Published online Aug 21, 2024. doi: 10.3748/wjg.v30.i31.3717
Revised: July 10, 2024
Accepted: July 22, 2024
Published online: August 21, 2024
Processing time: 91 Days and 20.1 Hours
Small cell lung carcinoma (SCLC) is highly susceptible to metastasis in the early stages of the disease. However, the stomach is an uncommon site of metastasis in SCLC, and only a few cases of this type of metastasis have been reported. There
We report three cases of gastric metastasis from SCLC in this article. The first patient presented primarily with cough, hemoptysis, and epigastric fullness. The other two patients presented primarily with abdominal discomfort, epigastric distension, and pain. All patients underwent gastroscopy and imaging examinations. Meanwhile, the immunohistochemical results of the lesions in three pa
Here, we focused on summarizing the characteristics of gastric metastasis of SCLC to enhance clinicians' under
Core Tip: Due to the rarity of small cell lung carcinoma (SCLC) gastric metastasis, few cases have been reported internationally, and clinicians are not sufficiently aware of the clinical features of this group of diseases. In this article, we report three cases of SCLC gastric metastasis to summarize the characteristics of this type of disease and improve the under
- Citation: Yang S, He QY, Zhao QJ, Yang HT, Yang ZY, Che WY, Li HM, Wu HC. Gastric metastasis of small cell lung carcinoma: Three case reports and review of literature. World J Gastroenterol 2024; 30(31): 3717-3725
- URL: https://www.wjgnet.com/1007-9327/full/v30/i31/3717.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i31.3717
The incidence of small cell lung carcinoma (SCLC) has gradually increased in recent years. Approximately 70% of patients have already developed distant metastases at diagnosis[1]. The liver, brain, bones, adrenal glands, and lymph nodes are common sites of metastasis for this disease[2]. In contrast, SCLC metastasis to the stomach is very rare, and the related clinical features have not been systematically described, which makes it easily missed and misdiagnosed. Therefore, an in-depth understanding of the clinical features of these diseases is essential for early diagnosis and timely treatment.
Case 1: A 56-year-old male presented with a complaint of persistent cough and hemoptysis for one year and epigastric fullness for six months.
Case 2: A 67-year-old male presented with a complaint of persistent epigastric discomfort for one month.
Case 3: A 77-year-old male presented with a complaint of persistent subxiphoid swelling and pain for two months.
Case 1: One year previously, the patient was admitted to our hospital due to cough and hemoptysis. The patient was diagnosed with SCLC via bronchoscopic pathologic biopsy. Then, six months prior to the present presentation, the patient developed abdominal distension.
Case 2: The patient had a 1-month history of persistent subxiphoid fullness and discomfort, which was aggravated by eating and accompanied by nausea. No other specific discomfort was described.
Case 3: The patient had a 2-month history of persistent subxiphoid swelling and pain. No other specific discomfort was described.
The three patients had no previous abnormalities.
Case 1: The patient had a 25-year history of smoking, with an average of 40 cigarettes smoked per day.
Case 2: The patient had a 30-year history of smoking, with an average of 20 cigarettes smoked per day.
Case 3: The patient had a 50-year history of smoking, with an average of 35 cigarettes smoked per day.
Case 1: The physical examination was unremarkable.
Case 2: There was mild epigastric tenderness on abdominal examination.
Case 3: There was mild subxiphoid tenderness on abdominal examination.
Case 1: Laboratory examinations revealed that the patient's level of neuron-specific enolase (NSE) was slightly greater (39.960 ng/mL) and that the levels of serum tumor markers were elevated [carcinoembryonic antigen (CEA) 16.44 µg/L (< 5), CA199 111.70 U/mL (< 35), and CA125 283.3 U/mL (< 35)]. Other laboratory data revealed no abnormalities.
Case 2: Laboratory examination revealed no abnormalities.
Case 3: The patient's CA199 and CEA levels were elevated to 310.5 U/mL and 655.55 U/mL, respectively.
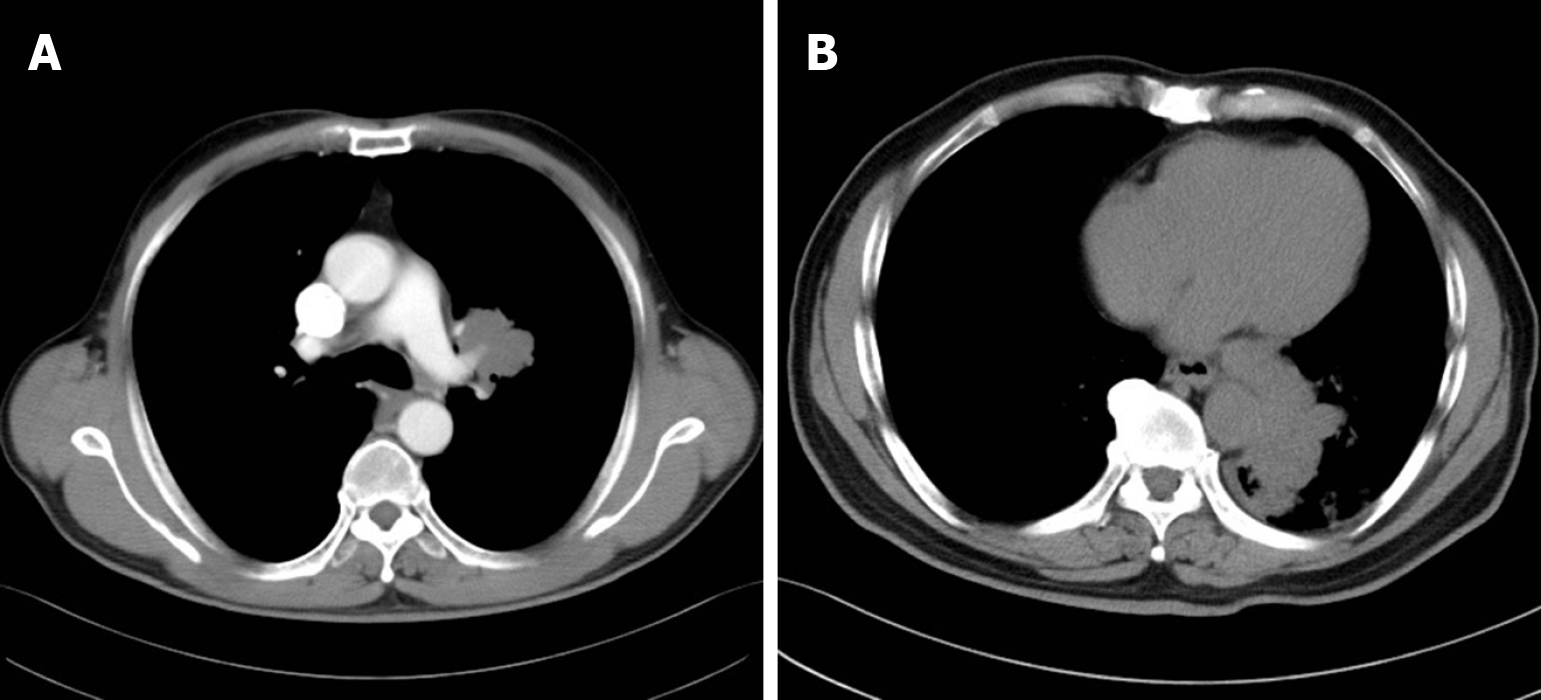
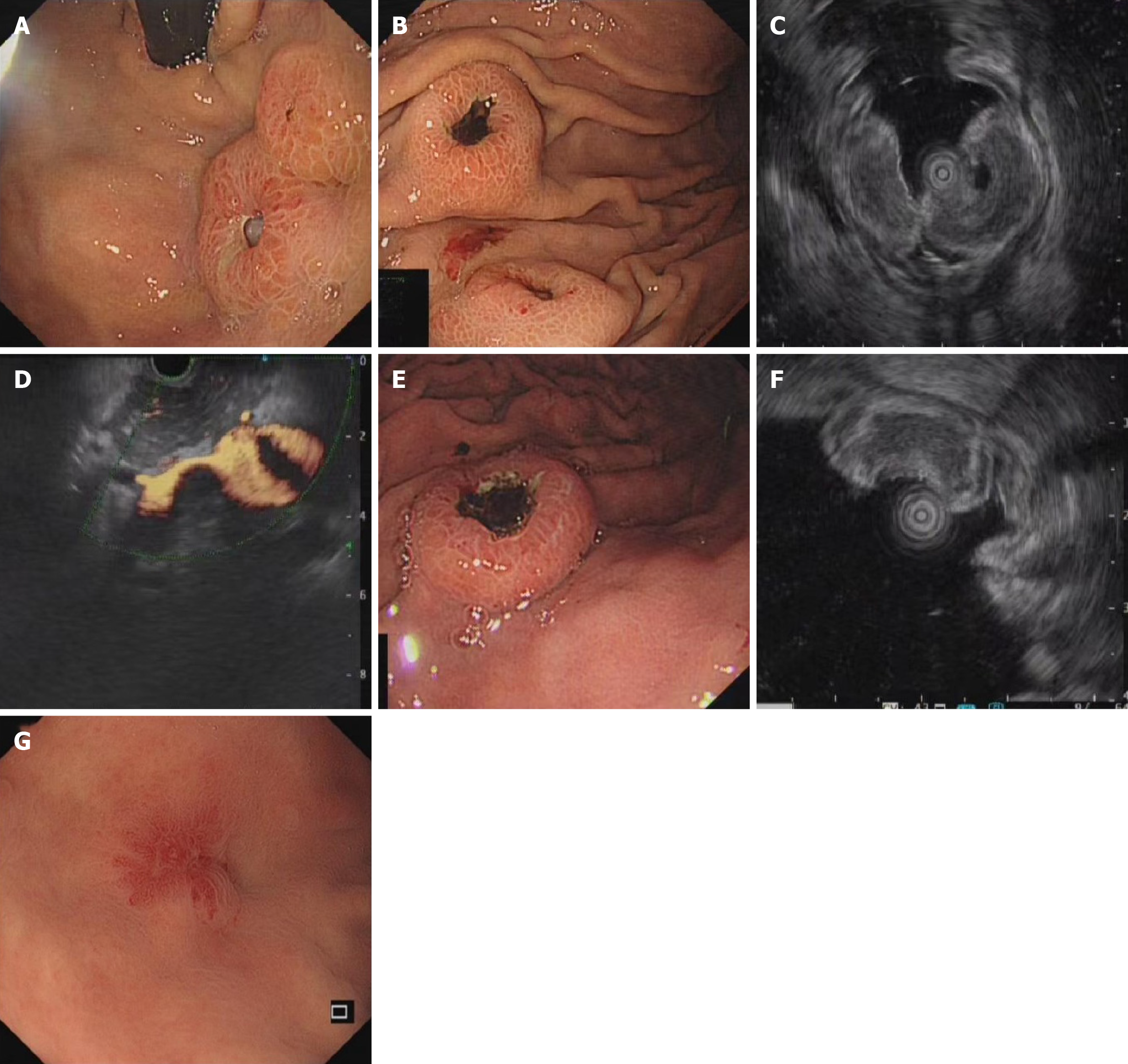
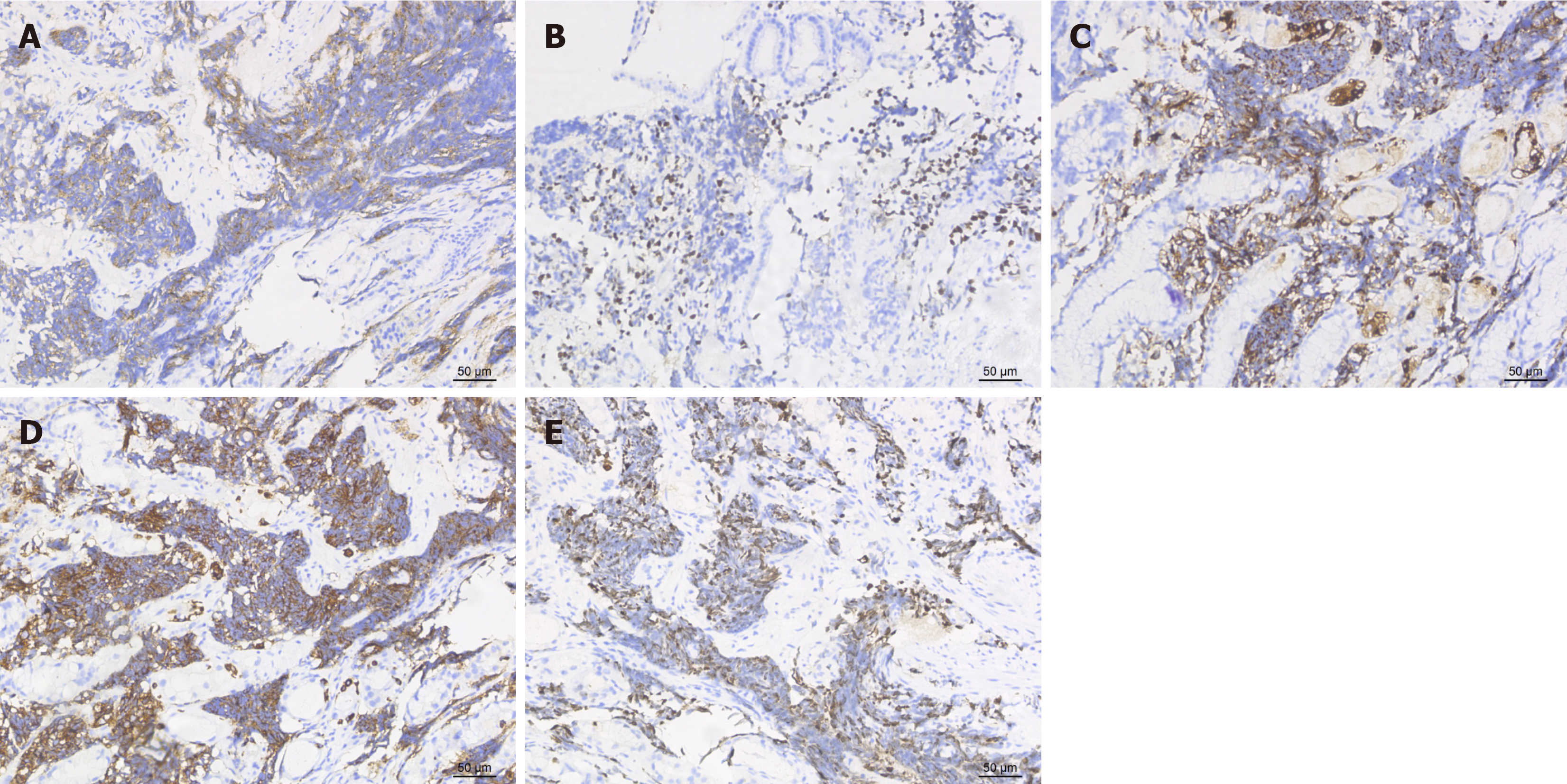
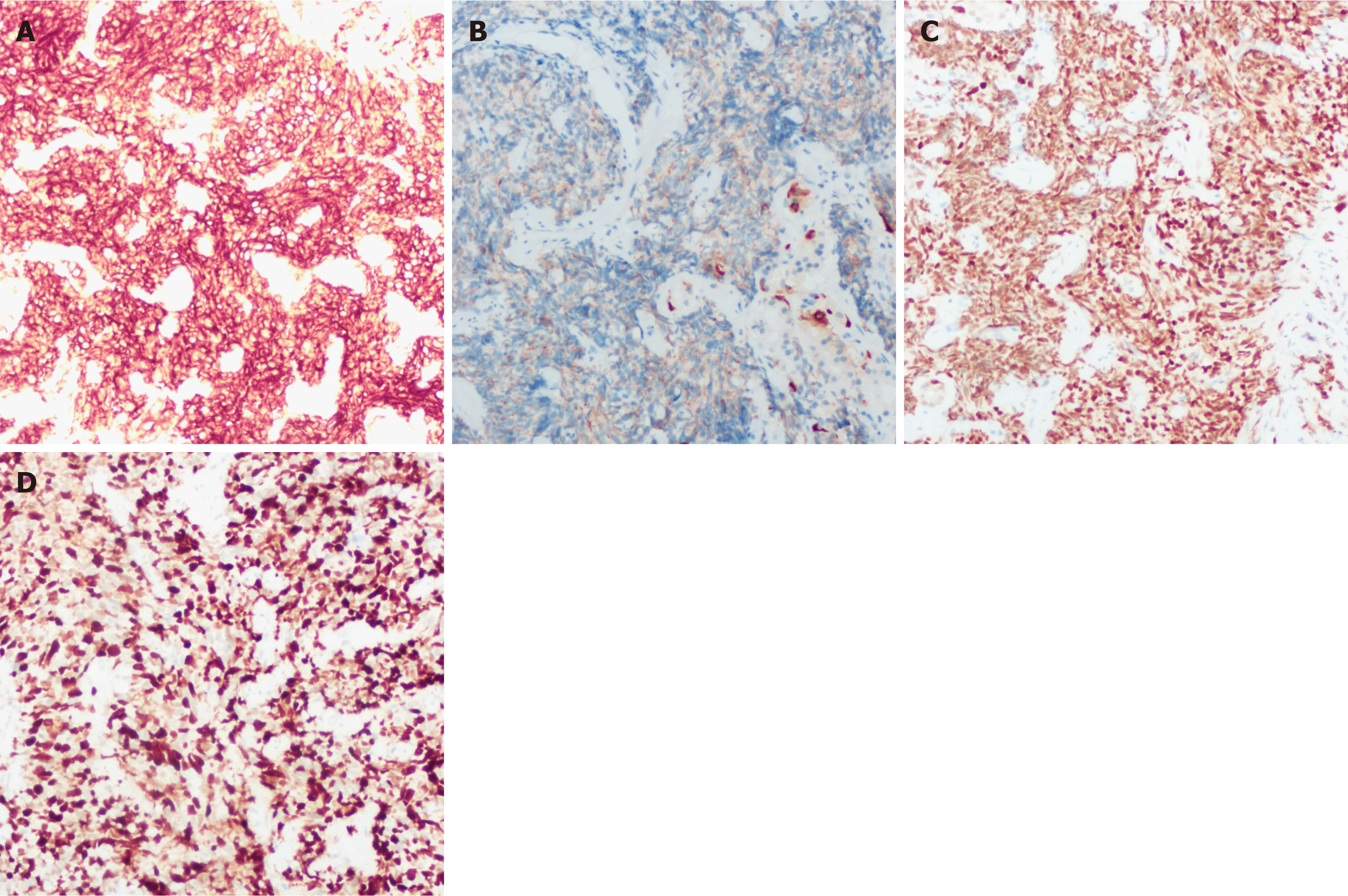
Case 1: In April 2020, the patient underwent a chest scan, which revealed centralized lung cancer in the left hilum (Figure 1A). The patient subsequently underwent immunohistochemistry after biopsy via bronchial fibroscopy, which revealed positivity for cytokine (CK), CD56, thyroid transcription factor (TTF-1), and chromogranin A (CgA) and negativity for CK7 and P40. Consequently, the diagnosis was SCLC. After developing abdominal discomfort, the patient returned to the hospital for gastroscopy, which revealed two masses approximately 1 cm in diameter in the body and fundus of the stomach, accompanied by central depression and ulcer formation (Figure 2A and B). Endoscopic ultrasound (EUS) revealed a hypoechoic lesion at the lesion site (Figure 2C) and portal vein thrombosis, which was thought to be due to metastasis (Figure 2D). Immunohistochemistry of the biopsy samples obtained from the gastric fundus revealed that the samples were positive for CK, CD56, TTF-1, CgA, and synaptophysin (Syn). The ki-67 index was 70% (Figure 3).
Case 2: Gastroscopy revealed a lesion at the greater curvature of the stomach measuring approximately 1.5 mm × 1.8 mm, with an ulcer forming on the surface, and biopsy of the primary lesion was performed (Figure 2E). EUS revealed hy
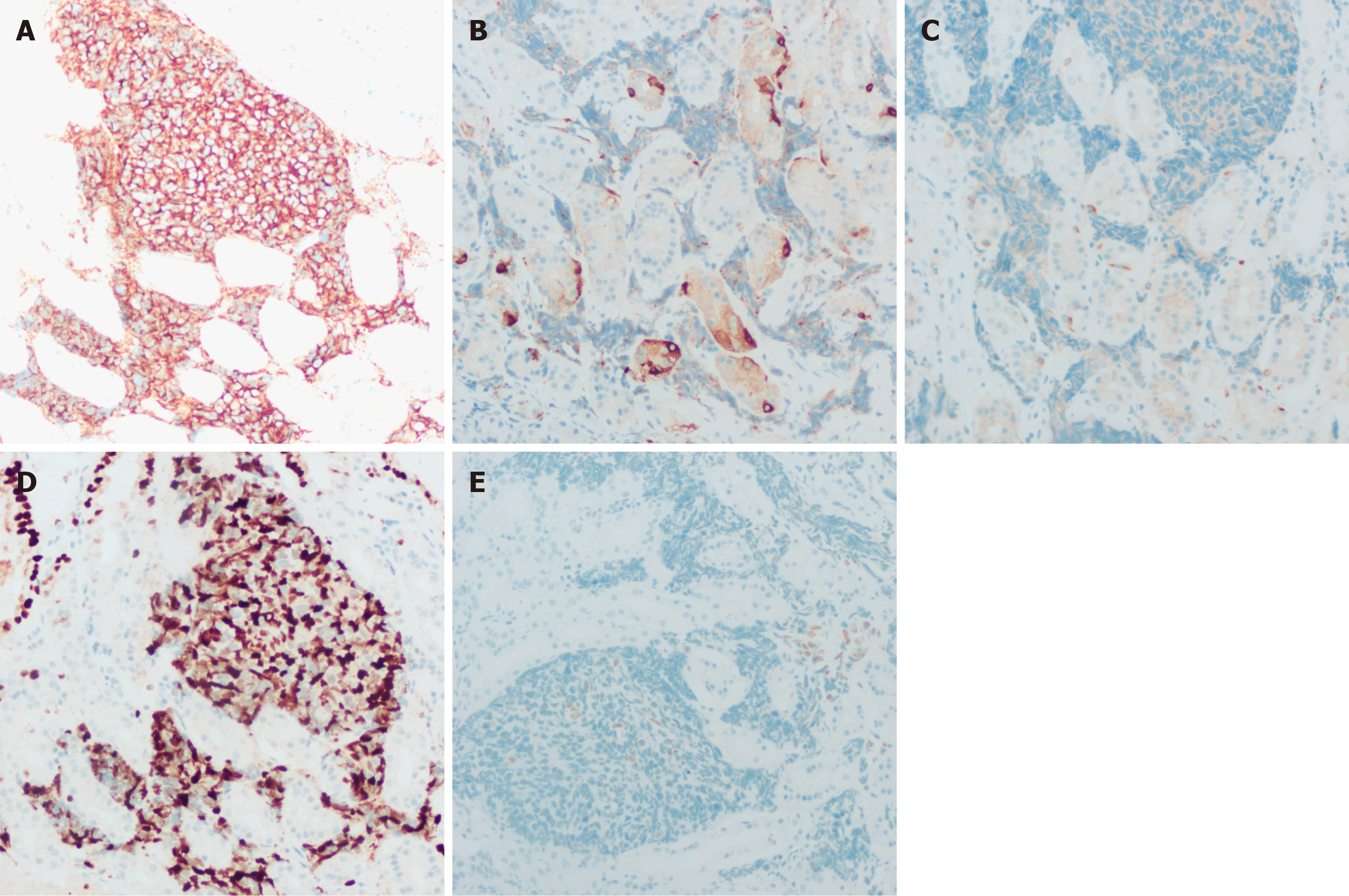
Case 3: Gastroscopy revealed a 4 mm × 5 mm red mucosal lesion in the fundus of the stomach with elevated margins and a central visible depression (Figure 2G). However, a CT scan at an outside hospital suggested multiple lesions in both lungs, possibly indicating tuberculosis or tumors, whereas an abdominal CT suggested multiple metastases in the liver. Immunohistochemistry of the liver biopsy sample revealed positivity for TTF-1, CA199, CD117, and CK and negativity for vimentin, villin, AFP, S100, CK20, hepatocytes, VD34, CK19, and CK7, which was considered to indicate a metastatic tract of small cell carcinoma to the liver. Histological examination of biopsy samples obtained from the gastric fundus revealed positive expression of CD56, CgA, CK, and TTF-1 and negative expression of Syn and Napsin-A. In addition, the ki-67 index was as high as 80% (Figure 5).
On the basis of CT, endoscopy, ultrasound, immunohistochemistry and other relevant examinations, the three patients in this study were ultimately diagnosed with gastric metastasis of SCLC.
All three patients refused to undergo the relevant treatment for personal reasons.
The three patients died between 3 and 6 months after diagnosis.
Lung cancer is the leading cause of cancer death, and approximately 50% of lung cancer patients may develop distant metastases at an early stage. The liver, adrenal glands, bone, brain, and lymph nodes are common sites of metastasis for lung cancer[3]. However, metastasis to the gastrointestinal tract is rare, and studies have shown that the rate of gast
The clinical presentation of patients with gastric metastases from SCLC is nonspecific. Patients usually present with digestive symptoms. For example, the patients in this case report presented mainly with symptoms of abdominal pain and bloating. Additionally, respiratory symptoms may be present. Previous studies have reported that symptoms arising from metastases tend to appear later than those arising from the primary tumor[8]. In contrast, in our case, two patients first presented with gastrointestinal symptoms but no obvious respiratory symptoms. Hence, when gastrointestinal metastasis occurs in SCLC patients, some patients will first present with signs and symptoms of the digestive system while not having any obvious respiratory signs and symptoms. Therefore, when diagnosing small cell carcinoma of the stomach, clinicians should consider whether the tumor has metastasized from other sites, especially the lung. This will reduce the incidence of clinical underdiagnosis and misdiagnosis.
Indeed, SCLC gastric metastases are usually first detected via gastroscopy and are further confirmed after gastroscopic biopsy. Owing to the low incidence of gastric metastasis in SCLC, few studies have been conducted on its endoscopic characterization. Among them are reports that the most common site of metastatic gastric cancer is often located in the mucosa and submucosa, which is related mainly to blood or lymphatic spread of the primary tumor through the sub
The cytomorphology of SCLC metastatic to the stomach closely resembles that of the original tumor. The main endoscopic manifestations are less cytoplasm, indistinct cell borders, fine nuclear chromatin, and absent or inconspicuous nucleoli. The tumor cells are round, elliptical, or spindle shaped[18]. Currently, immunohistochemistry is important for determining the tumor origin[19]. Syn, CD56, NSE, and CgA can be used as markers for pulmonary SCLC[20]. In our case, the immunohistochemistry results of all three patients were suggestive of CD56, CgA and TTF-1 positivity. The immunohistochemistry results of patient 1 suggested Syn positivity. Neuroendocrine tissues and tumors such as SCLC specifically express CD56, a cell surface protein[21,22]. Its expression rate in SCLC tissues is approximately 89%-100%, and the literature suggests that it can be used as a marker for SCLC[23,24]. More important than CD56 is TTF-1. TTF-1 is a transcription factor found mainly in brain, thyroid, and lung tissues, especially in SCLC[25]. Some researchers have reported that the rate of TTF-1 expression in SCLC is as high as 96%, which is significantly greater than that in extrapulmonary tissues[26]. Therefore, positivity for TTF-1 combined with the difference in its expression rate can be used to determine whether it originates from lung tumors. Additionally, previous studies have noted that TTF-1 and CK7 po
The three patients in this study died without treatment several months after the diagnosis of SCLC with gastric metastases. These findings indicate that the disease prognosis is poor. The therapeutic effects of the different treatment regimens could not be evaluated because the patients in this case did not undergo relevant treatment. When gastric metastasis occurs in SCLC, it often occurs at an advanced stage of the disease, often combined with metastases to other organs, and the opportunity for surgery is lost; thus, platinum-etoposide combination chemotherapy remains the treatment of choice[29,30]. In the future, as the molecular biology of SCLC is better understood, it may have a significant impact on improving the prognosis of this disease.
Gastric metastasis from SCLC has a low probability of occurring, but it is easily misdiagnosed and missed. Its clinical symptoms are nonspecific, and gastrointestinal symptoms may appear first; therefore, clinicians should be more vigilant. The diagnosis relies mainly on endoscopy, pathological biopsy, immunohistochemistry, and imaging examinations. Of these, the immunohistochemical results are critical. Chemotherapy is currently the first-line treatment option for patients with SCLC with gastric metastasis, but the clinical condition of such patients is more complicated, and a comprehensive treatment plan needs to be formulated on the basis of various factors. Early diagnosis and early individualized treatment can improve the survival rate of patients; Therefore, clinicians should improve their understanding of the diagnosis and treatment of SCLC gastric metastases.
The authors would like to thank each of the investigators who contributed to this article. We also thank the patients' families for their cooperation.
| 1. | Megyesfalvi Z, Tallosy B, Pipek O, Fillinger J, Lang C, Klikovits T, Schwendenwein A, Hoda MA, Renyi-Vamos F, Laszlo V, Rezeli M, Moldvay J, Dome B. The landscape of small cell lung cancer metastases: Organ specificity and timing. Thorac Cancer. 2021;12:914-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 2. | Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, Tsai JR, Huang MS. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Lermite E, Pessaux P, Du Plessis R, Bréhant O, Hennekinne-Mucci S, Michalak-Provost S, Arnaud JP. [Small bowel metastasis from primary lung carcinoma]. Gastroenterol Clin Biol. 2004;28:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982;49:170-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Gao S, Hu XD, Wang SZ, Liu N, Zhao W, Yu QX, Hou WH, Yuan SH. Gastric metastasis from small cell lung cancer: a case report. World J Gastroenterol. 2015;21:1684-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 7. | Peng Y, Liu Q, Wang Y, Song A, Duan H, Qiu Y, Li Q, Cui HJ. Pathological diagnosis and treatment outcome of gastric metastases from small cell lung cancer: A case report. Oncol Lett. 2019;18:1999-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 8. | Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy. 1996;28:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kim GH, Ahn JY, Jung HY, Park YS, Kim MJ, Choi KD, Lee JH, Choi KS, Kim DH, Lim H, Song HJ, Lee GH, Kim JH. Clinical and Endoscopic Features of Metastatic Tumors in the Stomach. Gut Liver. 2015;9:615-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Oh JC, Lee GS, Kim JS, Park Y, Lee SH, Kim A, Lee JM, Kim KS. [A case of gastric metastasis from small cell lung carcinoma]. Korean J Gastroenterol. 2004;44:168-171. [PubMed] |
| 11. | Guo HR, Yu J, Niu HR, Yang J. [A case of small cell lung cancer with gastric metastasis and literature review]. Xiandai Zhongliu Yixue. 2021;29:4400-4402. [DOI] [Full Text] |
| 12. | Mascarenhas Saraiva M, Ribeiro T, Moreira F, Lopes J, Corte Real A, Macedo G. A Gastric Lesion Revealing a Small-Cell Lung Cancer: A Rare Case. GE Port J Gastroenterol. 2022;29:220-222. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Casella G, Di Bella C, Cambareri AR, Buda CA, Corti G, Magri F, Crippa S, Baldini V. Gastric metastasis by lung small cell carcinoma. World J Gastroenterol. 2006;12:4096-4097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2764] [Article Influence: 460.7] [Reference Citation Analysis (3)] |
| 15. | Tan H. [Consensus and controversy on subtype classification of gastric neuroendocrine neoplasms]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:977-981. [PubMed] |
| 16. | Laffi A, Lania AGA, Ragni A, Di Vito V, Liccardi A, Rubino M, Sesti F, Colao A, Faggiano A; On Behalf Of The Nike Group. Gastric Neuroendocrine Tumors (g-NETs): A Systematic Review of the Management and Outcomes of Type 3 g-NETs. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 17. | Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3310] [Article Influence: 331.0] [Reference Citation Analysis (0)] |
| 19. | Rajasekaran Rathnakumar G, Gupta A, John F, Liu Y, Shakil S, Guevara E. A case of gastric small cell carcinoma with metastases to bone and liver. J Gastrointest Oncol. 2019;10:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Yun JP, Zhang MF, Hou JH, Tian QH, Fu J, Liang XM, Wu QL, Rong TH. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer. 2007;7:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Kaufmann O, Georgi T, Dietel M. Utility of 123C3 monoclonal antibody against CD56 (NCAM) for the diagnosis of small cell carcinomas on paraffin sections. Hum Pathol. 1997;28:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Kibbelaar RE, Moolenaar KE, Michalides RJ, Van Bodegom PC, Vanderschueren RG, Wagenaar SS, Dingemans KP, Bitter-Suermann D, Dalesio O, Van Zandwijk N. Neural cell adhesion molecule expression, neuroendocrine differentiation and prognosis in lung carcinoma. Eur J Cancer. 1991;27:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Messaritakis I, Stoltidis D, Kotsakis A, Dermitzaki EK, Koinis F, Lagoudaki E, Koutsopoulos A, Politaki E, Apostolaki S, Souglakos J, Georgoulias V. TTF-1- and/or CD56-positive Circulating Tumor Cells in patients with small cell lung cancer (SCLC). Sci Rep. 2017;7:45351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Švajdler M, Mezencev R, Šašková B, Ondič O, Mukenšnábl P, Michal M. Triple marker composed of p16, CD56, and TTF1 shows higher sensitivity than INSM1 for diagnosis of pulmonary small cell carcinoma: proposal for a rational immunohistochemical algorithm for diagnosis of small cell carcinoma in small biopsy and cytology specimens. Hum Pathol. 2019;85:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Zhang C, Schmidt LA, Hatanaka K, Thomas D, Lagstein A, Myers JL. Evaluation of napsin A, TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumors. Am J Clin Pathol. 2014;142:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ordóñez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Rossi G, Marchioni A, Romagnani E, Bertolini F, Longo L, Cavazza A, Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Deng S, Liu M, Xiao T, Gao J. Expression of neuro-oncological ventral antigen-1 in small-cell lung cancer and its value in pathological diagnosis. Transl Cancer Res. 2020;9:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Meijer JJ, Leonetti A, Airò G, Tiseo M, Rolfo C, Giovannetti E, Vahabi M. Small cell lung cancer: Novel treatments beyond immunotherapy. Semin Cancer Biol. 2022;86:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 30. | Zugazagoitia J, Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J Clin Oncol. 2022;40:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/