Published online Sep 7, 2024. doi: 10.3748/wjg.v30.i33.3823
Revised: August 2, 2024
Accepted: August 19, 2024
Published online: September 7, 2024
Processing time: 53 Days and 16.2 Hours
A growing body of research indicates significant differences between left-sided colon cancers (LCC) and right-sided colon cancers (RCC). Pan-immune-inflammation value (PIV) is a systemic immune response marker that can predict the prognosis of patients with colon cancer. However, the specific distinction between PIV of LCC and RCC remains unclear.
To investigate the prognostic and clinical significance of PIV in LCC and RCC patients.
This multicenter retrospective cohort study included 1510 patients with colon cancer, comprising 801 with LCC and 709 with RCC. We used generalized lifting regression analysis to evaluate the relative impact of PIV on disease-free survival (DFS) in these patients. Kaplan-Meier analysis, as well as univariate and multi
A total of 1510 patients {872 female patients (58%); median age 63 years [inter
These findings suggest that PIV may predict recurrence in patients with LCC but not RCC, underscoring the importance of tumor location when using PIV as a colon cancer biomarker.
Core Tip: This study underscores the critical role of tumor location in shaping the prognostic significance of the pan-immune-inflammation value in colon cancer patients. The findings reveal that a high pan-immune-inflammation value is strongly correlated with poorer disease-free survival in patients with left-sided colon cancer, while no such association was observed in right-sided colon cancer. These results suggest that integrating tumor location into prognostic evaluations could improve the identification of high-risk patients and facilitate more precise, personalized treatment strategies in clinical practice.
- Citation: Wang QY, Zhong WT, Xiao Y, Lin GL, Lu JY, Xu L, Zhang GN, Du JF, Wu B. Pan-immune-inflammation value as a prognostic biomarker for colon cancer and its variation by primary tumor location. World J Gastroenterol 2024; 30(33): 3823-3836
- URL: https://www.wjgnet.com/1007-9327/full/v30/i33/3823.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i33.3823
Inflammation plays a crucial role in tumor initiation, progression, and metastasis, in malignancies including colorectal cancer (CRC)[1,2]. As an emerging biomarker of the systemic inflammatory response (SIR), the pan-immune-inflammation value (PIV) has been demonstrated to predict prognosis[3,4] and sensitivity to anticancer treatment[5-7] in patients with various solid tumors in multiple meta-analyses and retrospective studies. In patients with CRC, an elevated PIV is independently associated with poor prognosis[8-10].
However, despite a growing body of research indicating significant distinctions between left-sided colon cancers (LCC) and right-sided colon cancers (RCC)[11-14], the existing research has concentrated on analyzing the role of PIV in CRC[8-10]. The utility and significance of PIV, as well as other SIR markers, may differ based on tumor location within the colon. Moreover, the majority of participants in these studies were recruited outside of China[8-10]. Only one study conducted in China reported a correlation between PIV and the clinical staging of CRC without exploring its relationship with prognosis[15]. These factors may lead to significant differences in PIV thresholds[8-10] and limit their clinical application. Given the geographic heterogeneity in the genomic landscape of CRC[16] and the variability in CRC location[11-14], such as LCC and RCC, PIV values may exhibit variations owing to these factors. Therefore, whether the findings of these studies apply to Chinese patients, particularly considering the differences between RCC and LCC, remains unknown.
To address these issues and assist clinicians in identifying potentially high-risk patients with colon cancer, we conducted a multicenter, retrospective cohort study in China. This study focused on patients with LCC and RCC who underwent radical surgery. The primary aim of this study was to assess the correlation between PIV and recurrence in these two groups.
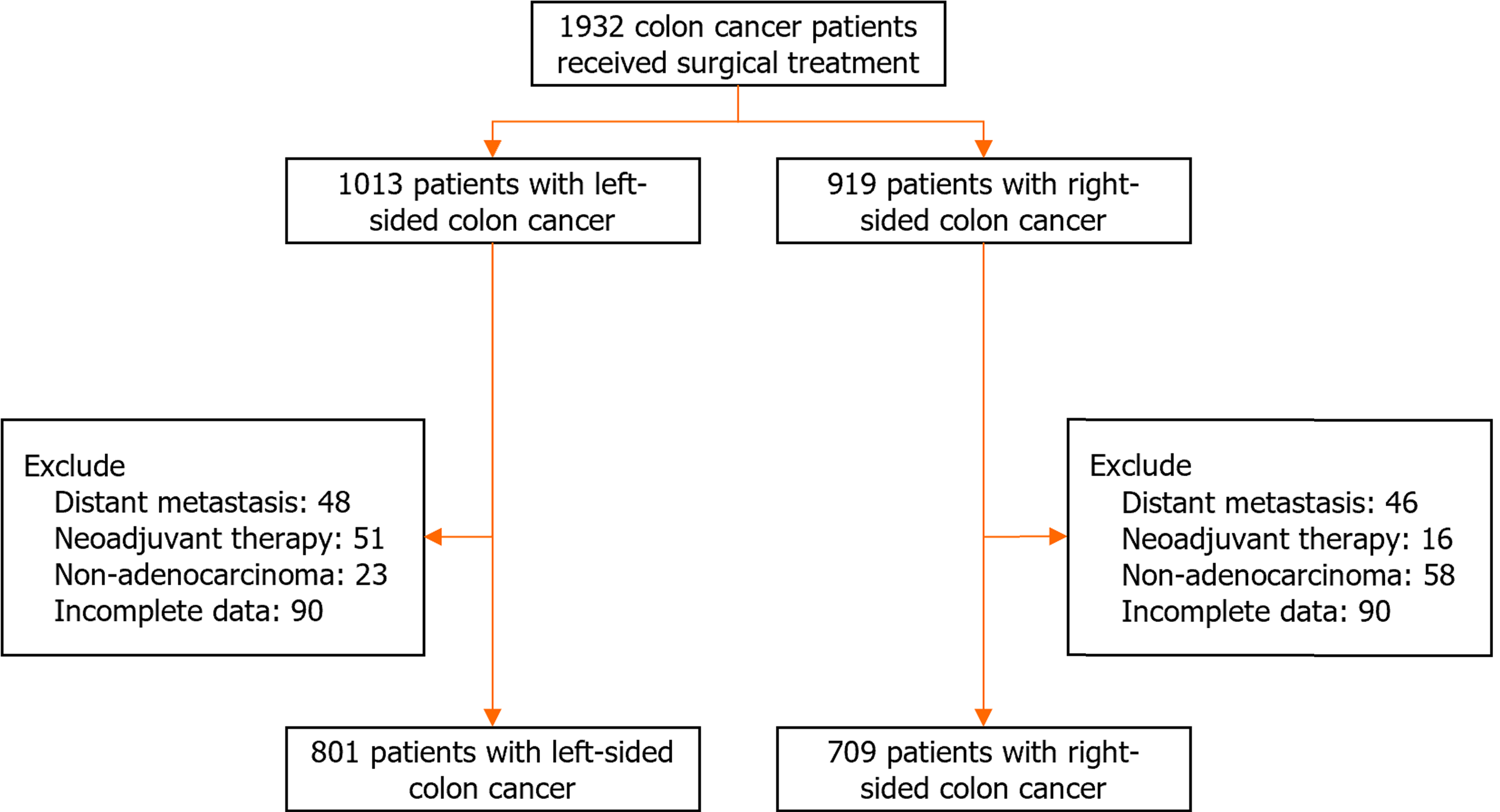
This study included patients with colon cancer treated at two institutes: The Peking Union Medical College Hospital from 2014 to 2021, and the 7th Medical Center of PLA General Hospital from 2015 to 2019. This study excluded patients with distant metastasis, non-adenocarcinoma, or those receiving neoadjuvant therapy, such as chemotherapy or chemoradiotherapy. A total of 1510 participants were included (Figure 1). A total of 180 participants were excluded from the analyses because of incomplete data such as clinicopathological characteristics and follow-up data.
The clinicopathological characteristics included sex, age, American Joint Committee on Cancer (AJCC) stage, tumor size, tumor grade [grades well and moderately differentiated (G1-2); grade poorly differentiated (G3)], tumor location, vascular invasion, perineural invasion, and DNA mismatch repair (MMR) [MMR-proficient (pMMR) and MMR-deficient (dMMR)]. Tumors located in the sigmoid, descending, and transverse colon near the spleen were defined as LCC. Tumors located in the transverse colon near the liver, ascending colon, and cecum were defined as RCC.
This study also included pre-operative tumor markers such as carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA). The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and PIV were calculated based on pre-operative peripheral blood data. The NLR was calculated as the neutrophil count divided by the lymphocyte count. The PLR was calculated as the platelet count divided by the lymphocyte count. The SII was calculated as NLR multiplied by the platelet count. PIV was calculated as the SII multiplied by the monocyte count. The time between radical surgery and recurrence during follow-up was defined as disease-free survival (DFS). Various thresholds were used to evaluate DFS[17]. Subsequently, the patients were cate
R version 4.2.1 was used for this study. The χ2 test and Fisher’s exact test were used to analyze categorical variables. The
Complete data from 1510 patients with colon cancer were analyzed in the present analysis (Supplementary Table 1). Of these patients, 872 (58%) were women, and the median age was 63 years [interquartile range (IQR): 54-71]. Median follow-up was 44.17 months (IQR: 29.67-62.32). We also compared the baseline characteristics of the included and excluded participants (Supplementary Table 2). No significant differences were observed between the two groups in terms of clinicopathological characteristics such as age, sex, AJCC stage, tumor grade, MMR, and tumor size.
Compared with patients with LCC, PIV was significantly higher in those with RCC [median (IQR): 214.34 (121.78-386.72) vs 175.87 (111.92-286.84); P < 0.001, Supplementary Table 1]. Similar results were observed for other SIR markers, such as NLR, PLR, and SII (Supplementary Table 1). To mitigate the potential influence of the clinical and pathological features of LCC and RCC on PIV, we employed propensity score matching by incorporating all relevant clinical and pathological indicators. In this propensity score-matched cohort, no statistically significant difference was observed at baseline between the 475 patients with LCC and 475 patients with RCC (Supplementary Table 3). There was no difference in PIV between patients with LCC and RCC [median (IQR): 182.42 (111.88-297.65) vs 189.45 (109.44-316.02); P = 0.987]. No differences were observed in NLR, and SII (Supplementary Table 3) as well. The patients with LCC had lower PLR [median (IQR): 144.85 (113.71-189.84) vs 165 (121.12-218.89); P < 0.001].
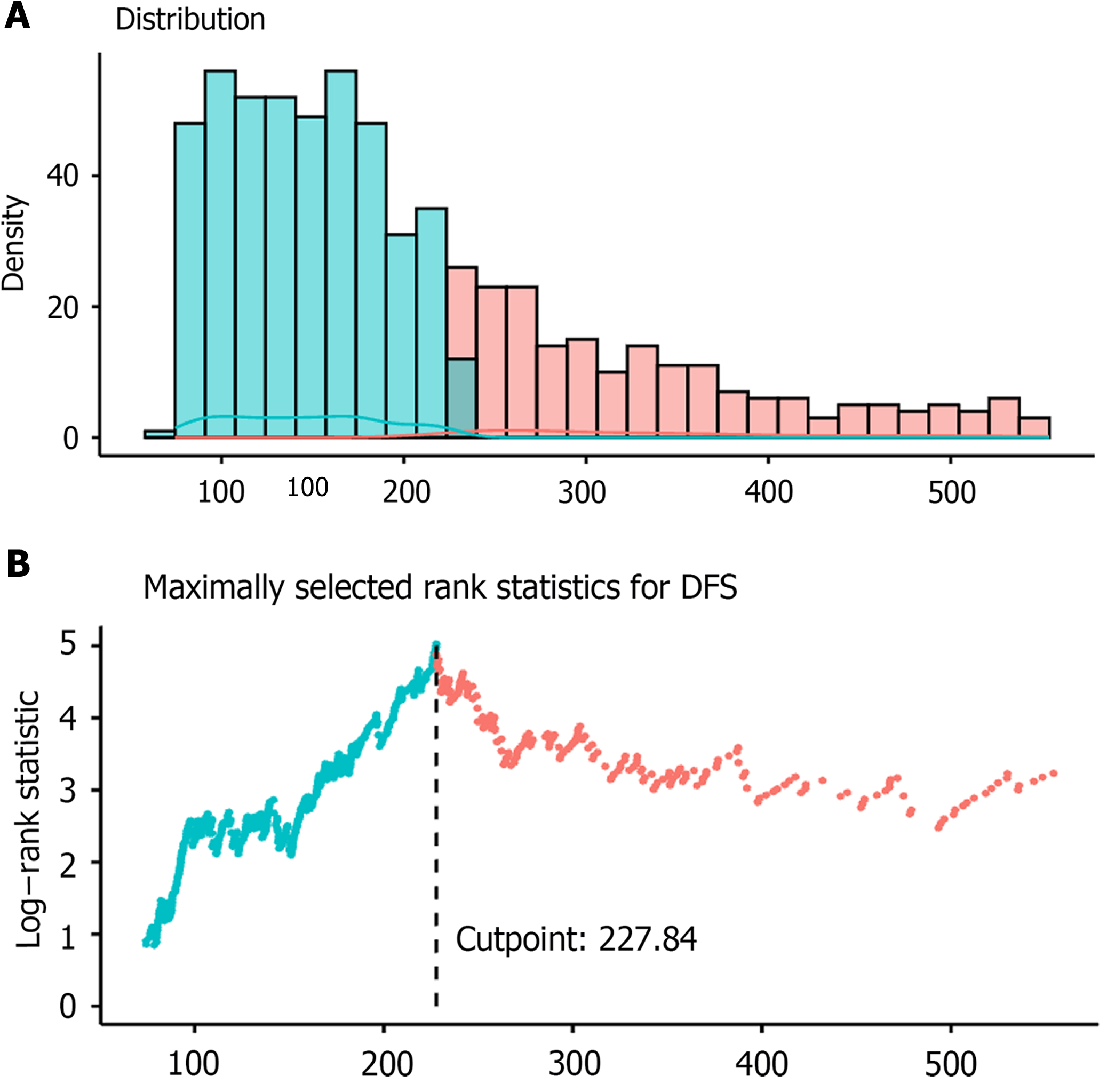
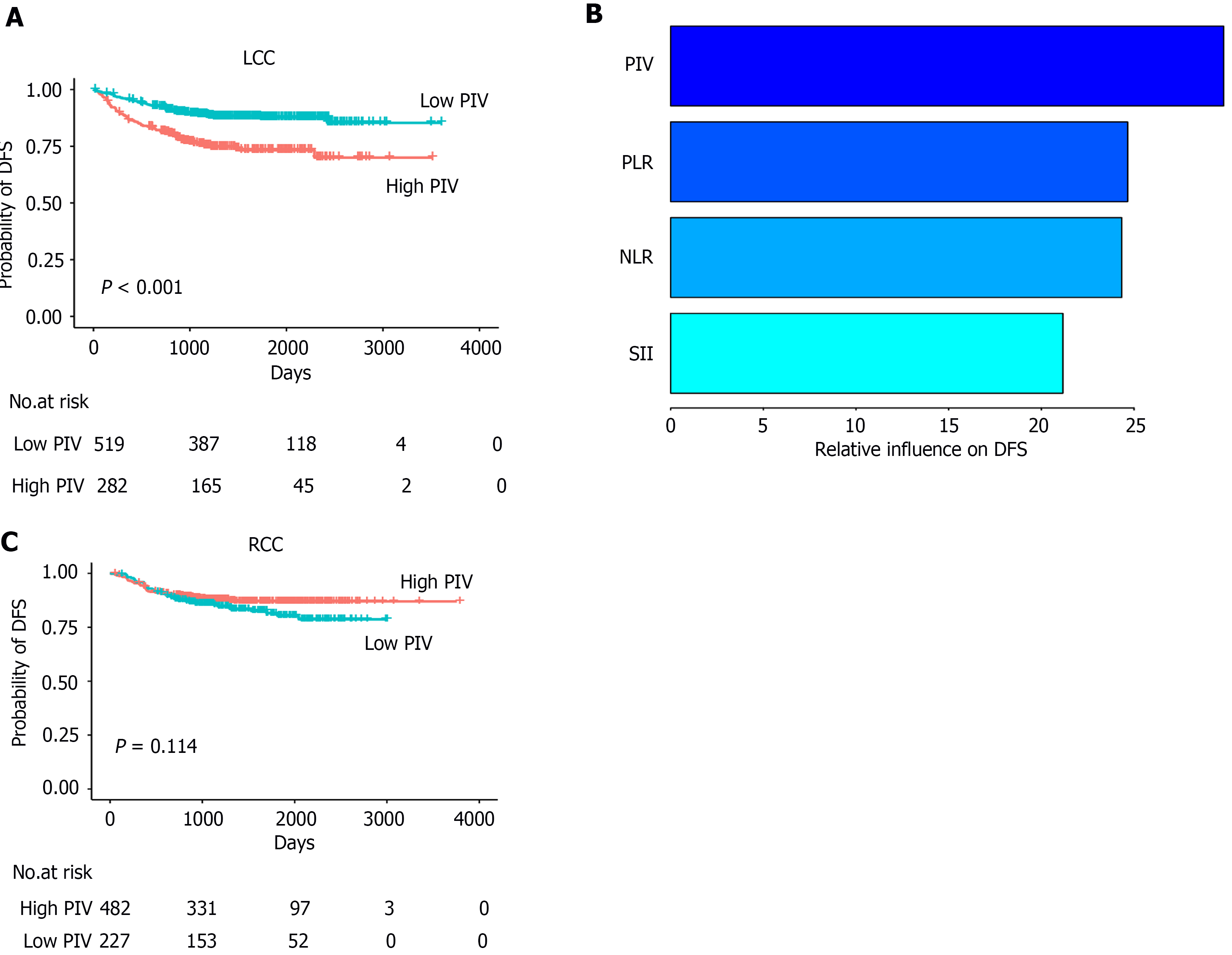
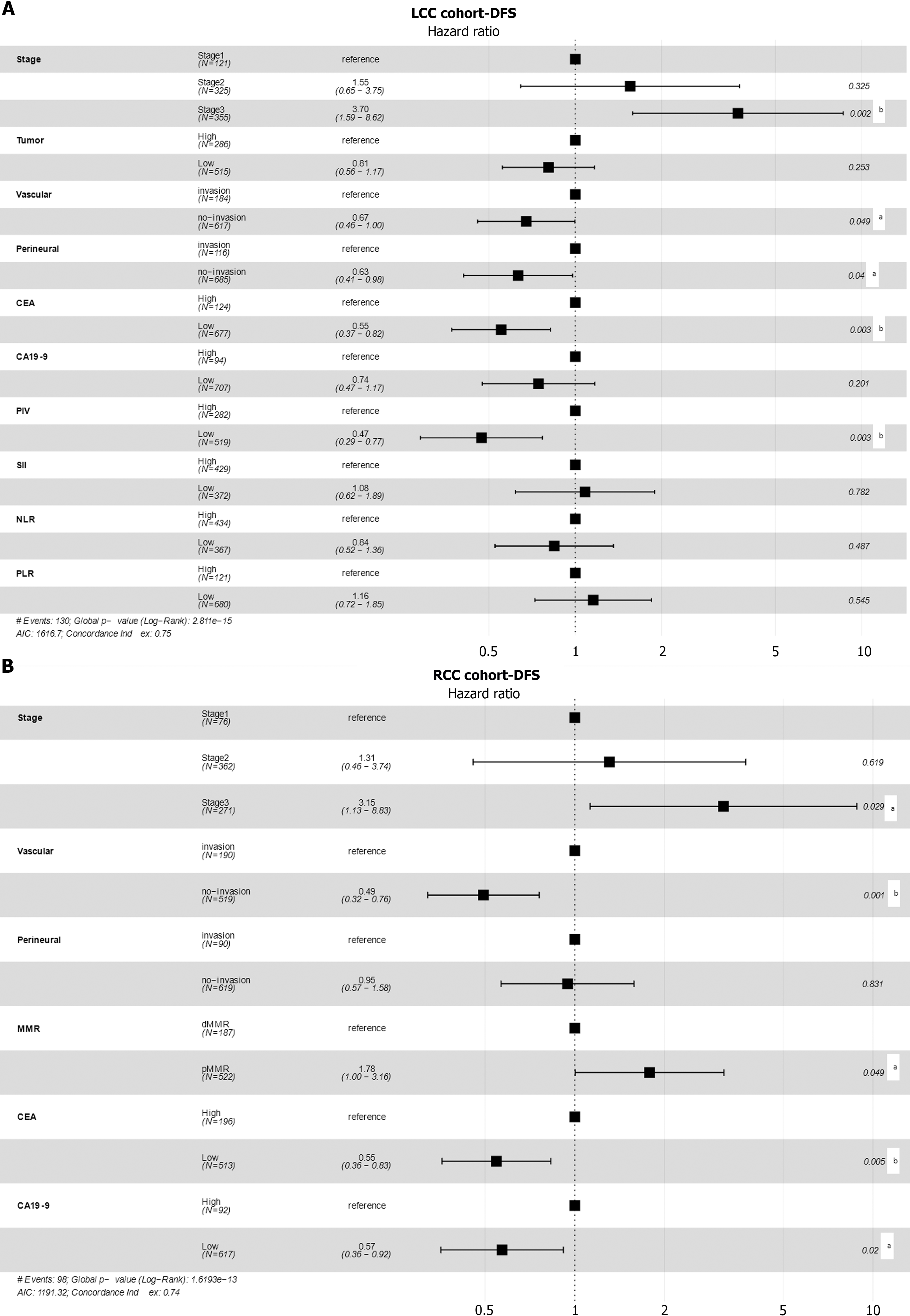
First, we evaluated the role of PIV in predicting the DFS in patients with LCC. For patients with LCC, the optimal cutoff value of PIV for DFS was determined to be 227.84 (Figure 2). We also evaluated the optimal cutoff values of other indicators for DFS and categorized them into two groups: High and low (Supplementary Table 4). A total of 282 patients (35%) had high PIV (Table 1). Kaplan-Meier analysis showed that the PIV-high group exhibited worse DFS than the PIV-low group [PIV-high: Adjusted hazard ratio (aHR) = 2.39, 95% confidence interval (CI): 1.70-3.38; P < 0.001; Figure 3A]. In univariate Cox regression analysis, high NLR, SII, and PLR were significantly associated with worse DFS (Table 2). Moreover, multivariate Cox regression analysis showed that among all SIR markers, only high PIV was independently associated with worse DFS (PIV-high: aHR = 2.12, 95%CI: 1.30-3.47; P = 0.003, Figure 4A). Additionally, the AJCC stage, vascular invasion, perineural invasion, and CEA levels were independently associated with DFS in patients with LCC (Table 2, Figure 4A). The generalized boosted regression model showed that PIV exhibited a higher relative influence on DFS than other SIR markers, such as NLR, PLR, and SII (Figure 3B).
| Variables | Patients | P value | ||
| All (n = 801) | PIV-high (n = 282) | PIV-low (n = 519) | ||
| Sex, n (%) | 0.044 | |||
| Female | 495 (62) | 188 (67) | 307 (59) | |
| Male | 306 (38) | 94 (33) | 212 (41) | |
| Age, median (IQR), years | 63 (54, 70) | 62.5 (54, 70.75) | 63 (54, 69) | 0.743 |
| Tumor grade, n (%) | 0.010 | |||
| G1-2 | 743 (93) | 252 (89) | 491 (95) | |
| G3 | 58 (7) | 30 (11) | 28 (5) | |
| Vascular, n (%) | 0.961 | |||
| Invasion | 184 (23) | 64 (23) | 120 (23) | |
| No-invasion | 617 (77) | 218 (77) | 399 (77) | |
| Perineural, n (%) | 0.727 | |||
| Invasion | 116 (14) | 43 (15) | 73 (14) | |
| No-invasion | 685 (86) | 239 (85) | 446 (86) | |
| MMR, n (%) | < 0.001 | |||
| dMMR | 85 (11) | 48 (17) | 37 (7) | |
| pMMR | 716 (89) | 234 (83) | 482 (93) | |
| T stage, n (%) | 0.001 | |||
| T1-2 | 143 (18) | 33 (12) | 110 (21) | |
| T3-4 | 658 (82) | 249 (88) | 409 (79) | |
| N stage, n (%) | 0.192 | |||
| N0 | 446 (56) | 152 (54) | 294 (57) | |
| N1 | 261 (33) | 89 (32) | 172 (33) | |
| N2 | 94 (12) | 41 (15) | 53 (10) | |
| NLR, median (IQR) | 2.13 (1.62, 2.92) | 3.06 (2.39, 4.15) | 1.8 (1.44, 2.23) | < 0.001 |
| PLR, median (IQR) | 141.67 (111.31, 183.98) | 182.76 (143.89, 238.09) | 127.47 (100, 155.05) | < 0.001 |
| SII, median (IQR) | 502.24 (357.54, 742.37) | 856.81 (650, 1299.84) | 402.6 (307.71, 508.28) | < 0.001 |
| PIV, median (IQR) | 175.87 (111.92, 286.84) | 361.93 (271.07, 644.13) | 133.11 (91.46, 172.42) | < 0.001 |
| CEA, median (IQR), ng/mL | 3.5 (2, 7.58) | 4.4 (2.3, 10.52) | 3.2 (1.88, 6.73) | < 0.001 |
| CA19-9, median (IQR), U/mL | 11.6 (7, 20.1) | 11.95 (6.73, 21.8) | 11.5 (7.2, 19.6) | 0.772 |
| Tumor size, median (IQR), cm | 4 (3, 5) | 5 (3.78, 6) | 4 (3, 5) | < 0.001 |
| Variables | LCC (n = 801) | RCC (n = 709) | ||||||
| aHR (95%CI)1 | P value1 | aHR (95%CI)2 | P value2 | aHR (95%CI)1 | P value1 | aHR (95%CI)2 | P value2 | |
| Sex | ||||||||
| Female | 1 (Reference) | NA | 1 (Reference) | NA | ||||
| Male | 1.17 (0.82-1.65) | 0.387 | 1.13 (0.76-1.68) | 0.541 | ||||
| Age, years | ||||||||
| < 65 | 1 (Reference) | NA | 1 (Reference) | NA | ||||
| ≥ 65 | 1.31 (0.93-1.85) | 0.124 | 1.44 (0.96-2.15) | 0.074 | ||||
| Tumor grade | ||||||||
| G1-2 | 1 (Reference) | NA | 1 (Reference) | NA | ||||
| G3 | 1.63 (0.92-2.90) | 0.094 | 0.88 (0.49-1.58) | 0.676 | ||||
| AJCC stage | ||||||||
| I | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA |
| II | 2.12 (0.89-5.06) | 0.089 | 1.55 (0.65-3.75) | 0.325 | 1.58 (0.56-4.50) | 0.390 | 1.31 (0.46-3.74) | 0.619 |
| III | 5.76 (2.52-13.15) | < 0.001 | 3.70 (1.59-8.62) | 0.002 | 5.10 (1.86-14.00) | 0.002 | 3.15 (1.13-8.83) | 0.029 |
| Tumor size | ||||||||
| < 5 cm | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | ||
| ≥ 5 cm | 1.61 (1.14-2.27) | 0.007 | 1.24 (0.86-1.80) | 0.253 | 0.88 (0.59-1.31) | 0.521 | ||
| Vascular | ||||||||
| No-invasion | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA |
| Invasion | 2.17 (1.52-3.10) | < 0.001 | 1.48 (1.00-2.19) | 0.049 | 3.01 (2.02-4.47) | < 0.001 | 2.02 (1.32-3.11) | 0.001 |
| Perineural | ||||||||
| No-invasion | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA |
| Invasion | 2.22 (1.49-3.32) | < 0.001 | 1.58 (1.02-2.45) | 0.040 | 2.24 (1.39-3.60) | < 0.001 | 1.06 (0.63-1.77) | 0.831 |
| MMR | ||||||||
| pMMR | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | ||
| dMMR | 0.74 (0.40-1.37) | 0.339 | 0.45 (0.25-0.78) | 0.005 | 0.56 (0.32-1.00) | 0.049 | ||
| CEA | ||||||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA |
| High | 2.80 (1.93-4.06) | < 0.001 | 1.81 (1.22-2.70) | 0.003 | 2.51 (1.69-3.73) | < 0.001 | 1.83 (1.20-2.79) | 0.005 |
| CA19-9 | ||||||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA |
| High | 2.02 (1.31-3.13) | 0.002 | 1.34 (0.85-2.11) | 0.201 | 2.65 (1.69-4.15) | < 0.001 | 1.75 (1.09-2.81) | 0.020 |
| PIV | ||||||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | ||
| High | 2.39 (1.70-3.38) | < 0.001 | 2.12 (1.30-3.47) | 0.003 | 0.72 (0.48-1.08) | 0.114 | ||
| SII | ||||||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | ||
| High | 1.89 (1.31-2.72) | < 0.001 | 0.92 (0.53-1.62) | 0.782 | 0.68 (0.44-1.04) | 0.076 | ||
| NLR | ||||||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | ||
| High | 1.72 (1.20-2.47) | 0.003 | 1.18 (0.74-1.90) | 0.487 | 1.37 (0.77-2.46) | 0.288 | ||
| PLR | ||||||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA | 1 (Reference) | NA | ||
| High | 1.73 (1.14-2.61) | 0.009 | 0.87 (0.54-1.38) | 0.545 | 1.58 (0.94-2.66) | 0.088 | ||
For patients with RCC, the optimal cutoff value of PIV for DFS was determined to be 145.99 (Supplementary Table 4). A total of 482 patients (68%) had a high PIV (Supplementary Table 5). Kaplan-Meier analysis did not reveal a significant association with DFS (PIV-high: aHR = 0.72, 95%CI: 0.48-1.08; P = 0.114; Figure 3C). Univariate Cox regression analysis also indicated that the NLR, PLR, and SII were not significantly associated with DFS (Table 2). Multivariate Cox regression analysis showed that the AJCC stage, CEA levels, CA19-9 levels, MMR, and vascular invasion were associated with DFS in patients with RCC (Table 2, Figure 4B).
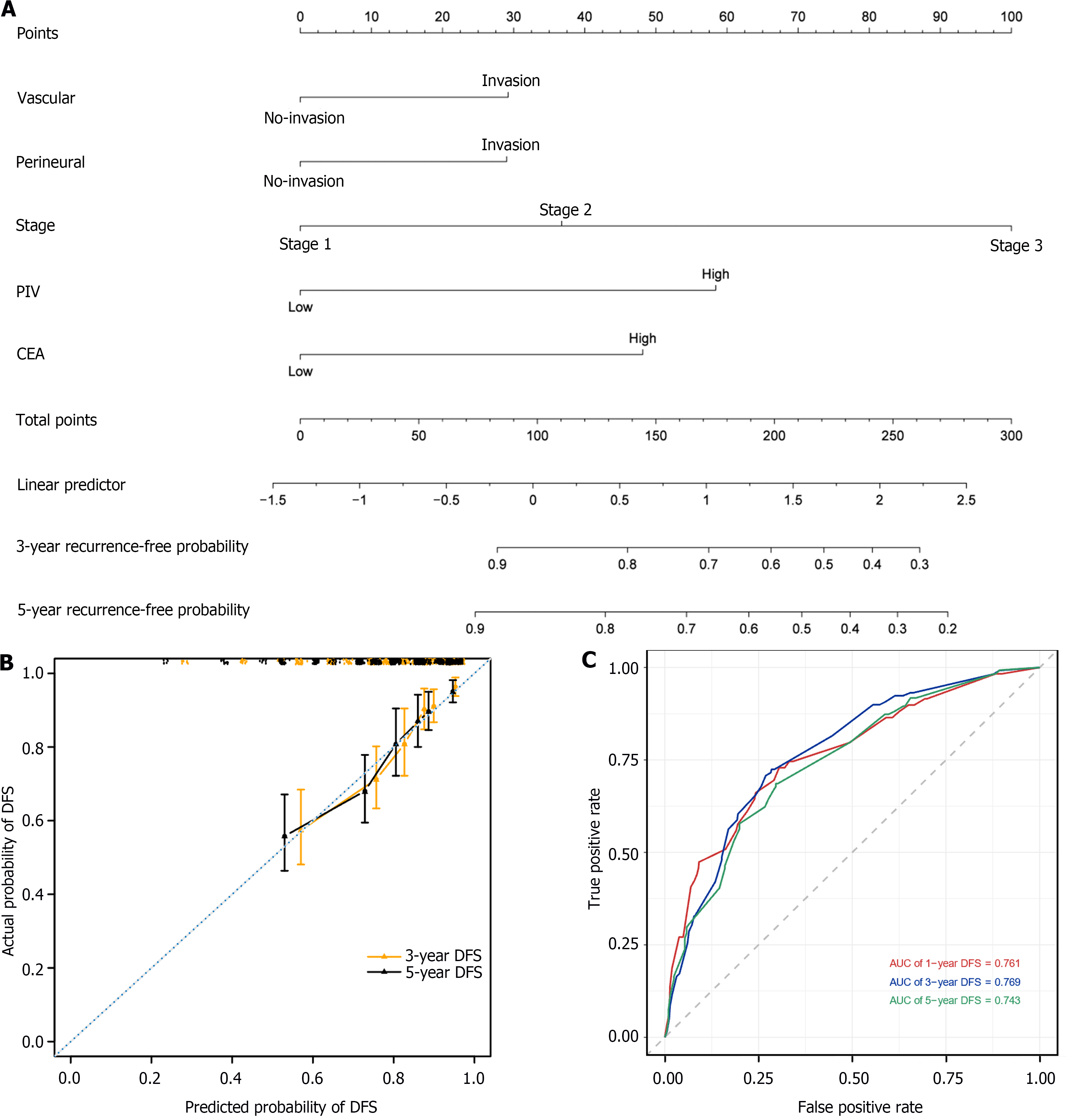
To accurately predict the prognosis of patients with LCC, we developed a nomogram to predict the probability of 3- and 5-year DFS using DFS-based multivariate Cox regression analysis in the LCC patient cohort. The nomogram included five independent prognostic factors: Vascular invasion, perineural invasion, stage, PIV, and CEA levels (Figure 5A). The predictive models exhibited substantial accuracy with a concordance index of 0.739 for DFS. The calibration curve sets showed good consistency between the actual and predicted probability of 3-year and 5-year DFS rates in the LCC patient cohort (Figure 5B). The 1-, 3-, and 5-year area under the receiver operating characteristic curve values of the nomogram for DFS were 0.761, 0.769, and 0.743, respectively (Figure 5C).
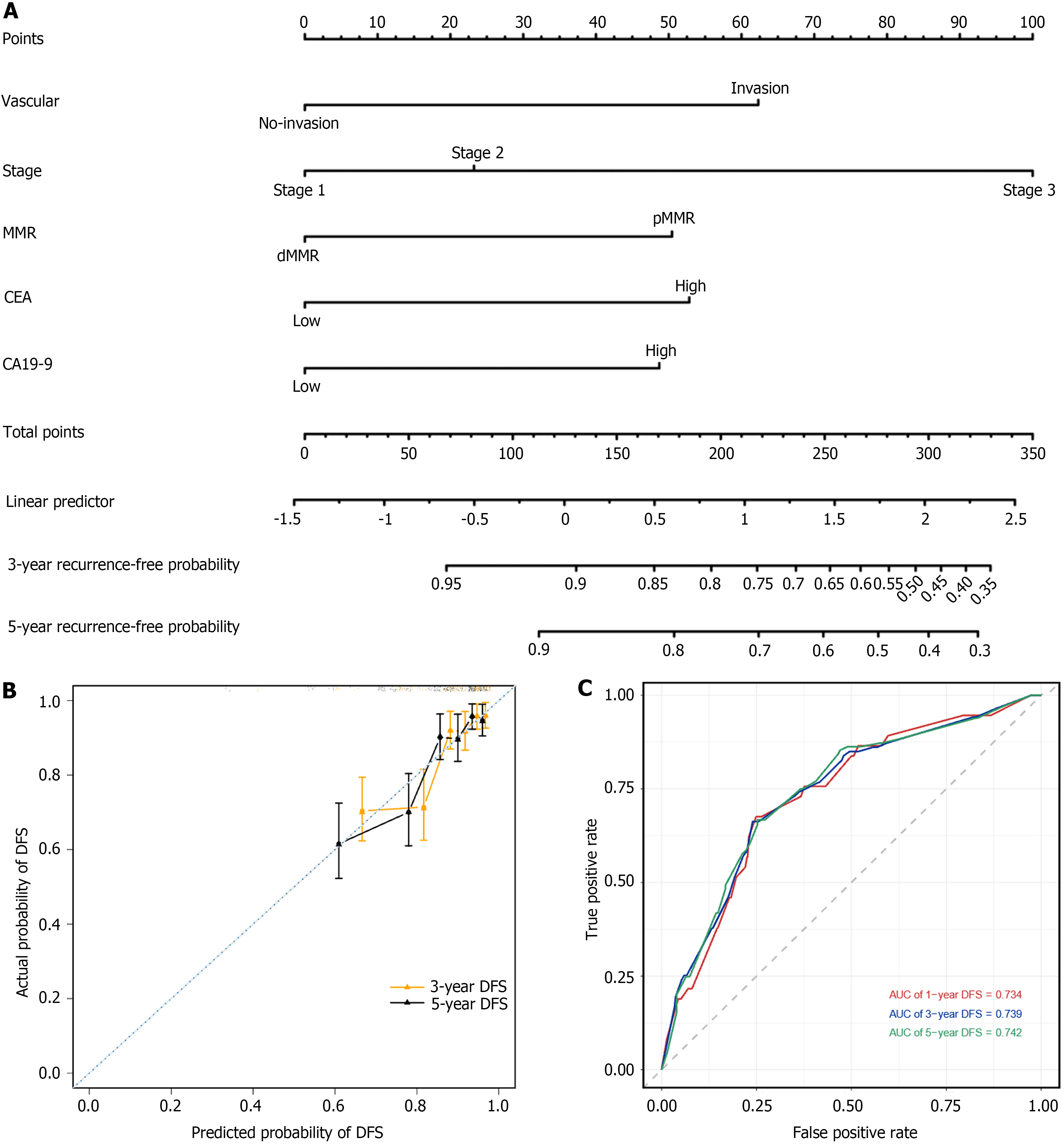
To accurately predict the prognosis of patients with RCC, we developed a nomogram to predict the probability of 3- and 5-year DFS using DFS-based multivariate Cox regression analysis in the RCC patient cohort. The nomogram included five independent prognostic factors: Vascular invasion, stage, MMR, CEA levels, and CA19-9 levels (Figure 6A). The predictive models exhibited substantial accuracy with a concordance index of 0.742 for DFS. The calibration curve sets showed good consistency between the actual and predicted probability of 3-year and 5-year DFS rates in the RCC patient cohort (Figure 6B). The 1-, 3-, and 5-year area under the receiver operating characteristic curve values of the nomogram for DFS were 0.734, 0.739, and 0.742, respectively (Figure 6C).
The clinicopathological characteristics of patients with LCC in the PIV-high and PIV-low groups were compared (Tables 1 and 3). Compared to PIV-low patients, a higher proportion of PIV-high patients were female (67% vs 59%; P = 0.044), had G3 tumors (11% vs 5%; P = 0.010), higher dMMR (17% vs 7%; P < 0.001), higher CEA levels [median (IQR): 4.4 (2.3-10.52) vs 3.2 (1.88-6.73); P < 0.001], larger tumor size [median (IQR): 5 (3.78-6) vs 4 (3-5); P < 0.001], and higher T stage (T3-4: 88% vs 79%; P = 0.001). Logistic multivariable analysis showed that LCC patients were more likely to have high PIV if they had larger tumor size [≥ 5 cm: Adjusted odds ratio (aOR) = 2.55; 95%CI: 1.85-3.51; P < 0.001], higher CEA levels (high: aOR = 1.94; 95%CI: 1.28-2.95; P = 0.002), and dMMR (dMMR: aOR = 2.53; 95%CI: 1.53-4.18; P < 0.001).
| Variables | LCC | RCC | ||
| aOR (95%CI) | P value | aOR (95%CI) | P value | |
| Sex | ||||
| Male | 1 (Reference) | NA | 1 (Reference) | NA |
| Female | 1.36 (0.98-1.88) | 0.062 | 1.49 (1.06-2.10) | 0.022 |
| Age | ||||
| < 65 | 1 (Reference) | NA | 1 (Reference) | NA |
| ≥ 65 | 1.00 (0.73-1.38) | 0.979 | 1.47 (1.04-2.07) | 0.028 |
| Tumor grade | ||||
| G1-2 | 1 (Reference) | NA | 1 (Reference) | NA |
| G3 | 1.45 (0.80-2.62) | 0.217 | 2.05 (1.17-3.60) | 0.013 |
| T stage | ||||
| T1-2 | 1 (Reference) | NA | 1 (Reference) | NA |
| T3-4 | 1.52 (0.96-2.41) | 0.076 | 2.07 (1.26-3.39) | 0.004 |
| N stage | ||||
| N0 | 1 (Reference) | NA | 1 (Reference) | NA |
| N1 | 0.97 (0.68-1.39) | 0.874 | 1.29 (0.85-1.95) | 0.237 |
| N2 | 1.58 (0.93-2.67) | 0.089 | 1.11 (0.63-1.945) | 0.715 |
| Tumor size | ||||
| < 5 cm | 1 (Reference) | NA | 1 (Reference) | NA |
| ≥ 5 cm | 2.55 (1.85-3.51) | < 0.001 | 2.19 (1.55-3.10) | < 0.001 |
| Vascular | ||||
| No-invasion | 1 (Reference) | NA | 1 (Reference) | NA |
| Invasion | 0.83 (0.56-1.25) | 0.379 | 0.86 (0.56-1.30) | 0.470 |
| Perineural | ||||
| No-invasion | 1 (Reference) | NA | 1 (Reference) | NA |
| Invasion | 1.09 (0.69-1.72) | 0.726 | 0.61 (0.36-1.02) | 0.059 |
| MMR | ||||
| pMMR | 1 (Reference) | NA | 1 (Reference) | NA |
| dMMR | 2.53 (1.53-4.18) | < 0.001 | 1.80 (1.18-2.76) | 0.006 |
| CEA | ||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA |
| High | 1.94 (1.28-2.95) | 0.002 | 1.20 (0.80-1.80) | 0.376 |
| CA19-9 | ||||
| Low | 1 (Reference) | NA | 1 (Reference) | NA |
| High | 1.01 (0.62-1.62) | 0.982 | 1.55 (0.87-2.74) | 0.134 |
Next, we compared baseline characteristics between the PIV-high and PIV-low groups of patients with RCC (Sup
To our knowledge, this is the largest multicenter retrospective study on Chinese patients with colon cancer who underwent curative surgery to evaluate the association between PIV and DFS. We analyzed the data from 1510 patients with colon cancer, focusing on the differences in PIV between patients with LCC and RCC. Our study found that an elevated PIV was an independent adverse factor for DFS in patients with LCC, but not in patients with RCC.
In our study, an elevated PIV was associated with worse DFS in LCC, which is consistent with the findings of other CRC studies[8-10]. Neutrophils have been associated with cancer development and metastasis by generating reactive oxygen species, suppressing the antitumor immune response, and influencing various paracrine signaling pathways[18,19]. Conversely, lymphocytes play a pivotal role as primary drivers of the antitumor immune response within the tumor microenvironment (TME)[20,21]. A growing body of research suggests that platelets play a role in promoting tumor initiation, progression, and metastasis through an intricate crosstalk with cancer cells[22,23]. Specifically, platelets can release numerous factors, including those that support survival, angiogenesis, and immunomodulation, without direct contact, contributing to the formation and maintenance of both primary and metastatic TME[23]. Similarly, monocytes can affect the TME through various mechanisms that induce immune tolerance, and angiogenesis, and increase tumor cell dissemination[24,25]. These findings underscore the multifaceted role of these immune and hematologic components in shaping the TME and modulating tumor biology.
However, PIV was not significantly associated with DFS in patients with RCC. Similar outcomes were noted when examining other SIR markers such as NLR, PLR, and SII. Another study highlighted the prognostic significance of NLR exclusively in LCC and its lack of predictive prognostic value in RCC[26]. These findings underscore the heterogeneity of the SIR across different colon cancer subtypes. Further investigations into the underlying mechanisms driving the observed differences in PIV and other SIR markers between LCC and RCC are required.
We found that patients with larger tumors, higher CEA levels, and dMMR were more likely to have elevated PIV, similar to observations in other studies on CRC[9,15]. Elevated PIV was independently associated with DFS even after adjusting for these and other factors. Additionally, PIV exhibited superior performance compared to other SIR markers (NLR, PLR, and SII) in predicting DFS in patients with LCC. In a study by Fucà et al[27] involving patients with metastatic CRC, the PIV score outperformed other SIR markers (NLR, PLR, and SII) in logistic regression. Similarly, among the various SIR markers (PLR, SII, PIV, and NLR), only PIV was an independent predictor of the prognosis of patients with HER2 (+) advanced breast cancer[28]. These findings suggest that PIV may be more promising than other SIR markers for predicting patient prognosis. This advantage may be attributed to the comprehensive nature of PIV, which encompasses all pro-inflammatory cells in the blood. This broader scope may contribute to more effective risk stratification than the NLR, PLR, or SII markers.
Consistent with prior studies[15,29], we found that patients with RCC were more likely to have elevated SIR markers (NLR, PLR, SII, and PIV) than patients with LCC. This observation may be attributed to the increased frequency of RCC with higher levels of pro-inflammatory factors, such as higher T and N stages, higher tumor grade, dMMR, higher CEA levels, and larger tumor size. However, no statistically significant difference in PIV between patients with LCC and those with RCC was observed after propensity score matching to eliminate baseline differences. This evidence suggests that baseline clinical and pathological features may influence the PIV variation between the two groups.
This study has certain limitations. First, retrospective studies may suffer from selection bias. Secondly, owing to the variance in treatment methods, patients with rectal cancer were excluded from this study. Therefore, further research is essential to evaluate the role of PIV in predicting the prognosis and sensitivity to neoadjuvant therapy in rectal cancer. Finally, we did not collect information on the patients’ adjuvant therapy, which may have introduced additional bias in the recurrence analyses.
In this multicenter retrospective study of patients with colon cancer who underwent surgery, we analyzed the role of PIV in predicting DFS in subgroups of patients with LCC and RCC. Our study suggests that elevated PIV may be an independent adverse factor for DFS in patients with LCC but not in those with RCC, highlighting the importance of considering tumor location when using PIV as a prognostic biomarker in colon cancer.
The authors acknowledge with gratitude all the staff who participated in this study.
| 1. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8560] [Article Influence: 475.6] [Reference Citation Analysis (0)] |
| 2. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8433] [Article Influence: 527.1] [Reference Citation Analysis (9)] |
| 3. | Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, Aksoy S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Hai-Jing Y, Shan R, Jie-Qiong X. Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: an updated meta-analysis of 30 studies. Front Nutr. 2023;10:1259929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 5. | Şahin AB, Cubukcu E, Ocak B, Deligonul A, Oyucu Orhan S, Tolunay S, Gokgoz MS, Cetintas S, Yarbas G, Senol K, Goktug MR, Yanasma ZB, Hasanzade U, Evrensel T. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep. 2021;11:14662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Corti F, Lonardi S, Intini R, Salati M, Fenocchio E, Belli C, Borelli B, Brambilla M, Prete AA, Quarà V, Antista M, Fassan M, Morano F, Spallanzani A, Ambrosini M, Curigliano G, de Braud F, Zagonel V, Fucà G, Pietrantonio F. The Pan-Immune-Inflammation Value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. 2021;150:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Feng J, Wang L, Yang X, Chen Q, Cheng X. Pretreatment Pan-Immune-Inflammation Value (PIV) in Predicting Therapeutic Response and Clinical Outcomes of Neoadjuvant Immunochemotherapy for Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2024;31:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Yang XC, Liu H, Liu DC, Tong C, Liang XW, Chen RH. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front Oncol. 2022;12:1036890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Seo YJ, Kim KE, Jeong WK, Baek SK, Bae SU. Effect of preoperative pan-immune-inflammation value on clinical and oncologic outcomes after colorectal cancer surgery: a retrospective study. Ann Surg Treat Res. 2024;106:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Efil SC, Guner G, Guven DC, Celikten B, Celebiyev E, Taban H, Akyol A, Isik A, Kilickap S, Yalcin S, Dizdar O. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin Res Hepatol Gastroenterol. 2023;47:102171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Lee MS, Menter DG, Kopetz S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw. 2017;15:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 12. | Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G, Barni S. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2017;3:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 565] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 13. | Imperial R, Ahmed Z, Toor OM, Erdoğan C, Khaliq A, Case P, Case J, Kennedy K, Cummings LS, Melton N, Raza S, Diri B, Mohammad R, El-Rayes B, Pluard T, Hussain A, Subramanian J, Masood A. Comparative proteogenomic analysis of right-sided colon cancer, left-sided colon cancer and rectal cancer reveals distinct mutational profiles. Mol Cancer. 2018;17:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Zhong ME, Chen Y, Xiao Y, Xu L, Zhang G, Lu J, Qiu H, Ge W, Wu B. Serum extracellular vesicles contain SPARC and LRG1 as biomarkers of colon cancer and differ by tumour primary location. EBioMedicine. 2019;50:211-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Zhao H, Chen X, Zhang W, Cheng D, Lu Y, Wang C, Li J, You L, Yu J, Guo W, Li Y, Huang Y. Pan-immune-inflammation value is associated with the clinical stage of colorectal cancer. Front Surg. 2022;9:996844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 16. | Guo L, Wang Y, Yang W, Wang C, Guo T, Yang J, Shao Z, Cai G, Cai S, Zhang L, Hu X, Xu Y. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023;165:414-428.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 17. | Lausen B, Schumacher M. Maximally Selected Rank Statistics. Biometrics. 1992;48:73-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 366] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 537] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 19. | Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 412] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 20. | Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117:451-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 21. | Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2244] [Cited by in RCA: 2468] [Article Influence: 224.4] [Reference Citation Analysis (0)] |
| 22. | Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131:1777-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 23. | Li S, Lu Z, Wu S, Chu T, Li B, Qi F, Zhao Y, Nie G. The dynamic role of platelets in cancer progression and their therapeutic implications. Nat Rev Cancer. 2024;24:72-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 24. | Goswami S, Anandhan S, Raychaudhuri D, Sharma P. Myeloid cell-targeted therapies for solid tumours. Nat Rev Immunol. 2023;23:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 25. | Ugel S, Canè S, De Sanctis F, Bronte V. Monocytes in the Tumor Microenvironment. Annu Rev Pathol. 2021;16:93-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 26. | Mazaki J, Katsumata K, Kasahara K, Tago T, Wada T, Kuwabara H, Enomoto M, Ishizaki T, Nagakawa Y, Tsuchida A. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20:922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, Marmorino F, Lonardi S, Rimassa L, Sartore-Bianchi A, Borelli B, Tampellini M, Bustreo S, Claravezza M, Boccaccino A, Murialdo R, Zaniboni A, Tomasello G, Loupakis F, Adamo V, Tonini G, Cortesi E, de Braud F, Cremolini C, Pietrantonio F. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. 2020;123:403-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 28. | Ligorio F, Fucà G, Zattarin E, Lobefaro R, Zambelli L, Leporati R, Rea C, Mariani G, Bianchi GV, Capri G, de Braud F, Vernieri C. The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Chang JS, Cheng HH, Huang SC, Lin HH, Chang SC, Lin CC. The impact of inflammatory markers on prognosis of stage II colon cancers depends on tumour sidedness. ANZ J Surg. 2023;93:182-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/