Published online Nov 14, 2024. doi: 10.3748/wjg.v30.i42.4544
Revised: August 26, 2024
Accepted: October 8, 2024
Published online: November 14, 2024
Processing time: 218 Days and 7.5 Hours
Acute pancreatitis (AP), the initially triggered inflammatory process in the pancreas, can be life-threatening. It has been reported that 15-lipoxygenase may promote the removal of damaged intracellular components, maintain intracellular homeostasis, and promote apoptosis by upregulating the activity of caspases. Despite an increased understanding of the lipoxygenase pathway in inflammation and immune diseases, the role of the Alox15 gene product in modulating the inflammatory changes during AP is not well defined.
To investigate the effect of Alox15 expression in cerulein-induced AP in rats.
Model rats were transfected with Alox15 by injecting a recombinant lentivirus vector encoding Alox15 into the left gastric artery before inducing AP. The expression of Alox15 was then assessed at the mRNA and protein levels.
Our in vivo results showed that serum amylase activity and pancreatic tissue water content were significantly reduced in Alox15-transfected rats. Further, the mRNA expression levels of tumor necrosis factor alpha, interleukin (IL)-1β, IL-6, and monocyte chemoattractant protein-1, as well as the protein expression of nuclear factor kappa B in pancreatic tissue were reduced. Additionally, we observed an upregulation of cleaved caspase-3 that implies an induction of apoptosis in pancreatic cells. The transfection of Alox15 resulted in a lower number of autophagic vacuoles in AP.
Our findings demonstrate a regulatory role of Alox15 in apoptosis and autophagy, making it a potential therapeutic target for AP.
Core Tip: Our in vivo data demonstrated for the first time that Alox15 transfection attenuated cerulein-induced acute pancreatitis (AP) in a murine model, highlighting its regulatory role in inflammation, apoptosis, and autophagy. These findings suggested that Alox15 may serve as a potential therapeutic target for the treatment of AP by modulating the key cellular processes involved in the pathology of this disease.
- Citation: Sun HW, Bai YY, Qin ZL, Li RZ, Madzikatire TB, Akuetteh PDP, Li Q, Kong HR, Jin YP. Transfection of 12/15-lipoxygenase effectively alleviates inflammatory responses during experimental acute pancreatitis. World J Gastroenterol 2024; 30(42): 4544-4556
- URL: https://www.wjgnet.com/1007-9327/full/v30/i42/4544.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i42.4544
Acute pancreatitis (AP) is a common inflammatory disease of the digestive tract, triggered by abnormal activation of enzymes in the pancreas, leading to pancreatic self-digestion and local tissue damage. The common causes for this disease include gallstones, alcoholism, and infections, which can lead to local or systemic complications[1]. Severe AP, especially when accompanied by organ failure or complications, is associated with a significant increase in mortality[2]. It has been shown that the complete resolution of inflammation and re-establishment of homeostasis is the ideal outcome of the defense mechanisms employed by the body against inflammation. To alleviate inflammation, our body must maintain a homeostasis of the internal environment. One of the primary mechanisms for attaining this goal is the generation of endogenous lipid mediators, with potent physiological properties like anti-inflammation, pro-resolving properties, phagocytosis, and the elimination of pathogens and apoptotic cells, preventing a progression from an acute to a chronic disease state and the induction of autoimmune disease[3-6].
Lipoxygenases are lipid-peroxidizing enzymes that oxidize polyunsaturated fatty acids to generate bioactive lipid metabolites such as leukotrienes, lipoxins, hepoxilins, resolvins, and protectins[7-12]. However, the pathophysiological role of lipoxygenases is not restricted to the synthesis of lipid mediators as they also play additional roles in other mechanisms such as autophagy, apoptosis, and ferroptosis[13-17]. Lipoxygenases are involved in regulating autophagy, promoting the removal of damaged intracellular components, maintaining intracellular homeostasis, and promoting apoptosis by upregulating the activity of caspases[18,19]. In addition, the activity of lipoxygenases is also associated with ferroptosis, an iron-dependent mode of programmed cell death. Lipoxygenases may also be involved in the regulation of iron-dependent death by affecting lipid peroxidation and cell membrane integrity[20]. Compelling evidence has already corroborated that lipoxygenases are involved in the pathogenesis of pancreatitis, particularly by regulating the activation of leukocytes and the release of inflammatory mediators. Murine 12/15-lipoxygenase (12/15-LO), encoded by the Alox15 gene and the human 15-lipoxygenase gene share multiple similarities, including expression patterns, biological effects, and 73% identical amino acid sequences[21,22]. It has been suggested that the names of the different lipoxygenase isoforms should follow their encoding genes. For example, all the 12/15-LO orthologs from various species should be named Alox15[23].
Advances in gene transfection, including chemical methods (such as calcium phosphate and liposomes), physical methods (like electroporation and microinjection), and viral vectors (which use modified viruses to deliver genes into cells), have facilitated the investigation of the role of Alox15 in vivo[24,25]. To explore the effects of Alox15 on inflammation in a murine model of AP, we employed a high transfection efficiency recombinant lentiviral vector encoding Alox15, which was delivered to the target tissue via the regional arterial infusion (RAI) technique. The RAI technique is a drug delivery system known for the delivery of drugs to the precise location of the disease, thus, ensuring increased concentration in the target tissue[26]. Therefore, the RAI technique was used to deliver the recombinant Alox15-lentiviral vector to the pancreas.
To date, the role of lipoxygenase enzymes in inflammatory diseases remains largely elusive due to the paucity of investigations[27,28]. Notably, the biological functions of Alox15 in AP have yet to be defined with clarity. We transfected rats with Alox15-lentivirus via a left gastric artery injection and investigated its effects in the cerulein-induced rat model of AP. Therefore, our present study elucidates the mechanisms by which Alox15 expression influences AP in vivo.
The materials used include cerulein, pentobarbital sodium, collagenase 1, and tublin (Sigma, St. Louis, MO, United States); PE-10 catheter (An Lai Software and Instruments Co. Ltd, Ningbo, China); amylase activity assay kit (Nanjing Jiancheng Biotechnology Research Institute, Nanjing, China); ELISA kit for interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) (eBioscience, California, United States); cleaved caspase 3 and NF-κB p-P65 antibodies (Cell Signaling Biotechnology, Danvers, MA, United States); TUNEL assay (Roche, Indianapolis, IN, United States); the primers of 15-LO, IL-1β, IL-6, TNF-α, and MCP-1 (Sangon Biotech, Shanghai, China); the rats Alox15-lentiviral vector and the control vector (Genechem Co., Ltd., Shanghai, China).
All animal experiment methods were approved by the animal ethics committee, Institutional Animal Committee, of Wenzhou Medical University (Wenzhou, China) and performed per humane care and use policies of laboratory animals.
Twenty-four healthy male SD rats, 10-12 weeks old (270-300 g), were housed individually in cages in room temperature (20-22 °C), maintained in climate-controlled condition using a 12-hour light-dark cycle. All rats were fed standard laboratory chow and fasted overnight before the experiment with water ad libitum.
Twenty-four male SD rats were randomly divided into 4 groups: The control group (no transfection of lentivirus, n = 6), the AP group (no transfection of lentivirus + cerulein-induced AP, n = 6), the Alox15-lentiviral vector group (transfection of Alox15 Lentivirus vector + cerulein-induced AP, n = 6) and the vector control group (transfection of control lentivirus vector + cerulein-induced AP, n = 6). In the Alox15-lentiviral vector group, 5 × 107Alox15 lentivirus vector transfected rats through left gastric artery infusion were included. In the control group and the AP group, the same volume of saline was injected via the left gastric artery. The control vector group included 5 × 107 control lentivirus vector transfected rats via left gastric artery infusion.
Alox15 lentivirus needs to be prepared first. Both Alox15 and control lentivirus vectors were bought from Shanghai Genechem Co., Ltd. (Shanghai, China). Alox15 gene was PCR amplified with specific primers and cloned into the lentiviral expression vector GV287 to create a recombinant plasmid. We carried out virus packaging in HEK-293 T cells co-transfected by Alox15 lentivirus. After 72 hours of transfection, viruses were extracted and viral titers were assessed.
Prior to all surgical procedures the animals were fasted for 12 hours with free access to water. Subcutaneous injection of 1.5% pentobarbital sodium (1 mg/kg) was employed to anesthetize the rats. Post gastric artery exploration, a sharp tip of PE-10 catheter (inner diameter 0.25 mm, outer diameter 0.5 mm) was inserted through the left gastric artery in retrograde into the celiac axis and was fixed for the concomitant infusion. All surgical procedures were performed under sterile conditions.
The dosage and the timing of delivery of Alox15 lentivirus vectors to rats were based on our preliminary experimental results. Alox15 lentivirus vectors and control lentivirus vectors [5 × 107 particle flux unit in 0.2 mL sterile phosphate buffered saline (PBS)] were delivered through the left gastric artery on day 5 before AP induction. An identical volume of PBS was administered into both the control and AP groups via the left gastric artery. Post transfection, the catheter was removed, the left gastric artery was ligated, and the incision was closed. The duration of the procedure was about 30 minutes. Transfection was determined at the end of the experiments as described below.
Five days following the left gastric artery infusion, AP was induced for six hours by supramaximal hourly intraperitoneal injection dosages of cerulein (50 ug/kg body weight, six times at 1-hour intervals)[29]. Cerulein was diluted in 0.9% saline. Saline was administered the same way in the control group. Rats were sacrificed 3 hours after the last cerulein injection. This time point corresponded to pancreatic tissue injury observed in this model, defined by pathological changes[29].
We used an overdose of 1.5% pentobarbital sodium (2 mg/kg) to sacrifice the rats. Blood samples were drawn from the aorta and centrifuged at 4 °C, while serum was kept at -80 °C until further analysis. The pancreas was extracted and divided into sections. The pancreas tail was snap-frozen in liquid nitrogen and kept at -80 °C for further measurement. The pancreatic body was utilised to quantify its tissue water content.
The pancreatic head tissue was immediately harvested, stored in 4% formaldehyde solution, embedded in paraffin, cut into sections (4 μm), and then stained with hematoxylin-eosin (HE) according to the manufacturer's protocol. According to Rongione et al[30], the pancreatic tissue pathological grading was performed in a blinded manner. using a scale of 0 to 4 based on the degree of inflammation, vacuolization, edema and necrosis.
Pancreatic tissue edema was evaluated by tissue water content. The pancreas was harvested immediately after sacrificing the rats, rinsed in PBS, dried, and weighed (wet weight). The pancreatic tissue was then stored at 95 °C to dry for 12 hours in an electric oven and then weighed again (dry weight). The pancreatic tissue water content was calculated using the formula: [water content = (wet weight – dry weight) / dry weight × 100%][31].
Serum amylase activity was determined by spectrophotometric assay using a Beckman Coulter AU5821 automatic biochemistry analyzer (Beckman Coulter, Inc., Fullerton, CA, United States) and was expressed in U/L.
Serum levels of TNF-α, IL-1β, IL-6, and MCP-1 were determined through ELISA using kits provided by the manufacturers.
Pancreatic tissue samples were homogenized in radioimmunoprecipitation assay lysis buffer supplemented with a protease inhibitor for total cell extraction. Protein lysates were separated by 10% sodium dodecyl SDS-PAGE and then exported to PVDF membranes. Membranes were blocked with 5% non-fat dried milk in Tris-buffered saline with Tween-20 (TBST) (0.1% Tween 20 in the Tris-buffered saline buffer) for 2 hours at room temperature. It was then incubated with one of the following primary antibodies overnight at a temperature of 4 °C: P-P65 (1:1000), or cleaved caspase-3 (1:500). The membranes were rinsed 3 times with TBST and then incubated with secondary antibody for 60 minutes at room temperature. Finally, they were washed 3 times for 10 minutes in TBST, and with the help of enhanced chemiluminescence target bands were developed and exposed on film. Tubulin was used as an endogenous control.
Immunofluorescence for Alox-15 in vivo was performed on snap-frozen sections of the pancreas from rats. Frozen sections were obtained from OCT-embedded pancreas tissue, and snap-frozen tissue was sectioned at 5-µm thickness. After blocking Fc receptor with 10% goat serum in PBS, frozen sections were incubated overnight with anti-Alox15 monoclonal Antibody (Invitrogen, MA5-25891, 1:100). After rinsing three times with PBS, the sections were incubated for 60 minutes with secondary antibodies Alexa Fluor® 488 to the primary antibodies. The images were acquired using a Leica SP8 LIGHTNING confocal microscope (Wetzlar, Germany). All of the preceding activities were carried out in the dark.
Determination of pancreatic acinar cell death was based on a TUNEL assay. As previously illustrated TUNEL assay was performed to analyze DNA breakages[29,32]. Pancreatic tissue was stored in 4% formaldehyde solution, embedded in paraffin, cut into 4 µm-thick and the sections adhered to glass slides.
According to the manufacturer’s protocol, sections were deparaffinized, hydrated, and stained for breaks in DNA using terminal deoxynucleotidyl transferase and fluorescein isothiocyanate-labeled dUTP (Roche, Indianapolis, IN, United States). The pancreatic sample was scanned from a glass slide. Ten images from different locations of the scanned slide were investigated by a digital camera under a magnification of 200 × and processed with an image-analysis software called (Panoramic+Viewer). Data were expressed as TUNEL positive cells per field.
A 1 mm3 portion of pancreatic tissue from each rat was fixed in 2.5% phosphate-buffered glutaraldehyde for 24 hours at 4 °C then rinsed with 0.1% sodium phosphate buffer for 15 minutes. The samples were then fixed in 1% osmium tetroxide for 2 hours at 4 °C. Then rinsed with 0.1% sodium phosphate buffer for 15 minutes. The samples were embedded in Epon-812 after being dehydrated in a series of grades of ethanol. A transmission electron microscope (Hitachi, Ltd., Tokyo, Japan) was used to analyse ultrathin sections that had been double-stained with uranyl acetate and lead citrate.
According to the manufacturer’s instruction, total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, United States). RNA concentration and quality were audited using a NANODROP 2000C spectrophotometer (ThermoFisher Scientific, Waltham, MA, United States). cDNA was synthesized from 1 ug of total RNA using a RevertAid RT Reverse Transcription Kit (ThermoFisher Scientific). Q-PCR was performed by SYBR Green I chimeric fluorescence method (HiScript II One Step qRT-PCR SYBR Green Kit, Vazyme). The conditions of Q-PCR were: 95 °C reaction for 10 minutes, 95 °C reaction for 15 seconds, and then 60 °C reaction for 1 minute, the number of reaction cycles was 45, and then the fluorescence signal was obtained at 60 °C. After the PCR reaction, 2-0Ct method was used to analyze the results of gene relative expression. The reaction was made in triplicate. The relative expression of mRNA of interest was expressed as 2 (−△△CT). Primers for IL-1β, IL-6, TNF-α, MCP-1, Alox15, and GAPDH are primarily designed using the Primer-BLAST tool on the website https: //www.ncbi.nlm.nih.gov/. By entering the target sequences and setting the appropriate primer design parameters, suitable primers can be identified. Finally, the Primer-BLAST tool is used to perform specificity checks to ensure that the designed primers do not bind to non-target sequences. The sequence of primers used for SYBR green Q-PCR is listed in Table 1. The expression level was normalized to GAPDH.
| Primer | Sequence | Nucleotide length | |
| IL-1β | Forward | 5′-CACCTTCTTTTCCTTCATCTTTG-3′ | 23 |
| Reverse | 5′-GTCGTTGCTTGTCTCTCCTTGTA-3′ | 23 | |
| IL-6 | Forward | 5′-TGATGGATGCTTCCAAACTGGA-3′ | 22 |
| Reverse | 5′-GAGCATTGGAAGTTGGGGTA-3′ | 20 | |
| TNF-α | Forward | 5′-ACTGAACTTCGGGGTGATTGG-3′ | 21 |
| Reverse | 5′-GCTTGGTGGTTTGCTACGAC-3′ | 20 | |
| MCP-1 | Forward | 5′-TCTCACTTGGTTCTGGTCCAGT-3′ | 22 |
| Reverse | 5′-GGCCTGTTGTTCACAGTTGCT-3′ | 21 | |
| Alox15 | Forward | 5′-GTCTACTCCACCACCTATTTTC-3′ | 22 |
| Reverse | 5′-CCTGTGCTCATTGCCTTGTC-3′ | 20 | |
| GAPDH | Forward | 5′-TGACTTCAACAGCGACACCCA-3′ | 21 |
| Reverse | 5′-GCTGTTGGGCTGTAGGGA-3′ | 18 |
The mean SD was calculated using the Statistical Package for the Social Sciences software SPSS version 18.0 (SPSS, Inc., Chicago, IL, United States). Student's t-test and one-way analysis of variance (ANOVA) were employed to analyse parameters of equal variances and normal distributions, followed by the Games-Howell test as a post hoc test. The Mann-Whitney U test was used for assessing parameters with disparate variances. Statistical significance was considered as P < 0.05 for all analyses.
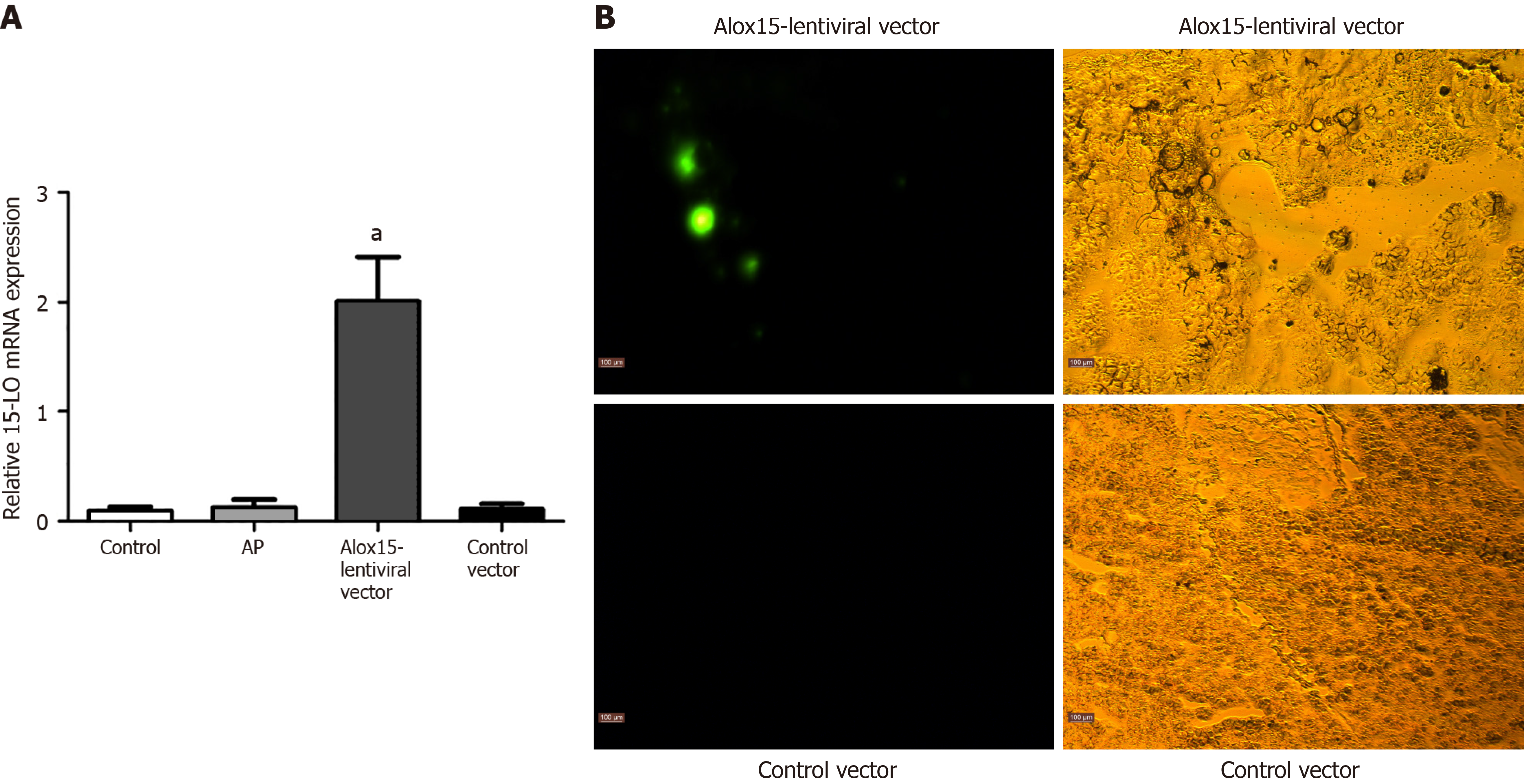
To quantify Alox15 mRNA expression in the pancreas, we employed reverse transcriptase-polymerase chain reaction (RT-PCR), which showed an elevated Alox15 expression in the Alox15-lentiviral vector group (Figure 1A). We used immunofluorescence microscopy to further confirm increased Alox15 expression (Figure 1B). Collectively, our data showed that Alox15-lentiviral vector transfection increased Alox15 expression in rats.
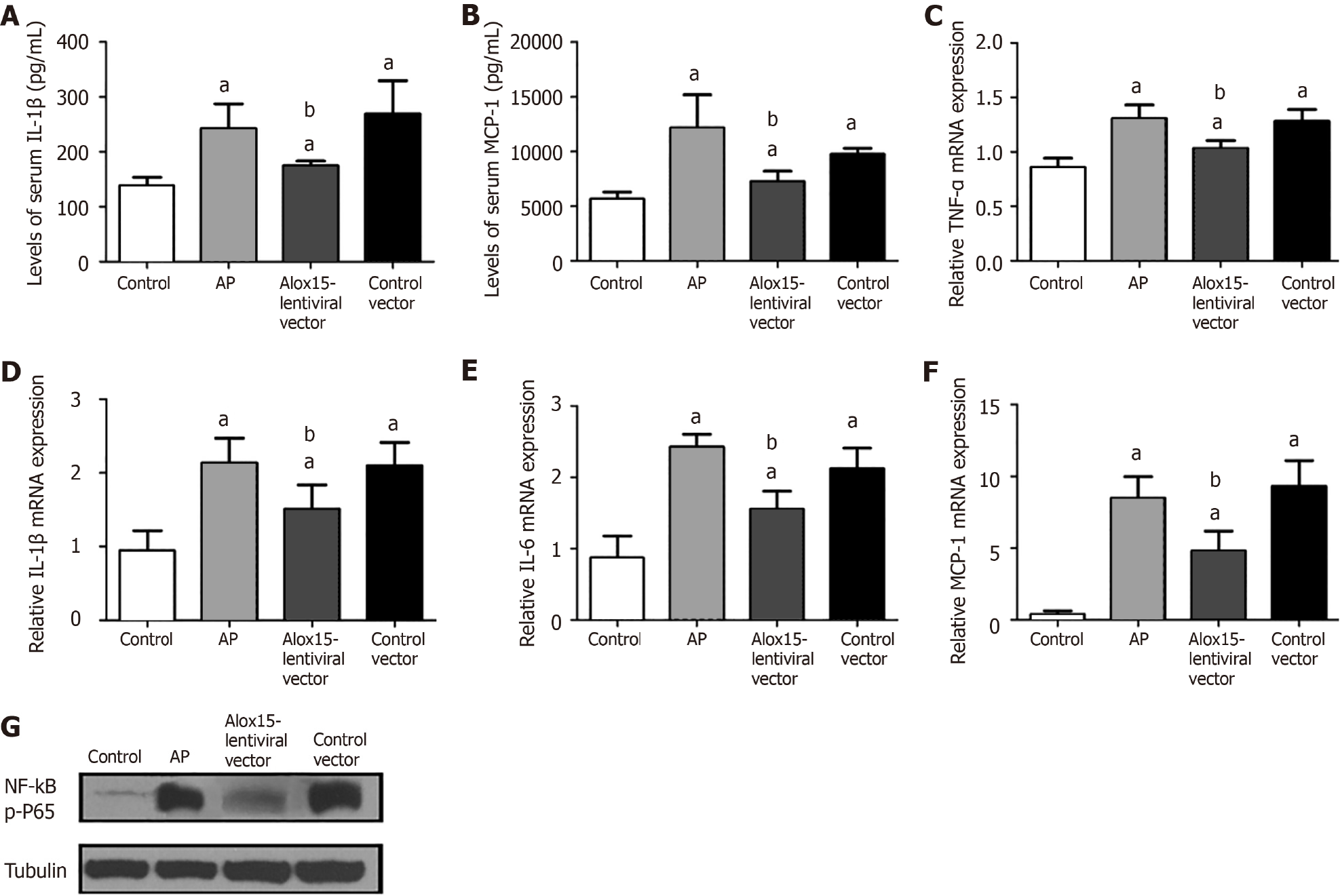
Following the induction of AP, we observed an increase in serum levels of IL-1β and MCP-1 in the control vector group. When compared to control rats, animals in the Alox15-lentiviral vector group exhibited a significant reduction in serum levels of IL-1β and MCP-1 (Figure 2A and B).
To assess the extent of inflammation in the pancreas, we measured the mRNA expression levels of inflammatory mediators, namely TNF-α, IL-1β, IL-6, and MCP-1 using RT-PCR. Cerulein treatment augmented the mRNA expression of these pro-inflammatory factors in the AP group and the control vector group but not in the Alox15-lentiviral vector group where their expression was reduced (Figure 2C-F). The rats in the AP and control vector groups exhibited similar mRNA expression profiles following the cerulein challenge.
Using western blot analysis, we assessed the expression of the inflammatory regulator phospho-p65 (p-P65) in pancreatic tissue (Figure 2G). The pancreatic tissue of cerulein-challenged AP rats exhibited a significant upregulation of p-P65 expression compared with the control group rats. Conversely, we observed reduced p-P65 expression in the pancreas of rats in the Alox15-lentiviral compared with the control vector group.
Taken together, the above results suggested an anti-inflammatory role of Alox15 in cerulein-induced inflammation in a rat model of AP and prompted us to investigate the underlying mechanism further and check if this translated to AP resolution.
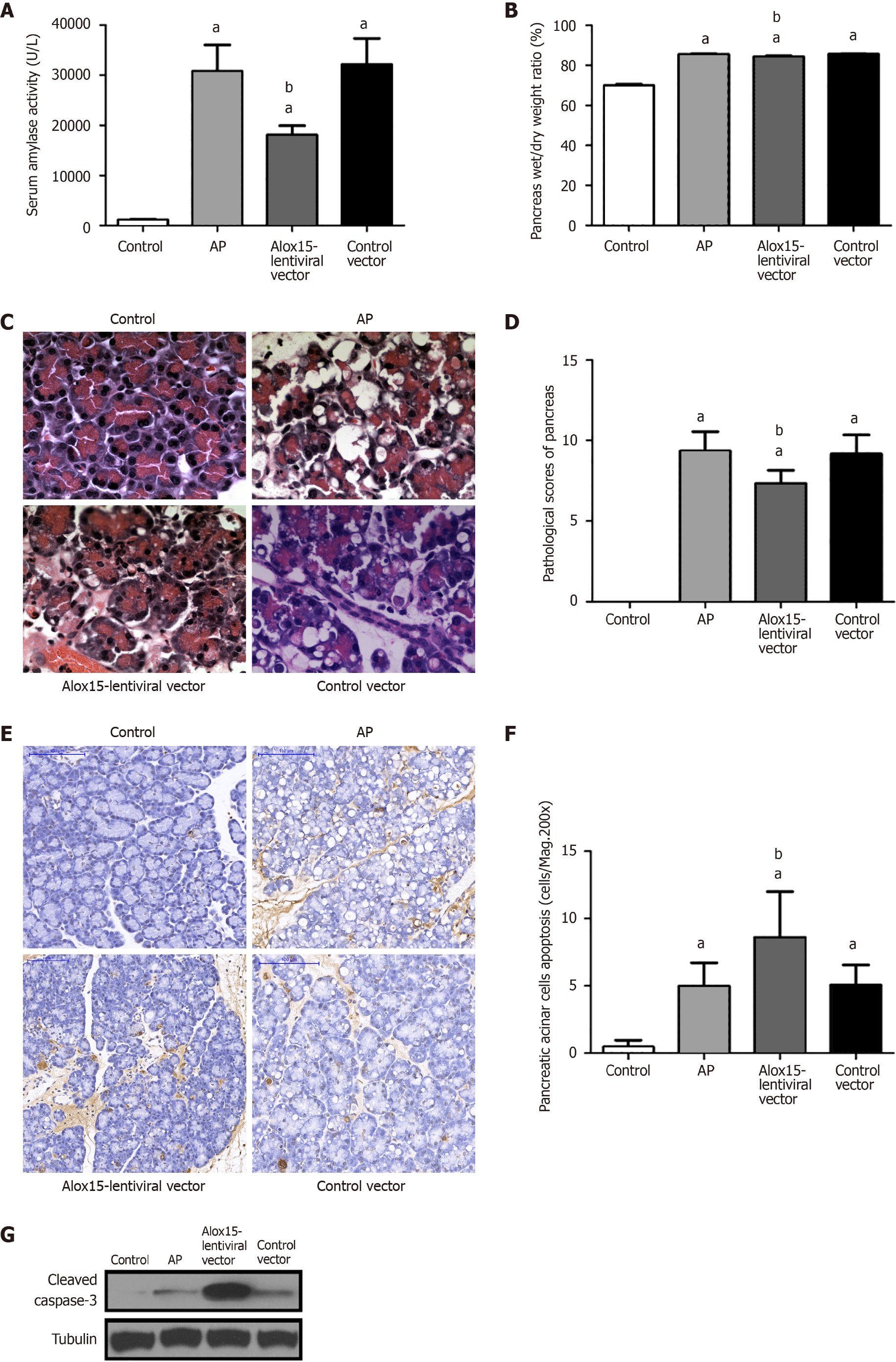
Following the induction of AP, serum amylase levels increased across all disease model groups when compared with the control group; however, these levels were significantly reduced in the Alox15-lentiviral vector group (Figure 3A). Pancreatic edema, measured by the wet-to-dry weight ratio of pancreatic tissue, was significantly higher in the AP, Alox15-lentiviral vector, and control vector groups compared with the control group. However, the wet-to-dry weight ratio of the Alox15-lentiviral vector group was lower than that of the control vector group (Figure 3B).
Histopathologic examination of the pancreas post-cerulein treatment revealed that rats in the AP group, the Alox15-lentiviral vector group, and the control vector group developed AP-associated morphological changes including inflammatory cell infiltration, vacuolization of the tissue cell cytoplasm, and acinar edema. Compared to the marked changes in the control vector group, these changes were alleviated in the pancreas of Alox15-lentiviral vector group rats (Figure 3C). Primarily, we observed moderate vacuolization in the Alox15-lentiviral vector group in contrast to the extensive vacuolization in the control vector group. We employed hematoxylin and eosin-based histochemical staining to quantitatively evaluate the severity of AP in pancreatic tissue sections. Compared to the control group, the AP group, Alox15-lentiviral vector group, and the control vector group had higher pathological scores. However, the Alox15-lentiviral vector group score was significantly lower than that of the AP and control vector groups. No difference in the pathological scores of the AP and control vector groups was observed (Figure 3D).
We evaluated the degree of apoptosis in pancreatic acinar cells using the terminal deoxynucleotidyl transferase dUTP nick end labeling assay at 200X magnification (Figure 3E). We observed an increase in apoptosis in tissue sections from the AP, Alox15-lentiviral vector, and control vector groups (cerulein-treated groups). Compared to the AP and control vector groups, the degree of apoptosis in the Alox15-lentiviral vector group was significantly higher (Figure 3F). The degree of apoptosis in the AP and control vector groups was similar and showed no significant difference. To further validate this, we quantified the expression of cleaved caspase-3 in pancreatic tissue protein extracts using western blotting (Figure 3G). Compared to the control group, the expression of cleaved caspase-3 was elevated in all cerulein-induced AP groups. While no significant difference was observed between the expression in the AP and the control vector groups, the Alox15-lentiviral vector group showed significantly upregulated levels of cleaved caspase-3. These data demonstrated that the increased expression of Alox15 promotes apoptosis of pancreatic acinar cells in a rat model of AP.
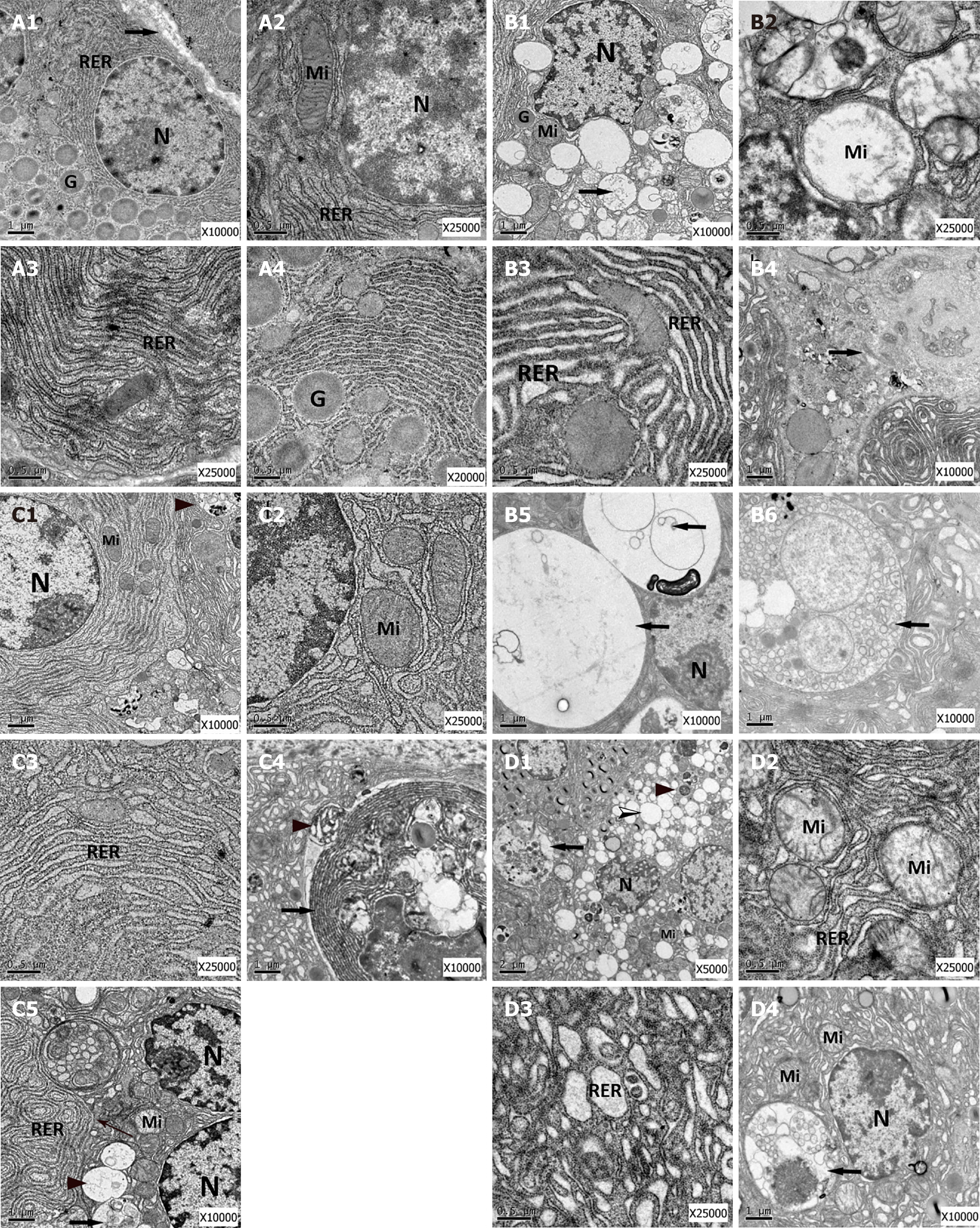
To analyze the effects of Alox15 upregulation on organelle morphology in pancreatic acinar cells, we used high-resolution transmission electron microscopy to acquire micrographs. As shown in Figure 4A, the morphology of intracellular structures in the control group (normal pancreatic acinar cells) comprised a round nucleus, a ribosome-rich cytoplasm, a ribosome-bound rough endoplasmic reticulum (RER), apical plasma membrane-oriented zymogen granules, and mitochondria with intact cristae.
Micrographs from the AP group (Figure 4A) showed typical characteristics of cells in AP. The pancreatic acinar cells were smaller in size with low levels of nuclear pyknosis. This was coupled with a significant reduction in zymogen granules and swollen mitochondria containing disrupted cristae. Further, compared to RER in the control group (Figure 4A), most of the RER architecture in the AP group changed to irregularly dilated sacs with few bound ribosomes. The pancreatic acinar cells in the AP group showed signs of necrosis. Additionally, they had an increased number of large autophagic vacuoles and autophagosomes in the cytoplasm.
The Alox15-lentiviral vector group micrographs showed minor ultrastructural changes in cell structures (Figure 4A). The nuclei of acinar cells showed minor pyknosis while the mitochondria exhibited slight swelling with partially disrupted cristae. In contrast to the AP and control vector groups, the RER did not show extensive dilatation and had fewer irregular sacs in the Alox15-lentiviral vector group micrographs (Figure 4A-C). Further, apoptotic bodies were present in the tissue. Compared to the vector control group, the cytoplasm had small-sized autophagosomes, autolysosomes, and autophagic vacuoles in the Alox15-lentiviral vector group micrographs (Figure 4A-C).
The micrographs acquired from the vector control group (Figure 4D) showed pancreatic acinar cells with generalized disorganization features, which were smaller in size. The nuclei exhibited mild pyknosis. The mitochondria were swollen, and the cristae were disrupted. The RER showed dilation and some parts had formed irregular sacs, showing a reduction in attached ribosomes. Additionally, the cytoplasm had an excessive amount of autolysosomes, autophagic vacuoles, and secondary lysosomes.
12/15-LO or Alox15 is a crucial enzyme in the lipoxin biosynthesis pathway and plays a functional role in its resolution[25]. Further, it plays a novel role in regulating autophagy and apoptosis[33,34]. In this report, we demonstrated for the first time, using in vivo data, that transfected Alox15 alleviates cerulein-induced AP in murine models. Three possible mechanisms may be attributed to this alleviation. First, we found that Alox15 upregulation inhibits inflammation by abrogating the expression nuclear factor kappa B in the pancreas, which reduces the pancreatic expression of IL-1β, IL-6, TNF-α, and MCP-1. Additionally, it reduces the plasma levels of IL-6 and MCP-1. Second, the expression of Alox15 activates caspase-3, which mediates apoptosis and protects pancreatic acinar cells from necrosis. Finally, as revealed by transmission electron micrographs, increased Alox15 expression mitigates organelle abnormalities, stabilizes the cellular structure, and minimizes the autophagic dysfunction in AP. Consequently, our present findings indicated that Alox15 attenuates cerulein-induced AP in rats via its regulation of inflammation, apoptosis, and autophagy.
Recently, lipoxygenases as well as their active metabolites have been revealed to participate in various types of programmed and unprogrammed cell death including apoptosis, autophagy, and ferroptosis[13,14,16]. Based on existing evidence, 12/15-LO signaling is thought to influence the apoptotic pathway[35]. However, emerging findings suggest that 12/15-LO is also involved in autophagy and ferroptosis[14,15,33,34].
Autophagy is an intercellular catabolic recycling pathway that regulates homeostasis by digesting cellular constitutes using autophagosome-contained lysosomal enzymes[36]. Autophagy primarily degrades macromolecular substances, including damaged organelles in cells, and maintains the intracellular environment in a steady state which is necessary for metabolic activities[36,37]. Autophagy can be classified into various categories in correspondence with the mode of transport of the cell materials to the lysosomes[38,39] that include but are not limited to chaperone-mediated autophagy, microautophagy, and macroautophagy. Of these categories, macroautophagy is the most common and degrades cell constituents such as mitochondria, ribosomes, and the endoplasmic reticulum by forming an enclosed lipid bilayer membrane called the autophagosome. The autophagosome fuses with lysosomes, forming an autolysosome, a monolayer membrane structure where degradation occurs[36].
The accumulation of different-sized vacuoles is an early and key pathological feature of pancreatitis. These vacuoles have been confirmed to contain activated trypsin[40]. Hashimoto et al[40] reported that cytoplasmic vacuoles induced in experimental AP are autophagic in origin, and positively correlate with trypsinogen activation and the onset of AP. Interestingly, during fasting, autophagy increases in the pancreas. However, it neither mediates trypsinogen activation nor leads to pancreatitis. When compared with fasting, autophagic vacuoles in pancreatitis are more substantial and emerge in larger numbers[41]. It remains unclear why fasting-mediated autophagic vacuoles do not induce trypsinogen activation in the pancreas like pancreatitis autophagic vacuoles. Mareninova et al[41] showed that the processing and activation of lysosomal proteases cathepsin L and cathepsin B were dysregulated, thus leading to impaired autophagy, which facilitated trypsinogen activation in pancreatitis. Impaired autophagy mediates two critical manifestations of AP, namely the accumulation of large vacuoles in acinar cells and intra-acinar trypsinogen activation[41-43].
Transmission electron microscopy is the gold standard for the morphological characterization of autophagy. A sustained increase in autophagic vacuoles, recognized as "autophagic stress," results from the disruption of finely balanced regulatory mechanisms and promotes programmed cell death and disease[44]. The results of our electron microscopy data showed that pancreatic acinar cell organelles from the AP and control vector groups were in disarray. Nuclear condensation, mitochondrial swelling, RER dilation and a large number of autophagosomes were apparent in the cytoplasm. However, autophagosomes in the Alox15-lentiviral vector group were smaller in size and occurred in lower numbers. The ultrastructural changes in the Alox15 Lentivirus group were characterized by minor mitochondrial swelling and mild endoplasmic reticulum dilation.
Taken together, our transmission electron microscopy findings provide evidence that the ultrastructural abnormalities of pancreatic acinar cells, and their organelles, induced by cerulein-mediated AP, were significantly reduced in rats exposed to Alox15. Thus, our data indicated that the transfection of Alox15 confers protection of pancreatic acinar cells. This is consistent with previous reports that 12/15-LO is required for physiological autophagy and that its inhibition or deficiency propagates autophagic dysfunction[15,43]. Compared with the AP and vector control groups, the cytoplasm in the Alox-15 transfection group pancreatic tissue showed small-sized autophagosomes, autolysosomes, and autophagic vacuoles via electron microscopy. These findings show that autophagic dysfunction is relieved by Alox15 transfection, thereby reducing trypsinogen activation and mitigating acinar cell damage, which could be essential for the abrogation of disease progression.
Apoptosis is a complex, fundamental, and conserved programmed cell death process initiated to remove unwanted and dysfunctional cells in organisms during development, homeostasis and disease[45,46]. The execution of apoptosis is largely dependent on the activation of caspases[47]. Of interest, the activation of caspase-3 is a critical step in the events of intrinsic apoptotic cell death[48]. Morphologically and biochemically, apoptosis differs from necrosis and is less “messy”[49]. Necrotic cell death involves the degradation or rupture of the plasma membrane leading to release or leakage of cellular contents. Conversely, apoptosis does not involve the release of cell constituents as plasma membrane integrity is maintained, and cell contents are degraded in enveloped sacs known as blebs or apoptotic bodies. Necrosis harms neighboring cells as the leaked contents trigger an inflammatory response. Therefore, when compared to apoptosis, necrosis is lethal" for the organism[50]. Parenchymal necrosis of the pancreas is known to cause tremendous complications in AP. Indeed, necrosis directly correlates with disease severity in experimental pancreatitis while apoptosis has an inverse correlation[50,51]. As such, the inhibition of necrosis coupled with the induction of apoptosis is a potential therapeutic strategy in AP[50-52]. The administration of caspase inhibitors has been demonstrated to worsen pancreatitis via the halting of apoptosis and stimulation of necrosis in the rat model[53]. Conversely, the upregulation of caspase activity and the consequent induction of apoptosis decreased necrosis in a mouse model of AP leading to disease resolution[53]. Therefore, caspases stimulate apoptosis and protect against pancreatitis by inhibiting necrosis. Emerging evidence has shown that 12/15-LO upregulates the activity of caspases 3 and 9 and is associated with the activation of apoptosis[17]. As demonstrated in our present study, Alox15 transfection induces the activation of caspase-3 and stimulates apoptosis, thereby attenuating AP, which is consistent with the above hypothesis. We speculate that this facilitates the switching from necrotic cell death (observed in the AP and control vector groups) to apoptotic cell death, which may also be partly responsible for the reduction of inflammation in the pancreas of cerulein-treated rats. Together, our present data reveal that 12/15-LO participates in the process of caspase-dependent apoptosis to protect pancreatic acinar cells.
Although we explored the role of Alox15 in AP, the present study has some limitations. First, disease progression in severe AP is extremely rapid, and it takes time for gene transfection to take effect. Thus, our future research direction will involve efforts to accelerate the onset of Alox15 expression to alleviate severe AP. Also, further research is needed to explore the specific molecular mechanisms employed by Alox15 to alleviate severe AP. Even though we referred to many experimental designs in the literature, the number of rats in our experiments described here was relatively small, and the reliability of the results may have been affected. In future experiments, agonists as well as inhibitors will be introduced to verify the mechanism of action of Alox15 in reducing cerulein-induced AP. These future experiments may explore the role of Alox15 in more depth by comparing the changes in inflammatory indices in agonist- and inhibitor-treated groups of Alox15 with those in the control group.
In summary, during AP progression, an upregulation of 12/15-LO attenuates AP in vivo. This upregulation of 12/15-LO has beneficial anti-inflammatory properties that downregulate AP-induced inflammation. One possible strategy involved in Alox15 upregulation-based pancreatic protection is likely to involve the modulation of apoptosis and autophagy in pancreatic acinar cells.
The authors would like to acknowledge and thank Joshua Banda and Roland Andersson for their help in article correction.
| 1. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 687] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 2. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 614] [Article Influence: 122.8] [Reference Citation Analysis (1)] |
| 3. | Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 5. | Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2011;25:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. 2015;1851:308-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 491] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 8. | Rådmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Haeggström JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866-5898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 687] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 10. | Romano M. Lipoxin and aspirin-triggered lipoxins. ScientificWorldJournal. 2010;10:1048-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Pace-Asciak CR. The hepoxilins and some analogues: a review of their biology. Br J Pharmacol. 2009;158:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922-5943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 779] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 13. | Li QQ, Li Q, Jia JN, Liu ZQ, Zhou HH, Mao XY. 12/15 lipoxygenase: A crucial enzyme in diverse types of cell death. Neurochem Int. 2018;118:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 13336] [Article Influence: 952.6] [Reference Citation Analysis (2)] |
| 15. | Morgan AH, Hammond VJ, Sakoh-Nakatogawa M, Ohsumi Y, Thomas CP, Blanchet F, Piguet V, Kiselyov K, O'Donnell VB. A novel role for 12/15-lipoxygenase in regulating autophagy. Redox Biol. 2015;4:40-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Vishnupriya P, Aparna A, Viswanadha VP. Lipoxygenase (LOX) Pathway: A Promising Target to Combat Cancer. Curr Pharm Des. 2021;27:3349-3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Choudhary R, Kumar M, Katyal A. 12/15-Lipoxygenase debilitates mitochondrial health in intermittent hypobaric hypoxia induced neuronal damage: An in vivo study. Redox Biol. 2022;49:102228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Cai W, Liu L, Shi X, Liu Y, Wang J, Fang X, Chen Z, Ai D, Zhu Y, Zhang X. Alox15/15-HpETE Aggravates Myocardial Ischemia-Reperfusion Injury by Promoting Cardiomyocyte Ferroptosis. Circulation. 2023;147:1444-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 280] [Article Influence: 93.3] [Reference Citation Analysis (8)] |
| 19. | Yin Z, Ding G, Chen X, Qin X, Xu H, Zeng B, Ren J, Zheng Q, Wang S. Beclin1 haploinsufficiency rescues low ambient temperature-induced cardiac remodeling and contractile dysfunction through inhibition of ferroptosis and mitochondrial injury. Metabolism. 2020;113:154397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Su Y, Zhao D, Jin C, Li Z, Sun S, Xia S, Zhang Y, Zhang Z, Zhang F, Xu X, Shao J, Zhang B, Zheng S. Dihydroartemisinin Induces Ferroptosis in HCC by Promoting the Formation of PEBP1/15-LO. Oxid Med Cell Longev. 2021;2021:3456725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 21. | Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68-69:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene. 2015;573:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Kocher A, Stamm T. Commentary on the article: Hughes M & Pauling JD. Exploring the patient experience of digital ulcers in systemic sclerosis. Semin Arthritis Rheum. 2018 Aug 11. pii: S0049-0172(18)30354-8. doi: 10.1016/j.semarthrit.2018.08.001. [Epub ahead of print] Review. Semin Arthritis Rheum. 2019;49:e9. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso JM, Badr KF. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci U S A. 1999;96:13375-13380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856-6865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Satoh H, Harada M, Tashiro S, Shiroya T, Imawaka H, Machii K. The effect of continuous arterial infusion of gabexate mesilate (FOY-007) on experimental acute pancreatitis. J Med Invest. 2004;51:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Miyata J, Yokokura Y, Moro K, Arai H, Fukunaga K, Arita M. 12/15-Lipoxygenase Regulates IL-33-Induced Eosinophilic Airway Inflammation in Mice. Front Immunol. 2021;12:687192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Sezin T, Ferreirós N, Jennrich M, Ochirbold K, Seutter M, Attah C, Mousavi S, Zillikens D, Geisslinger G, Sadik CD. 12/15-Lipoxygenase choreographs the resolution of IgG-mediated skin inflammation. J Autoimmun. 2020;115:102528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 229] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Frossard JL, Bhagat L, Lee HS, Hietaranta AJ, Singh VP, Song AM, Steer ML, Saluja AK. Both thermal and non-thermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 32. | Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 216] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1460] [Article Influence: 146.0] [Reference Citation Analysis (2)] |
| 34. | Hamaï A, Mehrpour M. [Autophagy and iron homeostasis]. Med Sci (Paris). 2017;33:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Tavakoli-Yaraki M, Karami-Tehrani F, Salimi V, Sirati-Sabet M. Induction of apoptosis by Trichostatin A in human breast cancer cell lines: involvement of 15-Lox-1. Tumour Biol. 2013;34:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2780] [Cited by in RCA: 2999] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 37. | Jones SA, Mills KH, Harris J. Autophagy and inflammatory diseases. Immunol Cell Biol. 2013;91:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298:C776-C785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Orenstein SJ, Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol. 2010;21:719-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Hashimoto D, Ohmuraya M, Hirota M, Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki K, Baba H, Mizushima N, Yamamura K. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Mareninova OA, Hermann K, French SW, O'Konski MS, Pandol SJ, Webster P, Erickson AH, Katunuma N, Gorelick FS, Gukovsky I, Gukovskaya AS. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 42. | Iwama H, Mehanna S, Imasaka M, Hashidume S, Nishiura H, Yamamura KI, Suzuki C, Uchiyama Y, Hatano E, Ohmuraya M. Cathepsin B and D deficiency in the mouse pancreas induces impaired autophagy and chronic pancreatitis. Sci Rep. 2021;11:6596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Yuan X, Wu J, Guo X, Li W, Luo C, Li S, Wang B, Tang L, Sun H. Autophagy in Acute Pancreatitis: Organelle Interaction and microRNA Regulation. Oxid Med Cell Longev. 2021;2021:8811935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Kiselyov K, Jennigs JJ Jr, Rbaibi Y, Chu CT. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3:259-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY). 2012;4:330-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 46. | Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2027] [Cited by in RCA: 1959] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 47. | Fritsch M, Günther SD, Schwarzer R, Albert MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H, Seeger JM, Lamkanfi M, Krönke M, Pasparakis M, Kashkar H. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 807] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 48. | Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 493] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 49. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5101] [Article Influence: 182.2] [Reference Citation Analysis (12)] |
| 50. | Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189-G196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Cao Y, Adhikari S, Clément MV, Wallig M, Bhatia M. Induction of apoptosis by crambene protects mice against acute pancreatitis via anti-inflammatory pathways. Am J Pathol. 2007;170:1521-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Orabi AI, Shah AU, Ahmad MU, Choo-Wing R, Parness J, Jain D, Bhandari V, Husain SZ. Dantrolene mitigates caerulein-induced pancreatitis in vivo in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G196-G204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370-3381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/