Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.108483
Revised: May 10, 2025
Accepted: July 1, 2025
Published online: July 21, 2025
Processing time: 94 Days and 1.4 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) is contraindicated for patients with cavernous transformation of the portal vein (CTPV) due to high surgery-related mortality risk. However, surgically assisted TIPS (SATIPS) can significantly reduce the risk.
To evaluate the clinical efficacy of SATIPS, this study was conducted.
One hundred and seven patients with CTPV and esophagogastric variceal bleeding were recruited from January 2023 to December 2024.The patients were recruited from three different hospitals. Overall, 54 patients received SATIPS treatment (SATIPS group), while 53 patients did not receive SATIPS and un
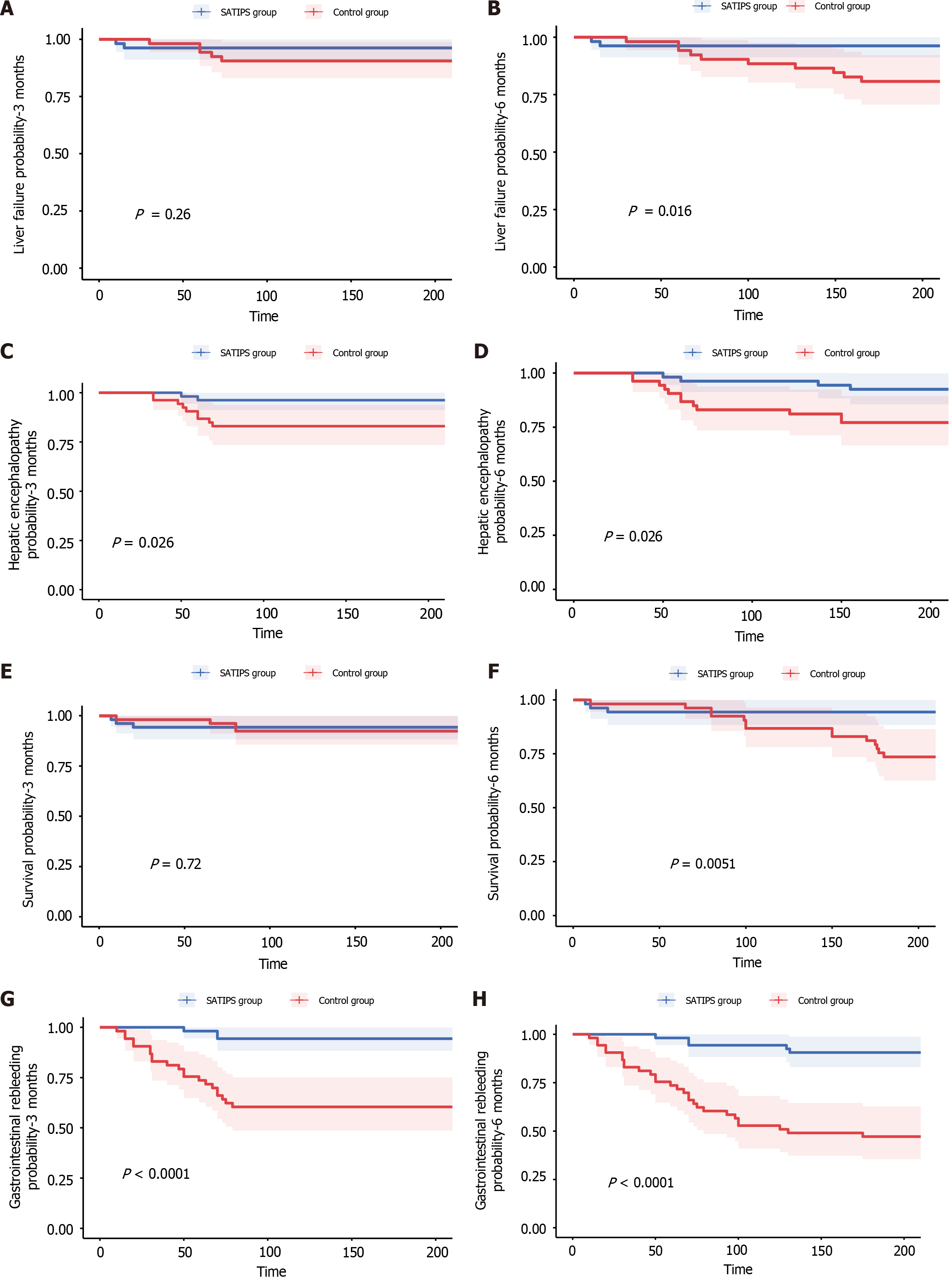
The survival rates for the SATIPS and control groups were 94.4% and 92.5% at 3 months (P value = 0.72) and 94.4% and 73.6% at 6 months (P value = 0.0051) respectively. The incidence of liver failure was 3.7% and 9.4% at 3 months (P value = 0.26) and 3.7% and18.9% at 6 months (P value = 0.016); the incidence of gastrointestinal bleeding was 5.6% and 37.7% at 3 months (P value < 0.001) and 9.3% and 47.2% (P value < 0.001) at 6 months; and the incidence of hepatic encephalopathy was 3.7% and 17.0% at 3 months (P value = 0.026) and 7.4% and 26.4% at 6 months (P value = 0.026) respectively.
For patients with CTPV, there were no optimal treatment. Regarding long-term efficacy, SATIPS can significantly reduce the rate of rebleeding, hepatic encephalopathy and liver failure, and is associated with better survival.
Core Tip: Cavernous transformation of portal vein (CTPV) is a serious structural change of portal vein. Conventional transjugular intrahepatic portosystemic shunt (TIPS) can not save the lives of patients with CTPV. This study was conducted through a retrospective case-control study. The result showed that surgically assisted TIPS can greatly improve the operation success rate of CTPV, and significantly improve the survival rate.
- Citation: Wu YF, Yue ZD, Fan ZH, Dong CB, Zhang Y, Li QM, Liu DF, Xu GZ, Wang DZ, Zhao HM, Wu ZP, Wang L. Clinical efficacy of surgically assisted transjugular intrahepatic portosystemic shunt for cavernous transformation of portal vein. World J Gastroenterol 2025; 31(27): 108483
- URL: https://www.wjgnet.com/1007-9327/full/v31/i27/108483.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i27.108483
The advancement of medical technology, coupled with a growing awareness of personal health, has led to an increase in the identification of patients suffering from liver cirrhosis and portal hypertension. In European and American regions, alcoholic cirrhosis is the predominant cause of liver cirrhosis, while hepatitis-related cirrhosis is more prevalent in Asia[1]. Portal hypertension can manifest through various clinical symptoms, including rupture and bleeding of esophageal and gastric fundus varices, intractable ascites, splenomegaly, and hypersplenism[2]. The advent of the transjugular intrahepatic portosystemic shunt (TIPS) has significantly benefited patients with portal hypertension by effectively lowering the portal pressure, thereby reducing the risk of variceal rupture and bleeding, alleviating intractable thoracic ascites, and enhancing the quality of life and survival rates of patients. However, not all patients with portal hypertension are candidates for TIPS. Some patients exhibit complete obliteration of the portal vein's normal structure, rendering TIPS unfeasible even in cases of severe hematemesis or melena that pose a threat to life. The complexity of portal vein lesions makes it challenging for physicians to accurately locate the portal vein trunk and its branches, resulting in a high-risk scenario with elevated failure and mortality rates associated with TIPS. In addressing the treatment challenges for this patient population, our center has explored various approaches. To navigate this technical limitation, we have considered surgical-assisted TIPS (SATIPS), initially proposed by Professor Rozenblit and Del Guercio[3]. However, it is noteworthy that this was primarily a case report rather than a controlled study assessing the clinical efficacy of the method. Jalaeian et al[4] compared the intrahepatic shunt function outcome and procedural complications of minilaparotomy-assisted transmesenteric-TIPS with the conventional transjugular method. However, their research did not include patients with cavernous transformation of the portal vein (CTPV), thereby failing to address the surgical challenges associated with TIPS for this patient group and the therapeutic benefits of SATIPS, which diverges from the focus of the current study. Wang et al[5] have utilized the transjugular extrahepatic portosystemic shunt (TEPS) as an alternative for patients with portal hypertension when vascular access for TIPS is unavailable. Compared with SATIPS, the procedure proved to be more challenging and had a higher risk of intraoperative abdominal bleeding. A detailed comparison of SATIPS with Grigory Rozenblit (surgically assisted portosystemic shunt), Hamed Jalaeian (surgically assisted portosystemic shunt), and TEPS is presented in Table 1. At our center, we have performed 54 SATIPS procedures with a corresponding control group and extensive data. Therefore, the present study sought to assess whether SATIPS treatment for patients with CTPV and portal hypertension improves survival rates.
| Intraoperative risk | Portal thrombosis | Study purpose | Comparison | |
| SATIPS | Medium | CTPV | To study the efficacy and feasibility of SATIPS in the treatment of CTPV | |
| Surgically assisted portosystemic shunt (Grigory Rozenblit) | Medium | None | To report a new technology | It reported the technology, but did not study the clinical efficacy |
| Surgically assisted portosystemic shunt (Hamed Jalaeian) | Medium | None | To study the effect of surgically assisted TIPS in the treatment of portal hypertension without portal vein thrombosis | The patients had no portal vein thrombosis, and routine TIPS did not require surgical intervention. To date, the study has limited significance |
| TEPS | Hard | CTPV | To study the effect of TEPS on CTPV | The risk of TEPS puncture is high, the risk of intraoperative bleeding is high, and the long-term stability of postoperative stent remains to be observed |
The study protocol was approved by the Ethics Committee of Beijing Shijitan Hospital Affiliated to Capital Medical University. All authors had access to the study data.
This multicenter retrospective case-control study analyzed 107 patients with CTPV and portal hypertension with esophagogastric variceal bleeding (EGVB). The patients were recruited from Beijing Shijitan Hospital, Capital Medical University, Xilin Gol League Central Hospital and The Second Hospital & Clinical Medical School, Lanzhou University. A total of 54 patients received SATIPS treatment (SATIPS group), while 53 patients did not receive SATIPS. These patients, for whom conventional TIPS was ineffective, were transitioned to prophylactic endoscopic sclerosing ligation (control group). TIPS was administered according to the TIPS treatment guidelines. Inclusion criteria: Patients aged 18-70 years; presenting with CTPV with ruptured esophageal and gastric varices; and those with good compliance and postoperative review. Exclusion criteria: Individuals under 18 or over 70 years; those requiring emergency surgery; patients with severe organ failure affecting the heart, lung, and kidney; and those with severe infections. All patients provided informed consent and agreed to adhere to all the protocols outlined in the study.
Surgical procedure: The surgical procedure was conducted by the surgeon at the center. The patient was placed in a supine position and intubated under general anesthesia, followed by routine disinfection once satisfactory anesthesia was achieved. The surgeon (positioned on the right side of the patient’s abdomen) made a median incision in the upper abdomen, extending from the lower costal margin to the top of the umbilicus. The skin, subcutaneous tissue, anterior sheath, rectus abdominis muscle, posterior sheath, transverse fascia of the abdomen, external fascia of the abdomen, and peritoneum were cut layer by layer. After exposing the abdominal tube, the distal branch of the small intestinal vein was isolated and ligated.
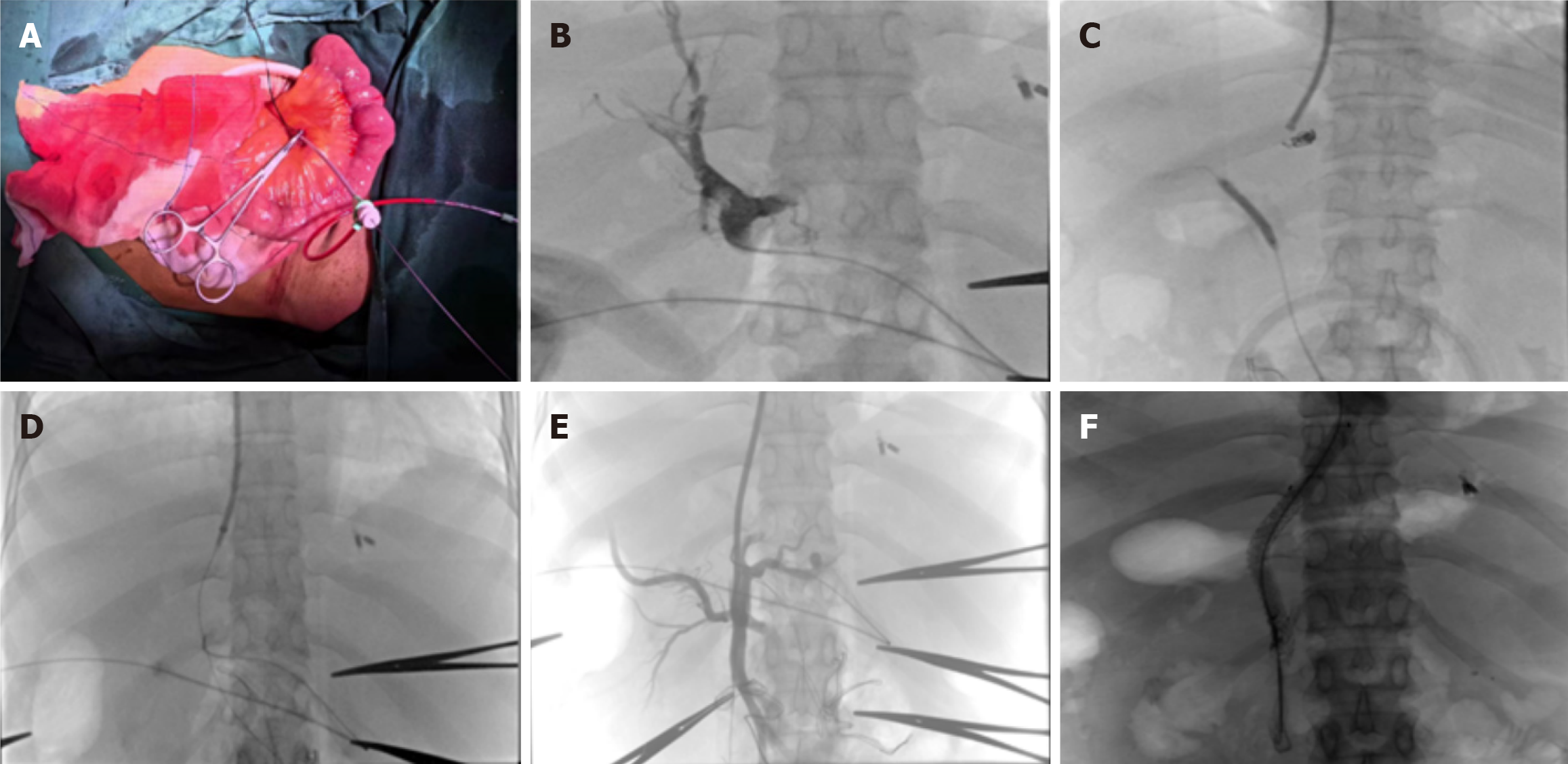
Interventional procedure: The interventional was carried out by the interventional physicians at the center. A puncture needle was used to access the proximal end of the small intestinal vein, followed by the insertion of a 5F vascular sheath. A super-smooth guide wire was then introduced, navigating through the superior mesenteric vein into the portal vein, where it was secured and marked for positioning. After successfully puncturing the right internal jugular vein with a jugular puncture needle, a 6F catheter sheath was placed, and a 5F pigtail catheter was advanced under the guidance of the guide wire. Inferior venography and hepatic venography were subsequently performed. The procedure continued with the insertion of the transjugular intrahepatic puncture system (HR China), successfully puncturing the pre-placed balloon through the inferior vena cava (IVC) (Ke Rui Chi, China) and into the portal static system. An end-to-side pore catheter was then inserted under the guide wire guidance, followed by portal venography. After positioning the cobra catheter into the abnormal esophageal and gastric varices, embolization material was slowly injected (COOK United States, Poco United States). The balloon was placed under the guide wire guidance (Ke Rui Chi China, ev3 United States), and the puncture canal between the IVC and the portal vein was pre-dilated using a pressure pump. Subsequently, a stent (Gore Corporation, second generation special stent, United States) was inserted. The fluoroscopic stent was not fully expanded, necessitating the use of a balloon to facilitate its expansion. A follow-up examination revealed that a portion of the contrast agent flowed from the portal vein stent into the IVC and subsequently into the right atrium. Intraoperative pictures are shown in Figure 1.
Data were analyzed using SPSS 17.0 software. The Measurement data were expressed as mean ± SD and compared using paired sample t-test. Categorical data were compared using the χ2 test or Fisher’s exact test. Factors influencing advantages were assessed through logistic analysis. Kaplan-Meier curves were plotted to estimate survival probabilities and the log-rank test was used to compare differences in survival time. P < 0.05 was considered statistically significant.
According to the research objectives and data characteristics, the re-survival time is divided into different intervals. Taking months as the unit, the survival rates of patients in different time periods are calculated, and the survival situations of the two groups of patients in each time interval are compared. In terms of the symptom incidence rate, starting from the end of the treatment, the time from the end of the treatment to the first occurrence of symptoms that meet the recurrence criteria is defined as the recurrence time. By comparing the recurrence time distribution, recurrence rate and other indicators of the two groups of patients, the treatment effect is analyzed.
No significant difference in height, age, and laboratory tests was found between the SATIPS and control groups (Table 2).
| Variable | SATIPS group | Control group | P value |
| Sex | 0.104 | ||
| Man | 30 | 22 | |
| Woman | 24 | 31 | |
| Age (years) | 52.1 ± 14.8 | 50.4 ± 15.8 | 0.962 |
| Etiology | 0.782 | ||
| Hepatitis B cirrhosis | 25 | 26 | |
| Hepatitis C cirrhosis | 5 | 6 | |
| Alcoholic cirrhosis | 10 | 7 | |
| Autoimmune cirrhosis | 5 | 3 | |
| Cholestatic cirrhosis | 4 | 2 | |
| Infection of the umbilical cord at birth | 5 | 8 | |
| Weight (kg) | 64.9 ± 8.6 | 70.6 ± 8.9 | 0.927 |
| Whether to cut the spleen | 0.368 | ||
| Yes | 13 | 15 | |
| No | 41 | 37 | |
| Height (cm) | 166.7 ± 6.6 | 169.5 ± 6.9 | 0.974 |
| Laboratory inspection | |||
| ALT (U/L) | 21.1 ± 14.1 | 25.7 ± 12.5 | 0.972 |
| AST (U/L) | 27.8 ± 18.4 | 30.9 ± 14.6 | 0.776 |
| PTA (%) | 64.4 ± 15.5 | 71.2 ± 14.3 | 0.566 |
| INR | 1.4 ± 0.5 | 1.3 ± 0.2 | 0.122 |
| Bilirubin (μmol/L) | 31.3 ± 21.7 | 22.9 ± 14.6 | 0.118 |
| Albumin (g/L) | 33.2 ± 5.1 | 35.6 ± 5.2 | 0.465 |
The survival rates for the SATIPS and control groups were 94.4% and 92.5% at 3 months (P value = 0.72) and 94.4% and 73.6% at 6 months (P value = 0.0051), respectively. The incidence of liver failure was 3.7% and 9.4% at 3 months (P value = 0.26) and 3.7% and18.9% at 6 months (P value = 0.016); the incidence of gastrointestinal bleeding was 5.6% and 37.7% at 3 months (P value < 0.001) and 9.3% and 47.2% (P value < 0.001) at 6 months; and the incidence of hepatic encephalopathy was 3.7% and 17.0% at 3 months (P value = 0.026) and 7.4% and 26.4% at 6 months (P value = 0.026) respectively. Notably, there were significant difference between the two groups (P value < 0.05; Tables 3 and 4, Figure 2).
| SATIPS group (%) | Control group (%) | P value | |
| Postoperative liver failure | |||
| 3 months | 3.7 | 9.4 | 0.26 |
| 6 months | 3.7 | 18.9 | 0.016 |
| Postoperative gastrointestinal rebleeding | |||
| 3 months | 5.6 | 37.7 | < 0.001 |
| 6 months | 9.3 | 47.2 | < 0.001 |
| Postoperative survival rate | |||
| 3 months | 94.4 | 92.5 | 0.72 |
| 6 months | 94.4 | 73.6 | 0.0051 |
| Postoperative hepatic encephalopathy | |||
| 3 months | 3.7 | 17.0 | 0.026 |
| 6 months | 7.4 | 26.4 | 0.026 |
| Parameter | Percentage (%) |
| Postoperative stent restenosis | |
| 3 months | 9.3 |
| 6 months | 13.0 |
| Number of stents | |
| One | 46.3 |
| Two | 53.7 |
In the SATIPS group, the preoperative IVC pressure was 6.6 ± 2.9 mmHg, significantly increasing to 10.1 ± 2.7 mmHg postoperatively (P value < 0.001). The preoperative portal pressure was 31.6 ± 5.1 mmHg, which significantly decreased to 18.4 ± 3.7 mmHg postoperatively (P value < 0.001). Additionally, the preoperative gradient of portal pressure was 24.8 ± 4.9 mmHg, which reduced to 8.4 ± 3.3 mmHg postoperatively (P value < 0.001; Table 5).
| Before operation | Post-operation | P value | |
| Inferior vena cava pressure | 6.6 ± 2.9 | 10.1 ± 2.7 | < 0.001 |
| Portal pressure | 31.6 ± 5.1 | 18.4 ± 3.7 | < 0.001 |
| PPG | 24.8 ± 4.9 | 8.4 ± 3.3 | < 0.001 |
Complications associated with surgery in the SATIPS group included: One case of abdominal wound infection and suppuration on the fifth postoperative day, stent stenosis on the seventh postoperative day increasing superior mesenteric vein pressure, subsequent rupture and bleeding of the superior mesenteric vein puncture site in one case. Furthermore, two cases experienced massive intra-abdominal hemorrhage occurred during the operation. In one case, the bleeding vessel was identified during the procedure and sutured to achieve hemostasis. In another case, the portal vein puncture site was suspected to be extrahepatic, and thus a covered stent was promptly placed to seal the bleeding site, successfully stopping the hemorrhage.
Cirrhosis is a chronic and progressive liver disease marked by widespread liver fibrosis, pseudolobular formation, and regenerated nodules. It is clinically characterized by portal hypertension and diminished hypohepatic function. Cirrhosis is caused by various factors, such as hepatitis B, hepatitis C, drug-induced damage, alcoholic liver disease, cholestatic conditions, autoimmune disorders, and Buga syndrome, among others. While portal hypertension can result from cirrhosis, it can also occur independently due to conditions[6].
Recently, the diagnosis of portal hypertension has become significantly more prevalent, and both healthcare professionals and patients are increasingly aware of this condition. Portal hypertension is characterized by elevated pressure in the portal vein due to hemodynamic changes in the portal system, which can lead to complications such as EGVB, refractory thoracic ascites, splenomegaly, and hypersplenism. The clinical manifestations are severe, posing risks to patient safety and adversely affecting the life quality[7]. Treatment options have expanded. For instance, EGVB can be managed through sclerotherapy and endoscopic variceal ligation, while percutaneous transhepatic variceal embolization is also a viable option[8,9]. Additionally, when combined with self-diversion techniques, ballon-occluded retrograde transvenous obliteration may be employed. A combination of diuretics, fluid intake regulation, catheterization, and drainage has been used to manage refractory hydrothorax and abdomen. For splenomegaly, conservative approaches may include regular use of medications aimed to boost white blood cell and platelet count or splenectomy. However, most of these interventions primarily address the symptoms rather than the underlying condition, and they do not alleviate portal vein pressure. To significantly lower portal vein pressure, you can further take TIPS or perform liver transplantation may be considered.
Most patients with portal hypertension prefer TIPS over liver transplantation due to its lower cost, making it more accessible for many families. TIPS provides an immediate reduction in portal pressure, which theoretically alleviates a range of symptoms associated with portal hypertension, although the extent of relief may vary among individuals[10,11]. The effectiveness of TIPS in managing EGVB is notable, with success rates exceeding 90%. While the effectiveness in treating refractory ascites is somewhat lower, it still achieves rates above 85%. Despite variations in symptom relief, most patients report a positive overall experience, characterized by reduced portal pressure, alleviated clinical symptoms, and an enhancement in both lifespan and quality of life. Therefore, TIPS is currently regarded as the primary treatment option for portal hypertension.
For portal vein thrombosis, treatment strategies differ based on the severity of the condition[12,13]. For mild portal vein thrombosis, where esophageal and gastric varices are not pronounced and there is no significant bleeding risk, anticoagulation may be considered[14,15]. Conversely, for severe portal vein thrombosis, particularly with recurrent EGVB and the presence of CTPV, TIPS may be contemplated[16-18]. However, it is noteworthy that CTPV alters the normal anatomy of the main portal vein and its branches, potentially affecting the structure of the superior mesenteric vein, which complicates the TIPS procedure and significantly increases the risk of intraoperative abdominal hemorrhage.
SATIPS offers a novel approach for patients with CTPV. This study compared the efficacy of SATIPS and non-surgical treatments in patients with portal vein spongiosis. The results indicated that SATIPS significantly enhanced survival rates and reduced the incidence of rebleeding within 6 months following treatment. During the 3-month observation period, there were no significant differences between the SATIPS group and the control group regarding the survival rate and incidence of post-treatment liver failure. However, during the 6-month observation period, patients in the SATIPS group had a higher survival rate and lower incidence of post-treatment liver failure. Moreover, the rebleeding of esophageal and gastric varices, as well as incidence of hepatic encephalopathy after treatment were significantly lower in the SATIPS group than in the control group at both the 3- and 6-month time points. In terms of long-term efficacy, SATIPS was associated with improved survival rates. Although there are certain surgical risks, the overall safety was controllable. In the future, researchers should aim to explore its efficacy in large-scale randomized clinical trial, and this technology should be optimized to promote its clinical absorption. As a challenging intervention for high-risk populations, SATIPS carries an inherent risk of complications. In this study, complications related to open abdominal surgery included abdominal wound infections, postoperative rupture and bleeding of the superior mesenteric vein, as well as severe intraoperative complications such as massive abdominal hemorrhage. Nevertheless, the patient's life was successfully salvaged through prompt and effective rescue measures. Compared to traditional TIPS, SATIPS increases the total hospitalization costs by 10%-20%, which is still affordable for most patients. Moreover, it generally extends the total hospital stay by 2-3 days compared to traditional TIPS. Compared to endoscopic treatment alone, SATIPS is undoubtedly more expensive, as the cost of TIPS surgery alone is higher than that of endoscopic sclerotherapy. However, the hospitalization time for SATIPS may not be necessarily longer because some patients experience suboptimal outcomes after endoscopic sclerotherapy and continue to suffer from recurrent bleeding, which prolongs their overall hospital stay. The high success rate, coupled with a low-risk profile, facilitated rapid patient recovery after surgery, bringing hope to individuals affected by portal vein spongiosis and portal hypertension.
SATIPS demonstrates unique advantages in treating portal vein spongiosis, characterized by a high rate of surgical success and a low rate of complications, while effectively alleviating the clinical symptoms. Regarding long-term efficacy, SATIPS can significantly reduce the rate of rebleeding, hepatic encephalopathy and liver failure, and is associated with better survival. The research findings have great guiding significance for clinical work.
| 1. | Bloom S, Kemp W, Lubel J. Portal hypertension: pathophysiology, diagnosis and management. Intern Med J. 2015;45:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Buob S, Johnston AN, Webster CR. Portal hypertension: pathophysiology, diagnosis, and treatment. J Vet Intern Med. 2011;25:169-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Rozenblit G, Del Guercio LR. Combined transmesenteric and transjugular approach for intrahepatic portosystemic shunt placement. J Vasc Interv Radiol. 1993;4:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Jalaeian H, Talaie R, D'Souza D, Taleb S, Noorbaloochi S, Flanagan S, Hunter D, Golzarian J. Minilaparotomy-Assisted Transmesenteric-Transjugular Intrahepatic Portosystemic Shunt: Comparison with Conventional Transjugular Approach. Cardiovasc Intervent Radiol. 2016;39:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Wang L, Peng Y, Qian Y, Liu Z, Du J, Meng Y, Feng L, Dai Z, Xu L. Transjugular extrahepatic portosystemic shunt (TEPS) creation in patients with complete occlusion of portal vein: Primary experience in a single medical center. Clin Res Hepatol Gastroenterol. 2022;46:101786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Guixé-Muntet S, Quesada-Vázquez S, Gracia-Sancho J. Pathophysiology and therapeutic options for cirrhotic portal hypertension. Lancet Gastroenterol Hepatol. 2024;9:646-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Shimizu T, Yoshioka M, Matsushita A, Ueda J, Kawashima M, Ono T, Kawano Y, Yoshida H. Esophagogastric Varix Caused by Extrahepatic Portal Vein Obstruction with Essential Thrombocythemia: A Case Report. J Nippon Med Sch. 2024;91:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Tranah TH, Nayagam JS, Gregory S, Hughes S, Patch D, Tripathi D, Shawcross DL, Joshi D. Diagnosis and management of ectopic varices in portal hypertension. Lancet Gastroenterol Hepatol. 2023;8:1046-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Zhang K, Sun X, Wang G, Zhang M, Wu Z, Tian X, Zhang C. Treatment outcomes of percutaneous transhepatic variceal embolization versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding. Medicine (Baltimore). 2019;98:e15464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Karagiannakis DS. Transjugular intrahepatic portosystemic shunt for recompensating decompensated cirrhosis? World J Gastroenterol. 2024;30:2621-2623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (2)] |
| 11. | de Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJC, Kramer M, Tjwa ETTL, Mostafavi N, Dijkgraaf MGW, van Delden OM, Beuers UHW, Coenraad MJ, Takkenberg RB. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. 2020;7:e000531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Hilscher MB, Wysokinski WE, Andrews JC, Simonetto DA, Law RJ, Kamath PS. Portal Vein Thrombosis in the Setting of Cirrhosis: Evaluation and Management Strategies. Gastroenterology. 2024;167:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Davis JPE, Lim JK, Francis FF, Ahn J. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2025;168:396-404.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | de Franchis R, editor. Portal Hypertension VII. Cham, Switzerland: Springer, 2022. [DOI] [Full Text] |
| 15. | Mendizabal M, Cançado GGL, Albillos A. Evolving portal hypertension through Baveno VII recommendations. Ann Hepatol. 2024;29:101180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 16. | Elkrief L, Hernandez-Gea V, Senzolo M, Albillos A, Baiges A, Berzigotti A, Bureau C, Murad SD, De Gottardi A, Durand F, Garcia-Pagan JC, Lisman T, Mandorfer M, McLin V, Moga L, Nery F, Northup P, Nuzzo A, Paradis V, Patch D, Payancé A, Plaforet V, Plessier A, Poisson J, Roberts L, Salem R, Sarin S, Shukla A, Toso C, Tripathi D, Valla D, Ronot M, Rautou PE; ERN RARE-LIVER and VALDIG, an EASL consortium. Portal vein thrombosis: diagnosis, management, and endpoints for future clinical studies. Lancet Gastroenterol Hepatol. 2024;9:859-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Meena BL, Sarin SK. Management of Portal vein Thrombosis in Cirrhosis. Semin Liver Dis. 2024;44:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Tadokoro T, Tani J, Manabe T, Takuma K, Nakahara M, Oura K, Mimura S, Fujita K, Nomura T, Morishita A, Kobara H, Himoto T, Ono M, Masaki T. Effectiveness of edoxaban in portal vein thrombosis associated with liver cirrhosis. Sci Rep. 2024;14:10784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |