Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.105895
Revised: April 5, 2025
Accepted: May 21, 2025
Published online: June 7, 2025
Processing time: 116 Days and 19.3 Hours
Visceral adipose tissue (VAT) plays a role in the pathogenesis of Crohn's disease (CD) and is associated with treatment outcomes following infliximab (IFX) therapy. We developed and validated the first delta-radiomics model to quantify VAT heterogeneity as a predictive biomarker for IFX response in patients with CD.
To develop a longitudinal computed tomography (CT)-based delta-radiomics model of VAT for predicting secondary loss of response (SLR) in patients with CD.
This retrospective study included 161 patients with CD who achieved clinical remission following IFX induction therapy between 2015 and 2023. All patients underwent CT enterography before IFX initiation and after completing induction therapy. VAT volume was delineated by two radiologists in consensus. Radiomics features were extracted from pre-treatment and post-induction CT images, and delta-radiomics features were calculated as follows: Delta features = Feature-post - Feature-pre. A radiomics model was constructed using logistic regression. Model performance was assessed using discrimination, calibration, and decision curve analyses.
Nine significant delta-radiomics features were used to develop the delta-radiomics model, yielding an area under the receiver operating characteristic curve (AUC) of 0.816 (95%CI: 0.737-0.896) in the training cohort and 0.750 (95%CI: 0.605-0.895) in the validation cohort. Multivariable logistic regression identified platelet count, Montreal behavior classification, and the VAT/subcutaneous adipose tissue volume ratio prior to treatment as independent risk factors for SLR. The combined model integrating clinical predictors and delta-radiomics features achieved superior predictive performance, with an AUC of 0.853 (95%CI: 0.786-0.921) in the training cohort and 0.812 (95%CI: 0.677-0.948) in the validation cohort.
We developed a predictive model based on longitudinal changes in VAT, demonstrating significant potential for identifying patients with CD at high risk of SLR to IFX therapy.
Core Tip: The treatment response to infliximab in patients with Crohn's disease (CD) is heterogeneous, with approximately 23%-46% of those achieving clinical remission after induction therapy experiencing secondary loss of response (SLR) within the first year. This significantly increases the risk of serious adverse outcomes. In this study, we developed a delta-radiomics model based on longitudinal changes in visceral adipose tissue assessed through computed tomography enterography. This model further integrates clinical and imaging biomarkers to identify patients with CD at high risk of SLR. This non-invasive approach holds promise as a valuable tool for optimizing personalized treatment regimens and guiding monitoring strategies.
- Citation: Li X, Song FL, He HF, Zeng SM, Feng ZC, Rong PF. Longitudinal computed tomography-based delta-radiomics of visceral adipose tissue predicts infliximab secondary loss of response in Crohn’s disease patients. World J Gastroenterol 2025; 31(21): 105895
- URL: https://www.wjgnet.com/1007-9327/full/v31/i21/105895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i21.105895
Crohn's disease (CD) is a chronic autoimmune inflammatory bowel disease (IBD) characterized by alternating relapsing and remitting symptoms throughout its course, often leading to progressive intestinal damage[1]. The primary clinical objective in managing CD is maintaining long-term asymptomatic remission and preventing serious complications[2,3]. Infliximab (IFX), a monoclonal antibody targeting tumor necrosis factor (TNF), has been shown to significantly improve intestinal mucosal healing and increase clinical remission rates in patients with CD[3,4]. However, approximately 23-46% of patients who achieve clinical remission following IFX induction therapy experience secondary loss of response (SLR) within the first year, increasing the risk of disease exacerbation and serious adverse outcomes[5].
Presently, the efficacy of IFX treatment is primarily monitored through periodic endoscopic evaluations. However, due to limitations such as invasiveness, cost, and patient discomfort, endoscopy is not suitable for all individuals. Although several risk factors-such as disease duration, C-reactive protein (CRP) levels, fecal calprotectin concentration, and the presence of creeping fat-have been identified in relation to IFX treatment response, many of these markers are subjective, and the underlying mechanisms remain poorly understood, which limits their widespread clinical applicability[6-8]. Therefore, there is a need for reliable predictive tools capable of identifying patients at risk of SLR to support personalized management strategies.
Growing evidence suggests that visceral adipose tissue (VAT) plays a critical role in the pathogenesis of CD and is strongly associated with disease complexity, prognosis, and the occurrence of complications[9]. VAT contributes to chronic intestinal inflammation by secreting pro-inflammatory cytokines such as TNF-α and various interleukins[10]. Several studies have employed fat attenuation techniques based on CT imaging to quantitatively assess VAT and derive metrics predictive of CD prognosis[11,12]. However, using single fat parameters to characterize the relationship between VAT and treatment response in CD has notable limitations. These methods often rely on conventional visual assessment, exhibit inter-observer variability, and lack sensitivity in detecting subtle changes in adipose tissue.
In contrast, more comprehensive analyses that capture microstructural alterations and functional impairments within VAT may offer deeper insights into therapeutic outcomes and disease progression in CD[13].
Radiomics, an emerging technique capable of non-invasively extracting extensive quantitative information from medical images, has demonstrated considerable potential in clinical diagnosis and treatment[14]. Previous studies have confirmed the significant clinical utility of VAT-based radiomic features in predicting CD progression[15]. However, in CD patients undergoing IFX induction therapy and achieving clinical remission, VAT exhibits tissue heterogeneity and subtle pathophysiological alterations that vary temporally and spatially before and after treatment. These changes may be indicative of differential therapeutic outcomes[9].
Delta-radiomic features enable precise quantification of longitudinal variations in subtle structural tissue characteristics between two time points[16], offering enhanced information for assessing the response of CD patients to IFX therapy. Compared to magnetic resonance enterography (MRE), CT provides superior spatial resolution for VAT quantification and broader clinical accessibility in routine practice, rendering it more feasible for delta-radiomic analysis. Despite this, no prior studies have assessed the potential clinical value of VAT radiomic features derived from longitudinal CT enterography for predicting SLR to IFX.
This study aims to develop a delta-radiomics model based on longitudinal changes in VAT and to integrate clinical and imaging biomarkers to investigate its predictive utility in identifying CD patients at high risk for SLR.
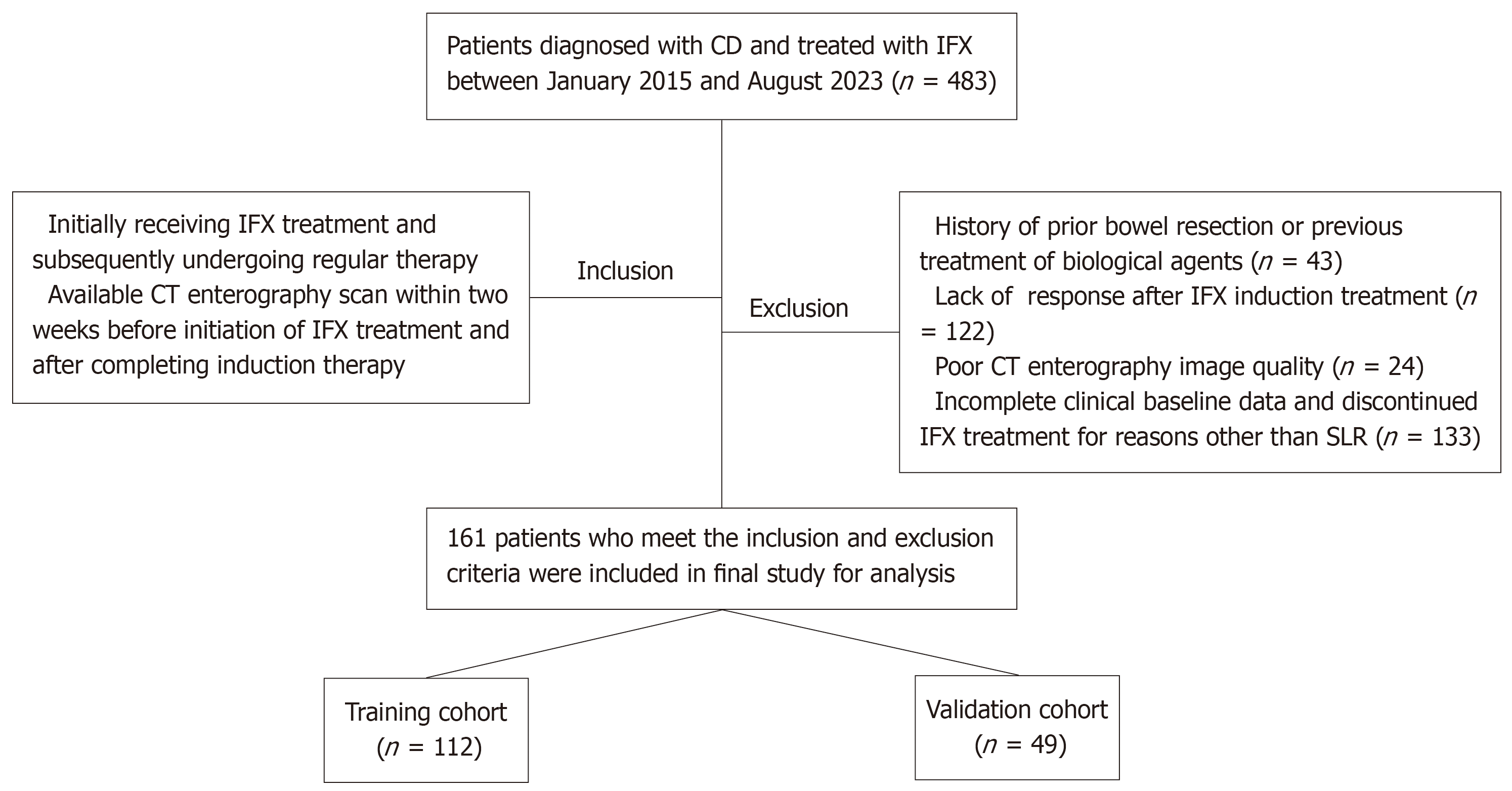
This retrospective study was approved by the Institutional Ethics Review Board of the Third Xiangya Hospital, Central South University, with the requirement for informed patient consent waived. Patients diagnosed with CD and treated with IFX between January 2015 and August 2023 at the institution were enrolled. CD diagnosis followed the European Crohn’s and Colitis Organization guidelines[3]. IFX was administered intravenously with a dosage of 5-10 mg/kg at weeks 0, 2, and 6, followed by maintenance dosing every 8 weeks.
Inclusion criteria: (1) Initial administration of IFX with subsequent regular continuation of therapy; and (2) Availability of CT enterography scans within two weeks prior to IFX initiation and following induction therapy (i.e., between weeks 0-2 and 2-6, with week 14 evaluation).
Exclusion criteria: (1) History of prior bowel resection or previous treatment of biological agents; (2) Lack of clinical response after IFX induction treatment; (3) Poor CT enterography image quality; and (4) Incomplete clinical baseline data and discontinued IFX treatment for reasons other than SLR.
The patient selection process is detailed in Figure 1. Enrolled patients were randomly assigned to the training or validation cohorts in a 7:3 ratio.
Baseline data included age, sex, height, weight, smoking history, neutrophil, lymphocyte, platelet counts, serum albumin, creatinine, erythrocyte sedimentation rate, and CRP levels. Additionally, CD location and behavior were documented according to the Montreal classification. Body mass index was calculated as weight (kg) divided by height squared (m²), and the neutrophil-lymphocyte ratio was calculated by dividing the neutrophil count by the lymphocyte count.
Generally, SLR is defined as the recurrence of clinical symptoms between 14 and 54 weeks following an initial response to IFX[17,18]. In this study, SLR outcomes were evaluated by an experienced multidisciplinary team based on clinical symptoms, radiological and endoscopic findings, and subsequent treatment modifications. These included initiating alternative biological agents, requiring immunosuppressants or corticosteroids, IFX dose escalation, and CD-related surgery. Additional criteria included a CD Activity Index > 150 or a reduction of less than 70% from baseline and/or evidence of mucosal recurrence, defined as a reduction in the Simple Endoscopic Score for CD (SES-CD) of < 50% or an SES-CD ≥ 3.
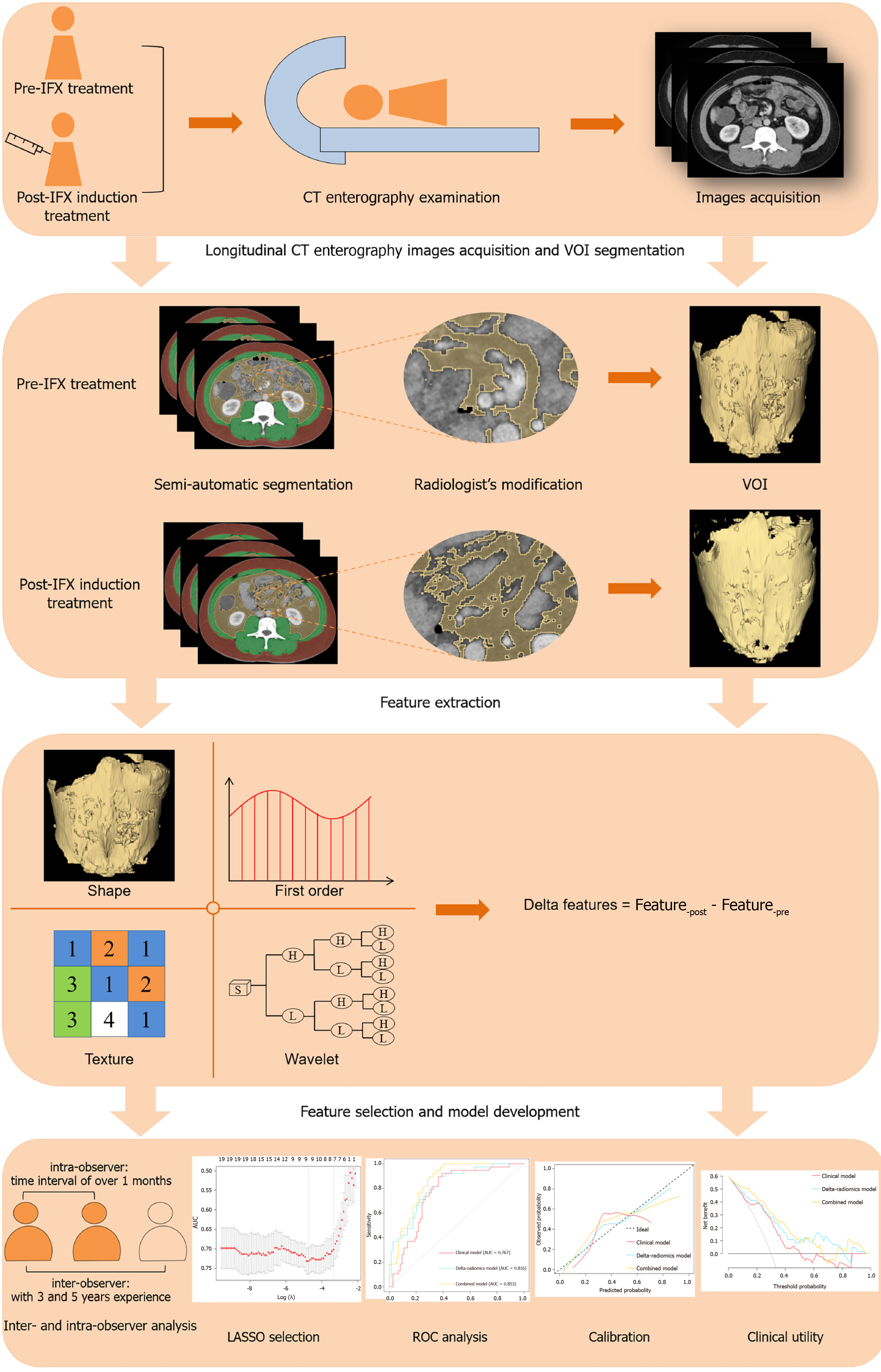
CT enterography examinations were performed using a 64-slice multidetector CT scanner (Philips Brilliance; Philips) or a 256-slice scanner (GE Revolution; GE Healthcare), following standardized bowel preparation protocols. Patients fasted prior to imaging and consumed 1200-1500 mL of 2.5% isotonic mannitol solution in three divided doses at 15-minute intervals before the scan.
Scanning was conducted in the supine position, from the diaphragm to the symphysis pubis. After the acquisition of non-enhanced images, a nonionic contrast agent (320 mg/mL; Loversol, Jiangsu Hengrui Medicine Corp Ltd) was administered intravenously at a dose of 1.5-2.0 mL/kg, with an average injection rate of 3.0-4.0 mL/s. Using automatic bolus-tracking, arterial phase images were obtained either 15 seconds after the attenuation value in the abdominal aorta reached 100 HU or with a fixed delay of 32-35 seconds following contrast administration. Portal venous phase images were acquired 35 seconds after the arterial phase, or with a total delay of 70 seconds post-injection.
Detailed scanner parameters are provided in Supplementary Table 1. All CT enterography scans were performed within two weeks before IFX initiation and following the completion of the induction phase. For radiomic analysis, images from the portal venous phase were selected.
Volumes of VAT used for radiomics analysis were defined from the top of the diaphragm to the superior margin of the symphysis pubis[15]. Based on tissue-specific attenuation thresholds ranging from -150 to -50 HU[19], two abdominal radiologists-blinded to all clinical information- initially delineated the VAT volumes using the 3D semi-automated segmentation module of an open-source software platform (3D Slicer V4.11.2, https://www.slicer.org/).
Subsequently, manual corrections and verification were performed to finalize the Volume of interests (VOIs). Any discrepancies between the two radiologists were thoroughly reviewed and resolved through consensus. A representative example of the VOI segmentation process is provided in Supplementary Figure 1.
To evaluate reproducibility, the two radiologists independently segmented 30 randomly selected cases after a one-month interval. The intra-class correlation coefficient (ICC) was calculated to assess both intra- and inter-observer reliability, with the methodology illustrated in Figure 2.
The Artificial Intelligent Kit (version 3.2.2; GE Healthcare) was employed to resample CT enterography images. To address differences in voxel resolution across various CT scanners, the voxel dimensions of the portal venous phase images were isotropically resampled to 1 mm × 1 mm × 1 mm (x, y, z) using a linear interpolation algorithm. Hounsfield unit values were discretized into 64 bins to standardize intensity levels.
Subsequently, 1702 radiomics features-including both pre-treatment and post-induction treatment data-were extracted for each patient using the “PyRadiomics” plugin in the 3D Slicer software. To quantify longitudinal changes in VAT, delta-radiomics features were calculated as the relative net differences between the pre-treatment feature values (Featurepre) and the post-induction treatment values (Featurepost) as follows: Delta features = Feature-post - Feature-pre. A total of 851 delta-radiomics features could be obtained per patient, encompassing shape features (n = 14), texture features (n = 75), histogram features (n = 18), and wavelet-based features (n = 744).
Additional adipose tissue parameters were incorporated into the study, including VAT volume (mm3), subcutaneous adipose tissue (SAT) volume (mm3), VAT/SAT volume ratio, VAT area (mm2) and SAT area (mm2) measured at the L3 Lumbar vertebral level, and VAT/SAT area ratio prior to treatment initiation and after induction therapy. All measu
To construct the clinical model, univariable and multivariable logistic regression analyses were performed in the training cohort to identify potential clinical predictors. Model performance was assessed using the minimal Akaike Information Criterion (AIC) to optimize fit.
A multi-step feature selection process was implemented to prevent overfitting in the delta-radiomics model. First, all delta-radiomics features were standardized using the z-score method to ensure data uniformity. Features with an ICC ≥ 0.75 were retained for further analysis. In the univariable analysis, features with P values < 0.05 were selected. The final set of significant features was determined using the least absolute shrinkage and selection operator (LASSO) method with tenfold cross-validation (Supplementary Figure 2), and these were used to construct a delta-radiomics score (delta-radscore).
A nomogram was then developed by integrating the selected clinical predictors with the delta-rad score through logistic regression. Model discrimination was evaluated using receiver operating characteristic (ROC) curve analysis, and the areas under the curves (AUCs) were compared using the DeLong test. Model calibration and clinical utility were assessed with a calibration and decision curve analysis (DCA).
Statistical analyses were conducted using R statistical software (version 4.3.1; https//www.r-project.org). Continuous variables were expressed as mean ± SD for normally distributed data or as median with interquartile range for non-normally distributed data. These were analyzed using the Student’s t-test and the Mann-Whitney U test, respectively. Categorical variables were presented as frequencies or percentages and compared using the χ2 test or Fisher’s exact test, as appropriate. A two-sided P value < 0.05 was considered statistically significant.
A total of 161 eligible patients were included in the final analysis (129 men, 32 women; mean age, 29 years). Demographic and clinical data for the training cohort (n = 112) and validation cohort (n = 49) are summarized in Table 1. Among them, 38 (33.9%) patients in the training cohort and 16 (32.7%) in the validation cohort experienced SLR to IFX during treatment. There were no significant differences in demographic or clinical characteristics between the two cohorts (all P > 0.05).
| Variables | Total (n = 161) | Training (n = 112) | Validation (n = 49) | P value |
| Age (years) | 29 (22, 38) | 27 (21, 38) | 31 (24, 38) | 0.308 |
| Male gender | 129 (80.1) | 90 (80.4) | 39 (79.6) | 0.999 |
| BMI (kg/m²) | 18.70 (16.60, 21.50) | 19.10 (16.60, 21.50) | 17.60 (16.60, 20.10) | 0.282 |
| Smoking | 49 (30.4) | 33 (29.5) | 16 (32.7) | 0.827 |
| Platelet count (× 109 cells/L) | 299 (299, 368) | 298 (227, 377) | 311 (234, 358) | 0.834 |
| Albumin (g/dL) | 35.2 (32.3, 40.1) | 35.2 (32.4, 39.9) | 35.0 (31.7, 40.1) | 0.827 |
| ESR (mm/hour) | 40.0 (22.0, 74.0) | 42.0 (22.0,75.0) | 37.0 (23.0,70.0) | 0.887 |
| CRP (mg/dL) | 20.2 (5.0, 50.0) | 21.6 (5.4, 49.9) | 17.6 (5.0, 51.2) | 0.779 |
| Cr (μmol/L) | 67 (59, 76) | 68 (61, 77) | 66 (58, 74) | 0.309 |
| NLR | 4.3 (2.7, 5.8) | 4.3 (2.7, 5.7) | 4.0 (2.7, 6.0) | 0.977 |
| CDAI | 159.0 (107.9, 234.2) | 158.7 (99.5, 235.4) | 159.0 (118.0, 230.0) | 0.786 |
| Montreal location classification | 0.380 | |||
| L1 (ileal disease) | 58 (36.0) | 41 (36.6) | 17 (34.7) | |
| L2 (colonic disease) | 15 (9.3) | 13 (11.6) | 2 (4.1) | |
| L3 (ileocolonic disease) | 88 (54.7) | 58 (51.8) | 30 (61.2) | |
| Montreal behaviour classification | 0.554 | |||
| B1 (non stricturing, non-penetrating) | 72 (44.7) | 47 (42.0) | 25 (51.0) | |
| B2 (stricturing) | 79 (49.1) | 58 (51.8) | 21 (42.9) | |
| B3 (penetrating) | 10 (6.2) | 7 (6.2) | 3 (6.1) | |
| Pre-IFX treatment | ||||
| SAT area (cm2/m2) | 20.0 (7.9, 33.4) | 21.7 (8.3, 33.4) | 19.2 (7.2, 33.4) | 0.511 |
| VAT area (cm2/m2) | 8.1 (3.2, 25.9) | 7.4 (3.2, 24.5) | 9.7 (3.1, 28.0) | 0.790 |
| SAT volume (cm3/m3) | 423.2 (166.0, 773.3) | 438.2 (170.7, 758.5) | 359.8 (155.1, 806.2) | 0.901 |
| VAT volume (cm3/m3) | 187.3 (83.1, 421.4) | 169.6 (79.15, 410.3) | 214.7 (93.6, 468.1) | 0.866 |
| VAT/SAT area ratio (≥ 1) | 45 (28.0) | 28 (25.0) | 17 (34.7) | 0.210 |
| VAT/SAT volume ratio (≥ 1) | 27 (16.8) | 19 (17.0) | 8 (16.3) | 0.921 |
| Post-IFX treatment | ||||
| SAT area (cm2/m2) | 22.7 (12.9, 35.6) | 23.9 (13.7, 39.7) | 19.8 (12.1, 31.7) | 0.068 |
| VAT area (cm2/m2) | 11.0 (5.2, 24.9) | 11.7 (6.1, 26.7) | 9.8 (4.2, 20.3) | 0.219 |
| SAT volume (cm3/m3) | 310.5 (166.1, 596.4) | 321.5 (181.8, 627.6) | 297.6 (156.8, 535.2) | 0.368 |
| VAT volume (cm3/m3) | 436.2 (244.5, 759.9) | 469.1 (272.9, 824.0) | 359.6 (214.8, 626.8) | 0.131 |
| VAT/SAT area ratio (≥ 1) | 37 (23.0) | 25 (22.3) | 12 (24.5) | 0.765 |
| VAT/SAT volume ratio (≥ 1) | 18 (11.2) | 12 (10.7) | 6 (12.2) | 0.778 |
In the training cohort, multivariable logistic regression analysis identified the following as independent predictors of SLR:
Platelet count [odds ratio (OR) = 1.005, 95%CI: 1.001-1.008, P = 0.011], Montreal behavior classification (OR = 2.307, 95%CI: 1.108-4.803, P = 0.025), VAT/SAT volume ratio before IFX treatment (OR = 3.977, 95%CI: 1.320-11.980, P = 0.014).
These three variables were incorporated into a clinical model developed using logistic regression (Table 2).
| Variables | Non-SLR (n = 74) | SLR (n = 38) | Univariable analysis | Multivariable analysis | ||
| OR (95CI) | P value | OR (95%CI) | P value | |||
| Age (years) | 27 (21, 38) | 27 (21, 38) | 1.004 (0.677-1.489) | 0.990 | ||
| Male gender | 61 (82.4) | 29 (76.3) | 1.456 (0.323-6.573) | 0.603 | ||
| BMI (kg/m2) | 19.25 (16.62, 21.10) | 18.95 (16.65, 23.02) | 0.968 (0.292-3.210) | 0.794 | ||
| Smoking | 23 (31.1) | 10 (26.3) | 1.263 (0.453-3.521) | 0.760 | ||
| Platelet count (× 109 cells/L) | 291 (197, 368) | 323 (272, 406) | 0.997 (0.021-46.938) | 0.049 | 1.005 (1.001-1.008) | 0.011 |
| Albumin (g/dL) | 35.7 (32.7, 40.3) | 34.3 (32.3, 39.7) | 1.020 (0.289-3.593) | 0.480 | ||
| ESR (mm/hour) | 39.5 (24.0, 74.8) | 47.0 (18.3, 75.5) | 1.000 (0.972-1.029) | 0.703 | ||
| CRP (mg/dL) | 22.1 (6.7, 51.2) | 16.1 (5.0, 44.4) | 1.006 (0.116-8.735) | 0.296 | ||
| Cr (μmol/L) | 69 (61,78) | 65 (58, 74) | 1.020 (0.078-13.332) | 0.167 | ||
| NLR | 4.3 (2.7, 5.7) | 4.3 (3.1, 5.8) | 1.033 (0.360-2.964) | 0.825 | ||
| CDAI | 156.5 (98.5, 242.4) | 166.4 (124.8, 233.5) | 1.001 (0.590-1.695) | 0.890 | ||
| Montreal location classification | 0.786 (0.090-6.874) | 0.401 | ||||
| L1 (ileal disease) | 29 (39.2) | 12 (31.5) | ||||
| L2 (colonic disease) | 8 (10.8) | 5 (13.2) | ||||
| L3 (ileocolonic disease) | 37 (50.5) | 21 (55.3) | ||||
| Montreal behaviour classification | 0.472 (0.007-32.379) | 0.031 | 2.307 (1.108-4.803) | 0.025 | ||
| B1 (non stricturing, non-penetrating) | 36 (48.6) | 11 (29.0) | ||||
| B2 (stricturing) | 35 (47.3) | 23 (60.5) | ||||
| B3 (penetrating) | 3 (4.1) | 4 (10.5) | ||||
| Pre-IFX treatment | ||||||
| SAT area (cm2/m2) | 21.2 (7.5, 33.4) | 21.7 (9.2, 33.4) | 0.999 (0.873-1.143) | 0.98 | ||
| VAT area (cm2/m2) | 6.9 (3.0, 21.6) | 9.7 (3.6, 26.9) | 0.994 (0.320-3.088) | 0.405 | ||
| SAT volume (cm3/m3) | 453.3 (165.2, 761.2) | 409.8 (179.3, 745.3) | 1.000 (0.840-1.190) | 0.815 | ||
| VAT volume (cm3/m3) | 168.5 (75.5, 394.8) | 234.1 (97.3, 415.2) | 0.999 (0.210-4.761) | 0.424 | ||
| VAT/SAT area ratio (≥ 1) | 17 (57.0) | 11 (28.9) | 1.366 (0.353-5.282) | 0.491 | ||
| VAT/SAT volume ratio (≥ 1) | 8 (10.8) | 11 (28.9) | 3.361 (0.034-330.690) | 0.016 | 3.977 (1.320-11.980) | 0.014 |
| Post-IFX treatment | ||||||
| SAT area (cm2/m2) | 26.4 (14.3, 39.8) | 20.2 (12.9, 39.3) | 1.007 (0.272-3.731) | 0.386 | ||
| VAT area (cm2/m2) | 11.8 (6.1, 28.6) | 11.1 (6.2, 23.0) | 1.009 (0.222-4.577) | 0.681 | ||
| SAT volume (cm3/m3) | 481.9 (289.0, 782.3) | 408.1 (271.2, 836.1) | 1.000 (0.226-4.427) | 0.435 | ||
| VAT volume (cm3/m3) | 319.2 (187.9, 624.6) | 434.9 (170.6, 612.7) | 1.000 (0.544-1.838) | 0.975 | ||
| VAT/SAT area ratio (≥ 1) | 18 (24.3) | 7 (18.4) | 0.703 (0.175-2.815) | 0.479 | ||
| VAT/SAT volume ratio (≥ 1) | 6 (8.1) | 6 (15.8) | 2.125 (0.193-23.398) | 0.215 | ||
A total of 851 delta-radiomics features were extracted from CT enterography images. Following intra- and inter-observer reliability assessment (ICCs > 0.75) and univariate correlation analysis, 193 delta-radiomics features showed statistically significant differences between the SLR and non-SLR groups in the training cohort (P < 0.05).
After applying LASSO selection, 9 delta-radiomics features with nonzero coefficients were obtained to construct a delta-radiomics model based on logistic regression. The model was expressed through the following formula:
Delta-radscore = -1.150
+ 0.452 × Original-Shape-MajorAxisLength
+ 0.448 × Original-Shape-MinorAxisLength
- 1.202 × Wavelet-LHH-Ngtdm-Busyness
- 0.584 × Wavelet-LLH-Gldm-LargeDependenceLowGrayLevelEmphasis
+ 0.436 × Wavelet-LHH-Ngtdm-Contrast
- 0.591 × Wavelet-LHL-Firstorder-Skewness
- 0.193 × Wavelet-HLL-Ngtdm-Contrast
+ 1.118 × Wavelet-HHL-Glcm-JointEnergy
- 0.125 × Wavelet-LLL-Glcm-DifferenceAverage
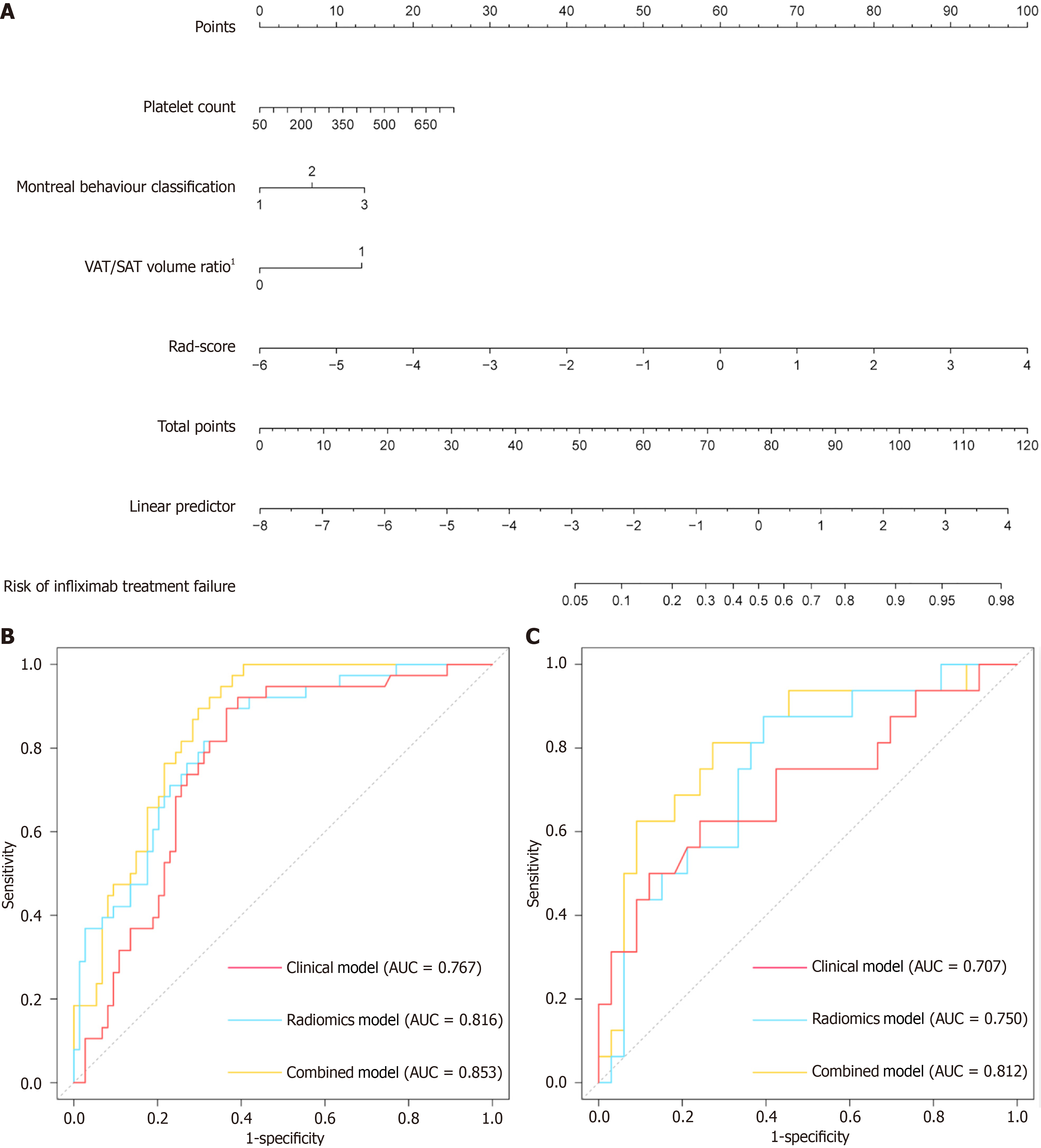
To integrate significant clinical parameters and delta-radscore, we further established a clinical-radiomics combined model. Based on the combined model, we developed a visual nomogram for simplified application in clinical practice (Figure 3A).
The predictive performance of each model was assessed using ROC analysis, yielding metrics such as the AUC, sen
| AUC (95%CI) | Sensitivity | Specificity | PPV | NPV | P value1 | Accuracy | |
| Training cohort (n = 112) | |||||||
| Clinical model | 0.767 (0.678-0.857) | 85.1% (63/74) | 36.8% (14/38) | 72.4% (63/87) | 56.0% (14/25) | - | 68.8% (77/112) |
| Radiomics model | 0.816 (0.737-0.896) | 86.5% (64/74) | 47.4% (18/38) | 76.2% (64/84) | 64.3% (18/28) | 0.133 | 73.2% (82/112) |
| Combined model | 0.853 (0.786-0.921) | 85.1% (63/74) | 52.6% (20/38) | 77.8% (63/81) | 64.5% (20/31) | 0.023 | 74.1% (83/112) |
| Validation cohort (n = 49) | |||||||
| Clinical model | 0.707 (0.539-0.876) | 96.97% (32/33) | 31.3% (5/16) | 74.4% (32/43) | 83.3% (5/6) | - | 75.5% (37/49) |
| Radiomics model | 0.750 (0.605-0.895) | 84.8% (28/33) | 50.0% (8/16) | 77.8% (28/36) | 61.5% (8/13) | 0.204 | 73.5% (36/49) |
| Combined model | 0.812 (0.677-0.948) | 93.9% (31/33) | 50.0% (8/16) | 79.5% (31/39) | 80.0% (8/10) | 0.048 | 79.6% (39/49) |
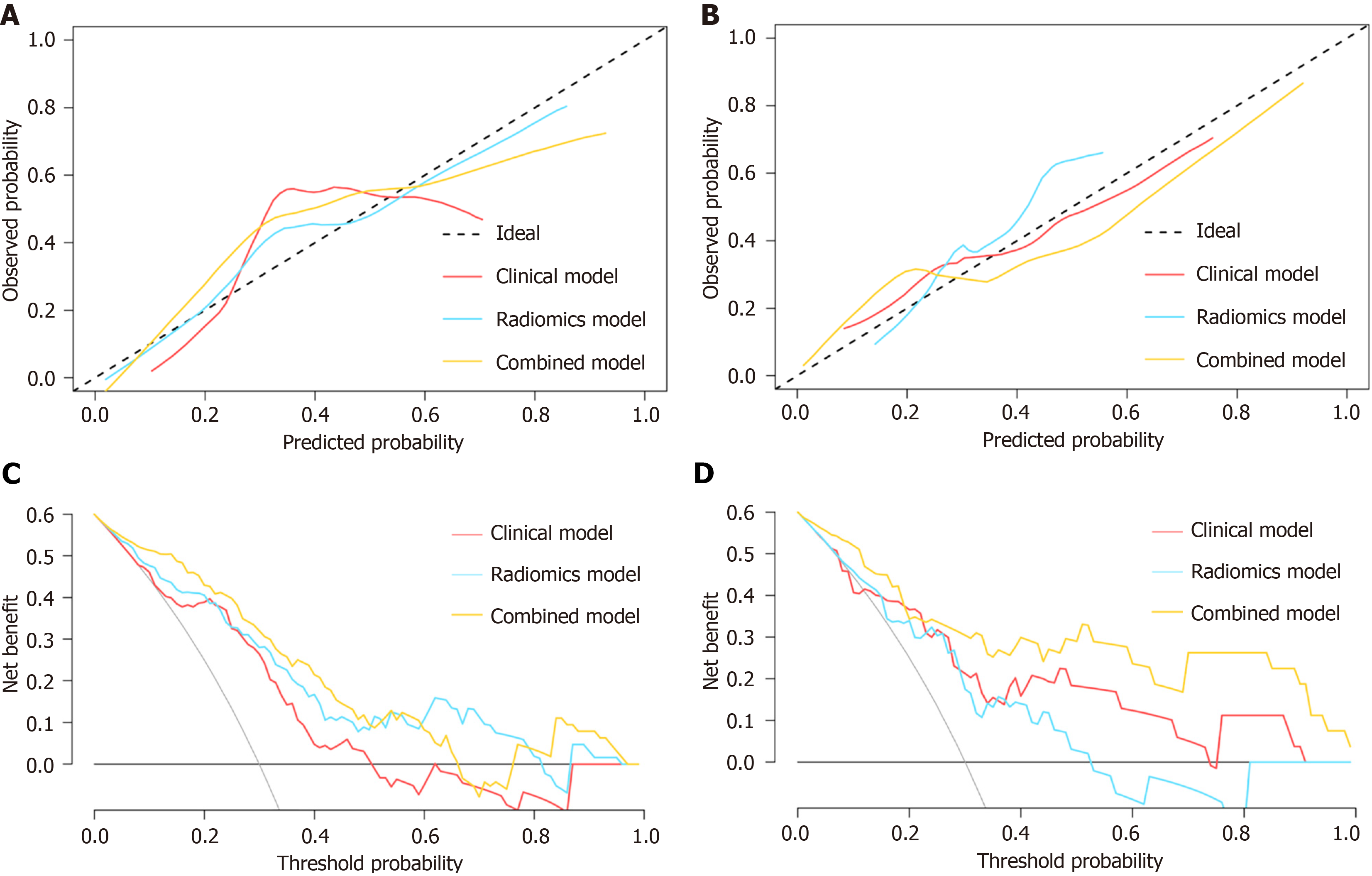
Calibration plots of the combined model showed excellent agreement between the predicted probabilities and observed outcomes in both the training (Figure 4A) and validation (Figure 4B) cohorts. DCA revealed that the combined model outperformed the clinical and delta-radiomics models, offering greater overall net benefit for predicting SLR to IFX treatment in CD patients across the most clinically relevant threshold probability ranges in both the training (Figure 4C) and validation (Figure 4D) cohorts.
Early and accurate identification of SLR to IFX treatment is critical for effectively managing CD. This study utilized baseline clinical parameters and longitudinal delta-radiomics features to develop and validate a predictive model for identifying patients at high risk of SLR. The model demonstrated satisfactory predictive performance in both the training and validation cohorts. Our findings further support the utility of delta-radiomics in capturing temporal and spatial alterations associated with pathophysiological and microstructural changes in VAT, providing valuable insights for predicting treatment response to IFX.
Numerous studies have established that VAT, a complex organ with multifaceted endocrine and immune functions, secretes various pro-inflammatory cytokines and plays a pivotal role in the pathogenesis of CD. It is strongly associated with disease progression and suboptimal therapeutic outcomes[20]. In CD, mesenteric adipose tissue exhibits structural disorganization and functional impairment, characterized by increased deposition of inflammatory mediators and infiltration of immune cells, thereby contributing to intestinal inflammation[21,22]. Radiomics techniques can non-invasively capture these pathophysiological alterations in VAT and transform them into quantifiable imaging features for analysis. A multicenter study by Li et al[15] demonstrated that a VAT-based radiomics model accurately identified patients at elevated risk of disease progression. Additionally, a VAT-based deep learning radiomics model effectively differentiated CD from ulcerative colitis[23], showing the presence of distinct microstructural and metabolic characteristics in VAT associated with CD.
Considering that IFX treatment may influence VAT evolution in CD patients[24], we conducted delta-radiomics analysis using CT enterography images obtained before and after IFX induction. Longitudinal changes in VAT between the pre-treatment and post-induction phases were extracted and quantified into nine delta-radiomics features using LASSO regression to predict long-term response to IFX. The resulting delta-radiomics model demonstrated strong predictive capability in identifying high-risk SLR patients. Notably, the nomogram derived from this model offers clinicians a practical tool for optimizing therapeutic strategies, thereby enabling personalized management of CD patients in clinical settings.
In our study, a VAT/SAT volume ratio ≥ 1.0 prior to IFX treatment independently predicted SLR in CD patients. This finding aligns with previous research. Gu et al[11] reported that a higher visceral fat index-the ratio of visceral to SAT-was associated with an increased risk of surgery within 6 months of initiating IFX therapy. Other studies have similarly shown that a higher baseline percentage of intra-abdominal VAT relative to total adipose tissue correlates with reduced rates of corticosteroid-free deep remission or endoscopic remission following biologic therapy[9], as well as with increased disease activity and the occurrence of comorbidities[25]. A prospective cohort study also reported that a higher VAT/SAT volume ratio was associated with more severe disease behavior[26].
Despite these findings, our study did not detect a significant correlation between the VAT/SAT area ratio and SLR to IFX. This discrepancy may be attributed to the limitation of measuring fat area from a single axial slice at the L3 vertebral level, which may not fully reflect overall fat distribution[27]. We also observed that patients with complicated disease behaviors, such as stricturing or penetrating phenotypes, were more likely to experience SLR. This is likely because these more complex disease forms are associated with heightened intestinal inflammation, and initiating anti-TNF therapy after developing such phenotypes may result in reduced efficacy and an increased risk of surgical intervention[28,29].
Abnormalities in platelet number and function have been well documented in IBD[30,31]. As pro-inflammatory cells, platelets release various inflammatory mediators that can initiate or amplify the inflammatory response through diverse cellular and molecular mechanisms, often involving classical immune cells in IBD[30]. Platelets play a critical role in both acute and chronic phases of inflammation. Furthermore, activated platelets secrete profibrogenic factors that contribute to the development of fibrosis in gastrointestinal disorders[32]. In patients with ulcerative colitis who have achieved mucosal healing, elevated platelet counts have been associated with a higher risk of disease recurrence[33].
The platelet-to-lymphocyte ratio has emerged as a promising biomarker for predicting the therapeutic response to anti-TNF treatment in both CD and ulcerative colitis[34,35]. Moreover, an elevated platelet-to-albumin ratio has been iden
Our study has several limitations. First, to enhance the stability and practical applicability of the model, we did not independently include radiomics features extracted from VAT before and after treatment; their clinical utility requires further investigation. Second, although this study confirmed the clinical value of longitudinal VAT changes derived from CT enterography for predicting IFX treatment outcomes using radiomics techniques, CT imaging raises concerns regarding radiation exposure, particularly in adolescent patients. While some studies have utilized MRE to analyze adipose tissue[37], most have been limited to assessments at the level of the third lumbar vertebra. Further research is needed to clarify MRE’s potential for evaluating the entire VAT compartment and its feasibility for radiomics applications, thereby helping to reduce radiation-related risks.
Additionally, our study's sample size was relatively limited due to strict inclusion and exclusion criteria. Although the model demonstrated robust performance in internal validation, this limitation may restrict its generalizability to broader patient populations. Multicenter prospective studies with larger cohorts must validate our findings and support clinical translation. Finally, although our LASSO-based model exhibited strong performance and practical utility, exploring alternative feature selection methods may yield further insights and should be considered in future research.
We developed and validated a comprehensive model that integrates delta-radiomics features derived from longitudinal changes in VAT with clinical predictors, enabling accurate identification of CD patients at high risk of SLR to IFX treatment. This model holds the potential for guiding individualized treatment planning and monitoring strategies in the clinical management of CD.
| 1. | Dolinger M, Torres J, Vermeire S. Crohn's disease. Lancet. 2024;403:1177-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 298] [Article Influence: 149.0] [Reference Citation Analysis (104)] |
| 2. | Cushing K, Higgins PDR. Management of Crohn Disease: A Review. JAMA. 2021;325:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 3. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 188] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 4. | Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn's disease. Aliment Pharmacol Ther. 2018;48:394-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 792] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 6. | Gisbert JP, Chaparro M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J Crohns Colitis. 2020;14:694-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 7. | Rimola J, Fernàndez-Clotet A, Capozzi N, Rojas-Farreras S, Alfaro I, Rodríguez S, Masamunt MC, Ricart E, Ordás I, Panés J. Pre-treatment magnetic resonance enterography findings predict the response to TNF-alpha inhibitors in Crohn's disease. Aliment Pharmacol Ther. 2020;52:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Yueying C, Jing F, Qi F, Jun S. Infliximab response associates with radiologic findings in bio-naïve Crohn's disease. Eur Radiol. 2023;33:5247-5257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Yarur AJ, Bruss A, Moosreiner A, Beniwal-Patel P, Nunez L, Berens B, Colombel JF, Targan SR, Fox C, Melmed GY, Abreu MT, Deepak P. Higher Intra-Abdominal Visceral Adipose Tissue Mass Is Associated With Lower Rates of Clinical and Endoscopic Remission in Patients With Inflammatory Bowel Diseases Initiating Biologic Therapy: Results of the Constellation Study. Gastroenterology. 2023;165:963-975.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Bassi M, Singh S. Impact of Obesity on Response to Biologic Therapies in Patients with Inflammatory Bowel Diseases. BioDrugs. 2022;36:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Gu P, Chhabra A, Chittajallu P, Chang C, Mendez D, Gilman A, Fudman DI, Xi Y, Feagins LA. Visceral Adipose Tissue Volumetrics Inform Odds of Treatment Response and Risk of Subsequent Surgery in IBD Patients Starting Antitumor Necrosis Factor Therapy. Inflamm Bowel Dis. 2022;28:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Sehgal P, Su S, Zech J, Nobel Y, Luk L, Economou I, Shen B, Lewis JD, Freedberg DE. Visceral Adiposity Independently Predicts Time to Flare in Inflammatory Bowel Disease but Body Mass Index Does Not. Inflamm Bowel Dis. 2024;30:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Zuo L, Li Y, Zhu W, Shen B, Gong J, Guo Z, Zhang W, Wu R, Gu L, Li N, Li J. Mesenteric Adipocyte Dysfunction in Crohn's Disease is Associated with Hypoxia. Inflamm Bowel Dis. 2016;22:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 6023] [Article Influence: 602.3] [Reference Citation Analysis (7)] |
| 15. | Li X, Zhang N, Hu C, Lin Y, Li J, Li Z, Cui E, Shi L, Zhuang X, Li J, Lu J, Wang Y, Liu R, Yuan C, Lin H, He J, Ke D, Tang S, Zou Y, He B, Sun C, Chen M, Huang B, Mao R, Feng ST. CT-based radiomics signature of visceral adipose tissue for prediction of disease progression in patients with Crohn's disease: A multicentre cohort study. EClinicalMedicine. 2023;56:101805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Huang Y, Zhu T, Zhang X, Li W, Zheng X, Cheng M, Ji F, Zhang L, Yang C, Wu Z, Ye G, Lin Y, Wang K. Longitudinal MRI-based fusion novel model predicts pathological complete response in breast cancer treated with neoadjuvant chemotherapy: a multicenter, retrospective study. EClinicalMedicine. 2023;58:101899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 108] [Reference Citation Analysis (1)] |
| 17. | Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 519] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 18. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1319] [Article Influence: 188.4] [Reference Citation Analysis (2)] |
| 19. | Montano-Loza AJ, Mazurak VC, Ebadi M, Meza-Junco J, Sawyer MB, Baracos VE, Kneteman N. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology. 2018;67:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Eder P, Adler M, Dobrowolska A, Kamhieh-Milz J, Witowski J. The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease. Cells. 2019;8:628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 21. | Gonçalves P, Magro F, Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis. 2015;21:453-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 22. | Weidinger C, Hegazy AN, Siegmund B. The role of adipose tissue in inflammatory bowel diseases. Curr Opin Gastroenterol. 2018;34:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Zhou Z, Xiong Z, Cheng R, Luo Q, Li Y, Xie Q, Xiao P, Hu D, Hu X, Shen Y, Li Z. Volumetric visceral fat machine learning phenotype on CT for differential diagnosis of inflammatory bowel disease. Eur Radiol. 2023;33:1862-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | Santos JCD, Malaguti C, Lucca FA, Cabalzar AL, Ribeiro TCDR, Gaburri PD, Chebli LA, Chebli JMF. Impact of biological therapy on body composition of patients with Chron's disease. Rev Assoc Med Bras (1992). 2017;63:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Büning C, von Kraft C, Hermsdorf M, Gentz E, Wirth EK, Valentini L, Haas V. Visceral Adipose Tissue in Patients with Crohn's Disease Correlates with Disease Activity, Inflammatory Markers, and Outcome. Inflamm Bowel Dis. 2015;21:2590-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, Schoeman S, Lim A, Bartholomeusz FD, Travis SPL, Andrews JM. Visceral Adipose Tissue Is Associated With Stricturing Crohn's Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm Bowel Dis. 2019;25:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 27. | Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (7)] |
| 28. | Moran GW, Dubeau MF, Kaplan GG, Yang H, Seow CH, Fedorak RN, Dieleman LA, Barkema HW, Ghosh S, Panaccione R; Alberta Inflammatory Bowel Disease Consortium. Phenotypic features of Crohn's disease associated with failure of medical treatment. Clin Gastroenterol Hepatol. 2014;12:434-42.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Yang T, Feng J, Yao R, Feng Q, Shen J. CT-based pancreatic radiomics predicts secondary loss of response to infliximab in biologically naïve patients with Crohn's disease. Insights Imaging. 2024;15:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 31. | Menchén L, Marín-Jiménez I, Arias-Salgado EG, Fontela T, Hernández-Sampelayo P, Rodríguez MC, Butta NV. Matrix metalloproteinase 9 is involved in Crohn's disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut. 2009;58:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Ripoche J. Blood platelets and inflammation: their relationship with liver and digestive diseases. Clin Res Hepatol Gastroenterol. 2011;35:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Nakarai A, Kato J, Hiraoka S, Takashima S, Inokuchi T, Takahara M, Sugihara Y, Harada K, Okada H. An Elevated Platelet Count Increases the Risk of Relapse in Ulcerative Colitis Patients with Mucosal Healing. Gut Liver. 2018;12:420-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Bertani L, Rossari F, Barberio B, Demarzo MG, Tapete G, Albano E, Baiano Svizzero G, Ceccarelli L, Mumolo MG, Brombin C, de Bortoli N, Bellini M, Marchi S, Bodini G, Savarino E, Costa F. Novel Prognostic Biomarkers of Mucosal Healing in Ulcerative Colitis Patients Treated With Anti-TNF: Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio. Inflamm Bowel Dis. 2020;26:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Soufli I, Hablal A, Bessaad S, Amri M, Labsi M, Boussa RS, Ameur F, Belguendouz H, Younes SA, Idris NS, Touil-Boukoffa C. Nitric Oxide, Neutrophil/Lymphocyte, and Platelet/Lymphocyte Ratios as Promising Inflammatory Biomarkers in Complicated Crohn's Disease: Outcomes of Corticosteroids and Anti-TNF-α Therapies. Inflammation. 2023;46:1091-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 36. | Stidham RW, Guentner AS, Ruma JL, Govani SM, Waljee AK, Higgins PD. Intestinal Dilation and Platelet:Albumin Ratio Are Predictors of Surgery in Stricturing Small Bowel Crohn's Disease. Clin Gastroenterol Hepatol. 2016;14:1112-1119.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Baum T, Cordes C, Dieckmeyer M, Ruschke S, Franz D, Hauner H, Kirschke JS, Karampinos DC. MR-based assessment of body fat distribution and characteristics. Eur J Radiol. 2016;85:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/