Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.107044
Revised: May 5, 2025
Accepted: June 13, 2025
Published online: July 14, 2025
Processing time: 119 Days and 15.3 Hours
Rotavirus (RV), a primary cause of diarrhea-related mortality in 2021, has been shown to damage intestinal epithelial cells while upregulating intestinal stem cells (ISCs) activities. ISCs within the crypt niche drive the continuous self-renewal of intestinal epithelium, preserving its barrier functions. Paneth cells secrete antimicrobial peptide and signaling molecules within the intestine crypt, thereby playing a crucial role in intestinal immune defense and providing ISCs functional support. However, the regulatory function of Paneth cells under pathological conditions, such as RV infection, remains unclear.
To determine the impact of RV infection on Paneth cells and how Paneth cells regulate ISCs during intestinal injury repair.
We constructed a reference genome for the RV enteric cytopathogenic human orphan virus strain and reanalyzed published single-cell RNA sequencing data to investigate Paneth cell responses to RV-induced intestinal injury. We derived Paneth-ISC communication networks using CellChat, tracked ISC differentiation with pseudotime analysis, and validated our findings in leucine-rich repeat-containing G protein-coupled receptor 5-enhanced green fluorescent protein-internal ribosomal entry site-Cre recombinase estrogen receptor variant 2 mice and organoids via immunofluorescence, flow cytometry, and reverse transcription quantitative polymerase chain reaction.
We found that RV directly infects Paneth cells, leading to a reduction in mature Paneth cells and an increase in kallikrein 1-high immature Paneth cells. Paneth-ISC communication was significantly enhanced. In particular, the bone morphogenic protein 7 (BMP7)-activin A receptor type 2B/BMP receptor type 1A-Smad pathway was upregulated post-infection, suggesting that Paneth cells suppress excessive ISC proliferation. Functional validation confirmed activation of this pathway.
Paneth cells regulate ISC proliferation during RV infection by activating BMP7 signaling, limiting excessive stem cell expansion and preserving crypt homeostasis for effective epithelial repair.
Core Tip: This study uncovers a novel regulatory role of Paneth cells in intestinal stem cell (ISC) homeostasis during rotavirus infection. While Paneth cells are known for supporting ISCs, we demonstrate that rotavirus directly infects Paneth cells, resulting in a loss of mature Paneth cells. Paneth-ISC communication is significantly enhanced post-infection, with upregulation of the bone morphogenic protein 7 (BMP7)-activin A receptor type 2B/BMP receptor type 1A-Smad pathway, which limits excessive ISC proliferation. These findings provide new insights into crypt homeostasis and suggest BMP7 signaling as a potential target for intestinal repair in viral infections.
- Citation: Bu XY, Tan HY, Wang AM, Wei MT, Pan S, Gao JZ, Li YH, Qian GX, Chen ZH, Ye C, Jia WD. Paneth cells inhibit intestinal stem cell proliferation through the bone morphogenic protein 7 pathway under rotavirus-mediated intestinal injury. World J Gastroenterol 2025; 31(26): 107044
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/107044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.107044
The intestinal epithelium is a highly dynamic tissue that maintains its barrier function against external environments through continuous self-renewal[1-3]. Intestinal stem cells (ISCs), located at the base of intestinal crypts, drive this renewal process by differentiating into various specialized epithelial cells, including Paneth cells adjacent to ISCs[4-6]. Paneth cells are crucial components of the intestinal innate immune system; they secrete antimicrobial peptides such as α-defensins and lysozymes (Lyz) into the crypt lumen, thereby protecting ISCs and preserving intestinal homeostasis[7-9]. Beyond their antimicrobial role, Paneth cells support ISCs by direct contact and by secreting signaling molecules like wingless-related integration site 3 (Wnt3), Notch, and R-spondin1, which enhance ISC proliferation and maintenance[10-13]. Additionally, Paneth cells provide metabolic support; their glycolytic production of lactate serves as a substrate for ISCs mitochondrial metabolism, bolstering ISCs function and differentiation[14-16]. However, the mechanisms by which Paneth cells regulate ISCs under pathological conditions remain incompletely understood.
Bone morphogenic protein (BMP) signaling regulates gastrointestinal morphogenesis, inflammatory responses, and ISCs homeostasis[17-19]. BMP signaling is generally considered a negative regulatory pathway that balances Wnt-driven epithelial self-renewal and proliferation, preventing excessive ISCs expansion[19-21]. Various BMP family members play crucial roles in inhibiting Wnt signaling, potentially exhibiting overlapping or distinct functions across different regions of the intestinal epithelium. For instance, BMP4 has been reported to alleviate colonic inflammation, BMP6 is associated with hepcidin and iron metabolism, and BMP7 can reduce pro-inflammatory cytokine production[22-24]. Studies have shown that BMP signaling activity is higher at the villus tip, forming a gradient along the crypt-villus axis[20]. However, increasing evidence suggests that BMP signaling exhibits distinct regulatory patterns in different intestinal regions; for example, BMP2 is primarily localized at the villus tip, while BMP4 is more prominent in stromal cells near the crypts[20,25-27]. The canonical BMP-Smad pathway inhibits ISCs proliferation by transcriptionally repressing stem cell genes, including leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5), independently of Wnt/β-catenin signaling[21]. Nonetheless, the spatiotemporal specificity of BMP signaling and its interaction networks with various epithelial cell subtypes require further investigation[28]. Furthermore, how the BMP pathway is dynamically regulated during the acute and recovery phases of intestinal injury remains to be fully elucidated.
Rotavirus (RV) is a major pathogen causing viral gastroenteritis, leading to intestinal epithelial damage and ranking as the primary cause of diarrhea-related mortality across all age groups in 2021[29,30]. Unlike chemically induced intestinal injury models, RV infection primarily damages intestinal villi without directly disrupting ISCs, making it an ideal model for studying cell-cell interactions within the crypt microenvironment, which may affect the activity of pathways including Wnt and BMP signaling[31,32]. Studies have shown that ISCs exhibit strong regenerative capacity during RV infection, suggesting that niche-supporting cells, including Paneth cells, may play a crucial role in crypt repair[33]. However, how Paneth cells influence stem cells in the pathological context of RV infection remains unclear.
Single-cell RNA sequencing enables precise characterization of intestinal epithelial cell (IEC) populations, revealing the unique gene expression profile of Paneth cells and identifying intercellular signaling networks. Here, we found that RV can directly infect Paneth cells and reduce their numbers. Furthermore, during RV-induced intestinal injury repair, Paneth cells regulate ISC homeostasis by inhibiting excessive proliferation through the BMP7 signaling pathway.
The wild type C57BL/6J mice and Lgr5-enhanced green fluorescent protein-internal ribosomal entry site-Cre recombinase estrogen receptor variant 2 (Lgr5-EGFP-CreERT2) mice and Lyz1-Cre mice were purchased from GemPharmatech (Nanjing, China). Mice were maintained in specific pathogen-free conditions under a strict 12-hour light cycle (lights on at 08:00 and off at 20:00). All animal studies were performed according to approved protocols by the Ethics Committee of the Institute of Health and Medicine, Hefei Comprehensive National Science Center.
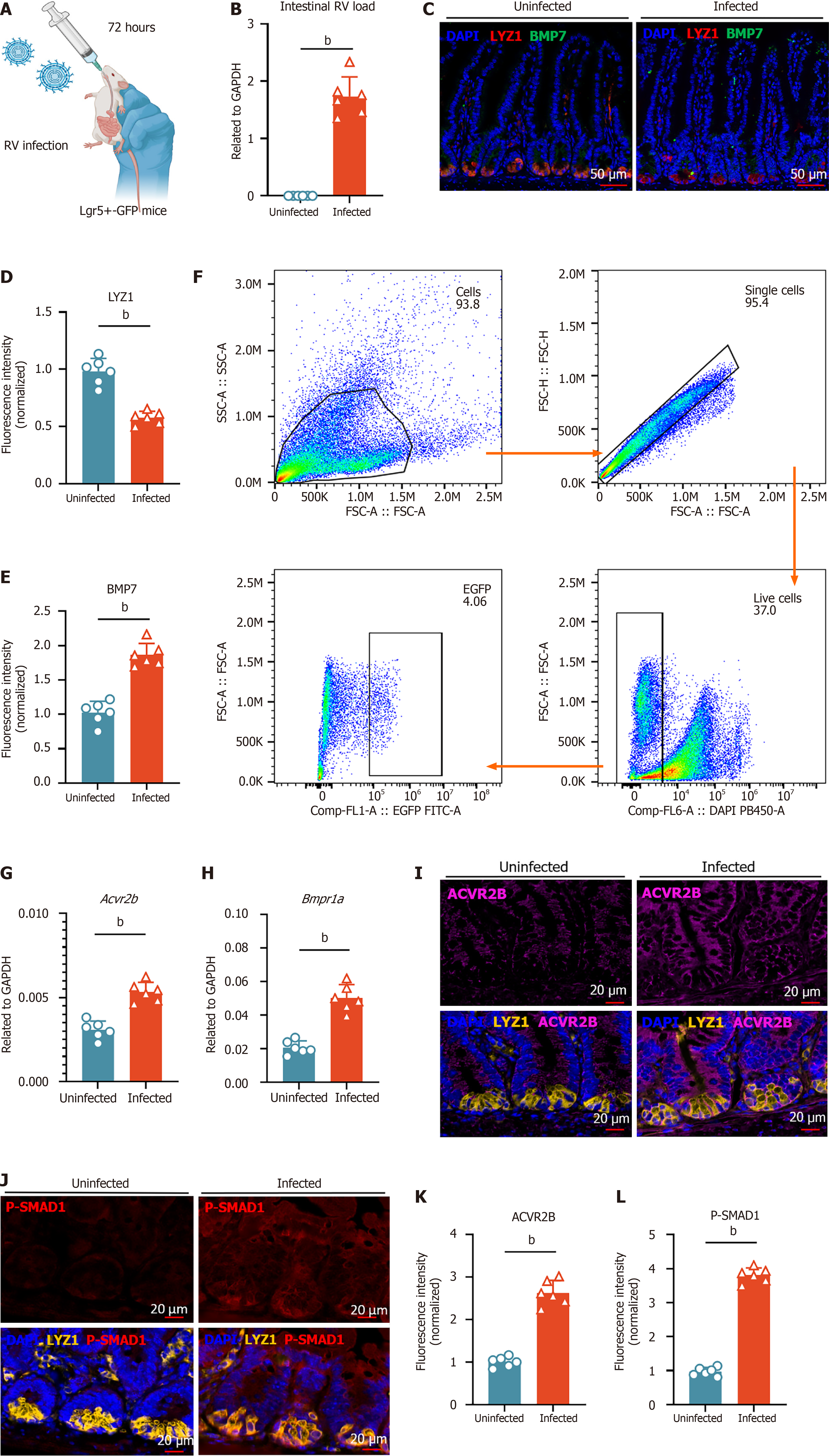
RV enteric cytopathogenic human orphan virus (EC) strain (provided by professor Zhu S, University of Science and Technology of China, China) was prepared following previously published protocols[34]. For RV infection in vivo, mice aged 5-8 weeks were orally inoculated by gavage with 150% diarrhea dose of EC virus in 200 μL sterile phosphate buffered saline (PBS). Mice were sacrificed 72 hours later, and small intestinal tissues were collected to detect viral loads and gene expression in intestinal tissues by reverse transcription quantitative polymerase chain reaction (RT-qPCR).
To determine the complete genome sequence of the RV EC strain, viral RNA was extracted from the intestinal tissues of infected mice. The RNA was reverse-transcribed into complementary DNA, which was then amplified using PCR to generate overlapping fragments covering the entire viral genome. These fragments were cloned into plasmid vectors, transformed into Escherichia coli for propagation, and subsequently sequenced using Sanger sequencing. The resulting sequences were assembled to construct the reference genome of the RV EC strain.
Single-cell RNA sequencing data (GSE169197), including samples from three control and three RV EC strain-infected C57BL/6 mice (8 weeks old), were obtained for analysis. Sequencing reads were demultiplexed and aligned to the murine reference genome (GRCm38) and the RV EC genome using the Cell Ranger pipeline (10 × Genomics, v7.1.0, CA, United States). Gene expression matrices were generated with Cell Ranger count, followed by quality control and downstream analysis in Seurat v4.1.3 (R v4.4), resulting in a total of 28477 analyzed cells. Cells with < 25% mitochondrial gene content were retained, and mitochondrial percentage along with total gene count per cell was regressed out during clustering. Uniform manifold approximation and projection was utilized for visualization and cell type identification. Cell identities were assigned based on canonical marker genes and validated using SingleR and reference datasets (e.g., scMCA). Each subcluster was analyzed independently by extracting the relevant cell population and reprocessing while regressing out mitochondrial content and replicate number. Differential expression analysis between conditions was conducted using the Wilcoxon rank-sum test, with multiple testing correction via the Benjamini-Hochberg method (false discovery rate < 0.05). Gene Ontology (GO) enrichment analysis was conducted using the clusterProfiler (v4.12.6) package to identify significantly enriched biological processes, molecular functions, and cellular components.
Cell-cell communication analysis was performed using the CellChat (v1.6.1) package to infer interactions between IECs. Murine-specific ligand-receptor interactions were predicted using the CellChatDB.mouse database. Enrichment scores for ligand-receptor interactions were calculated based on observed vs expected expression levels across cell populations. Pseudotime trajectory analysis was performed using the Monocle2 (v2.32.0), where cells were ordered in pseudotime using Discriminative Dimensionality Reduction with Trees for dimensionality reduction. The learn_graph function was used to construct cell fate trajectories, and Ridge plots were generated to visualize shifts in differentiation states between uninfected and infected conditions.
Mouse small intestine (formalin-fixed paraffin-embedded) from C57BL/6 mice, high definition spatial gene expression dataset by Space Ranger 3.0.0, 10 × Genomics (March 24, 2023). Intestine sample was derived from C57BL/6 male mice, aged 8 weeks. Space Ranger (v3.0) was used to process FASTQ files, map reads to the reference genome, detect tissue, align data to microscope and CytAssist images, and generate feature-barcode matrices for gene expression analysis.
Intestinal tissues were collected from wild-type C57BL/6J mice infected with RV and wild-type littermate controls. Tissue sections were immersed in ethylenediaminetetraacetic acid (EDTA) antigen retrieval buffer (pH = 8.0) for antigen retrieval. To block non-specific binding, sections were incubated with 3% bovine serum albumin for 30 minutes at room temperature. The primary antibody [anti-Lyz rabbit polyclonal antibody, PA5-16668, Thermo Fisher, MA, United States; anti-BMP7 rabbit monoclonal antibody, PA206692, Cusabio, TX, United States; anti-activin A receptor type 2B (anti-ACVR2B) rabbit monoclonal antibody, PA05805A0Rb, Cusabio, TX, United States; anti-phosphorylation-Smad1 rabbit polyclonal antibody, 28893-1-AP, Proteintech, Wuhan, China] was applied and incubated overnight at 4 °C. The next day, sections were incubated with the corresponding secondary antibodies for 50 minutes at room temperature. Cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole (Invitrogen, CA, United States) for 10 minutes at room temperature in the dark. Autofluorescence was quenched using a commercial autofluorescence quencher for 5 minutes, followed by a 10-minute rinse in distilled water. The sections were air-dried slightly and mounted using an anti-fade mounting medium. Fluorescence images were acquired using a DS-U3 confocal imaging system (Nikon, Japan).
For cells, total RNA was isolated using the TRNzol Universal reagent (Tiangen, Beijing, China) following the manufacturer’s protocol. Complementary DNA synthesis was performed with the PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan). Real-time PCR was conducted using SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara, Japan). Gene expression levels were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase and presented as 2-∆∆Ct. The primers used for mouse genes are as Table 1.
| Gene | Forward (5’ to 3’) | Reverse (5’ to 3’) |
| GAPDH | TGAGGCCGGTGCTGAGTATGTCG | CCACAGTCTTCTGGGTGGCAGTG |
| RT-RV | GAGAATGTTCAAGACGTACTCCA | CTGTCATGGTGGTTTCAATTTC |
| Acvr2b | AGGCAACTTCTGCAACGAG | CTTCCGATGACGATACATCCAG |
| Bmpr1a | AACAGCGATGAATGTCTTCGAG | GTCTGGAGGCTGGATTATGGG |
| Id1 | CGTGCGGAGCTTGTCTTTC | CCTCCTTCTCATAGGTCTGTGG |
| Olfm4 | CTGAACCTGACGGTCCGAGTA | TGCTAAGGACATTGTTTTGGTCT |
| Mki67 | ACCGTGGAGTAGTTTATCTGGG | TGTTTCCAGTCCGCTTACTTCT |
The small intestine was carefully dissected from Lgr5-EGFP-CreERT2 mice and thoroughly rinsed with ice-cold PBS to remove any luminal contents. The intestine was then inverted and cut into approximately 1 cm segments. To dissociate the epithelial cells, the tissue sections were incubated in Roswell Park Memorial Institute medium containing 2 mmol/L EDTA at 37 °C for 20 minutes with gentle shaking. After incubation, the supernatant containing the detached IECs was collected and filtered through a 100 μm cell strainer to eliminate larger tissue debris. The resulting cell suspension represented the IEC fraction. After isolating IECs from Lgr5-EGFP-CreERT2 mice, the cells were sorted using a fluorescence-activated cell sorting (FACS) Aria flow cytometer (Becton, Dickinson and Company, NJ, United States), with fluorescein isothiocyanate+ cells specifically selected as stem cells.
For surface staining, single-cell suspensions were incubated with FACS antibodies in FACS buffer, composed of PBS, 2% bovine serum albumin, and 5 mmol/L EDTA, for 20 minutes at 4 °C. The antibodies used for staining were Phycoerythrin-Cyanine 7-conjugated anti-CD27 (Biolegend, CA, United States)[11,35]. Flow cytometric analysis was conducted using a CytoFlex S cytometer (Beckman Coulter, CA, United States), and the resulting data were analyzed with FlowJo (v.10.0.7) software.
Small intestines were harvested from euthanized mice and flushed thoroughly with cold PBS to remove luminal contents. The tissue was opened longitudinally and washed again to eliminate residual mucus. The intestine was then cut into approximate 5 mm segments and incubated in PBS containing 10 mmol/L EDTA at 4 °C with gentle rotation for 30-45 minutes to dissociate the epithelium from the underlying mesenchyme. After incubation, tissue fragments were transferred to cold PBS without EDTA and vigorously shaken or pipetted to release intestinal crypts. The resulting suspension was passed through a 70 μm cell strainer into a 50 mL conical tube to remove villi and larger debris. Crypts were pelleted by centrifugation at 300 × g for 5 minutes at 4 °C and resuspended in ice-cold, 0.5 × Matrigel™ (Corning, NY, United States).
Crypts were adjusted to a final concentration of 5000-10000 crypts/mL, and 25-30 μL Matrigel droplets were plated in the center of each well of a pre-warmed 48-well plate. Plates were incubated at 37 °C for 20-30 minutes to allow Matrigel polymerization. Subsequently, 300 μL of pre-warmed IntestiCult™ organoid growth medium (mouse) (STEMCELL Technologies, Catalog #06005, Canada) was added to each well. Cultures were maintained at 37 °C in 5% CO2, with medium changes every 2-3 days. Mature organoids with cystic or budding morphology were typically observed within 5-7 days[36].
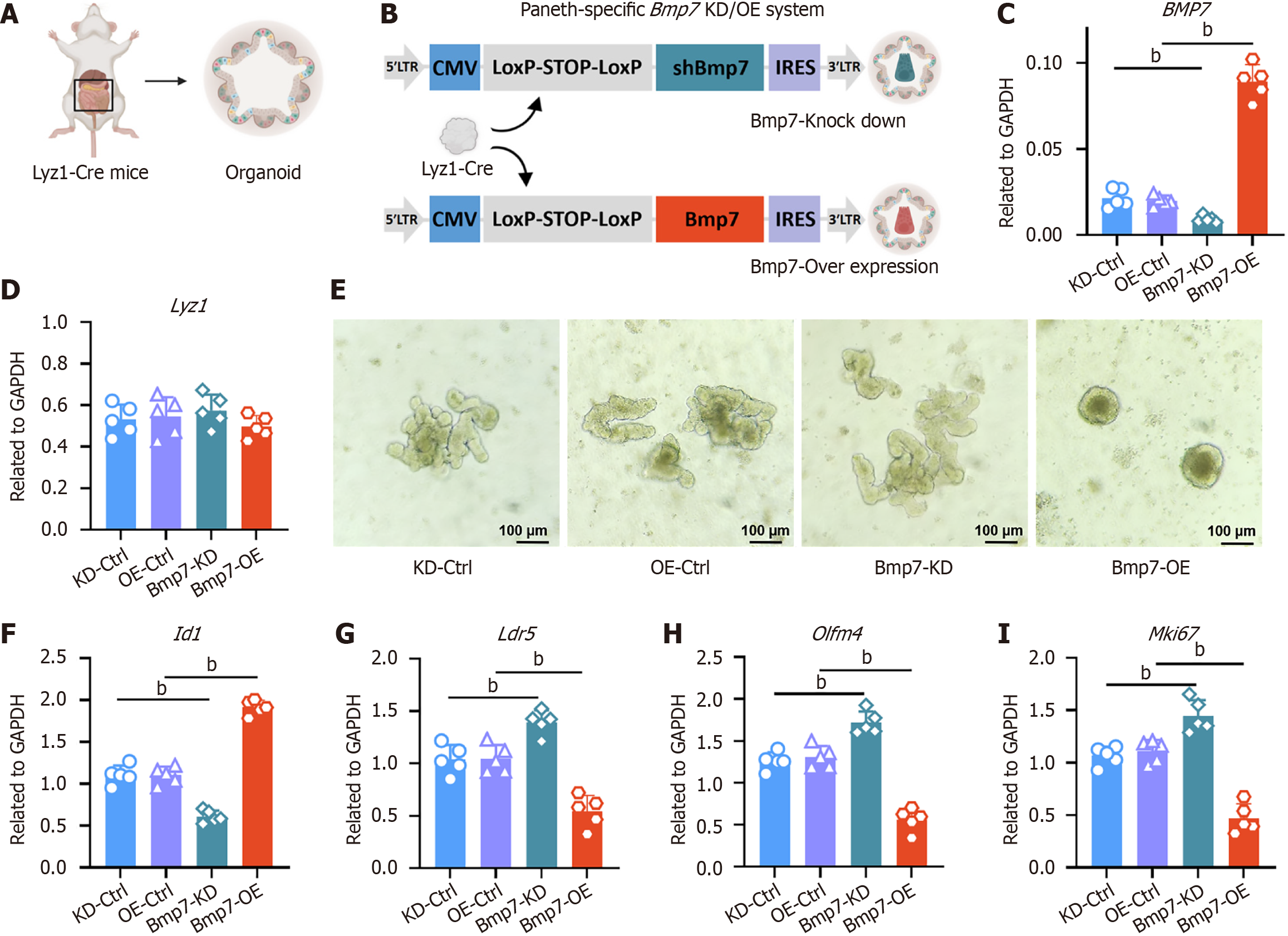
To achieve Paneth cell-specific modulation of Bmp7 in intestinal organoids, we constructed two Cre-inducible lentiviral vectors containing a LoxP-STOP-LoxP (LSL) cassette. For knockdown (KD), a plasmid small interfering RNA co-expression vector with recombination-based vector was used, in which the cytomegalovirus (CMV) promoter drives a triple polyadenylate stop cassette flanked by LoxP sites, followed by a short hairpin RNA targeting Bmp7 (shBmp7) (target sequence: 5’-GCGATTTGACAACGAGACCTT-3’) and an internal ribosome entry site (IRES)-GFP reporter. The control vector (KD-Ctrl) was identical except that shBmp7 was replaced with a non-targeting shRNA targeting luciferase sequence. For overexpression (OE), a plasmid lentivirus-CMV-based backbone was employed with the same LSL configuration, followed by the mouse Bmp7 open reading frame and an IRES-mCherry reporter. The control vector (OE-Ctrl) lacked the open reading frame and expressed IRES-mCherry only, to control for fluorescent marker effects.
Lentiviral particles were produced in human embryonic kidney 293T cells by co-transfection with packaging plasmids (psPAX2 and pMD2.G) using lipofectamine 3000 (Thermo Fisher, MA, United States). Supernatants were harvested at 48 and 72 hours, filtered through a 0.45 μm filter, and concentrated by ultracentrifugation at 25000 rpm for 2 hours at 4 °C. Organoids were enzymatically dissociated into single cells using TrypLE express (Gibco, MA, United States), resuspended in organoid medium containing 8 μg/mL polybrene, and incubated with viral particles for 6-8 hours. Cells were then embedded in Matrigel and cultured in IntestiCult™ Organoid Growth Medium (mouse) (STEMCELL Technologies, #06005, Canada) for recovery and expansion.
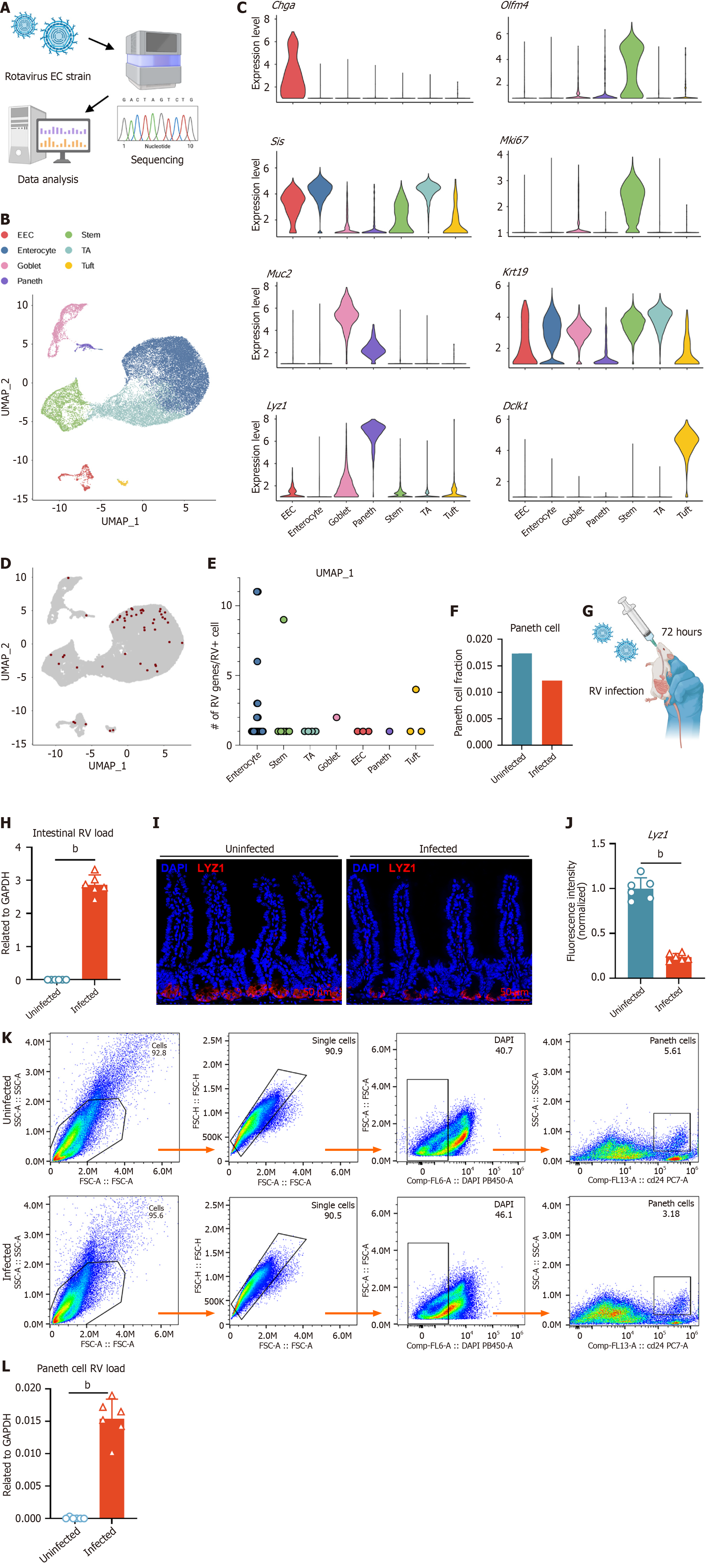
To investigate the underlying mechanisms of intestinal injury and repair, we established a RV infection model that induces villi damage without directly disrupting crypts (Figure 1A)[31,32]. We reanalyzed the single-cell RNA sequencing dataset GSE169197 by aligning the sequencing reads to the reference RV EC strain genome using Cell Ranger. Based on the marker gene expression specific to IECs, we identified seven distinct clusters: ISCs, Paneth cells, goblet cells, enteroendocrine cells, tuft cells, and enterocytes (Figure 1B and C). Spatial transcriptomic analysis further revealed the spatial distribution of marker gene expressions across the intestinal epithelium, providing a three-dimensional perspective on intestinal epithelial renewal. Lgr5, olfactomedin 4 (Olfm4), and Lyz1 were primarily expressed by crypt region cells, Krt19 and Sis were expressed by cells along the intestinal axis, while Muc2, Dclk1, and Chga were expressed in the epithelial layer cells (Supplementary Figure 1A)[37]. Alignment of single-cell RNA sequencing reads from the intestine with the RV genome reveals RV transcripts across multiple epithelial cell populations, including Paneth cells (Figure 1D and E). The proportion between uninfected and infected enterocytes significantly increased post-infection, while that for all other epithelial cell types, including Paneth cells, decreased (Figure 1F, Supplementary Figure 1B and
Previous studies have reported that RV can infect porcine Paneth cells, but its ability to infect murine Paneth cells remains unclear[38]. To validate the impact of RV infection on Paneth cells, we infected 5-week-old mice with RV and collected small intestinal tissues for analysis 72 hours post-infection (Figure 1G and H). Immunofluorescence (IF) staining showed a reduction in Paneth cell numbers after infection (Figure 1I and J). Flow cytometry analysis based on CD24 sorting confirmed a decreased proportion of Paneth cells in the infected group vs the control group (Figure 1K)[11,35]. Furthermore, qPCR analysis of sorted Paneth cells revealed low levels of RV transcript detection, demonstrating that Paneth cells can be directly infected by RV (Figure 1L).
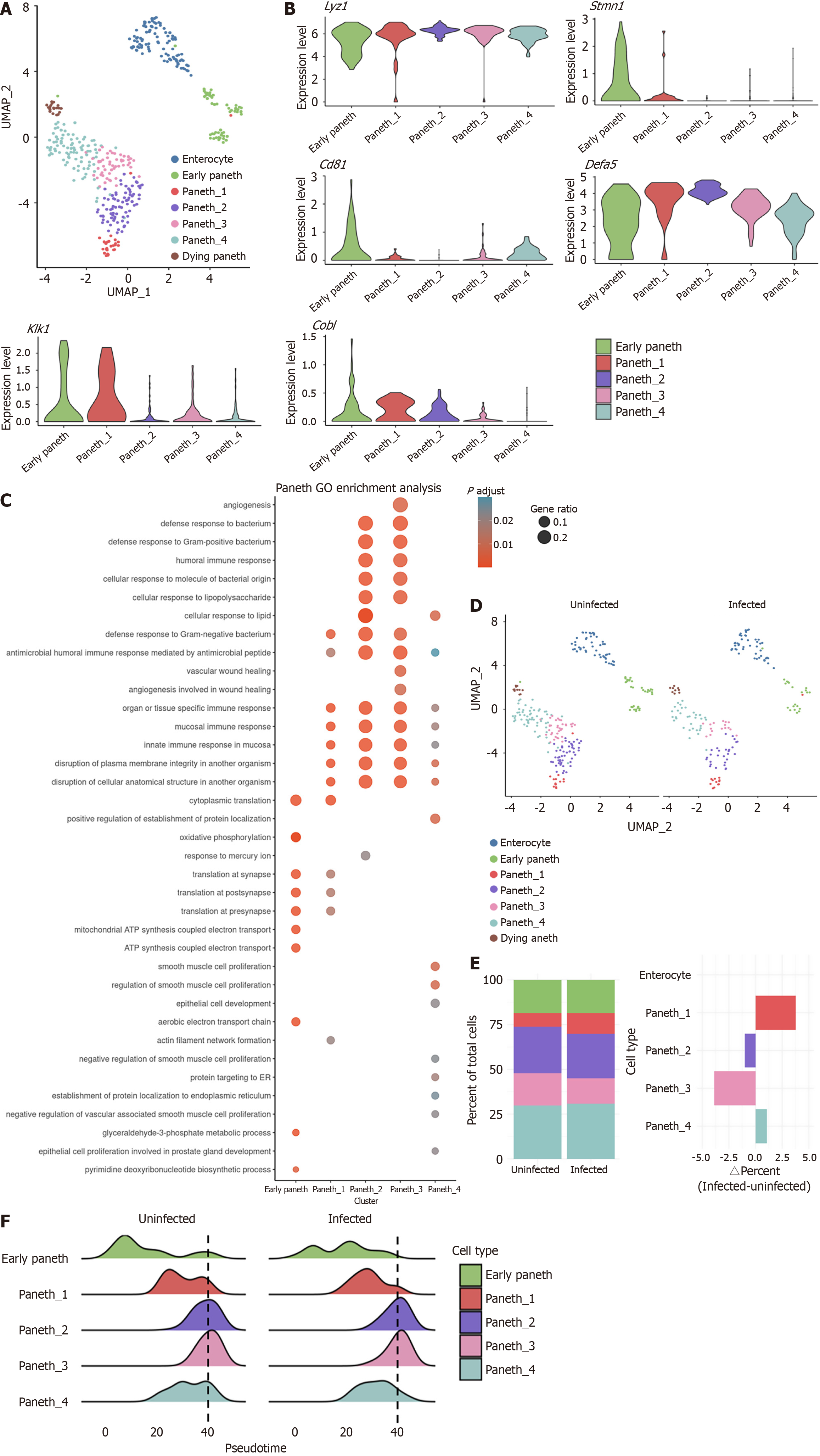
To further investigate the impact of RV infection on Paneth cells and intestinal injury repair, we performed unsupervised clustering to subclassify Paneth cells based on differentially expressed genes. This analysis identified seven subpopulations, including enterocyte (Sis_high), early Paneth (Lgr5+), Paneth_1-4, and dying Paneth with high expression of mitochondrial genes (Figure 2A and B). Among these subtypes, Paneth1 exhibited a tendency toward an inflammatory profile, characterized by the elevated expression of kallikrein 1 (Klk1). This serine protease hydrolyzes high-molecular-weight kininogen to release bradykinin, regulating vascular permeability, immune cell recruitment, and local inflammatory responses[39-41]. In contrast, Paneth2 exhibited high Cobl and low Cd81 expression, whereas Paneth4 showed the opposite pattern, and Paneth3 demonstrated low expression of both markers (Figure 2A and B)[42-44]. The varied expression patterns suggest functional diversity among Paneth cell subtypes.
GO enrichment analysis revealed that, early Paneth cells (Lgr5+) exhibited a pronounced proliferative signature, while Paneth1-4 were primarily involved in antimicrobial secretion. In addition, enterocyte (Sis_high) exhibited a strong intestinal epithelial phenotype (Supplementary Figure 2A and B). Further analysis suggested that Paneth1 and Paneth4 represent transitional states between early and mature Paneth cells, with Paneth2 and Paneth3 being more functionally mature (Figure 2C). Notably, although Paneth3 exhibited detectable RV transcripts, its antimicrobial pathway activity was lower than that of Paneth2. Instead, it demonstrated upregulation of vascular injury pathways. Given that Paneth cells have been reported to promote angiogenesis, this suggests a potential link between Paneth3 and intestinal repair (Supplementary Figure 2C)[45].
Quantifying the Paneth cell pre- to post-infection ratio, we observed that Paneth1 showed the largest increase, whereas Paneth3 exhibited the most significant decline (Figure 2D and E). Pseudotime trajectory analysis further demonstrated that early Paneth cells preferentially differentiated into mature Paneth cells, with a higher propensity to transition into Paneth1 under RV infection (Figure 2F). Collectively, these results suggest that RV-induced Paneth cell reduction is primarily due to the loss of mature Paneth cells, which may compensate by the differentiation of early Paneth cells into Klk1-high immature Paneth cells.
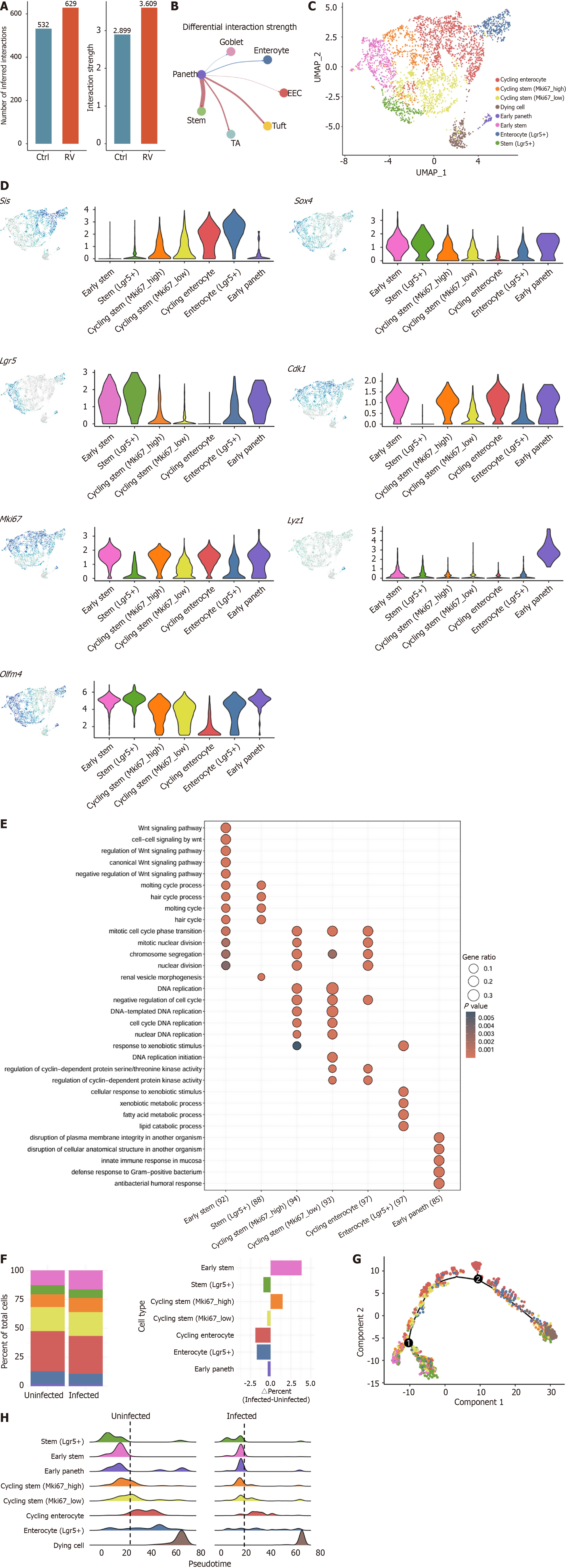
To investigate the impact of RV infection on intestinal epithelium communications, we used CellChat to detect biologically significant cell-cell communication networks (Figure 3A). The analysis revealed that both the number and strength of intercellular interactions increased post-infection, with the most significant enhancement observed between Paneth cells and ISCs (Figure 3B). To further characterize ISCs, we performed unsupervised clustering and identified seven distinct ISCs subpopulations with unique characteristics (Figure 3C and D). Among them, the stem (Lgr5+) population exclusively expressed Lgr5 but lacked marker of proliferation Ki-67 (Mki67) and cyclin-dependent kinase 1, representing a quiescent ISCs subset. In contrast, the early stem population highly expressed all 3 genes, representing a cycling ISCs subset initiating proliferation and differentiation. We further observed that as cycling stem cells differentiate into enterocytes, Lgr5 expression gradually decreases while Sis expression increases, aligning with previous reports on accelerated ISC proliferation and differentiation in response to intestinal injury[33,46]. Additionally, we identified an early Paneth cell cluster, characterized by co-expression of Lgr5 and Lyz1. Further validation by GO enrichment analysis confirmed the classification’s biological relevance (Figure 3E).
We observed a notable increase of uninfected-to-infected ratio in early stem cells and a relative decrease in stem (Lgr5+) cells, indicating that ISCs are in an active proliferative state following RV infection (Figure 3F, Supplementary Figure 3)[5,11,33]. Pseudotime trajectory analysis demonstrated that stem (Lgr5+) cells are positioned at the root of ISCs differentiation, representing the most primitive ISCs subset. As stemness gradually diminishes and epithelial differentiation characteristics increase, ISCs ultimately differentiate into various intestinal epithelial lineages (Figure 3G). Comparing the pseudotime ridge plots between uninfected and infected ISC populations, we observed that the infected stem (Lgr5+) cells exhibited an increased tendency to differentiate into early stem and cycling stem populations, with an overall shift in differentiation trajectories (Figure 3H). Collectively, these findings suggest that RV infection drives ISCs into a highly proliferative and differentiative state, with Lgr5+ Mki67- cells serving as the initial population in the ISCs differentiation trajectory.
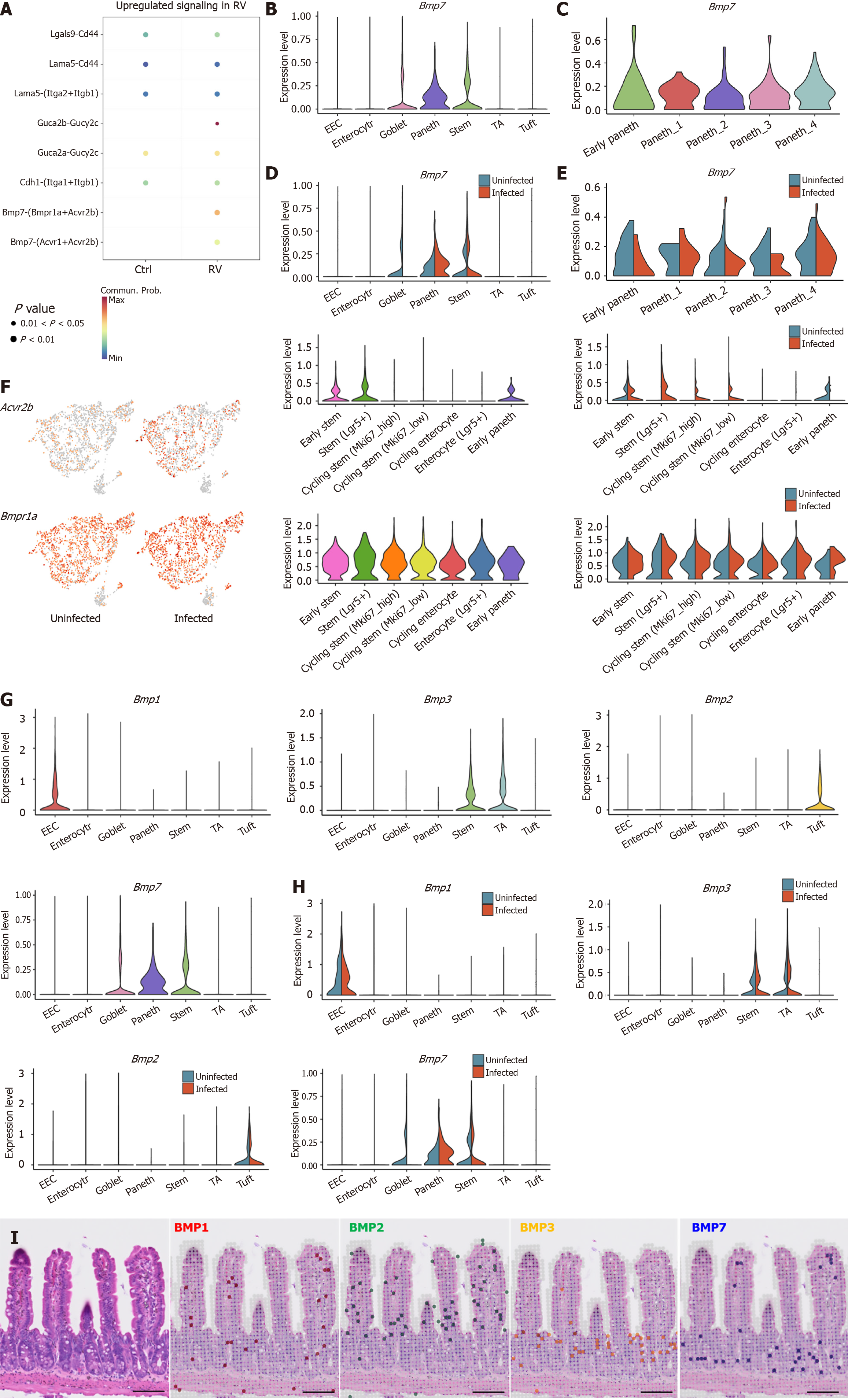
To explore the influence of Paneth cells on ISCs, CellChat analysis revealed that the Bmp7-Acvr2b pathway was upregulated in Paneth-ISC communication post-infection (Figure 4A). Further analysis confirmed that Bmp7 was specifically expressed in Paneth cells (Figure 4B). Previous studies have reported that BMP7 plays an anti-inflammatory role in the intestine, aligning with Paneth cell function, suggesting its expression may mediate part of Paneth cells’ anti-inflammatory activities (Figure 4C)[24,47]. Bmp7 was expressed across all Paneth subpopulations, with Paneth1 exhibiting the highest levels, supporting the hypothesis of its functional relevance. However, Bmp7 expression remained relatively stable across Paneth subtypes before and after infection, indicating that the regulation of Bmp7-Acvr2b signaling may primarily originate from ISCs rather than Paneth cells (Figure 4D and E). We then examined the expression dynamics of Acvr2b/BMP receptor type 1A (Bmpr1a) in ISCs before and after RV infection, revealing an upregulation of this signaling axis post-infection (Figure 4F). Specifically, Acvr2b was highly expressed in Lgr5+ ISCs, with a marked increase following infection. A similar trend was observed for Bmpr1a, a type I receptor. Given that the BMP signaling pathway inhibits ISCs proliferation, we propose that Paneth cells in the crypt suppress excessive Lgr5+ ISCs expansion via the Bmp7-Acvr2b/Bmpr1a signaling axis, thereby maintaining ISCs homeostasis[21,48]. Notably, Acvr2b expression was significantly downregulated in early Paneth cells post-infection, suggesting that as ISCs differentiate into Paneth cells, BMP7-mediated growth suppression may be partially lifted to facilitate the generation of new Paneth cells, which compensates for RV-induced Paneth cell loss.
Given that Paneth cells at the crypt base specifically express Bmp7, we further investigated the localization of various BMP family members in IECs. The results showed that enteroendocrine cells expressed Bmp1, ISCs and transit-amplifying cells highly expressed Bmp3, while tuft cells expressed Bmp2 (Figure 4G). Notably, these BMP gene expression levels remained unchanged before and after RV infection (Figure 4H). Finally, spatial transcriptomic analysis revealed that Bmp7 expression was primarily localized in the crypt region, whereas Bmp3 expression was enriched in migrating ISCs and transit-amplifying cells, with Bmp1 and Bmp2 expression were discovered throughout the intestinal epithelium (Figure 4I). These findings suggest that BMP signaling exhibits cell-type-specific spatial distribution within the intestinal epithelium, potentially playing distinct physiological roles in gut homeostasis and repair[27].
To further confirm that Paneth cells regulate ISC proliferation via BMP7 signaling during RV-induced intestinal injury, we infected Lgr5-enhanced green fluorescent protein-internal ribosomal entry site-Cre recombinase estrogen receptor variant 2 mice with RV (Figure 5A and B). IF staining before and after infection revealed that Bmp7 expression was highest in the crypts, which slightly increased post-infection (Figure 5C-E). Next, we isolated Lgr5+ ISCs using flow cytometry sorting. qPCR analysis revealed that Acvr2b and Bmpr1a expression levels in these ISCs were significantly upregulated post-infection (Figure 5F-H). Furthermore, multiplex IF analysis demonstrated increased activation of BMP receptors (ACVR2B) and downstream phosphorylation of Smad1 in crypts post-infection, indicating an upregulation of the BMP7-ACVR2B/BMPR1A signaling axis following RV infection (Figure 5I-L). Collectively, these findings demonstrate that Paneth cells suppress excessive Lgr5+ ISCs proliferation via the BMP7-ACVR2B/BMPR1A-Smad signaling pathway during RV-induced intestinal injury.
To investigate how Paneth cell-derived Bmp7 regulates ISCs, we generated intestinal organoids from jejunal crypts of Lyz1-Cre mice (Figure 6A). Paneth cell-specific Bmp7 modulation was achieved by lentiviral vectors: Plasmid small interfering RNA co-expression vector with recombination-CMV-LSL-shBmp7-IRES-GFP for KD and plasmid lentivirus-CMV-LSL-Bmp7-IRES-mCherry for OE (Figure 6B). qPCR analysis confirmed efficient Cre-mediated recombination, showing approximately 70% decrease (KD) and approximately 9-fold increase (OE) of Bmp7 expression compared to controls (Figure 6C). Expression of the Paneth cell marker Lyz1 was unaffected (Figure 6D), indicating stable Paneth cell identity and number.
Notable morphological differences were observed: Bmp7-KD organoids displayed extensive crypt budding with pronounced protrusions, indicating enhanced ISC proliferation and stemness. Conversely, Bmp7-OE organoids exhibited reduced budding, presenting a compact spherical structure indicative of decreased ISC activity (Figure 6E). These morphological changes correlated with altered BMP7 pathway activation, reflected by dose-dependent regulation of the downstream target gene Id1 (Figure 6F). Additionally, stemness and proliferation markers (Lgr5, Olfm4, Mki67) were significantly increased upon Bmp7 KD but suppressed in the OE group (Figure 6G-I).
Given that ISC proliferation depends heavily on Wnt signaling and Paneth cells are a source of Wnt ligands, we tested whether supplementing exogenous Wnt3a can override the suppressive effect of BMP7 (Supplementary Figure 4A and B). Jejunal organoids with Paneth cell-specific Bmp7-OE or OE-Ctrl were cultured ± 200 ng/mL recombinant Wnt3a. Bmp7 transcript levels remained stable, but Wnt3a partially rescued crypt budding and restored Lgr5, Olfm4, and Mki67 expression in Bmp7-OE organoids, while BMP pathway activity was unaffected (Supplementary Figure 4C-H). These results indicate that BMP and Wnt act in parallel yet independently to regulate ISC behavior, with BMP providing a counter-balance to Wnt-driven proliferation after injury. Collectively, these findings establish Paneth cell-derived Bmp7 as a critical inhibitor of ISC proliferation and stemness via BMP7 signaling, underscoring its pivotal role in maintaining intestinal epithelial homeostasis.
In this study, through single-cell and spatial transcriptomic analyses under RV infection, we identified significant alterations in the communication between Paneth cells and ISCs. Under normal physiological conditions, Paneth cells are recognized as key supporters of ISCs, maintaining their proliferation and differentiation. However, under pathological conditions induced by RV infection, we found that Paneth cells exert an inhibitory effect on ISCs via the BMP7-ACVR2B/BMPR1A signaling axis. Despite a reduction in the number and proportion of Paneth cells post-infection, their communication with ISCs was enhanced, suggesting that the crypt microenvironment may trigger compensatory or protective mechanisms to maintain intestinal homeostasis in response to pathogen invasion[7]. This finding aligns with previous studies showing that BMP signaling restricts excessive ISC proliferation at the crypt level, supporting the notion that Paneth cells not only sustain ISC activity under homeostatic conditions but also act as a “brake” through the BMP7-ACVR2B/BMPR1A pathway to prevent excessive ISC expansion during intestinal repair[48]. Further genetic modifications and in vivo/in vitro functional studies are required to validate the key regulatory nodes in this pathway and to elucidate its potential therapeutic implications in intestinal defense and repair. Interestingly, RV infection led to an increase in Acvr2b expression in Lgr5+ ISCs but a decrease in early Paneth cells, while Bmp7 secretion by Paneth cells remained relatively stable. This suggests that by dynamically regulating receptor expression, the crypt microenvironment fine-tunes signaling across different cell subtypes: On one hand, it sustains the inhibitory effect on Lgr5+ ISCs to prevent excessive proliferation; on the other hand, as ISCs differentiate into Paneth cells, BMP signaling is locally attenuated to ensure the replacement and maturation of new Paneth cells. This regulatory mechanism may provide an adaptive response to injury, preventing stem cell over proliferation while ensuring the replenishment of niche cells critical for crypt homeostasis.
Additionally, we examined the distribution of BMP family members in IECs, revealing that Bmp1, Bmp2, Bmp3, and Bmp7 exhibit distinct spatiotemporal expression patterns across different cell types. These findings highlight the spatial heterogeneity of BMP signaling along the crypt-villus axis and provide insights into the differential roles of BMP signaling in intestinal physiology. Prior studies have demonstrated that BMP7 plays diverse roles in immune modulation and inflammatory responses, and our study further identifies its high expression in Paneth cells, suggesting a unique role in maintaining crypt homeostasis and protecting against pathogens such as RV[24].
Our organoid experiments suggest that Paneth cell-derived BMP7 acts as a brake on ISC proliferation that functions in parallel with canonical Wnt signaling. Combined with prior evidence that Smad-mediated repression of Lgr5 occurs independently of Wnt/β-catenin signaling, these findings suggest a model in which BMP and Wnt represent two separable axes that converge on ISC transcriptional programmes to regulate regenerative output after epithelial injury[21]. The partial nature of the Wnt3a rescue further implies that BMP7 controls additional, Wnt-independent effectors, an area that merits systematic transcriptomic and functional dissection in future work to clarify how Paneth cells co-ordinate regeneration with growth restraint during intestinal repair.
RV infection-induced Paneth cell depletion was primarily attributed to the loss of mature Paneth cells, while the differentiation of early Paneth cells increased, accompanied by an expansion of the immature Klk1-high Paneth1 subset post-infection. This suggests that functional specialization and dynamic regulation exist within the Paneth cell population. The Klk1-high Paneth1 subset may play a more active role in pathogen defense and local inflammation regulation, potentially cooperating with BMP7 signaling[39,40]. To validate these hypotheses, future studies will generate a Paneth cell-specific Klk1 knockout mouse line and perform targeted proteomic and metabolomic analyses of Paneth subpopulations. Additionally, functional assays such as organoid co-culture systems and conditional in vivo depletion models will be employed to clarify the precise role of Paneth1 cells in intestinal injury repair.
Despite employing single-cell RNA sequencing, spatial transcriptomics, immunohistochemistry, and Paneth cell-specific Bmp7 KD and OE experiments to directly assess BMP7’s role, our study still lacks additional functional validations, such as conditional knockout of other key genes and targeted protein-level perturbations, to fully confirm the roles of identified pathways and cell subsets. Moreover, our analysis of public datasets has inherent limitations, including sample heterogeneity and limited sample size, which may affect the reproducibility of the results. Finally, species-specific differences between murine and human RV infection, particularly in Paneth cell tropism and infection efficiency, could limit the translational relevance of our findings. Future work should therefore incorporate rigorous in vivo functional studies, expand to larger, multi-center cohorts to mitigate database biases, and validate key observations in humanized mouse models or primary human intestinal tissues.
In summary, our study demonstrates that RV infection directly impacts Paneth cells, altering their numbers and function. We identified a BMP7-ACVR2B/BMPR1A-Smad signaling axis through which Paneth cells suppress excessive ISCs proliferation in response to RV-induced intestinal injury. Under this pathological context, the crypt microenvironment undergoes significant changes, and Paneth cells play a crucial role in limiting inflammatory damage and maintaining ISCs homeostasis. These findings provide new insights into the molecular mechanisms underlying intestinal repair and homeostasis and lay the foundation for future therapeutic strategies targeting BMP signaling or Paneth cell function in intestinal diseases.
In this study, we investigated the impact of RV infection on Paneth cells and their regulatory role in ISC homeostasis. By reanalyzing single-cell RNA sequencing data and performing spatial transcriptomic analysis, we confirmed that RV can directly infect Paneth cells, leading to a reduction in their numbers. This was further validated through IF staining, flow cytometry, and qPCR analysis, demonstrating that RV infection alters the crypt microenvironment. Beyond their well-established role in antimicrobial defense and ISC support, we found that Paneth cells modulate ISC proliferation during RV-induced intestinal injury repair. Specifically, our results highlight the involvement of the BMP7 signaling pathway in limiting excessive ISC expansion, suggesting a previously unappreciated mechanism through which Paneth cells contribute to epithelial regeneration under pathological conditions. Given that BMP signaling is known to balance Wnt-driven epithelial renewal, our findings provide new insights into the interplay between these pathways in response to viral infection. Overall, this study reveals a novel functional aspect of Paneth cells in maintaining ISC homeostasis during viral gastroenteritis. The ability of RV to infect Paneth cells and alter their regulatory function underscores the complexity of host-pathogen interactions within the intestinal niche. Future research should further explore the molecular mechanisms underlying BMP-mediated ISC regulation and investigate potential therapeutic strategies targeting Paneth cell function to enhance intestinal repair following viral infections.
| 1. | Kurashima Y, Kiyono H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu Rev Immunol. 2017;35:119-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 290] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 3. | Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018;39:677-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 668] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 4. | Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 728] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 5. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1385] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 6. | Beumer J, Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat Rev Mol Cell Biol. 2021;22:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 470] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 7. | Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 924] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 8. | Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 788] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 502] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 10. | Quintero M, Samuelson LC. Paneth Cells: Dispensable yet Irreplaceable for the Intestinal Stem Cell Niche. Cell Mol Gastroenterol Hepatol. 2025;19:101443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1999] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 12. | Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A. 2012;109:3932-3937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 711] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 14. | Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 611] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 15. | Igarashi M, Guarente L. mTORC1 and SIRT1 Cooperate to Foster Expansion of Gut Adult Stem Cells during Calorie Restriction. Cell. 2016;166:436-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 17. | Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Shroyer NF, Wong MH. BMP signaling in the intestine: cross-talk is key. Gastroenterology. 2007;133:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Que J. BMP Signaling in Development, Stem Cells, and Diseases of the Gastrointestinal Tract. Annu Rev Physiol. 2020;82:251-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104:15418-15423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 470] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 21. | Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H, Zhang B, Wang X, Yang X, Xie W, Li B, Han JJ, Chen YG. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat Commun. 2017;8:13824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 22. | Hu L, Xu J, Wang X, Feng L, Zhang C, Wang J, Wang S. Bone Morphogenetic Protein 4 Alleviates DSS-Induced Ulcerative Colitis Through Activating Intestinal Stem Cell by Target ID3. Front Cell Dev Biol. 2021;9:700864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 601] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 24. | Maric I, Poljak L, Zoricic S, Bobinac D, Bosukonda D, Sampath KT, Vukicevic S. Bone morphogenetic protein-7 reduces the severity of colon tissue damage and accelerates the healing of inflammatory bowel disease in rats. J Cell Physiol. 2003;196:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Zhang C, Feng Y, Yang H, Koga H, Teitelbaum DH. The bone morphogenetic protein signaling pathway is upregulated in a mouse model of total parenteral nutrition. J Nutr. 2009;139:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Hardwick JC, Van Den Brink GR, Bleuming SA, Ballester I, Van Den Brande JM, Keller JJ, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Xie Z, Zhou G, Zhang M, Han J, Wang Y, Li X, Wu Q, Li M, Zhang S. Recent developments on BMPs and their antagonists in inflammatory bowel diseases. Cell Death Discov. 2023;9:210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 28. | Lindholm HT, Parmar N, Drurey C, Campillo Poveda M, Vornewald PM, Ostrop J, Díez-Sanchez A, Maizels RM, Oudhoff MJ. BMP signaling in the intestinal epithelium drives a critical feedback loop to restrain IL-13-driven tuft cell hyperplasia. Sci Immunol. 2022;7:eabl6543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | GBD 2021 Diarrhoeal Diseases Collaborators. Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990-2021, for 204 countries and territories: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect Dis. 2025;25:519-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 84] [Article Influence: 84.0] [Reference Citation Analysis (1)] |
| 30. | Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Zou WY, Blutt SE, Zeng XL, Chen MS, Lo YH, Castillo-Azofeifa D, Klein OD, Shroyer NF, Donowitz M, Estes MK. Epithelial WNT Ligands Are Essential Drivers of Intestinal Stem Cell Activation. Cell Rep. 2018;22:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U, Estes MK. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 466] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 33. | Bomidi C, Robertson M, Coarfa C, Estes MK, Blutt SE. Single-cell sequencing of rotavirus-infected intestinal epithelium reveals cell-type specific epithelial repair and tuft cell infection. Proc Natl Acad Sci U S A. 2021;118:e2112814118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 34. | O'Neal CM, Crawford SE, Estes MK, Conner ME. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707-8717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | van Es JH, Wiebrands K, López-Iglesias C, van de Wetering M, Zeinstra L, van den Born M, Korving J, Sasaki N, Peters PJ, van Oudenaarden A, Clevers H. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc Natl Acad Sci U S A. 2019;116:26599-26605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Li R, Zan Y, Wang D, Chen X, Wang A, Tan H, Zhang G, Ding S, Shen C, Wu H, Zhu S. A mouse model to distinguish NLRP6-mediated inflammasome-dependent and -independent functions. Proc Natl Acad Sci U S A. 2024;121:e2321419121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 38. | Yan M, Su A, Pavasutthipaisit S, Spriewald R, Graßl GA, Beineke A, Hoeltig D, Herrler G, Becher P. Infection of porcine enteroids and 2D differentiated intestinal epithelial cells with rotavirus A to study cell tropism and polarized immune response. Emerg Microbes Infect. 2023;12:2239937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 39. | Seliga A, Lee MH, Fernandes NC, Zuluaga-Ramirez V, Didukh M, Persidsky Y, Potula R, Gallucci S, Sriram U. Kallikrein-Kinin System Suppresses Type I Interferon Responses: A Novel Pathway of Interferon Regulation. Front Immunol. 2018;9:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Yiu WH, Wong DW, Chan LY, Leung JC, Chan KW, Lan HY, Lai KN, Tang SC. Tissue kallikrein mediates pro-inflammatory pathways and activation of protease-activated receptor-4 in proximal tubular epithelial cells. PLoS One. 2014;9:e88894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Devetzi M, Goulielmaki M, Khoury N, Spandidos DA, Sotiropoulou G, Christodoulou I, Zoumpourlis V. Geneticallymodified stem cells in treatment of human diseases: Tissue kallikrein (KLK1)based targeted therapy (Review). Int J Mol Med. 2018;41:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Hasezaki T, Yoshima T, Mine Y. Anti-CD81 antibodies reduce migration of activated T lymphocytes and attenuate mouse experimental colitis. Sci Rep. 2020;10:6969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Alvarez KG, Goral L, Suwandi A, Lasswitz L, Zapatero-Belinchón FJ, Ehrhardt K, Nagarathinam K, Künnemann K, Krey T, Wiedemann A, Gerold G, Grassl GA. Human tetraspanin CD81 facilitates invasion of Salmonella enterica into human epithelial cells. Virulence. 2024;15:2399792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Beer AJ, González Delgado J, Steiniger F, Qualmann B, Kessels MM. The actin nucleator Cobl organises the terminal web of enterocytes. Sci Rep. 2020;10:11156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Hassan M, Moghadamrad S, Sorribas M, Muntet SG, Kellmann P, Trentesaux C, Fraudeau M, Nanni P, Wolski W, Keller I, Hapfelmeier S, Shroyer NF, Wiest R, Romagnolo B, De Gottardi A. Paneth cells promote angiogenesis and regulate portal hypertension in response to microbial signals. J Hepatol. 2020;73:628-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Koo BK, Clevers H. Stem cells marked by the R-spondin receptor LGR5. Gastroenterology. 2014;147:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Smoljan I, Detel D, Buljevic S, Erjavec I, Marić I. Therapeutic Potential of BMP7 in the Treatment of Osteoporosis Caused by the Interaction between Inflammation and Corticosteroids in Inflammatory Bowel Disease. Biomedicines. 2023;11:2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 835] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/