INTRODUCTION
This editorial reviews the potential role of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in the treatment of metabolic dysfunction-associated fatty liver disease, as discussed by Soresi and Giannitrapani[1] in World Journal of Gastroenterology. GLP-1RAs show promise in reducing fat and body weight, and improving insulin resistance (IR), which may play a role in protecting the liver from further damage. Although clinical trials have shown that GLP-1RAs, such as semaglutide, can reduce fatty liver, including histological signs of nonalcoholic steatohepatitis, their effects on fibrosis are limited. Therefore, combination therapies with different but complementary mechanisms of action are considered the best way to improve efficacy and slow disease progression. We demonstrate that although international drug agencies have not yet given clear indications, more treatment plans involving GLP-1RA combination therapies may emerge in the future.
The mechanism by which GLP-1RAs improve the sugar and fat metabolism of skeletal muscle cells was important in our previous research research[2,3]. Glucagon-like peptide (GLP) originates from the L cells of the intestine and exerts its effects by binding to glucagon-like peptide-1 receptor (GLP-1R). GLP-1 reduces glucagon secretion and plays a blood-sugar-lowering role by increasing insulin secretion dependent on postprandial glucose concentration. GLP-1RAs, with longer half-life and dipeptidyl peptidase-4 resistance, mimic the effects of GLP-1 and are extensively used in diabetes treatment[4]. Currently, the main GLP-1RAs on the market include liraglutide, exenatide and dulaglutide. Recent research has found that GLP-1RAs promote insulin secretion and regulate blood sugar, have cardiovascular benefits, kidney protection, and improve fatty liver, and cognitive function[5]. This editorial focuses on exploring the potential of GLP-1 analogs in the treatment of various diseases.
IMPACT OF GLP-1RAS ON THE CARDIOVASCULAR SYSTEM
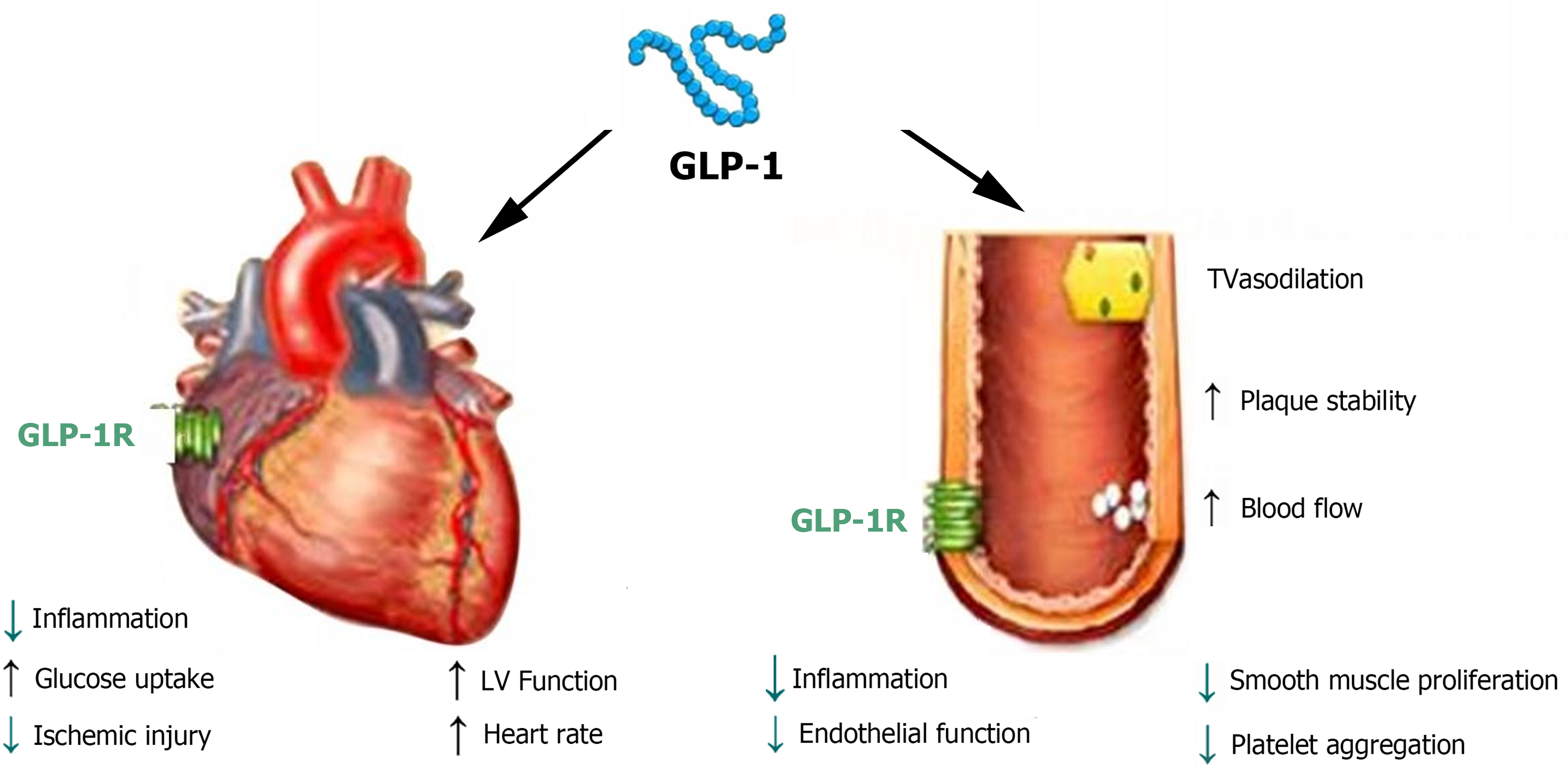
In the cardiovascular system, GLP-1Rs are expressed in both myocardial and endothelial cells[6], indicating that GLP-1Rs have direct and indirect effects on the heart and blood vessels (Figure 1). A multicenter long-term cardiovascular outcome trial confirmed that GLP-1RAs can reduce the mortality of cardiovascular diseases and are beneficial to the cardiovascular system, reducing the incidence of myocardial infarction or nonfatal stroke[7]. GLP-1RAs may improve endothelial cell function through anti-inflammatory and vasodilatory activities. The recent STEP-HFpEF trial[8] found that semaglutide significantly reduced heart-failure-related symptoms in patients with obesity-related preserved ejection fraction heart failure and type 2 diabetes mellitus (T2DM) within 1 year. In addition, the antiproliferative effect on smooth muscle cells and the anti-inflammatory effect on macrophages help prevent the occurrence and progression of atherosclerosis. Some researchers have proposed that GLP-1RAs can enhance insulin sensitivity in myocardial cells, increase the rate of glucose oxidation, reduce the rate of fatty acid oxidation, and enhance the energy metabolism of myocardial cells, thereby improving heart function[9]. An animal experiment of ischemia–reperfusion injury showed that early treatment with natural GLP-1 and GLP-1RAs can increase myocardial blood flow, reduce the area of myocardial infarction, and improve myocardial injury after ischemia–reperfusion. Cardiac calcium metabolism disorder is a sign of heart failure, and studies have shown that GLP-1RAs can regulate the downregulation of calcium ion levels caused by reduced phosphorylation of the ryanodine receptor and activation of calmodulin-dependent protein kinase II[10]. GLP-1RAs can improve myocardial remodeling by activating the NO synthase/cyclic GMP/cyclic GMP-dependent protein kinase signal transduction pathway. Although clinical studies have confirmed some cardiovascular protective effects of GLP-1RAs in diabetic patients, more basic research is needed to support the molecular mechanisms by which GLP-1RAs exert their cardiovascular protective effects.
Figure 1 Glucagon-like peptide-1 receptors are expressed in both cardiomyocytes and endothelial cells, exerting direct and indirect effects on the heart and blood vessels.
GLP-1: Glucagon-like peptide-1; GLP-1R: Glucagon-like peptide-1 receptor; LV: Left ventricle; TV: Tricuspid valve.
IMPACT OF GLP-1RAS ON BONE METABOLISM
Diabetes and osteoporosis are closely linked, with diabetes leading to fragile bones, increased fracture risk, and slow healing due to metabolic disorders caused by high blood sugar. Some blood-sugar-lowering medications, such as thiazolidinediones and insulin, may increase the risk of fractures by affecting bone metabolism[11]. Therefore, diabetes treatment strategies need to effectively control blood sugar while minimizing impacts on bone metabolic complications. GLP-1RAs can exert antiosteoporotic effects by controlling blood sugar levels, promoting bone formation, and inhibiting bone resorption, and primarily enhance bone formation through physiological effects that stimulate insulin secretion, inhibit glucagon secretion, and regulate gastric emptying[12]. GLP-1RAs may beneficially affect the skeleton of diabetic patients by interacting with the advanced glycation end products (AGEs)-RAGE-ROS pathway[13]. Thus, GLP-1RAs hold potential value in the treatment of diabetes and associated osteoporosis. Animal studies have shown that liraglutide can significantly reduce AGE and reduce bone absorption in a model of chronic diabetic bone injury complications in ApoE gene knockout mice[14,15]. However, the specific impact of GLP-1RAs on fracture risk and osteoporosis has not yet been clearly defined. Some studies have shown that GLP-1RAs have a good anabolic effect on bone metabolism, but the potential molecular mechanisms still need to be clarified[16].
IMPACT OF GLP-1RAS ON POLYCYSTIC OVARY SYNDROME
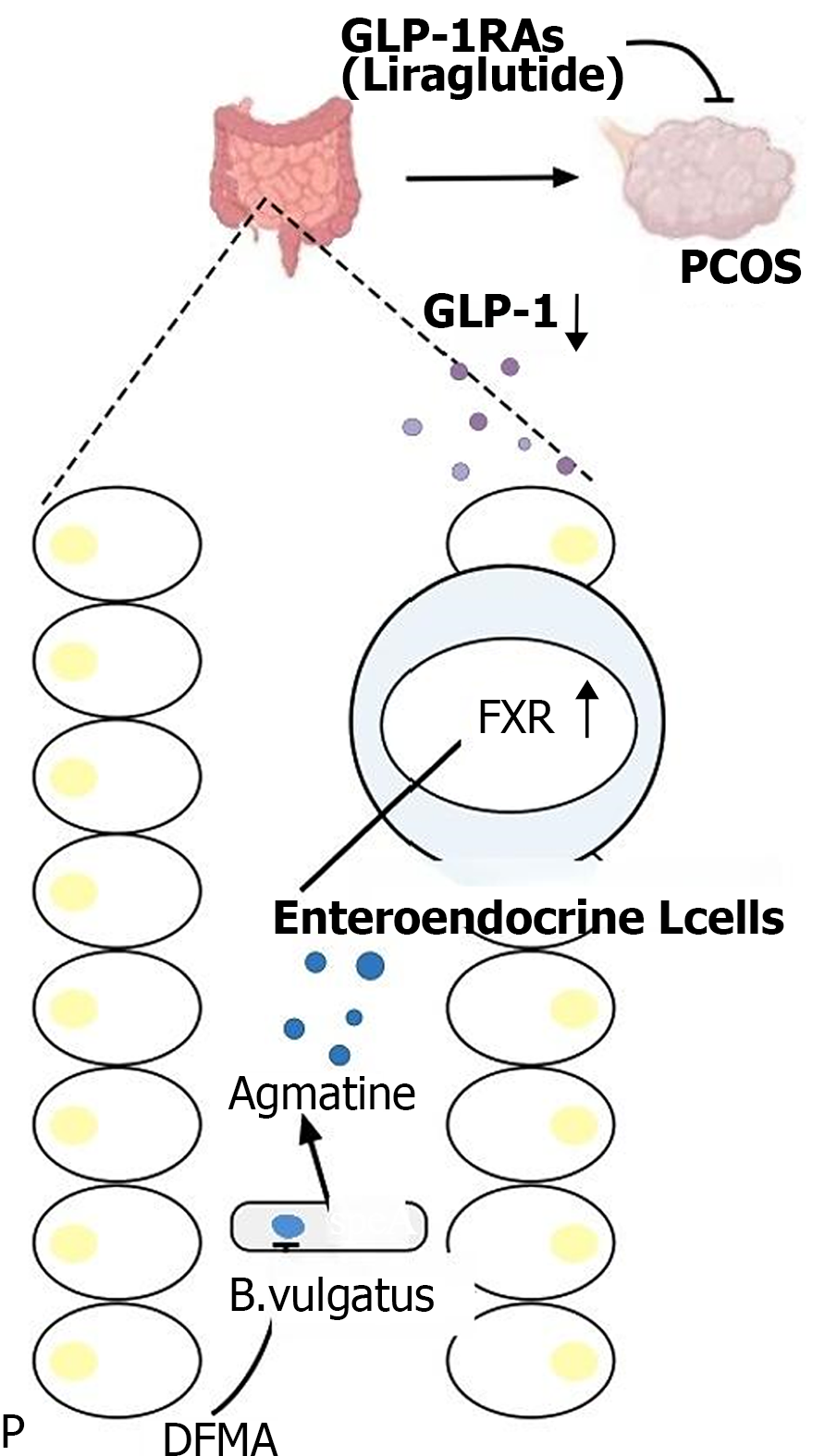
Polycystic ovary syndrome (PCOS), characterized by infrequent or absent ovulation, elevated androgen levels, polycystic ovaries, obesity, and infertility, is the most common endocrine and metabolic disorder in women of reproductive age[17]. Although the pathogenesis of PCOS is not fully understood, recent studies suggest a causal relationship between IR and all clinical symptoms of PCOS. The IR in PCOS disrupts the action of gonadotropins, enhances the sensitivity of theca cells to luteinizing hormone, leading to increased androgen production[18]. Concurrently, elevated insulin levels reduce the binding of sex-hormone-binding globulin in the liver to testosterone, resulting in hyperandrogenism[19]. High levels of free fatty acids, a primary manifestation of obesity, can exacerbate IR by activating serine/threonine kinases, which reduce the tyrosine phosphorylation of insulin receptor substrate-1[20]. Weight loss has been shown to improve reproductive function, hyperandrogenemia, and metabolism in women with PCOS. GLP-1RA, known for its weight loss and IR improvement effects, has been widely used in the treatment of PCOS. GLP-1RAs can reduce body weight in PCOS patients and lower androgen levels. However, research on the underlying mechanisms of GLP-1RAs in PCOS treatment has been limited. The proliferation and apoptosis of granulosa cells are fundamental to follicular development and atresia, with the forkhead box transcription factor O1 playing a crucial role in promoting PCOS follicular atresia and granulosa cell apoptosis[21]. Recent studies have found that the gut microbe Bacteroides vulgatus, through its metabolite difluoro methylarginine (DFMA), activates the farnesoid X receptor pathway, which subsequently suppresses GLP-1 secretion. This suppression leads to IR and ovarian dysfunction. However, the GLP-1RA liraglutide and the arginine decarboxylase inhibitor DFMA have been shown to improve ovarian dysfunction in mouse models that mimic PCOS[22], thereby presenting new potential targets for the treatment of PCOS (Figure 2). Other research has found that liraglutide can repair cognitive impairments in PCOS rat models by inhibiting overexpression of the Notch signaling pathway[23]. Therefore, based on preliminary research and clinical efficacy assessments, GLP-1RA has shown promising therapeutic effects in the treatment of PCOS.
Figure 2 Glucagon-like peptide-1 receptor agonists improve ovarian dysfunction in a mouse model of polycystic ovary syndrome.
GLP-1RA: Glucagon-like peptide-1 receptor agonists; GLP-1: Glucagon-like peptide-1; PCOS: Polycystic ovary syndrome; DFMA: Difluoro methylarginine.
GLP-1RA AND ALZHEIMER'S DISEASE
Alzheimer's disease (AD) is the most common neurodegenerative disease. Despite extensive research over the past few decades, the mechanisms of occurrence and development of AD have not been fully elucidated. Autopsy analysis has revealed that in the brains of AD patients with significantly reduced insulin receptor expression, IR also occurs as the disease progresses, indicating that defects in insulin signaling are related to the pathogenesis of AD[24]. Epidemiological studies have found a correlation between T2DM and AD[25]. Experimental studies have shown that most GLP-1 analogs can cross the blood–brain barrier and have a direct physiological effect on the human brain[26]. These effects include increasing the proliferation of neural progenitor cells, improving learning, and reducing the formation of plaques and inflammation in the brain. Experimental studies have also confirmed that GLP-1RAs can reduce the levels of AD pathological markers, such as oligomeric Aβ and Aβ plaque burden, reduce the activation of microglia, and improve memory[27]. Overexpression of GLP-1Rs in the hippocampus of mice has been found to increase neurite growth and improve spatial learning[28]. GLP-1RAs can reduce cell necrosis caused by glutamate, Fe2+ ions, and hypoxia, thereby protecting hippocampal neurons[29]. Calcium also plays an important role in neuronal plasticity and neurodegenerative diseases. In vitro experiments have found that pretreatment of rat hippocampal neurons with GLP-1 can weaken the response of Ca2+ ions to glutamate and weaken membrane depolarization, reducing the susceptibility of neurons to glutamate-induced death[30]. Studies have found that dulaglutide can protect learning and memory, reduce the hyperphosphorylation of tau protein, and its mechanism may be related to the phosphatidylinositol 3-kinase/serine-threonine kinase/glycogen synthase kinase-3β signaling pathway[30,31]. Based on the above analysis, although more clinical evidence is needed, the existing data has shown the potential application prospects and great research potential of GLP-1 and its analogs in the future treatment of AD.
IMPACT OF GLP-1RAS ON THE KIDNEYS
Diabetic kidney disease (DKD) is one of the most important microvascular complications of diabetes and is one of the important causes of chronic and end-stage kidney diseases. Its clinical manifestations are proteinuria and a decline in glomerular filtration rate. Even a slight increase in proteinuria or a decline in glomerular filtration rate is significantly related to an increased risk of cardiovascular diseases and cardiovascular death, so kidney protection is an important goal in the treatment of T2DM[32]. In addition to renin-angiotensin-aldosterone system (RAAS) inhibitors, there are currently no other drugs available for the treatment of DKD. GLP-1RAs have been proven to prevent the occurrence of proteinuria in diabetic patients and reduce the decline in glomerular filtration rate[33]. In humans, GLP-1R is present in cells of the proximal renal tubules and preglomerular vascular smooth muscle cells[34]. The FLOW trial demonstrated that semaglutide treatment significantly reduced the risk of major kidney disease events and cardiovascular death in patients with T2DM and chronic kidney disease. Studies have confirmed that GLP-1 can induce urinary sodium excretion and diuresis, and its mechanism may be to inhibit the sodium–hydrogen exchange protein located on the brush border of cells in the proximal renal tubules[35]. It rapidly downregulates the activity of Na+/K+-ATPase distributed on the brush border surface of the renal proximal tubules, causing pressure diuresis, which can partially explain the antihypertensive effect of GLP-1RAs[36]. The activation of the intrarenal RAAS is a well-known pathogenic medium of DKD. In experimental studies, GLP-1 and GLP-1RAs have been proven to reduce the concentration of angiotensin II. GLP-1 promotes the excretion of water and sodium by inhibiting the release of aldosterone and may also reduce hypertension, mesangial cell injury, and renal fibrosis induced by angiotensin II, thereby playing a role in protecting the kidneys[37]. GLP-1 reduces the production of reactive oxygen species in diabetic kidneys by activating the cyclic AMP-protein kinase A signal transduction pathway, inhibits oxidative stress, and protects the kidneys from oxidative damage. GLP-1RAs can significantly inhibit the activity of nuclear factor-κB in kidney tissue and significantly reduce expression of CD14, intercellular adhesion molecule-1, transforming growth factor-β1, and type IV collagen protein levels, indicating that GLP-1RAs can play a protective role in DKD by inhibiting the inflammatory response, and this effect is independent of the hypoglycemic, antihypertensive, and weight reduction effects[38-40]. The above data lay the foundation for future clinical research on whether GLP-1RAs can improve the prognosis of DKD patients.
CONCLUSION
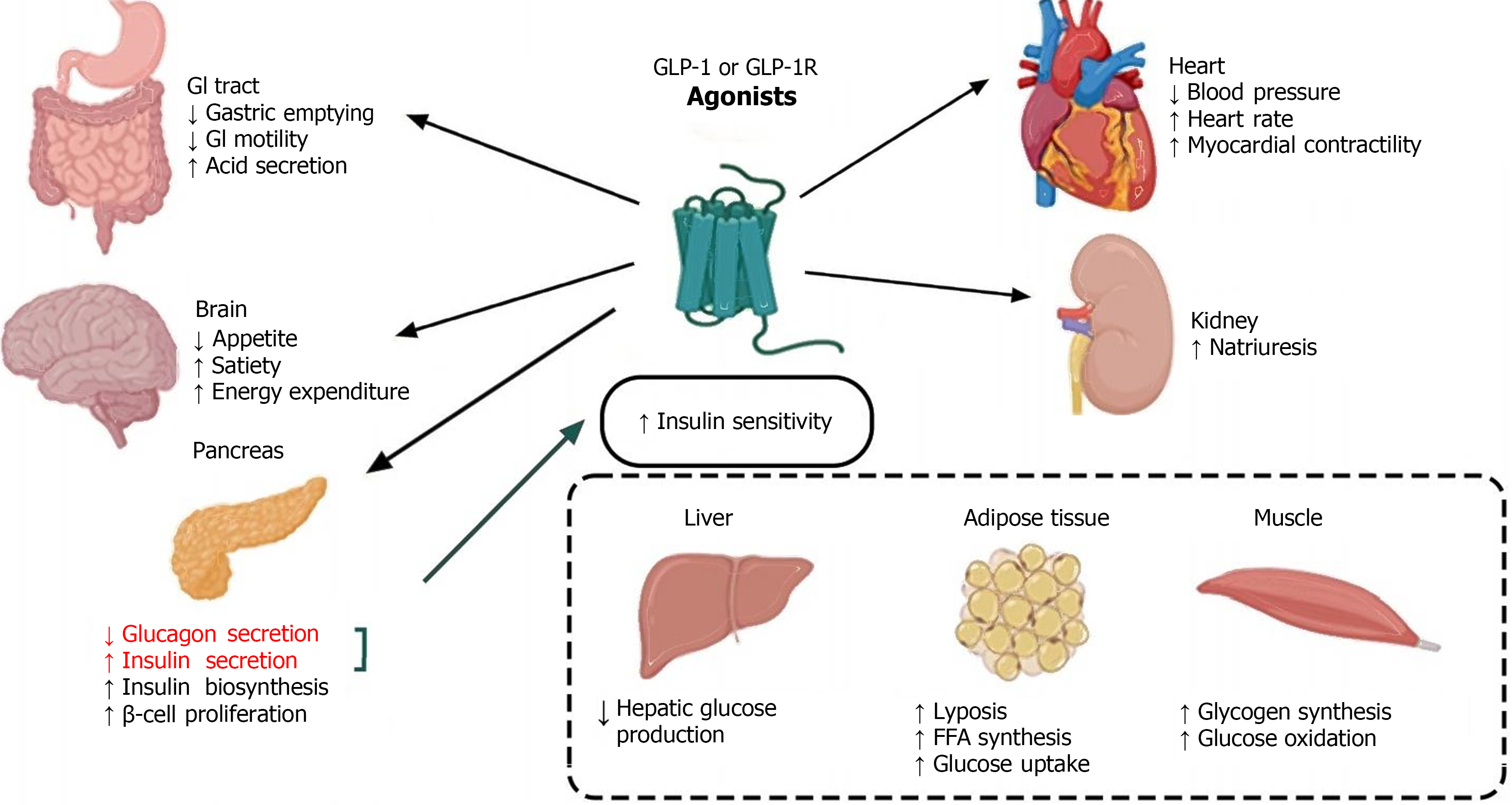
GLP-1RAs are innovative medications for treating T2DM, offering benefits such as reduced fasting glucose, lowered glycated hemoglobin, decreased blood pressure, and induced weight loss; all with a minimal risk of hypoglycemia. Their multifaceted action includes neuroprotection and cardiovascular and metabolic regulation, which emphasizes their potential in managing a variety of neurological and endocrine disorders. Despite ongoing debates about their tolerability and side effects, ongoing research is exploring oral formulations and enhancers to optimize their therapeutic profile. This editorial systematically reviews the broader impacts of GLP-1RAs, beyond glycemic control, and their therapeutic potential across different diseases (Figure 3), aiming to pave the way for future investigative directions.
Figure 3 Glucagon-like peptide-1 receptor agonists are extensively involved in various systemic diseases and hold therapeutic potential for a range of conditions.
GLP-1R: Glucagon-like peptide-1 receptor; GLP-1: Glucagon-like peptide-1; GI: Gastrointestinal.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade B
P-Reviewer: Li SY S-Editor: Lin C L-Editor: A P-Editor: Cai YX