Published online Jan 14, 2024. doi: 10.3748/wjg.v30.i2.184
Peer-review started: October 30, 2023
First decision: November 29, 2023
Revised: December 12, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 14, 2024
Processing time: 73 Days and 19.5 Hours
Resistance to clarithromycin (CLA) and levofloxacin (LFX) of Helicobacter pylori (H. pylori) is increasing in severity, and successful eradication is essential. Presently, the eradication success rate has greatly declined, leaving a large number of patients with previous treatment histories.
To investigate secondary resistance rates, explore risk factors for antibiotic resistance, and assess the efficacy of susceptibility-guided therapy.
We recruited 154 subjects positive for Urea Breath Test who attended The First Affiliated Hospital of China Medical University between July 2022 and April 2023. Participants underwent a string test after an overnight fast. The gastric juice was obtained and transferred to vials containing storage solution. Subsequently, DNA extraction and the specific DNA amplification were performed using quantitative polymerase chain reaction (qPCR). Demographic information was also analyzed as part of the study. Based on these results, the participants were administered susceptibility-guided treatment. Efficacy was compared with that of the empiric treatment group.
A total of 132 individuals tested positive for the H. pylori ureA gene by qPCR technique. CLA resistance rate reached a high level of 82.6% (n = 109), LFX resistance rate was 69.7% (n = 92) and dual resistance was 62.1% (n = 82). Gastric symptoms [odds ratio (OR) = 2.782; 95% confidence interval (95%CI): 1.076-7.194; P = 0.035] and rural residence (OR = 5.152; 95%CI: 1.407-18.861; P = 0.013) were independent risk factors for secondary resistance to CLA and LFX, respectively. A total of 102 and 100 individuals received susceptibility-guided therapies and empiric treatment, respectively. The antibiotic susceptibility-guided treatment and empiric treatment groups achieved successful eradication rates of 75.5% (77/102) and 59.0% (59/411) by the intention-to-treat (ITT) analysis and 90.6% (77/85) and 70.2% (59/84) by the per-protocol (PP) analysis, respectively. The eradication rates of these two treatment strategies were significantly different in both ITT (P = 0.001) and PP (P = 0.012) analyses.
H. pylori presented high secondary resistance rates to CLA and LFX. For patients with previous treatment failures, treatments should be guided by antibiotic susceptibility tests or regional antibiotic resistance profile.
Core Tip: Decreased success eradication rates of Helicobacter pylori (H. pylori) have received much attention in recent years, mainly due to the increasing resistance to antibiotics. Focus has begun to be placed on the efficacy of antibiotic susceptibility-guided eradication. This study revealed that the secondary resistance rate of H. pylori to clarithromycin and levofloxacin in Province Liaoning, was higher than the national average. Antibiotic susceptibility-guided eradication therapy is more effective than empiric treatment. It provided a reference for eradication therapy in regions in the northeast of China.
- Citation: Wang YM, Chen MY, Chen J, Zhang XH, Feng Y, Han YX, Li YL. Success of susceptibility-guided eradication of Helicobacter pylori in a region with high secondary clarithromycin and levofloxacin resistance rates. World J Gastroenterol 2024; 30(2): 184-195
- URL: https://www.wjgnet.com/1007-9327/full/v30/i2/184.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i2.184
Approximately half of the world's population is infected with Helicobacter pylori (H. pylori), a bacterium that contributes to various stomach disorders, such as atrophic gastritis, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma[1]. H. pylori has also been implicated in the development of extra-digestive disorders, such as iron deficiency anemia, idiopathic thrombocytopenic purpura, nonalcoholic fatty liver disease, and Alzheimer’s disease (AD)[2-4]. H. pylori also elicits immune responses. Several researchers have investigated the association of H. pylori infection with mean platelet volume and neutrophil/lymphocyte ratio values in both adults and children[5,6]. H. pylori, classified as a group I carcinogen of gastric cancer (GC), could promote the development of precancerous gastric lesions[7,8]. Successful H. pylori eradication might serve as a long-term preventive measure against GC among populations at high risk of the disease[9-11]. Strategies for the effective H. pylori treatment are essential for the prevention of GC.
Recently, the eradication rates of standard triple therapies containing clarithromycin (CLA) and regimens based on levofloxacin (LFX) have declined substantially[12-14]. This marked reduction in eradication efficacy resulted mainly from the rising prevalence of antibiotic resistance[15]. Bismuth quadruple therapy was recommended by guidelines and used clinically, yet still with unsatisfactory eradication effects[16]. A rapid increase in the prevalence of H. pylori resistance to CLA and LFX has been observed[17], which might be closely related to previous treatment failure. It has been pointed out that with more than two previous histories of treatments, resistance to CLA and LFX could increase by more than 50%[18]. Current consensus recommended antibiotic susceptibility tests before prescriptions in populations with several treatment failures[19]. Besides, in areas characterized by high CLA and LFX resistance, it is essential to detect antibiotic resistance of H. pylori before prescription. Epidemiological data on secondary H. pylori resistance are crucial for decision-making within respective areas when local patients are unavailable for prior tests.
However, culturing and antibiotic susceptibility tests require significant investments in time and specialized laboratory equipment, limiting their wide clinical application, likewise[20]. Furthermore, molecular tests, such as quantitative polymerase chain reaction (qPCR), hold promise for clinical application in detecting H. pylori infection and antibiotic resistance in gastric mucosal specimens[21,22]. For focal distribution within the gastric mucosa, H. pylori is prone to false-negative results when biopsies are performed at several sites under gastroscopy. Conversely, the presence of bacteria and shed epithelial cells in gastric fluid achieved using the more available and less invasive string-test than the conventional method of extracting mucosal samples through endoscopic biopsies[21].
Moreover, the identification of risk factors for antibiotic resistance of H. pylori is valuable for identifying high-risk populations susceptible to antibiotic-resistant strains. This information holds value in clinical settings, enabling to provide prescriptions suitable for a specific region, thereby reducing the incidence of secondary antibiotic resistance. Several studies have investigated risk factors, such as age, gender, and previous antibiotic use[23-25]. However, conclusions remained contentious, displaying discrepancies across different geographical regions.
In the current study, we calculated the secondary CLA and LFX resistance rate of H. pylori strains in Liaoning Province, a region in the northeast of China, based on qPCR by string-test. Risk factors for antibiotic resistance were also evaluated. Besides, we assessed the eradication rate of susceptibility-guided therapy eradication and compared the outcomes with the empiric treatment eradication rate, in order to improve the eradication rate in Liaoning Province.
This trial was conducted at the Department of Gastroenterology of the First Affiliated Hospital of China Medical University from July 2022 to April 2023. It was approved by the Human Ethics Review Committee of the First Affiliated Hospital of China Medical University (2021325). Written informed consent was obtained from all participants. Participants were drawn from various areas of Liaoning Province, and the study was conducted at a tertiary hospital. Subjects positive for H. pylori and aged ≥ 18 years were recruited. Exclusion criteria included administration of antibiotics in the past month, administration of proton pump inhibitors in the past half month, acute respiratory infections, recent gastrointestinal bleeding, esophageal and gastric varices, dysphagia, and esophageal cancer. Basic information on participants was obtained from the HIS Information System Technical Support Services, including gender, current smoking conditions, current drinking conditions, body mass index (BMI), hypertension, diabetes mellitus, gastrointestinal symptoms, family members with H. pylori infection, family histories of GC, and living area. The definition of current smoking and drinking in the present study was that participants engaged in smoking and/or drinking behavior in accordance with their personal smoking and/or drinking habits during the first 4 wk before conducting the string test. Gastrointestinal symptoms included abdominal pain, fullness, heartburn, dysphagia, and anorexia.
Urea Breath Test (UBT) was performed with The Kit For 13C-Urea Breath Test (Haiderun Pharmaceutical Group Co. Ltd., Beijing, China). After the initial baseline breath samples were collected, participants fasting for at least two hours ingested 100-mg 13C-labelled reagent. Breath samples exhaled after 30 min were analyzed by the WLD600C13C Analyzer (Haiderun Pharmaceutical Group Co. Ltd., Beijing, China).
A positive indicator of H. pylori infection was determined according to the manufacturer's instructions. Participants positive for UBT underwent the string-test. A gelatine capsule containing a 90-cm-long string of absorbent cotton (Shenzhen Hongmed-Infagen Co. Ltd., China) was swallowed by subjects after an overnight fast, along with 300 mL of water. One hour later, researchers withdrew the string, cut it at the designated position with a pair of sterile scissors, and discarded the proximal section to preclude oral contamination. The gastric-fluid-soaked portion was transferred into storage solution supplied in vials. All samples were sent to Shenzhen Hongmed-Infagen Co. Ltd. at ambient temperature for processing.
Genomic DNA was extracted by following the manufacturer's guidelines with H. pylori DNA extraction kit (Hongmed-Infagen Co. Ltd.). The Real-time PCR System Gentier 96R (Tianlong Technology Co. Ltd.) carried on the amplification to detect the presence of the specific ureA gene and the point mutations of 23S rRNA (A2142G, A2143G, and A2142C) and gyrA (260T, 261A, 261G, 271A, 271T, and 272G), which represented CLA and quinolone resistance, respectively. The cycling program included an initial cycle of 2 min at 42°C, then 2 min at 95°C, proceeded by 40 denaturation cycles of 10s at 95°C and 45s at 58°C for extension and annealing.
The susceptibility-guided treatment group enrolled participants with antibiotic susceptibility results. They received bismuth-based quadruple therapy based on The Fifth Chinese National Consensus Report on the management of H. pylori infection[19]. The quadruple therapy consisted of bismuth 200 mg bid, PPI (rabeprazole 20 mg or ilaprazole 5 mg) bid, and two types of antibiotics. Antibiotics were selected according to the antibiotic susceptibility outcomes, two of the following: Amoxicillin 1000 mg bid, CLA 500 mg bid, furazolidone 100 mg bid, LFX 500 mg qd, minocycline hydro
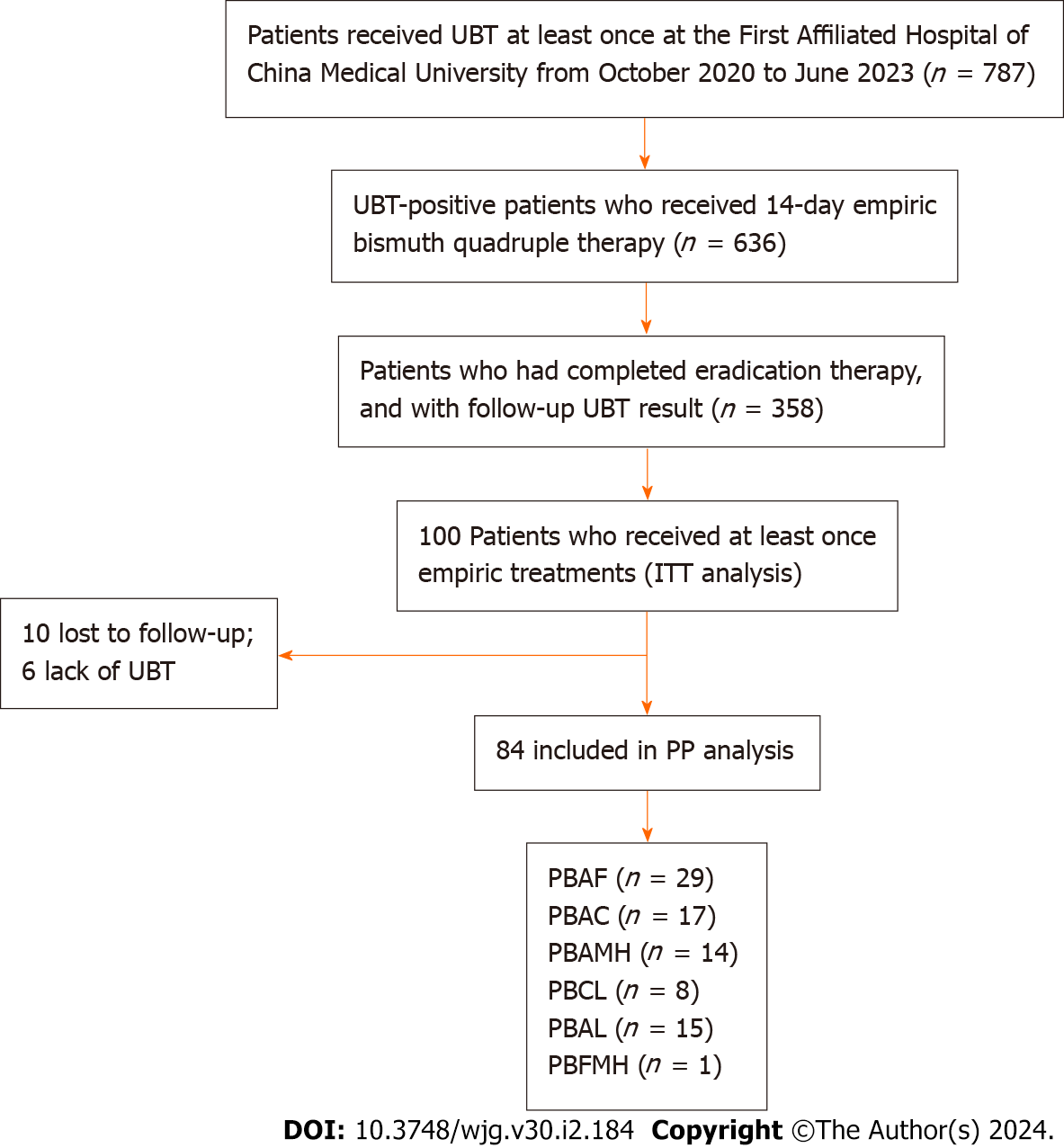
We retrospectively reviewed UBT and treatment records of the First Affiliated Hospital of China Medical University from October 2020 to June 2023 to assess the H. pylori eradication rates. Researchers reviewed treatment regimens and screened patients who received therapies at least once. Of the patients screened, those who met all the following criteria were recruited in the empiric treatment group: with both pre- and post-treatment UBT results, a complete course of bismuth quadruple therapy, and being prescribed a PPI of either rabeprazole or ilaprazole. Eradication rates of H. pylori were evaluated by intention-to-treat (ITT; including individuals enrolled in studies analyzing eradication therapies) and per-protocol (PP; eradication therapies) analyses.
The statistical software SPSS (version 18.0; SPSS Inc., Chicago, IL, United States) was used to perform all the statistical analyses. Categorical data were presented as numbers and percentages. Continuous data were presented as mean ± SD. The demographic data on participants were processed using descriptive statistical analysis. Chi-square test was applied to compare differences between groups. Fisher’s exact test was applied when over 20% of the expected counts were below 5. The factors that could influence antibiotic resistance were analyzed by univariate analysis. A binary logistic regression model was used to calculate the odds ratios (ORs) and 95% confidence intervals (95%CIs) of different variables related to antibiotic resistance. P value less than 0.05 in two tails was considered statistically significant.
In total, 154 participants with positive UBT results were recruited in the present study. A total of 132 (85.7%) H. pylori ureA-positive subjects were evaluated, of which group baseline characteristics were summarized in Table 1. Most participants are from urban areas (103; 78.0%), and nearly half are females (71; 53.8%). The mean age and standard deviation of 132 adults was 52.7 ± 12.6, with a range from 18 to 78 years old. Most participants (83; 62.9%) have gastric symptoms, but only a minority of individuals whose family members were with H. pylori infection (41; 31.1%) and who had a family history of GC (16; 12.1%). Almost half (61; 46.2%) were overweight in terms of BMI. Besides, a small proportion of participants conducted current drinking, current smoking, were diagnosed with hypertension, and were diagnosed with diabetes, in descending order of 19.7%, 17.4%, 16.7%, and 11.4%, respectively.
| Factors | n | % |
| Sex (female) | 71 | 53.8 |
| Age (yr, ≥ 50) | 85 | 64.4 |
| BMI (kg/m2, ≥ 25) | 61 | 46.2 |
| Current smoking | 23 | 17.4 |
| Current drinking | 26 | 19.7 |
| Hypertension (yes) | 22 | 16.7 |
| Diabetes (yes) | 15 | 11.4 |
| Gastrointestinal symptoms (yes) | 83 | 62.9 |
| Family members with Helicobacter pylori infection (yes) | 41 | 31.1 |
| Family histories of gastric cancer (yes) | 16 | 12.1 |
| Residence region (urban) | 103 | 78.0 |
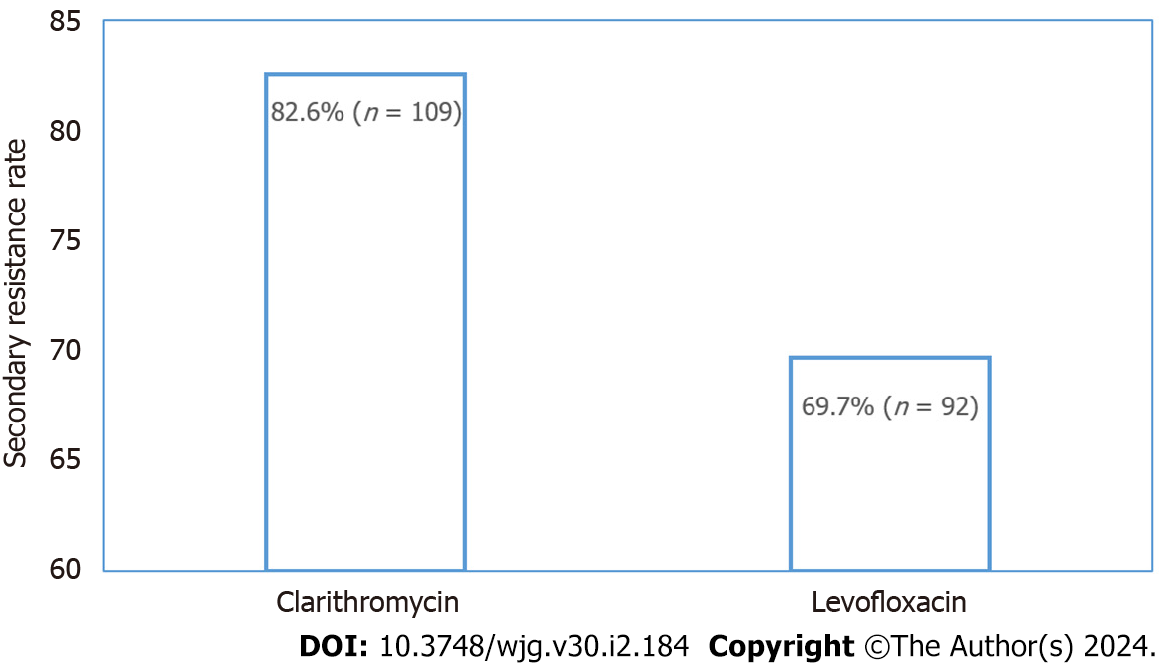
The secondary resistance rates to CLA and LFX were observed in 82.6% (n = 109) and 69.7% (n = 92) of the ureA positive subjects, respectively, which represented quite high levels (Figure 1). Of these, 82 isolates were resistant to both antibiotics, with a dual resistance rate of 62.1%. A total of 28.0% (n = 37) of the population was monoresistant, inferior to that of the dual-resistant population. Among these 37 subjects, the greatest number was resistant to CLA (27; 20.5%), leaving 10 subjects resistant to LFX. The least number of subjects were sensitive to both antibiotics, at 9.9% of 13 ones. Antibiotic resistance patterns of H. pylori are shown in Table 2.
| Type of resistance | Overall (n = 132) | |
| Number of strains | Resistance rate (%) | |
| No resistance | 13 | 9.9 |
| Monoresistance | 37 | 28.0 |
| LFX | 10 | 7.6 |
| CLA | 27 | 20.5 |
| Double resistance | 82 | 62.1 |
| CLA + LFX | 82 | 62.1 |
The chi-square results indicated that subjects with gastrointestinal symptoms and CLA-resistance differed significantly (P = 0.034). Another significant difference was observed between LFX-resistance and residential region (P = 0.008). As for the other factors, CLA-resistance differed from age and current smoking status, whereas LFX-resistance differed from age. However, these differences were not significant, with P values at 0.068, 0.076, and 0.060, respectively. No other associations were discovered between antibiotic resistance and patient characteristics, including gender, current drinking conditions, BMI, hypertension, diabetes mellitus, family members with H. pylori infection, and family histories of GC (P > 0.05; Table 3).
| Characteristics | Clarithromycin | P value | Levofloxacin | P value | ||
| Resistant | Sensitive | Resistant | Sensitive | |||
| Age (yr) | ||||||
| ≥ 50 | 20 | 11 | 0.068 | 64 | 21 | 0.060 |
| < 50 | 35 | 74 | 28 | 19 | ||
| Gender | ||||||
| Male | 48 | 13 | 0.275 | 43 | 18 | 0.854 |
| Female | 61 | 10 | 49 | 22 | ||
| Current smoking | ||||||
| Yes | 20 | 3 | 0.076 | 15 | 8 | 0.607 |
| No | 89 | 20 | 77 | 32 | ||
| Current drinking | ||||||
| Yes | 24 | 2 | 0.246 | 17 | 9 | 0.593 |
| No | 85 | 21 | 75 | 31 | ||
| BMI (kg/m2) | ||||||
| ≥ 24 | 51 | 10 | 0.772 | 45 | 16 | 0.345 |
| < 24 | 58 | 13 | 47 | 24 | ||
| Hypertension | ||||||
| Yes | 20 | 2 | 0.363 | 18 | 4 | 0.175 |
| No | 89 | 21 | 74 | 36 | ||
| Diabetes melllitus | ||||||
| Yes | 11 | 4 | 0.297 | 10 | 5 | 0.772 |
| No | 98 | 19 | 82 | 35 | ||
| Symptoms | ||||||
| Yes | 73 | 10 | 0.034 | 61 | 22 | 0.217 |
| No | 36 | 13 | 31 | 18 | ||
| Family members with Helicobacter pylori infection | ||||||
| Yes | 34 | 7 | 0.943 | 27 | 14 | 0.519 |
| No | 75 | 16 | 65 | 26 | ||
| Family histories of gastric cancer | ||||||
| Yes | 15 | 1 | 0.303 | 10 | 6 | 0.565 |
| No | 94 | 22 | 82 | 34 | ||
| Residence region | ||||||
| Urban | 83 | 20 | 0.255 | 66 | 37 | 0.008 |
| Rural | 26 | 3 | 26 | 3 | ||
Furthermore, age, current smoking status, gastric symptoms, and residence region were included in the binary logistic regression analysis to assess their association with antibiotic resistance. The analysis revealed that patients with gastrointestinal symptoms were more likely to develop CLA-resistance (OR = 2.782; 95%CI: 1.076-7.194; P = 0.035). Patients living in rural areas were more likely to develop resistance to LFX than those living in urban areas (OR = 5.152; 95%CI: 1.407-18.861; P=0.013; Table 4).
| Variables | Clarithromycin | Levofloxacin | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | ||||
| < 50 | 1 | 1 | ||
| ≥ 50 | 2.379 (0.924-6.125) | 0.072 | 1.972 (0.888-4.379) | 0.095 |
| Current smoking | ||||
| No | 1 | 1 | ||
| Yes | 1.151 (0.293-4.517) | 0.841 | 0.546 (0.192-1.557) | 0.258 |
| Symptoms | ||||
| No | 1 | 1 | ||
| Yes | 2.782 (1.076-7.194) | 0.035 | 1.851 (0.823-4.166) | 0.137 |
| Residence region | ||||
| Urban | 1 | 1 | ||
| Rural | 1.852 (0.489-7.010) | 0.364 | 5.152 (1.407-18.861) | 0.013 |
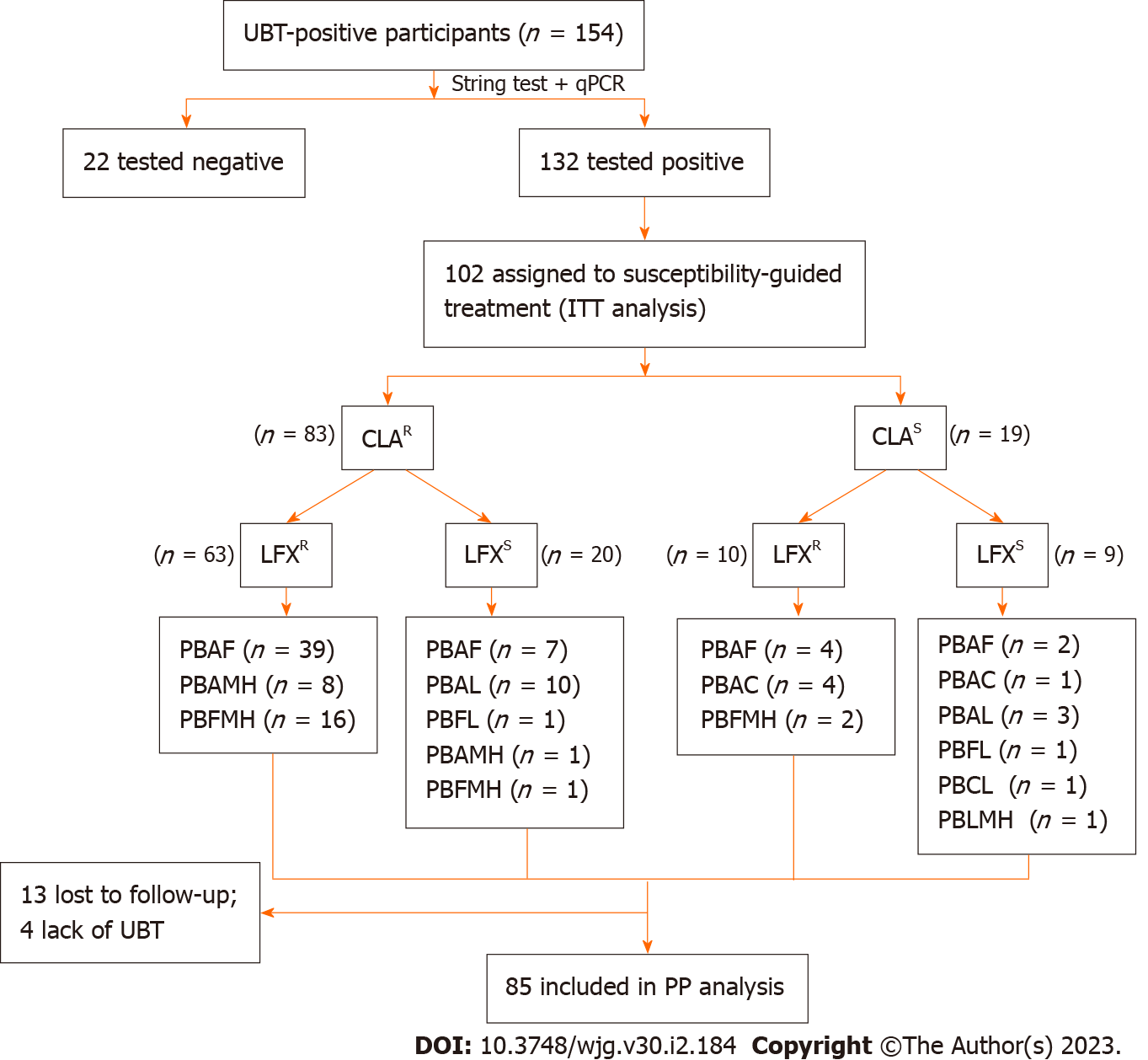
A total of 102 and 100 individuals received susceptibility-guided therapies with antimicrobial susceptibility tests and empiric treatment, respectively. Demographic information of the two groups was summarized in Table 5. All of them were included in the ITT analysis. Among the 102 individuals with susceptibility-guided therapies, 13 were lost to follow-up and 4 did not retest UBT. Overall, 85 of them with follow-up UBT values were included in the PP analysis. Different bismuth-based quadruple therapies regimens were shown in Figure 2. For the empiric treatment group, we screened 100 records from the hospital database that met the aforementioned criteria. With 10 missing following-up and 6 Lacking UBT, 84 participants were finally included in the PP analysis. Therapy regimens were shown in Figure 3. For the antibiotic-susceptibility-guided treatment and empiric treatment groups, the eradication rates in the ITT analyses were 75.5% (77/102) and 59.0% (59/100), respectively. In the PP analyses, the eradication rates were 90.6% (77/85) and 70.2% (59/84), respectively (Table 6). In addition, a significantly difference was found between these two treatment strategies both in the ITT (P = 0.001) and PP analyses (P = 0.012).
| Characteristics | All (n, %) | Susceptibility-guided treatment (n, %) | Empiric treatment (n, %) | P value |
| Age (yr) | 0.448 | |||
| < 50 | 57 (33.7) | 31 (54.4) | 26 (45.6) | |
| ≥ 50 | 112 (66.3) | 54 (48.2) | 58 (51.8) | |
| Gender | 0.812 | |||
| Male | 78 (46.2) | 40 (51.3) | 38 (48.7) | |
| Female | 91 (53.8) | 45 (49.5) | 46 (50.5) |
| Susceptibility-guided treatment group | Empiric treatment group | P value | |
| ITT analysis | 75.5% (77/102) | 59.0% (59/100) | 0.001 |
| PP analysis | 90.6% (77/85) | 70.2% (59/84) | 0.012 |
CLA is the first-line antibiotic for H. pylori eradication[19]. When the efficacy of CLA-based regimens has been declining, LFX has been adopted as a second-line treatment option[26]. Unfortunately, H. pylori has exhibited growing resistances to both drugs, contributing to a global increase in treatment failure rates[27]. In recent years, the incidence of secondary resistance in China has increased due to the extensive use of antibiotics[28]. Limited studies estimated secondary resistance rates in Liaoning, a province in the northeast of China[29]. Owing to the easier and less invasive nature of the string-test and the more economical and simpler approaches of molecular biology methods, we detected the point mutations of 23S rRNA (A2142G, A2143G, and A2142C) and gyrA (260T, 261A, 261G, 271A, 271T, and 272G) by the above methods for H. pylori antibiotic susceptibility status determination. The results finally confirmed a pretty high rate of secondary resistance to CLA (82.6%) and LFX (69.7%) among the 132 ureA positive subjects (132/154; 85.7%).
The failure of the standard triple therapy eradication was mostly attributed to CLA resistance[16]. The eradication rate of first-line treatment in CLA-resistant cases was even as low as 59.4%, calculated by a meta-analysis, compared to 90.1% in sensitive ones[30]. A secondary CLA-resistance rate of 76.9% has been recorded in China[28], but with variation across geographical regions. In this study, the secondary CLA-resistance rate was 82.6%, ranking second after Lanzhou (93.8%)[31], similar to Beijing (83.3%)[32] and Nanjing (77.8%)[33], while surpassing those in Shanghai (67.4%)[34], Nanchang (58.3%)[35], and Shenzhen (34.3%)[36]. The secondary resistance to CLA in China has demonstrated an increasing prevalence over time[32]. The significant upward trend in secondary resistance was observed after CLA-based treatments[32,33], indicating a correlation with the frequency of therapy[18]. The rate in Liaoning achieved a high level, probably attributed to the regional administration of CLA. CLA has been in frequent use in China since 1995, particularly for respiratory diseases prevalent in the colder regions, such as Liaoning and Beijing[37,38]. It provides a plausible explanation for the high rate of secondary CLA-resistance in the north of China. The Maastricht V consensus[16] suggest that if the CLA resistance rate exceeds 15% in a certain region, PPI-CLA-containing triple therapy without prior susceptibility tests should be abandoned[39]. Patients with previous CLA-containing therapy failure should not be readministered unless supported by confirmed susceptibility tests.
The LFX-resistance rate reached up to 69.7%, similar to that in Beijing (73.3%)[32] and Lanzhou (64.6%)[31], all surpassing the average of 61.6% in China[28]. These high resistance rates might be attributed to the following reasons. LFX is widely used in China, with fewer restrictions than in western countries, is more frequently used for urogenital diseases and respiratory infection, and is widely used as a non-prescription drug, even in animal aquaculture and husbandry[40]. According to consensus recommendations, regimens containing LFX could be used as rescue treatments in regions with high CLA-resistance[16]. We observed such a high resistance rate to LFX that cautions should be taken when prescribing it in clinical settings. However, in Beijing, despite the high resistance rate to LFX, the final eradication rate after the LFX-based triple regimen still exceeded 80%[32]. This might demonstrate that the ultimate therapeutic effect does not depend solely on the resistance or susceptibility status in vitro of H. pylori to LFX.
In terms of resistance patterns, dual-resistant subjects accounted for the majority of the 132 participants (82; 62.1%), followed by mono-resistant subjects (28.0%). This indicated that in the studied area, the probability of eradication success might be improved if susceptibility tests have been confirmed.
Regarding the risk factors associated with antibiotic resistance in this study, binary logistic regression analyses revealed gastric symptoms as an independent risk predictor for CLA resistance. Patients with gastric symptoms, including abdominal pain, fullness, and heartburn, had a significantly higher probability of developing resistance to CLA than those without symptoms (OR = 2.782; 95%CI: 1.076-7.194; P = 0.035). Our findings were consistent with those of a trial conducted in Yangzhou, China[23]. Based on these results, we recommended that patients presenting with gastric discomfort undergo antibiotic susceptibility tests and receive precise treatments. If patients are unavailable for testing, gastric symptoms are an indication for physicians to avoid prescribing CLA-containing regimens. Additionally, rural residence was observed as an independent risk factor for LFX resistance. The LFX-resistance rate was significantly higher in rural residents than in urban residents (OR = 5.152; 95%CI: 1.407-18.861; P = 0.013). This might be explained by the casual and frequent use of LFX and the limited knowledge of H. pylori in rural areas. Thus, the administration of LFX to rural residents should be considered with caution. Physicians should preach to the public, especially rural residents, about preventing the misuse of antibiotics.
Among the risk factors investigated in the present study, age and current smoking status failed to exhibit significantly associations with CLA or LFX resistance. P values for age and CLA resistance, smoking and CLA resistance, and age and LFX resistance showed 0.068, 0.076, and 0.060, respectively, which were near critical values. Some articles reached the conclusion that age was an independent risk factor for antibiotic resistance[31,34,41]. In our study, the LFX resistance rate was higher in the age group above 50 (64/82), suggesting a potential age-related trend. However, in the CLA-resistant population, no age-related trends were observed, presumably associated with other variables, such as the method of classifying age groups. As for the association between smoking and CLA-resistance, it remained unclear and required further investigations.
From the perspective of treatment efficacy, our study achieved acceptable eradication rates (90.6% by ITT and 75.5% by PP), similar to the results of other studies based on susceptibility-guided treatments[42,43]. These outcomes were significantly higher than those in the empiric treatment group (70.2% by ITT and 59.0% by PP) (P = 0.001 by ITT and P = 0.012 by PP). Achieving such a satisfactory result demonstrated that it is feasible for patients with previous eradication failures to perform antibiotic susceptibility tests based on qPCR by string-test.
The present study has limitations. Firstly, the analysis and derivation of antibiotic resistance data were based on data from a single hospital in Liaoning Province. A degree of bias might exist, although participants attending this large tertiary hospital come from across the province. Furthermore, certain risk factors approached critical values without reaching significance. Future studies should include larger sample sizes. In the future, we aim to conduct multicenter studies across various regions of the province to obtain a more comprehensive profile of antibiotic resistance in Liaoning. We will also endeavor to expand our sample size to provide more specific population characteristics. Nonetheless, as one of the few articles demonstrating secondary resistance in the northeast of China, this study still offered valuable insights into the possibility of eradication success in patients who had failed previous treatments once or more times.
Secondary resistance rates to CLA and LFX in Liaoning were high, both exceeding the average resistance rates in China. Patients with gastric symptoms and residing in rural areas were at higher risk of developing resistance to CLA and LFX, respectively. For patients who have failed previous eradications, antibiotic-guided treatments based on susceptibility results were more effective than empiric treatments. Therefore, it is crucial for clinicians to provide a regional treatment regimen based on regional antibiotic resistance patterns, combined with the patients’ previous antibiotic exposure, the presence of gastric symptoms, and their region of residence.
Decreased success eradication rates of Helicobacter pylori (H. pylori) have received much attention in recent years, mainly due to the increasing resistance to antibiotics. Hence, the rates and patterns of resistance of H. pylori to antibiotics need to be explored.
Susceptibility-guided therapy based on antibiotic susceptibility test improved H. pylori eradication rates. We evaluated the antibiotic resistance rate of H. pylori, performed precision treatment therapies, and compared the efficacy with empiric treatment therapies. It provided a reference for eradication therapy in regions in the northeast of China.
To investigate secondary resistance rates, explore risk factors for antibiotic resistance, and assess the efficacy of susceptibility-guided therapy.
We observed antibiotic resistance rates of H. pylori and performed a single-center, clinical trial with the susceptibility-guided eradication regimen.
Clarithromycin (CLA) and levofloxacin (LFX) resistance rates were 82.6% and 69.7%, respectively. Gastric symptoms and rural residence were independent risk factors for secondary resistance to CLA and LFX, respectively. The overall susceptibility-guided eradication rates calculated using intention-to-treat and per-protocol analyses were 90.6% and 75.5%, respectively, both higher than rates with empiric treatment therapies.
H. pylori presented high secondary resistance rates to CLA and LFX. For patients with previous treatment failures, treatments guided by antibiotic susceptibility tests showed good eradication efficacy.
Large-scale, multi-center observed researches in various regions of the province are needed to obtain a more comprehensive profile of antibiotic resistance in Liaoning. It will be necessary to compare the safety, medication adherence and cost-effectiveness of the susceptibility-guided eradication regimen with the empiric eradication regimen in the future.
The authors are grateful to Professor Zhou BS for his help in the statistical analysis of this paper.
| 1. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2211] [Article Influence: 245.7] [Reference Citation Analysis (3)] |
| 2. | Dogan A, Ekinci O, Ebinc S. Effect of Helicobacter pylori infection on the first-line treatment outcomes in patients with immune thrombocytopenic purpura. Eur Rev Med Pharmacol Sci. 2022;26:3995-4000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Mavilia-Scranton MG, Wu GY, Dharan M. Impact of Helicobacter pylori Infection on the Pathogenesis and Management of Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2023;11:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Xie J, Cools L, Van Imschoot G, Van Wonterghem E, Pauwels MJ, Vlaeminck I, De Witte C, El Andaloussi S, Wierda K, De Groef L, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling. J Extracell Vesicles. 2023;12:e12306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 5. | Sahin Y, Gubur O, Tekingunduz E. Relationship between the severity of Helicobacter pylori infection and neutrophil and lymphocyte ratio and mean platelet volume in children. Arch Argent Pediatr. 2020;118:e241-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (2)] |
| 6. | Guclu M, Faruq Agan A. Association of Severity of Helicobacter pylori Infection with Peripheral Blood Neutrophil to Lymphocyte Ratio and Mean Platelet Volume. Euroasian J Hepatogastroenterol. 2017;7:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Díaz P, Valenzuela Valderrama M, Bravo J, Quest AFG. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front Microbiol. 2018;9:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020;158:527-536.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 9. | Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, You J, Wong BCY, Chen J, Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology. 2022;163:154-162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 10. | Chiang TH, Chang WJ, Chen SL, Yen AM, Fann JC, Chiu SY, Chen YR, Chuang SL, Shieh CF, Liu CY, Chiu HM, Chiang H, Shun CT, Lin MW, Wu MS, Lin JT, Chan CC, Graham DY, Chen HH, Lee YC. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut. 2021;70:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 11. | Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69:2113-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 324] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 12. | Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (7)] |
| 13. | Graham DY. Transitioning of Helicobacter pylori Therapy from Trial and Error to Antimicrobial Stewardship. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Xie Y, Song C, Cheng H, Xu C, Zhang Z, Wang J, Huo L, Du Q, Xu J, Chen Y, Zhang X, Zhang G, Yang G, Zuo X, Guo T, Lu Y, Wang F, Wang X, Zhuang K, Chen S, Liu W, Lu N; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Long-term follow-up of Helicobacter pylori reinfection and its risk factors after initial eradication: a large-scale multicentre, prospective open cohort, observational study. Emerg Microbes Infect. 2020;9:548-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Kim BJ, Kim HS, Song HJ, Chung IK, Kim GH, Kim BW, Shim KN, Jeon SW, Jung YJ, Yang CH, Kim JH, Kim TH, Kim SG, Shin WG, Kim SM, Han SW, Lee JH, Kim KH, Park SK, Park BJ, Lee J, Kim JG; Korean College of Helicobacter and Upper Gastrointestinal Research. Online Registry for Nationwide Database of Current Trend of Helicobacter pylori Eradication in Korea: Interim Analysis. J Korean Med Sci. 2016;31:1246-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 2086] [Article Influence: 231.8] [Reference Citation Analysis (1)] |
| 17. | Graham DY, Moss SF. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When, How, Why. Am J Gastroenterol. 2022;117:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Jiang Z, Qian X, Wang Z, Dong Y, Pan Y, Zhang Z, Wang S. Antibiotic resistance of Helicobacter pylori isolated from patients in Nanjing, China: A cross-section study from 2018 to 2021. Front Cell Infect Microbiol. 2022;12:970630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 20. | Li H, Shen Y, Song X, Tang X, Hu R, Marshall BJ, Tang H, Benghezal M. Need for standardization and harmonization of Helicobacter pylori antimicrobial susceptibility testing. Helicobacter. 2022;27:e12873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Han X, Yu X, Gao X, Wang X, Tay CY, Wei X, Lai B, Marshall BJ, Zhang X, Chua EG. Quantitative PCR of string-test collected gastric material: A feasible approach to detect Helicobacter pylori and its resistance against clarithromycin and levofloxacin for susceptibility-guided therapy. Helicobacter. 2023;28:e12985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 22. | Wang YH, Li Z, Wang L, Zhu-Ge LY, Zhao RL, Wu S, Wang Y, An Y, Xie Y. A systematic review and meta-analysis of genotypic methods for detecting antibiotic resistance in Helicobacter pylori. Helicobacter. 2018;23:e12467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 23. | Zhang Y, Feng X, Bian L, Zhang Y, Li Q, Xu Y, She Q, Yan C, Lu G, Wu J, Xiao W, Ding Y, Deng B. Antibiotic Resistance of Helicobacter pylori and Related Risk Factors in Yangzhou, China: A Cross-Sectional Study. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Huang JG, Lim SYS, Aw MM, Quak SH. Antibiotic resistance patterns and therapeutic outcomes of pediatric Helicobacter pylori infection in a high-migrant Singaporean cohort. Helicobacter. 2022;27:e12868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Kwon YM, Kim SJ, Lee JG, Lee SP. Effects of prior antibiotic use on clarithromycin resistance in Helicobacter pylori. Helicobacter. 2023;28:e12974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Fallone CA, Moss SF, Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology. 2019;157:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 27. | Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 28. | Zhong Z, Zhang Z, Wang J, Hu Y, Mi Y, He B, Zhang Y, Zhang X, Xia X, Huang H, Lai Y, Lin M, Su C, Wu Z, Lu L, Zhang B, Huang S, Zhong C, Zeng X, Peng Y, Chen G, Zhang H, Zhou G, Liu S, Yang C, Yan L, Chen A, Zhang G, Xu P, Wang S, Zheng P, Xu S, Gao H. A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter pylori strains in China. Am J Cancer Res. 2021;11:5027-5037. [PubMed] |
| 29. | Wang Y, Li Y, Gong Y, Dong Y, Sun J, Chen M. Antibiotic resistance characteristics and risk factors analysis of Helicobacter pylori strains isolated from patients in Liaoning Province, an area in North China. PeerJ. 2023;11:e15268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Zou Y, Qian X, Liu X, Song Y, Song C, Wu S, An Y, Yuan R, Wang Y, Xie Y. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: A systematic review and meta-analysis. Helicobacter. 2020;25:e12714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Xu H, Yun J, Li R, Ma X, Gou L, Che T, Zhang D. Antibiotics Resistance Prevalence of Helicobacter pylori Strains in Northwest China. Infect Drug Resist. 2022;15:5519-5528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 32. | Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Jiang ZD, He BS, Zhang ZY, Wang SK, Ran D, Wang ZB. Analysis of the Primary and Post-Treatment Antibiotic Resistance of Helicobacter pylori in the Nanjing Area. Curr Pharm Biotechnol. 2021;22:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Wang J, Xie X, Zhong Z, Yuan H, Xu P, Gao H, Lai Y. Prevalence of antibiotic resistance of Helicobacter pylori isolates in Shanghai, China. Am J Transl Res. 2022;14:7831-7841. [PubMed] |
| 35. | Liu DS, Wang YH, Zhu ZH, Zhang SH, Zhu X, Wan JH, Lu NH, Xie Y. Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob Resist Infect Control. 2019;8:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Lyu T, Cheung KS, Ni L, Guo J, Mu P, Li Y, Yang Q, Yu X, Lyu Z, Wu J, Guo H, Leung WK, Seto WK. High prevalence and risk factors of multiple antibiotic resistance in patients who fail first-line Helicobacter pylori therapy in southern China: a municipality-wide, multicentre, prospective cohort study. J Antimicrob Chemother. 2020;75:3391-3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Liu X, Shen X, Chang H, Huang G, Fu Z, Zheng Y, Wang L, Li C, Liu L, Shen Y, Yang Y. High macrolide resistance in Streptococcus pyogenes strains isolated from children with pharyngitis in China. Pediatr Pulmonol. 2009;44:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Megraud F, Bruyndonckx R, Coenen S, Wittkop L, Huang TD, Hoebeke M, Bénéjat L, Lehours P, Goossens H, Glupczynski Y; European Helicobacter pylori Antimicrobial Susceptibility Testing Working Group. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 39. | Graham DY, Liou JM. Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship. Clin Gastroenterol Hepatol. 2022;20:973-983.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 40. | Chen J, Li P, Huang Y, Guo Y, Ding Z, Lu H. Primary Antibiotic Resistance of Helicobacter pylori in Different Regions of China: A Systematic Review and Meta-Analysis. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 41. | Dang NQH, Ha TMT, Nguyen ST, Le NDK, Nguyen TMT, Nguyen TH, Pham TTH, Tran VH. High rates of clarithromycin and levofloxacin resistance of Helicobacter pylori in patients with chronic gastritis in the south east area of Vietnam. J Glob Antimicrob Resist. 2020;22:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Gao C, Fan YH. Effect and Safety of Helicobacter pylori Eradication Treatment Based on Molecular Pathologic Antibiotic Resistance in Chinese Elderly People. Infect Drug Resist. 2022;15:3277-3286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 43. | Gao C, Du SY, Fang L, Fan YH, Song AP, Chen H. Eradication Treatment of Helicobacter pylori Infection Based on Molecular Pathologic Antibiotic Resistance. Infect Drug Resist. 2020;13:69-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin Y, Turkey; Ulasoglu C, Turkey S-Editor: Lin C L-Editor: A P-Editor: Yu HG