Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.109285
Revised: May 30, 2025
Accepted: June 23, 2025
Published online: July 14, 2025
Processing time: 65 Days and 22.2 Hours
Tenofovir amibufenamide (TMF) has shown antiviral efficacy comparable to tenofovir disoproxil fumarate (TDF) in chronic hepatitis B (CHB), with improved renal and bone safety profiles. While TDF is recognized for its lipid-lowering properties, the long-term effects of TMF on lipid metabolism remain unclear.
To assess lipid changes and long-term safety of TMF in CHB, including outcomes after TDF-to-TMF switch over 144 weeks.
This retrospective analysis utilized data from a phase III randomized, double-blind trial involving 53 patients with CHB treated with either TMF 25 mg (n = 39) or TDF 300 mg (n = 14) once daily for 96 weeks. Following this blinded phase, all participants entered an open-label extension in which they received TMF until week 144. This design enabled assessment of both the comparative effects of TMF and TDF and the impact of switching from TDF to TMF, thereby reflecting real-world treatment scenarios. Virological, biochemical and imaging evaluations were performed throughout the study.
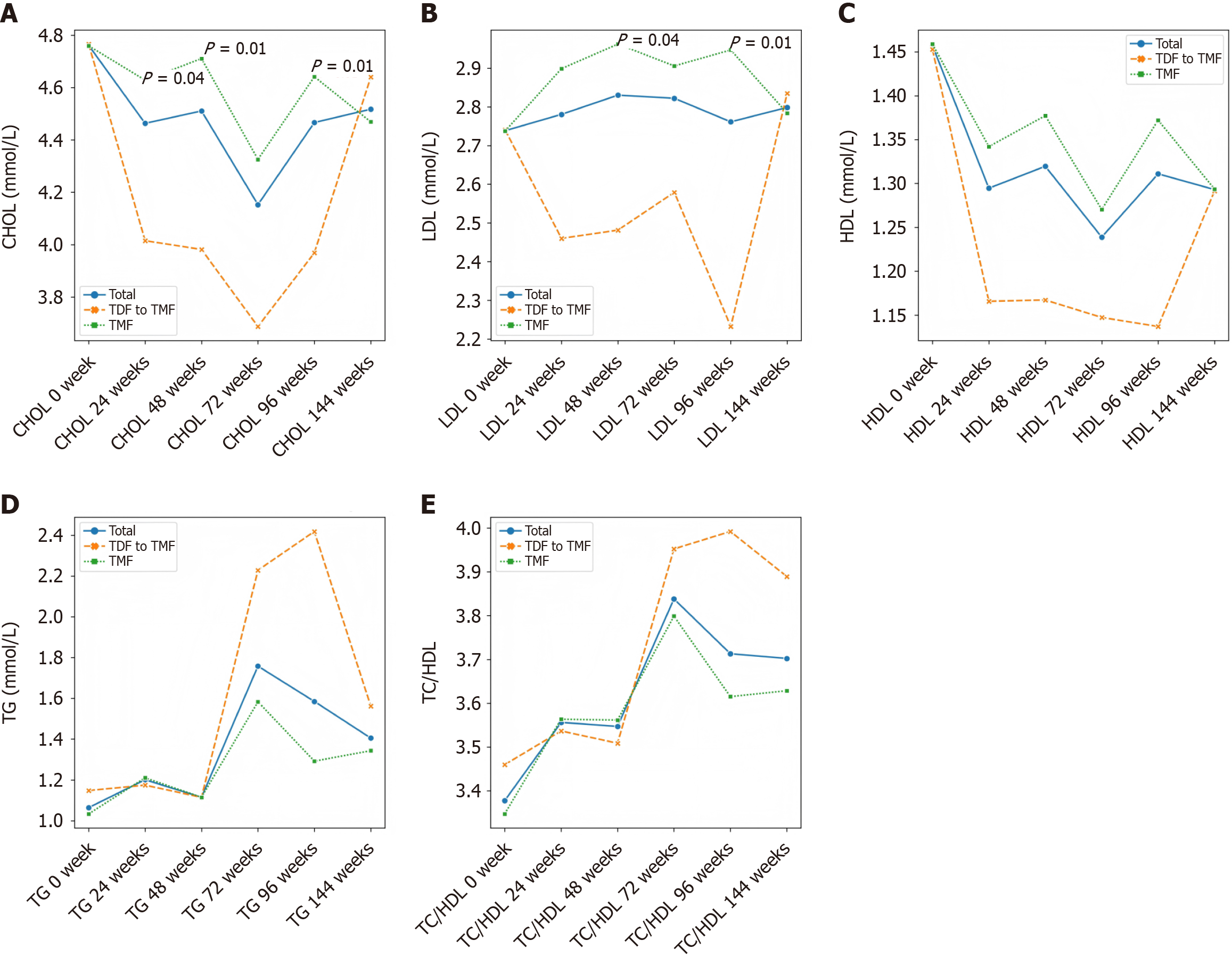
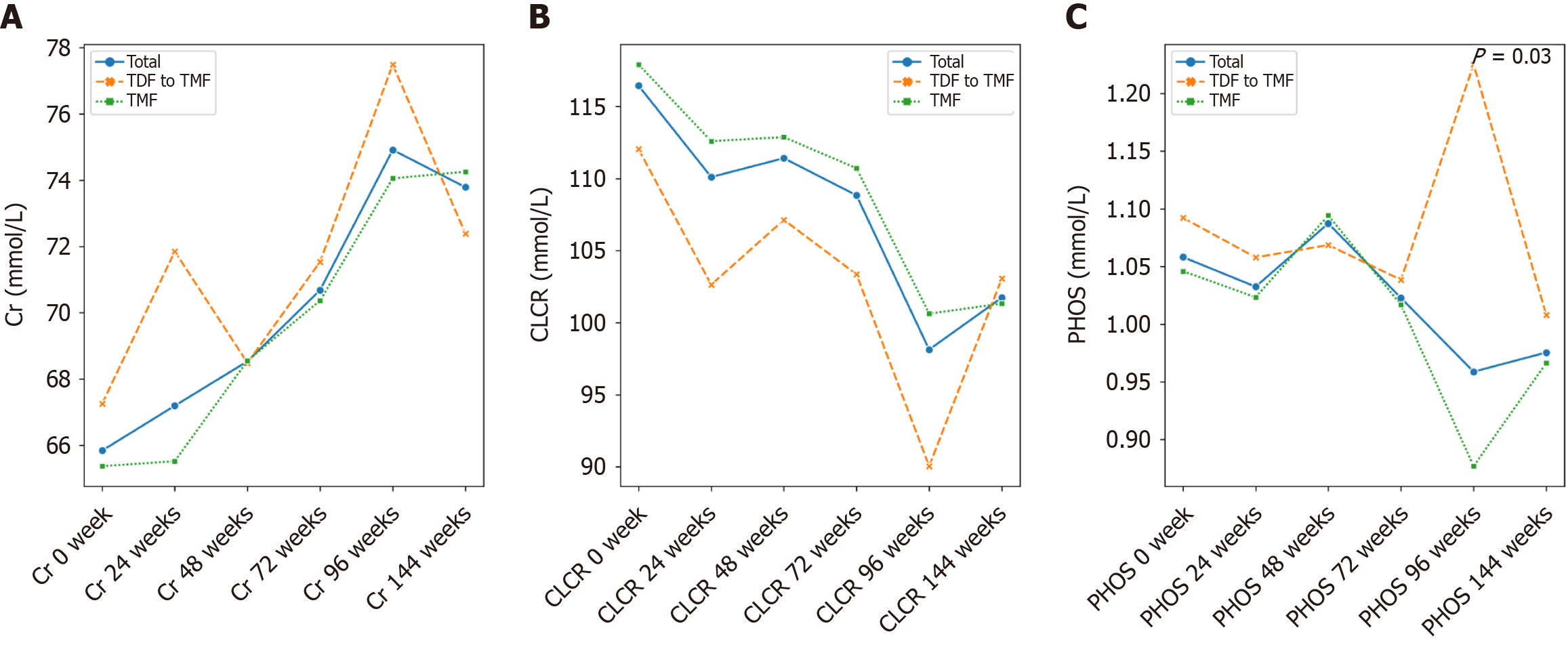
At week 96, both groups achieved comparable virological suppression and maintained stable hepatic and renal function. However, total cholesterol and low-density lipoprotein cholesterol levels were significantly higher in the TMF group compared to the TDF group (P = 0.012 and P = 0.040, respectively). TDF was associated with a transient increase in serum phosphate (P = 0.030). After switching to TMF, lipid profiles in the former TDF group gradually aligned with those of the continuous TMF group by week 144, with no lipid abnormalities observed in either group.
TMF provides sustained antiviral efficacy and maintains a favourable long-term lipid and renal safety profile. These findings support TMF as a viable first-line therapy and a switch option for CHB management in clinical practice.
Core Tip: This 144-week dual-phase study investigated the long-term lipid and safety profile of tenofovir amibufenamide (TMF) in chronic hepatitis B patients. Through a randomized, double-blind comparison with tenofovir disoproxil fumarate (TDF) followed by a TDF-to-TMF switch, the trial revealed comparable antiviral efficacy, improved renal and bone safety, and a moderate elevation in lipid levels with TMF that stabilized after switch therapy. These findings support TMF as a viable first-line and sequential therapy option in real-world chronic hepatitis B management.
- Citation: Zeng ZH, Liu JQ, Zhang M, Qiu CL, Xu ZY. Tenofovir amibufenamide in chronic hepatitis B: Lipid changes and 144-week safety with tenofovir disoproxil fumarate-to-tenofovir amibufenamide switch. World J Gastroenterol 2025; 31(26): 109285
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/109285.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.109285
Chronic hepatitis B (CHB) remains a major global health burden, contributing to approximately 55500 deaths annually[1]. Oral nucleoside analogs (NAs) effectively suppress hepatitis B virus (HBV) replication, thereby reducing the risk of liver injury, cirrhosis, hepatic failure and hepatocellular carcinoma (HCC) and improving long-term prognosis[2]. According to current Chinese guidelines, entecavir (ETV), tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF) and tenofovir amibufenamide (TMF) are recommended as first-line NAs for CHB management[3]. Although NAs offer durable antiviral efficacy, hepatitis B surface antigen (HBsAg) clearance is rare, necessitating prolonged or lifelong therapy. This raises concerns about long-term metabolic effects, particularly on bone mineral density, renal function and lipid metabolism.
ETV and TDF have both demonstrated efficacy in suppressing HBV, reversing liver fibrosis and reducing HCC incidence. ETV has a favourable safety profile and a low 5-year resistance rate of 1.2%[4]. TDF achieves higher virologic suppression and a lower risk of HCC than ETV but is associated with long-term adverse effects on bone and renal metabolism, as well as reductions in serum lipid levels[5].
TAF, a novel tenofovir prodrug, results in approximately 90% lower plasma tenofovir concentrations than TDF and is not renally eliminated. Clinical studies have shown that TAF offers comparable antiviral efficacy with improved bone and renal safety. However, its effects on lipid profiles remain debatable[6].
TMF is a newly developed tenofovir prodrug that differs from TAF by an additional methyl group, enhancing lipophilicity and cellular uptake. Preclinical studies suggest greater biological activity than TAF, and clinical trials have demonstrated similar antiviral efficacy with improved renal and bone safety compared to TDF. TMF was approved in China in June 2021. However, its impact on blood lipid profiles still needs to be explored. Therefore, we conducted this clinical trial to evaluate the effect of TMF on lipid profiles in CHB patients, with prolonged 144-week outcomes[3].
This retrospective analysis was based on a randomized, controlled phase III clinical trial conducted between July 18, 2018, and April 9, 2020. A total of 84 patients with confirmed CHB were randomized to receive either (TMF, 25 mg once daily) or TDF (300 mg once daily) for 96 weeks. At the end of the double-blind phase, all patients originally assigned to TDF were switched to TMF, and treatment continued through week 144 in an open-label extension.
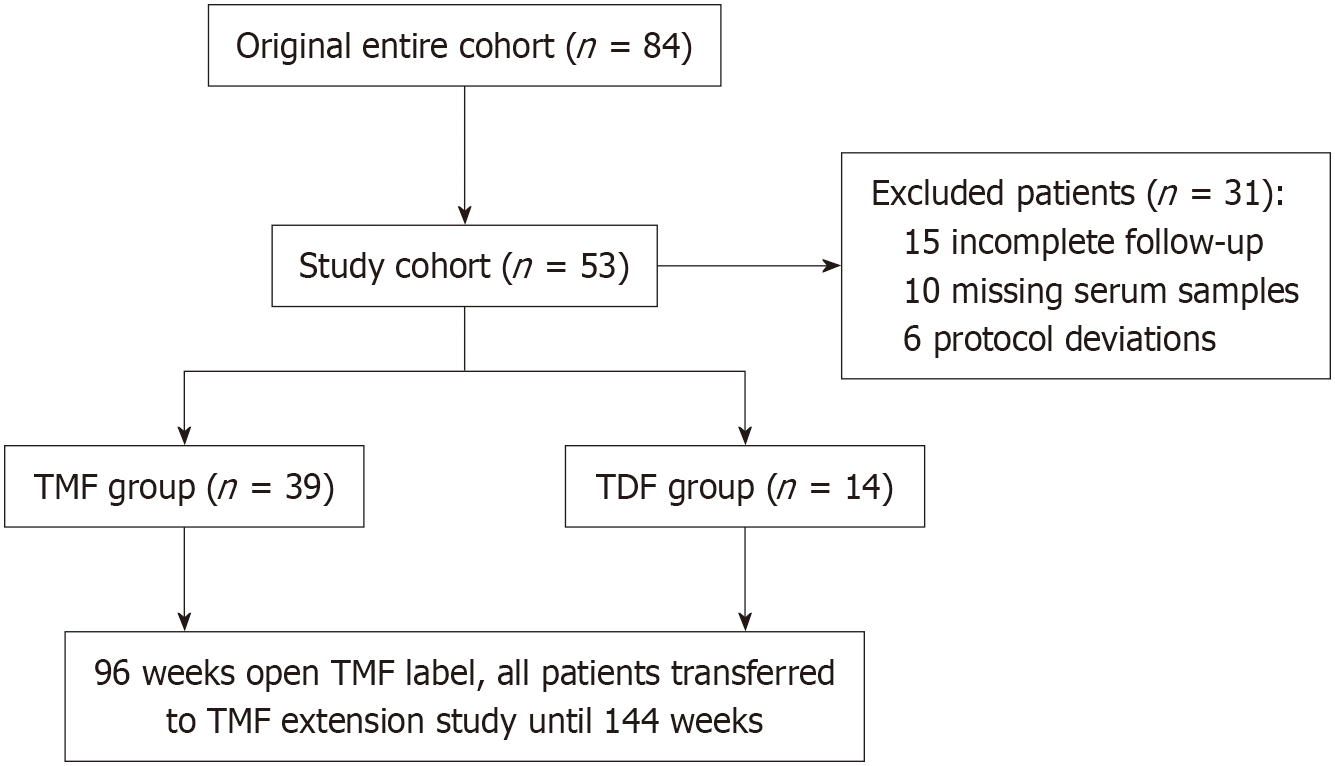
Of the 84 initially enrolled patients, 31 were excluded due to incomplete follow-up (n = 15), missing serum samples (n = 10), or protocol deviations (n = 6), leaving 53 patients for the final analysis. The details are shown in Figure 1.
Inclusion criteria: (1) Male participants aged 18-65 years or non-pregnant, non-lactating females of childbearing potential with a negative serum pregnancy test; (2) Chronic HBV infection, defined as HBsAg positivity for ≥ 6 months; (3) Hepatitis B e antigen (HBeAg)-positive or HBeAg-negative with HBV DNA ≥ 2 × 104 IU/mL and alanine aminotransferase (ALT) > 1 × upper limit of normal (ULN) but ≤ 10 × ULN at screening; and (4) Either treatment-naive (< 12 weeks of prior NA therapy) or treatment-experienced ( ≥ 12 weeks), provided other eligibility criteria were met.
Exclusion criteria: (1) Pregnancy, lactation, or intent to conceive; (2) Coinfection with hepatitis C virus, human immunodeficiency virus or hepatitis delta virus; (3) Imaging-confirmed HCC; (4) Decompensated liver disease (e.g., ascites, hepatic encephalopathy, variceal bleeding); (5) Abnormal haematologic or biochemical values [e.g., haemoglobin < 10 g/dL, platelets ≤ 50 × 109/L, ALT or aspartate aminotransferase (AST) > 10 × ULN, total bilirubin > 2.5 × ULN, albumin < 3.0 g/dL]; (6) History of solid organ or bone marrow transplantation; (7) Significant comorbidities affecting renal, cardiovascular, or neurological function; (8) Severe bone disease or malignancy within the past five years; (9) Use of immunomodulatory or nephrotoxic medications; (10) Known hypersensitivity to study drugs or their components; and (11) Substance abuse likely to affect treatment adherence.
The trial was registered at ClinicalTrials.gov (No. NCT03903796) and conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Ethics Committee of the Second Xiangya Hospital, and all procedures complied with relevant guidelines and regulations.
Demographic variables collected included age, sex, body mass index and the initiation date of antiviral therapy. Fasting lipid profiles were obtained for total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL), measured using standardized enzymatic assays. Serum samples were collected after a minimum 12-hour fast. Additional laboratory assessments included HBV-DNA, HBV-RNA, HBV-microRNA-3, HBsAg, HBeAg, haemoglobin, white blood cell count, platelet count, serum phosphate, AST, ALT, uric acid, creatinine, creatinine clearance rate and fasting blood glucose. Liver stiffness measurement and the fibrosis-4 index were assessed via transient elastography. All data were collected and analyzed by the Hepatology Institute of the Second Xiangya Hospital, Central South University. This retrospective study received ethical approval from the Ethics Committee of the Second Xiangya Hospital, Central South University.
Clinical raw data were collected and organized in Microsoft Excel and then imported into Python 3.8 for statistical analysis. Continuous variables were presented as mean ± SE. For normally distributed data, Student’s t-test was used for two-group comparisons, while one-way analysis of variance (ANOVA) was used for comparisons among multiple groups. Categorical variables were summarized as frequencies (percentages) and analyzed using the χ2 test. Student’s t-test, one-way ANOVA, and the χ2 test were conducted using the scipy.stats module. Dynamic changes in each indicator were visualized using the seaborn module to plot curves. Pearson correlation coefficient (R value) was calculated to evaluate associations between variables. Scatter plots and regression lines were used to illustrate relationships between liver fibrosis markers. A significance level of P < 0.05 was considered statistically significant, with a test level of α = 0.05.
Of the 53 enrolled patients, the mean age was 36.89 years, and 67.9% were male. No statistically significant differences (P > 0.05) were observed between the TDF and TMF groups in baseline laboratory parameters, including lipid profile (TC, TG, HDL and LDL), HBV-DNA, HBV-RNA, HBV-microRNA-3, HBsAg, HBeAg, or other laboratory indices. Detailed baseline characteristics are provided in Table 1.
| Mark | Total (n = 53) | TDF (n = 14) | TMF (n = 39) | P value |
| Gender (M/F) | 36/17 | 8/6 | 28/11 | 0.314 |
| Age | 36.89 ± 1.44 | 34.50 ± 1.95 | 37.74 ± 1.83 | 0.327 |
| BMI 0 week | 22.83 ± 0.67 | 22.01 ± 1.02 | 23.16 ± 0.85 | 0.448 |
| WBC 0 week | 6.13 ± 0.22 | 6.27 ± 0.45 | 6.08 ± 0.26 | 0.715 |
| Hb 0 week | 149.02 ± 2.06 | 147.79 ± 3.88 | 149.46 ± 2.45 | 0.723 |
| PLT 0 week | 214.62 ± 10.01 | 234.00 ± 15.58 | 207.67 ± 12.31 | 0.250 |
| ALT 0 week | 129.10 ± 17.09 | 176.42 ± 53.02 | 112.12 ± 13.01 | 0.097 |
| AST 0 week | 79.79 ± 9.70 | 99.85 ± 28.31 | 73.10 ± 8.91 | 0.236 |
| Cr 0 week | 65.84 ± 2.08 | 67.25 ± 5.17 | 65.37 ± 2.22 | 0.699 |
| CLCR 0 week | 116.45 ± 3.46 | 112.04 ± 7.11 | 117.91 ± 3.98 | 0.468 |
| PHOS 0 week | 1.06 ± 0.03 | 1.09 ± 0.07 | 1.05 ± 0.03 | 0.459 |
| GLU 0 week | 4.97 ± 0.11 | 4.73 ± 0.11 | 5.06 ± 0.14 | 0.176 |
| TG 0 week | 1.06 ± 0.07 | 1.15 ± 0.20 | 1.03 ± 0.06 | 0.477 |
| CHOL 0 week | 4.76 ± 0.15 | 4.77 ± 0.20 | 4.76 ± 0.19 | 0.984 |
| HDL 0 week | 1.46 ± 0.05 | 1.45 ± 0.10 | 1.46 ± 0.06 | 0.960 |
| LDL 0 week | 2.74 ± 0.11 | 2.74 ± 0.17 | 2.74 ± 0.14 | 0.989 |
| FIB-4 0 week | 1.46 ± 0.18 | 1.28 ± 0.29 | 1.52 ± 0.22 | 0.576 |
| CAP 0 week | 204.50 ± 5.74 | 212.07 ± 13.44 | 201.38 ± 5.99 | 0.403 |
| RNA 0 week | 4.14 ± 0.39 | 3.99 ± 0.86 | 4.18 ± 0.44 | 0.845 |
| DNA 0 week | 6.81 ± 0.17 | 7.20 ± 0.28 | 6.67 ± 0.21 | 0.181 |
| miR3 0 week | 5.38 ± 0.29 | 5.33 ± 0.60 | 5.40 ± 0.34 | 0.919 |
| HBsAg 0 week | 2.94 ± 0.12 | 3.12 ± 0.25 | 2.88 ± 0.14 | 0.381 |
| HBeAg 0 week | 0.98 ± 0.27 | 1.33 ± 0.54 | 0.85 ± 0.31 | 0.441 |
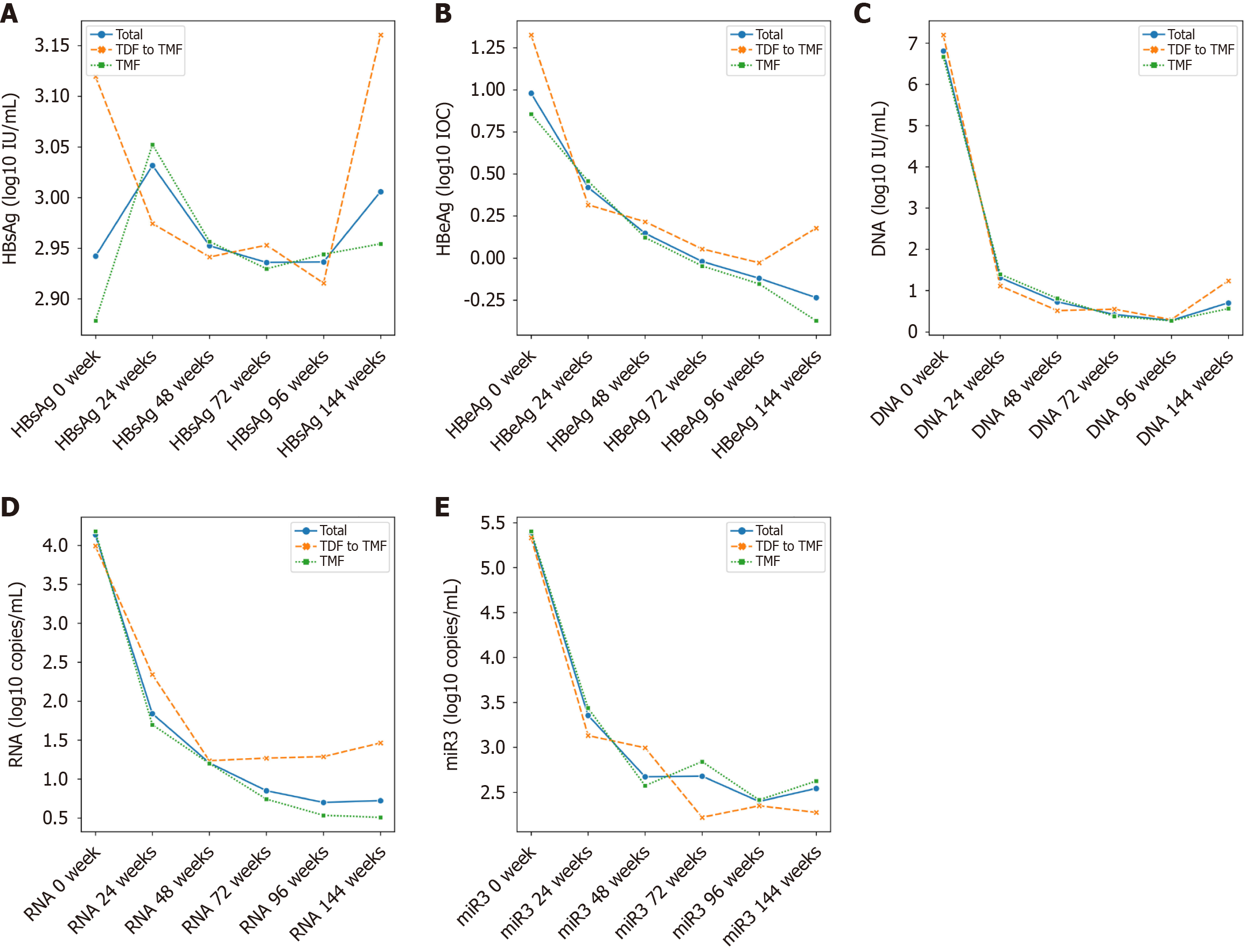
After 96 weeks of treatment, both treatment groups demonstrated significant suppression of HBV replication, as reflected by significant reductions in HBV-DNA, HBV-RNA and HBV-microRNA-3 levels from baseline (Tables 2 and 3). No significant differences were observed between the TDF and TMF groups in HBV-DNA, HBV-RNA, HBV-microRNA-3, HBsAg, or HBeAg levels. The details are shown in Table 4 and Figure 2.
| TMF | 0 week | 96 weeks | (0 week vs 96 weeks) |
| WBC | 6.08 ± 0.26 | 6.53 ± 0.28 | 0.247149198 |
| Hb | 149.46 ± 2.45 | 149.21 ± 2.78 | 0.944983486 |
| PLT | 207.67 ± 12.31 | 213.10 ± 11.67 | 0.74947324 |
| ALT | 112.12 ± 13.01 | 23.48 ± 2.31 | 3.09 × 10-9 |
| AST | 73.10 ± 8.91 | 23.22 ± 1.21 | 4.05 × 10-7 |
| Cr | 65.37 ± 2.22 | 74.05 ± 2.12 | 0.006031745 |
| CLCR | 117.91 ± 3.98 | 100.63 ± 3.44 | 0.001543066 |
| PHOS | 1.05 ± 0.03 | 0.88 ± 0.02 | 1.82 × 10-5 |
| GLU | 5.06 ± 0.14 | 5.65 ± 0.42 | 0.186321806 |
| TG | 1.03 ± 0.06 | 1.29 ± 0.13 | 0.069367579 |
| CHOL | 4.76 ± 0.19 | 4.64 ± 0.14 | 0.613234644 |
| HDL | 1.46 ± 0.06 | 1.37 ± 0.07 | 0.35635419 |
| LDL | 2.74 ± 0.14 | 2.95 ± 0.13 | 0.276270192 |
| FIB-4 | 1.52 ± 0.22 | 1.28 ± 0.24 | 0.475793108 |
| CAP | 201.38 ± 5.99 | 202.80 ± 6.26 | 0.870684054 |
| miR3 | 5.40 ± 0.34 | 2.41 ± 0.42 | 4.27 × 10-7 |
| RNA | 4.18 ± 0.44 | 0.53 ± 0.23 | 5.19 × 10-10 |
| DNA | 6.67 ± 0.21 | 0.27 ± 0.16 | 1.69 × 10-28 |
| HBsAg | 2.88 ± 0.14 | 2.94 ± 0.10 | 0.696890055 |
| HBeAg | 0.85 ± 0.31 | -0.15 ± 0.18 | 0.005855419 |
| TDF | 0 week | 96 weeks | (0 week vs 96 weeks) |
| WBC | 6.27 ± 0.45 | 6.87 ± 0.63 | 0.443861557 |
| Hb | 147.79 ± 3.88 | 147.50 ± 5.80 | 0.967641272 |
| PLT | 234.00 ± 15.58 | 239.14 ± 11.38 | 0.791953625 |
| ALT | 176.42 ± 53.02 | 20.62 ± 3.63 | 0.00922277 |
| AST | 99.85 ± 28.31 | 22.75 ± 1.15 | 0.011908537 |
| Cr | 67.25 ± 5.17 | 77.49 ± 5.63 | 0.192832703 |
| CLCR | 112.04 ± 7.11 | 90.04 ± 6.85 | 0.036435267 |
| PHOS | 1.09 ± 0.07 | 1.23 ± 0.27 | 0.611661657 |
| GLU | 4.73 ± 0.11 | 4.77 ± 0.14 | 0.80941735 |
| TG | 1.15 ± 0.20 | 2.42 ± 1.32 | 0.332101848 |
| CHOL | 4.77 ± 0.20 | 3.97 ± 0.19 | 0.008550427 |
| HDL | 1.45 ± 0.10 | 1.14 ± 0.10 | 0.033296713 |
| LDL | 2.74 ± 0.17 | 2.23 ± 0.17 | 0.046729094 |
| FIB-4 | 1.28 ± 0.29 | 1.12 ± 0.39 | 0.742777384 |
| CAP | 212.07 ± 13.44 | 185.17 ± 6.02 | 0.09703147 |
| miR3 | 5.33 ± 0.60 | 2.35 ± 0.69 | 0.003107141 |
| RNA | 3.99 ± 0.86 | 1.29 ± 0.68 | 0.024764947 |
| DNA | 7.20 ± 0.28 | 0.29 ± 0.29 | 4.24 × 10-12 |
| HBsAg | 3.12 ± 0.25 | 2.92 ± 0.13 | 0.473322675 |
| HBeAg | 1.33 ± 0.54 | -0.03 ± 0.32 | 0.040841537 |
| 96 weeks | TDF | TMF | P value |
| WBC | 6.87 ± 0.63 | 6.53 ± 0.28 | 0.578 |
| Hb | 147.50 ± 5.80 | 149.21 ± 2.78 | 0.769 |
| PLT | 239.14 ± 11.38 | 213.10 ± 11.67 | 0.214 |
| ALT | 20.62 ± 3.63 | 23.48 ± 2.31 | 0.531 |
| AST | 22.75 ± 1.15 | 23.22 ± 1.21 | 0.830 |
| Cr | 77.49 ± 5.63 | 74.05 ± 2.12 | 0.485 |
| CLCR | 90.04 ± 6.85 | 100.63 ± 3.44 | 0.152 |
| PHOS | 1.23 ± 0.27 | 0.88 ± 0.02 | 0.026 |
| GLU | 4.77 ± 0.14 | 5.65 ± 0.42 | 0.236 |
| TG | 2.42 ± 1.32 | 1.29 ± 0.13 | 0.163 |
| CHOL | 3.97 ± 0.19 | 4.64 ± 0.14 | 0.011 |
| HDL | 1.14 ± 0.10 | 1.37 ± 0.07 | 0.077 |
| LDL | 2.23 ± 0.17 | 2.95 ± 0.13 | 0.005 |
| FIB-4 | 1.12 ± 0.39 | 1.28 ± 0.24 | 0.739 |
| CAP | 185.17 ± 6.02 | 202.80 ± 6.26 | 0.105 |
| miR3 | 2.35 ± 0.69 | 2.41 ± 0.42 | 0.937 |
| RNA | 1.29 ± 0.68 | 0.53 ± 0.23 | 0.186 |
| DNA | 0.29 ± 0.29 | 0.27 ± 0.16 | 0.936 |
| HBsAg | 2.92 ± 0.13 | 2.94 ± 0.10 | 0.874 |
| HBeAg | -0.03 ± 0.32 | -0.15 ± 0.18 | 0.724 |
Lipid parameters remained stable in the TMF group over 96 weeks (Table 2). In contrast, patients receiving TDF showed significant reductions in TG, TC and LDL levels (Table 3). By week 24, a significant difference in TC was noted between the two groups, and by week 96, both TC and LDL levels were significantly lower in the TDF group compared to the TMF group (P = 0.01) (Figure 3 and Table 4).
After the week 48 switch from TDF to TMF, all patients continued TMF 25 mg once daily until week 144. Lipid levels (TG, HDL, TC, LDL) were monitored at weeks 72, 96 and 144. No significant changes were observed in lipid parameters compared to baseline, and no cases of dyslipidemia were reported during TMF treatment (Table 5).
| 144 weeks | TDF to TMF | TMF | P value |
| WBC | 6.20 ± 0.37 | 6.48 ± 0.27 | 0.558 |
| Hb | 145.57 ± 4.64 | 148.31 ± 2.92 | 0.764 |
| PLT | 240.50 ± 12.49 | 224.31 ± 12.05 | 0.405 |
| ALT | 32.71 ± 10.93 | 28.27 ± 5.24 | 0.257 |
| AST | 24.91 ± 3.67 | 25.01 ± 2.14 | 0.845 |
| Cr | 72.38 ± 4.95 | 74.26 ± 2.26 | 0.414 |
| CLCR | 103.07 ± 6.79 | 101.33 ± 3.50 | 0.154 |
| PHOS | 1.01 ± 0.04 | 0.97 ± 0.03 | 0.485 |
| GLU | 5.15 ± 0.17 | 5.82 ± 0.36 | 0.887 |
| TG | 1.56 ± 0.38 | 1.34 ± 0.14 | 0.904 |
| CHOL | 4.64 ± 0.23 | 4.47 ± 0.16 | 0.561 |
| HDL | 1.29 ± 0.12 | 1.29 ± 0.06 | 0.937 |
| LDL | 2.83 ± 0.22 | 2.78 ± 0.13 | 0.220 |
| FIB-4 | 15.38 ± 14.59 | 1.08 ± 0.13 | 0.739 |
| CAP | 182.22 ± 9.02 | 189.22 ± 7.8 | 0.175 |
| miR3 | 2.27 ± 0.72 | 2.62 ± 0.44 | 0.896 |
| RNA | 1.46 ± 0.79 | 0.51 ± 0.25 | 0.986 |
| DNA | 1.24 ± 0.76 | 0.56 ± 0.33 | 0.531 |
| HBsAg | 3.16 ± 0.17 | 2.95 ± 0.10 | 0.849 |
| HBeAg | 0.18 ± 0.39 | -0.37 ± 0.18 | 0.865 |
NAs inhibit HBV replication by blocking the reverse transcription of pre-genomic RNA into HBV DNA and are widely used for the treatment of chronic HBV infection globally[2,7]. However, NAs do not eliminate covalently closed circular DNA from infected hepatocyte nuclei[8]. Most patients experience viral rebound upon treatment discontinuation, necessitating long-term or even lifelong therapy, posing substantial medical and economic burdens. The long-term safety of NAs has become a growing concern, particularly due to TDF’s potential nephrotoxic effects[9]. TAF, a prodrug of tenofovir with improved renal and bone safety profiles, has demonstrated non-inferior antiviral efficacy compared to TDF in patients with CHB[10]. However, emerging evidence suggests that patients who switch from TDF to TAF may experience weight gain and increased fasting LDL cholesterol, raising concerns about the potential impact of TAF on lipid metabolism[11].
TMF, a methylated derivative of TAF, offers enhanced plasma stability and lipid solubility[12]. In a 24-week clinical trial, TMF yielded a superior virological response in treatment-naive patients with CHB compared to TAF (92% vs 74%, P = 0.033), without adverse effects on renal function or lipid profiles[13]. Our findings align with this conclusion (Figure 4). After 96 weeks of treatment, both TMF and TDF groups achieved significant virological suppression and normalization of ALT and AST levels relative to baseline (Tables 2 and 3). Furthermore, although no clinical abnormalities were observed, a reduction in creatinine clearance rate in the TDF group may indicate early renal impairment, underscoring the need for long-term safety monitoring.
In this study, we analyzed real-world data from patients with CHB who received either TMF or TDF for 96 weeks, followed by TMF treatment up to 144 weeks. During the initial 96-week period, significant differences in lipid profiles were observed between the two groups at several time points. Specifically, TC levels were significantly lower in the TDF group at weeks 24, 48 and 96, and LDL levels were also significantly lower at weeks 48 and 96 compared to the TMF group (Supplementary Table 1). However, by week 144, no statistically significant differences in lipid parameters were observed between the groups. Although the early lipid-lowering effects of TDF reached statistical significance, they appeared to have a limited long-term impact and lacked clear clinical relevance, as the lipid profiles converged over time. This suggests that TDF’s transient influence on lipid metabolism does not translate into a sustained or meaningful reduction in cardiovascular risk. These findings are consistent with previous studies reporting no significant difference in the TC to HDL cholesterol (TC/HDL) ratio between TDF and TAF treatment groups, as reductions in TC and HDL levels occurred concurrently in the TDF group. TDF has been reported to modulate serum cholesterol levels through the upregulation of the cluster of differentiation 36/peroxisome proliferators-activated receptor-α axis[14]. However, the molecular mechanism by which TDF treatment regulates HDL levels still requires further investigation. While the mean levels of LDL and TG also showed a decreasing trend during TDF treatment, these differences did not reach statistical significance, possibly due to the limited sample size. Notably, our results revealed a significant reduction in HDL levels during the 96-week TDF treatment period, suggesting that TDF may exert a broader inhibitory effect on lipid metabolic pathways. Plasma lipid parameters are widely recognized as important indicators for assessing cardiovascular risk. According to a recent large-scale epidemiological study, the TC/HDL ratio is considered a more reliable predictor of cardiovascular events than LDL levels alone[15]. Specifically, adding LDL to the TC/HDL ratio does not enhance its predictive value. In our cohort, we also observed no significant difference in the TC/HDL ratio between patients treated with TDF and those treated with TAF for 48 weeks, further supporting the notion that the lipid-modulating effects of these therapies may not substantially alter cardiovascular risk profiles.
After 96 weeks, all patients were switched to TMF 25 mg once daily and continued treatment until week 144, with lipid parameters monitored at weeks 72, 96 and 144. The results showed no statistically significant differences in TG, HDL, cholesterol and LDL levels compared to baseline. Additionally, no patients experienced lipid abnormalities during TMF treatment.
These findings suggest that TMF effectively maintains stable blood lipid levels over the long-term and does not induce dyslipidemia. This may be particularly beneficial for individuals with underlying lipid metabolism concerns, as maintaining stable lipid profiles is essential for cardiovascular health. Moreover, the results underscore the potential safety and efficacy of TMF in preserving lipid homeostasis, supporting its broader clinical application in the management of CHB. However, further research with larger cohorts and extended follow-up is warranted to confirm these findings and to fully elucidate the long-term metabolic safety profile of TMF, which could support further clinical applications[2,16,17]. TDF and TMF are widely used as first-line NAs for antiviral therapy in CHB[18]. However, current evidence regarding the effects of TMF on lipid metabolism is limited, and findings related to TAF remain inconclusive. Moreover, the molecular mechanisms by which NAs influence lipid metabolism are not yet fully understood. Therefore, multicenter, large-scale prospective studies are needed to clarify the metabolic impact of antiviral therapies, elucidate their underlying biological mechanisms and assess their long-term clinical significance.
Our findings indicate that TMF offers antiviral efficacy comparable to TDF while maintaining stable lipid profiles, suggesting it may be a safer alternative for patients requiring lifelong therapy, particularly those with pre-existing metabolic risk factors. Although TDF is associated with lipid-lowering effects, its long-term use is limited by concerns related to renal and bone toxicity. In contrast, TMF achieves effective viral suppression without inducing the lipid-lowering effects that may be undesirable in patients with normal or low baseline lipid levels.
This 144-week study further supports the long-term safety and efficacy of TMF, demonstrating that it sustains virological suppression while preserving lipid homeostasis. The transient lipid-lowering effects observed with TDF were not maintained following the switch to TMF, highlighting TMF as a potentially more suitable option for patients requiring prolonged antiviral treatment without compromising metabolic health.
Nonetheless, this study has certain limitations, most notably the relatively small sample size, which may limit the statistical power to detect subtle differences in lipid profiles. Future multicenter studies with larger and more diverse cohorts are warranted to validate and extend these findings.
This study supports TMF as a first-line antiviral agent for CHB, offering a favorable balance of virological efficacy, renal safety, and lipid profile stability, especially for long-term therapy.
We thank the clinical staff at the Second Xiangya Hospital for their assistance with patient management and data collection. We also acknowledge the statistical support provided by the Department of Public Health, Central South University.
| 1. | Wang Y, Li J, Wang S, Pang Y, Liu P, Xie B, Dou S, Yang T, Liu X, Shi Y, Chen D. The hepatitis B virus promotes the progression of non-alcoholic fatty liver disease through incomplete autophagy. Free Radic Biol Med. 2023;204:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Hwang EG, Jung EA, Yoo JJ, Kim SG, Kim YS. Risk of dyslipidemia in chronic hepatitis B patients taking tenofovir alafenamide: a systematic review and meta-analysis. Hepatol Int. 2023;17:860-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Liu Z, Jin Q, Zhang Y, Gong G, Wu G, Yao L, Wen X, Gao Z, Huang Y, Yang D, Chen E, Mao Q, Lin S, Shang J, Gong H, Zhong L, Yin H, Wang F, Hu P, Wu Q, Pan C, Jia W, Li C, Sun C, Niu J, Hou J; TMF Study Group. 96-Week Treatment of Tenofovir Amibufenamide and Tenofovir Disoproxil Fumarate in Chronic Hepatitis B Patients. J Clin Transl Hepatol. 2023;11:649-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Shaheen AA, AlMattooq M, Yazdanfar S, Burak KW, Swain MG, Congly SE, Borman MA, Lee SS, Myers RP, Coffin CS. Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers. Aliment Pharmacol Ther. 2017;46:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Zhang Q, Liang J, Yin J, Jiang Y, Yu N, Liao X, Zhao S, Wu L, Fan R. Real-life impact of tenofovir disoproxil fumarate and entecavir therapy on lipid profile, glucose, and uric acid in chronic hepatitis B patients. J Med Virol. 2022;94:5465-5474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Sun YT, Chen QQ. Review on article of effects of tenofovir alafenamide and entecavir in chronic hepatitis B virus patients. World J Hepatol. 2024;16:109-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Wang H, Nam SY, Jo J. Effect of chronic viral hepatitis and metabolic factors on renal cancer risk in a large cohort in Republic of Korea. Prev Med. 2023;175:107714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Ailioaie LM, Litscher G. Curcumin and Photobiomodulation in Chronic Viral Hepatitis and Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:7150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Yim HJ, Seo YS, Kim JH, Kim W, Jung YK, Jang JY, Lee SH, Kim YS, Kim CW, Kim HS, Shim JJ, Cho EY, Kim IH, Lee BS, Lee JH, Kim BS, Jang JW, Lee HW, Kwon JH, Kim MY, Song DS, Park JG, Lee YS, Yoon EL, Lee HA, Kang SH, Yang JM. Switching to Besifovir in Patients with Chronic Hepatitis B Receiving Tenofovir Disoproxil Fumarate: A Randomized Trial. Clin Mol Hepatol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Akdemir Kalkan I, Karasahin O, Sarigul F, Altunisik Toplu S, Aladag M, Akgul F, Mete AO, Golbol A, Nazik S, Kömür S, Merve Oren M, Yildiz Y, Demir Y, Ayhan M, Tasova Y, Bayındır Y, Dal T, Celen MK. Comparison of Tenofovir Alafenamide and Entecavir Therapy in Patients with Chronic Hepatitis B Initially Treated with Tenofovir Disoproxil: A Retrospective Observational Survey. Hepat Mon. 2022;21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Ogawa E, Nakamuta M, Koyanagi T, Ooho A, Furusyo N, Kajiwara E, Dohmen K, Kawano A, Satoh T, Takahashi K, Azuma K, Yamashita N, Yamashita N, Sugimoto R, Amagase H, Kuniyoshi M, Ichiki Y, Morita C, Kato M, Shimoda S, Nomura H, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Switching to tenofovir alafenamide for nucleos(t)ide analogue-experienced patients with chronic hepatitis B: week 144 results from a real-world, multi-centre cohort study. Aliment Pharmacol Ther. 2022;56:713-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Peng WT, Jiang C, Yang FL, Zhou NQ, Chen KY, Liu JQ, Peng SF, Fu L. Tenofovir amibufenamide vs tenofovir alafenamide for treating chronic hepatitis B: A real-world study. World J Gastroenterol. 2023;29:5907-5918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (2)] |
| 13. | Li L, Zhou J, Li Y, Wang F, Zhang D, Wang M, Tao Y, Chen E. Effectiveness and safety of tenofovir amibufenamide and its comparison with tenofovir alafenamide in patients with chronic hepatitis B: results from a retrospective real-world study. Front Pharmacol. 2023;14:1165990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Nakamura A, Miyoshi H, Kimura M, Maehara O, Yamada R, Kitagataya T, Yamamoto K, Shigesawa T, Nakamura A, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Sakamoto N; NORTE Study Group. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J Gastroenterol. 2021;56:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 15. | Zhou YG, Tian N, Xie WN. Total cholesterol to high-density lipoprotein ratio and nonalcoholic fatty liver disease in a population with chronic hepatitis B. World J Hepatol. 2022;14:791-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Qiu X, Yin Y, Zhang S, Liu W. Effectiveness and safety of tenofovir amibufenamide and tenofovir alafenamide in treating elderly patients diagnosed with decompensated hepatitis B cirrhosis: a retrospective cohort study. Front Pharmacol. 2025;16:1545108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Ma HN, Cao KS, Liu YM, Chen C, Zhang H, Tang FS. Tenofovir amibufenamide: A potential alternative for chronic hepatitis B treatment. World J Gastroenterol. 2025;31:102580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (1)] |
| 18. | Li Y, Lin Y, Gou G, Cui D, Gao X, Xu G, Zu H, Dang S. Effectiveness and Safety of Tenofovir Amibufenamide in the Treatment of Chronic Hepatitis B: A Real-world, Multicenter Study. J Clin Transl Hepatol. 2025;13:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/