INTRODUCTION
Liver cancer (LC) comprises a variety of malignant tumors that develop from liver cells or the structural liver components. The most prevalent form is hepatocellular carcinoma (HCC), which develops from hepatocytes, the primary functional cells of the liver. This type accounts for approximately 80%-90% of all primary LC cases[1-3]. Other LC types include intrahepatic cholangiocarcinoma, a malignant tumor of the bile ducts within the liver, as well as rare forms like angiosarcomas and hepatoblastomas. Intrahepatic cholangiocarcinoma is less common than HCC, but it is often diagnosed at later stages, resulting in poorer outcomes. Angiosarcomas are infrequent malignancies, typically linked to exposure to industrial toxins, while hepatoblastomas affect mainly children[4,5].
It is essential to distinguish primary LCs from secondary metastatic tumors, which originate in other organs (e.g., colorectal or breast cancer) and spread to the liver. These secondary cancers can obscure the primary site, requiring different diagnostic methods and therapeutic approaches[6]. LC continues to represent a significant clinical challenge, especially due to the HCC silent nature in its early stages. Despite growing therapeutic options, the disease is still often diagnosed late, limiting the effectiveness of available treatments[4,5].
Understanding the molecular and immunological mechanisms that underlie HCC becomes relevant and critical. This review offers a detailed exploration of the molecular and immunological landscape of HCC, emphasizing the central role of immune dysregulation in its development and progression. Special focus is given to galectins (Gal), mainly Gal-1 and Gal-3, as key players in modulating tumor immunity and promoting oncogenic processes. Their multifaceted roles as immune regulators, biomarkers, and therapeutic targets may open new avenues in the management of HCC. By shedding light on these mechanisms, this review aims to enhance current understanding and stimulate further research into personalized and more effective treatment strategies[7-11].
EPIDEMIOLOGY OF LC
In 2020, an estimated 905700 patients were diagnosed with LC, and 830200 deaths were attributed to this malignancy worldwide, making it the sixth most commonly diagnosed cancer and globally the third-leading cause of cancer-related deaths. The worldwide age-standardized rates were 9.5 per 100000 people for new cases and 8.7 per 100000 for deaths[1]. LC incidence and mortality were highest in Eastern Asia (17.8 and 16.1 per 100000, respectively), followed by Northern Africa and South-Eastern Asia. It ranked among the top three cancer-related deaths in 46 countries and among the top five in 90 countries, with notable burdens in some regions such as Mongolia, Vietnam, Egypt, and Cambodia[1].
LC is more common among males than females, with male-to-female age-standardized rate ratios ranging from 1.2 to 3.6 across different regions. Risk factors such as older age, chronic hepatitis infections, alcohol consumption, metabolic syndrome, and exposure to aflatoxins disproportionately affect some populations, including those in low-income and middle-income countries[1,3].
There are significant epidemiological variations associated with the Human Development Index (HDI). Countries with a high HDI are projected to experience the largest absolute increases in LC cases and deaths by 2040 (+55.7% and +57.6%, respectively). In contrast, low and medium HDI regions are expected to experience the highest relative increases, particularly in low HDI countries where cases may nearly double (+99.9%) and deaths rise by over 100%. These trends are mainly driven by demographic factors like population growth and aging, underscoring the need for targeted public health strategies in resource-limited settings to improve prevention, early detection, and lastly treatment[1].
MAIN RISK FACTORS FOR LC DEVELOPMENT
HBV and hepatitis C virus (HCV) infections are primary risk factors for HCC development. Chronic liver inflammation induced by these viral infections can lead to cirrhosis, ultimately increasing the LC risk. HBV is mainly prevalent in East Asia and sub-Saharan Africa, while HCV is widespread across Europe, America, and parts of Asia. Persistent infection leads to liver fibrosis and dysfunction, which heightens the risk of malignant transformation[2,6].
Another relevant risk factor for HCC is excessive alcohol consumption and associated-liver diseases. Chronic alcohol use promotes steatosis, inflammation, and progression to cirrhosis. Nonalcoholic fatty liver disease (NAFLD), which is increasingly common in developed countries and frequently associated with progression to nonalcoholic steatohepatitis (NASH), is closely linked to metabolic syndrome, obesity, and type 2 diabetes and especially cirrhosis and cirrhosis with HCC[12].
Cirrhosis is one of the most substantial risk factors for HCC, representing the terminal stage of liver damage. It is characterized by scar tissue formation (fibrosis) and the loss of normal liver architecture. Patients with cirrhosis have a significantly higher risk of developing LC as chronic injury and subsequent regeneration often result in genetic mutations that promote carcinogenesis[13]. Exposure to aflatoxins, produced by the Aspergillus fungus, is mainly hazardous in regions with inadequate food storage practices. These toxins cause DNA damage that can lead to mutations in genes regulating cell growth and apoptosis, increasing the likelihood of HCC development[14].
ROLE OF GENETIC AND EPIGENETIC MECHANISMS IN HEPATOCARCINOGENESIS
Hepatocarcinogenesis involves complex interactions between genetic and epigenetic alterations. Escape from TGF-β1-induced apoptosis and severe degradation of the pRB protein during the early carcinogenesis stage act synergistically to promote proliferation and transformation of altered hepatocytes into tumor cells. Inactivation of the p16INK4A and TP53 genes disrupts regulatory pathways critical for controlling cell proliferation and apoptosis, supporting malignant transformation. Epigenetic changes, such as the hypermethylation of RASSF1A and GSTP1, contribute to the silencing of tumor suppressor genes, exacerbating hepatocyte transformation under chronic liver damage conditions[15-18]. Activation of the NRF2-KEAP1 pathway represents an additional and significant molecular mechanism. Early mutations in NRF2 are common in precancerous lesions, while later-stage HCC often involves KEAP1 sequestration by p62, emphasizing distinct mechanisms at different stages of tumor progression. The Wnt/β-catenin pathway, frequently dysregulated through CTNNB1 mutations, promotes uncontrolled cellular growth, especially in later stages of hepatocarcinogenesis. Finally, signaling via the IGF-II/IGF-1R axis has been shown to promote tumor cell proliferation, migration, and survival, representing a promising therapeutic target in HCC treatment[16-18].
ADVANCES IN UNDERSTANDING MOLECULAR MARKERS AND PERSONALIZED THERAPIES
Recent advances in molecular profiling and biomarker research have reshaped the landscape of HCC diagnosis and treatment. Several serum biomarkers are already in clinical use, including alpha-fetoprotein (AFP), AFP-L3, des-gamma-carboxy prothrombin, glypican-3, and Golgi protein 73. These markers (used singly or in combination) can support early detection and monitoring. Notably, the GALAD score, which incorporates age, gender, AFP, AFP-L3, and des-gamma-carboxy prothrombin, has shown high diagnostic performance, with sensitivity of 80% and specificity near 90%, outperforming single markers especially in detecting small tumors[19,20].
Parallel to diagnostic developments, molecular profiling has enabled more tailored therapeutic approaches. Genetic alterations such as TP53 and CTNNB1 mutations, epigenetic modifications, and transcriptomic signatures increasingly guide treatment decisions. Personalized treatment approaches supported by these molecular features, such as VEGF inhibitors, PD-1/PD-L1 checkpoint inhibitors, and combination regimens, have demonstrated promising results in selected subgroups of patients[21-23].
Despite these advancements, HCC early diagnosis and treatment remain major challenges. Early stages of the disease are frequently asymptomatic, and most patients are diagnosed at advanced stages when therapeutic options are limited. Nevertheless, one of the major limitations to precision medicine in HCC remains the pronounced intratumoral heterogeneity. Tumors often consist of genetically diverse subclones, which evolve under selective pressure from therapy, leading to resistance and disease progression. This underscores the need for longitudinal sampling, multiregion profiling, and integration of liquid biopsy data to guide therapy. Therefore, while substantial progress has been made, future efforts should focus on overcoming heterogeneity to fully realize the potential of individualized HCC treatment[24-28].
LC IMMUNOPATHOGENESIS: INSIGHTS INTO IMMUNE-RELATED MECHANISMS
Liver fibrosis, as a pathological response to prolonged liver injury, is characterized by excessive production and accumulation of extracellular matrix (ECM) components and abnormal connective tissue growth. Chronic damage disrupts hepatocyte membranes, triggering necrosis, apoptosis, and scarring of liver tissue. In response to injury hepatocytes release damage-associated molecular patterns, which activate quiescent hepatic stellate cells (HSCs) that change into fibrogenic cells, driving the overproduction of ECM proteins, predominantly type I and type III collagen, as well as fibronectin. This disrupts the balance between matrix metalloproteinases (MMPs), which degrade ECM, and their tissue inhibitors, leading to ECM accumulation and scar formation. These changes compromise the structural integrity of the liver and impair its normal function. As previously reported, cirrhosis as an advanced stage of chronic liver disease, is characterized by disrupted liver architecture, nodule formation, vascular organization, and ECM deposition[29-35].
HCC represents a significant challenge in the oncology field, surely by its highly aggressive nature but especially for its exceptional capability for immune evasion and the complex and dynamic interplay of immune cells within the tumor microenvironment (TME). This peculiar environment serves both as a catalyst for tumor progression and a critical barrier to therapeutic efficacy. Mechanistically, chronic liver inflammation, a central driver of HCC, activates stromal and immune cells, leading to the secretion of cytokines/chemokines, growth factors, prostaglandins, and proangiogenic factors. These factors synergistically promote angiogenesis and the establishment of an immunosuppressive milieu that supports tumor growth. Notably, each immune component plays a pivotal role in shaping the HCC trajectory through distinct mechanisms.
In detail, the persistent stimulation of inflammatory signaling cascades results in the excessive production of reactive oxygen species (ROS) and nitrogen species[36]. The progression of immune evasion is characterized by the expansion of immunosuppressive cell populations, impairments in tumor antigen presentation/processing, a disrupted equilibrium between proinflammatory/anti-inflammatory cytokines, modifications in immune checkpoint pathways, and interactions between inhibitory immune receptors and their ligands[37].
Studies using genetic mouse models have highlighted NF-κB, HIF-1α, and STAT-3 as key molecular regulators that bridge the connection between inflammation and cancer[38]. Upon the tumor rise activated Kupffer cells shift to a protumorigenic state recruiting various liver immune cells, especially monocytes which subsequently differentiate into dendritic cells (DCs) and macrophages[39]. Tumor-associated macrophages (TAMs) are among the most influential players in the TME, polarizing into two phenotypes: M1 and M2.
M1 macrophages (induced by IFN-γ, TNF-α, LPS, and GM-CSF) exhibit anticancer effects, actively inhibiting tumor development by releasing nitric oxide, ROS, and proinflammatory cytokines such as interleukin (IL)-1, IL-6, IL-12, CXCL5 and CXCL8/10 and lastly recruiting type 1 helper T (Th1) cells. Conversely, M2 macrophages, which are highly abundant in HCC tissues, are driven by TGF-β, M-CSF, IL-10, IL-4, and IL-13. The M2 macrophages promote tumor growth and angiogenesis by suppressing effector T cell infiltration and activating Th2 immune response through production of arginase I, IL-1, IL-6, and IL-10[39,40].
Regarding the DCs, their role is essential for bridging innate and adaptive immunity. However, in the HCC TME the DC function is severely impaired, with reduced antigen presentation and cytokine secretion limiting their ability to activate T and natural killer cells effectively, thereby tipping the balance toward immune tolerance[41].
Neutrophils, traditionally viewed as infection fighters, have a complex role in cancer and their recruitment into tumors relies on G-CSF, IL-1, and the CXCL2-CXCR2 axis. Hypoxia, an HCC hallmark, prompts tumor cells to release CXCL5, activating NF-κB and PI3K/AKT pathways, which attract neutrophils. In TGF-β rich environments, neutrophils adopt a protumor N2 phenotype, expressing markers such as MMP9, CCL2, CCL5, and CD95, which facilitate tumor growth and immune suppression. Conversely, TGF-β-deficient neutrophils accelerate tumor progression through high expression of CCL17 and low levels of IL-12, ICAM-1, and TNF-α. Neutrophils directly interact with HCC cells by producing ROS, causing DNA damage and genetic instability, and activation of the PI3K/AKT and MAPK pathways, which amplify protumorigenic effects. Additionally, neutrophils impair natural killer cells and lymphocyte cytolytic activity and release proinflammatory molecules, such as cytokines, chemokines, and exosomes, to indirectly drive tumor progression[42].
Exploring the adaptive immunity, T cells are the cornerstone of specific immunity and central to anti-tumor defense. However, in HCC their function is seriously compromised. Chronic antigen exposure leads to T cell exhaustion and dysfunction, characterized by reduced cytotoxic activity and the upregulation of inhibitory receptors like PD-1 and CTLA-4. Regulatory T cells (Tregs) exacerbate this immune suppression, favoring a permissive environment for tumor growth. Activated through interactions with specific ligands, alongside the involvement of IL-10 and TGF-β signaling pathways, Tregs inhibit T cell proliferation and reduce cytokine production. The CD4+CD25+Foxp3+ subset of Tregs specifically diminishes the cytotoxic activity of CD8+ T cells via inhibitory molecules such as CTLA-4 and Lag-3 and by inhibiting the synthesis and release of perforin and granzymes. Furthermore, they downregulate cytokines essential for CD8+ T cell activation, including TNF-α and IFN-γ[43].
In addition the immune cells can contribute to ECM remodeling by activating stromal cells or producing MMPs. Activated HSCs, along with Kupffer cells, macrophages, and platelets, secrete TGF-β, further amplifying HSC activation. This increases the production of MMPs (e.g., MMP2, MMP9, MMP13) and their inhibitors, such as TIMP1[44]. Moreover, inflammation plays a central role in HCC angiogenesis by promoting the release of proangiogenic cytokines from immune cells and growth factors from activated HSCs. This inflammatory environment, coupled with oxidative stress and hypoxia, drives the development of new blood vessels.
As one of the most vascular tumors, HCC relies on angiogenesis for growth, progression, and metastasis. Neovascularization supports tumor nutrition, facilitates portal vein collateral circulation, and accelerates the transition from fibrosis to HCC. Additionally, it contributes to hypoxia, necrosis, and interstitial hypertension while fostering an immunosuppressive microenvironment by suppressing antigen-presenting cells and immune cells or enhancing Tregs, myeloid-derived suppressor cells, and TAMs, which further promote angiogenesis[45].
Finally, the gut-liver axis adds another layer of complexity to the immune landscape of HCC. The gut microbiota profoundly influences systemic immunity, and dysbiosis can exacerbate inflammation and immune dysfunction in the liver. The gut-liver axis, rooted in the anatomical and functional connection between the gastrointestinal tract and liver via portal circulation, is regulated by complex metabolic, immune, and neuroendocrine interactions. Emerging evidence from animal and human studies highlights the critical role of gut microbiota in HCC development. Key contributing mechanisms include: (1) “Leaky gut,” endotoxemia, and TLR activation; (2) Dysbiosis and bacterial metabolite production; and (3) Immunomodulation[46].
GALS
Overview
Gals are carbohydrate binding proteins showing high affinity to glycosylated proteins and glycosylated lipids[47]. Gal localization differs from cell to cell, so they can be present intracellularly in cytosol, cytoskeleton, mitochondria, nucleus, lysosomes, and plasma membrane, or they can be secreted in the extracellular environment[48]. In addition they can be divided according to the presence in cells.
Gal-1 and Gal-3 are ubiquitous molecules, present in almost every single type of cell and tissue in the human body. The existence of other types of Gal is often limited to only one cell type[49]. Gals are involved in different physiological processes such as intracellular communication, proliferation, differentiation, apoptosis, autophagy, and phagocytosis[50]. However, nowadays it is well known that Gals play such a relevant role in pathogenesis of different pathological processes, mainly cancer and inflammatory disorders[51]. The explanation for such a significant participation of Gals in host various processes lies in the fact that the glycan structure recognized by Gals can be expressed on the surface of different proteins and lipids. Protein and lipid transformation are under control of several intracellular and extracellular signals, metabolic disorder, cytokines, and growth factors[52]. In that way Gals can stimulate or inhibit the function of different human molecules, and they can perform it in a nonspecific way[47].
Gals in immune response
Many studies confirmed that Gals can affect the function of immune cells. In a model of myocardial infarction, Gal-3 induced polarization of macrophages into the M2 phenotype that is critical in the process of resolving inflammation and stimulating fibrosis[53]. On the other hand intracellular Gal-3 works in a proinflammatory way by enhanced production of IL-1β via NLRP3 activation or by facilitating phagocytosis in M1 macrophages[54,55]. Regarding the adaptive immune response, Gal-3 can work as an anti-inflammatory mediator and reduce the activity of CD8 coreceptor and thus limit TCR signaling[56]. On the contrary, cytoplasmatic Gal-9 stimulates TCR signaling and can be involved in the pathogenesis of inflammatory and autoimmune diseases[57]. Gal-3 can also interfere with the humoral immune response by suppressing differentiation of B cells[58]. According to the possibility of changing the function of components of innate and specific immunity, Gals can stimulate or inhibit immune responses in different situations[47].
Clinical studies have further examined the involvement of Gals in various diseases. The study performed by Milosevic et al[59] showed that systemic levels of Gal-3 are significantly lower in patients with gonarthrosis in comparison with healthy individuals, and more importantly its predominance over the level of proinflammatory cytokines suggests an immunosuppressive role of Gal-3 in the decrease of cartilage damage. Research focused on the association between Gals and psychiatric disorders has revealed increased Gal-3 in schizophrenia remission, while decreased Gal-3 exacerbated schizophrenia in comparison with controls points to the impact of this cytokine in schizophrenia pathophysiology[60]. Moreover, mutual engagement of Gal-3 with other proinflammatory cytokines probably exerts indirect control of cognition in schizophrenia[61].
A different study investigated patients with end-stage renal disease with HCV infection and suggested that increased Gal-3 together with IL-6 may be responsible for limiting the proinflammatory process and less liver destruction[62]. Gals were investigated in patients with ulcerative colitis and metabolic syndrome. Jovanovic et al[63] claimed that patients in the terminal phase of the metabolic syndrome have more severe forms of ulcerative colitis, followed by lower levels of sera Gal-1 and increased proinflammatory cytokines, thus pointing to a potential significant role of this Gal in this comorbidity. Finally, the same authors revealed that the predominance of Gal-3 and IL-10 over proinflammatory cytokines and a higher percentage of Gal-3 expressing innate and adaptive immune cells in lamina propria in patients with metabolic syndrome probably can reduce ongoing inflammation in ulcerative colitis[64].
Gals in tumor biology
Besides evidential involvement of Gals in inflammatory and autoimmune diseases, their role in carcinogenesis and tumor progression cannot be neglected. Changes in Gal expression are associated with cell proliferation, migration, invasion, and suppression of antitumor immune response during carcinogenesis[65,66]. A recent study conducted on patients with colorectal cancer (CRC) shed light on Gal-3 and its relevant prognostic role in this cancer[67]. Higher Gal-3 levels measured in feces of patients with more severe forms of CRC had a positive correlation between fecal Gal-3 and disease severity, tumor progression and AFP and CEA, characterizing Gal-3 as a promising biomarker for the monitoring of CRC severity and progression[67]. Investigation of the role of Gal-1 in CRC revealed an increased feces level of Gal-1 in more severe CRC stage and a predomination of Gal-1 over proinflammatory cytokines, suggesting its immunosuppressive role in the limitation of the antitumor immune response[68]. Moreover, systemic Gal-1 was significantly higher in the serum of patients with CRC patients with diagnosed anemia, implicating the potential involvement of Gal-1 in the pathogenesis of anemia[69]. Overall, these studies point to the fact that Gal can be classified as powerful immunomodulators, meaning that depending on the surroundings, they can act in a proinflammatory or anti-inflammatory way.
GAL-3
Structure and functions
Gal-3 is a distinctive member of the Gal protein family, defined by its extended N-terminal domain (110-130 amino acids) containing tandem repeats and a C-terminal carbohydrate recognition domain (CRD). The CRD adopts a β-sandwich structure formed by five-stranded and six-stranded β-sheets, enabling specific binding to carbohydrates such as lactose and N-acetyl-lactosamine[70]. Unlike most Gals Gal-3 exists as a monomer in solution but oligomerizes upon binding multivalent glycans, a process facilitated by its N-terminal domain[71,72]. This oligomerization enables glycan crosslinking and transmembrane signaling, underscoring the functional diversity of Gal-3[73].
Notably, Gal-3 lacks a signal sequence for classical secretion and relies on non-classical pathways for extracellular release[74]. Intracellularly, Gal-3 plays critical roles in pre-mRNA splicing, apoptosis regulation, and signal transduction[73,75,76]. Extracellularly, Gal-3 crosslinks glycans on the cell surface, forming lattices that modulate receptor activation, endocytosis, and interactions with the ECM (e.g., laminins and integrins). These interactions regulate cellular adhesion, migration, and signaling, affecting processes like T cell receptor dynamics and growth factor receptor regulation[77]. Additionally, Gal-3 functions as a chemokine-like molecule, promoting monocyte and macrophage migration via G-protein-coupled pathways[78] and modulating immune cell differentiation and activation and cytokine production[47].
Gal-3 can exhibit both proinflammatory and anti-inflammatory effects, depending on the specific context and physiological conditions[47,79]. The functional versatility of Gal-3 stems from its structural adaptability, enabling interactions with diverse ligands and regulatory pathways. Its roles in apoptosis resistance, immune modulation, and ECM remodeling emphasize its significance in both physiological processes and pathological conditions, especially inflammation, fibrosis, and cancer[77].
Gal-3 in oncogenesis and tumor progression
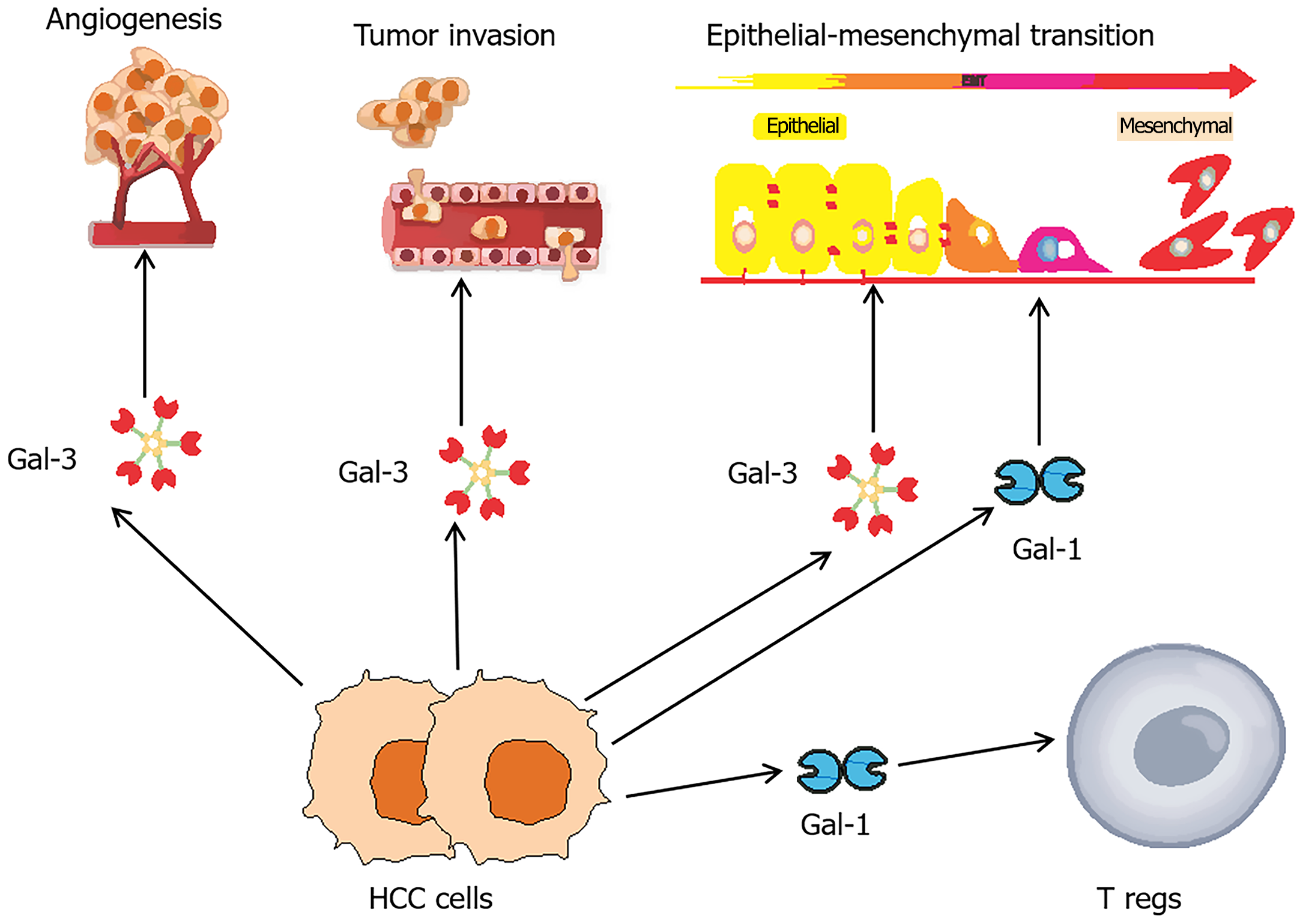
Gal-3 is a key regulator in cancer progression, influencing processes such as cell adhesion, proliferation, immune evasion, inflammation, angiogenesis, apoptosis, and metastasis (Figure 1). It modulates TME, enhancing tumor cell survival and immune suppression often through interactions with mesenchymal stromal cells. The diverse functions of Gal-3 depend on its subcellular localization, with nuclear Gal-3 regulating gene expression and cytoplasmic or extracellular Gal-3 promoting apoptosis resistance, cell adhesion, and TME interactions[80]. By inhibiting apoptosis via pathways involving p53, RAS, and BCL2 and forming glycan lattices that facilitate metastasis and receptor signaling, Gal-3 drives oncogenic processes. Additionally, Gal-3 impairs immune responses and supports angiogenesis through interactions with immune receptors, integrins, and TAMs, underscoring its central role in tumor growth and progression[77,81].
Figure 1 Galectins in key phases of hepatocellular carcinoma tumorigenesis.
Galectins (Gal) play pivotal roles in the tumorigenesis of hepatocellular carcinoma (HCC), influencing critical processes such as angiogenesis, invasion, metastasis, and immune modulation. Among these Gal-3 has been extensively studied for its role in promoting angiogenesis and enhancing the invasive and metastatic potential of HCC. Both Gal-1 and Gal-3 are implicated in epithelial-mesenchymal transition, a crucial step in the metastatic progression of HCC. Additionally, HCC cells releasing Gal-1 can modulate the immune microenvironment by stimulating regulatory T cells, leading to immunosuppressive effects and the apoptosis of anti-tumor effector T cells. HCC: Hepatocellular carcinoma; Gal: Galectin; Tregs: Regulatory T cells.
Role of Gal-3 in liver pathology
Gal-3 is a pivotal mediator in the development and progression of various liver diseases, including NAFLD, NASH, chronic viral hepatitis, biliary diseases, and liver fibrosis, and the regulation of hepatic progenitor cells. Its role spans critical processes such as lipid metabolism, inflammation, and fibrosis[82]. In NAFLD and NASH Gal-3 facilitates lipid accumulation and inflammatory signaling through pathways like CD36-mediated fatty acid uptake and NLRP3 inflammasome activation[83,84]. In chronic HBV and HCV, Gal-3 upregulation is associated with disease progression, while it exhibits a protective effect under certain conditions[82]. In primary biliary cholangitis, Gal-3 activation of NLRP3 inflammasome and Th17 pathways drives epithelial damage and inflammation[54].
A central role of Gal-3 in liver fibrosis has been established, with the protein driving HSC activation, collagen deposition, and tissue remodeling[85]. Through interactions with TGF-β signaling and profibrotic macrophages, Gal-3 fosters fibrogenesis[86]. In the context of liver cirrhosis, Gal-3 has been identified as a biomarker that when used in conjunction with other scoring systems can effectively differentiate advanced disease stages and forecast the likelihood of post-transplant infectious complications[87].
Therapeutic strategies targeting Gal-3 have shown promise with inhibitors like modified citrus pectins (e.g., belapectin) and β-thiodigalactosides[82,88]. Challenges remain in optimizing delivery methods and addressing the differential roles of intracellular and extracellular Gal-3[82]. These findings underscore the relevance of Gal-3 as both biomarker and therapeutic target, warranting further research to fully elucidate its roles and clinical potential in liver pathology.
Role of Gal-3 in HCC biology
Gal-3, a protein expressed in both healthy and cancerous tissues[89], has been the focus of early research exploring its role in HCC. Hsu et al[90] were among the first to report that Gal-3 is missing in normal hepatocytes as observed in liver biopsies and HCC cell lines, but it is significantly upregulated in HCC and strongly expressed in regenerating nodules of cirrhotic liver, suggesting its association with heightened mitotic activity or early neoplastic transformation. Subsequent studies, including those by Chung et al[91], confirmed marked Gal-3 upregulation in HCC tissues through cDNA microarray analysis, reinforcing its potential role in tumor biology.
Matsuda et al[92] further demonstrated that Gal-3 is expressed in 65% of HCC tumors, correlating with histological differentiation, vascular invasion, and worse prognosis, especially in patients with elevated nuclear expression. Additionally, Matsuda et al[92] reported that serum Gal-3 levels were significantly elevated in HCC compared to chronic liver disease, further highlighting its potential as a diagnostic marker. These early findings laid the groundwork for understanding the involvement of Gal-3 in HCC pathogenesis and its potential as a biomarker and prognostic factor.
As previously reported, HCC relies on angiogenesis for its progression, with tumor capillary endothelial cells deriving from liver sinusoidal endothelial cells through a capillarization process. This transformation is critical for tumor blood vessel formation[93]. A study by Jia et al[94] conducted a comparative protein expression analysis between these cell types and showed that Gal-3 was significantly upregulated in tumor capillary endothelial cells. For the first time the authors proposed a role for Gal-3 in vascular development within HCC.
Another study highlighted the role of Gal-3 in epidermal growth factor-mediated apoptosis in HCC, demonstrating that high epidermal growth factor concentrations inhibit proliferation and induce apoptosis in HepG2 cells by downregulating Gal-3, potentially through reduced AKT and ERK phosphorylation, with Gal-3 overexpression counteracting these effects[95]. Complementing these findings, silencing Gal-3 using small interfering RNA in HepG2 cells not only reduced proliferation, colony formation, migration, and invasion but also downregulated urokinase-type plasminogen activator receptor and its downstream signaling pathways, including AKT and MEK/ERK, underscoring the critical role of Gal-3 in modulating key tumorigenic processes[96].
Gal-3 serves as a downstream regulator of the transcription factor Runx2, playing a pivotal role in promoting epithelial-mesenchymal transition (EMT), vasculogenic mimicry, and HCC progression[97]. Additionally, Gal-3 significantly contributes to lymphatic metastasis by interacting with annexin A7, thereby influencing tumor cell proliferation, attachment, migration, and invasion[98]. Moreover, Gal-3 promotes HCC cell migration and invasion by inducing RhoA GTPase activity, MLC2 phosphorylation, and actin cytoskeleton rearrangement, as shown in both in vitro and in vivo models. Of note, in the same study, Gal-3 deficiency in mice resulted in smaller tumor burden, decreased cell proliferation, increased apoptosis, and a less invasive tumor phenotype, highlighting its potential as a therapeutic target to prevent HCC progression and metastasis[99].
Tummala et al[100] emphasized the pivotal role of Gal-3 as a paracrine signal originating from transformed hepatocytes, promoting hepatic progenitor cell stemness, expansion, and their progression toward aggressive HCC. Pharmacological or genetic inhibition of Gal-3 significantly suppressed HCC development, and its elevated expression in human HCC was associated with poorer survival outcomes, highlighting its viability as a therapeutic target[100].
Targeting the effects of Gal-3 in HCC tumorigenesis, Wang et al[101] demonstrated that the CRD of Gal-3 (Gal3C) exhibited significant anti-tumor activity by specifically inhibiting the activity of endogenous full-length Gal-3 and reducing cell viability, migration, and invasion. Recombinant Gal3C disrupted integrin clustering, dephosphorylated the FAK/SRC pathway, and downregulated NDRG1, a key protein implicated in tumor progression[101]. Further advancing this approach, the fusion protein PK5-RL-Gal3C was developed by linking the fifth Kringle domain of plasminogen to the N-terminus of Gal3C. This engineered protein not only enhanced the effects of Gal3C but also inhibited angiogenesis via the HIF1α/VEGF and Ang-2 pathways, induced cell cycle arrest and apoptosis, and significantly improved survival in tumor-bearing models[102]. These findings highlighted the potential of innovative therapies targeting Gal-3 to combat HCC progression and metastasis.
Gal-3 as HCC prognostic and surveillance biomarker
Gal-3 has emerged as a potential biomarker in HCC due to its strong association with disease progression and prognosis. Elevated serum Gal-3 levels are consistently observed in patients with HCC[103-105], correlating with poor prognostic factors such as portal vein invasion and metastatic disease[104]. Additionally, higher Gal-3 levels are linked to reduced overall survival (OS) and worsened clinical parameters, including elevated Child-Pugh scores and Cancer of the Liver Italian Program scores[106]. These findings highlight the utility of Gal-3 as a prognostic and monitoring biomarker in patients with HCC patients.
Despite its prognostic value, Gal-3 has limitations in distinguishing HCC from cirrhosis. Studies consistently report no significant differences in serum Gal-3 levels between these two conditions, making it unsuitable for diagnostic or surveillance purposes in patients with cirrhosis[103-105]. This lack of specificity restricts its diagnostic use in clinical practice. In addition, the prognostic relevance of Gal-3 is significant in HCV-related liver disease[103,106]. A report suggested that Gal-3 levels were significantly higher in HCV-associated cirrhosis and HCC compared with HBV-associated cases[103]. These findings suggest that Gal-3 may have greater prognostic value in the context of HCV infection, further emphasizing its role in liver disease progression.
However, in a study evaluating HBV cases, Gal-3 demonstrated a sensitivity of 80% and a specificity of 93% in predicting HCC, suggesting its potential in the context of HBV infection[107]. Therefore, while serum Gal-3 lacks the specificity to differentiate HCC from cirrhosis, its association with poor prognostic factors, elevated levels in HCV-related and HBV-related liver disease, and its predictive value in some contexts establish it as a valuable biomarker for assessing disease progression and survival outcomes in LC.
As previously mentioned, Gal-3 is elevated in the tissue of patients with HCC compared with healthy tissue[90], serving as a pivotal starting point for understanding its role in tumor progression and clinical outcomes. The tissue Gal-3 expression not only underscores its involvement in tumor biology but also highlights its potential utility in prognostic assessment and disease monitoring. A study by Kong et al[108] demonstrated that Gal-3 expression is significantly elevated in HCC tumor tissues compared with adjacent non-tumor tissues. Higher Gal-3 levels were closely associated with lymph-vascular invasion, poor histological differentiation, and reduced OS. Furthermore, Gal-3 was identified as an independent prognostic factor, with elevated expression correlating with poorer patient outcomes.
Song et al[109] identified Gal-3 as a key regulator of metastasis-related processes in HCC, including angiogenesis and EMT via activation of the PI3K-AKT-GSK-3β-β-catenin signaling cascade. In human HCC samples, the β-catenin/TCF4 transcriptional complex was found to target IGFBP3 and vimentin, mediating angiogenesis and EMT, respectively. In animal models Gal-3 promoted tumorigenesis and metastasis through β-catenin signaling, and its molecular inhibition synergized with sorafenib to enhance antitumor effects. These findings highlighted the Gal-3-β-catenin-IGFBP3/vimentin axis as a central pathway in HCC metastasis and a promising target for therapeutic interventions and biomarker development[109].
Additionally, the LGALS3 gene, which encodes Gal-3, has been identified as a key hub gene strongly linked to poor disease-free and OS in HCC, underscoring its critical role in tumor progression. Through comprehensive data integration and protein expression validation, this research further established Gal-3 as a promising prognostic biomarker and therapeutic target in HCC[110]. Finally, a recent meta-analysis of 33 studies involving 43 cohorts and 4168 patients with liver diseases highlighted the prognostic significance of Gal-3 in HCC. High Gal-3 expression in tissues was strongly associated with worse OS as well as positive vascular invasion[111].
Taken together, Gal-3 plays a multilayered role in HCC pathogenesis by supporting tumor cell survival, promoting angiogenesis, modulating immune responses, and easing metastatic dissemination. Its consistently elevated levels in HCC underscore its potential as a clinically meaningful biomarker for disease prognosis and monitoring as well as a promising therapeutic target. Numerous studies have confirmed that increased Gal-3 expression correlates with vascular invasion, EMT, high recurrence risk, and decreased OS[104,108,109].
Furthermore, Gal-3 has been identified as an independent prognostic factor[108,110], and its mechanistic involvement in oncogenic signaling pathways, apoptosis resistance, and immune modulation further substantiates its biological relevance[97-99,109]. Gal-3 has demonstrated encouraging results in select patient populations. Notably, in HBV-associated HCC, Gal-3 achieved sensitivity and specificity values of 80% and 93%, respectively[107]. However, broader analyses have revealed a significant overlap in Gal-3 levels between malignant and non-malignant liver diseases, mainly cirrhosis and chronic hepatitis, which limits its specificity and utility as a stand-alone diagnostic biomarker[103-105]. Additionally, the clinical Gal-3 application is constrained by variability in detection techniques and the lack of standardized cutoff values. Gal-3 is commonly quantified through immunohistochemistry in tissue samples and serum ELISAs. However, differences in antibody selection, assay sensitivity, scoring methodologies, and laboratory protocols contribute to inconsistencies across studies. The lack of universally accepted thresholds, especially for serum Gal-3, further complicates its clinical interpretability and comparability.
In summary while Gal-3 may not fully satisfy the stringent criteria required for independent diagnostic deployment, primarily due to specificity limitations and methodological heterogeneity, it fulfills key characteristics of a robust prognostic and disease-monitoring biomarker. Its incorporation into multiparametric panels, in combination with imaging and clinical scoring systems, may enhance diagnostic accuracy and support more refined patient stratification in clinical practice. Future research should focus on standardizing assay protocols, validating cutoff values, and conducting large-scale prospective studies to optimize its clinical translation and broaden its applicability in personalized medicine frameworks.
FUNCTIONS OF OTHER GALECTINS IN LC
Protective vs procarcinogenic roles of Gal-1 in HCC
The mRNA levels of Gal-1 are significantly elevated in primary HCC tissues compared with adjacent non-tumorous liver tissues and healthy liver samples[112]. Additionally, increased Gal-1 expression has been observed in metastatic lesions of patients with HCC, highlighting its relevance in cancer progression[113]. This lectin also plays a pivotal role in the tumor stroma. Activated HSCs exhibit markedly high Gal-1 levels. Conversely, suppression of Gal-1 expression in HSCs leads to a decrease in TGF-β and alpha-smooth muscle actin expression[112,114]. In cell culture studies Gal-1 mRNA levels are notably higher in invasive and poorly differentiated HCC cell lines (such as JHH-6 and HLF) compared with well-differentiated LC cells (Huh7, HepG2) or embryonic liver cells[115,116].
Gal-1 overexpression has a significant impact on various aspects of HCC progression, including cell migration, invasion, polarization, growth, proliferation, metastatic spread, and patient survival[111,117]. Elevated levels of Gal-1 are strongly associated with reduced OS and poorer relapse-free survival (RFS) in patients with HCC. While high Gal-1 expression does not correlate significantly with tumor size, it is notably linked to advanced tumor-node-metastasis (TNM) stages. Furthermore, high Gal-1 expression is significantly associated with positive vascular invasion but shows no significant correlation with tumor differentiation grade[111].
Supporting these findings, studies on athymic mice injected with HepG2 cells overexpressing Gal-1 demonstrated increased tumor volume and a higher number of tumor-draining lymph nodes compared with mice without Gal-1 overexpression. Similarly, the overexpression of Gal-1 in Huh7 and Hep3B cells enhances the invasiveness of cancer cells. In in vitro models, Gal-1 facilitates HCC cell invasion, while in vivo it promotes lung metastasis[7].
Gal-1 plays a dual role in promoting cancer and suppressing immune responses in HCC[118]. Gal-1 overexpression induces immunosuppressive effects by promoting HSC-mediated T cell apoptosis and increasing the production of IFN-γ and IL-10[119]. Notably, Gal-1 is highly expressed in Tregs, with expression levels further elevated upon activation[120]. Analysis of tissue microarrays from patients with HCC revealed a significant correlation between Gal-1 expression and the presence of FoxP3+ Tregs[120]. Patients with elevated levels of both Gal-1 and FoxP3+ Tregs exhibited higher recurrence rates and poorer OS. This interaction likely contributes to the suppression of anti-tumor immune responses in TME[120].
Additionally, Gal-1 overexpression in HSCs has been linked to reduced CD3+ T cell counts and a poor prognosis in patients with HCC. However, the expression of miR-22 can inhibit Gal-1 in HSCs, counteracting its immunosuppressive effects and favoring a less supportive TME for tumor progression[7]. The role of Gal-1 in inflammation-induced HCC has been extensively studied, providing insights into its dual functions during disease progression. Gal-1 expression is significantly elevated in chronic liver diseases associated with HCC development, suggesting its involvement in both protective and procarcinogenic processes[121]. Notably, the decreased Gal-1 leads to increased liver injury, fibrosis, and inflammation, which are critical precursors to tumorigenesis[122].
Further research suggests that Gal-1 exerts a protective effect in the early HCC stages by mitigating liver damage and inflammation. However, as HCC progresses, its role shifts toward promoting tumor growth and progression[122]. This dual function underscores the complex involvement of Gal-1 in HCC pathogenesis, influencing both the start and advancement of the disease. Elevated levels of oncogenic markers and modulators of tumor development, such as osteopontin, further emphasize its role in promoting malignancy at later stages[122].
Importantly, Gal-1 plays a crucial role in regulating liver cell proliferation and contributes significantly to HCC progression. Gal-1 enhances cell adhesion and serves as a glycan-dependent matricellular modulator in hepatoblastoma HepG2 cells. One of its key functions is as an inducer of EMT, a critical process that facilitates HCC invasion and metastasis[117]. Through a PI3K/AKT-dependent pathway, Gal-1 overexpression leads to elevated levels of the transcription factor Snail, which represses E-cadherin, a pivotal marker of epithelial integrity and a major driver of EMT[117]. Additionally, Gal-1 overexpression in HepG2 cells contributes to increased resistance to anoikis and the loss of apico-basal polarity-hallmarks of EMT. These mechanisms highlight the pivotal role of Gal-1 in promoting the invasive and metastatic potential of HCC cells (Figure 1)[123].
Overview of Gal-4 expression in HCC
Gal-4, a tandem-repeat Gal characterized by two CRDs, is predominantly expressed in epithelial cells along the gastrointestinal tract[124]. Studies using Northern blot analysis have shown that Gal-4 mRNA is more abundant in HCC compared with adjacent non-tumorous liver tissues or normal liver tissues from individuals without HCC. However, in rapidly proliferating HCC cell lines such as HuH-7 and HepG2, Gal-4 mRNA levels were either undetectable or minimal[117].
Elevated Gal-4 expression has been closely associated with favorable outcomes in patients with HCC. High expression levels correlated with improved OS and RFS[111]. Clinically, Gal-4 is linked to smaller tumor sizes, early TNM stages, well-differentiated tumors, and the absence of vascular invasion[111]. Interestingly, despite these associations with favorable characteristics, higher serological levels of Gal-4 have also been detected in more aggressive HCC forms, highlighting its complex role in the progression and prognosis of the disease[125].
Functional role of Gal-9 in HCC cell behavior
High expression of Gal-9 has been significantly associated with favorable clinical outcomes in patients with HCC. Elevated levels of Gal-9 correlated with improved OS and RFS. Clinically, Gal-9 expression was significantly linked to early TNM stages and the absence of vascular invasion. However, it showed no significant association with tumor size or differentiation grade[113]. Zhang et al[126] investigated the role of Gal-9 using in vitro models and immunohistochemical analyses of HCC tissues. The findings revealed that silencing Gal-9 expression in HepG2 cells through siRNA-mediated approaches resulted in reduced cell aggregation and increased cell proliferation and adhesion to the ECM. Additionally, Gal-9 suppression enhanced tumor cell-endothelial adhesion and promoted trans-endothelial invasion of HepG2 cells, suggesting that Gal-9 plays a critical role in maintaining cellular cohesion and limiting metastatic potential in HCC[118].
CONCLUSION
Gals, particularly Gal-1, Gal-3, Gal-4, and Gal-9, have emerged as pivotal regulators in the complex pathobiology of HCC. These multifunctional glycan-binding proteins orchestrate a broad range of cellular processes, including inflammation, immune modulation, angiogenesis, EMT, tumor proliferation, and metastatic dissemination. The dynamic and context-dependent nature of Gal functions (exerting both protumorigenic and anti-tumorigenic effects depending on their cellular localization and microenvironmental cues) highlights their dual role in HCC development and progression. Growing evidence supports the relevance of Gal not only as key modulators within the TME but also as potentially powerful biomarkers associated with prognosis, disease progression, treatment response, and immune evasion. Elevated serum or tissue expression levels of certain Gals, particularly Gal-3 and Gal-1, have been correlated with poor clinical outcomes, advanced disease stages, vascular invasion, and immune suppression. In contrast Gal-4 and Gal-9 expression has been associated with favorable prognostic indicators in specific patient subsets, further underlining the heterogeneity of Gal biology in HCC.
Although the therapeutic landscape of HCC has evolved significantly with the advent of molecular-targeted agents and immunotherapy, the prognosis for many patients remains dismal. The integration of Gal-targeted strategies, including Gal-3 inhibitors and fusion proteins such as Gal3C derivatives, may open new avenues for enhancing treatment efficacy and overcoming therapeutic resistance. Moreover, the incorporation of Gal profiling into diagnostic and prognostic frameworks may refine risk stratification and enable more personalized treatment approaches. Despite these promising insights, further large-scale, well-designed clinical studies are warranted to validate the clinical utility of Gal as biomarkers and to optimize the efficacy and safety of Gal-targeted therapies. A deeper understanding of their molecular interactions and context-specific roles will be essential to fully harness their therapeutic potential in HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Serbia
Peer-review report’s classification
Scientific Quality: Grade A, Grade B
Novelty: Grade B, Grade B
Creativity or Innovation: Grade A, Grade B
Scientific Significance: Grade B, Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Bian JM; Rodrigues de Bastos D S-Editor: Li L L-Editor: Filipodia P-Editor: Yu HG