Published online Jan 7, 2025. doi: 10.3748/wjg.v31.i1.96199
Revised: September 5, 2024
Accepted: October 12, 2024
Published online: January 7, 2025
Processing time: 224 Days and 3.6 Hours
Simulated microgravity environment can lead to gastrointestinal motility distur
To investigate the effects of Bifidobacterium lactis (B. lactis) BLa80 on the intestinal flora of rats in simulated microgravity and on the gastrointestinal motility-related SCF/c-kit pathway.
The internationally recognized tail suspension animal model was used to simulate the microgravity environment, and 30 rats were randomly divided into control group, tail suspension group and drug administration tail suspension group with 10 rats in each group for a total of 28 days. The tail group was given B. lactis BLa80 by intragastric administration, and the other two groups were given water intragastric administration, the concentration of intragastric administration was 0.1 g/mL, and each rat was 1 mL/day. Hematoxylin & eosin staining was used to observe the histopathological changes in each segment of the intestine of each group, and the expression levels of SCF, c-kit, extracellular signal-regulated kinase (ERK) and p-ERK in the gastric antrum of each group were detected by Western blotting and PCR. The fecal flora and mucosal flora of rats in each group were detected by 16S rRNA.
Simulated microgravity resulted in severe exfoliation of villi of duodenum, jejunum and ileum in rats, marked damage, increased space between villi, loose arrangement, shortened columnar epithelium of colon, less folds, narrower mucosal thickness, reduced goblet cell number and crypts, and significant improvement after probiotic intervention. Simulated microgravity reduced the expressions of SCF and c-kit, and increased the expressions of ERK and P-ERK in the gastric antrum of rats. However, after probiotic intervention, the expressions of SCF and c-kit were increased, while the expressions of ERK and P-ERK were decreased, with statistical significance (P < 0.05). In addition, simulated microgravity can reduce the operational taxonomic unit (OTU) of the overall intestinal flora of rats, B. lactis BLa80 can increase the OTU of rats, simulated microgravity can reduce the overall richness and diversity of stool flora of rats, increase the abundance of firmicutes in stool flora of rats, and reduce the abundance of Bacteroides in stool flora of rats, most of which are mainly beneficial bacteria. Simulated microgravity can increase the overall richness and diversity of mucosal flora, increase the abundance of Bacteroides and Desulphurides in the rat mucosal flora, and decrease the abundance of firmicutes, most of which are proteobacteria. After probiotics intervention, the overall Bacteroidetes trend in simulated microgravity rats was increased.
B. lactis BLa80 can ameliorate intestinal mucosal injury, regulate intestinal flora, inhibit ERK expression, and activate the SCF/c-kit signaling pathway, which may have a facilitating effect on gastrointestinal motility in simulated microgravity rats.
Core Tip: Previous studies have found that the simulated microgravity environment can cause gastrointestinal motility disorders in rats, but the mechanism is not clear. Considering that changes in intestinal mechanical barrier and biological barrier as well as stem cell factor (SCF)/c-kit signaling pathway related to Cajal stromal cells are closely related to gastrointestinal motility, we used protein molecular biology tests and bioinformatics methods to identify the effects and mechanisms of simulated microgravity environment on gastrointestinal motility in rats. And the effect of Bifidobacterium lactis (B. lactis) BLa80 after intervention. This study demonstrated for the first time that B. lactis BLa80 may affect the changes of gastrointestinal motility by improving intestinal mucosal barrier, improving intestinal flora dysregulation, and up-regulating SCF/c-kit signaling pathway.
- Citation: Zhang P, Zhu Y, Chen P, Zhou T, Han ZY, Xiao J, Ma JF, Ma W, Zang P, Chen Y. Effects of Bifidobacterium lactis BLa80 on fecal and mucosal flora and stem cell factor/c-kit signaling pathway in simulated microgravity rats. World J Gastroenterol 2025; 31(1): 96199
- URL: https://www.wjgnet.com/1007-9327/full/v31/i1/96199.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i1.96199
With the rapid development of human spaceflight, space medicine research is also facing major opportunities and challenges, and it is of great significance to ensure that astronauts can carry their missions in the space environment for a long time and in a more stable manner. In the spaceflight environment, microgravity is an extremely special factor that affects various organ systems of the human body, and in recent years, the research on the cardiovascular system, bones and other aspects of the microgravity environment has been relatively mature[1,2]. In recent years, research on the cardiovascular system and bones under microgravity environment is more mature. As for the digestive system, there are still many uncharted areas that deserve further exploration and research in terms of a series of physiological and/or pathological changes in the digestive system caused by microgravity factors.
Previous studies have found that microgravity or simulated microgravity environments can lead to damage to the body's intestinal mucosal barrier, dysbiosis of intestinal flora, and other alterations affecting gastrointestinal functions[3-5]. Interstitial cells of Cajal (ICCs) are the activation cells of gastrointestinal smooth muscle and the activation device of gastrointestinal slow wave[6]. Tyrosine kinase receptor (c-kit) is an important protein expressed on the surface of ICCs, which plays a key role in maintaining the phenotype and development of ICCs[7]. Stem cell factor (SCF) is a natural ligand of c-kit. The combination of SCF and c-kit can activate tyrosine kinase, phosphorylate c-kit, bind and activate a variety of second messengers, and produce a series of physiological functions[8]. SCF/C-kit signaling pathway can determine the differentiation of ICCs, maintain the growth and development of ICCs, and affect the number, distribution and function of ICCS. SCF/c-kit pathway has various regulatory effects on ICC, and its abnormality can lead to gastrointestinal motility dysfunction or other diseases[9]. Bifidobacterium lactis (B. lactis) BLa80 is a probiotic that has the ability to increase the expression of anti-inflammatory factors, stabilize the structure of the intestinal microbial community, and improve intestinal immunity[10]. Therefore, B. lactis BLa80 is expected to be used as a modifier to improve gastrointestinal motility disorders, intestinal mucosal barrier damage and intestinal flora dysbiosis in a simulated microgravity environment.
In this study, the internationally recognized rat tail suspension model was used to simulate the spaceflight microgravity environment[11]. So as to explore the changes in fecal flora and mucosal flora, as well as the changes in SCF/c-kit signaling pathway in Cajal mesenchymal stromal cells related to gastrointestinal motility in the rats exposed to the simulated microgravity environment and to study the effects of the intervention of B. lactis BLa80 and the value of its application.
Thirty healthy male SD rats of SPF grade, weighing 250-290 g, were provided by Beijing Sipeifu Biotechnology Co., LTD. The rats were housed in the SPF-grade animal room of the Aerospace Training Center, placed in the room temperature of 22 ± 1 °C, alternating between light and dark for 12 hours, and fed freely on standard pellet diets and drinking water.
B. lactis BLa80 was purchased from We care probiotics (Suzhou) Co.
Acrylamide, Bisacrylamide, Protein Molecular Weight Marker (Bio-rad, 161-0374, United States); Horseradish Peroxidase Labeled Goat Anti-Rabbit IgG (Beijing Zhongsui Jinqiao Biotechnology Co., Ltd., ZB-2301); Horseradish Peroxidase Labeled Goat Anti-Mouse IgG (Beijing Zhongsui Jinqiao Biotechnology Co., Ltd., ZB-2305); ECL Luminescent Liquid (Perkin Elme, United States, NEL105001EA); Protease Inhibitor (Roche, Sweden, 04693116001); Phosphorylated Protease Inhibitor (Roche, Sweden, 04906845001); Trizol Kit (Invitrogen); M-MLV Reverse Transcription Kit (Promega); TGuide S96 Fecal Genomic DNA Kit [Tengen Biochemical Technology (Beijing) Co., Ltd., DP812]; KOD OneTM PCR Master Mix (Beijing Bailinke Biotechnology Co., Ltd., KMM-101); EB (Qingdao Topband Biotechnology Co., Ltd., E1020-100 mL); 250 bp DNA Ladder Marker (Beijing Liuhe Tong Economic and Trade Co., Ltd., 3424A); KOD FX Neo (TOYOBO) (Beijing Bailink Biotechnology Co., Ltd., KFX-201S).
Stabilized pressure and current electrophoresis instrument (United States Bio-Rad, Powerpac HQ); vertical electrophoresis tank (United States Bio-Rad, MP3); semi-dry trans-electrophoresis transfer instrument (United States Bio-Rad, Trans-Blot SD); computer image analyzer (United States, Image-Pro Plus Analysis Soft ware); 4 °C low-temperature High-speed centrifuge (Thermo, MR 23i); room temperature high-speed centrifuge (Germany eppendorf, 5417C); electronic balance (Germany Sartouris, BS224S); UV spectrophotometer (Germany eppendorf, Biophotometer); micro-sampler (Germany eppendorf, eppendorf Research plus); micro-sampler (Germany eppendorf, eppendorf Research plus); thermostatic water bath (United States SHELLAB, W20M-2); liquid nitrogen and portable specimen storage tank (YDS-30-125); Real-Time PCR instrument (United States ABI 7500 fast); digital gel imaging system (BINTA); nucleic acid UV spectrophotometer (Germany) Biophotometer.
Wash the surface of the tail of SD rats with soapy water to remove grease, dander and dirt and blow dry with a hair dryer; use two pieces of medical tape to stick on both sides of the rat's tail, leaving a little distance at the end of the end of the tail, about 0.5 cm; and then use two pieces of medical tape to fix it transversely, fixed at the root and middle of the rat's tail to prevent the medical tape from falling off, and use the extra part of the medical tape in the previous section to fix the chain, and if necessary, use one more piece of 1 tape to fix it transversely; after completion, hang the end of the chain on the runner of the tail suspension device, and depending on the angle of the rat's torso and the level (head-down position 30° tail suspension). Use another 1 piece of medical tape to fix the chain horizontally; after completion, hang the end of the chain on the rotor of the tail suspension device, and adjust the length of the chain according to the angle between the rat's torso and the horizontal plane (head lowered by 30° tail suspension), and check the suspension situation regularly every day, and if there is any change in the angle, it is only necessary to adjust the length of the chain.
All rats were randomly divided into 3 groups: 10 rats in the normal ground control group, 10 rats in the tail-hanging simulated microgravity group, and 10 rats in the probiotic tail-hanging simulated microgravity group. All rats were kept for a total of 28 days. The rats in the probiotic tail-hanging simulated microgravity group were gavaged with 1 mL of B. lactis per rat per day, and the remaining two groups were gavaged with 1 mL of water per rat per day.
The experiment was conducted for 28 days, the rats in the probiotic hanging group were given B. lactis BLa80 by gavage every day, and the rats in the control group were given water by gavage for 28 days, the concentration of the gavage was 0.1 g/mL, 1 mL/day for each rat. At the end of 28 days of the experiment, the rats were fasted for about 12 hours, and cervical vertebrae dislocated and executed, and the rats were extracted from the stomach, duodenum, jejunum, ileum, colon, and feces, and all of the extracted materials were operated on ice, and then stored in 4% paraformaldehyde after being washed with ice-cold PBS buffer. After the stomach, duodenum, jejunum, ileum and colon were taken, they were washed with ice-cold PBS buffer, and then part of them were put into -80 °C for storage, and part of them were fixed in 4% paraformaldehyde for storage. The whole experimental procedure was carried operational taxonomic unit (OTU) in the animal room (SPF class) of the China Astronaut Research and Training Center, approved by the Ethics Committee of the China Astronaut Research and Training Center, Ethics Committee No. ACC-IACUC-2021-014.
One cm duodenum, jejunum, ileum and colon tissues were taken, immersed and fixed in formaldehyde, embedded in paraffin and sectioned, stained with hematoxylin and observed under light microscope.
Gastric sinus tissue was taken, quickly ground and added to the cell lysis solution, then left on ice for 20 minutes, centrifuged at 4 °C, 10000 r/minute for 10 minutes, and the protein content of the supernatant was determined by BCA protein analysis kit. After buffer dilution, the lysates were heated for 5 minutes. The lysates were separated by 12% SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride membranes, which were closed using skim milk powder with 5% TBS-T and shaken for 60 minutes at room temperature. The membranes were incubated with primary antibodies [extracellular signal-regulated kinase (ERK), p-ERK] at 4 °C overnight, and the membranes were washed three times with TBST shaking for 5 minutes each time to remove the residual primary antibodies, incubated with secondary antibodies for 1.5 hours, and the membranes were washed three times with TBST shaking for 5 minutes each time to remove the residual secondary antibodies, and the proteins were visualized with enhanced chemiluminescence reagents. The intensity of the bands was analyzed semi-quantitatively using ImageJ v1.48 software.
Gastric tissue was removed from the refrigerator at -80 °C, transferred to a dry specimen bottle, added 0.3 mL of RNA lysis solution, homogenized lysed samples, waited for 5 minutes at room temperature, added 0.3 mL of dilution solution, blown with a pipette gun to mix, and then left to stand at room temperature for 3 minutes. centrifuged at 4 °C (12000 r/minute) for 10 minutes, and then the supernatant was carefully aspirated into the tube with a pipette gun. Remove 2 μg of total RNA from the sample to be tested, and add Random Primers, Oligo (d T) 15 Primer, and Nuclease-Free Water to 10 μL. React at 70 °C for 5 minutes, and then insert into crushed ice to bring the temperature down. Add the reaction solution and make up to 20 μL with Nuclease-Free Water and mix well, then perform reverse transcription. The primer sequences and amplification lengths are shown in Table 1. After amplification, the Cq values of the target genes in each sample were obtained, the RQ values were calculated, and the obtained data were organized and statistically analyzed.
| Primer sequences | Primer | Products |
| SCF forward primer | 5'-CAATGGACAGCAATGGCACT-3' | 104 bp |
| SCF reverse primer | 5'-ACTGCCCTTGTAAGACTTGA-3' | |
| c-kit forward primer | 5'-CGCAGCTTCCTTATGACCAC-3' | 101 bp |
| c-kit reverse primer | 5'-AGTGGCCTCAACTACCTTCC-3' | |
| GAPDH forward primer | 5'-CAACTCCCTCAAGATTGTCA-3' | 128 bp |
| GAPDH reverse primer | 5'-GGCATGGACTGTGGTCATGA-3' |
The feces and colon of rats in each group were taken, transferred to sterile freezing tubes, placed in -80 °C refrigerator for storage, genomic DNA was extracted and PCR amplified, PCR products were mixed and purified, libraries were constructed, sequencing was performed, and relevant information was analyzed.
SPSS 26.0 software and GraphPad Prism 8 software were used for statistical analysis and graphing, and the results of the measurement data conforming to the normal distribution are expressed as mean ± SD, and the t-test was used for the measurements that were normally distributed and with uniformity of variance between the two groups, and the Mann-Whitney U test was used for the measurements that didn't conform to the normal distribution and Welch's correction was used for the measurements that didn't conform to the normal distribution. Welch's correction was used when the variance of normal distribution was not uniform; one-way ANOVA was used for comparisons between multiple groups for normal distribution with uniform variance, followed by Tukey's test for two-by-two comparisons, and Kraskal-Wallis rank-sum test was used for failure to conform to normal distribution, followed by Duun's test for two-by-two comparisons. The Welch's ANOVA test was used when the variance of the normal distribution did not match, and P < 0.05 indicated statistical significance.
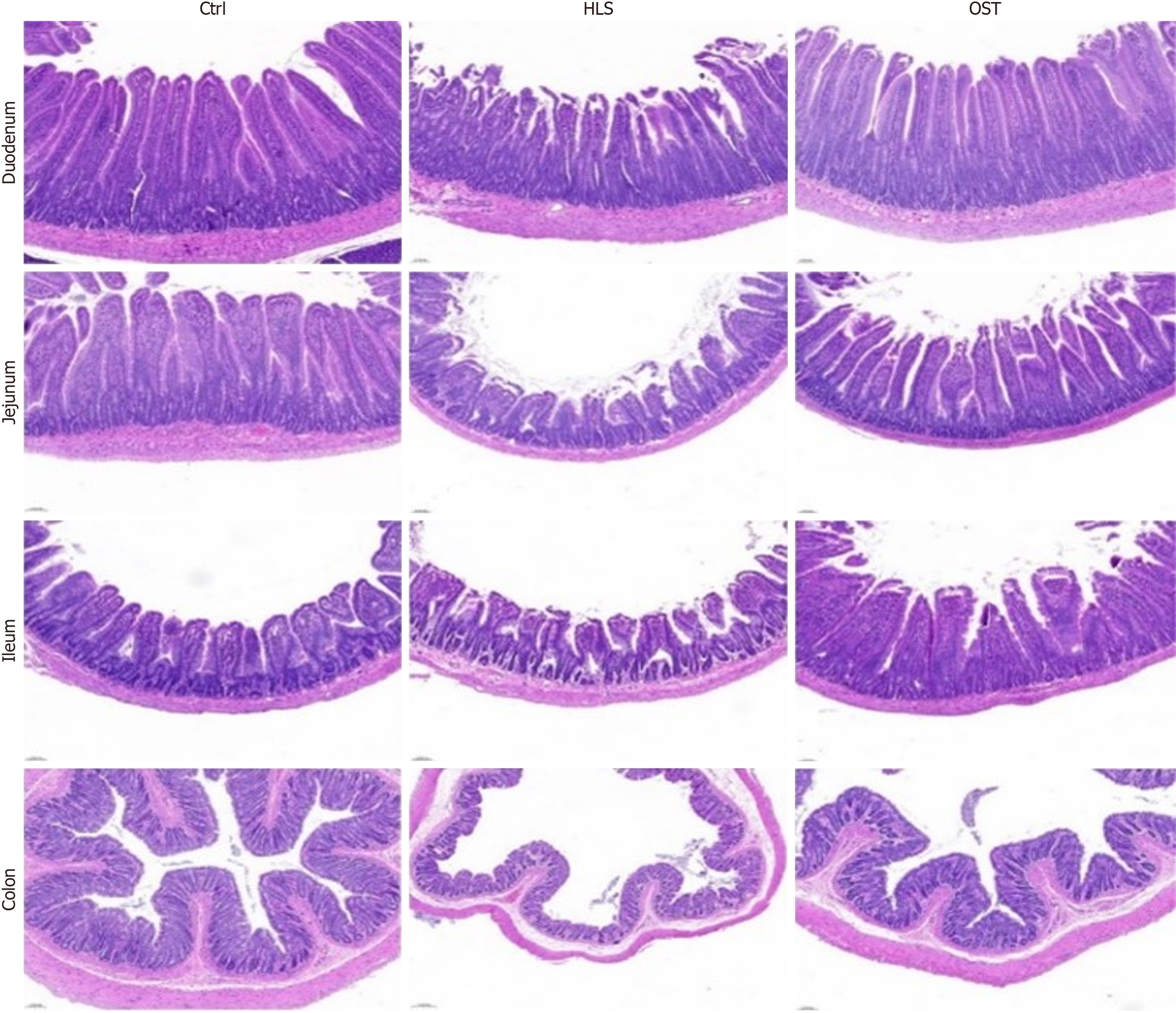
Three groups of rat duodenum, jejunum, ileum, and colon tissues were hematoxylin and eosin stained (Figure 1), and Ctrl, HLS, and OST represented the control group, tail-hanging group, and tail-hanging to probiotics group, respectively. Compared with the control group, the villi of the duodenum, jejunum and ileum in the tail-hanging group were loosely arranged, the gaps between the villi were enlarged, and the villi were severely detached and showed obvious breakage. The colonic columnar epithelium became shorter, with fewer folds, sparse lamina propria, and fewer cup cells; compared with the tail-hanging group, the duodenum, jejunum, and ileum in the probiotic tail-hanging group were tightly arranged, with less villi shedding, and with normal number of colonic cup cells and crypts.
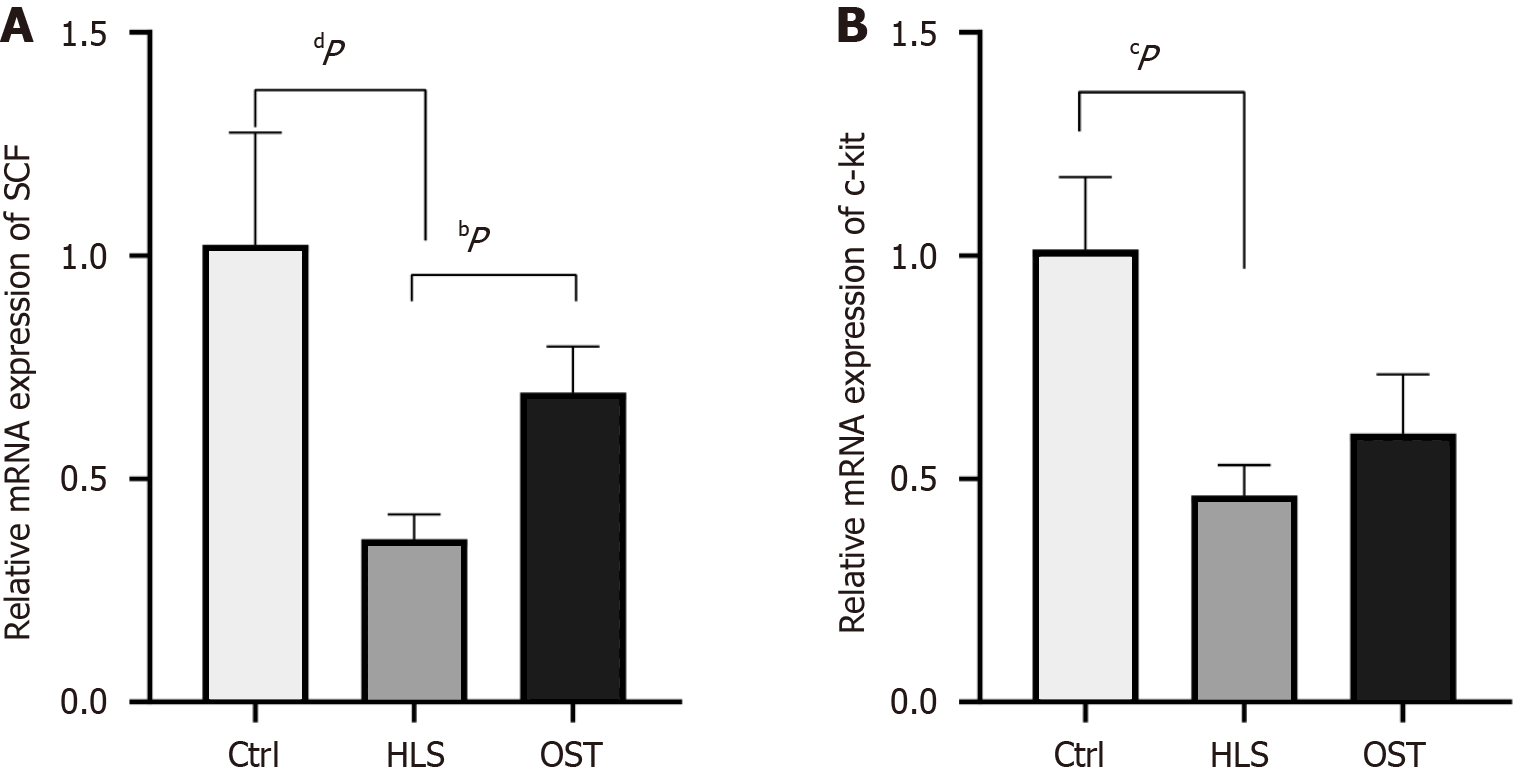
Real-time fluorescence quantitative PCR was applied to detect the expression of c-kit mRNA and SCF mRNA in the gastric sinus tissues of the three groups of rats. The results showed that the expression of c-kit mRNA and SCF mRNA in the tail-hanging group was significantly lower compared with that in the control group (P < 0.0001; P < 0.001), and the expression of c-kit mRNA and SCF mRNA in the tail-hanging group presented a trend of elevation with significant difference (P < 0.01), and that in the tail-hanging group the expression of SCF mRNA in the probiotics The expression of SCF mRNA in the tail-hanging group showed an elevated trend with significant difference (P < 0.01), The expression of c-kit mRNA in the probiotic tail-hanging group showed an elevated trend, but there was no significant difference (P > 0.05; Figure 2).
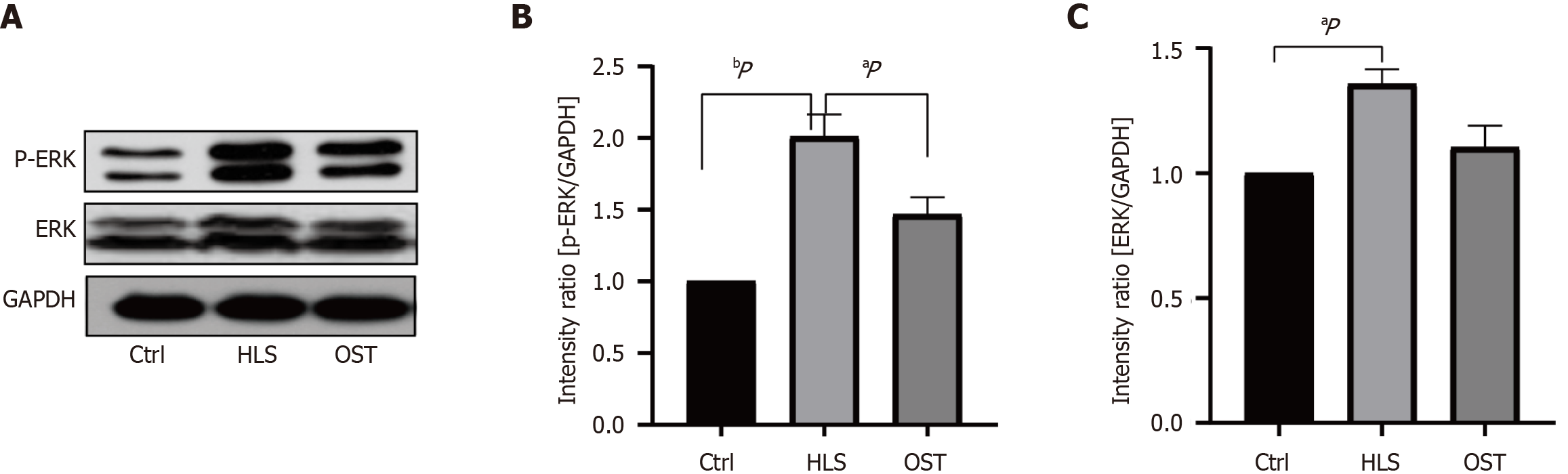
As can be seen in Figure 3, ERK and p-ERK were significantly up-regulated (P < 0.05) in the dangling tail group compared to the ground group, and ERK and p-ERK were significantly down-regulated (P < 0.05) in the probiotic tail dangling group compared to the dangling tail group (Figure 3).
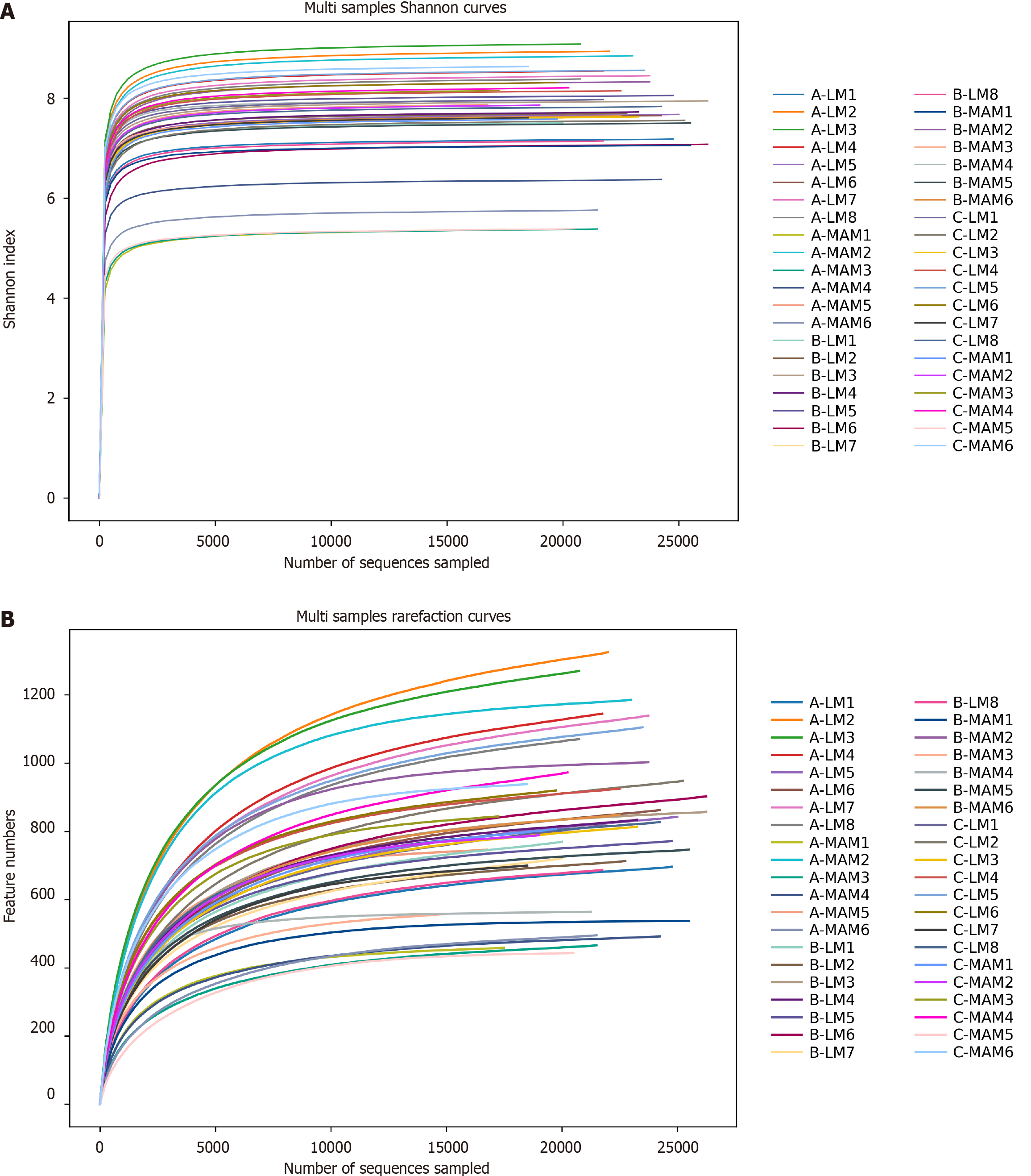
Sequencing depth assessment: As shown in Figure 4, the Shannon index curve (Figure 4A) and Rarefaction curve (Figure 4B) are used to assess the depth of sequencing, which can be used to compare the richness and diversity of species in different samples, and can also be used to indicate whether the sample size is reasonable. The horizontal coordinate represents the amount of randomly selected sequencing data, and the vertical coordinates are the Shannon index, and the number of observed species, respectively. As the amount of sequencing increases, the curve flattens OTU, indicating that the depth of sequencing is sufficient to reflect most of the microbial information in the sample, and that the sampling size is reasonable, and that adding more samples will only produce a small number of new species.
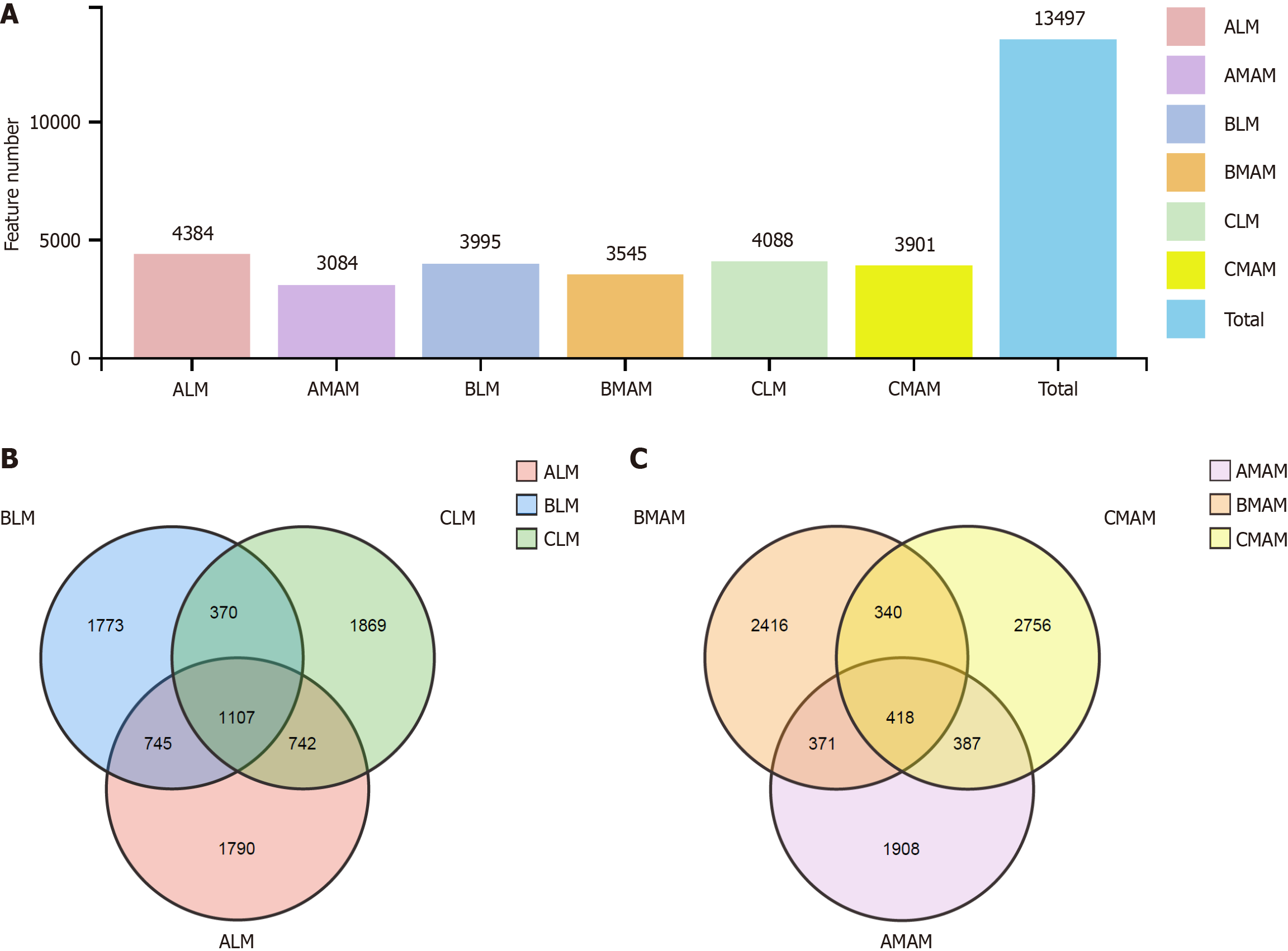
As shown in Figure 5, bar graphs (Figure 5A)and Venn diagrams (Figure 5B and C) were used to visualize the shared and specific OTUs between intestinal luminal microbiata (LM) and mucosa-associated microbiota (MAM), ALM and AMAM denote the intestinal luminal flora and mucosal flora in the control group respectively; BLM and BMAM respectively denote the intestinal luminal flora and mucosal flora of rats in the tail-hanging group; and CLM and CMAM denote the intestinal luminal flora and mucosal flora of rats in the probiotic tail-hanging group respectively. There were 4384 OTUs for ALM, 3995 OTUs for BLM, and 4088 OTUs for CLM, and a total of 1107 OTUs between them; 3084 OTUs for AMAM, 3545 OTUs for BMAM and 3901 OTUs for CMAM. CMAM had 3901, with a total of 418 OTUs between them. There were differences between the control, tail-hanging, and probiotic tail-hanging groups, and for the overall OTU trend, tail-hanging decreased the overall OTU values of the rats compared to the control group, while the probiotic tail-hanging group increased the overall OTU values of the rats. In addition, the total number of characteristics of mucosal flora was smaller than that of fecal flora, in which the tail-hanging group decreased the OTUs of fecal flora and increased the OTUs of mucosal flora in rats, and the probiotic tail-hanging group had a trend of increase for both fecal flora and mucosal flora.
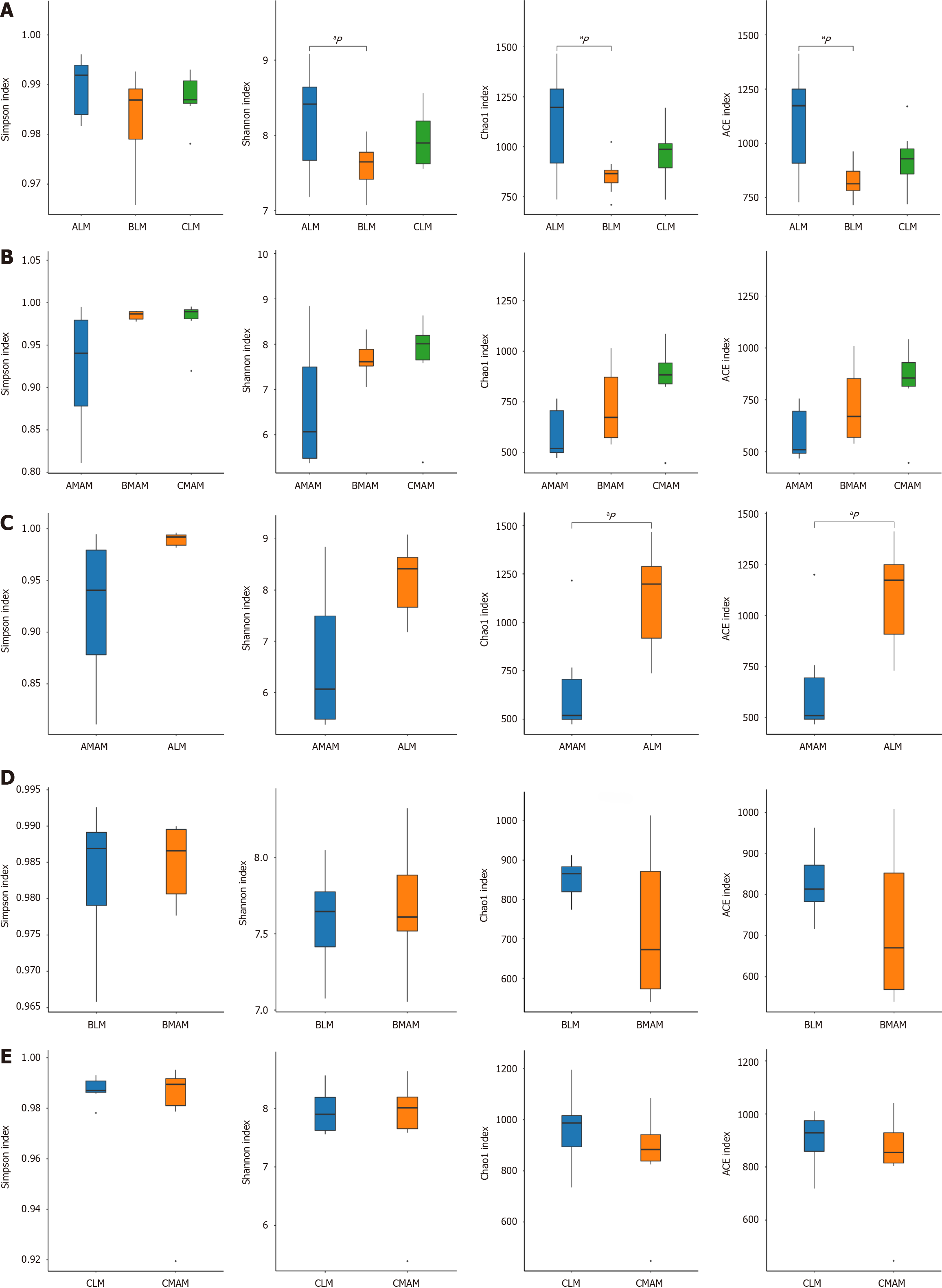
The alpha diversity index assesses the richness and diversity of sample species. the Chao1 and Ace indices can be used to assess species richness; Shannon and Simpson are used to measure diversity.
Part A in Figure 6 indicates whether there was a difference in fecal flora (LM) between the three groups (Figure 6A); Part B indicates whether there was a difference in mucosal flora (MAM) among the three groups (Figure 6B); Part C indicates whether there was a difference between fecal flora (ALM) of the control group and mucosal flora (AMAM) of the control group (Figure 6C); Part D indicates whether there is a difference between fecal flora (BLM) of the tail hanging group and mucosal flora (BMAM) of the tail hanging group whether there is a difference between them (Figure 6D); Part E indicates whether there is a difference between the fecal flora (CLM) of the probiotic hanging tail group and the mucosal flora (CMAM) of the probiotic hanging tail group (Figure 6E). From part A, it can be seen that the Shannon index, Chao1 and ACE index of the fecal flora of the tail-hanging group were significantly lower compared with that of the control group, and the difference was statistically significant (P < 0.05), indicating that the richness and diversity of the rat fecal flora in the tail-hanging group showed a decreasing tendency compared with that of the control group, and that the probiotic tail-hanging group was elevated compared with the tail-hanging group, but there was no significant difference; From part B It can be seen that the Shannon, Simpson, Chao1 and ACE indices were elevated in the hanging tail group compared with the control group of mucosal flora, and the difference was not statistically significant (P > 0.05), and the abundance and diversity of mucosal flora of the hanging tail group were slightly increased compared with that of the control group, but it was not statistically significant. This indicates that tail hanging decreases the richness and diversity of fecal flora and increases the richness and diversity of mucosal flora in rats, which may be a transient flora shift or flora alteration in response to stress caused by tail hanging. In the probiotic tail-hanging group, the trend of each index was higher relative to the tail-hanging group, indicating that B. lactis BLa80 increases the abundance and diversity of fecal flora and mucosal flora in simulated rats. From C, it can be concluded that the ACE and Chao1 indices of the fecal flora were significantly higher than those of the mucosal flora in the control group compared to the mucosal flora, and the differences were statistically significant (P < 0.05), but the differences in Shannon and Simpson indices were not statistically significant (P > 0.05), indicating that there is a difference in abundance between fecal flora and mucosal flora in the rats themselves. From D, it can be concluded that the Shannon, Simpson, Chao1 and ACE indices between the fecal flora and mucosal flora of the hanging tail group were not significantly different (P > 0.05). From E, it can be concluded that there was also no significant difference in Shannon, Simpson, Chao1 and ACE indices between fecal flora and mucosal flora in the probiotic hanging tail group (P > 0.05).
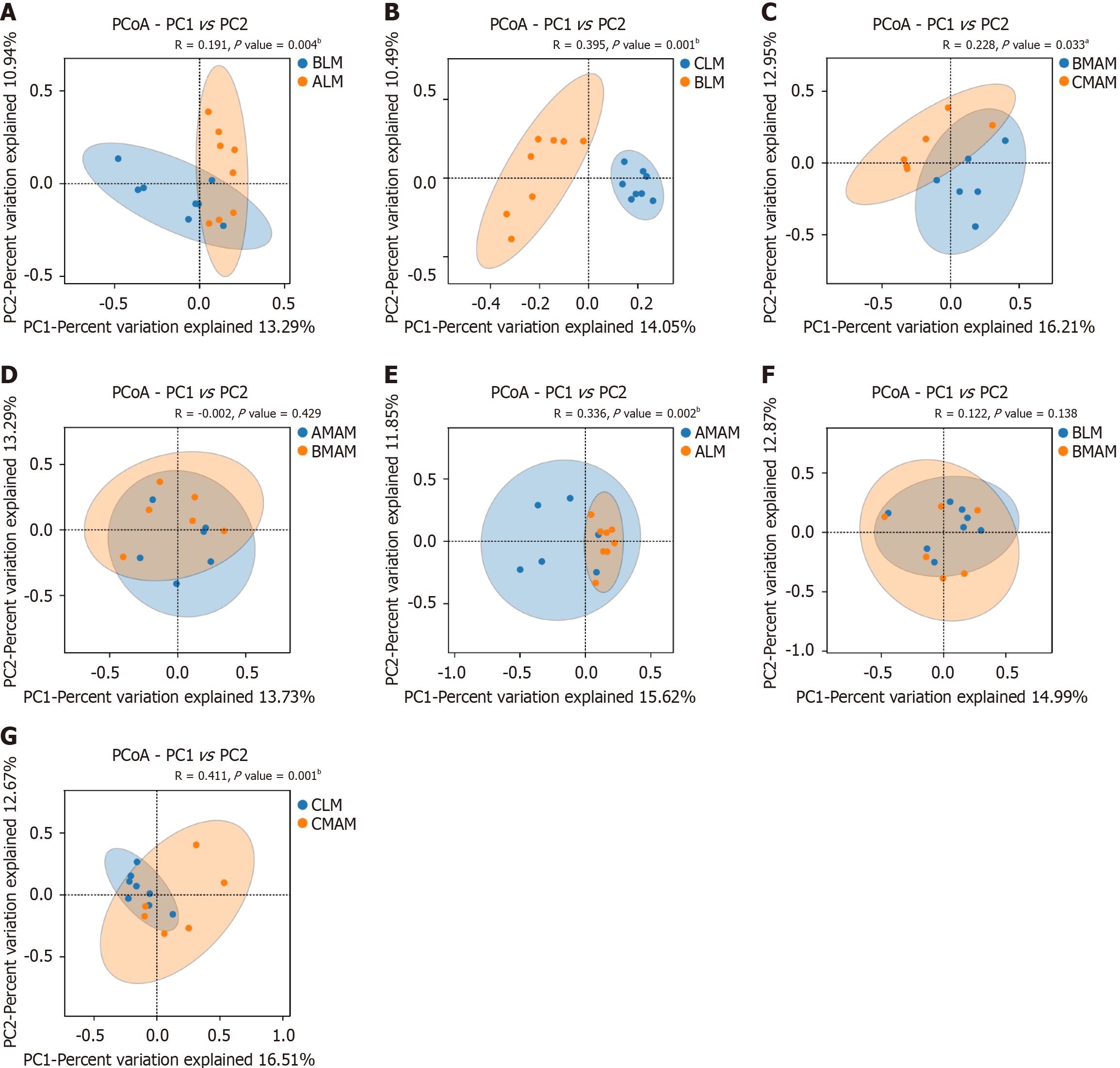
There was a significant difference in community structure between the fecal flora (ALM) of the control group and the fecal flora (BLM) of the tail-hanging group (P = 0.004; Figure 7A). As obtained from part B, there was a significant difference in community structure between the fecal flora (BLM) of the tail-hanging group and the fecal flora (CLM) of the probiotic tail-hanging group (P = 0.001; Figure 7B). As obtained from part C, there was a significant difference in community structure between the mucous membrane flora (BMAM) of the control group and the mucous membrane flora (CMAM) of the probiotic tail-hanging group (P = 0.033; Figure 7C). There was a significant difference (P = 0.033) in the community structure between the mucosal flora (BMAM) and the probiotic tail-hanging mucosal flora (CMAM). There was no significant difference (P = 0.429) in the community structure between the mucosal flora (AMAM) of the control group and the mucosal flora (BMAM) of the tail-hanging group (Figure 7D).
There was a significant difference between the fecal flora (ALM) and the mucosal flora (AMAM) of the control group (P = 0.002; Figure 7E). There was no significant difference in the community structure between the fecal flora (BLM) and the mucosal flora (BMAM) of the tail-hanging group (P = 0.138; Figure 7F). There was a significant difference in the community structure between the fecal flora (CLM) and the mucosal flora (CMAM) of the probiotic tail-hanging group (P = 0.001; Figure 7G). The community structure of the fecal flora was significantly different between the fecal flora and the mucosal flora (CMAM) of the probiotic tail-hanging group (P = 0.001).
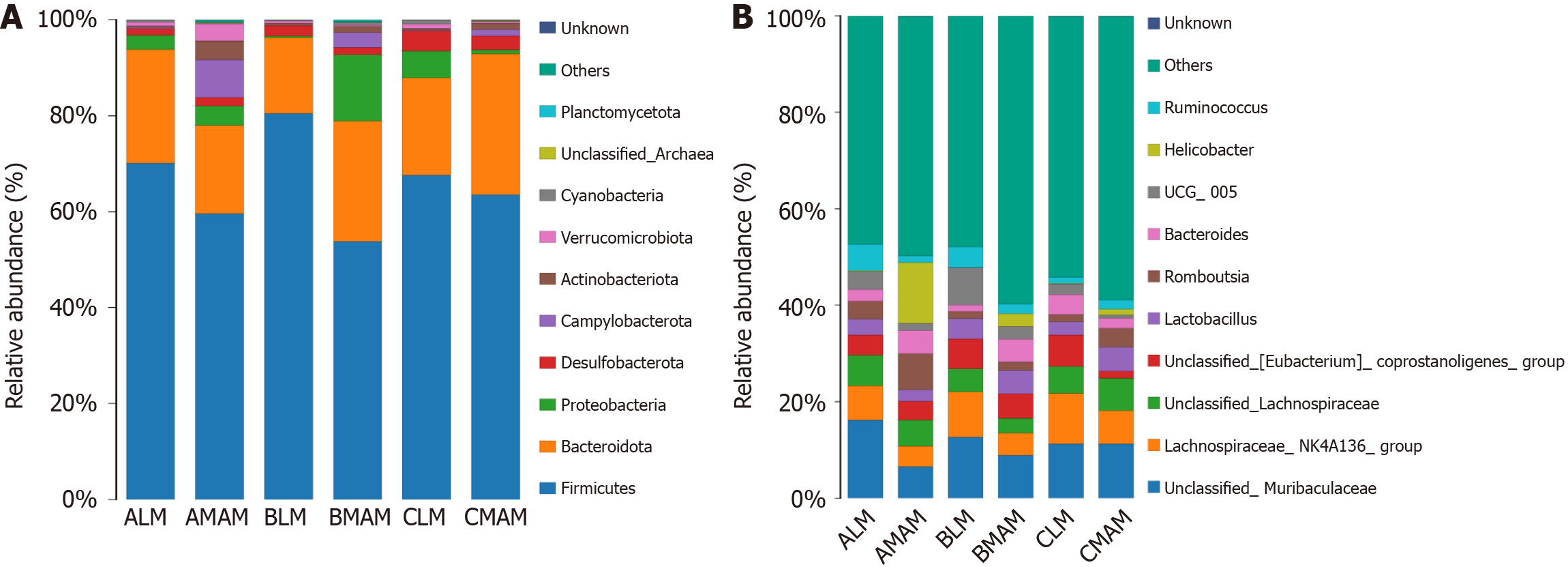
The samples from each group of control fecal flora (ALM), hanging tail group fecal flora (BLM), probiotic hanging tail group fecal flora (CLM), control mucosal flora (AMAM), hanging tail group mucosal flora (BMAM), probiotic hanging tail mucosal flora (CMAM) were annotated for the species at the phylum and genus level, and the samples from each group of the relative abundance of each species were selected based on the distribution of relative abundance of species, the top 10 species ranked by each group of the relative abundance of the species at the phylum and genus levels, the crop species group abundance histogram (Figure 8). Based on the distribution of relative abundance of species, the top 10 species in each group at phylum and genus level were selected, and the abundance histogram of crop species groups was presented (Figure 8).
Phylum level, as shown in Figure 8A, the intestinal flora of these 6 groups was dominated by Firmicutes, Bacteroidota, Proteobacteria, Campylobacterota, Desulfobacterota, Actinobacteriota, Verrucomicrobiota, Cyanobacteria, unclassified Archaea, and Planctomycetota. The top three ALM abundance ratios were 72.0% for the Firmicutes, 22.2% for the Bacteroidota, and 2.7% for the Proteobacteria. The top three abundance ratios for the AMA were 58.8% for the Firmicutes, 16.2% for the Bacteroidota, and 12.6% for the Campylobacterota. The top three abundance ratios for the BLM were 78.1% for the Firmicutes, 17.5% for the Bacteroidota, and 1.9% for the Desulfobacterota. The top three abundance ratios for the BMAM were 53.3% Firmicutes, 28.6% Bacteroidota, and 11.8% Proteobacteria. The CLM top three abundance ratios were 68.6% Firmicutes, 19.5% Bacteroidota, and 5.0% Proteobacteria. The CMAM top three abundance ratios were66.9% Firmicutes,25.5% Bacteroidota, and2.9% Desulfobacterota (Figure 8A).
Genus level, as shown in Figure 8B, the intestinal flora of these six groups is dominated by unclassified_Muribaculacea, Lachnospiraceae_NK4A136_group, unclassified_Lachnospiraceae, unclassified_(Eubacterium) coprostanoligenes_group, Lactobacillus, Romboustia, Bacteroides, UCG 005, Helicobacter, Ruminococcus comprise. The top three groups unclassified_Muribaculaceae, Lachnospiraceae_NK4A136_group, unclassified_Lachnospiraceae, and the top three abundances of the ALM group accounted for 16.1%, 7.1%, and 6.3%, respectively; the top three abundances of the AMAM were 6.5%, 4.2%, and 5.4%; BLM top three abundances were 12.6%, 9.3%, and 4.8%; BMAM top three abundances were 8.9%, 4.5%, and 3.0%; CLM top three abundances were 11.3%, 6.8%, and 6.7%, and CMAM top three abundances were 11.2%, 6.8%, and 6.7% (Figure 8B).
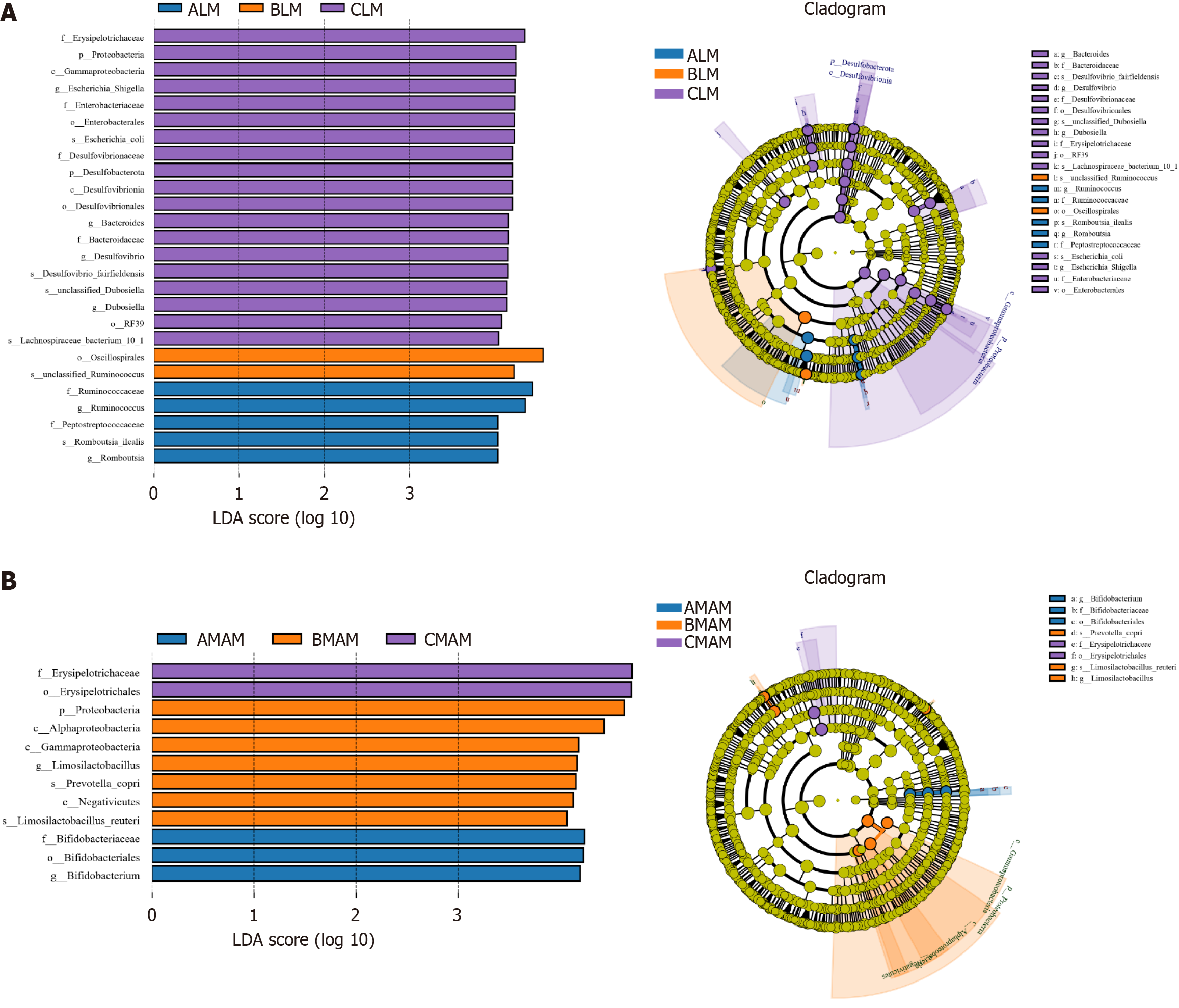
Species with the greatest impact on grouping in the ALM, BLM & CLM groups, and the AMAM, BMAM & CMAM groups were analyzed with LEfSe, i.e., species that were statistically different between groups (LDA threshold > 4), and taxonomic levels were chosen from phylum to species level.
As shown in Figure 9A, between the ALM, BLM, and CLM groups, a total of 26 species had statistically significant contributions to the species differences among the three groups, including five species that were significantly elevated in the ALM group, including Ruminococcaceae, Ruminococcus spp, Peptostreptococcaccac, Romboussia ilealis, and Romboutsia; and two species were significantly enriched in the BLM, including Oscillospirales, unclassified Ruminococcus; 19 species were significantly enriched in CLM, including Erysipelotrichaceae, Protobacteria, Enterobacterlacede, and Bacteroides (Figure 9A).
As shown in Figure 9B, between AMAM, BMAM and CMAM groups, a total of 12 species had statistically significant contributions to the species differences among the three groups, of which three species were significantly elevated in the AMAM group, including Bifidobacteriaceae, Bifidabacteriales, Bifidobacterium; seven species were significantly enriched in BMAM, including Proteobacteria, Alphaprotcobacteria, and Gammaproteobacteria; and two species were significantly enriched in CMAM enriched in CMAM, including Erysipelotrichaceae, Erysipelotrichales (Figure 9B).
With the rapid development of China's manned spaceflight industry, research on aerospace medicine is also facing major opportunities and challenges, and it is of great significance to ensure that astronauts can carry their missions more stably for a longer period of time in the spaceflight environment. In the spaceflight environment, microgravity is an extremely special factor that affects various organ systems of the human body, and in recent years, research on the cardiovascular system, bones and other aspects of the microgravity environment has been relatively mature[12-14]. While in the digestive system, microgravity causes a series of physiological and/or pathological changes in the digestive system, and there are still a lot of uncharted areas worthy of further exploration and research[15]. The present study was conducted using an internationally recognized rat model. In this study, the internationally recognized rat tail suspension model was used to simulate the spaceflight microgravity environment[16]. This study uses the internationally recognized rat tail suspension model to simulate the microgravity environment of spaceflight. Based on the theory of "microbe-brain-gut axis", we investigated some pathological and/or physiological alterations of the digestive system caused by microgravity, and explored the effects of simulated microgravity on the intestinal mucosal barrier, gastrointestinal dynamics-related SCF/c-kit pathway, and intestinal microecology of rats, as well as the mechanism of their interactions.
B. lactis BLa80 is a gram-positive polymorphic bacillus, which has been shown in many studies to have the ability to regulate diarrhea and constipation and enhance intestinal immunity[10]. B. lactis BLa80 is a Gram-positive polymorphic bacillus that has been shown in several studies to be effective in regulating diarrhea and constipation as well as enhancing intestinal immunity; it has a strong inhibitory and co-polymerizing ability against a variety of harmful bacteria, such as Escherichia coli, Salmonella, and Staphylococcus aureus, and it can increase the expression of anti-inflammatory factors, stabilize the intestinal microbial community structure, and significantly enhance the cellular immune function, humoral immunity, monocyte-macrophage function, and natural killer cell activity[17,18]. It has been widely reported in improving acute diarrhea symptoms and reducing inflammatory response in ulcerative colitis[10], but its effect on the digestive system in simulated microgravity environment has been less studied. Therefore, the present study focused on exploring the effects and mechanisms of B. lactis BLa80 on intestinal mucosal barrier, intestinal microorganisms, and gastrointestinal function in rats from the perspective of simulated microgravity.
First of all, the intestinal mucosal barrier is an important part of the human digestive system, which protects the health of the organism by maintaining the stability of the intestinal internal environment and preventing the entry of harmful substances into the body[19]. Intestinal barriers include mechanical barriers, immune barriers, chemical barriers, and microbial barriers. Mechanical barriers, also known as physical barriers, are the most basic line of defense, and their physiological structure is the mucosal epithelium, lamina propria, and mucosal muscularis layer, so the integrity of the intestinal structure is particularly important[20,21]. The integrity of the intestinal structure is therefore particularly important. Whether the intestinal tract is able to perform its proper function is related to the normal structure of the intestinal mucosa, the thickness of the intestinal mucosa, the length and tightness of the villi, and the number of cup-shaped cells, etc[22]. The thickness of the intestinal mucosa and the length and compactness of the villi determine the digestive and absorptive functions of the intestinal tract, and the cuprocytes play an important role in the secretion of mucus, the regulation of immune response, and the maintenance of intestinal microbial balance. The thickness of the intestinal mucosa, the length and tightness of the villi and the number of cuprocytes can be observed to show the changes in the digestive function of the intestinal mucosa and the degree of damage. Narrower mucosal thickness, shorter villi and fewer cup cells may lead to decreased digestive ability, shorter food retention time and imbalance of intestinal flora, which may affect intestinal digestion and absorption[23]. The present study was conducted in the duodenum, jejunum, and the intestine. In this study, the changes in tissue morphology and structure were observed by hematoxylin and eosin staining of tissue sections of duodenum, jejunum, ileum and colon using microscope. The results found that, compared with the ground group, the villi of duodenum, jejunum and ileum in the hanging group were severely detached and appeared obviously broken, the gap between the villi increased and the arrangement was loose, the columnar epithelium of the colon became shorter, the folds became fewer, the thickness of the mucosa became narrower, the number of cup cells and the crypts were reduced, and the weight of rats was obviously reduced after hanging, and there was diarrhea and other conditions; while there was a significant improvement in the probiotic hanging group, and the villi of duodenum, jejunum and ileum were arranged more tightly, and the mucosa was more compact, with the number of cup cells and crypts decreased. The villi of the intestine were more closely arranged, the villi shedding was lighter, the length of villi was longer, the number of cup cells and crypts of the colon tissue was normal, and the body weight was significantly improved after probiotic intervention compared with that of the tail hanging group, which indicated that the simulated microgravity would cause serious damage to the intestinal mucosa of the rats, with the thickness of the mucosa becoming narrower, the shedding of villi being impaired, and the reduction of the cup cells and crypts would result in the destruction of the intestinal mechanical barrier, and the cellular gaps of the intestine would be enlarged, and the defense ability decreased, and the displacement of intestinal flora could easily occur, leading to weight loss, diarrhea and other gastrointestinal dynamics disorders. After the intervention of B. lactis BLa80, it improved the pathological symptoms of the intestinal mucosal tissue of rats in the simulated microgravity environment, and it played a certain repairing effect on the intestinal mucosal barrier of the rats in the simulated microgravity environment.
Secondly, in addition to the aforementioned histomorphometric changes in the intestinal segments, the changes in gastrointestinal dynamics under exposure to simulated microgravity environment are also one of the focuses of the study. Previous studies at home and abroad have shown that exposure to simulated microgravity environment can cause changes in gastrointestinal motility, but the specific mechanism has not been clarified. Modern studies have found that ICCs are closely related to gastrointestinal (GI) motility[24,25]. ICCs are gastrointestinal pacemaker cells that initiate and propagate gastrointestinal electrical rhythms, and many GI dysfunctions are associated with damage to or loss of ICCs[26]. ICCs are the initiators and transmitters of gastrointestinal electrical rhythms. c-kit is a specific marker for Cajal mesenchymal stromal cells, and almost all Cajal mesenchymal stromal cells can specifically express the c-kit receptor. SCF is a natural ligand for the c-kit receptor, and specific binding of the two activates the SCF/c-kit signaling pathway, which is important for the maintenance of the phenotype, development and maturation of ICCs[27]. ERK, as a central part of the mitogen-activated protein kinase signaling pathway, is able to regulate cell proliferation, development, and inflammatory responses, and is also able to influence the SCF/c-kit signaling pathway, a pathway that is tightly linked to Cajal mesenchymal stromal cells and their activities in the digestive system[28,29]. The results of the present study showed that both SCF and c-kit were significantly decreased in the gastric antrum of rats in the tail-hanging simulated microgravity group compared to the ground group, and that SCF and c-kit would be increased in the gastric antrum of the tail-hanging rats after B. lactis BLa80 intervention. In addition, it was also found that ERK and p-ERK in the gastric sinus of hanging-tailed rats were up-regulated to varying degrees compared with the ground group, and the expression of ERK and p-ERK was reduced in rats exposed to simulated microgravity environment after B. lactis BLa80 intervention. The above results indicate that exposure to simulated microgravity reduces the expression of proteins related to the SCF/c-kit signaling pathway and decreases the activity of Cajal mesenchymal stromal cells, and that after B. lactis BLa80 intervention, it is likely to inhibit the ERK pathway by modulating ERK phosphorylation and activate the SCF/c-kit signaling pathway, so that the activity of Cajal mesenchymal stromal cells is enhanced, thus participating in the rat's. This may play an ameliorative role in simulating gastrointestinal motility disorders in rats exposed to microgravity environment.
Finally, microgravity also affects the composition of gut microbes, disrupting intestinal homeostasis and increasing susceptibility to intestinal diseases, which may affect astronauts' organic health[30-32]. Previous studies have found that the space microgravity environment for astronauts affects the specific gravity of the phylum Mycobacterium anisopliae and the phylum Thick-walled bacteria[33]. However, there are few studies on the difference between the effects of fecal flora (LM) and mucosal flora (MAM) in microgravity environment. In this study, we first analyzed the clustering of OTUs of rat intestinal flora, and each OTU can be considered to represent a species, and found that tail-hanging decreased the overall OTUs of rat intestinal flora, and probiotics increased the overall OTUs of tail-hanging rats. In addition, tail-hanging decreased the OTUs of fecal flora and increased the OTUs of mucosal flora in rats, which may be attributed to the simulation of the intestinal mucosal barrier resulting from the exposure to the microgravity environment. This may be due to the destruction of the intestinal mucosal barrier caused by exposure to simulated microgravity environment, resulting in increased permeability of the intestinal mucosa, and a large number of luminal flora transmitting through the intestinal mucosa, and ultimately reaching and immobilizing in the deeper or middle layer of the intestinal mucosa, which may be one of the causes of the dysregulation of the intestinal immune system and the occurrence of diarrhea, constipation, gastrointestinal dysfunction, etc., in rats[34]. According to the α-diversity analysis, tail-hanging decreased the abundance and diversity of fecal flora and increased the abundance and diversity of mucosal flora in rats, which also indicated that the stress response caused by tail-hanging probably damaged the intestinal mucosal barrier, resulting in the displacement of the intestinal flora and or alteration of the flora in rats. The fecal flora and mucosal flora of the probiotic tail-hanging group were both on the trend of increasing, suggesting that B. lactis BLa80 increases the abundance and diversity of the intestinal flora of tail-hanging rats. Based on the β-diversity analysis, it was found that there was a significant difference between the fecal flora and mucosal flora of rats per se, whereas there was no significant difference between the fecal flora and mucosal flora of rats exposed to simulated microgravity environment, which once again demonstrated that tail-hanging causes the displacement of fecal flora to mucosal flora to occur in rats. Regarding the differential species, at the phylum level, this study found that the top few between the three groups were composed of Phylum Thicket, Phylum Anaplasma, Phylum Aspergillus, and Phylum Actinomycetes, and the percentage of the top two in the control group reached more than 90, while most of the other conditionally pathogenic bacteria were less than 0.1%, which was similar to the intestinal flora structure of the healthy population. It was found that exposure to simulated microgravity increased the abundance of thick-walled bacilli and decreased the abundance of anamorphic bacilli in the rat fecal flora, which is consistent with previous United States National Aeronautics and Space Administration findings[33]. In addition, this study found that simulated microgravity environmental exposure increased the abundance of Bacteroidetes anomalies and Desulfovibrio flora and decreased the abundance of Firmicutes flora in the mucosal flora of rats, whereas the probiotic dangling group was trending toward an increase in Bacteroidetes anomalies for the overall level. At the genus level, this study found that all were composed of unclassified_Muribaculaceae, Lachnospiraceae_NK4A136_group, unclassified _(Eubacterium)_coprostanoligenes_group, etc. According to the LeFse analysis, it was found that the species phyla differed excessively between the groups, and the fecal flora of rats exposed to simulated microgravity environment had a reduced species diversity with significant differences, and was mostly dominated by a reduction in the number of beneficial flora, on the contrary, the mucosal flora of rats exposed to simulated microgravity environment had an increased species diversity with significant differences, and was mostly dominated by Proteobacteria.
In summary, simulated microgravity causes damage to the intestinal mucosal barrier, downregulation of the SCF/c-kit signaling pathway in Cajal mesenchymal stromal cells associated with gastrointestinal motility, and disorders of the intestinal flora in rats, and there is a significant difference between fecal and mucosal flora in rats per se, so future interventions can be considered as whether they are targeted at fecal or mucosal flora, and simulated microgravity reduces the OTU of the intestinal flora of the overall rat population, and B. lactis BLa80 increases OTU in rats, and the abundance and diversity of the intestinal flora in rats. B. lactis BLa80 increased the OTU of rats, and simulated micro
B. lactis BLa80 can reduce the damage of intestinal epithelial mucosal barrier and increase the expression of SCF/c-kit signaling pathway in Cajal mesenchymal stromal cells associated with gastrointestinal motility by regulating the structure, abundance and diversity of intestinal flora in rats under simulated microgravity, thereby improving the gastrointestinal motility disorders of rats in a tail-hanging environment. B. lactis BLa80's "intestinal flora-intestinal barrier-gastrointestinal motility" pathway, which targets the intestinal flora and its metabolites, may provide a new idea for the prevention and treatment of gastrointestinal dysfunction under the simulated microgravity environment.
B. lactis BLa80 can ameliorate intestinal mucosal injury, regulate intestinal flora, inhibit ERK expression, and activate the SCF/c-kit signaling pathway, which may have a facilitating effect on gastrointestinal motility in simulated microgravity rats.
| 1. | Hughson RL, Helm A, Durante M. Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat Rev Cardiol. 2018;15:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 2. | Moosavi D, Wolovsky D, Depompeis A, Uher D, Lennington D, Bodden R, Garber CE. The effects of spaceflight microgravity on the musculoskeletal system of humans and animals, with an emphasis on exercise as a countermeasure: a systematic scoping review. Physiol Res. 2021;70:119-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 3. | Yang JQ, Jiang N, Li ZP, Guo S, Chen ZY, Li BB, Chai SB, Lu SY, Yan HF, Sun PM, Zhang T, Sun HW, Yang JW, Zhou JL, Yang HM, Cui Y. The effects of microgravity on the digestive system and the new insights it brings to the life sciences. Life Sci Space Res (Amst). 2020;27:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Siddiqui R, Akbar N, Khan NA. Gut microbiome and human health under the space environment. J Appl Microbiol. 2021;130:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 5. | Shi J, Wang Y, He J, Li P, Jin R, Wang K, Xu X, Hao J, Zhang Y, Liu H, Chen X, Wu H, Ge Q. Intestinal microbiota contributes to colonic epithelial changes in simulated microgravity mouse model. FASEB J. 2017;31:3695-3709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 6. | Radu P, Zurzu M, Paic V, Bratucu M, Garofil D, Tigora A, Georgescu V, Prunoiu V, Popa F, Surlin V, Strambu V. Interstitial Cells of Cajal-Origin, Distribution and Relationship with Gastrointestinal Tumors. Medicina (Kaunas). 2022;59:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 7. | Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 8. | Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 701] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 9. | Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 621] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 10. | Dong Y, Liao W, Tang J, Fei T, Gai Z, Han M. Bifidobacterium BLa80 mitigates colitis by altering gut microbiota and alleviating inflammation. AMB Express. 2022;12:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 11. | Fu ZH, Wang Z, Wu J, Yang HY, Zhang X, Gao F, Li J. [A modified protocol for generating the simulated weightlessness rat model]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35:189-192. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 12. | Iandolo D, Strigini M, Guignandon A, Vico L. Osteocytes and Weightlessness. Curr Osteoporos Rep. 2021;19:626-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 13. | Norsk P. Adaptation of the cardiovascular system to weightlessness: Surprises, paradoxes and implications for deep space missions. Acta Physiol (Oxf). 2020;228:e13434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 14. | Pramanik J, Kumar A, Panchal L, Prajapati B. Countermeasures for Maintaining Cardiovascular Health in Space Missions. Curr Cardiol Rev. 2023;19:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 15. | Prakash M, Fried R, Götze O, May F, Frings-Meuthen P, Mulder E, Valentini J, Fox M, Fried M, Schwizer W, Misselwitz B. Microgravity Simulated by the 6° Head-Down Tilt Bed Rest Test Increases Intestinal Motility but Fails to Induce Gastrointestinal Symptoms of Space Motion Sickness. Dig Dis Sci. 2015;60:3053-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 16. | Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985). 2002;92:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 684] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 17. | Zhu M, Zhu J, Fang S, Zhao B. Complete Genome Sequence of Bifidobacterium animalis subsp. lactis BLa80, a Strain Isolated from Human Breast Milk. Microbiol Resour Announc. 2023;12:e0046522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 18. | Xu B, Liang S, Zhao J, Li X, Guo J, Xin B, Li B, Huo G, Ma W. Bifidobacterium animalis subsp. lactis XLTG11 improves antibiotic-related diarrhea by alleviating inflammation, enhancing intestinal barrier function and regulating intestinal flora. Food Funct. 2022;13:6404-6418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 19. | Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal Barrier in Human Health and Disease. Int J Environ Res Public Health. 2021;18:12836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (1)] |
| 20. | Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (2)] |
| 21. | Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3-20; quiz 21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1309] [Article Influence: 77.0] [Reference Citation Analysis (2)] |
| 22. | Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 1313] [Article Influence: 164.1] [Reference Citation Analysis (1)] |
| 23. | Okumura R, Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm Regen. 2018;38:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 24. | Huizinga JD, Hussain A, Chen JH. Interstitial cells of Cajal and human colon motility in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G552-G575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 25. | Mostafa RM, Moustafa YM, Hamdy H. Interstitial cells of Cajal, the Maestro in health and disease. World J Gastroenterol. 2010;16:3239-3248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 26. | Friedmacher F, Rolle U. Interstitial cells of Cajal: clinical relevance in pediatric gastrointestinal motility disorders. Pediatr Surg Int. 2023;39:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 27. | Tsai M, Valent P, Galli SJ. KIT as a master regulator of the mast cell lineage. J Allergy Clin Immunol. 2022;149:1845-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Choi S, Yeum CH, Kim YD, Park CG, Kim MY, Park JS, Jeong HS, Kim BJ, So I, Kim KW. Receptor tyrosine and MAP kinase are involved in effects of H(2)O(2) on interstitial cells of Cajal in murine intestine. J Cell Mol Med. 2010;14:257-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Dong F, Yang S, Sun H, Yan J, Guo X, Li D, Zhou D. Persistent mechanical stretch-induced calcium overload and MAPK signal activation contributed to SCF reduction in colonic smooth muscle in vivo and in vitro. J Recept Signal Transduct Res. 2017;37:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Jollet M, Nay K, Chopard A, Bareille MP, Beck A, Ollendorff V, Vernus B, Bonnieu A, Mariadassou M, Rué O, Derbré F, Goustard B, Koechlin-Ramonatxo C. Does Physical Inactivity Induce Significant Changes in Human Gut Microbiota? New Answers Using the Dry Immersion Hypoactivity Model. Nutrients. 2021;13:3865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Wang J, Han C, Lu Z, Ge P, Cui Y, Zhao D, Yang X, Wu B, Qiang L, Zhang Y, Chai Q, Lei Z, Li L, Hua Liu C, Zhang L. Simulated microgravity suppresses MAPK pathway-mediated innate immune response to bacterial infection and induces gut microbiota dysbiosis. FASEB J. 2020;34:14631-14644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Sun P, Yang J, Wang B, Ma H, Zhang Y, Guo J, Chen X, Zhao J, Sun H, Yang J, Yang H, Cui Y. The effects of combined environmental factors on the intestinal flora of mice based on ground simulation experiments. Sci Rep. 2021;11:11373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, McKenna MJ, Meydan C, Mishra T, Nasrini J, Piening BD, Rizzardi LF, Sharma K, Siamwala JH, Taylor L, Vitaterna MH, Afkarian M, Afshinnekoo E, Ahadi S, Ambati A, Arya M, Bezdan D, Callahan CM, Chen S, Choi AMK, Chlipala GE, Contrepois K, Covington M, Crucian BE, De Vivo I, Dinges DF, Ebert DJ, Feinberg JI, Gandara JA, George KA, Goutsias J, Grills GS, Hargens AR, Heer M, Hillary RP, Hoofnagle AN, Hook VYH, Jenkinson G, Jiang P, Keshavarzian A, Laurie SS, Lee-McMullen B, Lumpkins SB, MacKay M, Maienschein-Cline MG, Melnick AM, Moore TM, Nakahira K, Patel HH, Pietrzyk R, Rao V, Saito R, Salins DN, Schilling JM, Sears DD, Sheridan CK, Stenger MB, Tryggvadottir R, Urban AE, Vaisar T, Van Espen B, Zhang J, Ziegler MG, Zwart SR, Charles JB, Kundrot CE, Scott GBI, Bailey SM, Basner M, Feinberg AP, Lee SMC, Mason CE, Mignot E, Rana BK, Smith SM, Snyder MP, Turek FW. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science. 2019;364:eaau8650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 616] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 34. | Peritore-Galve FC, Kaji I, Smith A, Walker LM, Shupe JA, Washington MK, Algood HMS, Dudeja PK, Goldenring JR, Lacy DB. Increased intestinal permeability and downregulation of absorptive ion transporters Nhe3, Dra, and Sglt1 contribute to diarrhea during Clostridioides difficile infection. Gut Microbes. 2023;15:2225841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/