Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.109239
Revised: May 28, 2025
Accepted: June 16, 2025
Published online: July 21, 2025
Processing time: 78 Days and 8.4 Hours
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are chronic gastrointestinal disorders with an increasing global prevalence and significant healthcare impact. The exact etiology of this condition remains unclear. Neutrophils play a critical role in IBD pathogenesis. Translocator protein (TSPO), a mitochondrial protein linked to immune responses, has demon
To investigate the role of TSPO in IBD pathogenesis, particularly in neutrophils.
Bioinformatics analyses of Gene Expression Omnibus datasets (GE75214, GSE94648, GSE156776) assessed TSPO expression in IBD patients. TSPO expre
Bioinformatics analysis revealed elevated TSPO expression in the intestinal mucosa and peripheral blood of patients with IBD, especially in neutrophils. This was confirmed by quantitative real-time polymerase chain reaction and immunohistochemical staining, which showed a significant upregulation of TSPO in active IBD. Neutrophils from patients with UC and CD exhibited higher TSPO expression, which correlated with increased ROS production and NET formation. In a mouse model of dextran sodium sulfate-induced chronic colitis, TSPO was upregulated in the colonic neutrophils and brain tissues, indicating its systemic involvement. PET-CT imaging showed enhanced TSPO uptake in the inflamed colon and brain regions, particularly in the microglia, highlighting neuroinflammation.
TSPO is significantly upregulated in neutrophils in IBD and contributes to intestinal inflammation. Its elevated expression in gut highlights its potential as a promising therapeutic target for IBD.
Core Tip: This research recognizes translocator protein (TSPO) as a novel biomarker and promising therapeutic target for inflammatory bowel diseases (IBD). Elevated TSPO expression was observed in neutrophils of IBD patients, along with increased reactive oxygen species production and neutrophil extracellular trap formation. Additionally, upregulated TSPO in both the inflamed colon and brain tissue suggests its contributions to autoimmune inflammation and neuroinflammation. These findings recommend TSPO as a potential target for IBD treatment, highlighting its role in both intestinal and neurological inflammatory processes.
- Citation: He Q, Wu XH, Jiang DL, Lin RT, Xie F, Guan YH, Fei AH. Translocator protein facilitates neutrophil-mediated mucosal inflammation in inflammatory bowel diseases. World J Gastroenterol 2025; 31(27): 109239
- URL: https://www.wjgnet.com/1007-9327/full/v31/i27/109239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i27.109239
Inflammatory bowel diseases (IBD), primarily ulcerative colitis (UC) and Crohn’s disease (CD), are relapsing-remitting inflammatory disorders of the gastrointestinal tract and are the main causes of gut environment disorders. The global prevalence of IBD is predicted to steadily increase over the next decade and impose a heavy healthcare burden[1]. However, the exact etiology of IBD remains unclear and is believed to be multifactorial. Genetic factors, health-related behaviors, environmental changes, intestinal flora, and immune disorders are involved in and functionally interconnected with the pathogenesis of IBD[2,3]. Neutrophils, which are crucial players in innate immunity in IBD, exhibit huge complexities with heterogeneous populations and dual functions in intestinal homeostasis[4]. Neutrophils are the earliest activated cells during innate immune responses and could rapidly migrate to the inflammatory intestinal tissues to exert immune responses. The degree of neutrophil infiltration is a key biomarker for assessing the activity and resolution of intestinal inflammation[5]. Neutrophils function via two primary mechanisms: They release reactive oxygen species (ROS), form neutrophil extracellular traps (NETs) to eliminate pathogenic microorganisms and limit further inflammatory progression. For example, CD177+ neutrophils display strong bactericidal and tissue repair abilities, offering rapid protection during the early stages of intestinal mucosal inflammation[6]. Neutrophils can also recruit and induce other immune cells to the inflamed intestinal tissue to accelerate the restoration of inflammation or amplify the immune cascade response. Neutrophils can release azurophilic granules that attract monocytes, which subsequently differentiate into macrophages and adopt M1 or M2 phenotypes, thereby driving either pro-inflammatory or anti-inflammatory responses[7]. However, as functionally diverse innate immune cells, the specific mechanisms through which neutrophils contribute to IBD remain unclear.
The translocator protein (TSPO), an 18-kilodalton transmembrane protein, mainly located on the outer membrane of mitochondrial. Because of its interaction with benzodiazepines, it is also referred to as the peripheral-type benzodia
Therefore, we aimed to investigate the role of TSPO in IBD pathogenesis and identify a promising molecular target for the exploration of new therapeutic strategies for IBD.
Patients diagnosed as IBD were enrolled from the Department of Gastroenterology, Shanghai East Hospital, Tongji University of School Medicine, while healthy controls (HC) were enrolled from individuals attending the hospital’s out-patient department for routine physical examination between October 2021 and November 2023. Colon samples were collected from IBD patients, including those with active CD (A-CD) (n = 47), CD in remission (R-CD) (n = 18), active UC (A-UC) (n = 40), and UC in remission (R-UC) (n = 25), and HC (n = 35) who had an endoscopic examination as part of endoscopic examination.
Fasting blood samples anticoagulated with ethylenediamine tetraacetic acid were collected from IBD patients, comprising A-CD (n = 19), R-CD (n = 9), A-UC (n = 12), and R-UC (n = 6), and HC (n = 17). Diagnoses were established through a combination of clinical symptoms, endoscopic and radiologic findings, and histological results, following the American Gastroenterological Association Institute Guideline[20]. Simple Endoscopic Score for CD (CD-SES) and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) were used as the assessment for the severity of endoscopic findings as per previously established criteria[21,22]. CD-SES was based on four key endoscopic features: (1) Presence of ulcer; (2) Extent of the surface area affected by ulcers; (3) Non-ulcerative lesions; and (4) Presence of stenosis. UCEIS evaluation focused on vascular patterns, degree of bleeding, erosions, and ulcerations. This study was approved by the Ethics Committee of Shanghai East Hospital of Tongji University (No. 2024YS-232). All participants provided written informed consent before being included in the study.
Male C57BL/6 mice were obtained, and their genotypes were confirmed by the Shanghai Model Organisms Center, Inc. (Shanghai, China). All mice were bred and housed in specific pathogen-free conditions at the Experimental Animal Centre of Tongji University School of Medicine (Shanghai, China). All mice had unrestricted access to filtered air, sterile water, and autoclaved food, and were kept in individually ventilated cages under a 12-hour light-dark cycle. Male mice aged 8–10 weeks were used in the experiments. The animal protocols and procedures in this study were approved by the Biological Research Ethics Committee of Tongji University (No. TJBB05424103).
Chronic colitis was induced in wild-type (WT) mice using dextran sodium sulfate (DSS) as previously described[23]. In brief, WT mice were randomly assigned to experimental and control groups (n = 6 per group). Mice in the experimental group were administered 2% DSS (Sigma-Aldrich, St. Louis, MO, United States) in the drinking water for 7 days, followed by sterile water for 14 days. This cycle was repeated for three times until the end of the experiment. Mice were monitored daily for diarrhea, weight changes, and rectal bleeding. All mice were sacrificed at 8th week.
RNA was isolated using the TRIzol reagent (Life Technologies, Grand Island, NY, United States), and complementary DNA synthesis was performed using the 5 × All-in-One RT MasterMix Kit (Applied Biological Materials, Richmond, BC, Canada), following manufacturer’s protocol. Polymerase chain reaction (PCR) amplification was performed in triplicate under these conditions: (1) 25 °C for 1 minute; (2) Followed by 42 °C for 15 minutes; and (3) 85 °C for 5 minutes. The reactions were run on a 7900HT Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, United States) via the TB Green Premix Ex Taq PCR Kit (TaKaRa, Dalian, China). The quantitative real-time PCR (qRT-PCR) cycling conditions were as previously outlined: (1) An initial step at 95 °C for 30 seconds; (2) Followed by 40 cycles of 95 °C for 5 seconds; and (3) 60 °C for 30 seconds. TSPO primers used for qRT-PCR were as follows: Human TSPO, F: TCCTACCTGGTCTGGAAAGAGC and R: CCAGCAGGAGATCCACCAAGG. Gene expression levels were normalized against the housekeeping gene GAPDH, and relative quantification was determined performed using the 2-△△Ct method.
Peripheral neutrophils were isolated from anticoagulated blood by density gradient centrifugation. Briefly, peripheral blood was mixed with an equal volume of phosphate-buffered saline and layered onto Ficoll-PaqueTM PLUS (GE Healthcare, Uppsala, Sweden). After centrifugation at 2000 rpm for 30 minutes at 20 °C, neutrophils were retrieved from the lower layer after lysing red blood cells with Lysing Buffer (BD Biosciences, San Diego, CA, United States) according to the manufacturer’s protocol. Neutrophils were then resuspended in RPMI-1640 medium (Gibco Life Technologies, Grand Island, NY, United States) and 10% fetal bovine serum for further use. Neutrophil viability and morphology were assessed using the trypan blue exclusion test using a Neubauer counting chamber and visualized under a light microscope. Flow cytometry confirmed the cell purity (≥ 95%) before any experiments.
The effects of PK11195 on ROS production by neutrophil was assessed as follows: (1) Isolated neutrophils from HC (n = 6), A-CD (n = 6) and A-UC (n = 6) were incubated with PK11195 (Sigma-Aldrich; 100 nM) for 3 hours[24]; (2) Followed by stimulation with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich; 100 ng/mL) for 30 minutes; and (3) ROS levels in the supernatant were quantified using the Amplex Red Hydrogen Peroxide Assay Kit (Invitrogen) according to the manufacturer’s protocols, and the optical density was measured using a microplate spectrophotometer (BioTek, Winooski, VT, United States).
Coverslips were pre-coated with poly-L-lysine (0.5 mg/mL) to facilitate neutrophil adhesion. Experiments were performed in 24-well sterile plates containing poly L-lysine-coated coverslips. Neutrophils from HC (n = 6), A-CD (n = 6) and A-UC (n = 6) were pre-incubated with or without PK11195 (100 nM) for 30 minutes, followed by stimulation with PMA (100 ng/mL) for 3 hours to induce NET formation. Cells were fixed with 4% paraformaldehyde and stained with Hoechst 33342 (1:1000) to visualize the decondensed chromatin network. Uninduced neutrophils from the same sample were used as the negative control for this group. Images were captured under identical imaging conditions using a fluorescence microscope (AF6000, Leica, Wetzlar, Germany). The fluorescence intensity of NETs was measured using a fluorometric plate reader (Clariostar plus, BMG Labtech, Ortenberg, Germany), and NETs were considered positive when the fluorescence intensity was ≥ the mean of the negative control plus 2 standard deviations.
Human colon sections: Endogenous peroxidase was blocked by 3% hydrogen peroxide for 25 minutes. Sections underwent blocking with 3% bovine serum albumin (BSA) for 30 minutes, followed by incubation with rabbit anti-TSPO mAb (Abcam, Cambridge, United States, 1:100) overnight at 4 °C. After washing, sections were incubated with horseradish peroxidase (HRP)-conjugated goat anti-immunoglobulin G (IgG) (Servicebio, Wuhan, China, 1:200) at room temperature for 50 minutes. The 3,3′-Diaminobenzidine (DAB) staining was performed, followed by hematoxylin counterstaining. Sections were dehydrated, mounted, and imaged by a light microscope.
Mouse brain sections: Mice were perfused transcardially with 4% paraformaldehyde under anesthesia. Brains were harvested and sectioned into 5-μm slices. Following deparaffinization and rehydration, the slides were incubated with 0.3% Triton X-100 for 10 minutes and blocked by 3% BSA for 25 minutes. Immunohistochemistry was performed using rabbit anti-TSPO mAb (Abcam, 1:100) overnight at 4 °C. Negative controls utilized normal serum in lieu of primary antibody. The cells were incubated with HRP-conjugated goat anti-rabbit IgG (Servicebio, Wuhan, China, 1:200) at room temperature for 50 minutes. DAB staining was performed, followed by hematoxylin counterstaining. The sections were dehydrated, mounted, and imaged by a light microscope.
Colon sections: After endogenous peroxidase blockade (3% hydrogen peroxide, 25 minutes) and blocking (3% BSA, 30 minutes), sections were incubated with rabbit anti-TSPO mAb (Abcam, 1:500) and mouse anti-myeloperoxidase mAb (Servicebio, 1:800) overnight at 4 °C. Then slides were incubated with Cy3-conjugated goat anti-rabbit IgG (Servicebio, 1:300) and Alexa foluor 488-conjugated goat anti-mouse IgG (Servicebio, 1:400) for 1 hour at room temperature. Coverslips were mounted using the Slowfade Gold antifade mountant with 4'-6-diamidino-2-phenylindole (DAPI) (Servicebio, 1:1000) for DNA counterstaining. The stained samples were visualized using a fluorescence microscope.
Brain sections: Mice were perfused transcardially with 4% paraformaldehyde under anesthesia. Brains were harvested and sectioned into 5-μm slices. After endogenous peroxidase blockade (3% hydrogen peroxide, 25 minutes) and blocking (3% BSA, 30 minutes), sections were incubated with rabbit anti-TSPO mAb (Abcam, 1:500), rabbit anti-IBA1 mAb (Servicebio, 1:2000), or rabbit anti-glial fibrillary acidic protein mAb (Servicebio, 1:2000) overnight at 4 °C. Then slides were incubated with Cy3-conjugated goat anti-rabbit IgG (Servicebio, 1:300) and Alexa foluor 488-conjugated goat anti-rabbit IgG (Servicebio, 1:400) for 1 hour at room temperature. Coverslips were mounted using the Slowfade Gold antifade mountant with DAPI (Servicebio, 1:1000) for DNA counterstaining. The stained samples were visualized using a fluorescence microscope.
Micro-PET-CT imaging of mice was performed as described previously[13]. In brief, mice were anesthetized with 2% isoflurane in 100% oxygen (1 L/minute) via vaporizer (Molecular Imaging Products Company, United States). Animals were positioned supine on the imaging bed and maintained under 1% isoflurane in 100% oxygen (1 L/minute) during PET-CT. [18F] labeled N,N-diethyl-2-(2-[4-(2-fluoroethoxy)phenyl]-5,7-dimethylpyrazolo[1,5-alpha]pyrimidine-3-yl)acetamide (DPA-714) PET imaging was obtained for 10 minutes at 40 minutes post administration of [18F]DPA-714 (approximately 5 μCi/g body weight). PET images were acquired using a micro-PET Inveon system (Siemens Medical Solution, Germany) with an average FWHM of 1.65 mm ± 0.06 mm. The images were subsequently reconstructed using ordered subsets expectation-maximization reconstruction with two iterations, a zoom factor of 1, and a matrix size of 128 × 128. The reconstructed PET images were subsequently analyzed using PMOD 4.5 software (PMOD Technologies Ltd., Zurich, Switzerland). Images were manually fused with the PMOD mouse template (Ma-Benveniste-Mirrione), and the cortical brain regions of interest were collected. Radiotracer uptake was quantified using the standardized uptake value.
Raw microarray data of CEL file format were obtained from Gene Expression Omnibus (GEO) database. Data preprocessing and analysis were performed in R (version 4.4.1). The raw CEL files were processed using the oligo package. Background correction, normalization, and summarization of probe intensities were carried out using the Robust Multi-array Average method. After normalization, the package ggplot2 was used for the visualization of gene expression.
Data were analyzed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, United States). Non-normally distributed variables (Shapiro-Wilk, P < 0.05) were compared via Mann–Whitney U tests with Bonferroni correction. For two-group comparisons, P values < 0.05 were considered significant. For multiple group comparisons, Bonferroni correction was applied. Specifically, for three-group comparisons, adjusted P values < 0.0167 (0.05/3) were considered significant. For five-group comparisons, significance thresholds varied according to the numbers of comparisons. For analysis involving TSPO expression in colon and neutrophils, adjusted P values < 0.0083 (0.05/6) were considered significant. For TSPO expression in peripheral immune cells, adjusted P values < 0.005 (0.05/10) were considered significant. In comparison of six experimental groups against a reference control (medium), adjusted P values < 0.0083 (0.05/6) were considered significant. Results are presented as median with interquartile range. variables that met assumptions of normality and equal variances were analyzed using t-tests and are presented as mean ± SE. Spearman’s rank correlation was used to evaluate the relationship between TSPO and the severity of endoscopic findings.
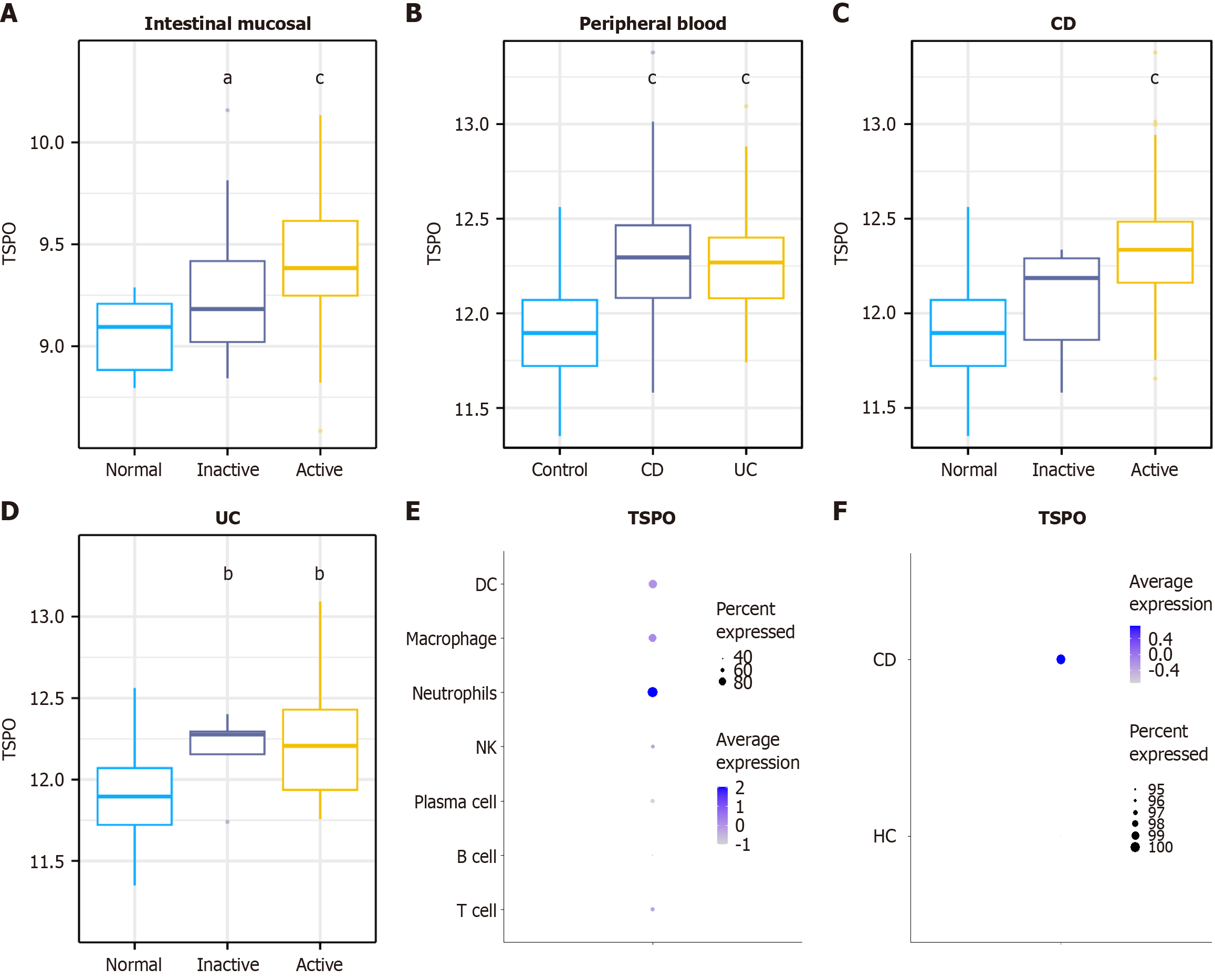
Considering the close relationship between TSPO and immune system, we used biometric analysis to explore the relationship between TSPO and IBD. Multiple GEO datasets from IBD patients were analyzed. As shown in Figure 1A, a marked increase in TSPO expression from HC to active disease was observed in both patients with CD and UC. Further analysis of a GEO dataset from the peripheral blood of IBD patients and HC showed significantly higher TSPO expression in both IBD patients and UC than in HC (Figure 1B). In patients with CD, TSPO expression progressively increased to remission and active disease compared with that in HC (Figure 1C). In patients with UC, TSPO expression was elevated in both the remission and active phases compared with that in HC; however, no significant difference was found between the two disease phases (Figure 1D).
Given the broad expression of TSPO, single-cell sequencing datasets were examined to determine its expression in immune cells. The results revealed high TSPO expression in myeloid cell populations, particularly in neutrophils, macrophages, and dendritic cells, with neutrophils exhibiting the highest expression (Figure 1E). Additional analysis suggested a trend towards increased TSPO expression in neutrophils from patients with CD compared with those from HC (Figure 1F). These findings indicate that TSPO is highly expressed in neutrophils and upregulated in the inflammatory environment of IBD.
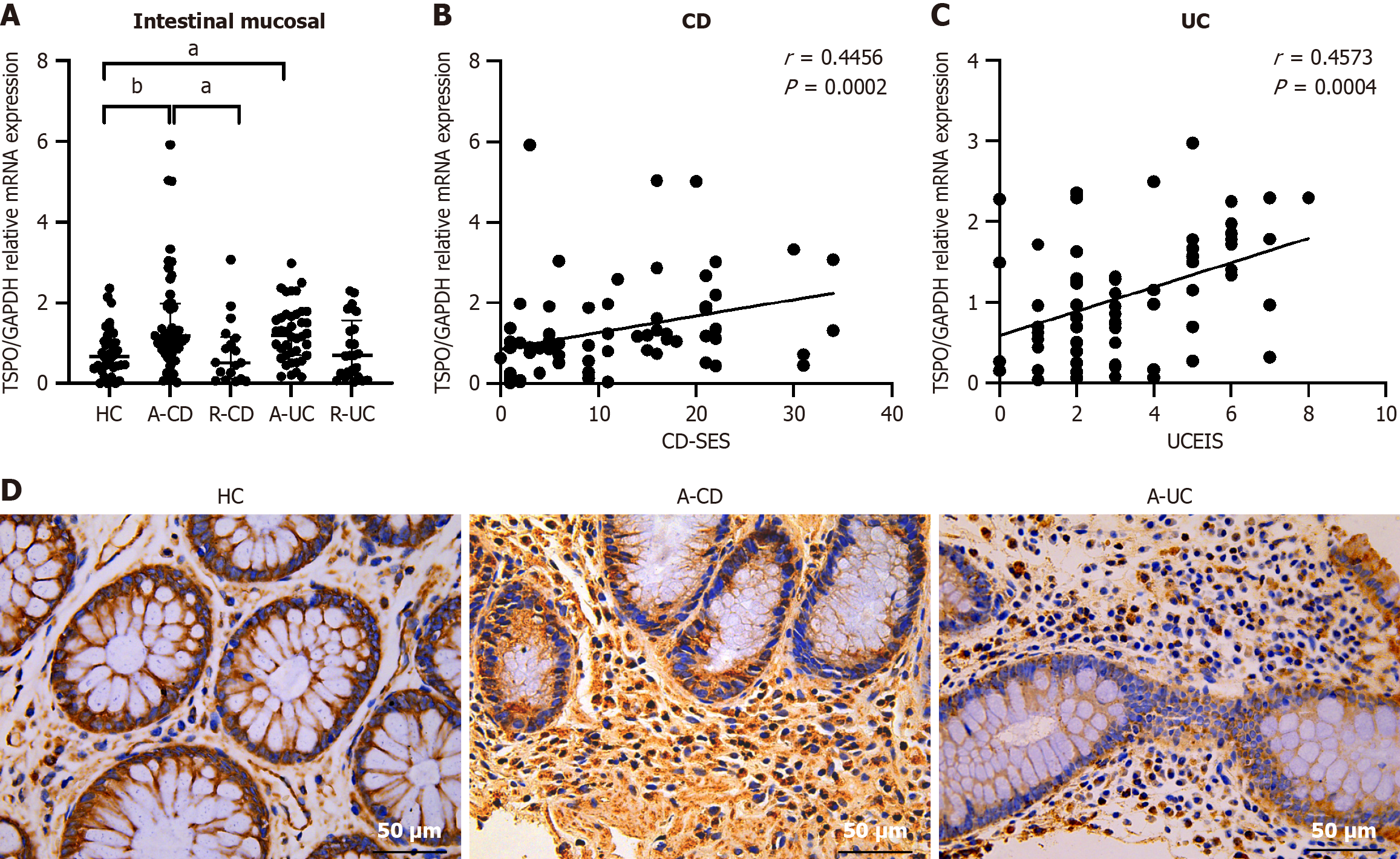
A total of 135 IBD patients (A-CD, n = 47; R-CD, n = 18; A-UC, n = 40; R-UC, n = 25) and 35 HC were recruited. Supplementary Table 1 presents the clinical data of all patients, including age, sex, lesion location, course of the disease, and therapy. No significant difference has been observed in the in clinical data between these groups. Based on the results of previous bioinformatics analyses, we examined the role of TSPO in IBD pathogenesis. The qRT-PCR analysis revealed a significant increase in TSPO expression in the inflamed mucosa of active IBD patients compared with that in HC [A-CD: 1.18 (0.76-1.98) vs HC: 0.66 (0.40-1.04), U = 469, adjusted P = 0.006, A-UC: 1.20 (0.70-1.71), U = 418, adjusted P = 0.018], while no significant difference has been observed between HC and remission groups. Interestingly, TSPO expression in A-CD was significantly higher than that in R-CD (A-CD: 1.18 (0.76-1.98) vs 0.51 (0.11-1.15), U = 236, adjusted P = 0.036), while the expression of TSPO did not differ between A-UC and R-UC (Figure 2A). To further assess the clinical relevance of TSPO in IBD pathogenesis, correlation analysis was performed between TSPO expression levels in the colonic mucosa and disease activity scores, specifically the CD-SES in CD and UCEIS in UC. A positive association was observed between TSPO expression levels and the CD-SES (r = 0.4456, P = 0.0002) as well as UCEIS (r = 0.4573, P = 0.0004) (Figure 2B and C). Immunohistochemical staining of the colonic mucosa corroborated these results, showing a marked increase in TSPO expression in the inflamed colonic mucosa of active IBD patients [A-CD: 78.50 (61.50-84.00) vs HC: 19.00 (11.50-36.50), U = 1, adjusted P = 0.012, A-UC: 56.00 (45.00-76.75), U = 3, adjusted P = 0.045], while the expression did not differ between A-CD and A-UC (Figure 2D and Supplementary Figure 1A). These findings align with those of previous bioinformatics analyses and illustrating significant TSPO upregulation in active IBD and is positively correlates with clinical severity.
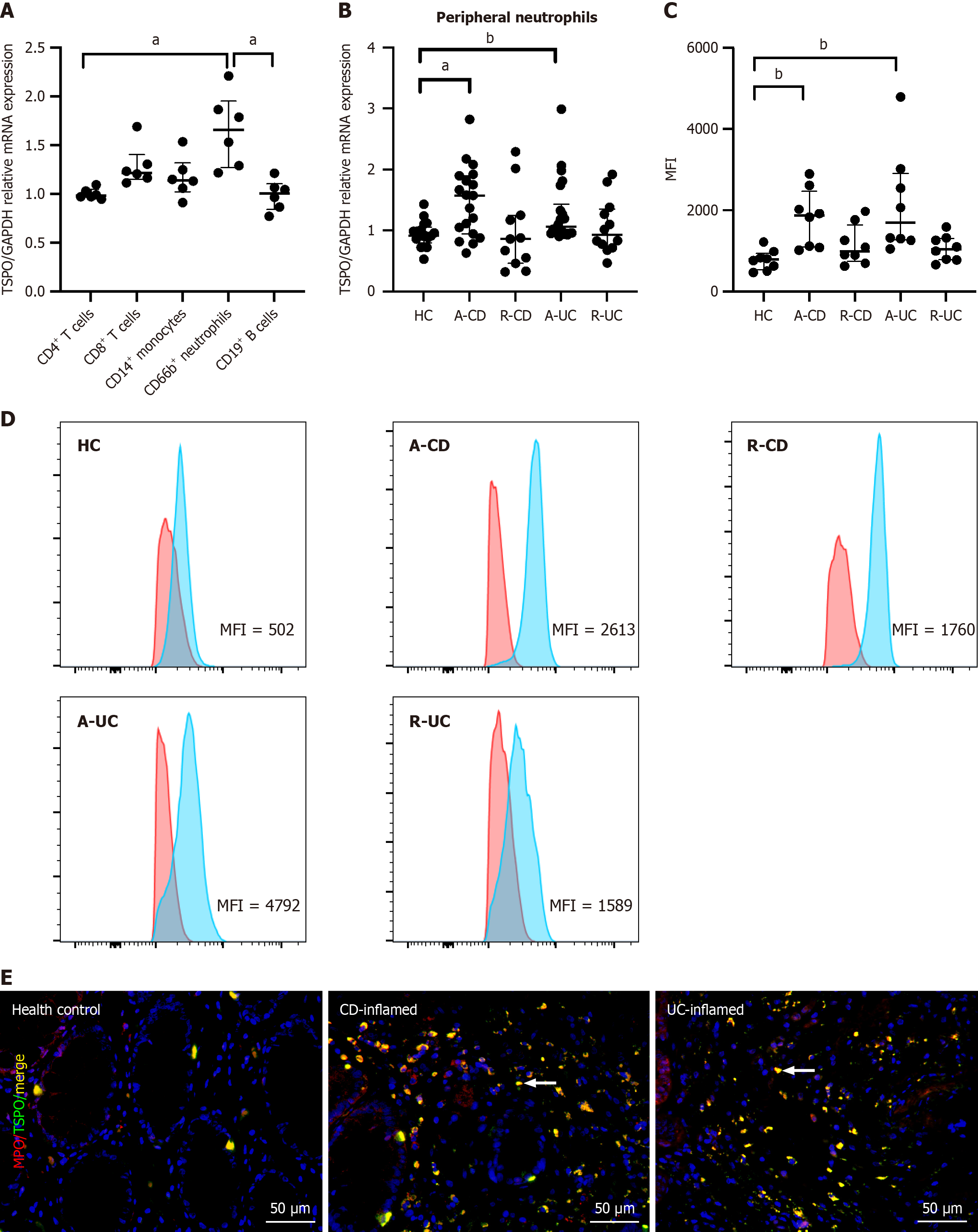
Considering the widespread expression of TSPO, we assessed its phenotypic expression in various immune cells. CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD66b+ neutrophils, and CD19+ B cells were isolated from the peripheral blood of HC, and TSPO expression was analyzed. Neutrophils exhibited notably high TSPO levels compared to CD4+ T cells [1.66 (1.27-1.95) vs 0.98 (0.97-1.05), U = 0, adjusted P = 0.02] and CD19+ B cells [1.01 (0.84-1.10), U = 0, adjusted P = 0.02], whereas no significant differences were found when compared with the remaining immune cells (Figure 3A). Addi
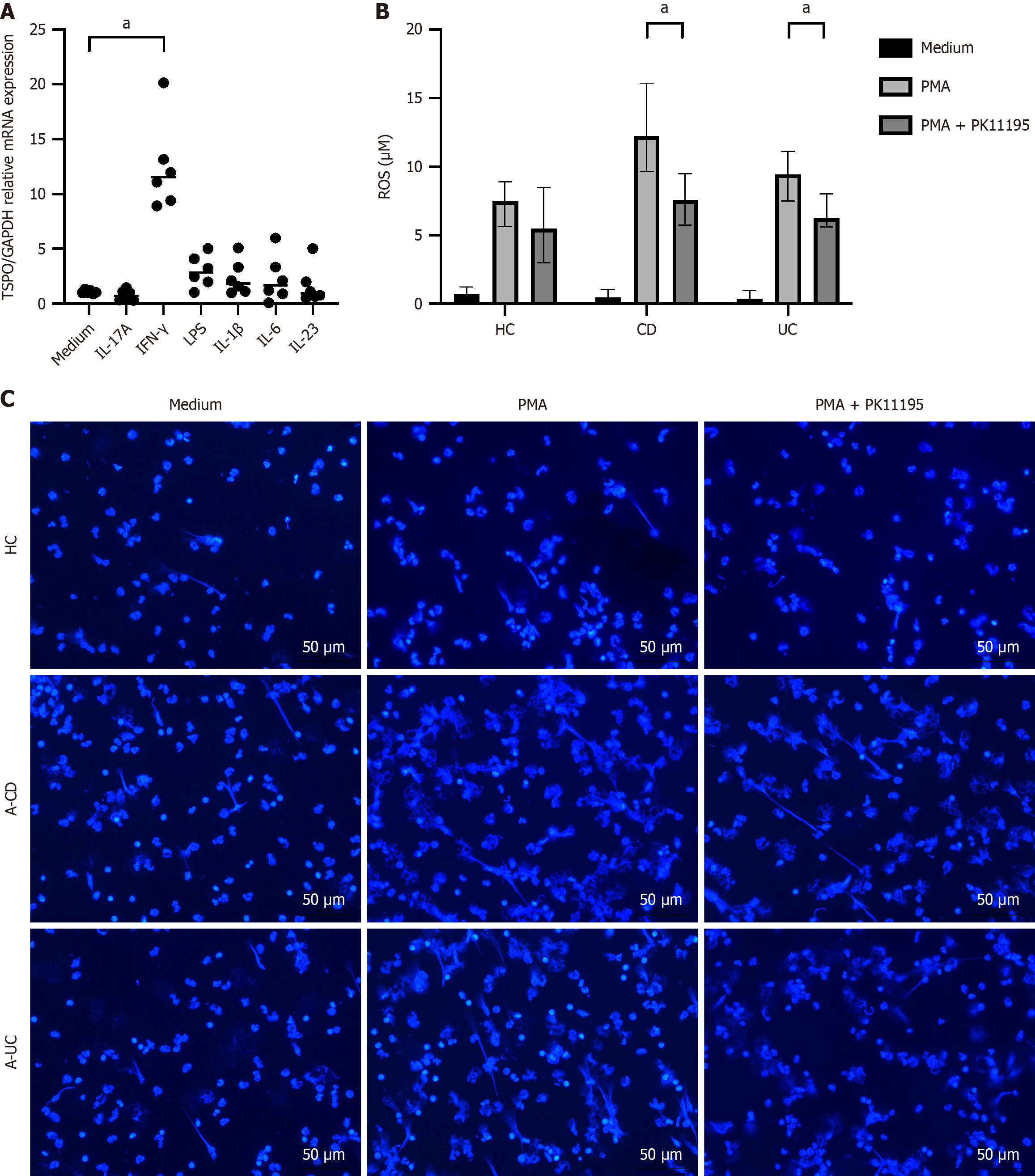
To further investigate the effects of TSPO on neutrophil function and the potential molecular pathways involved, we stimulated neutrophils with various inflammatory mediators or cytokines to assess the changes in TSPO expression. The results demonstrated that multiple factors, including interferon-γ, can enhance TSPO expression in neutrophils [11.54 (9.27-14.91), U = 0, adjusted P = 0.012] (Figure 4A). Further experiments involving the stimulation of peripheral blood neutrophils with TSPO antagonists showed that the addition of PMA and PMA combined with PK11195 (a TSPO antagonist) increased the capacity of neutrophils to generate ROS and form NETs (Figure 4B and C, Supplementary Figure 1B). For ROS production, A-IBD treated with PMA and PK11195 also showed significantly lower levels compared to those treated with PMA alone [A-CD: 7.60 (5.75-9.53) vs 12.25 (9.67-16.08), U = 9, adjusted P = 0.04, A-UC: 6.30 (5.60-8.00) vs 9.45 (7.52-11.13), U = 8, adjusted P = 0.03]. For NETs formation, A-CD treated with PMA and PK11195 showed significant reduction than those treated with PMA alone [29.00 (22.00-31.00) vs 36.00 (30.50-49.50), U = 7.5, adjusted P = 0.042]; meanwhile, A-UC treated with PMA and PK11195 showed a decreasing trend compared to A-UC treated with PMA alone. These findings indicated that TSPO was involved in the regulation of neutrophil bactericidal function, thereby contributing to the pathogenesis of intestinal inflammation.
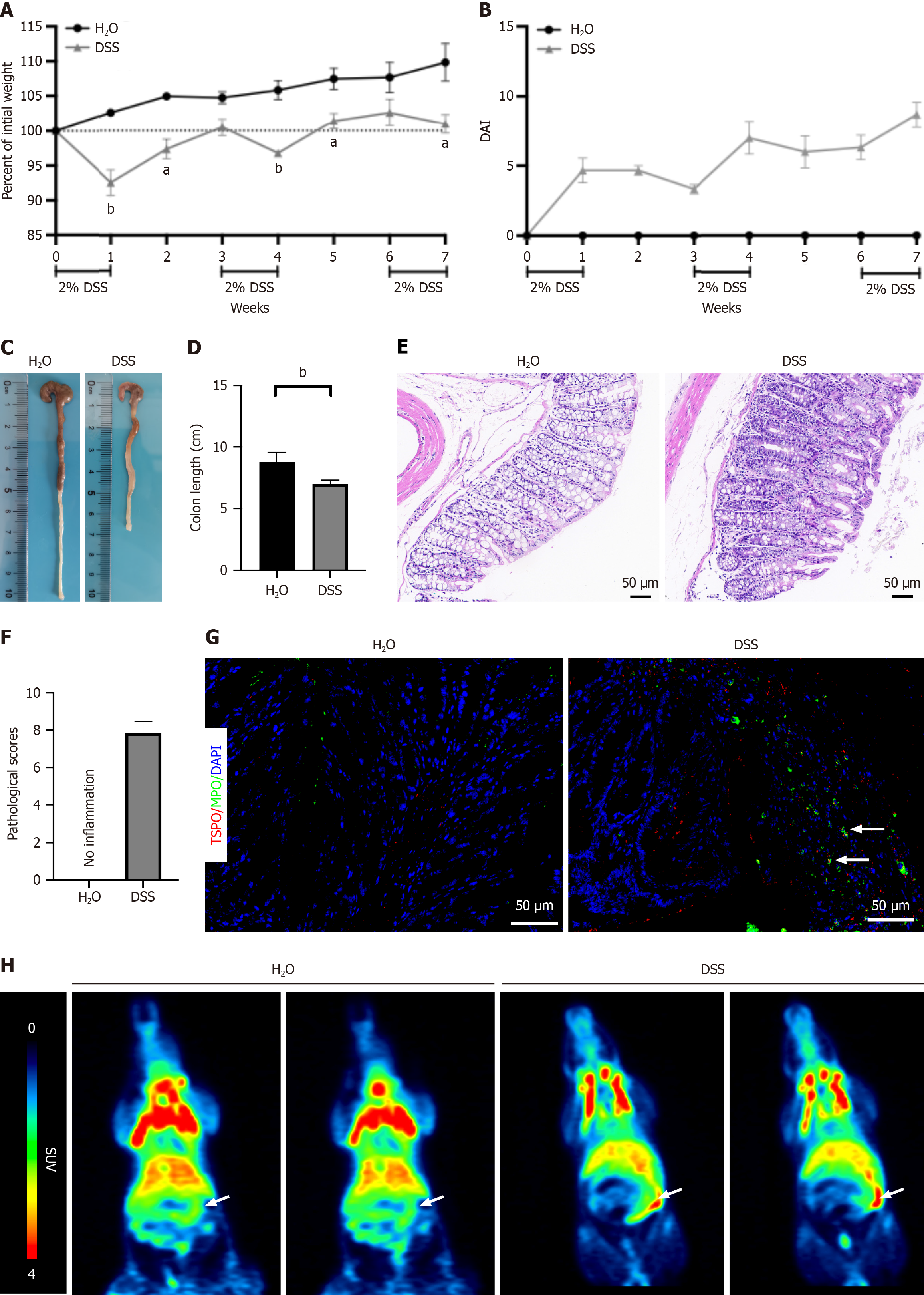
To unravel the intrinsic functions of TSPO in colitis pathogenesis in vivo, we utilized a chronic colitis model induced by DSS in WT mice, as described previously. As shown in Figure 5A-D, mice in the colitis group exhibited more pronounced weight loss and higher disease activity indices than the WT controls, along with shorter and thicker colons (6.87 cm ± 0.76 cm vs 8.95 cm ± 0.77 cm, P = 0.002). Histological examination of colonic tissues by hematoxylin and eosin staining revealed severe pathological changes in the colitis group, characterized by substantial disruption of the colonic architecture and extensive infiltration of inflammatory cells (Figure 5E and F), confirming the successful establishment of the chronic colitis model. Additionally, we observed a notable upregulation of TSPO in the colonic neutrophils of mice with colitis compared with that in controls (Figure 5G). PET-CT scanning using the TSPO-specific ligand [18F]DPA-714 produced consistent results, indicating markedly increased TSPO expression in the colitis group (1.970 ± 0.888 vs 3.728 ± 0.486, P = 0.0346) (Figure 5H, Supplementary Figure 2). These findings provide further in vivo evidence that TSPO is significantly upregulated during intestinal mucosal inflammation, indicating its involvement in IBD pathogenesis.
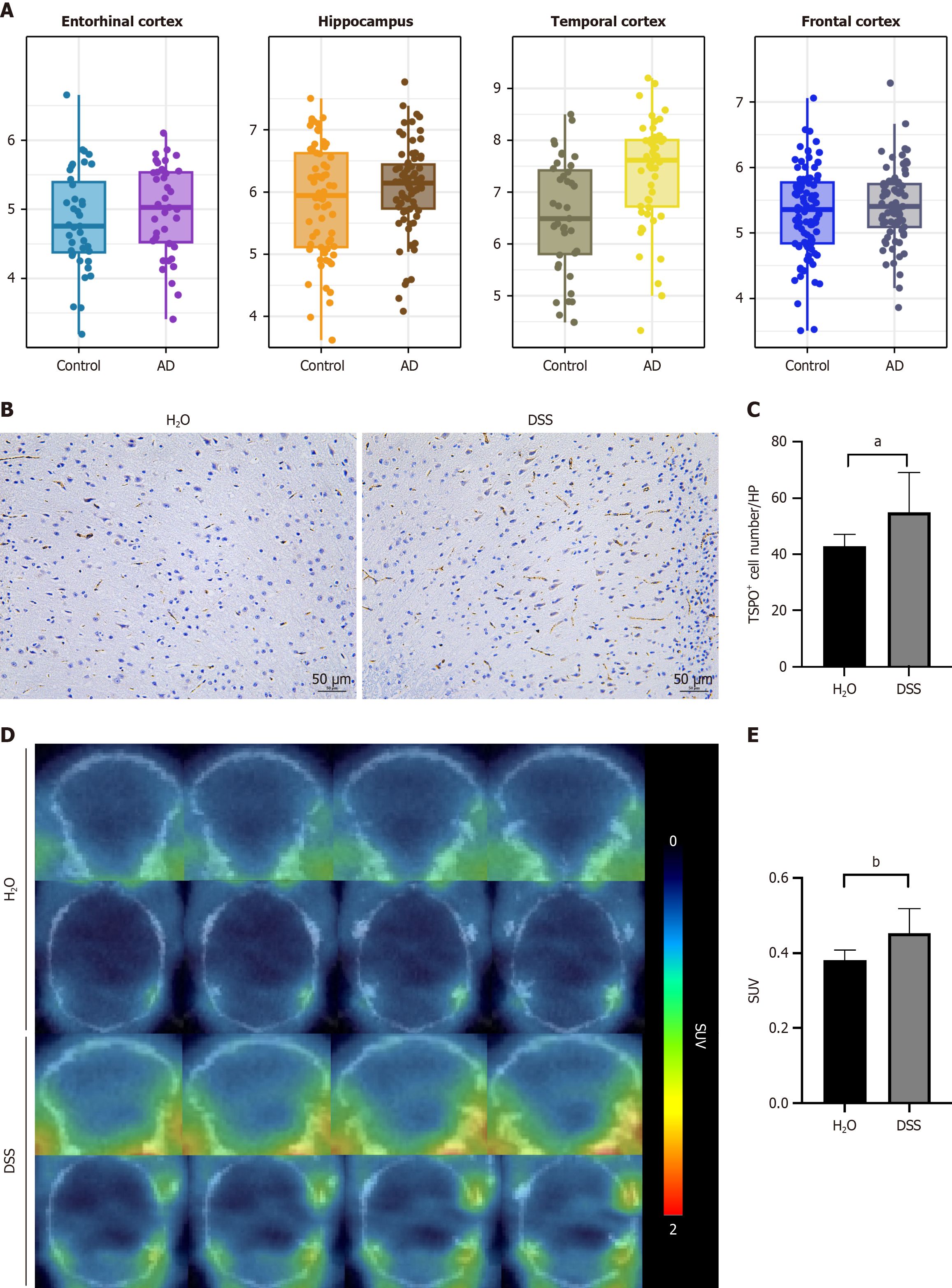
Given the close relationship between TSPO and immune cells and considering that AD is closely linked to autoimmune responses, we employed bioinformatics analysis to evaluate TSPO expression in the brains of AD patients compared with that in HC. The results revealed an increasing trend in TSPO expression across four brain regions: (1) The entorhinal cortex; (2) Hippocampus; (3) Temporal cortex; and (4) Frontal cortex. Notably, in the temporal cortex, TSPO expression was significantly upregulated in AD patients compared with that in HC (Figure 6A). Therefore, we examined TSPO expression in the brain following DSS-induced chronic colitis. Elevated TSPO expression was detected in the brains of colitis mice than in those of the controls (57.60 ± 11.41 vs 42.40 ± 5.90, P = 0.031) (Figure 6B and C). PET-CT using TSPO specific ligand ([18F]DPA-714) showed same trend in the brain tissues (0.385 ± 0.022 vs 0.470 ± 0.061, P = 0.0043) (Figure 6D and E).
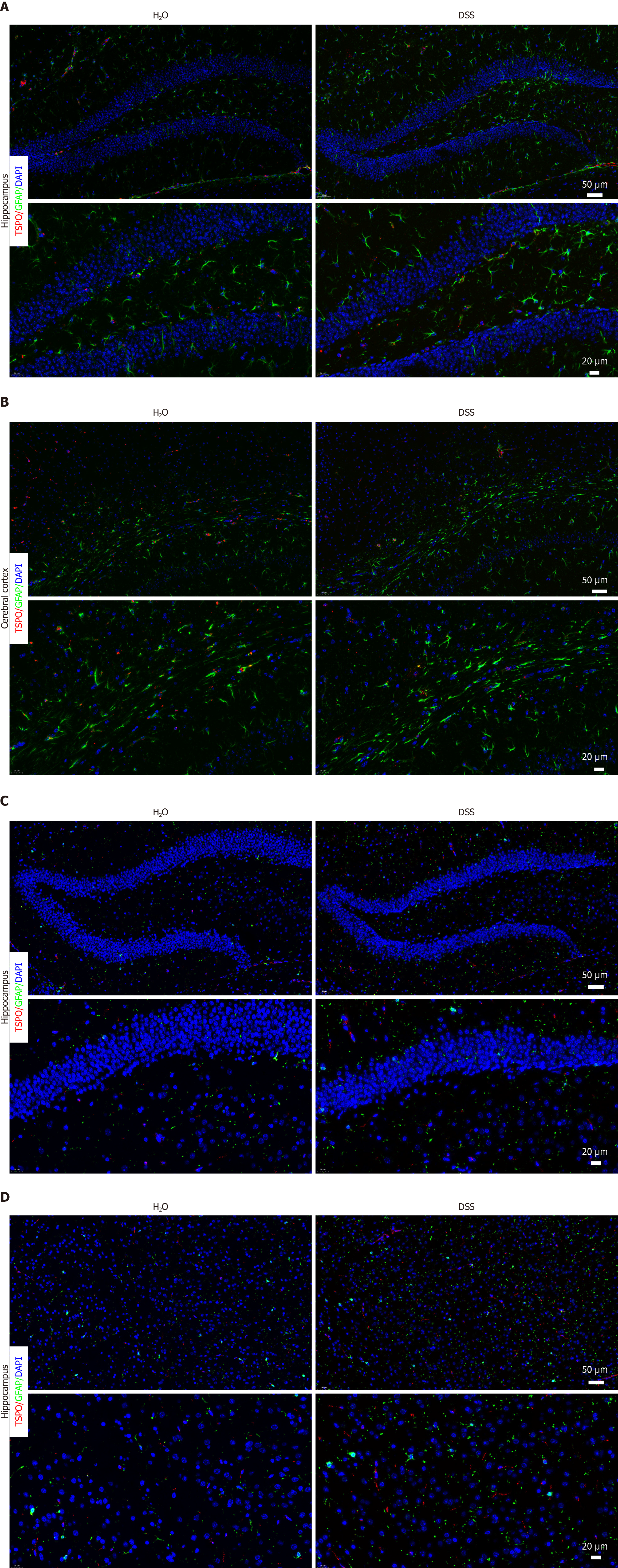
Considering the potential impact of colitis on AD progression, we examined the cellular changes related to neuroinflammation in the brain. Interestingly, when we analyzed the changes in TSPO expression in astrocytes and microglia, we observed significant alterations in TSPO expression in the two cell types closely associated with neuroinflammation. A slight increase in astrocyte populations was observed after colitis, whereas no significant difference in TSPO expression were observed in astrocytes (Figure 7A and B). Moreover, TSPO expression in the microglia was markedly increased, accompanied by a slight increase in microglial cell number (Figure 7C and D). Thus, these results demonstrated a significant upregulation of TSPO in the brain after colitis and aggravated neuroinflammation in the brain.
Strong evidence has revealed a close association between defects in neutrophil function and intestinal inflammation[25], while the specific mechanism remains unclear. In this study, we observed that TSPO was significantly associated with IBD disease activity and may modulate neutrophil function under colitis condition. In addition, an in vivo colitis mouse model showed elevated TSPO expression in neutrophils, suggesting that TSPO contributes to the disruption of intestinal immune homeostasis in IBD by regulating neutrophil activation. Assessment of TSPO expression in the mouse brain revealed a significant upregulation following DSS-induced colitis, accompanied by marked changes in the quantity of microglia and astrocytes in the brains of mice with colitis. Based on these results, we propose that TSPO participates in the regulation of gut homeostasis by modulating neutrophil function, which, in turn, influences neuroinflammation.
TSPO participates in many critical physiological processes, including cellular bioenergetics, metabolism, immune regulation, cellular proliferation, and apoptosis[26], suggesting that it may participate in diseases related to immune activity. Previous researches have shown that high levels of TSPO are observed in various types of cancer cells, suggesting that TSPO plays a role in cellular proliferation and carcinogenesis. A study using primary microglia pola
Interestingly, TSPO has been demonstrated to be closely related to neuroinflammation, and TSPO-PET has already been used as an indicator of microglial activity, showing significant changes in AD[31]. In addition, several studies have shown that the gut-brain axis can influence the progression of neurodegeneration in patients with AD via neuroimmune pathways. Considering the dual role of TSPO in colitis and neuroinflammation of AD, we examined the expression of TSPO in the brains of patients with AD via bioinformatics analysis. High levels of TSPO were observed in various brain regions compared with healthy people, which is consistent with previous researches[32]. Based on these findings and the established close association between TSPO and colitis, we hypothesized that TSPO influences neuroinflammation in a colitis environment. We assessed TSPO expression in the brain of mice and found a significant increase in its expression following colitis. Further results showed slight increase in the cell numbers of astrocytes and microglia, which have close association with neuroinflammation and show earlier changes before Aβ plague deposition. These results are partly consistent with previous studies[33,34]. Based on these results, we propose that TSPO may play a role in neuroinflammation, potentially through its regulation of neutrophil activity, thereby influencing the gut-brain axis. However, further evidence is warranted to confirm this hypothesis.
This study has some limitations. First, the analysis on TSPO expression was limited by the small sample size in several analysis (e.g., single-cell RNA sequencing, patients samples, and colitis mice model), due to data and experiment constraints. Future studies involving large-scale IBD cohorts and single-cell analyses are warranted to further elucidate the interactions between TSPO and neutrophils. In addition, in-depth investigations into the mechanisms linking TSPO, neutrophils, and neuroinflammation are needed to establish a stronger theoretical foundation for understanding IBD and AD.
TSPO influences the development and progression of IBD by modulating neutrophil function, thereby affecting neuroinflammation. The potential link between IBD and neuroinflammation offers new perspectives for future research, particularly in the context of the gut-brain axis. Future studies should explore the mechanisms underlying the interaction between TSPO, IBD, and neuroinflammation and assess the therapeutic potential of targeting TSPO as a dual-disease target.
The authors appreciate the excellent technical assistance of the staff at the Department of General Medicine, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and the Department of Gastroenterology, Shanghai East Hospital, Tongji University of School Medicine, and the PET Centre, Huashan Hospital, Fudan University.
| 1. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 2015] [Article Influence: 183.2] [Reference Citation Analysis (1)] |
| 2. | Petagna L, Antonelli A, Ganini C, Bellato V, Campanelli M, Divizia A, Efrati C, Franceschilli M, Guida AM, Ingallinella S, Montagnese F, Sensi B, Siragusa L, Sica GS. Pathophysiology of Crohn's disease inflammation and recurrence. Biol Direct. 2020;15:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 3. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 1145] [Article Influence: 190.8] [Reference Citation Analysis (0)] |
| 4. | Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, Khoyratty TE, van Grinsven E, González-Hernández S, Nicolás-Ávila JÁ, Vicanolo T, Maccataio A, Benguría A, Li JL, Adrover JM, Aroca-Crevillen A, Quintana JA, Martín-Salamanca S, Mayo F, Ascher S, Barbiera G, Soehnlein O, Gunzer M, Ginhoux F, Sánchez-Cabo F, Nistal-Villán E, Schulz C, Dopazo A, Reinhardt C, Udalova IA, Ng LG, Ostuni R, Hidalgo A. Co-option of Neutrophil Fates by Tissue Environments. Cell. 2020;183:1282-1297.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 5. | Gao H, Peng K, Shi Y, Zhu S, Sun R, Xu C, Liu P, Pang Z, Zhu L, Chen W, Feng B, Wu H, Zhou G, Li M, Li J, Ding B, Liu Z. Development and validation of a novel criterion of histologic healing in ulcerative colitis defined by inflammatory cell enumeration in lamina propria mucosa: A multicenter retrospective cohort in China. Chin Med J (Engl). 2024;137:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Zhou G, Yu L, Fang L, Yang W, Yu T, Miao Y, Chen M, Wu K, Chen F, Cong Y, Liu Z. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 7. | Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371:551-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 8. | Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Jin GL, Liu HP, Huang YX, Zeng QQ, Chen JX, Lan XB, Xin ZM, Xiong BJ, Yue RC, Yu CX. Koumine regulates macrophage M1/M2 polarization via TSPO, alleviating sepsis-associated liver injury in mice. Phytomedicine. 2022;107:154484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Shi Y, Cui M, Ochs K, Brendel M, Strübing FL, Briel N, Eckenweber F, Zou C, Banati RB, Liu GJ, Middleton RJ, Rupprecht R, Rudolph U, Zeilhofer HU, Rammes G, Herms J, Dorostkar MM. Long-term diazepam treatment enhances microglial spine engulfment and impairs cognitive performance via the mitochondrial 18 kDa translocator protein (TSPO). Nat Neurosci. 2022;25:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Fu Y, Wang D, Wang H, Cai M, Li C, Zhang X, Chen H, Hu Y, Zhang X, Ying M, He W, Zhang J. TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma. Neuro Oncol. 2020;22:240-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Xie Q, Li Z, Liu Y, Zhang D, Su M, Niitsu H, Lu Y, Coffey RJ, Bai M. Translocator protein-targeted photodynamic therapy for direct and abscopal immunogenic cell death in colorectal cancer. Acta Biomater. 2021;134:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Salerno S, Viviano M, Baglini E, Poggetti V, Giorgini D, Castagnoli J, Barresi E, Castellano S, Da Settimo F, Taliani S. TSPO Radioligands for Neuroinflammation: An Overview. Molecules. 2024;29:4212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Werry EL, Bright FM, Piguet O, Ittner LM, Halliday GM, Hodges JR, Kiernan MC, Loy CT, Kril JJ, Kassiou M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int J Mol Sci. 2019;20:3161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 15. | Cumbers GA, Harvey-Latham ED, Kassiou M, Werry EL, Danon JJ. Emerging TSPO-PET Radiotracers for Imaging Neuroinflammation: A Critical Analysis. Semin Nucl Med. 2024;54:856-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Blevins LK, Crawford RB, Azzam DJ, Guilarte TR, Kaminski NE. Surface translocator protein 18 kDa (TSPO) localization on immune cells upon stimulation with LPS and in ART-treated HIV(+) subjects. J Leukoc Biol. 2021;110:123-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Bernards N, Pottier G, Thézé B, Dollé F, Boisgard R. In vivo evaluation of inflammatory bowel disease with the aid of μPET and the translocator protein 18 kDa radioligand [18F]DPA-714. Mol Imaging Biol. 2015;17:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Jimenez IA, Stilin AP, Morohaku K, Hussein MH, Koganti PP, Selvaraj V. Mitochondrial translocator protein deficiency exacerbates pathology in acute experimental ulcerative colitis. Front Physiol. 2022;13:896951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Ostuni MA, Issop L, Péranzi G, Walker F, Fasseu M, Elbim C, Papadopoulos V, Lacapere JJ. Overexpression of translocator protein in inflammatory bowel disease: potential diagnostic and treatment value. Inflamm Bowel Dis. 2010;16:1476-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT; AGA Institute Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lémann M, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Bernhardt CA, Mary JY, Sandborn WJ. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61:535-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 22. | Kedia S, Virmani S, K Vuyyuru S, Kumar P, Kante B, Sahu P, Kaushal K, Farooqui M, Singh M, Verma M, Bajaj A, Markandey M, Sachdeva K, Das P, Makharia GK, Ahuja V. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022;71:2401-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 23. | Wu X, Chen H, Gao X, Gao H, He Q, Li G, Yao J, Liu Z. Natural Herbal Remedy Wumei Decoction Ameliorates Intestinal Mucosal Inflammation by Inhibiting Th1/Th17 Cell Differentiation and Maintaining Microbial Homeostasis. Inflamm Bowel Dis. 2022;28:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | de Lima CB, Tamura EK, Montero-Melendez T, Palermo-Neto J, Perretti M, Markus RP, Farsky SH. Actions of translocator protein ligands on neutrophil adhesion and motility induced by G-protein coupled receptor signaling. Biochem Biophys Res Commun. 2012;417:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Danne C, Skerniskyte J, Marteyn B, Sokol H. Neutrophils: from IBD to the gut microbiota. Nat Rev Gastroenterol Hepatol. 2024;21:184-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 125] [Reference Citation Analysis (1)] |
| 26. | Nutma E, Ceyzériat K, Amor S, Tsartsalis S, Millet P, Owen DR, Papadopoulos V, Tournier BB. Cellular sources of TSPO expression in healthy and diseased brain. Eur J Nucl Med Mol Imaging. 2021;49:146-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 27. | Zhou D, Ji L, Chen Y. TSPO Modulates IL-4-Induced Microglia/Macrophage M2 Polarization via PPAR-γ Pathway. J Mol Neurosci. 2020;70:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Zhang X, Han J, Xu Y, Cai M, Gao F, Han J, Wang D, Fu Y, Chen H, He W, Zhang J. TSPO Deficiency Exacerbates GSDMD-Mediated Macrophage Pyroptosis in Inflammatory Bowel Disease. Cells. 2022;11:856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Kupa LVK, Drewes CC, Barioni ED, Neves CL, Sampaio SC, Farsky SHP. Role of Translocator 18 KDa Ligands in the Activation of Leukotriene B4 Activated G-Protein Coupled Receptor and Toll Like Receptor-4 Pathways in Neutrophils. Front Pharmacol. 2017;8:766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Betlazar C, Middleton RJ, Banati R, Liu GJ. The Translocator Protein (TSPO) in Mitochondrial Bioenergetics and Immune Processes. Cells. 2020;9:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Xiang X, Wind K, Wiedemann T, Blume T, Shi Y, Briel N, Beyer L, Biechele G, Eckenweber F, Zatcepin A, Lammich S, Ribicic S, Tahirovic S, Willem M, Deussing M, Palleis C, Rauchmann BS, Gildehaus FJ, Lindner S, Spitz C, Franzmeier N, Baumann K, Rominger A, Bartenstein P, Ziegler S, Drzezga A, Respondek G, Buerger K, Perneczky R, Levin J, Höglinger GU, Herms J, Haass C, Brendel M. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. 2021;13:eabe5640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 32. | Zhang L, Hu K, Shao T, Hou L, Zhang S, Ye W, Josephson L, Meyer JH, Zhang MR, Vasdev N, Wang J, Xu H, Wang L, Liang SH. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm Sin B. 2021;11:373-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 33. | Dong L, Shen Y, Li H, Zhang R, Yu S, Wu Q. Shared Genes of PPARG and NOS2 in Alzheimer's Disease and Ulcerative Colitis Drive Macrophages and Microglia Polarization: Evidence from Bioinformatics Analysis and Following Validation. Int J Mol Sci. 2023;24:5651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 34. | Lorenzini L, Zanella L, Sannia M, Baldassarro VA, Moretti M, Cescatti M, Quadalti C, Baldi S, Bartolucci G, Di Gloria L, Ramazzotti M, Clavenzani P, Costanzini A, De Giorgio R, Amedei A, Calzà L, Giardino L. Experimental colitis in young Tg2576 mice accelerates the onset of an Alzheimer's-like clinical phenotype. Alzheimers Res Ther. 2024;16:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/