Published online Nov 14, 2024. doi: 10.3748/wjg.v30.i42.4523
Revised: September 24, 2024
Accepted: October 18, 2024
Published online: November 14, 2024
Processing time: 94 Days and 6.2 Hours
The prognosis of critically ill patients is closely linked to their gastrointestinal (GI) function. The acute GI injury (AGI) grading system, established in 2012, is extensively utilized to evaluate GI dysfunction and forecast outcomes in clinical settings. In 2021, the GI dysfunction score (GIDS) was developed, building on the AGI grading system, to enhance the accuracy of GI dysfunction severity assess
To compare the predictive capabilities of GIDS and the AGI grading system for 28-day mortality in critically ill patients.
A retrospective study was conducted at the general intensive care unit (ICU) of a regional university hospital. All data were collected during the first week of ICU admission. The primary outcome was 28-day mortality. Multivariable logistic regression analyzed whether GIDS and AGI grade were independent risk factors for 28-day mortality. The predictive abilities of GIDS and AGI grade were compa
The incidence of AGI in the first week of ICU admission was 92.13%. There were 85 deaths (47.75%) within 28 days of ICU admission. There was no initial 24-hour difference in GIDS between the non-survival and survival groups. Both GIDS (OR 2.01, 95%CI: 1.25-3.24; P = 0.004) and AGI grade (OR 1.94, 95%CI: 1.12-3.38; P = 0.019) were independent predictors of 28-day mortality. No significant difference was found between the predictive accuracy of GIDS and AGI grade for 28-day mortality during the first week of ICU admission (Z = -0.26, P = 0.794).
GIDS within the first 24 hours was an unreliable predictor of 28-day mortality. The predictive accuracy for 28-day mortality from both systems during the first week was comparable.
Core Tip: Gastrointestinal (GI) function plays a crucial role in the prognosis of critically ill patients. The acute GI injury (AGI) grade and GI dysfunction score (GIDS) are valuable tools for assessing the severity of GI dysfunction and predicting mortality in this patient population. Our study revealed that both GIDS and AGI grade during the first week of intensive care unit (ICU) admission independently predicted 28-day mortality. However, GIDS within the first 24 hours did not prove to be a reliable predictor. The ability to predict 28-day mortality based on the maximum values of GIDS and AGI grade during the initial week of ICU admission was comparable.
- Citation: Shen C, Wang X, Xiao YY, Zhang JY, Xia GL, Jiang RL. Comparing gastrointestinal dysfunction score and acute gastrointestinal injury grade for predicting short-term mortality in critically ill patients. World J Gastroenterol 2024; 30(42): 4523-4531
- URL: https://www.wjgnet.com/1007-9327/full/v30/i42/4523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i42.4523
Gastrointestinal (GI) dysfunction is prevalent among intensive care patients, affecting approximately 60% of this group, and is strongly linked to adverse outcomes[1,2]. GI dysfunction impacts prognosis by influencing multiple physiological systems, including circulation, respiratory function, liver function, coagulation, kidney function, and immune function. In cases of severe illness, disruption of the epithelial-microbial balance triggers inflammatory cascades, potentially leading to multiple organ dysfunction syndrome[3]. Therefore, timely assessment and monitoring of GI dysfunction are crucial strategies to guide interventions that improve outcomes.
The GI tract performs not only digestive and absorptive roles but also functions in immunity, endocrinology, and as a physical barrier, making its evaluation through a single marker, symptom, or sign complex[4]. The most broadly endorsed definition and scoring system for GI dysfunction was introduced by the European Society of Intensive Care Medicine (ESICM) in 2012. ESICM defined acute GI injury (AGI) and categorized it into four severity grades[5]. Subsequent research has validated the effectiveness of the AGI grade in reflecting the severity of GI dysfunction and predicting mortality in intensive care units (ICUs)[6-9]. However, the AGI grading system has been criticized for its subjective nature and complexity[6,8,10]. To improve accuracy in assessing the severity of GI dysfunction, reduce subjectivity, and enhance reproducibility, a five-grade GI dysfunction score (GIDS) based on the AGI framework was developed in 2021[11].
The prediction of short-term mortality and the evaluation of disease severity continue to be key clinical concerns for ICU physicians. Since the GIDS was introduced as an enhancement of the AGI grading system, limited studies have assessed its comparative effectiveness in these areas. This has somewhat limited the promotion and adoption of the new scoring system.
Accordingly, we undertook a retrospective analysis to determine whether both GIDS and AGI grade are independent risk factors for 28-day mortality, to evaluate their comparative effectiveness in predicting 28-day mortality, and to assess how well GIDS and AGI grade can delineate disease severity and predict 28-day mortality.
This retrospective, observational study was conducted in the general ICU of the First Affiliated Hospital of Zhejiang Chinese Medical University. Patients admitted to the ICU from January 1, 2023, to March 31, 2024, were consecutively recruited. The exclusion criteria for patients included: (1) Being under 18 years of age; (2) An Acute Physiology and Chronic Health Evaluation II (APACHE II) score below 10; (3) An ICU stay shorter than 72 hours; (4) cessation of active treatment; (5) Terminal malignancy; (6) Inability to assess GIDS or AGI grade for any reason; and (7) Incomplete data.
The sample size was calculated using the test for two receiver operating characteristic (ROC) curves procedure in PASS software (version 15.0.5) based on parameters from a previous study[12]. Estimates for the areas under the ROC curve for the AGI grading system and GIDS on the first day of ICU admission in predicting 28-day mortality were 0.57 and 0.70, respectively[12]. To achieve a target power of 90% and a type I error rate of 5%, analysis required 172 patients.
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University. Given the observational nature of this study, informed consent was waived.
The AGI grade diagnostic criteria are outlined in Supplementary Table 1, based on the 2012 recommendations by the ESICM[5]. The GIDS was estimated using the scoring scale detailed in Supplementary Table 2, proposed by Reintam et al[11] in 2021. The GI symptoms and signs considered in these two scoring systems adhere to definitions established by the ESICM in 2012 (Supplementary Table 3)[5]. Additionally, feeding intolerance syndrome (FI) was identified if a patient did not achieve at least 20 kcal/kg BW/day via the enteral route within 72 hours of a feeding attempt or if enteral feeding was discontinued for any clinical reason[5].
Given that scoring systems in the first 24 hours of ICU admission do not accurately reflect changes in GI function as the disease progresses, two attending physicians assessed each patient’s daily AGI grade and GIDS for the first week based on electronic medical records and critical care flow sheets. The initial assessment of the AGI grade started 72 hours post-admission. In cases of disagreement between the two assessors, both were required to provide detailed justifications for their scores. This process was conducted in the presence of an associate chief physician or chief physician, who made the final decision on the AGI grade or GIDS. All researchers involved underwent systematic training before the assessments to minimize information bias. The results were kept confidential during the evaluation process to ensure objectivity.
The remaining data were collected retrospectively from clinical electronic medical records and critical care flow sheets within the first week of ICU admission. The collected baseline demographic and clinical data included patients’ age, sex, previous chronic illnesses, the primary reason for ICU admission, and whether the patient had undergone abdominal surgery prior to ICU admission. Laboratory tests recorded on the day of admission included blood platelet count, C-reactive protein, blood glucose, albumin, lactate, and procalcitonin. Additionally, the study documented whether enteral nutrition was initiated within 48 hours of ICU admission and the occurrence of sepsis, acute kidney injury, and mechanical ventilation (MV) support during the ICU stay. APACHE II and sequential organ failure assessment (SOFA) scores were assessed daily, noting the highest values within the first 24 hours and the first week of admission. The primary outcome was 28-day mortality, defined as all-cause mortality within 28 days of ICU admission. Secondary outcomes included the length of stay in the ICU and days on MV. For patients discharged alive before 28 days, follow-up for primary outcomes was conducted via telephone.
Quantitative variables with a normal distribution are presented as mean ± SD and were analyzed using the student’s t-test. In contrast, quantitative variables that are not normally distributed are represented as median (interquartile range) and were analyzed using the Mann-Whitney test or the Kruskal-Wallis test, depending on the data. Categorical variables are expressed as absolute numbers and percentages and were analyzed using the χ2 test or Fisher’s exact test, as appropriate. Kappa statistics were applied to evaluate inter-rater reliability. Univariate analyses were initially conducted to identify risk factors associated with 28-day mortality. Variables with a P value of less than 0.2 in these analyses were included in the multivariate logistic regression to assess the impact of GI dysfunction on 28-day mortality. The ROC curve and the area under the ROC curve (AUC) were used to compare the effectiveness of four scoring systems-the GIDS, AGI grading system, SOFA score, and APACHE II score-in predicting 28-day mortality. Differences between the AUCs of two curves were evaluated using DeLong’s test. Further univariate analyses, stratified by the severity of GI dysfunction, were performed to examine the relationship between AGI grade or GIDS and disease severity. A P value of less than 0.05 was considered statistically significant. Data analysis was conducted using IBM SPSS software (version 26.0, SPSS, Chicago, IL, United States).
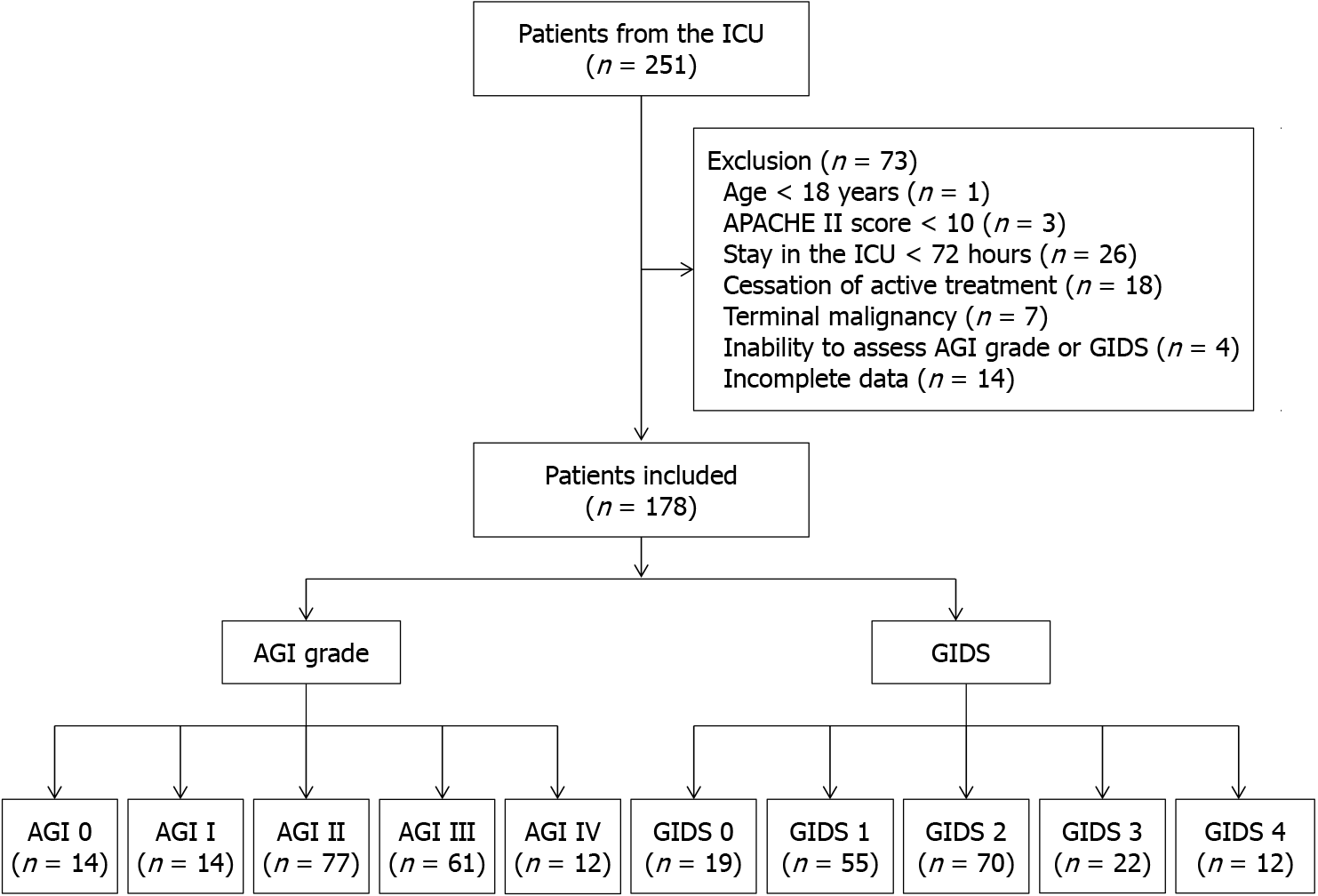
A total of 251 individuals admitted to the ICU from January 1, 2023, to March 31, 2024, were screened. Of these, 73 patients were excluded; the specific numbers and reasons for exclusion are detailed in the flow diagram (Figure 1). Consequently, 178 cases were included in the study. The inter-rater kappa values for GIDS and AGI grade assessments were 0.802 and 0.771, respectively.
The characteristics of the 178 patients are shown in Table 1. The median age was 76 years (IQR 67-83 years), with 60.67% being male. A total of 85 patients (47.75%) died within 28 days of ICU admission. The maximum GIDS, AGI grade, SOFA score, and APACHE II score were significantly higher in the non-survival group compared to the survival group during the first week of ICU admission (P < 0.001). Additionally, the maximum SOFA and APACHE II scores within the first 24 hours of ICU admission were significantly higher in the non-survival group than in the survival group (P < 0.001 and P = 0.017, respectively). No difference in GIDS was observed between these two groups within the first 24 hours of ICU admission.
| Characteristics | All (n = 178) | Survivors (n = 93) | Non-survivors (n = 85) | Statistic | P value |
| Age, years | 76.00 (67.00, 83.00) | 75.00 (63.00, 83.00) | 77.00 (68.00, 83.00) | Z = -1.23 | 0.218 |
| Male, n (%) | 108 (60.67) | 55 (59.14) | 53 (62.35) | χ² = 0.19 | 0.661 |
| Pre-existing diseases | |||||
| Diabetes mellitus, n (%) | 56 (31.46) | 30 (32.26) | 26 (30.59) | χ² = 0.06 | 0.811 |
| Hyptertension, n (%) | 95 (53.37) | 46 (49.46) | 49 (57.65) | χ² = 1.20 | 0.274 |
| Coronary heart disease, n (%) | 40 (22.47) | 17 (18.28) | 23 (27.06) | χ² = 1.96 | 0.161 |
| Stroke, n (%) | 25 (14.04) | 16 (17.20) | 9 (10.59) | χ² = 1.61 | 0.204 |
| Principal pathology | |||||
| Cardiovascular, n (%) | 16 (8.99) | 7 (7.53) | 9 (10.59) | χ² = 0.51 | 0.476 |
| Neurological, n (%) | 16 (8.99) | 10 (10.75) | 6 (7.06) | χ² = 0.74 | 0.389 |
| GI/pancreatic/liver, n (%) | 23 (12.92) | 14 (15.05) | 9 (10.59) | χ² = 0.79 | 0.375 |
| Pulmonary, n (%) | 76 (42.70) | 35 (37.63) | 41 (48.24) | χ² = 2.04 | 0.153 |
| Renal, n (%) | 13 (7.30) | 8 (8.60) | 5 (5.88) | χ² = 0.49 | 0.486 |
| Metabolic disorders, n (%) | 6 (3.37) | 3 (3.23) | 3 (3.53) | χ² = 0.00 | > 0.999 |
| Heart rate1, beats/minute | 98.47 ± 21.55 | 97.96 ± 21.65 | 99.02 ± 21.55 | t= -0.33 | 0.743 |
| C-reactive protein1, mg/L | 64.63 (23.67, 138.11) | 54.43 (18.23, 144.60) | 82.74 (40.36, 132.42) | Z = -1.19 | 0.235 |
| Glucose1, mmol/L | 9.31 (7.08, 13.00) | 8.97 (7.11, 12.10) | 9.42 (7.00, 14.65) | Z = -0.48 | 0.629 |
| Albumin1, g/L | 28.20 (25.10, 32.03) | 28.90 (25.80, 32.90) | 27.20 (24.50, 29.30) | Z = -2.73 | 0.006 |
| Serum lactate1, mmol/L | 1.70 (1.20, 2.98) | 1.60 (0.90, 2.40) | 1.90 (1.30, 3.30) | Z = -2.36 | 0.018 |
| Procalcitonin1, ng/mL | 0.68 (0.20, 2.81) | 0.58 (0.18, 3.35) | 0.78 (0.29, 2.45) | Z = -0.89 | 0.373 |
| Sepsis, n (%) | 43 (24.16) | 21 (22.58) | 22 (25.88) | χ² = 0.26 | 0.607 |
| Abdominal surgery, n (%) | 25 (14.04) | 16 (17.20) | 9 (10.59) | χ² = 1.61 | 0.204 |
| Acute kidney injure, n (%) | 93 (52.25) | 39 (41.94) | 54 (63.53) | χ² = 8.30 | 0.004 |
| Mechanical ventilation, n (%) | 145 (81.46) | 63 (67.74) | 82 (96.47) | χ² = 24.27 | < 0.001 |
| Start EN within 48 hours, n (%) | 138 (77.53) | 73 (78.49) | 65 (76.47) | χ² = 0.10 | 0.747 |
| SOFA score2, points | 7.00 (5.00, 11.00) | 6.00 (4.00, 9.00) | 9.00 (6.00, 11.00) | Z = -4.45 | < 0.001 |
| SOFA score3, points | 11.00 (7.00, 15.00) | 8.00 (6.00, 11.00) | 14.00 (11.00, 16.00) | Z = -8.28 | < 0.001 |
| APACHE II score2, points | 19.00 (15.00, 24.00) | 18.00 (13.00, 22.00) | 21.00 (16.00, 25.00) | Z = -2.38 | 0.017 |
| APACHE II score3, points | 22.00 (17.00, 28.00) | 18.00 (14.00, 22.00) | 26.00 (23.00, 32.00) | Z = -7.20 | < 0.001 |
| AGI grade3, grade | 2.00 (2.00, 3.00) | 2.00 (1.00, 2.00) | 3.00 (2.00, 3.00) | Z = -6.85 | < 0.001 |
| GIDS2, points | 1.00 (0.00, 1.00) | 1.00 (0.00, 1.00) | 1.00 (0.00, 1.00) | Z = -1.15 | 0.249 |
| GIDS3, points | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 2.00 (2.00, 3.00) | Z = -6.90 | < 0.001 |
Variables with P values less than 0.2, including coronary heart disease, primary pulmonary pathology, albumin levels, serum lactate, acute kidney injury, MV, SOFA score, APACHE II score, GIDS, and AGI grade, were selected for inclusion in the multivariate logistic regression analysis (Table 2). This analysis used 28-day mortality as the dependent variable. Due to the high correlation between GIDS and AGI grade, these were incorporated separately into two different models of the multivariate logistic regression to mitigate potential covariance issues (GIDS in model 1 and AGI grade in model 2). Acute kidney injury, a component of the APACHE II score, was not included. In model 1, SOFA score (OR 1.32, 95%CI: 1.13-1.54; P < 0.001) and GIDS (OR 2.01, 95%CI: 1.25-3.24; P = 0.004) were identified as independent predictors of 28-day mortality. In Model 2, SOFA score (OR 1.29, 95%CI: 1.11-1.52; P = 0.001) and AGI grade (OR 1.94, 95%CI: 1.12-3.38; P = 0.019) were also found to be independent predictors of 28-day mortality.
| Variables | Model 1 | Model 2 | ||
| P value | OR (95%CI) | P value | OR (95%CI) | |
| Pre-existing disease-coronary heart disease | 0.152 | 2.12 (0.76-5.92) | 0.185 | 1.99 (0.72-5.53) |
| Principal pathology- Pulmonary | 0.119 | 2.05 (0.83-5.05) | 0.139 | 1.96 (0.80-4.76) |
| Albumin | 0.609 | 0.98 (0.91-1.05) | 0.733 | 0.99 (0.92-1.06) |
| Serum lactate | 0.316 | 0.91 (0.77-1.09) | 0.283 | 0.91 (0.76-1.08) |
| Mechanical ventilation | 0.129 | 3.16 (0.72-13.95) | 0.107 | 3.37 (0.77-14.77) |
| SOFA score1 | < 0.001 | 1.32 (1.13-1.54) | 0.001 | 1.29 (1.11-1.52) |
| APACHE II score1 | 0.070 | 1.08 (0.99-1.18) | 0.058 | 1.09 (1.00-1.18) |
| GIDS1 | 0.004 | 2.01 (1.25-3.24) | / | / |
| AGI grade1 | / | / | 0.019 | 1.94 (1.12-3.38) |
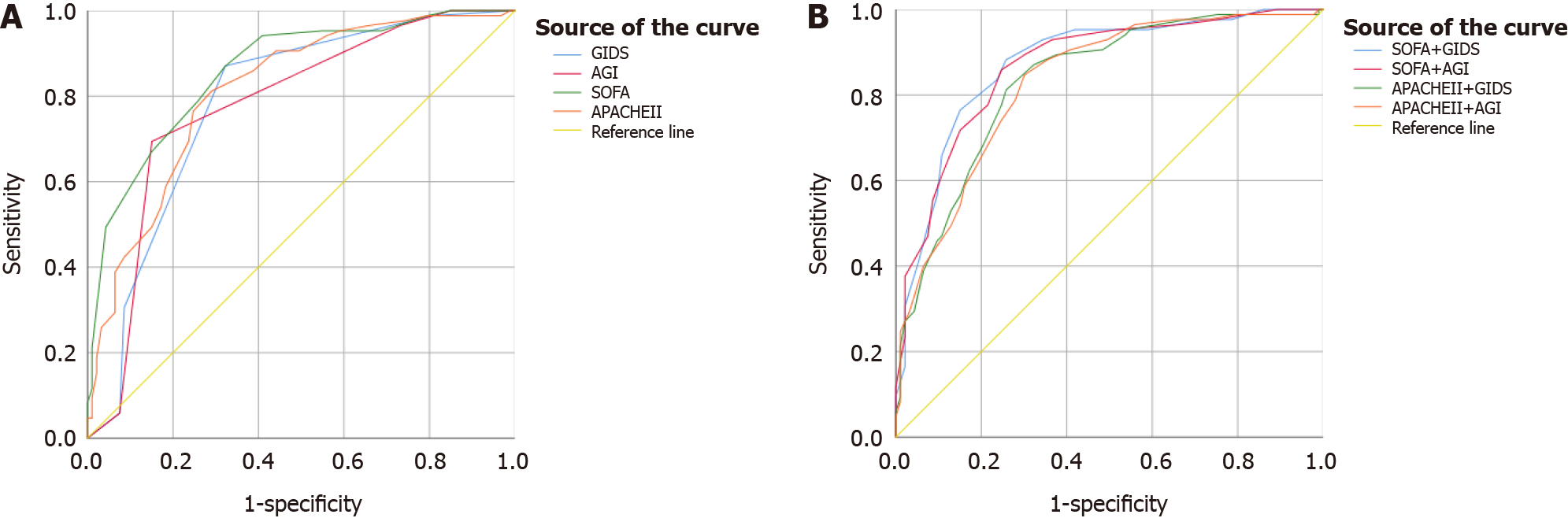
The AUCs for predicting 28-day mortality based on the highest recorded values of GIDS and AGI grade were 0.785 and 0.779, respectively (Figure 2A and Table 3). Statistical analysis showed no significant difference in the predictive power of these two scoring systems (Z = -0.26, P = 0.794). Compared to the GIDS (Z = -2.01, P= 0.045) and the AGI grade (Z = -2.43, P = 0.015), the SOFA score demonstrated a superior ability to predict 28-day mortality. The APACHE II score did not show any predictive advantage over GIDS and AGI grade. The optimal cut-off value for GIDS was between 1 and 2 points, with a sensitivity of 87.06% and a specificity of 67.74%. For the AGI grade, the cut-off was between grades 2 and 3, showing a sensitivity of 69.41% and a specificity of 84.95% (Table 3).
| Scoring system | AUC | P value | 95%CI | Cut-off value | Sensitivity (%) | Specificity (%) | YI | PV+ (%) | PV- (%) |
| GIDS1 | 0.785 | < 0.001 | 0.720-0.851 | 1.5 | 87.06 | 67.74 | 0.548 | 71.15 | 85.14 |
| AGI1 | 0.779 | < 0.001 | 0.713-0.845 | 2.5 | 69.41 | 84.95 | 0.544 | 80.82 | 75.24 |
| SOFA1 | 0.859 | < 0.001 | 0.805-0.913 | 9.5 | 87.06 | 67.74 | 0.548 | 71.15 | 85.14 |
| APACHE II1 | 0.812 | < 0.001 | 0.749-0.875 | 21.5 | 81.18 | 70.97 | 0.521 | 71.88 | 80.49 |
| SOFA + GIDS2 | 0.874 | < 0.001 | 0.822-0.927 | 11.5 | 88.24 | 74.19 | 0.624 | 75.76 | 87.34 |
| SOFA + AGI2 | 0.869 | < 0.001 | 0.816-0.921 | 12.5 | 85.88 | 75.27 | 0.612 | 76.04 | 85.37 |
| APACHE II + GIDS2 | 0.833 | < 0.001 | 0.774-0.892 | 23.5 | 81.18 | 74.19 | 0.554 | 74.19 | 81.18 |
| APACHE II + AGI2 | 0.831 | < 0.001 | 0.771-0.890 | 23.5 | 84.71 | 69.89 | 0.546 | 72.00 | 83.33 |
Combining the GIDS with the SOFA score significantly enhanced the predictive ability of GIDS for 28-day mortality (Z = -2.93, P = 0.003). However, pairing it with the APACHE II score showed no such improvement (Z = -1.17, P = 0.244). Similarly, integrating the AGI grade with the SOFA score significantly bolstered the AGI grade’s ability (Z = -3.21, P = 0.001), but combining it with the APACHE II score did not yield significant results (Z = -1.29, P = 0.198) (Figure 2B and Table 3).
Following the ROC curve analysis, the maximum values of GIDS and AGI grade recorded within the first week of ICU admission were converted into binary variables (Supplementary Table 4). In the GIDS 2-4 category, 28-day mortality was 71.15%, with a median SOFA score of 13 and a median APACHE II score of 24. For patients in the AGI grades III-IV category, 28-day mortality rose to 80.82%, with median scores of 14 for SOFA and 26 for APACHE II. The duration of MV showed no statistically significant differences between the scales. Notably, patients in the GIDS 2-4 group and those in AGI grades III-IV experienced shorter ICU stays compared to those in the GIDS 0-1 group (P = 0.006) and AGI grades 0-II (P < 0.001).
In this study, we found that both GIDS and AGI grade during the first week of ICU admission were independent risk factors for 28-day mortality. However, GIDS measured within the first 24 hours did not prove to be a reliable predictor. The ability to predict 28-day mortality based on the maximum values of GIDS and AGI grade during the initial week of ICU admission was comparable. The SOFA score demonstrated superior predictive accuracy compared to both GIDS and AGI grade. The predictive effectiveness of GIDS or AGI grade was significantly improved when combined with the SOFA score. Stratifying GIDS into categories of 0-1 and 2-4, and AGI grade into 0-II and III-IV, effectively identified disease severity and predicted 28-day mortality.
GI dysfunction is frequently observed in ICU settings. One study noted a 59.1% prevalence of GI symptoms on the first day of ICU admission[1]. Further investigations have shown that 88.13% of patients suffer from AGI within the first week of their ICU stay[9], and another study reported that 86.7% of critically ill COVID-19 patients experienced AGI during their ICU treatment[13]. In our study, the incidence of AGI within the first week of ICU admission was particularly high at 92.13%, potentially linked to factors such as the median age of the patients (76 years), the inclusion of patients with GI, pancreatic, or liver pathology (12.92%), and those undergoing post-abdominal surgery (14.04%). The diminished GI reserves in older adults make them particularly vulnerable to even minor insults, leading to rapid emergence of compensatory disorders[14]. Age is a known factor that increases the likelihood of GI symptoms[1].
A prospective study involving 276 patients found that the GIDS was effective in predicting 28-day mortality, both on the first day and throughout the first week of ICU admission[12]. Our observations suggest that GIDS during the first week of ICU admission, rather than within the first 24 hours, serves as an independent predictor. This finding may be due to the delayed manifestation of GI symptoms, which are often secondary to the primary reasons for ICU admission. A large-scale study with 1312 participants indicated that the proportion of patients experiencing at least two GI symptoms progressively increased each day during the first week of ICU admission[1]. Similarly, a study of 150 critically ill COVID-19 patients revealed that the incidence of GI symptoms was significantly higher on the fourth day compared to the first day of ICU stay[15]. The limited treatment options available for GI dysfunction[16] and the inherent delays in the effectiveness of interventions further underscore the importance of evaluating GI dysfunction throughout the first week to more accurately assess disease severity.
According to the AGI grading system and the definition of FI established by the ESICM in 2012, the classification of AGI grade III requires observing whether GI function can be restored after intervention, and the FI definition often involves a 72-hour period of unsuccessful enteral feeding attempts. In this study, AGI was evaluated between 72 hours and one week after ICU admission, with the highest AGI grade recorded reflecting the most severe level of GI dysfunction. Consistent with previous research[7,9,17], our findings confirm a strong correlation between AGI grade and 28-day mortality.
To our knowledge, this is the first proposal that the predictive capacity for 28-day mortality using the maximum values of the GIDS and AGI grade during the initial seven days of ICU admission is comparable. However, the AGI grade often relies on subjective assessments and can be influenced by local feeding practices and management strategies[11,18]. As a refinement of the AGI grading system, the GIDS incorporates more objective parameters and places less emphasis on FI. In clinical practice, the GIDS is no more complicated than the AGI grading system, and its routine use in the ICU is highly feasible due to its simplicity, clinical relevance, rapid assessment, standardization, ease of training, and integration with existing scoring systems.
Since the GIDS and AGI grade reflect the status of multiple organ systems only partially and indirectly, compared to the SOFA score, it follows that the SOFA score has greater predictive accuracy for 28-day mortality. The predictive accuracy of both GIDS and AGI grade is significantly enhanced when combined with the SOFA score, rather than the APACHE II score, likely due to the former’s superior predictive capacity for mortality as noted in previous studies[19,20]. We found that the APACHE II score did not offer any statistically significant predictive advantage over GIDS and AGI grade. Despite its frequent use in ICU settings, the APACHE II score’s key advantage is its ability to quickly assess a patient’s initial condition. In contrast, the SOFA score focuses on dynamic monitoring of multiple organ systems, providing a more comprehensive picture of the patient’s clinical course and status changes.
In critically ill patients, SOFA and APACHE II scores are widely used to evaluate illness severity and predict outcomes[21-24]. The GIDS and AGI grades are less dependent on laboratory tests, allowing them to be performed quickly at the bedside and enabling dynamic monitoring of patient status. Reclassifying AGI into two levels-AGI I and II (indicative of self-limiting conditions and GI dysfunction) and AGI III and IV (representing GI failure and life-threatening conditions)-effectively differentiates disease severity and short-term prognosis, a distinction not as apparent with the traditional four-grade AGI classification[6,7]. Similarly, categorizing GIDS into two groups, GIDS 0-1 and GIDS 2-4, has proven effective in identifying variations in disease severity and predicting 28-day mortality. Clinically, a GIDS score above 1 or an AGI grade above 2 indicates a more severe condition and a higher risk of mortality within 28 days. The shorter ICU stay observed in patients with severe GI dysfunction, compared to those with milder forms, may be attributable to the higher mortality rates associated with more severe dysfunction.
Nevertheless, this study has several limitations. First, it is a single-center retrospective study with a relatively small sample size, underscoring the need for a prospective study encompassing a larger cohort. Second, despite efforts to minimize it, subjectivity remains in the application of the GIDS and AGI grading systems. Third, the primary outcome measured was 28-day mortality; additional research focusing on longer-term outcomes, such as 60- and 90-day mortality, is necessary. Finally, the study did not differentiate between primary and secondary GI dysfunction.
The GIDS and AGI grade during the first week of ICU admission were established as independent risk factors for 28-day mortality in critically ill patients. Moreover, the ability to predict 28-day mortality based on the highest values of GIDS and AGI grade during the first week of ICU stay was found to be comparable. However, the GIDS measured within the first 24 hours of ICU admission did not reliably predict 28-day mortality.
| 1. | Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Reintam Blaser A, Poeze M, Malbrain ML, Björck M, Oudemans-van Straaten HM, Starkopf J; Gastro-Intestinal Failure Trial Group. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39:899-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 3. | Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 4. | Reintam Blaser A, Bachmann KF, Deane AM. Gastrointestinal function in critically ill patients. Curr Opin Clin Nutr Metab Care. 2023;26:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (2)] |
| 6. | Ding L, Chen HY, Wang JY, Xiong HF, He WH, Xia L, Lu NH, Zhu Y. Severity of acute gastrointestinal injury grade is a good predictor of mortality in critically ill patients with acute pancreatitis. World J Gastroenterol. 2020;26:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Li H, Zhang D, Wang Y, Zhao S. Association between acute gastrointestinal injury grading system and disease severity and prognosis in critically ill patients: A multicenter, prospective, observational study in China. J Crit Care. 2016;36:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Klanovicz TM, Franzosi OS, Nunes DSL, Loss SH, Batassini É, Turra EE, Teixeira C, Vieira SRR. Acute gastrointestinal failure is associated with worse hemodynamic and perfusion parameters over 48 h after admission in patients with septic shock: Retrospective cohort study. Nutr Clin Pract. 2023;38:617-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Hu B, Sun R, Wu A, Ni Y, Liu J, Guo F, Ying L, Ge G, Ding A, Shi Y, Liu C, Xu L, Jiang R, Lu J, Lin R, Zhu Y, Wu W, Xie B. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit Care. 2017;21:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Asrani VM, Brown A, Huang W, Bissett I, Windsor JA. Gastrointestinal Dysfunction in Critical Illness: A Review of Scoring Tools. JPEN J Parenter Enteral Nutr. 2020;44:182-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Reintam Blaser A, Padar M, Mändul M, Elke G, Engel C, Fischer K, Giabicani M, Gold T, Hess B, Hiesmayr M, Jakob SM, Loudet CI, Meesters DM, Mongkolpun W, Paugam-Burtz C, Poeze M, Preiser JC, Renberg M, Rooijackers O, Tamme K, Wernerman J, Starkopf J. Development of the Gastrointestinal Dysfunction Score (GIDS) for critically ill patients - A prospective multicenter observational study (iSOFA study). Clin Nutr. 2021;40:4932-4940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 12. | Liu X, Wang Q, Yang D, Fu M, Yang M, Bi Y, Wang C, Song X. Association between Gastrointestinal Dysfunction Score (GIDS) and disease severity and prognosis in critically ill patients: A prospective, observational study. Clin Nutr. 2023;42:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 13. | Sun JK, Liu Y, Zou L, Zhang WH, Li JJ, Wang Y, Kan XH, Chen JD, Shi QK, Yuan ST. Acute gastrointestinal injury in critically ill patients with COVID-19 in Wuhan, China. World J Gastroenterol. 2020;26:6087-6097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Lovat LB. Age related changes in gut physiology and nutritional status. Gut. 1996;38:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Lakenman PLM, van Schie JC, van der Hoven B, Baart SJ, Eveleens RD, van Bommel J, Olieman JF, Joosten KFM. Nutritional intake and gastro-intestinal symptoms in critically ill COVID-19 patients. Clin Nutr. 2022;41:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Reintam Blaser A, Preiser JC, Fruhwald S, Wilmer A, Wernerman J, Benstoem C, Casaer MP, Starkopf J, van Zanten A, Rooyackers O, Jakob SM, Loudet CI, Bear DE, Elke G, Kott M, Lautenschläger I, Schäper J, Gunst J, Stoppe C, Nobile L, Fuhrmann V, Berger MM, Oudemans-van Straaten HM, Arabi YM, Deane AM; Working Group on Gastrointestinal Function within the Section of Metabolism, Endocrinology and Nutrition (MEN Section) of ESICM. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 17. | Zhang D, Fu R, Li Y, Li H, Li Y, Li H. Comparison of the clinical characteristics and prognosis of primary versus secondary acute gastrointestinal injury in critically ill patients. J Intensive Care. 2017;5:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Martinez J, Rodriguez Hovnanian KM, Martinez EE. Biomarkers and Functional Assays of Epithelial Barrier Disruption and Gastrointestinal Dysmotility in Critical Illness-A Narrative Review. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Piton G, Chaignat C, Giabicani M, Cervoni JP, Tamion F, Weiss E, Paugam-Burtz C, Capellier G, Di Martino V. Prognosis of cirrhotic patients admitted to the general ICU. Ann Intensive Care. 2016;6:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Tekin B, Kiliç J, Taşkin G, Solmaz İ, Tezel O, Başgöz BB. The Comparison of scoring systems: SOFA, APACHE-II, LODS, MODS, and SAPS-II in critically ill elderly sepsis patients. J Infect Dev Ctries. 2024;18:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 21. | Naved SA, Siddiqui S, Khan FH. APACHE-II score correlation with mortality and length of stay in an intensive care unit. J Coll Physicians Surg Pak. 2011;21:4-8. [PubMed] |
| 22. | Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1444] [Article Influence: 32.1] [Reference Citation Analysis (33)] |
| 23. | Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 1900] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 24. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 8114] [Article Influence: 270.5] [Reference Citation Analysis (11)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/