Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4864
Revised: September 25, 2024
Accepted: November 4, 2024
Published online: December 14, 2024
Processing time: 208 Days and 3.8 Hours
Dysfunction of the intestinal barrier is a prevalent phenomenon observed across a spectrum of diseases, encompassing conditions such as mesenteric artery disse
Core Tip: Lipocalin-2, also referred to as neutrophil gelatinase-associated lipocalin or 24p3, plays a pivotal role in diverse physiological processes and pathological injuries. In this review, we delineate its structure and the mechanisms underlying its action, elucidate its multifaceted interplay with the immune environment and its relevance to gastrointestinal disorders, thereby providing insights into potential avenues using targeted therapies directed to this multifunctional protein.
- Citation: Zhang ZX, Peng J, Ding WW. Lipocalin-2 and intestinal diseases. World J Gastroenterol 2024; 30(46): 4864-4879
- URL: https://www.wjgnet.com/1007-9327/full/v30/i46/4864.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i46.4864
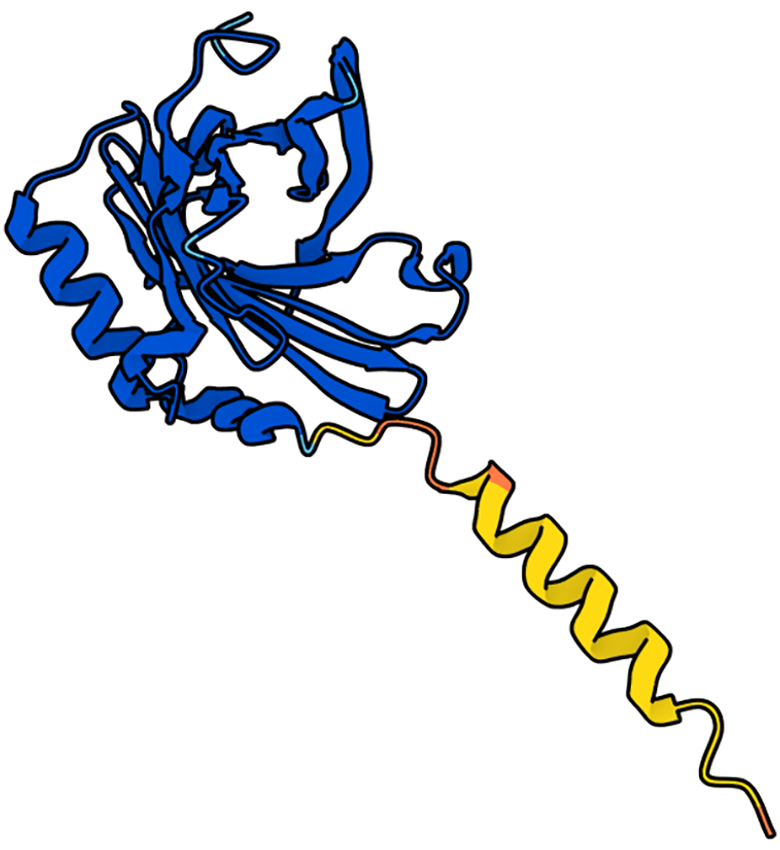
Lipocalin-2 (LCN2), also referred to as neutrophil gelatinase-associated lipocalin (NGAL) or 24p3, was initially identified in neutrophils in 1994 by Kjeldsen et al[1]. The human LCN2 protein shares homology with the rat 24p5-associated protein and the mouse 24p3 protein, resulting in a similar tertiary structure despite lower amino acid sequence identity[2]. LCN2 has a highly conserved structure characterized by an eight-stranded β-barrel, forming a closed calyx structure in the antiparallel direction, which serves as its ligand-binding site. This closed calyx structure of LCN2 is notably polarized, enabling it to bind to larger and more hydrophobic ligands, thus facilitating a wide array of complex biological functions[3].
In this comprehensive review, our objective is to delve deeply into the intricate role of LCN2 in immune regulation, elucidating its multifaceted functions within the immune system. Additionally, we explore the existing body of literature concerning LCN2 and its influence on intestinal barrier function, revealing its intricate involvement in maintaining barrier integrity and regulating permeability. Moreover, we will critically analyze the diverse roles attributed to LCN2 in a spectrum of intestinal diseases, thereby shedding light on its intricate contributions to disease pathogenesis and progression. By synthesizing these insights, we aim to provide a nuanced understanding of the complex interplay between LCN2 and intestinal health, thereby offering valuable insights into potential therapeutic strategies targeting this multifunctional protein.
LCN2 was initially isolated from neutrophils but has since been identified as being secreted by a variety of cell types, including adipocytes, hepatocytes, epithelial cells, macrophages, cardiomyocytes, and others[4-8]. In animals, LCN2 exists in various forms, such as monomers, homodimers, and heterodimers, each exerting distinct effects on cell proliferation, differentiation, and disease pathogenesis[9]. The functional diversity observed in different forms of LCN2 can be attributed to its ability to bind various ligands, forming complexes with unique functions[3]. For example, LCN2 is capable of binding to iron carrier proteins, and the presence or absence of iron within these complexes can profoundly alter their biological activity[10]. Moreover, LCN2 may interact with six distinct receptors, including NGAL receptor, low-density lipoprotein-related protein 2 (LRP2), LRP6, melanocortin 4 receptor (MC4R), MC1R, and MC3R[11]. However, the precise receptor-ligand interactions and the variable affinity of LCN2 for these receptors in different diseases or developmental processes remain incompletely understood. Furthermore, the downstream signaling pathways activated upon LCN2 receptor binding have not yet been fully elucidated. Further investigations into these aspects are crucial for obtaining a comprehensive understanding of LCN2-mediated cellular responses and their potential therapeutic implications in various pathological conditions. The crystal structure of LCN2 is demonstrated in Figure 1[12].
The elucidation of the LCN2 receptor and its downstream signaling mechanisms remains an active area of research[11]. In this review, we aim to provide a concise overview of how LCN2 interacts with its receptors and elicits various downstream cellular functions. The first identified LCN2 receptor, also known as solute carrier family 22 member 17 (SLC22A17) or NGAL receptor[13], has been associated with endocytosis, although the specific substrates and transport mechanisms involved have yet to be fully elucidated[14]. In cancer, for example, tumor cells exploit the interaction between LCN2 and SLC22A17 to scavenge iron from the iron-deficient environment of the cerebrospinal fluid, promoting their own growth while simultaneously impairing macrophage function[15]. In addition to SLC22A17, LRP2 has been identified as another receptor for LCN2. LRP2 binds to LCN2 on the surface of proximal tubular epithelial cells, facilitating its endocytosis and subsequent retrieval from the glomerular filtrate. This process ultimately leads to the degradation of LCN2[16]. Notably, blocking the recycling of LCN2 through LRP2-mediated endocytosis represents a potential therapeutic approach for the treatment of iron overload conditions caused by genetic disorders or repeated blood transfusions[17]. In summary, while significant progress has been made in understanding the interactions between LCN2 and its receptors, further research is warranted to delineate the precise mechanisms underlying LCN2-mediated cellular responses. Such insights hold promise for the development of novel therapeutic strategies targeting LCN2 signaling pathways in various pathological conditions.
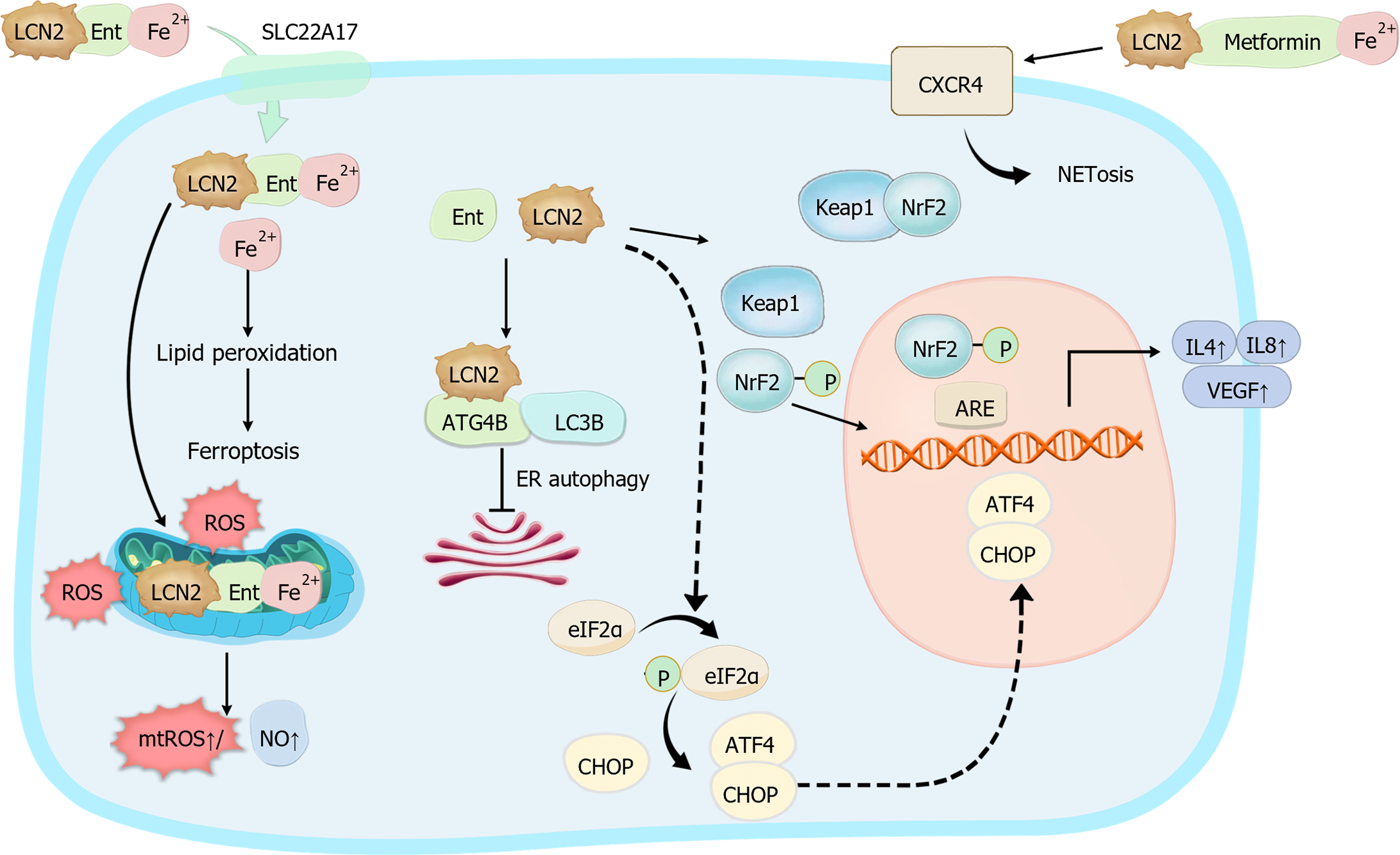
The iron transport capacity of LCN2 has been the subject of extensive investigation[9], yet numerous functional and mechanistic controversies persist. Recent advancements in cutting-edge scientific techniques, such as X-ray diffraction, sequencing, and proteomics analysis, have offered insights into the molecular mechanisms underlying LCN2-mediated iron ion transport. However, the complexity of iron transport pathways is further complicated by the formation of ternary complexes involving LCN2, iron ions, and small molecular weight iron carriers, which are chelating compounds produced and secreted by bacteria to sequester iron. In this intricate process, iron ions can bind to small molecular weight iron carriers associated with LCN2, resulting in the formation of ternary complexes comprising LCN2, the iron carrier, and iron ions[18]. Upon interaction with cell surface receptors, these ternary complexes are internalized via endocytosis, facilitating the release of bound iron into the cells and subsequently increasing the intracellular iron concentration. Recent studies have also revealed an additional layer of complexity, wherein metformin, which is conventionally known for its antidiabetic properties, can function as an iron carrier. Metformin forms a complex with iron and LCN2, further diversifying the repertoire of LCN2-associated iron transport mechanisms[19]. Conversely, binary complexes of LCN2 with iron carriers can also be internalized by cells, thereby translocating intracellular iron to the extracellular compartment and consequently reducing the intracellular iron content. These findings underscore the multifaceted nature of LCN2-mediated iron transport pathways and highlight the need for further research to elucidate the precise mechanisms governing these processes. These insights not only deepen our understanding of iron homeostasis but also have potential implications for the development of novel therapeutic strategies targeting iron-related disorders[20].
As a secreted protein, LCN2 is widely expressed across various cell types, and its pivotal role in innate immunity was initially recognized[21]. During bacterial infections, there is a notable increase in the plasma level of LCN2, which is primarily attributed to its secretion by immune cells. The antimicrobial mechanism of LCN2 involves the formation of ternary complexes with iron carrier proteins produced by bacteria, which sequester iron ions, thereby reducing the concentration of free iron ions in the plasma. This process effectively hampers bacterial uptake of iron, consequently inhibiting bacterial growth[22]. Furthermore, LCN2 exerts its antimicrobial effects through various mechanisms. It dose-dependently downregulates toll-like receptor 4 signaling, targeting the Janus kinase/signal transducer and activator of transcription (STAT) pathway, thus impeding bacterial infection[23]; additionally, LCN2 can bolster the host defense against bacterial pathogens by stimulating macrophages to produce reactive oxygen species (ROS) and nitric oxide, which are potent antimicrobial agents[24]. Notably, studies by Wang et al[25] demonstrated that in an Escherichia coli-induced infection model, LCN2 knockout mice exhibited disrupted neutrophil maturation and impaired migratory capacity. These effects may be attributed to the reduced production of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and chemokines such as monocyte chemoattractant protein-1[25]. Collectively, these findings underscore the multifaceted role of LCN2 in orchestrating the host immune response against bacterial infections, highlighting its importance as a potential therapeutic target in combating infectious diseases.
Indeed, LCN2 performs context-dependent functions on the basis of its cellular source and specific physiological or pathological conditions. In bacterial-induced inflammation, LCN2 secreted by neutrophils and hepatocytes primarily acts to combat infection, demonstrating an anti-infective role[26,27]. Conversely, during kidney injury, LCN2 production by macrophages and renal epithelial cells is induced by cytokines such as interleukin (IL)-10 and IL-1β, facilitating renal cell regeneration and mitigating kidney injury[28,29]. However, the role of LCN2 in cancer is multifaceted. While LCN2 production by neutrophils and macrophages can exacerbate malignancy and promote tumor progression and metastasis in certain tumors[7,30], conflicting findings suggest a potentially inhibitory role in macrophage function, with exacerbation of inflammatory injury and contributions to conditions such as ulcerative colitis (UC), thereby exacerbating intestinal damage[31,32].
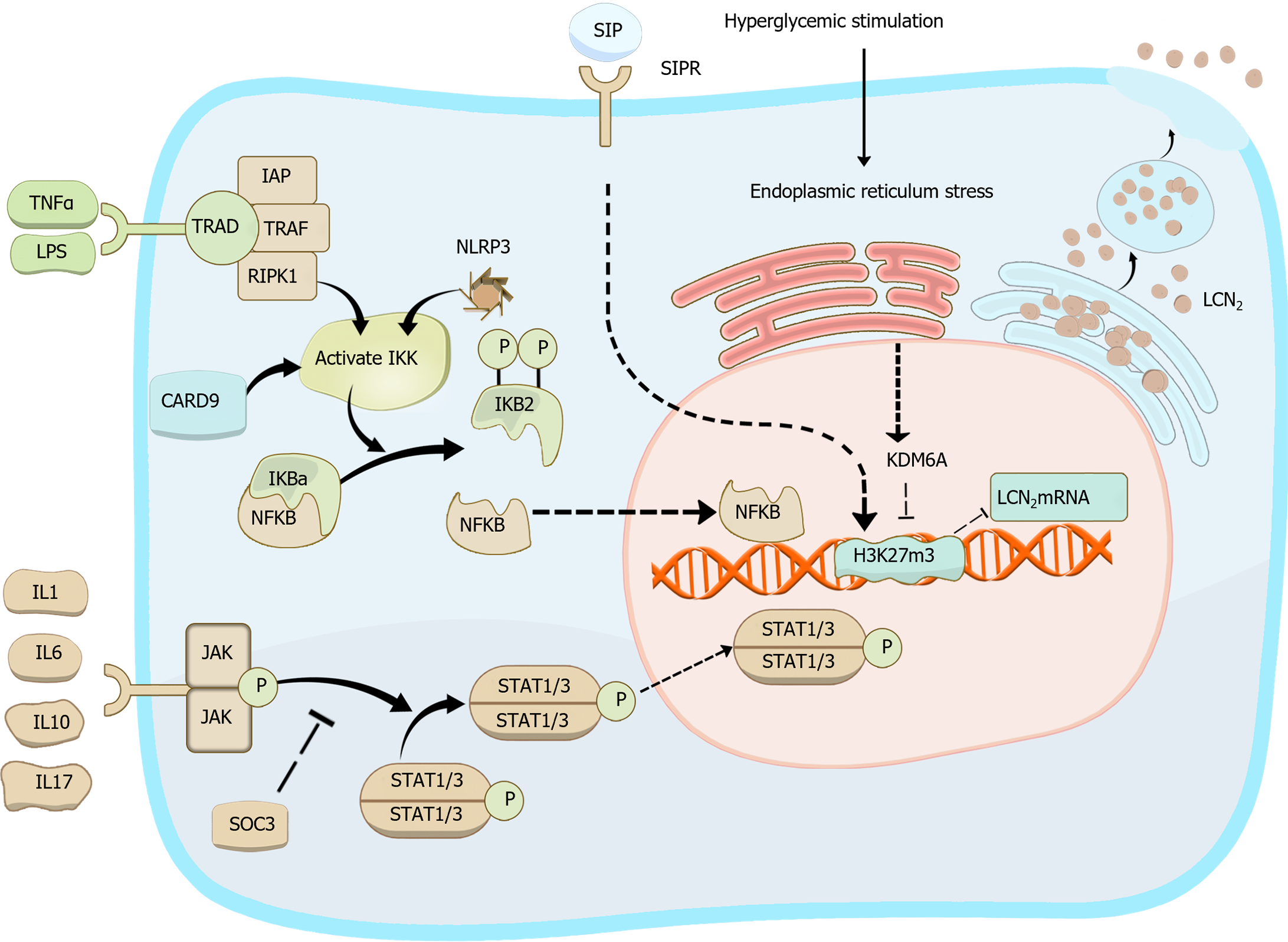
In this review, we delve more deeply into the intricate interactions between LCN2 and immune cells, including neutrophils, macrophages, T cells, and B cells. We aim to elucidate how this communication evolves in response to the cellular state and the dynamic disease microenvironment, drawing upon the latest literature and advances in LCN2 research. By synthesizing these insights, we seek to elucidate the specific mechanisms underpinning the complex and often paradoxical functions of LCN2. Additionally, we aim to outline the progress made in future research directions, thereby providing valuable insights into the evolving landscape of LCN2-mediated immune modulation and corresponding implications for therapeutic interventions in various disease contexts. Figure 2 illustrates the regulation of LCN2 biosynthesis and summarizes the influencing factors of LCN2 expression alterations across various models.
In the intricate landscape of immune regulation, LCN2 has emerged as a central player, engaging in complex interactions with various immune elements. Not only is LCN2 expression tightly regulated by a diverse array of immune cells and cytokines, but it also exerts regulatory effects on the levels of numerous cytokines, thus intricately modulating immune cell function. The signaling pathways mediated by LCN2 drive immune cell function in a highly context-dependent manner, resulting in cell type-specific responses under different physiological and pathological conditions. For example, LCN2 may promote proinflammatory responses in certain immune cells while exerting anti-inflammatory effects in others, highlighting the nuanced and dynamic nature of its immunomodulatory functions. Understanding the intricate crosstalk between LCN2 and immune elements holds significant promise for revealing the underlying mechanisms governing immune dysregulation in various disease states. By elucidating the latest insights into LCN2-mediated immune modulation, future research endeavors can pave the way for the development of targeted therapeutic strategies aimed at restoring immune homeostasis and ameliorating disease progression.
Neutrophils constitute the highest percentage of immune cells in the body[33] and play pivotal roles in conditions such as sepsis, tumors, ischemia-reperfusion injuries, and other inflammatory processes[34-36]. The interaction between LCN2 and neutrophils has been documented since the last century, with LCN2 initially isolated from secreted granules of neutrophils[1]. Recent findings underscore the close relationship between LCN2 and neutrophil activation and function[37]. Moreover, LCN2 produced by neutrophils exerts diverse effects on various organs and tissues, largely contingent upon the physiological state of the disease[30].
In sepsis resulting from various injuries, inflammatory factors stimulate neutrophils to produce and secrete LCN2[38]. Neutrophil-derived LCN2 forms an immune complex with Ent, effectively controlling infection by chelating iron[39]. However, increased expression of neutrophil LCN2 in the early stages of sepsis-associated acute respiratory distress syndrome has been correlated with poor patient prognosis and may also induce iron-mediated cell death in cardiomyocytes, contributing to cardiac insufficiency[40,41]. In chronic rhinosinusitis with nasal polyps, the IL-17A/TNF-α-neutrophil-LCN2 axis exacerbates inflammatory progression by promoting IL-8 expression in nasal polyps[42]. Tissue-infiltrating neutrophils secrete elevated levels of LCN2, which induces iron-mediated cell death in adipocytes and exacerbates malignancy in patients[30]. Furthermore, neutrophil-derived LCN2 polarizes macrophages toward a “reparative” phenotype, promoting cardiac remodeling in patients with infarction[43]. Moreover, LCN2 produced by other tissues can influence neutrophil function. LCN2 serves as a migratory activator of neutrophils[44]; for example, alcohol upregulates LCN2 protein expression in hepatocytes via the IL-6/STAT3 pathway, leading to neutrophil recruitment in the liver and subsequent T-cell depletion[45]. These findings underscore the multifaceted role of LCN2 in regulating immunity across different physiological and pathological states, particularly in a neutrophil-dependent manner.
Neutrophil extracellular traps (NETs) represent a well-established form of neutrophil activation[46]. In bacterial infections, NETs generated by neutrophils can transport LCN2 to the site of inflammation and infection, exerting an anti-infective role. While knockdown of LCN2 in neutrophils does not impact NET production, antimicrobial efficacy is significantly diminished[27]. This phenomenon may be attributed to bacterial Ent production, which suppresses neutrophil ROS levels and NET formation via the iron-chelating activity against bacterial Ent-mediated iron sequestration[47]. Recent research has revealed the role of LCN2-metformin-iron complexes synthesized by renal parenchymal cells in exacerbating acute kidney injury by binding to the C-X-C chemokine receptor type 4 receptor on the surface of neutrophils, thereby inducing NETosis[19]. These findings suggest a potentially self-sustaining, mutually reinforcing interplay between LCN2 and NETs. For example, in psoriasis, LCN2 produced by keratinocytes stimulates NET production by neutrophils, thereby fueling skin inflammation via toll-like receptor 4/IL-36 crosstalk and the downstream MyD88/nuclear factor-kappaB (NF-κB) signaling pathway. Concurrently, NETs stimulate keratinocytes to increase LCN2 expression and secretion[48]. Furthermore, the combined assessment of NETs and LCN2 has emerged as a prognostic biomarker for various diseases. Elevated NETs and LCN2 are significantly associated with severe acute kidney injury induced by coronavirus disease 2019[49]. NETs and LCN2 contribute to pathological changes in ocular graft-versus-host disease[50], and they are significantly associated with the presence of peripheral artery disease[51]. Despite these findings, the precise mechanism governing the interaction between LCN2 and NETs remains elusive. Additionally, the mechanisms underlying the NET-mediated upregulation of LCN2 production and secretion, as well as the manner in which LCN2 promotes NET production, warrant further investigation.
Macrophages serve as crucial regulators of the immune system, and their functions extend beyond classical phagocytosis to include the extensively studied phenomenon of macrophage polarization[52]. The interplay between LCN2 and macrophages is intricate, with LCN2 potentially acting as a liaison between macrophages and other cell types or as a key mediator facilitating interactions with macrophages. Recent research suggests a close association between LCN2 and macrophage polarization, which is primarily mediated through the regulation of iron metabolism.
LCN2 expression in macrophages is typically induced by inflammation[21]. The systemic inflammatory cascade associated with sepsis induction also triggers LCN2 expression as a first-line response of the innate immune system[53]. During acute lung inflammation, the number of LCN2-positive macrophages significantly increases, leading to intracellular iron overload and the polarization of alveolar macrophages toward the M1 phenotype, exacerbating oxidative stress[54]. Studies on LCN2-deficient mice and macrophages have revealed their reduced resistance to Salmonella infection, as evidenced by increased bacterial loads; decreased levels of nitric oxide, TNF-α, and IL-6; and elevated levels of IL-10. LCN2 downregulates IL-10 expression and enhances the bactericidal function of macrophages by promoting macrophage iron efflux[55]. The iron-loading status and cellular source of LCN2 modulate its biological function during renal failure in sepsis. The secretion of iron-free LCN2 by renal tubular epithelial cells is associated with renal injury, whereas increased release of iron-conjugated LCN2 (hLCN2) by macrophages is linked to renal recovery[56]. This mechanism may involve hLCN2-induced activation of the integrative stress response, mediated by the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 pathway, which further upregulates the expression of SLC7A11 through phosphorylated eukaryotic translation initiation factor 2A and increased ROS generation, enhancing cellular resistance to iron-induced cell death[10]. Furthermore, IL-10-treated macrophages produce hLCN2 and simultaneously activate the LCN2 receptor SLC22A17[28]. This receptor-mediated mechanism provides iron to cells, promoting cell migration and matrix adhesion[57,58].
LCN2 production by macrophages is regulated by a variety of self-expressed proteins, which subsequently influence the functions of downstream tissues and organs. For example, macrophage-expressed KDM6A exacerbates diabetic retinopathy by promoting LCN2 expression and impairing glycolytic processes in photoreceptor cells through the removal of trimethyl groups from histone H3K27[59]. Additionally, macrophage-expressed caspase-recruitment domain 9 upregulates LCN2 expression via the NF-κB pathway, subsequently increasing matrix metalloproteinase 9 expression. This upregulation contributes to the deterioration of cardiac function and adverse myocardial remodeling after myocardial infarction, which is mediated by the interaction between LCN2 and matrix metalloproteinase 9[60]. Macrophages also exacerbate chemokine ligand 4-induced liver injury in an LCN2-dependent manner[61]. Furthermore, macrophage-derived LCN2 plays a pivotal role in renal fibrosis, leading to proteinuria and renal injury by modulating the salbutamol/chemokine ligand 5/IL4 pathway[62]. However, these findings contrast with those of Horckmans et al[43], who demonstrated that LCN2 production by neutrophils after infarction induces macrophage differentiation toward the highly phagocytic MertK-M2c phenotype, consequently enhancing cardiac healing. This discrepancy may be attributed to the different cellular sources of LCN2 in various disease models, which dictate the polarization of M0 macrophages toward either proinflammatory macrophages, exacerbating tissue damage[63], or reparative macrophages, promoting phagocytosis[31,43].
As LCN2 is a secreted protein expressed in multiple tissues, the interplay between macrophages and LCN2 produced by various tissues in different disease states has garnered considerable attention. For example, dysfunctional adipocytes secrete LCN2, promoting the inflammatory activation of macrophages[64]. TNF-α induces LCN2 expression and secretion in adipocytes in a NACHT, LRR, and PYD domains-containing protein 3-dependent manner, subsequently promoting the activation of the NACHT, LRR, and PYD domains-containing protein 3 inflammasome in macrophages[65]. LCN2 also plays a pivotal role in mediating communication between macrophages and other immune cells. It serves as a central mediator facilitating crosstalk between neutrophils and hepatic macrophages by inducing chemokine signaling, particularly through the downstream receptor C-X-C chemokine receptor type 2, thereby exacerbating steatohepatitis[44].
LCN2 has a complex relationship with macrophages in cancer[66]. Sphingosine-1-phosphate released from apoptotic cancer cells enhances the production of LCN2 by tumor-associated macrophages upon signaling through its receptor sphingosine-1-phosphate receptor 1. Subsequently, LCN2 released by tumor-associated macrophages promotes tumor growth by inducing the expression of the lymphangiogenic factor vascular endothelial growth factor-C[67]. In metastatic meningiomas, while LCN2 expression by macrophages and neutrophils remains relatively stable, there is a marked increase in LCN2 expression by cancer cells. This upregulation is mediated by cytokines such as IL-6, IL-8, and IL-1β in the tumor microenvironment, which induce LCN2 expression in cancer cells through STAT and NF-κB transcriptional promoters. Additionally, cancer cells acquire iron from the cerebrospinal fluid in an LCN2-dependent manner, facilitating their growth while concurrently inhibiting the iron uptake and iron-dependent functional activity of macrophages[15].
Orabona et al[68] were the first to report that T lymphocytes can upregulate LCN2 expression in response to IL-9 stimulation. This regulatory relationship is mediated by STAT3, and the upregulation of LCN2 expression occurs gradually, reaching its peak 36-72 hours after stimulation. Additionally, as mentioned earlier, tumor cells can activate downstream LCN2 expression via STAT3, resulting in T-cell depletion and facilitating hepatocellular carcinoma metastasis[45]. In colorectal cancer (CRC), CD4+ T cells induce apoptosis by producing LCN2, thereby reducing the concentration of intracellular iron ions in T cells and compromising their antitumor capabilities. Concurrently, LCN2 overexpression promotes cholesterol metabolism and accelerates tumor metastasis. Moreover, iron transferred from T cells enters the tumor microenvironment, promoting tumor cell proliferation[69]. This finding parallels the role of LCN2 in macrophages[7], although further investigation is needed to determine whether the underlying mechanism is identical.
Silencing LCN2 expression has been shown to inhibit tumor proliferation, whereas reducing LCN2 expression enhances RSL3-induced ferroptosis in acute T lymphoblastic leukemia[70]. Inhibition of phosphoglycerate mutase 1 exerts antitumor effects by inducing energy stress and ROS-dependent protein kinase B inhibition. Consequently, this downregulates LCN2 expression in tumor cells, promoting ferroptotic cell death and CD8+ T-cell infiltration in hepatocellular carcinoma cells[71]. However, previous studies have presented contrasting findings, where Gprc5a-/-Lcn2-/- mice exhibited a heightened ability to promote lung adenocarcinoma proliferation. Additionally, these mice presented a decreased abundance of CD4+ T cells and increased levels of protumor inflammatory signals[72]. This apparent contradiction could stem from variations in the systemic and local tissue effects of LCN2 or differences in the source of LCN2. Further investigations are warranted to elucidate the underlying mechanisms driving these observations.
An intricate immune dialog between T cells and macrophages is orchestrated by LCN2. In the context of Mycobacterium tuberculosis infection, LCN2-mediated modulation of ROS levels culminates in the downregulation of major histocompatibility complex class I molecules in macrophages, subsequently attenuating CD8+ T-cell activation during the infection process[73]. Furthermore, LCN2 assumes multifaceted roles in tandem with T cells. Notably, Furutani et al[72] demonstrated the therapeutic potential of a novel proresolving lipid mediator mimetic, 3-oxa-PD1n-3, which was administered via the intrathecal injection of docosapentaenoic acid. This intervention effectively curtails LCN2 production in cutaneous T-cell lymphomas, thereby mitigating pruritus through the modulation of synaptic transmission[74]. Nevertheless, the intricacies of these interactions necessitate deeper mechanistic elucidation.
B lymphocytes play pivotal roles in orchestrating immune responses through their diverse effector functions, including the production of immunoglobulins for antigen neutralization, the modulation of immune responses, and antigen recognition and presentation to stimulate other effector cells. Despite their central importance in immune regulation, the direct interaction between LCN2 and B lymphocytes remains relatively understudied in current research endeavors[75]. Expanding our understanding of this potential interaction could offer valuable insights into the nuanced interplay between LCN2 and the adaptive immune system, warranting further investigation in future studies.
Recent studies have shown that inhibiting B-cell activation can reduce the secretion of LCN2 and slow the progression of lupus nephritis[76]. Moreover, limiting B-cell activation has been found to decrease LCN2 secretion, thereby alleviating renal ischemia-reperfusion injury[77]. Additionally, symptomatic patients with B-cell-derived non-Hodgkin’s lymphoma exhibit higher levels of LCN2 than normal individuals do[78]. Despite these significant findings, none of these studies have explored the direct interaction between LCN2 and B cells. Investigating these interactions should be a priority for future research to better understand their roles in disease pathogenesis and potential therapeutic interventions.
In future studies, investigating the iron-carrying status of LCN2 and its cellular origin is imperative. Additionally, LCN2 produced by the same cells in different diseases may manifest diverse roles. Taking a holistic approach, we should explore the intricate crosstalk between LCN2 and immune elements, with a focus on the disease context. This comprehensive analysis will be pivotal in unraveling the role of LCN2 in immune regulation. The mechanisms of LCN2 Interaction with cells are demonstrated in Figure 3.
Gastrointestinal diseases encompass a range of multifactorial triggers marked by compromised barrier function and heightened permeability of the gastrointestinal mucosa. These conditions often manifest symptoms such as digestive and absorption dysfunction, along with bacterial overgrowth, culminating in damage across multiple tissues. The impacts of LCN2 on gastrointestinal diseases are likely intricate and influenced by various factors, including the underlying cause of gastrointestinal damage and the microenvironment surrounding the affected tissues.
Autoimmune enteropathies are a group of rare and incurable conditions that include inflammatory bowel diseases (IBDs) [Crohn’s disease (CD); UC], intestinal Behçet’s disease, and others. LCN2 plays a pivotal role in the pathogenesis of these disorders. Research has confirmed elevated LCN2 expression in intestinal Behçet’s disease, but the corresponding specific mechanisms of action have yet to be clarified[79]. Current research has focused primarily on the interaction between LCN2 and IBD.
IBDs, which represent a relatively prevalent subset of gastrointestinal autoimmune conditions, are characterized by a propensity for pan-enteric involvement[80]. The pathological manifestations of IBD arise from a vicious cycle driven by an imbalance between intestinal mucosal damage and mucosal immunity[81]. Numerous studies have identified LCN2 as an associated gene in IBD[82], highlighting its potential utility as a diagnostic marker of CD[83]. The expression levels of LCN2 are correlated with disease activity and immune infiltration in UC[84,85], and LCN2 has emerged as a marker for UC-associated carcinoma[86]. Additionally, some studies suggest its involvement as a potential mediator of iron-induced cell death[87]. Furthermore, elevated levels of LCN2 have been consistently observed in the feces of IBD patients, suggesting that LCN2 is a promising noninvasive screening indicator[88]. The increased expression of LCN2 in UC is believed to be regulated by proinflammatory cytokines such as IL-17A, IL-22, and TNF-α, potentially via TNF-α-mitogen-activated protein kinases-NADPH oxidase 1 axis-derived ROS[84,89].
IBD often coincides with alterations in the composition of the intestinal microbiota[90]. LCN2 may modulate IBD by influencing the intestinal microbiota. Previous studies have demonstrated that LCN2 exerts protective effects against early-onset colitis and tumor formation by modifying the composition of the intestinal microbiota, particularly Lachnospiraceae and Alistipes spp.[91]. Additionally, LCN2 can promote cell migration, contributing to mucosal regeneration and tissue repair[92]. LCN2 deficiency has been associated with alterations in the microbiota-derived metabolome, characterized by reduced production of short-chain fatty acids and short-chain fatty acid-producing microorganisms, thereby exacerbating the intestinal inflammation induced by a high-fat diet[93]. However, conflicting findings have emerged regarding the role of LCN2 in IBD progression. For example, some studies suggest that LCN2 may aggravate IBD by binding to Ent and reducing myeloperoxidase activity[94]. Moreover, a lack of suppressor of cytokine signaling 3 in myeloid cells has been shown to increase LCN2 expression in neutrophils, exacerbating dextran sulfate sodium-induced colitis. Conversely, suppressor of cytokine signaling 3 has been implicated in preventing immune system activation in IBD[95]. Although LCN2 is widely recognized as a marker gene for IBD, its mechanistic involvement in IBD and the inconsistencies in its functional roles warrant further investigation.
Infectious diseases affecting the gastrointestinal tract encompass a spectrum of conditions induced by bacterial and viral pathogens, commonly presenting with symptoms such as abdominal pain, diarrhea, and vomiting. Among its initially recognized functions[21,22], LCN2 exhibits potent antimicrobial properties. During bacterial enteritis, LCN2 levels notably increase[96]. However, in cases of enteritis stemming from viral infections, LCN2 levels remain largely unchanged. Consequently, LCN2 has emerged as a valuable tool for discerning the etiology of pathogen-induced gastrointestinal inflammation[88]. Elevated expression of LCN2 in intestinal epithelial cells is facilitated by IL-17/IL-22 signaling following bacterial infection. Notably, a deficiency in LCN2 promotes the proliferation of Salmonella typhimurium, an enterobacterium, within the inflamed gut milieu. This exacerbates susceptibility to further bacterial invasion[97], exemplified by heightened susceptibility to Escherichia coli infection[98].
The bacteriostatic action of LCN2 primarily stems from its role in restricting bacterial access to and utilization of iron. By facilitating internal iron export in macrophages, LCN2 increases the production of proinflammatory mediators while concurrently suppressing IL-10 levels, thereby fostering host-mediated bacterial clearance and elimination. Furthermore, LCN2 orchestrates intercellular interactions within the immune milieu. Deficiencies in LCN2 compromise neutrophil chemotaxis and migration and disrupt the normal secretion of inflammatory cytokines by macrophages[25], thereby compromising intestinal barrier integrity[99]. However, this protective effect is limited to infections caused by Gram-negative bacteria that rely exclusively on iron carriers susceptible to LCN2 binding. Conversely, LCN2 has no discernible effect on bacteria, such as Staphylococcus aureus, which employ alternative iron acquisition mechanisms unaffected by LCN2[21]. LCN2 secreted by colonic epithelial cells confers anti-inflammatory effects by augmenting phagocytic activity and bolstering bacterial clearance[100]. Moreover, in LCN2-deficient mice, alterations in the composition of the ileal intestinal flora manifest, notably marked by a surge in injury-associated segmented filamentous bacteria. This surge correlates closely with mucosal cell layer injury, provoking an antimicrobial response within the intestinal epithelium and influencing helper T cell polarization[101].
LCN2 expression was markedly elevated in patients with Helicobacter pylori (H. pylori)-infected gastritis, whereas quercetin treatment led to decreased LCN2 expression in H. pylori-infected GES-1 cells. Mechanistically, SP1 was found to bind to the LCN2 promoter, thereby facilitating its transcription. Quercetin intervention mitigated M1 macrophage polarization via modulation of the SP1/LCN2 axis and subsequently attenuated H. pylori-induced apoptosis and inflammatory injury in gastric epithelial cells[102]. However, LCN2 has also been demonstrated to exert a proinflammatory effect in cases of low-grade intestinal inflammation[103].
The association between LCN2 and CRC has been extensively investigated in the context of intestinal tumors. As early as 1996, studies revealed high expression of LCN2 in colon cancer, including precancerous lesions[104]. Through various bioanalytical and proteomic approaches, researchers have consistently identified LCN2 as a potential biomarker for CRC development[105-108], which can be used in the diagnosis of CRC[109]. Its expression levels hold promise for CRC diagnosis and may serve as indicators for predicting metastasis and poor prognosis[110,111]. Notably, a recent study demonstrated correlations between LCN2 gene expression and the abundance of CRC-associated bacteria, such as Lactococcaceae and Veronica spp.[112].
In primary CRC, LCN2 mediates apoptosis by modulating TNF-α apoptosis-inducing ligand and its cell-surface-expressed death receptors 4/5. Silencing LCN2 expression leads to the upregulation of death receptor 5 expression, enhancing cancer cell sensitivity to chemotherapy via the p38 mitogen-activated protein kinases/C/EBP homologous protein pathway[113]. Deletion of the PKP3 gene results in increased LCN2 levels, rendering colon cancer cells resistant to 5-fluorouracil[114]. Conversely, LCN2 has been reported to inhibit the progression of duodenal tumors while promoting the growth of small intestinal tumors[115]. These conflicting findings underscore the need for further in-depth exploration of the functions of LCN2 in organisms.
In vitro experiments using various colon cancer cell lines have revealed differential expression levels of LCN2, which are correlated with variations in migration and invasion capacities[116]. Elevated LCN2 expression in CRC is often associated with a greater risk of distant metastasis. Kim et al[117] demonstrated that LCN2 negatively modulates epithelial-mesenchymal transition in CRC cells by modulating glucose metabolism, with reduced LCN2 expression enhancing the migratory and invasive potential of cancer cells. Further investigations indicated that mesenchymal stem cells engineered to overexpress LCN2 could target and deliver LCN2 to tumor sites, effectively suppressing liver metastasis in colon cancer[118]. Recent studies have confirmed that LCN2, which acts upstream of the NF-κB/Snail pathway, significantly inhibits epithelial-mesenchymal transition induction and metastasis both in vitro and in vivo by attenuating NF-κB/Snail pathway activation[119]. The outcomes and mechanisms of LCN2 in different organ and diseases are demonstrated in Table 1.
| Organ/site | Disease | Outcome | Mechanism | Ref. |
| Eye | Retinal inflammation | Overexpression of LCN2 in RPE cells manifested protection from cell death | LCN2 induced expression of antioxidant enzymes HMOX1 and SOD2 in RPE cells and inhibited the cytotoxic effects of H2O2 and lipopolysaccharide | [120] |
| Age-related macular degeneration | In RPE cells, elevated LCN2 expression disrupts autophagy, enhances inflammasome activity, induces oxidative stress, triggers ferroptosis, and exacerbates cellular damage, thereby impacting iron homeostasis | LCN2 forms a complex with ATG4B, designated as LCN2-ATG4B-LC3-II, modulating ATG4B activity and LC3-II lipidation to regulate autophagy | [121] | |
| Brain | Cerebral ischemia/reperfusion injury | After stroke in mice with middle cerebral artery occlusion, astrocyte LCN2 levels surged within 24 hours. Mice lacking LCN2 had smaller infarcts and better neurocognitive recovery poststroke | 24p3R inhibition reduced pyroptosis and pro-inflammatory cytokine secretion via LCN2, an effect reversed by Nigrostatin-induced NLRP3 activation. Pyroptosis in astrocytes was exacerbated by rLCN2 in Lcn2-/- mice | [122] |
| Cancer brain metastasis | LCN2 drives neuroinflammation, promoting brain metastasis in melanoma and breast cancer | Signals from the primary tumor activate astrocytes, which attract immunosuppressive granulocytes to brain metastases, a process that increases LCN2 levels. Targeting LCN2 genetically reduces neuroinflammation and brain metastasis | [123] | |
| Neurodegenerative diseases | LCN2 in astrocytes can shift their state from neurotoxic to neuroprotective, possibly preventing neuronal death and halting neurodegenerative disease progression | Activated astrocytes secrete LCN2, which binds to the 24p3R receptor in an autocrine loop, further activating astrocytes through the NF-κB pathway | [124] | |
| Kidney | Active lupus nephritis | Biomarker | LCN2 is highly secreted by renal tubular epithelial cells in response to various renal injuries, leading to elevated levels in blood and urine, and can serve as a biomarker for multiple kidney injuries and prognosis | [125] |
| Acute kidney injury | [126] | |||
| Kidney transplant | [127] | |||
| Liver | NASH | In patients with NASH, LCN2 expression was significantly associated with inflammation and fibrosis | LCN2 serves as a central mediator in hepatic stellate cell activation during leptin-deficient obesity, via the α-SMA/MMP9/STAT3 pathway, worsening NASH | [128] |
| SREBP-1c mediates LCN2 expression, linking to NASH pathogenesis. SREBP-1c deletion downregulates LCN2, alleviating NASH severity. It also couples iron and lipid metabolism via LCN2, impacting NASH development | [129] | |||
| Hepatocellular carcinoma | Elevated LCN2 expression promotes hepatocellular carcinoma cell proliferation | Targeting PGAM1 suppresses hepatocellular carcinoma growth by inhibiting LCN2 and PD-L1 expression through an energy stress/ROS-mediated reduction in AKT activity, thereby enhancing CD8+ T-cell infiltration | [71] | |
| Pancreas | Pancreatic ductal adenocarcinoma | Overexpression of LCN2 markedly inhibits pancreatic cancer cell adhesion and invasion | LCN2 diminishes pancreatic cancer cell adhesion and invasion by partially suppressing focal adhesion kinase tyrosine-397 phosphorylation and inhibits angiogenesis by impeding VEGF production | [130] |
| Lcn2 deficiency in PDAC mouse models retarded weight gain, adiposity, and tumor progression and enhanced survival | Pancreatic stromal cells, devoid of LCN2 synthesis, express the LCN2 receptor SLC22A17. LCN2 binding to SLC22A17 on cancer-associated fibroblasts stimulates secretion of inflammatory mediators and MMP9, enhancing immune cell infiltration and tumor progression in the pancreatic cancer microenvironment | [131] | ||
| Artery | Atherosclerosis | LCN2 enhances HDL metabolism and facilitates macrophage reverse cholesterol transport | LCN2 enhances HDL metabolism and attenuates atherogenesis by inhibiting Nedd4-1-mediated SR-BI ubiquitination at lysines 500 and 508 | [132] |
| Bone | Osteoporosis | LCN2, acting as a downstream factor, induces iron overload in osteocytes, promoting their apoptosis | Repin1 knockdown reduced LCN2 expression, mitigating the toxic effects of intracellular iron overload | [133] |
| Other | Chronic stress | Peripheral and central inhibition of the LCN2 pathway significantly alleviated CRS-induced behavioral deficits | LCN2’s anxiogenic effects are linked to its modulation of neuronal activities in the medial prefrontal cortex. Under CRS, hepatic tissues are identified as the main source of serum LCN2, released via the vagal efferent pathway from the dorsal motor vagal nucleus | [134] |
The status of LCN2 in relation to intestinal barrier function remains to be further elucidated. LCN2 has been shown to protect intestinal barrier function against invasion by Escherichia coli[99]. In CD and UC, LCN2 acts as a proinflammatory cytokine, exacerbating destruction of the intestinal barrier[87]. The precise relationship between LCN2 and gastrointestinal diseases remains incompletely understood, particularly concerning its involvement in complex conditions such as intestinal fistula, intestinal ischemia/reperfusion injury, and other forms of intestinal damage. A comprehensive exploration of the mechanisms underlying the actions of LCN2 in intestinal injury is essential for gaining insights into its roles in various diseases. Additionally, future research should focus on investigating drug therapies targeting LCN2 to explore potential therapeutic options for these conditions.
A wealth of literature on LCN2 has highlighted its utility as an indicator of disease activity and prognosis in bacterial infections and IBD, owing to its heightened expression during the acute phase. Initially recognized as an anti-infective protein secreted by neutrophils for the chelation of bacterial ectoferric iron, the role of LCN2 has since been extensively investigated in various diseases. However, differing cellular sources of LCN2 may involve specific mechanisms within distinct disease microenvironments, yielding varying or even conflicting results. This variability may be attributed to the iron-carrying state of LCN2, necessitating further research to elucidate its immunomodulatory role in diverse scenarios.
Moreover, recent studies have shed light on the ability of LCN2 to bind other small molecules, subsequently binding iron ions. For example, the LCN2-metformin-iron complex has been implicated in exacerbating renal ischemia-reperfusion injury[19]. These findings suggest that LCN2 may interact with conventional small molecule drugs to yield unforeseen effects, suggesting intriguing avenues for future research. Investigations of whether LCN2 can serve as a drug carrier to deliver therapeutics to damaged sites or function in vivo with the aid of cellular carriers (e.g., mesenchymal stem cells) or drug carriers (e.g., hydrogels and chitosan) represent promising future directions. However, the specific intracellular mechanisms of LCN2 action remain incompletely understood, and the interaction between LCN2 and the intestinal flora warrants further elucidation. Therefore, unraveling the potential cellular and molecular mechanisms of LCN2 in various diseases constitutes a vast area for future exploration.
We express our gratitude to Dr. Xin-Yu Wang and Dr. Cheng-Nan Chu for providing assistance in the design of the study.
| 1. | Kjeldsen L, Bainton DF, Sengeløv H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799-807. [PubMed] |
| 2. | Jaberi SA, Cohen A, D'Souza C, Abdulrazzaq YM, Ojha S, Bastaki S, Adeghate EA. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed Pharmacother. 2021;142:112002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 3. | Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39:1935-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077-42083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Xu MJ, Feng D, Wu H, Wang H, Chan Y, Kolls J, Borregaard N, Porse B, Berger T, Mak TW, Cowland JB, Kong X, Gao B. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: a critical role for IL-6/STAT3. Hepatology. 2015;61:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Cowland JB, Sørensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J Immunol. 2003;171:6630-6639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Mertens C, Mora J, Ören B, Grein S, Winslow S, Scholich K, Weigert A, Malmström P, Forsare C, Fernö M, Schmid T, Brüne B, Jung M. Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology. 2018;7:e1408751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, Espevik T, Frøland SS, Husberg C, Christensen G, Dickstein K, Kjekshus J, Øie E, Gullestad L, Aukrust P. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu Rev Nutr. 2017;37:103-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Meier JK, Schnetz M, Beck S, Schmid T, Dominguez M, Kalinovic S, Daiber A, Brüne B, Jung M. Iron-Bound Lipocalin-2 Protects Renal Cell Carcinoma from Ferroptosis. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Schröder SK, Gasterich N, Weiskirchen S, Weiskirchen R. Lipocalin 2 receptors: facts, fictions, and myths. Front Immunol. 2023;14:1229885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 12. | Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24593] [Cited by in RCA: 26641] [Article Influence: 5328.2] [Reference Citation Analysis (55)] |
| 13. | Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 538] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Yee SW, Giacomini KM. Emerging Roles of the Human Solute Carrier 22 Family. Drug Metab Dispos. 2021;50:1193-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Chi Y, Remsik J, Kiseliovas V, Derderian C, Sener U, Alghader M, Saadeh F, Nikishina K, Bale T, Iacobuzio-Donahue C, Thomas T, Pe'er D, Mazutis L, Boire A. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. 2020;369:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 16. | Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 609] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 17. | Barasch J, Hollmen M, Deng R, Hod EA, Rupert PB, Abergel RJ, Allred BE, Xu K, Darrah SF, Tekabe Y, Perlstein A, Wax R, Bruck E, Stauber J, Corbin KA, Buchen C, Slavkovich V, Graziano J, Spitalnik SL, Bao G, Strong RK, Qiu A. Disposal of iron by a mutant form of lipocalin 2. Nat Commun. 2016;7:12973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1169] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 19. | Cai Z, Wu X, Song Z, Sun S, Su Y, Wang T, Cheng X, Yu Y, Yu C, Chen E, Chen W, Yu Y, Linkermann A, Min J, Wang F. Metformin potentiates nephrotoxicity by promoting NETosis in response to renal ferroptosis. Cell Discov. 2023;9:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 515] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 21. | Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1423] [Article Influence: 64.7] [Reference Citation Analysis (5)] |
| 22. | Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1038] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 23. | Chen PC, Ho CH, Fan CK, Liu SP, Cheng PC. Antimicrobial Peptide LCN2 Inhibited Uropathogenic Escherichia coli Infection in Bladder Cells in a High-Glucose Environment through JAK/STAT Signaling Pathway. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Hop HT, Arayan LT, Huy TXN, Reyes AWB, Baek EJ, Min W, Lee HJ, Rhee MH, Watanabe K, Chang HH, Kim S. Lipocalin 2 (Lcn2) interferes with iron uptake by Brucella abortus and dampens immunoregulation during infection of RAW 264.7 macrophages. Cell Microbiol. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Wang Q, Li S, Tang X, Liang L, Wang F, Du H. Lipocalin 2 Protects Against Escherichia coli Infection by Modulating Neutrophil and Macrophage Function. Front Immunol. 2019;10:2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 520] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 27. | Li H, Feng D, Cai Y, Liu Y, Xu M, Xiang X, Zhou Z, Xia Q, Kaplan MJ, Kong X, Gao B. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology. 2018;68:1604-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Jung M, Sola A, Hughes J, Kluth DC, Vinuesa E, Viñas JL, Pérez-Ladaga A, Hotter G. Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 2012;81:969-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Vinuesa E, Sola A, Jung M, Alfaro V, Hotter G. Lipocalin-2-induced renal regeneration depends on cytokines. Am J Physiol Renal Physiol. 2008;295:F1554-F1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Wang D, Li X, Jiao D, Cai Y, Qian L, Shen Y, Lu Y, Zhou Y, Fu B, Sun R, Tian Z, Zheng X, Wei H. LCN2 secreted by tissue-infiltrating neutrophils induces the ferroptosis and wasting of adipose and muscle tissues in lung cancer cachexia. J Hematol Oncol. 2023;16:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 31. | Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K, Mesteri I, Nairz M, Boon L, Spiel A, Fuhrmann V, Strobl B, Müller M, Schenk P, Weiss G, Knapp S. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest. 2013;123:3363-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Liu Z, Cominelli F, Di Martino L, Liu R, Devireddy N, Devireddy LR, Wald DN. Lipocalin 24p3 Induction in Colitis Adversely Affects Inflammation and Contributes to Mortality. Front Immunol. 2019;10:812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Liew PX, Kubes P. The Neutrophil's Role During Health and Disease. Physiol Rev. 2019;99:1223-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 839] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 34. | Zhang H, Wang Y, Qu M, Li W, Wu D, Cata JP, Miao C. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin Transl Med. 2023;13:e1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 35. | Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 531] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 36. | Chu C, Wang X, Yang C, Chen F, Shi L, Xu W, Wang K, Liu B, Wang C, Sun D, Ding W. Neutrophil extracellular traps drive intestinal microvascular endothelial ferroptosis by impairing Fundc1-dependent mitophagy. Redox Biol. 2023;67:102906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 37. | An X, Qin J, Hu X, Zhou Y, Fu B, Wei H. Overexpression of lipocalin 2 in PBX1-deficient decidual NK cells promotes inflammation at the maternal-fetal interface. Am J Reprod Immunol. 2023;89:e13676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 38. | Luchtefeld M, Preuss C, Rühle F, Bogalle EP, Sietmann A, Figura S, Müller W, Grote K, Schieffer B, Stoll M. Gp130-dependent release of acute phase proteins is linked to the activation of innate immune signaling pathways. PLoS One. 2011;6:e19427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, Viltard M, Williams D, Paragas N, Leete T, Kulkarni R, Li X, Lee B, Kalandadze A, Ratner AJ, Pizarro JC, Schmidt-Ott KM, Landry DW, Raymond KN, Strong RK, Barasch J. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6:602-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 40. | Novak T, Crawford JC, Hahn G, Hall MW, Thair SA, Newhams MM, Chou J, Mourani PM, Tarquinio KM, Markovitz B, Loftis LL, Weiss SL, Higgerson R, Schwarz AJ, Pinto NP, Thomas NJ, Gedeit RG, Sanders RC Jr, Mahapatra S, Coates BM, Cvijanovich NZ, Ackerman KG, Tellez DW, McQuillen P, Kurachek SC, Shein SL, Lange C, Thomas PG, Randolph AG. Transcriptomic profiles of multiple organ dysfunction syndrome phenotypes in pediatric critical influenza. Front Immunol. 2023;14:1220028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Huang Y, Zhang N, Xie C, You Y, Guo L, Ye F, Xie X, Wang J. Lipocalin-2 in neutrophils induces ferroptosis in septic cardiac dysfunction via increasing labile iron pool of cardiomyocytes. Front Cardiovasc Med. 2022;9:922534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 42. | Zhang C, Wang H, Hu L, Zhang Q, Chen J, Shi L, Song X, Liu J, Xue K, Wang J, Wang D, Sun X. Lipocalin-2 promotes neutrophilic inflammation in nasal polyps and its value as biomarker. Allergol Int. 2024;73:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 43. | Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 350] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 44. | Ye D, Yang K, Zang S, Lin Z, Chau HT, Wang Y, Zhang J, Shi J, Xu A, Lin S, Wang Y. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J Hepatol. 2016;65:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 45. | Qiu X, Zhou J, Xu H, Li Y, Ma S, Qiao H, Zeng K, Wang Q, Ouyang J, Liu Y, Ding J, Liu Y, Zhang J, Shi M, Liao Y, Liao W, Lin L. Alcohol reshapes a liver premetastatic niche for cancer by extra- and intrahepatic crosstalk-mediated immune evasion. Mol Ther. 2023;31:2662-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Sun S, Duan Z, Wang X, Chu C, Yang C, Chen F, Wang D, Wang C, Li Q, Ding W. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 2021;12:606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 47. | Saha P, Yeoh BS, Olvera RA, Xiao X, Singh V, Awasthi D, Subramanian BC, Chen Q, Dikshit M, Wang Y, Parent CA, Vijay-Kumar M. Bacterial Siderophores Hijack Neutrophil Functions. J Immunol. 2017;198:4293-4303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Shao S, Fang H, Dang E, Xue K, Zhang J, Li B, Qiao H, Cao T, Zhuang Y, Shen S, Zhang T, Qiao P, Li C, Gudjonsson JE, Wang G. Neutrophil Extracellular Traps Promote Inflammatory Responses in Psoriasis via Activating Epidermal TLR4/IL-36R Crosstalk. Front Immunol. 2019;10:746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 49. | Henry BM, de Oliveira MHS, Cheruiyot I, Benoit J, Rose J, Favaloro EJ, Lippi G, Benoit S, Pode Shakked N. Cell-Free DNA, Neutrophil extracellular traps (NETs), and Endothelial Injury in Coronavirus Disease 2019- (COVID-19-) Associated Acute Kidney Injury. Mediators Inflamm. 2022;2022:9339411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | An S, Raju I, Surenkhuu B, Kwon JE, Gulati S, Karaman M, Pradeep A, Sinha S, Mun C, Jain S. Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: Implications for novel biomarkers and therapeutic strategies. Ocul Surf. 2019;17:589-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 51. | Buso G, Faggin E, Bressan A, Galliazzo S, Cinetto F, Felice C, Fusaro M, Erdmann A, Pauletto P, Rattazzi M, Mazzolai L. Biomarkers of Neutrophil Activation in Patients with Symptomatic Chronic Peripheral Artery Disease Predict Worse Cardiovascular Outcome. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 52. | Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4781] [Article Influence: 398.4] [Reference Citation Analysis (1)] |
| 53. | Saha P, Chassaing B, Yeoh BS, Viennois E, Xiao X, Kennett MJ, Singh V, Vijay-Kumar M. Ectopic Expression of Innate Immune Protein, Lipocalin-2, in Lactococcus lactis Protects Against Gut and Environmental Stressors. Inflamm Bowel Dis. 2017;23:1120-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | An HS, Yoo JW, Jeong JH, Heo M, Hwang SH, Jang HM, Jeong EA, Lee J, Shin HJ, Kim KE, Shin MC, Roh GS. Lipocalin-2 promotes acute lung inflammation and oxidative stress by enhancing macrophage iron accumulation. Int J Biol Sci. 2023;19:1163-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 55. | Nairz M, Schroll A, Haschka D, Dichtl S, Sonnweber T, Theurl I, Theurl M, Lindner E, Demetz E, Aßhoff M, Bellmann-Weiler R, Müller R, Gerner RR, Moschen AR, Baumgartner N, Moser PL, Talasz H, Tilg H, Fang FC, Weiss G. Lipocalin-2 ensures host defense against Salmonella Typhimurium by controlling macrophage iron homeostasis and immune response. Eur J Immunol. 2015;45:3073-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Mertens C, Kuchler L, Sola A, Guiteras R, Grein S, Brüne B, von Knethen A, Jung M. Macrophage-Derived Iron-Bound Lipocalin-2 Correlates with Renal Recovery Markers Following Sepsis-Induced Kidney Damage. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 698] [Article Influence: 33.2] [Reference Citation Analysis (10)] |
| 58. | Rehwald C, Schnetz M, Urbschat A, Mertens C, Meier JK, Bauer R, Baer P, Winslow S, Roos FC, Zwicker K, Huard A, Weigert A, Brüne B, Jung M. The iron load of lipocalin-2 (LCN-2) defines its pro-tumour function in clear-cell renal cell carcinoma. Br J Cancer. 2020;122:421-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Wen Y, Chen X, Feng H, Wang X, Kang X, Zhao P, Zhao C, Wei Y. Kdm6a deficiency in microglia/macrophages epigenetically silences Lcn2 expression and reduces photoreceptor dysfunction in diabetic retinopathy. Metabolism. 2022;136:155293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Liu Y, Shao YH, Zhang JM, Wang Y, Zhou M, Li HQ, Zhang CC, Yu PJ, Gao SJ, Wang XR, Jia LX, Piao CM, Du J, Li YL. Macrophage CARD9 mediates cardiac injury following myocardial infarction through regulation of lipocalin 2 expression. Signal Transduct Target Ther. 2023;8:394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 61. | Huang WJ, Qiu BJ, Qi XS, Chen CY, Liu WM, Zhou SA, Ding M, Lu FF, Zhao J, Tang D, Zhou X, Fu GB, Wang ZY, Ma HQ, Wu YL, Wu HP, Chen XS, Yu WF, Yan HX. CD24(+)LCN2(+) liver progenitor cells in ductular reaction contributed to macrophage inflammatory responses in chronic liver injury. Cell Biosci. 2023;13:184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 62. | Bonnard B, Ibarrola J, Lima-Posada I, Fernández-Celis A, Durand M, Genty M, Lopez-Andrés N, Jaisser F. Neutrophil Gelatinase-Associated Lipocalin From Macrophages Plays a Critical Role in Renal Fibrosis Via the CCL5 (Chemokine Ligand 5)-Th2 Cells-IL4 (Interleukin 4) Pathway. Hypertension. 2022;79:352-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Cheng L, Xing H, Mao X, Li L, Li X, Li Q. Lipocalin-2 promotes m1 macrophages polarization in a mouse cardiac ischaemia-reperfusion injury model. Scand J Immunol. 2015;81:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Sciarretta F, Ceci V, Tiberi M, Zaccaria F, Li H, Zhou ZY, Sun Q, Konja D, Matteocci A, Bhusal A, Verri M, Fresegna D, Balletta S, Ninni A, Di Biagio C, Rosina M, Suk K, Centonze D, Wang Y, Chiurchiù V, Aquilano K, Lettieri-Barbato D. Lipocalin-2 promotes adipose-macrophage interactions to shape peripheral and central inflammatory responses in experimental autoimmune encephalomyelitis. Mol Metab. 2023;76:101783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 65. | Javaid HMA, Ko E, Joo EJ, Kwon SH, Park JH, Shin S, Cho KW, Huh JY. TNFα-induced NLRP3 inflammasome mediates adipocyte dysfunction and activates macrophages through adipocyte-derived lipocalin 2. Metabolism. 2023;142:155527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 66. | Živalj M, Van Ginderachter JA, Stijlemans B. Lipocalin-2: A Nurturer of Tumor Progression and a Novel Candidate for Targeted Cancer Therapy. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 67. | Jung M, Ören B, Mora J, Mertens C, Dziumbla S, Popp R, Weigert A, Grossmann N, Fleming I, Brüne B. Lipocalin 2 from macrophages stimulated by tumor cell-derived sphingosine 1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci Signal. 2016;9:ra64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Orabona C, Dumoutier L, Renauld JC. Interleukin-9 induces 24P3 lipocalin gene expression in murine T cell lymphomas. Eur Cytokine Netw. 2001;12:154-161. [PubMed] |
| 69. | Che R, Wang Q, Li M, Shen J, Ji J. Quantitative Proteomics of Tissue-Infiltrating T Cells From CRC Patients Identified Lipocalin-2 Induces T-Cell Apoptosis and Promotes Tumor Cell Proliferation by Iron Efflux. Mol Cell Proteomics. 2024;23:100691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 70. | Tian C, Zheng M, Lan X, Liu L, Ye Z, Li C. Silencing LCN2 enhances RSL3-induced ferroptosis in T cell acute lymphoblastic leukemia. Gene. 2023;879:147597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 71. | Zheng Y, Wang Y, Lu Z, Wan J, Jiang L, Song D, Wei C, Gao C, Shi G, Zhou J, Fan J, Ke A, Zhou L, Cai J. PGAM1 Inhibition Promotes HCC Ferroptosis and Synergizes with Anti-PD-1 Immunotherapy. Adv Sci (Weinh). 2023;10:e2301928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 72. | Treekitkarnmongkol W, Hassane M, Sinjab A, Chang K, Hara K, Rahal Z, Zhang J, Lu W, Sivakumar S, McDowell TL, Kantrowitz J, Zhou J, Lang W, Xu L, Ochieng JK, Nunomura-Nakamura S, Deng S, Behrens C, Raso MG, Fukuoka J, Reuben A, Ostrin EJ, Parra E, Solis LM, Spira AE, McAllister F, Cascone T, Wistuba II, Moghaddam SJ, Scheet PA, Fujimoto J, Kadara H. Augmented Lipocalin-2 Is Associated with Chronic Obstructive Pulmonary Disease and Counteracts Lung Adenocarcinoma Development. Am J Respir Crit Care Med. 2021;203:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Choi JA, Cho SN, Lee J, Son SH, Nguyen DT, Lee SA, Song CH. Lipocalin 2 regulates expression of MHC class I molecules in Mycobacterium tuberculosis-infected dendritic cells via ROS production. Cell Biosci. 2021;11:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Furutani K, Chen O, McGinnis A, Wang Y, Serhan CN, Hansen TV, Ji RR. Novel proresolving lipid mediator mimetic 3-oxa-PD1n-3 docosapentaenoic acid reduces acute and chronic itch by modulating excitatory and inhibitory synaptic transmission and astroglial secretion of lipocalin-2 in mice. Pain. 2023;164:1340-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Abdelhamid L, Luo XM. Retinoic Acid, Leaky Gut, and Autoimmune Diseases. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Chalmers SA, Glynn E, Garcia SJ, Panzenbeck M, Pelletier J, Dimock J, Seccareccia E, Bosanac T, Khalil S, Harcken C, Webb D, Nabozny G, Fine JS, Souza D, Klein E, Herlitz L, Ramanujam M, Putterman C. BTK inhibition ameliorates kidney disease in spontaneous lupus nephritis. Clin Immunol. 2018;197:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Möckel T, Boegel S, Schwarting A. Transcriptome analysis of renal ischemia/reperfusion (I/R) injury in BAFF and BAFF-R deficient mice. PLoS One. 2023;18:e0291619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Hidman J, Larsson A, Thulin M, Karlsson T. Increased plasma endostatin and GDF15 in indolent non-Hodgkin lymphoma. Ups J Med Sci. 2023;128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Gul FC, Cicek D, Kaman D, Demir B, Nazik H. Changes of serum lipocalin-2 and retinol binding protein-4 levels in patients with psoriasis and Behçet's disease. Eur J Dermatol. 2015;25:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313-321.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 867] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 81. | Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1495] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 82. | Abella V, Scotece M, Conde J, Gómez R, Lois A, Pino J, Gómez-Reino JJ, Lago F, Mobasheri A, Gualillo O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers. 2015;20:565-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 83. | Alarfaj SJ, Mostafa SA, Negm WA, El-Masry TA, Kamal M, Elsaeed M, El Nakib AM. Mucosal Genes Expression in Inflammatory Bowel Disease Patients: New Insights. Pharmaceuticals (Basel). 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Stallhofer J, Friedrich M, Konrad-Zerna A, Wetzke M, Lohse P, Glas J, Tillack-Schreiber C, Schnitzler F, Beigel F, Brand S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm Bowel Dis. 2015;21:2327-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Bao W, Wang L, Liu X, Li M. Predicting diagnostic biomarkers associated with immune infiltration in Crohn's disease based on machine learning and bioinformatics. Eur J Med Res. 2023;28:255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 86. | Kou F, Cheng Y, Shi L, Liu J, Liu Y, Shi R, Peng G, Li J. LCN2 as a Potential Diagnostic Biomarker for Ulcerative Colitis-Associated Carcinogenesis Related to Disease Duration. Front Oncol. 2021;11:793760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Deng L, He S, Li Y, Ding R, Li X, Guo N, Luo L. Identification of Lipocalin 2 as a Potential Ferroptosis-related Gene in Ulcerative Colitis. Inflamm Bowel Dis. 2023;29:1446-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 88. | Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 455] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 89. | Makhezer N, Ben Khemis M, Liu D, Khichane Y, Marzaioli V, Tlili A, Mojallali M, Pintard C, Letteron P, Hurtado-Nedelec M, El-Benna J, Marie JC, Sannier A, Pelletier AL, Dang PM. NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol. 2019;12:117-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 90. | Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 623] [Article Influence: 103.8] [Reference Citation Analysis (1)] |
| 91. | Moschen AR, Gerner RR, Wang J, Klepsch V, Adolph TE, Reider SJ, Hackl H, Pfister A, Schilling J, Moser PL, Kempster SL, Swidsinski A, Orth Höller D, Weiss G, Baines JF, Kaser A, Tilg H. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe. 2016;19:455-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 92. | Playford RJ, Belo A, Poulsom R, Fitzgerald AJ, Harris K, Pawluczyk I, Ryon J, Darby T, Nilsen-Hamilton M, Ghosh S, Marchbank T. Effects of mouse and human lipocalin homologues 24p3/lcn2 and neutrophil gelatinase-associated lipocalin on gastrointestinal mucosal integrity and repair. Gastroenterology. 2006;131:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Qiu X, Macchietto MG, Liu X, Lu Y, Ma Y, Guo H, Saqui-Salces M, Bernlohr DA, Chen C, Shen S, Chen X. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int J Obes (Lond). 2021;45:143-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 94. | Yeoh BS, Aguilera Olvera R, Singh V, Xiao X, Kennett MJ, Joe B, Lambert JD, Vijay-Kumar M. Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am J Pathol. 2016;186:912-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Zhou L, Yan Z, Yang W, Buckley JA, Al Diffalha S, Benveniste EN, Qin H. Socs3 expression in myeloid cells modulates the pathogenesis of dextran sulfate sodium (DSS)-induced colitis. Front Immunol. 2023;14:1163987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 96. | Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 97. | Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Bäumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 98. | Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:1834-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 377] [Article Influence: 18.9] [Reference Citation Analysis (9)] |
| 99. | Zhang K, Chen J, Liang L, Wang Z, Xiong Q, Yu H, Du H. Lcn2 deficiency accelerates the infection of Escherichia coli O157:H7 by disrupting the intestinal barrier function. Microb Pathog. 2023;185:106435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 100. | Toyonaga T, Matsuura M, Mori K, Honzawa Y, Minami N, Yamada S, Kobayashi T, Hibi T, Nakase H. Lipocalin 2 prevents intestinal inflammation by enhancing phagocytic bacterial clearance in macrophages. Sci Rep. 2016;6:35014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Klüber P, Meurer SK, Lambertz J, Schwarz R, Zechel-Gran S, Braunschweig T, Hurka S, Domann E, Weiskirchen R. Depletion of Lipocalin 2 (LCN2) in Mice Leads to Dysbiosis and Persistent Colonization with Segmented Filamentous Bacteria. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Wang Z, Zhou X, Hu X, Zheng C. Quercetin ameliorates Helicobacter pylori-induced gastric epithelial cell injury by regulating specificity protein 1/lipocalin 2 axis in gastritis. J Appl Toxicol. 2024;44:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 104. | Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 336] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 105. | Fung KY, Priebe I, Purins L, Tabor B, Brierley GV, Lockett T, Nice E, Gibbs P, Tie J, McMurrick P, Moore J, Ruszkiewicz A, Burgess A, Cosgrove LJ. Performance of serum lipocalin 2 as a diagnostic marker for colorectal cancer. Cancer Biomark. 2013;13:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Barresi V, Di Gregorio C, Reggiani-Bonetti L, Ieni A, Ponz-De Leon M, Barresi G. Neutrophil gelatinase-associated lipocalin: a new prognostic marker in stage I colorectal carcinoma? Hum Pathol. 2011;42:1720-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | McLean MH, Thomson AJ, Murray GI, Fyfe N, Hold GL, El-Omar EM. Expression of neutrophil gelatinase-associated lipocalin in colorectal neoplastic progression: a marker of malignant potential? Br J Cancer. 2013;108:2537-2541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Gawel DR, Lee EJ, Li X, Lilja S, Matussek A, Schäfer S, Olsen RS, Stenmarker M, Zhang H, Benson M. An algorithm-based meta-analysis of genome- and proteome-wide data identifies a combination of potential plasma biomarkers for colorectal cancer. Sci Rep. 2019;9:15575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Martí J, Fuster J, Solà AM, Hotter G, Molina R, Pelegrina A, Ferrer J, Deulofeu R, Fondevila C, García-Valdecasas JC. Prognostic value of serum neutrophil gelatinase-associated lipocalin in metastatic and nonmetastatic colorectal cancer. World J Surg. 2013;37:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Lin HM, Chatterjee A, Lin YH, Anjomshoaa A, Fukuzawa R, McCall JL, Reeve AE. Genome wide expression profiling identifies genes associated with colorectal liver metastasis. Oncol Rep. 2007;17:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Maier HT, Aigner F, Trenkwalder B, Zitt M, Vallant N, Perathoner A, Margreiter C, Moser P, Pratschke J, Amberger A. Up-regulation of neutrophil gelatinase-associated lipocalin in colorectal cancer predicts poor patient survival. World J Surg. 2014;38:2160-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Dayama G, Priya S, Niccum DE, Khoruts A, Blekhman R. Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Med. 2020;12:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 113. | Kim SL, Min IS, Park YR, Lee ST, Kim SW. Lipocalin 2 inversely regulates TRAIL sensitivity through p38 MAPK-mediated DR5 regulation in colorectal cancer. Int J Oncol. 2018;53:2789-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Raghavan R, Koyande N, Beher R, Chetlangia N, Ramadwar M, Pawade S, Thorat R, van Hengel J, Sklyarova T, van Roy F, Dalal SN. Plakophilin3 loss leads to increased adenoma formation and rectal prolapse in APC(min) mice. Biochem Biophys Res Commun. 2022;586:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Reilly PT, Teo WL, Low MJ, Amoyo-Brion AA, Dominguez-Brauer C, Elia AJ, Berger T, Greicius G, Pettersson S, Mak TW. Lipocalin 2 performs contrasting, location-dependent roles in APCmin tumor initiation and progression. Oncogene. 2013;32:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y, Kim JS. Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int J Cancer. 2006;118:2490-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 117. | Kim SL, Lee ST, Min IS, Park YR, Lee JH, Kim DG, Kim SW. Lipocalin 2 negatively regulates cell proliferation and epithelial to mesenchymal transition through changing metabolic gene expression in colorectal cancer. Cancer Sci. 2017;108:2176-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Harati MD, Amiri F, Jaleh F, Mehdipour A, Harati MD, Molaee S, Bahadori M, Shokrgozar MA, Jalili MA, Roudkenar MH. Targeting delivery of lipocalin 2-engineered mesenchymal stem cells to colon cancer in order to inhibit liver metastasis in nude mice. Tumour Biol. 2015;36:6011-6018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Feng M, Feng J, Chen W, Wang W, Wu X, Zhang J, Xu F, Lai M. Lipocalin2 suppresses metastasis of colorectal cancer by attenuating NF-κB-dependent activation of snail and epithelial mesenchymal transition. Mol Cancer. 2016;15:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 120. | Parmar T, Parmar VM, Perusek L, Georges A, Takahashi M, Crabb JW, Maeda A. Lipocalin 2 Plays an Important Role in Regulating Inflammation in Retinal Degeneration. J Immunol. 2018;200:3128-3141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Gupta U, Ghosh S, Wallace CT, Shang P, Xin Y, Nair AP, Yazdankhah M, Strizhakova A, Ross MA, Liu H, Hose S, Stepicheva NA, Chowdhury O, Nemani M, Maddipatla V, Grebe R, Das M, Lathrop KL, Sahel JA, Zigler JS Jr, Qian J, Ghosh A, Sergeev Y, Handa JT, St Croix CM, Sinha D. Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD. Autophagy. 2023;19:92-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 122. | Li J, Xu P, Hong Y, Xie Y, Peng M, Sun R, Guo H, Zhang X, Zhu W, Wang J, Liu X. Lipocalin-2-mediated astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3 inflammasome activation in cerebral ischemia/reperfusion injury. J Neuroinflammation. 2023;20:148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 123. | Adler O, Zait Y, Cohen N, Blazquez R, Doron H, Monteran L, Scharff Y, Shami T, Mundhe D, Glehr G, Kanner AA, Horn S, Yahalom V, Haferkamp S, Hutchinson JA, Bleckmann A, Nahary L, Benhar I, Yust Katz S, Pukrop T, Erez N. Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2. Nat Cancer. 2023;4:401-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 124. | Jung BK, Ryu KY. Lipocalin-2: a therapeutic target to overcome neurodegenerative diseases by regulating reactive astrogliosis. Exp Mol Med. 2023;55:2138-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 125. | Li Y, Tang C, Vanarsa K, Thai N, Castillo J, Lea GAB, Lee KH, Kim S, Pedroza C, Wu T, Saxena R, Mok CC, Mohan C. Proximity extension assay proteomics and renal single cell transcriptomics uncover novel urinary biomarkers for active lupus nephritis. J Autoimmun. 2024;143:103165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 126. | Stanski NL, Rodrigues CE, Strader M, Murray PT, Endre ZH, Bagshaw SM. Precision management of acute kidney injury in the intensive care unit: current state of the art. Intensive Care Med. 2023;49:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 127. | Swolinsky JS, Hinz RM, Markus CE, Singer E, Bachmann F, Halleck F, Kron S, Naik MG, Schmidt D, Obermeier M, Gebert P, Rauch G, Kropf S, Haase M, Budde K, Eckardt KU, Westhoff TH, Schmidt-Ott KM. Plasma NGAL levels in stable kidney transplant recipients and the risk of allograft loss. Nephrol Dial Transplant. 2024;39:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 128. | Kim KE, Lee J, Shin HJ, Jeong EA, Jang HM, Ahn YJ, An HS, Lee JY, Shin MC, Kim SK, Yoo WG, Kim WH, Roh GS. Lipocalin-2 activates hepatic stellate cells and promotes nonalcoholic steatohepatitis in high-fat diet-fed Ob/Ob mice. Hepatology. 2023;77:888-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 129. | Lee EH, Lee JH, Kim DY, Lee YS, Jo Y, Dao T, Kim KE, Song DK, Seo JH, Seo YK, Seong JK, Moon C, Han E, Kim MK, Ryu S, Shin M, Roh GS, Jung HR, Osborne TF, Ryu D, Jeon TI, Im SS. Loss of SREBP-1c ameliorates iron-induced liver fibrosis by decreasing lipocalin-2. Exp Mol Med. 2024;56:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 130. | Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, Ramachandran V, Sung B, Chakraborty A, Bresalier RS, Logsdon C, Aggarwal BB, Krishnan S, Guha S. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100-6108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 131. | Gomez-Chou SB, Swidnicka-Siergiejko AK, Badi N, Chavez-Tomar M, Lesinski GB, Bekaii-Saab T, Farren MR, Mace TA, Schmidt C, Liu Y, Deng D, Hwang RF, Zhou L, Moore T, Chatterjee D, Wang H, Leng X, Arlinghaus RB, Logsdon CD, Cruz-Monserrate Z. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017;77:2647-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 132. | Hu S, Zhu Y, Zhao X, Li R, Shao G, Gong D, Hu C, Liu H, Xu K, Liu C, Xu M, Zhao Z, Li T, Hu Z, Shao M, Liu J, Li X, Wu H, Li J, Xu Y. Hepatocytic lipocalin-2 controls HDL metabolism and atherosclerosis via Nedd4-1-SR-BI axis in mice. Dev Cell. 2023;58:2326-2337.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 133. | Xia Y, Ge G, Xiao H, Wu M, Wang T, Gu C, Yang H, Geng D. REPIN1 regulates iron metabolism and osteoblast apoptosis in osteoporosis. Cell Death Dis. 2023;14:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 134. | Yan L, Yang F, Wang Y, Shi L, Wang M, Yang D, Wang W, Jia Y, So KF, Zhang L. Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice. Nat Commun. 2024;15:3034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/