Published online Jan 7, 2025. doi: 10.3748/wjg.v31.i1.101463
Revised: October 7, 2024
Accepted: October 30, 2024
Published online: January 7, 2025
Processing time: 84 Days and 20.6 Hours
A dual therapy regimen containing amoxicillin is a common treatment option for the eradication of Helicobacter pylori (H. pylori). While substantial research sup
To evaluate efficacy and safety of VA dual therapy as first-line or rescue treatment for H. pylori in elderly patients.
As a real-world retrospective study, data were collected from elderly patients aged 60 years and above who accepted VA dual therapy (vonoprazan 20 mg twice daily + amoxicillin 1000 mg thrice daily for 14 days) for H. pylori eradication in the Department of Gastroenterology at Peking University First Hospital between June 2020 and January 2024. H. pylori status was evaluated by 13C-urease breath test 6 weeks after treatment. All adverse events (AEs) during treatment were recorded.
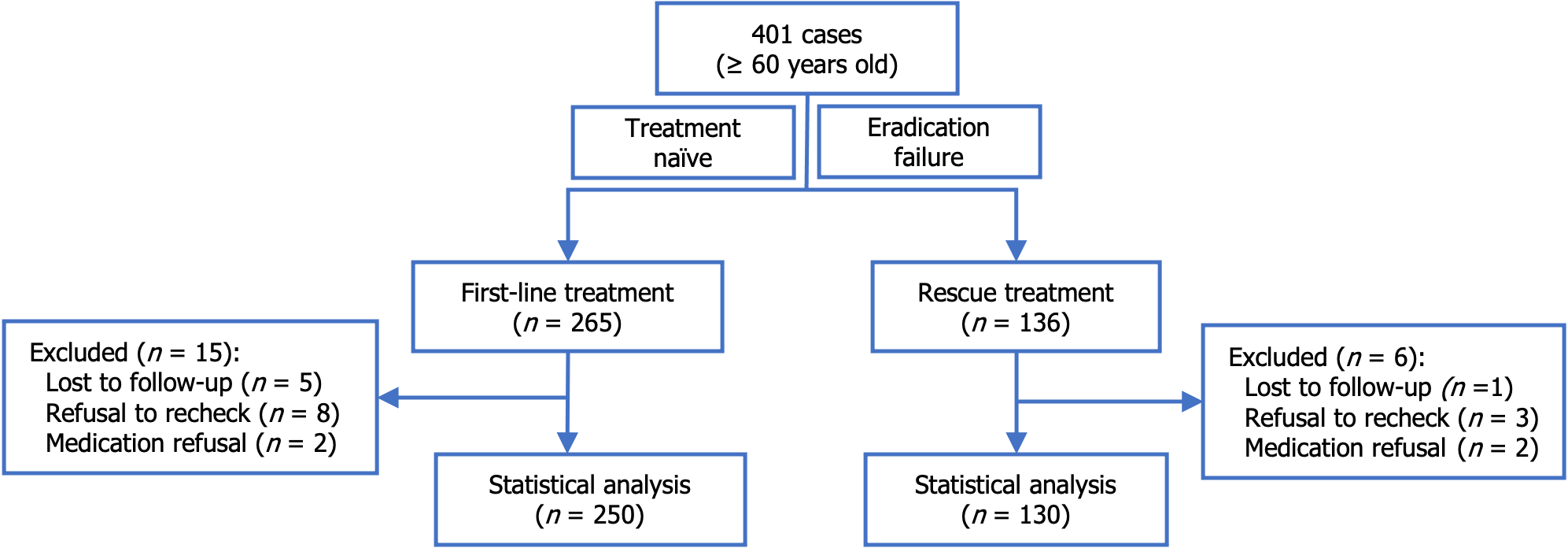
In total, 401 cases were screened. Twenty-one cases were excluded due to loss to follow-up, lack of re-examination, or unwillingness to take medication. The total of 380 included cases comprised 250 who received VA dual therapy as first-line treatment and 130 who received VA dual therapy as rescue treatment. H. pylori was successfully eradicated in 239 cases (95.6%) in the first-line treatment group and 116 cases (89.2%) in the rescue treatment group. The overall incidence of AEs was 9.5% for both groups. Specifically, 9.2% of patients experienced an AE in the first-line treatment group and 10.0% in the rescue treatment group. Five patients discontinued treatment due to AE, with a discontinuation rate of 1.3%. No serious AE occurred.
The VA dual therapy regimen as a first-line treatment and a rescue therapy was effective and safe for elderly patients aged 60 and older.
Core Tip: Vonoprazan-amoxicillin (VA) dual treatment is as effective as traditional bismuth-based quadruple therapy for the eradication of Helicobacter pylori (H. pylori) in the general population. However, the safety of this regimen in the elderly population is unknown. This real-world study retrospectively analyzed data from elderly patients treated for H. pylori infection. VA dual therapy demonstrated good safety and efficacy in the elderly patients with an eradication rate similar to that of the general population. The findings provided evidence supporting the use of VA dual therapy in elderly patients.
- Citation: Gao W, Li JW, Ye H, Zhang XZ, Liu JX, Cheng H. Real-world evidence on the efficacy and safety of vonoprazan-amoxicillin dual therapy for Helicobacter pylori treatment in elderly patients. World J Gastroenterol 2025; 31(1): 101463
- URL: https://www.wjgnet.com/1007-9327/full/v31/i1/101463.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i1.101463
Dual therapy combining acid suppressants, such as vonoprazan or proton pump inhibitors, with amoxicillin has garnered significant attention for the treatment of Helicobacter pylori (H. pylori) infection. This regimen is a safe and effective option due to few drug interactions and a low incidence of adverse events (AEs) compared to more complex regimens[1,2]. While substantial research supports the efficacy and safety of dual therapy in the general population, there is still a lack of studies specifically focusing on its safety in elderly patients.
The elderly population often faces additional challenges, including multiple chronic conditions and the need for long-term medication. These challenges make them more vulnerable to drug interactions and adverse reactions[3]. Therefore, it is crucial to conduct in-depth safety studies that focus on elderly patients to ensure that a treatment approach is appropriate for them.
Our study aimed to gain a comprehensive understanding of the efficacy and safety of vonoprazan and amoxicillin (VA) dual therapy specifically in the population older than 60 years. The results from this study can provide valuable insights into the applicability and tolerability into VA dual therapy for H. pylori eradication in this sensitive population.
A retrospective real-world study was carried out at the Department of Gastroenterology, Peking University First Hospital, Beijing, China. The data collection period was from June 2020 to January 2024. The study included elderly patients (aged ≥ 60 years) who received VA dual therapy as either a first-line or rescue treatment. The primary objective was to assess the eradication rate, while secondary objectives included the incidence of AE and treatment adherence. The study was reviewed and approved by the Ethics Committee of Peking University First Hospital (Approval No. 2023Y009-001).
H. pylori infection was diagnosed as positive through the 13C-urease breath test (13C-UBT) (75 mg 13C-urea; Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., Shenzhen, China). To assess treatment efficacy, H. pylori status was reassessed via the 13C-UBT at least 6 weeks following the completion of therapy. The VA dual therapy regimen, consisting of vonoprazan (20 mg per tablet; Takeda Pharmaceutical Co., Tokyo, Japan) and amoxicillin (500 mg per capsule; The United Laboratories International Holdings Limited, Hong Kong, China), involved the administration of vonoprazan 20 mg twice daily and amoxicillin 1000 mg three times per day for a duration of 14 days. It was recommended to take the vonoprazan half an hour prior to breakfast and dinner, while the amoxicillin was recommended to be taken right after breakfast, lunch, and dinner.
Continuous variables were expressed as the mean ± SD, while categorical variables were presented as counts and absolute relative frequencies, displayed as percentages (%) with their 95%CI, where applicable. Statistical significance was determined at P < 0.05 (two-tailed).
Descriptive statistics and Student’s t-tests were performed using Microsoft Excel (Version 16.85; Microsoft Corporation), assuming equal variance and applying two-tailed tests when appropriate. The calculation of 95%CI and comparison of proportions were conducted using R (The R Foundation for Statistical Computing, Vienna, Austria) with the RStudio IDE (Posit Software, PBC). The Pearson’s χ2 test was performed via the chisq.test () function, while the Fisher’s exact test was calculated by the fisher.test () function. The prop.test () function was employed for two-sample proportion tests.
A total of 401 cases involving patients aged 60 years or older were assessed. Among these cases, 265 patients received VA dual therapy as first-line treatment, and 136 patients received VA dual therapy as rescue treatment. Twenty-one cases were excluded due to loss to follow-up, failure to re-examine H. pylori infection via13C-UBT, or unwillingness to comply with medication instruction. Ultimately, 380 cases were included in the study, with 250 cases included in the first-line treatment group and 130 cases in the rescue treatment group (Figure 1). The demographic and clinical characteristics of the patients are summarized in Table 1.
| Characteristics | First-line treatment, n = 250 | Rescue treatment, n = 130 | P value |
| Age in years | 65.3 ± 4.4 | 65.1 ± 3.6 | 0.6000 |
| Range | 60-82 | 60-76 | |
| Sex as M/F | 106/144 | 60/70 | 0.5500 |
| Body weight in kg | 65.5 ± 11.3 | 65.8 ± 10.8 | 0.8000 |
| BMI in kg/m2 | 24.0 ± 3.4 | 23.7 ± 2.9 | 0.3700 |
| Cigarette smoking | 31 (12.4) | 15 (11.5) | 0.9300 |
| Alcohol drinking | 35 (14.0) | 27 (20.8) | 0.1200 |
| Family history of gastric cancer | 15 (6.0) | 15 (11.5) | 0.0900 |
| Endoscopy diagnosis | |||
| Gastritis | 193 (77.2) | 97 (77.2) | 0.6600 |
| CSG | 108 (43.2) | 32 (24.6) | 0.0006c |
| CAG | 85 (34.0) | 65 (50.0) | 0.0035b |
| Peptic ulcer | 51 (20.4) | 27 (20.8) | 1.0000 |
| Gastric ulcer | 14 (5.6) | 11 (4.4) | 0.4000 |
| Duodenal ulcer | 27 (10.8) | 16 (6.4) | 0.7900 |
| Complex (gastric and duodenal) ulcer | 10 (4.0) | 0 (0.0) | 0.0180a |
| Gastric cancer | 1 (0.4) | 1 (0.8) | 1.0000 |
| MALToma | 3 (1.2) | 0 (0.0) | 0.5500 |
| Gastric hyperplastic polyp | 2 (0.8) | 5 (3.8) | 0.0490a |
| Combined diseases | 1.8 ± 1.4 | 1.8 ± 1.4 | 0.9020 |
| 0 | 53 (21.2) | 26 (20.0) | 0.8900 |
| 1 | 61 (24.4) | 34 (26.1) | 0.8000 |
| 2 | 64 (25.6) | 35 (26.9) | 0.8800 |
| ≥ 3 | 72 (28.8) | 35 (16.9) | 0.7900 |
| Combined medicine | 1.3 ± 1.6 | 1.5 ± 1.7 | 0.3400 |
| 0 | 99 (39.6) | 45 (34.6) | 0.4000 |
| 1 | 59 (23.6) | 36 (27.7) | 0.4500 |
| 2 | 40 (16.0) | 17 (13.1) | 0.5400 |
| ≥ 3 | 52 (20.8) | 32 (24.6) | 0.4700 |
| Comorbidity | |||
| Hypertension | 89 (35.6) | 49 (37.7) | 0.7700 |
| Diabetes mellitus | 45 (18.0) | 20 (15.4) | 0.6200 |
| Hyperlipidemia | 88 (35.2) | 33 (25.4) | 0.0700 |
| Heart disease | 32 (12.8) | 15 (11.5) | 0.8500 |
| Lung disease | 10 (4.0) | 5 (3.8) | 1.0000 |
| Liver disease | 28 (11.2) | 12 (9.2) | 0.6800 |
| Renal disease | 13 (5.2) | 7 (5.4) | 1.0000 |
| Cerebrovascular disease | 10 (4.0) | 7 (5.4) | 0.6000 |
| Autoimmune disease | 24 (9.6) | 9 (6.9) | 0.4500 |
| Hypersensitivity disease | 4 (1.6) | 4 (3.1) | 0.4500 |
| Other malignant tumor | 25 (10.0) | 19 (14.6) | 0.2400 |
| Adherence | 247 (98.8) | 128 (98.5) | 1.0000 |
| Adverse events | 23 (9.2); 95%CI: 6.2%-13.4% | 13 (10.0); 95%CI: 4.8%-15.2% | 1.0000 |
| Eradication rate | 95.6% (239/250); 95%CI: 93.0%-98.1% | 89.2% (116/130); 95%CI: 83.9%-94.6% | 0.0300a |
There were no statistically significant differences in demographic data between the first-line treatment group and the rescue treatment group. The mean age of the 250 patients in the first-line treatment group was 65.3 years. Among these, 197 patients (78.8%) had comorbid conditions and 151 patients (60.4%) were taking other medications simultaneously, including statins, antihypertensives, and hypoglycemic agents. In the rescue treatment group, which included 130 patients, the mean age was 65.1 years. Of these, 104 (80.0%) had comorbidities and 90 patients (69.2%) were taking other medications concurrently.
Chronic superficial gastritis was more commonly observed in patients receiving VA dual therapy as first-line treatment, whereas the more advanced chronic atrophic gastritis was more frequently observed in the rescue treatment group. Patients with more severe forms of gastritis may have been more inclined to pursue rescue treatment after the failure of initial therapy. There were no significant differences between the two groups concerning overall ulcer disease, although a higher incidence of complex ulcers was noted in the first-line treatment group. There were more patients with a gastric hyperplastic polyp diagnosis in the rescue treatment group.
The eradication rate was 95.6% (239/250, 95%CI: 93.0%-98.1%) in the first-line treatment group (Table 2). A total of 4 patients discontinued treatment, with 3 patients experiencing treatment failure (2 patients discontinued treatment due to AE and 1 patient due to drinking alcohol). One patient who had discontinued after 4 days of treatment due to a rash achieved successful treatment (Table 3). Comparison between patients with successful and failed treatment showed a higher proportion of chronic atrophic gastritis (63.6% vs 32.6%, P = 0.049) and poor adherence (72.7% vs 99.6%, P = 0.00025; Table 2) in the failed group.
| Characteristic | Total, n = 250 | VA success, n = 239 | VA failure, n = 11 | P value |
| Age in years | 65.3 ± 4.4 | 65.4 ± 4.4 | 63.4 ± 4.1 | 0.1500 |
| Range | 60-82 | 60-82 | 60-72 | |
| Sex as M/F | 106/144 | 103/136 | 3/8 | 0.3600 |
| Body weight in kg | 65.5 ± 11.3 | 65.4 ± 11.1 | 67.4 ± 14.4 | 0.6400 |
| BMI in kg/m2 | 24.0 ± 3.4 | 23.99 ± 11.1 | 24.8 ± 3.7 | 0.5000 |
| Cigarette smoking | 31 (12.4) | 30 (12.5) | 1 (9.1) | 1.0000 |
| Alcohol drinking | 35 (14.0) | 34 (14.2) | 1 (9.1) | 1.0000 |
| Family history of gastric cancer | 15 (6.0) | 14 (5.9) | 1 (9.1) | 0.5000 |
| Endoscopy diagnosis | ||||
| Gastritis | 193 (77.2) | 183 (76.6) | 10 (90.9) | 0.7000 |
| CSG | 108 (43.2) | 105 (43.9) | 3 (27.3) | 0.3500 |
| CAG | 85 (34.0) | 78 (32.6) | 7 (63.6) | 0.0490a |
| Peptic ulcer | 51 (20.4) | 50 (20.9) | 1 (9.1) | 0.4700 |
| Gastric ulcer | 14 (5.6) | 14 (5.9) | 0 | 1.0000 |
| Duodenal ulcer | 27 (10.8) | 26 (10.9) | 1 (9.1) | 1.0000 |
| Complex (gastric and duodenal) ulcer | 10 (4.0) | 10 (4.2) | 0 | 1.0000 |
| Gastric cancer | 1 (0.4) | 1 (0.4) | 0 | 1.0000 |
| MALToma | 3 (1.2) | 3 (1.3) | 0 | 1.0000 |
| Gastric hyperplastic polyp | 2 (0.8) | 2 (0.8) | 0 | 1.0000 |
| Combined diseases | 1.8 ± 1.4 | 1.8 ± 1.4 | 1.5 ± 1.4 | 0.4300 |
| 0 | 53 (21.2) | 50 (20.9) | 3 (27.3) | 0.7100 |
| 1 | 61 (24.4) | 57 (23.8) | 4 (36.4) | 0.4700 |
| 2 | 64 (25.6) | 63 (26.4) | 1 (9.1) | 0.3000 |
| ≥ 3 | 72 (28.8) | 69 (28.9) | 3 (27.3) | 1.0000 |
| Combined medicine | 1.3 ± 1.6 | 1.4 ± 1.6 | 0.7 ± 1.2 | 0.1100 |
| 0 | 99 (39.6) | 92 (38.5) | 7 (63.6) | 0.1200 |
| 1 | 59 (23.6) | 57 (23.8) | 2 (18.2) | 1.0000 |
| 2 | 40 (16.0) | 40 (16.7) | 0 | 0.2200 |
| ≥ 3 | 52 (20.8) | 50 (20.9) | 2 (18.2) | 0.1300 |
| Comorbidity | ||||
| Hypertension | 89 (35.6) | 86 (36.0) | 3 (27.3) | 0.7500 |
| Diabetes mellitus | 45 (18.0) | 44 (18.4) | 1 (9.1) | 0.6900 |
| Hyperlipidemia | 88 (35.2) | 85 (35.6) | 3 (27.3) | 0.7500 |
| Heart disease | 32 (12.8) | 32 (13.4) | 0 | 0.3800 |
| Lung disease | 10 (4.0) | 10 (4.2) | 0 | 1.0000 |
| Liver disease | 28 (11.2) | 26 (10.9) | 2 (18.2) | 0.3500 |
| Renal disease | 13 (5.2) | 12 (5.0) | 1 (9.1) | 0.4500 |
| Cerebrovascular disease | 10 (4.0) | 10 (4.2) | 0 | 1.0000 |
| Autoimmune disease | 24 (9.6) | 22 (9.2) | 2 (18.2) | 0.2900 |
| Hypersensitivity disease | 4 (1.6) | 4 (1.7) | 0 | 1.0000 |
| Malignant tumor | 25 (10.0) | 22 (9.2) | 3 (27.3) | 0.0900 |
| Compliance | 246 (98.4) | 238 (99.6) | 8 (72.7) | 0.0003c |
| Adverse events | 23 (9.2); 95%CI: 6.2%-13.4% | 21 (8.8) | 2 (18.2) | 0.2900 |
| Eradication rate | 95.6% (239/250); 95%CI: 93.0%-98.1% |
| Group | No. | Sex | Age in years | Duration of medication in days | Symptom of AE | Successful eradication | Cure rate |
| First-line treatment, | 008 | M | 67 | 10 | Forgot1 | No | 1/4 |
| 079 | F | 62 | 7 | Abdominal pain | No | ||
| 090 | F | 72 | 7 | Skin rash | No | ||
| 242 | F | 79 | 4 | Skin rash | Yes | ||
| Rescue treatment, n = 2 | 073 | F | 68 | 10 | Abdominal discomfort | Yes | 2/2 |
| 122 | F | 60 | 10 | Skin rash | Yes |
The eradication rate was 89.2% (116/130, 95%CI: 83.9%-94.6%) in the rescue treatment group. Comparison between patients with successful and failed treatment showed a higher proportion of gastric ulcer in the failed group (28.6% vs 6.0%, P = 0.018). There was no difference in adherence and AE between patients with successful and failed treatment (Table 4). Two patients who discontinued treatment after 10 days due to AE achieved successful treatment (Table 3).
| Characteristic | Total, n = 130 | VA success, n = 116 | VA failure, n = 14 | P value |
| Age in years | 65.1 ± 3.6 | 65.0 ± 3.6 | 66.3 ± 3.3 | 0.180 |
| Range | 60-76 | 60-76 | 60-72 | |
| Sex as M/F | 60/70 | 53/63 | 7/7 | 0.780 |
| Weight in kg | 65.8 ± 10.8 | 65.4 ± 10.4 | 68.8 ± 13.6 | 0.390 |
| BMI in kg/m2 | 23.7 ± 2.9 | 23.6 ± 2.8 | 24.7 ± 4.0 | 0.320 |
| Cigarette smoking | 15 (11.5) | 11 (9.5) | 4 (28.6) | 0.058 |
| Alcohol drinking | 27 (20.8) | 24 (20.7) | 3 (21.4) | 1.000 |
| Family history of gastric cancer | 15 (11.5) | 12 (10.3) | 3 (21.4) | 0.210 |
| Endoscopy diagnosis | ||||
| Gastritis | 97 (77.2) | 90 (77.6) | 7 (50.0) | 0.045a |
| CSG | 32 (24.6) | 30 (25.9) | 2 (14.3) | 0.520 |
| CAG | 65 (50.0) | 60 (51.7) | 5 (35.7) | 0.400 |
| Peptic ulcer | 27 (20.8) | 21 (18.1) | 6 (42.8) | 0.070 |
| Gastric ulcer | 11 (4.4) | 7 (6.0) | 4 (28.6) | 0.018a |
| Duodenal ulcer | 16 (6.4) | 14 (12.1) | 2 (14.3) | 0.680 |
| Complex (gastric and duodenal) ulcer | 0 | 0 | 0 | 1.000 |
| Gastric cancer | 1 (0.8) | 0 | 1 (7.1) | 0.110 |
| MALToma | 0 | 0 | 0 | 1.000 |
| Gastric hyperplastic polyp | 5 (3.8) | 5 (4.3) | 0 | 1.000 |
| Combined diseases | 1.8 ± 1.4 | 1.7 ± 1.3 | 2.1 ± 2.0 | 0.540 |
| 0 | 26 (20.0) | 24 (20.7) | 2 (14.3) | 0.740 |
| 1 | 34 (26.1) | 28 (24.1) | 6 (42.8) | 0.190 |
| 2 | 35 (26.9) | 34 (29.3) | 1 (7.1) | 0.110 |
| ≥ 3 | 35 (16.9) | 30 (25.9) | 5 (35.7) | 0.520 |
| Combined medicine | 1.5 ± 1.7 | 1.5 ± 1.7 | 1.9 ± 2.4 | 0.490 |
| 0 | 45 (34.6) | 40 (34.5) | 5 (35.7) | 1.000 |
| 1 | 36 (27.7) | 33 (28.4) | 3 (21.4) | 0.760 |
| 2 | 17 (13.1) | 15 (12.9) | 2 (14.3) | 1.000 |
| ≥ 3 | 32 (24.6) | 28 (24.1) | 4 (28.6) | 0.750 |
| Comorbidity | ||||
| Hypertension | 49 (37.7) | 43 (37.1) | 6 (42.8) | 0.770 |
| Diabetes mellitus | 20 (15.4) | 17 (14.7) | 3 (21.4) | 0.450 |
| Hyperlipidemia | 33 (25.4) | 29 (25.0) | 4 (28.6) | 0.750 |
| Heart disease | 15 (11.5) | 13 (11.2) | 2 (14.3) | 0.660 |
| Lung disease | 5 (3.8) | 5 (4.3) | 0 | 1.000 |
| Liver disease | 12 (9.2) | 9 (7.8) | 3 (21.4) | 0.120 |
| Renal disease | 7 (5.4) | 7 (6.0) | 0 | 1.000 |
| Cerebrovascular disease | 7 (5.4) | 7 (6.0) | 0 | 1.000 |
| Autoimmune disease | 9 (6.9) | 9 (7.8) | 0 | 0.600 |
| Hypersensitivity disease | 4 (3.1) | 4 (3.4) | 0 | 1.000 |
| Malignant tumor | 19 (14.6) | 17 (14.7) | 2 (14.3) | 1.000 |
| Compliance | 128 (98.5) | 114 (98.3) | 14 (100) | 1.000 |
| Adverse events | 13 (10.0); 95%CI: 4.8%-15.2% | 13 (11.2) | 0 | 0.360 |
| Eradication rate | 89.2% (116/130); 95%CI: 83.9-94.6% |
The eradication rate of the first-line treatment group was significantly higher than that of the rescue treatment group (95.6% vs 89.2%, P = 0.03; Table 1).
Of the 380 patients, 375 (98.7%) demonstrated good adherence, which was defined as taking more than 80% of all prescribed tablets (Table 2). All 5 patients (5/380, 1.3%) who discontinued treatment did so due to side effects. Among them, 3 patients successfully achieved H. pylori eradication.
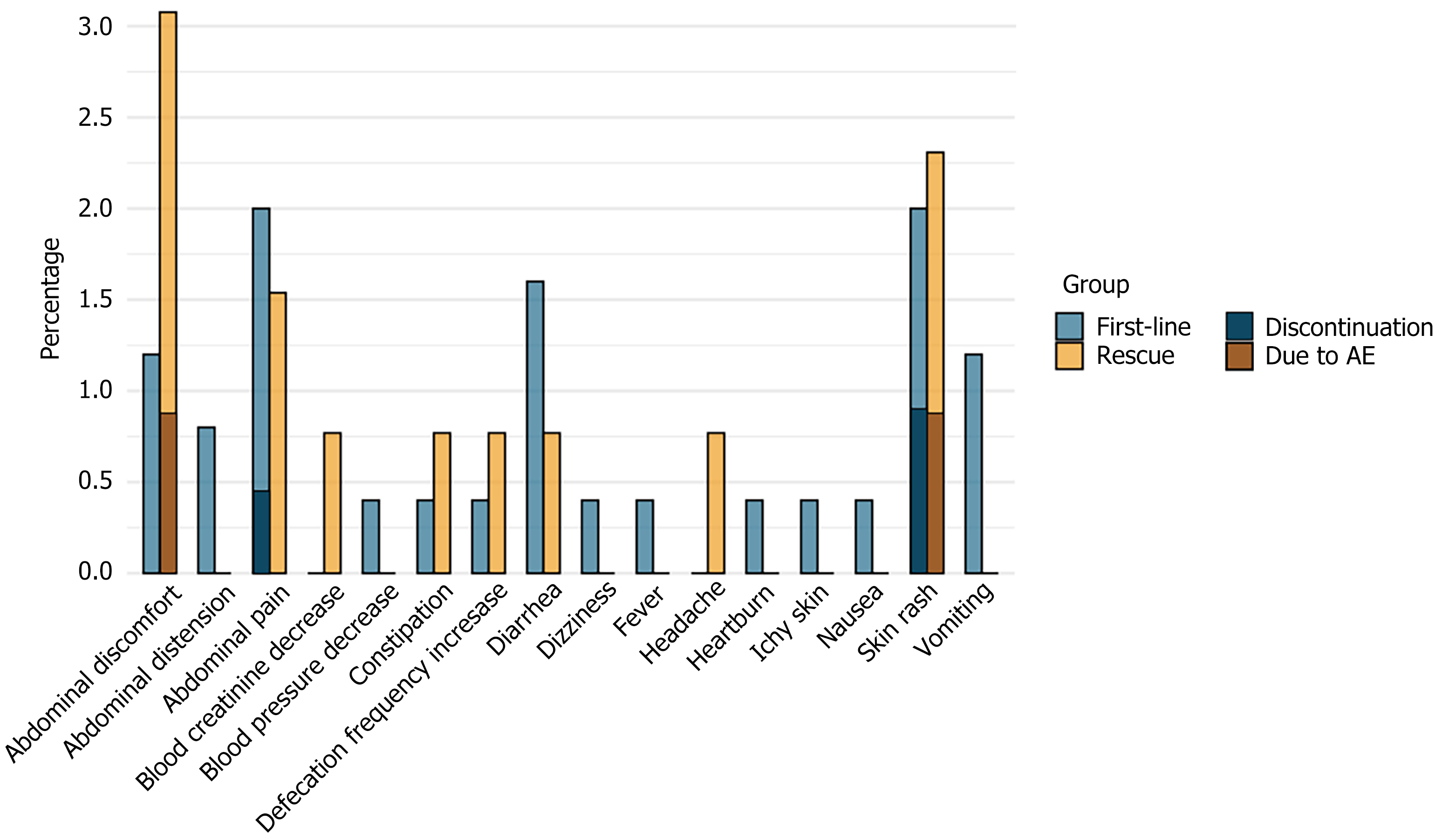
A total of 36 patients (9.5%) experienced AE. The most common AEs were skin rash, abdominal pain, abdominal discomfort, and diarrhea. No severe AE occurred during the treatment. Among the 5 patients who discontinued treatment due to AE, 3 cases were from the first-line treatment group and 2 cases were from the rescue treatment group. In the first-line treatment group, 2 patients discontinued treatment after 7 days of medication due to AE, leading to treatment failure. The other 3 patients (1 patient discontinued after 4 days of medication and 2 patients after 10 days of medication) had successful eradication (Table 4). The distribution of various AEs and the AEs that led to treatment discontinuation in both the first-line and rescue treatment groups are illustrated in Figure 2 and Table 5.
| Symptom | First-line, n = 250 | Rescue, n = 130 | Total, n = 380 | Nonadherence due to AE | Failed in treatment |
| Skin rash | 5 (2.0) | 3 (2.3) | 8 (2.1) | 3 | 1 |
| Abdominal pain | 5 (2.0) | 2 (1.5) | 7 (1.8) | 1 | 1 |
| Abdominal discomfort | 3 (1.2) | 4 (3.1) | 7 (1.8) | 1 | 0 |
| Diarrhea | 4 (1.6) | 1 (0.8) | 5 (1.3) | 0 | 0 |
| Vomiting | 3 (1.2) | 0 | 3 (0.8) | 0 | 0 |
| Increased bowl movement | 1 (0.4) | 1 (0.8) | 2 (0.5) | 0 | 0 |
| Abdominal distension | 2 (0.8) | 0 | 2 (0.5) | 0 | 0 |
| Constipation | 1 (0.4) | 1 (0.8) | 2 (0.5) | 0 | 0 |
| Heartburn | 1 (0.4) | 0 | 1 (0.3) | 0 | 0 |
| Itchy skin | 1 (0.4) | 0 | 1 (0.3) | 0 | 0 |
| Nausea | 1 (0.4) | 0 | 1 (0.3) | 0 | 0 |
| Fever | 1 (0.4) | 0 | 1 (0.3) | 0 | 0 |
| Headache | 0 | 1 (0.8) | 1 (0.3) | 0 | 0 |
| Dizziness | 1 (0.4) | 0 | 1 (0.3) | 0 | 0 |
| Blood pressure decrease | 1 (0.4) | 0 | 1 (0.3) | 0 | 0 |
| Total AE | 23 (9.2) | 13 (10.0) | 36 (9.5) | 5 | 2 |
| Adherence | 247 (98.8) | 128 (98.5) | 375 (98.7) |
The global increase of the elderly population has led to a rise in frailty, which is significantly impacting the risk of inappropriate drug prescriptions. It is crucial to select and dose medications with care in the elderly population to strike a balance between effectiveness, safety, and tolerability. Antimicrobial treatments can lead to severe AEs, particularly during a long course of medication or in the context of existing medical conditions[4,5].
It is necessary and beneficial to eradicate H. pylori in elderly patients. Studies have indicated that the cumulative incidence of gastric precancerous lesions significantly decreases following H. pylori eradication, with particularly notable benefits for the elderly[6,7]. A study from Hong Kong showed that elderly patients who underwent H. pylori eradication therapy had a significantly lower incidence of gastric cancer compared to the general population, particularly 10 years after treatment[8]. A retrospective study from Japan focusing on elderly patients over 80 years of age showed that H. pylori eradication therapy with proton pump inhibitors or vonoprazan-containing triple regimen was generally safe and well-tolerated in elderly patients. The study also found it was effective in preventing and treating peptic ulcers and associated complications such as bleeding and perforation[9].
Currently, reports on the efficacy and safety of treatment for elderly patients have mostly focused on quadruple or triple therapy regimens[10,11]. However, there have been no studies on VA dual therapy in the elderly population. In a recent study on triple and quadruple therapies in Europe[12], data from the European Registry on H. pylori Management spanning from 2013 to 2022 were compared for treatment outcomes between older (≥ 60 years) and younger (18-59 years) patients. Older patients, who had more concomitant medications and penicillin allergies, reported fewer AEs. First-line treatment effectiveness was 90% for older patients and 88% for younger patients (P < 0.05), while second-line treatment was equally effective at 84% for both groups. Triple therapies were less effective (< 90%), and quadruple therapies achieved the optimal results. Overall, older adults had a favorable safety profile, and there were no significant differences in treatment effectiveness between age groups. Due to low amoxicillin resistance and the simple composition of the regimen, high-dose dual therapy, such as VA dual therapy, has attracted significant attention[13].
Du et al[14] systematically reviewed 15 studies involving 4568 patients in the general population to assess the efficacy and safety of VA dual therapy as first-line treatment. The pooled eradication rates were 85.0% based on intention-to-treat analysis and 90.0% by per-protocol analysis. The therapy showed higher efficacy than proton pump inhibitor-based triple therapy but lower efficacy than vonoprazan-containing quadruple therapy. AEs were mild and occurred in 17.5% of cases. There was a high adherence rate of 96%. Current studies indicate that VA dual therapy demonstrates good safety in the general population[15-20].
In our previous study conducted from November 2013 to May 2017, rabeprazole-amoxicillin dual therapy was evaluated in patients aged ≥ 60 years or in patients with multiple comorbidities[21]. The first-line treatment achieved a 90.9% eradication rate, with mild AEs occurring in 11.1% of patients and a discontinuation rate due to side effects of 6.1% (93.9% in adherence). In this study of VA dual therapy in elderly individuals, the eradication rate of the first-line treatment group was 95.6%, while the eradication rate of the rescue treatment group was 89.2%. However, the general significance of the effectiveness of VA dual therapy still needs to be studied because a recent randomized clinical trial conducted in Europe and the United States demonstrated an unsatisfactory efficacy[22]. The potential hypotheses for the inability to attain high cure rates include: (1) Low antibiotic concentrations in the stomach; (2) Genetic heterogeneity; (3) Failure to achieve an intragastric pH level conducive to amoxicillin’s effectiveness in eradicating the infection; and (4) Lack of pilot studies or attempts to optimize treatment regimens[23].
A total of 98.7% of the patients had good adherence, with an AE rate of 9.5% and a discontinuation rate due to side effects of 1.3%. Based on the results of this study, the efficacy and incidence of AEs due to VA dual therapy in elderly patients were similar to those previously reported in the general population.
This study had several limitations. As a retrospective analysis, it lacked the ability to randomize and control for confounding factors, which could influence the outcomes. The reliance on existing medical records may lead to incomplete or inaccurate data, and potential selection bias could not be excluded. To avoid the influence of confounding factors in retrospective studies, a prospective, multicenter, randomized controlled clinical trial may yield more definitive results. Additionally, while the sample size of 380 cases provides valuable insights, it may still limit the generalizability of the findings to the broader population, particularly in specific subgroups. Currently, most studies on the efficacy of VA dual therapy were conducted in China, and their results were relatively consistent with traditional quadruple regimens. There are fewer reports from Western countries. Chey et al[22] reported findings from populations in the United States and Europe, showing that the eradication effect was poorer in Western populations. Since VA dual therapy is a relatively new regimen, more studies, especially basic research, may be needed to clarify the differences observed in different populations. However, as a real-world study, the results reflect routine clinical practice, offering a certain degree of representativeness despite the absence of randomization.
The results of this study showed that VA dual therapy demonstrated good efficacy and safety in H. pylori treatment of elderly patients. The safety and efficacy are similar to the general population. Additional large-scale cohort studies are needed to confirm these results.
| 1. | Hu Y, Huang XH, Zhou B, Liu ML, Liu YF, Yu T, Sun P, Tan BB, Hu Y, Cheng F, Pan XL, Hong JB, Shu X, Zhu Y, Lu NH. Vonoprazan and amoxicillin dual therapy for 14 days as the first-line treatment of Helicobacter pylori infection: A non-inferiority, randomized clinical trial. Helicobacter. 2024;29:e13045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 2. | Gao W, Teng G, Wang C, Xu Y, Li Y, Cheng H. Eradication rate and safety of a "simplified rescue therapy": 14-day vonoprazan and amoxicillin dual regimen as rescue therapy on treatment of Helicobacter pylori infection previously failed in eradication: A real-world, retrospective clinical study in China. Helicobacter. 2022;27:e12918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 3. | Jonaitis P, Nyssen OP, Saracino IM, Fiorini G, Vaira D, Pérez-Aísa Á, Tepes B, Castro-Fernandez M, Pabón-Carrasco M, Keco-Huerga A, Voynovan I, Lucendo AJ, Lanas Á, Martínez-Domínguez SJ, Almajano EA, Rodrigo L, Vologzanina L, Brglez Jurecic N, Denkovski M, Bujanda L, Mahmudov U, Leja M, Lerang F, Babayeva G, Bordin DS, Gasbarrini A, Kupcinskas J, Gridnyev O, Rokkas T, Marcos-Pinto R, Phull PS, Smith SM, Tonkić A, Boltin D, Buzás GM, Šembera Š, Şimşek H, Matysiak-Budnik T, Milivojevic V, Marlicz W, Venerito M, Boyanova L, Doulberis M, Capelle LG, Cano-Català A, Moreira L, Mégraud F, O'Morain C, Gisbert JP, Jonaitis L; Hp-EuReg investigators. Comparison of the management of Helicobacter pylori infection between the older and younger European populations. Sci Rep. 2023;13:17235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 4. | Pea F. Antimicrobial treatment of bacterial infections in frail elderly patients: the difficult balance between efficacy, safety and tolerability. Curr Opin Pharmacol. 2015;24:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Soenen S, Rayner CK, Jones KL, Horowitz M. The ageing gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2016;19:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 6. | Toyokawa T, Suwaki K, Miyake Y, Nakatsu M, Ando M. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J Gastroenterol Hepatol. 2010;25:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 7. | Mizukami K, Kodama M, Fukuda M, Hirashita Y, Tsutsumi K, Fukuda K, Ogawa R, Okamoto K, Okimoto T, Murakami K. Comparison of the improvement in gastric mucosal tissue after Helicobacter pylori eradication between young and elderly people. Arab J Gastroenterol. 2023;24:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 8. | Leung WK, Wong IOL, Cheung KS, Yeung KF, Chan EW, Wong AYS, Chen L, Wong ICK, Graham DY. Effects of Helicobacter pylori Treatment on Incidence of Gastric Cancer in Older Individuals. Gastroenterology. 2018;155:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 9. | Iwata E, Sugimoto M, Asaoka D, Hojo M, Ito M, Kitazawa N, Kurihara N, Masaoka T, Mizuno S, Mori H, Nagahara A, Niikura R, Ohkusa T, Sano M, Shimada Y, Suzuki H, Takeuchi Y, Tanaka A, Tokunaga K, Ueda K, Sakaki N, Takahashi S, Kawai T. Characteristics of Helicobacter pylori Eradication Therapy in Patients 80 Years or Older Living in a Metropolitan Area: A Multicenter Retrospective Study. Helicobacter. 2024;29:e13125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 10. | Yang Q, He C, Hu Y, Hong J, Zhu Z, Xie Y, Shu X, Lu N, Zhu Y. 14-day pantoprazole- and amoxicillin-containing high-dose dual therapy for Helicobacter pylori eradication in elderly patients: A prospective, randomized controlled trial. Front Pharmacol. 2023;14:1096103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 11. | Gao C, Fan YH. Effect and Safety of Helicobacter pylori Eradication Treatment Based on Molecular Pathologic Antibiotic Resistance in Chinese Elderly People. Infect Drug Resist. 2022;15:3277-3286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 12. | Jonaitis P, Kupcinskas J, Gisbert JP, Jonaitis L. Helicobacter pylori Eradication Treatment in Older Patients. Drugs Aging. 2024;41:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 13. | Gao CP, Zhang D, Zhang T, Wang JX, Han SX, Graham DY, Lu H. PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter. 2020;25:e12692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 14. | Du RC, Hu YX, Ouyang Y, Ling LX, Xu JY, Sa R, Liu XS, Hong JB, Zhu Y, Lu NH, Hu Y. Vonoprazan and amoxicillin dual therapy as the first-line treatment of Helicobacter pylori infection: A systematic review and meta-analysis. Helicobacter. 2024;29:e13039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (1)] |
| 15. | Zhang J, Zhang H, Zhu XJ, Yao N, Yin JM, Liu J, Dan HJ, Pang QM, Liu ZH, Shi YQ. Efficacy and safety of vonoprazan and high-dose amoxicillin dual therapy in eradicating Helicobacter pylori: A systematic review and meta-analysis. Int J Antimicrob Agents. 2024;64:107331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 16. | Ju KP, Kong QZ, Li YY, Li YQ. Low-dose or high-dose amoxicillin in vonoprazan-based dual therapy for Helicobacter pylori eradication? A systematic review and meta-analysis. Helicobacter. 2024;29:e13054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 17. | Liu L, Shi H, Shi Y, Wang A, Guo N, Li F, Nahata MC. Vonoprazan-based therapies versus PPI-based therapies in patients with H. pylori infection: Systematic review and meta-analyses of randomized controlled trials. Helicobacter. 2024;29:e13094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (1)] |
| 18. | Zhou BG, Jiang X, Ding YB, She Q, Li YY. Vonoprazan-amoxicillin dual therapy versus bismuth-containing quadruple therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. Helicobacter. 2024;29:e13040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 19. | Chen PY, Tsai FP, Chen MJ, Yang HY, Wu MS, Liou JM. Vonoprazan-based versus proton pump inhibitor-based therapy in Helicobacter pylori eradication: an updated systematic review and meta-analysis of randomised trials. Gut. 2024;73:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 20. | Hsu PI, Chen KY, Tai WC, Yang JC, Tsay FW, Liu YH, Chen CL, Lee CL, Yeh HZ, Kuo CH, Chuah SK, Lee HC, Shie CB, Shiu SI, Kao JY, Yamaoka Y, Graham DY, Wu DC; Taiwan Acid-related Disease (TARD) Study Group. Hybrid, High-Dose Dual and Bismuth Quadruple Therapies for First-Line Treatment of Helicobacter pylori Infection in Taiwan: A Multicenter, Open-Label, Randomized Trial. Am J Gastroenterol. 2023;118:1184-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 21. | Gao W, Ye H, Deng X, Wang C, Xu Y, Li Y, Zhang X, Cheng H. Rabeprazole-amoxicillin dual therapy as first-line treatment for H pylori eradication in special patients: A retrospective, real-life study. Helicobacter. 2020;25:e12717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 22. | Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology. 2022;163:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (2)] |
| 23. | Graham DY. Why the Vonoprazan Helicobacter pylori Therapies in the US-European Trial Produced Unacceptable Cure Rates. Dig Dis Sci. 2023;68:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/