Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3403
Revised: June 4, 2024
Accepted: July 10, 2024
Published online: July 28, 2024
Processing time: 140 Days and 21.9 Hours
There is currently a shortage of accurate, efficient, and precise predictive instruments for rectal neuroendocrine neoplasms (NENs).
To develop a predictive model for individuals with rectal NENs (R-NENs) using data from a large cohort.
Data from patients with primary R-NENs were retrospectively collected from 17 large-scale referral medical centers in China. Random forest and Cox proportional hazard models were used to identify the risk factors for overall survival and progression-free survival, and two nomograms were constructed.
A total of 1408 patients with R-NENs were included. Tumor grade, T stage, tumor size, age, and a prognostic nutritional index were important risk factors for prognosis. The GATIS score was calculated based on these five indicators. For overall survival prediction, the respective C-indexes in the training set were 0.915 (95% confidence interval: 0.866-0.964) for overall survival prediction and 0.908 (95% confidence interval: 0.872-0.944) for progression-free survival prediction. According to decision curve analysis, net benefit of the GATIS score was higher than that of a single factor. The time-dependent area under the receiver operating characteristic curve showed that the predictive power of the GATIS score was higher than that of the TNM stage and pathological grade at all time periods.
The GATIS score had a good predictive effect on the prognosis of patients with R-NENs, with efficacy superior to that of the World Health Organization grade and TNM stage.
Core Tip: We utilized the data of 1408 patients with rectal neuroendocrine neoplasms from a large multicenter database of 17 large-scale Chinese medical centers. We found that tumor grade, T stage, tumor size, age, and a prognostic nutritional index were independent predictors of prognosis in patients with rectal neuroendocrine neoplasms. In addition, we constructed the GATIS score for overall survival and progression-free survival in these patients, which had a C-index of 0.915 for overall survival and 0.908 for progression-free survival; moreover, it showed a better predictive power than that of the TNM stage and pathological grade.
- Citation: Zeng XY, Zhong M, Lin GL, Li CG, Jiang WZ, Zhang W, Xia LJ, Di MJ, Wu HX, Liao XF, Sun YM, Yu MH, Tao KX, Li Y, Zhang R, Zhang P. GATIS score for predicting the prognosis of rectal neuroendocrine neoplasms: A Chinese multicenter study of 12-year experience. World J Gastroenterol 2024; 30(28): 3403-3417
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3403
Neuroendocrine neoplasms (NENs) are rare tumors that originate from embryonic neuroendocrine cells and are characterized by neuroendocrine markers[1,2]. They can occur in various organs throughout the body, with the rectum being a common site of incidence (1.04 per 100000 people)[1,2]. Depending on the presence of hormone-related symptoms, NENs can be divided into functional and non-functional types. Rectal NENs (R-NENs) are mostly non-functional[3]. The overall prognosis of R-NENs is favorable, with a 5-year survival rate as high as 90%[4].
Currently, World Health Organization (WHO) classification and TNM staging are the two most commonly used criteria for the prognosis of NENs[5,6]. According to the morphology and malignant potential of the tumor cells, the WHO 2010 criteria classify R-NENs as G1, G2, or G3. G1 and G2 are neuroendocrine tumors (NETs) with low malignant potential, whereas G3 is usually a neuroendocrine carcinoma (NEC) or mixed adenoneuroendocrine carcinoma (MANEC) with high malignant potential[5]. In recent years, the WHO 2019 classification has also classified some NETs with high malignant potential as G3 to better stratify the prognosis; however, the evaluation criteria remain controversial[7]. Additionally, the WHO classification criteria are not effective in predicting the prognosis of patients with R-NENs[8,9]. TNM staging has a distinct grading system for NET, whereas NEC/MANEC suggests the utilization of colorectal cancer criteria, which are complex in classification and have limited predictive power for patient prognosis. Consequently, developing an effective and precise prognostic assessment tool to guide the clinical diagnosis and treatment of R-NENs is essential.
Nomogram models based on multivariate regression analysis and integrating multiple predictors are useful for the prognostic evaluation of various malignant tumors[10-12]. However, due to the low incidence of R-NENs, a nomogram model with a large sample size is lacking. In this study, we retrospectively collected R-NENs data from 17 large referral hospitals in China and constructed a prognostic model using the Cox proportional hazard model and random forest method. Internal and external validations were conducted to assist in prognosis prediction, diagnosis, and treatment of patients with R-NENs.
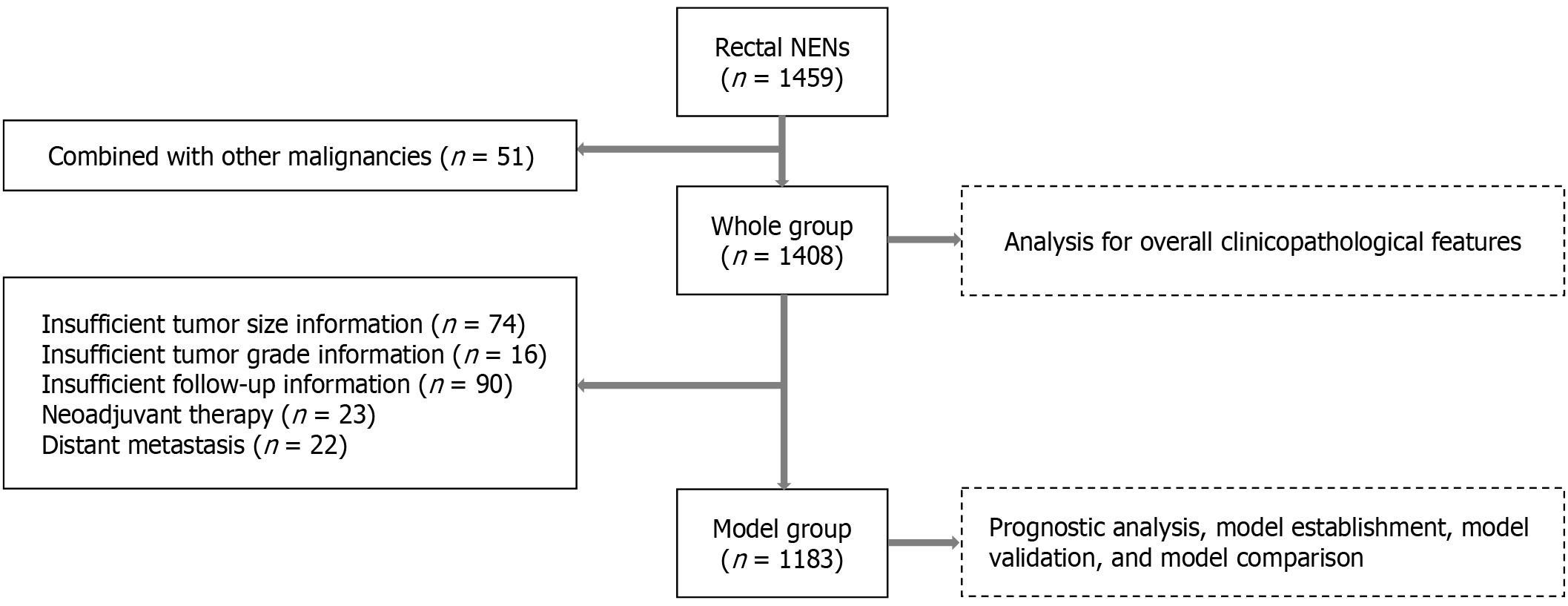
Data of patients with R-NENs admitted to 17 major referral hospitals in China between January 2010 and April 2022 were retrospectively collected. The inclusion criterion was a pathological diagnosis of R-NENs, while the exclusion criterion was simultaneous or metachronous malignancies at other sites (Figure 1). Patient data were collected and analyzed, and the following patients were also excluded during the nomogram construction phase: (1) Patients with metastases at first diagnosis; (2) Patients undergoing neoadjuvant therapy; and (3) Patients with incomplete clinicopathological and follow-up information (Figure 1).
The clinicopathological characteristics of the patients, including sex, age, preoperative hematologic examination, surgical information, postoperative complications, and pathological conditions were collected. Preoperative hematologic examination indicators were collected from patients at the initial diagnosis or within seven days before surgery. The prognostic nutritional index (PNI) was calculated as serum albumin plus five times the peripheral blood lymphocyte count. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were determined as the ratios of peripheral blood neutrophils and platelets-to-lymphocytes, respectively. The WHO 2010 standard was used to classify R-NENs, while the American Joint Committee on Cancer 8th TNM staging criteria was used for NET, NEC, and MANEC staging[5,6]. Each center conducted individual follow-ups, with the last follow-up in July 2022. The primary outcome measures were overall survival (OS) and progression-free survival (PFS), defined as the time from initial diagnosis to death from any cause or final follow-up, and the time from initial diagnosis to disease progression, patient death (whichever occurred earlier), or final follow-up.
SPSS version 25.0 (IBM, Armonk, New York, United States) was used for statistical analysis. Normally distributed data are expressed as mean ± SD, whereas non-normally distributed data are expressed as median and interquartile range. The t-test was used to compare normally distributed data, and the Mann-Whitney U-test was used to determine non-normally distributed data. Truncated values, such as PNI, NLR, and PLR, were determined using the X-tile software. Kaplan-Meier and Log-rank tests were employed to map and compare prognostic differences in patients. The Cox proportional hazards model was used to identify independent risk factors for OS and PFS, and the random forest model was used to assess the correlation between each factor and prognosis. Both the random forest and nomogram models were constructed using R 4.0.0. Statistical significance was set at P < 0.05 (two-tailed). The statistical methods used in this study were reviewed by Yong Gan from the Huazhong University of Science and Technology.
According to the inclusion-exclusion criteria, a total of 1408 patients with R-NENs were included in this study, of whom 591 (42.0%) were female and 817 (58.0%) were male, with a mean age of 51.9 ± 12.1 years. Twenty-three (1.6%) patients received neoadjuvant therapy and 1360 (96.6%) patients underwent complete tumor resection, of which 650 (47.8%) underwent surgery and 710 (52.2%) underwent endoscopic resection. The mean tumor size was 1.3 ± 1.2 cm. The pathological classification was NET in 1307 (92.8%), NEC in 71 (5.0%), MANEC in 14 (1.0%), and unknown in 16 (1.1%) patients. In total, 1149 (81.6%) were classified as G1, 158 (11.2%) as G2, 85 (6.0%) as G3, and 16 (1.1%) as unknown. Of the total group, 1150 (81.7%) had T1, 140 (9.9%) had T2, 82 (5.8%) had T3, and 36 (2.6%) had T4 disease. A total of 103 patients (7.3%) had lymph node metastases, and 61 (4.3%) had lymphovascular invasion. Ninety patients (6.4%) received postoperative adjuvant therapy. Table 1 summarizes the demographic and clinicopathological characteristics of the patients.
| All (n = 1408) | n | |
| Sex | 1408 | |
| Female | 591 (42.0) | |
| Male | 817 (58.0) | |
| Age, year | 51.9 (12.1) | 1408 |
| Size (cm) | 1408 | |
| ≤ 1 | 872 (61.9) | |
| 1-2 | 291 (20.7) | |
| > 2 | 171 (12.1) | |
| NA | 74 (5.3) | |
| Size (cm) | 1.3 (1.2) | 1334 |
| Neoadjuvant therapy | 1408 | |
| No | 1385 (98.4) | |
| Yes | 23 (1.6) | |
| Complete resection | ||
| No | 48 (3.4) | |
| Yes | 1360 (96.6) | |
| Pathological type | 1408 | |
| NET | 1307 (92.8) | |
| NEC | 71 (5.0) | |
| MANEC | 14 (1.0) | |
| NA | 16 (1.1) | |
| Pathological grade, | 1408 | |
| G1 | 1149 (81.6) | |
| G2 | 158 (11.2) | |
| G3 | 85 (6.0) | |
| NA | 16 (1.1) | |
| T stage | 1408 | |
| T1 | 1150 (81.7) | |
| T2 | 140 (9.9) | |
| T3 | 82 (5.8) | |
| T4 | 36 (2.6) | |
| Lymphatic metastasis | 1408 | |
| No | 1305 (92.7) | |
| Yes | 103 (7.3) | |
| TNM stage | 1408 | |
| I | 1133 (80.5) | |
| II | 130 (9.2) | |
| III | 92 (6.5) | |
| IV | 53 (3.8) | |
| Lymph vascular invasion | 1408 | |
| No | 1347 (95.7) | |
| Yes | 61 (4.3) | |
| CgA | 1408 | |
| Negative | 1046 (74.3) | |
| Positive | 362 (25.7) | |
| Syn | 1408 | |
| Negative | 381 (27.1) | |
| Positive | 1027 (72.9) | |
| Adjuvant therapy | 1408 | |
| No | 1318 (93.6) | |
| Yes | 90 (6.4) | |
Patients who did not receive neoadjuvant therapy, did not have distant metastases at diagnosis, or had complete data were screened for nomogram construction (Figure 1). A total of 1183 patients with R-NENs were included in this study. The median follow-up time for the entire group was 34 months, and 44 patients (3.7%) died during the follow-up period. The 1-year, 3-year, and 5-year OS rates were 98.9%, 96.2%, and 94.7%, respectively. The patients were randomly divided into a training dataset (819 cases, 69.2%) and a validation set (364 cases, 30.8%) in a 7:3 ratio. The clinicopathological characteristics of the two groups of patients are shown in Table 2. There was no significant difference at baseline.
| All | Training set | Validation set | P value | |
| n = 1183 | n = 819 | n = 364 | ||
| Sex | 0.402 | |||
| Female | 504 (42.6) | 356 (43.5) | 148 (40.7) | |
| Male | 679 (57.4) | 463 (56.5) | 216 (59.3) | |
| Age, year | 51.7 (12.0) | 51.5 (12.0) | 52.3 (12.0) | 0.291 |
| Size, year | 1.1 (1.0) | 1.1 (1.0) | 1.2 (1.2) | 0.187 |
| Size (cm) | 0.300 | |||
| ≤ 1 | 819 (69.2) | 568 (69.4) | 251 (69.0) | |
| 1-2 | 246 (20.8) | 176 (21.5) | 70 (19.2) | |
| > 2 | 118 (10.0) | 75 (9.2) | 43 (11.8) | |
| PLR | 126.1 (52.3) | 126.6 (54.3) | 124.9 (47.6) | 0.588 |
| NLR | 2.3 (5.1) | 2.2 (2.5) | 2.5 (8.4) | 0.559 |
| PNI | 54.1 (6.8) | 54.2 (6.9) | 53.9 (6.5) | 0.455 |
| Pathological type | 0.824 | |||
| NET | 1134 (95.9) | 786 (96.0) | 348 (95.6) | |
| NEC | 44 (3.7) | 29 (3.5) | 15 (4.1) | |
| MANEC | 5 (0.4) | 4 (0.5) | 1 (0.3) | |
| Pathological grade | 0.876 | |||
| G1 | 1020 (86.2) | 705 (86.1) | 315 (86.5) | |
| G2 | 114 (9.6) | 81 (9.9) | 33 (9.1) | |
| G3 | 49 (4.1) | 33 (4.0) | 16 (4.4) | |
| T stage | 0.358 | |||
| T1 | 1010 (85.4) | 707 (86.3) | 303 (83.2) | |
| T2 | 111 (9.4) | 74 (9.0) | 37 (10.2) | |
| T3 | 45 (3.8) | 29 (3.5) | 16 (4.4) | |
| T4 | 17 (1.4) | 9 (1.1) | 8 (2.2) | |
| Lymphatic metastasis | 0.290 | |||
| No | 1107 (93.6) | 771 (94.1) | 336 (92.3) | |
| Yes | 76 (6.4) | 48 (5.9) | 28 (7.7) | |
| TNM stage | 0.227 | |||
| I | 996 (84.2) | 699 (85.3) | 297 (81.6) | |
| II | 108 (9.1) | 71 (8.7) | 37 (10.2) | |
| III | 79 (6.7) | 49 (6.0) | 30 (8.2) | |
| Lymph vascular invasion | 0.252 | |||
| No | 1149 (97.1) | 799 (97.6) | 350 (96.2) | |
| Yes | 34 (2.9) | 20 (2.4) | 14 (3.8) | |
| CgA | 0.100 | |||
| Negative | 871 (73.6) | 615 (75.1) | 256 (70.3) | |
| Positive | 312 (26.4) | 204 (24.9) | 108 (29.7) | |
| Syn | 0.393 | |||
| Negative | 297 (25.1) | 212 (25.9) | 85 (23.4) | |
| Positive | 886 (74.9) | 607 (74.1) | 279 (76.6) | |
| Adjuvant therapy | 0.286 | |||
| No | 1125 (95.1) | 783 (95.6) | 342 (94.0) | |
| Yes | 58 (4.9) | 36 (4.4) | 22 (6.0) |
The median follow-up time of patients in the training dataset was 34 months, and the 1-year, 3-year, and 5-year OS rates were 98.5%, 95.4%, and 94.2%, respectively. The optimal cut-off values of the PLR, NLR, and PNI hematologic indicators for prognosis according to X-tile were 128.00, 1.45, and 46.57, respectively. A univariate analysis suggested that age, PNI, tumor size, pathological type and grade, T stage, lymph node metastasis, TNM stage, lymphovascular invasion, CgA staining, and adjuvant therapy were associated with prognosis. In the multivariate analysis, considering the colli
| Univariate analysis1 | Multivariate analysis1 | Univariate analysis2 | Multivariate analysis2 | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (male) | 1.148 (0.588-2.243) | 0.686 | 1.168 (0.648-2.106) | 0.605 | ||||
| Age (> 60) | 2.194 (1.115-4.317) | 0.023 | 2.875 (1.505-5.495) | < 0.001 | ||||
| PLR (> 128.00) | 1.592 (0.818-3.100) | 0.171 | 1.313 (0.668-2.577) | 0.402 | ||||
| NLR (> 1.45) | 1.629 (0.740-3.585) | 0.226 | 2.322 (1.106-4.874) | 0.007 | ||||
| PNI (> 46.57) | 0.144 (0.071-0.295) | 0.023 | 0.233 (0.109-0.499) | < 0.001 | 0.130 (0.043-0.392) | < 0.001 | 0.365 (0.135-0.583) | 0.010 |
| Size (cm) | ||||||||
| ≤ 1 | - | Ref. | - | Ref. | - | Ref. | ||
| 1-2 | 6.190 (2.591-14.790) | < 0.001 | 1.706 (0.538-5.413) | 0.364 | 4.960 (1.956-12.580) | 0.001 | ||
| > 2 | 13.439 (5.563-32.468) | < 0.001 | 3.349 (1.314-8.533) | 0.011 | 10.240 (5.200-25.200) | < 0.001 | ||
| Pathological type | ||||||||
| NET | - | Ref. | - | Ref. | ||||
| NEC | 31.177 (14.961-64.968) | < 0.001 | 15.121 (5.590-59.102) | < 0.001 | ||||
| MANEC | 27.899 (6.399-121.627) | < 0.001 | 26.252 (6.203-85.32) | < 0.001 | ||||
| Pathological grade | ||||||||
| G1 | - | Ref. | - | Ref. | - | Ref. | - | Ref. |
| G2 | 6.380 (2.582-15.768) | 0.001 | 4.211 (1.603-11.059) | 0.004 | 8.680 (2.830-20.602) | < 0.001 | 2.937 (1.011-8.535) | 0.048 |
| G3 | 48.175 (21.495-107.969) | < 0.001 | 30.681 (10.821-86.988) | < 0.001 | 25.362 (6.362-62.325) | < 0.001 | 7.126 (2.685-18.913) | < 0.001 |
| T stage | ||||||||
| T1 | - | Ref. | - | Ref. | - | Ref. | ||
| T2 | 3.234 (1.169-8.945) | 0.024 | 13.370 (3.794-47.086) | < 0.001 | 2.136 (1.236-5.362) | 0.025 | ||
| T3 | 17.473 (7.846-38.915) | < 0.001 | 12.630 (2.635-18.354) | < 0.001 | 6.653 (2.368-15.362) | 0.001 | ||
| T4 | 23.081 (8.328-63.964) | < 0.001 | 26.368 (5.364-53.258) | < 0.001 | 8.365 (3.325-13.325) | < 0.001 | ||
| Lymph node metastasis (yes) | 11.387 (5.786-22.409) | < 0.001 | 12.440 (4.172-37.112) | < 0.001 | ||||
| TNM stage | ||||||||
| I | - | Ref. | - | Ref. | ||||
| II | 3.280 (1.188-9.056) | 0.022 | 6.839 (1.692-27.650) | 0.006 | ||||
| III | 14.524 (7.080-29.797) | < 0.001 | 17.792 (5.982-52.932) | < 0.001 | ||||
| Lymph vascular invasion (yes) | 5.753 (2.013-16.447) | 0.001 | 5.899 (1.213-28.703) | < 0.001 | ||||
| CgA (positive) | 1.992 (1.012-3.919) | 0.046 | 1.188 (0.608-2.322) | 0.512 | ||||
| Syn (positive) | 1.161 (0.541-2.492) | 0.702 | 0.960 (0.483-1.908) | 0.919 | ||||
| Adjuvant therapy (yes) | 10.775 (5.161-22.499) | < 0.001 | 13.110 (3.737-45.962) | < 0.001 | ||||
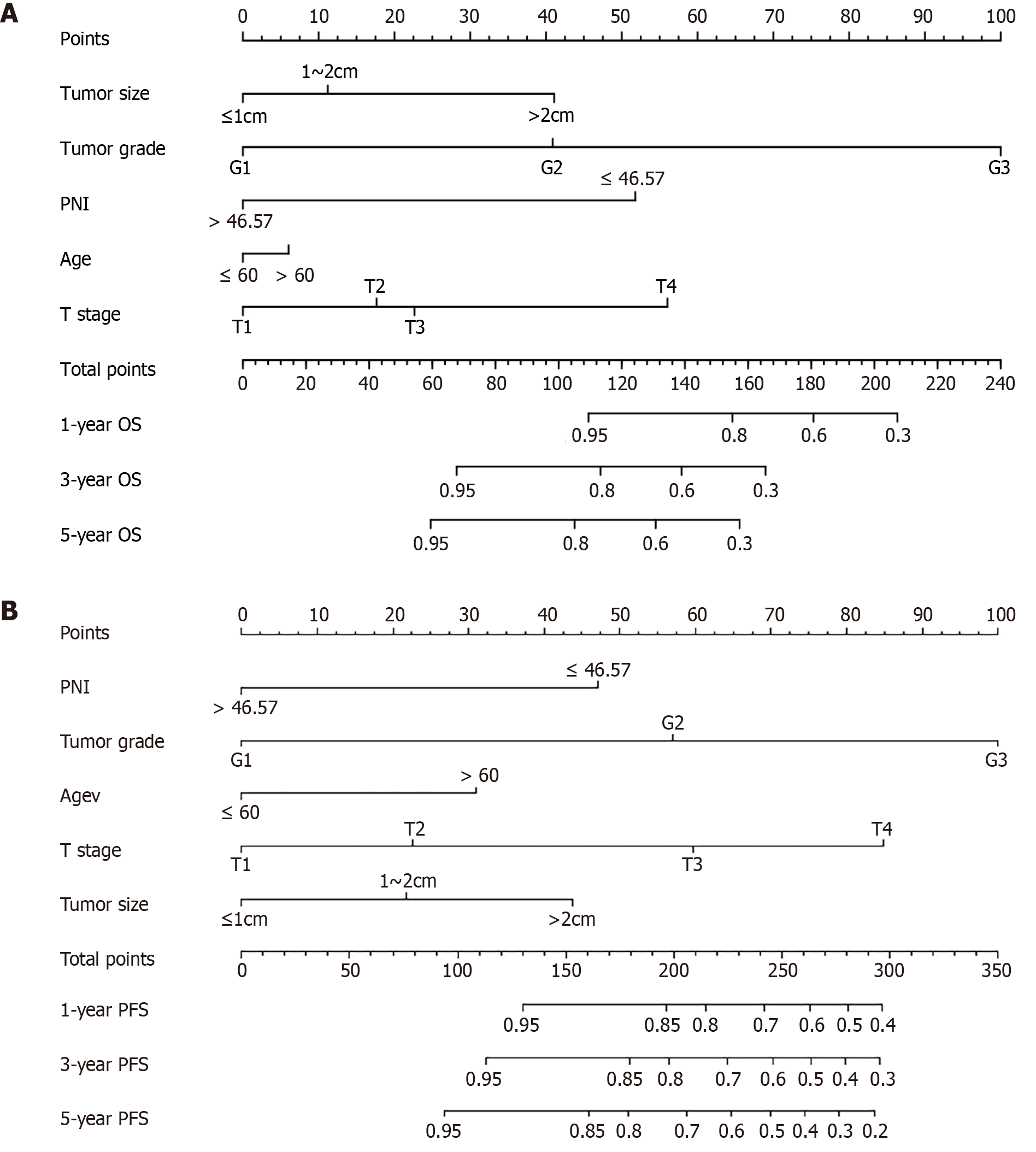
The random forest model was used for further screening and 1000 random trees were used. The model tended to stabilize when the size of the training tree reached approximately 100. The importance of each clinicopathological factor was assessed and ranked. The five indicators of minimal depth were tumor grade, T stage, tumor size, patient age, and PNI. After considering the screening results of the two models, the five indicators, namely, tumor grade, T stage, tumor size, age, and PNI, were included in the construction of the prognostic nomogram for OS in patients (Figure 2A).
Bootstrap resampling (1000 iterations) was used to verify the accuracy of the GATIS score; the C-index of OS in the training set was 0.915 (95%CI: 0.866-0.964), while the C-index in the validation set was 0.812 (0.702-0.923). The similarity between the actual and predicted survival rates based on the GATIS scores was verified using a calibration plot. The predicted 3-year and 5-year OS rates were consistent with the actual survival rates within a 10% error range indicated by the dotted line. Similar results were obtained for the validation set (Figure 3A-D). The decision curve analysis of the training dataset showed that the net benefit of the GATIS score was greater than that of a single factor (Figure 3E). The time-dependent area under the receiver operating characteristic curve (TD-AUC) showed that the predictive power of the GATIS score for OS was higher than that of the TNM stage and pathological grade at all time points (Figure 3F).
A univariate analysis suggested that age, NLR, PNI, tumor size, pathological type and grade, T stage, lymph node metastasis, TNM stage, lymphovascular invasion, and adjuvant therapy were associated with PFS. In the multivariate analysis, considering the collinearity of the TNM stage with T stage and LNM, TNM stage was not included. A multivariate analysis showed that PNI (HR = 0.365, 95%CI: 0.135-0.583, P = 0.010), pathological grade (G2, HR = 2.937, 95%CI: 1.011-8.535, P = 0.048; G3, HR = 7.126, 95%CI: 2.685-18.913, P < 0.001) and T stage (T2, HR = 2.136, 95%CI: 1.236-5.362, P = 0.025; T3, HR = 6.653, 95%CI: 2.368-15.362, P = 0.001; T4, HR = 8.365, 95%CI: 3.325-13.325, P < 0.001) were independent factors for PFS. The results of the log-rank test and Cox proportional hazards regression analysis for PFS are shown in Table 3.
The random forest model was used for further screening and 1000 random trees were used. The model tended to stabilize when the size of the training tree reached approximately 100. The importance of each clinicopathological factor was assessed and ranked. The five indicators of minimal depth were tumor grade, T stage, tumor size, patient age, and PNI. After considering the screening results of the two models, the five indicators of tumor grade, T stage, tumor size, age, and PNI were included in the construction of the prognostic nomogram of PFS for R-NENs patients (Figure 2B).
Bootstrap resampling (1000 iterations) was used to verify the accuracy of the GATIS score; the C-index of PFS in the training set was 0.908 (95%CI: 0.872-0.944), while the C-index in the validation set was 0.865 (0.756-0.909). The similarity between the actual and predicted survival rates based on the GATIS scores was verified using a calibration plot. The predicted 3-year and 5-year PFS rates were consistent with the actual survival rates within the 10% error range indicated by the dotted line. Similar results were obtained for the validation set (Figure 4A-D). The decision curve analysis curve of the training dataset showed that the net benefit of the GATIS score was greater than that of a single factor (Figure 4E). The TD-AUC results showed that the predictive power of the GATIS score for PFS was higher than that of the TNM stage and pathological grade at all time points (Figure 4F).
Rectal NETs are rare and more common in the elderly, with a poorer prognosis in younger individuals[13,14]. There is no evidence of sex or familial aggregation. Generally, R-NENs have a small diameter, and most patients are classified as G1, with a good prognosis[13,14]. The 5-year survival rate can reach 90%[4]. In this study, the average tumor size was 1.3 cm, and 81.6% of the patients had a pathological grade of G1. The 5-year OS rate was 94.7%, which is consistent with that in previous reports.
Due to their rarity and limited sample size, the prognostic factors of R-NENs, particularly the preoperative hematologic factors, have not been fully elucidated. In this study, we found that the PNI was an independent prognostic factor in patients with R-NENs. PNI, a nutrition-related indicator, has been confirmed to be inversely correlated with patient prognosis in various tumors[15,16]. Our findings suggest that individuals with an elevated preoperative PNI, indicating adequate nutritional status, exhibit a reduced risk of mortality. This finding suggests that preoperative nutritional improvement in patients with R-NENs can help improve their prognosis. Additionally, we found that a large tumor diameter and a high pathological grade were poor prognostic factors, which is consistent with previous studies[17,18].
A random forest is an ensemble algorithm composed of decision trees belonging to the bagging (Bootstrap Aggregation) method of ensemble learning. It is composed of numerous decision trees with no correlation between them. When a new input sample is presented for classification, each decision tree in the forest is judged and classified separately, and the most frequent result is considered the final result. A random forest can assess feature importance, interactions between features, and balance errors; however, it may overfit noisy classification or regression problems. It has been used for the prediction and model construction of various tumors with satisfactory results[19-21]. To the best of our knowledge, this is the first randomized forest study on patients with R-NENs. We analyzed the correlation between clinicopathological factors and patient’ prognoses and determined the importance of these factors. Five representative indicators were identified: Tumor grade, T stage, tumor size, age, and PNI.
WHO classification and TNM staging are currently the most commonly used prognostic evaluation systems for NENs; however, several studies have shown that their predictive efficacy for R-NENs is limited. Predictive models, including scoring models and nomograms, have been widely studied and applied in the clinical practice for NENs; however, there are few studies on nomogram models for R-NENs, most of which have small sample sizes and do not consider preoperative hematologic factors[8,9,22,23]. In this study, we combined the results of the Cox proportional hazard and random forest models and constructed a prognostic nomogram model for OS and PFS in R-NENs patients according to the five screened variables. Good prediction performance was achieved in both the training and validation sets, and the calibration analysis showed that there was a good fit between the predicted and actual survival rates. In addition, we compared the predictive effect of the GATIS score with that of the WHO and TNM stages. The TD-AUC results showed that the predictive efficacy of the GATIS score was better than that of the WHO and TNM stages at multiple follow-up time points, suggesting that the GATIS scoring tool may provide a more accurate assessment of the prognosis of R-NENs patients.
This study had some limitations. First, the follow-up time of this study was still short, and a longer follow-up is needed to verify the validity of the GATIS score. Second, with the advancement of molecular and gene detection technologies, more prognostic factors (e.g., genes or biological markers) may be identified. These variables were not included in the GATIS score, and further research is required to identify them. Nevertheless, our study provides a useful tool for the prognostic assessment of R-NENs after complete resection, offering a reference for diagnosis and treatment based on a large sample size of patients with R-NENs.
In conclusion, our study showed that the overall prognosis of patients with R-NENs was favorable, and that tumor size, tumor pathological grade, age, T stage, and preoperative PNI were important factors affecting the prognosis of patients. The GATIS score based on the Cox HR model and random forest had a good predictive effect on the prognosis of patients with R-NENs, and its efficacy was superior than that of the WHO grade and TNM stage. The GATIS score can be used to guide individualized treatment strategies and predict the individualized survival outcomes of patients with R-NENs, to help improve the prognostic evaluation, strengthen patient stratification in clinical trials, and make prognosis-based decisions for patients with R-NENs.
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2665] [Article Influence: 296.1] [Reference Citation Analysis (5)] |
| 2. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 3. | Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, Cremonesi M, de Herder WW, Dromain C, Falconi M, Fani M, Fanti S, Hicks RJ, Kabasakal L, Kaltsas G, Lewington V, Minozzi S, Cinquini M, Öberg K, Oyen WJG, O'Toole D, Pavel M, Ruszniewski P, Scarpa A, Strosberg J, Sundin A, Taïeb D, Virgolini I, Wild D, Herrmann K, Yao J. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 4. | Chagpar R, Chiang YJ, Xing Y, Cormier JN, Feig BW, Rashid A, Chang GJ, You YN. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Rindi G, Arnold R, Bosman F, Al E, WHO classification of tumours of the digestive system: 4th edition. France: International Agency for Research on Cancer, 2010. |
| 6. | Amin MB, Gress DM, Meyer Vega LR, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Compton CC. AJCC Cancer Staging Manual. Eighth Edition. New York: Springer, 2016: 203-220. |
| 7. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2758] [Article Influence: 459.7] [Reference Citation Analysis (3)] |
| 8. | Chen Q, Chen J, Deng Y, Zhang Y, Huang Z, Zhao H, Cai J. Nomogram for the prediction of lymph node metastasis and survival outcomes in rectal neuroendocrine tumour patients undergoing resection. J Gastrointest Oncol. 2022;13:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Feng X, Wei G, Wang W, Zhang Y, Zeng Y, Chen M, Chen Y, Chen J, Zhou Z, Li Y. Nomogram for individually predicting overall survival in rectal neuroendocrine tumours. BMC Cancer. 2020;20:865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Liu B, Li K, Ma R, Zhang Q. Two web-based dynamic prediction models for the diagnosis and prognosis of gastric cancer with bone metastases: evidence from the SEER database. Front Endocrinol (Lausanne). 2023;14:1136089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Lin Y, Wang M, Jia J, Wan W, Wang T, Yang W, Li C, Chen X, Cao H, Zhang P, Tao K. Development and validation of a prognostic nomogram to predict recurrence in high-risk gastrointestinal stromal tumour: A retrospective analysis of two independent cohorts. EBioMedicine. 2020;60:103016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Mjaess G, Peltier A, Roche JB, Lievore E, Lacetera V, Chiacchio G, Beatrici V, Mastroianni R, Simone G, Windisch O, Benamran D, Fourcade A, Nguyen TA, Fournier G, Fiard G, Ploussard G, Roumeguère T, Albisinni S, Diamand R. A Novel Nomogram to Identify Candidates for Focal Therapy Among Patients with Localized Prostate Cancer Diagnosed via Magnetic Resonance Imaging-Targeted and Systematic Biopsies: A European Multicenter Study. Eur Urol Focus. 2023;9:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, Cassese G, Pagano G, Tropeano FP, Luglio G, De Palma GD. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Srirajaskanthan R, Clement D, Brown S, Howard MR, Ramage JK. Optimising Outcomes and Surveillance Strategies of Rectal Neuroendocrine Neoplasms. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Sun H, Wang H, Pan H, Zuo Y, Zhao R, Huang R, Xue Y, Song H. CD19 (+) B Cell Combined with Prognostic Nutritional Index Predicts the Clinical Outcomes of Patients with Gastric Cancer Who Underwent Surgery. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Jia J, Zhang L, Wang T, Yang W, Lyu J, Zeng X, Li X, Zeng X, Liu W, Tao K, Zhang P. Association between preoperative skeletal muscle mass depletion and poor relapse-free survival in patients with gastrointestinal stromal tumors after complete resection. Nutrition. 2022;98:111636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Li YW, He YP, Liu FQ, Peng JJ, Cai SJ, Xu Y, Wang MH. Grade G2 Rectal Neuroendocrine Tumor Is Much More Invasive Compared With G1 Tumor. Front Oncol. 2021;11:646536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Duan X, Zhao M, Zhang S, Xu Z, Mi L, Shi J, Ma X, Liu Y, Li N, Yin X, Han X, Han G, Wang J, Xu J, Yin F. Effects of tumor distance from anal verge on survival outcomes for rectal NENs and lymphatic metastasis risk score for colorectal NENs. Int J Colorectal Dis. 2020;35:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Muñoz AJ, Souto JC, Lecumberri R, Obispo B, Sanchez A, Aparicio J, Aguayo C, Gutierrez D, Palomo AG, Fanjul V, Del Rio-Bermudez C, Viñuela-Benéitez MC, Hernández-Presa MÁ. Development of a predictive model of venous thromboembolism recurrence in anticoagulated cancer patients using machine learning. Thromb Res. 2023;228:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 20. | Gitto S, Interlenghi M, Cuocolo R, Salvatore C, Giannetta V, Badalyan J, Gallazzi E, Spinelli MS, Gallazzi M, Serpi F, Messina C, Albano D, Annovazzi A, Anelli V, Baldi J, Aliprandi A, Armiraglio E, Parafioriti A, Daolio PA, Luzzati A, Biagini R, Castiglioni I, Sconfienza LM. MRI radiomics-based machine learning for classification of deep-seated lipoma and atypical lipomatous tumor of the extremities. Radiol Med. 2023;128:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 21. | Tian S, Yu R, Zhou F, Zhan N, Li J, Wang X, Peng X. Prediction of HER2 status via random forest in 3257 Chinese patients with gastric cancer. Clin Exp Med. 2023;23:5015-5024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Li J, Huang L, Liao C, Liu G, Tian Y, Chen S. Two machine learning-based nomogram to predict risk and prognostic factors for liver metastasis from pancreatic neuroendocrine tumors: a multicenter study. BMC Cancer. 2023;23:529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 23. | Zheng H, Zhao Y, He Q, Hao H, Tian Y, Zou B, Jiang L, Qiu X, Zhou Y, Li Z, Xu Y, Zhao G, Xue F, Li S, Fu W, Li Y, Zhou X, Li Y, Zhu Z, Chen J, Xu Z, Cai L, Li E, Li H, Xie J, Zheng C, Lu J, Li P, Huang C. Multi-institutional development and validation of a nomogram to predict recurrence after curative resection of gastric neuroendocrine/mixed adenoneuroendocrine carcinoma. Gastric Cancer. 2021;24:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/