Published online Dec 28, 2024. doi: 10.3748/wjg.v30.i48.5162
Revised: September 17, 2024
Accepted: October 22, 2024
Published online: December 28, 2024
Processing time: 125 Days and 6.1 Hours
Diagnosing laryngopharyngeal reflux (LPR) is challenging due to overlapping symptoms. While proton pump inhibitors (PPIs) are commonly prescribed, reliable predictors of their responsiveness are unclear. Reflux monitoring technologies like dual potential of hydrogen (pH) sensors and multichannel intraluminal impedance-pH (MII-pH) could improve diagnosis. Research suggests that a composite pH parameter, defined by ≥ 2 pharyngeal acid reflux (PAR) episodes and/or excessive esophageal acid reflux (EAR), predicts PPI efficacy. The criteria for PAR episodes, a pharyngeal pH drop of ≥ 2 units to < 5 within 30 seconds during esophageal acidification, showed strong interobserver reliability. We hypothesized that PAR episodes alone might also predict PPI responsiveness.
To investigate whether PAR episodes alone predict a positive response to PPI therapy.
Patients suspected of having LPR were prospectively recruited from otolaryngologic clinics in three Taiwanese tertiary centers. They underwent a 24-hour esophagopharyngeal pH test using either 3-pH-sensor or hypopha
A total of 522 patients (mean age 52.3 ± 12.8 years, 54% male) were recruited. Of these, 190 (mean age 51.5 ± 12.4 years, 61% male) completed the treatment, and 89 (47%) responded to PPI therapy. Response rates were highest in the PAR alone group (73%, n = 11), followed by EAR alone (59%, n = 68), both pH (+) (56%, n = 18), and both pH (-) (33%, n = 93). Multivariate analysis adjusting for age, sex, body mass index, and endoscopic esophagitis showed that participants with PAR alone, EAR alone, and both pH (+) were 7.4-fold (P = 0.008), 4.2-fold (P = 0.0002), and 3.4-fold (P = 0.03) more likely to respond to PPI therapy, respectively, compared to the both pH (-) group. Secondary analyses using the definition of ≥ 1 PAR episode were less robust.
In the absence of proven hypopharyngeal predictors, this post-hoc analysis found that baseline ≥ 2 PAR episodes alone are linked to PPI responsiveness, suggesting the importance of hypopharyngeal reflux monitoring.
Core Tip: This study examines the link between pharyngeal acid reflux (PAR) episodes and the effectiveness of proton pump inhibitor (PPI) therapy in laryngopharyngeal reflux (LPR) patients. Using specific potential of hydrogen (pH) criteria for PAR episodes detected by hypopharyngeal multichannel intraluminal impedance-pH, researchers found that patients with ≥ 2 baseline PAR episodes had a significantly higher response rate (73%) to PPI therapy compared to those without acidic reflux (33%). These findings underscore the importance of hypopharyngeal reflux monitoring, as PAR episodes appear to be crucial in predicting PPI efficacy. Hence, the authors recommend a personalized approach to LPR diagnosis and treatment in order to enhance patient outcomes.
- Citation: Chen YY, Wang CC, Chuang CY, Tsou YA, Peng YC, Chang CS, Lien HC. Link between pharyngeal acid reflux episodes and the effectiveness of proton pump inhibitor therapy. World J Gastroenterol 2024; 30(48): 5162-5173
- URL: https://www.wjgnet.com/1007-9327/full/v30/i48/5162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i48.5162
Laryngopharyngeal reflux (LPR) is a prevalent condition characterized by the backflow of stomach contents into the larynx and pharynx, leading to symptoms such as chronic cough, throat clearing, and hoarseness[1]. Diagnosing LPR poses significant challenges due to its overlapping symptoms with other upper respiratory conditions and the lack of a definitive diagnostic gold standard[2]. These challenges complicate the identification of effective treatments and contribute to ongoing difficulties in managing the condition[3].
Proton pump inhibitors (PPIs) are commonly used as a first-line treatment for LPR, but their effectiveness has been debated. Despite their widespread use, the high cost and inconsistent patient responses to PPIs raise concerns about their overall efficacy[4]. Advanced reflux monitoring techniques, such as multichannel intraluminal impedance-potential of hydrogen (MII-pH) and hypopharyngeal MII-pH (HMII-pH), have been introduced to improve diagnosis and better identify patients who may benefit from PPI therapy[5,6]. However, identifying reliable predictors of therapeutic success remains an ongoing challenge[7].
Recent studies have highlighted the potential diagnostic value of pharyngeal acid reflux (PAR) episodes, particularly when a patient has ≥ 2 PAR episodes combined with excessive esophageal acid reflux (EAR)[8]. Defined by a significant pH drop of ≥ 2 units to below 5 within 30 seconds in the pharynx during esophageal acidification[9], PAR episodes have shown promise as a predictor of PPI response. Despite technological advancements, such as the use of HMII-pH to validate the aforementioned criteria[9] and the high accuracy of a deep learning artificial intelligence model for detecting these episodes[10], the role of PAR as an independent predictor of PPI therapy success remains underexplored. In this study, we hypothesized that response rates to PPI therapy would be higher in patients with ≥ 2 PAR episodes alone, compared to those of patients with normal acid exposure in both the esophagus and hypopharynx.
This study was a post-hoc analysis of data from a prospective multicenter cohort study previously conducted across three tertiary medical centers in Taiwan, including Taichung Veterans General Hospital, China Medical University Hospital, and Chung Shan Medical University Hospital. The comprehensive details of the study’s design, objectives, methodology, and protocols have been thoroughly documented elsewhere[8]. The study’s protocol received approval from the Institutional Review Board of Taichung Veterans General Hospital (Approval No. C06254-2) and was conducted in adherence to the Declaration of Helsinki principles.
Individuals aged 20 to 70 who presented at the otolaryngology departments of the involved hospitals from January 2010 through February 2019 were evaluated for inclusion. Eligibility was determined based on: (1) Experiencing major symptoms indicative of chronic laryngitis, including hoarseness, cough, throat clearing, a sensation of a lump in the throat, and throat pain of at least moderate severity lasting three months or more; and (2) Exhibiting laryngoscopic findings consistent with reflux, such as posterior laryngitis, edema, and erythema. Exclusion criteria were the presence of any diagnosed non-reflux-related conditions that could account for the symptoms (Supplementary Table 1).
Dr. Wang, an experienced laryngologist, performed nasolaryngoscopies on all study participants using a Pentax VNL-1171K device (Pentax, Tokyo, Japan) to identify laryngeal signs of reflux, quantified using the reflux finding score, and to exclude upper airway cancers. Additionally, each participant underwent an upper gastrointestinal endoscopy with Olympus GIFXQ-240 or GIFXQ-260 models (Olympus, Tokyo, Japan) to rule out malignancies and identify any signs of reflux esophagitis, classified as grade B or above by the Los Angeles classification.
Participants first had an interview, laryngoscopy, and upper gastrointestinal endoscopy to assess study eligibility. Those who qualified then underwent esophageal manometry with an 8-channel pneumohydraulic perfused manometric assembly (Dentsleeve Pty Ltd, Adelaide, South Australia) after fasting overnight. The station pull-through technique was used to measure resting pressures, and the upper esophageal sphincters (UES) and lower esophageal sphincters (LES) were located in a supine position.
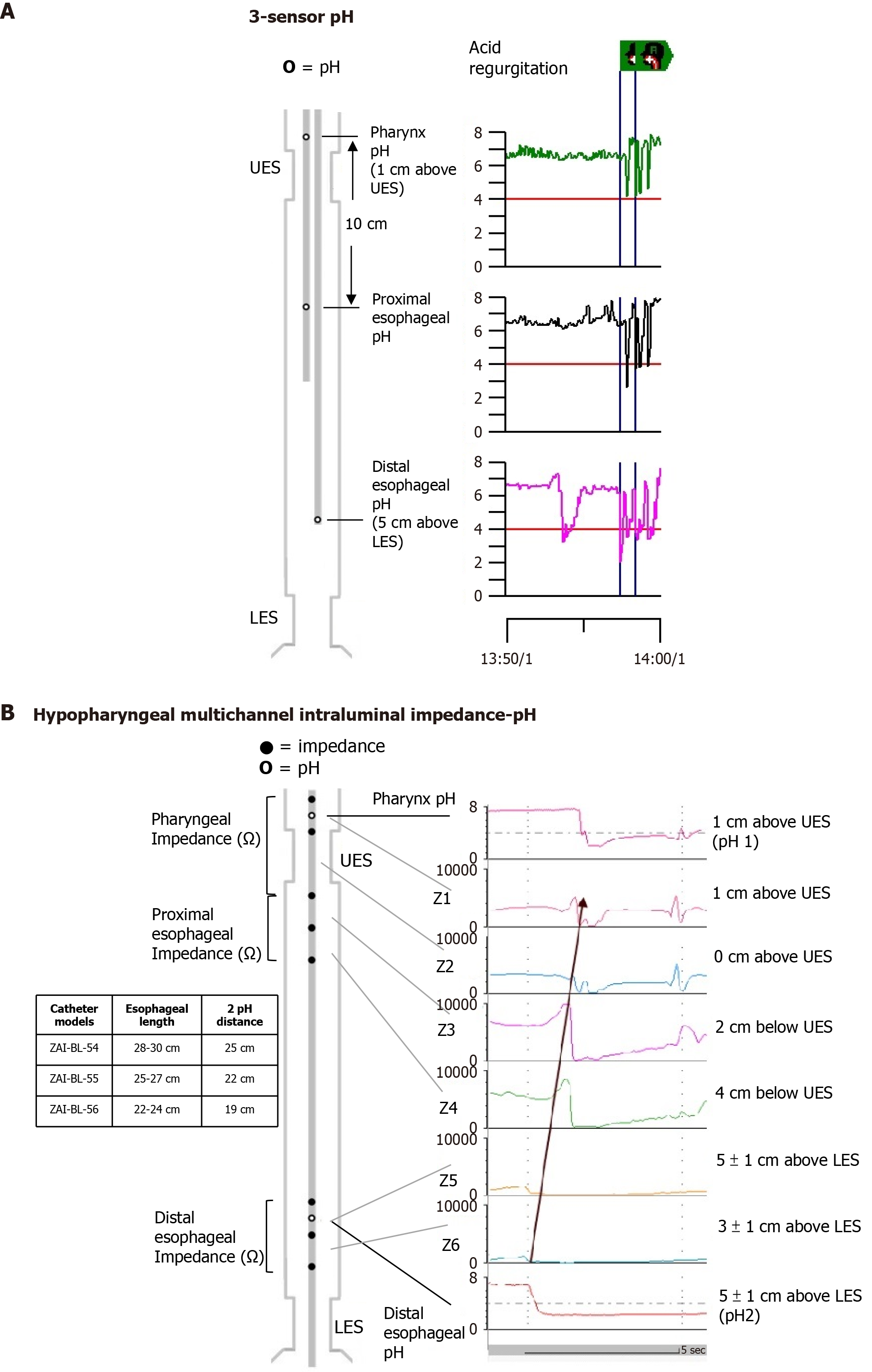
Primary esophageal peristalsis and acid sensitivity were evaluated by swallowing 5 mL of water ten times and undergoing the Bernstein test. For acid reflux monitoring, a 3-pH-sensor or a HMII-pH catheter was used after discontinuing PPIs for at least seven days. The placement of the hypopharyngeal pH sensor and esophageal pH sensor was determined by the manometric locations of UES and LES. The 3-pH-sensor catheters, featuring three antimony sensors within a bifurcated probe (Sandhill Scientific, Highlands Ranch, CO, United States), were configured with the proximal pH sensor placed 1 cm above the UES, the distal sensor 5 cm above the LES, and the middle sensor located 10 cm below the proximal sensor (Figure 1A). For HMII-pH monitoring, catheter size selection was based on esophageal length (models ZAI-BL-54, -55, and -56; Sandhill Scientific), allowing for precise positioning of the proximal pH probe 1 cm above the UES and the distal probe approximately 5 cm ± 1 cm above the LES. This setup positioned three pairs of impedance electrodes at the pharynx, proximal esophagus, and distal esophagus (Figure 1B).
The methodology for ambulatory simultaneous esophagopharyngeal reflux monitoring has been previously documented[8]. PAR episode analysis was independently conducted, with consensus reviews by two experienced specialists (Lien HC and Chen YY), who were unaware of patient details. The strict criteria for defining PAR episodes, with minor modifications from those proposed by Williams et al[11], required a decrease in pharyngeal pH by ≥ 2 units reaching a nadir of < 5 within 30 seconds during esophageal acidification. The rationale for using nadir pH < 5 instead of < 4 as the threshold was to increase diagnostic sensitivity and to minimize the impact of pepsin activity in damaging the laryngopharyngeal mucosa[12]. The 3-step method used to identify individual PAR episodes based on these criteria has demonstrated good interobserver reliability[9]. Additionally, 80% of the PAR signals are HMII-pH-proven PAR episodes, with strong interobserver reproducibility, as described previously[9]. Reflux episodes during meal times were excluded from the analysis.
For the 3-pH-sensor data, exclusions included irrelevant liquid swallows, slow pH drifts, isolated pharyngeal pH drops, and artifacts[13,14]. PAR episodes were more accurately identified using a proximal esophageal pH sensor for better reflux tracking[14]. With HMII-pH catheters, impedance sensors differentiated between retrograde PAR episodes and antegrade swallowing events. A PAR episode confirmed by HMII-pH was defined as a retrograde 50% decrease in baseline impedance, starting from the more distal esophageal channel (located 3 cm ± 1 cm above the upper margin of the LES) to the more proximal pharyngeal channel (situated 1 cm above the upper margin of the UES)[15], during the period of retrograde esophagopharyngeal pH drops[9]. Additionally, a PAR episode was only recognized if the nadir in both pharyngeal impedance sensors was less than 1200 ohms, preceded by a retrograde impedance drop in full column reflux of the esophagus, and if no swallow occurred during the pharyngeal impedance drop[16]. A PAR episode was abnormal if it occurred at least twice in a 24-hour period using either 3-pH-sensor or HMII-pH catheters[5,8,14]. Non-acid reflux episodes in the hypopharynx, particularly those with a pH greater than 5, may also contribute to symptom development[17] but were not evaluated in this study. This is partly due to overestimation by automated analyses[18] and the lack of consensus among experts on interpreting pharyngeal non-acid reflux[15], with around 70% being falsely identified as non-acid reflux[19]. Additionally, these episodes may be more relevant to anti-reflux surgery than to acid suppression therapy. Abnormal EAR was characterized by an excessive percentage of time with a pH < 4 in the distal esophagus, defined as ≥ 4.2% over 24 hours, ≥ 6.3% in an upright position, or ≥ 1.2% in a supine position[20].
After pH testing, participants were given Nexium (40 mg) (AstraZeneca Pharmaceuticals, Södertälje, Sweden) before breakfast and dinner for 12 weeks. Researchers and participants remained unaware of the pH test results. Adherence, side effects, and additional medication use were monitored during follow-ups at weeks 4, 8, and 12. Treatment success was defined as a ≥ 50% reduction in main laryngeal symptoms at these intervals[21]. Additionally, patient-reported outcomes were measured using the gastroesophageal reflux disease analyzer (GERDyzer) at the study’s start and end, a tool that assesses LPR-related quality of life using a 10-item scale[22].
Participants were divided into four groups based on reflux status: PAR alone, EAR alone, both pH (+), and both pH (-) (non-reflux controls). Group comparisons involved demographic, clinical, and physiological data using Kruskal-Wallis and χ2 tests for continuous and dichotomous variables, respectively. Outcomes were analyzed per protocol, adjusted for demographic and clinical factors, and included a sensitivity analysis using ≥ 1 PAR episode as a cut-off for pathological reflux. Multivariate logistic regression was used to identify predictors of a positive PPI response, with statistical significance set at P < 0.05.
Assuming response rates of 60% for participants with positive pH and 30% for those with negative pH based on previous data[8], a sample size of at least 150 achieves a statistical power of 96.5% with a composite pH (+) to pH (-) ratio of 1:1 in this study, at a significance level of 0.025 (https://clincalc.com/Stats/Sample-Size.aspx).
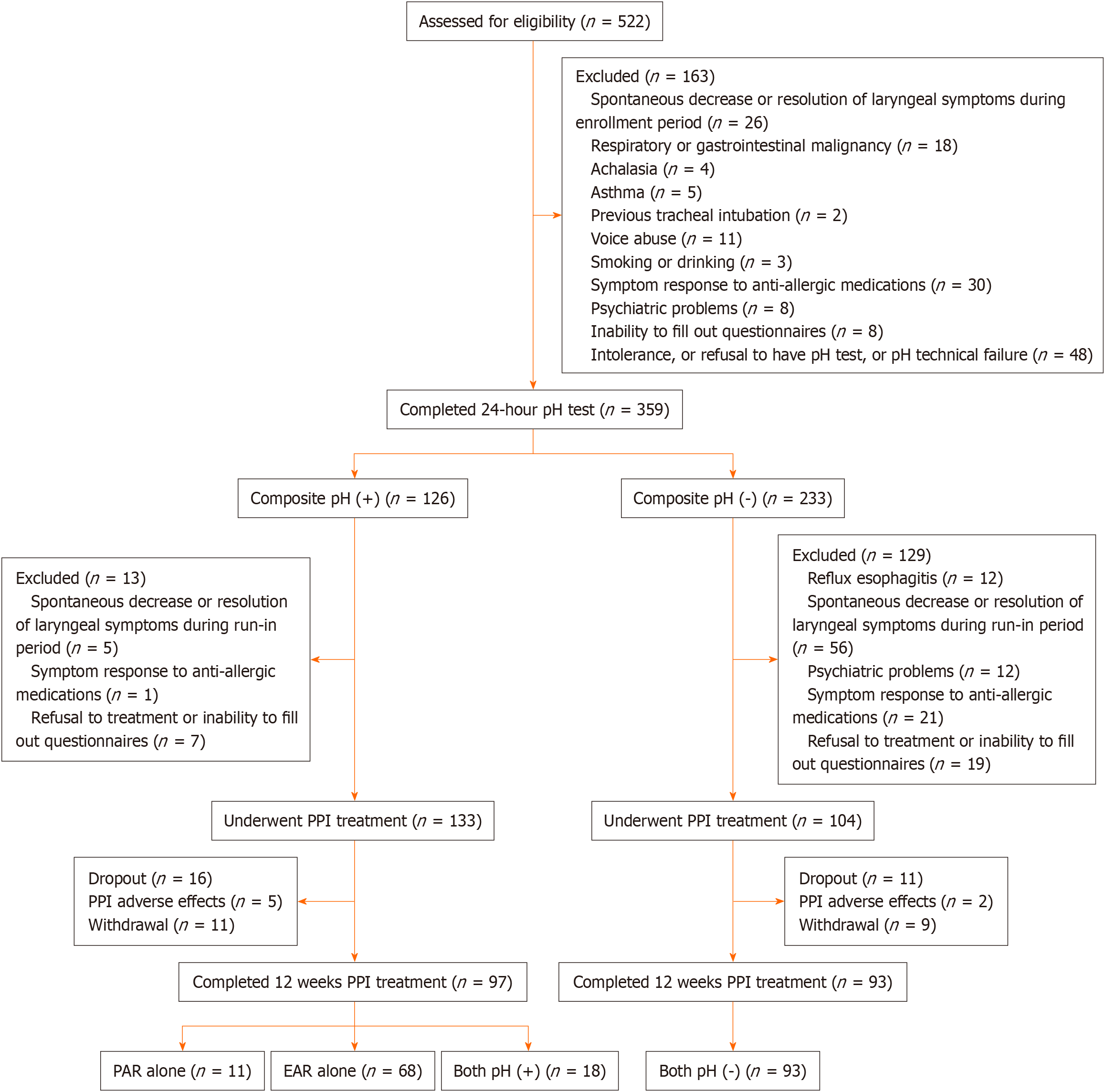
We enrolled 522 patients (mean age 52.3 ± 12.8 years, 54% male) from otolaryngologic clinics with suspected LPR. Following extensive exclusions due to non-reflux causes, refusal, intolerance, and ineligibility, 217 underwent esophagopharyngeal pH testing and esomeprazole treatment. Attrition included 27 participants due to dropout (n = 20) or adverse effects (n = 7) such as constipation, diarrhea, headache and dyspepsia. A total of 190 participants (mean age 51.5 ± 12.4 years, 61% male) completed the study, which involved either 3-pH-sensor (n = 93) or HMII-pH monitoring (n = 97) and the subsequent 12-week treatment course. Among them, 11 had PAR alone, 68 had EAR alone, 18 had both pH (+), and 93 had both pH (-) (Figure 2).
Participants’ baseline characteristics (Table 1) showed consistent age and body mass index (BMI) across groups, except for slightly younger age and lower BMI in the PAR alone and both pH (-) groups. Sex distribution varied, with the EAR alone group having a higher male percentage (74%) and the PAR alone group having a lower one (36%). Those with both pH (+) were less likely to seek otolaryngologist care (50%) and had longer symptom duration. Primary laryngeal symptoms, acid suppressive therapy history, and comorbidities were similar across groups. Cough was predominant in the PAR alone group, while heartburn was more common in the both pH (+) group.
| Characteristic | PAR1 alone | EAR2 alone | Both pH (+) | Both pH (-) | P value (4-group comparison) |
| Demographics | |||||
| Age in years, n (%) | 47.2 (16.4) | 53.9 (10.8)9 | 54.8 (15.5) | 49.7 (12.1) | 0.1 |
| Male sex, n (%) | 4 (36)11 | 50 (74)9 | 11 (61) | 50 (54) | 0.03 |
| BMI in kg/m2, (n (%) | 22.7 (2.7)12 | 24.7 (3.8)9 | 25.0 (2.9) | 23.5 (3.5) | 0.08 |
| ENT first visit, n (%) | 9 (82) | 50 (74) | 9 (50)10 | 76 (82) | 0.03 |
| Clinical presentations | |||||
| Major laryngeal symptom, n (%) | |||||
| Globus sensation, n (%) | 1 (9) | 17 (25) | 6 (33) | 26 (28) | 0.5 |
| Throat pain, n (%) | 2 (18) | 18 (26) | 4 (22) | 18 (19) | 0.7 |
| Hoarseness, n (%) | 3 (27) | 18 (26) | 5 (28) | 30 (32) | 0.9 |
| Cough, n (%) | 5 (45)8 | 11 (16) | 2 (11) | 8 (9) | 0.008 |
| Throat clearing, n (%) | 0 (0) | 3 (4) | 1 (6) | 11 (12) | 0.2 |
| Symptom duration in months, median (IQR) | 13 (4, 24)12 | 18 (7, 48)13 | 30 (13, 90)10 | 12 (6, 36) | 0.03 |
| Typical reflux symptoms3, n (%) | 5 (45) | 34 (50) | 13 (72) | 47 (51) | 0.4 |
| Previous acid suppressive therapy use, n (%) | 5 (45) | 43 (63) | 13 (72) | 52 (57) | 0.3 |
| Diabetes mellitus, n (%) | 1 (9) | 2 (3) | 1 (6) | 5 (5) | 0.8 |
| Hypertension, n (%) | 1 (9) | 16 (24) | 3 (17) | 17 (18) | 0.5 |
| Post nasal drip, n (%) | 4 (36) | 29 (43) | 9 (50) | 39 (42) | 0.9 |
| Endoscopic findings | |||||
| Reflux esophagitis, n (%) | < 0.0001 | ||||
| No reflux esophagitis, n (%) | 4 (36) | 15 (22) | 4 (22) | 28 (30) | |
| Grade A, n (%) | 4 (36) | 36 (53) | 11 (61) | 65 (70) | |
| Grade B, n (%) | 3 (27) | 11 (16) | 3 (17) | 0 (0.0) | |
| Grade C, n (%) | 0 (0) | 6 (9) | 0 (0) | 0 (0) | |
| Grade D, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Barrett’s esophagus, n (%) | 0 (0) | 5 (7)9 | 2 (11)10 | 0 (0) | 0.03 |
| Hiatus hernia, n (%) | 1 (9) | 11 (16) | 5 (28)10 | 7 (8) | 0.08 |
| Peptic ulcer, n (%) | 3 (27) | 5 (7) | 2 (11) | 13 (14) | 0.2 |
| Helicobacter pylori, n (%) | 3 (27) | 12 (18) | 5 (28) | 14 (15) | 0.6 |
| Reflux finding score4, median (IQR) | 6 (3, 11) | 7 (5, 9) | 5 (3, 7) | 7 (4, 10) | 0.4 |
| Patient report outcome, median (IQR) | |||||
| Reflux symptom index total score5, median (IQR) | 16 (11, 20) | 16 (12, 21) | 20 (10, 28) | 16 (11, 22) | 0.5 |
| GERDyzer total score6, median (IQR) | 45 (31, 49) | 38 (19, 49) | 35 (20, 50) | 36 (25, 50) | 0.7 |
| Heartburn frequency7, median (IQR) | 1 (0, 4) | 2 (0, 4) | 3 (1, 4)10 | 1 (0, 3) | 0.1 |
| Heartburn severity7, median (IQR) | 2 (0, 3) | 2 (0, 3) | 3 (1, 4) | 2 (0, 3) | 0.2 |
| Acid regurgitation frequency7, median (IQR) | 3 (1, 4) | 2 (0, 4) | 3 (1, 4) | 2 (1, 4) | 0.8 |
| Acid regurgitation severity7, median (IQR) | 2 (1, 4) | 2 (0, 4) | 3 (1, 4) | 3 (1, 3) | 0.8 |
Reflux esophagitis (Los Angeles classification) occurred in one-sixth to one-fourth of participants with pH abnormalities. Barrett’s esophagus and hiatal hernia prevalence was highest in the both pH (+) group, followed by EAR alone, PAR alone, and both pH (-) control groups. Reflux finding score items were similar among groups, except for slight differences between EAR alone and both pH (-) for subglottic edema and thick endolaryngeal mucus (Supplementary Table 2).
Among the 190 participants who completed treatment, 89 (47%) responded to PPI therapy. Univariate logistic regression revealed significant baseline predictors of PPI response (Supplementary Table 3). After adjusting for age, sex, BMI, and reflux esophagitis, the PAR alone group showed a higher PPI response (73%) compared to the both pH (-) controls group (33%), with an adjusted odds ratio (aOR) of 7.4 [95% confidence interval (CI): 1.7-32.7; P = 0.008]. Similar trends were seen in the EAR alone group (59% vs 33%; aOR = 4.2; 95%CI: 2.0-8.8; P = 0.0002) and the both pH (+) groups (56% vs 33%; aOR = 3.4; 95%CI: 1.1-10.0; P = 0.03) (Table 2, Supplementary Table 3).
| Outcome | PAR1 alone | EAR2 alone | Both pH (+) | Both pH (-) | P value (4-group comparison) |
| Week 4 | |||||
| Symptom improvement, median (IQR) | 40 (5, 70)3 | 30 (0, 60)4 | 30 (0, 40)5 | 0 (0, 30) | 0.001 |
| ≥ 50% improvement, n (%) | 5 (45) | 28 (41)4 | 4 (22) | 19 (20) | 0.02 |
| Change of the GERDyzer total score, median (IQR) | -11 (-21, -3) | -14 (-26, -1)4 | -9 (-19, -4) | -5 (-12, 0) | 0.02 |
| Week 8 | |||||
| Symptom improvement, median (IQR) | 70 (20, 80)3 | 50 (30, 80)4 | 35 (0, 60) | 20 (0, 50) | 0.0007 |
| ≥ 50% improvement, n (%) | 8 (73)3 | 40 (59)4 | 7 (39) | 31 (33) | 0.003 |
| Change of the GERDyzer total score, median (IQR) | -23 (-39, -13)3 | -18 (-28, -1)4 | -18 (-23, -10) | -8 (-16, 0) | 0.006 |
| Week 12 | |||||
| Symptom improvement, median (IQR) | 85 (20, 99)3 | 60 (10, 85)4 | 50 (0, 90) | 30 (0, 60) | 0.002 |
| ≥ 50% improvement, n (%) | 8 (73)3 | 40 (59)4 | 10 (56) | 31 (33) | 0.003 |
| Change of the GERDyzer total score, median (IQR) | -26 (-28, -19)3 | -20 (-30, -3)4 | -16 (-26, -8) | -9 (-18, -1) | 0.01 |
Improvement in individual laryngeal symptoms and typical reflux symptom scores also varied among groups (Supplementary Table 4 and Supplementary Table 5), with significant improvements of 80% (4/5) and 73% (8/11) noted in cough symptoms in the PAR alone and EAR alone groups, respectively. Sensitivity analysis indicated a response rate of 53% in the PAR alone group using a ≥ 1 PAR episode as a pathological cut-off (Supplementary Table 6).
The GERDyzer scores showed that the PAR alone group had significant post-treatment quality of life improvements compared to the both pH (-) control group. Similar positive trends were also seen in the EAR alone and both pH (+) groups (Supplementary Figure 1).
The PAR alone group showed significantly lower acid exposure time (%AET) across all positions compared to the EAR alone or both pH (+) groups, with no notable differences from the both pH (-) group, except for a higher %AET in the supine position (Table 3). The EAR alone and both pH (+) groups recorded more acid reflux events in the distal esophagus than the both pH (-) group, with higher numbers also compared to PAR alone using 3-pH-sensor (Supplementary Table 7). In proximal recordings, the PAR alone and both pH (+) groups had higher reflux event numbers than the both pH (-) group, while the EAR alone group’s numbers were higher only in the HMII-pH system (Supplementary Table 7).
| Finding | PAR1 alone | EAR2 alone | Both pH (+) | Both pH (-) | P value (4-group comparison) |
| 24-hour pH findings | |||||
| Distal esophagus | |||||
| Total time pH < 4, median (IQR) | 1.2 (0.9, 2.1)6,7 | 6.1 (4.5, 8.5)4 | 7.6 (4.7, 11.9)5 | 0.6 (0.1, 1.5) | < 0.0001 |
| Upright time pH < 4, median (IQR) | 2.2 (1.3, 3.4)6,7 | 8.1 (6.1, 12.1)4 | 9.1 (7.4, 14.3)5 | 0.9 (0.2, 2.3) | < 0.0001 |
| Supine time pH < 4, median (IQR) | 0.0 (0.0, 0.2)3,6,7 | 1.3 (0.0, 4.5)4 | 1.4 (0.2, 10.1)5 | 0.0 (0.0, 0.0) | < 0.0001 |
| Pharynx | |||||
| Number of PAR event, total, median (IQR) | 5 (2, 10)3,6 | 0 (0, 0)4,8 | 3 (2, 6)5 | 0 (0, 0) | < 0.0001 |
| Number of PAR event, upright, median (IQR) | 5 (2, 9)3,6 | 0 (0, 0)4,8 | 3 (2, 5)5 | 0 (0, 0) | < 0.0001 |
| Number of PAR event, supine, median (IQR) | 0 (0, 1)3,6 | 0 (0, 0)4,8 | 0 (0, 0)5 | 0 (0, 0) | 0.0002 |
| Manometric findings | |||||
| Lower esophageal sphincter, median (IQR), mmHg | 19 (15, 27)6,7 | 13 (10, 20)8 | 9 (7, 11)5 | 16 (10, 27) | 0.0002 |
| Upper esophageal sphincter, median (IQR), mmHg | 32 (22, 87) | 30 (18, 48)4,8 | 15 (7, 28)5 | 38 (25, 54) | 0.0002 |
| Ineffective esophageal motility, n (%) | 1 (14) | 14 (33) | 3 (27) | 13 (24) | 0.6 |
| Esophageal sensation | |||||
| Bernstein test, n (%) | 4 (36) | 21 (31)4 | 9 (50)5 | 14 (15) | 0.01 |
| Symptom index, n (%) | 3 (27) | 24 (35)4,8 | 12 (67)5 | 17 (18) | 0.0003 |
Manometric results showed the highest LES resting pressure in the PAR alone group, with no significant difference from the both pH (-) group (Table 3). UES resting pressures were similar between the PAR alone and both pH (-) groups, and were lowest in the both pH (+) group. There were no notable differences in ineffective esophageal motility across the groups. The EAR alone and both pH (+) groups had higher positive Bernstein test results compared to the both pH (-) group, with the PAR alone group showing a non-significant trend toward higher results.
The rate of a positive symptom index during 24-hour testing was highest in the both pH (+) group, with no significant difference between the PAR alone and both pH (-) groups.
Our study assessed the efficacy of PPI therapy in managing PAR and found a significant correlation between baseline PAR episodes and positive responses to PPI therapy. Using specific pH criteria for PAR episodes-a pharyngeal pH drop of ≥ 2 units to < 5 within 30 seconds during esophageal acidification detected by HMII-pH-patients with ≥ 2 PAR episodes alone (but not ≥ 1) showed a higher response rate to 12 weeks of esomeprazole treatment. Specifically, 73% experienced a ≥ 50% reduction in primary laryngeal symptoms, highlighting the unique pathophysiological role of PAR in LPR and the need for specialized diagnostic approaches.
The American College of Gastroenterology recently recommended upfront reflux testing before PPI therapy for patients suspected of having LPR but who lack typical symptoms. In particular, impedance-pH catheter use was encouraged[23,24]. Diagnosing PAR episodes is difficult due to a lack of consensus among experts[15]. Using the HMII-pH technique, which has a high sampling rate of 50 Hz, we examined the criteria for PAR episodes by tracking refluxate along the entire esophagus to the hypopharynx and found good reproducibility[9]. Moreover, 80% of PAR episodes detected by 3-pH-sensor signals can be identified by HMII-pH[9]. In the current study, we classified patients by acid reflux status and discovered that, although rare, PAR episodes correlated with cough symptoms (Table 1) and could predict PPI therapy outcomes (Supplementary Table 4). Our findings emphasize the value of monitoring both esophageal and hypopharyngeal reflux using a composite pH parameter to assess suspected LPR[8]. This approach challenges the diagnostic modality that relies solely on esophageal monitoring by demonstrating the potential of PAR episodes to predict PPI effectiveness.
Despite the suboptimal use of conventional side-hole water-perfused manometry in our study, we found significant differences in resting pressures of the LES and UES across four different reflux categories. These differences may partly explain the varying response rates to PPI therapy among the groups: the PAR alone group had the highest response, followed by EAR alone, both pH (+), and both pH (-) groups. The both pH (+) group had the lowest resting pressures of both LES and UES, which could contribute to esophageal and hypopharyngeal refluxate (Table 3). Although the PPI response rate was 56% in the both pH (+) group, i.e. significantly higher than the 33% in the both pH (-) group, it was lower than the 73% in the PAR alone group, which had the highest LES and UES pressures among the three abnormal pH groups. The suboptimal response to high-dose PPI therapy in the both pH (+) group compared to the PAR alone group may be partly due to irritation from a larger volume of non-acidic refluxate, which is not alleviated by PPI therapy. Although PAR episodes often occur alongside excessive pathological esophageal reflux, they do not always coincide[9]. The underlying mechanisms of the high PPI response rate in the PAR alone group needs further investigation. This phenomenon could be due to a small refluxate volume in the context of normal UES resting pressures, normal esophageal acid exposure, and potentially impaired UES reflexes[25,26], suggesting the possibility of a distinct pathophysiological phenotype.
In our cohort, a significant number of patients (n = 68) with pathological reflux who presented with EAR alone positively responded to PPI therapy for both LPR and typical reflux symptoms (Supplementary Table 5). This highlights the importance of monitoring distal esophageal reflux and challenges the earlier concept of relying solely on PAR episodes for diagnosing suspected LPR[27]. Finally, our findings support a vagally mediated reflexogenic mechanism in this subset of patients[8,28]. However, one possible explanation for the slightly lower, though not statistically significant, PPI response rate of 59% in the EAR alone group compared to 73% in the PAR alone group could be an underestimation of PAR episodes in the EAR alone group due to day-to-day variation, resulting in the misclassification of the both pH (+) group.
Our study has several merits. First, the presence of ≥ 2 PAR episodes alone might represent a distinct pathophysiological phenotype of LPR characterized by normal UES resting pressures and potentially impaired UES reflexes. This distinction could serve as a biomarker for identifying patients who are more likely to respond to PPI therapy. Second, including PAR episodes alone increased the sensitivity for predicting PPI responders from 56% to 65%. This suggests that relying solely on distal esophageal pH metrics may overlook patients with significant hypopharyngeal reflux. Third, the study findings underscore the need for HMII-pH technology. This implies that there needs to be a shift from traditional esophageal pH monitoring or MII-pH to a more comprehensive evaluation that includes hypopharyngeal reflux, ultimately leading to better-targeted therapies for LPR. Fourth, our findings suggest that a more tailored approach to LPR management is required. By identifying specific reflux phenotypes such as PAR alone, clinicians can tailor treatment strategies, potentially combining PPIs with other interventions to address each patient's unique pathophysiology. This personalized approach could lead to improved patient outcomes, avoid unnecessary treatments for those less likely to benefit, and result in more effective long-term management of LPR.
However, our study also has limitations. First, the post-hoc analysis study design in Taiwanese tertiary centers and the small sample size of patients with PAR alone may limit the robustness and generalizability of our findings. However, the latter may reflect the rarity of PAR episodes, which could still be clinically important in a small subset of patients. Second, the diagnostic criteria of PAR episodes using 3-pH-sensor and hypopharyngeal impedance-pH technologies have not been accepted universally, even though they have been validated with good inter-observer reproducibility. Third, the diagnostic criteria of PAR episodes used in this study may not fully capture the complexity of reflux in LPR. For instance, pharyngeal non-acid reflux with pH > 5 could contribute to symptoms[29], or measuring mean nocturnal baseline impedance from pH-impedance could enhance diagnostic accuracy[30,31], or prolonged wireless pH monitoring could improve the diagnostic yield of conclusive GERD[32]. Therefore, refined diagnostic protocols are needed to validate and extend our results.
Our study found a significant link between baseline PAR episodes and the effectiveness of PPI therapy in LPR patients. Patients with ≥ 2 PAR episodes alone showed a 73% response rate to esomeprazole and exhibited a distinct pathophysiological phenotype, suggesting that PAR could be a biomarker for predicting PPI response in the heterogeneous LPR population. Incorporating PAR monitoring using HMII-pH technology provides a more comprehensive evaluation of reflux, thereby improving the identification of PPI responders. These findings provide evidence in favor of developing tailored treatment strategies for LPR and underscore the importance of precise diagnostic protocols to enhance patient outcomes. Further research is needed to refine these criteria and deepen our understanding of the relationship between reflux and LPR symptoms.
We deeply appreciate Miss Chong for her secretarial work and the Biostatistics Task Force at Taichung Veterans General Hospital for their expert guidance. A part of this study was presented at Digestive Disease Week in 2023.
| 1. | Lechien JR, Vaezi MF, Chan WW, Allen JE, Karkos PD, Saussez S, Altman KW, Amin MR, Ayad T, Barillari MR, Belafsky PC, Blumin JH, Johnston N, Bobin F, Broadhurst M, Ceccon FP, Calvo-Henriquez C, Eun YG, Chiesa-Estomba CM, Crevier-Buchman L, Clarke JO, Dapri G, Eckley CA, Finck C, Fisichella PM, Hamdan AL, Hans S, Huet K, Imamura R, Jobe BA, Hoppo T, Maron LP, Muls V, O'Rourke AK, Perazzo PS, Postma G, Prasad VMN, Remacle M, Sant'Anna GD, Sataloff RT, Savarino EV, Schindler A, Siupsinskiene N, Tseng PH, Zalvan CH, Zelenik K, Fraysse B, Bock JM, Akst LM, Carroll TL. The Dubai Definition and Diagnostic Criteria of Laryngopharyngeal Reflux: The IFOS Consensus. Laryngoscope. 2024;134:1614-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 2. | Chen JW, Vela MF, Peterson KA, Carlson DA. AGA Clinical Practice Update on the Diagnosis and Management of Extraesophageal Gastroesophageal Reflux Disease: Expert Review. Clin Gastroenterol Hepatol. 2023;21:1414-1421.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 3. | Lechien JR, Akst LM, Hamdan AL, Schindler A, Karkos PD, Barillari MR, Calvo-Henriquez C, Crevier-Buchman L, Finck C, Eun YG, Saussez S, Vaezi MF. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol Head Neck Surg. 2019;160:762-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (35)] |
| 4. | Francis DO, Rymer JA, Slaughter JC, Choksi Y, Jiramongkolchai P, Ogbeide E, Tran C, Goutte M, Garrett CG, Hagaman D, Vaezi MF. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Hoppo T, Sanz AF, Nason KS, Carroll TL, Rosen C, Normolle DP, Shaheen NJ, Luketich JD, Jobe BA. How much pharyngeal exposure is "normal"? Normative data for laryngopharyngeal reflux events using hypopharyngeal multichannel intraluminal impedance (HMII). J Gastrointest Surg. 2012;16:16-24; discussion 24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Lechien JR. Clinical Update Findings about pH-Impedance Monitoring Features in Laryngopharyngeal Reflux Patients. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Krause AJ, Yadlapati R. Review article: Diagnosis and management of laryngopharyngeal reflux. Aliment Pharmacol Ther. 2024;59:616-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Lien HC, Wang CC, Kao JY, Yeh HZ, Hsu JY, Lee SW, Chuang CY, Tsou YA, Wang JD, Vaezi MF, Chang CS. Distinct Physiological Characteristics of Isolated Laryngopharyngeal Reflux Symptoms. Clin Gastroenterol Hepatol. 2020;18:1466-1474.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (35)] |
| 9. | Chen YY, Wang CC, Lin YC, Kao JY, Chuang CY, Tsou YA, Fu JC, Yang SS, Chang CS, Lien HC. Validation of Pharyngeal Acid Reflux Episodes Using Hypopharyngeal Multichannel Intraluminal Impedance-pH. J Neurogastroenterol Motil. 2023;29:49-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Fu J, Lee P, Wang C, Lin Y, Chuang C, Tsou Y, Chen Y, Yang S, Lien H. A cascade deep learning model for diagnosing pharyngeal acid reflux episodes using hypopharyngeal multichannel intraluminal Impedance-pH signals. Intelligence-Based Med. 2023;8:100131. [DOI] [Full Text] |
| 11. | Williams RB, Ali GN, Wallace KL, Wilson JS, De Carle DJ, Cook IJ. Esophagopharyngeal acid regurgitation: dual pH monitoring criteria for its detection and insights into mechanisms. Gastroenterology. 1999;117:1051-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Johnston N, Bulmer D, Gill GA, Panetti M, Ross PE, Pearson JP, Pignatelli M, Axford SE, Dettmar PW, Koufman JA. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Harrell SP, Koopman J, Woosley S, Wo JM. Exclusion of pH artifacts is essential for hypopharyngeal pH monitoring. Laryngoscope. 2007;117:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Maldonado A, Diederich L, Castell DO, Gideon RM, Katz PO. Laryngopharyngeal reflux identified using a new catheter design: defining normal values and excluding artifacts. Laryngoscope. 2003;113:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Zerbib F, Roman S, Bruley Des Varannes S, Gourcerol G, Coffin B, Ropert A, Lepicard P, Mion F; Groupe Français De Neuro-Gastroentérologie. Normal values of pharyngeal and esophageal 24-hour pH impedance in individuals on and off therapy and interobserver reproducibility. Clin Gastroenterol Hepatol. 2013;11:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Kawamura O, Aslam M, Rittmann T, Hofmann C, Shaker R. Physical and pH properties of gastroesophagopharyngeal refluxate: a 24-hour simultaneous ambulatory impedance and pH monitoring study. Am J Gastroenterol. 2004;99:1000-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Tutuian R, Mainie I, Agrawal A, Adams D, Castell DO. Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest. 2006;130:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Kang HJ, Park JM, Choi SY, Kim SI, Lee YC, Eun YG, Ko SG. Comparison Between Manual and Automated Analyses in Multichannel Intraluminal Impedance: pH Monitoring for Laryngopharyngeal Reflux. Otolaryngol Head Neck Surg. 2022;166:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Li JR, Wang JS, Wu MK, Zhao J, Guo HG. Classification of the non-acid laryngopharyngeal reflux. Chin Med J (Engl). 2020;134:984-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325-332. [PubMed] |
| 21. | Qadeer MA, Phillips CO, Lopez AR, Steward DL, Noordzij JP, Wo JM, Suurna M, Havas T, Howden CW, Vaezi MF. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:2646-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Wu CP, Liang WM, Wang CC, Chang CS, Yeh HZ, Hsu JY, Ko CW, Lee SW, Chang SC, Sung FC, Lien HC. The suitability of the GERDyzer instrument in pH-test-proven laryngopharyngeal reflux patients. Medicine (Baltimore). 2016;95:e4439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Gyawali CP, Carlson DA, Chen JW, Patel A, Wong RJ, Yadlapati RH. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol. 2020;115:1412-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 24. | Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol. 2022;117:27-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 579] [Article Influence: 144.8] [Reference Citation Analysis (1)] |
| 25. | Szczesniak MM, Williams RB, Brake HM, Maclean JC, Cole IE, Cook IJ. Upregulation of the esophago-UES relaxation response: a possible pathophysiological mechanism in suspected reflux laryngitis. Neurogastroenterol Motil. 2010;22:381-386, e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Babaei A, Venu M, Naini SR, Gonzaga J, Lang IM, Massey BT, Jadcherla S, Shaker R. Impaired upper esophageal sphincter reflexes in patients with supraesophageal reflux disease. Gastroenterology. 2015;149:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 422] [Article Influence: 17.6] [Reference Citation Analysis (36)] |
| 28. | Yadlapati R, Katzka DA. Laryngopharyngeal Reflux Is an Eternally Rolling Boulder. Clin Gastroenterol Hepatol. 2020;18:1431-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Carroll TL, Fedore LW, Aldahlawi MM. pH Impedance and high-resolution manometry in laryngopharyngeal reflux disease high-dose proton pump inhibitor failures. Laryngoscope. 2012;122:2473-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ribolsi M, Luca Guarino MP, Balestrieri P, Altomare A, Tullio A, Petitti T, Cicala M. The Results From Up-Front Esophageal Testing Predict Proton Pump Inhibitor Response in Patients With Chronic Cough. Am J Gastroenterol. 2021;116:2199-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Kurylo CM, Eastwood D, Blumin JH, Johnston N, Bock JM. Correlation of Esophageal Mean Nocturnal Baseline Impedance With Markers of Laryngopharyngeal Reflux. Laryngoscope. 2023;133:1927-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Krause AJ, Greytak M, Kaizer AM, Carlson DA, Chan WW, Chen CL, Gyawali CP, Jenkins A, Pandolfino JE, Polamraju V, Wong MW, Yadlapati R. Diagnostic Yield of Ambulatory Reflux Monitoring Systems for Evaluation of Chronic Laryngeal Symptoms. Am J Gastroenterol. 2024;119:627-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/