Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.106113
Revised: April 9, 2025
Accepted: June 26, 2025
Published online: July 14, 2025
Processing time: 145 Days and 5.8 Hours
Hepatocellular carcinoma (HCC) represents the most prevalent form of primary liver cancer, characterized by high mortality rates, frequent recurrence and metastasis, poor clinical prognosis, and a complex pathogenesis with limited therapeutic options. Autophagy plays a pivotal role in the immune response and functions as a lysosome-mediated degradation mechanism essential for recycling cellular components and eliminating aggregated proteins, damaged organelles, and invasive pathogens, thereby maintaining cellular function and dynamic homeostasis. Additionally, autophagy regulates several critical proteins and signaling pathways, including mammalian target of the rapamycin (mTOR), Beclin-1, the phosphatidylinositol 3-kinase/protein kinase B/mTOR signaling pathway, the Hippo/yes-associated protein signaling pathway, and the Janus kinase/signal transducer of activation signaling pathway. This regulatory capa
Core Tip: In this paper, we examine the mechanisms of autophagy, key proteins and signaling pathways involved, and the modulation of autophagy by Chinese medicine monomers in the prevention and treatment of hepatocellular carcinoma. This review aims to provide valuable insights for the development of Chinese medicine strategies against hepatocellular carcinoma and to inform the rational use of these therapies in clinical practice.
- Citation: Zheng SH, Xue TY, Wang QY, Ye YA, Zhang P. Chinese medicine monomers for hepatocellular carcinoma: New ideas related to autophagy. World J Gastroenterol 2025; 31(26): 106113
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/106113.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.106113
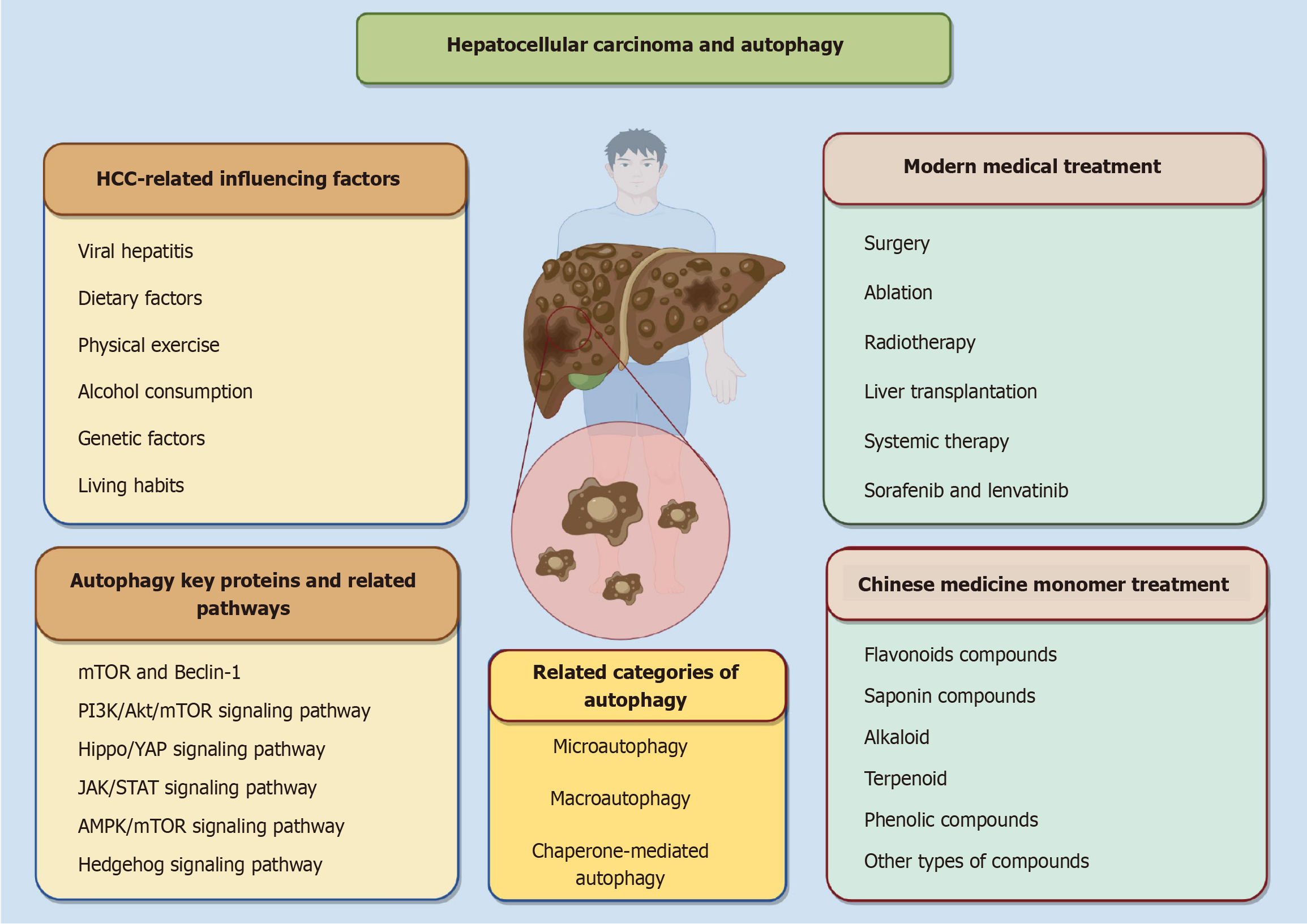
In China, primary liver cancer ranks second in terms of tumor-related deaths and fourth in terms of prevalence among malignant tumors, thereby posing a significant global health threat[1-3]. China is a large country with one-third of the world’s population infected with hepatitis B[4,5]. With hepatitis B vaccination, the prevalence of hepatitis B in China has shown a decreasing trend, but the burden of hepatitis B virus-related disease remains high due to the large population base and the large number of chronically infected individuals[6]. With a five-year survival rate that is among the lowest for malignant tumors[7], primary liver cancer has already accounted for over 900000 new cases and a sharp increase in mortality to 830000 deaths in 2020[8]. In 2019, there will be about 747000 cases of hepatocellular carcinoma (HCC) globally, a 70% increase since 1990, and 480000 of those alone will die from HCC[9]. Primary liver cancer is primarily composed of three distinct types: HCC, intrahepatic cholangiocarcinom, and combined hepatocellular-cholangiocarcinoma, with HCC being the predominant type in clinical cases[10,11]. China is responsible for approximately 50% of the global incidence of new HCC cases[12]. The leading etiological factor for HCC is viral hepatitis, followed by non-alcoholic fatty liver disease associated with metabolic syndrome and excessive alcohol consumption[13]. Contemporary medical practice posits that the management of HCC encompasses various modalities such as surgery, ablation, radiotherapy, liver transplantation, and systemic therapy, among others[14-17]. The selection of appropriate clinical interventions for HCC patients is contingent upon their specific clinical staging, with liver transplantation and surgery serving as primary treatments for individuals in the early stages of HCC. Notably, early-stage HCC patients have demonstrated a 5-year survival rate of 70%, thereby instilling considerable optimism among patients worldwide who are afflicted with early-stage HCC[18,19]. However, due to the fact that the clinical symptoms of early HCC patients are generally not obvious, many HCC patients are diagnosed in advanced or even terminal stages, which do not meet the indications for surgical treatment, so they can only seek systemic therapy and other systemic therapies, but did not achieve better results[20,21]. The subsequent introduction of sorafenib and lenvatinib has led to a new era of targeted therapy for HCC, which has greatly improved the outcome of patients with advanced HCC in first-line treatment[22-24]. Although the emergence of these modern medical treatments and western medicines is a major advancement in the treatment of HCC, they still face the problems of unsatisfactory clinical efficacy and prognosis, limited patient tolerance, and shorter extension of median survival. Therefore, there is an urgent need to develop new therapeutic options to improve the survival of HCC patients. And Chinese medicine monomer plays a key positive role in the treatment of HCC, with the characteristics of high safety and strong targeting, which plays an irreplaceable role in improving the quality of life of HCC patients, improving clinical symptoms and delaying the recurrence and metastasis of tumors.
Autophagy, as an intracellular biological process, is a type of programmed cell death, the main purpose of which is to maintain the cell’s material circulation, energy supply, and self-renewal, etc. This process plays an important role in the removal of intracellular wastes and the maintenance of energy homeostasis in living organisms[25,26]. In cells, autophagy plays a positive protective role, but excessive autophagy or disruption of the autophagic machinery leads to cell death[27]. Several studies have corroborated the critical role of active autophagy modulation in the prevention and treatment of HCC[28,29]. Autophagy has emerged as a pivotal research focus for both current and prospective strategies in the prevention and treatment of HCC within the domain of Chinese medicine. Nevertheless, its precise mechanisms remain inadequately understood. In recent years, the exploration of anti-tumor properties of Chinese medicine monomers has intensified, positioning these compounds as a significant area of interest in oncology research. This is largely due to their capacity to modulate autophagy-related pathways or induce autophagy-mediated cell death in HCC. In this study, we searched the PubMed and Web of Science databases for high-quality literature using keywords such as “Chinese medicine”, “autophagy” and “hepatocellular carcinoma” (Table 1). Articles reviewed spanned the period from 2000 to 2025, while meta-analyses or systematic reviews were excluded. This paper systematically examines the interplay between autophagy-related pathways and HCC and delineates specific therapeutic protocols involving Chinese medicine monomers that regulate autophagy to intervene in HCC. The aim is to furnish a theoretical foundation and reference framework for the development and clinical application of Chinese medicine monomers in the prevention and management of HCC (Figure 1).
| Number | Search terms |
| #1 | Chinese medicine monomers[MeSH Terms] |
| #2 | Traditional Chinese medicine monomers[Title/Abstract] OR Chinese medicinal herb monomers[Title/Abstract] OR TCM monomers[Title/Abstract] OR herbal medicine monomers[Title/Abstract] OR medicine herb monomers[Title/ Abstract] OR Chinese herb medicine monomers[Title/Abstract] |
| #3 | #1 OR #2 |
| #4 | Hepatocellular carcinoma[MeSH Terms] |
| #5 | Liver cancer[Title/Abstract] OR HCC[Title/Abstract] OR LC[Title/Abstract] OR hepatic cancer[Title/Abstract] OR primary liver cancer[Title/Abstract] OR liver hepatocellular carcinoma[Title/Abstract] OR LIHC[Title/Abstract] |
| #6 | #4 OR #5 |
| #7 | Autophagy[MeSH Terms] |
| #8 | Cellular autophagy[Title/Abstract] |
| #9 | Hepatic autophagy[Title/Abstract] |
| #10 | #7 OR #8 OR #9 |
| #11 | #3 AND #6 AND #10 |
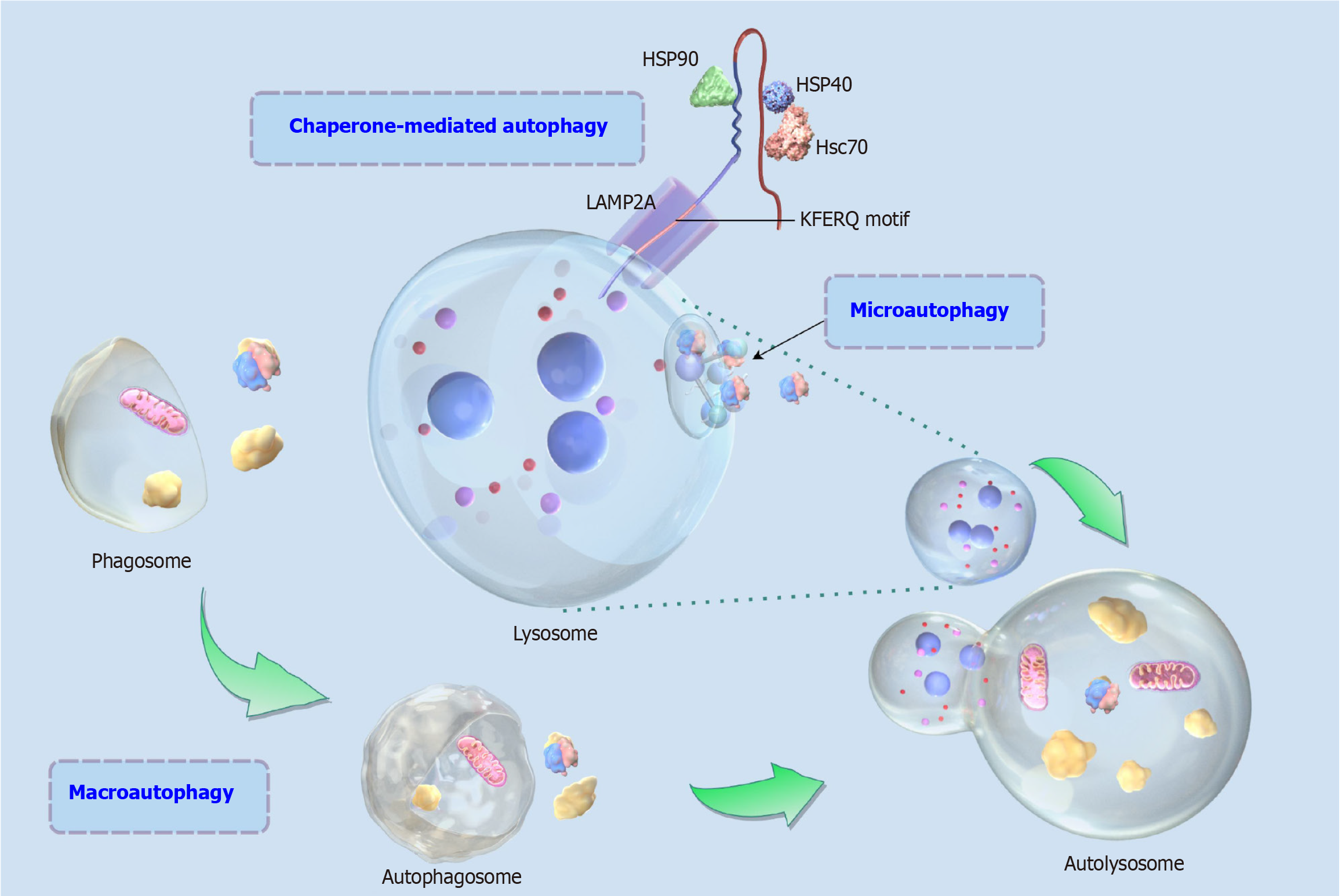
Autophagy plays a unique role in the immune response, especially in the innate immune response, where it helps to remove invading microorganisms and damaged organelles, thereby maintaining cell and tissue health. Autophagy is a complex cellular process containing a series of molecular and organelle interactions, and autophagy also serves as a highly conserved eukaryotic recycling and degradation process by engulfing damaged cytoplasmic proteins or organelles and encapsulating them into vesicles, fusing them with lysosomes to form autolysosome and degrading and recycling and reutilizing their internal metabolites, and participating in a wide range of biological processes such as stress response, programmed cell death, etc.[30-32]. Autophagy is a self-protection mechanism for the abnormalities of the energy metabolism of the organism or for the sensory stimuli of the outside world, and it plays a key role in the maintenance of the intracellular homeostasis[33]. Autophagy is divided into various types, according to the specificity of the degraded substrate can be divided into non-selective autophagy and selective autophagy, and according to the pathway of the substrate transported to the lysosome can be divided into microautophagy, macroautophagy and chaperone-mediated autophagy (Figure 2), of which macroautophagy is the most widely studied type, and is also the most distinctive mechanism in the eukaryotic cells, which can transport raw materials in the cytoplasm through the vesicles to lysosome, and then be degraded by lysosome hydrolysis enzymes into useful compounds for the cell to be recycled again[34,35]. Macroautophagy is usually the main form in which autophagy occurs, so we also define macroautophagy as autophagy.
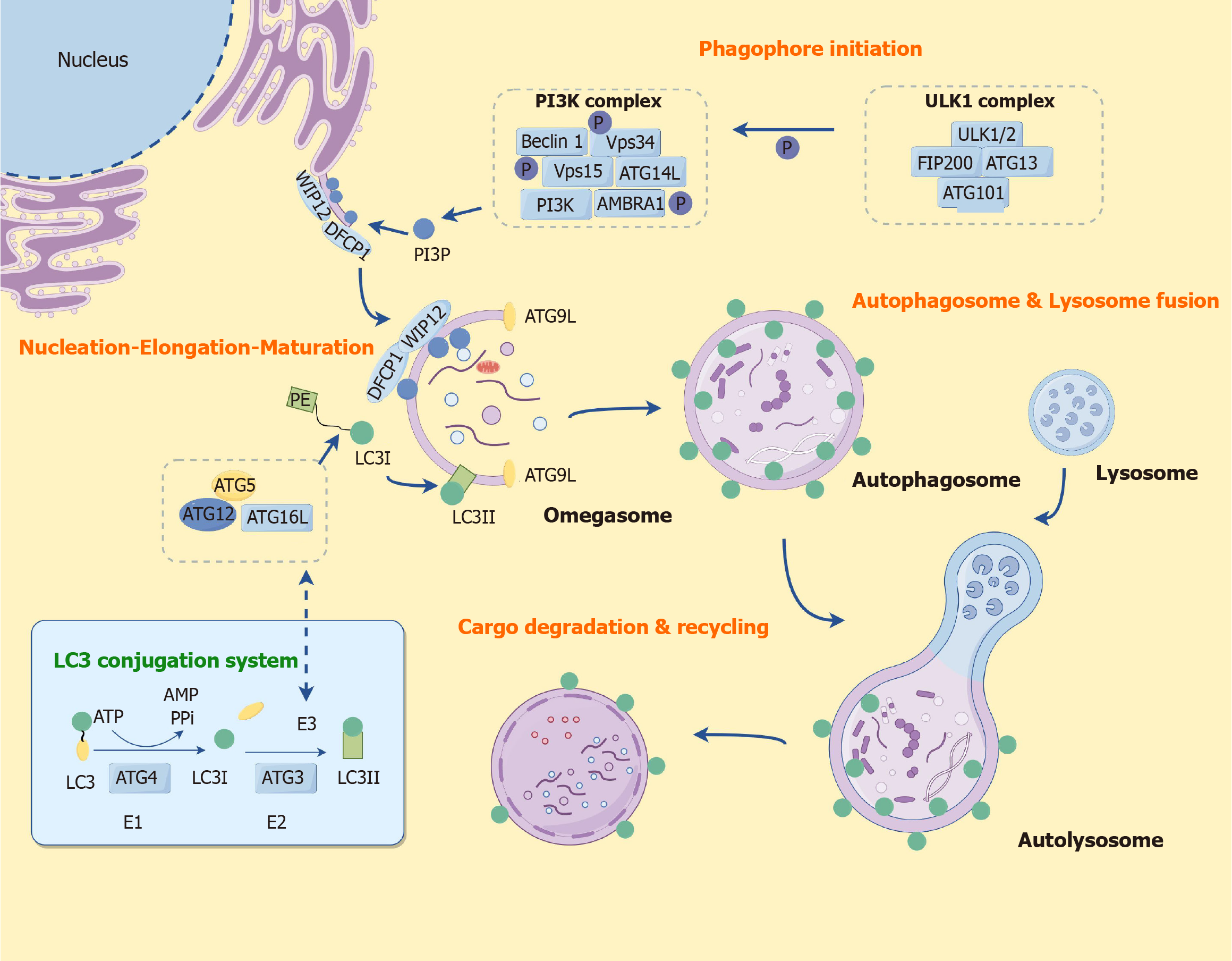
Initially, during the initiation of autophagy, a double-membrane-bound vacuole, referred to as an autophagosome, is generated. This autophagosome persistently fuses with the lysosome to facilitate the complete degradation of substrates, thereby enabling their reutilization by the organism[36]. Autophagosomes originate from phagosomes, which are membranes formed by a family of autophagy-related genes that are evolutionarily conserved across humans and yeast[37]. Autophagy is initiated by the UNC-51-like kinase 1 (ULK1) complex, and the subsequent phosphatidylinositol 3-kinase (PI3K) complex produces the phosphatidylinositol-3-phosphate required for autophagy, with WIPI2 and double FYVE domain containing protein 1 mediating the formation of phagocytic vesicles, and autophagy-related protein 12 (ATG12) forms a covalent linkage with ATG5 and a complex with ATG16L, the ATG12-ATG5-ATG16L preautophagosome structure. It participates in the membrane expansion of autophagic vesicles and binds to phosphatidylethanolamine (PE) to form lipid-soluble LC3II. LC3II binds to the membrane of autophagic vesicles and participates in the membrane expansion of autophagic vesicles and encapsulation of substances. Autophagosomes and lysosomes bind to each other to form autophagic lysosomes and degrade the cargo, which is recycled again by the cells. During the initiation phase of autophagy, the ULK1 complex (including ULK1, ATG13, FAK family kinase-interacting protein of 200 kDa, and ATG101) is activated. This complex plays a key role in the formation of autophagosomes[38-41]. Notably, PI3K complexes are also closely associated with autophagy initiation, and the ability of PI3K complex to produce phosphatidylinositol-3-phosphate required for autophagy promotes the formation of phagophore assembly site and facilitates the progression of the autophagy process[42,43]. Moreover, autophagosome formation is closely related to the Beclin-1 complex in addition to the ULK1 complex[44]. Beclin-1 forms complexes with proteins such as VPS34, VPS15, and ATG14, which are involved in the formation of membrane structures in autophagic vesicles. The ATG protein family (ATG12, ATG5, ATG16L, etc.) plays a key role in the formation of autophagic vesicles, with ATG12 forming a covalent linkage with ATG5 and a complex with ATG16L, the ATG12-ATG5-ATG16L preautophagosomal structure, which participates in the membrane expansion of phagophore assembly site[45,46].
The ubiquitin-binding proteins p62 and LC3 are key proteins in autophagosome formation and are recruited into the autophagosome membrane through binding interactions with LC3. LC3-PE[47] lipidation system is the hydrolysis of LC3 by ATG4 protein to generate water-soluble LC3I, which in turn is transferred to ATG3, and then ATG12-ATG5-ATG16L complex and PE bind to each other to form the lipid-soluble LC3II. LC3II attaches to autophagosome membranes and autophagy precursors until lysosomal and autophagosome are fused together, LC3II also binds to the membrane of autophagic vesicles and participates in the membrane expansion of autophagic vesicles and the encapsulation of substances, thus LC3II/LC3I is an important marker for observing the occurrence of autophagy[48,49]. The LC3-PE lipidation system, the ATG12-ATG5-ATG16L complex, and the PI3K complex interact synergistically with each other and play irreplaceable roles in the extension and closure of phagophore assembly site. After the formation of autophagosomes, through the assistance of some lysosome-associated membrane proteins, autophagosomes combine with lysosomes to eventually form autolysosome, and the substances encircling the autolysosome are degraded by lysosomal hydrolases into carbohydrates, fatty acids and amino acids, etc., which are recycled again by the cells, forming a virtuous cycle[50]. The degraded products are transported to other organelles via transport proteins for cellular reuse and participation in cellular metabolic processes such as new protein synthesis. Autophagy exerts the effect of keeping cells alive by completely degrading cellular material into metabolites that can be used in biosynthetic processes or energy production[51]. More importantly, autophagy-related signaling pathways[52,53] represent an adaptive response to maintain cellular homeostasis in response to cellular stress and play a key role in the regulation of autophagy (Figure 3).
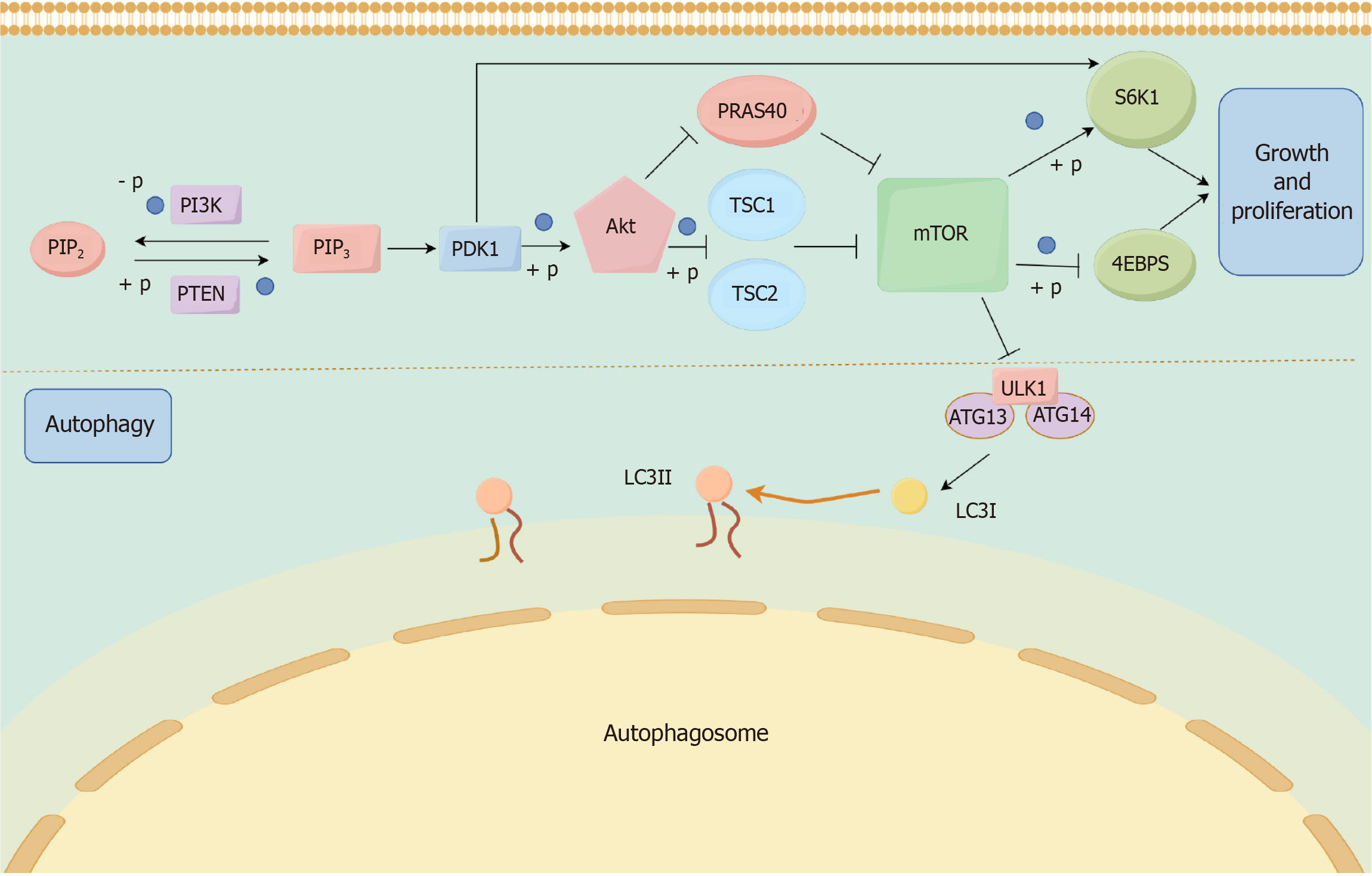
As one of the key regulators of autophagy, mammalian target of the rapamycin (mTOR) can regulate cell growth and metabolism by inhibiting the initiation of autophagy. mTOR down-regulation is one of the initiating signals of autophagy, so mTOR is both a classical autophagy negative regulator protein and a core hub for regulating autophagy. mTOR is a highly conserved serine/threonine protein kinase belonging to the PI3K-associated kinase family and exists in two complexes, mTOR complex 1 (mTORC1) and mTORC2[54]. In general, mTORC1 can act as a major regulator of cell growth and proliferation by promoting anabolic processes and limiting catabolic processes (e.g., autophagy, etc.), whereas the mTORC2 complex regulates cytoskeletal structure and cell survival[55,56]. When nutrition is sufficient, mTOR can inactivate autophagy-related genes, thus exerting an inhibitory effect on autophagy. However, when nutrition is deficient, the activity of mTOR is reduced by the class I PI3K-protein kinase B (Akt) pathway and autophagy is inhibited. mTORC1 inhibits catabolic processes, which include autophagy. In contrast to mTORC1, a better-studied autophagy regulator, the function of mTORC2 has not been fully characterized[57]. mTORC1 is a major regulator associated with protein synthesis and degradation. mTORC1 plays a key role, first, as a key complex in the inhibition of autophagy, and second, mTORC1 is also able to increase ribosome production to promote protein synthesis[58]. As a major upstream kinase for the activity of the autophagy-related protein ULK1, the downstream target of mTOR signaling is RPS6Kβ1, which also further promotes protein synthesis and plays a role in promoting cell proliferation[59]. Different signaling pathways converge on the PI3K lipid kinase complex and the ULK1 protein kinase complex. mTORC1 exerts a regulatory function on autophagy by effectively regulating the activity of the ULK1 complex. Upon activation of mTORC1, autophagy is inhibited due to mTORC1-mediated inhibitory phosphorylation of ULK1. However, under different cellular stresses, mTORC1 activity was inhibited, which increased ULK1 complex activity and induced autophagy[60]. When nutrition is sufficient, mTORC1 directly binds and phosphorylates ULK1 and ATG13, inhibiting their activities, whereas when nutrition is deficient or mTORC1 is inactivated, the dephosphorylated ULK1 complex is activated, which initiates autophagy and plays a regulatory role.
Beclin-1 is a key autophagy-associated protein that plays a critical role in the initiation and regulation of the autophagic process and participates in the autophagy complex to regulate the formation of autophagic vesicles, thus ensuring the normalization of autophagy[61,62]. For cell survival and maintenance of homeostasis, the role of Beclin-1 has important biological significance. As a direct homolog of ATG6, Beclin-1 is involved in the initiation of autophagic vesicles. It is a central player in the regulation of autophagy by forming complexes with other autophagy-related proteins and promoting the activation of VPS34, which initiates the formation of autophagic vesicles[63,64]. Activation of Beclin-1/VPS34 complex kinase activity further promotes the production of phosphatidylinositol 3-phosphate, which facilitates autophagosome maturation, cargo recruitment, and lipid membrane extension[65]. Moreover, during the initiation of autophagy, Beclin-1 also interacts with the UV radiation resistance-associated gene, which in turn forms a complex that promotes the formation of membrane structures of autophagic vesicles[66]. There are also related studies that found that Beclin-1 is not only involved in autophagy process in cell biology, but also related to anti-tumor[67] and regulation of apoptosis[68] and other processes.
The PI3K/Akt/mTOR signaling pathway (Figure 4)[69,70] is an important pathway in the regulation of cell metabolism, growth and survival, and is also closely related to the autophagy process. The activity level of this signaling pathway directly affects the initiation and inhibition of autophagy, and it is mainly composed of PI3K, Akt and mTOR. PI3K, as a class of intracellular lipoprotein kinase, can be stimulated and activated by cytokines and growth factors, and its type I PI3K activation catalyzes the generation of phosphatidylinositol 3,4,5-trisphosphate from phosphatidylinositol bisphosphate, and phosphatidylinositol 3,4,5-trisphosphate can further activate Akt. Akt, on the other hand, is one of the most critical signaling targets downstream of PI3K. After activation, Akt continues to activate downstream target proteins, which further promotes the activation of the downstream signaling node mTOR. 4E-binding protein 1 and S6 kinase beta 1 act as direct substrates for mTORC1 and regulate the initiation and extension of protein translation through phosphorylation of mTORC1, thereby participating in cellular responses and coordinating cell growth and proliferation[71,72]. And in the course of HCC, aberrant activation of the mTORC1-4E-binding protein 1/S6 kinase beta 1 signaling axis is one of the key mechanisms driving liver tumorigenesis, progression, and treatment resistance. Notably, the primary mechanism by which Akt activates mTOR is through inhibition of the tuberous sclerosis complex (TSC), a potent inhibitor of mTOR, and direct phosphorylation[73]. Akt-mediated phosphorylation of TSC relieved the inhibition of mTOR, thus exerting an indirect activation of mTOR[74]. In addition, Akt can also play a role in activating mTOR by inhibiting the negatively regulated proline-rich AKT substrate of 40-kDa and phosphorylation[75-77]. And then as mTOR is activated, the corresponding cascade effect will be activated to inhibit the progression of autophagy. As an important aggregation point of multiple regulatory cellular autophagy signaling pathways, mTOR has been a central factor in generating the autophagic process and is able to negatively regulate the occurrence of autophagy[78,79]. In summary, the PI3K/Akt signaling pathway acts as an upstream regulator of mTOR to regulate cellular autophagy through activation of mTOR, and the PI3K/Akt/mTOR signaling pathway plays a key role in cell growth and survival, which directly affects the autophagic process of cells. Both over-activation or inhibition of this signaling pathway may be associated with a variety of diseases, including HCC, metabolic diseases, and so on. Therefore, the PI3K/Akt/mTOR signaling pathway has also become an important target for therapeutic research and drug development.
Yes-associated protein (YAP) in the Hippo signaling pathway is an important transcription factor in this signaling pathway, which mainly regulates cell proliferation, apoptosis, and effectively maintains cellular homeostasis, and the dysregulation of this pathway contributes to the development of many types of cancer, including HCC[80]. Dysregulation or overexpression of the Hippo/YAP signaling pathway has been found to be able to cause liver growth, increase in tumor cells, and so on[81]. Hippo/YAP, as an important cell signaling pathway, is involved in the regulation of biological processes such as apoptosis, differentiation, proliferation, and tissue development. Recent studies have shown that the Hippo/YAP signaling pathway is also highly correlated with autophagy and plays an important role in cell metabolism and survival[82]. The Hippo signaling pathway is mainly composed of three kinase cascades, YAP/transcriptional coactivator with PDZ-binding motif, mammalian Sterile 20-like kinase 1/2 (MST1/2) and large tumor suppressor 1 and 2 (LATS1/2). Upon activation of the Hippo signaling pathway, MST1/2 is able to interact with the cofactor Sav1 and activate LATS1/2, which in turn promotes LATS1/2 phosphorylation and can inhibit YAP activity[83,84]. It is worth our attention that MST1/2 phosphorylates LATS1/2 in many ways[85,86], MST1/2 can phosphorylate Mps One binder 1, and then Mps One binder 1 binds to its own inhibitory domain of LATS1/2 to activate LATS1/2. Secondly, MST1/2 is also capable of phosphorylating LATS1/2 directly at the C-terminal hydrophobic group, which ultimately exerts its effect. YAP[87,88], a key downstream effector of the Hippo signaling pathway, is located in the cytoplasm and is phosphorylated in the inactivated state. However, when the Hippo signaling pathway is activated, the activity of YAP is inhibited. Meanwhile, it was found that the downregulation of YAP was associated with the activation of autophagy, specifically, the degradation of YAP could promote the initiation and execution of autophagy[89,90]. Overall, there is a complex interaction between autophagy and the Hippo/YAP signaling pathway, which is biologically important for maintaining cell survival and metabolic homeostasis, and this physiological function and the specific mechanism still require more in-depth studies in the future.
Janus kinase (JAK), as a non-tyrosine receptor kinase, mainly includes four members, tyrosine kinase 2 and JAK1-3. And signal transducer of activation (STAT) as a downstream target of JAK, the main members include seven different isoforms of STAT1-STAT4, STAT5a/b and STAT6. Upon phosphorylation, JAK is able to activate the downstream target STAT, which in turn leads to the dimerization of phosphorylated STAT to transfer to the nucleus to bind to specific DNA regulatory sequences, thereby further regulating gene expression and transcription[91]. Nowadays, the JAK/STAT signaling pathway has been shown to be a new autophagy regulator that can inhibit factors that regulate or transcriptionally activate autophagy, such as Beclin-1, Bcl-2/adenovirus E1B 19 kDa interacting protein 3, and hypoxia-inducible factor-1alpha, and thus exert a therapeutic effect on disease[92,93]. Upon stimulation by activating factors, JAK protein kinase becomes activated and subsequently phosphorylates STAT proteins. These activated STAT proteins then translocate into the nucleus, where they regulate gene expression through multimer formation - a process intricately linked to the initiation of autophagy. STAT3 serves as a key downstream effector within the JAK/STAT signaling pathway and plays a significant role in modulating autophagy[94,95]. Studies have indicated that high glucose-induced activation of the JAK/STAT pathway in podocytes inhibits autophagy, thereby impeding the efficient clearance of damaged organelles and proteins, increasing apoptosis, and ultimately exacerbating disease progression[96]. Despite these findings, the precise mechanistic relationship between the JAK/STAT signaling pathway and autophagy remains incompletely understood. Future research, involving more comprehensive animal experiments or clinical trials, is essential to elucidate this interaction and provide novel insights and references for clinical applications.
AMP-activated protein kinase (AMPK), as an important protein kinase in organisms, can maintain organismal and cellular energy homeostasis by regulating a variety of metabolic pathways and, as a major energy-sensitive kinase, plays an important role in the regulation of cellular energy homeostasis. Most notably, AMPK was able to activate the phosphorylation of the autophagy-related gene ULK1, or directly phosphorylate core autophagy components such as Beclin-1, ATG9 and VPS34, effectively promoting autophagy[97-99]. The important energy receptor AMPK can also be regulated by ATP/AMP, which is stimulated and activated in response to AMP overaccumulation or ATP deficiency, thus further participating in organismal autophagy. As an ATP receptor, mTOR is also a major regulator involved in the regulation of autophagy. Under nutrient-rich conditions, mTOR is able to promote anabolism and block the catabolic process, which ultimately promotes cell proliferation and growth and exerts the effect of inhibiting autophagy[100]. Moreover, mTOR is a downstream target of AMPK, and activated AMPK promotes autophagy by regulating downstream signaling pathways and ultimately negatively regulating mTOR expression[101,102]. AMPK activates autophagy by directly phosphorylating ULK1, such as Ser317/Ser777, and is also able to deregulate its inhibitory effect on ULK1 by inhibiting mTORC1, which synergistically promotes the initiation of autophagy under energetic stress. In addition, Ser317 phosphorylation promotes ULK1 kinase activity and autophagosome formation and enhances the interaction of ULK1 with downstream autophagy proteins such as FAK family kinase-interacting protein of 200 kDa, while Ser777 phosphorylation stabilizes the assembly of the ULK1 complex and prevents its inhibition by mTORC1. Finally, related studies also found that AMPK was able to directly phosphorylate mTOR and further lead to a decrease in the level of autophosphorylation, promoting the initiation of autophagy[103].
The hedgehog signaling pathway was first discovered by Nüsslein-Volhard and Wieschaus[104] when they screened for mutated genes in Drosophila, which caused Drosophila embryos to take on the shape of spiny blobs like hedgehogs, hence the name “Hedgehog”. The hedgehog signaling pathway, as one of the key signaling pathways for embryonic development and differentiation[105,106], also plays an important role in the regulation of organismal autophagy[107,108]. The hedgehog signaling pathway is mainly composed of the transmembrane protein Smo, the hedgehog signaling pathway ligand Hh, the nuclear transcription factor Gli, the transmembrane protein receptor Ptch, the repressor factor Sufu, the kinesin-like protein Cos2, and the serine/threonine protein kinase Fu[109]. And up to now, three hedgehog homologous genes have been identified in mammals, namely Indian hedgehog, Sonic hedgehog, and desert hedgehog. Relevant scholars have long found that the nuclear transcription factor Gli can significantly enhance the activities of caspase-9 and caspase-3 by decreasing the expression of cyclinE2 and cyclinD1, which ultimately interferes with apoptosis and proliferation of HCC cells[110]. The Gli inhibitor GANT61, on the other hand, inhibits the hedgehog signaling pathway while inducing autophagy, which plays a role in attenuating hepatic fibrosis, and also improves renal and pulmonary fibrosis, with remarkable efficacy. What’s more, GANT61 can inhibit cell proliferation and induce apoptosis while activating autophagy[111]. Some other scholars have demonstrated that the Gli inhibitor GANT61 also induces enhanced autophagy in the organism by inhibiting the hedgehog signaling pathway, whereas Hh agonists or Hh ligands inhibit the enhancement of autophagy by modulating the hedgehog signaling pathway[112]. The hedgehog signaling pathway has a key role in regulating intracellular autophagy, but the regulation of autophagy is not exactly the same in different diseases, and the related molecular regulatory mechanisms are not fully understood, which requires further in-depth studies[113].
As a key ingredient of the traditional Chinese herb Oldenlandia diffusa, the total flavonoids of Oldenlandia diffusa have significant anti-tumor effects. Chen et al[114] have demonstrated through in vivo and in vitro models that flavonoids of Oldenlandia diffusa can induce autophagy and apoptosis in HCC cells by activating the protein kinase R-like endoplasmic reticulum kinase-eukaryotic translation initiation factor 2α-activating transcription factor 4 signaling pathway and inducing endoplasmic reticulum stress, which ultimately exerts an inhibitory effect on HCC proliferation. Quercetin, as a plant flavonoid, is widely found in various herbal medicines, such as chrysanthemum and senna, etc. Quercetin is also a natural antioxidant with very rich biological activities[115]. One study found that after quercetin intervention, tumor weight and volume in HCC model mice were significantly reduced, the proportion of CD206+ cells was reduced, while the proportion of CD86+ cells was significantly increased, and the levels of pro-inflammatory factors such as interleukin-17A, tumor necrosis factor-α, and interleukin-6 were also significantly inhibited, which ultimately confirmed that quercetin exerts a positive therapeutic effect on HCC by regulating macrophage polarization and promoting autophagy through the nuclear factor-kappaB signaling pathway[116]. Other researchers have found that quercetin can exert inhibitory effects on the growth of human HCC cells in a time and dose-dependent manner through in vitro experiments[117]. Through relevant functional tests, it was found that quercetin can effectively activate the mitogen-activated protein kinases (MAPK) pathway and inhibit the AKT/mTOR pathway, and ultimately stimulate autophagy, which stimulation of autophagy may be the key mechanism of action of quercetin in inducing apoptosis in HCC tumor cells. Isoquercitrin (ISO) is a flavonoid compound present within Chinese medicines such as mulberry leaves and honeysuckle, with biological activities such as anti-allergy, anti-tumor[118] and anti-virus. Shui et al[119] applied ISO to Huh7 and HepG2 human HCC cells and found that exposure to ISO inhibited cell colony growth and viability and triggered autophagy dysregulation while activating the AMPK/mTOR/p70S6K pathway, which ultimately demonstrated that ISO could induce cellular autophagy to exert a potential anti-HCC effect.
The flavonoid baicalin, found mainly in the herb Scutellaria baicalensis, is one of the main active ingredients in the root of Scutellaria baicalensis, and has potential preventive effects on the development and progression of various chronic liver diseases[120]. Tan et al[121] found that baicalin-treated tumor-associated macrophages cultured with HCC cells were able to significantly reduce the proliferation of HCC cells, and that baicalin directly induced the repolarization of M2 macrophages and tumor-associated macrophages, but this particular effect was closely related to the transcriptional activation of the RelB/p52 pathway and the elevation of autophagy, which ultimately clarified the significant inhibitory effect of baicalin on HCC. Baicalein (BA), another key ingredient of Scutellaria baicalensis, differs from baicalin in its uses, biological activities and chemical structure, and is commonly used in the treatment of a variety of diseases with anti-tumor and anti-hepatotoxic effects[122]. Wang et al[123] found that BA intervention in HCC HepG2 cells effectively prevented colony formation and was able to significantly induce autophagosome formation after one day of intervention, while at the same time, BA significantly up-regulated the expression of microtubule-associated proteins 1A/1B-light chain 3-II in HepG2 cells in a time-dependent and concentration-dependent manner. There are also related experiments[124] showing that BA can trigger protective autophagy in HCC cells and can induce apoptosis through endoplasmic reticulum stress, which ultimately exerts anti-HCC effects, and the further combination of autophagy inhibitors with BA is likely to be a new approach for HCC prevention or treatment in the future. Niu et al[125] also found in in vitro and in vivo experiments that BA was also able to significantly inhibit hepatitis B virus secretion and prevent HCC by inhibiting the CCDC88A-AKT mTOR signaling pathway and activating autophagy, thereby exerting an antiviral effect. Luteolin, which is found in a variety of plants, also possesses a variety of pharmacological activities, especially its antitumor properties[126]. Cao et al[127] concluded that luteolin can be widely used as an autophagy modulator for the treatment of HCC. Luteolin rapidly increased the number of intracellular autophagosomes and further promoted the conversion of LC3B-I to LC3B-II, and facilitated the expression of Beclin-1. Meanwhile, some other studies have found that luteolin can act directly on liver tumor cells, inhibit HCC cell growth, regulate autophagy and induce apoptosis, and also further affect immunogenic cell death, thus exerting anti-HCC effects[128].
Nowadays, apigenin, which is widely distributed in nature, has become a key ingredient in anti-cancer immunotherapy and also has significant anti-viral effects[129,130]. Apigenin was able to reduce the viability of SK-Hep1 and SMMC-7721 cells in a dose-dependent manner, which inhibited the invasion and migration of HCC cells, and apigenin also triggered the autophagy of HCC cells by regulating the expression of autophagy-related genes, further exerting a therapeutic effect[131]. Muscone, as a Chinese medicine monomer, Qi et al[132] found that muscone was able to increase the autophagy and apoptosis rates of HCC cells by modulating the AMP kinase/mTORC1 signaling pathway thereby inducing autophagy, which ultimately promotes apoptosis through endoplasmic reticulum stress, thus exerting an anti-HCC effect. Prunella vulgaris total flavonoids are derived from the Chinese medicine Xia Gu Cao. It was found that Prunella vulgaris total flavonoids significantly reduced tumor weight and volume, inhibited the activity of SMMC-7721 cells, suppressed autophagy and promoted apoptosis in HCC cells by activating the PI3K/Akt/mTOR signaling pathway in HCC mice[133]. Isoginkgetin (ISK) is a natural biflavonoid isolated from the traditional Chinese herb Ginkgo biloba, which is often used clinically for the treatment of circulatory disorders. But nowadays, Yao et al[134] have found that ISK can inhibit the migration and proliferation of HCC cells through in vitro experiments. After the intervention of ISK, LC3II expression and autophagy will be significantly increased, and the knockdown of autophagy-related genes ULK1 or ATG5 will alleviate the ISK-induced cell death. The Chinese medicine monomer delphinidin is uniquely effective in the prevention and treatment of tumors, such as HCC[135], breast cancer[136] and lung cancer[137]. It was found that delphinidin could effectively block autophagic flux by inhibiting the expression of DEAD box RNA helicase 17 and multidrug resistance protein 1, inducing autophagic pathway blockage and promoting apoptosis in HCC cells, which had good anti-HCC effects[138]. Some of the studies also found that the growth inhibitory effect of the flavonoid Kaempferol on HCC cells at different time intervals was time- and dose-dependent, and that Kaempferol was also able to reduce the motility of Hep3B cells and stall the cell cycle in the G0/G1 phase, ultimately reversing the protective effect of endoplasmic reticulum stress inhibitors by inducing cellular autophagy and apoptosis[139]. In addition, Han et al[140] found that the flavonoid epimedium extract is also capable of exerting a therapeutic effect on HCC by activating extracellular signal-regulated kinase (ERK)/ULK1/nuclear receptor coactivator 4 (NCOA4)-mediated iron autophagy, which serves as a novel regulatory mechanism. Icariin is also a common flavonoid, and studies have confirmed that Icariin can promote autophagy and apoptosis in HCC cells through the lncRNA LOXL1-AS1-mediated β-catenin signaling pathway, exerting anti-HCC effects[141]. Finally, the prenylated flavonoid icaritin, as a multifunctional flavonoid natural product, was also confirmed in relevant clinical trials to exert anti-HCC activity by modulating the receptor for advanced glycation end-products/high mobility group box 1 signaling pathway and further regulating the apoptosis/autophagy crosstalk[142].
Platycodin D (PD) is a saponin analog isolated from the traditional Chinese medicine Platycodon grandiflorum, which is used as a natural product in clinical studies for a variety of biological activities such as anti-tumor, cough suppressant, immunomodulation and analgesia[143]. By observing the accumulation of autophagosomes, up-regulation of LC3-II, and cytoplasmic vacuoles, Li et al[144] found that PD could trigger protective autophagy in HCC HepG2 cells, in which the activation of ERK played a key role, which provides a new solution for the prevention and treatment of HCC in the future. At the same time, these investigators also found that PD was also involved in inducing autophagy in BEL-7402 cells, as evidenced by an increase in the number of MDC-positive cells and the formation of cytoplasmic vacuoles, and that intervention with an autophagy inhibitor was able to potentiate PD-induced apoptosis and inhibition of cell proliferation[145]. Solamargine is an alkaloidal glycoside extracted from the traditional Chinese medicine lobelia, and as a traditional herbal-derived compound, it has been shown to induce apoptosis in a variety of cancer cells[146,147]. A systematic review also found that solamargine is able to exert anticancer activity by affecting multiple biological pathways, including the mitochondrial pathway, tumor suppressor pathway, and cell survival pathway[148]. Some scholars found that the oncogenic factor leukaemia inhibitory factor would be abnormally elevated in HCC tissues and would be down-regulated to a certain extent after intervention with solamargine, which was later also confirmed to be able to effectively induce autophagy and apoptosis of HCC cells both in vivo and in vitro, thus further inhibiting the proliferation of HCC[149].
Dioscin[150], as a natural biologically active saponin analog with many biological activities, has been widely focused on by researchers around the globe, exerting significant therapeutic effects on human malignant tumors, organ damage, and metabolic disorders. Mao et al[151] found that dioscin significantly induced cell autophagy, apoptosis and DNA damage in HepG2 and SMMC7721 cells, exerted inhibitory effects on cell migration and proliferation, and also further restored liver function and Ki67 levels and changed body weight of rats, which had a significant inhibitory effect on primary HCC in rats. Saikosaponin D, a triterpenoid saponin isolated from the traditional plant saikosaponin, was able to induce autophagy, apoptosis, and differentiation in different cancer cells, as well as significantly reduce side effects and enhance anticancer effects[152]. Tian et al[153] found that the number of radiation-induced autophagosomes was significantly increased in HCC cells after saikosaponin D intervention by laser confocal microscopy and transmission electron microscopy, while the intervention of mTOR agonist significantly reduced the formation of autophagosomes in HCC cells, and it is precisely by inducing the formation of autophagy that saikosaponin D enhances the radiosensitization of the cells and inhibits the growth of HCC cells. Anemarrhena asphodeloides Bunge is a commonly used plant-based Chinese medicine with a history of more than 2000 years, and timosaponin-AIII (TSAIII), a biologically active steroidal saponin, was isolated from this plant-based Chinese medicine, and now TSAIII is mostly used for anti-tumor therapy in clinical practice[154]. Wang et al[155] found that TSAIII was able to degrade X-linked inhibitor of apoptosis protein through the autophagy-lysosome pathway and induce apoptosis in HCC cells through a p53-dependent mechanism, resulting in favorable therapeutic effects. Deapioplatycodin D is a triterpenoid saponin extracted from the root of Platycodon grandiflorum, and Li et al[156] similarly found that deapioplatycodin D increased changes in autophagy-associated protein levels and induced cellular senescence, which exerted an inhibitory effect on HCC cell proliferation.
The natural alkaloid lycorine, which is found in the plant Allium sativum in the family Alliaceae, has great therapeutic potential. Lycorine is highly specific for a wide range of cancers in vitro and in vivo, as well as for a variety of drug-resistant cancer cells[157]. Some researchers found that lycorine induced autophagy and apoptosis effects in HCC cells in vivo and in vitro, and through molecular validation of the mechanism, it was found that the reduction of tongue cancer resistance-associated protein 1 level and lycorine-induced autophagy in HCC cells were closely related, which ultimately confirmed that lycorine promotes HCC cell autophagy and apoptosis, thus exerting a significant anti-HCC effect[158]. Berberine[159], which is mainly found in the traditional Chinese medicine Huanglian and other berberis plants, can significantly improve liver injury, and can also effectively inhibit the proliferation of various types of tumor cells and prevent their metastasis. In vitro and in vivo experimental studies revealed that after interfering with ATG5, a key gene for autophagy, berberine-induced cell death in human HCC cells was attenuated, and berberine was also able to modulate the mTOR signaling pathway by inhibiting Akt activity, up-regulating P38 MAPK signaling, and activating Beclin-1, thereby further inducing MHCC97-L and HepG2 cells to undergo autophagic death[160]. Other researchers have found through in-depth studies of berberine that it can inhibit the viability of HCC cells in a time- and dose-dependent manner and exert therapeutic effects through cellular autophagy and apoptosis, and that berberine also significantly inhibits CD147 expression in HCC cells in a dose-dependent manner[161].
Stachydrine hydrochloride is the main bioactive ingredient extracted from the traditional Chinese medicine Leonurus japonicus Houtt. It has blood-activating, diuretic and uterine contracting effects. Bao et al[162] demonstrated through in vitro studies that stachydrine hydrochloride could effectively promote the expression of LC-3B and p62, and exerted anti-HCC effects by inducing autophagy, cell cycle arrest, which in turn further promoted cellular senescence. Fangchinoline[163], originally isolated from the dried root of Stephaniae tetrandrine, has a remarkable pharmacological profile, including some anticancer activity in selected preclinical models and tumor cell lines, and can participate in and modulate the activation of various key oncogenic molecules to exert therapeutic effects. Relevant studies have shown that fangchinoline, although unable to induce apoptosis in PLC/PRF/5 and HepG2 cells, can play an anti-HCC role through the regulation of autophagy, the main process of which is firstly, the nuclear translocation of p53 stimulates the autophagy induction of fangchinoline, and then transcriptionally activates the key gene of autophagy, sestrin2, which ultimately initiates the autophagy[164].
Glycyrrhetinic acid (GA), one of the key ingredients of the traditional Chinese herb licorice (Glycyrrhiza uralensis Fisch), also has significant anti-HCC effects and is widely used in drug preparation. GA was able to significantly reduce the viability of HCC cells, and when autophagy was inhibited with chloroquine, the viability of the cells was significantly decreased, but the expression of cleaved caspases was significantly increased, while after the intervention of GA in HepG2 cells, ERK was activated, which ultimately triggered the protective autophagy of HCC cells[165]. Alantolactone is derived from Inula helenium, and Kang et al[166] found that apoptosis could be induced in liver tumor cells through phosphatase and tensin homolog (PTEN)-induced putative kinase 1-mediated mitotic inhibition and reactive oxygen species-mediated inhibition of AKT signaling, and that mitochondrial autophagy, as a type of cargo-specific autophagy, plays a key role in this apoptotic cell death as well. Cinobufagin is a natural active ingredient isolated from the traditional Chinese herb Venenum bufonis, which is capable of reversing multidrug resistance in tumor cells by modulating autophagy[167]. Using transcriptome analysis, some scholars found that cinobufagin was able to trigger protective autophagy and exerted an inhibitory effect on apoptosis in HCC Huh-7 cells and HepG2 by acting on the PI3K/Akt/mTOR signaling pathway, and also up-regulated the expression of autophagy-associated protein 12-5 and Beclin1 to promote cellular autophagy[168].
Celastrol, a terpenoid extracted from the traditional Chinese medicine Lei Gong Teng, is an emerging anticancer agent with significant broad-spectrum anticancer activity[169]. Han et al[170] found that in human HCC cells HepG2, celastrol was able to induce the accumulation of hypoxia-inducible factor-1alpha protein, which increased the expression of Bcl-2/adenovirus E1B 19 kDa interacting protein 3, thus further inducing autophagy and ultimately exerting therapeutic effects. Aloin is a Chinese Pharmacopoeia quality standard compound with a wide range of pharmacological activities such as anti-tumor[171]. In vivo and in vitro experiments revealed that aloin was able to inhibit the expression of circ_0011385, and also promoted cell autophagy and apoptosis, which ultimately exerted an inhibitory effect on the invasion and proliferation of HCC through the regulation of the circ_0011385/miR-149-5p/WT1 axis[172]. Carnosic acid (CA) is a terpene compound isolated from Rosemary, which has antibacterial and antioxidant properties and is widely used in drug discovery and nutritional healthcare industries[173]. Through cellular experiments, Gao et al[174] found that after CA intervention, PTEN and PI3K were not affected, but the levels of phosphorylated Akt and mTOR were reduced, which ultimately confirmed that CA could significantly induce autophagic cell death in HepG2 cells by inhibiting the Akt/mTOR pathway. Bruceae fructus is a fruit-based Chinese medicine, which is effective in clearing heat and removing toxins, killing worms, and stopping dysentery, and brusatol, as its key Chineses medicine monomer, has a strong anticancer potential. Ye et al[175] jointly found that brusatol could activate autophagy in HCC cells, and the autophagy inhibitor chloroquine could reverse brusatol-induced apoptosis in Bel7404 cells. The final results showed that brusatol induced autophagy by acting on the PI3K/Akt/mTOR pathway, and exerted the effects of promoting HCC cell apoptosis and inhibiting tumor cell proliferation. The electrophilic sesquiterpenes isolated from Eupatorium chinense L. are key herbal monomers for HCC treatment, Zhu et al[176] found through in vivo experiments that Eupatorium chinense L. significantly inhibited the growth of HCC in xenograft tumor mouse model and were able to promote apoptosis through mitochondrial dysfunction, and also promoted ferrocyte apoptosis through NCOA4-mediated ferritin phagocytosis. It is worthy of our attention that Tian et al[177] conducted an in-depth study on the terpene Euphorbia fischeriana Steud root extract and performed in vitro and in vivo assays, and found that it regulates NCOA4-mediated ferrophagocytic proteins by targeting the PI3K/Akt pathway, which further induces an increase in iron eosinophils and exerts an anti HCC effects.
Resveratrol is a naturally occurring polyphenolic compound found in a variety of medicinal plants. It is a secondary metabolite produced by plants under stress, is readily absorbed orally, and metabolized and excreted in the urine and feces. Current research has confirmed that resveratrol has a therapeutic effect on cancer[178], diabetes[179] and vascular disease[180], but there are some potential adverse effects[181]. Relevant studies found that resveratrol-induced exosomes could inhibit the malignant phenotype of Huh7 cells by suppressing the activation of autophagy and nuclear translocation of β-catenin, and also inhibit exosome secretion by down-regulating the expression of Rab27a, which could inhibit the migration and proliferation of Huh7 cells and inhibit the further development of HCC[182]. Wu et al[183] also found that resveratrol can effectively stimulate autophagy, regulate the immune system and regulate the intestinal microbiota, in addition to the potential therapeutic effect on HCC, there is a certain preventive effect on malignant diseases, such as gastric cancer, lung cancer and thyroid cancer. Salidroside (Sal) is a key compound isolated from various Rhodiola rosea plants and has a wide range of biological activities including anti-tumor, anti-viral, anti-inflammatory, and immunostimulatory activities, and Sal also has an anti-aging effect and is able to prolong the human lifespan by interfering with the activity of heat shock protein 90[184,185]. Jiang et al[186] performed a cellular assay and found that Sal was able to up-regulate the expression levels of Beclin-1, Bax, LC3II, caspase-9, and caspase-3 proteins, as well as down-regulate the expression levels of p62, LC3I, Bcl-2, proteins, in 97H cells, which resulted in a significant enhancement of autophagy and apoptosis, while at the same time, the Sal also inhibited the activation of the PI3K/Akt/mTOR signaling pathway, and significantly increased the phosphorylation of PI3K, Akt, and mTOR proteins when combined with autophagy inhibitors.
Plumbagin (PL) is an active anthraquinone compound, Lin et al[187] found that PL could significantly inhibit tumor growth in an in vitro HCC nude mouse model study, and also found by cellular experiments that after PL intervention, the expression of factors related to tumor autophagy and apoptosis, such as ATG5, Beclin1, ATG7, and LC3, was significantly inhibited in SMMC-7721 cells. were significantly inhibited. The Chinese medicine monomer emodin[188], a natural anthraquinone derivative, is present within many widely used Chinese medicines, such as Rhubarb and Huzhang, but may have some hepatic and renal toxicity when used for long periods of time or in excessive amounts. Emodin was able to induce autophagy to promote the degradation of β-catenin and Snail, and induced autophagy and suppressed epithelial-mesenchymal transition through inhibition of Wnt/β-catenin and PI3K/Akt/mTOR pathways, which ultimately blocked cellular metastasis of HCC[189]. In addition, relevant studies have found that emodin can mediate cellular autophagy by regulating the miR-371a-5p/PTEN axis, which ultimately exerts an inhibitory effect on liver tumors[190]. Gamabufotalin and bufalin, both Chinese medicine monomers, are found in the skin glands of the toad, and although they have certain biological activities, they may also cause certain toxic side effects in humans when applied clinically. Gamabufotalin was able to up-regulate the expression of LC3II/LC3I and down-regulate the expression of p62, and promoted cytoprotective autophagy through the mTOR/ULK1 signaling pathway, which significantly inhibited the growth of HCC xenograft tumors without significant toxicity to normal tissues[191]. Tsai et al[192] found that bufalin was able to trigger DNA breaks and apoptosis in SK-HEP-1 cells, whereas through acidic vesicular organelles, double-membrane vacuoles and cleavage of microtubule-associated protein 1 light chain 3, triggered autophagic cell death in SK-HEP-1 cells. Other researchers conducted cellular experiments and found that bufalin was able to act synergistically with the JNK pathway, promoting the expression of ATG8, BECN-1, MAPK, and tumor necrosis factor, and down-regulating the expression of Bid and Bcl-2, exerting the effect of inducing autophagy in liver tumor cells[193].
Chen et al[194] found that the herbal monomer artesunate could inhibit the degradation of autophagosomes in HCC cells, which was mainly manifested by the increase in the number of double-membrane vacuoles and GFP-LC3B puncta, the enhancement of SQSTM1/p62 expression, the elevated expression of LC3B protein, and ultimately exerted the anti-HCC effect by inhibiting lysosomal autophagy. Dihydroartemisinin, the active metabolite of artemisinin and its derivatives, has a wide range of anticancer activities and can exert synergistic antitumor effects with a variety of clinical drugs[195]. Here, a study confirms that dihydroartemisinin effectively modulates autophagy and inhibits the proliferation of HepG2215 cells in a time- and dose-dependent manner, whereas reactive oxygen species production induces mitochondrial and nuclear DNA damage[196]. Gomisin N (GN) as a common herbal monomer is derived from Schisandra chinensis. Existing studies confirmed that GN significantly reduced the protein levels of phospho-PI3K, phospho-Akt, and Mcl-1 in HCCLM3 and HepG2 cells, and more importantly, GN was further able to activate mTOR while inhibiting the activity of ULK1, exerting an inhibitory effect on autophagy in liver tumor cells[197]. The herbal monomer cryptotanshinone (CPT) inhibits tumor cell proliferation, migration, and invasion, and its efficacy can be significantly enhanced by combining it with antitumor drugs. Luo et al[198] found that CPT was able to elevate the expression of ATG5 and Beclin1 and the conversion of LC3II, while effectively reducing the expression of p62/SQSTM1, exerting the effect of inducing autophagy in HCC cells and protecting HCC cells from cell death, and discovering the potential anti-tumor mechanism of CPT.
Esculetin is a natural dihydroxycoumarin, which is mainly extracted from the Chinese herb Fraxinus rhynchophylla Hance, and has biological properties such as anti-tumor, anti-inflammatory and anti-diabetic[199]. One experiment found that esculetin was able to change the level of cellular autophagy and iron metabolism, promoted ferritin-associated phenomena, and significantly inhibited the migration and proliferation of HCCLM3 and HUH7 cells, altered the level of cellular oxidative stress, and ultimately reduced the size of the tumor cells, exerting a significant inhibitory effect on hepatic malignant tumors[200]. The Chinese medicine monomer gambogenic acid is extracted from the plant Gamboge and also has excellent anti-tumor properties[201,202]. Gambogenic acid can effectively inhibit the degradation of autophagosomes and exert the effect of inhibiting protective autophagy in BEL-7402/ADM cells, and also further promote adriamycin-induced apoptosis, thus increasing the sensitivity of adriamycin to BEL-7402/ADM cells[203]. Hydroxysafflor yellow A (HSYA), a natural active ingredient extracted from the traditional Chinese medicine safflower, blocks autophagic flux to promote apoptosis in liver cancer and also inhibits cell proliferation[204]. Chen et al[205] found that the expression of Beclin 1 and LC3II was significantly increased and the levels of p62 and phosphorylated-ERK1/2 were significantly decreased in HepG2 cells treated with HSYA, and it was precisely by inhibiting the phosphorylation of ERK and promoting the expression of Beclin 1 in HCC cells that HSYA regulated autophagy, thus exerting a positive therapeutic effect. Gamabufotalin, a key compound extracted from the traditional Chinese medicine Chansu, also possesses anti-HCC activity, and Zheng et al[206] found that it significantly enhances cell autophagy and apoptosis within tumors, and prevents further development of HCC by down-regulating STAMBPL1 and reducing mTOR signaling.
In conclusion, the monomers and active ingredients of Chinese medicine have the potential to elucidate the specific mechanisms and material basis by which Chinese medicine regulates autophagy to prevent and manage HCC. These ingredients primarily include flavonoid compounds, saponin compounds, alkaloids, terpenoids, phenolic compounds, and other types of compounds. Currently, research on the regulation of autophagy by Chinese medicine monomers is limited and warrants further investigation.
The complexity of Chinese medicine formulations, the absence of standardized dosages, and significant regional variations in ingredient efficacy present substantial challenges for in-depth research. Consequently, researchers have increasingly focused on the structural diversity and active roles of Chinese medicine monomers. With robust governmental support for the modernization of Chinese medicine, alongside the promotion of its clinical applications and the advancement of research, the study of Chinese medicine monomers has emerged as a primary approach for investigating the anti-disease mechanisms of Chinese medicine. What is more worthy of our attention is that Chinese medicine contains a unique theoretical system and practical experience, but in the context of the high development of modern medicine, the modernization of Chinese medicine still needs to achieve a breakthrough through scientific clinical trial[207]. Firstly, clinical trial can transform traditional experience into quantifiable evidence and promote the theory of Chinese medicine to leap from empirical medicine to evidence-based medicine. Secondly, the establishment of efficacy evaluation standards through clinical trial and the realization of quality control by combining core technologies such as fingerprint mapping[208] can ensure the safety and reproducibility of Chinese medicine treatment and lay a solid foundation for future development. Finally, systematic clinical trial can also screen the active ingredients and optimize the compounding scheme, as well as further clarify their indications and mechanisms of action, so as to promote the transformation of individual ingredients of Chinese medicine into internationally recognized innovative medicines.
HCC primarily manifests as lesions in the liver; however, its pathological impact extends beyond the hepatic system, affecting multiple bodily systems and organs, thereby inflicting significant psychological and physiological harm on patients. The investigation into the effects of Chinese medicine monomers and their active constituents on HCC is fundamentally an exploration of their pharmacological mechanisms of action on the disease. This approach integrates traditional Chinese medicine with Western medical methodologies to elucidate therapeutic mechanisms. Numerous studies highlighted in this paper underscore the pivotal role of autophagy in HCC progression, suggesting that autophagy may emerge as a novel therapeutic target for HCC prevention and treatment. Recent advancements indicate that the modulation of autophagy by Chinese medicine monomers can substantially influence the pathological progression of HCC, offering valuable insights for the clinical application of Chinese medicine in the precise prevention and treatment of HCC.
Despite advancements, significant gaps remain in the regulation of autophagy through Chinese medicine for the prevention and treatment of HCC. Firstly, while evidence-based treatment is a distinctive strength of Chinese medicine, there is a disconnect between the integration of western medical concepts of “disease” and the Chinese medicine concept of “evidence”. Many current studies rely on Western medicine modeling methods, neglecting the evidence-based classification inherent to Chinese medicine, and fail to establish a fundamental connection between disease and symptomatology. Consequently, future research should focus on comprehensive experimental studies that explore the relationship between HCC-related targets and evidence-based diagnosis. Secondly, the current research on the regulation of autophagy by Chinese medicine monomers against HCC mainly focuses on the study of a single monomer, and there is a lack of research on the regulation of autophagy by the combined use of Chinese medicine monomers to prevent and control HCC, and whether the combined use of different Chinese medicine monomers or their active ingredients acting on the same target can increase the efficacy also needs to be further explored. Thirdly, most of the current studies focus on animal experiments and in vitro cellular experiments, and lack clinical trials and real-world studies to clarify the specific efficacy and mechanism of action of Chinese medicine monomers and active ingredients on clinical patients. Finally, the mechanism of autophagy in HCC is complex and involves many related protein factors and signaling pathways, so it is difficult to completely clarify the specific mechanism of action of Chinese medicine monomers in intervening in HCC by regulating autophagy. Therefore, the research on autophagy of Chinese medicine monomers and Chinese medicine formulas should be strengthened to clarify the specific mechanism of action of Chinese medicine formulas in treating HCC by regulating autophagy, and the existing Chinese medicine formulas should be disassembled or adjusted, so as to provide experimental data and theoretical support for the clinical application of Chinese medicine.
In light of the rapid advancements in modern technology, it is imperative for researchers to integrate the guiding principles of Chinese medicine theory with comprehensive investigations into the specific mechanisms by which Chinese medicine monomers regulate autophagy in the treatment of HCC. Furthermore, researchers should leverage contemporary methodologies and technologies, such as spatial transcriptomics[209] and artificial intelligence[210], to conduct multidisciplinary studies on the anti-HCC properties of Chinese medicine. This approach aims to elucidate the underlying mechanisms, optimize prescriptions, and achieve precise dosing of the active ingredients in Chinese medicine monomers. Ultimately, the goal is to develop clinically accurate, effective, and internationally recognized treatments that contribute to global health.
We sincerely acknowledge the colleagues for their support in inspiring debates on this topic. We thank the reviewers for their comments and suggestions, which helped improve the manuscript.
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13328] [Article Influence: 1332.8] [Reference Citation Analysis (4)] |
| 2. | Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2611] [Article Influence: 373.0] [Reference Citation Analysis (2)] |
| 3. | Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng J, Feletto E, Canfell K, Qu C, Chen W. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 2021;148:1051-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1261] [Article Influence: 157.6] [Reference Citation Analysis (6)] |
| 5. | Chen S, Li J, Wang D, Fung H, Wong LY, Zhao L. The hepatitis B epidemic in China should receive more attention. Lancet. 2018;391:1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Shan S, Zhao X, Jia J. Comprehensive approach to controlling chronic hepatitis B in China. Clin Mol Hepatol. 2024;30:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 7. | Storandt MH, Mahipal A, Tella SH, Kommalapati A, Jin Z. Systemic Therapy in Advanced Hepatocellular Carcinoma: Patient Selection and Key Considerations. J Hepatocell Carcinoma. 2022;9:1187-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68755] [Article Influence: 13751.0] [Reference Citation Analysis (201)] |
| 9. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13226] [Cited by in RCA: 12082] [Article Influence: 2013.7] [Reference Citation Analysis (42)] |
| 10. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56702] [Article Influence: 7087.8] [Reference Citation Analysis (135)] |
| 11. | Wege H, Schulze K, von Felden J, Calderaro J, Reig M; rare liver tumors working group of the European Reference Network on Hepatological Diseases (ERN RARE-LIVER). Rare variants of primary liver cancer: Fibrolamellar, combined, and sarcomatoid hepatocellular carcinomas. Eur J Med Genet. 2021;64:104313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 5055] [Article Influence: 631.9] [Reference Citation Analysis (2)] |
| 13. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 853] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 14. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4370] [Article Influence: 546.3] [Reference Citation Analysis (6)] |
| 15. | Yu Y, Feng M. Radiotherapy for Hepatocellular Carcinoma. Semin Radiat Oncol. 2018;28:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1589] [Article Influence: 397.3] [Reference Citation Analysis (42)] |
| 17. | Machairas N, Tsilimigras DI, Pawlik TM. State-of-the-art surgery for hepatocellular carcinoma. Langenbecks Arch Surg. 2021;406:2151-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Gofton C, Agar M, George J. Early Implementation of Palliative and Supportive Care in Hepatocellular Carcinoma. Semin Liver Dis. 2022;42:514-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4479] [Article Influence: 895.8] [Reference Citation Analysis (4)] |
| 20. | Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 502] [Reference Citation Analysis (0)] |
| 21. | Thomas MB, O'Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4122] [Article Influence: 515.3] [Reference Citation Analysis (5)] |
| 23. | Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, Inaba Y, Kuromatsu R, Kobayashi M, Okusaka T, Tamai T, Kitamura C, Saito K, Haruna K, Okita K, Kumada H. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 24. | Shi T, Iwama H, Fujita K, Kobara H, Nishiyama N, Fujihara S, Goda Y, Yoneyama H, Morishita A, Tani J, Yamada M, Nakahara M, Takuma K, Masaki T. Evaluating the Effect of Lenvatinib on Sorafenib-Resistant Hepatocellular Carcinoma Cells. Int J Mol Sci. 2021;22:13071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Wen X, Yang Y, Klionsky DJ. Moments in autophagy and disease: Past and present. Mol Aspects Med. 2021;82:100966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 957] [Article Influence: 319.0] [Reference Citation Analysis (0)] |
| 27. | Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023;14:648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 645] [Reference Citation Analysis (0)] |
| 28. | Wong MM, Chan HY, Aziz NA, Ramasamy TS, Bong JJ, Ch'ng ES, Armon S, Peh SC, Teow SY. Interplay of autophagy and cancer stem cells in hepatocellular carcinoma. Mol Biol Rep. 2021;48:3695-3717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Akkoç Y, Gözüaçık D. Autophagy and liver cancer. Turk J Gastroenterol. 2018;29:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, Harper JW, Jäättelä M, Johansen T, Juhasz G, Kimmelman AC, Kraft C, Ktistakis NT, Kumar S, Levine B, Lopez-Otin C, Madeo F, Martens S, Martinez J, Melendez A, Mizushima N, Münz C, Murphy LO, Penninger JM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Santambrogio L, Scorrano L, Simon AK, Simon HU, Simonsen A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Kroemer G. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1297] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 31. | Nanayakkara R, Gurung R, Rodgers SJ, Eramo MJ, Ramm G, Mitchell CA, McGrath MJ. Autophagic lysosome reformation in health and disease. Autophagy. 2023;19:1378-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 32. | Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 2187] [Article Influence: 312.4] [Reference Citation Analysis (0)] |
| 33. | Li W, He P, Huang Y, Li YF, Lu J, Li M, Kurihara H, Luo Z, Meng T, Onishi M, Ma C, Jiang L, Hu Y, Gong Q, Zhu D, Xu Y, Liu R, Liu L, Yi C, Zhu Y, Ma N, Okamoto K, Xie Z, Liu J, He RR, Feng D. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021;11:222-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 339] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 34. | Chen Y, Lin L, Tao X, Song Y, Cui J, Wan J. The role of podocyte damage in the etiology of ischemia-reperfusion acute kidney injury and post-injury fibrosis. BMC Nephrol. 2019;20:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Lei Y, Klionsky DJ. Transcriptional regulation of autophagy and its implications in human disease. Cell Death Differ. 2023;30:1416-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 36. | Lőrincz P, Juhász G. Autophagosome-Lysosome Fusion. J Mol Biol. 2020;432:2462-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 37. | Almacellas E, Pelletier J, Day C, Ambrosio S, Tauler A, Mauvezin C. Lysosomal degradation ensures accurate chromosomal segregation to prevent chromosomal instability. Autophagy. 2021;17:796-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 631] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 39. | Levin-Konigsberg R, Grinstein S. Phagosome-endoplasmic reticulum contacts: Kissing and not running. Traffic. 2020;21:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Lin MG, Hurley JH. Structure and function of the ULK1 complex in autophagy. Curr Opin Cell Biol. 2016;39:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 41. | Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 42. | Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 1208] [Article Influence: 201.3] [Reference Citation Analysis (0)] |
| 43. | Zhao L, You W, Sun D, Xu H, You X, Xu H, Wu Z, Xie Z, Liang Y. Vps21 Directs the PI3K-PI(3)P-Atg21-Atg16 Module to Phagophores via Vps8 for Autophagy. Int J Mol Sci. 2022;23:9550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 44. | Cao W, Li J, Yang K, Cao D. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. 2021;108:304-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 45. | Zhou Y, Manghwar H, Hu W, Liu F. Degradation Mechanism of Autophagy-Related Proteins and Research Progress. Int J Mol Sci. 2022;23:7301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 46. | Fu J, Zhao L, Pang Y, Chen H, Yamamoto H, Chen Y, Li Z, Mizushima N, Jia H. Apicoplast biogenesis mediated by ATG8 requires the ATG12-ATG5-ATG16L and SNAP29 complexes in Toxoplasma gondii. Autophagy. 2023;19:1258-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Vujić N, Bradić I, Goeritzer M, Kuentzel KB, Rainer S, Kratky D, Radović B. ATG7 is dispensable for LC3-PE conjugation in thioglycolate-elicited mouse peritoneal macrophages. Autophagy. 2021;17:3402-3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Fleming A, Bourdenx M, Fujimaki M, Karabiyik C, Krause GJ, Lopez A, Martín-Segura A, Puri C, Scrivo A, Skidmore J, Son SM, Stamatakou E, Wrobel L, Zhu Y, Cuervo AM, Rubinsztein DC. The different autophagy degradation pathways and neurodegeneration. Neuron. 2022;110:935-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 336] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 49. | Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176:11-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 2235] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 50. | Memisoglu G, Eapen VV, Yang Y, Klionsky DJ, Haber JE. PP2C phosphatases promote autophagy by dephosphorylation of the Atg1 complex. Proc Natl Acad Sci U S A. 2019;116:1613-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1187] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 52. | Patel AS, Morse D, Choi AM. Regulation and functional significance of autophagy in respiratory cell biology and disease. Am J Respir Cell Mol Biol. 2013;48:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Chen C, Gao H, Su X. Autophagy-related signaling pathways are involved in cancer (Review). Exp Ther Med. 2021;22:710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Shi B, Ma M, Zheng Y, Pan Y, Lin X. mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cell Physiol. 2019;234:12562-12568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 55. | Sabatini DM. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci U S A. 2017;114:11818-11825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 56. | Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1563] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 57. | Sciarretta S, Forte M, Frati G, Sadoshima J. New Insights Into the Role of mTOR Signaling in the Cardiovascular System. Circ Res. 2018;122:489-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 58. | Ghosh R, Pattison JS. Macroautophagy and Chaperone-Mediated Autophagy in Heart Failure: The Known and the Unknown. Oxid Med Cell Longev. 2018;2018:8602041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Datan E, Shirazian A, Benjamin S, Matassov D, Tinari A, Malorni W, Lockshin RA, Garcia-Sastre A, Zakeri Z. mTOR/p70S6K signaling distinguishes routine, maintenance-level autophagy from autophagic cell death during influenza A infection. Virology. 2014;452-453:175-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Al-Bari MAA, Xu P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann N Y Acad Sci. 2020;1467:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 61. | Xu HD, Qin ZH. Beclin 1, Bcl-2 and Autophagy. Adv Exp Med Biol. 2019;1206:109-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 62. | Ye J, Zhang J, Zhu Y, Wang L, Jiang X, Liu B, He G. Targeting autophagy and beyond: Deconvoluting the complexity of Beclin-1 from biological function to cancer therapy. Acta Pharm Sin B. 2023;13:4688-4714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 63. | Ohashi Y. Activation Mechanisms of the VPS34 Complexes. Cells. 2021;10:3124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Hill SM, Wrobel L, Rubinsztein DC. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2019;26:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 65. | Mathre S, Reddy KB, Ramya V, Krishnan H, Ghosh A, Raghu P. Functional analysis of the biochemical activity of mammalian phosphatidylinositol 5 phosphate 4-kinase enzymes. Biosci Rep. 2019;39:BSR20182210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Song Y, Quach C, Liang C. UVRAG in autophagy, inflammation, and cancer. Autophagy. 2020;16:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Wijshake T, Zou Z, Chen B, Zhong L, Xiao G, Xie Y, Doench JG, Bennett L, Levine B. Tumor-suppressor function of Beclin 1 in breast cancer cells requires E-cadherin. Proc Natl Acad Sci U S A. 2021;118:e2020478118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 68. | Prerna K, Dubey VK. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int J Biol Macromol. 2022;204:258-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 69. | Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G, Liu J, Zhang J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol. 2020;104:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 70. | Xiu AY, Ding Q, Li Z, Zhang CQ. Doxazosin Attenuates Liver Fibrosis by Inhibiting Autophagy in Hepatic Stellate Cells via Activation of the PI3K/Akt/mTOR Signaling Pathway. Drug Des Devel Ther. 2021;15:3643-3659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 71. | Danesh Pazhooh R, Rahnamay Farnood P, Asemi Z, Mirsafaei L, Yousefi B, Mirzaei H. mTOR pathway and DNA damage response: A therapeutic strategy in cancer therapy. DNA Repair (Amst). 2021;104:103142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac Cancer. 2020;11:511-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 73. | Lin CP, Traets JJH, Vredevoogd DW, Visser NL, Peeper DS. TSC2 regulates tumor susceptibility to TRAIL-mediated T-cell killing by orchestrating mTOR signaling. EMBO J. 2023;42:e111614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 74. | Zhang F, Peng W, Zhang J, Wang L, Dong W, Zheng Y, Wang Z, Xie Z, Wang T, Wang C, Yan Y. PARK7 enhances antioxidative-stress processes of BMSCs via the ERK1/2 pathway. J Cell Biochem. 2021;122:222-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Lv D, Guo L, Zhang T, Huang L. PRAS40 signaling in tumor. Oncotarget. 2017;8:69076-69085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 76. | Zhou Q, Tang S, Zhang X, Chen L. Targeting PRAS40: a novel therapeutic strategy for human diseases. J Drug Target. 2021;29:703-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Jhanwar-Uniyal M, Dominguez JF, Mohan AL, Tobias ME, Gandhi CD. Disentangling the signaling pathways of mTOR complexes, mTORC1 and mTORC2, as a therapeutic target in glioblastoma. Adv Biol Regul. 2022;83:100854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 78. | Wang Y, Gao G, Wu Y, Wang Y, Wu X, Zhou Q. S100A4 Silencing Facilitates Corneal Wound Healing After Alkali Burns by Promoting Autophagy via Blocking the PI3K/Akt/mTOR Signaling Pathway. Invest Ophthalmol Vis Sci. 2020;61:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1951] [Article Influence: 325.2] [Reference Citation Analysis (0)] |
| 80. | Matthaios D, Tolia M, Mauri D, Kamposioras K, Karamouzis M. YAP/Hippo Pathway and Cancer Immunity: It Takes Two to Tango. Biomedicines. 2021;9:1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Nishina H. Physiological and pathological roles of the Hippo-YAP/TAZ signaling pathway in liver formation, homeostasis, and tumorigenesis. Cancer Sci. 2022;113:1900-1908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Wang D, He J, Huang B, Liu S, Zhu H, Xu T. Emerging role of the Hippo pathway in autophagy. Cell Death Dis. 2020;11:880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 83. | Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 717] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 84. | Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 85. | Zheng A, Chen Q, Zhang L. The Hippo-YAP pathway in various cardiovascular diseases: Focusing on the inflammatory response. Front Immunol. 2022;13:971416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 86. | Driskill JH, Pan D. The Hippo Pathway in Liver Homeostasis and Pathophysiology. Annu Rev Pathol. 2021;16:299-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 87. | Cunningham R, Hansen CG. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin Sci (Lond). 2022;136:197-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 199] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 88. | Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X. An overview of signaling pathways regulating YAP/TAZ activity. Cell Mol Life Sci. 2021;78:497-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 89. | Jin L, Chen Y, Cheng D, He Z, Shi X, Du B, Xi X, Gao Y, Guo Y. YAP inhibits autophagy and promotes progression of colorectal cancer via upregulating Bcl-2 expression. Cell Death Dis. 2021;12:457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 90. | Zhao M, Zhang Y, Jiang Y, Wang K, Wang X, Zhou D, Wang Y, Yu R, Zhou X. YAP promotes autophagy and progression of gliomas via upregulating HMGB1. J Exp Clin Cancer Res. 2021;40:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 91. | Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 901] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 92. | You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H, Han W. The role of STAT3 in autophagy. Autophagy. 2015;11:729-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 434] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 93. | Wang M, Zhang Y, Liu M, Jia Y, He J, Xu X, Shi H, Zhang Y, Zhang J, Liu Y. Inhibition of STAT3 signaling as critical molecular event in HUC-MSCs suppressed Glioblastoma Cells. J Cancer. 2023;14:611-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Shen S, Niso-Santano M, Adjemian S, Takehara T, Malik SA, Minoux H, Souquere S, Mariño G, Lachkar S, Senovilla L, Galluzzi L, Kepp O, Pierron G, Maiuri MC, Hikita H, Kroemer R, Kroemer G. Cytoplasmic STAT3 represses autophagy by inhibiting PKR activity. Mol Cell. 2012;48:667-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 95. | Du Toit A. Autophagy: STAT3 maintains order. Nat Rev Mol Cell Biol. 2012;13:754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Chen D, Liu Y, Chen J, Lin H, Guo H, Wu Y, Xu Y, Zhou Y, Zhou W, Lu R, Zhou J, Wu J. JAK/STAT pathway promotes the progression of diabetic kidney disease via autophagy in podocytes. Eur J Pharmacol. 2021;902:174121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 97. | Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1847] [Cited by in RCA: 2420] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 98. | Weerasekara VK, Panek DJ, Broadbent DG, Mortenson JB, Mathis AD, Logan GN, Prince JT, Thomson DM, Thompson JW, Andersen JL. Metabolic-stress-induced rearrangement of the 14-3-3ζ interactome promotes autophagy via a ULK1- and AMPK-regulated 14-3-3ζ interaction with phosphorylated Atg9. Mol Cell Biol. 2014;34:4379-4388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 99. | Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 664] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 100. | Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1689] [Article Influence: 153.5] [Reference Citation Analysis (0)] |
| 101. | Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3114] [Cited by in RCA: 3092] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 102. | Wataya-Kaneda M. Mammalian target of rapamycin and tuberous sclerosis complex. J Dermatol Sci. 2015;79:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Chun Y, Kim J. AMPK-mTOR Signaling and Cellular Adaptations in Hypoxia. Int J Mol Sci. 2021;22:9765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 104. | Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2958] [Cited by in RCA: 2936] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 105. | Li D, Cheng S, Zhang W, Wang M, Sun C, Zhang C, Wang Y, Jin J, Zhang Y, Li B. Hedgehog-Gli1 signaling regelates differentiation of chicken (Gallus gallus) embryonic stem cells to male germ cells. Anim Reprod Sci. 2017;182:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Chen H, Zuo Q, Wang Y, Ahmed MF, Jin K, Song J, Zhang Y, Li B. Regulation of Hedgehog Signaling in Chicken Embryonic Stem Cells Differentiation Into Male Germ Cells (Gallus). J Cell Biochem. 2017;118:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Yao Y, Li T, Yu T, Yang X, Wang Y, Cai J, Cheng SY, Liu C, Yue S. Hedgehog signal activates AMPK via Smoothened to promote autophagy and lipid degradation in hepatocytes. Biochem Cell Biol. 2023;101:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 108. | Zhang Y, Xin W, Hu X, Wang H, Ye X, Xu C, Nan Y, Wu Z, Ju D, Fan J. Inhibition of Hedgehog signaling ameliorates foam cell formation by promoting autophagy in early atherosclerosis. Cell Death Dis. 2023;14:740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 109. | Jeng KS, Sheen IS, Leu CM, Tseng PH, Chang CF. The Role of Smoothened in Cancer. Int J Mol Sci. 2020;21:6863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Zhang D, Liu J, Wang Y, Chen J, Chen T. shRNA-mediated silencing of Gli2 gene inhibits proliferation and sensitizes human hepatocellular carcinoma cells towards TRAIL-induced apoptosis. J Cell Biochem. 2011;112:3140-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Li J, Zhang L, Xia Q, Fu J, Zhou Z, Lin F. Hedgehog signaling inhibitor GANT61 induces endoplasmic reticulum stress-mediated protective autophagy in hepatic stellate cells. Biochem Biophys Res Commun. 2017;493:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 112. | Wang Y, Han C, Lu L, Magliato S, Wu T. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology. 2013;58:995-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 113. | Zeng X, Ju D. Hedgehog Signaling Pathway and Autophagy in Cancer. Int J Mol Sci. 2018;19:2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 114. | Chen H, Shang X, Yuan H, Niu Q, Chen J, Luo S, Li W, Li X. Total flavonoids of Oldenlandia diffusa (Willd.) Roxb. suppresses the growth of hepatocellular carcinoma through endoplasmic reticulum stress-mediated autophagy and apoptosis. Front Pharmacol. 2022;13:1019670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Hu Y, Li R, Jin J, Wang Y, Ma R. Quercetin improves pancreatic cancer chemo-sensitivity by regulating oxidative-inflammatory networks. J Food Biochem. 2022;46:e14453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 116. | Wu R, Zhou T, Xiong J, Zhang Z, Tian S, Wang Y, Chen J, Tian X. Quercetin, the Ingredient of Xihuang Pills, Inhibits Hepatocellular Carcinoma by Regulating Autophagy and Macrophage Polarization. Front Biosci (Landmark Ed). 2022;27:323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 117. | Ji Y, Li L, Ma YX, Li WT, Li L, Zhu HZ, Wu MH, Zhou JR. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J Nutr Biochem. 2019;69:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 118. | Liu J, Ren L, Wang H, Li Z. Isoquercitrin Induces Endoplasmic Reticulum Stress and Immunogenic Cell Death in Gastric Cancer Cells. Biochem Genet. 2023;61:1128-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 119. | Shui L, Wang W, Xie M, Ye B, Li X, Liu Y, Zheng M. Isoquercitrin induces apoptosis and autophagy in hepatocellular carcinoma cells via AMPK/mTOR/p70S6K signaling pathway. Aging (Albany NY). 2020;12:24318-24332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Yang JY, Li M, Zhang CL, Liu D. Pharmacological properties of baicalin on liver diseases: a narrative review. Pharmacol Rep. 2021;73:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 121. | Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015;6:e1942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 122. | Gupta S, Buttar HS, Kaur G, Tuli HS. Baicalein: promising therapeutic applications with special reference to published patents. Pharm Pat Anal. 2022;11:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Wang YF, Li T, Tang ZH, Chang LL, Zhu H, Chen XP, Wang YT, Lu JJ. Baicalein Triggers Autophagy and Inhibits the Protein Kinase B/Mammalian Target of Rapamycin Pathway in Hepatocellular Carcinoma HepG2 Cells. Phytother Res. 2015;29:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 124. | Wang Z, Jiang C, Chen W, Zhang G, Luo D, Cao Y, Wu J, Ding Y, Liu B. Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. Biomed Res Int. 2014;2014:732516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Niu YJ, Ai X, Lin XT, Xu WM, Lao SY, Tian ZC, Zhu HY, Zhou W, Huang H, Shi XL. Baicalein inhibits hepatitis B virus through the coiled coil domain containing protein 88A (CCDC88A)-dependent autophagy pathway. Phytomedicine. 2025;140:156577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 126. | Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother. 2019;112:108612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 606] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 127. | Cao Z, Zhang H, Cai X, Fang W, Chai D, Wen Y, Chen H, Chu F, Zhang Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell Physiol Biochem. 2017;43:1803-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 128. | Han Y, Xiao Y, Yu L, Chen J, Yang X, Cui H, Liang J. Advances in the Mechanism of Luteolin against Hepatocellular Carcinoma Based on Bioinformatics and Network Pharmacology. J Cancer. 2023;14:966-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Xu L, Zaky MY, Yousuf W, Ullah A, Abdelbaset GR, Zhang Y, Ahmed OM, Liu S, Liu H. The Anticancer Potential of Apigenin Via Immunoregulation. Curr Pharm Des. 2021;27:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 130. | Lee IG, Lee J, Hong SH, Seo YJ. Apigenin's Therapeutic Potential Against Viral Infection. Front Biosci (Landmark Ed). 2023;28:237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 131. | Zeng J, Xie H, Zhang ZL, Li ZX, Shi L, Wu KY, Zhou Y, Tian Z, Zhang Y, Zhou W, Shen WG. Apigenin regulates the migration, invasion, and autophagy of hepatocellular carcinoma cells by downregulating YAP. Neoplasma. 2022;69:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 132. | Qi W, Li Z, Yang C, Jiangshan Dai J, Zhang Q, Wang D, Wu C, Xia L, Xu S. Inhibitory mechanism of muscone in liver cancer involves the induction of apoptosis and autophagy. Oncol Rep. 2020;43:839-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 133. | Song YG, Kang L, Tian S, Cui LL, Li Y, Bai M, Fang XY, Cao LH, Coleman K, Miao MS. Study on the anti-hepatocarcinoma effect and molecular mechanism of Prunella vulgaris total flavonoids. J Ethnopharmacol. 2021;273:113891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 134. | Yao J, Tang S, Shi C, Lin Y, Ge L, Chen Q, Ou B, Liu D, Miao Y, Xie Q, Tang X, Fei J, Yang G, Tian J, Zeng X. Isoginkgetin, a potential CDK6 inhibitor, suppresses SLC2A1/GLUT1 enhancer activity to induce AMPK-ULK1-mediated cytotoxic autophagy in hepatocellular carcinoma. Autophagy. 2023;19:1221-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 135. | Lim WC, Kim H, Ko H. Delphinidin inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma cells. J Cell Biochem. 2019;120:9887-9899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 136. | Han B, Meng F, Niu Y, Liu H, Li J, Peng X. Effect of Delphinidin on Metabolomic Profile in Breast Carcinogenesis. Anticancer Agents Med Chem. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Kang SH, Bak DH, Chung BY, Bai HW, Kang BS. Delphinidin enhances radio-therapeutic effects via autophagy induction and JNK/MAPK pathway activation in non-small cell lung cancer. Korean J Physiol Pharmacol. 2020;24:413-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 138. | Sun S, Xu K, Yan M, Cui J, Zhu K, Yang Y, Zhang X, Tang W, Huang X, Dou L, Chen B, Lin Y, Zhang X, Man Y, Li J, Shen T. Delphinidin induces autophagic flux blockage and apoptosis by inhibiting both multidrug resistance gene 1 and DEAD-box helicase 17 expressions in liver cancer cells. J Pharm Pharmacol. 2023;75:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 139. | Sharma N, Gupta M, Anand P, Akhter Y, Al-Dayan N, Majed HA, Biswas S, Ali S, Sarwat M. Mechanistic Insight into the Autophagic and Apoptotic Activity of Kaempferol on Liver Cancer Cells. Onco Targets Ther. 2024;17:579-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 140. | Han L, Tian X, Yang X, Li T, Wang S, Bao Y, Meng X. The pathogenesis of hepatocellular carcinoma: ERK/ULK1/NCOA4-mediated inhibition of iron autophagy, and Epimedium extract targeted modulation of this pathway to treat hepatocellular carcinoma. Phytomedicine. 2025;141:156666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 141. | Gao S, Zhang W, Dai J, Hu W, Xu Y, Yang H, Ye B, Ouyang H, Tang Q, Zhao G, Zhu J. Icariin mediates autophagy and apoptosis of hepatocellular carcinoma cells induced by the β-catenin signaling pathway through lncRNA LOXL1-AS1. Naunyn Schmiedebergs Arch Pharmacol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Bailly C. Molecular and cellular basis of the anticancer activity of the prenylated flavonoid icaritin in hepatocellular carcinoma. Chem Biol Interact. 2020;325:109124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 143. | Li Q, Yang T, Zhao S, Zheng Q, Li Y, Zhang Z, Sun X, Liu Y, Zhang Y, Xie J. Distribution, Biotransformation, Pharmacological Effects, Metabolic Mechanism and Safety Evaluation of Platycodin D: A Comprehensive Review. Curr Drug Metab. 2022;23:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 144. | Li T, Tang ZH, Xu WS, Wu GS, Wang YF, Chang LL, Zhu H, Chen XP, Wang YT, Chen Y, Lu JJ. Platycodin D triggers autophagy through activation of extracellular signal-regulated kinase in hepatocellular carcinoma HepG2 cells. Eur J Pharmacol. 2015;749:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 145. | Li T, Xu XH, Tang ZH, Wang YF, Leung CH, Ma DL, Chen XP, Wang YT, Chen Y, Lu JJ. Platycodin D induces apoptosis and triggers ERK- and JNK-mediated autophagy in human hepatocellular carcinoma BEL-7402 cells. Acta Pharmacol Sin. 2015;36:1503-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 146. | Ge J, Wang P, Ma H, Zhang J. Solamargine Inhibits Prostate Cancer Cell Growth and Enhances the Therapeutic Efficacy of Docetaxel via Akt Signaling. J Oncol. 2022;2022:9055954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 147. | Furtado RA, Ozelin SD, Ferreira NH, Miura BA, Almeida Junior S, Magalhães GM, Nassar EJ, Miranda MA, Bastos JK, Tavares DC. Antitumor activity of solamargine in mouse melanoma model: relevance to clinical safety. J Toxicol Environ Health A. 2022;85:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Kalalinia F, Karimi-Sani I. Anticancer Properties of Solamargine: A Systematic Review. Phytother Res. 2017;31:858-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 149. | Yin S, Jin W, Qiu Y, Fu L, Wang T, Yu H. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J Hematol Oncol. 2022;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 150. | Bandopadhyay S, Anand U, Gadekar VS, Jha NK, Gupta PK, Behl T, Kumar M, Radha, Shekhawat MS, Dey A. Dioscin: A review on pharmacological properties and therapeutic values. Biofactors. 2022;48:22-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 151. | Mao Z, Han X, Chen D, Xu Y, Xu L, Yin L, Sun H, Qi Y, Fang L, Liu K, Peng J. Potent effects of dioscin against hepatocellular carcinoma through regulating TP53-induced glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis, autophagy, and DNA damage. Br J Pharmacol. 2019;176:919-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 152. | Manoharan S, Deivendran B, Perumal E. Chemotherapeutic Potential of Saikosaponin D: Experimental Evidence. J Xenobiot. 2022;12:378-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 153. | Tian YD, Lin S, Yang PT, Bai MH, Jin YY, Min WL, Ma HB, Wang BF. Saikosaponin-d Increases the Radiosensitivity of Hepatoma Cells by Adjusting Cell Autophagy. J Cancer. 2019;10:4947-4953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Liu Z, Cao Y, Guo X, Chen Z. The Potential Role of Timosaponin-AIII in Cancer Prevention and Treatment. Molecules. 2023;28:5500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 155. | Wang N, Feng Y, Zhu M, Siu FM, Ng KM, Che CM. A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim Biophys Acta. 2013;1833:2890-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 156. | Li Y, Xiao P, Sun Y, Li Y, Zhao H, Sun J, Wang X, Han X, Jin N, Li X, Bao Y. Deapioplatycodin D promotes cell senescence induced by P21 through the mediation of incomplete mitophagy via BNIP3L. Biomed Pharmacother. 2024;178:117215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 157. | Roy M, Liang L, Xiao X, Feng P, Ye M, Liu J. Lycorine: A prospective natural lead for anticancer drug discovery. Biomed Pharmacother. 2018;107:615-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 158. | Yu H, Qiu Y, Pang X, Li J, Wu S, Yin S, Han L, Zhang Y, Jin C, Gao X, Hu W, Wang T. Lycorine Promotes Autophagy and Apoptosis via TCRP1/Akt/mTOR Axis Inactivation in Human Hepatocellular Carcinoma. Mol Cancer Ther. 2017;16:2711-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 159. | Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020;14:564-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 336] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 160. | Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, Tsao SW. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem. 2010;111:1426-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 161. | Hou Q, Tang X, Liu H, Tang J, Yang Y, Jing X, Xiao Q, Wang W, Gou X, Wang Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011;102:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 162. | Bao X, Liu Y, Huang J, Yin S, Sheng H, Han X, Chen Q, Wang T, Chen S, Qiu Y, Zhang C, Yu H. Stachydrine hydrochloride inhibits hepatocellular carcinoma progression via LIF/AMPK axis. Phytomedicine. 2022;100:154066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 163. | Mérarchi M, Sethi G, Fan L, Mishra S, Arfuso F, Ahn KS. Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models. Molecules. 2018;23:2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 164. | Wang N, Pan W, Zhu M, Zhang M, Hao X, Liang G, Feng Y. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br J Pharmacol. 2011;164:731-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 165. | Tang ZH, Li T, Chang LL, Zhu H, Tong YG, Chen XP, Wang YT, Lu JJ. Glycyrrhetinic Acid triggers a protective autophagy by activation of extracellular regulated protein kinases in hepatocellular carcinoma cells. J Agric Food Chem. 2014;62:11910-11916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 166. | Kang X, Wang H, Li Y, Xiao Y, Zhao L, Zhang T, Zhou S, Zhou X, Li Y, Shou Z, Chen C, Li B. Alantolactone induces apoptosis through ROS-mediated AKT pathway and inhibition of PINK1-mediated mitophagy in human HepG2 cells. Artif Cells Nanomed Biotechnol. 2019;47:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 167. | Dai CL, Zhang RJ, An P, Deng YQ, Rahman K, Zhang H. Cinobufagin: a promising therapeutic agent for cancer. J Pharm Pharmacol. 2023;75:1141-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 168. | Xu Z, Bao J, Jin X, Li H, Fan K, Wu Z, Yao M, Zhang Y, Liu G, Wang D, Yu X, Guo J, Xu R, Gong Q, Wang F, Wang J. The Effects of Cinobufagin on Hepatocellular Carcinoma Cells Enhanced by MRT68921, an Autophagy Inhibitor. Am J Chin Med. 2023;51:1595-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 169. | Wang C, Dai S, Zhao X, Zhang Y, Gong L, Fu K, Ma C, Peng C, Li Y. Celastrol as an emerging anticancer agent: Current status, challenges and therapeutic strategies. Biomed Pharmacother. 2023;163:114882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 170. | Han X, Sun S, Zhao M, Cheng X, Chen G, Lin S, Guan Y, Yu X. Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1α protein synthesis. PLoS One. 2014;9:e112470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 171. | Yang Y, Wu JJ, Xia J, Wan Y, Xu JF, Zhang L, Liu D, Chen L, Tang F, Ao H, Peng C. Can aloin develop to medicines or healthcare products? Biomed Pharmacother. 2022;153:113421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 172. | Fu D, Ji Q, Wang C, Yu L, Yu R. Aloin decelerates the progression of hepatocellular carcinoma through circ_0011385/miR-149-5p/WT1 axis. Cell Cycle. 2021;20:2476-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 173. | Birtić S, Dussort P, Pierre FX, Bily AC, Roller M. Carnosic acid. Phytochemistry. 2015;115:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 174. | Gao Q, Liu H, Yao Y, Geng L, Zhang X, Jiang L, Shi B, Yang F. Carnosic acid induces autophagic cell death through inhibition of the Akt/mTOR pathway in human hepatoma cells. J Appl Toxicol. 2015;35:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 175. | Ye R, Dai N, He Q, Guo P, Xiang Y, Zhang Q, Hong Z, Zhang Q. Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;105:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 176. | Zhu ZH, Xu XT, Shen CJ, Yuan JT, Lou SY, Ma XL, Chen X, Yang B, Zhao HJ. A novel sesquiterpene lactone fraction from Eupatorium chinense L. suppresses hepatocellular carcinoma growth by triggering ferritinophagy and mitochondrial damage. Phytomedicine. 2023;112:154671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 177. | Tian X, Liu F, Zhao J, Liu Q, Wang H, Liu Y, Zhang J, Zhang Y, Yang Y, Shi S, Jiang S. Mechanism and molecular targets of Euphorbia fischeriana Steud root extract in hepatocellular carcinoma. J Ethnopharmacol. 2025;346:119707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 178. | Ren B, Kwah MX, Liu C, Ma Z, Shanmugam MK, Ding L, Xiang X, Ho PC, Wang L, Ong PS, Goh BC. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021;515:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (1)] |
| 179. | Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 180. | Breuss JM, Atanasov AG, Uhrin P. Resveratrol and Its Effects on the Vascular System. Int J Mol Sci. 2019;20:1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 181. | Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH, Nasrallah GK, Pintus G. Potential Adverse Effects of Resveratrol: A Literature Review. Int J Mol Sci. 2020;21:2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 486] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 182. | Tong K, Wang P, Li Y, Tong Y, Li X, Yan S, Hu P. Resveratrol Inhibits Hepatocellular Carcinoma Progression through Regulating Exosome Secretion. Curr Med Chem. 2024;31:2107-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 183. | Wu SX, Xiong RG, Huang SY, Zhou DD, Saimaiti A, Zhao CN, Shang A, Zhang YJ, Gan RY, Li HB. Effects and mechanisms of resveratrol for prevention and management of cancers: An updated review. Crit Rev Food Sci Nutr. 2023;63:12422-12440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 184. | Zhang X, Xie L, Long J, Xie Q, Zheng Y, Liu K, Li X. Salidroside: A review of its recent advances in synthetic pathways and pharmacological properties. Chem Biol Interact. 2021;339:109268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 185. | Zhang J, Li Z, Song J, Zhou L, Chen X, Ge W, Dong T, Luo Y, Mao T, Li Z, Tan D, Rasmussen LJ, Bohr VA, Tong X, Dai F. Salidroside promotes healthy longevity by interfering with HSP90 activity. Geroscience. 2024;46:1641-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 186. | Jiang B, Feng L, Yang T, Guo W, Li Y, Wang T, Liu C, Su H. Combination of chloroquine diphosphate and salidroside induces human liver cell apoptosis via regulation of mitochondrial dysfunction and autophagy. Mol Med Rep. 2023;27:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 187. | Lin Y, Chen Y, Wang S, Ma J, Peng Y, Yuan X, Lv B, Chen W, Wei Y. Plumbagin induces autophagy and apoptosis of SMMC-7721 cells in vitro and in vivo. J Cell Biochem. 2019;120:9820-9830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 188. | Dong X, Fu J, Yin X, Cao S, Li X, Lin L; Huyiligeqi, Ni J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother Res. 2016;30:1207-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 553] [Article Influence: 55.3] [Reference Citation Analysis (7)] |
| 189. | Qin B, Zeng Z, Xu J, Shangwen J, Ye ZJ, Wang S, Wu Y, Peng G, Wang Q, Gu W, Tang Y. Emodin inhibits invasion and migration of hepatocellular carcinoma cells via regulating autophagy-mediated degradation of snail and β-catenin. BMC Cancer. 2022;22:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 190. | Wu W, Lu P, Huang Y, Zhu Z, Li C, Liu Y. Emodin regulates the autophagy via the miR-371a-5p/PTEN axis to inhibit hepatic malignancy. Biochem Biophys Res Commun. 2022;619:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 191. | Liu L, Shi D, Xia ZY, Wang BW, Wang XL, Wang XT, Wang GL, Li MJ, Zheng QS, Li D, Li BH. Gamabufotalin Induces Apoptosis and Cytoprotective Autophagy through the mTOR Signaling Pathway in Hepatocellular Carcinoma. J Nat Prod. 2023;86:966-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 192. | Tsai SC, Yang JS, Peng SF, Lu CC, Chiang JH, Chung JG, Lin MW, Lin JK, Amagaya S, Wai-Shan Chung C, Tung TT, Huang WW, Tseng MT. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol. 2012;41:1431-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 193. | Hsu CM, Tsai Y, Wan L, Tsai FJ. Bufalin induces G2/M phase arrest and triggers autophagy via the TNF, JNK, BECN-1 and ATG8 pathway in human hepatoma cells. Int J Oncol. 2013;43:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 194. | Chen W, Ma Z, Yu L, Mao X, Ma N, Guo X, Yin X, Jiang F, Wang Q, Wang J, Fang M, Lin N, Zhang Y. Preclinical investigation of artesunate as a therapeutic agent for hepatocellular carcinoma via impairment of glucosylceramidase-mediated autophagic degradation. Exp Mol Med. 2022;54:1536-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 195. | Dai X, Zhang X, Chen W, Chen Y, Zhang Q, Mo S, Lu J. Dihydroartemisinin: A Potential Natural Anticancer Drug. Int J Biol Sci. 2021;17:603-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 196. | Shi X, Wang L, Ren L, Li J, Li S, Cui Q, Li S. Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phytother Res. 2019;33:1413-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 197. | Zhu PL, Lam DF, Li JK, Fu XQ, Yin CL, Chou JY, Wang YP, Liu YX, Chen YJ, Wu JY, Wu Y, Bai JX, Liang C, Yu ZL. Gomisin N Exerts Anti-liver Cancer Effects and Regulates PI3K-Akt and mTOR-ULK1 Pathways in Vitro. Biol Pharm Bull. 2020;43:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 198. | Luo Y, Song L, Wang X, Huang Y, Liu Y, Wang Q, Hong M, Yuan Z. Uncovering the Mechanisms of Cryptotanshinone as a Therapeutic Agent Against Hepatocellular Carcinoma. Front Pharmacol. 2020;11:1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 199. | Zhang L, Xie Q, Li X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother Res. 2022;36:279-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 200. | Xiu Z, Li Y, Fang J, Han J, Li S, Li Y, Yang X, Song G, Li Y, Jin N, Zhu Y, Zhu G, Sun L, Li X. Inhibitory Effects of Esculetin on Liver Cancer Through Triggering NCOA4 Pathway-Mediation Ferritinophagy in vivo and in vitro. J Hepatocell Carcinoma. 2023;10:611-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 201. | Wang M, Tu Y, Liu C, Cheng H, Zhang M, Li Q. Gambogenic Acid Inhibits Invasion and Metastasis of Melanoma through Regulation of lncRNA MEG3. Biol Pharm Bull. 2023;46:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 202. | Ma X, Xu M, Zhang X, Wang X, Su K, Xu Z, Wang X, Yang Y. Gambogenic acid inhibits proliferation and ferroptosis by targeting the miR-1291/FOXA2 and AMPKα/SLC7A11/GPX4 axis in colorectal cancer. Cell Biol Int. 2023;47:1813-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 203. | Wang M, Zhan F, Cheng H, Li Q. Gambogenic Acid Inhibits Basal Autophagy of Drug-Resistant Hepatoma Cells and Improves Its Sensitivity to Adriamycin. Biol Pharm Bull. 2022;45:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 204. | Wu N, Li J, Luo H, Wang D, Bai X. Hydroxysafflor yellow A promotes apoptosis via blocking autophagic flux in liver cancer. Biomed Pharmacother. 2021;136:111227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 205. | Chen Z, Liu L, Liu Y, Wang S, Zhang S, Dong R, Xu M, Ma Y, Wang J, Zhang Q, Wei P. Hydroxysafflor yellow A induces autophagy in human liver cancer cells by regulating Beclin 1 and ERK expression. Exp Ther Med. 2020;19:2989-2996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 206. | Zheng P, Xu D, Cai Y, Zhu L, Xiao Q, Peng W, Chen B. A multi-omic analysis reveals that Gamabufotalin exerts anti-hepatocellular carcinoma effects by regulating amino acid metabolism through targeting STAMBPL1. Phytomedicine. 2024;135:156094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 207. | Xu F, Zhang H, Chen J, Zhan J, Liu P, Liu W, Qi S, Mu Y. Recent progress on the application of compound formulas of traditional Chinese medicine in clinical trials and basic research in vivo for chronic liver disease. J Ethnopharmacol. 2024;321:117514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 208. | Wendl MC, Waterston RH. Generalized gap model for bacterial artificial chromosome clone fingerprint mapping and shotgun sequencing. Genome Res. 2002;12:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 209. | Vu T, Vallmitjana A, Gu J, La K, Xu Q, Flores J, Zimak J, Shiu J, Hosohama L, Wu J, Douglas C, Waterman ML, Ganesan A, Hedde PN, Gratton E, Zhao W. Spatial transcriptomics using combinatorial fluorescence spectral and lifetime encoding, imaging and analysis. Nat Commun. 2022;13:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 210. | Sahu A, Mishra J, Kushwaha N. Artificial Intelligence (AI) in Drugs and Pharmaceuticals. Comb Chem High Throughput Screen. 2022;25:1818-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/