Published online Jun 28, 2024. doi: 10.3748/wjg.v30.i24.3120
Revised: June 5, 2024
Accepted: June 13, 2024
Published online: June 28, 2024
Processing time: 65 Days and 0.1 Hours
Immune checkpoint inhibitors (ICIs) are widely used due to their effectiveness in treating various tumors. Immune-related adverse events (irAEs) are defined as adverse effects resulting from ICI treatment. Gastrointestinal irAEs are a common type of irAEs characterized by intestinal side effects, such as diarrhea and colitis, which may lead to the discontinuation of ICIs.
Core Tip: Immune checkpoint inhibitor (ICI)-related gastritis is rare but may lead to serious complications such as gastrorrhagia. The strategies such as early identification, pathological diagnosis, management interventions, and immunotherapy reactivation are discussed to enable clinicians to better manage ICI-related gastritis and improve the prognosis of these patients.
- Citation: Yu LL, He ZL, Qian XL. Managing immune checkpoint inhibitor-associated gastritis: Insights and strategies. World J Gastroenterol 2024; 30(24): 3120-3122
- URL: https://www.wjgnet.com/1007-9327/full/v30/i24/3120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i24.3120
I am writing to express my concern regarding the paper titled “Immune checkpoint inhibitor-associated gastritis: Patterns and management” by Lin et al[1], recently published in the World Journal of Gastroenterology. The authors systematically summarize the occurrence patterns and management strategies of immune checkpoint inhibitor (ICI)-associated gastritis, providing important evidence for research and practice in this field.
Over the last decade, the emergence of ICI therapy has revolutionized the treatment of a growing number of malignancies[2]. Immune-related adverse events (irAE) are side effects that resemble autoimmune responses in the patients receiving ICIs[3]. ICI-related gastritis, although rare, may lead to serious complications, such as gastrorrhagia. The most common abnormality reported on endoscopy is erythema, followed by erosions. Other findings include granularity, sloughing, exudates, ulcers, atrophy, and rarely, severe hemorrhagic gastritis[4-6].
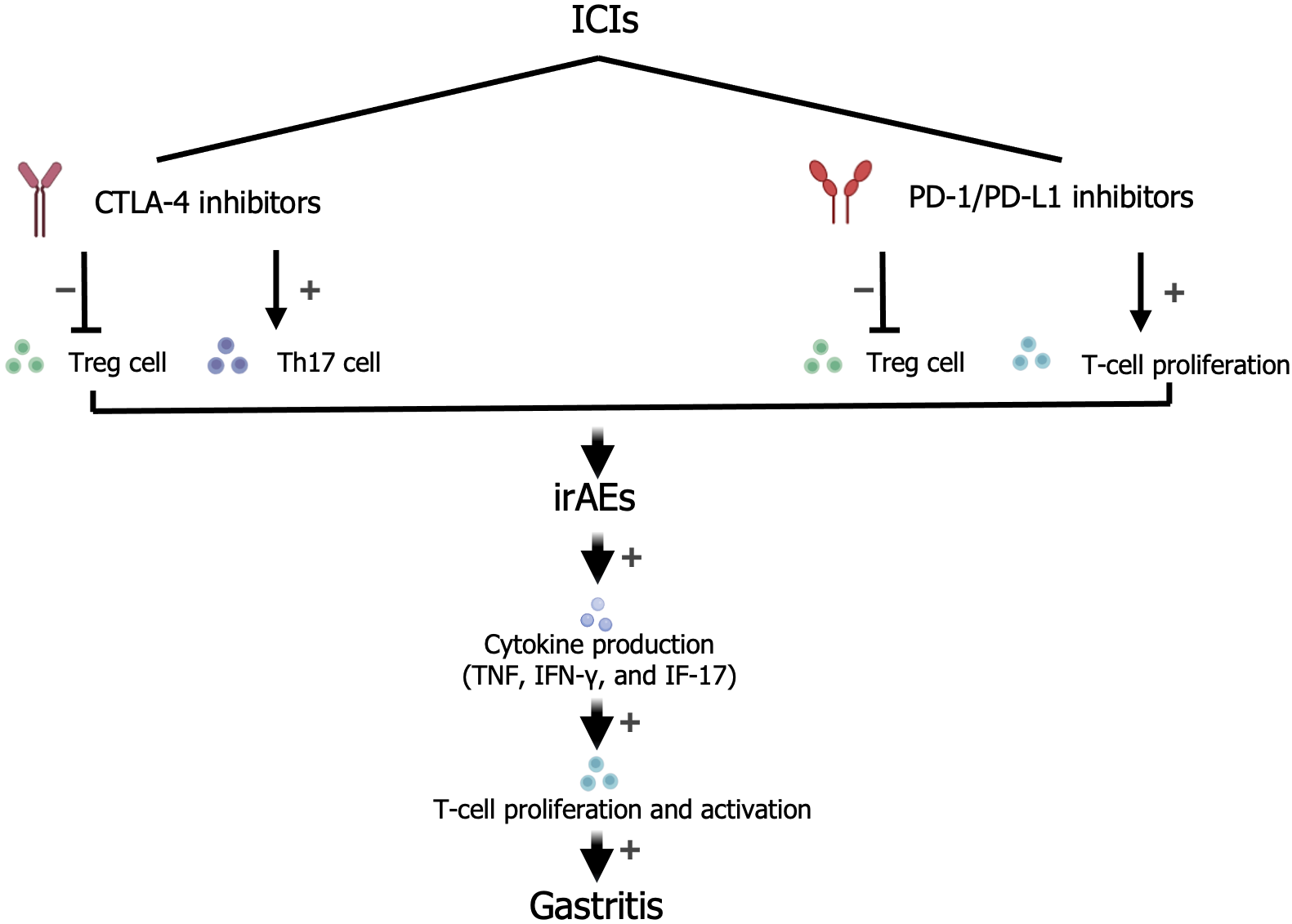
A common mechanism (Figure 1) by which ICIs exert their effects involves activation of effector T cells by inhibition of programmed death 1, programmed death-ligand 1, and cytotoxic T-lymphocyte antigen 4[7]. It is also proposed that the proliferation of activated T cells and increased cytokine production, caused by a lack of self-tolerance, may result in irAEs[8,9]. However, the detailed mechanisms underlying irAEs remain unclear. Therefore, the treatment decisions for ICI-related gastritis are based on individual clinical presentations.
However, the article does not adequately address individualized treatment options for diverse patient populations. Considering the patient's immune status, gastritis severity, and other factors, the formulation of an individualized treatment plan is particularly important. For example, before starting an ICI, autoantibody screening may be considered for patients with a personal or familial history of autoimmune disease or those presenting with signs or symptoms suggestive of an underlying autoimmune disease. This precaution is due to their enhanced risk of developing a full-blown autoimmune disease post-treatment[10]. Second, the article neglects to mention the evaluation and monitoring strategies prior to ICI therapy when discussing preventive measures, which are important for reducing the risk of gastritis.
Additionally, the issue of re-provocation after ICI treatment warrants attention, particularly the risk of recurrence of gastritis. Upon complete resolution of irAEs, the resumption of immunotherapy is crucial for treatment and prognosis despite the risk of irAE relapse.
In the future, we need to continue to deepen our understanding of irAE gastritis, enabling timely and appropriate diagnosis and treatment and providing clinicians with guidance for the treatment of ICI-related gastritis to improve patient prognosis.
We acknowledge all the authors whose publications are used as references in our article.
| 1. | Lin J, Lin ZQ, Zheng SC, Chen Y. Immune checkpoint inhibitor-associated gastritis: Patterns and management. World J Gastroenterol. 2024;30:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (33)] |
| 2. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10716] [Article Influence: 765.4] [Reference Citation Analysis (34)] |
| 3. | Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 4. | Rao BB, Robertson S, Philpott J. Checkpoint Inhibitor-Induced Hemorrhagic Gastritis with Pembrolizumab. Am J Gastroenterol. 2019;114:196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Nishimura Y, Yasuda M, Ocho K, Iwamuro M, Yamasaki O, Tanaka T, Otsuka F. Severe Gastritis after Administration of Nivolumab and Ipilimumab. Case Rep Oncol. 2018;11:549-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Kobayashi M, Yamaguchi O, Nagata K, Nonaka K, Ryozawa S. Acute hemorrhagic gastritis after nivolumab treatment. Gastrointest Endosc. 2017;86:915-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Chan KK, Bass AR. Autoimmune complications of immunotherapy: pathophysiology and management. BMJ. 2020;369:m736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4982] [Article Influence: 622.8] [Reference Citation Analysis (0)] |
| 9. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3470] [Article Influence: 433.8] [Reference Citation Analysis (0)] |
| 10. | Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suárez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 999] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/