Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4958
Revised: October 25, 2024
Accepted: November 1, 2024
Published online: December 14, 2024
Processing time: 120 Days and 15.3 Hours
The presence of Helicobacter pylori (H. pylori) infection has been indicated to have a protective influence on esophageal cancer (EC) in some studies, but its specific impact on the risk of esophageal squamous cell carcinoma and esophageal adenocarcinoma remains inconclusive. This manuscript comment addresses the recent study by López-Gómez et al. Despite it was a retrospective observational study without a control group, this study revealed a notably low prevalence of H. pylori infection among EC patients, indicating a potential association between H. pylori and EC in Spain. It is important to note that the relationship between H. pylori and the risk of EC varies geographically. We also conducted a meta-analysis focusing on this association in Asian populations to offer precise clinical insights. However, no significant correlation between H. pylori infection and EC was identified, suggesting that the perceived protective effect of H. pylori against EC may have been overestimated in the Asian population.
Core Tip: Helicobacter pylori (H. pylori) has been implicated in the development of gastric cancer and its eradication is widely accepted as a treatment approach. However, recent research indicates that H. pylori may also play a role in maintaining the balance of gastroesophageal junction cells and could potentially have a protective effect against esophageal cancer (EC). Previous meta-analyses have shown that the relationship between H. pylori and the risk of esophageal squamous cell carcinoma or esophageal adenocarcinoma varies across different regions. This manuscript discusses a recent study by López-Gómez et al. Additionally, an updated meta-analysis focusing on the potential link between H. pylori and EC in Asian populations has been conducted to offer more precise clinical recommendations for countries like China and other Asian nations.
- Citation: Liu J, Liu YL. Should we pay more attention to the potential link between Helicobacter pylori and esophageal cancer in Asian countries. World J Gastroenterol 2024; 30(46): 4958-4963
- URL: https://www.wjgnet.com/1007-9327/full/v30/i46/4958.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i46.4958
Helicobacter pylori (H. pylori) is a type of Gram-negative bacterium that has been inhabiting the gastric epithelium of humans for over 58000 years, predating their migration from east Africa[1]. While this bacterium is present in approximately half of the global population, its prevalence in developed nations has been on the decline[2]. In 1994, H. pylori was classified as a group I carcinogen due to substantial evidence linking it to noncardia gastric adenocarcinoma[3]. H. pylori has the capability to induce gastric atrophy in humans, thereby elevating the risk of gastric cancer[4]. The esophageal cancer (EC) comprises two primary histological subtypes: (1) Esophageal squamous cell carcinoma (ESCC); and (2) Esophageal adenocarcinoma (EAC). However, the impact of H. pylori on the risk of developing ESCC and/or EAC remains a topic of ongoing investigation.
H. pylori infection has been suggested to exhibit a “protective effect” against EC. The prevalence of H. pylori has decreased in Western nations since the 20th century, coinciding with a rise in EC incidence. While prior meta-analyses have examined the association between these factors[5-8], conflicting viewpoints still exist. Currently, the precise relationship between H. pylori and EC remains unclear, with contradictory evidence regarding its potential protective or detrimental impact on EAC.
Recent publications have addressed the correlation between H. pylori and EC, offering new insights for further investigation. However, the specific relationship in Asian regions remains unclear. This manuscript discusses the article by López-Gómez et al[9] and performs a meta-analysis examining the association between H. pylori infection and EC risk in Asian populations. The aim of this manuscript is to provide targeted clinical recommendations for H. pylori eradication therapy in high-risk EC populations in China and other Asian nations.
A study conducted by López-Gómez et al[9] revealed a notably low prevalence of prior H. pylori infection among individuals diagnosed with esophageal carcinoma. Despite being a single-arm retrospective observational study lacking a control group, this research offers insights into a potential link between H. pylori and esophageal carcinoma in Spain from a unique perspective. A previous systematic review and meta-analysis[6] demonstrated a statistically significant relationship between H. pylori and ESCC in Western countries, although this analysis employed a broader definition of H. pylori positivity and included only four studies. Notably, another meta-analysis by Xie et al[7] suggested that H. pylori infection significantly reduced the risk of EAC in Western populations. Various hypotheses have been proposed to explain this association, including alterations in esophageal microbiota due to proton pump inhibitors (PPIs), H. pylori-induced atrophy and loss of acid parietal cells in the antrum, induction of apoptosis in gastric adenocarcinoma cells progressing from Barrett’s esophagus (BE) through the Fas apoptotic pathway, and reduction in ghrelin synthesis leading to secondary effects on central obesity and gastroesophageal reflux disease[10-12].
PPIs are frequently recommended for the treatment of acid-related gastrointestinal disorders and are a component of the multidrug regimen for eradicating H. pylori[13]. However, prolonged use of PPIs could alter the microbial composition in the esophagus, potentially contributing to the development of BE and EC, although conflicting findings have been reported[14]. A recent meta-analysis of 25 cohort studies from 23 publications identified an elevated risk of EC with PPI use[15]. Conversely, a meta-analysis encompassing 65 studies conducted by Zhang et al[16] did not find a significant association between PPI use and EC. Therefore, further robust evidence is required to ascertain the potential role of PPIs in the link between H. pylori infection and EC.
The prevalence of H. pylori infection varies significantly across different geographical regions. While H. pylori infection has been shown to reduce the risk of EAC in Western populations, no significant association was found in terms of the risk of ESCC when data from Eastern and Western populations were combined. Upon conducting a stratified analysis based on study location, no significant link between H. pylori infection and ESCC risk was observed in Western subjects. However, a notable association between H. pylori infection and a decreased risk of ESCC was identified in East Asian populations[7]. Furthermore, a meta-analysis[17] revealed no significant relationship between H. pylori infection and ESCC risk in the general population, while a lower risk was noted in the Middle East. Another meta-analysis[8] indicated that the presence of H. pylori infection and cytotoxin-associated gene A protein (CagA)-positive strains has been associated with a decreased risk of developing EAC in the general population, while no significant correlation between H. pylori infection/CagA-positive strains and ESCC was detected. It is worth noting that CagA-positive strains may demonstrate a positive correlation with ESCC in non-Asian populations and an inverse correlation in Asian populations.
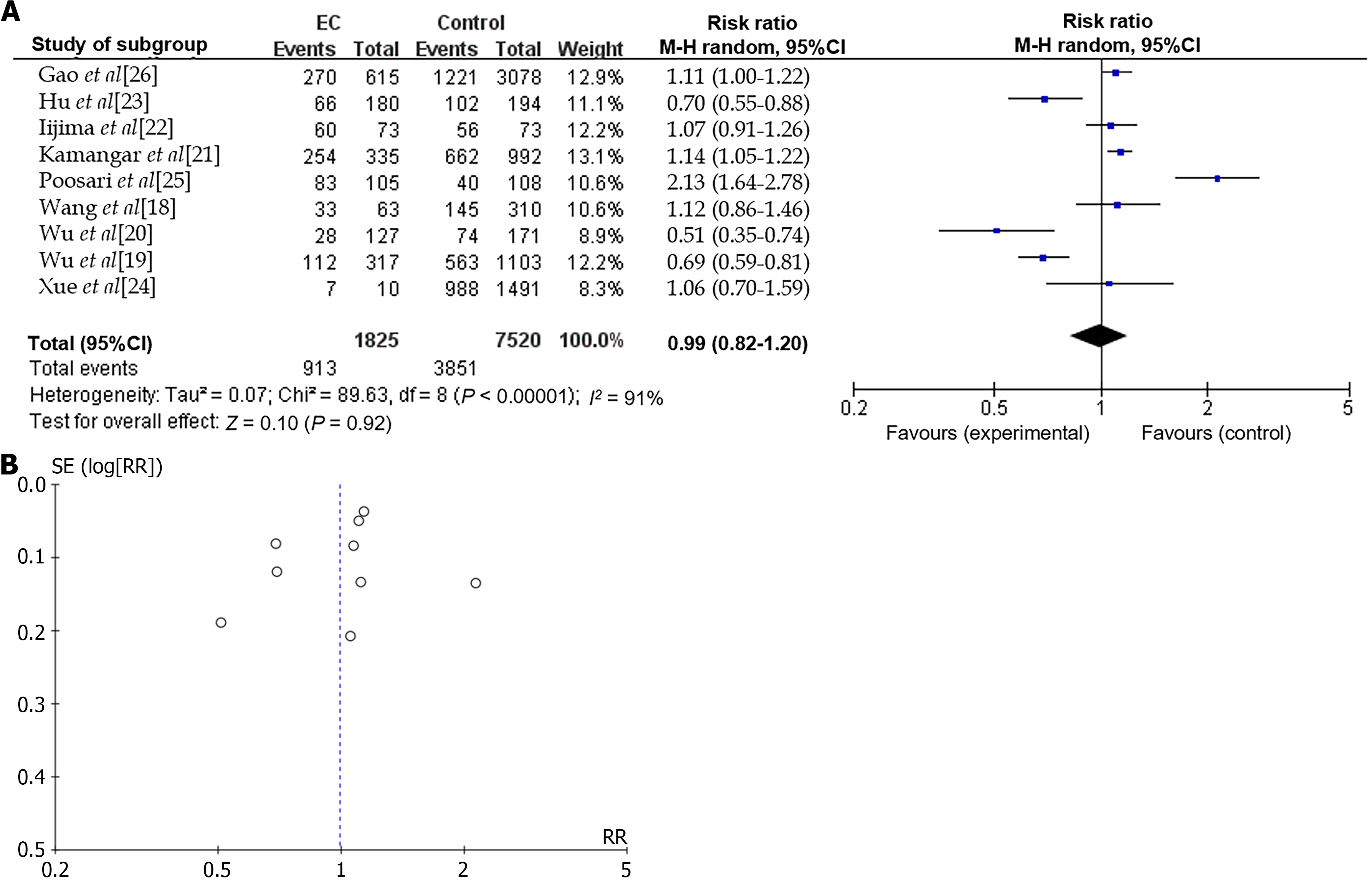
In this manuscript, a meta-analysis was also conducted to examine the relationship between H. pylori infection and EC (EAC and ESCC) in Asian populations. The aim of this updated meta analysis was to offer clinical recommendations regarding H. pylori eradication therapy for high-risk populations of EC in China and other Asian countries. Seven studies conducted in Asian countries were included to investigate the potential link between H. pylori infection and EC (Table 1)[18-26]. The results of the meta-analysis indicated that there was no statistically significant association between H. pylori infection and EC in the Asian population (risk ratio = 0.99, 95%CI: 0.82-1.20, P = 0.92) with significant heterogeneity I2 = 91% (Figure 1)[18-26].
| Ref. | Publication year | Study country | Study type | Study object | Case | Control | ||
| H. pylori + | H. pylori - | H. pylori + | H. pylori - | |||||
| Wang et al[18] | 2003 | China | Case-control | ESSC | 33 | 30 | 145 | 165 |
| Wu et al[19] | 2009 | China | Case-control | ESSC | 112 | 205 | 563 | 540 |
| Wu et al[20] | 2005 | China | Case-control | ESSC | 28 | 99 | 74 | 97 |
| Kamangar et al[21] | 2007 | China | Case-control | ESSC | 254 | 81 | 662 | 330 |
| Iijima et al[22] | 2007 | Japan | Case-control | ESSC | 60 | 13 | 56 | 17 |
| Hu et al[23] | 2009 | China | Case-control | ESSC | 66 | 114 | 102 | 92 |
| Xue et al[24] | 2013 | China | Cohort | ESSC | 7 | 3 | 988 | 503 |
| Poosari et al[25] | 2023 | Thailand | Case-control | Esophageal cancer | 83 | 22 | 40 | 68 |
| Gao et al[26] | 2022 | China | Case-control | Adenocarcinoma of the esophagogastric junction | 270 | 345 | 1221 | 1857 |
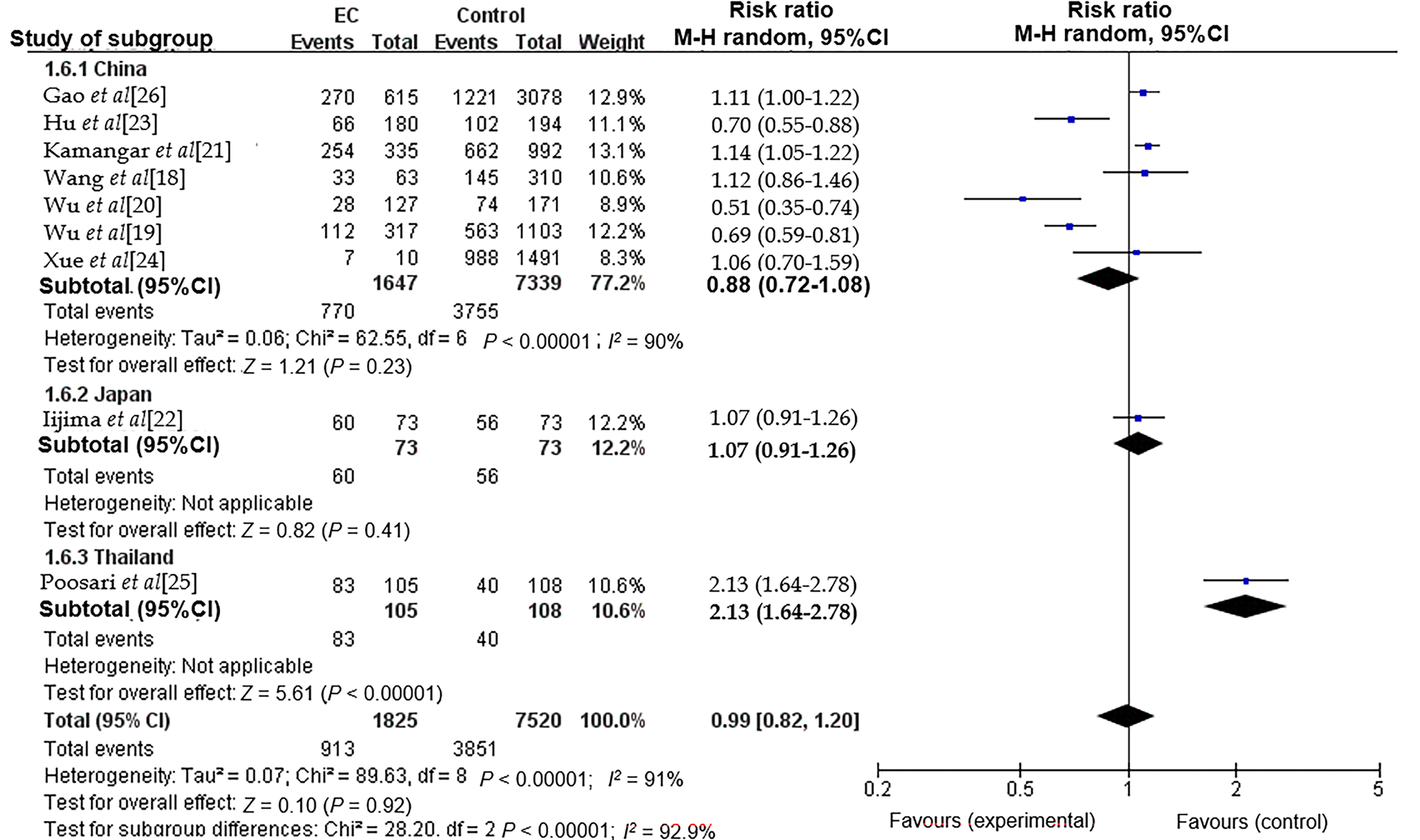
The association between H. pylori infection and EC exhibited significant heterogeneity, as indicated by an I2 value of 91%. This variability may be attributed to several factors, including differences in population characteristics, variations in the number of cases and controls, the methodologies employed for H. pylori detection, and the overall study design. We furtherly examined the possible causes of heterogeneity by using subgroup analysis. Subgroup analyses were performed based on different countries in the current investigation (Figure 2), while no significant reduction in heterogeneity was observed within the Asian population, consistent with previous meta-analysis[7]. Thus, further research is necessary to elucidate the underlying causes of this pronounced heterogeneity.
The relationship between the risk of ESCC/EAC and H. pylori infection demonstrates regional variability. While H. pylori infection may decrease the risk of EC in some countries, the perceived “protective” effect of this association has been found to be overestimated in Asian populations.
We thanks to Zhang X (Department of Gastroenterology, Lu'an Hospital of Anhui Medical university, Lu'an People's Hospital of Anhui Province) for his strong support in revising this letter.
| 1. | Malfertheiner P, Schulz C, Hunt RH. Helicobacter pylori Infection: A 40-Year Journey through Shifting the Paradigm to Transforming the Management. Dig Dis. 2024;42:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Chen YC, Malfertheiner P, Yu HT, Kuo CL, Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, Wu CY, Lin JT, O'Morain C, Megraud F, Lee WC, El-Omar EM, Wu MS, Liou JM. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology. 2024;166:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 278] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 3. | Lim MCC, Jantaree P, Naumann M. The conundrum of Helicobacter pylori-associated apoptosis in gastric cancer. Trends Cancer. 2023;9:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Mu T, Lu ZM, Wang WW, Feng H, Jin Y, Ding Q, Wang LF. Helicobacter pylori intragastric colonization and migration: Endoscopic manifestations and potential mechanisms. World J Gastroenterol. 2023;29:4616-4627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413-1417, 1417.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila). 2008;1:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 7. | Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, Shao L, Zou DH, Yu XM, Mao WM. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19:6098-6107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Nie S, Chen T, Yang X, Huai P, Lu M. Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus. 2014;27:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | López-Gómez M, Morales M, Fuerte R, Muñoz M, Delgado-López PD, Gómez-Cerezo JF, Casado E. Prevalence of Helicobacter pylori infection among patients with esophageal carcinoma. World J Gastroenterol. 2024;30:3479-3487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (7)] |
| 10. | Castaño-Rodríguez N, Goh KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7:15957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 11. | Doorakkers E, Lagergren J, Santoni G, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of Barrett's esophagus and esophageal adenocarcinoma. Helicobacter. 2020;25:e12688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Murphy G, Kamangar F, Albanes D, Stanczyk FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Dawsey SM, Freedman ND. Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut. 2012;61:1533-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett's oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16:2905-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Pei Z, Yang L, Peek RM, Jr Levine SM, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005;11:7277-7283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 15. | Tran TH, Myung SK, Trinh TTK. Proton pump inhibitors and risk of gastrointestinal cancer: A metaanalysis of cohort studies. Oncol Lett. 2024;27:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Zhang ML, Fan YX, Meng R, Cai WK, Yin SJ, Zhou T, Huang YH, Wang P, Jiang FF, Yang M, He GH. Proton Pump Inhibitors and Cancer Risk: An Umbrella Review and Meta-analysis of Observational Studies. Am J Clin Oncol. 2022;45:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Gao H, Li L, Zhang C, Tu J, Geng X, Wang J, Zhou X, Jing J, Pan W. Systematic Review with Meta-analysis: Association of Helicobacter pylori Infection with Esophageal Cancer. Gastroenterol Res Pract. 2019;2019:1953497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Wang KX, Wang XF, Peng JL, Cui YB, Wang J, Li CP. Detection of serum anti-Helicobacter pylori immunoglobulin G in patients with different digestive malignant tumors. World J Gastroenterol. 2003;9:2501-2504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wu IC, Wu DC, Yu FJ, Wang JY, Kuo CH, Yang SF, Wang CL, Wu MT. Association between Helicobacter pylori seropositivity and digestive tract cancers. World J Gastroenterol. 2009;15:5465-5471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Wu DC, Wu IC, Lee JM, Hsu HK, Kao EL, Chou SH, Wu MT. Helicobacter pylori infection: a protective factor for esophageal squamous cell carcinoma in a Taiwanese population. Am J Gastroenterol. 2005;100:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH, Perez-Perez GI, Abnet CC, Zhao P, Mark SD, Taylor PR, Dawsey SM. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer. 2007;96:172-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Iijima K, Koike T, Abe Y, Inomata Y, Sekine H, Imatani A, Nakaya N, Ohara S, Shimosegawa T. Extensive gastric atrophy: an increased risk factor for superficial esophageal squamous cell carcinoma in Japan. Am J Gastroenterol. 2007;102:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Hu HM, Kuo CH, Lee CH, Wu IC, Lee KW, Lee JM, Goan YG, Chou SH, Kao EL, Wu MT, Wu DC. Polymorphism in COX-2 modifies the inverse association between Helicobacter pylori seropositivity and esophageal squamous cell carcinoma risk in Taiwan: a case control study. BMC Gastroenterol. 2009;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Xue L, Xing L, Wang J, Shen H, Cui J, Mi J, Wang J, Misumi J, Zhang X. Serum pepsinogens and Helicobacter pylori are not associated with esophageal squamous cell carcinoma in a high-risk area in China. Tumori. 2013;99:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Poosari A, Nutravong T, Namwat W, Sa-Ngiamwibool P, Ungareewittaya P, Boonyanugomol W. Relationship between Helicobacter Pylori Infection and the Risk of Esophageal Cancer in Thailand. Asian Pac J Cancer Prev. 2023;24:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Gao P, Cai N, Yang X, Yuan Z, Zhang T, Lu M, Jin L, Ye W, Suo C, Chen X. Association of Helicobacter pylori and gastric atrophy with adenocarcinoma of the esophagogastric junction in Taixing, China. Int J Cancer. 2022;150:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/