Published online Feb 28, 2024. doi: 10.3748/wjg.v30.i8.956
Peer-review started: October 16, 2023
First decision: December 21, 2023
Revised: January 3, 2024
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: February 28, 2024
Processing time: 132 Days and 21.6 Hours
The prevalence of sarcopenia in patients undergoing liver transplantation (LT) remains to be determined partly because of different diagnostic criteria. Sarcopenia has recently been recognized as a new prognostic factor for predicting outcomes in LT candidates.
To estimate the prevalence of sarcopenia and evaluate its clinical effect on LT candidates.
This systematic search was conducted in PubMed, Web of Science, Embase, and Cochrane Library for original English-language articles that investigated the prevalence and influence of sarcopenia in patients undergoing LT from database inception to November 30, 2022. Cohort studies of the definition of sarcopenia that estimate sarcopenia prevalence and evaluate its effect on clinical outcomes and the risk of mortality were included.
Twenty-five studies involving 7760 patients undergoing LT were included. The pooled prevalence of sarcopenia in patients undergoing LT was 40.7% [95% confidence intervals (95%CI): 32.1–49.6]. The 1-, 3-, and 5-year cumulative probabilities of post-LT survival in patients with preoperative sarcopenia were all lower than those without sarcopenia (P < 0.05). Sarcopenia was associated with an increased risk of post-LT mortality in patients undergoing LT (adjusted hazard ratio: 1.58; 95%CI: 1.21–2.07). Patients with preoperative sarcopenia had a longer intensive care unit stay, a high risk ratio of sepsis, and serious post-LT complications than those without sarcopenia.
Sarcopenia is prevalent in a substantial proportion of patients undergoing LT and is strongly and independently associated with higher a risk of mortality risk.
Core Tip: The prevalence and effect of sarcopenia on patients undergoing liver transplantation (LT) remains to be determined partly because of different diagnostic criteria. Twenty-five studies involving 7760 patients undergoing LT were included in this meta-analysis. The pooled prevalence of sarcopenia in patients undergoing LT was 40.7%. Sarcopenia was associated with an increased risk of post-LT mortality in patients undergoing LT.
- Citation: Jiang MJ, Wu MC, Duan ZH, Wu J, Xu XT, Li J, Meng QH. Prevalence and clinical impact of sarcopenia in liver transplant recipients: A meta-analysis. World J Gastroenterol 2024; 30(8): 956-968
- URL: https://www.wjgnet.com/1007-9327/full/v30/i8/956.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i8.956
According to the Global Burden of Disease project, liver disease accounts for approximately 2 million deaths annually worldwide, including one million patients who died from complications of cirrhosis and one million patients who died from liver cancer and viral hepatitis[1]. Liver transplantation (LT) has become the standard treatment for patients with decompensated end-stage liver disease (ESLD)[2]. However, less than 10% of global organ transplantation needs are met at current rates of transplantation[1]. With the widespread shortage of human organs, rigorous selection of LT candidates is essential[3]. Therefore, choosing which patients are clinically suitable for LT is one of the hardest challenges for clinicians. Waiting-list mortality and post-LT survival are key determinant factors in waiting-list placement[3]. The model for ESLD (MELD) score is the most common tool used to predict outcomes in patients for LT[4]. Although the MELD score has a strong predictive value for pre-LT outcomes, it underestimates disease severity in approximately 15%–20% of patients with cirrhosis, leading to an inaccurate prediction of post-LT outcomes[4]. A common but often overlooked complication in patients with ESLD is malnutrition[5]. Undeniably, patients who are malnourished are more likely to suffer from adverse outcomes and have a high mortality risk[6,7]. Given the importance of nutritional status, an appropriate nutritional assessment must be established to determine the effect of nutritional status on patients undergoing LT.
Sarcopenia is a progressive and generalized loss of skeletal muscle mass, strength, and function and is a major component of malnutrition[8]. A previous study discovered that patients with cirrhosis have a high protein oxidation rate and a low carbohydrate oxidation rate, leading to an imbalance in skeletal muscle protein synthesis and breakdown[9]. Hepatocellular dysfunction and portosystemic shunting also result in biochemical and hormonal perturbations in patients with ESLD that contribute to sarcopenia[7]. The prevalence of sarcopenia in patients with ESLD ranges from 30% to 70%, depending on the liver disease etiology, disease stage, and diagnostic criteria[10]. Regardless of how sarcopenia is defined, it is a robust predictor of clinically relevant adverse outcomes, including poor quality of life, mortality in patients on the LT waitlist, longer stays in the hospital or intensive care unit, high incidence of infection following LT, and higher overall healthcare costs[11].
Over the past few years, sarcopenia has become a topic of prolific exploration in patients with ESLD[11]. A meta-analysis published in 2016 indicated that sarcopenia was associated with post-LT mortality; however, overlapping patients were included in this article[3]. Since then, several large and rigorously designed studies with long-term follow-up have been published. Considering the large variability in the prevalence of sarcopenia, the effect of sarcopenia on a broader range of clinically important LT-related outcomes remains unclear. Thus, this meta-analysis aimed to systematically evaluate the literature about patients who underwent LT to summarize the diagnostic criteria for sarcopenia, estimate its prevalence, and assess its effect on clinical outcomes.
This meta-analysis was conducted based on the PRISMA checklist and was registered in PROSPERO (CRD42022379765).
A systematic search was conducted in PubMed, Web of Science, Embase, and Cochrane Library for original English-language articles that investigated the prevalence and effect of sarcopenia on patients undergoing LT from database inception to November 30, 2022. The search keywords and search strategies for all the included databases are shown in Supplementary Table 1. To find additional potential studies, the reference lists of the included articles were also manually searched. In addition, the study only included human studies.
The inclusion criteria were as follows: (1) Patients who underwent LT; (2) a definite diagnosis of sarcopenia based on either muscle mass or muscle function parameters; (3) studies that included the prevalence of sarcopenia in patients who underwent LT and clinical outcomes for post-LT patients; and (4) prospective or retrospective cohort studies. The exclusion criteria were as follows: (1) Case reports or review articles; (2) unclear definition of sarcopenia; (3) studies that only examined the effect of sarcopenia on pre-LT patients or patients awaiting LT; (4) duplicate studies; and (5) studies with insufficient data or unclear study information. Two reviewers independently read the full text and screened the articles that met the inclusion and exclusion criteria. The discrepancies between reviewers regarding inclusion were settled through consensus or by consulting with third-party experts.
Data were independently extracted and coded from each included study by two researchers (Jiang MJ and Wu MC) using an Excel spreadsheet. The basic information gathered from the studies included in this analysis encompasses the first author, year of publication, study design, study location, age or sex distribution, total number of participants, definition of sarcopenia, crude prevalence of sarcopenia, clinical characteristics, including the etiology of liver disease, liver function, presence of hepatocellular carcinoma, follow-up duration, and relevant outcomes. The quality of the included studies was also scored by at least two authors (Jiang MJ, Wu MC, Duan ZH, and Xiao TX) independently using the Newcastle–Ottawa Scale (NOS; 9 items in total, out of 9 points)[12]. Disagreements were resolved by consensus or discussion with a third author (Wu J, Li J, and Meng QH).
The prevalence of sarcopenia was determined through a meta-analysis. Subgroup data were collected according to the method used to define sarcopenia, sex, region, etiology of liver disease, and severity of liver disease. The primary outcome of this meta-analysis was mortality risk in patients undergoing LT with sarcopenia. The effect of sarcopenia on the incidence of post-LT survival was evaluated using the pooled unadjusted hazard ratio (HR) or adjusted HR and 95% confidence intervals (95%CI). The 90-day, 1-year, 3-year, and 5-year cumulative mortality were also pooled for patients with and without sarcopenia using the Freeman–Tukey double arcsine transformation method[13]. Continuous outcome data evaluated using homogenous metrics (e.g., same test instrument) were summarized as weighted mean difference (WMD) and 95%CI. Dichotomous variables were tested using both risk ratios (RR) and 95%CI. Random-effects meta-regression was used to test the difference between two groups. Sensitivity analysis was used to evaluate the stability of the model. Egger’s and Begg’s tests combined with the observation funnel plot were used to evaluate publication bias. Heterogeneity was statistically assessed using the I2 measurement and Cochran’s Q statistic. A random effects model was used if heterogeneity was high (P value of Q statistic ≤ 0.1 or I2 ≥ 50%). Stata 16 software was used for the meta-analysis. Two-sided P < 0.05 was considered statistically significant.
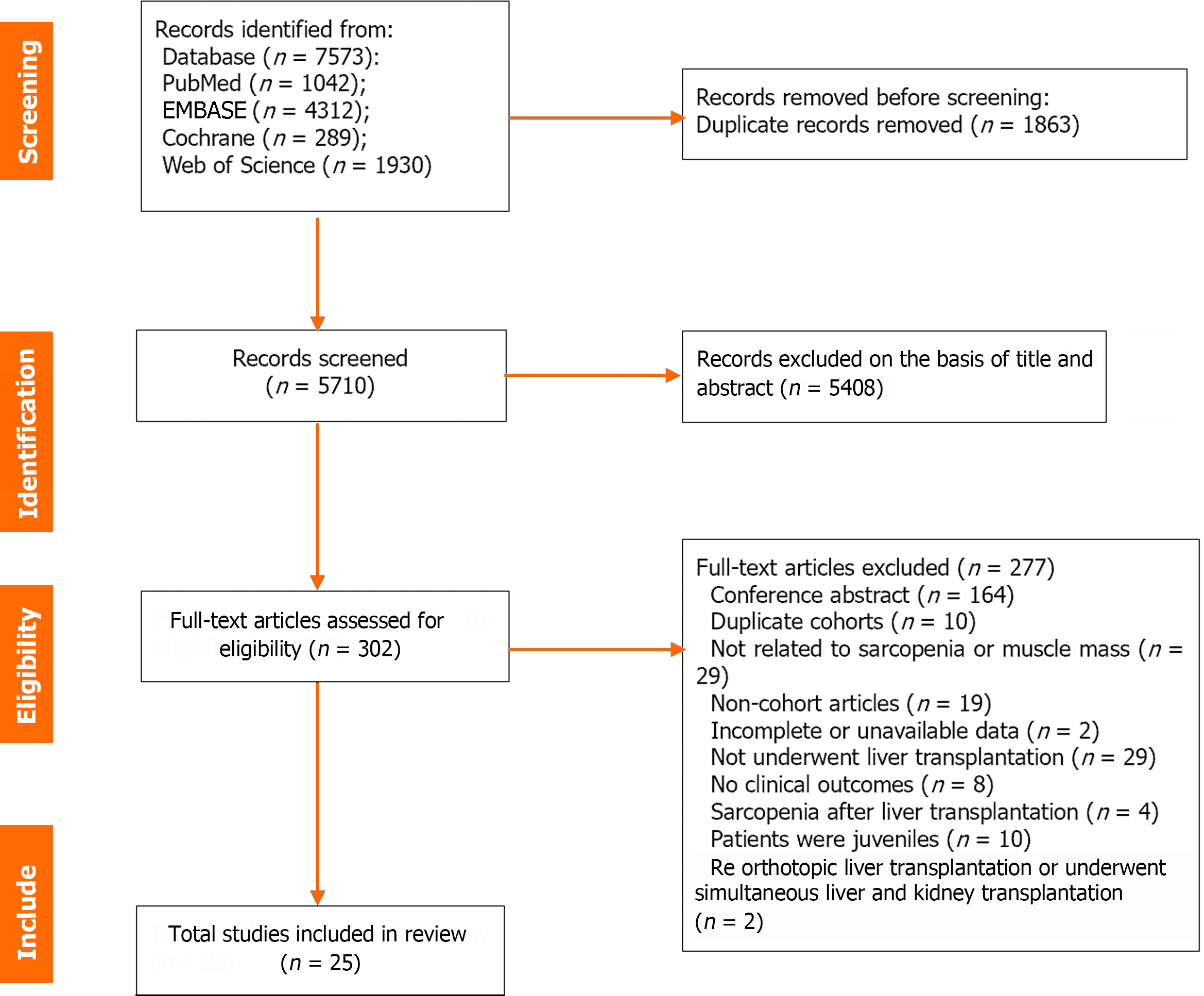
A detailed flowchart of the literature search is shown in Figure 1. Of the 7573 records identified from four databases (PubMed, n = 1,042; EMBASE, n = 4312; Cochrane Library, n = 289; Web of Science, n = 1930), 1863 duplicates and 5408 ineligible titles/abstracts were excluded. Of the other 302 articles that underwent full-text review, 25 retrospective cohort studies with data on 7760 patients were included. The detailed characteristics of all the studies in this meta-analysis are summarized in Table 1. Overall, 10 of the 25 studies were from Asia, 6 from Europe, 5 from North America, and 2 from Africa. Only one study was from Australia, and one was a multicenter international study. The number of patients of the included studies ranged from 47 to 2816. The mean age of the patients ranged from 41.6 to 57.0 years among the included researches. All studies were rated high quality with an NOS score of ≥ 7 (Supplementary Table 2).
| Ref. | Country | Methods for measuring muscle mass | Definition of sarcopenia | Sample size (n) | Mean age (yr) | Male (%) | HCC (%) | BMI (Kg/m2) | Follow-up time | NOS |
| Kumar et al[2], 2020 | India | CT: L3-SMI | L3-SMI < 52.4 cm2/m2 for male and < 38.5 cm2/m2 for female | 115 | 46.30 ± 10.20 | 90.4 | - | 24.5 ± 4.3 | 90 d | 7 |
| Bhanji et al[8], 2019 | United States | CT: L3-SMI | L3-SMI < 50 cm2/m2 in men and < 39 cm2/m2 in women | 293 | 51.95 ± 11.00 | 70.3 | 30.4 | 27.51 ± 5.73 | 13 | 8 |
| Shafaat et al[26], 2023 | United States | CT: L3-SMI | L3-SMI < 50 cm2/m2 in men, < 39 cm2/m2 in women | 454 | 57.00 ± 8.89 | 65.0 | - | 29.0 ± 5.7 | 11yr | 9 |
| Sim et al[27], 2022 | Korea | CT: L3-SMI | L3-SMI < 39.9cm2/m2 in men, < 28.9 cm2/m2 in women | 2816 | 53.00 ± 7.41 | 75.0 | 52.8 | 24.2 ± 3.1 | 7.8 yr | 8 |
| Prakash et al[28], 2022 | India | CT: L4-PMTH | L4-PMTH < 1 mm/m for men and < 10.4 mm/m for women | 51 | 46.40 ± 9.10 | 96.1 | 37.3 | 23.34 ± 5.1 | - | 7 |
| Wu et al[29], 2021 | Taiwan China | CT: L3-PMI | L3-PMI < 2.63 cm2/m2 for female | 122 | 52.32 ± 7.98 | 55.0 | 24.6 | 24.83 ± 4.45 | 10 yr | 8 |
| Kuo et al[30], 2019 | United States | CT: L3-SMI | L3-SMI < 48 cm2/m2 for men | 126 | 53.00 ± 8.89 | 63.0 | 14.0 | 28 ± 6.67 | 10.6 yr | 8 |
| Golse et al[31] 2017 | France | CT: L3-4 PMA | L3-4 PMA < 1464 mm2 in women and < 1561 mm2 in men | 256 | 5.003 ± 10.50 | 76.6 | 40.0 | 25.3 ± 10.5 | 8 yr | 8 |
| Beumer et al[32], 2022 | Multicenter international study | CT: L3-SMI | L3-SMI < 37 cm2/m2 for women with a BMI < 25 kg/m2 or 42 cm2/m2 for women with a BMI ≥ 25 kg/m2, and < 45 cm2/m2 for men with a BMI < 25 kg/ m2, or < 51 cm2/m2 for men with a BMI ≥ 25 kg/m2 | 528 | 57.00 ± 9.00 | 86.0 | 100.0 | 26.67 ± 5.02 | 5 yr | 8 |
| Pinto Dos Santos et al[33], 2020 | Germany | CT: L3-PSMI | L3-PSMI < 18.6 cm2/m2 | 368 | 56.80 ± 9.70 | 69.3 | 44.6 | 25.2 ± 4.37 | 10 yr | 7 |
| Izumi et al[34], 2017 | Japan | CT: L3-PMI | L3-PMI < 612.5 mm2/m2 in men and < 442.9 mm2/m2 in women | 47 | 54.00 ± 10.00 | 51.1 | 23.4 | - | 120 d | 7 |
| Hamaguchi et al[35], 2017 | Japan | CT: L3-SMI | L3-SMI < 40.31 cm2/m2 in men and < 30.88 cm2/m2 in women | 250 | 54.00 ± 14.07 | 44.8 | 33.0 | 22.7 ± 3.63 | 5 yr | 8 |
| Irwin et al[36], 2021 | South Africa | CT: L3-SMI | L3-SMI < 39 cm2/m2 for women and < 50 cm2/m2 for men | 106 | - | 60.4 | - | - | 1 yr | 9 |
| Tan et al[14], 2022 | China | CT: L3-PMI | L3-PMI < 6.25 cm2/m2 for man | 70 | 41.60 ± 9.70 | 100.0 | - | 22.9 ± 2.9 | 10 yr | 8 |
| Masuda et al[37], 2014 | Japan | CT: L3-PMA | < 800 cm2 for men and < 380 cm2 for women | 204 | 54.32 ± 9.60 | 50.49 | - | 23.6 ± 3.4 | 8 yr | 7 |
| Miarka et al[38], 2021 | Poland | CT: L3-SMI | L3-SMI < 50 cm2/m2 for men and 39 cm2/m2 for women | 98 | 55.00 ± 8.00 | 76.5 | 26.5 | 27 ± 4 | 1224 d | 7 |
| Montano-Loza et al[39], 2014 | Canada | CT: L3-SMI | L3-SMI ≤ 41 cm2/m2 for women and ≤ 53 cm2/m2 for men with a BMI ≥25 kg/m2 and L3 SMI ≤ 43 cm2/m2 for patients with a BMI < 25 kg/m2 | 248 | 55.00 ± 1.00 | 68.0 | 39.0 | 27.19 ± 2.23 | 5 yr | 9 |
| Kalafateli et al[23], 2017 | United Kingdom | CT: L3-PMI | L3-PMI < 340 mm2/m2 for men and < 264 mm2/m2 for women | 232 | 54.00 ± 12.00 | 69.8 | 25.0 | 25 ± 6.43 | 1 yr | 8 |
| Czigany et al[40], 2020 | Germany | CT: L3-SMI | L3-SMI < 50 cm2/m2 in men and < 39 cm2/m2 in women | 225 | 54.00 ± 12.00 | 66.7 | 28.0 | 27 ± 5 | 90 d | 8 |
| Hey et al[41], 2022 | Australia | DEXA: APLM | APLM < 7.26 kg/m2 for male and < 5.5 kg/m2 for female | 469 | 55.00 ± 10.59 | 72.1 | 26.0 | - | 1 yr | 8 |
| Lindqvist et al[42], 2019 | Sweden | CT: L3-SMI | L3-SMI < 43 cm2/m2 for men with BMI < 25 kg/m2 and < 53 cm2/m2 for BMI > 25 kg/m2 and < 41 cm2/m2 for women in all BMI ranges | 53 | 57.00 ± 11.11 | 69.8 | 52.8 | 25.1 ± 5.3 | 1 yr | 8 |
| Riccardo et al[43], 2019 | Japan | CT: L3-SMI | L3-SMI < 42 cm2/m2 for men and L3-SMI < 38 cm2/m2 for women | 138 | 57.00 ± 13 | 56.5 | - | 24.8 ± 5 | 10 yr | 8 |
| Andrea et al[21], 2013 | United States | CT: L3-4 SMI | L3-4 SMI ≤ 38.5 cm2/m2 for women and ≤ 52.4 cm2/m2 for men | 338 | 55.00 ± 10.00 | 65.9 | - | 28 ± 6 | 1021.2 d | 9 |
| Young et al[44], 2018 | Korea | CT: L3-PMTH | L3-PMTH < 15.5 mm/m | 92 | 53.33 ± 5.75 | 67.4 | 100.0 | 24.17 ± 2.81 | 36 months | 8 |
| Hassan et al[45], 2022 | Egypt | CT: L3-SMI | L3-SMI < 52.4 cm2/m2 for male and < 38.5 cm2/m2 for female | 61 | 48.70 ± 12.50 | 75,4 | - | 23.9 ± 3.9 | 6 months | 7 |
All the studies included in this meta-analysis used a low muscle mass as the basis of diagnosis. No studies have used low muscle strength or low physical performance as diagnostic criteria. Twenty-four studies used skeletal muscle area-based CT to diagnose sarcopenia, and only one study used dual-energy X-ray absorptiometry (DEXA) to assess sarcopenia. Fifteen studies reported the cross-sectional muscle area with the third lumbar-skeletal muscle index (L3-SMI), whereas the psoas muscle area or psoas muscle index (PMI) was reported in nine studies. Twenty-three studies used different diagnostic criteria based on sex. As the most used diagnostic method for sarcopenia, the cutoff values of L3-SMI ranged from 39.0-52.4 cm2/m2 in men and from 28.9-42 cm2/m2 in women. A summary of the diagnostic criteria used to assess sarcopenia in the included studies is presented in Table 1.
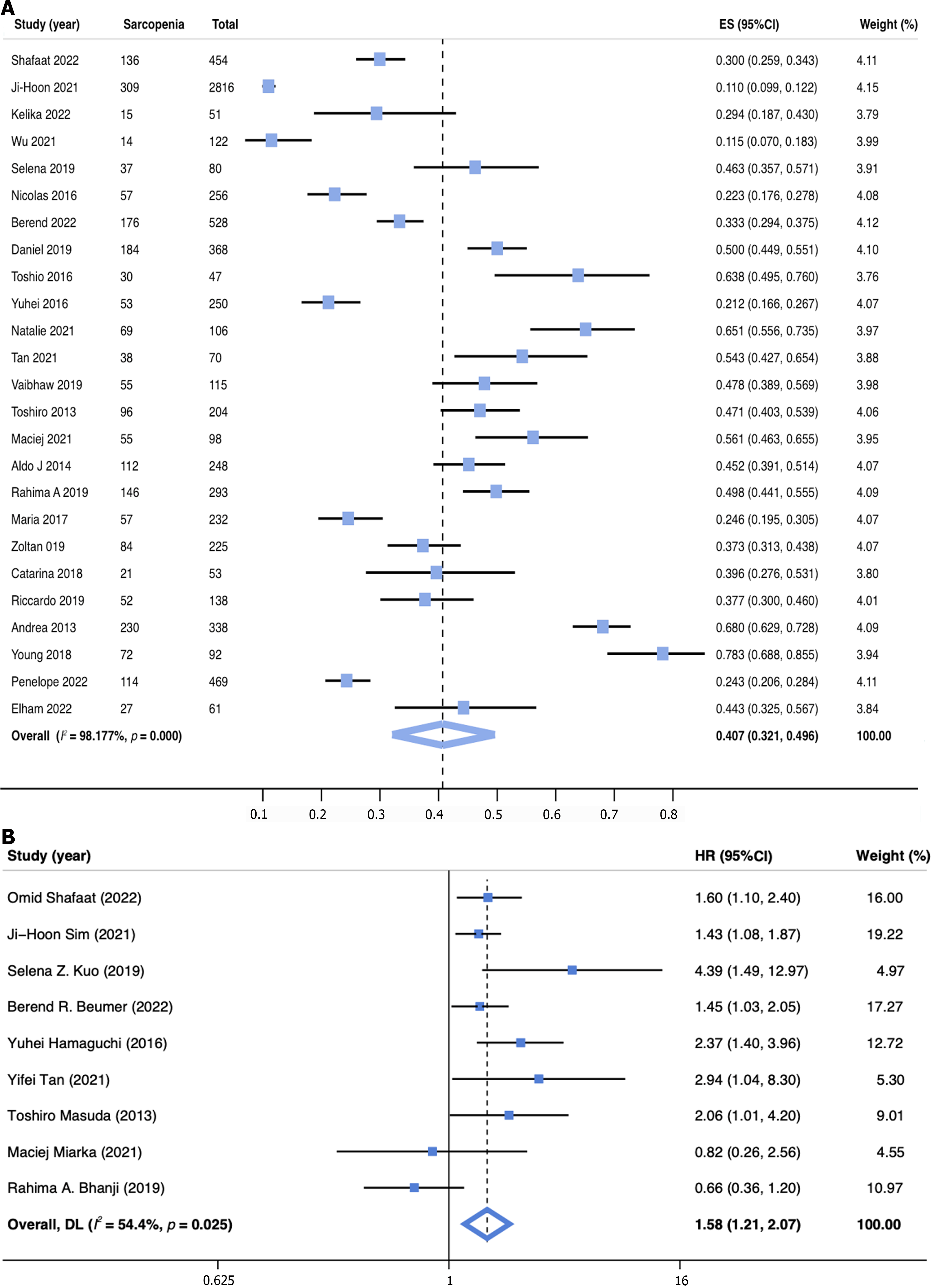
The prevalence of sarcopenia was reported in 25 studies (n = 7760) and ranged from 11.0% to 78.3%, yielding a pooled prevalence of 40.7% (95%CI: 32.1-49.6; Figure 2A). Given the significant heterogeneity, subgroup analyses of sarcopenia rates were conducted for different definitions, sexes, regions, basic diseases, and Child–Pugh class (Table 2). The subgroup analysis based on the definition of sarcopenia showed that the prevalence of sarcopenia was 41.5% (95%CI: 29.6–53.9) when defined by L3-SMI, 36.4% (95%CI: 16.2–59.5) by L3-PMI, and 41.5% (95%CI: 26.7–57.1) by other definitions. A subgroup analysis by sex was also performed, which revealed that male patients had a higher pooled prevalence of sarcopenia (43.3%, 95%CI: 31.1–55.9) than female patients (33.1%, 95%CI: 21.6–45.6). Moreover, 23 studies were analyzed by regional subgroup, and Africa had the highest pooled prevalence of sarcopenia among patients undergoing LT (57.6%, 95%CI: 50.0–65.1). Among the different primary liver diseases, patients with alcoholic liver disease had the highest prevalence of sarcopenia (52.2%, 95%CI: 36.2–68.2). Finally, the prevalence for patients with Child–Pugh class C (54.3%, 95%CI: 43.9–64.8) was higher than those with Child–Pugh class B (38.9%, 95%CI: 30.8–47.0) or A (30.4%, 95%CI: 26.0–35.0).
| Subgroup | Studies (n) | Sarcopenia (n) | Prevalence [% (95%CI)] | I2 (%) | P value |
| Definition of sarcopenia | |||||
| L3-SMI | 15 | 1562 | 41.5 (29.6-53.9) | 98.6 | < 0.001a |
| L3-PMI | 4 | 139 | 36.4 (16.2-59.5) | 95.6 | < 0.001a |
| Others | 6 | 538 | 41.5 (26.7-57.1) | 97.0 | < 0.001a |
| Sex | |||||
| Male | 17 | 1280 | 43.3 (31.1-55.9) | 98.9 | < 0.001a |
| Female | 15 | 366 | 33.1 (21.6-45.6) | 95.7 | < 0.001a |
| World region | |||||
| Europe | 6 | 458 | 37.7 (26.6-49.5) | 94.0 | < 0.001a |
| Asia | 10 | 734 | 38.8 (23.3-55.5) | 98.3 | < 0.001a |
| North America | 5 | 661 | 47.8 (33.4-62.4) | 96.7 | < 0.001a |
| Africa | 2 | 96 | 57.6 (50.0-65.1) | 0.0 | < 0.001a |
| Disease types | |||||
| Viral | 8 | 407 | 32.3 (18.9-47.2) | 97.0 | < 0.001a |
| ALD | 11 | 477 | 52.2 (36.2-68.2) | 96.8 | < 0.001a |
| NAFLD | 5 | 54 | 47.2 (25.7-68.6) | 85.6 | < 0.001a |
| AIH/PSC/PBC | 4 | 141 | 33.6 (19.0-48.2) | 63.5 | 0.042a |
| HCC | 10 | 399 | 35.9 (18.6-53.2) | 98.4 | < 0.001a |
| Other | 7 | 123 | 41.2 (22.6-59.8) | 92.2 | < 0.001a |
| Child-Pugh class | |||||
| A | 4 | 121 | 30.4 (26.0-35.0) | 44.6 | 0.144 |
| B | 5 | 157 | 38.9 (30.8-47.0) | 56.8 | 0.055 |
| C | 7 | 446 | 54.3 (43.9-64.8) | 88.2 | < 0.001a |
The 90-day, 1-year, 3-year, and 5-year cumulative probabilities of post-LT survival in patients with sarcopenia were 92.9% (95%CI: 88.9–96.9), 79.8% (95%CI: 72.8–86.8), 74.3% (95%CI: 68.0–80.5), and 63.6% (95%CI: 56.5–70.6), respectively (Supplementary Figures 1-4). By comparison, they were 96.5% (95%CI: 94.7–98.3), 92.7% (95%CI: 90.2–96.2), 93.4% (95%CI: 90.6–96.2), and 79.5% (95%CI: 73.2–85.8), respectively, in patients without sarcopenia (Supplemen
| Survival [% (95%CI)] | With sarcopenia | Without sarcopenia | P value |
| 90-day | 92.9 (88.9-96.9), 5 studies 422 patients | 96.5 (94.7-98.3), 5 studies 891 patients | 0.289 |
| 1-year | 79.8 (72.8-86.8), 10 studies 894 patients | 92.7 (90.2-95.2), 10 studies 3475 patients | 0.015a |
| 3-year | 74.3 (68.0-80.5) 4 studies 185 patients | 93.4 (90.6-96.2) 4 studies 291 patients | 0.003a |
| 5-year | 63.6 (56.5-70.6) 8 studies 730 patients | 79.5 (73.2-85.8) 8 studies 1316 patients | 0.006a |
Based on univariate analysis of data from 9 studies (n = 4845), sarcopenia was associated with an increased risk of post-LT mortality, with a pooled unadjusted HR of 1.72 (95%CI: 1.33–2.24, I2 = 60.7%, P = 0.009; Supplementary Figure 5). In data from multivariate analysis (nine studies, n = 4430), sarcopenia was still significantly associated with increased post-LT mortality with a pooled adjusted HR of 1.58 (95%CI: 1.21–2.07, I2 = 54.4%, P = 0.025; Figure 2B).
Seven studies involving 1,369 patients reported data on the length of ICU stay that were available for meta-analysis. Patients with preoperative sarcopenia had longer ICU stays than those without sarcopenia post-LT (WMD: 4.503, 95%CI: 2.218–6.788, P < 0.001) (Supplementary Figure 6). Six studies involving 1001 patients reported data on the length of stay (LOS) and were included in the meta-analysis. LOS was not different in patients with or without preoperative sarcopenia (WMD: 9.352, 95%CI: 2.557–15.261, P = 0.162; Supplementary Figure 7). Data from four studies involving 606 patients were available for meta-analysis of developing sepsis post-LT, showing that patients with preoperative sarcopenia had a higher risk of sepsis post-LT than those without sarcopenia (RR: 2.00, 95%CI: 1.143–3.503, P = 0.015; Supple
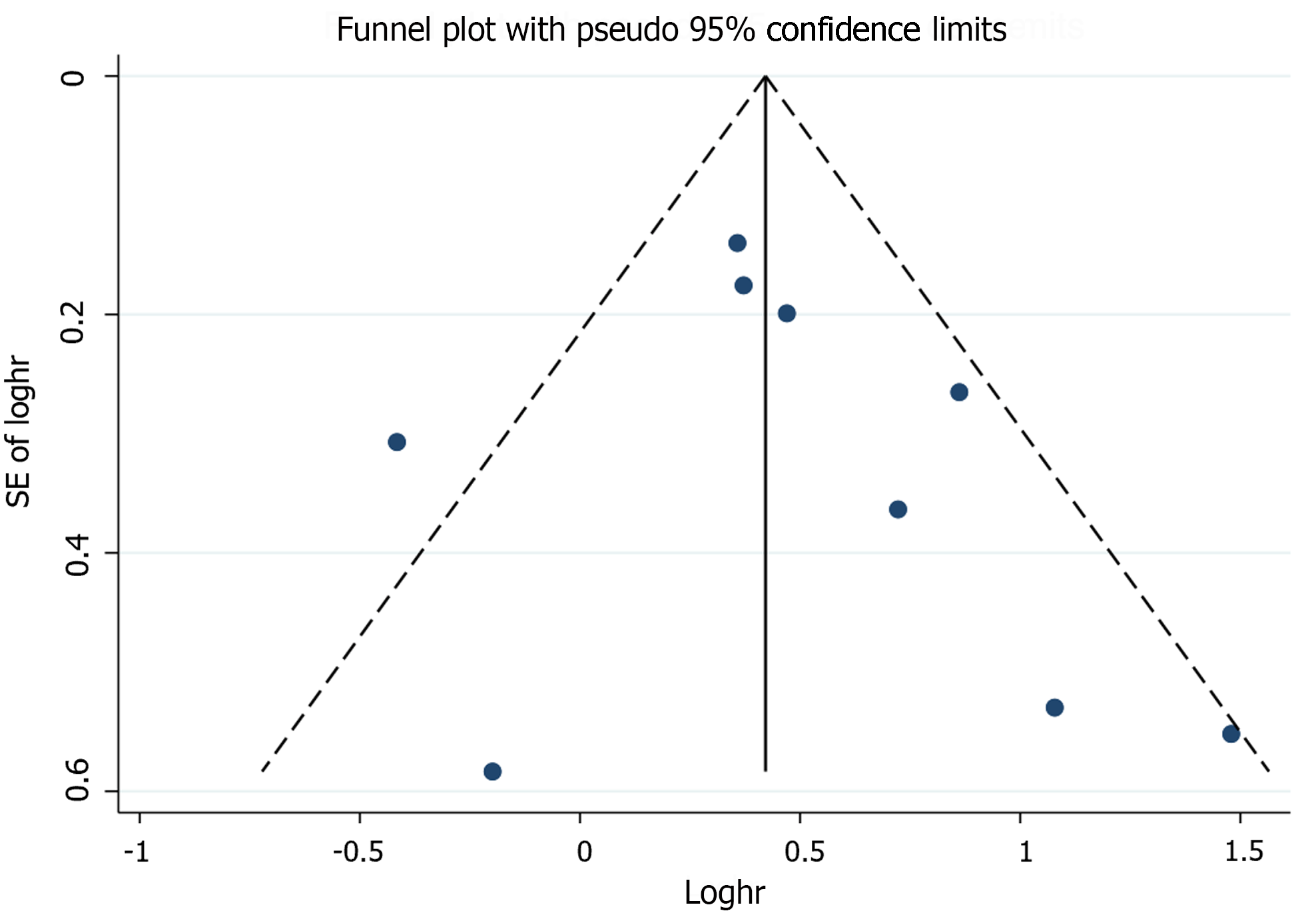
Sensitivity analyses that excluded one study at a time and then pooled the remaining studies showed adjusted HRs ranging from 1.49 to 1.70, suggesting that our results were robust (Supplementary Figure 10). Meta-regression analyses showed no association of pooled adjusted HR with sample size (P = 0.819), percentage of male patients (P = 0.660), average follow-up time (P = 0.746), different regions (P = 0.786), diagnostic method (P = 0.553), and NOS (P = 0.865; Supplementary Table 3). The funnel plot was symmetrical (Figure 3). Egger’s (P = 0.526) and Begg’s (P = 0.348) tests suggested no significant statistical evidence of publication bias.
LT is the only potential treatment for ESLD[14]. To increase the survival rates of LT candidates, preoperative risk assessment for risk is essential. Most patients with ESLD who undergo LT are physically deconditioned, with low functional capacity, malnutrition, sarcopenia, and frailty[10,15]. Sarcopenia is a common symptom of ESLD that strongly affects adverse outcomes and mortality in this population[11]. Although a meta-analysis also evaluated the association between skeletal muscle mass and mortality in patients with cirrhosis, studies of post-LT patients were excluded[5]. Another systematic review reported sarcopenia-impaired outcomes in patients awaiting or undergoing LT; many patients evaluated for LT but did not undergo LT were also included in this meta-analysis[3]. However, since then, a number of large, rigorously designed, and long-term follow-up studies have provided updated data. Therefore, in this study, a more comprehensive search was performed, a much larger pool of potential studies was screened, studies with overlapping cohorts and only waiting-LT patients were excluded, and a comprehensive range of subgroup and sensitivity analyses was performed to summarize the prevalence, post-LT survival, and outcomes of sarcopenia in patients who underwent LT.
As a major component of malnutrition, sarcopenia is a strong predictor of morbidity and mortality in patients with ESLD[16]. Despite the importance of sarcopenia, no consensus has been established regarding how to accurately measure and define sarcopenia in clinical settings[17]. The European Working Group on Sarcopenia defined sarcopenia as “a progressive and generalized skeletal muscle disorder associated with an increased likelihood of adverse outcomes including falls, fractures, disability, and mortality”, combining both muscle mass and muscle strength or muscle performance[18]. In this study, 24 studies used skeletal muscle area measured by CT scans as the method to diagnose sarcopenia, and only one study used DEXA to assess sarcopenia. None of the included studies used low muscle strength or low physical performance as diagnostic criteria in this meta-analysis. While most working groups recommend considering both muscle mass and muscle function for the diagnosis of sarcopenia, most studies in patients with liver disease have investigated sarcopenia using measures of muscle mass alone[10,18,19]. Based on the available data on liver disease, some guidance developed a consensus definition for the operationalization of sarcopenia in liver disease as the phenotypic manifestation of loss of muscle mass alone[10,11]. In the future, muscle strength or physical performance should also be included in the diagnosis of sarcopenia, and consistent tests should be conducted to diagnose sarcopenia.
Owing to the varied definitions of sarcopenia, a wide range of the prevalence of sarcopenia in patients undergoing LT was reported[3]. In this meta-analysis, the overall pooled prevalence of sarcopenia for patients who underwent LT was 40.6%. In another meta-analysis, the prevalence of sarcopenia ranged from 22.2% to nearly 70% in patients undergoing LT or evaluated for LT[3]. A previous meta-analysis excluded post-LT patients, and sarcopenia affected 37.5% of patients with cirrhosis[5]. The etiology of liver disease has been associated with differences in the prevalence of sarcopenia[10,20]. Our finding is consistent with previous studies that have shown that patients with alcohol-associated liver disease (ALD) had a lower baseline muscle mass[20,21]. A previous study reported that sarcopenia is related to the severity of liver disease as estimated by the Child–Pugh class[22]. In another study, the muscle mass index was negatively correlated with the Child–Pugh score[16]. In this meta-analysis, patients with ALD and those in the Child–Pugh C class had the highest prevalence of sarcopenia of > 50%. Our study is in line with the results from a recent study that showed that sarcopenia is common in patients with ESLD and worsens with the progression of liver disease[16]. All studies that reported the prevalence of sarcopenia separately for different sexes included in this meta-analysis indicated a higher prevalence among men. Sex is believed as the most important factor influencing muscle mass in the general population[16]. Therefore, future studies to define sarcopenia are needed for clinical application with consideration of sex, age, ethnicity, and basic diseases.
The North American Expert Opinion Statement on Sarcopenia in LT recommends using sarcopenia to predict the prognosis of LT[11]. However, various clinical outcomes, such as overall mortality in evaluated patients, waitlist mortality in listed patients, post-LT mortality in patients undergoing LT, and short-term vs long-term outcomes, confound the comparison between published studies and the development of generalized definitions[11]. Our studies mainly focused on post-LT mortality and complications. From this meta-analysis, no difference in the 90-day cumulative probabilities of survival post-LT was found between patients with or without sarcopenia. However, the 1-, 3-, and 5-year cumulative probabilities of post-LT survival in patients with sarcopenia were all lower than those in patients without sarcopenia. In our meta-analysis, sarcopenia was associated with a pooled HR of 1.58 (95%CI: 1.21–2.07) for post-LT mortality similar to a prior meta-analysis with a pooled HR of 1.84 (95%CI: 1.11–3.05) for post-LT mortality[3]. Although multiple studies have shown sarcopenia to be associated with post-LT mortality[3,23,24], data reporting preoperative sarcopenia associated with adverse post-LT outcomes are limited. In this meta-analysis, patients with preoperative sarcopenia had longer ICU stays than those without sarcopenia post-LT. In addition, patients with preoperative sarcopenia had a higher RR of sepsis and serious post-LT complications than those without sarcopenia. This indicates that preoperative sarcopenia may play an important role in the clinical outcomes of patients undergoing LT. Given the lack of an objective metric of sarcopenia, some guidelines do not recommend using sarcopenia as a contraindication against LT[10,11]. However, sarcopenia may guide the decision about LT in an attempt to minimize liver-related complications and optimize overall patient recovery. Therefore, it is important to incorporate sarcopenia into the management and treatment of LT candidates to optimize nutrition and physical activity[25].
This study has several limitations. First, the wide inclusion criteria in this study generated significant heterogeneity that could not be explained. A random-effects model with subgroup analyses was used whenever possible to minimize the effect of heterogeneity. Subgroup analysis, meta-regression, and sensitivity analysis were used to identify the possible sources of heterogeneity. Second, significant heterogeneity in the means of defining sarcopenia and the diagnostic criteria employed was noted among the included studies. Thus, future studies should build uniform cutoff thresholds based on ethnicity to assess sarcopenia. Third, the included articles and all diagnostic protocols lacked an assessment of muscle strength and physical performance. Future prospective studies using the criteria including muscle mass and muscle strength/physical performance must determine whether the predictive power is improved after employing a more comprehensive algorithm to diagnose sarcopenia. Fourth, the number of studies on some variables for clinical outcomes was limited, so the application and promotion of the combined results were also restricted to a certain extent. Fifth, the etiology of liver diseases is an important factor associated with mortality. However, we could not analyze the effect of liver disease etiology on our results because the HR in each study was not reported separately according to etiology. Finally, although the quality of the included studies was evaluated using the NOS statement entries during the search and screening processes, some subjectivity remained in the evaluation of the literature because of the lack of accepted quality evaluation criteria, which may lead to some selection bias in the included studies.
This meta-analysis demonstrated that sarcopenia affects 40% of LT recipients. This study also showed that sarcopenia was associated with a 1.58-fold higher risk of post-LT mortality. Sarcopenia was also associated with long-term survival rates and adverse post-LT outcomes. Because of the high prevalence and adverse post-LT outcomes, sarcopenia should be considered a part of the initial evaluation of LT candidates. More future studies are needed are needed to incorporate sarcopenia or muscle mass index/function into a formal prognostic scale for LT patients.
Liver transplantation (LT) has become the standard treatment for patients with end-stage liver disease (ESLD). With the widespread shortage of human organs, rigorous selection of LT candidates is essential. Over the past few years, sarcopenia has become a topic of prolific exploration in patients with ESLD. Sarcopenia has recently been recognized as a new prognostic factor for predicting outcomes in LT candidates. Therefore, this study aimed to estimate the prevalence of sarcopenia and evaluate its clinical effect on LT candidates.
As a major component of malnutrition, sarcopenia is a strong predictor of morbidity and mortality in patients with ESLD. However, the link between sarcopenia and LT candidates is not well studied.
This meta-analysis aimed to systematically evaluate the literature about patients who underwent LT to summarize the diagnostic criteria for sarcopenia, estimate its prevalence, and assess its effect on clinical outcomes.
This systematic search was conducted in PubMed, Web of Science, EMBASE, and Cochrane Library for original English-language articles that investigated the prevalence and influence of sarcopenia on patients undergoing LT from database inception to November 30, 2022. The prevalence of sarcopenia was determined through a meta-analysis. The effect of sarcopenia on the incidence of post-LT survival was evaluated using the pooled unadjusted hazard ratio (HR) or adjusted HR and 95% confidence intervals.
Twenty-five studies involving 7760 patients undergoing LT were included. The pooled prevalence of sarcopenia in patients undergoing LT was 40.7%. The 1-, 3-, and 5-year cumulative probabilities of post-LT survival in patients with preoperative sarcopenia were all lower than those without sarcopenia (P < 0.05). Sarcopenia was associated with an increased risk of post-LT mortality in patients undergoing LT. Patients with preoperative sarcopenia had a longer intensive care unit stay, a high risk ratio of sepsis, and serious post-LT complications than those without sarcopenia.
Sarcopenia is prevalent in a substantial proportion of patients undergoing LT. This study also showed that sarcopenia was associated with a 1.58-fold higher risk of post-LT mortality. Sarcopenia was also associated with long-term survival rates and adverse post-LT outcomes.
Because of the high prevalence and adverse post-LT outcomes, sarcopenia should be considered a part of the initial evaluation of LT candidates. More studies are needed to incorporate sarcopenia into a formal prognostic scale for LT recipients.
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2472] [Article Influence: 353.1] [Reference Citation Analysis (1)] |
| 2. | Kumar V, Benjamin J, Shasthry V, Subramanya Bharathy KG, Sinha PK, Kumar G, Pamecha V. Sarcopenia in Cirrhosis: Fallout on Liver Transplantation. J Clin Exp Hepatol. 2020;10:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant. 2016;16:2277-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 4. | van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA, Polak WG, Porte RJ, van Hoek B, van den Berg AP, Metselaar HJ, IJzermans JNM. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, Engelmann C, Zhang P, Jeong JY, van Vugt JLA, Xiao H, Deng H, Gao X, Ye Q, Zhang J, Yang L, Cai Y, Liu N, Li Z, Han T, Kaido T, Sohn JH, Strassburg C, Berg T, Trebicka J, Hsu YC, IJzermans JNM, Wang J, Su GL, Ji F, Nguyen MH. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;76:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 283] [Article Influence: 70.8] [Reference Citation Analysis (2)] |
| 6. | Sam J, Nguyen GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 752] [Article Influence: 107.4] [Reference Citation Analysis (2)] |
| 8. | Bhanji RA, Montano-Loza AJ, Watt KD. Sarcopenia in Cirrhosis: Looking Beyond the Skeletal Muscle Loss to See the Systemic Disease. Hepatology. 2019;70:2193-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Meng QH, Wang JH, Yu HW, Li J, Feng YM, Hou W, Zhang J, Zhang Q, Wang X, Liu Y. Resting energy expenditure and substrate metabolism in Chinese patients with acute or chronic hepatitis B or liver cirrhosis. Intern Med. 2010;49:2085-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 482] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 11. | Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology. 2019;70:1816-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 12. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17259] [Article Influence: 663.8] [Reference Citation Analysis (0)] |
| 13. | Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1058] [Cited by in RCA: 1687] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 14. | Tan Y, Duan T, Li B, Zhang B, Zhu Y, Yan K, Song J, Lv T, Yang J, Jiang L, Wen T, Yan L. Sarcopenia defined by psoas muscle index independently predicts long-term survival after living donor liver transplantation in male recipients. Quant Imaging Med Surg. 2022;12:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Pollok JM, Tinguely P, Berenguer M, Niemann CU, Raptis DA, Spiro M; ERAS4OLT. org collaborative. Enhanced recovery for liver transplantation: recommendations from the 2022 International Liver Transplantation Society consensus conference. Lancet Gastroenterol Hepatol. 2023;8:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 16. | Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM, Zhang LY, Tan W, Shi PM, Yu H, Zhang CQ, Xie WF. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12:1948-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 17. | Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats AJS, Crivelli AN, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C; GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 795] [Cited by in RCA: 710] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 18. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 1750] [Article Influence: 250.0] [Reference Citation Analysis (0)] |
| 19. | Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300-307.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2739] [Cited by in RCA: 4588] [Article Influence: 764.7] [Reference Citation Analysis (0)] |
| 20. | Welch N, Dasarathy J, Runkana A, Penumatsa R, Bellar A, Reen J, Rotroff D, McCullough AJ, Dasarathy S. Continued muscle loss increases mortality in cirrhosis: Impact of aetiology of liver disease. Liver Int. 2020;40:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | DiMartini A, Cruz RJ Jr, Dew MA, Myaskovsky L, Goodpaster B, Fox K, Kim KH, Fontes P. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O'Beirne J, Patch D, Pinzani M, Morgan MY, Agarwal B, Yu D, Burroughs AK, Tsochatzis EA. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, Tomiyama K, Yagi S, Mori A, Uemoto S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 25. | Waits SA, Englesbe MJ. Making Progress Toward Frailty Remediation in End-Stage Liver Disease. Transplantation. 2016;100:2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Shafaat O, Liu Y, Jackson KR, Motter JD, Boyarsky BJ, Latif MA, Yuan F, Khalil A, King EA, Zaheer A, Summers RM, Segev DL, McAdams-DeMarco M, Weiss CR. Association between Abdominal CT Measurements of Body Composition before Deceased Donor Liver Transplant with Posttransplant Outcomes. Radiology. 2023;306:e212403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Sim JH, Kwon HM, Kim KW, Ko YS, Jun IG, Kim SH, Kim KS, Moon YJ, Song JG, Hwang GS. Associations of sarcopenia with graft failure and mortality in patients undergoing living donor liver transplantation. Liver Transpl. 2022;28:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 28. | Prakash K, Sam AF, K N, Tandon N. Effect of Preoperative Sarcopenia, Malnutrition and Functional status on Postoperative Morbidity Following Liver Transplantation. Prog Transplant. 2022;32:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Wu MY, Lim WX, Cheng YF, Chang CD, Hsu HW, Lin CC, Chen CL, Chang WC, Yu CY, Tsang LL, Chuang YH, Ou HY. Sarcopenia adversely impacts postoperative complications in living-donor liver transplantation recipients. Sci Rep. 2021;11:19247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Kuo SZ, Ahmad M, Dunn MA, Montano-Loza AJ, Carey EJ, Lin S, Moghe A, Chen HW, Ebadi M, Lai JC. Sarcopenia Predicts Post-transplant Mortality in Acutely Ill Men Undergoing Urgent Evaluation and Liver Transplantation. Transplantation. 2019;103:2312-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, Castaing D, Antonini T, Coilly A, Samuel D, Cherqui D, Vibert E. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017;23:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 32. | Beumer BR, van Vugt JLA, Sapisochin G, Yoon P, Bongini M, Lu D, Xu X, De Simone P, Pintore L, Golse N, Nowosad M, Bennet W, Tsochatzis E, Koutli E, Abbassi F, Claasen MPAW, Merli M, O'Rourke J, Gambato M, Benito A, Majumdar A, Tan EK, Ebadi M, Montano-Loza AJ, Berenguer M, Metselaar HJ, Polak WG, Mazzaferro V, IJzermans JNM; Collaborators. Impact of muscle mass on survival of patients with hepatocellular carcinoma after liver transplantation beyond the Milan criteria. J Cachexia Sarcopenia Muscle. 2022;13:2373-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Pinto Dos Santos D, Kloeckner R, Koch S, Hoppe-Lotichius M, Zöller D, Toenges G, Kremer WM, Zimmermann T, Mittler J, Lang H, Düber C, Galle PR, Weinmann A, Sprinzl MF. Sarcopenia as prognostic factor for survival after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2020;32:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Izumi T, Watanabe J, Tohyama T, Takada Y. Impact of psoas muscle index on short-term outcome after living donor liver transplantation. Turk J Gastroenterol. 2016;27:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Okajima H, Uemoto S. Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation. 2017;101:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Irwin NEA, Fabian J, Hari KR, Lorentz L, Mahomed A, Botha JF. Myosteatosis, the More Significant Predictor of Outcome: An Analysis of the Impact of Myosteatosis, Sarcopenia, and Sarcopenic Obesity on Liver Transplant Outcomes in Johannesburg, South Africa. Exp Clin Transplant. 2021;19:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, Uchiyama H, Ikeda T, Baba H, Maehara Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 38. | Miarka M, Gibiński K, Janik MK, Główczyńska R, Zając K, Pacho R, Raszeja-Wyszomirska J. Sarcopenia-The Impact on Physical Capacity of Liver Transplant Patients. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB, Kneteman N. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 40. | Czigany Z, Kramp W, Bednarsch J, van der Kroft G, Boecker J, Strnad P, Zimmermann M, Koek G, Neumann UP, Lurje G. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am J Transplant. 2020;20:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Hey P, Hoermann R, Gow P, Hanrahan TP, Testro AG, Apostolov R, Sinclair M. Reduced upper limb lean mass on dual energy X-ray absorptiometry predicts adverse outcomes in male liver transplant recipients. World J Transplant. 2022;12:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Lindqvist C, Brismar TB, Majeed A, Wahlin S. Assessment of muscle mass depletion in chronic liver disease: Dual-energy x-ray absorptiometry compared with computed tomography. Nutrition. 2019;61:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Pravisani R, Soyama A, Isola M, Sadykov N, Takatsuki M, Hidaka M, Adachi T, Ono S, Hara T, Hamada T, Baccarani U, Risaliti A, Eguchi S. Chronological changes in skeletal muscle mass following living-donor liver transplantation: An analysis of the predictive factors for long-term post-transplant low muscularity. Clin Transplant. 2019;33:e13495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Kim YR, Park S, Han S, Ahn JH, Kim S, Sinn DH, Jeong WK, Ko JS, Gwak MS, Kim GS. Sarcopenia as a predictor of post-transplant tumor recurrence after living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Sci Rep. 2018;8:7157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 45. | Hassan EA, Makhlouf NA, Ibrahim ME, Dabbous HM, Salah MA, Aboalam HS, Mohamed MZ, Fadel BA, Salama MAR. Impact of Sarcopenia on Short-Term Complications and Survival After Liver Transplant. Exp Clin Transplant. 2022;20:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabbous H, Egypt S-Editor: Lin C L-Editor: A P-Editor: Cai YX