Published online Feb 14, 2024. doi: 10.3748/wjg.v30.i6.579
Peer-review started: November 14, 2023
First decision: December 5, 2023
Revised: December 16, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 14, 2024
Processing time: 82 Days and 15.3 Hours
Helicobacter pylori (H. pylori) infection has been well-established as a significant risk factor for several gastrointestinal disorders. The urea breath test (UBT) has emerged as a leading non-invasive method for detecting H. pylori. Despite numerous studies confirming its substantial accuracy, the reliability of UBT results is often compromised by inherent limitations. These findings underscore the need for a rigorous statistical synthesis to clarify and reconcile the diagnostic accuracy of the UBT for the diagnosis of H. pylori infection.
To determine and compare the diagnostic accuracy of 13C-UBT and 14C-UBT for H. pylori infection in adult patients with dyspepsia.
We conducted an independent search of the PubMed/MEDLINE, EMBASE, and Cochrane Central databases until April 2022. Our search included diagnostic accuracy studies that evaluated at least one of the index tests (13C-UBT or 14C-UBT) against a reference standard. We used the QUADAS-2 tool to assess the methodological quality of the studies. We utilized the bivariate random-effects model to calculate sensitivity, specificity, positive and negative test likelihood ratios (LR+ and LR-), as well as the diagnostic odds ratio (DOR), and their 95% confidence intervals. We conducted subgroup analyses based on urea dosing, time after urea administration, and assessment technique. To investigate a possible threshold effect, we conducted Spearman correlation analysis, and we generated summary receiver operating characteristic (SROC) curves to assess heterogeneity. Finally, we visually inspected a funnel plot and used Egger’s test to evaluate publication bias.
The titles and abstracts of 4621 studies were screened; 79 articles were retrieved and selected for full-text reading. Finally, 60 studies were included in the diagnostic test accuracy meta-analysis. Our analysis demonstrates superior diagnostic accuracy of 13C-UBT over 14C-UBT, indicated by higher sensitivity (96.60% vs 96.15%), specificity (96.93% vs 89.84%), likelihood ratios (LR+ 22.00 vs 10.10; LR- 0.05 vs 0.06), and area under the curve (AUC; 0.979 vs 0.968). Notably, 13C-UBT's DOR (586.47) significantly outperforms 14C-UBT (DOR 226.50), making it the preferred diagnostic tool for dyspeptic individuals with H. pylori infection. Correlation analysis revealed no threshold effect (13C-UBT: r = 0.48; 14C-UBT: r = -0.01), and SROC curves showed consistent accuracy. Both 13C-UBT and 14C-UBT showed high AUC values (13C-UBT 0.979; 14C-UBT 0.968) near 1.00, reinforcing their excellent accuracy and endorsing both as reliable diagnostic tools in clinical practice.
In summary, our study has demonstrated that 13C-UBT has been found to outperform the 14C-UBT, making it the preferred diagnostic approach. Additionally, our results emphasize the significance of carefully considering urea dosage, assessment timing, and measurement techniques for both tests to enhance diagnostic precision. Nevertheless, it is crucial for researchers and clinicians to evaluate the strengths and limitations of our findings before implementing them in practice.
Core Tip: The urea breath test (UBT) is a pivotal noninvasive method for detecting Helicobacter pylori (H. pylori); however, its reliability is challenging. This meta-analysis aimed to compare the precision of the 13C-UBT and 14C-UBT in diagnosing H. pylori among adults with dyspepsia, providing insights to enhance clinical strategies.
- Citation: Lemos FFB, Castro CT, Silva Luz M, Rocha GR, Correa Santos GL, de Oliveira Silva LG, Calmon MS, Souza CL, Zarpelon-Schutz AC, Teixeira KN, Queiroz DMM, Freire de Melo F. Urea breath test for Helicobacter pylori infection in adult dyspeptic patients: A meta-analysis of diagnostic test accuracy. World J Gastroenterol 2024; 30(6): 579-598
- URL: https://www.wjgnet.com/1007-9327/full/v30/i6/579.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i6.579
Helicobacter pylori (H. pylori) is a spiral-shaped, gram-negative microaerophilic bacterium that infects approximately 43% of the global population[1]. While the majority of infected individuals remain asymptomatic, chronic gastritis inevitably ensues, leading to a significant burden of morbidity and mortality[2,3]. Adults who are infected with H. pylori are at increased risk of developing peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue lymphoma[4-6]. To address this, current guidelines advocate for either a test-and-treat or a scope-and-treat approach in managing uninvestigated dyspepsia, underscoring the importance of timely diagnosis and intervention[7,8].
Diagnostic testing for H. pylori infection typically involves two primary categories: Invasive (endoscopic) and non-invasive testing, depending on the application of upper endoscopy[9]. For individuals aged 50 years or older or those with alarm features, the recommended standard diagnostic approach involves upper endoscopy, followed by histopathological examination (HE) or rapid urease test (RUT), and occasionally, culture[8]. In contrast, in dyspeptic patients under 50 years without specific risk factors or alarm symptoms, non-invasive methods such as urea breath testing (UBT), stool antigen testing, and serology are preferred[8,10].
Among non-invasive diagnostic techniques, the UBT has emerged as a prominent method. This approach capitalizes on the urease activity of H. pylori, initiating the hydrolysis of ingested urea and consequent release of labeled carbon dioxide[11]. Two commonly utilized isotopic variants, 13C-UBT and 14C-UBT, offer distinctive features. In 13C-UBT, a stable isotope (carbon-13) is employed, and breath samples are collected and analyzed for labeled carbon dioxide using various methods such as mass spectrometry and infrared spectrometry[12]. This method presents important advantages, notably the absence of ionizing radiation, rendering it suitable for repeated application and applicable in vulnerable populations, including pregnant women and children[11,13]. In contrast, 14C-UBT utilizes a radioactive isotope (carbon-14) and primarily relies on scintillation counting for detection[14,15]. Despite its historical use, concerns regarding radiation exposure have diminished its preference in contemporary clinical practice.
In a prior meta-analysis, Ferwana et al[16] assessed the diagnostic accuracy of the UBT, encompassing both 13C-UBT and 14C-UBT, for detecting H. pylori infection in adult dyspeptic patients. Despite its high accuracy, the reliability of UBT results was constrained by significant unexplained heterogeneity, persisting even after subgroup analysis[16]. This pattern persisted in subsequent studies, with Zhou et al[17] finding analogous challenges in calculating pooled estimates of diagnostic accuracy for 14C-UBT. Moreover, a subsequent systematic review emphasized that the variability in thresholds and reference standards across studies limited the data available for pooling accuracy measures at specific UBT thresholds[18].
These findings underscore the need for a rigorous statistical synthesis to clarify and reconcile the diagnostic accuracy of the UBT for the diagnosis of H. pylori infection, addressing challenges identified in prior research. To address this gap in the evidence, we conducted a systematic review and meta-analysis to determine the diagnostic accuracy of the UBT for H. pylori infection in adult patients with dyspepsia.
This study adhered to the guidelines outlined in the PRISMA-DTA[19]. These guidelines encompass a 27-item checklist and a 3-phase flowchart, both designed to enhance the transparency of systematic review reporting. Accordingly, our study protocol has been officially registered in the PROSPERO database under the registration number CRD42023449854.
This search strategy was designed following the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Version 2.0, 2022)[20]. We performed independent, computer-assisted searches of the: (1) PubMed/MEDLINE; (2) EMBASE; and (3) Cochrane Library databases. MeSH (Medical Subject Headings) and Emtree (EMBASE Subject Headings) index terms and free-text words were combined. Search terms included "urea breath test," "breath test," "13C-urea breath test," "14C-urea breath test," "13C-UBT," "14C-UBT," "Helicobacter pylori," "H. pylori," and "dyspepsia." Boolean operators (AND, OR) were also used to narrow or broaden the search as required. No language restriction was applied. To identify additional studies, reference lists were also scanned. Finally, we conducted a “citing reference” search (by searching articles which cited the included studies) in PubMed/MEDLINE and EMBASE. Following the search, all identified citations were collated and uploaded into the Rayyan (https://www.rayyan.ai/) tool, and all duplicates were removed.
Two independent reviewers, Lemos FFB and Calmon MS, screened the references against predefined eligibility criteria. In the case of disagreement, a 3rd researcher, Silva Luz M, was consulted. Full-text papers were obtained for references considered relevant. If any study was not retrieved, the authors were contacted. Two authors, Lemos FFB and Correa Santos GL, independently screened the full-text papers against the eligibility criteria. In the case of disagreement, consensus was reached.
We included diagnostic accuracy studies that evaluated at least one of the index tests (13C- or 14C-UBTs) against a reference standard (biopsy fragments followed by culture or HE or RUT and/or not serology/stool antigen-based tests in adult dyspeptic patients. Exclusion criteria were as follows: (1) Studies that enrolled children or adolescents under 18 years of age; (2) studies that included only patients with acute upper gastrointestinal bleeding; (3) studies that enrolled subjects who presented for reasons other than dyspeptic symptoms, complicated dyspeptic cases that need surgery, those who received previous therapy for H. pylori within the last 3 months, or long-term use of corticosteroids and immunosuppressant drugs; (4) screening studies; (5) studies that did not report true positive, false positive, false negative, and true negative data and the threshold used for the index tests; (6) case-control studies because these are prone to bias[21]; and (7) full-text articles not available or articles not available in English, Spanish, or Portuguese.
Two review authors, Rocha GR and Correa Santos GL, independently extracted data from each included study using a pre-piloted data extraction form. In case of discrepancies, a 3rd researcher, Lemos FFB, was consulted. The extracted data included: (1) Information about the studies, such as the first author, publication year, and country; (2) details about the study design, including the type of study (prospective and retrospective cohort studies, cross-sectional studies, or randomized clinical trials), the reference standards used, blinding of the index test and reference standard, and the flow and timing (retrospective/prospective); (3) participant information, i.e., the total number of participants and population characteristics (age, mean ± SD, sex, and disease prevalence); (4) reference standard details, including the time interval between the index test and the reference standard; index test information, including the model (13C- or 14C-UBT), cut-off values, urea dosing, time for measurement after urea administration (min), and measurement technique; and (5) diagnostic accuracy data, including the number of true positives, false positives, false negatives, and true negatives.
Two independent reviewers, Silva Luz M and de Oliveira Silva LG, conducted critical appraisal using the QUADAS-2 tool. In cases of disagreement, they consulted a 3rd researcher, Lemos FFB. The QUADAS-2 tool is applied in four phases[21]: Summarizing the review question, tailoring the tool and producing review-specific guidance, constructing a flow diagram for the primary study, and evaluating bias and applicability. This tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed for the risk of bias, and the first three domains are also evaluated for concerns regarding applicability. It's important to note that "risk of bias" refers to internal validity, i.e., whether there are systematic errors in conducting the study with respect to the specific domain, while "applicability concern" pertains to external validity, i.e., whether there are concerns that the population, index test, or reference standard used in the studies align with the review question. Signaling questions were also included to assist in assessing the risk of bias.
Eligible studies were subjected to data extraction, and we organized the data into 2 × 2 tables. In our analysis, we selected only the optimal threshold values for H. pylori positivity in cases where multiple thresholds were presented. We added 0.5 to values equal to zero to ensure computational stability and prevent potential issues[22].
To address the anticipated diversity in meta-analyses of diagnostic accuracy studies, we utilized the random-effects model to calculate sensitivity, specificity, positive and negative test likelihood ratios (LR+ and LR-), as well as the diagnostic odds ratio (DOR)[23]. We also determined the corresponding 95% confidence intervals (95%CIs). The results of the 13C- and 14C-UBT are presented separately. Subgroup analyses were conducted with a focus on urea dosing, time for measurement after urea administration (in minutes), and the assessment technique employed. To investigate the possibility of a threshold effect in the analysis, we conducted Spearman correlation analysis. A substantial threshold effect was recognized when the correlation coefficient reached or exceeded 0.6[24].
We performed a bivariate random-effects meta-analysis and generated summary receiver operating characteristic (SROC) curves to visually assess heterogeneity. Furthermore, these curves allowed us to predict accuracy by summarizing diagnostic performance as the area under the curve (AUC)[25]. We categorized accuracy levels as follows: fail (0.50-0.60), poor (0.61-0.70), fair (0.71-0.80), good (0.81-0.90), and excellent (0.91-1.00)[22].
To evaluate publication bias, we conducted a visual inspection of a funnel plot and employed Egger’s tests for statistical assessment. The creation of this plot and the assessment of the risk of data due to missing data required a minimum of ten studies.
All analyses were performed using R version 4.2.1, an environment for statistical computing in Vienna, Austria, utilizing the “meta” package (version 6.5-0), “dmetar” package (version 0.1.0), and the “mada” package (version 0.5.11).
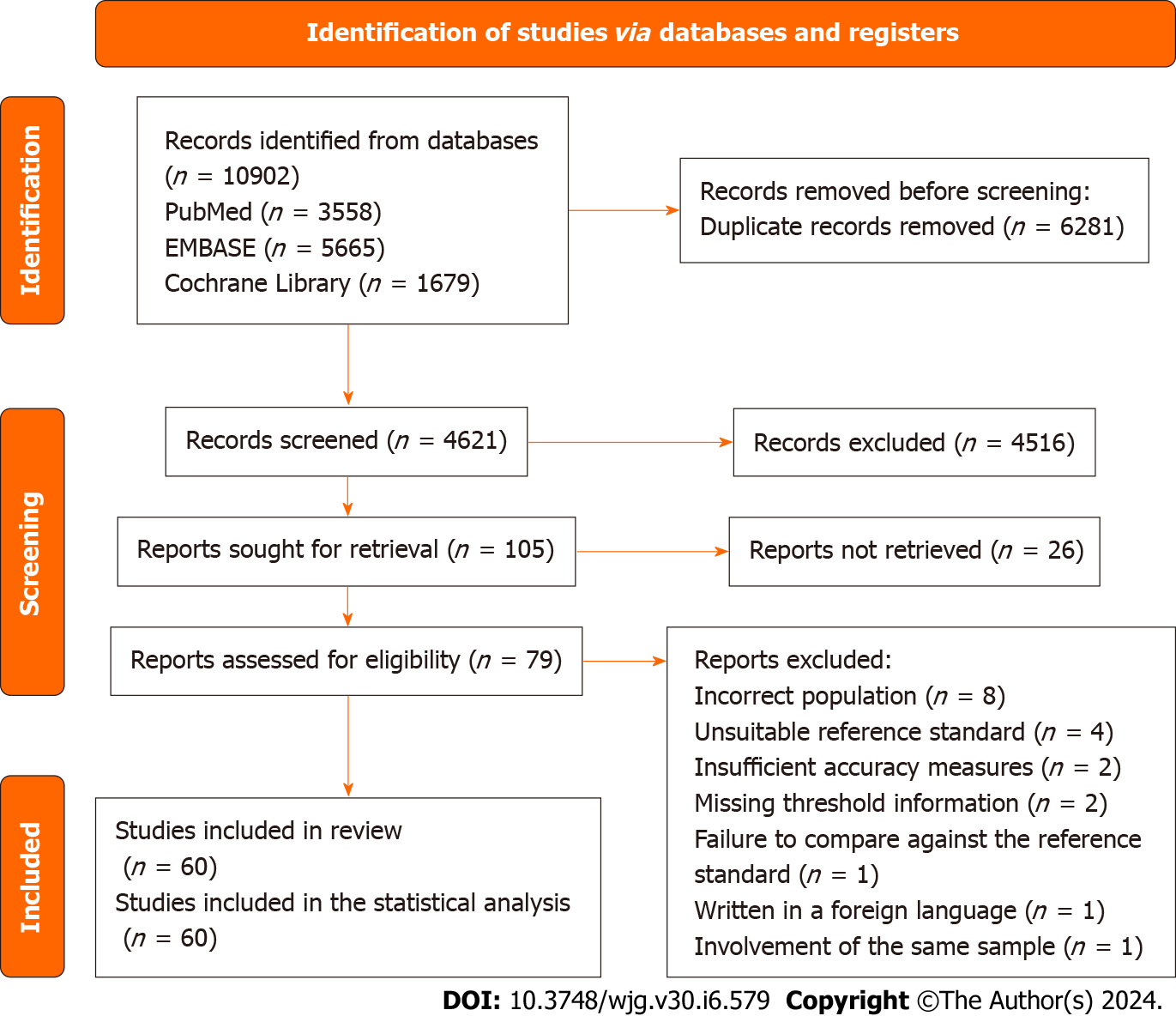
Database searches initially yielded 10902 reports, from which duplicates were removed. No additional references were discovered through alternative search methods. Subsequently, the titles and abstracts of 4621 studies underwent screening, resulting in the retrieval and selection of 79 articles for full-text examination. Ultimately, 60 studies fulfilled the inclusion criteria. The reasons for exclusion were as follows: Incorrect population (n = 8), unsuitable reference standard (n = 4), insufficient accuracy measures (n = 2), missing threshold information (n = 2), failure to compare against the reference standard (n = 1), writing in a foreign language (n = 1), and involvement of the same sample (n = 1). Figure 1 illustrates the flow of information through various phases of the systematic review.
Among all the studies, 39 (comprising 65%) employed the 13C-UBT as their primary diagnostic test, featuring a median population size of 200 individuals (lower to upper quartile: 84.5-254). Cross-sectional study design was predominant, making up 97.5% of the total, while only one study (2.5%) adopted a randomized controlled trial approach. For the 13C-UBT, the median pre-test probability was 51.2% (lower to upper quartile: 47.8-67.6). Various reference standards were used, with the most common being "H. pylori culture (HpC) or (HE and RUT)," accounting for 22.5% of cases. Other reference standards included "HE and RUT" (17.5%), "HE" (12.5%), "HE or HpC" (12.5%), "HE or (RUT and serology)" (5%), "RUT" (5%), "HE, HpC, and RUT" (5%), and "HpC" (2.5%). Some studies also combined reference standards, such as "RUT or HE" and “(HE, HpC, RUT) at least two positives," each constituting 2.5% of the sample, as shown in Table 1.
| Ref. | Country | Design | Population, n | Prevalence (%) | Reference standard | Index test (model) | Optimal cut-off | Urea dosing (mg) | Time after administration (min) | Measurement tecnique | TP | FP | TN | FN |
| Wang et al[29], 2021 | China | Cross-sectional | 217 | 65.9 | HE | 13C-UBT | 10.4‰ DOB | 50 | 30 | IS | 120 | 14 | 60 | 23 |
| Alzoubi et al[33], 2020 | Jordan | Cross-sectional | 30 | 56.7 | RUT or HE | 13C-UBT | 4‰ DOB | 75 | 30 | IS | 16 | 3 | 10 | 1 |
| Nawacki et al[34], 2018 | Poland | Cross-sectional | 50 | 36.0 | RUT | 13C-UBT | 9.5‰ DOB | NR | 30 | IS | 16 | 0 | 32 | 2 |
| Som et al[27], 2014 | India | Cross-sectional | 83 | 59.0 | RUT | 13C-UBT | 1.47‰ | 75 | Multiple times | ICOS | 49 | 0 | 34 | 0 |
| Bruden et al[35], 2011 | United States | Cross-sectional | 280 | 53.2 | HE or (HpC and RUT) | 13C-UBT | 7 DOB | NR | NR | NR | 139 | 16 | 115 | 10 |
| Peng et al [36], 2009 | Taiwan | Cross-sectional | 100 | 53.0 | HpC or (HE and RUT) | 13C-UBT | 4.8‰ DOB | 100 | 15 | IS | 53 | 7 | 40 | 0 |
| Jordaan et al[37], 2008 | South Africa | Cross-sectional | 103 | 58.3 | HE | 13C-UBT | 4.5‰ DOB | 75 | NR | GCMS | 55 | 3 | 40 | 5 |
| Gatta et al[26], 2006 | Italy | RCT | 100 | 43.0 | HE and RUT | 13C-UBT | 4.40‰-6.26‰ DOB | 25 | 30 | IRMS | 43 | 0 | 57 | 0 |
| Peng et al[38], 2005 | Taiwan | Cross-sectional | 50 | 36.0 | HpC or (HE and RUT) | 13C-UBT | 5‰ DOB | 100 | 15 | IRMS | 18 | 0 | 32 | 0 |
| Kato et al[39], 2004 | Japan | Cross-sectional | 254 | 51.1 | HpC or (HE and RUT) | 13C-UBT | 2.5‰ DOB | 100 | 20 | IRMS | 252 | 5 | 242 | 6 |
| Ohara et al[40], 2004 | Japan | Cross-sectional | 254 | 51.2 | HpC or (HE and RUT) | 13C-UBT | 2.5‰ DOB | 100 | Multiple times | IRMS | 127 | 2 | 122 | 3 |
| Chen et al[41], 2003 | Taiwan | Cross-sectional | 554 | 66.6 | HpC or (HE and RUT) | 13C-UBT | 3.5‰ DOB | 100 | 20 | IS | 361 | 6 | 179 | 8 |
| Valdepérez et al[42], 2003 | Spain | Cross-sectional | 85 | 76.8 | HE and RUT | 13C-UBT | NR | 100 | 30 | NR | 61 | 0 | 19 | 2 |
| Gatta et al[43], 2003 | Italy | Cross-sectional | 200 | 56.5 | HpC or (HE and RUT) | 13C-UBT | 3.11‰-6.84‰ DOB | 75 | 30 | IRMS | 113 | 0 | 87 | 0 |
| Wong et al[44], 2003 | China | Cross-sectional | 200 | 49.5 | HE and RUT | 13C-UBT | 2.1‰ DOB | 50 | 20 | IRMS | 99 | 0 | 101 | 0 |
| Ng et al[45], 2002 | China | Cross-sectional | 213 | 54.9 | HE and RUT | 13C-UBT | 4.0‰-6.5‰ DOB | 75 | 30 | IRMS | 112 | 2 | 94 | 5 |
| Wong et al[46], 2001 | China | Cross-sectional | 101 | 48.1 | HE and RUT | 13C-UBT | 7.0-8.0‰ DOB | 50 | 20 | IRMS | 99 | 4 | 103 | 0 |
| Wong et al[47], 2001 | China | Cross-sectional | 294 | 55.4 | HE, HpC, CLO (RUT), in-house RUT, PCR, UBT (at least four positive) | 13C-UBT | 5‰ DOB | 75 | 30 | IRMS | 151 | 4 | 127 | 12 |
| Shirin et al[48], 2001 | United States | Cross-sectional | 97 | 47.4 | HE and RUT | 13C-UBT | Positive: > 6‰ DOB (> 2 points)/negative: < 3‰ DOB (> 2 points) | 75 | 5 | MCS | 45 | 2 | 49 | 1 |
| Pilotto et al[49], 2000 | Italy | Cross-sectional | 96 | 51.0 | HE, HpC, and RUT | 13C-UBT | 5‰ DOB | 100 | 30 | IRMS | 49 | 2 | 45 | 0 |
| Sheu et al[50], 2000 | Taiwan | Cross-sectional | 177 | 47.5 | HE or HpC | 13C-UBT | 3.5 DOB | 50 | 15 | IS | 81 | 1 | 92 | 3 |
| Wong et al[51], 2000 | China | Cross-sectional | 202 | 56.4 | HE and RUT | 13C-UBT | 4.5‰ DOB | 75 | 30 | IRMS | 108 | 2 | 86 | 6 |
| Hahn et al[52], 2000 | United States | Cross-sectional | 67 | 6.0 | HE and at least two positives of (definitive presence of H. pylori organisms in HE, UBT, Serology) | 13C-UBT | 2.4‰ DOB | 125 | 30 | IRMS | 4 | 9 | 54 | 0 |
| Chen et al[53], 2000 | Japan | Cross-sectional | 162 | 83.3 | HE and Serology | 13C-UBT | 2.5‰ DOB | 100 | 20 | IRMS | 135 | 1 | 26 | 0 |
| Peng et al[54], 2000 | Taiwan | Cross-sectional | 136 | 59.6 | HpC or (HE and RUT) | 13C-UBT | 4.8‰ DOB | 100 | 15 | IRMS | 76 | 6 | 49 | 5 |
| Riepl et al[55], 2000 | Germany | Cross-sectional | 84 | 35.7 | HE, HpC, and RUT | 13C-UBT | 6.5‰ DOB | 75 | 15 | IS | 30 | 0 | 54 | 0 |
| D'Elios et al[56], 2000 | Italy | Cross-sectional | 256 | 45.3 | HE | 13C-UBT | 4‰ DOB | 75 | 30 | IRMS | 113 | 2 | 138 | 3 |
| van der Hulst et al[57], 1999 | Italy | Cross-sectional | 544 | 52.2 | HE or HpC | 13C-UBT | 7.5‰ DOB ± 0.8 | 100 | 30 | LOGES | 260 | 21 | 239 | 24 |
| Leodolter et al[58], 1999 | Germany | Cross-sectional | 320 | 48.1 | HpC or (HE and RUT) | 13C-UBT | 4‰ DOB | 75 | 30 | IRMS | 142 | 2 | 164 | 12 |
| Mock et al[59], 1999 | Canada | Cross-sectional | 98 | 19.8 | HE or (RUT and Serology) | 13C-UBT | 3‰ DOB | 75 | 30 | IRMS | 17 | 2 | 75 | 2 |
| Mock et al[59], 1999 | Korea | Cross-sectional | 107 | 68.2 | HE or (RUT and Serology) | 13C-UBT | 3‰ DOB | 75 | 30 | IRMS | 69 | 1 | 33 | 4 |
| Perri et al[60], 1998 | Belgium | Cross-sectional | 172 | 73.3 | HE or HpC | 13C-UBT | 1.15‰ DOB | 75 | 60 | IRMS | 121 | 1 | 45 | 5 |
| Ohara et al[61], 1998 | Japan | Cross-sectional | 213 | 77.5 | HpC or at least two positives of (HE, RUT, Serology) | 13C-UBT | 2.5‰ DOB | 100 | 20 | IRMS | 162 | 1 | 47 | 3 |
| Leodolter et al[62], 1998 | Germany | Cross-sectional | 40 | 50.0 | HpC or (HE and RUT) | 13C-UBT | 4‰ DOB | 75 | 10 | IRMS | 20 | 0 | 20 | 0 |
| Andersen et al[63], 1998 | Denmark | Cross-sectional | 97 | 54.6 | HE or HpC | 13C-UBT | 5‰ DOB | 100 | Multiple times | IRMS | 46 | 4 | 40 | 7 |
| Ellenrieder et al[64], 1997 | Germany | Cross-sectional | 132 | 43.2 | (HE, HpC, RUT) at least two positives | 13C-UBT | 3.5‰ DOB | NR | 30 | IS | 52 | 8 | 67 | 5 |
| Epple et al[65], 1997 | Germany | Cross-sectional | 126 | 61.1 | HE | 13C-UBT | 1.3‰ DOB | 75 | 30 | IRMS | 74 | 7 | 42 | 3 |
| Labenz et al[66], 1996 | Germany | Cross-sectional | 70 | 67.1 | HE or HpC | 13C-UBT | 4‰ DOB | 75 | 30 | IRMS | 46 | 0 | 23 | 1 |
| Logan et al[67], 1991 | England | Cross-sectional | 56 | 68.0 | HE | 13C-UBT | 4.5‰ DOB | 125 | Multiple times | IRMS | 32 | 1 | 15 | 2 |
| Dill et al[68], 1990 | Scotland | Cross-sectional | 69 | 49.3 | HpC | 13C-UBT | 3‰ c-PDR | 250 | 20 | IRMS | 33 | 0 | 35 | 1 |
On the other hand, the 14C-UBT accounted for 35% of the total (21 studies) with a median population size of 108.5 (lower to upper quartile: 63.5-125.5). For the 14C-UBT, the median pre-test probability was 64.9% (lower to upper quartile: 43.6-73.1). Various reference standards were employed in these studies, with "HE" being the most prevalent, accounting for 38.1% of cases. Other reference standards included "HE and RUT" (14.3%) and "HpC or HE" (9.5%). Some studies also used combinations of reference standards, such as "HE, RUT, Serology (at least two positive)" and "HpC or [HE and (RUT or Gram staining)]," each comprising 4.8% of the sample, as detailed in Table 2.
| Ref. | Country | Design | Population, n | Prevalence (%) | Reference standard | Index test (model) | Optimal cut-off | Urea dosing (μCi) | Time after administration (min) | Measurement tecnique | TP | TN | FP | FN |
| Han et al[30], 2023 | China | Cross-sectional | 205 | 42.4 | HE and RUT | 14C-UBT | 100 dpm | 0.75 | 20 | SC | 83 | 3 | 115 | 4 |
| Wang et al[29], 2021 | China | Cross-sectional | 267 | 71.9 | HE | 14C-UBT | 238 dpm | 0.75 | 25 | NR | 158 | 12 | 63 | 34 |
| Miftahussurur[69], 2021 | Indonesia | Cross-sectional | 55 | 23.6 | HE | 14C-UBT | 57 cpm | 1 | 10 | HA | 12 | 1 | 41 | 1 |
| Cosgun et al[70], 2016 | Turkey | Cross-sectional | 126 | 92.1 | HpC or HE | 14C-UBT | NR | 1 | 10 | HA | 112 | 7 | 3 | 4 |
| Atli et al[71], 2012 | Turkey | Cross-sectional | 100 | 35.0 | HE | 14C-UBT | Positive: > 50 cpm/suspicious: 25-50 cpm /negative: < 25 dpm | 1 | 10 | HA | 32 | 4 | 61 | 3 |
| Alarcón-Rivera et al[72], 2011 | Mexico | Cross-sectional | 84 | 70.2 | HE | 14C-UBT | Positive: > 50 ppm/indeterminate: 25-50 ppm/negative: < 25 ppm | 1 | 10-15 | HA | 56 | 1 | 24 | 3 |
| Mansour-Ghanaei et al[73], 2011 | Iran | Cross-sectional | 125 | 56.8 | HE, RUT, Serology (at least two positive) | 14C-UBT | 50 cpm | 1 | 15 | HA | 67 | 0 | 54 | 4 |
| Ozdemir et al[74], 2008 | Turkey | Cross-sectional | 89 | 66.3 | HE, RUT, PCR (at least two positive) | 14C-UBT | Positive: > 50 cpm/equivocal: 25-50 cpm; negative: < 25 dpm | 1 | 10 | HA | 57 | 0 | 30 | 2 |
| Rasool et al[75], 2007 | Pakistan | Cross-sectional | 94 | 64.9 | RUT | 14C-UBT | 50 cpm | 1 | 10 | β-SC | 60 | 3 | 30 | 1 |
| Gurbuz et al[76], 2005 | Turkey | Cross-sectional | 65 | 44.6 | HE | 14C-UBT | Positive: > 50 cpm/suspicious: 25-50 cpm /negative: < 25 dpm | 1 | 10 | HA | 26 | 8 | 28 | 3 |
| Gatta et al[31], 2003 | Italy | Cross-sectional | 117 | 65.0 | HpC or (HE and RUT) | 14C-UBT | 130-136 dpm (dpm at sample-dpm at T0) | 1 | 12.5 | LSC | 73 | 2 | 39 | 3 |
| González et al[77], 2003 | Chile | Cross-sectional | NR | 71.9 | Two or more positives | 14C-UBT | 200 dpm | 1 | 10 | LSC | 61 | 14 | 11 | 3 |
| Oztürk et al[78], 2009 | Turkey | Cross-sectional | 75 | 65.8 | HE | 14C-UBT | 100 dpm | 1 | 10 | LSC | 48 | 5 | 20 | 0 |
| Gomes et al[79], 2002 | Brazil | Cross-sectional | 137 | 83.9 | HE and RUT | 14C-UBT | 1000 cpm | 5 | 15 | LSC | 114 | 1 | 21 | 1 |
| Desroches et al[80], 1997 | Canada | Cross-sectional | 56 | 80.4 | HE or HpC | 14C-UBT | 0.33‰ AS (14CO2 specific activity) | 5 | 20 | LSC | 44 | 0 | 11 | 1 |
| Allardyce et al[81], 1997 | New Zealand | Cross-sectional | 63 | 34.9 | HE and RUT | 14C-UBT | 49 dpm | 1 | 30 | β-SC | 22 | 2 | 39 | 0 |
| Faigel et al[82], 1996 | United States | Cross-sectional | 50 | 42.6 | HE or RUT | 14C-UBT | Positive: > 200 dpm in any sample/borderline: 100-200 dpm (as the peak count)/negative: < 100 dpm (in all samples) | 1 | Multiple times | LSC | 18 | 1 | 26 | 2 |
| Goh et al[83], 1995 | Malaysia | Cross-sectional | 63 | 50.8 | HpC or [HE and (RUT or Gram staining)] | 14C-UBT | 1275 dpm | 5 | 15 | LSC | 32 | 0 | 31 | 0 |
| Kao et al[84], 1993 | China | Cross-sectional | 184 | 53.8 | HpC or RUT | 14C-UBT | 150‰ | 5 | 10 | LSC | 99 | 14 | 71 | 0 |
| Vivas et al[85], 1993 | Venezuela | Cross-sectional | 15 | 53.3 | HE | 14C-UBT | 100 dpm | 1 | 20 | β-SC | 8 | 1 | 6 | 0 |
| Novis et al[28], 1991 | Israel | Cross-sectional | 64 | 80.3 | HE | 14C-UBT | 4,7‰ | 10 | Multiple times | LSC | 59 | 3 | 12 | 2 |
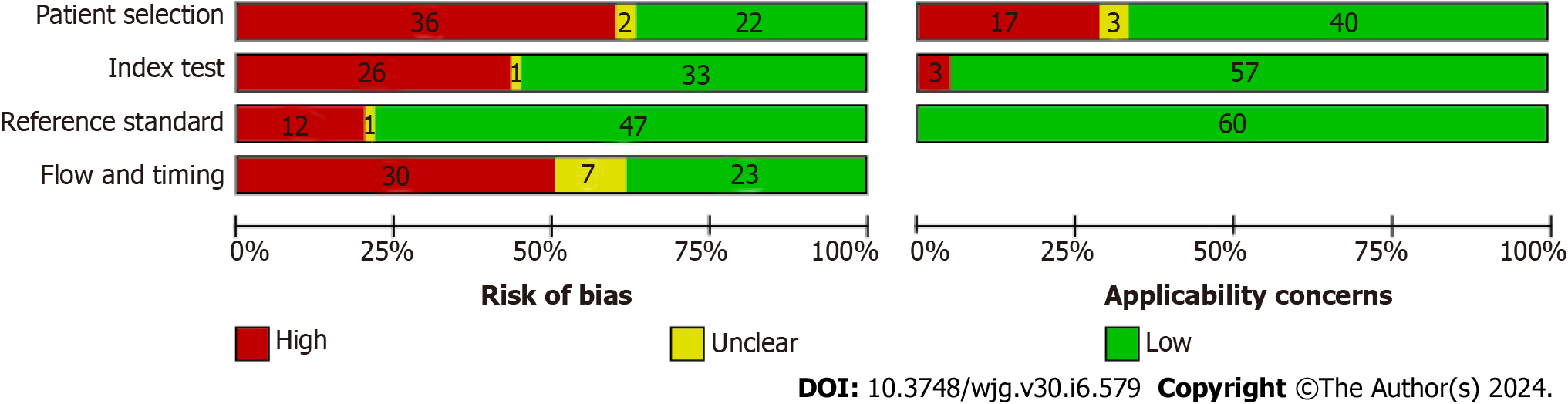
Supplementary Figure 1 and Figure 2 provide a visual representation of the comprehensive methodological quality assessment of the included studies. In the patient selection domain, 22 studies (35.5%) were categorized as having a low risk of bias, 36 studies (58.1%) were associated with a high risk of bias, and 2 studies (3.2%) were considered to have an unclear risk of bias. In terms of patient selection applicability, 40 studies (64.5%) exhibited low concern, 17 studies (27.4%) showed high concern, and 3 studies (4.8%) had unclear concern.
Within the index test selection domain, 33 studies (53.2%) were rated as having a low risk of bias, 26 studies (41.9%) were identified with a high risk, and 1 study (1.6%) had an unclear risk of bias. Concerning index test applicability, 57 studies (91.9%) displayed low concern, while 3 studies (4.8%) raised high concern.
In the reference standard domain, 47 studies (75.8%) demonstrated a low risk of bias, 12 studies (19.4%) showed a high risk of bias, and 1 study (1.6%) had an unclear risk of bias. Notably, none of the studies raised concerns about the applicability of the reference standard.
Lastly, in the flow and timing domain, 23 studies (37.1%) were associated with a low risk of bias, 23 studies (37.1%) exhibited a high risk of bias, and 7 studies (11.3%) had an unclear risk of bias.
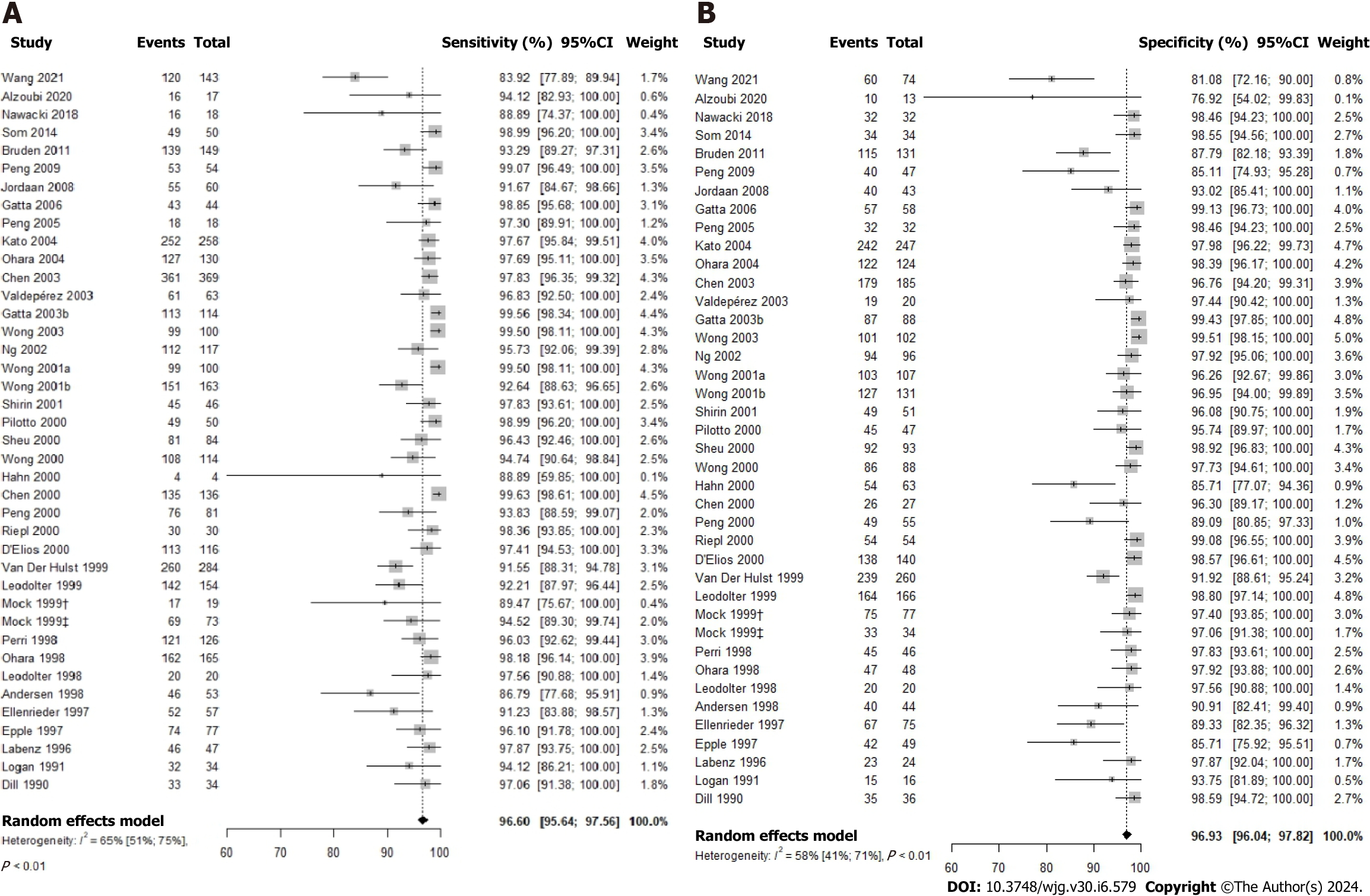
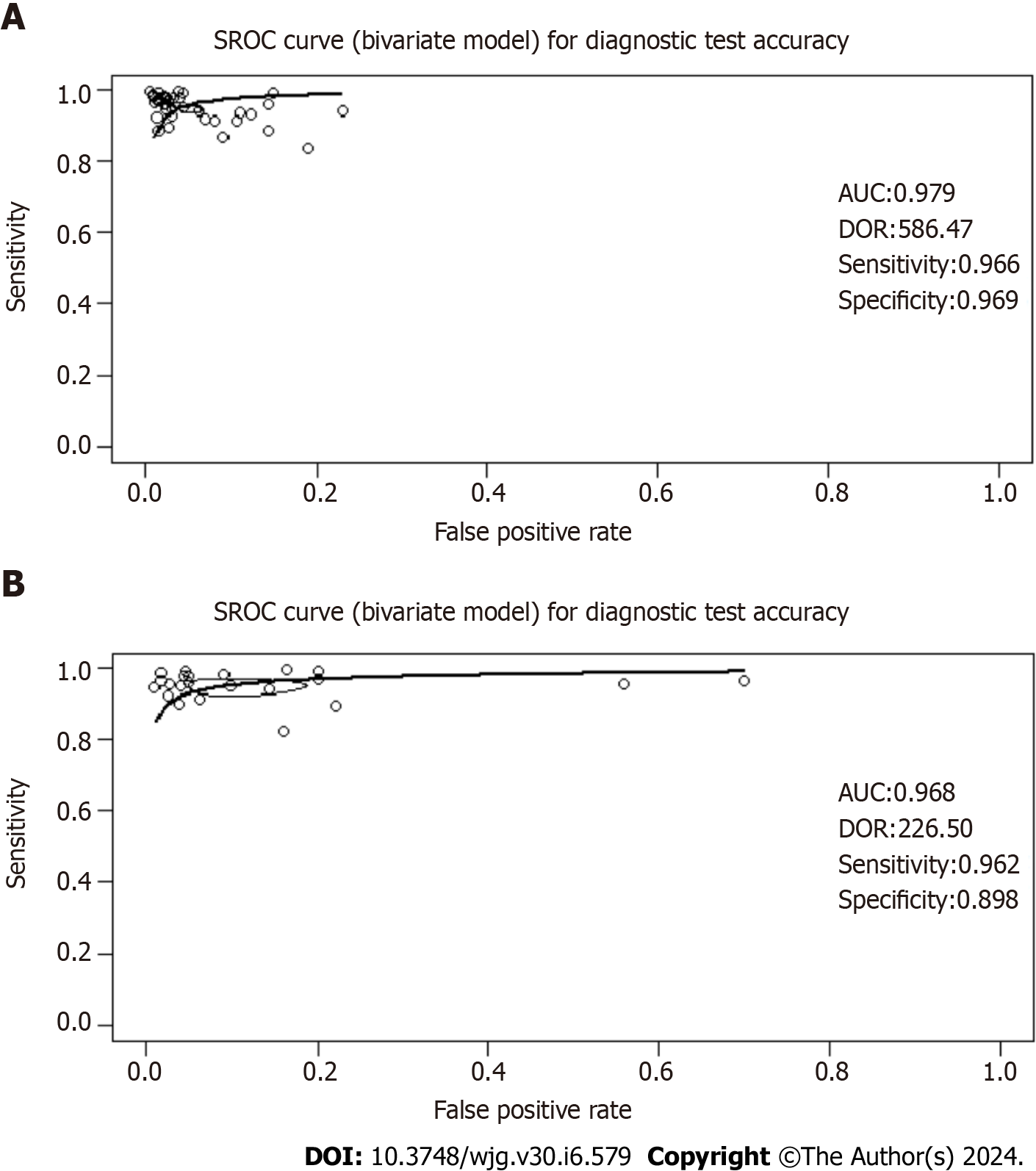
The 13C-UBT test was evaluated for its diagnostic accuracy in 39 studies via a comprehensive meta-analysis The results demonstrated a high sensitivity of 96.60% (95%CI: 95.64-97.56; P value < 0.01; I2 = 65.0%) and an equally impressive specificity of 96.93% (95%CI: 96.04-97.82; P value < 0.01; I2 = 58.0%) for this test (Figure 3). Additionally, the DOR was calculated at 586.47 (95%CI: 340.03-1011.51), with a positive likelihood ratio (LR+) of 22.00 (95%CI: 15.60-30.10) and a negative likelihood ratio (LR-) of 0.05 (95%CI: 0.04-0.06) as presented in Supplementary Table 1.
Among the thirty-six studies that documented the urea dosage, a 25 mg urea dose demonstrated notably high sensitivity (98.85%; 95%CI: 95.68-100.00) and specificity (99.13%; 95%CI: 96.73-100.00), as illustrated in Supplementary Figure 2. Increasing the urea dose to 50 mg across four studies resulted in a sensitivity of 95.28% (95%CI: 88.51-100.00) and a specificity of 94.91% (95%CI: 87.67-100.00). Seventeen studies explored the use of 75 mg of urea in the 13C-UBT, revealing a sensitivity of 96.47% (95%CI: 95.14-97.79) and a specificity of 98.33% (95%CI: 97.59-99.07). In cases where 100 mg of urea was used (in 12 studies), the 13C-UBT demonstrated a sensitivity of 97.31% (95%CI: 95.92-98.70) and a specificity of 96.08% (95%CI: 94.34-97.82). Two studies employing 125 mg of urea showed a sensitivity of 93.76% (95%CI: 86.13-100.00) and a specificity of 88.66% (95%CI: 81.07-96.25). Lastly, in a single study using 250 mg of urea, the 13C-UBT exhibited a sensitivity of 97.06% (95%CI: 91.38-100.00) and a specificity of 98.59% (95%CI: 94.72-100.00).
Among the 36 studies that provided information on the time after urea administration, optimal sensitivity (98.87%; 95%CI: 98.14-99.60) and specificity (98.14%; 95%CI: 96.98-99.30) were achieved when the assessment was conducted 20 min after urea administration [in 7 studies (Supplementary Figure 3)]. Notably, there were variations in sensitivity and specificity for different time intervals following urea administration.
For tests conducted 5 min post-urea administration (in one study), sensitivity was 97.83% (95%CI: 93.61-100.0), and specificity was 96.08% (95%CI: 90.75-100.00). Tests performed 10 min after urea administration (based on one study) yielded a sensitivity of 97.56% (95%CI: 90.88-100.0) and a specificity of 97.56% (95%CI: 90.88-100.00).
Similarly, in the case of tests carried out at 15 min post-urea administration (as reported in five studies), sensitivity averaged at 97.61% (95%CI: 95.68-99.55), with specificity at 95.85% (95%CI: 91.33-100.00). Longer intervals, such as 30 min and 60 min, as well as tests conducted at multiple time points after urea administration, displayed some variability. For instance, tests performed 30 min after urea administration (in 19 studies) had a sensitivity of 95.15% (95%CI: 93.30-96.92) and a specificity of 96.18% (95%CI: 94.48-97.87). A single study conducting tests 60 min post-urea administration reported a sensitivity of 96.03% (95%CI: 92.62-99.44) and a specificity of 97.83% (95%CI: 93.61-100.00). In the case of four studies investigating multiple time points after urea administration, the sensitivity was 96.13% (95%CI: 92.13-100.0), and the specificity was 97.95% (95%CI: 96.08-99.81).
In our analysis of 38 studies that included data on the 13C-UBT assessment technique, Integrated Cavity Output Spectrometry (ICOS) for measuring CO2 Isotope Ratios exhibited exceptional performance. ICOS demonstrated a sensitivity of 98.99% (95%CI: 96.20-100.00) and a specificity of 98.55% (95%CI: 94.56-100.00), as visualized in Supplementary Figure 4. In contrast, Infrared spectrometry, assessed in 8 studies, displayed a sensitivity of 94.72% (95%CI: 90.91-98.54) and a specificity of 98.55% (95%CI: 88.17-98.22).
Gas chromatography-mass spectrometry, investigated in a single study, yielded a sensitivity of 91.67% (95%CI: 84.67-98.66) and a specificity of 93.02% (95%CI: 85.41-100.00). Isotope-ratio mass spectrometry, scrutinized in 17 studies, demonstrated a sensitivity of 97.37% (95%CI: 96.45-98.28) and a specificity of 98.38% (95%CI: 84.67-98.66). Molecular correlation spectrometry, examined in a solitary study, exhibited a sensitivity of 97.83% (95%CI: 93.61-100.00) and a specificity of 96.08% (95%CI: 90.75-100.00). Similarly, Laser opto-galvanic effect spectroscopy, reported in one study, recorded a sensitivity of 91.65% (95%CI: 88.31-94.78) and a specificity of 91.92% (95%CI: 88.61-98.21).
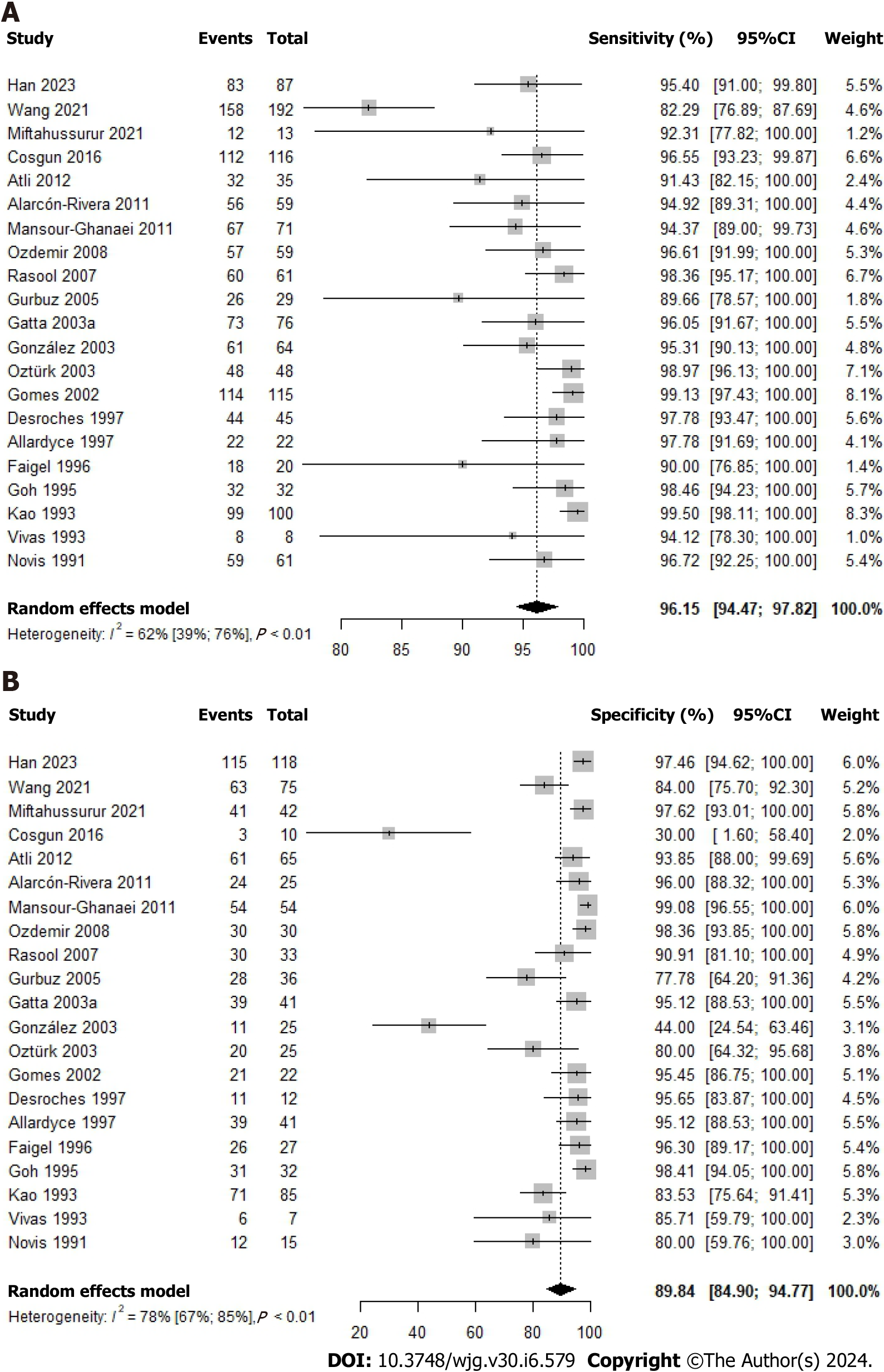
A total of 21 studies investigated the diagnostic accuracy of the 14C-UBT, revealing a combined sensitivity of 96.15% (95%CI: 94.47-97.82; P value < 0.01; I2 = 62.0%) and specificity of 89.84% (95%CI: 84.90-94.77; P value < 0.01; I2 = 78.0%), as depicted in Figure 4. Within this dataset, a DOR of 226.50 (95%CI: 102.57-500.15), a positive likelihood ratio (LR+) of 10.10 (95%CI: 5.74-16.90), and a negative likelihood ratio (LR-) of 0.06 (95%CI: 0.04-0.08) were observed, as summarized in Supplementary Table 1.
Twenty-one studies investigated varying urea dosages in the context of the 14C-UBT. Among these, the use of a 5 µCi marked urea dose, as examined in four studies, demonstrated exceptional sensitivity (99.21%; 95%CI: 98.20-100.00) and specificity (93.43%; 95%CI 86.45-100.00), as depicted in Supplementary Figure 5. Elevating the urea dose to 10 µCi, as explored in a single study, resulted in a sensitivity of 96.72% (95%CI: 92.15-100.00) and a specificity of 80.00% (95%CI: 56.76-100.00). Conversely, when employing 1 µCi of marked urea (in 14 studies), the 14C-UBT exhibited a sensitivity of 96.78% (95%CI: 95.46-98.09) and a specificity of 87.19% (95%CI: 59.76-95.81). Lastly, two studies using 0.75 µCi of urea reported a sensitivity of 88.94% (95%CI: 76.10-100.00) and a specificity of 91.32% (95%CI: 78.18-100.00).
When considering the time for measurement after urea administration, an analysis of all included studies consistently revealed the highest sensitivity (98.39%; 95%CI: 96.36-100.00) and specificity (98.71%; 95%CI: 96.58-100.00) when the tests were conducted 15 minutes after urea administration, as illustrated in Supplementary Figure 6.
In studies conducted shortly after urea administration (within 10 minutes, n = 9), the sensitivity was consistently high at 97.83% (95%CI: 96.34-99.33), while specificity was somewhat lower at 79.90% (95%CI: 66.15-93.65). A single study, conducted at 12.5 minutes post-administration, reported a sensitivity of 96.05% (95%CI: 91.67-100.00) and a specificity of 95.12% (95%CI: 88.53-100.00). Studies conducted between 10- and 15-minutes post-urea administration (n = 3) showed a sensitivity of 94.92% (95%CI: 89.31-100.00) and a specificity of 96.00% (95%CI: 88.32-100.00).
However, longer intervals (20, 25, and 30 min), as well as tests conducted at various time points after urea administration, exhibited more variability. For instance, studies conducted at 20 min post-administration (n = 3) showed a sensitivity of 96.52% (95%CI: 93.50-97.55) and a specificity of 97.23% (95%CI: 94.48-99.97). A single study conducted at 25 min post-urea administration reported a sensitivity of 82.29% (95%CI: 76.89-87.69) and a specificity of 84.00% (95%CI: 75.70-92.30). A study conducted at 30 minutes post-administration yielded a sensitivity of 97.78% (95%CI: 91.69-100.00) and a specificity of 95.12% (95%CI: 88.53-100.00). In the case of two studies that investigated multiple time points after urea administration, the sensitivity was 96.03% (95%CI: 91.79-100.00), and the specificity was 91.02% (95%CI: 76.07-100.00).
In the assessment of 20 studies with available data on the assessment technique, it was observed that liquid scintillation counting yielded a higher sensitivity of 98.79% (95%CI: 97.90-99.69) while maintaining a specificity of 87.24% (95%CI: 77.69-96.79). Conversely, Solid Scintillation UBT (scintillation counting) demonstrated higher specificity, reaching 97.46% (95%CI: 94.62-100.00), with a sensitivity of 95.40% (95%CI: 91.00-99.80), as illustrated in Supplementary Figure 7.
In contrast, the Heliprobe Analyser, assessed in 7 studies, displayed a sensitivity of 95.41% (95%CI: 93.32-97.50) and a specificity of 88.10% (95%CI: 74.43-100.00). Ultimately, the use of Beta-scintillation counter for the assessment of 14C-UBT resulted in a sensitivity of 98.11% (95%CI: 95.33-100.00) and a specificity of 93.47% (95%CI: 88.11-98.82).
Spearman’s correlation analysis for studies evaluating 13C-UBT revealed a correlation coefficient (r) of 0.48, indicating the absence of a threshold effect. Similarly, 14C-UBT studies exhibited a negligible correlation (r = -0.01), also suggesting the absence of a threshold effect. Visual inspection of the SROC curves did not reveal any significant heterogeneity. Both the 13C-UBT (AUC = 0.979; Figure 5A) and the 14C-UBT (AUC = 0.968; Figure 5B) displayed excellent diagnostic accuracy.
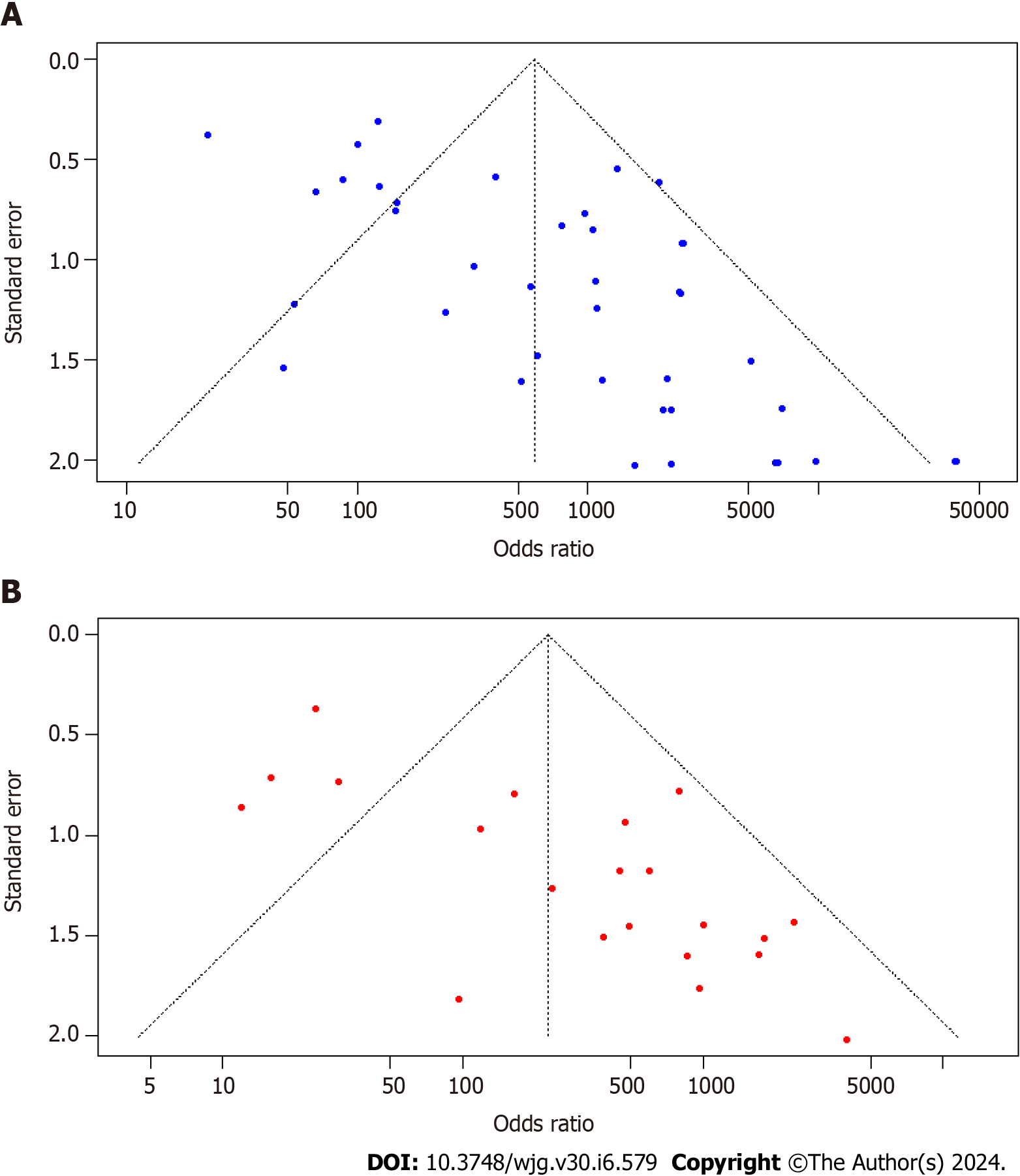
The funnel plot visualization exposed asymmetry in both the 13C-UBT (Figure 6A) and 14C-UBT (Figure 6B) models. Additionally, Egger's test confirmed the presence of publication bias in both tests. The intercept was 2.54 with a P value < 0.001 for 13C-UBT and 3.04 with a P value < 0.001 for 14C-UBT.
Our analysis has revealed that the 13C-UBT outperforms the 14C-UBT in terms of diagnostic accuracy, as evidenced by the following values: DOR, Likelihood Ratios (LR+ and LR-), and AUC values. Specifically, the 13C-UBT has sensitivity and specificity values of 96.60% (95%CI: 95.64-97.56; P value < 0.01; I2 = 65.0%) and 96.93% (95%CI: 96.04-97.82; P value < 0.01; I2 = 58.0%), respectively. In contrast, the 14C-UBT has sensitivity and specificity values of 96.15% (95%CI: 94.47-97.82; P value < 0.01; I2 = 62.0%) and 89.84% (95%CI: 84.90-94.77; P value < 0.01; I2 = 78.0%). The LR+ values for the 13C-UBT and
Furthermore, the DOR values show a substantial difference between the two tests. The 13C-UBT yields a significantly higher DOR of 586.47 compared to the 14C-UBT's DOR of 226.50. These results indicate that the 13C-UBT is statistically superior at distinguishing dyspeptic individuals with and without H. pylori infection, making it the preferred diagnostic tool in this clinical context.
Finally, it is essential to emphasize that our correlation analysis, utilizing both the 13C-UBT (r = 0.48) and the 14C-UBT (r = -0.01), yielded no evidence of a threshold effect. Visual examination of the SROC curves revealed no heterogeneity, indicating consistent accuracy assessments across the studies. Additionally, both the 13C-UBT and the 14C-UBT displayed remarkably high AUC values: 0.979 for the 13C-UBT and 0.968 for the 14C-UBT, which approaching 1.00 reinforces the excellent accuracy of these tests in detecting H. pylori infection in individuals with dyspepsia. These findings strongly support the reliability of the 13C-UBT and the 14C-UBT as valuable diagnostic tools in clinical practice.
Our analysis highlights the critical importance of selecting the appropriate urea dose when conducting the 13C-UBT for diagnosing H. pylori infection. While the 25 mg urea dose displays the highest sensitivity (98.85%) and specificity (99.13%), concerns regarding the generalizability of these results arise due to the fact that these findings are primarily based on a single study[26]. In contrast, the use of 75 mg and 100 mg doses is supported by a larger body of evidence, maintaining excellent diagnostic accuracy with sensitivity and specificity exceeding 96%. Conversely, higher doses, such as 125 mg or 250 mg, exhibit a modest reduction in accuracy, particularly in terms of specificity. These findings strongly advocate for the consideration of 75 mg and 100 mg doses when aiming to optimize both sensitivity and specificity.
A crucial factor affecting the performance of the 13C-UBT is the timing of the assessment following urea administration. Our observations reveal that the optimal sensitivity and specificity, both exceeding 98%, are achieved at the 20-minute mark post-urea administration. Tests conducted at shorter intervals, such as 5 min and 10 min, also demonstrate high sensitivity and specificity, albeit slightly lower than the 20-min assessment. Conversely, assessments at 15 min maintain excellent accuracy, with sensitivity close to 98% and specificity around 95%. However, assessments at longer intervals, such as 30 min, 60 min, and multiple time points, exhibit some variability, with sensitivity and specificity values slightly lower than the 20-min assessment. These results highlight the 20-min assessment as the most reliable time point, offering a balance between high sensitivity and specificity. Nevertheless, the test remains accurate when conducted at shorter intervals.
The choice of assessment technique is also crucial for test accuracy. ICOS is the most accurate technique, with a sensitivity of 98.99% and a specificity of 98.55%. However, it is important to note that ICOS was evaluated in a single study[27], potentially limiting the generalizability of these results. To address this limitation, Isotope-ratio mass spectrometry is a more advisable option. In contrast, Infrared spectrometry, gas chromatography-mass spectrometry, isotope-ratio mass spectrometry, molecular correlation spectrometry, and Laser opto-galvanic effect spectroscopy yield varying levels of sensitivity and specificity. These findings underscore the significance of selecting the right assessment technique. While ICOS may be preferred when available due to its exceptional accuracy, other factors such as cost, availability, and local expertise should also be considered when making this choice.
Our research indicates that the urea dosage utilized in the 14C-UBT can also impact test accuracy. Specifically, a urea dose of 5 µCi was examined in four studies and was found to possess exceptional sensitivity (99.21%) and specificity (93.43%). These findings underscore the potential benefits of employing a 5 µCi dose for the 14C-UBT, as it offers a high level of accuracy in detecting H. pylori infection. However, increasing the urea dose to 10 µCi, as investigated in a single study[28], resulted in a slightly lower sensitivity (96.72%) and a specificity of 80.00%. This suggests that while higher urea dosages may still provide reliable results, they may be associated with a decrease in specificity, which could lead to more false-positive results.
On the other hand, the use of 1 µCi of marked urea, which was the most commonly used dosage in 14 studies, resulted in a sensitivity of 96.78% and a specificity of 87.19%. This indicates that a 1 µCi dose remains a viable option for the 14C-UBT, offering a good balance between sensitivity and specificity. Two recent studies using 0.75 µCi of urea reported a sensitivity of 88.94% and a specificity of 91.32%, suggesting that even lower urea doses can provide reasonable diagnostic accuracy[29,30].
Regarding the time for measurement, tests conducted 15 min after urea administration consistently exhibited the highest sensitivity (98.39%) and specificity (98.71%). This indicates that the 15-min time point is optimal for maximizing test accuracy. Tests conducted within 10 min post-administration maintained high sensitivity (97.83%) but had a somewhat lower specificity (79.90%). A single study conducted at 12.5 min post-administration reported favorable sensitivity (96.05%) and specificity (95.12%)[31]. In contrast, longer intervals (20, 25, and 30 min) showed more variability, with varying levels of sensitivity and specificity. This suggests that measurements taken beyond 15 min may not be as reliable for H. pylori detection. Clinicians should carefully consider the timing of the 14C-UBT to ensure accurate results, with a preference for the 15-min mark when feasible.
Lastly, our analysis of assessment techniques uncovered differences in sensitivity and specificity. Liquid scintillation counting demonstrated the highest sensitivity (98.79%) but had a specificity of 87.24%. In contrast, Solid Scintillation UBT (scintillation counting) showed higher specificity (97.46%) at the expense of sensitivity (95.40%). The Heliprobe Analyser and Beta-scintillation counter also demonstrated moderate sensitivity and specificity. When choosing the assessment technique, the trade-off between sensitivity and specificity should be considered in relation to the clinical context. For instance, if high sensitivity is paramount to avoid missing positive cases, liquid scintillation counting may be the preferred method. Conversely, if high specificity is crucial to minimize false positives, solid scintillation counting could be a better choice.
This meta-analysis adhered to established guidelines and rigorous methodological principles, enhancing the validity and reliability of our findings. We used a bivariate random-effects model to calculate sensitivity, specificity, likelihood ratios, and the DOR, alongside generating SROC curves for a comprehensive statistical analysis of the included studies. Subgroup analyses based on urea dosing, measurement timing, and assessment technique were conducted to explore potential sources of variation, while Spearman correlation analysis was used to assess the threshold effect's impact on diagnostic accuracy. Additionally, we assessed publication bias through visual inspections of funnel plots and Egger's tests.
However, it's important to acknowledge inherent limitations in our analysis. These include potential language bias, reliance on available data, and challenges associated with the inherent heterogeneity in diagnostic accuracy studies. Although we did not impose language restrictions in our search, the inclusion of studies conducted in English, Spanish, or Portuguese may introduce language bias[32]. The exclusion of studies due to unavailability of full-text articles or articles not in these specified languages could potentially lead to the omission of essential data.
Furthermore, the quality of our meta-analysis is closely tied to the quality of the primary studies we included. Biases within these primary studies can affect our analysis outcomes. In particular, we have concerns regarding the inclusion of patients, as there was no reported consecutive patient inclusion in some studies, and the index test was not always performed using a pre-specified threshold. Moreover, the diversity in diagnostic accuracy studies can present challenges when consolidating results, and despite subgroup analyses, residual heterogeneity may impact the broad applicability of our findings. Encouragingly, the visual examination of the SROC curves indicates consistent accuracy assessments across the included studies. Nevertheless, it is imperative to underscore that the reliability of our meta-analysis hinges on the data provided in these included studies. The absence or inconsistency of critical data points can significantly affect the precision of our analysis. Researchers and clinicians should consider these strengths and limitations when applying our findings in their practice.
In summary, our study offers crucial insights for selecting optimal diagnostic methods to detect H. pylori infection in clinical settings. We found that the 13C-UBT outperforms the 14C-UBT in terms of diagnostic accuracy, making it the preferred diagnostic approach. Furthermore, our findings highlight the significance of precise considerations when choosing urea dosage, assessment timing, and measurement techniques for both the 13C-UBT and 14C-UBT, thus enhancing diagnostic precision. These insights provide practical guidance to healthcare practitioners when choosing the most suitable diagnostic method for H. pylori infection, tailored to their specific clinical context. Factors like diagnostic accuracy, cost, and availability should be carefully weighed in this decision-making process. Our findings also have the potential to contribute significantly to the standardization of testing procedures, ensuring consistent and reliable results, especially for patients with dyspepsia or suspected H. pylori infection. Nevertheless, it's essential for researchers and clinicians to consider the strengths and limitations when applying our findings in their practice.
The urea breath test (UBT) has become a widely accepted non-invasive method for detecting Helicobacter pylori (H. pylori). While numerous studies have confirmed its high accuracy, its reliability is often hindered by inherent limitations.
In a previous investigation, the diagnostic accuracy of the UBT, which encompasses both 13C-UBT and 14C-UBT, was evaluated in adult patients with dyspepsia to determine the presence of H. pylori infection. Although the test demonstrated a high degree of precision, its reliability was compromised by significant and unexplained heterogeneity, which persisted even after conducting subgroup analyses. This trend continued in subsequent studies, with similar challenges encountered in determining pooled estimates of diagnostic accuracy for 14C-UBT. Furthermore, a subsequent systematic review revealed that the variability in thresholds and reference standards across studies limited the available data for pooling accuracy measures at specific UBT thresholds. These findings underscore the need for a rigorous statistical synthesis to clarify and reconcile the diagnostic accuracy of the UBT for the diagnosis of H. pylori infection, addressing challenges identified in prior research.
To evaluate and contrast the diagnostic accuracy of 13C-UBT and 14C-UBT for H. pylori infection in adult patients with dyspepsia.
We conducted independent searches of PubMed/MEDLINE, EMBASE, and Cochrane Central databases until April 2022, focusing on diagnostic accuracy studies that evaluated at least one of the index tests (13C-UBT or 14C-UBT) against a reference standard. We utilized the QUADAS-2 tool to assess the methodological quality of the studies, and we calculated sensitivity, specificity, positive and negative test likelihood ratios (LR+ and LR-), as well as the diagnostic odds ratio (DOR) and their 95% confidence intervals using the bivariate random-effects model. We conducted subgroup analyses based on urea dosing, time after urea administration, and assessment technique. To investigate a possible threshold effect, we conducted Spearman correlation analysis, and we generated summary receiver operating characteristic (SROC) curves to assess heterogeneity. Lastly, we visually inspected a funnel plot and used Egger’s test to evaluate publication bias.
A screening of 4621 studies led to the selection of 60 articles for inclusion in a diagnostic test accuracy meta-analysis after full-text reading. Our analysis highlights the superior diagnostic accuracy of 13C-UBT compared to 14C-UBT, as evidenced by higher sensitivity (96.60% vs 96.15%), specificity (96.93% vs 89.84%), likelihood ratios (LR+ 22.00 vs 10.10; LR- 0.05 vs 0.06), and AUC values (0.979 vs 0.968). Particularly noteworthy is the significantly higher DOR of 13C-UBT (586.47) compared to 14C-UBT (DOR 226.50), establishing 13C-UBT as the preferred diagnostic tool for individuals with dyspepsia and H. pylori infection. Correlation analysis indicated no threshold effect for both 13C-UBT (r = 0.48) and 14C-UBT (r = -0.01), and the SROC curves consistently demonstrated accurate performance for both tests. The high AUC values (13C-UBT: 0.979; 14C-UBT: 0.968), nearing 1.00, further affirm the excellent accuracy of both UBT variants, solidifying their reliability as diagnostic tools in clinical practice.
Our study establishes 13C-UBT as the superior diagnostic approach over 14C-UBT. Furthermore, our findings underscore the critical importance of meticulously considering factors such as urea dosage, assessment timing, and measurement techniques for both tests to optimize diagnostic accuracy. However, it is paramount for researchers and clinicians to thoroughly evaluate the strengths and limitations of our conclusions before integrating them into clinical practice.
Future research should focus on improving the comprehension, practicality, and dependability of UBTs for H. pylori infection. This endeavor involves refining techniques, examining sources of variability, exploring threshold effects, conducting longitudinal and comparative investigations, addressing biases, and assessing cost-effectiveness.
| 1. | Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 282] [Reference Citation Analysis (0)] |
| 2. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1475] [Cited by in RCA: 1259] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 3. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 557] [Article Influence: 185.7] [Reference Citation Analysis (1)] |
| 4. | Narayanan M, Reddy KM, Marsicano E. Peptic Ulcer Disease and Helicobacter pylori infection. Mo Med. 2018;115:219-224. [PubMed] |
| 5. | Usui Y, Taniyama Y, Endo M, Koyanagi YN, Kasugai Y, Oze I, Ito H, Imoto I, Tanaka T, Tajika M, Niwa Y, Iwasaki Y, Aoi T, Hakozaki N, Takata S, Suzuki K, Terao C, Hatakeyama M, Hirata M, Sugano K, Yoshida T, Kamatani Y, Nakagawa H, Matsuda K, Murakami Y, Spurdle AB, Matsuo K, Momozawa Y. Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N Engl J Med. 2023;388:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 6. | Lemos FFB, de Castro CT, Calmon MS, Silva Luz M, Pinheiro SLR, Faria Souza Mendes Dos Santos C, Correa Santos GL, Marques HS, Delgado HA, Teixeira KN, Souza CL, Oliveira MV, Freire de Melo F. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J Gastroenterol. 2023;29:2202-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM; Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM). Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 8. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 834] [Article Influence: 208.5] [Reference Citation Analysis (0)] |
| 9. | Talebi Bezmin Abadi A. Diagnosis of Helicobacter pylori Using Invasive and Noninvasive Approaches. J Pathog. 2018;2018:9064952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Kayali S, Aloe R, Bonaguri C, Gaiani F, Manfredi M, Leandro G, Fornaroli F, Di Mario F, De' Angelis GL. Non-invasive tests for the diagnosis of helicobacter pylori: state of the art. Acta Biomed. 2018;89:58-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 11. | Sankararaman S, Moosavi L. Urea Breath Test. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] |
| 12. | Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut. 1999;45 Suppl 1:I18-I22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Balon H, Gold CA, Dworkin HJ, McCormick VA, Freitas JE. Procedure guideline for carbon-14-urea breath test. Society of Nuclear Medicine. J Nucl Med. 1998;39:2012-2014. [PubMed] |
| 15. | Balon HR, Roff E. C-14-urea breath test: a new product and a word of caution. J Nucl Med. 1998;39:1306. [PubMed] |
| 16. | Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, Altayar O, Limburg PJ, Murad MH, Knawy B. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (4)] |
| 17. | Zhou Q, Li L, Ai Y, Pan Z, Guo M, Han J. Diagnostic accuracy of the (14)C-urea breath test in Helicobacter pylori infections: a meta-analysis. Wien Klin Wochenschr. 2017;129:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, Yaghoobi M, Gurusamy KS. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 2315] [Article Influence: 289.4] [Reference Citation Analysis (0)] |
| 20. | Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 1st ed. New Jersey: John Wiley, 2023. [DOI] [Full Text] |
| 21. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10323] [Article Influence: 688.2] [Reference Citation Analysis (3)] |
| 22. | Reiman MP, Thorborg K, Goode AP, Cook CE, Weir A, Hölmich P. Diagnostic Accuracy of Imaging Modalities and Injection Techniques for the Diagnosis of Femoroacetabular Impingement/Labral Tear: A Systematic Review With Meta-analysis. Am J Sports Med. 2017;45:2665-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Macaskill P, Takwoingi Y, Deeks JJ, Gatsonis C. Understanding meta-analysis. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 1st ed. Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y, editors. New Jersey: John Wiley, 2023: 203-247. [DOI] [Full Text] |
| 24. | Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part I. General Guidance and Tips. Korean J Radiol. 2015;16:1175-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 25. | Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J Radiol. 2015;16:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 394] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 26. | Gatta L, Ricci C, Tampieri A, Osborn J, Perna F, Bernabucci V, Vaira D. Accuracy of breath tests using low doses of 13C-urea to diagnose Helicobacter pylori infection: a randomised controlled trial. Gut. 2006;55:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (3)] |
| 27. | Som S, Maity A, Banik GD, Ghosh C, Chaudhuri S, Daschakraborty SB, Ghosh S, Pradhan M. Excretion kinetics of 13C-urea breath test: influences of endogenous CO2 production and dose recovery on the diagnostic accuracy of Helicobacter pylori infection. Anal Bioanal Chem. 2014;406:5405-5412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Novis BH, Gabay G, Leichtmann G, Peri M, Bernheim J, Pomeranz IS. Two point analysis 15-minute 14C-urea breath test for diagnosing Helicobacter pylori infection. Digestion. 1991;50:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Wang X, Zhang S, Chua EG, He Y, Li X, Liu A, Chen H, Wise MJ, Marshall BJ, Sun D, Tay CY. A re-testing range is recommended for (13)C- and (14)C-urea breath tests for Helicobacter pylori infection in China. Gut Pathog. 2021;13:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 30. | Han YH, Zhang W, Wang YT, Xiong ZJ, Du Q, Xie Y, Lu H. Performance evaluation of a novel 14C-urea breath test (solid scintillation) for the diagnosis of helicobacter pylori infection. Medicine (Baltimore). 2023;102:e33107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Gatta L, Ricci C, Stanghellini V, Alì A, Menegatti M, Morselli Labate AM, Corinaldesi R, Miglioli M, Vaira D. Best cut-off values for [14C]-urea breath tests for Helicobacter pylori detection. Scand J Gastroenterol. 2003;38:1144-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Stern C, Kleijnen J. Language bias in systematic reviews: you only get out what you put in. JBI Evid Synth. 2020;18:1818-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Alzoubi H, Al-Mnayyis A, Al Rfoa I, Aqel A, Abu-Lubad M, Hamdan O, Jaber K. The Use of (13)C-Urea Breath Test for Non-Invasive Diagnosis of Helicobacter pylori Infection in Comparison to Endoscopy and Stool Antigen Test. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Nawacki Ł, Czyż A, Bryk P, Kozieł D, Stępień R, Głuszek S. Can urea breath test (UBT) replace rapid urea test (RUT)? Pol Przegl Chir. 2018;90:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Bruden DL, Bruce MG, Miernyk KM, Morris J, Hurlburt D, Hennessy TW, Peters H, Sacco F, Parkinson AJ, McMahon BJ. Diagnostic accuracy of tests for Helicobacter pylori in an Alaska Native population. World J Gastroenterol. 2011;17:4682-4688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Peng NJ, Lai KH, Lo GH, Hsu PI. Comparison of noninvasive diagnostic tests for Helicobacter pylori infection. Med Princ Pract. 2009;18:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Jordaan M, Laurens JB. Diagnosis of Helicobacter pylori infection with the (13)C-urea breath test by means of GC-MS analysis. J Sep Sci. 2008;31:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Peng NJ, Lai KH, Liu RS, Lee SC, Tsay DG, Lo CC, Tseng HH, Huang WK, Lo GH, Hsu PI. Capsule 13C-urea breath test for the diagnosis of Helicobacter pylori infection. World J Gastroenterol. 2005;11:1361-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Kato S, Nakayama K, Minoura T, Konno M, Tajiri H, Matsuhisa T, Iinuma K; Japanese pediatric Helicobacter study group. Comparison between the 13C-urea breath test and stool antigen test for the diagnosis of childhood Helicobacter pylori infection. J Gastroenterol. 2004;39:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Ohara S, Kato M, Saito M, Fukuda S, Kato C, Hamada S, Nagashima R, Obara K, Suzuki M, Honda H, Asaka M, Toyota T. Comparison between a new 13C-urea breath test, using a film-coated tablet, and the conventional 13C-urea breath test for the detection of Helicobacter pylori infection. J Gastroenterol. 2004;39:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Chen TS, Chang FY, Chen PC, Huang TW, Ou JT, Tsai MH, Wu MS, Lin JT. Simplified 13C-urea breath test with a new infrared spectrometer for diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Valdepérez J, Vicente R, Novella MP, Valle L, Sicilia B, Yus C, Gomollón F. [Is the breath test reliable in primary care diagnosis of Helicobacter pylori infection?]. Aten Primaria. 2003;31:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Gatta L, Vakil N, Ricci C, Osborn JF, Tampieri A, Perna F, Miglioli M, Vaira D. A rapid, low-dose, 13C-urea tablet for the detection of Helicobacter pylori infection before and after treatment. Aliment Pharmacol Ther. 2003;17:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Wong WM, Lam SK, Lai KC, Chu KM, Xia HH, Wong KW, Cheung KL, Lin SK, Wong BC. A rapid-release 50-mg tablet-based 13C-urea breath test for the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Ng FH, Lai KC, Wong BC, Wong WM, Wong SY, Chow KC, Yuen ST, Leung SY, Lam SK. [13C]-urea breath test without prior fasting and without test meal is accurate for the detection of Helicobacter pylori infection in Chinese. J Gastroenterol Hepatol. 2002;17:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Wong WM, Wong BC, Li TM, Wong KW, Cheung KL, Fung FM, Xia HH, Lam SK. Twenty-minute 50 mg 13C-urea breath test without test meal for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001;15:1499-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Wong BC, Wong WM, Wang WH, Tang VS, Young J, Lai KC, Yuen ST, Leung SY, Hu WH, Chan CK, Hui WM, Lam SK. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001;15:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Shirin H, Kenet G, Shevah O, Wardi J, Birkenfeld S, Shahmurov M, Bruck R, Niv Y, Moss SF, Avni Y. Evaluation of a novel continuous real time (13)C urea breath analyser for Helicobacter pylori. Aliment Pharmacol Ther. 2001;15:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Pilotto A, Franceschi M, Leandro G, Rassu M, Zagari RM, Bozzola L, Furlan F, Bazzoli F, Di Mario F, Valerio G. Noninvasive diagnosis of Helicobacter pylori infection in older subjects: comparison of the 13C-urea breath test with serology. J Gerontol A Biol Sci Med Sci. 2000;55:M163-M167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Sheu BS, Lee SC, Lin PW, Wang ST, Chang YC, Yang HB, Chuang CH, Lin XZ. Carbon urea breath test is not as accurate as endoscopy to detect Helicobacter pylori after gastrectomy. Gastrointest Endosc. 2000;51:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Wong WM, Wong BC, Wong KW, Fung FM, Lai KC, Hu WH, Yuen ST, Leung SY, Lau GK, Lai CL, Chan CK, Go R, Lam SK. (13)C-urea breath test without a test meal is highly accurate for the detection of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2000;14:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Hahn M, Fennerty MB, Corless CL, Magaret N, Lieberman DA, Faigel DO. Noninvasive tests as a substitute for histology in the diagnosis of Helicobacter pylori infection. Gastrointest Endosc. 2000;52:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Chen X, Haruma K, Kamada T, Mihara M, Komoto K, Yoshihara M, Sumii K, Kajiyama G. Factors that affect results of the 13C urea breath test in Japanese patients. Helicobacter. 2000;5:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Peng NJ, Hsu PI, Lee SC, Tseng HH, Huang WK, Tsay DG, Ger LP, Lo GH, Lin CK, Tsai CC, Lai KH. A 15-minute [13C]-urea breath test for the diagnosis of Helicobacter pylori infection in patients with non-ulcer dyspepsia. J Gastroenterol Hepatol. 2000;15:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Riepl RL, Folwaczny C, Otto B, Klauser A, Blendinger C, Wiebecke B, König A, Lehnert P, Heldwein W. Accuracy of 13C-urea breath test in clinical use for diagnosis of Helicobacter pylori infection. Z Gastroenterol. 2000;38:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | D'Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Usefulness of (13)C-urea breath test in the diagnosis of gastric helicobacter pylori infection. Int J Immunopathol Pharmacol. 2000;13:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | van der Hulst RW, Hensen EF, van der Ende A, Kruizinga SP, Homan A, Tytgat GN. [Laser-assisted 13C-urea breath test; a new noninvasive detection method for Helicobacter pylori infection]. Ned Tijdschr Geneeskd. 1999;143:400-404. [PubMed] |
| 58. | Leodolter A, Domínguez-Muñoz JE, Von Arnim U, Malfertheiner P. Citric acid or orange juice for the 13C-urea breath test: the impact of pH and gastric emptying. Aliment Pharmacol Ther. 1999;13:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Mock T, Yatscoff R, Foster R, Hyun JH, Chung IS, Shim CS, Yacyshyn B. Clinical validation of the Helikit: a 13C urea breath test used for the diagnosis of Helicobacter pylori infection. Clin Biochem. 1999;32:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Perri F, Clemente R, Festa V, Quitadamo M, Conoscitore P, Niro G, Ghoos Y, Rutgeerts P, Andriulli A. Relationship between the results of pre-treatment urea breath test and efficacy of eradication of Helicobacter pylori infection. Ital J Gastroenterol Hepatol. 1998;30:146-150. [PubMed] |
| 61. | Ohara S, Kato M, Asaka M, Toyota T. The UBiT-100 13CO2 infrared analyzer: comparison between infrared spectrometric analysis and mass spectrometric analysis. Helicobacter. 1998;3:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Leodolter A, Domínguez-Muñoz JE, von Arnim U, Manes G, Malfertheiner P. 13C-urea breath test for the diagnosis of Helicobacter pylori infection. A further simplification for clinical practice. Scand J Gastroenterol. 1998;33:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Andersen LP, Kiilerick S, Pedersen G, Thoreson AC, Jørgensen F, Rath J, Larsen NE, Børup O, Krogfelt K, Scheibel J, Rune S. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Ellenrieder V, Glasbrenner B, Stoffels C, Weiler S, Bode G, Möller P, Adler G. Qualitative and semi-quantitative value of a modified 13C-urea breath test for identification of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1997;9:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Epple HJ, Kirstein FW, Bojarski C, Frege J, Fromm M, Riecken EO, Schulzke JD. 13C-urea breath test in Helicobacter pylori diagnosis and eradication. Correlation to histology, origin of 'false' results, and influence of food intake. Scand J Gastroenterol. 1997;32:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Labenz J, Bärsch G, Peitz U, Aygen S, Hennemann O, Tillenburg B, Becker T, Stolte M. Validity of a novel biopsy urease test (HUT) and a simplified 13C-urea breath test for diagnosis of Helicobacter pylori infection and estimation of the severity of gastritis. Digestion. 1996;57:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Logan RP, Polson RJ, Misiewicz JJ, Rao G, Karim NQ, Newell D, Johnson P, Wadsworth J, Walker MM, Baron JH. Simplified single sample 13Carbon urea breath test for Helicobacter pylori: comparison with histology, culture, and ELISA serology. Gut. 1991;32:1461-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Dill S, Payne-James JJ, Misiewicz JJ, Grimble GK, McSwiggan D, Pathak K, Wood AJ, Scrimgeour CM, Rennie MJ. Evaluation of 13C-urea breath test in the detection of Helicobacter pylori and in monitoring the effect of tripotassium dicitratobismuthate in non-ulcer dyspepsia. Gut. 1990;31:1237-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Miftahussurur M. Noninvasive Helicobacter pylori Diagnostic Methods in Indonesia. Gut Liver. 2020;14:553-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Cosgun Y, Yildirim A, Yucel M, Karakoc AE, Koca G, Gonultas A, Gursoy G, Ustun H, Korkmaz M. Evaluation of Invasive and Noninvasive Methods for the Diagnosis of Helicobacter Pylori Infection. Asian Pac J Cancer Prev. 2016;17:5265-5272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 71. | Atli T, Sahin S, Arslan BU, Varli M, Yalcin AE, Aras S. Comparison of the C14 urea breath test and histopathology in the diagnosis of Helicobacter pylori in the elderly. J Pak Med Assoc. 2012;62:1061-1065. [PubMed] |
| 72. | Alarcón-Rivera G, Vázquez-Jiménez G, de la Cruz-Patiño E, Abarca M, Leyva E, Delgado F, Ruíz-Juárez I, Grube-Pagola P, Roesch-Dietlen F, Remes-Troche JM. [Comparative analysis between breath test, serological immunoassay and rapid-urease test for detection of Helicobacter pylori infection in Mexican patients with non-investigated dyspepsia]. Rev Gastroenterol Mex. 2011;76:322-329. [PubMed] |
| 73. | Mansour-Ghanaei F, Sanaei O, Joukar F. Clinical Validation of an Office-Based C-UBT (Heliprobe) for H. pylori Diagnosis in Iranian Dyspeptic Patients. Gastroenterol Res Pract. 2011;2011:930941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Ozdemir E, Karabacak NI, Degertekin B, Cirak M, Dursun A, Engin D, Unal S, Unlü M. Could the simplified (14)C urea breath test be a new standard in noninvasive diagnosis of Helicobacter pylori infection? Ann Nucl Med. 2008;22:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Rasool S, Abid S, Jafri W. Validity and cost comparison of 14carbon urea breath test for diagnosis of H Pylori in dyspeptic patients. World J Gastroenterol. 2007;13:925-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Gurbuz AK, Ozel AM, Narin Y, Yazgan Y, Baloglu H, Demirturk L. Is the remarkable contradiction between histology and 14C urea breath test in the detection of Helicobacter pylori due to false-negative histology or false-positive 14C urea breath test? J Int Med Res. 2005;33:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | González P, Galleguillos C, Massardo T, Rivera M, Morales A, Smok G, Moyano L, Pimentel C, Alay R, Otárola S. Could the [14C]urea breath test be proposed as a 'gold standard' for detection of Helicobacter pylori infection ? Med Sci Monit. 2003;9:CR363-CR368. [PubMed] |
| 78. | Oztürk E, Yeşilova Z, Ilgan S, Ozgüven M, Dağalp K. Performance of acidified 14C-urea capsule breath test during pantoprazole and ranitidine treatment. J Gastroenterol Hepatol. 2009;24:1248-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Gomes AT, Coelho LK, Secaf M, Módena JL, Troncon LE, Oliveira RB. Accuracy of the 14C-urea breath test for the diagnosis of Helicobacter pylori. Sao Paulo Med J. 2002;120:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Desroches JJ, Lahaie RG, Picard M, Morais J, Dumont A, Gaudreau C, Picard D, Chartrand R. Methodological validation and clinical usefulness of carbon-14-urea breath test for documentation of presence and eradication of Helicobacter pylori infection. J Nucl Med. 1997;38:1141-1145. [PubMed] |
| 81. | Allardyce RA, Chapman BA, Tie AB, Burt MJ, Yeo KJ, Keenan JI, Bagshaw PF. 37 kBq 14C-urea breath test and gastric biopsy analyses of H. pylori infection. Aust N Z J Surg. 1997;67:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Faigel DO, Childs M, Furth EE, Alavi A, Metz DC. New noninvasive tests for Helicobacter pylori gastritis. Comparison with tissue-based gold standard. Dig Dis Sci. 1996;41:740-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Goh KL, Parasakthi N, Peh SC, Ong KK. 14C-urea breath test: a useful non-invasive test in the diagnosis of Helicobacter pylori infection. Med J Malaysia. 1995;50:208-211. [PubMed] |
| 84. | Kao CH, Huang CK, Wang SJ, Hsu CY, Lin WY, Chen GH. Accuracy of a rapid 10-minute carbon-14 urea breath test for the diagnosis of Helicobacter pylori-associated peptic ulcer disease. Eur J Nucl Med. 1993;20:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang YQ, China S-Editor: Lin C L-Editor: A P-Editor: Zhao YQ