Published online Aug 21, 2024. doi: 10.3748/wjg.v30.i31.3668
Revised: July 11, 2024
Accepted: August 2, 2024
Published online: August 21, 2024
Processing time: 154 Days and 21.2 Hours
Gut microbiota (GM) affects the progression and response to treatment in liver diseases. The GM composition is diverse and associated with different etiologies of liver diseases. Notably, alterations in GM alterations are observed in patients with portal hypertension (PH) secondary to cirrhosis, with hepatitis B virus (HBV) infection being a major cause of cirrhosis in China. Thus, understanding the role of GM alterations in patients with HBV infection-related PH is essential.
To evaluate GM alterations in patients with HBV-related PH after transjugular intrahepatic portosystemic shunt (TIPS) placement.
This was a prospective, observational clinical study. There were 30 patients (with a 100% technical success rate) recruited in the present study. Patients with esophagogastric variceal bleeding due to HBV infection-associated PH who underwent TIPS were enrolled. Stool samples were obtained before and one month after TIPS treatment, and GM was analyzed using 16S ribosomal RNA amplicon sequencing.
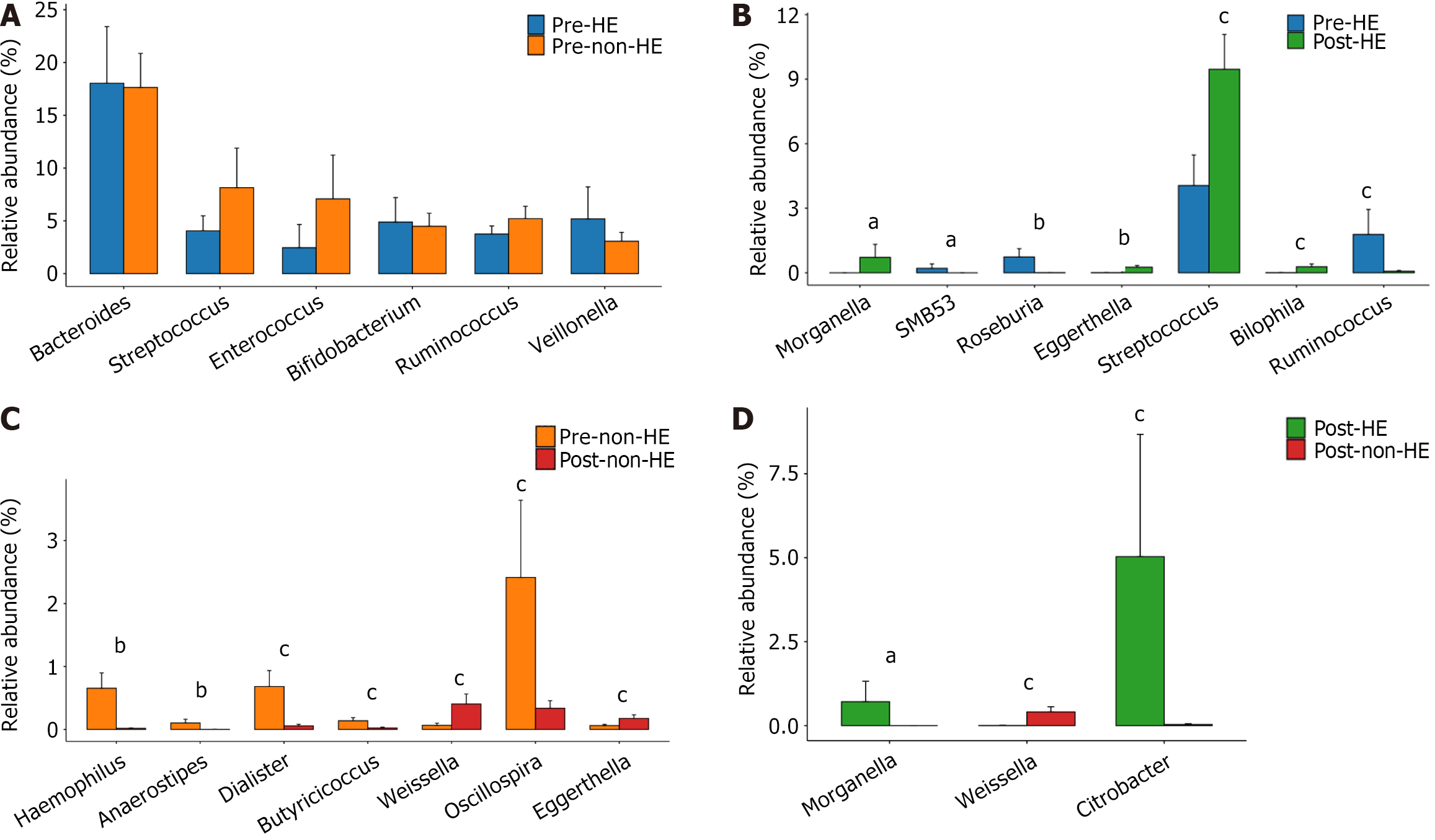
One month after TIPS placement, 8 patients developed hepatic encephalopathy (HE) and were assigned to the HE group; the other 22 patients were assigned to the non-HE group. There was no substantial disparity in the abundance of GM at the phylum level between the two groups, regardless of TIPS treatment (all, P > 0.05). However, following TIPS placement, the following results were observed: (1) The abundance of Haemophilus and Eggerthella increased, whereas that of Anaerostipes, Dialister, Butyricicoccus, and Oscillospira declined in the HE group; (2) The richness of Eggerthella, Streptococcus, and Bilophila increased, whereas that of Roseburia and Ruminococcus decreased in the non-HE group; and (3) Members from the pathogenic genus Morganella appeared in the HE group but not in the non-HE group.
Intestinal microbiota-related synergism may predict the risk of HE following TIPS placement in patients with HBV-related PH. Prophylactic microbiome therapies may be useful for preventing and treating HE after TIPS placement.
Core Tip: Gut microbiota (GM) alterations have been demonstrated in patients with portal hypertension, influencing hepatic encephalopathy (HE) development. The present study analyzes the changes in GM composition in Chinese patients with hepatitis B virus related portal hypertension before and after transjugular intrahepatic portosystemic shunt (TIPS). The results revealed that patients without HE exhibited higher GM-related synergism than those with HE, regardless of TIPS placement. Thus, the synergism of intestinal microbiota may serve as a useful indicator to predict the risk of HE after TIPS placement.
- Citation: Zhao HW, Zhang JL, Liu FQ, Yue ZD, Wang L, Zhang Y, Dong CB, Wang ZC. Alterations in the gut microbiome after transjugular intrahepatic portosystemic shunt in patients with hepatitis B virus-related portal hypertension. World J Gastroenterol 2024; 30(31): 3668-3679
- URL: https://www.wjgnet.com/1007-9327/full/v30/i31/3668.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i31.3668
The gut microbiota (GM) represents the largest group of symbiotic microbiota in the human body, affecting human health and the progression of many diseases and playing an essential part in the development, pathophysiology, and therapeutic response of liver diseases[1-3]. Further, GM can affect the liver and central nervous system via metabolites, hormones, neurotransmitters, and inflammatory factors, known as the gut-liver-brain axis[4-7]. Notably, patients with portal hypertension (PH) secondary to liver cirrhosis exhibit intestinal mucosal edema, increased intestinal permeability, intestinal microbiota translocation, and changes in intestinal microecological structure and function[8,9]. The degree of hepatic encephalopathy (HE), which is associated with an overall reduction in autochthonous microbial population and a rise in pathogenic bacterial abundance[10] is directly correlated with the severity of cirrhosis and the degree of GM dysbiosis[6,11]. Treatments that target the GM, such as rifaximin and fecal bacteria transplantation, can markedly reduce the incidence of HE[12-14]. Application of the transjugular intrahepatic portosystemic shunt (TIPS) is the most effective treatment for PH-associated complications and is recommended by the current guidelines. Decompression of PH post-TIPS placement alleviates intestinal mucosal edema and improves the composition of the intestinal flora[15-17]. However, HE is the most common complication after TIPS placement, with an incidence of 35%-50% within six months, seriously affecting the quality of life of patients[18,19].
The signatures of the intestinal flora in HE patients vary according to the etiology of cirrhosis. Hepatitis B virus (HBV) infection is the primary cause of cirrhosis in China, while alcoholic liver disease and nonalcoholic fatty liver disease are the leading causes in Western countries[20-22]. Moreover, the GM is closely related to HBV immune tolerance and anti-HBV infection. Specifically, GM and its metabolites affect the host immune response to HBV, thus affecting the level of HBV replication[3,23-25]. However, there is currently no research on GM alterations in patients with HBV-related PH after TIPS placement. Therefore, in the present study, we analyzed alterations in the GM in patients with HBV-related PH before and after TIPS placement.
The present research was conducted following the ethical standards outlined in the 1964 Declaration of Helsinki and its subsequent amendments, or similar ethical standards. Moreover, this study received approval from the Ethics Committee/Institutional Review Board of Beijing Shijitan Hospital (2019 Scientific Research Review Approval No. 2). Before the procedures, all participants or their authorized representatives provided written informed consent.
This study was conducted at Beijing Shijitan Hospital and Beijing Tongren Hospital between January 2019 and December 2021. Interventional radiologists, gastroenterologists, pathologists, and hepatobiliary specialists selected the study participants. The criteria for inclusion were as follows: (1) Age, 18-75 years; (2) Diagnosis of liver cirrhosis caused by HBV infection based on laboratory tests, clinical signs, histology, or abdominal imaging; and (3) Esophagogastric variceal bleeding due to PH as an indication for TIPS placement or prophylactic TIPS placement. The exclusion criteria were as follows: (1) Noncirrhotic PH; (2) Previous history of HE; (3) Refractory ascites; (4) Portal vein thrombosis accounts for more than 50% of portal vein lumen; (5) Cavernous transformation of the portal vein; (6) Budd-Chiari syndrome; (7) Previous TIPS placement or surgical shunting; (8) Hepatocellular carcinoma or other malignancies; (9) Child-Pugh score > 13 or model for end-stage liver disease score > 18; (10) Active infection or sepsis; (11) History of gastrointestinal surgery; and (12) Unavailability of stool samples. Participants were required to withdraw from the study if they were lost to follow-up or received liver transplantation.
The procedures for TIPS placement were performed according to expert consensus[26]. A TIPS-specific stent (diameter, 8 mm; Viatorr; Gore Medical Flagstaff, Newark, DE, United States) was placed in all patients. Third-generation cepha
Doppler ultrasonography and abdominal enhanced computed tomography scans were performed 1, 3, and 6 months after TIPS placement to evaluate the stent patency. HE was graded in accordance with the West Haven criteria[27]. Fecal samples (3-5 g) were collected using sterile sampling tubes 1-3 days before and 1 month after TIPS placement and stored at -80 °C within 2 hours after sampling. The clinical follow-up data were recorded simultaneously.
Genesky Biotechnologies Inc., (Shanghai, China) conducted 16S rRNA amplicon sequencing analysis to determine the GM of the fecal samples. In summary, the total genomic DNA was obtained by utilizing the FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA, United States), following the directions provided by the supplier. A Nanodrop 2000 and Qubit 3.0 Spectrophotometer were used to measure the concentration and purity of genomic DNA, while agarose gel electrophoresis was used to assess the integrity of the DNA. With the primers 341F (5’-CCTACGGGNGGCWGCAG-3’) and 805R (5’-GACTACHVGGGTATCTAATCC-3’), the 16S rRNA gene’s V3-V4 hypervariable sections were amplified. An Illumina NovaSeq 6000 sequencer (Illumina, San Diego, CA, United Staes) was then used to sequence the amplified material.
QIIME2 was used to process raw read sequences. The cut-adapt plugin was used to trim the primer and adaptor sequences. For quality assurance and the detection of amplicon sequence variants (ASVs), the DADA2 plugin was utilized. A pretrained Naive Bayes classifier trained on Greengenes (version 13.8) was used to assign taxonomy to typical ASV sequences with a confidence threshold of 0.8[28,29].
The statistical analyses were conducted using SPSS (version 20.0; IBM, Armonk, NY, United States). The means ± SD were used to express quantitative data, while percentages were used to indicate categorical variables. Pearson’s χ2 test and the independent-sample t-test were used to assess differences between the two groups. To compare two paired samples, a paired-sample t-test was employed, and to analyze multiple samples, a one-way analysis of variance (ANOVA) was employed. The ACE, Chao1, Shannon, and Simpson indices were used to reflect the diversity differences, and the Wilcoxon rank-sum test was used to compare them in an alpha diversity study. ANOSIM was used to calculate beta diversity. The analysis of the variations in the microbiomes between the groups was done using the MetaStat approach. The Wilcoxon rank-sum and Kruskal-Wallis tests were run using the linear discriminant analysis (LDA) effect size algorithm to compare numerous abundant taxa. Each differential taxon’s LDA score was determined using the LDA; the difference screening thresholds were set at an LDA score > 2 and a P value < 0.05. The study employed redundancy analysis to assess the impact of confounding variables on the composition of the microbiome. To examine the relation
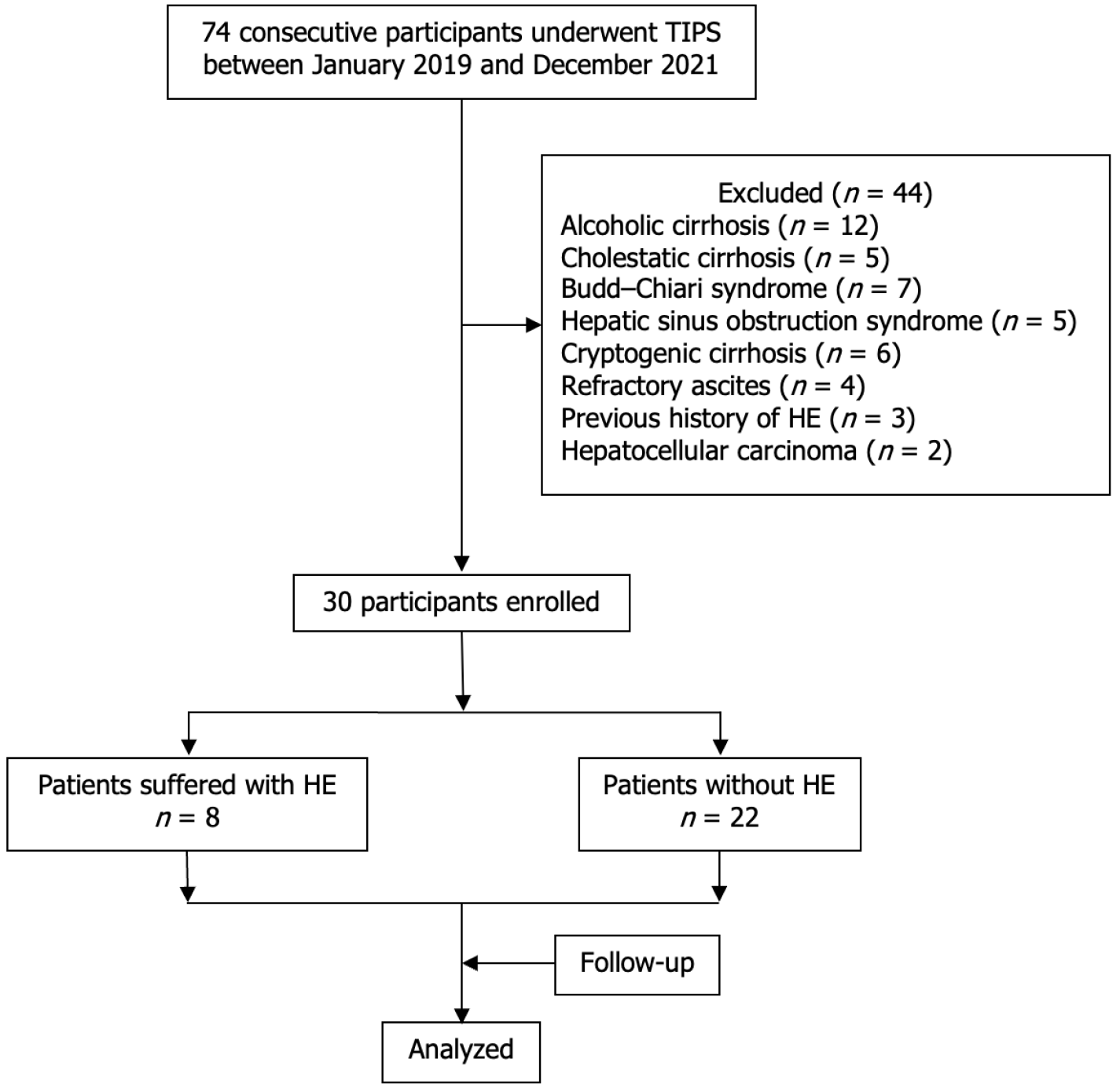
Between January 2019 and December 2021, 74 consecutive patients underwent TIPS placement. Among them, 30 were eventually enrolled, while (Figure 1). The technical success rate was 100% (30/30 patients). Eight (26.67%) of the enrolled patients, including four men and four women with a mean age of 53 ± 17.66 years (range, 28-71 years), experienced HE (five with grade I and three with grade II) within 1 month of TIPS placement and were assigned to the HE group. The remaining 22 (73.33%) patients without HE were assigned to the non-HE group and included 15 men and 7 women with a mean age of 56.18 ± 18.81 years (range, 35-78 years). There were no significant differences between the baseline clinical characteristics of the two groups (all, P > 0.05, Table 1). The portal venous pressure decreased from 34.85 ± 6.62 mmH2O (25-51 mmH2O) to 22.70 ± 5.10 mmH2O (11-30 mmH2O) (P < 0.01) in the non-HE group and from 33.88 ± 5.08 mmH2O (25-51 mmH2O) to 23. 8 ± 2.36 mmH2O (29-43 mmH2O) (P < 0.01) in the HE group. Patients with HE were treated with rifaximin, lactulose, or ornithine aspartate, and the symptoms disappeared within 3-7 days. The follow-up period was 11.5 ± 2.05 months (range, 8-15 months), and no patient experienced TIPS dysfunction, esophagogastric variceal rebleeding, or other complications during the follow-up.
| Item | Non-HE group (n = 22) | HE group (n = 8) | P value |
| Sex | |||
| Male | 15 | 4 | 0.36 |
| Female | 7 | 4 | |
| Age, years | 56.18 ± 18.81 (35-78) | 53 ± 17.66 (28-71) | 0.76 |
| Portal vein thrombosis | |||
| Yes | 12 | 5 | 0.70 |
| No | 10 | 3 | |
| Child-Pugh score | |||
| A | 10 | 4 | 0.39 |
| B | 12 | 3 | |
| C | 0 | 1 | |
| HVPG, mmHg | 14.26 ± 9.15 (2-25) | 11.75 ± 6.92 (4-22) | 0.45 |
| MELD score | 8.33 ± 5.52 (0.31-20.95) | 5.42 ± 2.83 (1.80-10.22) | 0.08 |
| Total bile acid, μmol/L | 30.93 ± 20.64 (5.00-69.80) | 52.58 ± 46.28 (8.60-152.70) | 0.51 |
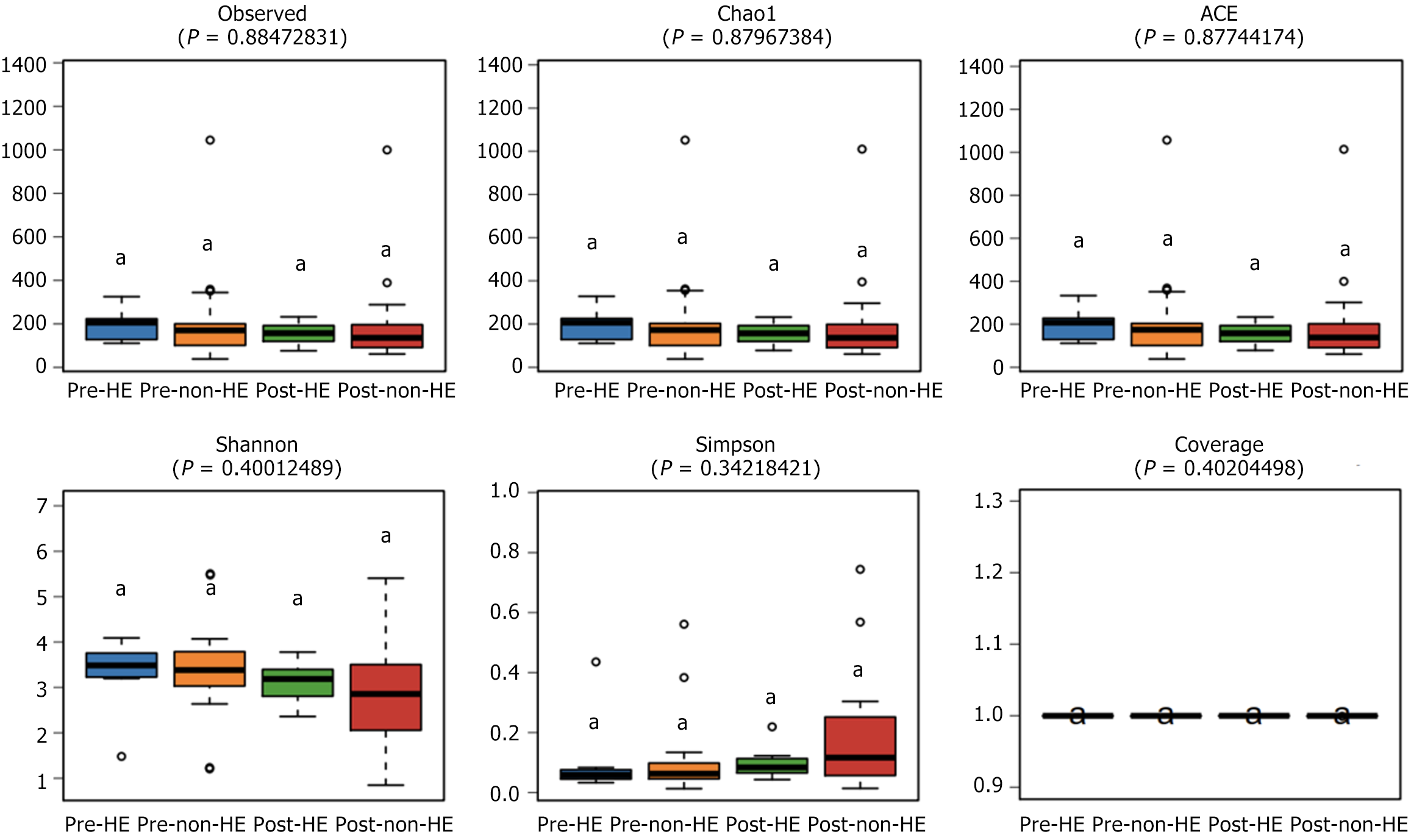
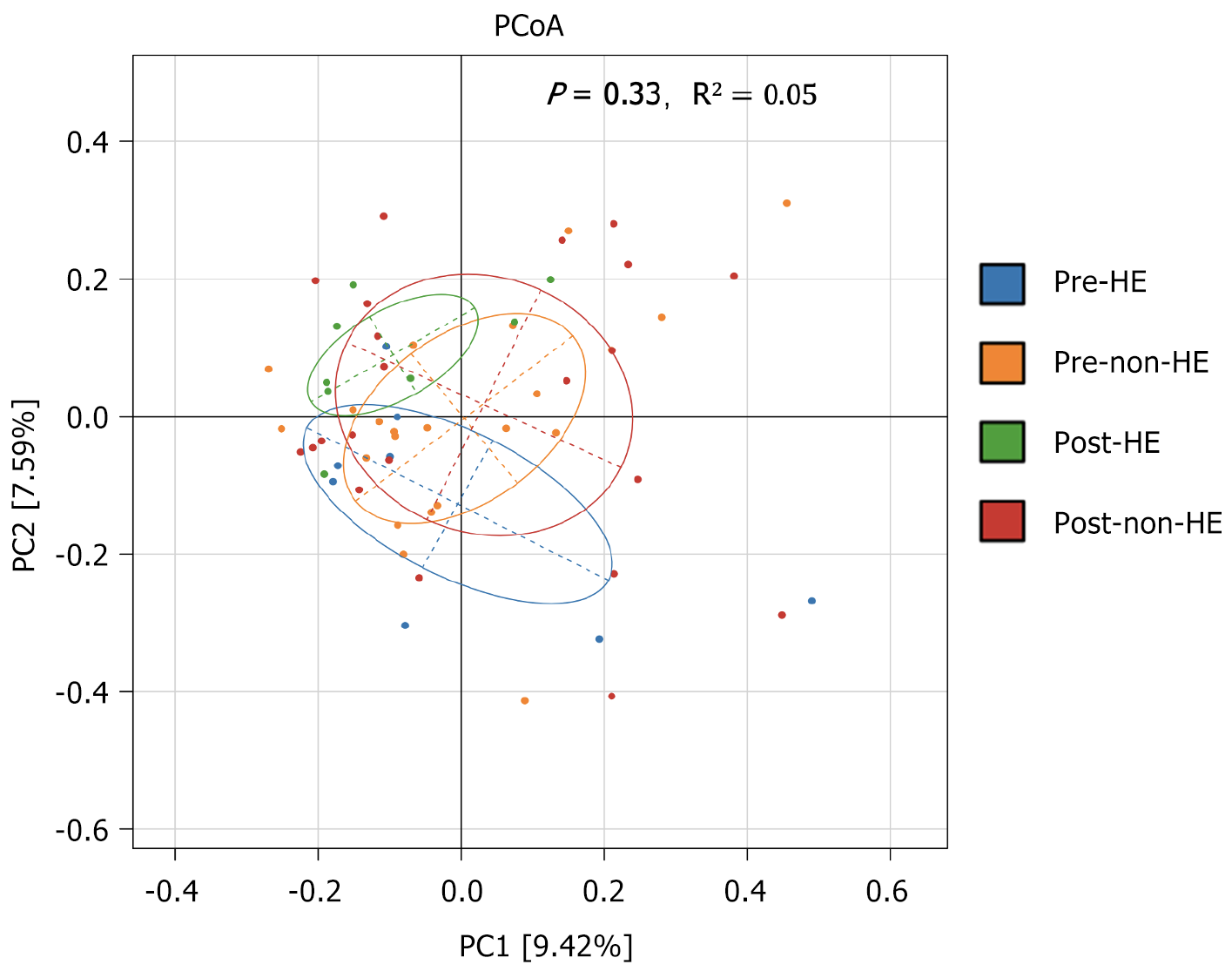
The alpha diversity of the samples was determined to compare the gut microbial composition pre- and post-TIPS placement, between and within the two groups. Before and after TIPS, there were no discernible changes between the two groups. Moreover, none of the four indices revealed any significant changes within the groups (Chao1, P = 0.88; ACE, P = 0.88; Shannon, P = 0.40; and Simpson, P = 0.34; Figure 2). Comparison of the GM abundance among the groups using principal coordinate analysis of ASV counts was used to compare the GM abundance across the groups; the results showed no discernible difference between the two groups before and after TIPS placement (R2 = 0.05, P = 0.33; Figure 3).
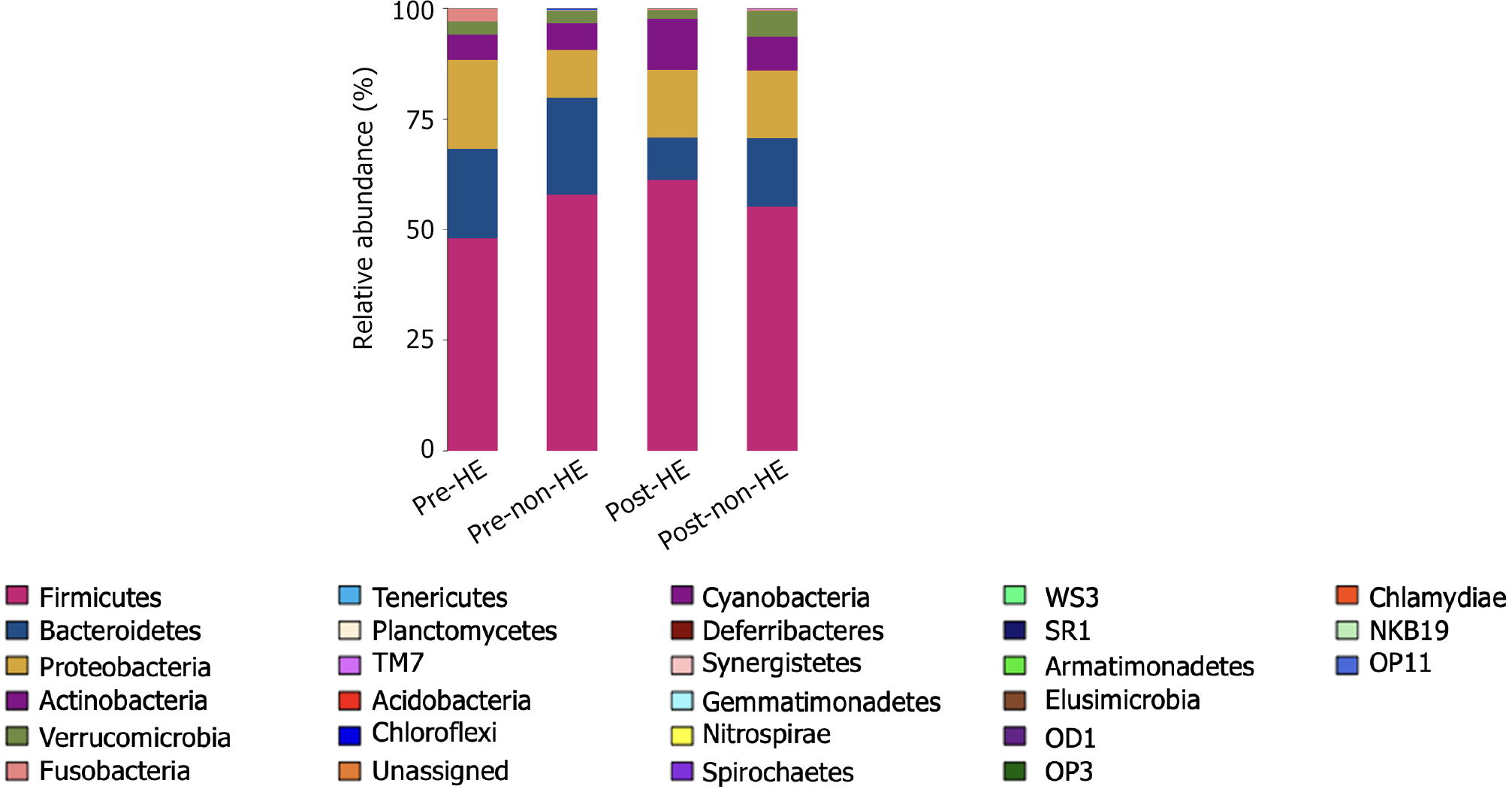
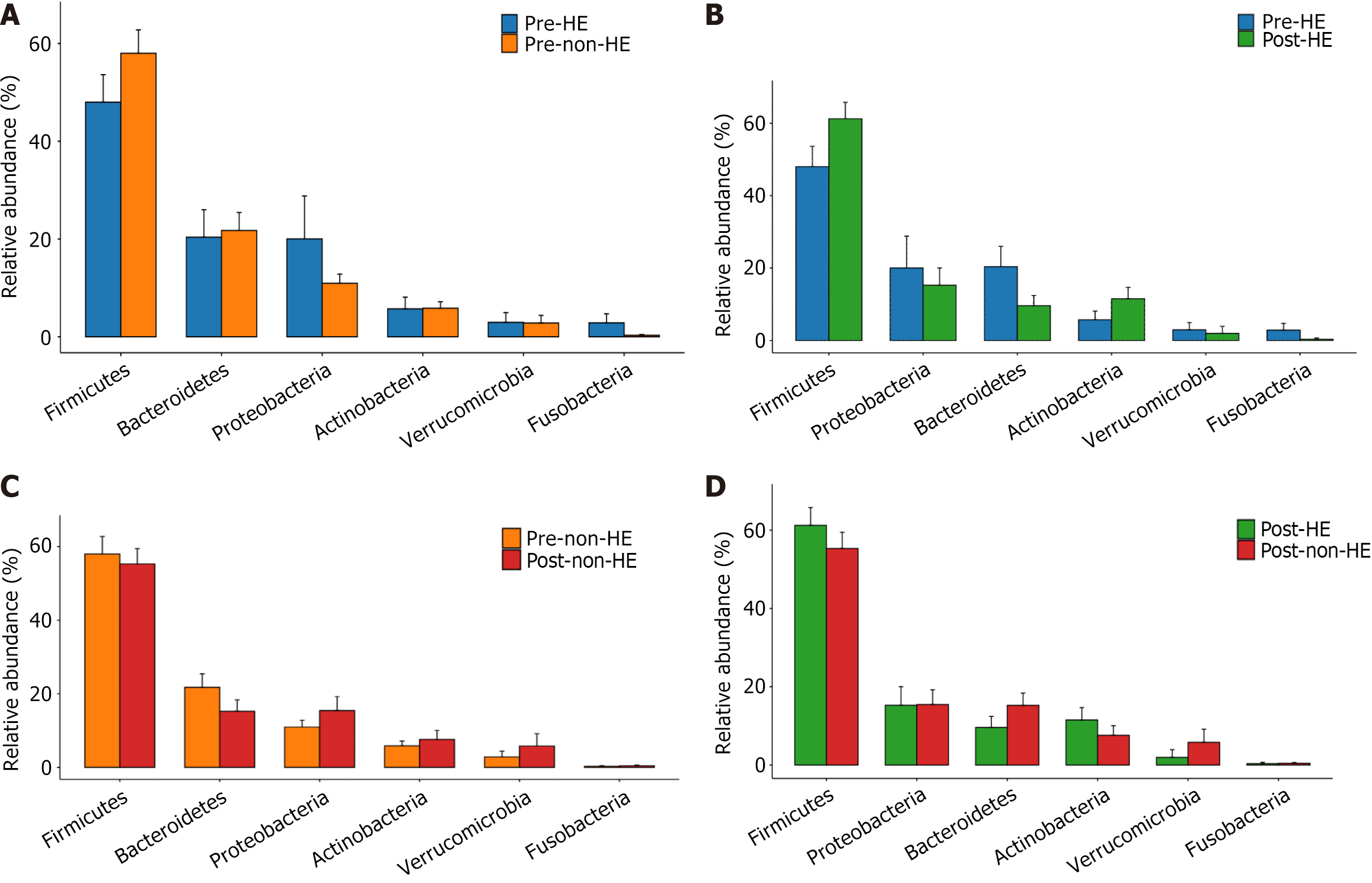
At the phylum level, the intestinal microbiota of both groups, pre- and post-TIPS placement, was composed primarily of members from Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteria (Figure 4), with no significant changes in their relative abundance levels (all, P > 0.05). Furthermore, there were no significant differences between the two groups, regardless of the TIPS treatment (all, P > 0.05; Figure 5). At the genus level, 19 taxa with an abundance level greater than 1% were detected in the HE group before TIPS placement, whereas 21 taxa were detected after TIPS placement. In the non-HE group, 20 taxa with an abundance level greater than 1% were observed before TIPS placement; this number decreased to 17 after TIPS placement.
Before TIPS placement, there was no discernible difference between the two groups. Members of the pathogenic genus Morganella, however, only showed up in the HE group following TIPS placement, not in the non-HE group. In addition, the abundance of the beneficial genus Weissella was greater in the non-HE group than in the HE group, whereas the abundance of the indigenous genus Citrobacter exhibited an opposite trend (all, P > 0.05). Intra-group comparison indicated that the abundance of the pathogenic bacteria Haemophilus and certain indigenous taxa, including Anaerostipes, Dialister, Butyricicoccus, and Oscillospira, decreased in the non-HE group after TIPS placement, whereas the abundance of the genera Weissella and Eggerthella increased. In the HE group, the abundance of the indigenous bacteria Roseburia and Ruminococcus decreased, whereas that of the pathogenic bacteria Eggerthella, Streptococcus, and Bilophila increased after TIPS; simultaneously, Morganella appeared, and SMB53 disappeared (Figure 6).
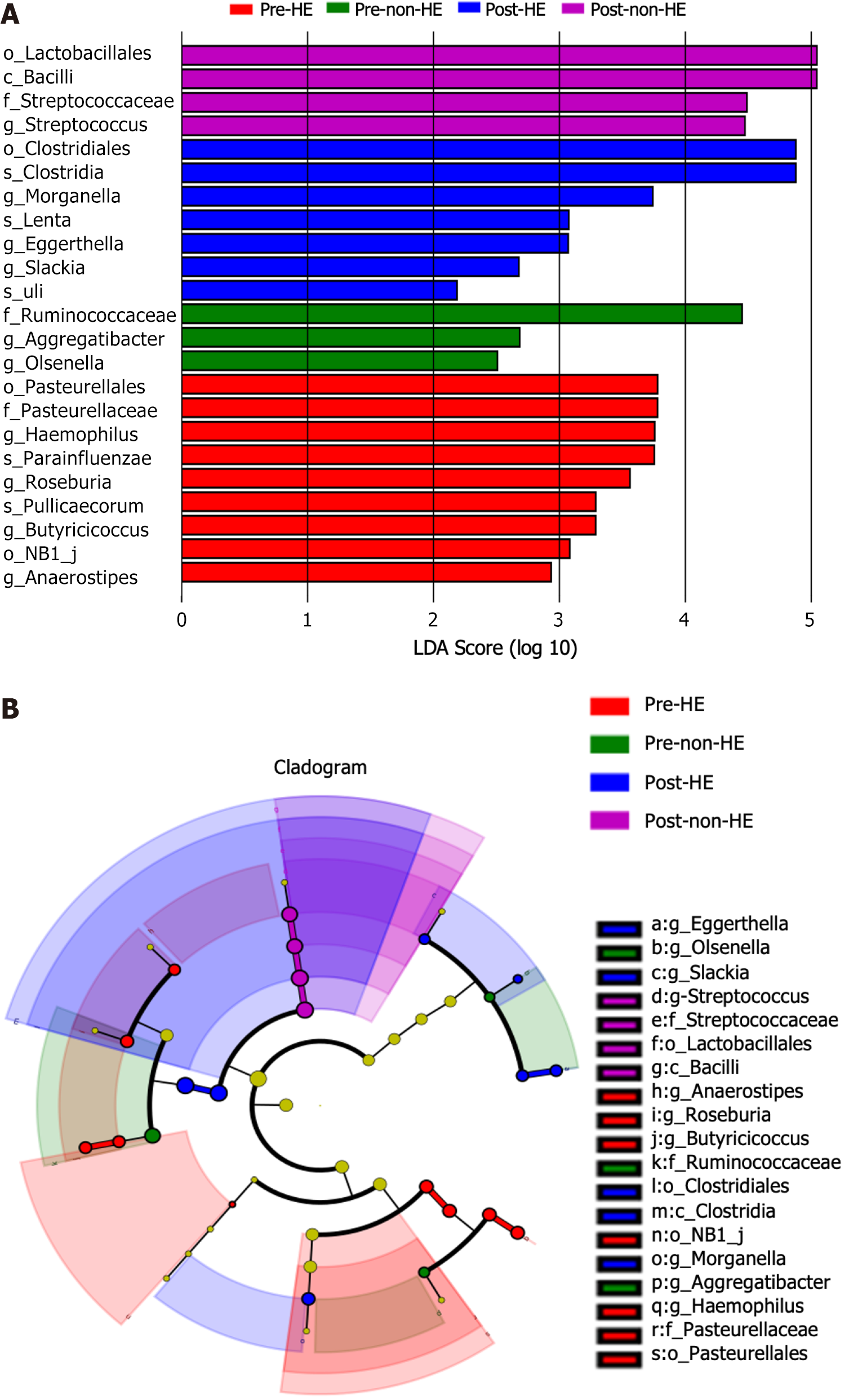
LDA effect size revealed one differential taxon at the class level, one at the order level, one at the family level, and three at the genus level in the non-HE group (LDA score > 2.0; P < 0.05). In the HE group, there were three differential taxa at the order level, one at the family level, seven at the genus level, and four at the species level (LDA score > 2.0; P < 0.05; Figure 7). The differential taxa included Clostridiales, Clostridia, Morganella, Lenta, Eggerthella, Slackia, and Uli in the HE group post-TIPS placement, whereas Lactobacillales, Bacilli, and Streptococcaceae, along with its subordinate genus Streptococcus, were detected in the non-HE group post-TIPS placement.
In the HE group, 308 pairs of bacteria showed a positive correlation and 35 pairs showed a negative correlation at the genus level before TIPS placement. After TIPS placement, 162 pairs of bacteria showed a positive correlation, and 43 pairs showed a negative correlation negatively correlated, respectively, after TIPS placement (all, P < 0.05). In the non-HE group, the number of positively correlated bacterial pairs before TIPS placement was 2787 and that of the negatively correlated bacterial pairs was 48. After TIPS placement, 1777 pairs were positively correlated, while 67 pairs were negatively correlated (all, P < 0.05). Thus, the microbiota synergism decreased after TIPS placement in both groups; the interaction in the HE group was weaker than that in the non-HE group before and after TIPS placement (Supplementary Figure 1).
The relationships between the 20 most abundant and differentially expressed bacteria within the two groups after TIPS placement were analyzed. In the HE group, a positive correlation was observed between Clostridia and its subordinate taxon Clostridiales, Lenta and Eggerthella, Blautia and Clostridia as well as its subordinate taxon Clostridiales, Veillonella and Slackia, and Prevotella and Morganella after TIPS placement. In contrast, a negative correlation was observed between Lactobacillus and Slackia, Dorea and Uli, and Citrobacter and Clostridia as well as Clostridiales (all, P < 0.05).
In the non-HE group, there was a positive correlation between Bacilli and Lactobacillales. Additionally, Streptococcaceae and its subordinate genus Streptococcus were correlated with Lactobacillus, Eubacterium, Veillonella, and Bifidobacterium after TIPS placement. Moreover, Enterococcus was negatively correlated with Streptococcaceae and Streptococcus, while Parabacteroides was negatively correlated with Lactobacillales and Bacilli (all, P < 0.05) (Table 2).
| HE group | Non-HE group | ||
| Positive correlation | |||
| Clostridia | Clostridialesa | Bacilli | Lactobacillalesa |
| Lenta | Eggerthellaa | Streptococcacea, Streptococcus | Lactobacillusc, Eubacteriumc, Bifidobacteriumc, |
| Veillonella | Slackiab | ||
| Prevotella | Morganellab | ||
| Blautia | Clostridiab, Clostridialesb | ||
| Negative correlation | |||
| Lactobacillus | Slackiac | Enterococcus | Streptococcaceaeb, Streptococcusb |
| Dorea | Ulic | Parabacteroides | Lactobacillalesb, Bacillib |
| Citrobacter | Clostridiac, Clostridialesc | ||
The GM is a complex ecosystem composed of different bacteria that have symbiotic relationships with each other and other microbes[30]. Recently, several studies have indicated alternations in the abundance of “beneficial” and potentially “pathogenic” intestinal bacteria during chronic HBV infection. Nevertheless, the exact alterations in intestinal bacteria after HBV infection remain unknown[31,32]. In this prospective study, we analyzed the composition and alterations of the GM in patients with HBV-related PH before and after TIPS placement and found no significant differences in the diversity and abundance of intestinal bacteria between the two groups, regardless of TIPS placement. We also observed no change within both groups before and after TIPS placement.
Firmicutes and Bacteroides are the dominant phyla in the intestinal microbiota, followed by Actinobacteria and Proteobacteria, which together account for more than 97% of total GM[33]. Consistent with the results of a previous report, we found that the intestinal microbiota of both groups before and after TIPS placement was primarily composed of Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteria at the phylum level in the current study[34].
The current investigation found that before TIPS installation, there was no difference in the genus-level composition of the intestinal microbiota between the two groups. However, in the HE group, after TIPS placement, the abundance of Haemophilus and Eggerthella (potentially pathogenic bacteria) increased, that of Anaerostipes, Dialister, Butyricicoccus, and Oscillospira (indigenous bacteria) decreased, and members from Morganella appeared. Notably, Morganella can produce urease, which promotes the production of blood ammonia, thereby increasing the incidence of HE[35,36]. In the non-HE group, after TIPS placement, the abundance of Eggerthella, Streptococcus, and Bilophila (potential pathogenic bacteria) increased, that of Roseburia and Ruminococcus decreased (indigenous bacteria). Wei et al[32] reported that compared with healthy participants, patients with HBV-related cirrhosis exhibit an increase in the abundance of members from Enterobacteriaceae, Veillonellaceae, and Streptococcaceae. In another study, the abundance of members from Enterobacteriaceae increased with the severity of HBV-related liver diseases, including asymptomatic HBV carriage, chronic HBV, and decompensated HBV cirrhosis, whereas that of bifidobacteria and lactic acid bacteria decreased[37]. The abundance of potentially pathogenic bacteria, especially those from the families Veillonellaceae, Enterobacteriaceae, Alcaligenaceae, and Porphyromonadaceae, higher in patients with HBV-related decompensated cirrhosis is than in patients with compensated cirrhosis[38]. In the present study, alterations in GM were observed in both the HE and non-HE groups after TIPS place
Alterations in the intestinal microflora and colonization by opportunistic pathogenic microflora increase the risk of comorbidities in patients with HBV-related liver disease[3,39]. The decreased synergism between bacteria also indicates bacterial dysbiosis. In our study, a notable positive relationship was detected before TIPS placement, particularly in the non-HE group. However, this relationship weakened after TIPS placement in both groups, indicating that the synergism among different bacteria was weakened. Synergism was detected not only among harmful or beneficial bacteria independently but also between harmful and beneficial bacteria. Hence, intestinal flora dysbiosis was more prominent in patients with HE. Lactobacillus and Bifidobacterium are associated with intestinal benefits that influence host immunity. Hence, they ameliorate intestinal dysbiosis and strengthen the intestinal barrier integrity in patients with chronic HBV infections[10,39,40]. In the non-HE group, we observed synergism of Lactobacillus with Streptococcus and Bifidobacterium with Eubacterium. Furthermore, we primarily observe a negative correlation, suggesting antagonism, between beneficial and pathologic bacteria.
There are various limitations. First, the sample size was small, and follow-up was limited. Second, fecal samples were only collected one month post-TIPS application; thus, alterations in intestinal flora over time after TIPS treatment could not be assessed. Third, this study was descriptive, and the correlation between the intestinal flora and HBV infection status was not analyzed. Finally, the correlation between the intestinal flora and clinical parameters was not analyzed. These aspects should be examined in future research to validate the findings of the present study.
Diverse GM compositions associated with different liver disease etiologies have been widely reported. The present study demonstrated that while the diversity of intestinal microbiota was not altered after TIPS treatment in HBV-related PH, non-HE patients without HE exhibited higher GM-related synergism than patients with HE regardless of TIPS treatment. This suggests that synergism of intestinal microbiota may predict the risk of HE after TIPS treatment. Further studies are needed to verify the findings of the present study and to evaluate the prophylactic effects of personalized microbiome-based therapies formulated based on the differences in the gut microbial compositions after TIPS application.
We extend our gratitude to the biomedical statistician Jing Dong for the statistical review of the study.
| 1. | Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 865] [Cited by in RCA: 1175] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 2. | Dazıroğlu MEÇ, Yıldıran H. Intestinal dysbiosis and probiotic use: its place in hepatic encephalopathy in cirrhosis. Ann Gastroenterol. 2023;36:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Kassa Y, Million Y, Gedefie A, Moges F. Alteration of Gut Microbiota and Its Impact on Immune Response in Patients with Chronic HBV Infection: A Review. Infect Drug Resist. 2021;14:2571-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Bajaj JS, Shamsaddini A, Fagan A, McGeorge S, Gavis E, Sikaroodi M, Brenner LA, Wade JB, Gillevet PM. Distinct gut microbial compositional and functional changes associated with impaired inhibitory control in patients with cirrhosis. Gut Microbes. 2021;13:1953247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Chen Z, Ruan J, Li D, Wang M, Han Z, Qiu W, Wu G. The Role of Intestinal Bacteria and Gut-Brain Axis in Hepatic Encephalopathy. Front Cell Infect Microbiol. 2020;10:595759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Shahbazi A, Sepehrinezhad A, Vahdani E, Jamali R, Ghasempour M, Massoudian S, Sahab Negah S, Larsen FS. Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Huang L, Yu Q, Peng H, Zhen Z. Alterations of gut microbiome and effects of probiotic therapy in patients with liver cirrhosis: A systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e32335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 9. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1618] [Article Influence: 134.8] [Reference Citation Analysis (43)] |
| 10. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 879] [Article Influence: 73.3] [Reference Citation Analysis (1)] |
| 11. | Bajaj JS, Vargas HE, Reddy KR, Lai JC, O'Leary JG, Tandon P, Wong F, Mitrani R, White MB, Kelly M, Fagan A, Patil R, Sait S, Sikaroodi M, Thacker LR, Gillevet PM. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:756-765.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Tun KM, Hong AS, Batra K, Naga Y, Ohning G. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Hepatic Encephalopathy and Clostridioides difficile Infection in Patients With Cirrhosis. Cureus. 2022;14:e25537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Hassouneh R, Bajaj JS. Gut Microbiota Modulation and Fecal Transplantation: An Overview on Innovative Strategies for Hepatic Encephalopathy Treatment. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (3)] |
| 14. | Afecto E, Ponte A, Fernandes S, Silva J, Gomes C, Correia J, Carvalho J. Fecal microbiota transplantation in hepatic encephalopathy : a review of the current evidence and future perspectives. Acta Gastroenterol Belg. 2021;84:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1990] [Article Influence: 248.8] [Reference Citation Analysis (2)] |
| 16. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 17. | Vizzutti F, Schepis F, Arena U, Fanelli F, Gitto S, Aspite S, Turco L, Dragoni G, Laffi G, Marra F. Transjugular intrahepatic portosystemic shunt (TIPS): current indications and strategies to improve the outcomes. Intern Emerg Med. 2020;15:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Zuo L, Lv Y, Wang Q, Yin Z, Wang Z, He C, Guo W, Niu J, Bai W, Li K, Yu T, Yuan X, Chen H, Liu H, Xia D, Wang E, Luo B, Li X, Yuan J, Han N, Nie Y, Fan D, Han G. Early-Recurrent Overt Hepatic Encephalopathy Is Associated with Reduced Survival in Cirrhotic Patients after Transjugular Intrahepatic Portosystemic Shunt Creation. J Vasc Interv Radiol. 2019;30:148-153.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 19. | Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 20. | Liu Q, Liu S, Chen L, Zhao Z, Du S, Dong Q, Xin Y, Xuan S. Role and effective therapeutic target of gut microbiota in NAFLD/NASH. Exp Ther Med. 2019;18:1935-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Biolato M, Manca F, Marrone G, Cefalo C, Racco S, Miggiano GA, Valenza V, Gasbarrini A, Miele L, Grieco A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:509-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 909] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 23. | Hua X, Feng H. Changes in intestinal microbiota of HBV-associated liver cirrhosis with/without hepatic encephalopathy. Medicine (Baltimore). 2022;101:e29935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Yi X, Yuan Y, Li N, Yi L, Wang C, Qi Y, Gong L, Liu G, Kong X. A mouse model with age-dependent immune response and immune-tolerance for HBV infection. Vaccine. 2018;36:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, Chen XX, Xiao CX, Yu XF, Xu HZ, Xu LZ, Tang YN, Zhou F, Wang XL, Chen MY, Chen LG, Hong MZ, Ren JL, Pan JS. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Chinese College of Interventionalists. [CCI clinical practice guidelines: management of TIPS for portal hypertension (2019 edition)]. Linchuang Gandan Zazhi. 2019;35:2694-2699. [DOI] [Full Text] |
| 27. | American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 28. | Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12449] [Cited by in RCA: 13309] [Article Influence: 1901.3] [Reference Citation Analysis (0)] |
| 29. | Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18515] [Cited by in RCA: 20091] [Article Influence: 2009.1] [Reference Citation Analysis (14)] |
| 30. | Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Kang Y, Cai Y. Gut microbiota and hepatitis-B-virus-induced chronic liver disease: implications for faecal microbiota transplantation therapy. J Hosp Infect. 2017;96:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Wei X, Yan X, Zou D, Yang Z, Wang X, Liu W, Wang S, Li X, Han J, Huang L, Yuan J. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. 2013;13:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 395] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 34. | Li M, Li K, Tang S, Lv Y, Wang Q, Wang Z, Luo B, Niu J, Zhu Y, Guo W, Bai W, Wang E, Xia D, Wang Z, Li X, Yuan J, Yin Z, Trebicka J, Han G. Restoration of the gut microbiota is associated with a decreased risk of hepatic encephalopathy after TIPS. JHEP Rep. 2022;4:100448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 35. | Minnullina L, Pudova D, Shagimardanova E, Shigapova L, Sharipova M, Mardanova A. Comparative Genome Analysis of Uropathogenic Morganella morganii Strains. Front Cell Infect Microbiol. 2019;9:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Wang Q, Wang B, Saxena V, Miles L, Tiao J, Mortensen JE, Nathan JD. The gut-liver axis: impact of a mouse model of small-bowel bacterial overgrowth. J Surg Res. 2018;221:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Yun Y, Chang Y, Kim HN, Ryu S, Kwon MJ, Cho YK, Kim HL, Cheong HS, Joo EJ. Alterations of the Gut Microbiome in Chronic Hepatitis B Virus Infection Associated with Alanine Aminotransferase Level. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Paratore M, Santopaolo F, Cammarota G, Pompili M, Gasbarrini A, Ponziani FR. Fecal Microbiota Transplantation in Patients with HBV Infection or Other Chronic Liver Diseases: Update on Current Knowledge and Future Perspectives. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Chen C, Li L, Wu Z, Chen H, Fu S. Effects of lactitol on intestinal microflora and plasma endotoxin in patients with chronic viral hepatitis. J Infect. 2007;54:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (3)] |
| 40. | Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes. 2011;2:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/