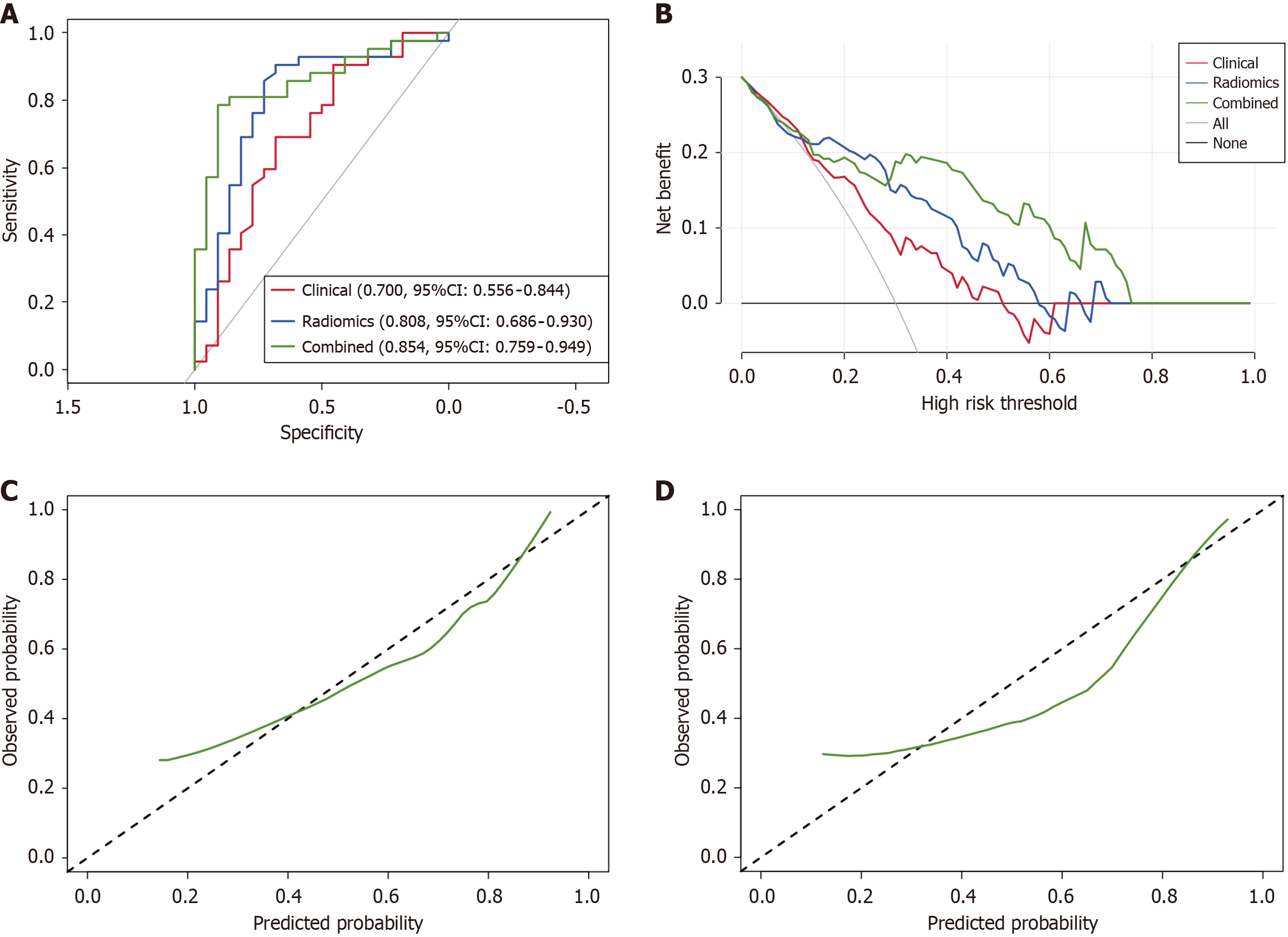
To further improve the predictive performance of the radiomics predictive model, we introduced the clinically independent predictors into the radiomics predictive model. Hence, a combined predictive model was developed via multivariate logistic regression analysis based on the training cohort. The AUCs of the combined predictive model were 0.871 (95%CI: 0.824-0.929) and 0.854 (95%CI: 0.759-0.949) in the training and validation cohorts, respectively.
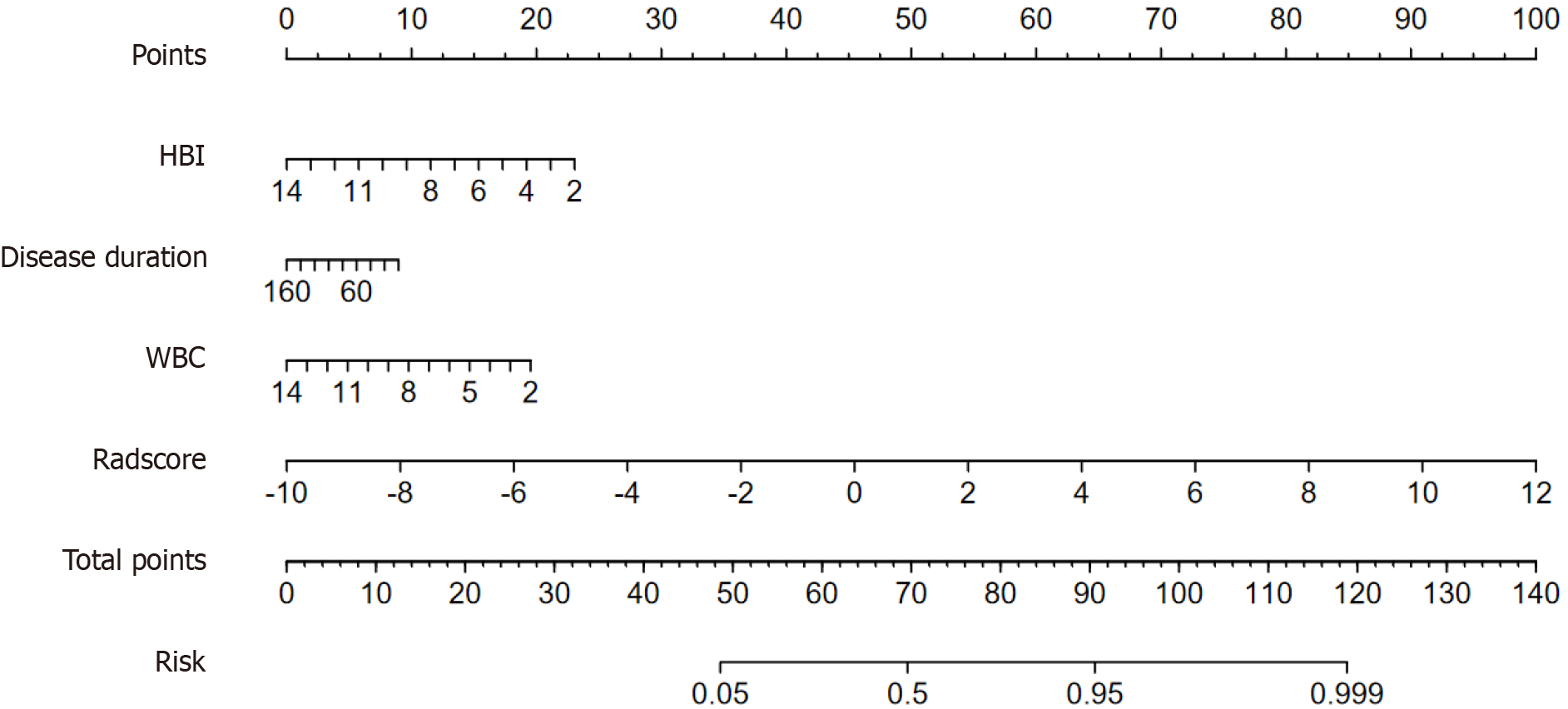
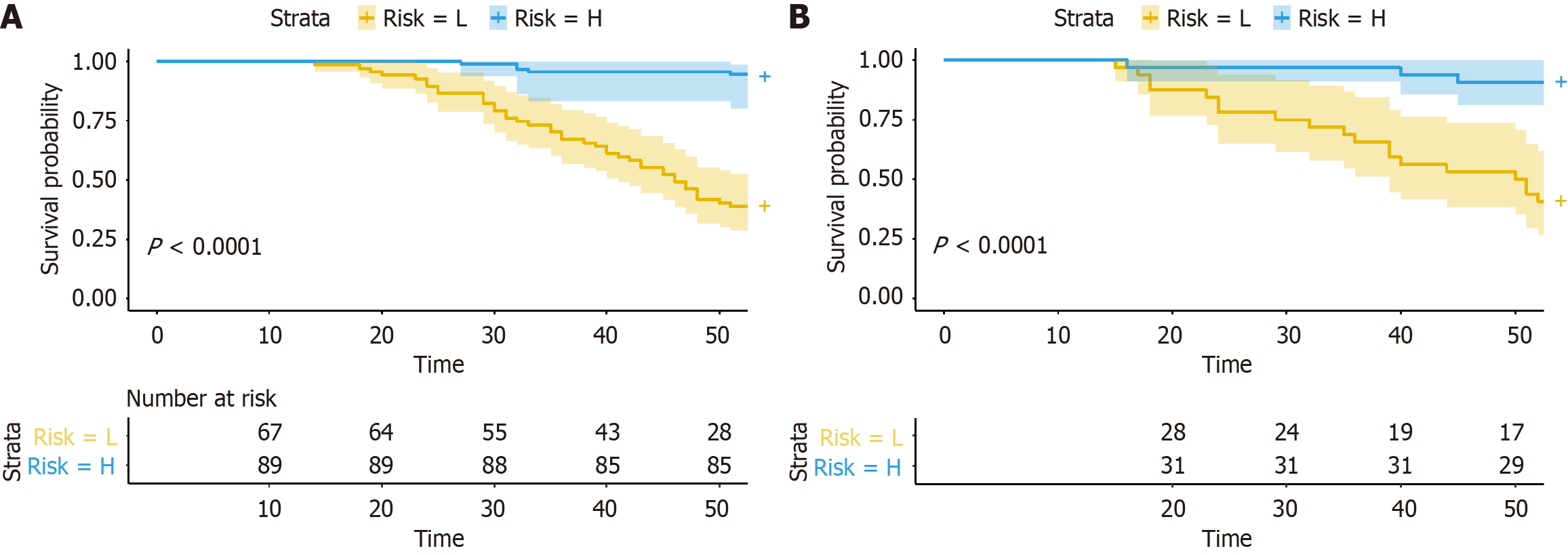
As shown in Table 3 and Figure 3A, the predictive performance of the combined predictive model was significantly better than the radiomics and clinical predictive model (0.854 vs 0.808 vs 0.700). Delong test showed that the AUCs between the combined model and radiomics or clinical model were statistically significant in the training cohort (P = 0.019 and P < 0.001), and the same result was observed in the validation cohort between the combined and clinical model (P = 0.029). However, there was no statistically significant between the combined and radiomics predictive model in the validation cohort (P = 0.356). DCA (Figure 3B) revealed that the combined predictive model outperformed than other models in clinical net benefit. In addition, calibration curve (Figure 3C and D) showed that there is a good consistency of the combined model between the prediction accuracy and the actual situation in both training and validation cohorts. Thus, confirming the superiority of the combined predictive model. To enhance clinical applicability of the combined predictive model, we further visualized this model as a nomogram (Figure 4). Moreover, we also stratified the risk of SLOR in IFX-treated CD patients by calculating the optimal cutoff value based on the predictive probability derived from the combined predictive model via X-tile software, thus resulting in a cutoff value of 0.751, and patients were categorized into high-risk and low-risk subgroups. Kaplan-Meier curves revealed that there were statistically significant in the occurrence of SLOR between the high-risk and low-risk subgroups in the training and validation cohorts (both P < 0.01; Figure 5).
| Model | Clinical | Radiomics | Combined | |
| Training cohort | AUC | 0.720 (95%CI: 0.635-0.804) | 0.818 (95%CI: 0.752-0.884) | 0.871 (95%CI: 0.824-0.929) |
| SEN | 0.633 | 0.817 | 0.762 | |
| SPE | 0.745 | 0.727 | 0.872 | |
| ACC | 0.667 | 0.776 | 0.795 | |
| Validation cohort | AUC | 0.700 (95%CI: 0.556-0.844) | 0.808 (95%CI: 0.686-0.930) | 0.854 (95%CI: 0.759-0.949) |
| SEN | 0.595 | 0.857 | 0.691 | |
| SPE | 0.727 | 0.727 | 0.910 | |
| ACC | 0.645 | 0.813 | 0.766 | |
We developed and validated a non-invasive clinical-radiomics model based on CTE for predicting SLOR of IFX in CD. This model demonstrated satisfactory predictive performance by integrating radiomics features of the intestinal wall and creeping fat with clinically independent predictors associated with SLOR. DCA and Kaplan-Meier curves confirmed its’ clinical utility.
CD is closely correlated to the excessive expression of TNF-α. Once activated, TNF-α binds to specific receptors, triggering inflammatory responses and cytotoxicity. It stimulates neutrophils, enhances the expression of nitric oxide and adhesion molecules, disrupts cellular immune function and results in gastrointestinal inflammatory lesions. Inflammation increases intestinal permeability and compromises mucosal barrier function, exposing other intestinal sections to microbiota and metabolites[18]. This process triggers immune responses and pathological changes in mesenteric fat, leading to the development of creeping fat. As one of the significant differences between CD and other gastrointestinal diseases, creeping fat is not merely a simple accumulation of adipose tissue but rather a unique pathological structure with pronounced inflammatory characteristics. Molecular analysis of creeping fat revealed that the expression levels of inflammation-associated genes are significantly elevated compared to those in mesenteric fat from healthy individuals, while genes involved in lipid metabolism are concurrently downregulated[19]. Additionally, the proportion of non-adipocytes within creeping fat is markedly higher than in normal mesenteric fat, with increased infiltration by immune cells such as macrophages and T lymphocytes, which results in the impairment of the intestinal mucosal barrier, bacterial translocation, and further exacerbates intestinal inflammation and fibrosis[20,21]. Creeping fat serves as a rich reservoir for pro-inflammatory and pro-fibrotic cytokines in CD, including TNF-α, IL-6, and IL-10[22]. Excessively high concentrations of TNF-α hinder IFX from fully neutralizing it, thereby preventing the achievement of inflammation control. Furthermore, cytokines such as IL-6 and IL-10 within creeping fat may activate alternative signaling pathways, such as JAK/STAT, bypassing the TNF-α-dependent inflammatory cascade and hindering IFX from fully inhibiting the inflammatory response. Notably, creeping fat contains a higher proportion of M2 macrophages compared to M1 macrophages. M2 macrophages contribute to intestinal fibrosis through the secretion of profibrotic cytokines, which may reduce drug penetration into the deeper layers of the intestinal wall and diminish the local concentration of IFX at the site of lesions[2,23].
Previous study have reported the value of radiomics analysis based on the intestinal segment with the most severe inflammation as being a predictor of SLOR[15]. However, predicting SLOR based solely on the intestinal segment with the most severe inflammation has significant limitations. It’s not only overlooks the overall burden of intestinal inflammation, dynamic changes, and other lesion characteristics but also tends to produce biased and inaccurate results. Wang et al[24] identified patients with CD who were resistant to IFX via a CT based radiomics signature of visceral adipose tissue and bowel lesions. Visceral adipose tissue is a part of systemic fat but is not a pathological change specific to CD that directly reflects localized inflammation around the intestinal tract, as creeping fat does. Furthermore, although visceral adipose tissue produces proinflammatory factors, its direct impact in CD is limited. In contrast, creeping fat produces proinflammatory factors, such as TNF-α, that act directly on the intestines to influence the local inflammatory response[22]. Another study reported that R2* is correlated with intestinal inflammation in CD patients, and MRE-based radiomics signature can facilitate individualized identification of SLOR to IFX in patients with CD[25]. Although there is no radiation, MRE also has its shortcomings. Some CD patients have contraindications for MRE (such as pacemakers, claustrophobia), or unable to cooperate with long-term examinations (such as children, patients with pain), are not suitable for MRE examination. In addition, given that MRE has not yet been widely adopted in some medical centers and there exist variations in the standardized protocols for MRE across different centers, the breadth and consistency of its data sources are inferior to those of CTE.
Our study highlighted the effectiveness of a combined predictive model, which integrates radiomics features of the inflamed intestine and creeping fat with clinically independent predictors in predicting SLOR of IFX in CD. The combined model demonstrated superior performance compared to standalone clinical and radiomics models, achieving the highest AUC in both the training and validation cohorts. DeLong test indicated statistically significant in AUCs between the combined and radiomics models in the training cohort (P = 0.019), but no significant difference was observed in the validation cohort (P = 0.356). It may due to the reduced sensitivity because the relatively small sample size in the validation cohort. By determining an optimal cutoff value (0.751), patients were stratified into high-risk and low-risk subgroups. Kaplan-Meier survival analysis revealed a statistically significant in SLOR incidence between the two subgroups in both the training and validation cohorts (P < 0.01), underscoring the clinical application prospect of the combined model in risk stratification and individualized patient management. For CD patients predicted by the combined model to have a high risk of SLOR (probability ≥ 0.751), it may be necessary to adjust the treatment strategy as follows: Firstly, enhance monitoring and shorten the follow-up interval, such as evaluating clinical symptoms, IFX concentration, fecal calprotectin, and other relevant indicators every 4-6 weeks. Additionally, increase the frequency of endoscopic or imaging assessments to detect early signs of inflammation recurrence. Moreover, adjusting the IFX dosage or shortening the dosing interval if necessary. For low-risk patients (probability < 0.751), the standard treatment regimen can be maintained, including conducting routine tests according to the guideline-recommended follow-up schedule.
Our study has several limitations: Firstly, although most of the routine clinical baseline data were included, specific biomarkers such as fecal calprotectin, which have certain pathological indication value, were not included in this study because they are not part of the routine clinical testing items and a large number of patients were missing this data. In the future, a prospective cohort study protocol will be designed to systematically collect multi-dimensional biomarker data to optimize the combination of clinical predictors. Secondly, the current technical process adopts a cascaded architecture of segmentation and prediction, which may cause the step-by-step attenuation of feature information. For this purpose, an end-to-end deep learning architecture is proposed to be constructed. By jointly optimizing the dual tasks of image segmentation and therapeutic effect prediction, a high degree of synergy between feature extraction and prediction tasks can be achieved. Furthermore, although cases from two centers were included, the sample size (n = 220) was still insufficient, which may affect the generalization performance of the model. Subsequently, a multi-center study is planned to be conducted, including at least 1000 case samples, in order to improve and evaluate the model's performance.
We developed and validated a CTE-based radiomics model to identify CD patients at high risk of SLOR via integrative analysis of the intestinal wall and creeping fat, which can be a valuable reference for clinicians before initiating IFX treatment, thus enabling timely adjustments of treatment plans and facilitating personalized management of CD patients.
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1971] [Article Influence: 219.0] [Reference Citation Analysis (113)] |
| 2. | Lissner D, Schumann M, Batra A, Kredel LI, Kühl AA, Erben U, May C, Schulzke JD, Siegmund B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Biancheri P, Di Sabatino A, Rovedatti L, Giuffrida P, Calarota SA, Vetrano S, Vidali F, Pasini A, Danese S, Corazza GR, MacDonald TT. Effect of tumor necrosis factor-α blockade on mucosal addressin cell-adhesion molecule-1 in Crohn's disease. Inflamm Bowel Dis. 2013;19:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 5. | Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, Loebstein R, Chowers Y, Eliakim R, Kopylov U, Ben-Horin S. Optimizing Anti-TNF-α Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2016;14:550-557.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40-7; quiz 48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Zhang QW, Shen J, Zheng Q, Ran ZH. Loss of response to scheduled infliximab therapy for Crohn's disease in adults: A systematic review and meta-analysis. J Dig Dis. 2019;20:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Siegmund B. Mesenteric fat in Crohn's disease: the hot spot of inflammation? Gut. 2012;61:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Li Y, Zhu W, Zuo L, Shen B. The Role of the Mesentery in Crohn's Disease: The Contributions of Nerves, Vessels, Lymphatics, and Fat to the Pathogenesis and Disease Course. Inflamm Bowel Dis. 2016;22:1483-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 10. | Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, Schoeman S, Lim A, Bartholomeusz FD, Travis SPL, Andrews JM. Visceral Adipose Tissue Is Associated With Stricturing Crohn's Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm Bowel Dis. 2019;25:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 11. | Büning C, von Kraft C, Hermsdorf M, Gentz E, Wirth EK, Valentini L, Haas V. Visceral Adipose Tissue in Patients with Crohn's Disease Correlates with Disease Activity, Inflammatory Markers, and Outcome. Inflamm Bowel Dis. 2015;21:2590-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, Coffey JC, Rieder F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn's Disease. Inflamm Bowel Dis. 2019;25:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 13. | Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med. 2017;38:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Li H, Feng J, Suo S, Feng Q, Shen J. A Novel Radiomics Nomogram for the Prediction of Secondary Loss of Response to Infliximab in Crohn's Disease. J Inflamm Res. 2021;14:2731-2740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Yueying C, Jing F, Qi F, Jun S. Infliximab response associates with radiologic findings in bio-naïve Crohn's disease. Eur Radiol. 2023;33:5247-5257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 16. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1508] [Article Influence: 167.6] [Reference Citation Analysis (0)] |
| 17. | Jeffrey AW, Abu-Rgeef R, Picardo S, Menon S, So K, Venugopal K. Safety and efficacy of transitioning inflammatory bowel disease patients from intravenous to subcutaneous infliximab: a single-center real-world experience. Ann Gastroenterol. 2023;36:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Zulian A, Cancello R, Ruocco C, Gentilini D, Di Blasio AM, Danelli P, Micheletto G, Cesana E, Invitti C. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study. PLoS One. 2013;8:e78495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 20. | Zielińska A, Siwiński P, Sobolewska-Włodarczyk A, Wiśniewska-Jarosińska M, Fichna J, Włodarczyk M. The role of adipose tissue in the pathogenesis of Crohn's disease. Pharmacol Rep. 2019;71:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity. 2019;50:992-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 543] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 22. | Yin Y, Xie Y, Ge W, Li Y. Creeping fat formation and interaction with intestinal disease in Crohn's disease. United European Gastroenterol J. 2022;10:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 23. | Kredel LI, Jödicke LJ, Scheffold A, Gröne J, Glauben R, Erben U, Kühl AA, Siegmund B. T-cell Composition in Ileal and Colonic Creeping Fat - Separating Ileal from Colonic Crohn's Disease. J Crohns Colitis. 2019;13:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Luo Z, Zhou Z, Zhong Y, Zhang R, Shen X, Huang L, He W, Lin J, Fang J, Huang Q, Wang H, Zhang Z, Mao R, Feng ST, Li X, Huang B, Li Z, Zhang J, Chen Z. CT-based radiomics signature of visceral adipose tissue and bowel lesions for identifying patients with Crohn's disease resistant to infliximab. Insights Imaging. 2024;15:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Feng J, Feng Q, Chen Y, Yang T, Cheng S, Qiao Y, Shen J. MRI-Based Radiomic Signature Identifying Secondary Loss of Response to Infliximab in Crohn's Disease. Front Nutr. 2021;8:773040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/