Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1237
Peer-review started: December 6, 2023
First decision: January 4, 2024
Revised: January 6, 2024
Accepted: February 4, 2024
Article in press: February 4, 2024
Published online: March 7, 2024
Processing time: 90 Days and 23.4 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a highly fatal disease with limited effective treatment especially after first-line chemotherapy. The human epidermal growth factor receptor 2 (HER-2) immunohistochemistry (IHC) positive is associated with more aggressive clinical behavior and shorter overall survival in PDAC.
We present a case of multiple metastatic PDAC with IHC mismatch repair proficient but HER-2 IHC weakly positive at diagnosis that didn’t have tumor regression after first-line nab-paclitaxel plus gemcitabine and PD-1 inhibitor treatment. A novel combination therapy PRaG 3.0 of RC48 (HER2-antibody-drug conjugate), radio
PRaG 3.0 might be a novel strategy for HER2-positive metastatic PDAC patients who failed from previous first-line approach and even PD-1 immunotherapy but needs more data in prospective trials.
Core Tip: Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death worldwide. Herein, we present a case of multiple metastatic PDAC with immunohistochemistry (IHC) mismatch repair proficient but human epidermal growth factor receptor 2 (HER-2) IHC weakly positive at diagnosis that didn’t have tumor regression after first-line nab-paclitaxel plus gemcitabine and PD-1 inhibitor treatment. A novel combination therapy PRaG 3.0 of RC48 (HER2-antibody-drug conjugate), radiotherapy, PD-1 inhibitor, granulocyte-macrophage colony-stimulating factor and interleukin-2 was then applied as second-line therapy and the patient had confirmed good partial response with progress-free-survival of 6.5 months. We proposed that PRaG 3.0 might be a good therapeutic strategy for HER2-positive metastatic PDAC patients who failed from previous first-line approach and even PD-1 immunotherapy.
- Citation: Kong YH, Xu ML, Zhang JJ, Chen GQ, Hong ZH, Zhang H, Dai XX, Ma YF, Zhao XR, Zhang CY, Chen RZ, Xing PF, Zhang LY. PRaG 3.0 therapy for human epidermal growth factor receptor 2-positive metastatic pancreatic ductal adenocarcinoma: A case report. World J Gastroenterol 2024; 30(9): 1237-1249
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1237
Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 90% of pancreatic tumors. Due to it is typically diagnosed at an advanced stage and the tumor metastasis has been happened, the prognosis of PDAC is very poor with average survival time less than one year[1]. Modified FOLFIRINOX (fluorouracil, folinic acid, irinotecan, and oxaliplatin) or AG (gemcitabine and albumin-bound paclitaxel) was recommended as first-line therapy for metastatic PDAC. However, PDAC demonstrated significant resistance to these chemotherapies. Besides, the effectiveness of novel immunotherapies has been limited to certain tumor types classified as highly "immunogenic", such as lung cancer and melanoma; while PDAC with a unique immunosuppressive microenvironment and a low tumor mutational burden[2], has typically resisted to immunotherapies, as demonstrated in majority of phase I and II clinical trials.
The human epidermal growth factor receptor 2 (HER-2) protein regulates cell proliferation, apoptosis, differentiation and angiogenesis. HER-2 overexpression is linked to tumorigenesis, more aggressive clinical behavior, and shorter overall survival (OS) in a variety of human malignancies. About 7% to 58% of pancreatic tumors exhibit an overexpression of the HER-2 gene[3]. Although HER-2 targeting therapy showed efficacy in diverse malignancies, including breast, gastric, and lung cancers, the clinical trials of targeted HER-2 therapy, including trastuzumab, did not improve OS nor progress-free-survival (PFS) in metastatic PDAC[4]. New combination treatment approaches for HER2-positive metastatic PDAC are thus needed.
Here, we present a case of liver multiple metastatic PDAC patient with immunohistochemistry (IHC) microsatellite stability (MSS) and HER-2 positive. She failed from first-line AG chemotherapy and PD-1 inhibitor, but received a notable response after changing to a novel combination treatment of HER-2 antibody-drug conjugate (ADC), PD-1 inhibitor, hypofractionated radiotherapy, sequential granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-2), which was named as PRaG 3.0 therapy.
A 53-year-old Chinese woman presented with abdominal pain and diagnosed with advanced pancreatic cancer.
The patient was diagnosed with metastatic pancreatic cancer in October 2021. She was initially treated with nab-paclitaxel plus gemcitabine and tislelizumab. She received two cycles of therapy and enhanced computed tomography (CT) was evaluated to find no decrease in both pancreatic tumor and hepatic metastases, leading to overall response as stable disease. Allergic reaction occurred with severe rash and itching in the second cycle of therapy, which was considered to be related to chemotherapy.
The patient had a history of pancreatitis in 2020, no history of hypertension, diabetes, or heart disease.
No personal and family history of tumors.
Slight tenderness in the left abdomen.
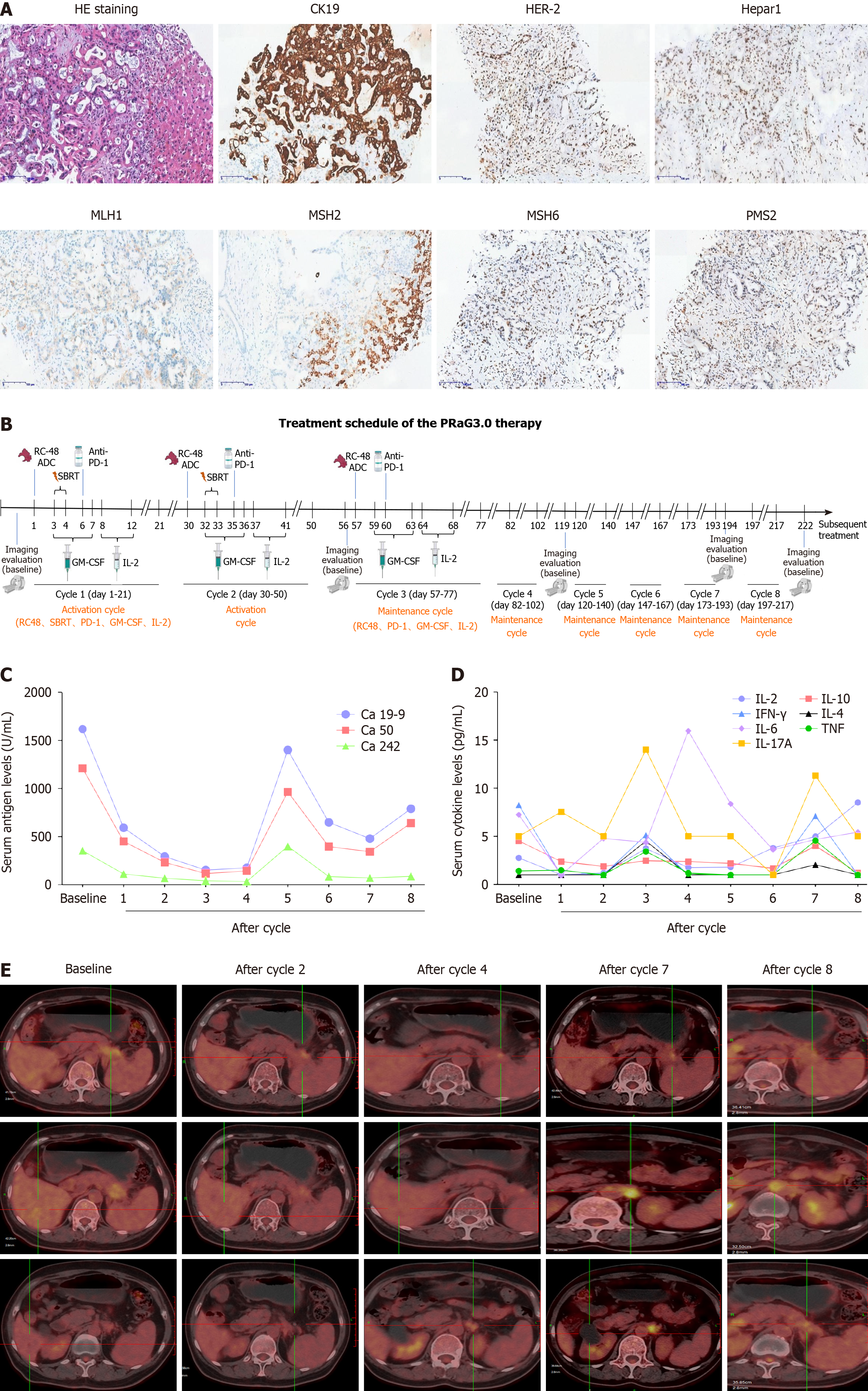
Liver mass puncture pathology showed pancreaticobiliary tumor, with IHC: HER-2(+), Hepar1(-), GPC3(-), CD34(+), CK19(+), CK(+), CK8/18(+), EGFR(1+), Ki67(+20%), MLH1(+), MSH2(+), PMS2(+), MSH6(+) (Figure 1A).
Mass shadow in the pancreatic tail with splenic artery invasion was shown in CT scan. Multiple liver abnormal signal focus and multiple enlarged retroperitoneal lymph nodes were observed, which were considered as metastases.
Based on the above clinical history and findings, the patient was diagnosed with HER-2 positive metastatic PDAC.
The patient came to started PRAG 3.0 therapy (NCT05115500) from 12-17-2021. Disitamab vedotin (RC48, a HER2-ADC) 110 mg (2 mg/kg) was intravenously administered on day 1, and then stereotactic body radiotherapy (SBRT) (8 Gy × 2 fractions) was delivered to the pancreatic lesion on day 3 and 4. GM-CSF (molgramostim) 200 μg was injected subcutaneously daily for five days concurrently with SBRT (day 3-7), and then recombinant human IL-2 200 million IU was injected subcutaneously daily for five days sequential after GM-CSF (day 8-12). Anti-PD-1 antibody penpulimab was intravenously administered within one week after completion of SBRT. The PRAG 3.0 therapy was repeated every 21 d for a cycle (the protocol of PRAG 3.0 is shown in Figure 1B). After two activation cycles, she received maintenance cycle. In each maintenance cycle, RC48 (2 mg/kg) was intravenously administered on day 1, GM-CSF (molgramostim) 200 μg was injected subcutaneously daily for five days (day 3-7) and then recombinant human IL-2 200 million IU was injected subcutaneously daily for five days sequential after GM-CSF (day 8-12). Anti-PD-1 antibody penpulimab was intravenously administered every 21 d for a cycle.
After two activation cycles of PRaG 3.0, the unirradiated liver metastatic lesions had a radiographic response of partial response (PR), and fluorodeoxyglucose (FDG) metabolism was significantly lower than before. The unirradiated paraaortic lymph nodes had no significant changes in size but deceased in FDG metabolism. The irradiated primary tumor reduced in size markedly and decreased in FDG metabolism (Figure 1E). Serum carbohydrate antigen 19-9 (Ca 19-9) decreased from 1617 to 176 U/mL, Ca 50 decreased from 1210 to 116 U/mL, Ca 242 decreased from 353.5 to 33.4 U/mL (Figure 1C). After four cycles, the radiographic evaluation confirmed good PR with further shrinkage of liver lesions. The paraaortic lymph nodes had no significant changes in size but increased in FDG metabolism (Figure 1E). After seven cycles, the paraaortic lymph nodes were slightly larger and higher in standard uptake value (SUV) metabolism. After eight cycles, new lesions of paraaortic lymph nodes were found with high SUV metabolism (Figure 1E) and the patient was evaluated progressive disease (PD) with PFS of 6.5 months and overall survival (OS) of 14.2 months. The serum Ca 19-9, Ca 50 and Ca 242 levels rebounded suddenly after the fifth cycle, and then decreased after six and seven cycles (Figure 1C). The patient got grade 1 fatigue, alopecia, and transient hyperthyroidism, which was self-recovered without medication. There were no grade 2 or above treatment-related adverse events at any point during PRaG 3.0 treatment.
PDAC is the fourth leading cause of cancer-related death worldwide. AG and FOLFIRINOX have been established as standard first-line treatment in metastatic PDAC. First-line AG chemotherapy was reported to lead to a more prolonged OS than single-agent gemcitabine (median OS, 8.5 months vs 6.7 months; P < 0.001) in metastatic PDAC in the phase III MPACT trial[5]. Our case failed from first-line AG chemotherapy with grade 3 toxicity. She also received PD-1 treatment meanwhile with no satisfactory response. Indeed, MSS PDAC has little response to single-agent anti-PD-1 therapy, while the results of the combination of anti-PD-1 with anti-CTLA-4 were also disappointing. In a phase II trial, durvalumab (a PD-L1 inhibitor) alone or in combination with tremilimumab (a CTLA-4 inhibitor) was given to 65 patients with refractory metastatic PDAC[6]. The results showed that the median OS was 3.6 months vs 3.1 months. It is considered that PDAC’s immune-quiescent and -suppressive tumor microenvironment is responsible for its resistance to immune checkpoint inhibitors. Therapies that can convert its immunological “cold” to “hot” status may be effective for improving treatments outcomes.
PRaG therapy was an innovative oncology treatment modality that was first proposed in 2019, which consisted of a combination of PD-1 inhibitor (P), radiotherapy (Ra), and GM-CSF (G), for the treatment of chemo-refractory patients with metastatic solid tumors. In our previous study, PRaG therapy showed continuously synergistic anti-tumor immune effect[7]. Thus, for HER-2 IHC positive metastatic solid tumor patients, we proposed PRaG 3.0 therapy (NCT05115500) this time.
Reprograming the tumor microenvironment, radiotherapy may enhance the anti-PD-1 effects by inducing both anti-tumor immune and immunosuppressive cells. For example, SBRT treatment can increase the percentage of PD-1+T effectors in PDAC tumors, however, the amount and function of these effectors are likely constrained by overwhelming myeloid suppressor burden[8], suggesting that more comprehensive therapies are necessary for best benefit. In PRaG therapy, GM-CSF is important for the maturation of dendritic cells and enhancing tumor antigens presentation to the immune system. Additionally, GM-CSF increases IL-2-activated killer activity and antibody-dependent cytotoxicity[9].
In PRaG 3.0, we added HER2 ADC (RC48) and IL-2 to the original PRaG regimen. RC48 (Disitamab vedotin) is a novel ADC with a humanized monoclonal antibody targeting HER-2 conjugated to a small molecule toxin monomethyl auristatin E, a synthetic antineoplastic agent. RC48 has revealed a promising efficacy with acceptable safety in patients with HER-2 positive advanced tumors[10]. IL-2 can further activate natural killer cells to kill PDAC cells. The five components in PRaG 3.0 cooperate with each other to form a multi-target therapeutic system. The combination of RC48 and PD-1/PD-L1 demonstrated synergistic efficacy and exerted long-lasting anti-tumor immunity in pre-clinical study[11]. SBRT can release tumor antigens and convert tumors into an in-situ vaccine, synergizing with PD-1/PD-L1 inhibitors. GM-CSF and IL-2 are immunomodulatory cytokines, promoting antigen-presenting cell activities and amplifying T cell immune response. In our case, the patient failed from previous first-line AG and anti-PD-1 treatment. The HER-2 was weakly expressed in tumor tissues of this patient, which indicated that she might benefit from combination therapy. Actually, she received indeed an exerted favorable response from the novel combination therapy with mild adverse reactions. It indicated that PRaG 3.0 therapy had altered the patient’s responsiveness to PD-1 inhibitor.
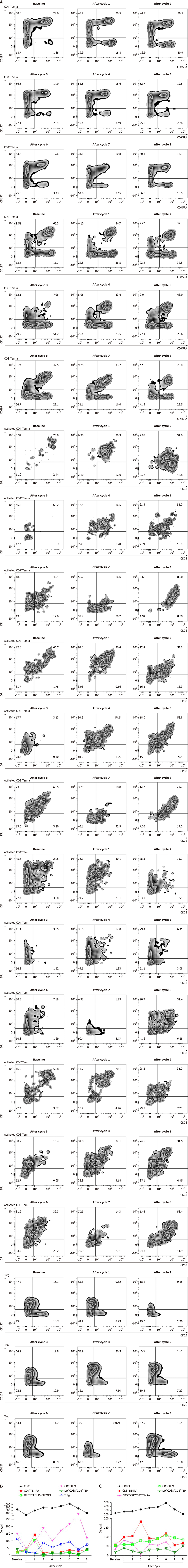
The levels of cytokines like IL-2, IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor and interferon-γ in peripheral blood were evaluated but no changes related to treatment efficacy were observed (Figure 1D). The percentage of peripheral effector memory CD3+CD197-CD45RA+CD8+T cells (Temra) (Figure 2A) and CD4+Temra in peripheral blood were increased after first two treatment cycles which contain radiotherapy but deceased to the baseline during the maintenance cycles without radiotherapy. The activated human leukocyte antigens (HLA)-DR+CD38+CD4+Temra, activated HLA-DR+CD38+CD8+Temra, the CD3+CD8+CD45RA-CD197-HLA-DR+CD38+ activated memory effector T cells (Tem) and activated CD3+CD4+CD45RA-CD197-HLA-DR+CD38+ Tem increased after the first treatment cycle but fell back afterwards. T cells cloned rapidly when activated and differentiated toward the shortly-lived effector cells or the memory progenitor cells (MPECs). MPECs then differentiated toward the central memory T cells and Tem, for a long-lasting immune response. Some subsets expressed CD45RA and become effector memory CD45RA re-expressing T cells (Temra), which were terminally differentiated. With high cytotoxicity and low proliferation, Temra’s function was still controversial[12]. Tregs’ percentage decreased significantly after treatment, which may predict a good treatment effect. The changing of peripheral blood of T cell subsets was displayed in Figure 2B and C, which were more easily obtained than tissue samples but it was challenging for analysis due to their complexities. The exact relationship between the lymphocyte subsets and treatment response and their specific mechanism needs further investigation.
To our knowledge, it was the first time to combine RC48 with radiotherapy and anti-PD-1 therapy, which showed a significant reduction in irradiated and unirradiated lesions with PFS for 6.5 months after PRAG 3.0 therapy on a HER-2 positive metastatic PDAC patient who failed from previous first-line AG and PD-1 treatment. The efficacy and safety of this treatment pattern and its potential effects on peripheral lymphocyte subsets need further analyzed and confirmed in the open-label prospective study (NCT05115500).
We are grateful to patient and her family.
| 1. | Singh RR, O'Reilly EM. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs. 2020;80:647-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Wu J, Cai J. Dilemma and Challenge of Immunotherapy for Pancreatic Cancer. Dig Dis Sci. 2021;66:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, Yang Q, Wang Y, Han W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018;9:838-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 4. | Mao T, Zhang X, Xu H, Ge W, Li S, Ma J, Yue M, Xue S, Cui J, Wang L. HDACs/mTOR inhibitor synergizes with pyrotinib in HER2-positive pancreatic cancer through degradation of mutant P53. Cancer Cell Int. 2022;22:380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 5139] [Article Influence: 395.3] [Reference Citation Analysis (12)] |
| 6. | O'Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, Fisher G, Hezel A, Chang SC, Vlahovic G, Takahashi O, Yang Y, Fitts D, Philip PA. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 563] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 7. | Kong Y, Zhao X, Xu M, Pan J, Ma Y, Zou L, Peng Q, Zhang J, Su C, Xu Z, Zhou W, Peng Y, Yang J, Zhou C, Li Y, Guo Q, Chen G, Wu H, Xing P, Zhang L. PD-1 Inhibitor Combined With Radiotherapy and GM-CSF (PRaG) in Patients With Metastatic Solid Tumors: An Open-Label Phase II Study. Front Immunol. 2022;13:952066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Mills BN, Qiu H, Drage MG, Chen C, Mathew JS, Garrett-Larsen J, Ye J, Uccello TP, Murphy JD, Belt BA, Lord EM, Katz AW, Linehan DC, Gerber SA. Modulation of the Human Pancreatic Ductal Adenocarcinoma Immune Microenvironment by Stereotactic Body Radiotherapy. Clin Cancer Res. 2022;28:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Palameta S, Manrique-Rincón AJ, Toscaro JM, Semionatto IF, Fonseca MC, Rosa RSM, Ruas LP, Oliveira PSL, Bajgelman MC. Boosting antitumor response with PSMA-targeted immunomodulatory VLPs, harboring costimulatory TNFSF ligands and GM-CSF cytokine. Mol Ther Oncolytics. 2022;24:650-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, Shi B, Liu J, He Z, Yu G, Ying J, Han W, Hu C, Ling Y, Chi Z, Cui C, Si L, Fang J, Zhou A, Guo J. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res. 2021;27:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | Huang L, Wang R, Xie K, Zhang J, Tao F, Pi C, Feng Y, Gu H, Fang J. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res Treat. 2022;191:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Verma K, Ogonek J, Varanasi PR, Luther S, Bünting I, Thomay K, Behrens YL, Mischak-Weissinger E, Hambach L. Human CD8+ CD57- TEMRA cells: Too young to be called "old". PLoS One. 2017;12:e0177405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cochior D, Romania S-Editor: Zhang H L-Editor: A P-Editor: Zheng XM