Published online Jun 14, 2024. doi: 10.3748/wjg.v30.i22.2902
Revised: May 6, 2024
Accepted: May 28, 2024
Published online: June 14, 2024
Processing time: 100 Days and 2 Hours
Remarkable progress over the last decade has equipped clinicians with many options in the treatment of inflammatory bowel disease. Clinicians now have the unique opportunity to provide individualized treatment that can achieve and sustain remission in many patients. However, issues of primary non-response (PNR) and secondary loss of response (SLOR) to non-tumour necrosis factor inhibitor (TNFi) therapies remains a common problem. Specific issues include the choice of optimization of therapy, identifying when dose optimization will recapture response, establishing optimal dose for escalation and when to switch therapy.
To explores the issues of PNR and SLOR to non-TNFi therapies.
This review explores the current evidence and literature to elucidate management options in cases of PNR/SLOR. It will also explore potential predictors for response following SLOR/PNR to therapies including the role of therapeutic drug monitoring (TDM).
In the setting of PNR and loss of response to alpha-beta7-integrin inhibitors and interleukin (IL)-12 and IL-23 inhibitors dose optimization is a reasonable option to capture response. For Janus kinase inhibitors dose optimization can be utilized to recapture response with loss of response.
The role of TDM in the setting of advanced non-TNFi therapies to identify patients who require dose optimization and as a predictor for clinical remission is not yet established and this remains an area that should be addressed in the future.
Core Tip: In the setting of primary non-response (PNR) and loss of response (LOR) to alpha-beta7-integrin inhibitors and interleukin (IL)-12 and IL-23 inhibitors dose optimization is a reasonable option to capture response. For Janus kinase inhibitors dose optimization can be utilized to recapture response with LOR is less successful in the setting of PNR. The role of therapeutic drug monitoring in the setting of non-tumour necrosis factor inhibitor therapies to identify patients who require dose optimization and as a predictor for clinical remission is not yet established and this remains an area that should be addressed in the future research.
- Citation: Vootukuru N, Vasudevan A. Approach to loss of response to advanced therapies in inflammatory bowel disease. World J Gastroenterol 2024; 30(22): 2902-2919
- URL: https://www.wjgnet.com/1007-9327/full/v30/i22/2902.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i22.2902
It has been over two decades since tumor necrosis factor antagonists revolutionized the management of inflammatory bowel disease (IBD) and allowed for the achievement of sustained disease remission with relatively few side effects from treatment including in people who were previously refractory to medical therapy[1]. Many advanced therapies have subsequently been approved for use in IBD with varying mechanisms of action, including alpha-beta7-integrin inhibitors, interleukin (IL)-12 and IL-23 inhibitors, Sphingosine-1-phosphate (S1P) receptor modulators and Janus kinase (JAK) inhibitors[2]. With the shift in the treatment paradigm favoring earlier utilization of advanced therapies, there is added complexity in determining the best methods for optimizing, switching, and escalating medical therapy to achieve the best outcomes. With the growing therapeutic armamentarium, it is important that the choice of therapy is individualized and both patient and disease related factors are considered to improve the likelihood of achieving disease remission while being tolerable for the patient. Yet, there is complexity in identifying the most appropriate individualized treatment for patients, with no single factor being able to identify which agent is most suitable. Similarly, identifying patients who are not responding to a treatment early, and deciding the best course of action can also be challenging. Determining why a particular therapy was not effective can be useful in deciding the best approach to a patient with an inadequate response to treatment and what further measures should be taken. Treatment non-response can be classified into two broad categories-primary non-response (PNR) which refers to a lack of clinical response during initial treatment or secondary non-response which refers to an initial response to therapy follow by a loss of response (LOR) over time[1]. This review will focus on the management options before class switching for clinicians faced with PNR and secondary LOR of non-tumour necrosis factor inhibitor (TNFi) based advanced therapy.
This review aims to explore the efficacy of dose intensification and in-class switching in cases of PNR and LOR with non-TNFi advanced therapy. A literature search was performed in PubMed and Ovid Medline up to November 2023 for original articles and reviews under the subject headings “inflammatory bowel disease,” “Crohn’s disease,” “CD,” “ulcerative colitis,” “JAK Inhibitors,” “Tofacitinib,” “Upadacitinib,” “Ustekinumab,” “anti- IL- 12/23p40,” “alpha-beta7-integrin inhibitors,” “Vedolizumab,” “Sphingosine-1-phosphate,” “ozanimod,” “dose escalation,” “dose intensification,” “re-induction,” “drug levels,” “TDM,” and their synonyms .In addition, the reference lists from the selected articles were reviewed to identify additional studies of potential interest. Only studies conducted in adults were included.
There is no clear consensus on the definition of PNR but in general PNR refers to a failure to display improvement in clinical signs or symptoms during the induction phase[3-5] with significant variability in time to clinical response noted between therapeutics[6,7]. The time of expected response varies with different therapies, but it is usually considered PNR if there is a lack of response to induction treatment or within 14 weeks of commencing therapy[6,7]. Our understanding of the mechanism of PNR comes from experiences with anti-tumour necrosis factors (TNFs). The two major recognised mechanisms of PNR to anti-TNFs are pharmacokinetic (due to rapid drug clearance resulting in low trough levels) and pharmacodynamic (mechanistic) failure[8], which refers to failure due to inflammation mediated by alternative pathways to the mechanism targeted by the allocated therapy[9]. Secondary non-response or LOR describes the clinical phenomenon whereby patients who initially respond to advanced therapy then subsequently lose response[10]. As with PNR, our understanding of the mechanisms leading to LOR are primarily derived from experiences with anti-TNFs. The main causes of LOR with anti-TNFs are suboptimal drug concentrations due to low trough level drug concentrations and/or anti-drug antibodies or mechanistic failure due to disease transitioning to another pathway of inflammation[11,12].
Predictive factors for PNR and LOR appear to be similar with different agents and seem to relate to the underlying inflammatory burden. For vedolizumab the GEMINI trials demonstrated that less severe disease activity at baseline was associated with higher likelihood of remission in IBD[13,14] which has since been reflected in real word studies[15-23]. Furthermore, an association of elevated inflammatory markers with lower rates of clinical response and remission has also been described[13-15,24-26]. A post-hoc analysis of the GEMINI trials reported higher rates of rates of induction and maintenance of clinical remission among TNF antagonist naïve patients[27,28] which has been confirmed by real world trials[17,21,23,26,29-32]. Patients who achieve early response to vedolizumab also appear to be more likely to have a long-term response[15,16,30,33,34]. Similar to vedolizumab, a higher rate of clinical response and remission is expected with ustekinumab in both Crohn’s and ulcerative colitis (UC) among patients with less severe disease activity at baseline[35-44] with an elevated C-reactive protein (CRP) associated with lower rates of clinical remission[45,46]. With ustekinumab therapy, failure of both TNF and vedolizumab was associated with lower rates of clinical remission[38,47,48]. As with other advanced therapy, patients lower baseline disease activity were more likely to achieve remission with tofacitinib[49-51] whereas higher CRP levels[50,52] and prior TNF[50,53] or biologic therapy[51,54] was associated with lower rates of clinical response. Interestingly, younger patients were less likely to demonstrate clinical response or remission[50,52] with tofacitinib. For ozanimod, a similar rate of clinical remission of UC is seen among patients with prior biologic use with a slower rate of onset[55,56] whereas lower rates of clinical remission are seen with etrasimod among patients with prior biologic or JAK-inhibitor exposure[57].
The approach to patients with suspicion for PNR or LOR requires detailed assessment to determine if worsening symptoms are caused by increased IBD activity and then to determine the possible causes of PNR or LOR[10,58]. Disease activity is assessed through assessing clinical symptoms aided by evaluation with a combination of objective measures such as laboratory testing, endoscopy, and cross-sectional imaging[10,58]. It is essential at this stage that alternative causes for presumed PNR and LOR are excluded such as co-infection[59], poor adherence[60], improper drug storage[61], irritable bowel disease, bacterial overgrowth and bile acid malabsorption[58]. There is a consensus in guidelines for reactive therapeutic drug monitoring (TDM) in patients who fail to respond to anti-TNF therapy[11,62] however the role of TDM with other advanced therapies is less clearly defined. Thereafter the clinician is faced by three main methods of recapturing response-treatment escalation, addition of immunomodulator therapy, switching to a different therapy with a similar mechanism (in class switch) or switching to a therapy with a different mechanism (out of class switch).
Vedolizumab is a full human IgG1 monoclonal antibody which targets α4β7 integrin, modulating lymphocyte trafficking in the gut[63]. Vedolizumab is administered intravenously (at a dose of 300 mg) with induction does at week 0 and 2 and maintenance doses thereafter at an interval of 4, 6 or 8 weekly[13,14]. The seminal GEMINI trials established the role of vedolizumab in IBD with clinical response rates of 47.1% and 25.7% in the treatment of UC and Crohn’s disease (CD) respectively[13,14]. Despite the recognised efficacy of vedolizumab, eventual LOR to treatment is common, with rates reported to be 47.9 per 100 person-years in Crohn’s and 39.8 per 100 person-years in UC[64]. Where mechanistic failure is thought to be unlikely clinicians will most often dose escalate from 300 mg every 8 weeks to every 4 week and less commonly every 6 weeks to attempt to induce or recapture remission[65]. Yet it is unclear if vedolizumab levels can be utilised to identify patients who will not respond to dose escalation and hence require therapy class-switching (Table 1).
| Ref. | Number | Disease | PNR and/or SLOR | Study design | Intervention | Follow-up | Outcome | Result |
| Trials: Vedolizumab dose escalation | ||||||||
| Loftus et al[66] | 32 | UC | LOR | Single-arm open label-multicentre | 4 weekly 300 mg | 28 weeks | Clinical response/clinical remission | 53.1% (19% with response prior to escalation)/25.0% (6% in remission prior) |
| Vermeire et al[67] | 57 | Crohn’s | LOR | Single-arm open label-multicentre | 4 weekly 300 mg | 28 weeks | Clinical Response/Clinical Remission | 54.4% (39% with response prior to escalation)/22.8% (4% in remission prior) |
| Vaughn et al[68] | 58 | Crohn’s or UC | LOR | Retrospective cohort study-multicentre | 4-7 weekly 300 mg | 15 weeks | Clinical Response | 62.0% |
| Gouynou et al[69] | 23 | Crohn’s or UC | PNR/LOR | Retrospective cohort study-single centre | NS-increased frequency | 9 months | Clinical response | 52.2% |
| Outtier et al[70] | 59 | Crohn’s or UC | LOR | Prospective observational study-multicentre | 4 weekly 300 mg | 8 weeks | Clinical response | 54.2% |
| Kolehmainen et al[71] | 36 | Crohn’s or UC | PNR/LOR | Retrospective cohort study-single centre | NS-increased frequency | 12 months | Clinical response | 33.3% |
| Perry et al[23] | 24 | UC | PNR/Partial Responder | Retrospective cohort study-single centre | 4 weekly 300 mg | 51 weeks | Clinical response/corticosteroid free remission | 41.7%/41.7% |
| Christensen et al[72] | 43 | Crohn’s or UC | NS | Prospective cohort study-single centre | 4 or 6 weekly 300 mg | 26 weeks | Clinical response/clinical remission | 58.1%/55.8% |
| Dreesen et al[74] | 16 | Crohn’s or UC | NS | Retrospective cohort study-single centre | 4 weekly 300 mg | 14 weeks (UC), 22 weeks (Crohn’s) | Clinical response | 56.3% |
| Kopylov et al[73] | 48 | Crohn’s or UC | NS | Retrospective cohort study-multicentre | 4 weekly 300 mg | 52 weeks | Clinical response | 62.5% |
| Williet et al[75] | 15 | Crohn’s or UC | PNR | Prospective cohort study-single centre | 4 weekly 300 mg | 36 weeks | Clinical response | 53.8% |
| Attauabi et al[76] | 37 | Crohn’s or UC | LOR | Retrospective cohort study-2 centre | 4-7 weekly 300 mg | < 70 weeks | Clinical remission | 62.2% |
| Jairath et al[77] | 55 | UC | PNR | Open label multicentre RCT | 4 weekly 300mg or 600 mg | 30 weeks | Clinical response/clinical remission | 30.9/9.1% |
| Trials: Ustekinumab frequency | ||||||||
| Dalal et al[41] | 75 | Crohn’s | LOR | Retrospective cohort study-single centre | 4 or 6 weekly 90 mg | 12 months | Corticosteroid free clinical remission | 54.7% |
| Derikx et al[48] | 47 | Crohn’s | NS | Retrospective cohort study-single centre | 4 or 6 weekly 90 mg | 8.9 months | Corticosteroid free remission | 29.6% |
| Bundschuh et al[98] | 27 | Crohn’s | LOR | Retrospective cohort study | 4 or 6 weekly 90 mg | NS | Clinical response | 54.5% |
| Haider et al[100] | 15 | Crohn’s | PNR | Retrospective cohort study-single centre | 4 weekly 90 mg | 78 weeks | Clinical response/clinical remission | 46.6%/33.3% |
| Fumery et al[101] | 100 | Crohn’s | Partial Response/LOR | Retrospective cohort study-single centre | 4 weekly 90 mg | 2.4 months | Clinical response/clinical remission | 61%/31% |
| Ollech et al[102] | 51 | Crohn’s | NS | Retrospective cohort study-single centre | 4 weekly 90 mg | 5.9 months | Clinical remission | 27.5% |
| Dalal et al[96] | 157 (Crohn’s: 117, UC: 40) | Crohn’s or UC | Partial Response/LOR | Retrospective cohort study-single centre | 4 or 6 weekly 90 mg | 12 months | Steroid free clinical remission | Crohn’s 57.3%/UC 52.5% |
| Rowbotham et al[107] | 24 | UC | NS | Randomised-withdrawal maintenance study | 8 weekly 90 mg | 16 weeks | Clinical remission | 58.3% |
| Trials: Ustekinumab reinduction | ||||||||
| Sedano et al[109] | 15 | Crohn’s | Partial Response/LOR | Retrospective cohort study-single centre | IV reinduction | 14.9 weeks | Clinical response/clinical remission | 66.7%/53.3% |
| Heron et al[110] | 65 | Crohn’s | Partial Response/LOR | Retrospective cohort study - multicentre | IV reinduction | 14 weeks | Clinical Remission with either biochemical and endoscopic response or remission | 31.0% |
| Bermejo et al[111] | 43 | Crohn’s | LOR | Retrospective cohort study-multicentre | IV re-induction | 16 weeks | Clinical response/clinical remission | 52.8%/43.3% |
| Ten Bokkel et al[112] | 29 | Crohn’s | LOR | Prospective cohort study-multicentre | IV re-induction | 52 weeks | Clinical remission | 44.8% |
| Trials: Ustekinumab increased frequency and/or reinduction | ||||||||
| Cohen et al[99] | 68 | Crohn’s | PNR/Partial Response | Retrospective cohort study-single centre | IV induction + 4 or 6 weekly 90 mg | 3-6 months | Clinical response/clinical remission | 79.4%/30.9% |
| Yao et al[114] | 128 | Crohn’s | Partial Response/LOR | Retrospective cohort study-single centre | 4 weekly 90 mg +/- IV Re-induction | 3 months | Clinical remission | 62.9% Shortening/69.6% re-induction |
| Hudson et al[113] | 18 | Crohn’s | SLOR | Retrospective case series-single centre | IV re-induction +/- 4 or 6 weekly 90 mg | 4-8 weeks | Clinical remission or response | 83.3% |
| Ramaswamy et al[106] | 31 | Crohn’s | Partial response/LOR | Retrospective cohort study-single centre | 4 weekly 90 mg +/- IV re-induction | 12 weeks | Clinical response | 64.5% |
| Chaparro et al[40] | 60 | Crohn’s | PNR/LOR | Retrospective cohort study-multicentre | 4 weekly 90 mg /IV re-induction | NS | Clinical remission | 78.3% |
| Ma et al[115] | 24 | Crohn’s | LOR | Retrospective cohort study-multicentre | 4 or 6 weekly 90 mg +/- IV reinduction | NS | Clinical response | 54.2% |
| Young et al[97] | 21 | Crohn’s | PNR/LOR/partial response | Retrospective cohort study-single centre | 4 or 6 weekly 90 mg +/- IV induction | 177 days | Clinical response | 52.4% |
| Johnson et al[103] | 229 | Crohn’s | PNR/LOR | Retrospective cohort study-multicentre | 4 or 6 weekly 90 mg + IV reinduction/IV reinduction | NS | Clinical response | 45.9% |
| Olmedo et al[104] | 91 | Crohn’s | PNR/LOR | Retrospective cohort study-multicentre | 4 or 6 weekly 90 mg + IV reinduction | 16 weeks | Steroid free clinical response/Steroid free clinical remission | 62.6%/25.3% |
| Kopylov et al[105] | 142 | Crohn’s | NS | Retrospective cohort study-multicentre | 4 or 6 weekly 90 mg +/- IV induction | 16 weeks | Clinical response/clinical remission | 51.4%/38.7% |
| Trials: Tofacitinib dose escalation | ||||||||
| Ma et al[51] | 71 | UC | LOR | Prospective cohort study-multicentre | 10 mg BD | NS | Clinical response | 54.9% |
| Honap et al[52] | 19 | UC | LOR | Retrospective cohort study-multicentre | 10 mg BD | NS | Clinical response | 47.4% |
| Sandborn et al[150] | 57 | UC | LOR | Prospective cohort study-multicentre | 10 mg BD | 12 months | Clinical response/clinical remission | 64.9%/49.1% |
| Trials: Upadacitinib dose escalation | ||||||||
| Sandborn et al[151] | 60 | Crohn’s | NS (inadequate response) | Phase II placebo controlled RCT | 12 mg BD/24 mg BD IR | 52 weeks | Clinical remissions | 15% 12 mg BD/24 mg BD 39% |
| Panaccione et al[153] | 190 | UC | LOR/inadequate response | Prospective cohort study | 30 mg ER daily | 48 weeks | Clinical remission | 27.9% |
Dose escalation: Observational data suggest that dose escalation of vedolizumab is effective in overcoming PNR and secondary LOR. The GEMINI long-term safety trials confirmed that vedolizumab 300mg dose escalation to 4 weekly restored clinical remission following LOR in UC and Crohn’s[66,67] in a clinical trial setting. A retrospective study of 192 IBD patients among whom 58 patients were dose escalated (largely to 4 weekly vedolizumab) for secondary LOR or subclinical disease reported a clinical response rate of 62%[68]. Another observational study of 23 IBD patients who underwent dose optimisation of vedolizumab for primary or secondary LOR showed that increased vedolizumab dosing frequency resulted in a treatment response in more than half of IBD[69]. Similar findings establishing the efficacy of vedolizumab dose intensification were described in further recent retrospective and prospective studies[23,68-76]. This has been confirmed in a systematic review by Peyrin-Biroulet et al[64] which reports that dose intensification of vedolizumab following secondary LOR restores clinical response in more than half of IBD patients on maintenance vedolizumab therapy.
However, a recent randomized control trial (RCT) including 278 UC patients reported that among patients with early nonresponse to vedolizumab (at week 6) and high drug clearance, vedolizumab dose escalation ranging from 300 mg to 600 mg every 4 to 6 weeks did not lead to higher rates of clinical remission and response. In fact, Jairath et al reported that approximately 10% of patients with early non-response achieved clinical remission at week 30 irrespective of the dose received[77]. The findings of this RCT may explain the heterogeneity of data regarding the correlation of vedolizumab trough levels with remission and support the fact that time on therapy with careful monitoring may be sufficient to ensure adequate response is eventually achieved rather than switching therapies.
Role of TDM: In 2017, post-hoc analysis of the GEMINI trial reported that higher vedolizumab serum concentrations were associated with higher remission rates after induction therapy in patients with moderately to severely active UC or CD[32]. These finding were confirmed in a prospective trial of 51 IBD patients which showed that vedolizumab trough levels were higher at weeks 6 and 22 in patients with combined clinical and endoscopic remission[78]. The correlation between vedolizumab levels and response has subsequently been described in further studies including an association with endoscopic response and histological healing[74,79-81]. Furthermore, observational studies describe the role of early vedolizumab trough level as a predictor for clinical[78,82-84] and histological remission[85] and the need for dose intensification within 6 months[75]. Interestingly a multicentre retrospective study of 58 patients with IBD with secondary LOR to vedolizumab reports reported an odds ratio of 3.7 for clinical response to dose escalation with vedolizumab concentration < 7.4 µg/mL compared to a vedolizumab concentration ≥ 7.4 µg/mL[68] and a small retrospective study of 23 patients showed that early changes in the pharmacokinetic profile of vedolizumab may predict recapture of response after dose optimization[69]. A prospective study of 47 primary non-responders to vedolizumab with IBD who were dose-escalated reported that all patients with vedolizumab trough levels < 19.0 mg/mL at week 6 required dose escalation and achieved clinical response 4 weeks later[75]. Furthermore, Singh et al[86] undertook a meta-analysis in UC patients which reported vedolizumab trough concentration ≥ 18.5-20.8 μg/mL at week 6, and ≥ 9.0-12.6 μg/mL during maintenance may be associated with clinical remission at week 14 and clinical/endoscopic/biochemical response or remission with maintenance therapy respectively. A systematic review by Cao et al[87] suggested a blood concentration of vedolizumab surpassing 25.0 μg/mL indicated mucosal healing in UC patients under maintenance therapy but was unable to provide a clear predictive cut-off value of blood concentration on mucosal healing or endoscopic remission under induction therapy in IBD reporting a range between 8.0 and 28.9 μg/mL.
Given these findings, it would appear TDM may have a role in monitoring response to treatment in vedolizumab and in determining mechanistic failure. However, more recent observational data has not found an association with trough vedolizumab levels and clinical remission[88,89]. A prospective study of 159 patients with IBD did not find a correlation between trough vedolizumab concentration and clinical remission among patients on maintenance therapy[89]. Furthermore, the utility of vedolizumab trough levels to guide dose escalation was explored by a multicentre retrospective study which found no difference in vedolizumab trough levels prior to optimisation among those reaching clinical remission compared with those with active disease after dose escalation[81]. Similar findings were reported in a prospective study that found baseline trough levels of vedolizumab were not predictive of clinical and biological response at weeks 4 and 8 to dose escalation[70].
Predictors of failure to respond to dose escalation: With this apparent equipoise, the role of vedolizumab drug mo
Further RCTs are required to clearly delineate the optimal frequency and dose for optimisation of vedolizumab therapy. With regards to TDM, further research is required to establish if TDM could aid in management of patients on vedolizumab and identify potential predictors for response to dose escalation. Currently, it appears that a strategy of persisting with therapy and possible dose escalation in patients with early nonresponse to therapy or following secondary LOR to treatment can overcome LOR to therapy.
Ustekinumab is a humanised monoclonal antibody targeting the p40 subunit of IL-12 and IL-23[90]. The landmark UNITI trials established the utility of Ustekinumab in Crohn’s disease and UC[90,91]. LOR also occurs with ustekinumab therapy[92,93] and is estimated to occur in 21% per person-year on standard dosing and 25% per person- year for dose interval shortened therapy[94]. Approximately 20% of patients will require dose-interval shortening during the maintenance therapy[95]. The optimal management of primary nonresponse and secondary LOR to standard ustekinumab dosing remains unclear[96]. Potential approaches include empiric dose intensification through reduction of dose interval, re-induction to recapture clinical response in both CD and UC or use of TDM as outlined below[41] (Table 1).
Dose-escalation: Treatment intensification with 4 weekly or 6 weekly ustekinumab to capture response in Crohn’s disease is an established management strategy for LOR to therapy[41,48,97-106]. A multicentre study of 100 patients with active CD showed clinical remission at a median follow-up of 2.4 months in approximately 30% of patients following treatment intensification with ustekinumab 90 mg every 4 weeks for LOR or incomplete response[101]. Similar findings were also described in a recent retrospective study of 110 patients with CD which reported that shortening ustekinumab 90 mg dose interval to 4 weekly among 55 patients with PNR or LOR achieved clinical remission in 28% and endoscopic remission in 36% of patients at a median follow-up of 5.9 months[102]. Furthermore, Dalal et al reported in a retrospective study of 123 patients with Crohn’s disease that dose intensification to both 4 weekly and 6 weekly is clinically effective with 50% of patients in both groups achieving corticosteroid-free clinical remission within 12 months[41]. The efficacy of treatment intensification of ustekinumab was further demonstrated in a recent large retrospective multicentre study including 1113 CD patients treated with ustekinumab which reported among 77 patients who experienced loss of remission and underwent dose optimisation 57% achieved clinical response and among 152 patients who were dose-optimized because of primary nonresponse or incomplete response to ustekinumab approximately 40% achieved clinical response[103].
While there is less evidence for dose intensification in UC, it still appears robust with a single retrospective cohort study including 123 patients with CD and 40 patients with UC which described corticosteroid free clinical remission rates > 50% among all CD and UC patients at 12 months after ustekinumab dose intensification and ≥ 40% at 24 months[96]. Rowbotham et al[107] report 58% rate of clinical remission at week 16 in UC patients with increase in frequency of ustekinumab to 8 weekly from 12 weekly.
Re-induction: Re-induction following LOR to ustekinumab in Crohn’s is another strategy that can be used and is supported by several observational studies[105,108-113]. A retrospective study of 65 patients with Crohn’s reported that clinical remission was achieved at week 14 in approximately 30% of patients even among those already on escalated maintenance dosing of ustekinumabevery 4 weeks[110]. A recent retrospective observational study of 128 patients with Crohn’s which compared dose optimisation of ustekinumab by shortening interval or through intravenous reinduction reported greater increases in ustekinumab trough level and higher rates of clinical and endoscopic remission at 3 months with intravenous reinduction compared with shortening of drug intervals[114]. Similar findings were described in a retrospective observational study which reported that among patients with severe CD optimization of ustekinumab with 2 initial intravenous inductions was more effective than standard with clinical response and clinical remission rates of 92% and 88% respectively[115]. The findings suggest that even a temporary increase in the dose of ustekinumab therapy may be sufficient to recapture response to ustekinumab treatment so should be considered for patients losing response to therapy.
While more data is needed to delineate efficacy of dose optimisation of ustekinumab with reinduction as opposed to interval shortening and the role of dose optimisation, the findings of meta-analyses by Meserve et al[116] and Yang et al[94] provide strong evidence for a benefit to recapture clinical response with dose escalation in Crohn’s disease following LOR or inadequate response.
Switch from subcutaneous to intravenous therapy or risankizumab: Switching from subcutaneous to intravenous ustekinumab or to therapies with a similar mechanism is an evolving area of practice with potential to overcome LOR to ustekinumab[117]. Argüelles-Arias et al[117] describe a clinical remission rate of approximately 43% with the use of intravenous ustekinumab maintenance following LOR to subcutaneous dosing. This is not unexpected given the established role of re-induction of ustekinumab[105,108-112] however further data is needed to support this switch from subcutaneous to intravenous ustekinumab. A switch from ustekinumab to risankizumab which isa selective inhibitor of the p40 subunit of IL-23 has shown potential in inducing early response in cases of treatment failure with ustekinumab as reported in recent case report[118].
Role of TDM: Despite some contrary findings regarding the association between trough levels and Crohn’s disease response (clinical or biochemical)[119-123], there is robust evidence to suggest that ustekinumab trough levels correlate with clinical, biomarker and/or endoscopic response in Crohn’s[124-139]. This was confirmed in meta-analysis which showed higher median ustekinumab trough concentrations occur in individuals who achieve clinical remission compared with those who do not achieve remission[140].
In UC, a single prospective study by Adedokun et al[129] evaluates ustekinumab levels in UC describing dose-proportional serum concentrations of ustekinumab and association of serum concentrations with clinical and histologic response as well as normalization of inflammation markers.
However, despite these findings the role of TDM to guide ustekinumab therapy is limited. The significant variations between studies in reported ustekinumab levels to achieve response in conjunction with the heterogeneity in methods of reporting ustekinumab levels do not currently permit a clear cutoff value for defining a response to therapy[140]. Furthermore, there is only sparse data evaluating ustekinumab levels following dose escalation[110,123,133,141] and endoscopic remission was associated with an increase in ustekinumab levels in only one of these observational studies[133]. Interestingly, Hanžel et al[133] reported that patients with ustekinumab concentrations < 3.5 mg/L following dose optimisation were unlikely to achieve endoscopic or biochemical remission. There is currently a lack of data regarding optimal drug levels and drug level response to dose escalation with ustekinumab and further clinical studies are required in order to guide treatment.
Predictors for failure to respond to dose escalation: Given the current lack of sufficient data to utilise ustekinumab levels to guide therapy, factors including patient and disease characteristics may potentially be used to identify patients at risk of mechanistic failure. Dalal et al[41] reported that perianal disease, pre-intensification Harvey-Bradshaw Index, current opioid use, and current corticosteroid use were associated with ustekinumab failure after dose intensification in Crohn’s disease. Heron et al[110] did not identify any predictors of clinical response or remission to ustekinumab reinduction and Cohen et al[99] described response to initial ustekinumab induction therapy as the only independent predictor of response to ustekinumab dose escalation. Therefore, while factor such as perianal disease and disease severity should be considered when considering dose-escalation of ustekinumab for LOR, there is insufficient evidence for these predictors to identify patients unlikely to respond to dose optimisation. Further research is required to establish predictors of response to ustekinumab dose escalation or reinduction as well as identifying a drug level that can reliably delineate patients with clinical remission. As a result, dose escalation or reinduction in patients with early nonresponse to ustekinumab therapy or following secondary LOR is a viable management option.
In recent times the JAK inhibitors have emerged as efficacious therapy in IBD[142-144]. Tofacitinib is an oral, small molecule JAK inhibitor which inhibits all JAKs but preferentially inhibits JAK1 and JAK3[145] and upadacitinib is an oral selective reversible inhibitor of JAK1[146]. The landmark OCTAVE and U-ACHIEVE/U-ACCOMPLISH trials established the efficacy of tofacitinib and upadacitinib respectively in induction and maintenance of remission in UC[142,143] and Loftus et al[144] established the efficacy of upadacitinib in induction and maintenance therapy in Crohn’s disease. Yet despite their efficacy, a significant portion of patients experience primary or secondary LOR with JAK Inhibitor therapy. There is a reported PNR rate of approximately 20% and LOR rate of 39% per person year in UC patients treated with tofacitinib[142,147]. With upadacitinib treatment there is a PNR rate of approximately 50% in Crohn’s and 65%-75% in UC[143,144] (Table 1).
Dose escalation of tofacitinib: Higher numerical rates of remission (total mayo score ≤ 2) in UC have been noted at 40.6% with 10 mg twice daily dosing of tofacitinib compared to 34.3% with 5 mg twice daily tofacitinib during maintenance therapy[142]. Furthermore, dose-escalation of tofacitinib from 5 mg twice daily to 10 mg twice daily whilst on maintenance therapy can be effective in recapturing response to tofacitinib in patients with UC[51-53,148,149]. In fact, the OCTAVE long-term extension study reported that dose escalation to 10 mg bowel disease (BD) following treatment failure with 5 mg BD tofacitinib recaptured clinical response in approximately 65% of patients and clinical remission in approximately 50% at 12 months of escalated therapy[148]. Similarly in a retrospective study of patients with UC, Honap et al[52] and Ma et al[51] described recapture of response with dose-escalation of tofacitinib to 10mg BD in approximately half of patients who had lost response. However, in a post hoc analysis which evaluated tofacitinib treatment persistence in this same group described discontinuation among the dose escalation group of approximately 49% with a median time to discontinuation of 4.4 years[53]. In the setting of PNR, extended induction therapy from 8 weeks to 16 weeks of tofacitinib 10 mg BD is able to capture clinical response in 52.2% of patients at week 16[150].
Dose escalation of upadacitinib: The U-ACHIEVE and U-ENDURE trials both reported higher rates of remission with 30 mg upadacitinib compared to 15 mg upadacitinib[143,144]. Furthermore, early data from the phase 2 CELEST study in Crohn’s disease reported that patients with inadequate response obtained clinical remission and endoscopic response with upadacitinib dose escalation[151]. Reassuringly, the long-term extension study of CELESTE described clinical remission at 30 months in 55% of patients dose escalated from 15 mg to 30 mg maintenance[152]. Panacionne et al also noted the efficacy of dosing escalation of upadacitinib to 30 mg daily for LOR or inadequate response with clinical remission following escalation in 30% of UC patients at 48 weeks[153]. Extended induction therapy for PNR from 8 weeks to 16 weeks of upadacitinib 45 mg daily is able to capture clinical response in 46.6% of patients at week 16[154].
Switch between JAK inhibitors: Furthermore, the addition of upadacitinib as a treatment option for UC permits within drug class switching following treatment failure of tofacitinib with PNR or LOR. Two small case series have reported clinical remission with upadacitinib in UC patients with PNR or LOR to tofacitinib[155,156]. Furthermore a prospective study of 26 patients with IBD reported that upadacitinib was effective in inducing clinical and biochemical remission following primary or secondary nonresponse to tofacitinib[157]. There are currently no published studies assessing the efficacy of switching from upadacitinib to tofacitinib following PNR or LOR.
Role of TDM: Given the recent integration of the JAK Inhibitors in IBD therapeutic armamentarium the role of drug monitoring with JAK-Inhibitors is unclear[158]. Early pharmacokinetic studies of tofacitinib in UC reported that while plasma tofacitinib concentrations increased proportionately with dose there was no difference in tofacitinib concentrations at baseline versus at the end of induction at week 8 and that tofacitinib concentrations did not differ with clinical remission at specific doses[159].
Further studies are needed to elucidate the role of TDM and the association between drug levels and clinical remission. Additional clinical research will also be essential to establish predictors for mechanistic failure with JAK-inhibitors to guide treatment decisions. However currently dose escalation of both tofacitinib and upadacitinib or in-class switching both represent potential methods of recapturing response.
Ozanimod and etrasimod are selective S1P receptor modulators of S1P1 and S1P5[160] and S1P1, S1P4 and S1P5[57] respectively. S1P receptor modulators have recently emerged as therapeutic options for induction and maintenance of remission in UC[57,161]. There are no studies evaluating management of LOR and secondary nonresponse with S1P receptor modulators.
As with TNF-inhibitor therapy, patients with suspected PNR and LOR to non-TNFi advanced therapy require detailed assessment to exclude other causes of symptoms and to assess disease activity[10,58].
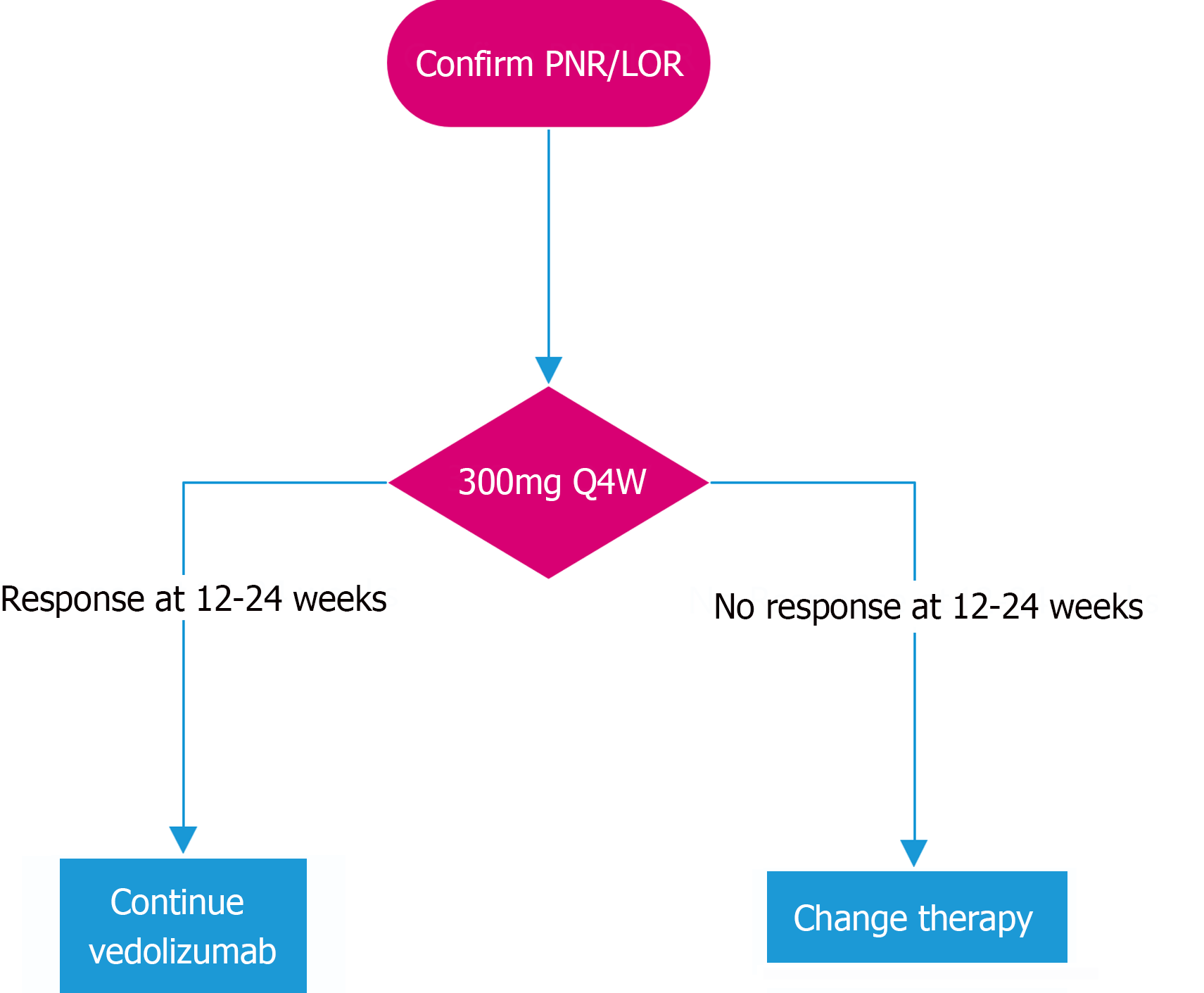
In PNR and LOR to vedolizumab therapy, it appears reasonable to increase frequency of vedolizumab dosing[23,68-77]. While increasing the frequency of vedolizumab 300 mg to 6 weekly and 4 weekly may both be effective strategies[68,72,76], we favour an increase to 4 weekly dosing, if available, in order to maximise likelihood of capturing response and minimise risk of prolonging futile therapy. The role for TDM prior to vedolizumab dose optimisation is not yet established but may be used depending on availability. There is significant heterogeneity in reported drug levels which correlate with clinical remission[75,86,87] and conflicting data regarding the utility of drug levels to predict clinical remission[88,89] and response to dose escalation[70,81]. We propose assessing for response to vedolizumab at approximately 12-24 weeks to allow adequate time for effect of vedolizumab dose escalation[68,70,72,74]. If clinical response is not captured by 24 weeks, we suggest switching therapy (Figure 1).
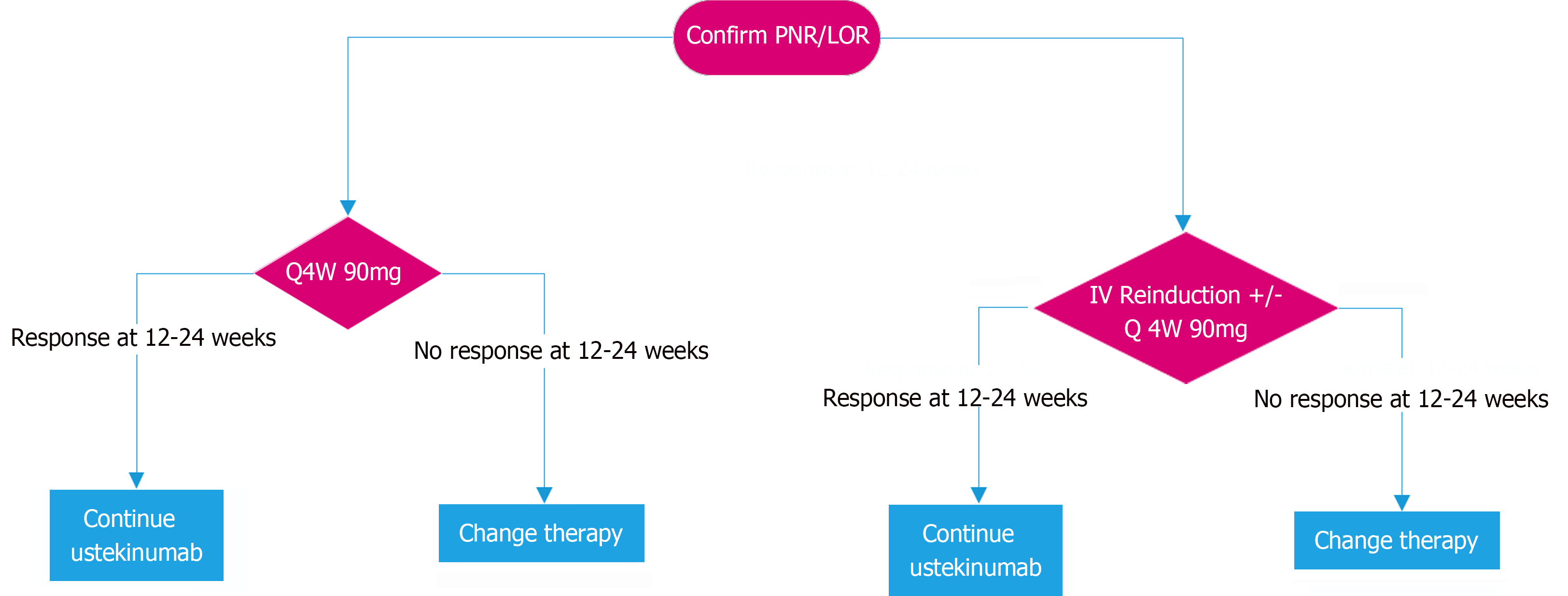
Once PNR/LOR is confirmed with ustekinumab therapy, options to capture response include increasing dose frequency, intravenous reinduction or a combination of both. While increasing the frequency of ustekinumab 90 mg to 4 weekly or 6 weekly is an effective method of capturing response in the setting of PNR/LOR[41,48,96,98,100-102,107], we favour an increase to 4 weekly dosing, if available, in order to maximise likelihood of capturing response with a view to consider reducing dose at a later stage if remission is achieved and sustained. Intravenous reinduction of ustekinumab is effective for recapturing response in LOR but not PNR[109-112] and consequently in the setting of PNR intravenous reinduction should be combined with increased frequency of ustekinumab[97,99,103-106,113,114]. While ustekinumab levels appear to be positively correlated with response[124-139] there are significant variations in the reporting of levels to achieve clinical remission, which make it more difficult to use levels to guide therapy[140]. Response to ustekinumab should be assessed at approximately 12-24 weeks to allow adequate time for the effect of dose escalation to be assessed[97,99,104-107,109-111,114]. If clinical response is not captured at 24 weeks, we suggest confirming LOR with objective measures and consideration for switching to an alternate therapy (Figure 2).
In the setting of PNR to tofacitinib extended induction to 16 weeks from 8 weeks may be an effective means of inducing clinical response[150]. Where patients have LOR with tofacitinib, dose optimisation to 10 mg BD is an effective means of recapturing response[51,52,148]. We suggest assessing for response can be performed as early as 8 weeks after treatment adjustment given the rapid onset of action of tofacitinib, although some people may take many months to respond as response rates continue to increase up until 12 months after dose escalation of tofacitinib[142,148]. If clinical response is captured at reassessment, we suggest continuing tofacitinib therapy otherwise we suggest utilisation of an alternate therapy.
In the setting of PNR to upadacitinib extended induction to 16 weeks from 8 weeks may be an effective means of inducing clinical response[154]. Following LOR with upadacitinib, dose optimisation to 30 mg daily effectively recaptures response in about[152,153]. An assessment for response can occur as early as 8 weeks, although some patients may take longer to respond and response rates continue to increase to 52 weeks of additional treatment at 30 mg daily, so consideration in the clinical context of whether a patient should continue treatment would be on a case by case basis[144]. We suggest continuing upadacitinib therapy if response is captured at the time of reassessment, otherwise an alternate therapy should be considered.
With significant shifts in the treatment paradigm of IBD over the last decade, there remains many unanswered questions regarding the optimal treatment algorithm with non-TNF-i advanced therapy. In this review we propose practical algorithms for the management of PNR and secondary LOR to non-TNF-i advanced therapy. Further clinical research and real-world experience is required to optimise these treatment pathways and to establish the role of TDM to better identify patients who will not respond to dose optimisation. This knowledge will help minimise risk of prolonging futile therapy with dose escalation while also ensuring advanced therapies are not prematurely discarded in the absence of evidence of irrecoverable non-response.
| 1. | State M, Negreanu L. Defining the Failure of Medical Therapy for Inflammatory Bowel Disease in the Era of Advanced Therapies: A Systematic Review. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Katsanos KH, Papamichael K, Feuerstein JD, Christodoulou DK, Cheifetz AS. Biological therapies in inflammatory bowel disease: Beyond anti-TNF therapies. Clin Immunol. 2019;206:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Wong U, Cross RK. Primary and secondary nonresponse to infliximab: mechanisms and countermeasures. Expert Opin Drug Metab Toxicol. 2017;13:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Attauabi M, Dahl EK, Burisch J, Gubatan J, Nielsen OH, Seidelin JB. Comparative onset of effect of biologics and small molecules in moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. EClinicalMedicine. 2023;57:101866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Vasudevan A, Gibson PR, van Langenberg DR. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? World J Gastroenterol. 2017;23:6385-6402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 8. | Sparrow MP, Papamichael K, Ward MG, Riviere P, Laharie D, Paul S, Roblin X. Therapeutic Drug Monitoring of Biologics During Induction to Prevent Primary Non-Response. J Crohns Colitis. 2020;14:542-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Gisbert JP, Chaparro M. Primary Failure to an Anti-TNF Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-TNF Agent) or Swap (for Another Mechanism of Action)? J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Wang LF, Chen PR, He SK, Duan SH, Zhang Y. Predictors and optimal management of tumor necrosis factor antagonist nonresponse in inflammatory bowel disease: A literature review. World J Gastroenterol. 2023;29:4481-4498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 11. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1722] [Article Influence: 246.0] [Reference Citation Analysis (0)] |
| 12. | Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 519] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 13. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1635] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 14. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1965] [Article Influence: 151.2] [Reference Citation Analysis (1)] |
| 15. | Amiot A, Grimaud JC, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R, Marteau P, Vaysse T, Bourrier A, Nancey S, Laharie D, Allez M, Savoye G, Moreau J, Gagniere C, Vuitton L, Viennot S, Aubourg A, Pelletier AL, Bouguen G, Abitbol V, Bouhnik Y; Observatory on Efficacy and of Vedolizumab in Patients With Inflammatory Bowel Disease Study Group; Groupe d'Etude Therapeutique des Affections Inflammatoires du tube Digestif. Effectiveness and Safety of Vedolizumab Induction Therapy for Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2016;14:1593-1601.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 16. | Amiot A, Serrero M, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R, Marteau P, Vaysse T, Bourrier A, Nancey S, Laharie D, Allez M, Savoye G, Moreau J, Vuitton L, Viennot S, Aubourg A, Pelletier AL, Bouguen G, Abitbol V, Gagniere C, Bouhnik Y; OBSERV-IBD study group and the GETAID. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2017;46:310-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, Whitehead D, Hudesman D, Lukin D, Swaminath A, Shmidt E, Wang S, Boland BS, Chang JT, Kane S, Siegel CA, Loftus EV, Sandborn WJ, Sands BE, Colombel JF. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn's Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 18. | Chaparro M, Garre A, Ricart E, Iborra M, Mesonero F, Vera I, Riestra S, García-Sánchez V, Luisa De Castro M, Martin-Cardona A, Aldeguer X, Mínguez M, de-Acosta MB, Rivero M, Muñoz F, Andreu M, Bargalló A, González-Muñoza C, Pérez Calle JL, García-Sepulcre MF, Bermejo F, Huguet JM, Cabriada JL, Gutiérrez A, Mañosa M, Villoria A, Carbajo AY, Lorente R, García-López S, Piqueras M, Hinojosa E, Arajol C, Sicilia B, Conesa AM, Sainz E, Almela P, Llaó J, Roncero O, Camo P, Taxonera C, Domselaar MV, Pajares R, Legido J, Madrigal R, Lucendo AJ, Alcaín G, Doménech E, Gisbert JP; GETECCU study group. Short and long-term effectiveness and safety of vedolizumab in inflammatory bowel disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2018;48:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Baumgart DC, Bokemeyer B, Drabik A, Stallmach A, Schreiber S; Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice--a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Kopylov U, Ron Y, Avni-Biron I, Koslowsky B, Waterman M, Daher S, Ungar B, Yanai H, Maharshak N, Ben-Bassat O, Lichtenstein L, Bar-Gil Shitrit A, Israeli E, Schwartz D, Zittan E, Eliakim R, Chowers Y, Ben-Horin S, Dotan I. Efficacy and Safety of Vedolizumab for Induction of Remission in Inflammatory Bowel Disease-the Israeli Real-World Experience. Inflamm Bowel Dis. 2017;23:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Bamias G, Kokkotis G, Gizis M, Kapizioni C, Karmiris K, Koureta E, Kyriakos N, Leonidakis G, Makris K, Markopoulos P, Michalopoulos G, Michopoulos S, Papaconstantinou I, Polymeros D, Siakavellas SI, Triantafyllou K, Tsironi E, Tsoukali E, Tzouvala M, Viazis N, Xourafas V, Zacharopoulou E, Zampeli E, Zografos K, Papatheodoridis G, Mantzaris G. Predictors of Response to Vedolizumab in Patients with Ulcerative Colitis: Results from the Greek VEDO-IBD Cohort. Dig Dis Sci. 2022;67:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Mühl L, Becker E, Müller TM, Atreya R, Atreya I, Neurath MF, Zundler S. Clinical experiences and predictors of success of treatment with vedolizumab in IBD patients: a cohort study. BMC Gastroenterol. 2021;21:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Perry C, Fischer K, Elmoursi A, Kern C, Currier A, Kudaravalli P, Akanbi O, Tripathi N, Yarra P, Su L, Flomenhoft D, Stromberg A, Barrett TA. Vedolizumab Dose Escalation Improves Therapeutic Response in a Subset of Patients with Ulcerative Colitis. Dig Dis Sci. 2021;66:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Shelton E, Allegretti JR, Stevens B, Lucci M, Khalili H, Nguyen DD, Sauk J, Giallourakis C, Garber J, Hamilton MJ, Tomczak M, Makrauer F, Burakoff RB, Levine J, de Silva P, Friedman S, Ananthakrishnan A, Korzenik JR, Yajnik V. Efficacy of Vedolizumab as Induction Therapy in Refractory IBD Patients: A Multicenter Cohort. Inflamm Bowel Dis. 2015;21:2879-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 25. | Eriksson C, Marsal J, Bergemalm D, Vigren L, Björk J, Eberhardson M, Karling P, Söderman C; SWIBREG Vedolizumab Study Group, Myrelid P, Cao Y, Sjöberg D, Thörn M, Karlén P, Hertervig E, Strid H, Ludvigsson JF, Almer S, Halfvarson J. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scand J Gastroenterol. 2017;52:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 26. | Shmidt E, Kochhar G, Hartke J, Chilukuri P, Meserve J, Chaudrey K, Koliani-Pace JL, Hirten R, Faleck D, Barocas M, Luo M, Lasch K, Boland BS, Singh S, Vande Casteele N, Sagi SV, Fischer M, Chang S, Bohm M, Lukin D, Sultan K, Swaminath A, Hudesman D, Gupta N, Kane S, Loftus EV Jr, Sandborn WJ, Siegel CA, Sands BE, Colombel JF, Shen B, Dulai PS. Predictors and Management of Loss of Response to Vedolizumab in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:2461-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Feagan BG, Rubin DT, Danese S, Vermeire S, Abhyankar B, Sankoh S, James A, Smyth M. Efficacy of Vedolizumab Induction and Maintenance Therapy in Patients With Ulcerative Colitis, Regardless of Prior Exposure to Tumor Necrosis Factor Antagonists. Clin Gastroenterol Hepatol. 2017;15:229-239.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 28. | Sands BE, Sandborn WJ, Van Assche G, Lukas M, Xu J, James A, Abhyankar B, Lasch K. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease in Patients Naïve to or Who Have Failed Tumor Necrosis Factor Antagonist Therapy. Inflamm Bowel Dis. 2017;23:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Narula N, Peerani F, Meserve J, Kochhar G, Chaudrey K, Hartke J, Chilukuri P, Koliani-Pace J, Winters A, Katta L, Shmidt E, Hirten R, Faleck D, Parikh MP, Whitehead D, Boland BS, Singh S, Sagi SV, Fischer M, Chang S, Barocas M, Luo M, Lasch K, Bohm M, Lukin D, Sultan K, Swaminath A, Hudesman D, Gupta N, Shen B, Kane S, Loftus EV, Siegel CA, Sands BE, Colombel JF, Sandborn WJ, Dulai PS. Vedolizumab for Ulcerative Colitis: Treatment Outcomes from the VICTORY Consortium. Am J Gastroenterol. 2018;113:1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Stallmach A, Langbein C, Atreya R, Bruns T, Dignass A, Ende K, Hampe J, Hartmann F, Neurath MF, Maul J, Preiss JC, Schmelz R, Siegmund B, Schulze H, Teich N, von Arnim U, Baumgart DC, Schmidt C. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease - a prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44:1199-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Dulai P, Meserve J, Hartke J, Chilukuri P, Chaudrey K, Koliani-pace J, Kochhar G, Parikh M, Shmidt E, Hirten R, Luo M, Barocas M, Lasch K, Sultan K, Swaminath A, Bohm M, Lukin D, Hudesman D, Shen B, Siegel C, Sands B, Colombel J, Kane S, Loftus E, Singh S, Sandborn W, Boland B. DOP023 Predictors of clinical and endoscopic response with vedolizumab for the treatment of moderately-severely active ulcerative colitis: results from the US VICTORY consortium. J Crohns Colitis. 2017;11:S40-41. [DOI] [Full Text] |
| 32. | Rosario M, French JL, Dirks NL, Sankoh S, Parikh A, Yang H, Danese S, Colombel JF, Smyth M, Sandborn WJ, Feagan BG, Reinisch W, Sands BE, Sans M, Fox I. Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn's Disease. J Crohns Colitis. 2017;11:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 33. | Kim J, Yoon H, Kim N, Lee KM, Jung SA, Choi CH, Kim ES, Jung Y, Eun CS, Kim TO, Kang SB, Kim YS, Seo GS, Lee CK, Im JP, Park SJ, Park DI, Ye BD. Clinical Outcomes and Response Predictors of Vedolizumab Induction Treatment for Korean Patients With Inflammatory Bowel Diseases Who Failed Anti-TNF Therapy: A KASID Prospective Multicenter Cohort Study. Inflamm Bowel Dis. 2021;27:1931-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Haga K, Shibuya T, Osada T, Sato S, Fukuo Y, Kobayashi O, Yamada T, Asaoka D, Ito K, Nomura K, Haraikawa M, Nomura O, Fukushima H, Murakami T, Ishikawa D, Hojo M, Nagahara A. Early Clinical Remission Is a Predictor of Long-Term Remission with the Use of Vedolizumab for Ulcerative Colitis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Ma C, Fedorak RN, Kaplan GG, Dieleman LA, Devlin SM, Stern N, Kroeker KI, Seow CH, Leung Y, Novak KL, Halloran BP, Huang VW, Wong K, Blustein PK, Ghosh S, Panaccione R. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn's disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther. 2017;45:1232-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (6)] |
| 36. | Scribano ML, Aratari A, Neri B, Bezzio C, Balestrieri P, Baccolini V, Falasco G, Camastra C, Pantanella P, Monterubbianesi R, Tullio A, Saibeni S, Papi C, Biancone L, Cosintino R, Faggiani R. Effectiveness of ustekinumab in patients with refractory Crohn's disease: a multicentre real-life study in Italy. Therap Adv Gastroenterol. 2022;15:17562848211072412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Ma C, Fedorak RN, Kaplan GG, Dieleman LA, Devlin SM, Stern N, Kroeker KI, Seow CH, Leung Y, Novak KL, Halloran BP, Huang VW, Wong K, Blustein PK, Ghosh S, Panaccione R. Long-term Maintenance of Clinical, Endoscopic, and Radiographic Response to Ustekinumab in Moderate-to-Severe Crohn's Disease: Real-world Experience from a Multicenter Cohort Study. Inflamm Bowel Dis. 2017;23:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Amiot A, Filippi J, Abitbol V, Cadiot G, Laharie D, Serrero M, Altwegg R, Bouhnik Y, Peyrin-Biroulet L, Gilletta C, Roblin X, Pineton de Chambrun G, Vuitton L, Bourrier A, Nancey S, Gornet JM, Nahon S, Bouguen G, Viennot S, Pariente B, Fumery M; UC-USK-GETAID Study Group. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. 2020;51:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Murate K, Maeda K, Nakamura M, Sugiyama D, Wada H, Yamamura T, Sawada T, Mizutani Y, Ishikawa T, Furukawa K, Ohno E, Honda T, Kawashima H, Miyahara R, Ishigami M, Nishikawa H, Fujishiro M. Endoscopic Activity and Serum TNF-α Level at Baseline Are Associated With Clinical Response to Ustekinumab in Crohn's Disease Patients. Inflamm Bowel Dis. 2020;26:1669-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Chaparro M, Baston-Rey I, Fernández-Salgado E, González García J, Ramos L, Diz-Lois Palomares MT, Argüelles-Arias F, Iglesias Flores E, Cabello M, Rubio Iturria S, Núñez Ortiz A, Charro M, Ginard D, Dueñas Sadornil C, Merino Ochoa O, Busquets D, Iyo E, Gutiérrez Casbas A, Ramírez de la Piscina P, Boscá-Watts MM, Arroyo M, García MJ, Hinojosa E, Gordillo J, Martínez Montiel P, Velayos Jiménez B, Quílez Ivorra C, Vázquez Morón JM, María Huguet J, González-Lama Y, Muñagorri Santos AI, Amo VM, Martín-Arranz MD, Bermejo F, Martínez Cadilla J, Rubín de Célix C, Fradejas Salazar P, San Román AL, Jiménez N, García López S, Figuerola A, Jiménez I, Martínez Cerezo FJ, Taxonera C, Varela P, de Francisco R, Monfort D, Molina Arriero G, Hernández Camba A, García-Alonso FJ, Van Domselaar M, Pajares Villarroya R, Núñez A, Rodríguez Moranta F, Marín-Jiménez I, Robles Alonso V, Martín Rodríguez MDM, Camo-Monterde P, García Tercero I, Navarro Llavat M, Arias García L, Hervías Cruz D, Sulleiro S, Novella C, Vispo E, Barreiro-de Acosta M, Gisbert JP. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn's Disease Patients: The SUSTAIN Study. Inflamm Bowel Dis. 2022;28:1725-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Dalal RS, Njie C, Marcus J, Gupta S, Allegretti JR. Predictors of Ustekinumab Failure in Crohn's Disease After Dose Intensification. Inflamm Bowel Dis. 2021;27:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Hong SJ, Krugliak Cleveland N, Akiyama S, Zullow S, Yi Y, Shaffer SR, Malter LB, Axelrad JE, Chang S, Hudesman DP, Rubin DT. Real-World Effectiveness and Safety of Ustekinumab for Ulcerative Colitis From 2 Tertiary IBD Centers in the United States. Crohns Colitis 360. 2021;3:otab002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Iborra M, Beltrán B, Fernández-Clotet A, Iglesias-Flores E, Navarro P, Rivero M, Gutiérrez A, Sierra-Ausin M, Mesonero F, Ferreiro-Iglesias R, Hinojosa J, Calvet X, Sicilia B, González-Muñoza C, Antolín B, González-Vivo M, Carbajo AY, García-López S, Martín-Cardona A, Surís G, Martin-Arranz MD, de Francisco R, Cañete F, Domènech E, Nos P; GETECCU group (Grupo Español de trabajo de Enfermedades de Crohn y Colitis Ulcerosa). Real-world long-term effectiveness of ustekinumab in Crohn's disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2020;52:1017-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Iborra M, Beltrán B, Fernández-Clotet A, Gutiérrez A, Antolín B, Huguet JM, De Francisco R, Merino O, Carpio D, García-López S, Mesonero F, Navarro P, Ferreiro-Iglesias R, Carbajo AY, Rivero M, Gisbert JP, Piñero-Pérez MC, Monfort D, Bujanda L, García-Sepulcre MF, Martín-Cardona A, Cañete F, Taxonera C, Domènech E, Nos P; GETECCU Group (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa). Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn's disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2019;50:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Chaparro M, Garre A, Iborra M, Sierra-Ausín M, Barreiro-de Acosta M, Fernández-Clotet A, de Castro L, Boscá-Watts M, Casanova MJ, López-García A, Lorente R, Rodríguez C, Carbajo AY, Arroyo MT, Gutiérrez A, Hinojosa J, Martínez-Pérez T, Villoria A, Bermejo F, Busquets D, Camps B, Cañete F, Manceñido N, Monfort D, Navarro-Llavat M, Pérez-Calle JL, Ramos L, Rivero M, Angueira T, Camo Monterde P, Carpio D, García-de-la-Filia I, González-Muñoza C, Hernández L, Huguet JM, Morales VJ, Sicilia B, Vega P, Vera I, Zabana Y, Nos P, Suárez Álvarez P, Calviño-Suárez C, Ricart E, Hernández V, Mínguez M, Márquez L, Hervías Cruz D, Rubio Iturria S, Barrio J, Gargallo-Puyuelo C, Francés R, Hinojosa E, Del Moral M, Calvet X, Algaba A, Aldeguer X, Guardiola J, Mañosa M, Pajares R, Piqueras M, García-Bosch O, López Serrano P, Castro B, Lucendo AJ, Montoro M, Castro Ortiz E, Mesonero F, García-Planella E, Fuentes DA, Bort I, Delgado-Guillena P, Arias L, Iglesias A, Calvo M, Esteve M, Domènech E, Gisbert JP. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-world Evidence from the ENEIDA Registry. J Crohns Colitis. 2021;15:1846-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Oh K, Hong HS, Ham NS, Lee J, Park SH, Yang SK, Yoon H, Kim YS, Choi CH, Ye BD; Korean Association for the Study of Intestinal Diseases. Real-world effectiveness and safety of ustekinumab induction therapy for Korean patients with Crohn's disease: a KASID prospective multicenter study. Intest Res. 2023;21:137-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 47. | Ando K, Fujiya M, Ueno N, Motoya S, Nasuno M, Tanaka H, Ito T, Maemoto A, Sakurai K, Katsurada T, Orii F, Ashida T, Hirayama D, Nakase H. EP1317: real-world clinical outcomes and predictive factors accounting for short- to medium-term effectiveness of ustekinumab in treating ulcerative colitis: A Japan-based study. Gastroenterology. 2022;162:S1099. [DOI] [Full Text] |
| 48. | Derikx LAAP, Plevris N, Su S, Gros B, Lyons M, Siakavellas SI, Constantine-Cooke N, Jenkinson P, O'Hare C, Noble C, Arnott ID, Jones GR, Lees CW. Rates, predictive factors and effectiveness of ustekinumab intensification to 4- or 6-weekly intervals in Crohn's disease. Dig Liver Dis. 2023;55:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Mukherjee A, Tsuchiwata S, Nicholas T, Cook JA, Modesto I, Su C, D'Haens GR, Sandborn WJ. Exposure-Response Characterization of Tofacitinib Efficacy in Moderate to Severe Ulcerative Colitis: Results From Phase II and Phase III Induction and Maintenance Studies. Clin Pharmacol Ther. 2022;112:90-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Sandborn WJ, Armuzzi A, Liguori G, Irving PM, Sharara AI, Mundayat R, Lawendy N, Woolcott JC, Danese S. Predictors of Sustained Response With Tofacitinib Therapy in Patients With Ulcerative Colitis. Inflamm Bowel Dis. 2022;28:1338-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Ma C, Panaccione R, Xiao Y, Khandelwal Y, Murthy SK, Wong ECL, Narula N, Tsai C, Peerani F, Reise-Filteau M, Bressler B, Starkey SY, Loomes D, Sedano R, Jairath V, Bessissow T; Canadian IBD Research Consortium. REMIT-UC: Real-World Effectiveness and Safety of Tofacitinib for Moderate-to-Severely Active Ulcerative Colitis: A Canadian IBD Research Consortium Multicenter National Cohort Study. Am J Gastroenterol. 2023;118:861-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 52. | Honap S, Chee D, Chapman TP, Patel M, Kent AJ, Ray S, Sharma E, Kennedy J, Cripps S, Walsh A, Goodhand JR, Ahmad T, Satsangi J, Irving PM, Kennedy NA; LEO [London, Exeter, Oxford] IBD Research Consortium. Real-world Effectiveness of Tofacitinib for Moderate to Severe Ulcerative Colitis: A Multicentre UK Experience. J Crohns Colitis. 2020;14:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 53. | Panaccione R, Abreu MT, Lazariciu I, Mundayat R, Lawendy N, Salese L, Woolcott JC, Sands BE, Chaparro M. Persistence of treatment in patients with ulcerative colitis who responded to tofacitinib therapy: data from the open-label, long-term extension study, OCTAVE open. Aliment Pharmacol Ther. 2022;55:1534-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Biemans VBC, Sleutjes JAM, de Vries AC, Bodelier AGL, Dijkstra G, Oldenburg B, Löwenberg M, van Bodegraven AA, van der Meulen-de Jong AE, de Boer NKH, Srivastava N, West RL, Römkens TEH, Horjus Talabur Horje CS, Jansen JM, van der Woude CJ, Hoekstra J, Weersma RK, van Schaik FDM, Hoentjen F, Pierik MJ; Dutch Initiative on Crohn and Colitis (ICC). Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51:880-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 55. | Sands B, Pondel M, Silver M, Petersen A, Wolf D, Panaccione R, Loftus E Jr, Colombel JF, Sturm A, D'Haens G. P031 Impact of Prior Biologic Exposure on Response to Ozanimod for Moderate-to-Severe Ulcerative Colitis in the Phase 3 True North Study. Am J Gastroenterol. 2021;116:S8. [PubMed] [DOI] [Full Text] |
| 56. | Colombel JF, Rubin DT, Vermeire S, Jain A, Canavan JB, Wu H, Lawlor G, Osterman MT, Dignass A, Regueiro R. P609 Impact of prior biologic exposure on durability of recaptured response to ozanimod during the True North open-label extension study. J Crohns Colitis. 2023;17:i738-739. [DOI] [Full Text] |
| 57. | Sandborn WJ, Vermeire S, Peyrin-Biroulet L, Dubinsky MC, Panes J, Yarur A, Ritter T, Baert F, Schreiber S, Sloan S, Cataldi F, Shan K, Rabbat CJ, Chiorean M, Wolf DC, Sands BE, D'Haens G, Danese S, Goetsch M, Feagan BG. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet. 2023;401:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 234] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 58. | Marsal J, Barreiro-de Acosta M, Blumenstein I, Cappello M, Bazin T, Sebastian S. Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front Med (Lausanne). 2022;9:897936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 59. | Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, Van der Woude J, Baert F, Eliakim R, Katsanos K, Brynskov J, Steinwurz F, Danese S, Vermeire S, Teillaud JL, Lémann M, Chowers Y. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 60. | van der Have M, Oldenburg B, Kaptein AA, Jansen JM, Scheffer RC, van Tuyl BA, van der Meulen-de Jong AE, Pierik M, Siersema PD, van Oijen MG, Fidder HH. Non-adherence to Anti-TNF Therapy is Associated with Illness Perceptions and Clinical Outcomes in Outpatients with Inflammatory Bowel Disease: Results from a Prospective Multicentre Study. J Crohns Colitis. 2016;10:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Rentsch C, Headon B, Ward MG, Gibson PR. Inadequate storage of subcutaneous biological agents by patients with inflammatory bowel disease: Another factor driving loss of response? J Gastroenterol Hepatol. 2018;33:10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 471] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 63. | Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 64. | Peyrin-Biroulet L, Danese S, Argollo M, Pouillon L, Peppas S, Gonzalez-Lorenzo M, Lytras T, Bonovas S. Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients With Crohn's Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:838-846.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 65. | Panaccione R, Lee WJ, Clark R, Kligys K, Campden RI, Grieve S, Raine T. Dose Escalation Patterns of Advanced Therapies in Crohn's Disease and Ulcerative Colitis: A Systematic Literature Review. Adv Ther. 2023;40:2051-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Loftus EV Jr, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis. 2017;11:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 67. | Vermeire S, Loftus EV Jr, Colombel JF, Feagan BG, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Crohn's Disease. J Crohns Colitis. 2017;11:412-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Vaughn BP, Yarur AJ, Graziano E, Campbell JP, Bhattacharya A, Lee JY, Gheysens K, Papamichael K, Osterman MT, Cheifetz AS, Cross RK. Vedolizumab Serum Trough Concentrations and Response to Dose Escalation in Inflammatory Bowel Disease. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Gouynou C, Pouillon L, Rousseau H, Zallot C, Baumann C, Peyrin-Biroulet L. Early changes in the pharmacokinetic profile of vedolizumab-treated patients with IBD may predict response after dose optimisation. Gut. 2019;68:178-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Outtier A, Wauters L, Rahier J, Bossuyt P, Colard A, Franchimont D, Lambrecht G, Macken E, Van Moerkercke W, Baert F, Humblet E, Van Hootegem P, Gils A, Ferrante M, Vermeire S, the Belgian IBD Research; Development Group. Effect of vedolizumab dose intensification on serum drug concentrations and regain of response in inflammatory bowel disease patients with secondary loss of response. GastroHep. 2021;3:63-71. [DOI] [Full Text] |
| 71. | Kolehmainen S, Ylisaukko-Oja T, Jokelainen J, Koivusalo M, Jokiranta TS, Sipponen T. Benefit of measuring vedolizumab concentrations in inflammatory bowel disease patients in a real-world setting. Scand J Gastroenterol. 2021;56:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Christensen B, Colman RJ, Micic D, Gibson PR, Goeppinger SR, Yarur A, Weber CR, Cohen RD, Rubin DT. Vedolizumab as Induction and Maintenance for Inflammatory Bowel Disease: 12-month Effectiveness and Safety. Inflamm Bowel Dis. 2018;24:849-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Kopylov U, Avni-Biron I, Ron Y, Koslowsky B, Waterman M, Daher S, Ungar B, Schwartz D, Zittan E, Openhaim M, Yanai H, Maharshak N, Bar Gil Shitrit A, Naftali T, Eliakim R, Chowers Y, Ben-Horin S, Dotan I. Effectiveness and safety of vedolizumab for maintenance treatment in inflammatory bowel disease-The Israeli real world experience. Dig Liver Dis. 2019;51:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Dreesen E, Verstockt B, Bian S, de Bruyn M, Compernolle G, Tops S, Noman M, Van Assche G, Ferrante M, Gils A, Vermeire S. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2018;16:1937-1946.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 75. | Williet N, Boschetti G, Fovet M, Di Bernado T, Claudez P, Del Tedesco E, Jarlot C, Rinaldi L, Berger A, Phelip JM, Flourie B, Nancey S, Paul S, Roblin X. Association Between Low Trough Levels of Vedolizumab During Induction Therapy for Inflammatory Bowel Diseases and Need for Additional Doses Within 6 Months. Clin Gastroenterol Hepatol. 2017;15:1750-1757.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 76. | Attauabi M, Vind I, Pedersen G, Bendtsen F, Seidelin JB, Burisch J. Short and long-term effectiveness and safety of vedolizumab in treatment-refractory patients with ulcerative colitis and Crohn's disease - a real-world two-center cohort study. Eur J Gastroenterol Hepatol. 2021;33:e709-e718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Jairath V, Yarur A, Osterman MT, James A, Balma D, Mehrotra S, Yang L, Yajnik V, Qasim Khan RM. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024;22:1077-1086.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Hanžel J, Sever N, Ferkolj I, Štabuc B, Smrekar N, Kurent T, Koželj M, Novak G, Compernolle G, Tops S, Gils A, Drobne D. Early vedolizumab trough levels predict combined endoscopic and clinical remission in inflammatory bowel disease. United European Gastroenterol J. 2019;7:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Pouillon L, Rousseau H, Busby-Venner H, De Carvalho Bittencourt M, Choukour M, Gauchotte G, Zallot C, Danese S, Baumann C, Peyrin-Biroulet L. Vedolizumab Trough Levels and Histological Healing During Maintenance Therapy in Ulcerative Colitis. J Crohns Colitis. 2019;13:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | Ungaro RC, Yarur A, Jossen J, Phan BL, Chefitz E, Sehgal P, Kamal K, Bruss A, Beniwal-Patel P, Fox C, Patel A, Bahur B, Jain A, Stein D, Naik S, Dubinsky MC. Higher Trough Vedolizumab Concentrations During Maintenance Therapy are Associated With Corticosteroid-Free Remission in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 81. | Ungar B, Malickova K, Hanžel J, Abu Arisha M, Paul S, Rocha C, Ben Shatach Z, Abitbol CM, Haj Natour O, Selinger L, Yavzori M, Fudim E, Picard O, Shoval I, Eliakim R, Kopylov U, Magro F, Roblin X, Chowers Y, Drobne D, Lukas M, Ben Horin S. Dose optimisation for Loss of Response to Vedolizumab- Pharmacokinetics and Immune Mechanisms. J Crohns Colitis. 2021;15:1707-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 82. | Osterman MT, Rosario M, Lasch K, Barocas M, Wilbur JD, Dirks NL, Gastonguay MR. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther. 2019;49:408-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 83. | Guidi L, Pugliese D, Panici Tonucci T, Bertani L, Costa F, Privitera G, Tolusso B, Di Mario C, Albano E, Tapete G, Gremese E, Papa A, Gasbarrini A, Rapaccini GL, Armuzzi A. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol J. 2019;7:1189-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Anbarserry D, Mosli M, Qari Y, Saadah O, Bokhary R, Esmat A, Alsieni M, Shaker A, Elango R, Alharthi S. The use of therapeutic drug monitoring for early identification of vedolizumab response in Saudi Arabian patients with inflammatory bowel disease. Sci Rep. 2023;13:1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 85. | Yacoub W, Williet N, Pouillon L, Di-Bernado T, De Carvalho Bittencourt M, Nancey S, Lopez A, Paul S, Zallot C, Roblin X, Peyrin-Biroulet L. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 86. | Singh S, Dulai PS, Vande Casteele N, Battat R, Fumery M, Boland BS, Sandborn WJ. Systematic review with meta-analysis: association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2019;50:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 87. | Cao WT, Huang R, Jiang KF, Qiao XH, Wang JJ, Fan YH, Xu Y. Predictive value of blood concentration of biologics on endoscopic inactivity in inflammatory bowel disease: A systematic review. World J Gastroenterol. 2021;27:886-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 88. | Plevris N, Jenkinson PW, Chuah CS, Lyons M, Merchant LM, Pattenden RJ, Arnott ID, Jones GR, Lees CW. Association of trough vedolizumab levels with clinical, biological and endoscopic outcomes during maintenance therapy in inflammatory bowel disease. Frontline Gastroenterol. 2020;11:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Sivridaş M, Creemers RH, Wong DR, Boekema PJ, Römkens TEH, Gilissen LPL, van Bodegraven AA, Loeff FC, Rispens T, Derijks LJJ. Therapeutic Drug Monitoring of Vedolizumab in Inflammatory Bowel Disease Patients during Maintenance Treatment-TUMMY Study. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 90. | Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P; UNITI–IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1445] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 91. | Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, Li K, Peyrin-Biroulet L, Van Assche G, Danese S, Targan S, Abreu MT, Hisamatsu T, Szapary P, Marano C; UNIFI Study Group. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019;381:1201-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 915] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 92. | Rubín de Célix C, Chaparro M, Gisbert JP. Real-World Evidence of the Effectiveness and Safety of Ustekinumab for the Treatment of Crohn's Disease: Systematic Review and Meta-Analysis of Observational Studies. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 93. | Taxonera C, Olivares D, López-García ON, Alba C. Meta-analysis: Real-world effectiveness and safety of ustekinumab in patients with ulcerative colitis. Aliment Pharmacol Ther. 2023;57:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 94. | Yang H, Li B, Guo Q, Tang J, Peng B, Ding N, Li M, Yang Q, Huang Z, Diao N, Zhu X, Deng J, Guo H, Hu P, Chao K, Gao X. Systematic review with meta-analysis: loss of response and requirement of ustekinumab dose escalation in inflammatory bowel diseases. Aliment Pharmacol Ther. 2022;55:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Zhdanava M, Zhao R, Manceur AM, Kachroo S, Lefebvre P, Pilon D. Persistence and Dose Escalation During Maintenance Phase and Use of Nonbiologic Medications Among Patients With Ulcerative Colitis Initiated on Ustekinumab in the United States. Crohns Colitis 360. 2023;5:otad045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 96. | Dalal RS, Pruce JC, Allegretti JR. Long-Term Outcomes After Ustekinumab Dose Intensification for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2023;29:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 97. | Young A, Tsistrakis S, Rubinov J, Kohan E, Hirten R, Ungaro R, Cohen B, Cho J, Sands BE, Colombel J, Oikonomou I. Ustekinumab Dose Intensification Can Be Effective in Crohnʼs Disease Patients Not Responding to Induction. Am J Gastroenterol. 2018;113:S351-352. [DOI] [Full Text] |
| 98. | Bundschuh M, Mcdonough G, Kumar P, Kozuch P, Shivashankar R. 777 Effect of Ustekinumab Dose Escalation on Recapturing Clinical Response in Patients With Crohn's Disease. Am J Gastroenterol. 2019;114:S452-452. [DOI] [Full Text] |
| 99. | Cohen E, Ha C, Syal G. 656 Ustekinumab Dose Escalation Is Effective in Treatment of Crohn's Disease. Am J Gastroenterol. 2019;114:S384-385. [DOI] [Full Text] |
| 100. | Haider SA, Yadav A, Perry C, Su L, Akanbi O, Kudaravalli P, Tripathi N, Hashim MA, Abdelsalam M, Hussein M, Elkheshen A, Patel V, Ali SE, Lamb L, Ingram K, Mayne C, Stuffelbeam AB, Flomenhoft D, Stromberg A, Barrett TA. Ustekinumab dose escalation improves clinical responses in refractory Crohn's disease. Therap Adv Gastroenterol. 2020;13:1756284820959245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Fumery M, Peyrin-Biroulet L, Nancey S, Altwegg R, Gilletta C, Veyrard P, Bouguen G, Viennot S, Poullenot F, Filippi J, Buisson A, Bozon A, Brazier F, Pouillon L, Flourie B, Boivineau L, Siproudhis L, Laharie D, Roblin X, Diouf M, Treton X. Effectiveness And Safety Of Ustekinumab Intensification At 90 Mg Every Four Weeks In Crohn's Disease: A Multicenter Study. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 102. | Ollech JE, Normatov I, Peleg N, Wang J, Patel SA, Rai V, Yi Y, Singer J, Dalal SR, Sakuraba A, Cohen RD, Rubin DT, Pekow J. Effectiveness of Ustekinumab Dose Escalation in Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2021;19:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 103. | Johnson AM, Barsky M, Ahmed W, Zullow S, Galati J, Jairath V, Narula N, Peerani F, Click BH, Coburn ES, Dang TT, Gold S, Agrawal M, Garg R, Aggarwal M, Mohammad D, Halloran B, Kochhar GS, Todorowski H, Ud Din NM, Izanec J, Teeple A, Gasink C, Muser E, Ding Z, Swaminath A, Lakhani K, Hogan D, Datta S, Ungaro RC, Boland BS, Bohm M, Fischer M, Sagi S, Afzali A, Ullman T, Lawlor G, Baumgart DC, Chang S, Hudesman D, Lukin D, Scherl EJ, Colombel JF, Sands BE, Siegel CA, Regueiro M, Sandborn WJ, Bruining D, Kane S, Loftus EV Jr, Dulai PS. The Real-World Effectiveness and Safety of Ustekinumab in the Treatment of Crohn's Disease: Results From the SUCCESS Consortium. Am J Gastroenterol. 2023;118:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 104. | Olmedo Martín RV, Vázquez Morón JM, Martín Rodríguez MDM, Lázaro Sáez M, Hernández Martínez Á, Argüelles-Arias F. Effectiveness and safety of ustekinumab dose escalation in Crohn's disease: a multicenter observational study. Rev Esp Enferm Dig. 2023;115:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Kopylov U, Hanzel J, Liefferinckx C, De Marco D, Imperatore N, Plevris N, Baston-Rey I, Harris RJ, Truyens M, Domislovic V, Vavricka S, Biemans V, Myers S, Sebastian S, Ben-Horin S, González Lama Y, Gilletta C, Ariella BS, Zelinkova Z, Weisshof R, Storan D, Zittan E, Farkas K, Molnar T, Franchimont D, Cremer A, Afif W, Castiglione F, Lees C, Barreiro-de Acosta M, Lobaton T, Doherty G, Krznaric Z, Pierik M, Hoentjen F, Drobne D. Effectiveness of ustekinumab dose escalation in Crohn's disease patients with insufficient response to standard-dose subcutaneous maintenance therapy. Aliment Pharmacol Ther. 2020;52:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 106. | Ramaswamy PK, Moattar H, Sawyer EA, James R, Edwards J, Shukla D. Mo1869 Efficacy of dose intensification of ustekinumab in crohn's disease. Gastroenterology. 2020;158:S956. [DOI] [Full Text] |
| 107. | Rowbotham DS, Scherl EJ, Sands BE, Panaccione R, Peyrin-biroulet L, Zhang H, Miao Y, Leong RW, Afif W, Arasaradnam RP, Danese S, Marano C. P508 Dose adjustment in patients with moderate to severe ulcerative colitis: results from year 3 of the UNIFI maintenance study long-term extension. J Crohns Colitis. 2021;15:S488-489. [DOI] [Full Text] |
| 108. | Bennett A, Evers Carlini L, Duley C, Garrett A, Annis K, Wagnon J, Dalal R, Scoville E, Beaulieu D, Schwartz D, Horst S. A Single Center Experience With Long-Term Ustekinumab Use and Reinduction in Patients With Refractory Crohn Disease. Crohns Colitis 360. 2020;2:otaa013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Sedano R, Guizzetti L, McDonald C, Jairath V. Intravenous Ustekinumab Reinduction Is Effective in Prior Biologic Failure Crohn's Disease Patients Already on Every-4-Week Dosing. Clin Gastroenterol Hepatol. 2021;19:1497-1498.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Heron V, Li Fraine S, Panaccione N, Restellini S, Germain P, Candido K, Bernstein CN, Bessissow T, Bitton A, Chauhan UK, Lakatos PL, Marshall JK, Michetti P, Seow CH, Rosenfeld G, Panaccione R, Afif W. Efficacy of Intravenous Ustekinumab Reinduction in Patients With Crohn's Disease With a Loss of Response. J Can Assoc Gastroenterol. 2022;5:208-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Bermejo F, Jiménez L, Algaba A, Vela M, Bastida G, Merino O, López-García A, Melcarne L, Rodríguez-Lago I, de la Maza S, Bouhmidi A, Barreiro-de Acosta M, López-Serrano P, Carrillo-Palau M, Mesonero F, Orts B, Bonillo D, Granja A, Guerra I. Re-induction With Intravenous Ustekinumab in Patients With Crohn's Disease and a Loss of Response to This Therapy. Inflamm Bowel Dis. 2022;28:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 112. | Ten Bokkel Huinink S, Biemans V, Duijvestein M, Pierik M, Hoentjen F, West RL, van der Woude CJ, de Vries AC. Re-induction with intravenous Ustekinumab after secondary loss of response is a valid optimization strategy in Crohn's disease. Eur J Gastroenterol Hepatol. 2021;33:e783-e788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Hudson J, Herfarth HH, Barnes EL. Letter: optimising response to ustekinumab therapy for patients with Crohn's disease. Aliment Pharmacol Ther. 2020;52:906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Yao J, Peng X, Zhong Y, Su T, Bihi A, Zhao J, Liu T, Wang W, Hu P, Zhang M, Zhi M. Extra intravenous Ustekinumab reinduction is an effective optimization strategy for patients with refractory Crohn's disease. Front Med (Lausanne). 2023;10:1105981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 115. | Ren H, Kang J, Wang J, Su J, Zou L, Yin A, Li J, Zhou Q, Wang W, Tang Z, Zhang J, Lu Y, Yang Y, Qiu C, Ding Y, Dong W, An P. Efficacy of Ustekinumab Optimization by 2 Initial Intravenous Doses in Adult Patients With Severe Crohn's Disease. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 116. | Meserve J, Ma C, Dulai PS, Jairath V, Singh S. Effectiveness of Reinduction and/or Dose Escalation of Ustekinumab in Crohn's Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2728-2740.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 117. | Argüelles-Arias F, Valdés Delgado T, Maldonado Pérez B, González Antuña J, Castro Laria L. Intravenous ustekinumab maintenance treatment in patients with loss of response to subcutaneous dosing. Therap Adv Gastroenterol. 2023;16:17562848231191670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 118. | Ziv Y, Swaminath A, Sultan K. Patient With Ulcerative Colitis and Failure of Ustekinumab Responds to Risankizumab: Similar Is Not Identical. Inflamm Bowel Dis. 2022;28:e140-e141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Kolar M, Pudilova K, Bortlik M, Duricova D, Malickova K, Lukas M, Hruba V, Machkova N, Vanickova R, Mitrova K, Vasatko M. P641 Ustekinumab efficiency as a higher-line therapy in association with serum levels in patients with Crohn’s disease. J Crohns Colitis. 2019;13:S438-439. [DOI] [Full Text] |
| 120. | Mechie NC, Burmester M, Mavropoulou E, Pilavakis Y, Kunsch S, Ellenrieder V, Amanzada A. Evaluation of ustekinumab trough levels during induction and maintenance therapy with regard to disease activity status in difficult to treat Crohn disease patients. Medicine (Baltimore). 2021;100:e25111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Afif W, Sattin B, Dajnowiec D, Khanna R, Seow CH, Williamson M, Karra K, Wang Y, Gao LL, Bressler B. Ustekinumab Therapeutic Drug Monitoring-Impact on Clinical Practice: A Multicenter Cross-Sectional Observational Trial. Dig Dis Sci. 2022;67:3148-3157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 122. | Pan Y, Ahmed W, Mahtani P, Wong R, Longman R, Jeremy Lukin D, Scherl EJ, Battat R. Utility of Therapeutic Drug Monitoring for Tumor Necrosis Factor Antagonists and Ustekinumab in Postoperative Crohn's Disease. Inflamm Bowel Dis. 2022;28:1865-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Nguyen KM, Mattoo VY, Vogrin S, Basnayake C, Connell WR, Ding NS, Flanagan E, Kamm MA, Lust M, Niewiadomski O, Schulberg JD, Wright EK. Relationship Between Serum Ustekinumab Trough Concentration and Clinical and Biochemical Disease Activity: A Real-World Study in Adult Patients with Crohn's Disease. Eur J Drug Metab Pharmacokinet. 2023;48:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 124. | Adedokun OJ, Xu Z, Gasink C, Jacobstein D, Szapary P, Johanns J, Gao LL, Davis HM, Hanauer SB, Feagan BG, Ghosh S, Sandborn WJ. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients With Crohn's Disease. Gastroenterology. 2018;154:1660-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 125. | Glassner K, Irani M, Malaty H, Abraham BP. P051 Real world experience in therapeutic drug monitoring in inflammatory bowel disease patients on ustekinumab. Gastroenterology. 2019;156:S36. [DOI] [Full Text] |
| 126. | Soufflet N, Boschetti G, Roblin X, Cuercq C, Williet N, Charlois AL, Duclaux-Loras R, Danion P, Mialon A, Faure M, Paul S, Flourie B, Nancey S. Concentrations of Ustekinumab During Induction Therapy Associate With Remission in Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2019;17:2610-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 127. | Battat R, Kopylov U, Bessissow T, Bitton A, Cohen A, Jain A, Martel M, Seidman E, Afif W. Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2017;15:1427-1434.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 128. | Verstockt B, Dreesen E, Noman M, Outtier A, Van den Berghe N, Aerden I, Compernolle G, Van Assche G, Gils A, Vermeire S, Ferrante M. Ustekinumab Exposure-outcome Analysis in Crohn's Disease Only in Part Explains Limited Endoscopic Remission Rates. J Crohns Colitis. 2019;13:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 129. | Adedokun OJ, Xu Z, Marano C, O'Brien C, Szapary P, Zhang H, Johanns J, Leong RW, Hisamatsu T, Van Assche G, Danese S, Abreu MT, Sands BE, Sandborn WJ. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:2244-2255.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 130. | Painchart C, Brabant S, Duveau N, Nachury M, Desreumaux P, Branche J, Gérard R, Prevost CLD, Wils P, Lambin T, Boualit M, Labalette M, Pariente B. Ustekinumab Serum Trough Levels May Identify Suboptimal Responders to Ustekinumab in Crohn's Disease. Dig Dis Sci. 2020;65:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 131. | Thomann AK, Schulte LA, Globig AM, Hoffmann P, Klag T, Itzel T, Teufel A, Schreiner R, Scheffe N, Ebert MP, Wehkamp J, Gauss A, Hasselblatt P, Klaus J, Reindl W. Ustekinumab serum concentrations are associated with clinical outcomes in Crohn's disease - a regional multi-center pilot study. Z Gastroenterol. 2020;58:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Gómez Espín R, Nicolás De Prado I, Gil Candel M, González Carrión M, Rentero Redondo L, Iniesta Navalón C. Association between ustekinumab trough concentrations and biochemical outcomes in patients with Crohn's disease. A real life study. Rev Esp Enferm Dig. 2021;113:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 133. | Hanžel J, Koželj M, Špes Hlastec A, Kurent T, Sever N, Zdovc J, Smrekar N, Novak G, Štabuc B, Grabnar I, Drobne D. Ustekinumab concentrations shortly after escalation to monthly dosing may identify endoscopic remission in refractory Crohn's disease. Eur J Gastroenterol Hepatol. 2021;33:e831-e836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Walshe M, Borowski K, Boland K, Rho S, Stempak JM, Silverberg MS. Ustekinumab induction concentrations are associated with clinical and biochemical outcomes at week 12 of treatment in Crohn's disease. Eur J Gastroenterol Hepatol. 2021;33:e401-e406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 135. | Yao JY, Zhang M, Wang W, Peng X, Zhao JZ, Liu T, Li ZW, Sun HT, Hu P, Zhi M. Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn's disease: a Chinese real-world study. BMC Gastroenterol. 2021;21:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 136. | Hirayama H, Morita Y, Imai T, Takahashi K, Yoshida A, Bamba S, Inatomi O, Andoh A. Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn's disease. BMC Gastroenterol. 2022;22:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 137. | Lao Domínguez FÁ, Fobelo Lozano MJ, Gutiérrez Pizarraya A, Castro Fernández M. Relationship between ustekinumab trough concentrations and both clinical and biological remission in patients with Crohn's disease. Gastroenterol Hepatol. 2023;46:631-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 138. | McDonald C, Kerr H, Gibbons E, Lukose T, Cheriyan D, Harewood G, Patchett S, O'Toole A, Kelly O, Boland K. Higher Ustekinumab Levels in Maintenance Therapy are Associated with Greater Mucosal Healing and Mucosal Response in Crohn's Disease: An Experience of 2 IBD Centers. Inflamm Bowel Dis. 2024;30:423-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 139. | Alsoud D, De Hertogh G, Compernolle G, Tops S, Sabino J, Ferrante M, Thomas D, Vermeire S, Verstockt B. Real-world Endoscopic and Histological Outcomes Are Correlated with Ustekinumab Exposure in Patients with Ulcerative Colitis. J Crohns Colitis. 2022;16:1562-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 140. | Vasudevan A, Tharayil V, Raffals LH, Bruining DH, Becker M, Murad MH, Loftus EV Jr. Systematic Review and Meta-analysis: The Association Between Serum Ustekinumab Trough Concentrations and Treatment Response in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2024;30:660-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 141. | Heron V, Bessissow T, Bitton A, Lakatos P, Seidman E, Jain A, Battat R, Germain P, Lemieux C, Afif W. P533 Ustekinumab therapeutic drug monitoring in Crohn’s disease patients with loss of response. J Crohns Colitis. 2019;13:S379-380. [DOI] [Full Text] |
| 142. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1296] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 143. | Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 407] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 144. | Loftus EV Jr, Panés J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R, Reinisch W, Louis E, Chen M, Nakase H, Begun J, Boland BS, Phillips C, Mohamed MF, Liu J, Geng Z, Feng T, Dubcenco E, Colombel JF. Upadacitinib Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2023;388:1966-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 296] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 145. | Danese S, Grisham M, Hodge J, Telliez JB. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155-G162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 146. | De Vries LCS, Wildenberg ME, De Jonge WJ, D'Haens GR. The Future of Janus Kinase Inhibitors in Inflammatory Bowel Disease. J Crohns Colitis. 2017;11:885-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 147. | Taneja V, Systrom H, Walradt T, El-dallal M, Feuerstein JD. S0857 Loss of Response to Tofacitinib in Ulcerative Colitis Maintenance: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2020;115:S442-443. [DOI] [Full Text] |
| 148. | Sands BE, Armuzzi A, Marshall JK, Lindsay JO, Sandborn WJ, Danese S, Panés J, Bressler B, Colombel JF, Lawendy N, Maller E, Zhang H, Chan G, Salese L, Tsilkos K, Marren A, Su C. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51:271-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 149. | Sandborn WJ, Peyrin-Biroulet L, Sharara AI, Su C, Modesto I, Mundayat R, Gunay LM, Salese L, Sands BE. Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status. Clin Gastroenterol Hepatol. 2022;20:591-601.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 150. | Sandborn WJ, Peyrin-Biroulet L, Quirk D, Wang W, Nduaka CI, Mukherjee A, Su C, Sands BE. Efficacy and Safety of Extended Induction With Tofacitinib for the Treatment of Ulcerative Colitis. Clin Gastroenterol Hepatol. 2022;20:1821-1830.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 151. | Sandborn W, Panés J, Peyrin-biroulet L, Louis E, Greenbloom S, Zhou W, Zhou Q, Liu J, Lacerda AP. DOP79 Dose escalation of upadacitinib in Crohn’s disease patients with an inadequate response: Data from the randomised CELEST study. J Crohns Colitis. 2020;14:S117-118. [DOI] [Full Text] |
| 152. | D'Haens G, Panés J, Louis E, Lacerda A, Zhou Q, Liu J, Loftus EV Jr. Upadacitinib Was Efficacious and Well-tolerated Over 30 Months in Patients With Crohn's Disease in the CELEST Extension Study. Clin Gastroenterol Hepatol. 2022;20:2337-2346.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 153. | Panaccione R, Long M, Ran Z, Atreya R, Nakase H, Zhou W, Yao X, Ilo D, D’haens G. S805 Efficacy of Upadacitinib Dose Escalation in a Phase 3 Long-Term Extension Ulcerative Colitis Study. Am J Gastroenterol. 2022;117:e574-575. [DOI] [Full Text] |
| 154. | Vermeire S, Danese S, Zhou W, Klaff J, Ilo D, Yao X, Levy G, Higgins PDR, Loftus EV, Panaccione R. DOP41 Efficacy and safety of extended induction treatment with upadacitinib 45 mg once daily followed by maintenance upadacitinib 15 or 30 mg once daily in patients with moderately to severely active Ulcerative Colitis. J Crohns Colitis. 2022;16:i090-091. [DOI] [Full Text] |
| 155. | Radcliffe M, Long M, Barnes E, Herfarth H, Hansen J, Jain A. Efficacy of upadacitinib in patients previously treated with tofacitinib for the management of ulcerative colitis. Inflamm Bowel Dis. 2023;29:S82-82. [DOI] [Full Text] |
| 156. | Hosomi S, Nishida Y, Fujiwara Y. Efficacy of Upadacitinib as a Second-line JAK Inhibitor in Ulcerative Colitis: A Case Series. Intern Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 157. | Friedberg S, Choi D, Hunold T, Choi NK, Garcia NM, Picker EA, Cohen NA, Cohen RD, Dalal SR, Pekow J, Sakuraba A, Krugliak Cleveland N, Rubin DT. Upadacitinib Is Effective and Safe in Both Ulcerative Colitis and Crohn's Disease: Prospective Real-World Experience. Clin Gastroenterol Hepatol. 2023;21:1913-1923.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 158. | Restellini S, Afif W. Update on TDM (Therapeutic Drug Monitoring) with Ustekinumab, Vedolizumab and Tofacitinib in Inflammatory Bowel Disease. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 159. | Mukherjee A, Hazra A, Smith MK, Martin SW, Mould DR, Su C, Niezychowski W. Exposure-response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: Results from a dose-ranging phase 2 trial. Br J Clin Pharmacol. 2018;84:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 160. | Scott FL, Clemons B, Brooks J, Brahmachary E, Powell R, Dedman H, Desale HG, Timony GA, Martinborough E, Rosen H, Roberts E, Boehm MF, Peach RJ. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1 ) and receptor-5 (S1P5 ) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 161. | Sandborn WJ, Feagan BG, D'Haens G, Wolf DC, Jovanovic I, Hanauer SB, Ghosh S, Petersen A, Hua SY, Lee JH, Charles L, Chitkara D, Usiskin K, Colombel JF, Laine L, Danese S; True North Study Group. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2021;385:1280-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 376] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/