Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.108524
Revised: May 28, 2025
Accepted: July 3, 2025
Published online: July 21, 2025
Processing time: 96 Days and 6.5 Hours
This editorial is based on the network pharmacology and in vivo study by Qin
Core Tip: Ginseng and other complementary and alternative therapies are frequently used in patients with inflammatory bowel disease. While preclinical studies and in vivo models demonstrate the therapeutic potential of several traditional and herbal remedies, quality clinical trials are scarce. Clinicians should be aware of the current evidence base, including proposed mechanisms of action and available clinical data. This editorial provides a focused overview to support informed, evidence-based discussions with patients.
- Citation: Schildkraut T, Srinivasan AR, Nguyen A, Lai M, Vasudevan A. Complementary medicines and ginseng for inflammatory bowel disease-rooted in science, but will it bear fruit? World J Gastroenterol 2025; 31(27): 108524
- URL: https://www.wjgnet.com/1007-9327/full/v31/i27/108524.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i27.108524
We read with interest the study “Network pharmacology and in vivo study: Unravelling the therapeutic mechanisms of Panax ginseng in potentially treating ulcerative colitis” by Qin et al[1]. The authors demonstrated that panaxadiol (PD) reduced weight loss, disease activity index (DAI) scores and colon shortening in a mouse model of dextran sulfate sodium (DSS) induced ulcerative colitis (UC), and exerted anti-inflammatory and intestinal barrier-promoting effects via modulation of the mitogen-activated protein kinase (MAPK)/nuclear factor-κB (NF-κB) and AMP-activated protein kinase/nuclear factor erythroid 2-related factor 2/nicotinamide adenine dinucleotide (phosphate): Quinone oxidoreductase 1 pathways[1].
Inflammatory bowel disease (IBD) is becoming increasingly prevalent worldwide for reasons that are not fully elucidated, although its development is understood to entail a complex interaction of environmental, dietary, microbial and immune factors in genetically predisposed individuals[2]. Its pathogenesis is thought to involve the dysregulation of the gut microbiome, an enhanced intestinal immune response, and disruption of the intestinal epithelial barrier[3]. Standard medical therapy for IBD includes corticosteroids, thiopurines, 5-aminosalicylic acid (5-ASA), and advanced therapies which have revolutionized the treatment of IBD. However, these therapeutic options remain limited by tolerability, adverse effects, and loss of response in many cases. Therefore, there is ongoing demand for diversification of therapy choices, including the exploration of non-conventional therapies that some patients may perceive as more natural or less toxic[4].
Complementary and alternative medicines (CAMs) are nonmainstream or non-allopathic treatments used in conjunction with, or in lieu of conventional medical therapies. These include herbal therapies, exercise, and acupuncture. Interventions such as probiotics, fish oil, psychological interventions, and dietary therapies have also been trialed in IBD but are beyond the scope of this editorial. Ginseng, the root of Panax ginseng, is a commonly used traditional herbal medicine that has been shown to contain multiple active ingredients with immunomodulatory and anti-inflammatory actions in pre-clinical studies through a wide variety of potential mechanisms. Likewise, alternative interventions such as cannabis, Aloe vera, curcumin, and ginger constituents have been suggested to confer anti-inflammatory mechanisms. Despite a relative lack of quality clinical trials for these interventions, and no established guidelines to support their use, they have been utilized by many patients with chronic and inflammatory conditions. As such, there is a clear need for health professionals to understand the evidence to date and the potential mechanisms of commonly used CAMs.
Compared to conventional therapies, there is a lack of robust clinical data on the safety and efficacy of CAMs, which limits the clinicians’ ability to critically appraise their efficacy as an alternative or adjunct treatment option when managing patients with IBD[5]. Increasing clinician awareness of non-conventional therapies is essential, given that it is estimated that up to 50% of patients with IBD have used CAMs, many feel insufficiently informed regarding these therapies and covert use or reluctance to disclose use to clinicians is common[6-9]. Further, some CAM therapies are associated with intolerances and drug interactions, and their use has also been associated with reduced compliance with conventional therapy. This adds to the imperative for clinicians to be well informed about CAMs and capable of engaging in evidence-based discussions with patients regarding their use[5].
More frequently used CAM’s that have been investigated in randomized controlled trials (RCTs) for their potential therapeutic effects in IBD include cannabis, curcumin, Aloe vera, ginger, resveratrol, Indigo naturalis (IN), Artemisia absinthium (wormwood), Triticum aestivum (wheat grass), Andrographis paniculata (AP), and Tripterygium wilfordii (TW). A summary of the findings of these studies can found in Table 1. Currently, much of our understanding of these therapies is derived from basic science and animal model research. Mouse models of colitis, induced by interventions such as DSS, trinitrobenzenesulfonic acid, iodoacetamide, and high-fat diets, have been extensively employed to investigate the mechanisms and effects of ginseng and other CAMs. While these studies provide valuable insights, their findings should be interpreted with caution, as the complex pathophysiology of IBD may not be fully represented in animal colitis models, so human trials are needed to confirm these findings.
| Population, n | Interventions | Findings | Comments | Ref. | |
| Ginseng | UC patients with mild or moderate lesions within 15 cm of the anal verge, n = 120 | Intervention: High dose suppository preparation of Panax notoginseng combined with other herbs; control: Low dose suppository preparation of Panax notoginseng combined with other herbs | Lower clinical symptom scores; improved clinical efficacy; lower IL-6 and IL-23; improved endoscopic scores; lower recurrence rate | Unclear blinding of proceduralists/investigators; clinical efficacy based on author’s criteria of clinical efficacy; no definition of recurrence; no significant difference in adverse reaction rate | Zeng et al[38], 2022 |
| Cannabinoids | UC patients with Mayo ≥ 4 and ≤ 10, n = 32 | Intervention: CBD rich cannabis (160 mg/mL CBD, 40 mg/mL THC), n = 17; control: Placebo oil, n = 15 | Improved QoL; no difference in endoscopic scores or inflammatory markers | Gradual treatment dose escalation; intervention was well tolerated | Naftali et al[46], 2021 |
| UC patients with Mayo ≥ 4 and ≤ 10, n = 60 | Intervention: CBD-rich extract capsules (50-250 mg twice a day), n = 29; control: Placebo capsules, n = 31 | No significant difference in rates of remission; trend toward improved PGAS in PP analysis (82% had mild to normal disease at the end of treatment in the CBD-rich extract group vs 52% in the placebo group, P = 0.069; improved QoL | Disproportionate number of withdrawals and lower adherence in the CBD-rich extract group | Irving et al[45], 2018 | |
| CD patients with CDAI 200-450, n = 20 | Intervention: Cannabidiol oil (10 mg twice a day), n = 10; control: Placebo oil, n = 10 | No significant improvement in CDAI or laboratory tests | Intervention had good tolerability and safety profile | Naftali et al[44], 2017 | |
| Treatment refractory CD with CDAI > 200, n = 21 | Intervention: THC cigarettes (115 mg), n = 11; control: Placebo cigarettes, n = 10 | Greater improvements in CDAI scores (90% in the cannabis group versus 40% in the placebo group, P = 0.028); improved appetite and sleep; no significant difference in rates of remission | No significant side effects observed | Naftali et al[43], 2013 | |
| Curcumin | Quiescent UC, n = 89 | Intervention: Curcumin (2 g/day) + 5-ASA, n = 45); control: Placebo + 5-ASA, n = 44 | Reduced relapse rates in curcumin group (4.65% vs 20.51%, P = 0.049); improved clinical and endoscopic scores | Intervention was well tolerated | Hanai et al[47], 2006 |
| Active mild-to-moderate distal UC, n = 45 | Intervention: Curcumin enema (140 mg daily) + oral 5-ASA, n = 23; control: Placebo + 5-ASA, n = 22 | Improved clinical remission rate (71.4% vs 31.3%, P = 0.03) and endoscopic scores (85.7% vs 50%, P = 0.04) on PP analysis; no significant difference in treatment response, clinical remission or endoscopic scores on ITT analysis | Nearly 40% of patients in the curcumin group and 28% or patients in the placebo group did not complete the study period; no serious side effects observed | Singla et al[48], 2014 | |
| Active mild-to-moderate UC refractory to mesalamine treatment, n = 50 | Intervention: Curcumin capsules (3 g/day) + continue mesalamine, n = 26; control: Placebo capsules + continue mesalamine, n = 24 | Improved rates of clinical remission (53.8% in the curcumin group vs 0% in the placebo group, P = 0.01), clinical response (65.3% vs 12.5%, P < 0.001) and endoscopic remission (38% vs 0%, P = 0.043) | No adverse effects observed | Lang et al[49], 2015 | |
| Active mild-to-moderate UC, n = 62 | Intervention: Curcumin capsules (150 mg three times a day) + mesalamine, n = 29; control: Placebo capsules + mesalamine, n = 33 | There was no significant difference between curcumin and placebo in rates of clinical remission, clinical response, mucosal healing, and treatment failure | High attrition rate, 21 out of 62 patients (13/29 patients randomized to curcumin arm and 8/25 patients randomized to the placebo arm) did not complete the trial; no adverse clinical or biochemical effects were observed | Kedia et al[50], 2017 | |
| Ginger | Active mild-to-moderate UC, n = 46 | Intervention: Dried ginger powder capsules (2000 mg/day), n = 22; control: Placebo capsules, n = 24 | Improved SCCAIQ (7.6 ± 4.03 to 4.01 ± 1.23 in the ginger group vs 6.2 ± 3.22 to 5.55 ± 2.39, between group P < 0.017; improved stool frequency score (P = 0.041) and bowel distress and cramp scores (P = 0.029) | Minor adverse effects (heartburn) | Nikkhah-Bodaghi et al[53], 2019 |
| Resveratrol | Active mild-to-moderate UC, n = 50 | Intervention: Resveratrol capsules (500 mg daily), n = 25; control: Placebo capsules (containing MCT oil), n = 25 | Increased QoL scores in resveratrol group vs placebo (P ≤ 0.001); significant reduction in CRP, TNF-α levels, and NF-κB levels in the resveratrol group (P ≤ 0.01); no significant change in the placebo group | Reported significant reduction in SCCAIQ scores in resveratrol group vs placebo; within group P values comparing baseline to post-intervention measurements were not provided | Samsami-Kor et al[54], 2015 |
| Active mild-to-moderate UC, n = 56 | Intervention: Resveratrol capsules (500 mg daily), n = 28; control: Placebo capsules (containing MCT oil), n = 28 | Increased QoL scores in resveratrol group following intervention (P < 0.01) and compared to placebo (P < 0.001); significant reduction in MDA, superoxide dysmutase and TAC following intervention (P < 0.001) and compared to placebo (P < 0.001) | Reported significant reduction in SCCAIQ scores in resveratrol group vs placebo; within group P values comparing baseline to post-intervention measurements were not provided | Samsamikor et al[55], 2016 | |
| Artemisia absinthium | CD patients with CDAI > 200, n = 20 | Intervention: Dried powdered wormwood capsules (3 × 750 mg three times a day), n = 10; control: Placebo (unspecified form) | Improved rates of clinical response (P = 0.05); the authors reported a significant decrease in serum TNF-α levels; however, P value was recorded as 0.5 | No “out of line” side effects reported that could be attributed to wormwood administration as per the authors | Krebs et al[51], 2010 |
| CD patients with CDAI ≥ 170, n = 40 | Intervention: Wormwood powder capsules (2 × 250 mg three times a day; capsules also contained rose, cardamom, mastic resin), n = 20; control: Placebo capsules, n = 20 | Reduced occurrence of CD exacerbation during steroid wean (P value not reported) and higher rates of clinical symptom score improvement in wormwood group compared to placebo (P = 0.01); 10% in wormwood group vs 80% in placebo required steroid re-commencement (P value not reported) | Adverse events not reported by authors | Omer et al[52], 2007 | |
| IN | UC patients with Mayo ≥ 6, n = 86 | Intervention: Powdered IN capsules (0.5 g daily, n = 23), (1.0 g daily, n = 20), (2.0 g daily, n = 21); control: Placebo capsules, n = 22 | Dose dependent significant improvement in clinical response in IN group, 13.6% to placebo, 69.6% to 0.5 g IN (P = 0.0002), 75.0% to 1.0 g IN (P = 0001) and 81.0% to 2.0 g IN (P < 0.0001); significantly improved rates of clinical remission with 1.0 g IN (55%, P = 0.0004) and 2.0 g IN (38.1%, P = 0.0093) compared to placebo (4.5%); improved rate of mucosal healing compared to placebo; significant decrease in partial Mayo scores, increase in serum albumin | Proportions of treatment-related adverse effects were 14% in placebo, 26% in 0.5 g IN, 35% in 1.0 g IN, and 29% in 2.0 g IN; adverse effects included liver dysfunction affecting 10%-20% of patients receiving IN; the study was terminated early due to safety concerns regarding IN (external to the trial) | Naganuma et al[57], 2018 |
| Active mild-to-moderate UC with Lichtiger index 5-10 intolerant or refractory to existing treatments, n = 42 | Intervention: IN capsules (500 mg twice a day), n = 23; control: Placebo capsules, n = 19 | Significant clinical response (P = 0.001) in the IN group but not in placebo; higher rate of clinical response (26.3% in the placebo group vs 82.6% in the IN group, P = 0.0003); significant improvement in serum albumin in IN group | Significantly higher baseline mean Lichtiger index in the IN group compared to the placebo group (9.04 ± 1.92 vs 7.47 ± 1.43, P = 0.0053) | Uchiyama et al[59], 2020 | |
| Aloe vera | Active mild-to-moderate UC with SCCAI ≥ 3, n = 44 | Intervention: Oral Aloe vera gel (100 ml twice a day), n = 30; control: Placebo liquid preparation containing flavoring but no active agents, n = 14 | Small but statistically significant fall in median SCCAI in the Aloe vera group (P = 0.01) but not in placebo; greater rate of clinical response (47% in Aloe vera group vs 14% in placebo group, OR = 5.3, P = 0.048; significant improvement in histological scores in Aloe vera group (P = 0.031) but not in placebo | Only minor adverse effects observed, which were similar in the Aloe vera and placebo groups | Langmead et al[60], 2004 |
| Triticum aestivum | Active UC patients, n = 24 | Intervention: Wheat grass juice (100 ml), n = 12; control: Placebo juice (similar to wheat grass in appearance but not in taste or smell), n = 12 | Significantly differences in improvements in rectal bleeding (82% vs 58%, P = 0.025), DAI scores (91% vs 42%, P = 0.031), and PGAS (91% vs 42%, P = 0.031) | Blinding was limited but that inability to match the taste and smell of the control juice to wheat grass juice | Ben-Arye et al[61], 2002 |
| Andrographis paniculata | Active mild-to-moderate UC, n = 120 | Intervention: HMPL-004 (400 mg three times a day), n = 60; control: Mesalazine slow release granules (1500 mg three times a day), n = 60 | Overall clinical efficacy (remission + partial remission + improvement) in 76% in the HMPL-004 group and 82% in the mesalazine group (P < 0.001 compared to baseline for both groups, no significant difference between groups; no between group differences in overall endoscopic efficacy or histological scores | Adverse reactions in the HMPL-004 group were rate and limited to mild allergic reactions or rash | Tang et al[63], 2011 |
| Active mild-to-moderate UC with Mayo Score 4-10, n = 223 | Intervention: HMPL-004 capsules (1800 mg or 1200 mg per day in three divided doses), n = 74 in each intervention group; control: Placebo capsules, n = 75 | Improved rates of clinical response with 1800 mg daily (60%, P = 0.0183) but not 1200 mg daily of HMPL-004 (45%, P = 0.5924) compared to placebo (40%); mucosal healing improved from baseline in 50% of patients receiving 1800 mg HMPL-004 and in 33.3% of patients receiving placebo (P = 0.0404) | Similar rate of adverse effects in the HMPL-004 groups and placebo | Sandborn et al[64], 2013 | |
| Tripterygium wilfordii | CD patients who had undergone terminal ileectomy, partial colectomy or ileocolectomy with ileocolonic anastomosis and CDAI ≤ 150 in the preceding 2 weeks, n = 39 | Intervention: GTW tablets (1 mg/kg per day), n = 21; control: 5-ASA (4 g/day), n = 18 | Lower rate of clinical recurrence (5.3% vs 23.5%, P < 0.001) and endoscopic recurrence (21.1% vs 52.9%, P < 0.001) in GTW group compared to 5-ASA group in PP analysis; higher proportion in endoscopic remission and lower proportion in endoscopic recurrence (P < 0.001) in the GTW group compared to 5-ASA group in PP analysis | Single-blinded; no severe adverse events occurred, and no patients were withdrawn prematurely from the study due to adverse events. The adverse event rate was similar between groups | Ren et al[65], 2013 |
NF-κB, which is activated by phosphorylation as a downstream transcription factor of MAPK, modulates inflammation by inducing cytokines such as interleukin-6 (IL-6), IL-8, tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein. IL-6 also activates NF-κB in colon epithelial cells, which has been associated with increased expression of intercellular adhesion molecule-1, promoting recruitment of neutrophils and local inflammation[10]. Intestinal inflammation in IBD is associated with the presence of CD14 positive macrophages, dendritic cells, and elevated levels of reactive oxygen species and pro-inflammatory cytokines including IL-1, IL-6, TNF-α, and IL-1β[3,11]. Increased levels of TNF-α and NF-κB are found in intestinal biopsies of patients with IBD[10]. Overexpression of TNF-α promotes the expression of adhesion molecules that mediate activation and infiltration of lymphocytes at the site of inflammation and is associated with chronic inflammation and tissue damage[3]. Intestinal inflammation results in production of IL-12 and IL-23 by antigen-presenting cells. IL-12 promotes a T helper cell 1-mediated immune response, which is central to the pathogenesis of Crohn’s disease (CD), via activation of signal transducers and activator of transcription 4 (STAT4). The downstream effects of IL-23 include induction of inflammatory mediators via tyrosine kinase 2, Janus kinase 2 (JAK2), STAT3, and STAT4 pathways[3]. UC is characterized by an aberrant T helper cell 2 immune response, in which higher levels of IL-13 released from atypical natural killer cells are seen[2].
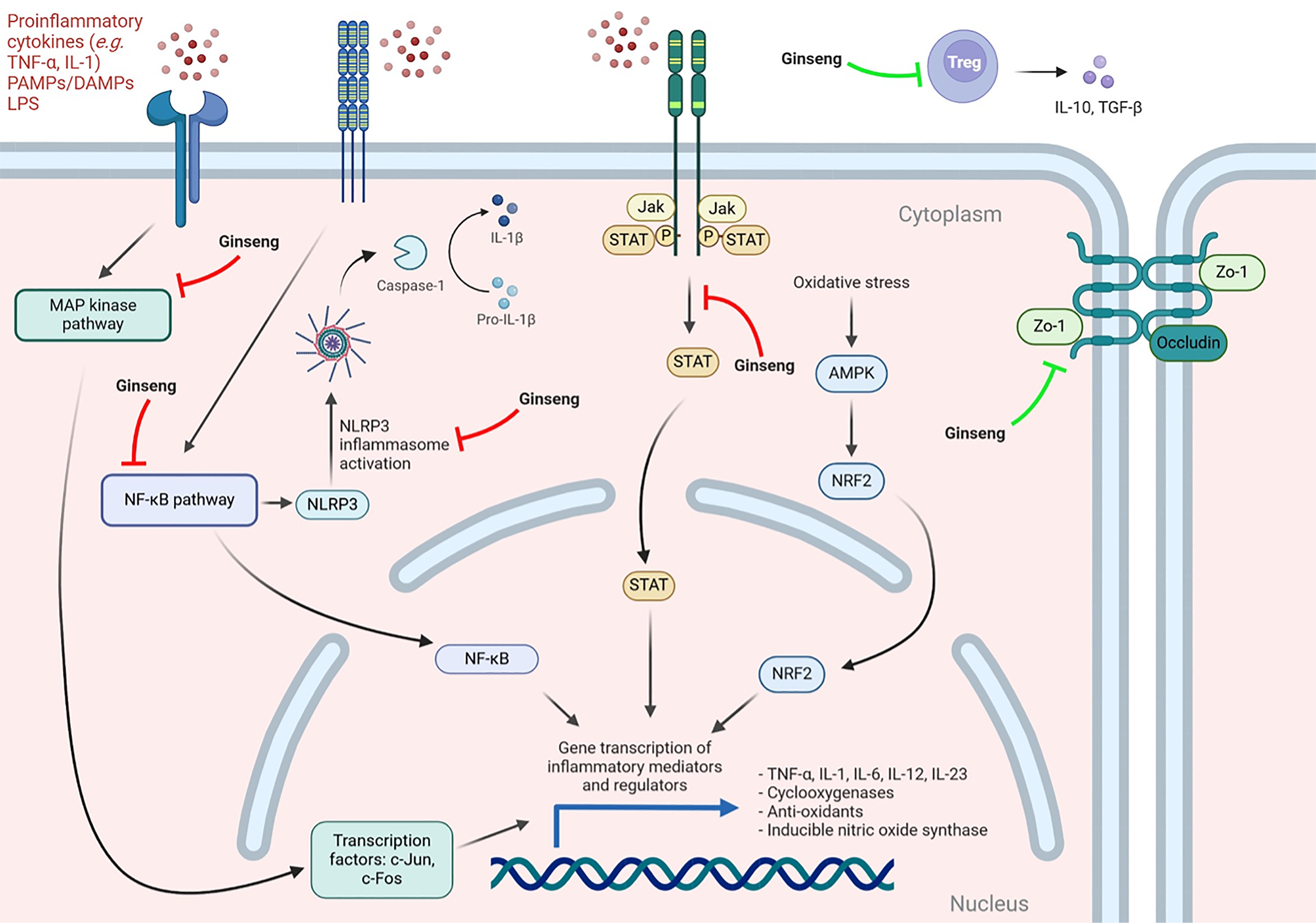
Ginsenosides, a family of over 300 known monomers, are the major active component of ginseng. Several pre-clinical studies have demonstrated their effect on inflammation and immune mediators through a spectrum of proposed mechanisms (Figure 1)[12]. The effects of various ginseng products observed in mouse models of colitis include inhibition of NF-κB and STAT3 signaling, reduced NOD-like receptor protein 3 (NLRP3) levels, and activation of the AMP-activated protein kinase pathway. Ginsenosides have also been implicated in the suppression of pro-inflammatory mediators and cytokines such as IL-6, cyclooxygenase and inducible nitric oxide synthase, and up-regulation of tight junction proteins such as zonula occludens-1 and occludin[13-20]. Ginsenoside Rg1 has been found to inhibit the T helper cell 17/Treg imbalance observed in induced colitis models, restoring IL-10 and factor forkhead box protein 3 expression[17]. Its ability to promote the expression of NLRP12, a negative regulator of innate immunity implicated in the modulation of colonic inflammation and gut microbiome diversity, has also been demonstrated[19,20]. MAPK, which increases adhesion and transmigration of lymphocytes and activates transcription factors c-Fos and c-Jun, thereby regulating the transcription of inflammatory mediators such as IL-6 and TNF-α, is also modulated by ginsenosides[21]. Qin et al[1] found that MAPK phosphorylation was increased with DSS exposure, but this effect was attenuated by PD. Elsewhere, PD has been reported to inhibit IL-1β production via modulation of zinc finger protein 91 and MAPKs in both in vitro and in vivo mouse models[12,22]. Improved histological and clinical metrics such as colon length, body weight, and decreased DAI have also been observed in mouse models[13,20,22,23].
Preclinical studies have also identified several mechanisms for the potential benefits of other CAMs commonly used in IBD. Cannabinoids, known for their analgesic properties, interact with cannabinoid receptors in the gastrointestinal tract and on immune cells, and may suppress inflammatory cytokines, adhesion molecules, and inflammatory cell migration and proliferation, as well as modulate cannabinoid receptor type 1-mediated colonic inflammation[24,25]. The endocannabinoid system has also been implicated in the inhibition of gastrointestinal motility and gastric acid and intestinal secretions[24].
Ginger and its bioactive phytochemicals have demonstrated antioxidant and anti-inflammatory effects, augmenting superoxide dismutase and glutathione peroxidase and inhibiting xanthine peroxidase, prostaglandin E2, nitric oxide, TNF-α, IL-1β, and NF-κB. It may also regulate the inflammatory response through suppression of the NLRP, Toll-like receptor, STAT, MAPK, and mechanistic target of rapamycin pathways[26]. Resveratrol, the polyphenolic organic compound found in red wine as well as various other plants, is thought to inhibit oxidative stress and inflammation through its effects on NF-κB, silent information regulator sirtuin 1, mechanistic target of rapamycin, and TNF-α. It may promote the integrity of the intestinal barrier by increasing expression of zonula occludens-1 phosphorylation of occludin and reducing oxidative damage[27,28]. Resveratrol may also affect the composition and function of the gut microbiome[27].
IN is a dry pigment used in traditional Chinese medicine for its anti-inflammatory effects, which are thought to be mediated through its modulation of the NF-κB and MAPK pathways, and suppression inflammatory cytokines such as IL-6, IL-8, and TNF-α[28]. Aloe vera gel, the mucilaginous aqueous extract of the leaf pulp of Aloe barbadensis, has been used in traditional medicine practices for its proposed anti-inflammatory and anti-oxidant benefits, although the mechanisms are largely unclear[29]. Its active components may possess topical anti-inflammatory, anti-septic, and analgesic properties, inhibit the cyclooxygenase pathway and suppress prostaglandin E2[30]. Aloe vera also contains elements with peroxidase activity, superoxide dysmutase enzymes and multiple antioxidants[31,32].
Other notable findings include the anti-inflammatory effects of curcumin, which are thought to be driven by inhibition of NF-κB, resulting in suppression of inflammatory cytokines including IL-1, IL-6, IL-8, and TNF-α[28,33]. Meanwhile, Artemisia absinthium has been shown to suppress TNF-α and other interleukins in vitro[34]. Likewise, AP, a herbal remedy used in a number of Asian cultures, has been demonstrated to suppress TNF-α, IL-1β, and NF-κB in vitro[35,36]. TW, another traditional Chinese remedy, exerts anti-inflammatory effects via T-cell and dendritic cell apoptosis and inhibition of cytokine transcription through NF-κB suppression[37]. Despite these effects being demonstrated in basic research and mouse models, clinical data in IBD remains limited.
Currently, there is a paucity of clinical trials evaluating the effects of ginseng in IBD. In one RCT, Panax notoginseng (dose not specified) in combination with several other herbal therapies was rectally administered (2 suppositories twice a day for 1 month, 1 suppository twice a day for 1 month and 1 suppository at night for one month) in 120 UC patients with mild or moderate lesions within 15 cm of the anal verge. Compared to a control group, who received a low dose version of the same preparation (80% compared with experimental group), high dose suppositories were associated with improved clinical symptom scores, endoscopic scores and inflammatory cytokine levels (exact values not provided, P < 0.001). The high dose preparation was also reported to result in significantly lower rates of disease recurrence compared to the low dose preparation (5% vs 33.3% at 6 months, 8.3% vs 40% at 8 months, 10% vs 46.7% at 12 months, P < 0.001)[38]. Major limitations of these findings include the lack of double-blinding and a failure to include a control group taking placebo or conventional therapy in the absence of Panax notoginseng. Further, no comment is made on other therapies or corticosteroids being taken by patients included in the study. Despite its widespread real-world use, there is a lack of quality studies investigating the effect of ginseng in patients with IBD, highlighting a need for further research.
Cannabis use for symptom management is frequently reported among IBD patients, with up to 15% of patients actively using it as treatment for a variety of symptoms including abdominal pain, nausea, and diarrhoea[39]. Cannabinoids are known to improve patient-reported symptoms such as appetite, sleep, and general well-being[40]. Its use has been linked to improved quality of life (QoL), general health perception, social function, and clinical response rates; however there is limited evidence of benefit in terms of rates of clinical remission or inflammatory markers[41]. There are, however, significant safety concerns with chronic cannabis use, including an association with worse disease prognosis in CD and need for surgery[42].
An RCT of 21 CD patients with CD activity index (CDAI) above 200 who did not respond to conventional therapy with oral corticosteroids, immunomodulators or anti-TNF-α, randomly assigned participants to a delta-9-tetrahydrocannabinol (THC)-containing cannabis cigarette intervention (0.5 g of dried cannabis flowers corresponding to 115 mg of THC, twice daily) or a placebo of cannabis cigarettes with the THC extracted (containing less than 0.4% THC and undetectable amounts of all other cannabinoids) over 8 weeks to assess induction of remission. Clinical response, as measured by CDAI scores, was observed in 90% of the intervention group and 10% of the placebo group (P = 0.028). There was no significant difference in clinical remission rates (5/11 or 45% in the cannabis group vs 1/10 in the placebo group, P = 0.43)[43]. Another RCT investigated the effect of low dose cannabidiol (CBD) (10 mg twice a day) on induction of remission in twenty patients with refractory CD with CDAI above 200 and found no significant effect on clinical response. Mean CDAI in the CBD group was 337 ± 108 at baseline and 220 ± 122 after 8 weeks of treatment, and in the placebo group, 308 ± 96 at baseline and 216 ± 121 at 8 weeks (P > 0.05)[44]. The effects of a CBD-rich botanical extract (50-250 mg twice a day) compared to placebo on disease remission were investigated in 60 UC patients with Mayo scores between 4 and 10. The authors found similar rates of remission in the two groups after 10 weeks of treatment (odds ratio: 0.82, P = 0.753), with a trend toward improved total and partial Mayo scores using per protocol (PP) analysis (treatment difference = -1.61, P = 0.068). Importantly, the data was limited by poor compliance with the treatment protocol in the intervention arm[45]. Most recently, a 2021 RCT investigating the effects of 8 weeks of THC cigarettes (containing 160 mg/mL CBD and 40 mg/mL THC) compared to placebo in 32 patients with UC with Mayo scores between 4 and 10 found improved QoL but no significant difference in endoscopic scores or inflammatory markers between the groups[46]. These findings, particularly those related to subjective outcomes in THC trials, should be interpreted with caution, as blinding may be compromised due to the psychoactive and sensory effects of the intervention.
Curcumin, an active derivative of turmeric commercially available in supplement form, has been widely adopted by patients with a range of inflammatory conditions. As adjunct therapy in IBD, curcumin has been shown to potentially reduced relapse rates, increase rates of remission and reduce disease activity scores. A double-blind RCT was conducted to investigate the effect of curcumin (2 g per day) as an adjunct to 5-ASA compared to placebo and 5-ASA on maintenance of remission over 6 months in 89 patients with quiescent UC. The addition of curcumin was found to reduce relapse rates (4.65% vs 20.51%, P = 0.049) and improve clinical (P = 0.038) and endoscopic scores (P = 0.0001) compared to placebo[47]. Another double-blind, single-center RCT of curcumin (140 mg) via daily enema administration as an adjunct to oral 5-ASA compared to placebo enema and oral 5-ASA in 45 patients with active distal UC found no statistically significant difference in rates of clinical remission (43.5% vs 22.7%, P = 0.14), clinical response (56.5% vs 36.4%, P = 0.18) or mucosal healing (52.5% vs 36.4%, P = 0.29) after 8 weeks using intention to treat analysis. However, significant improvements in both clinical response rate (71.4% in the treatment group vs 31.3% in the placebo group, P = 0.03) and endoscopic scores (85.7% in the treatment group vs 50% in the placebo group, P = 0.04) was demonstrated with PP analysis[48]. In a multicenter double-blind RCT of 50 patients with active mild-to-moderate UC refractory to treatment with maximum dose oral mesalamine, the addition of curcumin capsules (3 g per day) for induction of remission resulted in improved rates of clinical remission (53.8% vs 0%, P = 0.01), clinical response (65.3% vs 12.5%, P < 0.001) and endoscopic remission (38% vs 0%, P = 0.043) after 4 weeks of therapy[49]. Meanwhile, a further double blind RCT found no significant differences in clinical remission (31.3% vs 27.3%, P = 0.75), clinical response (20.7% vs 36.4%, P = 0.18), treatment failure (25% vs 18.5%,P = 0.59) or mucosal healing (34.5% vs 30.3%, P = 0.72) in patients with mild-to-moderately active UC receiving curcumin (150 mg three times a day) as an adjunct to mesalamine[50].
Artemisia absinthium, commonly referred to as wormwood, is a common traditional herbal remedy. In a RCT of 20 patients with active CD and CDAI above 200 receiving either powdered wormwood (3 × 750 mg three times a day) or placebo for 6 weeks, wormwood supplementation was associated with improved rates of clinical response as measured by number of patients with CDAI below 150 (6/10 in the wormwood group vs zero in the placebo group, P = 0.05). The authors reported a significant decrease in serum TNF-α level, however, the P value was recorded as 0.5[51]. Another RCT demonstrated greater rates of successful corticosteroid weaning with the use of wormwood in CD patients undergoing a corticosteroid taper. Forty patients with active CD with CDAI greater or equal to 170 while on corticosteroids were randomized to wormwood capsules (2 × 250 mg three times a day in combination with other herbal remedies) or placebo. Following a gradual wean and cessation of corticosteroid treatment, with other concomitant CD medication maintained at stable doses, patients receiving wormwood for 10 weeks were found to have a lower rate of exacerbation (10% vs 80%, P value not reported) and improved clinical symptom scores (65% vs 0%, P = 0.01) compared to placebo over an observation period of 20 weeks[52].
A single clinical trial investigating ginger in IBD has published its results thus far. In a 2019 RCT, 46 patients with active mild-to-moderate UC were randomized to treatment with ginger powder capsules (2000 mg per day) or placebo capsules and monitored for changes in disease activity, QoL and oxidative stress factors over 12 weeks. The study found significant reduction in malondialdehyde following the intervention, as well as statistically significant improvements in simple clinical colitis activity index questionnaire (SCCAIQ) scores (7.6 ± 4.03 to 4.01 ± 1.23 in the ginger group vs 6.2 ± 3.22 to 5.55 ± 2.39, between group P < 0.017), stool frequency scores (P = 0.041) and bowel distress and cramp scores (P = 0.029) compared to placebo[53].
One RCT randomized 50 patients with active mild-to-moderate UC to receive resveratrol (500 mg daily) or placebo capsules for 6 weeks. Their findings demonstrated improved QoL as measured by QoL scores and clinical colitis activity index scores in patients receiving resveratrol supplements[54]. QoL as measured by IBD questionnaire-9 scores increased from 32.72 ± 7.52 to 47.64 ± 8.59 in the resveratrol group (P < 0.01) and from 35.54 ± 9.50 to 41.08 ± 8.59 in the placebo group (between group P < 0.001). SCCAIQ scores decreased from 12.34 ± 2.51 to 8.14 ± 2.1 in the resveratrol group and 10.76 ± 2.55 to 9.34 ± 2.65 (within group P value not reported, between group P < 0.001). A similarly designed RCT by the same authors evaluating the effect of 500 mg daily of resveratrol in 56 patients with active mild-to-moderate UC resulted in consistent findings, with increased QoL scores in resveratrol group following intervention (34.85 ± 7.67 at baseline to 47.64 ± 8.59 following treatment, P < 0.01) and compared to placebo (35.67 ± 9.89 to 41.08 ± 8.59, P < 0.01) (between group P < 0.001). There were also significant reductions in malondialdehyde, superoxide dismutase, and total antioxidant capacity. There was greater improvement in SCCAIQ scores in the resveratrol group compared to placebo (11.67 ± 272 at baseline to 8.14 ± 2.1 following treatment vs 10.88 ± 2.69 to 9.34 ± 2.65, between group P < 0.001), although the significance of these findings is unclear as within group P values comparing baseline to post-treatment measurements in each group were not provided[55].
Several studies have suggested that IN may have clinical efficacy in IBD. A 2024 meta-analysis included nine studies reporting on 299 patients with IBD. Their findings suggested a pooled clinical response rate in UC patients of 0.796 (95% confidence interval: 0.7465-0.8379, I2 = 0) and pooled relative risk of clinical response in the two RCTs of 3.82 in the IN group compared to placebo[56]. One of the RCTs, a 2018 multicenter trial evaluating the efficacy and safety of IN in 86 patients with active UC found a dose dependent significant improvement in clinical response in patients receiving IN (13.6% with a clinical response to placebo; 69.6% to 0.5 g IN; 75.0% to 1.0 g IN; and 81.0% to 2.0 g IN) prior to its early termination due to a report of pulmonary hypertension possibly linked to IN. Rates of clinical remission were also significantly higher in the 1.0 g and 2.0 g IN groups, 55% (P = 0.0004) and 38.1% (P = 0.0093), respectively, compared to 4.5% remission in patients receiving placebo. IN was also associated with significantly improved rates of mucosal healing, 56.5% with 0.5 g IN (P = 0.0045), 60% with 1.0 g IN (P = 0.0032), 47.6% with 2.0 g IN (P = 0.0217), compared to 13.6% with placebo, and improved partial Mayo scores and serum albumin[57]. A post-hoc analysis of the trial highlighted that these benefits extend to patient subgroups with steroid-dependent disease, previous anti-TNF-α exposure, concomitant immunomodulator use and those with baseline Mayo scores ≥ 9[58]. Another multi-center RCT was conducted inter
One RCT conducted to investigate the effect of oral Aloe vera (100 mL twice daily) on induction of remission in UC patients with mild-to-moderately active disease and simple clinical colitis activity index (SCCAI) of 3 or more found no statistically significant difference in clinical remission (defined as SCCAI of ≤ 2) or clinical improvement (defined as SCCAI decrease of ≥ 3) rates compared to placebo after 4 weeks of treatment. There was, however, a small but statistically significant fall in median SCCAI in the Aloe vera group (6.5 to 6.0, P = 0.01) but not in placebo. Additionally, there was a significantly greater rate of clinical response in the Aloe vera group compared to placebo (47% vs 14%, odds ratio = 5.3, P = 0.048). There was significant improvement in histological scores in the Aloe vera group (P = 0.031) but not in placebo, without significant differences in rates of sigmoidoscopic remission or improvement[60].
Triticum aestivum, or wheat grass, is consumed in the form of juice for the treatment of various conditions. It has been theorized that this herbal remedy’s rich nutritional content and possible anti-oxidant properties may provide benefit for patients with IBD[61,62]. Its therapeutic potential was interrogated in a single RCT of 24 patients with active UC who were randomized to receive fresh wheat grass juice (100 mL daily) or placebo for 1 month. The authors found no significant difference between the groups on sigmoidoscopic evaluation. Patients receiving wheat grass reported greater improvements in rectal bleeding (82% vs 58%, P = 0.025) and DAI scores (91% vs 42%, P = 0.031), and there were greater improvements in physician global assessments scores in the wheat grass group compared to placebo (91% vs 42%, P = 0.031)[61]. These findings should be interpreted with caution, as blinding of participants was compromised due to distinguishable sensory characteristics of the intervention, potentially introducing bias.
In a multi-center RCT of 120 patients with active mild-to-moderate UC across five centers, HMPL-004, an extract of AP, was compared to mesalamine (4500 mg per day) for the treatment of mild-to-moderately active UC. HMPL-004 (1200 mg per day) was found to have similar efficacy to mesalamine (76% and 82%, respectively) over 8 weeks of treatment when measured as a combined clinical outcome of improvement, partial remission and remission. Endoscopic remission rates were also similar, with 28% and 24% in the HMPL-004 and mesalamine groups, respectively (P < 0.001, no significant between the group differences). Histologic improvement (by at least 25%) was seen in 53% and 40% of patients who had baseline and post-treatment biopsies, in the HMPL-004 and mesalamine groups, respectively (P < 0.001, no significant between group differences)[63]. Another multi-center RCT in 223 patients with active mild-to-moderate UC across 52 centers, demonstrated improved rates of clinical response with 8 weeks of 1800 mg daily of HMPL-004 (60%, P = 0.0183) but not 1200 mg daily of HMPL-004 (45%, P = 0.5924) compared to placebo (40%). There were no significant differences in rates of clinical remission. Mucosal healing was seen in 50% of patients receiving 1800 mg and 37.8% of patients receiving 1200 mg of HMPL-004, compared to 33.3% of patients receiving placebo, although the difference was only significant in the high dose group compared to placebo (P = 0.0404)[64].
One single-blind RCT investigated the efficacy of polyglycosides extracted from TW (GTW) (1 mg/kg per day) in the prevention of disease recurrence in post-operative CD patients with inactive disease (CDAI under 150 in the preceding 1-2 weeks) over 52 weeks. The comparator was 4 g per day of 5-ASA. The findings using PP analysis demonstrated a lower rate of clinical recurrence in the GTW group compared to 5-ASA (5.3% vs 23.5%, P < 0.001). This correlated with a lower rate of endoscopic recurrence in GTW group compared to 5-ASA group (21.1% vs 52.9%, P < 0.001). Patients receiving GTW were also found to have higher proportion in endoscopic remission (78.9% vs 47.1%, P < 0.001)[65]. Two other RCTs were available in Chinese and were not reviewed[66,67].
Despite a lack of high-quality clinical data, pre-clinical studies demonstrate effects of the active components of ginseng on several pathways that are targeted by conventional IBD therapies, listed in Table 2. These similarities in target site provide biologic plausibility of the therapeutic effects of CAMs in IBD, though further clinical studies are needed to confirm its therapeutic efficacy and translate these findings into clinical practice. Some notable findings are worth discussing in more detail.
| Mechanisms | Conventional therapies | Complementary and alternative medicines |
| NF-κB | Sulfasalazine, methotrexate, corticosteroids | Ginseng; curcumin; ginger; resveratrol; Indigo naturalis; Andrographis paniculata; Tripterygium wilfordii |
| JAK/STAT | JAK inhibitors | Alopolysaccharide (polymer from Aloe vera) |
| MAPK | Not targeted by current conventional therapies | Ginseng; ginger; Indigo naturalis |
| Intestinal epithelial barrier | Not targeted by current conventional therapies | Ginseng |
The NF-κB pathway is a key immunological regulator that has been found to be highly induced in IBD patients and is inhibited by conventional therapies, such as sulfasalazine, methotrexate and corticosteroids[11]. It also appears to be a key target of many of the CAMs used in IBD, including ginseng, curcumin, ginger, resveratrol, IN, AP, and TW[54,68]. The effects of PD are thought to be partly mediated through this pathway, as displayed in the findings by Qin et al[1]. Given the importance of this pathway in therapy and the move toward potential use of combination therapies, there is some plausibility in considering these therapies as adjunct treatments if further data confirms their safety and efficacy.
Interestingly, constituents of ginseng, ginger, and IN have been demonstrated to modulate MAPK. The p38 MAPK pathway is a signaling cascade that plays a central role in the regulation of inflammation and apoptosis and has been proposed as a potential therapeutic target for diseases including rheumatoid arthritis and IBD[69]. The development of small molecule drugs that inhibit p38 MAPK has been limited by serious safety concerns and clinical side effects including hepatotoxicity, cardiotoxicity, central nervous system toxicity, and immunosuppression, so the safety of CAMs targeting p38 MAPK should be explored further[21]. The JAK/STAT signaling is another relevant pathway in the pathogenesis and treatment of IBD and has garnered a lot of attention in IBD as therapies selectively targeting this pathway, such as upadacitinib, can be administered orally and have been shown to be efficacious in treating IBD[70]. Alopolysaccharide, a polymer from Aloe vera, was shown to result in JAK2 inhibition in vitro and in a UC animal model[71].
PD has been shown to maintain the integrity of the intestinal epithelial barrier by enhancing the expression of mucin and tight junction proteins[1]. This is a novel mechanism and has been proposed as a potential treatment strategy in IBD, given the possible role of intestinal permeability in worsening the inflammatory state[72,73]. Therefore, further research to clarify the role of this in reducing inflammation and potentially preventing or delaying the onset of IBD would be of value[74].
Given their multitude of shared biological pathways and potential mechanisms, the application of artificial intelligence and network pharmacology presents a promising avenue for identifying CAM therapies with a higher likelihood of efficacy. Artificial intelligence-driven models can facilitate the discovery of novel therapeutic compounds and molecular targets, optimize herbal formulations, and predict herb-drug interactions, thereby enhancing safety and effectiveness. By leveraging comprehensive herbal medicine databases from diverse regions, these tools can also improve clinical trial design and generate stronger evidence for the role of CAM in IBD[75,76].
Regulation, standardization, and safety of CAMs remain ongoing concerns. Evidence of efficacy is often lacking or not required, particularly due to the “low risk” classification of natural and herbal supplements, which currently occupy a regulatory grey zone between food and medicine. Regulatory frameworks vary internationally: In Canada, for example, safety and efficacy data are mandated, whereas in Australia, most herbal products are not subject to pre-market efficacy evaluation, with manufacturers responsible for quality and safety. In contrast, European regulations require both biological and toxicological data when medical claims are made. Inadequate regulation has enabled the marketing of CAMs with limited or no supporting evidence, often leading to insufficient safety monitoring and reduced confidence among consumers and health professionals. Safety issues have included toxicity, drug interactions, contamination, and undeclared ingredients. Globally, there is a clear need for standardized, robust processes for risk assessment, quality assurance, and post-marketing surveillance, and strengthening of authorities to enforce adherence to protocols and good manufacturing practices[77]. These regulatory and standardization challenges, compounded by the current lack of high-quality RCT evidence, remain key barriers to the safe, evidence-based integration of CAMs into mainstream healthcare.
The findings by Qin et al[1] add to a growing body of pre-clinical data demonstrating the therapeutic potential of ginseng and its constituents in IBD. Ginseng and other CAM’s are frequently used by patients hoping to improve their symptoms, reduce their reliance on conventional therapies and avoid side effects of prescribed medications. Despite their popularity, herbal remedies and supplements have not been rigorously investigated in clinical trials. Well-designed high quality RCTs and strengthened regulations are required to confirm the therapeutic benefits of these therapies and facilitate their safe and evidence-based integration into clinical practice.
| 1. | Qin Y, Zhang RY, Zhang Y, Zhao YQ, Hao HF, Wang JP. Network pharmacology and in vivo study: Unraveling the therapeutic mechanisms of Panax ginseng in potentially treating ulcerative colitis. World J Gastroenterol. 2025;31:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 2. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1130] [Article Influence: 94.2] [Reference Citation Analysis (31)] |
| 3. | Nakase H. Treatment of inflammatory bowel disease from the immunological perspective. Immunol Med. 2020;43:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Nguyen GC, Croitoru K, Silverberg MS, Steinhart AH, Weizman AV. Use of Complementary and Alternative Medicine for Inflammatory Bowel Disease Is Associated with Worse Adherence to Conventional Therapy: The COMPLIANT Study. Inflamm Bowel Dis. 2016;22:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Lin SC, Cheifetz AS. The Use of Complementary and Alternative Medicine in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2018;14:415-425. [PubMed] |
| 6. | Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:415-429.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 7. | Joos S, Rosemann T, Szecsenyi J, Hahn EG, Willich SN, Brinkhaus B. Use of complementary and alternative medicine in Germany - a survey of patients with inflammatory bowel disease. BMC Complement Altern Med. 2006;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Fernández A, Barreiro-de Acosta M, Vallejo N, Iglesias M, Carmona A, González-Portela C, Lorenzo A, Domínguez-Muñoz JE. Complementary and alternative medicine in inflammatory bowel disease patients: frequency and risk factors. Dig Liver Dis. 2012;44:904-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lippert A, Renner B. Herb-Drug Interaction in Inflammatory Diseases: Review of Phytomedicine and Herbal Supplements. J Clin Med. 2022;11:1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Vavricka SR, Galván JA, Dawson H, Soltermann A, Biedermann L, Scharl M, Schoepfer AM, Rogler G, Prinz Vavricka MB, Terracciano L, Navarini A, Zlobec I, Lugli A, Greuter T. Expression Patterns of TNFα, MAdCAM1, and STAT3 in Intestinal and Skin Manifestations of Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 665] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 12. | Zhao L, Zhang T, Zhang K. Pharmacological effects of ginseng and ginsenosides on intestinal inflammation and the immune system. Front Immunol. 2024;15:1353614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 13. | Ullah HMA, Saba E, Lee YY, Hong SB, Hyun SH, Kwak YS, Park CK, Kim SD, Rhee MH. Restorative effects of Rg3-enriched Korean Red Ginseng and Persicaria tinctoria extract on oxazolone-induced ulcerative colitis in mice. J Ginseng Res. 2022;46:628-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Saba E, Lee YY, Kim M, Hyun SH, Park CK, Son E, Kim DS, Kim SD, Rhee MH. A novel herbal formulation consisting of red ginseng extract and Epimedium koreanum Nakai-attenuated dextran sulfate sodium-induced colitis in mice. J Ginseng Res. 2020;44:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Saba E, Lee YY, Rhee MH, Kim SD. Alleviation of Ulcerative Colitis Potentially through th1/th2 Cytokine Balance by a Mixture of Rg3-enriched Korean Red Ginseng Extract and Persicaria tinctoria. Molecules. 2020;25:5230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Li S, Huo X, Qi Y, Ren D, Li Z, Qu D, Sun Y. The Protective Effects of Ginseng Polysaccharides and Their Effective Subfraction against Dextran Sodium Sulfate-Induced Colitis. Foods. 2022;11:890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Lee SY, Jeong JJ, Eun SH, Kim DH. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. 2015;762:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Liu C, Wang J, Yang Y, Liu X, Zhu Y, Zou J, Peng S, Le TH, Chen Y, Zhao S, He B, Mi Q, Zhang X, Du Q. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem Pharmacol. 2018;155:366-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Chen X, Xu T, Lv X, Zhang J, Liu S. Ginsenoside Rh2 alleviates ulcerative colitis by regulating the STAT3/miR-214 signaling pathway. J Ethnopharmacol. 2021;274:113997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Zhu G, Wang H, Wang T, Shi F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int Immunopharmacol. 2017;50:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Feng YJ, Li YY. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J Dig Dis. 2011;12:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Wang JY, Xing Y, Li MY, Zhang ZH, Jin HL, Ma J, Lee JJ, Zhong Y, Zuo HX, Jin X. Panaxadiol inhibits IL-1β secretion by suppressing zinc finger protein 91-regulated activation of non-canonical caspase-8 inflammasome and MAPKs in macrophages. J Ethnopharmacol. 2022;283:114715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Li J, Zhong W, Wang W, Hu S, Yuan J, Zhang B, Hu T, Song G. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS One. 2014;9:e87810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1655] [Cited by in RCA: 1573] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 25. | Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Ballester P, Cerdá B, Arcusa R, Marhuenda J, Yamedjeu K, Zafrilla P. Effect of Ginger on Inflammatory Diseases. Molecules. 2022;27:7223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 27. | Li M, Li P, Tang RX, Lu H. Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways. Food Sci Hum Wellness. 2022;11:22-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Dzwonkowski M, Bahirwani J, Rollins S, Muratore A, Christian V, Schneider Y. Selected Use of Complementary and Alternative Medicine (CAM) Agents in IBD. Curr Gastroenterol Rep. 2025;27:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 29. | Babalola WO, Ofusori DA, Awoniran P, Falana BA. Aloe vera gel attenuates acetic acid-induced ulcerative colitis in adult male Wistar rats. Toxicol Rep. 2022;9:640-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 31. | Esteban A, Zapata JM, Casano L, Martín M, Sabater B. Peroxidase activity in Aloe barbadensis commercial gel: probable role in skin protection. Planta Med. 2000;66:724-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Sabeh F, Wright T, Norton SJ. Isozymes of superoxide dismutase from Aloe vera. Enzyme Protein. 1996;49:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Picardo S, Altuwaijri M, Devlin SM, Seow CH. Complementary and alternative medications in the management of inflammatory bowel disease. Therap Adv Gastroenterol. 2020;13:1756284820927550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, Lee WJ, Park JS, Shin CY, Oh TY, Jun CD. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol. 2006;12:4850-4858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 35. | Parichatikanond W, Suthisisang C, Dhepakson P, Herunsalee A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int Immunopharmacol. 2010;10:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Chao WW, Kuo YH, Lin BF. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J Agric Food Chem. 2010;58:2505-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443-13450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Zeng L, Li X, Bai G, Liu Y, Lu Q. Rectal administration of Panax notoginseng and Colla Corii Asini suppositories in ulcerative colitis: clinical effect and influence on immune function. Am J Transl Res. 2022;14:603-611. [PubMed] |
| 39. | Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Doeve BH, van de Meeberg MM, van Schaik FDM, Fidder HH. A Systematic Review With Meta-Analysis of the Efficacy of Cannabis and Cannabinoids for Inflammatory Bowel Disease: What Can We Learn From Randomized and Nonrandomized Studies? J Clin Gastroenterol. 2021;55:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Weiss A, Friedenberg F. Patterns of cannabis use in patients with Inflammatory Bowel Disease: A population based analysis. Drug Alcohol Depend. 2015;156:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276-1280.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 44. | Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, Laish I, Benjaminov F, Konikoff FM. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn's Disease, a Randomized Controlled Trial. Dig Dis Sci. 2017;62:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 45. | Irving PM, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, Hart A, Murray C, Lindsay JO, Taylor A, Barron R, Wright S. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 46. | Naftali T, Bar-Lev Schleider L, Almog S, Meiri D, Konikoff FM. Oral CBD-rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn's Disease, a Randomised Controlled Trial. J Crohns Colitis. 2021;15:1799-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 439] [Article Influence: 22.0] [Reference Citation Analysis (3)] |
| 48. | Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P, Deb R, Tiwari V, Rohatgi S, Dhingra R, Kedia S, Sharma PK, Makharia G, Ahuja V. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis. 2014;8:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (3)] |
| 49. | Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, Ching JY, Cheong PK, Avidan B, Gamus D, Kaimakliotis I, Eliakim R, Ng SC, Ben-Horin S. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2015;13:1444-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 50. | Kedia S, Bhatia V, Thareja S, Garg S, Mouli VP, Bopanna S, Tiwari V, Makharia G, Ahuja V. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World J Gastrointest Pharmacol Ther. 2017;8:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 51. | Krebs S, Omer TN, Omer B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn's disease - A controlled clinical trial. Phytomedicine. 2010;17:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Omer B, Krebs S, Omer H, Noor TO. Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn's disease: a double-blind placebo-controlled study. Phytomedicine. 2007;14:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 53. | Nikkhah-Bodaghi M, Maleki I, Agah S, Hekmatdoost A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement Ther Med. 2019;43:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res. 2015;46:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res. 2016;47:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | Kakdiya R, Jha DK, Choudhury A, Jena A, Sharma V. Indigo naturalis (Qing dai) for inflammatory bowel disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2024;48:102250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 57. | Naganuma M, Sugimoto S, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, Tanaka S, Andoh A, Ohmiya N, Saigusa K, Yamamoto T, Morohoshi Y, Ichikawa H, Matsuoka K, Hisamatsu T, Watanabe K, Mizuno S, Suda W, Hattori M, Fukuda S, Hirayama A, Abe T, Watanabe M, Hibi T, Suzuki Y, Kanai T; INDIGO Study Group. Efficacy of Indigo Naturalis in a Multicenter Randomized Controlled Trial of Patients With Ulcerative Colitis. Gastroenterology. 2018;154:935-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 58. | Naganuma M, Sugimoto S, Fukuda T, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, Tanaka S, Andoh A, Ohmiya N, Saigusa K, Yamamoto T, Morohoshi Y, Ichikawa H, Matsuoka K, Hisamatsu T, Watanabe K, Mizuno S, Abe T, Suzuki Y, Kanai T; INDIGO Study Group. Indigo naturalis is effective even in treatment-refractory patients with ulcerative colitis: a post hoc analysis from the INDIGO study. J Gastroenterol. 2020;55:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Uchiyama K, Takami S, Suzuki H, Umeki K, Mochizuki S, Kakinoki N, Iwamoto J, Hoshino Y, Omori J, Fujimori S, Yanaka A, Mizokami Y, Ohkusa T. Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: An investigator-initiated multicenter double-blind clinical trial. PLoS One. 2020;15:e0241337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Langmead L, Feakins RM, Goldthorpe S, Holt H, Tsironi E, De Silva A, Jewell DP, Rampton DS. Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double-blind placebo-controlled trial. Scand J Gastroenterol. 2002;37:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Mishra N, Tripathi R, Pandey D, Shah K, Chauhan NS. Wheatgrass (Triticum aestivum): a miraculous microgreen: an overview. J Future Foods. 2025;5:239-247. [DOI] [Full Text] |
| 63. | Tang T, Targan SR, Li ZS, Xu C, Byers VS, Sandborn WJ. Randomised clinical trial: herbal extract HMPL-004 in active ulcerative colitis - a double-blind comparison with sustained release mesalazine. Aliment Pharmacol Ther. 2011;33:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Sandborn WJ, Targan SR, Byers VS, Rutty DA, Mu H, Zhang X, Tang T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am J Gastroenterol. 2013;108:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 65. | Ren J, Wu X, Liao N, Wang G, Fan C, Liu S, Ren H, Zhao Y, Li J. Prevention of postoperative recurrence of Crohn's disease: Tripterygium wilfordii polyglycoside versus mesalazine. J Int Med Res. 2013;41:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Tao QS, Ren JA, Ji ZL, Li JS, Wang XB, Jiang XH. [Maintenance effect of polyglycosides of Tripterygium wilfordii on remission in postoperative Crohn disease]. Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12:491-493. [PubMed] |
| 67. | Liao NS, Ren JA, Fan CG, Wang GF, Zhao YZ, Li JS. [Efficacy of polyglycosides of Tripterygium wilfordii in preventing postoperative recurrence of Crohn disease]. Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12:167-169. [PubMed] |
| 68. | Shayesteh F, Haidari F, Shayesteh AA, Mohammadi-Asl J, Ahmadi-Angali K. Ginger in patients with active ulcerative colitis: a study protocol for a randomized controlled trial. Trials. 2020;21:278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 69. | Yang F, Zhao LJ, Xu Q, Zhao J. The journey of p38 MAP kinase inhibitors: From bench to bedside in treating inflammatory diseases. Eur J Med Chem. 2024;280:116950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 70. | Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, Vetrano S, Vande Casteele N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 71. | Lin H, Honglang L, Weifeng L, Junmin C, Jiantao Y, Junjing G. The mechanism of alopolysaccharide protecting ulceralive colitis. Biomed Pharmacother. 2017;88:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Qiao Y, He C, Xia Y, Ocansey DKW, Mao F. Intestinal mucus barrier: A potential therapeutic target for IBD. Autoimmun Rev. 2025;24:103717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 73. | Mansouri P, Mansouri P, Behmard E, Najafipour S, Kouhpayeh A, Farjadfar A. Novel targets for mucosal healing in inflammatory bowel disease therapy. Int Immunopharmacol. 2025;144:113544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 74. | Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 2017;10:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 75. | Chu H, Moon S, Park J, Bak S, Ko Y, Youn BY. The Use of Artificial Intelligence in Complementary and Alternative Medicine: A Systematic Scoping Review. Front Pharmacol. 2022;13:826044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Ng JY, Cramer H, Lee MS, Moher D. Traditional, complementary, and integrative medicine and artificial intelligence: Novel opportunities in healthcare. Integr Med Res. 2024;13:101024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 77. | Komala MG, Ong SG, Qadri MU, Elshafie LM, Pollock CA, Saad S. Investigating the Regulatory Process, Safety, Efficacy and Product Transparency for Nutraceuticals in the USA, Europe and Australia. Foods. 2023;12:427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/