Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1011
Peer-review started: January 10, 2024
First decision: January 19, 2024
Revised: January 26, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 7, 2024
Processing time: 55 Days and 18.2 Hours
With continuous population and economic growth in the 21st century, plastic pollution is a major global issue. However, the health concern of microplastics/ nanoplastics (MPs/NPs) decomposed from plastic wastes has drawn public attention only in the recent decade. This article summarizes recent works dedicated to understanding the impact of MPs/NPs on the liver-the largest digestive organ, which is one of the primary routes that MPs/NPs enter human bodies. The interrelated mechanisms including oxidative stress, hepatocyte energy re-distribution, cell death and autophagy, as well as immune responses and inflammation, were also featured. In addition, the disturbance of microbiome and gut-liver axis, and the association with clinical diseases such as metabolic dysfunction-associated fatty liver disease, steatohepatitis, liver fibrosis, and cirrhosis were briefly discussed. Finally, we discussed potential directions in regard to this trending topic, highlighted current challenges in research, and proposed possible solutions.
Core Tip: The liver is heavily impacted by exposure to microplastics/nanoplastics (MPs/NPs). This editorial not only summarized the key molecular and cellular events in the liver triggered by MPs/NPs but also highlighted prospective research directions including translational and clinical studies for further investigation in this field.
- Citation: Chiang CC, Yeh H, Shiu RF, Chin WC, Yen TH. Impact of microplastics and nanoplastics on liver health: Current understanding and future research directions. World J Gastroenterol 2024; 30(9): 1011-1017
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1011
Plastic pollution has become one of the greatest challenges in the 21st century. The growing use of plastic materials nowadays has caused a serious burden not only to the environment but to human health. Microplastics (MPs, typically < 5 mm) and nanoplastics (NPs, typically < 1 μm) are small plastic particles manufactured by industry or degraded by physical and chemical processes[1,2]. These particles are now ubiquitously observed in the soil, drinking water, and even the air we breathe[3]. Furthermore, plastic particles can also enter the food chain and be biomagnified, which finally will return to our dining table and accumulate in the human body[4]. Despite a potential threat to human health, this critical issue has only attracted public awareness in recent years. Recent studies have indicated the occurrence and accumulation of MPs in the human body including blood, lungs, liver, and even in human placenta, which received considerable attention[5]. However, biomonitoring, translational, and clinical studies of human body burdens of MPs/NPs are still in their infancy.
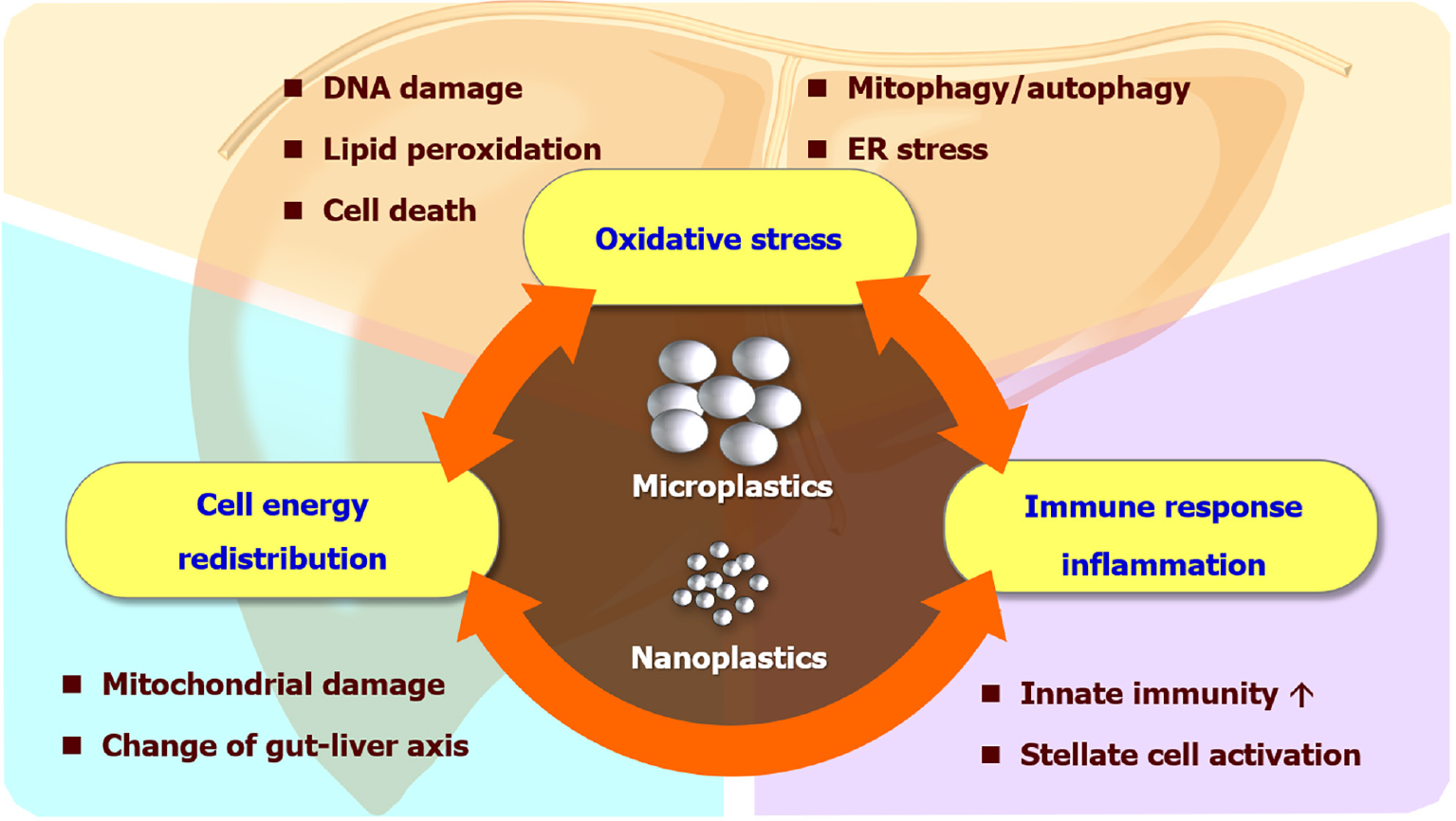
Among these, the liver is a major organ of the reticuloendothelial system, also known as the monocyte-phagocytic system, which contains gatekeeper cells like sinusoidal endothelial cells or Kupffer cells, capable of clearing foreign particles in blood circulation[6]. In addition, enterohepatic circulation includes the transportation of substances absorbed by enterocytes through portal flow and the passage of bile into the intestine via the biliary tracts[7]. This re-entry cycle can cause repeated exposure of MPs/NPs to hepatocytes and sequelae in the liver. Although in vitro and in vivo studies have demonstrated possible mechanisms that MPs can affect liver health (Figure 1), human studies are currently limited.
MPs/NPs can either generate extracellular reactive oxygen species (ROS) by weathering degradation like light or heat[8], or intracellular ROS by disrupting the mitochondrial membrane integrity and potential after internalization[3]. The redox imbalance can further cause DNA damage and genotoxicity, protein oxidation and misfolding, and lipid peroxidation with membrane instability. Metabolic dysfunction-associated fatty liver disease (MAFLD), or metabolic dysfunction-associated steatotic liver disease is a liver manifestation of metabolic syndrome which affects nearly one-third of the global adult population[9]. The theory of multiple blows is currently a recognized pathogenesis of MAFLD[10,11]. Although multiple hits like diet, obesity, insulin resistance, genetic factors, and gut dysbiosis have been found to contribute to MAFLD pathogenesis, environmental toxins or pollutants were barely mentioned in previous literature[12,13]. Recently, multiple models have demonstrated that the liver can be insulted by MPs through ROS generation, directly or indirectly resulting in MAFLD. In zebrafish models, combined exposure to a high-fat diet and MPs increased oxidative stress and upregulated lipogenic and inflammatory gene expression, which led to steatotic liver and altered behaviors[14]. Co-exposure of MPs with antibiotic pollutants in zebrafish exhibited significantly higher levels of lipid accumulation and inflammation in conjunction with oxidative stress production in their livers[15]. In mice, single-cell transcriptome analysis revealed that MPs triggered Kupffer cell and T cell activation in the high-fat diet context[16]. The study also showed MPs regulated PPAR signaling, chemical carcinogenesis-ROS pathways, and complement and blood coagulation cascade in the liver. In human pluripotent stem cell-derived liver organoids, MPs increased the gene and protein expression of hepatic HNF4A and CYP2E1, which control lipid metabolism, insulin signaling, and mitochondrial function[17]. The upregulation of the cytochrome p450 enzyme, CYP2E1, is responsible for the phase I metabolism of the liver and is highly linked to the occurrence of oxidative stress. Activated Kupffer cells can form extracellular traps of MPs/NPs, driving hepatocellular epithelial-mesenchymal transition and pro-inflammatory cytokine production through the ROS signaling pathway[18].
The energy metabolism affected by MPs/NPs is not merely limited to lipids. Exposure to MPs changes the purinergic metabolites in the liver, which suggests MPs can deplete the energy reserve of different organisms[19-21]. Additionally, the mRNA of ND5, an important protein subunit of the electron transport chain located at the inner membrane of mitochondria, was altered after exposure to NPs[22]. Since MPs/NPs can cause mitochondrial damage, it is expected that NPs interfere with the ability to produce ATPs and mobilize energy reserve, which is further echoed by liver and serum metabolite analyses related to tricarboxylic acid cycle and glycolysis[23,24]. Moreover, liver transcriptomic and metabolomic studies revealed MPs/NPs can perturb monosaccharide and lipid metabolism including pentose phosphate pathways and gluconeogenesis[25,26]. Not only do MPs/NPs inhibit building block synthesis and signal transduction, but they also damage intestinal function and suppress the absorption of nutrients[27]. Overall, these studies indicate that MPs/NPs can lead to energy deprivation in the liver.
A multitude of evidence suggests MPs/NPs drive cell death including apoptosis, pyroptosis, and ferroptosis. MPs activated hepatic intrinsic apoptosis signaling p53/Bcl-2/Bax signaling[28] and meanwhile stimulated the compensatory antioxidant Nrf2/Keap1 pathway[29]. Besides, studies showed MPs/NPs induced apoptosis by activating protein kinase RNA-like endoplasmic reticulum kinase (PERK) and mitogen-activated protein kinase pathways[30,31]. In addition, MPs/NPs induced hepatocyte pyroptosis by increasing NLRP3/ASC and caspase-1-dependent pathway[32,33]. Furthermore, MPs induced lipid peroxidation in the liver, which regulates ferroptosis-related proteins such as TFRC, FTH1, and GPX4[32]. MPs/NPs can also lead to hepatocyte autophagy by altering autophagosome LC3 and p62 ratios[33-35], and mitophagy by PERK pathway with increased ER stress[31]. A recent study demonstrated MPs triggered apoptosis and necroptosis in mouse liver through the ROS/PTEN/PI3K/AKT axis with excessive autophagy flux[36].
MPs/NPs promote inflammation and stimulate innate immune responses. After the 30 d exposure to MPs, the mouse liver showed severe vacuolar degeneration, hepatocyte edema, and inflammatory cell infiltration[29]. MPs/NPs increase cytokine expression and induce enzymatic activity related to inflammation[37,38]. The nuclear factor-kappaB (NF-κB) pathway is activated, which furthers the inflammatory response in the liver[39]. Exposure to MPs can recruit neutrophils, macrophages, and natural killer cells to the liver[39,40]. Among the infiltrative immune cells, Kupffer cells (liver-resident macrophages) play a central role in lipid metabolism and responses of hepatocytes to fat overload[41]. The activation of Kupffer cells by engulfing MPs/NPs will affect lipid metabolism, oxidize free fatty acids, and then produce excessive ROS and result in liver damage[41-43]. Furthermore, MPs polarized hepatic macrophages to pro-inflammatory M1 type and facilitated extracellular trap formation of neutrophils and macrophages[18,39,40,44]. Notably, one recent study suggested that polyethylene MPs impede the innate immune response in the liver by disrupting the extracellular matrix[45]. The contradictory result to previous research may need more future studies to confirm and clarify the underlying mechanism.
Most chronic hepatitis ultimately results in fibrosis and cirrhosis. One study showed that NPs can increase ROS and exacerbate high-fat diet-induced liver fibrosis[46]. Another study demonstrated the ROS generated by MPs can act on the TGF-β/Smad2/3 signaling axis in hepatocytes[18]. Also, ROS can cause DNA break and release from both hepatocyte nuclei and mitochondria, where in the cytoplasm the fragmented DNA sensing cGAS/STING cascade is triggered and the pro-fibrotic NF-κB pathway is activated[47]. In addition, co-exposure to cadmium and MPs promotes the extracellular release of ATP through the hemichannels of hepatocytes. The extracellular ATP activates hepatic stellate cells by interacting with P2X7 receptors and initiates fibrosis[48]. Interestingly, one retrospective study analyzing human liver tissue discovered six different MP polymers in the liver of individuals with cirrhosis, but not in those without underlying liver disease[49].
The pathogenesis of MPs/NPs may appear challenging and complicated offering a lot of research opportunities. Microbiome research has become one of the popular topics in the recent decade. Several studies have uncovered that MPs/NPs disturb the homeostasis of gut microbiota, which affects hepatic fat accumulation and steatohepatitis[15,50,51]. In zebrafish models, the abundance of Bacteroidetes and Proteobacteria decreased significantly and the abundance of Firmicutes increased significantly by polystyrene MPs[15,52]. On the contrary, polystyrene MP exposure decreased the relative abundances of Firmicutes and a-Proteobacteria in mouse intestines[51]. Conflicting results in different species require future studies for validation. However, high throughput sequencing of the 16S rRNA gene V3-V4 region revealed a significant change in the richness and diversity of gut microbiota in both polystyrene MP-exposed zebrafish and mice[51,52]. MPs/NPs-related dysbiosis may be a ”second hit” or be sensitized by other factors to cause intestinal barrier dysfunction (leaky gut) and liver inflammation[53-56]. In addition, MPs/NPs can leach out additives, flame retardants, dyes, and other organic compounds. The adsorbability, large surface area, and biodistribution characteristics of MPs/NPs also can accentuate the bioaccumulation and toxicity of heavy metals and organic compounds (Trojan-horse effect)[57,58]. This effect on hepatocytes is not only found in cell line experiments and model organisms[28,33,59-61] but also discovered in liver organoids from human embryonic stem cells and patient-derived-induced pluripotent stem cells[62,63], which may provide a powerful strategy for personalized toxicology evaluation. Furthermore, microfluidic technology has kept evolving in recent years with more efficient approaches for the identification, separation, and quantification of MPs[64]. Microfluidics is also widely applied to isolation, analysis, and parallel manipulation of single cells[65,66]. Combining these two research fields with microfluidics may take its advantage of manipulating small volumes of samples within micrometer-scale structures with a point-of-care potential. Lastly, the “long-term uncontrolled inflammation” by MPs/NPs can be a cause of tumor induction. Although one epidemiological study suggested polyvinyl chloride MPs exposure may increase the risk of liver cancers[67], it is uncertain whether the carcinogenic effect is caused by MPs or vinyl chloride monomer per se. Nevertheless, the more prominent existence of different MPs in cirrhotic patients than in healthy subjects implies that MPs/NPs may play a more important role in pre-cancerous lesions[49]. More pre-clinical and population-based research evidence is needed to delineate the correlation between MPs/NPs and liver cancers.
While trying to close the knowledge gap for plastic pollution, scientists are facing some specific challenges. The discrepant results among studies can be owing to various characterizations of MPs/NPs or different experimental protocols. Future experimental designs need to take the type, size, shape, and surface groups of MPs/NPs into consideration. It is also imperative to set exposure concentration and duration comparable to the realistic environment. Standardization of the materials and methods may yield more consistent results. Moreover, the current literature lacks clinical and epidemiological studies. Conducting human population studies can elucidate the association between the MPs/NPs exposure and health outcomes. With advanced analytical technologies, new experimental models, and well-informed interdisciplinary research collaborations, we expect to gain deeper insight into the risk of MPs/NPs to liver health, which will benefit the development of mitigation strategies and policies.
| 1. | Lambert S, Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere. 2016;145:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 614] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 2. | Koelmans AA, Besseling E, Shim WJ. Nanoplastics in the Aquatic Environment. Critical Review. In: Bergmann M, Gutow L, Klages M, editors. Marine Anthropogenic Litter. Cham: Springer International Publishing, 2015: 325-340. |
| 3. | Khan A, Jia Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. iScience. 2023;26:106061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 4. | Xu JL, Lin X, Wang JJ, Gowen AA. A review of potential human health impacts of micro- and nanoplastics exposure. Sci Total Environ. 2022;851:158111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 5. | Auguet T, Bertran L, Barrientos-Riosalido A, Fabregat B, Villar B, Aguilar C, Sabench F. Are Ingested or Inhaled Microplastics Involved in Nonalcoholic Fatty Liver Disease? Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Kalyane D, Maheshwari R, Raval N, Chauhan AS, Tekade RK. Chapter 9 - Transportation and Biointeraction Properties in Nanomaterials Across Biological Systems. In: Tekade RK, editor Basic Fundamentals of Drug Delivery: Academic Press, 2019: 343-368. |
| 7. | Grasela TH, Lukacova V, Morris DN, Clark RD, Andrews KA, Bolger MB. 4.04 - Human PK Prediction and Modeling. In: Chackalamannil S, Rotella D, Ward SE, editors. Comprehensive Medicinal Chemistry III. Oxford: Elsevier, 2017: 51-82. |
| 8. | Pannetier P, Cachot J, Clérandeau C, Faure F, Van Arkel K, de Alencastro LF, Levasseur C, Sciacca F, Bourgeois JP, Morin B. Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: Part I-adverse effects on fish cell line. Environ Pollut. 2019;248:1088-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1220] [Reference Citation Analysis (4)] |
| 10. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2300] [Article Influence: 230.0] [Reference Citation Analysis (1)] |
| 11. | Tilg H, Adolph TE, Moschen AR. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 12. | Ramírez-Mejía MM, Qi X, Abenavoli L, Romero-Gómez M, Eslam M, Méndez-Sánchez N. Metabolic dysfunction: The silenced connection with fatty liver disease. Ann Hepatol. 2023;28:101138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Klaunig JE, Li X, Wang Z. Role of xenobiotics in the induction and progression of fatty liver disease. Toxicol Res (Camb). 2018;7:664-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Boopathi S, Haridevamuthu B, Mendonca E, Gandhi A, Priya PS, Alkahtani S, Al-Johani NS, Arokiyaraj S, Guru A, Arockiaraj J, Malafaia G. Combined effects of a high-fat diet and polyethylene microplastic exposure induce impaired lipid metabolism and locomotor behavior in larvae and adult zebrafish. Sci Total Environ. 2023;902:165988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Zhou W, Shi W, Du X, Han Y, Tang Y, Ri S, Ju K, Kim T, Huang L, Zhang W, Yu Y, Tian D, Chen L, Wu Z, Liu G. Assessment of Nonalcoholic Fatty Liver Disease Symptoms and Gut-Liver Axis Status in Zebrafish after Exposure to Polystyrene Microplastics and Oxytetracycline, Alone and in Combination. Environ Health Perspect. 2023;131:47006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (15)] |
| 16. | Liu W, Li M, Guo H, Wei S, Xu W, Yan Y, Shi Y, Xu Z, Chang K, Wei G, Zhao S. Single-cell transcriptome analysis of liver immune microenvironment changes induced by microplastics in mice with non-alcoholic fatty liver. Sci Total Environ. 2024;912:168308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Cheng W, Li X, Zhou Y, Yu H, Xie Y, Guo H, Wang H, Li Y, Feng Y, Wang Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci Total Environ. 2022;806:150328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 18. | Wang S, Chen L, Shi X, Wang Y, Xu S. Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ Pollut. 2023;324:121388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7:46687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 652] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 20. | Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ Sci Technol. 2016;50:4054-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1355] [Article Influence: 135.5] [Reference Citation Analysis (1)] |
| 21. | Wright SL, Rowe D, Thompson RC, Galloway TS. Microplastic ingestion decreases energy reserves in marine worms. Curr Biol. 2013;23:R1031-R1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 22. | Brandts I, Teles M, Tvarijonaviciute A, Pereira ML, Martins MA, Tort L, Oliveira M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics. 2018;110:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 23. | Ye G, Zhang X, Liu X, Liao X, Zhang H, Yan C, Lin Y, Huang Q. Polystyrene microplastics induce metabolic disturbances in marine medaka (Oryzias melastigmas) liver. Sci Total Environ. 2021;782:146885. |
| 24. | Wei L, Liao P, Wu H, Li X, Pei F, Li W, Wu Y. Toxicological effects of cinnabar in rats by NMR-based metabolic profiling of urine and serum. Toxicol Appl Pharmacol. 2008;227:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Luo T, Wang C, Pan Z, Jin C, Fu Z, Jin Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ Sci Technol. 2019;53:10978-10992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 26. | Wang C, Hou M, Shang K, Wang H, Wang J. Microplastics (Polystyrene) Exposure Induces Metabolic Changes in the Liver of Rare Minnow (Gobiocypris rarus). Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317-E325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 156] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Sheng S, Han N, Wei Y, Wang J, Han W, Xing B, Xing M, Zhang W. Liver Injury Induced by Exposure to Polystyrene Microplastics Alone or in Combination with Cadmium in Mice Is Mediated by Oxidative Stress and Apoptosis. Biol Trace Elem Res. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Li S, Shi M, Wang Y, Xiao Y, Cai D, Xiao F. Keap1-Nrf2 pathway up-regulation via hydrogen sulfide mitigates polystyrene microplastics induced-hepatotoxic effects. J Hazard Mater. 2021;402:123933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 30. | Hu Q, Wang H, He C, Jin Y, Fu Z. Polystyrene nanoparticles trigger the activation of p38 MAPK and apoptosis via inducing oxidative stress in zebrafish and macrophage cells. Environ Pollut. 2021;269:116075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Pan L, Yu D, Zhang Y, Zhu C, Yin Q, Hu Y, Zhang X, Yue R, Xiong X. Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci Total Environ. 2021;781:146753. [DOI] [Full Text] |
| 32. | Mu Y, Sun J, Li Z, Zhang W, Liu Z, Li C, Peng C, Cui G, Shao H, Du Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere. 2022;291:132944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 33. | Zhong G, Rao G, Tang L, Wu S, Tang Z, Huang R, Ruan Z, Hu L. Combined effect of arsenic and polystyrene-nanoplastics at environmentally relevant concentrations in mice liver: Activation of apoptosis, pyroptosis and excessive autophagy. Chemosphere. 2022;300:134566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 34. | Kaloyianni M, Bobori DC, Xanthopoulou D, Malioufa G, Sampsonidis I, Kalogiannis S, Feidantsis K, Kastrinaki G, Dimitriadi A, Koumoundouros G, Lambropoulou DA, Kyzas GZ, Bikiaris DN. Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics. Toxics. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Missawi O, Venditti M, Cappello T, Zitouni N, Marco G, Boughattas I, Bousserrhine N, Belbekhouche S, Minucci S, Maisano M, Banni M. Autophagic event and metabolomic disorders unveil cellular toxicity of environmental microplastics on marine polychaete Hediste diversicolor. Environ Pollut. 2022;302:119106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Wang S, Wu H, Shi X, Wang Y, Xu S. Polystyrene microplastics with different sizes induce the apoptosis and necroptosis in liver through the PTEN/PI3K/AKT/autophagy axis. Sci Total Environ. 2023;899:165461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol. 2018;200:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 419] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 38. | Silvestre F. Signaling pathways of oxidative stress in aquatic organisms exposed to xenobiotics. J Exp Zool A Ecol Integr Physiol. 2020;333:436-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Zhao L, Shi W, Hu F, Song X, Cheng Z, Zhou J. Prolonged oral ingestion of microplastics induced inflammation in the liver tissues of C57BL/6J mice through polarization of macrophages and increased infiltration of natural killer cells. Ecotoxicol Environ Saf. 2021;227:112882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 40. | Ma S, Xiao Y, Zhang X, Xu Y, Zhu K, Zhang K, Li X, Zhou H, Chen G, Guo X. Dietary exposure to polystyrene microplastics exacerbates liver damage in fulminant hepatic failure via ROS production and neutrophil extracellular trap formation. Sci Total Environ. 2024;907:167403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Diehl KL, Vorac J, Hofmann K, Meiser P, Unterweger I, Kuerschner L, Weighardt H, Förster I, Thiele C. Kupffer Cells Sense Free Fatty Acids and Regulate Hepatic Lipid Metabolism in High-Fat Diet and Inflammation. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Rudolph J, Völkl M, Jérôme V, Scheibel T, Freitag R. Noxic effects of polystyrene microparticles on murine macrophages and epithelial cells. Sci Rep. 2021;11:15702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Prata JC. Microplastics and human health: Integrating pharmacokinetics. Crit Rev Environ Sci Technol. 2023;53:1489-1511. [DOI] [Full Text] |
| 44. | Yin K, Wang D, Zhang Y, Lu H, Hou L, Guo T, Zhao H, Xing M. Polystyrene microplastics promote liver inflammation by inducing the formation of macrophages extracellular traps. J Hazard Mater. 2023;452:131236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 141] [Reference Citation Analysis (0)] |
| 45. | Huang H, Hou J, Liao Y, Wei F, Xing B. Polyethylene microplastics impede the innate immune response by disrupting the extracellular matrix and signaling transduction. iScience. 2023;26:107390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 46. | Li L, Xu M, He C, Wang H, Hu Q. Polystyrene nanoplastics potentiate the development of hepatic fibrosis in high fat diet fed mice. Environ Toxicol. 2022;37:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 47. | Shen R, Yang K, Cheng X, Guo C, Xing X, Sun H, Liu D, Liu X, Wang D. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ Pollut. 2022;300:118986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 48. | Sun J, Qu H, Ali W, Chen Y, Wang T, Ma Y, Yuan Y, Gu J, Bian J, Liu Z, Zou H. Co-exposure to cadmium and microplastics promotes liver fibrosis through the hemichannels -ATP-P2X7 pathway. Chemosphere. 2023;344:140372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 49. | Horvatits T, Tamminga M, Liu B, Sebode M, Carambia A, Fischer L, Püschel K, Huber S, Fischer EK. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82:104147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 381] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 50. | Dong R, Zhou C, Wang S, Yan Y, Jiang Q. Probiotics ameliorate polyethylene microplastics-induced liver injury by inhibition of oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022;130:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631-632:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 674] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 52. | Jin Y, Xia J, Pan Z, Yang J, Wang W, Fu Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ Pollut. 2018;235:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 549] [Article Influence: 68.6] [Reference Citation Analysis (4)] |
| 53. | Okamura T, Hamaguchi M, Hasegawa Y, Hashimoto Y, Majima S, Senmaru T, Ushigome E, Nakanishi N, Asano M, Yamazaki M, Sasano R, Nakanishi Y, Seno H, Takano H, Fukui M. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ Health Perspect. 2023;131:27006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 54. | Lv W, Shen Y, Xu S, Wu B, Zhang Z, Liu S. Underestimated health risks: Dietary restriction magnify the intestinal barrier dysfunction and liver injury in mice induced by polystyrene microplastics. Sci Total Environ. 2023;898:165502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 55. | Luo T, Wang D, Zhao Y, Li X, Yang G, Jin Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci Total Environ. 2022;844:156884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Zheng H, Wang J, Wei X, Chang L, Liu S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci Total Environ. 2021;750:143085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 57. | Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. 2020;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 382] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 58. | Zhao WG, Tian YM, Zhao P, Zhao LA, Jin C. [Research Progress on Trojan-horse Effect of Microplastics and Heavy Metals in Freshwater Environment]. Huan Jing Ke Xue. 2023;44:1244-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Wang Q, Chen G, Tian L, Kong C, Gao D, Chen Y, Junaid M, Wang J. Neuro- and hepato-toxicity of polystyrene nanoplastics and polybrominated diphenyl ethers on early life stages of zebrafish. Sci Total Environ. 2023;857:159567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 60. | Menéndez-Pedriza A, Jaumot J, Bedia C. Lipidomic analysis of single and combined effects of polyethylene microplastics and polychlorinated biphenyls on human hepatoma cells. J Hazard Mater. 2022;421:126777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 61. | Luo T, Weng Y, Huang Z, Zhao Y, Jin Y. Combined hepatotoxicity of imidacloprid and microplastics in adult zebrafish: Endpoints at gene transcription. Comp Biochem Physiol C Toxicol Pharmacol. 2021;246:109043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Cheng W, Zhou Y, Xie Y, Li Y, Zhou R, Wang H, Feng Y, Wang Y. Combined effect of polystyrene microplastics and bisphenol A on the human embryonic stem cells-derived liver organoids: The hepatotoxicity and lipid accumulation. Sci Total Environ. 2023;854:158585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 63. | Liang S, Luo Y, Yi J, Feng L, Xu M, Yao R. Toxicity of microplastics and plastic additive co-exposure in liver Disse organoids from healthy donors and patient-derived induced pluripotent stem cells. 2022 Preprint. Available from: bioRxiv:2022.2009.2012.506301. [DOI] [Full Text] |
| 64. | Ece E, Hacıosmanoğlu N, Inci F. Microfluidics as a Ray of Hope for Microplastic Pollution. Biosensors (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 65. | Zare RN, Kim S. Microfluidic platforms for single-cell analysis. Annu Rev Biomed Eng. 2010;12:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 66. | Liu L, Zhang QH, Li RT. In Situ and Individual-Based Analysis of the Influence of Polystyrene Microplastics on Escherichia coli Conjugative Gene Transfer at the Single-Cell Level. Environ Sci Technol. 2023;57:15936-15944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 67. | Zarus GM, Muianga C, Brenner S, Stallings K, Casillas G, Pohl HR, Mumtaz MM, Gehle K. Worker studies suggest unique liver carcinogenicity potential of polyvinyl chloride microplastics. Am J Ind Med. 2023;66:1033-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Taiwan Society of Nephrology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu L, China S-Editor: Fan JR L-Editor: A P-Editor: Yu HG