Published online Apr 21, 2025. doi: 10.3748/wjg.v31.i15.104901
Revised: February 22, 2025
Accepted: March 26, 2025
Published online: April 21, 2025
Processing time: 102 Days and 22.8 Hours
The prevalence of intrahepatic cholangiocarcinoma (ICC) is increasing globally. Despite advancements in comprehending this intricate malignancy and for
Core Tip: The conversion treatment for unresectable intrahepatic cholangiocarcinoma (ICC) can reduce tumor burden and enhance the likelihood of surgical resection. Chemotherapy is still the base treatment for advanced ICC. However, locoregional therapies and systemic therapies are promising treatment strategies for advanced ICC, aiming to enhance tumor response and improve patient outcomes. Achieving an adequate future liver remnant is crucial to prevent posthepatectomy liver failure; techniques are being investigated to enhance future liver remnant and improve outcomes in patients with advanced ICC.
- Citation: Liu JJ, Zhou M, Yuan T, Huang ZY, Zhang ZY. Conversion treatment for advanced intrahepatic cholangiocarcinoma: Opportunities and challenges. World J Gastroenterol 2025; 31(15): 104901
- URL: https://www.wjgnet.com/1007-9327/full/v31/i15/104901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i15.104901
Intrahepatic cholangiocarcinoma (ICC) ranks as the second most prevalent primary liver cancer after hepatocellular carcinoma (HCC), constituting approximately 10%-15% of primary liver cancers[1]. Chronic inflammation typically occurs when ICC develops, leading to cholestasis and subsequent cholangiocyte injury[2]. The numerous risk factors for ICC include bile duct cysts, cholangitis, Caroli’s disease, hepatolithiasis, cirrhosis, viral hepatitis, parasitic infection, digestive diseases, and metabolic and endocrine disorders, among others[3]. As the main risk factors for ICC have become more common over the past few decades, mortality from this disease has tended to grow in a number of areas across the world[4].
Surgical treatment is the most effective approach for achieving long-term survival in patients with cholangiocarcinoma. The patient should undergo surgery if radical resection of cholangiocarcinoma is feasible and if there are no distant metastases or other surgical contraindications[5]. However, cholangiocarcinoma is typically asymptomatic in the initial stages, leading to a diagnosis only when the disease has progressed significantly, severely limiting therapy options and resulting in a poor outcome[6,7]. Only 20%-30% of patients have resectable disease[8]. Even in cases where surgical resection is performed with the goal of curing the disease, the 5-year overall survival (OS) rate is only 20%-35%[2].
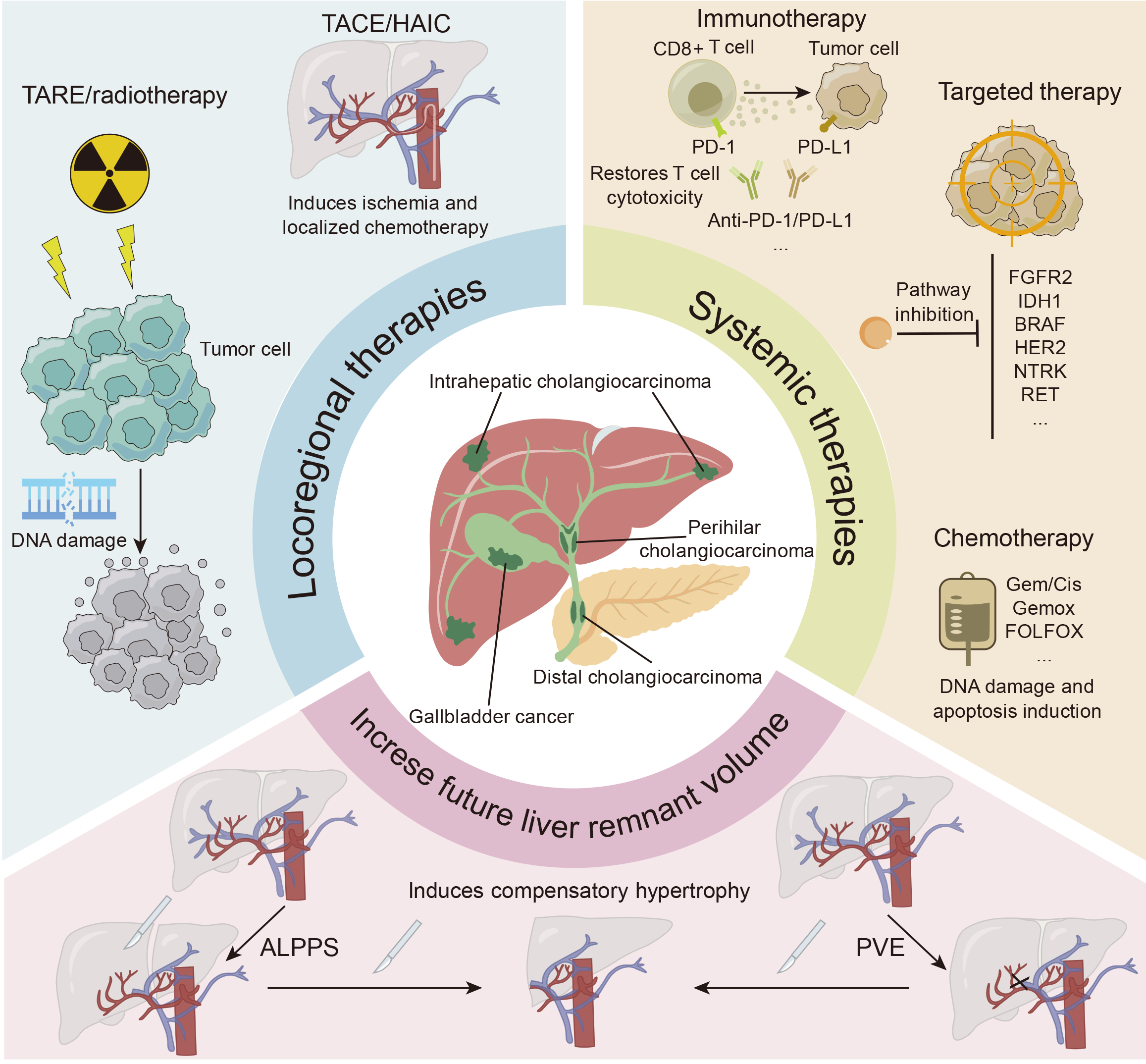
Nonsurgical therapy for ICC has advanced significantly in recent years. Chemotherapy, targeted therapy, immunotherapy, and their combination might yield favorable outcomes in the management of advanced or unresectable ICC; furthermore, localized interventions such as transarterial chemoembolization (TACE), hepatic arterial infusion (HAI) chemotherapy (HAIC), transarterial radioembolization (TARE), and radiotherapy are frequently utilized. Advancements in technology and pharmaceuticals, coupled with systemic therapies, not only yield superior outcomes in terms of tumor reduction but also increase patient survival. Conversion treatment is the process of transforming an unresectable ICC into a resectable ICC and then surgically excising the tumor. Several studies have shown that conversion treatment, which involves the use of systemic or local therapies, can increase the percentage of patients suitable for curative resection, hence considerably improving their long-term survival rates[9-13].
As methods of treatment improve, future research will focus mostly on identifying the most efficient conversion treatment procedures for advanced ICC, particularly to determine which patients are most likely to benefit from this strategy. A unified definition of conversion treatment and explicit criteria for patient selection are essential for the advancement of this therapeutic technique and the assurance of consistent clinical outcomes.
The conversion treatment method is a novel approach for unresectable malignancies that was originally suggested for HCC patients and aims to reduce the tumor burden by applying combination therapy, thereby making patients appropriate for surgical resection[14,15]. In recent years, conversion treatment has received increasing interest in the field of ICC, as increasing amounts of data indicate that multimodal strategies for treatment can enhance resectability and patient outcomes. In contrast to HCC, where conversion treatment is well established, ICC has historically exhibited low conversion rates; however, advancements in systemic and locoregional therapies are progressively altering this approach. A study showed that patients with advanced ICC who undergo surgical resection following successful conversion treatment have a two-year survival rate of 88%, with a median survival of 46 months[16].
In certain instances, radical excision may be unfeasible because of the advanced stage of the tumor and its infiltration into hepatic blood vessels[13]. Nonetheless, some patients may have the chance to undergo radical resection via conversion treatment to achieve tumor downstaging. Given the rapid development of antitumor therapies, a combination therapy approach may lead to tumor downstaging in patients with unresectable ICC, increase the likelihood of radical surgical resection, and prolong patient survival. The optimal conversion treatment results in a high objective response rate (ORR), minimal harmful effects on patients, and the ability to achieve conversion as rapidly as possible. In addition to improving survival, conversion treatment can also offer symptomatic relief by diminishing the tumor burden, potentially alleviating biliary obstruction, discomfort, and other symptoms related to ICC. Thus, this may result in an increase in quality of life, even when complete resection is not possible.
Conversion treatment typically involves the application of active treatment modalities (such as targeted therapy, immunotherapy, etc.) to manage or reverse tumor proliferation, which has significant potential for a cure or prolonged response. In contrast, palliative care primarily aims to alleviate symptoms and enhance quality of life[17]. It includes interventions such as percutaneous transhepatic biliary drainage and biliary stenting. It is typically employed for individuals with advanced ICC and has poor treatment efficacy on the tumor itself.
There are currently no clear criteria for the indications for conversion treatment in ICC. Some patients with advanced ICC who are unsuitable for surgical resection due to inadequate residual liver volume, impaired liver function, and other factors might be included in conversion treatment. The following section outlines the significant breakthroughs in locoregional and systemic as well as combination therapies for the conversion treatment of ICC.
In 2010, the ABC-02 study recommended the gemcitabine + cisplatin (GC) regimen as a first-line treatment option for advanced cholangiocarcinoma and reported an ORR of 26.1%[18]. In another phase III clinical study (KHBO1401-MITSUBA), the ORR for the GC regimen group in patients with advanced cholangiocarcinoma was 15%, with no instances of successful conversion treatment reported. In the GC + S-1 regimen, the ORR was 41.5%, with three patients successfully converted to surgical resection[19]. In addition to the GC regimen, the combination of gemcitabine and oxaliplatin (Gemox) is another therapy recommended by the National Comprehensive Cancer Network guidelines for advanced cholangiocarcinoma[20]. Despite the absence of a randomized trial evaluating the efficacy of the Gemox regimen against the GC regimen, Gemox was favored as a standard regimen in certain areas and was utilized as a reference regimen in multiple clinical trials for cholangiocarcinoma[21].
A retrospective study assessed the efficacy of neoadjuvant chemotherapy in managing initially unresectable ICC. Among the 74 advanced ICC patients, 39 (53%) successfully converted and underwent further resection following a median of six cycles of treatment[22]. The results revealed that individuals with locally advanced ICC who underwent surgical intervention after neoadjuvant chemotherapy presented comparable outcomes to those with initially resectable ICC who received surgery only[22]. A GC regimen combined with nab-paclitaxel was studied in a clinical trial. The survival results were encouraging, with a median OS of 19.2 months[23]. Twelve patients (20%) were successfully converted from unresectable to resectable and subsequently underwent surgery[23].
Owing to the different radiosensitivities of hepatic tissue and the limitations of radiotherapy technology, radiotherapy is infrequently utilized for the management of liver cancer. Nonetheless, advancements in imaging and radiation technologies, together with an enhanced comprehension of the radiation tolerance of normal liver tissue, have prompted an increasing number of researchers to use radiotherapy for the treatment of liver cancer[24,25]. The treatment effects observed in contemporary research exhibit considerable variability[26-28], potentially attributable to disparities in radiotherapy methods and study cohorts.
A retrospective analysis involved 79 patients with unresectable ICC, 70 of whom underwent systemic chemotherapy prior to radiation. The overall 3-year survival rate for the total group was 44%. Patients receiving a biologically equivalent radiation dose exceeding 80.5 Gy had a superior survival rate of 73% compared with 38%, and the local control (LC) rates were 78% and 45%, respectively[29]. Another phase II study evaluated the application of proton radiation in 92 patients with localized, unresectable ICC or HCC. Patients underwent 15 portions of proton treatment, culminating in a maximum cumulative dosage of 67.5 Gy equivalent. The ICC patients had a median tumor size of 6 cm, with 25% having more than one tumor and 30% having vascular thrombosis. The two-year LC and OS rates in the ICC group were 94% and 47%, respectively[30]. Data from preclinical and clinical studies have shown that radiation therapy enhances T cell infiltration, increases the quantity of lymphocytes that infiltrate tumors, and broadens the repertoire of T cell receptors[31]. This could serve as a potential method to augment the efficacy of immunotherapy, offering a combination therapy alternative for the conversion treatment of unresectable ICC.
TACE is a therapeutic approach for people with locally advanced malignancies that aims to administer locally high concentrations of chemotherapy to induce tumor ischemia[32,33]. TACE has been utilized in multiple liver malignancies, including ICC. In this method, the hepatic artery supplying blood to the tumor is accessible, typically through femoral artery access, and identified via conventional angiography[34]. TACE is performed by administering an emulsion containing chemotherapy drugs and an oily contrast medium such as lipiodol conventional TACE or special particles combined with chemotherapy drugs (drug-eluting bead-TACE). The chemotherapy drugs mixed with the oil-based contrast agent are injected into the hepatic artery during conventional TACE. The artery is subsequently embolized with embolic material, cutting off the tumor’s blood supply[35]. Common chemotherapy medications include cisplatin, mitomycin C, and doxorubicin[36]. However, the high liquidity of lipiodol leads to systemic toxicity. Consequently, alternative interventional embolic materials, including drug-eluting beads, have been studied to increase drug loading and release in TACE therapy, referred to as drug-eluting bead-TACE[37].
Compared with HCC, ICC has a limited arterial blood supply, making it challenging for TACE to achieve optimal embolization efficacy. The OS following TACE is limited, with considerable variation in patient selection, sample size, and research methodology across different studies. Overall, the median OS reported in various studies ranged from 6 to 21 months[37-43]. It has been reported that when combined with the GC regimen, the ORR of D-TACE can reach 68%, enabling the effective conversion of 25% of patients into operable patients. The median OS of the combined treatment group was 33.7 months[44].
The technique used in TARE parallels that utilized in TACE. This method uses microspheres tagged with the β-emitter yttrium-90 (Y-90) to direct radiation onto inoperable primary or metastatic liver cancers[45]. Owing to the preferential entrapment of Y-90 microspheres in tumor blood vessels, substantial doses of radiation can be delivered to the tumor while presenting acceptable radiation levels for adjacent normal liver tissue[46].
Mouli et al[47] reported that Y-90 radioembolization for unresectable ICC resulted in an overall objective tumor response in 98% of patients; the mean tumor shrinkage rate was 35%. According to the World Health Organization standards, 11 patients (25%) achieved a partial response, 33 patients (73%) demonstrated stable disease, and 1 patient (2%) experienced increasing disease. Five patients (11%) were downstaged to a resectable condition following treatment and successfully underwent R0 resection[47]. Similarly, in another study combining GC chemotherapy and Y-90 microspheres, 9 patients (22%) were able to be downstaged to surgery, and 8 of them (20%) successfully underwent R0 surgical resection, with a median OS of 46 months after surgery[16].
In previous studies on TARE, tumor size or load has typically not been regarded as an exclusion criterion. Several studies have included patients with tumors larger than 5 cm or exhibiting multifocal intrahepatic disease[48,49]. The inclusion of patients with significant tumor burdens indicates that TARE may serve as a feasible therapeutic alternative for conversion treatment in ICC. TARE may effectively reduce tumor size and enhance resectability, serving as a bridge to curative surgery for patients with originally unresectable ICC. Further research on TARE for the treatment of ICC can elucidate more patient tumor characteristics and identify more suitable treatment populations to improve treatment outcomes.
Compared with systemic chemotherapy, HAIC aims to provide substantial quantities of chemotherapeutic agents into the hepatic circulation, resulting in a more prominent local cytotoxic effect[50]. In HAIC, frequently utilized chemotherapeutic agents include floxuridine, gemcitabine, oxaliplatin, cisplatin, and their combinations[51-54]. Tumor size is not a contraindication for HAIC treatment, and certain trials have incorporated patients with tumors exceeding 10 cm in diameter[53,55].
A phase II clinical trial assessed the efficacy of HAI of floxuridine in conjunction with systemic chemotherapy for the treatment of 38 patients with unresectable ICC. The 6-month progression free survival (PFS) rate was 84.1%, 22 patients (58%) achieved a partial response, 32 patients (84%) achieved disease control, and 4 patients were successfully converted to surgical resection, with one achieving complete response[53]. In another study of HAI with gemcitabine in conjunction with oxaliplatin for the treatment of locally advanced ICC, 2 out of 12 patients achieved a partial response and underwent R0 resection surgery[56]. A study evaluated the therapeutic efficacy of HAIC vs first-line systemic chemotherapy for ICC, revealing superior outcomes for patients in the HAIC cohort compared with those receiving systemic chemotherapy[57]. In the subgroup analysis, patients with single tumors appeared to benefit from the consideration of HAIC for OS and PFS. These findings indicate that HAIC may exhibit superior efficacy in patients with early unresectable ICC. Nonetheless, additional prospective and randomized investigations are needed to validate this conclusion.
Targeted therapy is a treatment approach that has developed dramatically over the past decade. The mechanism of action involves inhibiting the signal transduction pathway during tumor proliferation by binding to the specific targets via chemical compounds, thereby inducing apoptosis in tumor cells, ultimately facilitating tumor stage reduction, and increasing the likelihood of surgical resection. In targeted therapy, key oncogenic targets in ICC tumor cells have been identified, and drugs to inhibit these targets have been developed. Mutations in genetic targets can be detected in nearly 40% of cholangiocarcinoma patients[58]. Common mutations may vary depending on where the tumor occurs. Mutations in isocitrate dehydrogenase-1 (IDH1) and rearrangements of fibroblast growth factor receptor 2 (FGFR2) are predominantly associated with ICC, whereas mutations in TP53 and KRAS, together with ERBB2 amplifications, are more frequently observed in extrahepatic cholangiocarcinoma (ECC)[21,59]. Mutations in the BAP1 and neurotropic tyrosine kinase receptor (NTRK) genes are commonly observed in ICC, whereas ECC typically shows abnormalities in the ELF3 and ARID1B genes, along with fusions of PRKACA and PRKACB[60-62].
FGFR abnormalities (fusion, mutation, and amplification) are crucial in the development of ICC; however, the mutation rate is low in ECC and gallbladder carcinoma[63]. Mutations in FGFR can result in dysregulated FGFR signaling, which may promote cancer by increasing cell proliferation, invasion, and angiogenesis[64]. Therapeutics targeting FGFR2 and its gene family have demonstrated significant potential in the treatment of ICC[65]. Pemigatinib, an inhibitor of FGFR1-3, has obtained Food and Drug Administration approval for the management of advanced cholangiocarcinoma[66]. Among patients with advanced cholangiocarcinoma who had FGFR2 rearrangements and fusion mutations, pemigatinib had an ORR of 35.5% in the FIGHT-202 study; 98% of these patients had ICC. Furthermore, among 107 advanced cholangiocarcinoma patients, two individuals who experienced tumor downstaging underwent effective surgical intervention, with a conversion rate of 1.8% and an average survival duration of 17.8 months[67].
IDH1 mutations are prevalent in cholangiocarcinoma, and occur more commonly in ICC than in ECC[68]. IDH1 is a member of the IDH protein family. Mutant IDH enzymes exhibit neomorphic activity, catalyzing the conversion of the physiological metabolite α-ketoglutarate to 2-hydroxyglutarate. 2-hydroxyglutarate functions as an oncometabolite, and its accumulation induces multiple epigenetic alterations[69]. Ivosidenib, a targeted agent for IDH1 mutations, prolongs OS among patients with advanced cholangiocarcinoma with IDH1 mutations[70]. In the ClarIDHy trial, patients with advanced cholangiocarcinoma who received ivosidenib exhibited a median OS of 10.3 months[71]. The findings of the trial supported the approval of this drug for clinical use[71].
The overexpression of human epidermal growth factor receptor 2 (HER2) is more prevalent in ECC (19.9%) than in ICC (4.8%)[72]. The overexpression of HER2 proteins promotes the formation of homodimers or heterodimers. Consequently, development and proliferation are predominantly facilitated by the mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways[73]. A phase II clinical trial (KCSG-HB19-14) demonstrated that trastuzumab in combination with oxaliplatin, leucovorin, and 5-fluorouracil (FOLFOX) chemotherapy resulted in an ORR of 29.4% in patients with HER2-positive advanced cholangiocarcinoma who had previously failed GC chemotherapy, with a median OS of 10.7 months and no reported cases of successful conversion[74].
The incidence of NTRK gene fusion in cholangiocarcinoma is less than 1%, and it is not commonly used as a regular screening marker[75]. However, some clinical trials have shown positive outcomes. Entrectinib and larotrectinib are NTRK inhibitors that have demonstrated success in treating patients with advanced malignancies that are NTRK fusion positive. Among patients with systemic treatment-treated NTRK fusion-positive advanced malignancies, those treated with entrectinib had an ORR of 57%[76]. Another clinical trial of larotrectinib for the treatment of NTRK fusion-positive malignancies reported an ORR of 75%[77].
In addition to single-target inhibitors, multitarget inhibitors have also shown efficacy in the treatment of ICC. Lenvatinib is a multitarget inhibitor of tyrosine kinase receptors that targets FGFR1-4, vascular endothelial growth factor receptor 1-3, and platelet-derived growth factors receptor-α, among others[78]. A phase II clinical trial demonstrated that lenvatinib monotherapy as a second-line treatment for advanced cholangiocarcinoma can achieve an ORR of 11.5% and a median survival duration of 7.35 months[79]. In 2021, the American Society of Clinical Oncology Annual Meeting reported a phase II clinical trial (NCT03951597) assessing the treatment of advanced ICC with lenvatinib combined with toripalimab and Gemox chemotherapy. In patients who completed the follow-up, the ORR was 80%, and the disease control rate (DCR) was 93.3%. Three patients were successfully converted and received surgical therapy[80]. Given the high ORR of 80%, it is important to interpret these results with caution. The limitations of this investigation, including sample size, bias in patient selection, treatment regimen variability, and study design, should be noted. In summary, while the high ORR is encouraging, it is crucial to emphasize that further studies are needed to confirm these findings in a broader patient population and with more standardized treatment protocols. For patients with ICC, the use of targeted therapies has new potential for conversion treatment. Many targeted therapies are in basic or clinical research, with several demonstrating promising antitumor efficacy; however, additional multicenter clinical trials are necessary to provide more reliable clinical evidence.
Immunotherapy has been investigated for ICC as a significant treatment option, and positive outcomes have been reported. As research on tumor immunity has advanced, an increasing number of studies have focused on programmed cell death 1 (PD-1), its ligand (PD-L1) inhibitors, and adoptive cell therapy (ACT). Research indicates that PD-L1 expression is increased in ICC tumor tissues, suggesting that PD-1/PD-L1 inhibitors could function as effective immunotherapies for ICC patients.
A clinical study of nivolumab performed a subgroup analysis of PD-L1 expression levels to evaluate its impact on median PFS. The findings indicated that patients exhibiting PD-L1 positivity (characterized by ≥ 1% of tumor cells exhibiting PD-L1 expression) experienced prolonged PFS[81]. Compared with chemotherapy alone, the findings of the TOPAZ-1 study indicated that the combination of durvalumab with GC enhanced OS, PFS, and the ORR in patients with unresectable advanced cholangiocarcinoma. The median OS for patients receiving combination therapy was 12.9 months, whereas it was 11.3 months for those receiving chemotherapy[82]. In another clinical trial, KEYNOTE-966, which had a median follow-up duration of 36.6 months, the median OS for the pembrolizumab cohort was 12.7 months, whereas it was 10.9 months for the placebo cohort[83]. The TOPAZ-1 and KEYNOTE-966 clinical trials confirmed the efficacy of immune checkpoint inhibitors targeting PD-1 and PD-L1 when combined with chemotherapy for advanced cholangiocarcinoma treatment. However, the variability in treatment protocols across studies, including differences in drug doses, treatment durations, and combination regimens, can impact the comparability and reliability of these results.
Early clinical results have been encouraging for ACT, a treatment based on the engineering and isolation of living T cells along with other immune cells[84]. This form of therapy has been extensively utilized in melanoma[85]. Kverneland et al[86] utilized ACT to treat three patients with advanced cholangiocarcinoma; one of the patients achieved a partial response. Furthermore, several reports exist regarding chimeric antigen receptor-modified T cell therapy for cholangiocarcinoma. A study targeting epidermal growth factor receptor (EGFR) in advanced cholangiocarcinoma included 19 patients with unresectable biliary system malignancies exhibiting high EGFR positivity (> 50% of cancer cells expressed EGFR). The findings indicated that one patient achieved a complete response, whereas the disease remained stable in 10 patients, yielding a median PFS of 4 months[87]. In recent years, ACT has demonstrated efficacy in various tumor treatments through optimization and improvement. However, its potential pivotal role in ICC conversion treatment requires further fundamental and clinical investigation.
Posthepatectomy liver failure (PHLF) following major liver resection is linked to a high mortality rate. An adequate future liver remnant (FLR) is a critical element in reducing the risk of PHLF[88]. The method of promoting an FLR increase prior to tumor resection is a prevalent approach for facilitating conversion treatment in patients with FLR deficiency. A study revealed that an FLR less than 25% triples the likelihood of postoperative hepatic dysfunction and serves as a predictor of morbidity and duration of hospitalization. Ninety percent of patients who underwent trisectionectomy with an FLR of 25% or less exhibited postoperative hepatic dysfunction, while none of those with an FLR over 25% did[89].
By encouraging FLR hypertrophy, causing shrinkage of the liver volume intended for resection, and refining patient selection, portal vein embolization (PVE) lowers the risk of major hepatectomy[90]. The duration necessary for the remnant liver to regenerate post-PVE is typically prolonged (approximately 4 to 6 weeks), and more than 20% of patients ultimately forfeit the opportunity to have surgery because of tumor advancement or inadequate FLR proliferation during the interim period[91,92]. Current treatment techniques for these patients include combined TACE, hepatic vein embolization, and other methods to increase FLR growth and control tumor development[93,94]. Presently, PVE is infrequently used in the management of cholangiocarcinoma[95,96], and additional clinical trials are needed to investigate its value in conversion treatment. Some experts suggest that PVE is particularly crucial when the FLR is projected to be less than 25% of the whole hepatic volume in a healthy liver, less than 30% in cases of chemotherapy-induced liver damage, or less than 40% in a liver weakened by underlying cirrhosis. However, PVE must be administered cautiously to patients with severe liver cirrhosis, older patients, and rapidly progressing tumors.
To prevent PHLF, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) have been implemented to promote hypertrophy of the FLR[97,98]. This novel approach was swiftly embraced by hepatobiliary surgery for managing advanced liver tumors owing to its extremely high R0 resection rate[99,100]. A propensity score matching analysis demonstrated a significantly greater OS for patients with locally advanced ICC in the ALPPS group than for those receiving palliative chemotherapy[101]. Similarly, to validate the use of ALLPS in conversion treatment, further research with a greater degree of evidence is necessary.
The immunogenic subtype of ICC is correlated with increased sensitivity to immune checkpoint inhibitors, indicating that antigen-presenting cells may play a role in T cell priming and activation[102]. A recent preclinical study revealed that CD40-mediated activation of antigen-presenting cells in ICC markedly improved the efficacy of anti-PD-1 therapy, offering a novel approach for combination immunotherapy[103]. Photodynamic therapy is an advanced treatment that involves the intravenous delivery of a photosensitizing chemical, which is then activated by light exposure at a certain wavelength, leading to ischemic necrosis[104]. Many researchers have investigated its ability to reshape the tumor environment and efficiently stimulate antitumor immunity[105,106]. Thymosin alpha 1 is endogenously present in the thymus and is essential for T cell maturation and differentiation[107]. Recent research has indicated that thymosin alpha 1 could markedly increase OS in HCC patients[108]. Its potential involvement in the conversion treatment of ICC warrants further investigation by researchers.
Endoluminal radiofrequency ablation (RFA) has emerged as a novel therapeutic in the past decade. A radiofrequency catheter is inserted into the bile duct during endoscopy. RFA can diminish the tumor burden by inducing localized tumor damage and may also contribute to the modulation of tumor immunity, hence offering survival advantages for patients who are ineligible for radical surgery[109]. Nonetheless, the impact of RFA on survival remains contentious[110]. In addition, studies have explored the role of microwave ablation in the treatment of advanced ICC[111,112]. To standardize therapy options and improve patient selection, more prospective trials are needed.
Combination therapy is an excellent approach that enhances efficacy, significantly surpassing that of monotherapy. The combination of many therapies can augment the immunogenicity of tumor cells and modify the tumor environment, yielding more promising outcomes. Researchers have recently assessed the safety and clinical efficacy of lenvatinib combined with durvalumab and FOLFOX-HAIC among individuals with unresectable ICC. The findings indicated that the ORR according to the mRECIST criteria was 65.2%, the median PFS was 11.9 months, and 3 patients (13%) demon
| Study design | Intervention | Patients, n | Conversion treatment rate (%) | Key findings | Ref. |
| Retrospective study | Lenvatinib + durvalumab + FOLFOX-HAIC | 23 | 13 | Lenvatinib + durvalumab + FOLFOX-HAIC showed high ORR (65.2% mRECIST, 39.1% RECIST 11), with a median OS of 17.9 months and PFS of 11.9 months, supporting its potential as a first-line option for unresectable ICC | Zhao et al[113], 2024 |
| Retrospective study | GC chemotherapy vs GC chemotherapy + PD-1 inhibitors vs GC chemotherapy + lenvatinib + PD-1 inhibitors | 22 vs 20 vs 53 | 0 vs 0 vs 3.8 | The triple-regimen group had the longest OS (39.6 months), significantly exceeding the dual-regimen (OS = 20.8 months) and chemo-only groups (OS = 13.1 months). ORR was 18.2% (chemo), 55.5% (dual), and 54.7% (triple), indicating superior efficacy of combination therapy for advanced ICC | Dong et al[114], 2024 |
| Retrospective study | Gemox-HAIC + Gem-SYS combined with lenvatinib and PD-1 inhibitor | 21 | 19 | Gemox-HAIC plus Gem-SYS with lenvatinib + PD-1 inhibitor achieved a median OS of 19.5 months in large unresectable ICC. ORR was 52.3%. The regimen was well tolerated, with no grade 5 AEs | Ni et all[128], 2024 |
| Retrospective study | Systemic chemotherapy vs systemic chemotherapy + PD-L1 inhibitors vs HAIC + lenvatinib + PD-L1 inhibitors | 50 vs 49 vs 42 | 0 vs 2 vs 9.5 | ORR (50.0%) and DCR (88.1%) were highest in the HAIC + lenvatinib + PD-L1 inhibitor group, surpassing systemic chemotherapy alone (ORR = 6.0%, DCR = 52.0%) and systemic chemotherapy + PD-L1 inhibitor (ORR = 18.4%, DCR = 73.5%). Fewer grade 3-4 AEs were reported in the HAIC + lenvatinib + PD-L1 inhibitor group, supporting its superiority over systemic chemotherapy alone for unresectable ICC | Lin et al[129], 2024 |
| Retrospective study | Chemotherapy vs chemotherapy + PD-1/L1 | 30 vs 51 | 0 vs 5.9 | The chemotherapy + anti-PD-1/PD-L1 group had significantly longer OS (11 months vs 8 months) than chemotherapy alone. ORR (29.4%) and DCR (78.4%) were also higher compared to chemotherapy alone (ORR = 13.3%, DCR = 73.3%), supporting its superior efficacy | Madzikatire et al[130], 2024 |
| Retrospective study | Radiotherapy vs EQD2 < 60 Gy + GC chemotherapy vs EQD2 ≥ 60 Gy + GC chemotherapy | 21 vs 70 vs 25 | 0 vs 8.6 vs 28 | Patients receiving EQD2 ≥ 60 Gy + chemotherapy had the highest curative resection rate (28%) and significantly better OS than those receiving lower-dose radiotherapy or radiotherapy alone. These findings suggest that high-dose radiotherapy combined with chemotherapy improves outcomes in locally advanced unresectable ICC | Im et al[131], 2024 |
| Retrospective study | SIRT using yttrium-90 | 28 | 34.5 | SIRT for localized and locally advanced ICCA achieved a radiologic response rate of 57.1%, with a median OS of 22.9 months. 34.5% of patients were successfully downstaged to surgery or transplant, leading to significantly longer OS, supporting SIRT as an effective treatment option for advanced ICC | Yu et al[48], 2024 |
| Retrospective study | GC chemotherapy vs HAIP chemotherapy | 76 vs 192 | 1.3 vs 6.8 | HAIP chemotherapy significantly improved survival in liver-confined unresectable ICCA compared to systemic chemotherapy. Median OS was 27.7 months with HAIP vs 11.8 months with GC chemotherapy | Franssen et al[132], 2024 |
| Retrospective study | PD-1 inhibitors + lenvatinib + Gemox chemotherapy | 53 | 11.3 | PD-1 inhibitor + lenvatinib + Gemox chemotherapy showed a median OS of 14.3 months in advanced ICC. ORR was 52.8% and DCR was 94.3%, demonstrating high anti-tumor activity. Tumor burden score, TNM stage, and PD-L1 expression were identified as independent prognostic factors for survival | Zhu et al[133], 2023 |
| Phase 2 clinical trial | Toripalimab + lenvatinib + Gemox chemotherapy | 30 | 10 | Toripalimab + lenvatinib + Gemox achieved an ORR of 80% and a DCR of 93.3% in advanced ICC. Median OS was 22.5 months, and PFS was 10.2 months. Patients with PD-L1 positivity (≥ 1%) showed a trend toward improved response | Shi et al[134], 2023 |
| Retrospective study | Yttrium-90 + gemcitabine, cisplatin, and capecitabine | 13 | 53.8 | Yttrium-90 TARE combined with gemcitabine, cisplatin, and capecitabine achieved a median OS of 29 months and PFS of 13 months in locally advanced ICC. 53.8% of patients were downstaged to surgery, leading to significantly improved OS. Complete and partial responses were observed in 38.5% and 46.2% of patients, respectively | Ahmed et al[135], 2023 |
| Retrospective study | TACE + TKIs + anti-PD-1 vs HAIC + TKIs + anti-PD-1 | 19 vs 39 | 0 vs 15.4 | The HAIC + TKIs + anti-PD-1 group achieved significantly higher ORR (RECIST: 48.7% vs 15.8%; mRECIST: 61.5% vs 21.1%) and DCR (82.1% vs 36.8%) compared to TACE + TKIs + anti-PD-1 in unresectable ICC | Zhang et al[136], 2022 |
| Retrospective study | TACE + lenvatinib | 44 | 63.6 | TACE combined with lenvatinib successfully downstaged 63.6% of patients with initially unresectable ICC to surgical resection. Among them, 82.1% achieved R0 resection. Patients who underwent successful downstaging had significantly better OS | Yuan et al[137], 2022 |
| Phase 2 clinical trial | Gem/Cis vs Gem/Cis-DEBIRI | 22 vs 24 | 8 vs 25 (downsizing to resection/ablation) | The Gem/Cis + DEBIRI group had significantly higher ORR at 2, 4, and 6 months compared to Gem/Cis alone. Downsizing to resection/ablation was more frequent (25% vs 8%). Median OS (33.7 months vs 12.6 months) were significantly improved, supporting Gem/Cis + DEBIRI as a safe and effective treatment option for unresectable ICC | Martin et al[44], 2022 |
| Retrospective study | Yttrium-90 | 136 | 8.1 | Yttrium-90 radioembolization achieved a median OS of 14.2 months in unresectable ICC. 8.1% of patients were downstaged to resection, with 72.7% achieving R0 resection. Post-resection median OS was 39.9 months, supporting Y90 as an effective treatment with potential for downstaging and long-term survival benefits | Gupta et al[138], 2022 |
| Retrospective study | Yttrium-90 | 81 | 3.7 | Yttrium-90 transarterial radioembolization achieved a median OS of 14.5 months in unresectable ICC, with objective response and DCRs of 41.8% and 83.6%, respectively | Bargellini et al[139], 2020 |
| Retrospective study | Yttrium-90 | 115 | 4 | Yttrium-90 radioembolization in unresectable ICC resulted in a median OS of 29 months from diagnosis. 4% of patients were downstaged to curative-intent resection, supporting yttrium-90 as a potential option for tumor control and downstaging | Buettner et al[140], 2020 |
| Phase 2 clinical trial | HAI floxuridine + systemic Gemox | 38 | 11 | HAI plus systemic Gemox achieved a median OS of 25.0 months and a median PFS of 11.8 months in unresectable ICC. 58% of patients achieved a partial response, and 4 patients (11%) were downstaged to resection, with 1 complete pathologic response. Patients with IDH1/2 mutations had significantly better two-year OS | Cercek et al[53], 2020 |
| Phase 2 clinical trial | SIRT + chemotherapy | 41 | 22 | SIRT combined with cisplatin and gemcitabine achieved a 39% response rate (RECIST) and a 98% DCR in unresectable ICC. Median PFS was 14 months, and median OS was 22 months. 22% of patients were downstaged to surgery, with 20% achieving R0 resection. These findings support SIRT plus chemotherapy as an effective treatment with potential for surgical downstaging | Edeline et al[16], 2020 |
| Retrospective study | HAI of gemcitabine plus oxaliplatin | 12 | 16.7 | HAI of gemcitabine + oxaliplatin for unresectable locally advanced ICC achieved a DCR of 91%. Median OS was 9.1 months, and time to progression was 20.3 months. Partial responses enabled R0 resection in 2 patients, supporting HAI as a promising and tolerable therapy for locally advanced ICC | Ghiringhelli et al[56], 2013 |
| Retrospective study | Drug eluting bead-TACE | 26 | 3.8 | Drug-eluting bead transarterial chemoembolization achieved a median OS of 11.7 months and PFS of 3.9 months. Local tumor control was achieved in 66% of DEB-TACE patients, with one patient successfully downstaged to resection. These findings suggest DEB-TACE is a safe and effective alternative for ICC | Kuhlmann et al[141], 2012 |
| Prospective multicenter study | Drug-eluting bead therapy loaded with irinotecan | 24 | 12.5 | Drug-eluting bead therapy achieved a median OS of 17.5 months, significantly longer than chemotherapy alone in unresectable ICC. One patient was successfully downstaged to resection. These findings suggest that drug-eluting bead therapy is a safe and effective adjunctive treatment for ICC, providing a survival advantage over chemotherapy alone | Schiffman et al[142], 2011 |
Advancements in different methods of treatment have increased hope in the management of ICC (Figure 1). Nonetheless, the optimal timing for surgical intervention following effective conversion treatment remains uncertain. Furthermore, considerations must include the duration required for the medicine to exhibit efficacy, potential adverse reactions, and the necessity for prior discontinuation of the medication. Some clinicians endorse prompt curative resection subsequent to successful conversion treatment. The degree of urgency is predicated on the potential risk of forfeiting surgical options should tumor reprogression occur. Nevertheless, in practical settings, many patients who achieve effective tumor control via conversion treatment are often reluctant to undergo surgery, preferring to sustain their current condition. This hesitance predominantly stems from apprehensions regarding surgical intervention and the possible disruption of the tumor microenvironment postoperatively, which may heighten the risk of tumor recurrence. The timing of surgery is predominantly contingent upon the physician’s experience. For patients with ICC, multidisciplinary teams (MDTs) may be helpful in determining the best time for surgery and conversion treatment. Owing to the essential contribution of MDTs in enhancing patient outcomes, the National Comprehensive Cancer Network and other authorities advocate the use of MDTs in the management of cholangiocarcinoma[20,115-117]. Future studies are needed to ascertain the optimal period for effective conversion treatment and to identify suitable biological markers for predicting treatment efficacy (Table 2).
| ClinicalTrials.gov reference | Study phase | Interventions | Primary endpoint | Status |
| NCT05400902 | Phase 2 | HAIC combined with tislelizumab and apatinib | ORR | Recruiting |
| NCT05535647 | Phase 2 | Regorafenib and HAIC | ORR | Not yet recruiting |
| FOLFOX | ||||
| NCT06239532 | Phase 2 | TAE + HAIC + tislelizumab + surufatinib | ORR | Recruiting |
| NCT05010668 | Phase 2 | Cryoablation combined with sintilimab plus lenvatinib | ORR | Recruiting |
| NCT04954781 | Phase 2 | TACE in combination with tislelizumab | ORR | Recruiting |
| NCT06298968 | Phase 2 | Combined therapy using GC, lenvatinib and adebrelimab | ORR | Recruiting |
| NCT04961970 | Phase 3 | HAIC with FOLFOX | OS | Recruiting |
| Systemic chemotherapy with GP | ||||
| NCT06335927 | Phase 2 | HAIC-Gemox + cadonilimab + regorafenib | ORR | Recruiting |
| NCT04238637 | Phase 2 | Y-90 SIRT + durvalumab | ORR | Recruiting |
| Y-90 SIRT + durvalumab + tremelimumab | ||||
| NCT05342194 | Phase 3 | Toripalimab, lenvatinib, and gemcitabine-based chemotherapy | OS | Not yet recruiting |
| Toripalimab, oral placebo, and gemcitabine-based chemotherapy | ||||
| Intravenous placebo, oral placebo, and gemcitabine-based chemotherapy | ||||
| NCT04299581 | Phase 2 | Cryoablation combined with anti-PD-1 antibody | ORR | Recruiting |
| NCT05781958 | Phase 2 | Cadonilimab combined with gemcitabine and cisplatin | ORR | Active, not recruiting |
| NCT05174650 | Phase 2 | Combined treatment with atezolizumab and derazantinib | ORR | Active, not recruiting |
| NCT05422690 | Phase 2 | Gemcitabine, cisplatin and durvalumab chemotherapy treatments with Y-90 | ORR | Recruiting |
| NCT04454905 | Phase 2 | Camrelizumab in combination with apatinib | PFS | Recruiting |
| NCT06648525 | Phase 2 | Adebrelimab + irinotecan liposomes + 5-fluorouracil + calcium folinate + lenvatinib | PFS | Not yet recruiting |
| Adebrelimab + irinotecan liposomes + 5-fluorouracil + calcium folinate | ||||
| NCT05738057 | Phase 2 | Combined therapy using D-TACE, gemcitabine and cisplatin, and camrelizumab | Conversion rate | Recruiting |
| NCT05835245 | Phase 2 | Cryoablation combined with sintilimab plus lenvatinib | ORR | Recruiting |
| NCT06058663 | Phase 1 | Radioembolization with tremelimumab and durvalumab | Incidence of treatment-emergent adverse events | Recruiting |
| NCT05655949 | Phase 2 | Gemcitabine + cisplatin + durvalumab + Y-90 selective internal radiation therapy | PFS | Recruiting |
| Incidence of grade 3 or higher treatment-related toxicity | ||||
| NCT06567600 | Phase 2 | Low-dose gemcitabine and cisplatin and PD-1/PD-L1 antibody | ORR | Not yet recruiting |
| NCT04634058 | Phase 2 | PD-L1 antibody combined with CTLA-4 antibody | ORR | Recruiting |
| NCT01862315 | Phase 2 | Hepatic arterial infusion with floxuridine and dexamethasone combined with systemic Gemox | PFS | Active, not recruiting |
| NCT05348811 | Phase 2 | HAIC combined with donafenib and sintilimab | ORR | Recruiting |
| NCT06192797 | Phase 2 | Combined HAIC, lenvatinib and pucotenlimab | Number of patients amendable to curative surgical interventions | Recruiting |
| NCT06192784 | Phase 2 | Combined DEB-TACE, lenvatinib and pucotenlimab | Number of patients amendable to curative surgical interventions | Recruiting |
| NCT04834674 | Phase 2 | DEB-TACE combined with apatinib and PD-1 antibody | ORR | Recruiting |
| PFS | ||||
| NCT05913661 | Phase 2 | Pemigatinib combined with PD-1 inhibitor | ORR | Recruiting |
On the basis of extensive research on the pathogenesis of ICC, personalized precision therapy based on immune and targeted therapy has emerged as a new treatment approach that has transformed clinical practice in recent years. Additionally, a number of studies have investigated biomarkers in order to better achieve the goal of precision treatment. It is becoming increasingly recognized that biomarkers, as potent tools for predicting treatment effects and patient prognosis, can be used to guide clinical practice and optimize the benefits of patient conversion treatment.
As mentioned above, the identification of sensitive biomarkers, such as FGFR2 fusions and IDH1 mutations, has become an important step in selecting ICC patients for targeted therapy. Furthermore, more genes are under investigation as biomarkers. In a previous study, researchers discovered that ARID1A is downregulated in ICC, which was correlated with unfavorable clinicopathological characteristics and poor prognosis, indicating that ARID1A may function as a prognosis indicator for ICC patients[118]. BAP1 is also regarded as a putative tumor suppressor in ICC and may function as a significant prognostic indicator and potential therapeutic target. Reduced BAP1 expression is strongly associated with OS and recurrence-free survival after surgery[119].
PD-1/PD-L1 expression is frequently utilized as a biomarker for immune checkpoint inhibitors. In the setting of ICC, PD-L1 expression is strongly linked with tumor invasion, tumor-node-metastasis stage, and other markers[120,121]. The immune system may successfully resume its attack on tumor cells by blocking the interaction between PD-1/PD-L1, and this therapeutic approach has emerged as a significant advancement in cancer treatment today[122].
Microsatellite instability has been investigated as a biomarker for the treatment of ICC[123], but the infrequency of microsatellite instability in ICC has hindered the ability to draw definitive conclusions about its occurrence and prognostic significance[124]. Furthermore, research has demonstrated that tumor mutation burden is an independent marker for the prognosis of ICC patients[125]. In addition to the biomarkers mentioned above, other factors that are being investigated include circulating tumor DNA and interleukin-6[126,127]. The identification of ICC biomarkers offers a basis for the development of clinical treatment strategies. Consequently, it is crucial to focus on the identification and investigation of ICC molecular targets and immune checkpoints to facilitate conversion treatment for patients with advanced ICC.
ICC remains an aggressive cancer that is almost always fatal and is becoming more common. Liver resection is one treatment that can improve long-term survival for people with ICC; however, the overall prognosis for these patients remains poor. Through ongoing advancements in tumor therapy, the stage of ICC can be reduced via systemic or local treatments in conjunction with techniques such as PVE and ALPPS, hence improving the resectability of ICC.
Conversion treatment has emerged as a viable option for patients with initially unresectable ICC, aiming to reduce the tumor burden and increase the likelihood of successful surgical resection. Although this approach has potential, there are still several challenges. The interventions used for ICC differ markedly among studies regarding combinations of medicines, dosing protocols, and patient criteria, resulting in diversity in clinical outcomes. The purpose of most related clinical trials is to explore the efficacy of new treatment options, and a small number of patients have unexpected conversion resections obtained during palliative treatment. Consequently, the treatment methods that yielded favorable outcomes in the trial have limited applicability for ICC conversion treatment. In conclusion, while conversion treatment may serve as a revolutionary treatment approach for patients with locally advanced ICC, considerable efforts are needed to produce more solid data. Reliable recommendations for the clinical implementation of conversion treatment in ICC can be established only through these procedures.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12654] [Article Influence: 6327.0] [Reference Citation Analysis (6)] |
| 2. | Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 313] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 3. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 550] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 4. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 5. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1456] [Article Influence: 121.3] [Reference Citation Analysis (4)] |
| 6. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1036] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 7. | Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, Factor VM, Thorgeirsson SS. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021-1031.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 440] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 8. | Akateh C, Ejaz AM, Pawlik TM, Cloyd JM. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J Hepatol. 2020;12:693-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 9. | Adamus N, Edeline J, Henriques J, Fares N, Lecomte T, Turpin A, Vernerey D, Vincens M, Chanez B, Tougeron D, Tournigand C, Assenat E, Delaye M, Manfredi S, Bouché O, Williet N, Vienot A, Blaise L, Mas L, Neuzillet C, Boilève A, Roth GS. First-line chemotherapy with selective internal radiation therapy for intrahepatic cholangiocarcinoma: The French ACABi GERCOR PRONOBIL cohort. JHEP Rep. 2025;7:101279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Igata Y, Kudo M, Kojima M, Kami S, Aoki K, Satake T, Kobayashi T, Sugimoto M, Kobayashi S, Konishi M, Gotohda N. Conversion surgery after gemcitabine and cisplatin plus durvalumab for advanced intrahepatic cholangiocarcinoma: A case report. World J Clin Cases. 2024;12:6721-6727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 11. | Zhao S, Zhang X, Luo J, Yan H, Zhang J, Lin R, Zhu K. Conversion therapy for unresectable intrahepatic cholangiocarcinoma using gemcitabine plus S-1 combined with PD-1 inhibitors: a case report. Front Oncol. 2024;14:1476593. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Zhang HW, Yu HB. Case report: Translational treatment of unresectable intrahepatic cholangiocarcinoma: Tislelizumab, Lenvatinib, and GEMOX in one case. Front Oncol. 2024;14:1428370. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Wang X, Li H, Sun H, Chen J, Lin H. Case Report: Camrelizumab combined with gemcitabine and oxaliplatin in the treatment of advanced intrahepatic cholangiocarcinoma: a case report and literature review. Front Immunol. 2023;14:1230261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Sun HC, Zhu XD. Downstaging Conversion Therapy in Patients With Initially Unresectable Advanced Hepatocellular Carcinoma: An Overview. Front Oncol. 2021;11:772195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 15. | Wang MD, Xu XJ, Wang KC, Diao YK, Xu JH, Gu LH, Yao LQ, Li C, Lv GY, Yang T. Conversion therapy for advanced hepatocellular carcinoma in the era of precision medicine: Current status, challenges and opportunities. Cancer Sci. 2024;115:2159-2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 16. | Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, Ayav A, Campillo-Gimenez B, Beuzit L, Pracht M, Lièvre A, Le Sourd S, Boudjema K, Rolland Y, Boucher E, Garin E. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 17. | Makki M, Bentaleb M, Abdulrahman M, Suhool AA, Al Harthi S, Ribeiro MA Jr. Current interventional options for palliative care for patients with advanced-stage cholangiocarcinoma. World J Clin Oncol. 2024;15:381-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (3)] |
| 18. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3333] [Article Influence: 208.3] [Reference Citation Analysis (15)] |
| 19. | Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, Seo S, Taketomi A, Takayama T, Yamaue H, Takahashi M, Sho M, Kamei K, Fujimoto J, Toyoda M, Shimizu J, Goto T, Shindo Y, Yoshimura K, Hatano E, Nagano H; Kansai Hepatobiliary Oncology Group (KHBO). Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30:102-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 161] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 20. | Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 21. | Yoo C, Hyung J, Chan SL. Recent Advances in Systemic Therapy for Advanced Intrahepatic Cholangiocarcinoma. Liver Cancer. 2024;13:119-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 23. | Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 24. | Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, Hallemeier CL, Koay EJ, Lasley F, Meyer J, Owen D, Pursley J, Schaub SK, Smith G, Venepalli NK, Zibari G, Cardenes H. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2022;12:28-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 25. | Li Y, Shimizu S, Mizumoto M, Iizumi T, Numajiri H, Makishima H, Li G, Sakurai H. Proton Beam Therapy for Multifocal Hepatocellular Carcinoma (HCC) Showing Complete Response in Pathological Anatomy After Liver Transplantation. Cureus. 2022;14:e25744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Smart AC, Goyal L, Horick N, Petkovska N, Zhu AX, Ferrone CR, Tanabe KK, Allen JN, Drapek LC, Qadan M, Murphy JE, Eyler CE, Ryan DP, Hong TS, Wo JY. Hypofractionated Radiation Therapy for Unresectable/Locally Recurrent Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2020;27:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Jackson MW, Amini A, Jones BL, Rusthoven CG, Schefter TE, Goodman KA. Treatment Selection and Survival Outcomes With and Without Radiation for Unresectable, Localized Intrahepatic Cholangiocarcinoma. Cancer J. 2016;22:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Jiang W, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, Zhang JY, Chen YX, Tan YS. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol. 2010;136:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, Kaseb AO, Bishop AJ, Swanick CW, Koay EJ, Thames HD, Hong TS, Das P, Crane CH. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol. 2016;34:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 30. | Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L, Murphy JE, Javle MM, Wolfgang JA, Drapek LC, Arellano RS, Mamon HJ, Mullen JT, Yoon SS, Tanabe KK, Ferrone CR, Ryan DP, DeLaney TF, Crane CH, Zhu AX. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 31. | Zhu M, Jin M, Zhao X, Shen S, Chen Y, Xiao H, Wei G, He Q, Li B, Peng Z. Anti-PD-1 antibody in combination with radiotherapy as first-line therapy for unresectable intrahepatic cholangiocarcinoma. BMC Med. 2024;22:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, Cucchetti A, Golfieri R. Transarterial Chemoembolization and Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma-a Systemic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2021;44:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Owen M, Makary MS, Beal EW. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers (Basel). 2023;15:2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 34. | Gorji L, Aoun H, Critchfield J, Al Hallak N, Beal EW. Locoregional Therapy for Intrahepatic Cholangiocarcinoma: The Role of Intra-Arterial Therapies. Cancers (Basel). 2023;15:4727. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. Hepatobiliary Surg Nutr. 2017;6:7-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Currie BM, Soulen MC. Decision Making: Intra-arterial Therapies for Cholangiocarcinoma-TACE and TARE. Semin Intervent Radiol. 2017;34:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Zhou TY, Zhou GH, Zhang YL, Nie CH, Zhu TY, Wang HL, Chen SQ, Wang BQ, Yu ZN, Wu LM, Zheng SS, Sun JH. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J Cancer. 2020;11:4534-4541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Luo J, Zheng J, Shi C, Fang J, Peng Z, Huang J, Sun J, Zhou G, Li T, Zhu D, Xu H, Hou Q, Ying S, Sun Z, Du H, Xie X, Cao G, Ji W, Han J, Gu W, Guo X, Shao G, Yu Z, Zhou J, Yu W, Zhang X, Li L, Hu H, Hu T, Wu X, Chen Y, Ji J, Hu W. Drug-eluting beads transarterial chemoembolization by CalliSpheres is effective and well tolerated in treating intrahepatic cholangiocarcinoma patients: A preliminary result from CTILC study. Medicine (Baltimore). 2020;99:e19276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Sun T, Zhang W, Chen L, Ren Y, Liu Y, Zheng C. A comparative study of efficacy and safety of transarterial chemoembolization with CalliSpheres and conventional transarterial chemoembolization in treating unresectable intrahepatic cholangiocarcinoma patients. J Cancer. 2022;13:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, Gruber-Rouh T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Liu D, Wang J, Ma Z, Zhang N, Zhao Y, Yang X, Wen Z, Xie H. Treatment of unresectable intrahepatic cholangiocarcinoma using transarterial chemoembolisation with irinotecan-eluting beads: analysis of efficacy and safety. Cardiovasc Intervent Radiol. 2022;45:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 42. | Herber S, Otto G, Schneider J, Manzl N, Kummer I, Kanzler S, Schuchmann A, Thies J, Düber C, Pitton M. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2007;30:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Martin RCG 2nd, Simo KA, Hansen P, Rocha F, Philips P, McMasters KM, Tatum CM, Kelly LR, Driscoll M, Sharma VR, Crocenzi TS, Scoggins CR. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann Surg Oncol. 2022;29:5462-5473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Kennedy A, Brown DB, Feilchenfeldt J, Marshall J, Wasan H, Fakih M, Gibbs P, Knuth A, Sangro B, Soulen MC, Pittari G, Sharma RA. Safety of selective internal radiation therapy (SIRT) with yttrium-90 microspheres combined with systemic anticancer agents: expert consensus. J Gastrointest Oncol. 2017;8:1079-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Tong AK, Kao YH, Too CW, Chin KF, Ng DC, Chow PK. Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. Br J Radiol. 2016;89:20150943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Mouli S, Memon K, Baker T, Benson AB 3rd, Mulcahy MF, Gupta R, Ryu RK, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 48. | Yu Q, Ungchusri E, Pillai A, Liao CY, Baker T, Fung J, DiSabato D, Zhang M, Liao C, Van Ha T, Ahmed O. Selective internal radiation therapy using yttrium-90 microspheres for treatment of localized and locally advanced intrahepatic cholangiocarcinoma. Eur Radiol. 2024;34:2374-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 49. | Gangi A, Shah J, Hatfield N, Smith J, Sweeney J, Choi J, El-Haddad G, Biebel B, Parikh N, Arslan B, Hoffe SE, Frakes JM, Springett GM, Anaya DA, Malafa M, Chen DT, Chen Y, Kim RD, Shridhar R, Kis B. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J Vasc Interv Radiol. 2018;29:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Massani M, Bonariol L, Stecca T. Hepatic Arterial Infusion Chemotherapy for Unresectable Intrahepatic Cholangiocarcinoma, a Comprehensive Review. J Clin Med. 2021;10:2552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Franssen S, Soares KC, Jolissaint JS, Tsilimigras DI, Buettner S, Alexandrescu S, Marques H, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Margonis GA, Weiss MJ, Bauer TW, Shen F, Poultsides GA, Marsh JW, Cercek A, Kemeny N, Kingham TP, D'Angelica M, Pawlik TM, Jarnagin WR, Koerkamp BG. Comparison of Hepatic Arterial Infusion Pump Chemotherapy vs Resection for Patients With Multifocal Intrahepatic Cholangiocarcinoma. JAMA Surg. 2022;157:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 52. | Ishii M, Itano O, Morinaga J, Shirakawa H, Itano S. Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PLoS One. 2022;17:e0266707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 53. | Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, Hauser HF, Do RKG, Lowery MA, Harding JJ, Varghese AM, Reidy-Lagunes D, Saltz L, Schultz N, Kingham TP, D'Angelica MI, DeMatteo RP, Drebin JA, Allen PJ, Balachandran VP, Lim KH, Sanchez-Vega F, Vachharajani N, Majella Doyle MB, Fields RC, Hawkins WG, Strasberg SM, Chapman WC, Diaz LA Jr, Kemeny NE, Jarnagin WR. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination With Systemic Gemcitabine and Oxaliplatin in Patients With Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 54. | Li S, Deng M, Wang Q, Mei J, Zou J, Lin W, Shi M, Chen M, Wei W, Guo R. Transarterial Infusion Chemotherapy with FOLFOX Could be an Effective and Safe Treatment for Unresectable Intrahepatic Cholangiocarcinoma. J Oncol. 2022;2022:2724476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 55. | Huang P, Huang X, Zhou Y, Yang G, Sun Q, Shi G, Chen Y. The Efficacy and Safety of Hepatic Arterial Infusion Chemotherapy Based on FOLFIRI for Advanced Intrahepatic Cholangiocarcinoma as Second-Line and Successive Treatment: A Real-World Study. Can J Gastroenterol Hepatol. 2022;2022:9680933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Yang Z, Fu Y, Wu W, Hu Z, Pan Y, Wang J, Chen J, Hu D, Zhou Z, Chen M, Zhang Y. Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front Pharmacol. 2023;14:1234342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 58. | Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 59. | Manne A, Woods E, Tsung A, Mittra A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front Oncol. 2021;11:768009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1940] [Cited by in RCA: 1777] [Article Influence: 296.2] [Reference Citation Analysis (0)] |
| 61. | Komuta M. Intrahepatic cholangiocarcinoma: Tumour heterogeneity and its clinical relevance. Clin Mol Hepatol. 2022;28:396-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Rodrigues PM, Olaizola P, Paiva NA, Olaizola I, Agirre-Lizaso A, Landa A, Bujanda L, Perugorria MJ, Banales JM. Pathogenesis of Cholangiocarcinoma. Annu Rev Pathol. 2021;16:433-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 63. | Aitcheson G, Mahipal A, John BV. Targeting FGFR in intrahepatic cholangiocarcinoma [iCCA]: leading the way for precision medicine in biliary tract cancer [BTC]? Expert Opin Investig Drugs. 2021;30:463-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 626] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 65. | Nishida N. The role of FGFR inhibitors in the treatment of intrahepatic cholangiocarcinoma-unveiling the future challenges in drug therapy. Hepatobiliary Surg Nutr. 2023;12:790-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Hoy SM. Pemigatinib: First Approval. Drugs. 2020;80:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 67. | Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1178] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 68. | Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol. 2019;10:751-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 69. | Lavacchi D, Caliman E, Rossi G, Buttitta E, Botteri C, Fancelli S, Pellegrini E, Roviello G, Pillozzi S, Antonuzzo L. Ivosidenib in IDH1-mutated cholangiocarcinoma: Clinical evaluation and future directions. Pharmacol Ther. 2022;237:108170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Ivosidenib Boosts OS in Cholangiocarcinoma. Cancer Discov. 2021;11:2953-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DVT, Borad MJ, Bridgewater JA, Harris WP, Murphy AG, Oh DY, Whisenant JR, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Chamberlain CX, Aguado-Fraile E, Choe S, Wu B, Liu H, Gliser C, Pandya SS, Valle JW, Abou-Alfa GK. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021;7:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 72. | Galdy S, Lamarca A, McNamara MG, Hubner RA, Cella CA, Fazio N, Valle JW. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36:141-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 73. | Zhu K, Yang X, Tai H, Zhong X, Luo T, Zheng H. HER2-targeted therapies in cancer: a systematic review. Biomark Res. 2024;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 74. | Lee CK, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, Kim MH, Park S, Kang B, Hong M, Kim JW, Park HS, Kang MJ, Park YN, Choi HJ. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol. 2023;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 75. | Taghizadeh H, Dong Y, Gruenberger T, Prager GW. Perioperative and palliative systemic treatments for biliary tract cancer. Ther Adv Med Oncol. 2024;16:17588359241230756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1235] [Article Influence: 176.4] [Reference Citation Analysis (0)] |
| 77. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 2074] [Article Influence: 259.3] [Reference Citation Analysis (0)] |
| 78. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4111] [Article Influence: 513.9] [Reference Citation Analysis (5)] |
| 79. | Ueno M, Ikeda M, Sasaki T, Nagashima F, Mizuno N, Shimizu S, Ikezawa H, Hayata N, Nakajima R, Morizane C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: primary analysis results. BMC Cancer. 2020;20:1105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 80. | Jian Z, Fan J, Shi GM, Huang XY, Wu D, Yang GH, Ji Y, Chen Y, Liang F, Lu JC, Ge NL, Sun HC, Huang XW, Qiu SJ, He YF, Yang XR, Xu Y, Gao Q, Sun J. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. J Clin Oncol. 2021;39:4094. [DOI] [Full Text] |
| 81. | Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, Schell MJ, Zhou JM, Mahipal A, Kim BH, Kim DW. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 82. | Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, Kitano M, Lee CK, Kim JW, Chen MH, Suksombooncharoen T, Ikeda M, Lee MA, Chen JS, Potemski P, Burris HA 3rd, Ostwal V, Tanasanvimon S, Morizane C, Zaucha RE, McNamara MG, Avallone A, Cundom JE, Breder V, Tan B, Shimizu S, Tougeron D, Evesque L, Petrova M, Zhen DB, Gillmore R, Gupta VG, Dayyani F, Park JO, Buchschacher GL Jr, Rey F, Kim H, Wang J, Morgan C, Rokutanda N, Żotkiewicz M, Vogel A, Valle JW. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. 2024;9:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 83. | Finn RS, Ueno M, Yoo C, Ren Z, Furuse J, Kelley RK, Chan SL, Edeline J, Klümpen H, Yau T, Verslype C, Ozaka M, Bouattour M, Park JO, Vogel A, Valle JW, Starkopf L, Malhotra U, Siegel AB, Qin S. Three-year follow-up data from KEYNOTE-966: Pembrolizumab (pembro) plus gemcitabine and cisplatin (gem/cis) compared with gem/cis alone for patients (pts) with advanced biliary tract cancer (BTC). J Clin Oncol. 2024;42:4093. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 84. | Olson DJ, Odunsi K. Adoptive Cell Therapy for Nonhematologic Solid Tumors. J Clin Oncol. 2023;41:3397-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 85. | Martín-Lluesma S, Svane IM, Dafni U, Vervita K, Karlis D, Dimopoulou G, Tsourti Z, Rohaan MW, Haanen JBAG, Coukos G. Efficacy of TIL therapy in advanced cutaneous melanoma in the current immuno-oncology era: updated systematic review and meta-analysis. Ann Oncol. 2024;35:860-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 86. | Kverneland AH, Chamberlain CA, Borch TH, Nielsen M, Mørk SK, Kjeldsen JW, Lorentzen CL, Jørgensen LP, Riis LB, Yde CW, Met Ö, Donia M, Svane IM. Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types. J Immunother Cancer. 2021;9:e003499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 87. | Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q, Wang Y, Jia H, Han W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin Cancer Res. 2018;24:1277-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 88. | Reese T, Gilg S, Böcker J, Wagner KC, Vali M, Engstrand J, Kern A, Sturesson C, Oldhafer KJ, Sparrelid E. Impact of the future liver remnant volume before major hepatectomy. Eur J Surg Oncol. 2024;50:108660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 89. | Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 90. | Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer. 2012;1:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Aloia TA. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: Portal Vein Embolization Should Remain the Gold Standard. JAMA Surg. 2015;150:927-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Piron L, Deshayes E, Escal L, Souche R, Herrero A, Pierredon-Foulongne MA, Assenat E, le Lam N, Quenet F, Guiu B. [Portal vein embolization: Present and future]. Bull Cancer. 2017;104:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 94. | Hwang S, Ha TY, Ko GY, Kwon DI, Song GW, Jung DH, Kim MH, Lee SK, Lee SG. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg. 2015;39:2990-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Nevermann N, Bode J, Vischer M, Krenzien F, Lurje G, Pelzer U, Fehrenbach U, Auer TA, Schmelzle M, Pratschke J, Schöning W. Perioperative outcome and long-term survival for intrahepatic cholangiocarcinoma after portal vein embolization and subsequent resection: A propensity-matched study. Eur J Surg Oncol. 2023;49:107100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Olthof PB, Aldrighetti L, Alikhanov R, Cescon M, Groot Koerkamp B, Jarnagin WR, Nadalin S, Pratschke J, Schmelze M, Sparrelid E, Lang H, Guglielmi A, van Gulik TM; Perihilar Cholangiocarcinoma Collaboration Group. Portal Vein Embolization is Associated with Reduced Liver Failure and Mortality in High-Risk Resections for Perihilar Cholangiocarcinoma. Ann Surg Oncol. 2020;27:2311-2318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Vennarecci G, Grazi GL, Sperduti I, Busi Rizzi E, Felli E, Antonini M, D'Offizi G, Ettorre GM. ALPPS for primary and secondary liver tumors. Int J Surg. 2016;30:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Balci D, Sakamoto Y, Li J, Di Benedetto F, Kirimker EO, Petrowsky H. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for cholangiocarcinoma. Int J Surg. 2020;82S:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Alvarez FA, Ardiles V, de Santibañes M, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg. 2015;261:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 100. | Schadde E, Malagó M, Hernandez-Alejandro R, Li J, Abdalla E, Ardiles V, Lurje G, Vyas S, Machado MA, de Santibañes E. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery. 2015;157:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Li J, Moustafa M, Linecker M, Lurje G, Capobianco I, Baumgart J, Ratti F, Rauchfuss F, Balci D, Fernandes E, Montalti R, Robles-Campos R, Bjornsson B, Topp SA, Fronek J, Liu C, Wahba R, Bruns C, Brunner SM, Schlitt HJ, Heumann A, Stüben BO, Izbicki JR, Bednarsch J, Gringeri E, Fasolo E, Rolinger J, Kristek J, Hernandez-Alejandro R, Schnitzbauer A, Nuessler N, Schön MR, Voskanyan S, Petrou AS, Hahn O, Soejima Y, Vicente E, Castro-Benitez C, Adam R, Tomassini F, Troisi RI, Kantas A, Oldhafer KJ, Ardiles V, de Santibanes E, Malago M, Clavien PA, Vivarelli M, Settmacher U, Aldrighetti L, Neumann U, Petrowsky H, Cillo U, Lang H, Nadalin S. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann Surg Oncol. 2020;27:1372-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 102. | Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, Elarouci N, Ayadi M, Adam R, Azoulay D, Castaing D, Vibert E, Cherqui D, Samuel D, Sa Cuhna A, Marchio A, Pineau P, Guettier C, de Reyniès A, Faivre J. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology. 2020;72:965-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 103. | Diggs LP, Ruf B, Ma C, Heinrich B, Cui L, Zhang Q, McVey JC, Wabitsch S, Heinrich S, Rosato U, Lai W, Subramanyam V, Longerich T, Loosen SH, Luedde T, Neumann UP, Desar S, Kleiner D, Gores G, Wang XW, Greten TF. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 104. | Kahaleh M, Mishra R, Shami VM, Northup PG, Berg CL, Bashlor P, Jones P, Ellen K, Weiss GR, Brenin CM, Kurth BE, Rich TA, Adams RB, Yeaton P. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 105. | Wang W, Gao Y, Xu J, Zou T, Yang B, Hu S, Cheng X, Xia Y, Zheng Q. A NRF2 Regulated and the Immunosuppressive Microenvironment Reversed Nanoplatform for Cholangiocarcinoma Photodynamic-Gas Therapy. Adv Sci (Weinh). 2024;11:e2307143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 106. | Huang YP, Wang YX, Zhou H, Liu ZT, Zhang ZJ, Xiong L, Zou H, Wen Y. Surufatinib combined with photodynamic therapy induces ferroptosis to inhibit cholangiocarcinoma in vitro and in tumor models. Front Pharmacol. 2024;15:1288255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 107. | Mao L. Thymosin alpha 1 - Reimagine its broader applications in the immuno-oncology era. Int Immunopharmacol. 2023;117:109952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 108. | Linye H, Zijing X, Wei P, Chao H, Chuan L, Tianfu W. Thymosin alpha-1 therapy improves postoperative survival after curative resection for solitary hepatitis B virus-related hepatocellular carcinoma: A propensity score matching analysis. Medicine (Baltimore). 2021;100:e25749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Xia M, Qin W, Hu B. Endobiliary radiofrequency ablation for unresectable malignant biliary strictures: Survival benefit perspective. Dig Endosc. 2023;35:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 110. | Jarosova J, Zarivnijova L, Cibulkova I, Mares J, Macinga P, Hujova A, Falt P, Urban O, Hajer J, Spicak J, Hucl T. Endoluminal radiofrequency ablation in patients with malignant biliary obstruction: a randomised trial. Gut. 2023;72:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 111. | Yang GW, Zhao Q, Qian S, Zhu L, Qu XD, Zhang W, Yan ZP, Cheng JM, Liu QX, Liu R, Wang JH. Percutaneous microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of advanced intrahepatic cholangiocarcinoma. Onco Targets Ther. 2015;8:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Zhang A, Liu B, Xu D, Sun Y. Advanced intrahepatic cholangiocarcinoma treated using anlotinib and microwave ablation: A case report. Medicine (Baltimore). 2019;98:e18435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Zhao R, Zhou J, Miao Z, Xiong X, Wei W, Li S, Guo R. Efficacy and safety of lenvatinib plus durvalumab combined with hepatic arterial infusion chemotherapy for unresectable intrahepatic cholangiocarcinoma. Front Immunol. 2024;15:1397827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 114. | Dong Z, Sui C, Lu J, Guo J, Duan K, Wang K, Geng L, Dai B, Yang J. Chemotherapy combined with lenvatinib and PD-1 may be a potential better alternative option for advanced unresectable intrahepatic cholangiocarcinoma: a retrospective real-world study. Front Immunol. 2024;15:1463574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 115. | Bourien H, Lamarca A, McNamara MG, Hubner RA, Valle JW, Edeline J. Druggable molecular alterations in bile duct cancer: potential and current therapeutic applications in clinical trials. Expert Opin Investig Drugs. 2021;30:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 412] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 117. | Cho MT, Gholami S, Gui D, Tejaswi SL, Fananapazir G, Abi-Jaoudeh N, Jutric Z, Samarasena JB, Li X, Valerin JB, Mercer J, Dayyani F. Optimizing the Diagnosis and Biomarker Testing for Patients with Intrahepatic Cholangiocarcinoma: A Multidisciplinary Approach. Cancers (Basel). 2022;14:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Yang SZ, Wang AQ, Du J, Wang JT, Yu WW, Liu Q, Wu YF, Chen SG. Low expression of ARID1A correlates with poor prognosis in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2016;22:5814-5821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 119. | Chen XX, Yin Y, Cheng JW, Huang A, Hu B, Zhang X, Sun YF, Wang J, Wang YP, Ji Y, Qiu SJ, Fan J, Zhou J, Yang XR. BAP1 acts as a tumor suppressor in intrahepatic cholangiocarcinoma by modulating the ERK1/2 and JNK/c-Jun pathways. Cell Death Dis. 2018;9:1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 120. | Dong Z, Liao B, Shen W, Sui C, Yang J. Expression of Programmed Death Ligand 1 Is Associated with the Prognosis of Intrahepatic Cholangiocarcinoma. Dig Dis Sci. 2020;65:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 121. | Wu H, Wei Y, Jian M, Lu H, Song Q, Hao L, Yue Y. Clinicopathological and Prognostic Significance of Immunoscore and PD-L1 in Intrahepatic Cholangiocarcinoma. Onco Targets Ther. 2021;14:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Chen F, Sheng J, Li X, Gao Z, Zhao S, Hu L, Chen M, Fei J, Song Z. Unveiling the promise of PD1/PD-L1: A new dawn in immunotherapy for cholangiocarcinoma. Biomed Pharmacother. 2024;175:116659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 123. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2213] [Article Influence: 368.8] [Reference Citation Analysis (1)] |
| 124. | Goeppert B, Roessler S, Renner M, Singer S, Mehrabi A, Vogel MN, Pathil A, Czink E, Köhler B, Springfeld C, Pfeiffenberger J, Rupp C, Weiss KH, Schirmacher P, von Knebel Doeberitz M, Kloor M. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br J Cancer. 2019;120:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 125. | Song JP, Liu XZ, Chen Q, Liu YF. High tumor mutation burden indicates a poor prognosis in patients with intrahepatic cholangiocarcinoma. World J Clin Cases. 2022;10:790-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (315)] |
| 126. | Choi WJ, Ivanics T, Gravely A, Gallinger S, Sapisochin G, O'Kane GM. Optimizing Circulating Tumour DNA Use in the Perioperative Setting for Intrahepatic Cholangiocarcinoma: Diagnosis, Screening, Minimal Residual Disease Detection and Treatment Response Monitoring. Ann Surg Oncol. 2023;30:3849-3863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 127. | Saeheng T, Karbwang J, Na-Bangchang K. Interleukin-6 and Lymphocyte-to-Monocyte Ratio Indices Identify Patients with Intrahepatic Cholangiocarcinoma. Biomedicines. 2024;12:844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 128. | Ni JY, Sun HL, Guo GF, Zhou X, Wei JX, Xu LF. Hepatic arterial infusion of GEMOX plus systemic gemcitabine chemotherapy combined with lenvatinib and PD-1 inhibitor in large unresectable intrahepatic cholangiocarcinoma. Int Immunopharmacol. 2024;140:112872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 129. | Lin YS, Li S, Yang X, Guo RP, Huang YH, Bai KH, Weng J, Yun JP. First-line hepatic arterial infusion chemotherapy plus lenvatinib and PD-(L)1 inhibitors versus systemic chemotherapy alone or with PD-(L)1 inhibitors in unresectable intrahepatic cholangiocarcinoma. J Cancer Res Clin Oncol. 2024;150:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 130. | Madzikatire TB, Heng S, Gu H, Shan Y, Lin E, Banda J, Debora A, Madziva BA, Bowa MJ, Mudhuri MG, Bwalya C. Real-world outcomes of chemotherapy plus immune checkpoint inhibitors versus chemotherapy alone in advanced, unresectable, and recurrent intrahepatic cholangiocarcinoma. Front Immunol. 2024;15:1390887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 131. | Im JH, Yu JI, Kim TH, Kim TG, Kim JW, Seong J. Combined High-Dose Radiotherapy with Sequential Gemcitabine-Cisplatin Based Chemotherapy Increase the Resectability and Survival in Locally Advanced Unresectable Intrahepatic Cholangiocarcinoma: A Multi-institutional Cohort Study. Cancer Res Treat. 2024;56:838-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 132. | Franssen S, Holster JJ, Jolissaint JS, Nooijen LE, Cercek A, D'Angelica MI, Homs MYV, Wei AC, Balachandran VP, Drebin JA, Harding JJ, Kemeny NE, Kingham TP, Klümpen HJ, Mostert B, Swijnenburg RJ, Soares KC, Jarnagin WR, Groot Koerkamp B. Gemcitabine with Cisplatin Versus Hepatic Arterial Infusion Pump Chemotherapy for Liver-Confined Unresectable Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2024;31:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Zhu C, Li H, Yang X, Wang S, Wang Y, Zhang N, Wang Y, Xue J, Zhang L, Ning C, Yang X, Xun Z, Chao J, Long J, Sang X, Zhu Z, Zhao H. Efficacy, safety, and prognostic factors of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment in advanced intrahepatic cholangiocarcinoma: a multicenter real-world study. Cancer Immunol Immunother. 2023;72:2949-2960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 134. | Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, Wang XY, Sun L, Ge NL, Huang XW, Qiu SJ, Yang XR, Gao Q, He YF, Xu Y, Sun J, Ren ZG, Fan J, Zhou J. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. 2023;8:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 135. | Ahmed O, Yu Q, Patel M, Hwang G, Pillai A, Liao CY, Fung J, Baker T. Yttrium-90 Radioembolization and Concomitant Systemic Gemcitabine, Cisplatin, and Capecitabine as the First-Line Therapy for Locally Advanced Intrahepatic Cholangiocarcinoma. J Vasc Interv Radiol. 2023;34:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 136. | Zhang N, Yu BR, Wang YX, Zhao YM, Zhou JM, Wang M, Wang LR, Lin ZH, Zhang T, Wang L. Clinical outcomes of hepatic arterial infusion chemotherapy combined with tyrosine kinase inhibitors and anti-PD-1 immunotherapy for unresectable intrahepatic cholangiocarcinoma. J Dig Dis. 2022;23:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 137. | Yuan P, Song J, Wang F, Zhu G, Chen B. Combination of TACE and Lenvatinib as a promising option for downstaging to surgery of initially unresectable intrahepatic cholangiocarcinoma. Invest New Drugs. 2022;40:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Gupta AN, Gordon AC, Gabr A, Kalyan A, Kircher SM, Mahalingam D, Mulcahy MF, Merkow RP, Yang AD, Bentrem DJ, Caicedo-Ramirez JC, Riaz A, Thornburg B, Desai K, Sato KT, Hohlastos ES, Kulik L, Benson AB, Salem R, Lewandowski RJ. Yttrium-90 Radioembolization of Unresectable Intrahepatic Cholangiocarcinoma: Long-Term Follow-up for a 136-Patient Cohort. Cardiovasc Intervent Radiol. 2022;45:1117-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 139. | Bargellini I, Mosconi C, Pizzi G, Lorenzoni G, Vivaldi C, Cappelli A, Vallati GE, Boni G, Cappelli F, Paladini A, Sciuto R, Masi G, Golfieri R, Cioni R. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc Intervent Radiol. 2020;43:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 140. | Buettner S, Braat AJAT, Margonis GA, Brown DB, Taylor KB, Borgmann AJ, Kappadath SC, Mahvash A, IJzermans JNM, Weiss MJ, Lamarca A, Bell JK, Valle JW, Hagendoorn J, Koerkamp BG, Sze DY, Lam MGEH. Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Analysis. J Vasc Interv Radiol. 2020;31:1035-1043.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 141. | Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, Fischer R. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Schiffman SC, Metzger T, Dubel G, Andrasina T, Kralj I, Tatum C, McMasters KM, Scoggins CR, Martin RC. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol. 2011;18:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/