Published online Jan 14, 2025. doi: 10.3748/wjg.v31.i2.101180
Revised: October 26, 2024
Accepted: November 19, 2024
Published online: January 14, 2025
Processing time: 102 Days and 23.4 Hours
Intrapancreatic fat deposition (IPFD) has garnered increasing attention in recent years. The prevalence of IPFD is relatively high and associated with factors such as obesity, age, and sex. However, the pathophysiological mechanisms underlying IPFD remain unclear, with several potential contributing factors, including oxida
Core Tip: Intrapancreatic fat deposition (IPFD) is a prevalent and clinically significant condition linked to exocrine pancreatic diseases, obesity, age, and sex. Although the mechanisms driving IPFD remain poorly understood, factors like oxidative stress, gut microbiota alterations, and hormonal imbalances are thought to contribute. Imaging is currently the main diagnostic method, but emerging biomarkers and scoring systems offer new diagnostic avenues. While there are no established treatments, glucagon-like peptide-1 receptor agonists and novel experimental therapies show promise. This review highlights recent insights into IPFD’s pathogenesis, diagnosis, and therapeutic potential, underscoring its clinical importance in pancreatic health management.
- Citation: Ye J, Wang JG, Liu RQ, Shi Q, Wang WX. Association between intra-pancreatic fat deposition and diseases of the exocrine pancreas: A narrative review. World J Gastroenterol 2025; 31(2): 101180
- URL: https://www.wjgnet.com/1007-9327/full/v31/i2/101180.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i2.101180
In recent years, obesity has emerged as a global issue, with the number of overweight and obese individuals increasing steadily across all age groups and socioeconomic strata, particularly in developed and some developing countries[1]. Research on obesity has revealed that the fat distribution pattern within the body plays a critical role in the development of obesity-related abnormalities in glucose and lipid metabolism. This uneven distribution can have multiple adverse effects on health. When triglyceride and free fatty acid (FFA) levels in the bloodstream exceed the metabolic capacity of adipose tissue, the excess lipids cannot be effectively processed or stored, leading to their accumulation in nonadipose tissues. This phenomenon, known as “ectopic fat deposition”, can occur in several vital organs - including the liver, heart, kidneys, and pancreas - potentially compromising their normal functions[2]. Among the various forms of ectopic fat deposition, fat accumulation in the pancreas has attracted increasing attention in recent years. This condition has been described in the literature using various terms, such as “pancreatic steatosis”[3,4], “fatty pancreas”[5,6], “pancreatic lipomatosis”[7,8], “fatty infiltration of the pancreas”[9,10], “nonalcoholic fatty pancreas disease”[11-13], and “intrapancreatic fat deposition”[14,15]. The use of these different terms reflects the diversity and complexity of the current under
| Name | Definition |
| Pancreatic steatosis | The description of fat accumulation in the pancreatic gland |
| Fatty pancreas | Fatty accumulation in the pancreas |
| Pancreatic lipomatosis | The most frequent benign pathologic condition of the adult pancreas |
| Fatty infiltration of the pancreas | Deposition of a large amount of fat in the pancreas |
| Nonalcoholic fatty pancreas disease | The accumulation of fat in pancreatic tissue (located within adipocytes) |
| Intra-pancreatic fat deposition | Diffuse presence of fat (measured on a continuous scale) within the pancreas; excludes peri-pancreatic (extra-lobular) fat |
In this paper, we use the term “intrapancreatic fat deposition (IPFD)” to describe the abnormal accumulation of fat in the pancreas, which is defined as the diffuse presence of fat within the pancreas, excluding peripancreatic (extralobular) fat[16]. IPFD is considered a manifestation of metabolic syndrome (MetS) within the pancreas and encompasses a spec
Currently, large-scale epidemiological data to accurately assess the prevalence of IPFD are lacking. However, a cross-sectional study conducted in Indonesia reported that the prevalence of IPFD was approximately 35%[20], whereas a prospective single-center study in the United States reported a prevalence rate of 27.8%[21]. In children, the estimated prevalence of IPFD is approximately 10%[22]. Another retrospective study involving 102 pediatric patients aged 5-18 years indicated that IPFD was relatively common in this group, with a prevalence rate of approximately 26.5%, and it was associated with comorbidities such as diabetes and dyslipidemia[23]. In a study from Japan, the detection rate of IPFD using abdominal ultrasound in a health screening population was reported to be 46.8%[24]. This high detection rate might have been influenced by the older age of the study participants (59.5 ± 13.2 years) and the inclusion of individuals who consumed alcohol. Another study conducted in Chile involving 203 patients without other pancreatic diseases reported that IPFD was detected in approximately 30% of the population[25]. In contrast, a population survey from Yangzhou, China, revealed a lower prevalence of IPFD, 2.7% among the 1228 individuals screened, which was lower than the rates reported in other studies conducted in China and other parts of Asia[26-30]. A meta-analysis of IPFD studies suggested that the overall prevalence was approximately 33% and strongly associated with MetS[31]. The variability in the prevalence of IPFD across different populations could be attributed to regional differences and the methods used for detection, but overall, approximately one-third of the population appears to have IPFD.
Smoking has been identified as a risk factor for IPFD. Research has demonstrated that nicotine exposure during breastfeeding can induce pancreatic steatosis in adult rats[32]. Furthermore, the prevalence of sedentary lifestyles and high-calorie diets has led to an increasing number of obese people. Multiple studies have indicated that body mass index (BMI) and obesity are associated with IPFD[33-35]. Some studies have suggested that the prevalence of IPFD increases with age[28,36] and that IPFD is associated with nonalcoholic fatty liver disease. Moreover, triglyceride, lipoprotein, and adiponectin levels are independent risk factors for non-alcoholic fatty pancreatic disease exacerbation[28]. Other studies have shown that racial and ethnic differences also play a role as risk factors for IPFD, with Hispanic individuals exhi
The pathophysiological mechanisms of IPFD are not yet fully understood. Current research suggests that this disease is primarily associated with excessive fatty deposition and fatty replacement in pancreatic tissue, which involves both intralobular fat and interlobular fat[16,17]. Several processes and components may contribute to the development of IPFD, including the accumulation of excess fat within pancreatic β-cells and acinar cells, the replacement of apoptotic acinar cells with intralobular fat, the transdifferentiation of acinar cells into adipocytes, and the presence of interlobular fat cells rich in triglycerides and pancreatic stellate cells.
Oxidative stress (OS) refers to a state of imbalance between oxidation and antioxidants in the body, which can disrupt normal oxidative protein folding. Endoplasmic reticulum (ER) stress is a cellular stress response caused by abnormal protein folding and modification in the ER. Under stress conditions, OS is also the cause of ER stress, which leads to the development of pathogenic states[41]. OS has been associated with MetS and diabetes. Both IPFD and nonalcoholic fatty liver disease are considered manifestations of MetS in different organs. In the “two-hit” hypothesis of nonalcoholic steatohepatitis, OS is viewed as the “second hit” (the “two-hit” theory suggests that peripheral insulin resistance (IR) leads to the accumulation of FFAs in the liver, causing liver steatosis, which constitutes the first hit; lipid accumulation sensitizes the liver to OS, triggering a series of liver toxicity events that cause inflammation, forming the second hit); therefore, OS may also play a crucial role in the development of IPFD[42,43]. OS can lead to the accumulation of misfolded proteins, inducing ER stress. The interaction between OS and ER stress plays a significant role in cellular homeostasis dysfunction and may be associated with accelerated lipid droplet (LD) formation in the pancreas[17,41]. ER stress is closely linked to fat metabolism and influences lipid synthesis, storage, and utilization by altering lipid metabolism and LD dynamics, which can lead to lipotoxicity and metabolic disturbances[44]. In mice with glucose metabolism disorders, many lipid compounds have been observed inside and outside pancreatic cells in the form of LDs, along with abnormal ER structural abnormalities and mitochondrial structural transformation. These findings suggest a potential correlation between IPFD and OS[45]. A 2021 study using a high-fat diet model in mice also suggested that ER stress might be involved in IPFD[46].
Gut microbiota have a significant impact on human health and disease. In recent years, many metabolic diseases, including obesity, type 2 diabetes, nonalcoholic liver disease, metabolic heart disease, and malnutrition, have been linked to gut microbiota. Anatomically, the pancreas is connected to the gastrointestinal tract via the pancreatic duct, where a vast array of microbiota resides, which may influence pancreatic homeostasis and the development of various pancreatic diseases[47]. A 2024 study of the gut-pancreas axis revealed that the IPFD was highly correlated with the metabolic products of gut microbiota, such as trimethylamine N-oxide and butyrate levels, suggesting a possible link between IPFD and gut microbiota[48].
IPFD also seems to be associated with hormonal imbalances. Adipose tissue is an important endocrine organ that secretes various adipokines to regulate metabolic homeostasis. In response to external stimuli, adipose tissue can undergo functional impairments, structural changes, and phenotypic alterations[49,50]. Studies have shown that the gut hormone guanylin peptide can prevent β-cell steatosis under lipotoxic conditions and reduce fat accumulation in the pancreas[51]. Guanylin peptide also has a complete expression system in the pancreas and is involved in regulating electrolyte homeo
Furthermore, previous studies have shown that the regulatory factor serine/threonine protein kinase 25, which is associated with ectopic fat storage, metabolic inflammation, and fibrosis, can exacerbate the severity of IPFD in mouse models, although the underlying mechanisms remain unclear[55]. Low levels of uric acid may also inhibit pancreatic steatosis via the glycerophospholipid metabolic pathway in mice[56].
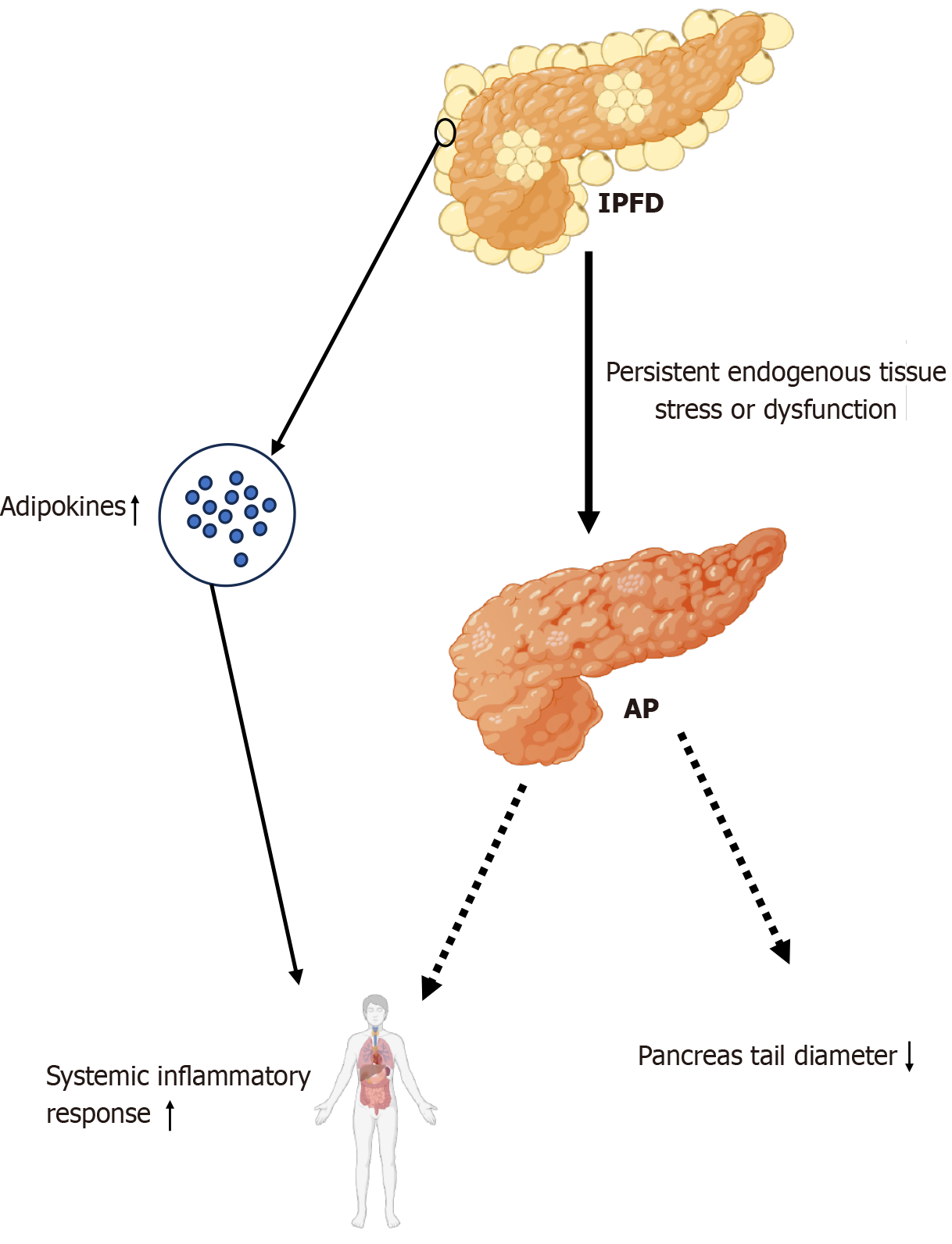
Excessive IPFD may disrupt normal pancreatic function and affect the secretion function of the pancreas. Now IPFD, as an increasingly common pancreatic disease, plays an important role in pancreatitis, the most common exocrine disease of the pancreas. In patients with pancreatitis, pancreatic fat deposition levels are elevated[52,57]. A 2021 study evaluated the association between IPFD and acute pancreatitis (AP), suggesting that IPFD might be a risk factor for AP and hypo
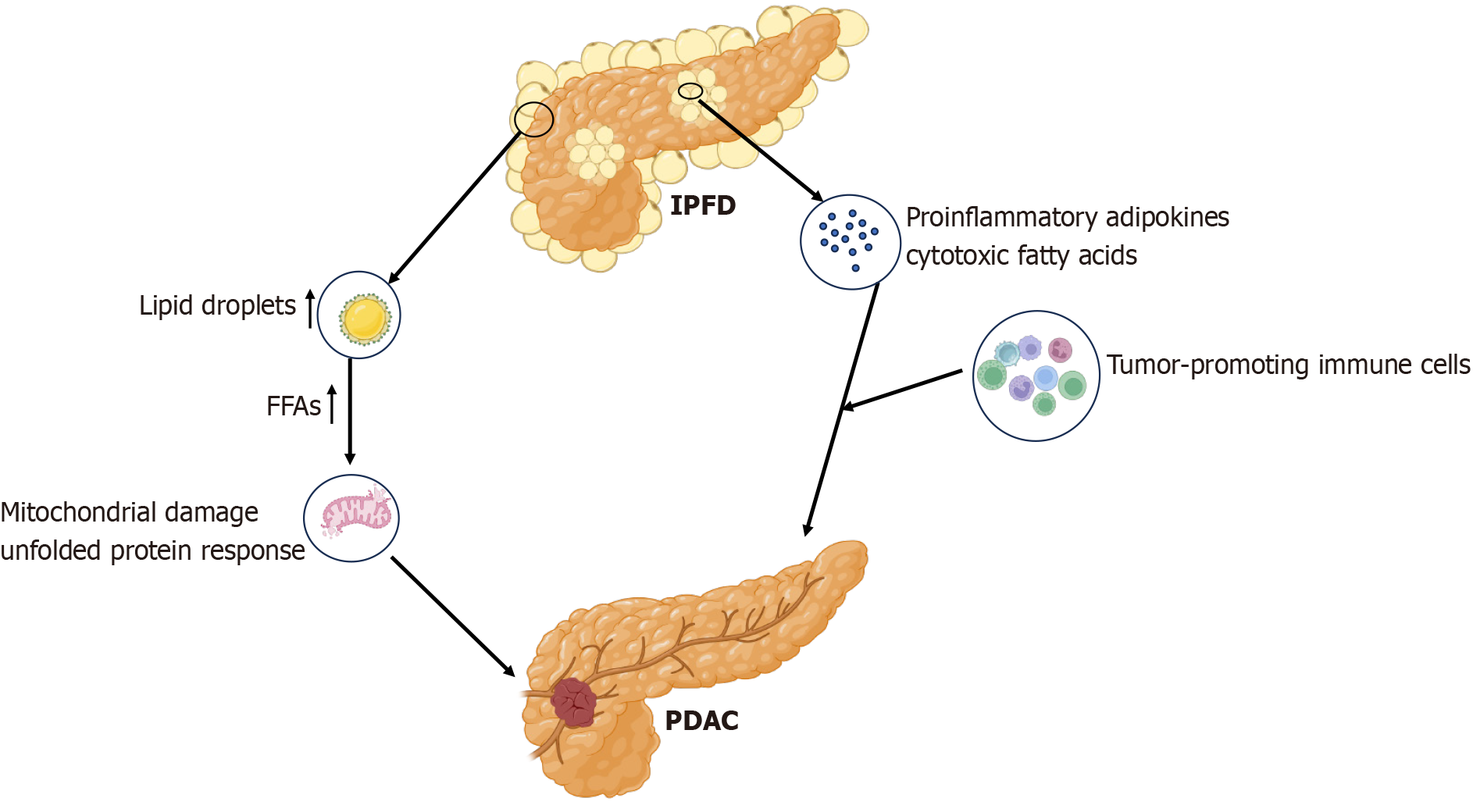
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic malignancy, accounting for approximately 90% of malignant pancreatic tumors. By 2030, PDAC is projected to become the second leading cause of cancer-related death[66]. IPFD is currently considered significantly associated with PDAC, and IPFD is believed to promote the spread of PDAC and worsen the prognosis of tumor patients[59,67-71]. A recent prospective cohort and Mendelian randomization study suggested that IPFD was a high-risk factor for PDAC and may not be related to overall body fat[14]. This association may involve inflammatory and cancer-promoting signals from local adipocytes. This study also revealed that the characteristics of pancreatic steatosis potentially differ from those of fat deposition in other organs[14]. Another study involving overweight patients revealed that pancreatic steatosis indicated by computed tomography (CT) before the diagnosis of PDAC was an independent risk factor for PDAC[72].
Fatty infiltration in the pancreas falls into one of two categories: Intralobular fatty infiltration and extralobular fatty infiltration. The lipid compositions of these types differ and play distinct roles in oncogenesis development and pro
LDs are organelles that regulate intracellular lipid homeostasis. In healthy tissues, intrapancreatic adipocytes and LDs store lipids for metabolic needs. In cases of obesity or visceral fat accumulation, LDs increase the intracellular levels of cytotoxic FFAs, inducing mitochondrial damage and the unfolded protein response[73]. Additionally, IPFD may drive the development of early pancreatic cancer, with inflammatory processes in the fat-rich pancreas being a crucial trigger for PDAC development[74,75]. High levels of proinflammatory adipokines and cytotoxic FFAs released by pancreatic fat cells contribute to the recruitment of tumor-promoting immune cells and create a microenvironment conducive to cancer formation. Furthermore, the size of LD pools in cancer cells is an important determinant of tumor metastatic potential. In addition, tumor lipid reprogrammed metabolism is potentially regulated by intrapancreatic adipocytes and abundant LDs[75,76] (Figure 2).
IPFD is also associated with impaired pancreatic exocrine function. Fecal elastase-1, a highly stable enzyme, is commonly used to assess pancreatic exocrine function. Individuals with impaired pancreatic exocrine function harbor significantly more pancreatic fat, which negatively correlated with fecal elastase levels in the tested population[77,78]. Lipids may affect acinar cells or replace lost acinar cells and necrotic apoptotic endocrine tissue, leading to pancreatic exocrine dysfunction[77].
Pancreatic fistula is a common and serious complication following pancreatic surgery. The development of postoperative pancreatic fistula is associated with the softness of the pancreatic parenchyma, and IPFD can increase pancreatic gland softness[79-81]. Additionally, MetS and type 2 diabetes have been linked to IPFD, as obesity - a condition closely asso
| Disease | Mechanism | Ref. |
| Pancreatitis | Inflammation induced by fat deposition and adipokine secreted by adipose tissue promote the occurrence and development of pancreatitis | Sbeit and Khoury[58], 2021; Tirkes et al[64], 2019 |
| Pancreatic cancer | The inflammatory process of the pancreas in the context of fatty pancreas is an important inducible factor, and the different types of fatty infiltration of the pancreas also play a role in the different processes of tumor development | Frendi et al[9], 2024; Lilly et al[75],2023 |
| Exocrine dysfunction | Lipids have affected acinar cells or replaced lost acinar cells and necrotic apoptotic endocrine tissue | Tahtacı et al[77], 2018 |
| POPF | IPFD increases the softness of the pancreatic glands and raises the risk of POPF | Gaujoux et al[79], 2010; Dei et al[81], 2022 |
| Type 2 diabetes mellitus | IPFD leads to dysfunction of islet beta cells, affecting insulin secretion and exacerbating insulin resistance | Lu et al[83], 2019; Chin et al[84], 2021 |
| Metabolic syndrome | IPFD is associated with components of metabolic syndrome such as obesity, hyperglycemia, dyslipidemia, etc | Smits and van Geenen[82], 2011 |
| Cardiovascular disease | IPFD is associated with abnormal fat distribution and metabolic disorders throughout the body, which may affect the development of atherosclerosis | Kim et al[85], 2014 |
At present, no universally accepted standard exists for diagnosing pancreatic fat content, and imaging remains the primary method for assessing and diagnosing IPFD. Histological examination of pancreatic samples has revealed fat cell infiltration and LDs localized within acinar cells[42,86,87]. However, due to the retroperitoneal location of the pancreas[31], pancreatic biopsy is challenging. Due to the uneven distribution of fat in the pancreas among IPFD patients, histological examinations might not have accurately reflected fat deposition throughout the entire pancreas. Furthermore, pancreatic tissue samples can be obtained only during pancreatic surgery, which is limited to specific indications. Most individuals at risk for IPFD have never undergone pancreatic surgery, making the specific evaluation of pancreatic fat content via histological methods difficult.
Imaging techniques, including abdominal ultrasound, endoscopic ultrasound, CT, proton magnetic resonance spec
CT can provide a (semi)quantitative analysis of pancreatic fat via attenuation of the pancreas, depending on the contrast with the spleen[94,95], with unenhanced CT often providing more accurate results than enhanced CT[96]. The combination of quantitative CT parameters, specifically pancreas attenuation and pancreas surface lobularity, enhances the accuracy of detecting pancreatic fat infiltration. In the validation cohort, this method yielded negative and positive predictive values of 91% and 70%, respectively[94]. A visceral fat tissue area threshold of ≥ 107.2 cm² may predict pancreatic fat degeneration[97]. CT attenuation indices and visual assessments of CT images have aided in studying the clinical relevance of IPFD with impaired glucose metabolism and type 2 diabetes[98,99]. Additionally, the CT fat volume fraction can automatically measure the severity of IPFD[100]. Moreover, quantitative CT assessments of pancreatic fat infiltration location and content are accurate[101], which could help in preoperative planning and postoperative complications[102-105]. Compared with other imaging methods, CT is more accurate and convenient for measuring pancreatic volume and abdominal fat content. However, CT involves potential radiation exposure and is relatively expensive, and it does not show a significant advantage over ultrasound in the quantitative assessment of pancreatic fat[18,97].
MRI is based on signal differences between water and fat. It offers better soft tissue contrast and higher sensitivity, playing an important role in the assessment and clinical application of IPFD. Chemical shift-encoded MRI is based on the difference in resonant frequencies caused by the different distributions of the surrounding electron clouds of hydrogen protons in water and adipose tissue, and its measured fat fraction is highly correlated with the actual fat fraction[16,36,106]. It maps the region of interest in the caput, corpus, and cauda of the pancreas to minimize the influence of other visceral fat. Compared with nuclear magnetic resonance spectroscopy, it needs less time and is more convenient. The proton density fat fraction is an objective measure of the intrinsic properties of tissues. The use of MRI to quantify the proton density fat fraction can standardize and objectively measure fat content, with good repeatability, and can reflect the overall fat content[107-109]. Moreover, multiparametric MRI of the pancreas provides more reliable and accurate fat quantification than conventional MRI[110]. 1H-magnetic resonance spectroscopy is considered the gold standard to noninvasively measure pancreatic fat content, with accuracy comparable to that of histological and biochemical markers[111]. Another new technique, 3D-IDEAL (iterative decomposition with echo asymmetry and least squares estimation), provides faster imaging, less signal contamination from surrounding fat, and reduced susceptibility to respiratory motion effects, potentially offering superior performance in evaluating fat content in small organs such as the pancreas[112,113]. However, MRI-related imaging technology is also associated with several problems, such as high costs and long scanning intervals, which affect its application.
With the increasing influence of artificial intelligence (AI) on diagnostic technologies, recent studies have proposed that AI-assisted MRI and CT models could be utilized as objective, automated, and reproducible tools for assessing pancreatic fat infiltration[114]. Additionally, several emerging biomarkers have been identified as potential screening tools for patients with IPFD. The level of serum fibroblast growth factor-21 (FGF-21), a cytokine intimately involved in glucose and lipid metabolism, has been shown to be closely associated with metabolic diseases. A previous study indicated that serum FGF-21 was strongly linked to IPFD and could serve as a reliable screening marker for the disease[115]. However, the transabdominal ultrasound used in the diagnosis of IPFD in this study can only be used to identify is the presence fat infiltration in the pancreas, not its quantity. Therefore, the correlation between the serum FGF-21 level and the degree of fat infiltration in the pancreas was difficult to explore in this study, and whether the serum FGF-21 level is a prognostic indicator for patients with an IPFD also needs to be further explored. In the lipidomic analysis of serum, triglycerides were identified as significant markers for the development of IPFD following AP, which can be used to assess and identify individuals at high risk of IPFD[116]. IPFD in healthy nonobese individuals in this study was not significantly associated with any markers of lipid metabolism, and new prospective longitudinal studies are needed to validate this finding and to focus on changes in IPFD over time and its association with markers of lipid metabolism. Furthermore, an Egyptian study revealed statistically significant differences in fatty acid-binding protein-1 (FABP-1) levels based on the presence of fatty deposits among fatty pancreas patients[117]. Thus, FABP-1 may be a direct and noninvasive predictor of IPFD[117]. Although the diagnosis of IPFD was graded in this study, verifying whether the level of FABP-1 was correlated with the severity of IPFD was difficult because the diagnosis was based on transabdominal ultrasound, and the correlation between the FABP level and the IPFD grade was not discussed. The hypertriglyceridemic waist phenotype, a clinical indicator of visceral fat accumulation that reflects visceral obesity and metabolic disorders, was found to be significantly associated with IPFD and could be a useful screening reference, particularly for Asians who are underweight but prone to developing visceral obesity[118]. A new diagnostic score that includes obesity, hyperlipidemia and fatty liver has also been developed to initially identify IPFD[119]. Common anthropometric parameters and biochemical markers also demonstrated good predictive value, including weight-related parameters such as BMI and triglyceride-related measures, with a combination of multiple parameters offering potentially greater predictive accuracy[120].
Currently, the clinical significance and related mechanisms of IPFD are not fully understood, and routine, effective management strategies are lacking. Lifestyle modification appears to be the most effective treatment approach available. Exercise is considered beneficial in reducing ectopic fat accumulation in the pancreas[121]. However, this finding was based on a small study focusing only on individuals with prediabetes and type 2 diabetes. The effects of exercise on pancreatic fat accumulation in other normal populations, as well as the optimal exercise duration and intensity, remain unstudied. Additionally, low-calorie diets have been found to help reduce pancreatic fat accumulation in type 2 diabetes patients[122]. Altering the intake of other nutrients also seemed to influence the incidence of IPFD. Lactoferrin, an iron-binding protein with numerous biological functions and significant immunomodulatory effects, was found to improve pancreatic fat levels and function and to mitigate weight gain when administered as a supplement[123]. Probiotics have shown great potential in inhibiting the development of IPFD and alleviating related metabolic and pathological abnormalities[124]. Moreover, reducing branched-chain amino acid intake might improve IPFD by activating the liver kinase B1/AMP-activated protein kinase pathway or inhibiting the mechanistic target of rapamycin complex 1 pathway[125]. However, these dietary changes have only been validated in animal models and lack supporting clinical data.
Lifestyle modifications appear to be a promising approach for the prevention and treatment of IPFD. However, existing studies examining the effects of lifestyle changes on pancreatic fat content have primarily focused on diabetic patients and animal models, leaving a gap in data across broader population samples. Additionally, risk factors for IPFD, such as smoking, high BMI, and obesity, are strongly linked to lifestyle habits, suggesting that future studies targeting lifestyle interventions could potentially enable early intervention and achieve preventive effects for IPFD.
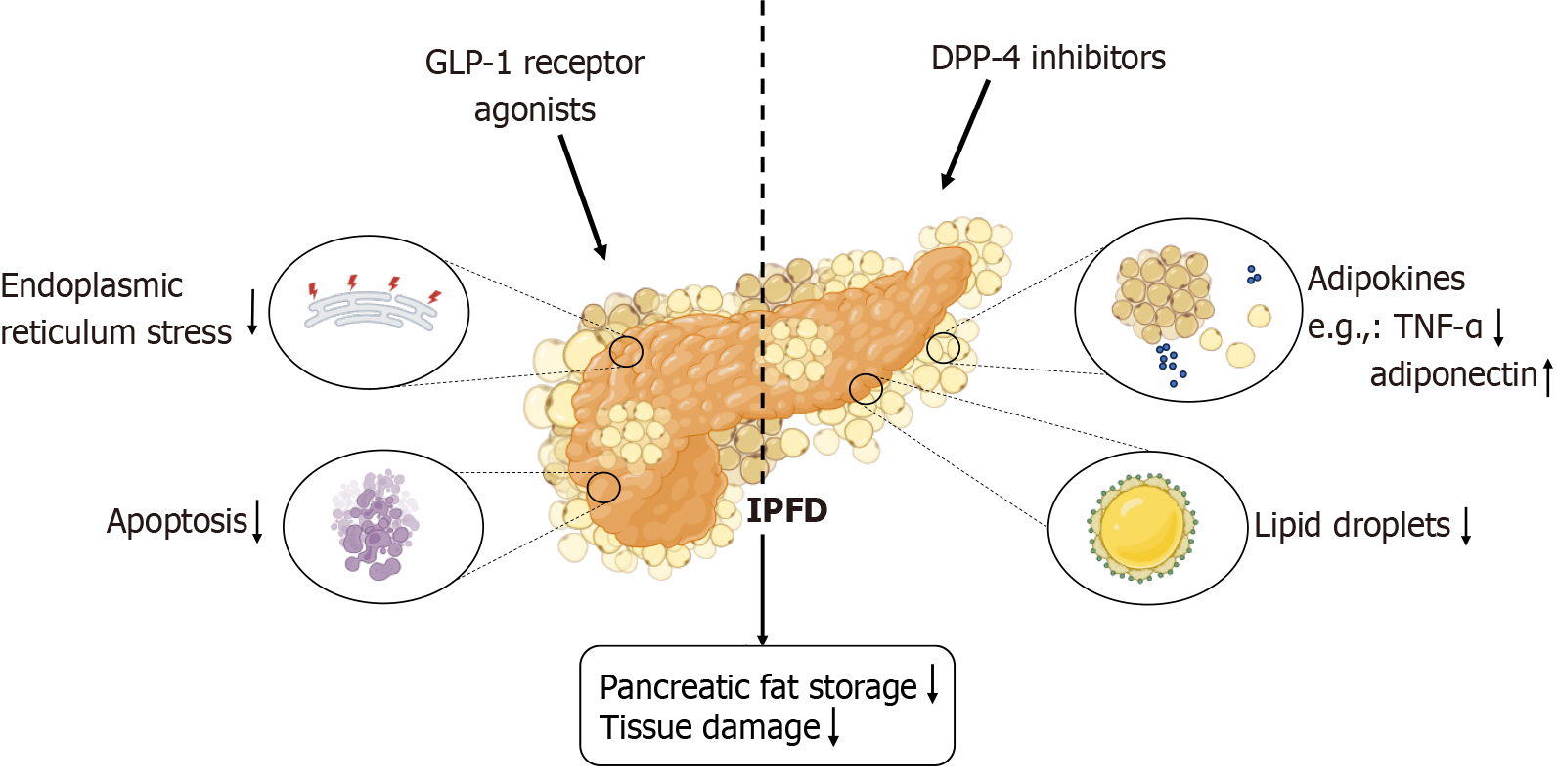
Evidence-based medicine for the pharmacological treatment of IPFD is lacking. Glucose-lowering drugs, lipid-regulating medications, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants are promising candidates for improving IPFD[126]. In preclinical studies, glucose-lowering drugs such as glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, lipid-regulating statins, pancreatic lipase inhibitors, somatostatin receptor agonists such as octreotide, and antioxidants such as ascorbic acid have shown favorable results[127]. Metformin reduced IPFD severity in animal models, but imaging studies before and after metformin administration did not reveal significant changes in the clinical setting[128]. Newer therapies [such as GLP-1 receptor agonists, DPP-4 inhibitors, and sodium-glucose co-transporter type 2 (SGLT-2) inhibitors] and classical therapies (such as statins, metformin, and angiotensin II receptor blockers) at least partially reduce pancreatic ER stress[129]. The administration of the GLP-1 receptor agonist liraglutide in high-fat diet-fed mouse models suggested that it might reduce IPFD by modulating ER stress pathways and downstream apoptosis signals[46,130,131]. A retrospective study in Japan suggested that SGLT-2 inhibitors might reduce pancreatic fat accumulation in type 2 diabetes patients, but this study had a small sample size and could not exclude the effects of other antidiabetic drugs on fat accumulation, indicating that the results were only suggestive[132,133]. A 2021 study of the SGLT-2 inhibitor empagliflozin revealed that it effectively reduced liver fat in mice and humans, but the changes in pancreatic fat were not statistically significant[134].
Additionally, some new drug therapies have appeared to offer new possibilities based on animal model studies. Oral glucosamine may provide some protection against fat accumulation and pancreatic tissue damage in rats fed a high-fat diet[135]. Sodium butyrate improved metabolic disorders as well as pancreatic lipid accumulation and uric acid metabo
Currently, there is no evidence-based medical guideline recommending specific, effective drugs for the treatment of IPFD. Among the potential therapeutic options, GLP-1 receptor agonists and DPP-4 inhibitors show the most promise, but further large-scale clinical studies are necessary to evaluate their effectiveness in treating IPFD. Such studies could facilitate the development of reliable therapies aimed at reducing pancreatic fat accumulation and mitigating the patho
| Treatment method | Category | Mechanism of action | Research evidence | Ref. |
| Low-calorie diets | Non-pharmacological | Reduces insulin secretion and decreases adipose tissue accumulation in the pancreas and body | Evidence indicates that low-carbohydrate diets effectively diminish pancreatic and visceral fat deposition in individuals with obesity and type 2 diabetes | Taylor et al[122], 2018 |
| Exercise | Non-pharmacological | Enhances overall insulin sensitivity and reduces pancreatic fat content | Numerous small clinical trials have demonstrated that physical activity can lower pancreatic fat deposition, regardless of the baseline glucose tolerance of participants | Heiskanen et al[121], 2018 |
| Lactoferrin | Non-pharmacological | Improves lipid profiles, pancreatic function, and histological integrity | Supplementation with lactoferrin has been shown to mitigate weight gain, improve lipid levels, and enhance pancreatic function in models fed a high-fat diet | Hassan et al[123], 2022 |
| Metformin | Pharmacological | Improves insulin resistance and reduces pancreatic and overall adipose tissue | In studies involving high-fat diet-fed mouse models, metformin significantly decreased pancreatic fat deposition and ameliorated insulin resistance | Souza-Mello et al[128], 2010 |
| GLP-1 receptor agonists | Pharmacological | Stimulates insulin secretion, inhibits glucagon release, promotes weight loss, and decreases pancreatic fat | A growing body of clinical and preclinical evidence supports the efficacy of GLP-1 receptor agonists in reducing fat accumulation in both the pancreas and liver while enhancing pancreatic function | Kuriyama et al[131], 2024; Vanderheiden et al[130], 2016; Fang et al[46], 2021 |
| SGLT-2 inhibitors | Pharmacological | Increases glucose excretion through urine, reduces lipogenesis, and improves pancreatic fat deposition | Research has demonstrated that SGLT-2 inhibitors, such as dapagliflozin, effectively improve body fat distribution and reduce pancreatic fat accumulation in patients with type 2 diabetes | Ghosh et al[133], 2022; Shi et al[132], 2023 |
| DPP-4 inhibitors | Pharmacological | Enhances insulin secretion from pancreatic beta cells, suppresses glucagon secretion from alpha cells, and improves fat accumulation | Clinical trials and animal studies indicate that the DPP-4 inhibitor sitagliptin can effectively manage pancreatic steatosis and prevent the progression of pancreatic diseases | Souza-Mello et al[128], 2010; Nag et al[127], 2024 |
| Statins | Pharmacological | Lowers blood lipid levels, inhibits pancreatic cell proliferation, and alleviates endoplasmic reticulum stress | Animal studies have demonstrated that statins significantly reduce pancreatic fat accumulation in models subjected to high-fat diets | Chen et al[138], 2014; Krisnamurti et al[137], 2022 |
| Angiotensin II receptor blockers | Pharmacological | Mitigates intracellular calcium overload and lipid accumulation, improving insulin sensitivity and preventing fat degeneration | Various animal studies suggest that angiotensin II receptor blockers can alleviate pancreatic steatosis by enhancing metabolic conditions in diabetic patients | Souza-Mello et al[128], 2010; Lee et al[129], 2023 |
The understanding of IPFD has remained insufficient. Although it is a very common condition, it has not been adequately addressed clinically. More multicenter, large-scale population surveys are needed to clarify the prevalence and related risk factors for IPFD in different regions and populations. Given the rise in obesity, the systemic metabolic disturbances caused by excessive fat accumulation warrant significant attention, especially because the pancreas is an important organ related to metabolism. The pathogenesis of IPFD is not fully understood, and further clinical and basic research is needed to explore its detailed mechanisms and validate its clinical significance. IPFD is highly related to exocrine pancreatic diseases, with excessive lipid accumulation in the pancreas creating an inflammatory microenvironment that promotes pancreatitis and pancreatic cancer, which further impairs pancreatic function and leads to more severe clinical outcomes. Furthermore, existing animal models of IPFD have primarily used high-fat diet models, and genetic models that specifically investigate the regulation of pancreatic fat cell accumulation are lacking. Future research might involve the development of relevant genetic animal models to further explore the mechanisms of IPFD.
Given the numerous and serious clinical consequences of IPFD, there is a pressing need for more accessible and efficient screening methods. These methods could complement traditional examinations to assess pancreatic fat content, facilitate timely identification of IPFD, and help prevent its worsening effects on the endocrine environment, thereby reducing the risk of progression to malignant disease. Furthermore, robust studies are needed to clarify specific pan
We thank each of the authors for their contributions to this article.
| 1. | GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403:2162-2203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 1513] [Article Influence: 756.5] [Reference Citation Analysis (0)] |
| 2. | Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1017] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 3. | Zhou J, Li ML, Zhang DD, Lin HY, Dai XH, Sun XL, Li JT, Song LY, Peng H, Wen MM. The correlation between pancreatic steatosis and metabolic syndrome in a Chinese population. Pancreatology. 2016;16:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Bi Y, Wang JL, Li ML, Zhou J, Sun XL. The association between pancreas steatosis and metabolic syndrome: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Fujii M, Ohno Y, Yamada M, Kamada Y, Miyoshi E. Impact of fatty pancreas and lifestyle on the development of subclinical chronic pancreatitis in healthy people undergoing a medical checkup. Environ Health Prev Med. 2019;24:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Otsuka N, Shimizu K, Taniai M, Tokushige K. Risk factors for fatty pancreas and effects of fatty infiltration on pancreatic cancer. Front Physiol. 2023;14:1243983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Coulier B. Pancreatic Lipomatosis: An Extensive Pictorial Review. J Belg Soc Radiol. 2016;100:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A, Claussen CD, Schick F. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Frendi S, Martineau C, Cazier H, Nicolle R, Chassac A, Albuquerque M, Raffenne J, Le Faouder J, Paradis V, Cros J, Couvelard A, Rebours V. Role of the fatty pancreatic infiltration in pancreatic oncogenesis. Sci Rep. 2024;14:6582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Tang Y, Wei Z, Li N, Jiang C, Liang C, Sun L, Tian L, Jin Z, Wu Z, Sun H. CT Quantitation and Prediction of the Risk of Type 2 Diabetes Mellitus in Non-Obese Patients with Pancreatic Fatty Infiltration. Diabetes Metab Syndr Obes. 2024;17:2619-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Filippatos TD. Non-Alcoholic Fatty Pancreas Disease: A Diagnosis of Increasing Importance. Angiology. 2022;73:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol. 2013;12:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Zhang P, Lv S, Su X, Du Y, Xu C, Jin Z. Ectopic fat deposition and its related abnormalities of lipid metabolism followed by nonalcoholic fatty pancreas. Endosc Ultrasound. 2022;11:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Yamazaki H, Streicher SA, Wu L, Fukuhara S, Wagner R, Heni M, Grossman SR, Lenz HJ, Setiawan VW, Le Marchand L, Huang BZ. Evidence for a causal link between intra-pancreatic fat deposition and pancreatic cancer: A prospective cohort and Mendelian randomization study. Cell Rep Med. 2024;5:101391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Schepis T, Tringali A, Spada C, Costamagna G, Boškoski I. Intrapancreatic Fat Deposition: Cause or Consequence of First Acute Pancreatitis Attack? Am J Gastroenterol. 2023;118:910-911. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Petrov MS, Taylor R. Intra-pancreatic fat deposition: bringing hidden fat to the fore. Nat Rev Gastroenterol Hepatol. 2022;19:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 17. | Petrov MS. Fatty change of the pancreas: the Pandora's box of pancreatology. Lancet Gastroenterol Hepatol. 2023;8:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Wagner R, Eckstein SS, Yamazaki H, Gerst F, Machann J, Jaghutriz BA, Schürmann A, Solimena M, Singer S, Königsrainer A, Birkenfeld AL, Häring HU, Fritsche A, Ullrich S, Heni M. Metabolic implications of pancreatic fat accumulation. Nat Rev Endocrinol. 2022;18:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 19. | Möller K, Jenssen C, Braden B, Hocke M, Hollerbach S, Ignee A, Faiss S, Iglesias-Garcia J, Sun S, Dong Y, Carrara S, Dietrich CF. Pancreatic changes with lifestyle and age: What is normal and what is concerning? Endosc Ultrasound. 2023;12:213-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Lesmana CR, Pakasi LS, Inggriani S, Aidawati ML, Lesmana LA. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol. 2015;15:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Sepe PS, Ohri A, Sanaka S, Berzin TM, Sekhon S, Bennett G, Mehta G, Chuttani R, Kane R, Pleskow D, Sawhney MS. A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc. 2011;73:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Pham YH, Bingham BA, Bell CS, Greenfield SA, John SD, Robinson LH, Eissa MA. Prevalence of Pancreatic Steatosis at a Pediatric Tertiary Care Center. South Med J. 2016;109:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Cho JY, You SK, Lim HH, Kim HJ. Clinical Significance of Pancreatic Fat in Children: A Single-Center Experience. Pancreas. 2022;51:972-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Okada K, Watahiki T, Horie K, Takayama T, Aida Y, To K, Shida T, Ishige K, Nishiyama H, Shoda J, Suzuki H. The prevalence and clinical implications of pancreatic fat accumulation identified during a medical check-up. Medicine (Baltimore). 2021;100:e27487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Berger Z, Orellana F, Cocio R, Torres F, Simian D, Araneda G, Toledo P. Pancreatic steatosis: A frequent finding in a Chilean population. Rev Gastroenterol Mex (Engl Ed). 2023;88:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Wang S, Xu L, Che X, Li C, Xu L, Hou K, Fan Y, Wen T, Qu X, Liu Y. E3 ubiquitin ligases Cbl-b and c-Cbl downregulate PD-L1 in EGFR wild-type non-small cell lung cancer. FEBS Lett. 2018;592:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Sotoudehmanesh R, Tahmasbi A, Sadeghi A, Hosseini H, Mohamadnejad M. The Prevalence of Nonalcoholic Fatty Pancreas by Endoscopic Ultrasonography. Pancreas. 2019;48:1220-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Weng S, Zhou J, Chen X, Sun Y, Mao Z, Chai K. Prevalence and factors associated with nonalcoholic fatty pancreas disease and its severity in China. Medicine (Baltimore). 2018;97:e11293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Wang CY, Ou HY, Chen MF, Chang TC, Chang CJ. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc. 2014;3:e000297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Qureshi S, Memon F, Chisti N, Ghazanfar S, Quraishy MS. Frequency of Nonalcoholic fatty pancreatic disease in patients with carcinoma pancreas presenting for upper abdominal Endoscopic Ultrasound in a tertiary care center. J Pak Med Assoc. 2022;72:2209-2212. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its clinical relevance: A systematic review, meta-analysis, and meta-regression. Metabolism. 2017;69:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 32. | Pietrobon CB, Lisboa PC, Bertasso IM, Peixoto TC, Soares PN, de Oliveira E, Rabelo K, de Carvalho JJ, Manhães AC, de Moura EG. Pancreatic steatosis in adult rats induced by nicotine exposure during breastfeeding. Endocrine. 2021;72:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Rossi AP, Fantin F, Zamboni GA, Mazzali G, Rinaldi CA, Del Giglio M, Di Francesco V, Barillari M, Pozzi Mucelli R, Zamboni M. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity (Silver Spring). 2011;19:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Targher G, Rossi AP, Zamboni GA, Fantin F, Antonioli A, Corzato F, Bambace C, Pozzi Mucelli R, Zamboni M. Pancreatic fat accumulation and its relationship with liver fat content and other fat depots in obese individuals. J Endocrinol Invest. 2012;35:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 35. | Jaghutriz BA, Wagner R, Heni M, Lehmann R, Machann J, Stefan N, Häring HU, Fritsche A. Metabolomic Characteristics of Fatty Pancreas. Exp Clin Endocrinol Diabetes. 2020;128:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Li J, Xie Y, Yuan F, Song B, Tang C. Noninvasive quantification of pancreatic fat in healthy male population using chemical shift magnetic resonance imaging: effect of aging on pancreatic fat content. Pancreas. 2011;40:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Lê KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, Hu HH, Nayak KS, Goran MI. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care. 2011;34:485-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Szczepaniak LS, Victor RG, Mathur R, Nelson MD, Szczepaniak EW, Tyer N, Chen I, Unger RH, Bergman RN, Lingvay I. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35:2377-2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Bhalla S, Kuchel GA, Pandol S, Bishehsari F. Association of Pancreatic Fatty Infiltration With Age and Metabolic Syndrome Is Sex-Dependent. Gastro Hep Adv. 2022;1:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Altinmakas E, Guler B, Copur S, Siriopol D, Sag AA, Guneyli S, Dogan H, Afsar B, Balik E, Covic A, Johnson RJ, Kanbay M. Determinants of Pancreatic Steatosis: A Retrospective Observational Study. Middle East J Dig Dis. 2021;13:343-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 41. | Fujii J, Homma T, Kobayashi S, Seo HG. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J Biol Chem. 2018;9:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 42. | Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J, Woodward T, Noh K, Raimondo M. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas. 2009;38:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Lin M, Weng SY, Chai KF, Mao ZJ. Lipidomics as a tool of predicting progression from non-alcoholic fatty pancreas disease to type 2 diabetes mellitus. RSC Adv. 2019;9:41419-41430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Kim G, Lee J, Ha J, Kang I, Choe W. Endoplasmic Reticulum Stress and Its Impact on Adipogenesis: Molecular Mechanisms Implicated. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 45. | Lipovšek S, Dolenšek J, Dariš B, Valladolid-Acebes I, Vajs T, Leitinger G, Stožer A, Skelin Klemen M. Western diet-induced ultrastructural changes in mouse pancreatic acinar cells. Front Cell Dev Biol. 2024;12:1380564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Fang T, Huang S, Chen Y, Chen Z, Chen J, Hu W. Glucagon Like Peptide-1 Receptor Agonists Alter Pancreatic and Hepatic Histology and Regulation of Endoplasmic Reticulum Stress in High-fat Diet Mouse Model. Exp Clin Endocrinol Diabetes. 2021;129:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 48. | Kirsoy F, Yalniz M, Bahçecioğlu İH, Artaş H, Türkoğlu S, Solmaz O, Tawheed A. The gut-pancreas axis: investigating the relationship between microbiota metabolites and pancreatic steatosis. Intern Emerg Med. 2024;19:1887-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185:419-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 643] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 50. | Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 607] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 51. | Otero A, Becerril S, Martín M, Cienfuegos JA, Valentí V, Moncada R, Catalán V, Gómez-Ambrosi J, Burrell MA, Frühbeck G, Rodríguez A. Effect of guanylin peptides on pancreas steatosis and function in experimental diet-induced obesity and after bariatric surgery. Front Endocrinol (Lausanne). 2023;14:1185456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 52. | Al-Ani Z, Ko J, Petrov MS. Intra-pancreatic fat deposition across the pancreatitis spectrum and the influence of gut hormones. Dig Liver Dis. 2023;55:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Sakata N, Yoshimatsu G, Kodama S. Development and Characteristics of Pancreatic Epsilon Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Napolitano T, Silvano S, Vieira A, Balaji S, Garrido-Utrilla A, Friano ME, Atlija J, Collombat P. Role of ghrelin in pancreatic development and function. Diabetes Obes Metab. 2018;20 Suppl 2:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Nuñez-Durán E, Chanclón B, Sütt S, Real J, Marschall HU, Wernstedt Asterholm I, Cansby E, Mahlapuu M. Protein kinase STK25 aggravates the severity of non-alcoholic fatty pancreas disease in mice. J Endocrinol. 2017;234:15-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Xiao Y, Han L, Wang H, Ke H, Xu S, Huang Z, Lyu G, Li S. Uric Acid Inhibits Mice Pancreatic Steatosis via the Glycerophospholipid Pathway. ACS Omega. 2024;9:21829-21837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 57. | Ko J, Al-Ani Z, Long K, Tarrant C, Skudder-Hill L, Petrov MS. Intrapancreatic, Liver, and Skeletal Muscle Fat Depositions in First Attack of Acute Pancreatitis Versus Health. Am J Gastroenterol. 2022;117:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 58. | Sbeit W, Khoury T. Fatty Pancreas Represents a Risk Factor for Acute Pancreatitis: A Pilot Study. Pancreas. 2021;50:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Dong X, Zhu Q, Yuan C, Wang Y, Ma X, Shi X, Chen W, Dong Z, Chen L, Shen Q, Xu H, Ding Y, Gong W, Xiao W, Wang S, Li W, Lu G. Associations of Intrapancreatic Fat Deposition With Incident Diseases of the Exocrine and Endocrine Pancreas: A UK Biobank Prospective Cohort Study. Am J Gastroenterol. 2024;119:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 60. | Sbeit W, Abu Elheja F, Msheiil B, Shahin A, Khoury S, Sbeit M, Khoury T. Fatty pancreas was associated with a higher acute pancreatitis Systemic Inflammatory Response Syndrome score at hospital admission. Eur J Gastroenterol Hepatol. 2023;35:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (2)] |
| 62. | Rafaqat S, Radoman-Vujačić I, Patoulias D, Khurshid H, Klisić A. Adipokines and their role in acute pancreatitis. J Med Biochem. 2024;43:512-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Ko J, Skudder-Hill L, Priya S, Kimita W, Bharmal SH, Petrov MS. Associations between Intra-Pancreatic Fat Deposition, Pancreas Size, and Pancreatic Enzymes in Health and after an Attack of Acute Pancreatitis. Obes Facts. 2022;15:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Tirkes T, Jeon CY, Li L, Joon AY, Seltman TA, Sankar M, Persohn SA, Territo PR. Association of Pancreatic Steatosis With Chronic Pancreatitis, Obesity, and Type 2 Diabetes Mellitus. Pancreas. 2019;48:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 65. | Tirkes T, Yadav D, Conwell DL, Territo PR, Zhao X, Persohn SA, Dasyam AK, Shah ZK, Venkatesh SK, Takahashi N, Wachsman A, Li L, Li Y, Pandol SJ, Park WG, Vege SS, Hart PA, Topazian M, Andersen DK, Fogel EL; Consortium for the Study of Chronic Pancreatitis, Diabetes, Pancreatic Cancer (CPDPC). Quantitative MRI of chronic pancreatitis: results from a multi-institutional prospective study, magnetic resonance imaging as a non-invasive method for assessment of pancreatic fibrosis (MINIMAP). Abdom Radiol (NY). 2022;47:3792-3805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 66. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 1299] [Article Influence: 259.8] [Reference Citation Analysis (0)] |
| 67. | Sreedhar UL, DeSouza SV, Park B, Petrov MS. A Systematic Review of Intra-pancreatic Fat Deposition and Pancreatic Carcinogenesis. J Gastrointest Surg. 2020;24:2560-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Khoury T, Sbeit W. Fatty Pancreas and Pancreatic Cancer: An Overlooked Association? J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Lipp M, Tarján D, Lee J, Zolcsák Á, Szalai E, Teutsch B, Faluhelyi N, Erőss B, Hegyi P, Mikó A. Fatty Pancreas Is a Risk Factor for Pancreatic Cancer: A Systematic Review and Meta-Analysis of 2956 Patients. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 70. | Desai V, Patel K, Sheth R, Barlass U, Chan YM, Sclamberg J, Bishehsari F. Pancreatic Fat Infiltration Is Associated with a Higher Risk of Pancreatic Ductal Adenocarcinoma. Visc Med. 2020;36:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Truong E, Pandol S, Jeon C. Uniting epidemiology and experimental models: pancreatic steatosis and pancreatic cancer. EBioMedicine. 2022;79:103996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Hoogenboom SA, Bolan CW, Chuprin A, Raimondo MT, van Hooft JE, Wallace MB, Raimondo M. Pancreatic steatosis on computed tomography is an early imaging feature of pre-diagnostic pancreatic cancer: A preliminary study in overweight patients. Pancreatology. 2021;21:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Ackerman D, Tumanov S, Qiu B, Michalopoulou E, Spata M, Azzam A, Xie H, Simon MC, Kamphorst JJ. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 2018;24:2596-2605.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (4)] |
| 74. | Chang ML. Fatty Pancreas-Centered Metabolic Basis of Pancreatic Adenocarcinoma: From Obesity, Diabetes and Pancreatitis to Oncogenesis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Lilly AC, Astsaturov I, Golemis EA. Intrapancreatic fat, pancreatitis, and pancreatic cancer. Cell Mol Life Sci. 2023;80:206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 76. | Rozeveld CN, Johnson KM, Zhang L, Razidlo GL. KRAS Controls Pancreatic Cancer Cell Lipid Metabolism and Invasive Potential through the Lipase HSL. Cancer Res. 2020;80:4932-4945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 77. | Tahtacı M, Algın O, Karakan T, Yürekli ÖT, Alışık M, Köseoğlu H, Metin MR, Bolat AD, Erel Ö, Ersoy O. Can pancreatic steatosis affect exocrine functions of pancreas? Turk J Gastroenterol. 2018;29:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Kromrey ML, Friedrich N, Hoffmann RT, Bülow R, Völzke H, Weiss FU, Lerch MM, Motosugi U, Kühn JP. Pancreatic Steatosis Is Associated With Impaired Exocrine Pancreatic Function. Invest Radiol. 2019;54:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 80. | Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, Kim SW. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2010;251:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Dei H, Natsume S, Okuno M, Kawakatsu S, Hosoda W, Matsuo K, Hara K, Ito S, Komori K, Abe T, Nagino M, Shimizu Y. Impact of pancreatic fat infiltration on postoperative pancreatic fistula occurrence in patients undergoing invagination pancreaticojejunostomy. HPB (Oxford). 2022;24:2119-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 82. | Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (2)] |
| 83. | Lu T, Wang Y, Dou T, Xue B, Tan Y, Yang J. Pancreatic fat content is associated with β-cell function and insulin resistance in Chinese type 2 diabetes subjects. Endocr J. 2019;66:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 84. | Chin SO, Hwang YC, Cho IJ, Jeong IK, Ahn KJ, Chung HY. Pancreatic fat accumulation is associated with decreased β-cell function and deterioration in glucose tolerance in Korean adults. Diabetes Metab Res Rev. 2021;37:e3425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Kim MK, Chun HJ, Park JH, Yeo DM, Baek KH, Song KH, Chung DJ, Kwon HS. The association between ectopic fat in the pancreas and subclinical atherosclerosis in type 2 diabetes. Diabetes Res Clin Pract. 2014;106:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH. Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond). 2010;34:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 87. | Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring). 2008;16:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 88. | Li S, Su L, Lv G, Zhao W, Chen J. Transabdominal ultrasonography of the pancreas is superior to that of the liver for detection of ectopic fat deposits resulting from metabolic syndrome. Medicine (Baltimore). 2017;96:e8060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 89. | Oh H, Park HJ, Oh J, Lee ES, Park SB, Cha MJ, Ahn S. Hyperechoic pancreas on ultrasonography: an analysis of its severity and clinical implications. Ultrasonography. 2022;41:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Oh J, Park HJ, Lee ES, Park SB, Choi BI, Ahn S. Severity of hyperechoic pancreas on ultrasonography as a risk factor for glycemic progression. Ultrasonography. 2021;40:499-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Suzuki H, Kawashima H, Ohno E, Ishikawa T, Hashimoto S, Nakamura M, Miyahara R, Ishigami M, Hirooka Y, Fujishiro M. What is the role of measuring shear wave dispersion using shear wave elastography in pancreatic parenchyma? J Med Ultrason (2001). 2020;47:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Kawamura A, Takakura K, Torisu Y, Kinoshita Y, Tomita Y, Nakano M, Yamauchi T, Suka M, Sumiyama K, Koido S, Saruta M. Impact of qualitative endoscopic ultrasonography on fatty pancreas at a referral medical center. JGH Open. 2022;6:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 93. | Muftah AA, Pecha RL, Riojas Barrett M, Abidi WM, Patel KK, Keihanian T, Othman MO. Pancreatic parenchymal changes seen on endoscopic ultrasound are dynamic in the setting of fatty pancreas: A short-term follow-up study. Pancreatology. 2022;22:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Previtali C, Sartoris R, Rebours V, Couvelard A, Cros J, Sauvanet A, Cauchy F, Paradis V, Vilgrain V, Dioguardi Burgio M, Ronot M. Quantitative imaging predicts pancreatic fatty infiltration on routine CT examination. Diagn Interv Imaging. 2023;104:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Fukuda Y, Yamada D, Eguchi H, Hata T, Iwagami Y, Noda T, Asaoka T, Kawamoto K, Gotoh K, Kobayashi S, Takeda Y, Tanemura M, Mori M, Doki Y. CT Density in the Pancreas is a Promising Imaging Predictor for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2017;24:2762-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 96. | Hori M, Onaya H, Hiraoka N, Yamaji T, Kobayashi H, Takahashi M, Mutoh M, Shimada K, Nakagama H. Evaluation of the degree of pancreatic fatty infiltration by area-based assessment of CT images: comparison with histopathology-based and CT attenuation index-based assessments. Jpn J Radiol. 2016;34:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Gursoy Coruh A, Uzun C, Akkaya Z, Halil Elhan A. The relation of CT quantified pancreatic fat index with visceral adiposity and hepatic steatosis. Turk J Surg. 2020;36:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, Kim YH, Kim JH, Yoon YS, Han HS, Kang HS. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology. 2014;271:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 99. | Ookura R, Usuki N. Visual assessment of pancreatic fat deposition: useful grading system and the relation to BMI and diabetes. Jpn J Radiol. 2023;41:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Tanabe M, Kunihiro Y, Higashi M, Ihara K, Tanabe M, Yagi T, Kobayashi T, Ueda T, Ito K. Pancreatic Steatosis Evaluated by Automated Volumetric CT Fat Fraction of the Pancreas: Association with Severity in COVID-19 Pneumonia. Tomography. 2022;8:2806-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Koç U, Taydaş O. Evaluation of pancreatic steatosis prevalence and anthropometric measurements using non-contrast computed tomography. Turk J Gastroenterol. 2020;31:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Tanaka K, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, Kobayashi D, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y. Pancreatic Fat and Body Composition Measurements by Computed Tomography are Associated with Pancreatic Fistula After Pancreatectomy. Ann Surg Oncol. 2021;28:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Chung MJ, Park SW, Lee KJ, Park DH, Koh DH, Lee J, Lee HS, Park JY, Bang S, Min S, Park JH, Kim SJ, Park CH. Clinical impact of pancreatic steatosis measured by CT on the risk of post-ERCP pancreatitis: a multicenter prospective trial. Gastrointest Endosc. 2024;99:214-223.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 104. | Kobayashi N, Shinohara H, Haruta S, Udagawa H, Ueno M. Reducing the risk of postoperative pancreatic fistula in radical gastrectomy: pre-assessment with computed tomography for the diagnosis of pancreatic steatosis. Langenbecks Arch Surg. 2022;407:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Sano S, Okamura Y, Ohgi K, Sugiura T, Ito T, Yamamoto Y, Ashida R, Sasaki K, Uesaka K. Histological pancreatic findings correlate with computed tomography attenuation and predict postoperative pancreatic fistula following pancreatoduodenectomy. HPB (Oxford). 2022;24:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 106. | Yang W, Xie Y, Song B, Xia C, Tang C, Li J. Effects of aging and menopause on pancreatic fat fraction in healthy women population: A strobe-compliant article. Medicine (Baltimore). 2019;98:e14451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Vieira J, Amorim J, Martí-Bonmatí L, Alberich-Bayarri Á, França M. Quantifying steatosis in the liver and pancreas with MRI in patient with chronic liver disease. Radiologia (Engl Ed). 2020;62:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 108. | Idilman IS, Yildiz AE, Karaosmanoglu AD, Ozmen MN, Akata D, Karcaaltincaba M. Proton density fat fraction: magnetic resonance imaging applications beyond the liver. Diagn Interv Radiol. 2022;28:83-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Kim J, Albakheet SS, Han K, Yoon H, Lee MJ, Koh H, Kim S, Suh J, Han SJ, Ihn K, Shin HJ. Quantitative MRI Assessment of Pancreatic Steatosis Using Proton Density Fat Fraction in Pediatric Obesity. Korean J Radiol. 2021;22:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Yoon JH, Lee JM, Lee KB, Kim SW, Kang MJ, Jang JY, Kannengiesser S, Han JK, Choi BI. Pancreatic Steatosis and Fibrosis: Quantitative Assessment with Preoperative Multiparametric MR Imaging. Radiology. 2016;279:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 111. | Gaborit B, Abdesselam I, Kober F, Jacquier A, Ronsin O, Emungania O, Lesavre N, Alessi MC, Martin JC, Bernard M, Dutour A. Ectopic fat storage in the pancreas using 1H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes (Lond). 2015;39:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 112. | Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring). 2010;18:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 113. | Hu HH, Smith DL Jr, Nayak KS, Goran MI, Nagy TR. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging. 2010;31:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 114. | Janssens LP, Takahashi H, Nagayama H, Nugen F, Bamlet WR, Oberg AL, Fuemmeler E, Goenka AH, Erickson BJ, Takahashi N, Majumder S. Artificial intelligence assisted whole organ pancreatic fat estimation on magnetic resonance imaging and correlation with pancreas attenuation on computed tomography. Pancreatology. 2023;23:556-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Han F, Yin L, Yu X, Xu R, Tian M, Liu X, Zhou L, Hu L, Gong W, Xiao W, Lu G, Yao G, Ding Y. High circulating fibroblast growth factor-21 levels as a screening marker in fatty pancreas patients. PeerJ. 2023;11:e15176. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 116. | Singh RG, Nguyen NN, Cervantes A, Cho J, Petrov MS. Serum lipid profile as a biomarker of intra-pancreatic fat deposition: A nested cross-sectional study. Nutr Metab Cardiovasc Dis. 2019;29:956-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 117. | Okasha HH, Hegazy MA, Shaker O, Elfatah YA, El-Sawy SS, Abdelfatah D, Abdellatef A. Study of non-alcoholic fatty pancreatic disease among the Egyptian population and the value of serum fatty acid binding protein-1 (FABP-1) as a non-invasive biomarker. Clin Res Hepatol Gastroenterol. 2024;48:102364. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 118. | Yu X, Wang D, Xiao W, Shi X, She Q, Sun H, Qi T, Xu R, Li G, Liu X, Gong W, Yan Z, Ding Y, Lu G. Relationship between fatty pancreas and hypertriglyceridemic waist phenotype: a cross-sectional study. Sci Rep. 2020;10:21937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Khoury T, Mari A, Sbeit W. A Novel Clinical Score Predicting the Presence of Fatty Pancreas. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Xiao Y, Wang H, Han L, Huang Z, Lyu G, Li S. Predictive value of anthropometric and biochemical indices in non-alcoholic fatty pancreas disease: a cross-sectional study. BMJ Open. 2024;14:e081131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 121. | Heiskanen MA, Motiani KK, Mari A, Saunavaara V, Eskelinen JJ, Virtanen KA, Koivumäki M, Löyttyniemi E, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: a randomised controlled trial. Diabetologia. 2018;61:1817-1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 122. | Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, Hollingsworth KG, Mathers JC, Sattar N, Lean MEJ. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018;28:547-556.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 123. | Hassan MA, Abedelmaksoud TG, Abd El-Maksoud AA. Effects of Lactoferrin Supplemented with Fermented Milk on Obesity-Associated Pancreatic Damage in Rats. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 124. | Matboli M, Al-Amodi HS, Hamady S, Ali M, Roushdy MM, Hasanin AH, Aboul-Ela YM, Albadawy R, Gomaa E, Kamel HFM, ELsawi HA, Farid LM, Abouelkhair MB, Elmakromy GM, Fawzy NM. Experimental investigation for nonalcoholic fatty pancreas management using probiotics. Diabetol Metab Syndr. 2024;16:147. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 125. | Lu J, Pan T, Gao J, Cai X, Zhang H, Sha W, Lei T. Reduced Branched-Chain Amino Acid Intake Improved High-Fat Diet-Induced Nonalcoholic Fatty Pancreas Disease in Mice. Pancreas. 2024;53:e157-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 126. | Petrov MS. The Pharmacological Landscape for Fatty Change of the Pancreas. Drugs. 2024;84:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 127. | Nag S, Mandal S, Mukherjee O, Majumdar T, Mukhopadhyay S, Kundu R. Vildagliptin inhibits high fat and fetuin-A mediated DPP-4 expression, intracellular lipid accumulation and improves insulin secretory defects in pancreatic beta cells. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 128. | Souza-Mello V, Gregório BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond). 2010;119:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 129. | Lee JW, Gu HO, Jung Y, Jung Y, Seo SY, Hong JH, Hong IS, Lee DH, Kim OH, Oh BC. Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation. Exp Mol Med. 2023;55:910-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 130. | Vanderheiden A, Harrison LB, Warshauer JT, Adams-Huet B, Li X, Yuan Q, Hulsey K, Dimitrov I, Yokoo T, Jaster AW, Pinho DF, Pedrosa I, Lenkinski RE, Pop LM, Lingvay I. Mechanisms of Action of Liraglutide in Patients With Type 2 Diabetes Treated With High-Dose Insulin. J Clin Endocrinol Metab. 2016;101:1798-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 131. | Kuriyama T, Ishibashi C, Kozawa J, Baden MY, Horii T, Niki A, Ozawa H, Hosokawa Y, Fujita Y, Sadahiro K, Satoh T, Hamaguchi T, Shimomura I. Effects of liraglutide on intrapancreatic fat deposition in patients with type 2 diabetes. Clin Nutr ESPEN. 2024;59:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Shi M, Zhang H, Wang W, Zhang X, Liu J, Wang Q, Wang Y, Zhang C, Guo X, Qiao Q, Cui C, Xu J, Wang J. Effect of dapagliflozin on liver and pancreatic fat in patients with type 2 diabetes and non-alcoholic fatty liver disease. J Diabetes Complications. 2023;37:108610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 133. | Ghosh A, Dutta K, Bhatt SP, Gupta R, Tyagi K, Ansari IA, Venugopal VK, Mahajan H, Pandey RM, Pandey S, Misra A. Dapagliflozin Improves Body Fat Patterning, and Hepatic and Pancreatic Fat in Patients With Type 2 Diabetes in North India. J Clin Endocrinol Metab. 2022;107:e2267-e2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 134. | Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, Soghomonian A, Houssays M, Varlet I, Eisinger M, Lasbleiz A, Peiretti F, Bornet CE, Lefur Y, Pini L, Rapacchi S, Bernard M, Resseguier N, Darmon P, Kober F, Dutour A. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol. 2021;20:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 135. | Barrientos C, Pérez A, Vázquez J. Ameliorative Effects of Oral Glucosamine on Insulin Resistance and Pancreatic Tissue Damage in Experimental Wistar rats on a High-fat Diet. Comp Med. 2021;71:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Adeyanju OA, Badejogbin OC, Areola DE, Olaniyi KS, Dibia C, Soetan OA, Oniyide AA, Michael OS, Olatunji LA, Soladoye AO. Sodium butyrate arrests pancreato-hepatic synchronous uric acid and lipid dysmetabolism in high fat diet fed Wistar rats. Biomed Pharmacother. 2021;133:110994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 137. | Krisnamurti DGB, Farida S, Putri RC, Fachri W, Purwaningsih EH. The effect of simvastatin-Acalypha indica Linn. combination on the improvement of fatty pancreas in rats induced with a high fructose and cholesterol diet. J Adv Vet Anim Res. 2022;9:346-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 138. | Chen ZY, Liu SN, Li CN, Sun SJ, Liu Q, Lei L, Gao LH, Shen ZF. Atorvastatin helps preserve pancreatic β cell function in obese C57BL/6 J mice and the effect is related to increased pancreas proliferation and amelioration of endoplasmic-reticulum stress. Lipids Health Dis. 2014;13:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 139. | Munika E, Gunawan S, Wuyung PE, Soetikno V. Beneficial Effects of 6-Gingerol on Fatty Pancreas Disease in High-Fat High-Fructose Diet-Induced Metabolic Syndrome Rat Model. Pancreas. 2024;53:e193-e198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 140. | Albadawy R, Hasanin AH, Agwa SHA, Hamady S, Mohamed RH, Gomaa E, Othman M, Yahia YA, Ghani AMA, Matboli M. Prospective insight into the role of benzyl propylene glycoside as a modulator of the cGAS-STING signaling pathway in the management of nonalcoholic fatty pancreas animal model. Biol Res. 2023;56:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/