Published online May 21, 2025. doi: 10.3748/wjg.v31.i19.105283
Revised: March 27, 2025
Accepted: April 27, 2025
Published online: May 21, 2025
Processing time: 124 Days and 15.2 Hours
Despite the promising prospects of utilizing artificial intelligence and machine learning (ML) for comprehensive disease analysis, few models constructed have been applied in clinical practice due to their complexity and the lack of reasonable explanations. In contrast to previous studies with small sample sizes and limited model interpretability, we developed a transparent eXtreme Gradient Boosting (XGBoost)-based model supported by multi-center data, using patients' basic information and clinical indicators to forecast the occurrence of anastomotic leakage (AL) after rectal cancer resection surgery. The model demonstrated robust predictive performance and identified clinically relevant thresholds, which may assist physicians in optimizing perioperative management.
To develop an interpretable ML model for accurately predicting the occurrence probability of AL after rectal cancer resection and define our clinical alert values for serum calcium ions.
Patients who underwent anterior resection of the rectum for rectal carcinoma at the Department of Digestive Surgery, Xijing Hospital of Digestive Diseases, Air Force Medical University, and Shaanxi Provincial People's Hospital, were retrospectively collected from January 2011 to December 2021,. Ten ML models were integrated to analyze the data and develop the predictive models. Receiver operating characteristic (ROC) curves, calibration curve, decision curve analysis, accuracy, sensitivity, specificity, positive predictive value, negative predictive value, and F1 score were used to evaluate model performance. We employed the SHapley Additive exPlanations (SHAP) algorithm to explain the feature importance of the optimal model.
A total of ten features were integrated to construct the predictive model and identify the optimal model. XGBoost was considered the best-performing model with an area under the ROC curve (AUC) of 0.984 (95%confidence interval: 0.972-0.996) in the test set (accuracy: 0.925; sensitivity: 0.92; specificity: 0.927). Furthermore, the model achieved an AUC of 0.703 in external validation. The interpretable SHAP algorithm revealed that the serum calcium ion level was the crucial factor influencing the predictions of the model.
A superior predictive model, leveraging clinical data, has been crafted by employing the most effective XGBoost from a selection of ten algorithms. This model, by predicting the occurrence of AL in patients after rectal cancer resection, has identified the significant role of serum calcium ion levels, providing guidance for clinical practice. The integration of SHAP provides a clear interpretation of the model's predictions.
Core Tip: Ten machine learning models were established using ten factors and interpreted using the SHapley Additive exPlanations model. Through model evaluation and comparison, we selected the best prediction model and performed external validation in multiple centers. We found for the first time that perioperative serum calcium ion level plays an important role in the occurrence of anastomotic leakage (AL) after anterior resection of rectal cancer, and proposed that preoperative serum calcium level lower than 2.1 and postoperative calcium level lower than 2.2 are clinical warning values for the occurrence of AL.
- Citation: Kang BY, Qiao YH, Zhu J, Hu BL, Zhang ZC, Li JP, Pei YJ. Serum calcium-based interpretable machine learning model for predicting anastomotic leakage after rectal cancer resection: A multi-center study. World J Gastroenterol 2025; 31(19): 105283
- URL: https://www.wjgnet.com/1007-9327/full/v31/i19/105283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i19.105283
Colorectal cancer is recognized as a critical public health issue, being the third most common cancer and the second leading cause of cancer-related mortality worldwide[1]. Rectal cancer is one of the most common and severe diseases that threatens human health worldwide as well[2]. And the global burden of rectal cancer is expected to increase by 2040[3]. Up to 20% of patients undergoing low anterior resection for rectal cancer experience anastomotic leakage (AL)[4]. AL is a severe complication after rectal cancer surgery, leading to increased permanent stoma formation and cancer recurrence[5,6]. Therefore, it is of great importance to investigate the risk factors for AL to reduce its incidence.
Machine learning (ML), an artificial intelligence (AI)-based predictive tool, holds significant advantages in dealing with the complex relationships between diseases and contributing factors in the medical field[7,8]. Currently, ML is exten
Despite the promising prospects of utilizing AI and ML for comprehensive disease analysis, few models have been applied in clinical practice due to their complexity and the lack of reasonable explanations[15,16]. In contrast to previous studies with small sample sizes and limited interpretability, we developed a transparent eXtreme Gradient Boosting (XGBoost)-based model supported by multi-center data, using patients' basic information and clinical indicators to forecast the occurrence of AL after rectal cancer resection. The model demonstrated robust predictive performance and identified clinically relevant thresholds, which may assist physicians in optimizing perioperative management.
This study encompassed 1818 patients diagnosed with rectal cancer, all of whom underwent anterior resection of the rectum for rectal carcinoma at the Department of Digestive Surgery, Xijing Hospital of Digestive Diseases, Air Force Medical University, from January 2011 to December 2021. The external validation data was collected from Shaanxi Provincial People's Hospital, encompassing 60 cases of patients who underwent anterior resection for rectal cancer from January 2021 to January 2024. The implementation of radical resection for rectal carcinoma strictly adhered to the treatment guidelines of the corresponding period, with standard surgical procedures employed. The case information came from electronic medical records. This study was a secondary analysis of retrospective data and was a retrospective, multi-cohort, observational study using de-identified data. Therefore, informed consent and research ethics committee approval were not required. The study protocol adhered to the ethical guidelines of the 1995 Declaration of Helsinki, and the previous study was approved by the ethics committee of the First Affiliated Hospital of Air Force Military Medical University (approval No. KY20212211-N-1).
Inclusion criteria: (1) Age ≥ 18 years; (2) Patients with confirmed AL after anterior resection of the rectum for rectal cancer; and (3) Primary rectal carcinoma confirmed by preoperative pathology.
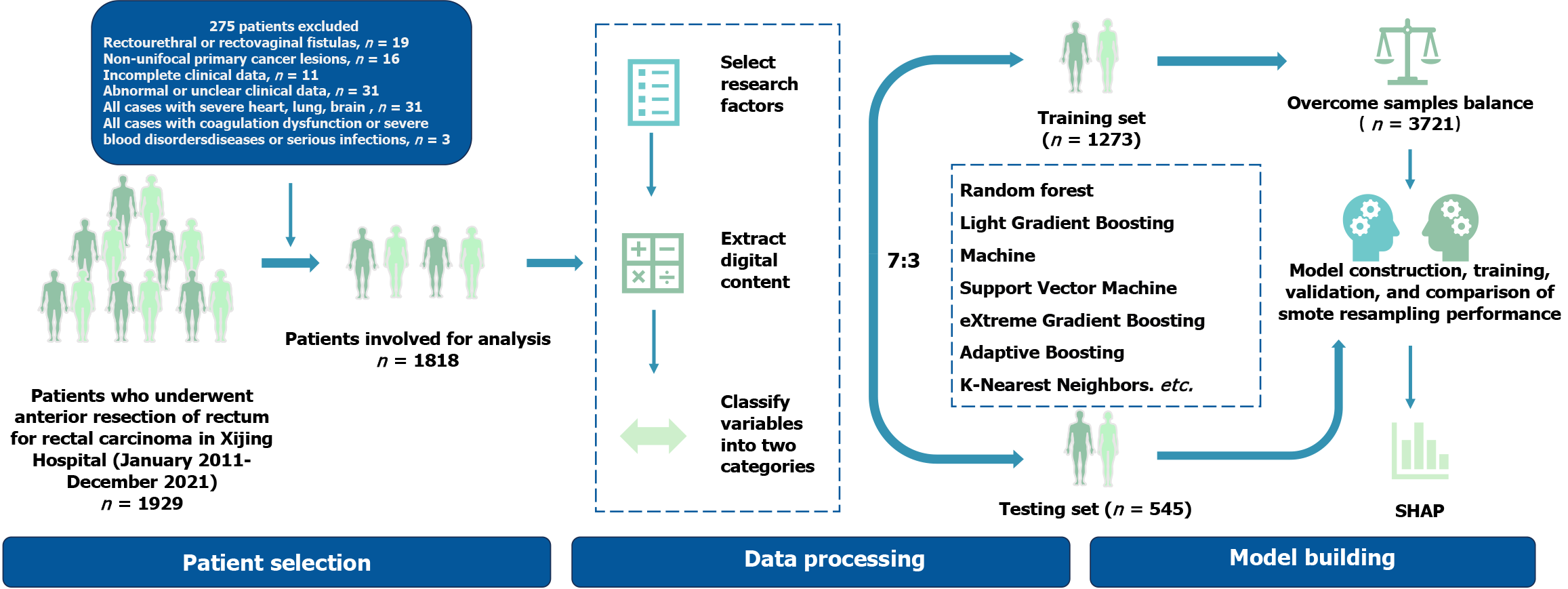
Exclusion criteria: (1) Non-unifocal primary cancer lesions; (2) Development of rectourethral or rectovaginal leakage; (3) Incomplete clinical data; (4) Abnormal or unclear clinical data; (5) All cases with severe heart, lung, and brain diseases or serious infections; and (6) All cases with coagulation dysfunction or severe blood disorders. The flowchart of patient selection and model construction is illustrated in Figure 1.
The diagnosis of rectal cancer was based on the 2021 guidelines from the National Comprehensive Cancer Network for the diagnosis and treatment of rectal cancer. The definition of AL adopted the criteria proposed by The International Study Group of Rectal Cancer in 2009, and patients with three grades of A, B, and C were included in the study[17]. Based on 28 continuous variables, the median was taken as the cutoff value for dichotomization and 11 categorical variables were analyzed in dummy form. Preoperative and postoperative patient data were collected three days prior to surgery and one day after surgery, respectively, and subsequently entered into the electronic medical records. The data did not contain missing values.
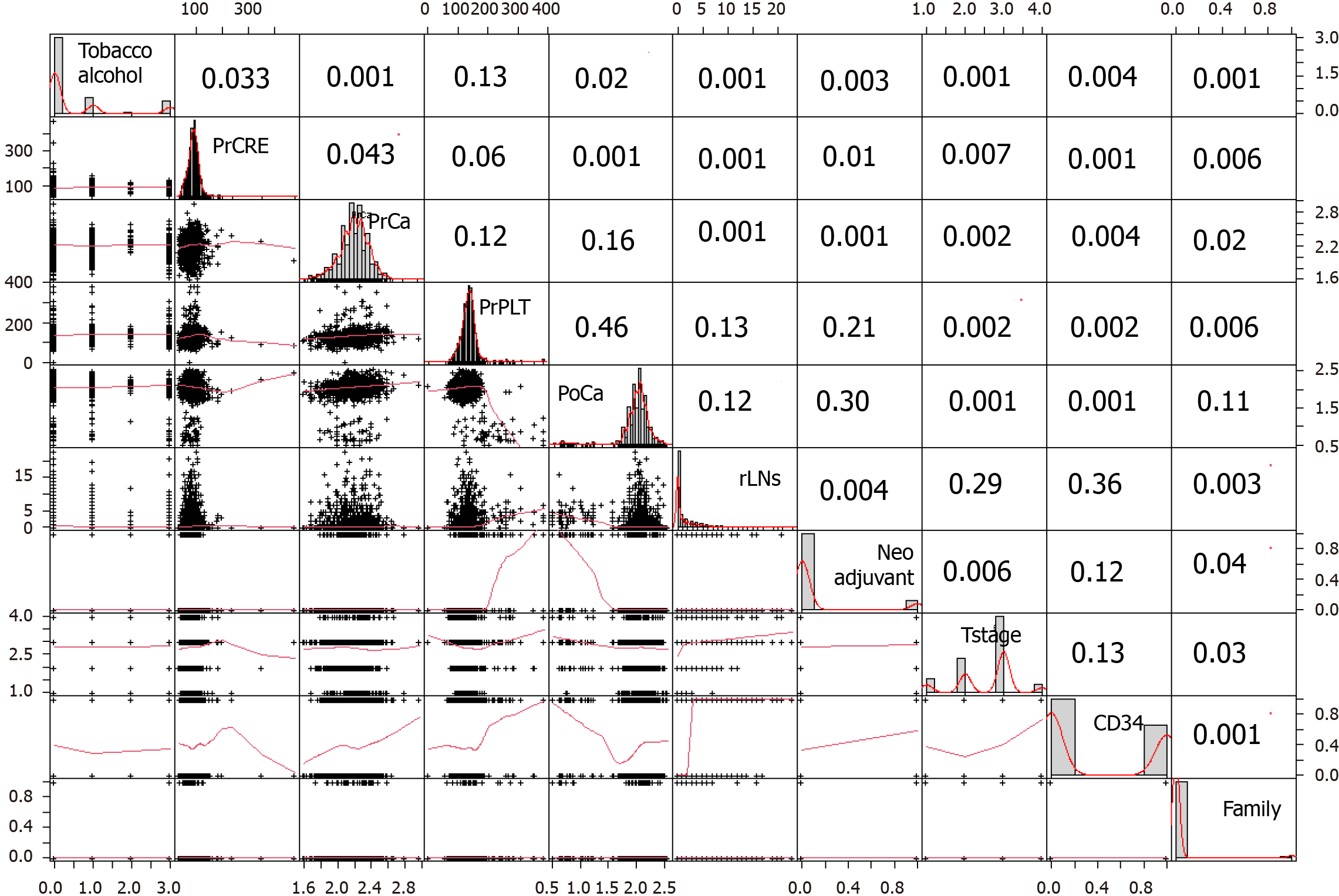
Based on patient information, clinical variables, and hematological indicators, we included 39 variables for analysis and screened for important factors using least absolute shrinkage and selection operator (LASSO) regression, stepwise Logistic regression, and Boruta regression. By employing LASSO regression, we used the optimal regularization lambda parameters, divided the entire dataset into 10 folds, and sequentially incorporated each variable into the model, ceasing the addition when the area under the receiver operating characteristic (ROC) curve (AUC) no longer increased, thereby conducting feature selection. Nine variables were considered significant in all regression analyses. Preoperative and postoperative serum creatinine levels, as well as postoperative cystatin C, were considered to be of significant importance in both regression models. These three variables all reflect renal function. To minimize potential interactions and considering that postoperative serum creatinine is more commonly used in clinical practice, we selected postoperative serum creatinine for inclusion in the model construction.
The patients (n = 1818) were randomly assigned to training and testing datasets in a 7:3 ratio. To evaluate the impact of data imbalance, models were constructed and evaluated using both raw data and SMOTE resampled data (n = 3721), and the most effective data processing method was selected. Additionally, the efficacy of the model will be rigorously evaluated through external validation processes to guarantee the precision and dependability of its prognostic capabilities. Subsequently, an optimal predictive model was developed utilizing 10 distinct ML algorithms: Logistic regression, support vector machine, gradient boosting machines, neural network, random forest, XGBoost, K-nearest neighbors, AdaBoost, light gradient boosting machine, and CatBoost. The performance of the models was rigorously assessed employing ROC curves, calibration curve, decision curve analysis (DCA), accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and F1 score.
SHAP, originally developed by Lee, is an approach grounded in cooperative game theory for elucidating ML models[18]. By assigning SHAP values that quantify the contribution of each predictor variable, SHAP creates a consistent framework ideal for assessing variable contributions within predictive models, thus mitigating the challenge of model opacity. Distinct from alternative methodologies, SHAP values offer local interpretability, enhancing the comprehension of model intricacies. Positive SHAP values indicate that the variable is more instrumental in predicting patients with AL, while negative values suggest that the variable is more conducive to predicting patients without AL.
Statistical analyses were performed by using R, version 4.4.1 (R, Foundation for Statistical Computing). SMOTE resampling was calculated using the R package DMwR. LASSO regularization model, Boruta regression, and Logistic regression were calculated using the R packages Boruta and glmnet. Ten ML models were calculated using the R packages e1071, gbm, caret, XGBoost, nnet, Adaboost, lightgbm, and Catboost. ROC curve, DCA, and calibration curve were calculated using the R packages pROC and rmda and risk regression. All continuous variables in this study were converted into categorical variables by median, and all categorical variables appeared in frequency and percentage form. The χ2 test was used to compare the differences between groups, and P < 0.05 was considered statistically significant. The kernelshap and shiny packages in R were utilized to ascertain the significance and hierarchy of variables within the model. The directional influence of each variable on the outcome was established based on SHAP values and detailed visual explanations were generated at the individual observation level.
This retrospective study included 1818 patients (average age, 61.6 years; range, 20-89 years) from January 2011 to December 2021, including 1119 males and 699 females, of whom 86 (4.73%) suffered from AL. The baseline data of included patients are shown in Table 1. Baseline data for the training set, validation set, and external validation are shown in Supplementary Tables 1-3.
| NAL (n = 1732) | AL (n = 86) | P value | |
| Sex = male | 1055 (60.9) | 64 (74.4) | 0.016 |
| Tobacco or alcohol | < 0.001 | ||
| No | 1256 (72.5) | 46 (53.5) | |
| Tobacco | 258 (14.9) | 18 (20.9) | |
| Alcohol | 25 (1.4) | 1 (1.2) | |
| All | 193 (11.1) | 21 (24.4) | |
| T stage | < 0.001 | ||
| T1 | 192 (11.1) | 2 (2.3) | |
| T2 | 453 (26.2) | 17 (19.8) | |
| T3 | 995 (57.4) | 43 (50.0) | |
| T4 | 92 (5.3) | 24 (27.9) | |
| N stage | 0.025 | ||
| N0 | 291 (16.8) | 12 (14.0) | |
| N1 | 1260 (72.7) | 57 (66.3) | |
| N2 | 181 (10.5) | 17 (19.8) | |
| Histological type | < 0.001 | ||
| Adenocarcinoma | 36 (2.1) | 0 (0.0) | |
| Adenosquamouscarcinoma | 0 (0.0) | 6 (7.0) | |
| Others | 1663 (96.0) | 80 (93.0) | |
| Multi | 1 (0.1) | 0 (0.0) | |
| Neuroendocrine | 17 (1.0) | 0 (0.0) | |
| Stromal | 15 (0.9) | 0 (0.0) | |
| Family = yes | 32 (1.8) | 10 (11.6) | < 0.001 |
| CD34 = yes | 647 (37.4) | 67 (77.9) | < 0.001 |
| FOBT = M1 | 1276 (73.7) | 64 (74.4) | 0.978 |
| Location = others | 76 (4.4) | 2 (2.3) | 0.517 |
| Age = high | 871 (50.3) | 39 (45.3) | 0.433 |
| Neoadjuvant = yes | 142 (8.2) | 57 (66.3) | < 0.001 |
There was no significant difference in age, location, or other aspects between the AL group and NAL group (P = 0.433, P = 0.517). In the univariate analysis, significant differences (P < 0.05) were observed between the AL group and the NAL group with respect to family history, tobacco and alcohol history, CD34, T stage, preoperative and postoperative blood calcium levels, preoperative platelet count, preoperative plasma albumin and globulin levels, positive lymph node count (rLNs), and neoadjuvant therapy. Upon further multivariate analysis, significant differences were observed between the AL group and the NAL group in family history, tobacco and alcohol history, CD34, T stage, preoperative and post
We trained 10 different ML prediction models on a dataset of 1273 raw samples and a dataset of 3721 samples, which were balanced using the SMOTE (Synthetic Least Squares) method and focused on 10 key factors. The performance of each model in the test set is shown in Table 2.
| Model | Accuracy | Sensitivity/PPV | Specificity/NPV | F1 |
| Logistic.R | 0.916 | 0.98 | 0.912 | 0.521 |
| SVM.R | 0.873 | 0.76 | 0.879 | 0.355 |
| GBM.R | 0.901 | 0.96 | 0.898 | 0.471 |
| NeuralNetwork.R | 0.89 | 0.98 | 0.885 | 0.455 |
| RandomForest.R | 0.974 | 0.56 | 0.994 | 0.667 |
| XGBoost.R | 0.925 | 0.92 | 0.927 | 0.708 |
| KNN.R | 0.919 | 0.88 | 0.921 | 0.5 |
| Adaboost.R | 0.903 | 0.8 | 0.908 | 0.43 |
| LightGBM.R | 0.916 | 0.92 | 0.915 | 0.5 |
| CatBoost.R | 0.906 | 0.96 | 0.904 | 0.485 |
| Logistic.S | 0.914 | 0.98 | 0.91 | 0.515 |
| SVM.S | 0.952 | 0.92 | 0.954 | 0.639 |
| GBM.S | 0.956 | 0.96 | 0.956 | 0.667 |
| NeuralNetwork.S | 0.958 | 0.96 | 0.958 | 0.676 |
| RandomForest.S | 0.95 | 0.76 | 0.96 | 0.585 |
| XGBoost.S | 0.939 | 0.96 | 0.938 | 0.593 |
| KNN.S | 0.952 | 0.84 | 0.958 | 0.618 |
| Adaboost.S | 0.804 | 0.92 | 0.798 | 0.301 |
| LightGBM.S | 0.927 | 0.96 | 0.925 | 0.545 |
| CatBoost.S | 0.943 | 0.96 | 0.942 | 0.608 |
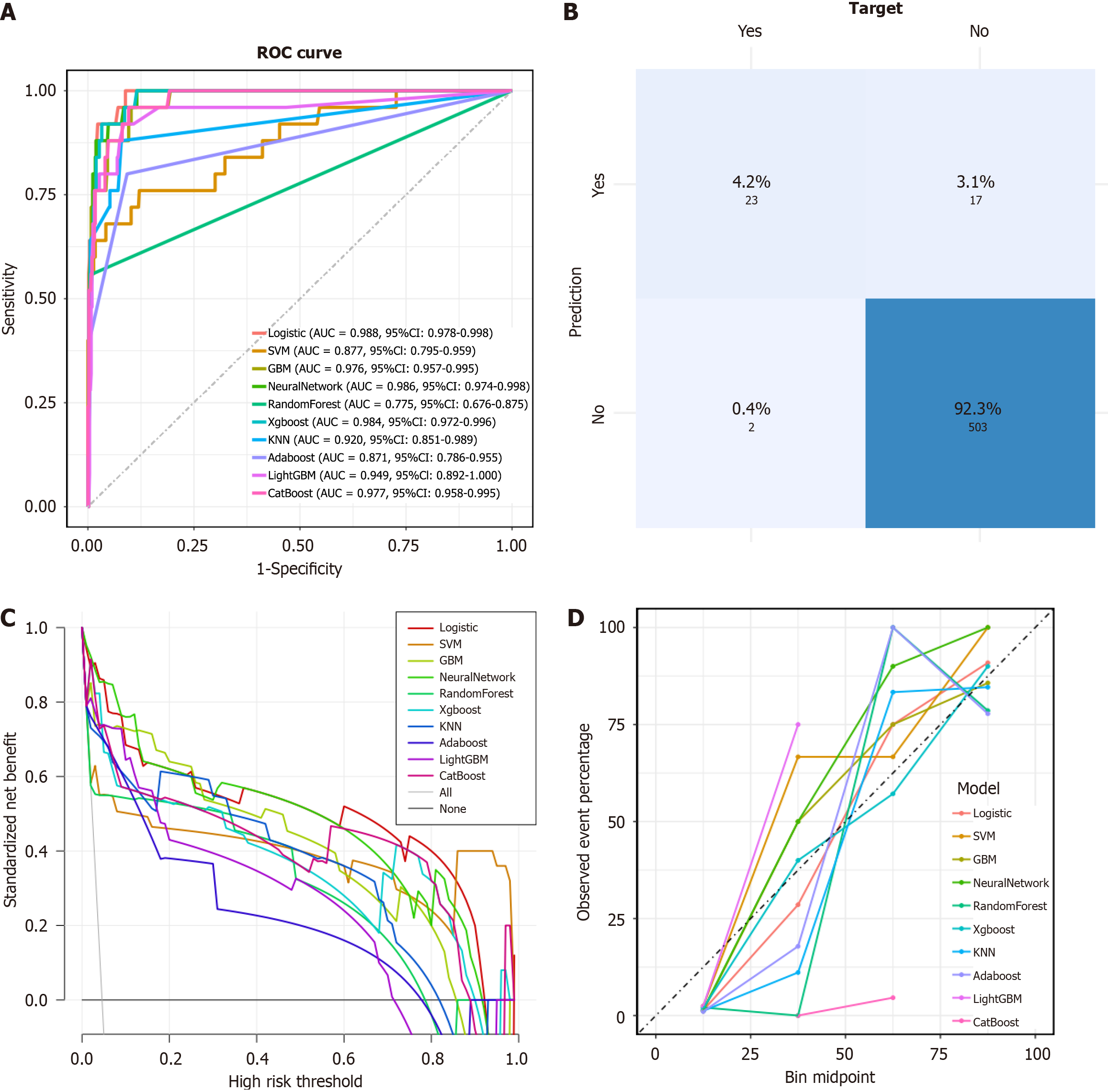
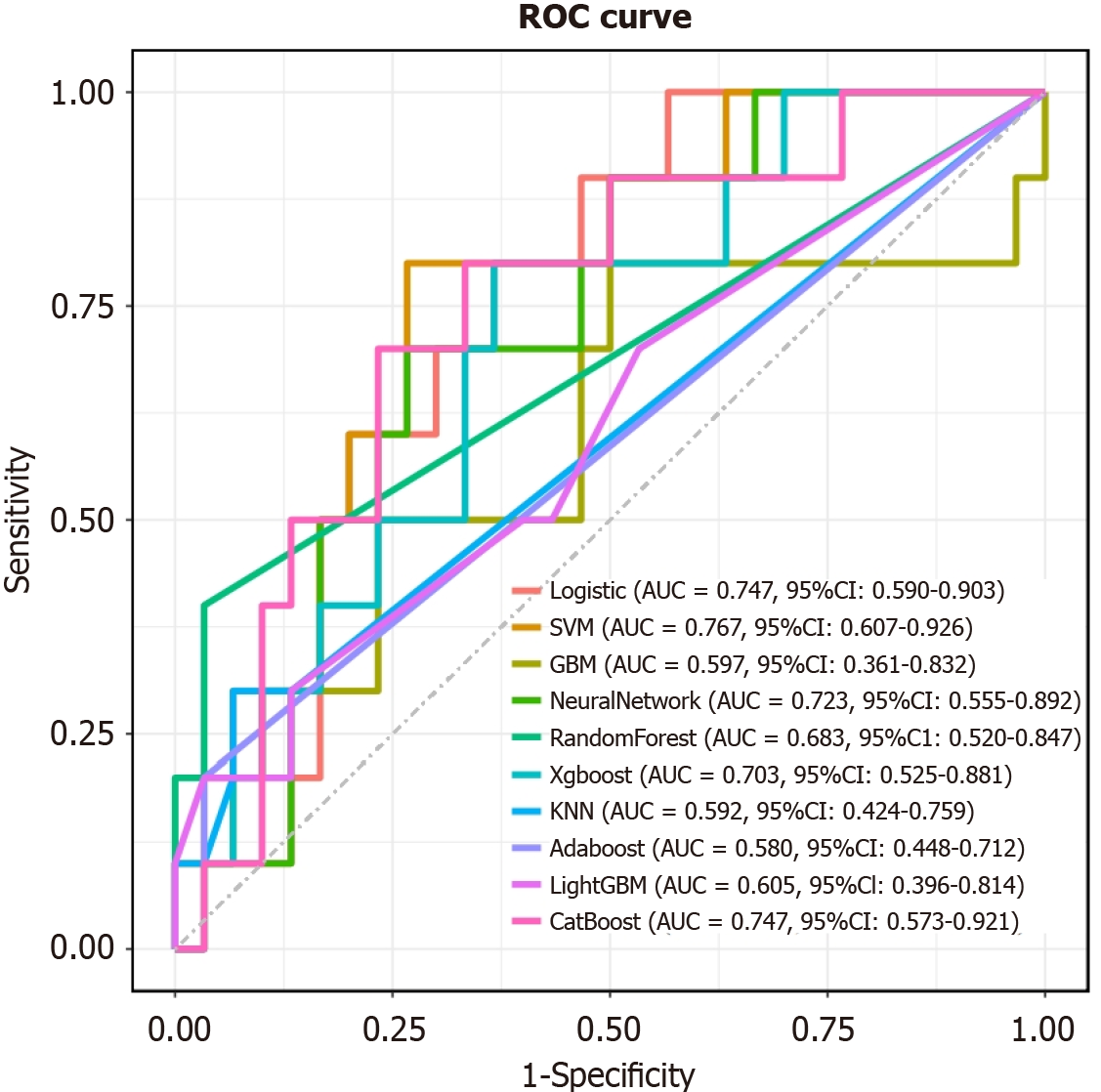
Most models demonstrated satisfactory predictive performance in terms of accuracy, sensitivity, specificity, PPV, NPV, and F1 score. When comparing the models derived from the raw dataset and the SMOTE-resampled dataset, it was observed that while the resampled group exhibited higher accuracy, the F1 score and calibration curve were inferior. The F1 score, as the harmonic mean of precision and recall, accurately shows a model's ability to predict minority classes[19]. The calibration curve, which compares predicted and actual outcomes, indicates how well the model performs in real-world scenarios[20]. When early stopping was applied, SMOTE resampling of the data led to worse F1 scores and calibration curves for AL patients. This suggests that resampling the minority data amplifies the noise and raises the risk of overfitting. XGBoost, Logistic regression, and Neural Network performed well on the ROC curve, with the highest AUC values of 0.988, 0.986 and 0.984, respectively, signifying their robust predictive capacity for AL. In terms of calibration curves and F1 scores, XGBoost outperformed the other two models, leading us to conclude that XGBoost is the optimal model in terms of performance for predicting AL occurrence, with the best PPV (0.92) and NPV (0.927). DCA is a method for evaluating the potential impact of predictive models on clinical decision-making and it assesses the clinical utility of a model by comparing the net benefits of different threshold probabilities for treatment decisions[21]. The calibration curve in predictive modeling serves as a graphical representation that delineates the agreement between the predicted probabilities and the observed outcomes, thereby assessing the model's accuracy[22]. Therefore, to further evaluate the fitting effect of the XGBoost model, we drew its DCA curve and calibration curve. The DCA curve shows that the XGBoost model can provide profitable predictive performance within a threshold probability range of around 0.05 to 0.9. Despite the presence of data imbalance, the model demonstrated good performance in calibration curves, ROC curves, and confusion matrices, indicating that it indeed possesses high predictive performance (Figures 3 and 4).
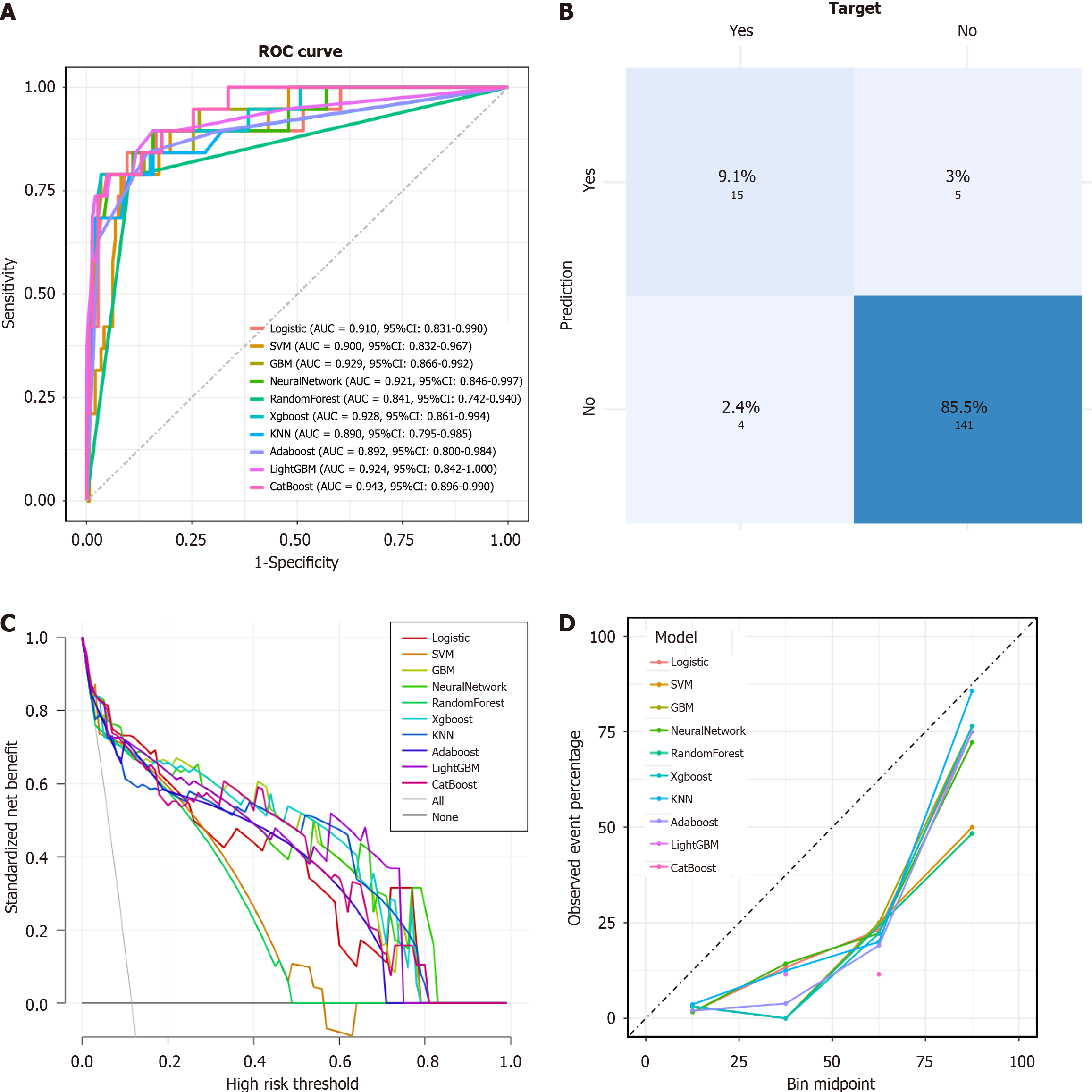
Although the model exhibited satisfactory predictive performance in the training and validation cohorts, the generalizability of findings from a single-center study is inherently constrained. To bolster the evidence for the model's predictive utility and to underscore the pivotal role of serum calcium ion concentrations, we pursued external validation. This rigorous assessment culminated in an AUC of 0.703 (95% confidence interval: 0.525 to 0.881) (Figure 5).
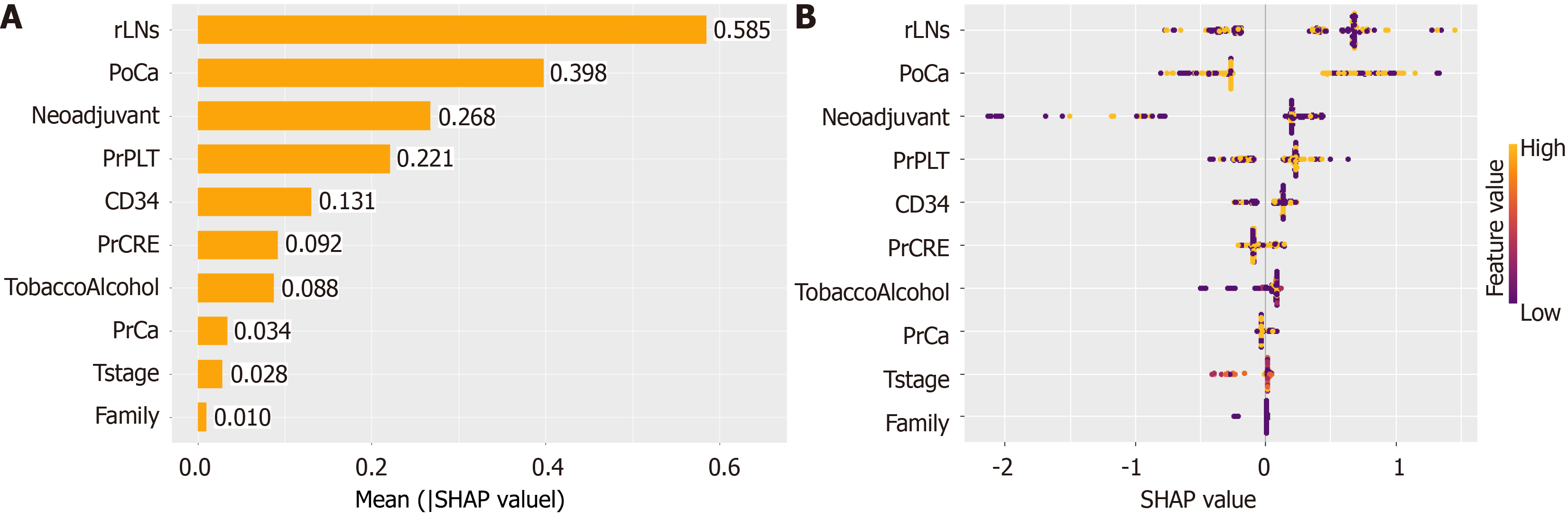
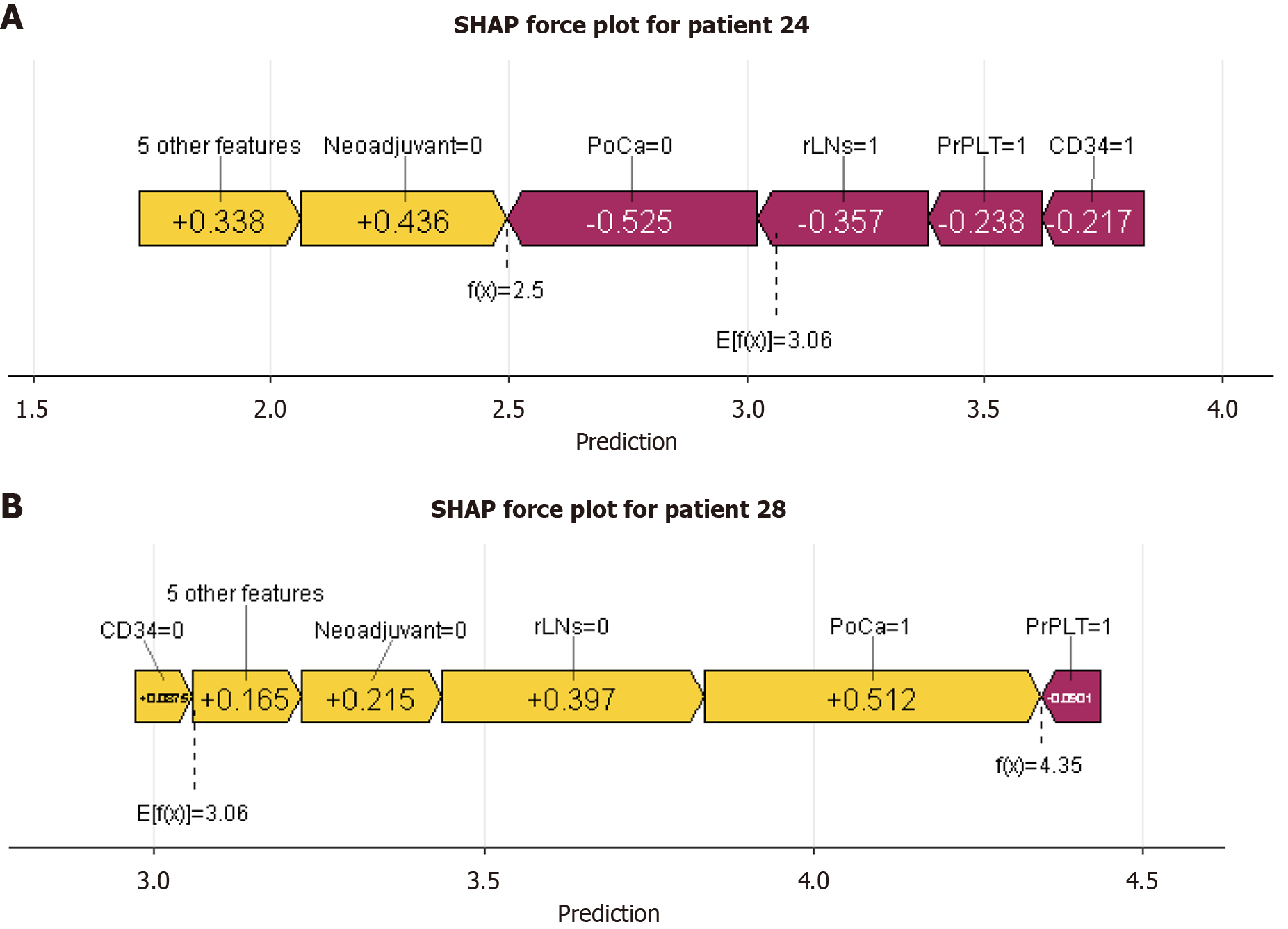
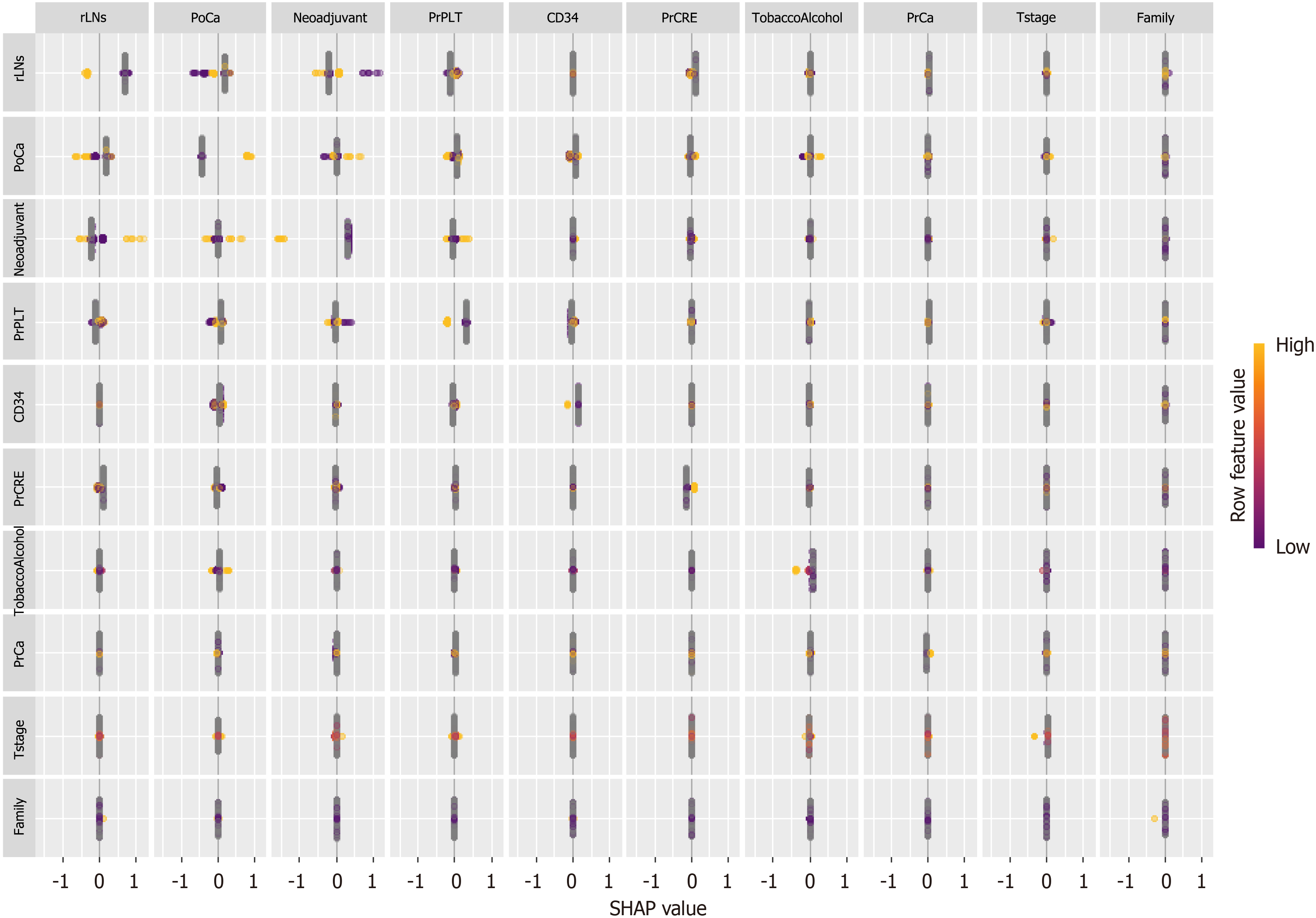
While ML models can achieve high predictive accuracy, their decision-making processes are often opaque, limiting their interpretability in clinical contexts. The application of SHAP has effectively demystified the "black box" nature of ML models, endowing them with interpretability. Through the lens of SHAP, we are able to gain a deeper comprehension of the predictive processes and, consequently, enhance the reliability of our models[23]. This approach allows for a more transparent understanding of how features contribute to the output and the individual characteristics of patients, which is crucial for the credibility and effectiveness of ML applications. In this study, we employed SHAP values to quantify the contribution of each variable to the predictive outcomes for patients (Figure 6). The greater the SHAP value, the more significant the increase in the incidence of AL that the variable was associated with. This method provided a robust framework for understanding the impact of individual factors on AL risk. Therefore, we analyzed one AL patient and one NAL patient separately (Figure 7). By using the interaction of significant factors diagram, we can clarify the effects of different factor combinations on the model and gain a deeper understanding of its behavior (Figure 8).
AL is a serious complication after anterior resection of rectal cancer, with a clinical incidence ranging from 3% to 20%, and can lead to severe mortality and poor prognosis[24]. In recent studies, AL has been confirmed to be associated with poor overall survival and disease-free survival[25]. Although new surgical methods have been recognized to be able to improve surgical outcomes[26], the occurrence of AL still poses a significant threat to patients. Therefore, reducing the occurrence of AL or intervening early in AL becomes a top priority.
In this study, to better predict the occurrence of AL and clarify the risk threshold for its development, we divided the clinical information, hematological indicators, and surgical details of 1818 patients into binary variables for analysis and external validation among 60 patients. This approach not only refined the model's predictive capabilities but also provides a clearer benchmark for clinical decision-making and perioperative care. And this study included 86 cases of AL, which is a larger number of positive cases compared to previous studies. This increased sample size of affected individuals contributes to a more accurate and reliable model. In the selection of patients, with the criterion that missing values should be less than 10%, we opted to exclude those with incomplete clinical data to ensure the most authentic dataset. To ensure data authenticity and minimize potential biases, we opted not to impute missing values and instead excluded incomplete records.
From a methodological perspective, in order to improve the accuracy of the model variables, we used three variable importance assessment methods and selected variables that are deemed significant by at least two of these methods for the construction of our ML model. Furthermore, to mitigate issues such as noise amplification and overfitting that may arise from SMOTE resampling, we constructed models using two sets of data: One before and one after SMOTE resampling. This approach enhanced the quality of the data, ensuring a more robust and reliable model. In the realm of ML, this study incorporated 10 distinct mainstream ML models. We employed a validation set to assess the models, conducting a comprehensive comparison that considered both graphical representations and data metrics. Ultimately, we determined that the XGBoost ML model demonstrated optimal performance across all assessed metrics. Moreover, this model demonstrated commendable predictive performance on imbalanced datasets as well, which aligned with the clinical reality of the low incidence of AL. Although the occurrence of AL is highly correlated with the medical environment and the surgical team at the time of surgery, the construction of this model can serve two main purposes. On the one hand, it allows for the estimation of the likelihood of an anastomotic leak in patients, guiding the medical team to pay closer attention to those at risk. On the other hand, it helps in identifying significant factors and determining clinical alert values, thereby improving patients outcomes.
From the importance ranking of features displayed by SHAP, which was used to assess the risk factors for the development of anastomotic leaks. the number of positive lymph nodes was considered the most significant prognostic factor, which was associated with tumor spread and metastasis, indicating the need for a more extensive lymph node dissection. In terms of hematological indicators, preoperative and postoperative serum calcium ion levels and preoperative platelet level were identified as the primary contributors. By synthesizing previous research and clinical experience, it is posited that both of these factors are intricately linked to the processes of infection and healing at the anastomotic site. A study in 2021 demonstrated that postoperative serum calcium ion level may be used to identify patients at risk for AL, and postoperative low serum calcium ion level can represent a risk factor for AL in digestive surgery[27]. In our study, we found that postoperative serum calcium ion levels have a greater impact on AL compared to preoperative serum calcium ion levels. On the other hand, this also highlights the importance of continuous blood calcium monitoring. In the context of coagulation, calcium ions, also known as clotting factor IV, can activate the intrinsic pathway of blood coagulation in conjunction with other coagulation factors, thereby accelerating the formation and activation of thrombin[28].
From a mechanistic perspective, existing studies have demonstrated that local calcium can modulate keratinocytes and fibroblasts, as well as contribute to the formation of the stratum corneum lipid barrier through signal transduction and gene expression[29]. Further research indicates that calcium flash (rapid calcium waves) dependent on TRP channels is involved in the early stages of wound healing, which could partially explain the poor intestinal anastomosis associated with low calcium levels[30]. Building on the aforementioned theories, researchers have utilized calcium silicate ceramics to stimulate adipose-derived stem cells, thereby promoting angiogenesis and enhancing skin wound healing, as confirmed in animal models[31]. Beyond these functions, calcium ions can also enhance the antimicrobial activity of antimicrobial dressings by damaging and destroying bacterial cell membranes, as well as by oxidizing bacterial media, which further contributes to bacterial killing[32,33]. Clinical applications bestow greater significance upon basic research, and studies on the role of calcium in wound healing have been performed in animal models[34]. Therefore, in response to the present clinical practice, we can initially maintain the normal serum calcium ion levels in patients to minimize the risk of anastomotic leaks. In the future, it may be possible to further develop calcium-rich dressings or sutures to promote the healing of anastomotic sites in patients. Preoperative systemic immune-inflammation index (SII) in patients with colorectal cancer is considered a marker for evaluating the systemic inflammatory status of patients and associated with patient prognosis[35]. SII is calculated with the formula SII = (P × N)/L, where P, N, and L refer to platelet, neutrophil, and lymphocyte counts, respectively. Therefore, we believe that elevated preoperative platelet counts may indicate a heightened systemic inflammatory response, which could increase the risk of AL in patients and reduce their prognosis.
In this research, we found that neoadjuvant therapy is a risk factor for anterior resection of rectal cancer, which is consistent with previous studies[36]. A new significant risk factor was identified: Perioperative serum calcium ion levels. Patients with preoperative serum calcium ion levels below 2.2 or postoperative serum calcium ion levels below 2.06 are considered to be at risk for AL. This finding can not only aid clinicians in identifying high-risk populations for AL, but also provide clinical alert values. We posit that calcium plays a crucial role in anastomotic healing by exerting antimicrobial effects, promoting hemostasis, and enhancing the function of keratinocytes and fibroblasts. Consequently, hypocalcemia significantly impacts the risk of AL in patients post-proctectomy for rectal cancer.
Our multicenter study has constructed an ML predictive model that exhibits superior predictive accuracy, de
However, our study was not without its limitations. Primarily, our retrospective study was inevitably subject to information bias, which included omissions and errors in data collection. Additionally, during the data processing phase, factors such as missing data also came into play. In addition, due to the limitations of our center's database, some important factors may be overlooked in model construction and multicenter validation, such as body mass index and the distance from the lower edge of the tumor to the dentate line. The lower AUC in the external validation may be attributed to the following reasons: (1) The external validation dataset is relatively small and there are differences in the baseline characteristics of patients from different centers; and (2) Variations exist in the testing conditions of hematological indicators across different centers, and during the study period, the testing techniques for hematological indicators have evolved. This omission may result in a less comprehensive model and could diminish its accuracy and stability. Finally, while this study provided an interactive, physician-friendly UI, there was still a gap before it meets clinical application standards. Therefore, we plan to develop a related app based on this model to facilitate its widespread use in clinical practice.
We have developed an ML predictive model based on perioperative serum calcium ion levels and other indices, which has shown excellent performance in predicting the occurrence of AL following anterior resection for rectal cancer. The application of SHAP has significantly enhanced the model's interpretability, playing a crucial role in both understanding the model and facilitating its clinical application. During the model construction, we have also identified the significant role of perioperative serum calcium ion and defined clinical alert values, which aids in the early warning of AL and provides prognostic information for patients.
| 1. | Thomas CE, Lin Y, Kim M, Kawaguchi ES, Qu C, Um CY, Lynch BM, Van Guelpen B, Tsilidis K, Carreras-Torres R, van Duijnhoven FJB, Sakoda LC, Campbell PT, Tian Y, Chang-Claude J, Bézieau S, Budiarto A, Palmer JR, Newcomb PA, Casey G, Le Marchandz L, Giannakis M, Li CI, Gsur A, Newton C, Obón-Santacana M, Moreno V, Vodicka P, Brenner H, Hoffmeister M, Pellatt AJ, Schoen RE, Dimou N, Murphy N, Gunter MJ, Castellví-Bel S, Figueiredo JC, Chan AT, Song M, Li L, Bishop DT, Gruber SB, Baurley JW, Bien SA, Conti DV, Huyghe JR, Kundaje A, Su YR, Wang J, Keku TO, Woods MO, Berndt SI, Chanock SJ, Tangen CM, Wolk A, Burnett-Hartman A, Wu AH, White E, Devall MA, Díez-Obrero V, Drew DA, Giovannucci E, Hidaka A, Kim AE, Lewinger JP, Morrison J, Ose J, Papadimitriou N, Pardamean B, Peoples AR, Ruiz-Narvaez EA, Shcherbina A, Stern MC, Chen X, Thomas DC, Platz EA, Gauderman WJ, Peters U, Hsu L. Characterization of Additive Gene-environment Interactions For Colorectal Cancer Risk. Epidemiology. 2025;36:126-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Lian J, Li J, Liu C, Luan B, Miao Y. Research progress of robot and laparoscope in postoperative complications of rectal cancer. J Robot Surg. 2024;18:135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | de'Angelis N, Schena CA, Azzolina D, Carra MC, Khan J, Gronnier C, Gaujoux S, Bianchi PP, Spinelli A, Rouanet P, Martínez-Pérez A, Pessaux P; Association Française de Chirurgie (AFC). Histopathological outcomes of transanal, robotic, open, and laparoscopic surgery for rectal cancer resection. A Bayesian network meta-analysis of randomized controlled trials. Eur J Surg Oncol. 2025;51:109481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; International TaTME Registry Collaborative. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg. 2019;269:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 5. | Zarnescu EC, Zarnescu NO, Costea R. Updates of Risk Factors for Anastomotic Leakage after Colorectal Surgery. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Su Y, Li Y, Zhang H, Yang W, Liu M, Luo X, Liu L. Machine learning model for prediction of permanent stoma after anterior resection of rectal cancer: A multicenter study. Eur J Surg Oncol. 2024;50:108386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Hu W, Jin T, Pan Z, Xu H, Yu L, Chen T, Zhang W, Jiang H, Yang W, Xu J, Zhu F, Dai H. An interpretable ensemble learning model facilitates early risk stratification of ischemic stroke in intensive care unit: Development and external validation of ICU-ISPM. Comput Biol Med. 2023;166:107577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262-e273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 744] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 9. | Shao S, Zhao Y, Lu Q, Liu L, Mu L, Qin J. Artificial intelligence assists surgeons' decision-making of temporary ileostomy in patients with rectal cancer who have received anterior resection. Eur J Surg Oncol. 2023;49:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 10. | Chen KA, Goffredo P, Butler LR, Joisa CU, Guillem JG, Gomez SM, Kapadia MR. Prediction of Pathologic Complete Response for Rectal Cancer Based on Pretreatment Factors Using Machine Learning. Dis Colon Rectum. 2024;67:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Stanzione A, Verde F, Romeo V, Boccadifuoco F, Mainenti PP, Maurea S. Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World J Gastroenterol. 2021;27:5306-5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (4)] |
| 12. | Liu Y, Shi J, Liu W, Tang Y, Shu X, Wang R, Chen Y, Shi X, Jin J, Li D. A deep neural network predictor to predict the sensitivity of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Cancer Lett. 2024;589:216641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Ma J, Bo Z, Zhao Z, Yang J, Yang Y, Li H, Yang Y, Wang J, Su Q, Wang J, Chen K, Yu Z, Wang Y, Chen G. Machine Learning to Predict the Response to Lenvatinib Combined with Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (1)] |
| 14. | Hu C, Li L, Huang W, Wu T, Xu Q, Liu J, Hu B. Interpretable Machine Learning for Early Prediction of Prognosis in Sepsis: A Discovery and Validation Study. Infect Dis Ther. 2022;11:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 15. | Rajkomar A, Dean J, Kohane I. Machine Learning in Medicine. N Engl J Med. 2019;380:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1894] [Article Influence: 270.6] [Reference Citation Analysis (3)] |
| 16. | Kagawa Y, Smith JJ, Fokas E, Watanabe J, Cercek A, Greten FR, Bando H, Shi Q, Garcia-Aguilar J, Romesser PB, Horvat N, Sanoff H, Hall W, Kato T, Rödel C, Dasari A, Yoshino T. Future direction of total neoadjuvant therapy for locally advanced rectal cancer. Nat Rev Gastroenterol Hepatol. 2024;21:444-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 17. | Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, Rahbari NN, Büchler MW, Weitz J; International Study Group of Rectal Cancer. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery. 2013;153:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Islam MM, Rahman MJ, Rabby MS, Alam MJ, Pollob SMAI, Ahmed NAMF, Tawabunnahar M, Roy DC, Shin J, Maniruzzaman M. Predicting the risk of diabetic retinopathy using explainable machine learning algorithms. Diabetes Metab Syndr. 2023;17:102919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 19. | Buchan K, Filannino M, Uzuner Ö. Automatic prediction of coronary artery disease from clinical narratives. J Biomed Inform. 2017;72:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Brien WF, Crawford L, Raby A, Richardson H. In-house calibration of the international sensitivity index or calibration curve for determination of the international normalized ratio. Arch Pathol Lab Med. 2004;128:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur Urol. 2018;74:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 885] [Article Influence: 110.6] [Reference Citation Analysis (2)] |
| 22. | Moosavi SM, Ghassabian S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In: Stauffer MT, editor. Calibration and Validation of Analytical Methods - A Sampling of Current Approaches London: Intech Open, 2018. [DOI] [Full Text] |
| 23. | Ladbury C, Zarinshenas R, Semwal H, Tam A, Vaidehi N, Rodin AS, Liu A, Glaser S, Salgia R, Amini A. Utilization of model-agnostic explainable artificial intelligence frameworks in oncology: a narrative review. Transl Cancer Res. 2022;11:3853-3868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Wan X, Wang G, Ren Y, Cheng Y, Zhao Y, Han G. A scoring system to predict the risk of anastomotic leakage after anterior resection for rectal cancer. J Surg Oncol. 2014;109:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse Effects of Anastomotic Leakage on Local Recurrence and Survival After Curative Anterior Resection for Rectal Cancer: A Systematic Review and Meta-analysis. World J Surg. 2017;41:277-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 26. | Abdelsamad A, Elsheikh A, Eltantawy M, Othman AM, Arif F, Atallah H, Elderiny H, Zayed H, Alshal MM, Ali MM, Elmorsi AH, Rashad S, Elagezy F, Gebauer F, Langenbach MR, Hamdy NM. A battle of surgical strategies: Clinically enlarged lateral lymph nodes in patients with locally advanced rectal cancer; extended mesorectal excision (e-TME) versus traditional surgery (TME-alone) a meta-analysis. Pathol Res Pract. 2025;269:155874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Budin C, Staniloaie D, Vasile D, Ilco A, Balan DG, Popa CC, Stiru O, Tulin A, Enyedi M, Miricescu D, Georgescu DE, Georgescu TF, Badiu DC, Mihai DA. Hypocalcemia: A possible risk factor for anastomotic leak in digestive surgery. Exp Ther Med. 2021;21:523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355-3362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle). 2014;3:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 972] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 30. | Subramaniam T, Fauzi MB, Lokanathan Y, Law JX. The Role of Calcium in Wound Healing. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 31. | Wang M, Zhan H, Wang J, Song H, Sun J, Zhao G. Calcium silicate-stimulated adipose-derived stem cells promote angiogenesis and improve skin wound healing. Aging (Albany NY). 2023;15:4746-4756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Souad G, Baghdadi C. Effect of calcium phosphate synthesis conditions on its physico-chemical properties and evaluation of its antibacterial activity. Mater Res Express. 2020;7:015040. |
| 33. | Tang NFR, Armynah B, Tahir D. Structural and optical properties of alginate-based antibacterial dressing with calcium phosphate and zinc oxide for biodegradable wound painting applications. Int J Biol Macromol. 2024;276:133996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Zhou Q, Kang H, Bielec M, Wu X, Cheng Q, Wei W, Dai H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr Polym. 2018;197:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 35. | Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 250] [Cited by in RCA: 532] [Article Influence: 59.1] [Reference Citation Analysis (12)] |
| 36. | Spinelli A, Foppa C, Carvello M, Sacchi M, De Lucia F, Clerico G, Carrano FM, Maroli A, Montorsi M, Heald RJ. Transanal Transection and Single-Stapled Anastomosis (TTSS): A comparison of anastomotic leak rates with the double-stapled technique and with transanal total mesorectal excision (TaTME) for rectal cancer. Eur J Surg Oncol. 2021;47:3123-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/