Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1815
Peer-review started: December 5, 2023
First decision: January 27, 2024
Revised: February 22, 2024
Accepted: March 13, 2024
Article in press: March 13, 2024
Published online: April 7, 2024
Processing time: 120 Days and 3.5 Hours
Colorectal cancer (CRC) is a complex disease with diverse etiologies and clinical outcomes. Despite considerable progress in development of CRC therapeutics, challenges remain regarding the diagnosis and management of advanced stage metastatic CRC (mCRC). In particular, the five-year survival rate is very low since mCRC is currently rarely curable. Over the past decade, cancer treatment has significantly improved with the introduction of cancer immunotherapies, specifically immune checkpoint inhibitors. Therapies aimed at blocking immune checkpoints such as PD-1, PD-L1, and CTLA-4 target inhibitory pathways of the immune system, and thereby enhance anti-tumor immunity. These therapies thus have shown promising results in many clinical trials alone or in combination. The efficacy and safety of immunotherapy, either alone or in combination with CRC, have been investigated in several clinical trials. Clinical trials, including KEYNOTE-164 and CheckMate 142, have led to Food and Drug Administration approval of the PD-1 inhibitors pembrolizumab and nivolumab, respectively, for the treatment of patients with unresectable or metastatic microsatellite instability-high or deficient mismatch repair CRC. Unfortunately, these drugs benefit only a small percentage of patients, with the benefits of immunotherapy remaining elusive for the vast majority of CRC patients. To this end, primary and secondary resistance to immunotherapy remains a significant issue, and further research is necessary to optimize the use of immunotherapy in CRC and identify biomarkers to predict the response. This review provides a comprehensive overview of the clinical trials involving immune checkpoint inhibitors in CRC. The underlying rationale, challenges faced, and potential future steps to improve the prognosis and enhance the likelihood of successful trials in this field are discussed.
Core Tip: Colorectal cancer (CRC) often eludes early detection, limiting the efficacy of existing chemotherapy and targeted therapies. This article delves into the realm of immune checkpoint inhibitors in CRC, dissecting their mechanisms and outcomes through a comprehensive review of clinical trials. It sheds light on the underlying rationale, challenges faced, and potential strategies to improve prognosis and trial success in this critical domain. Notably, while microsatellite instability-high CRC exhibits heightened responsiveness to checkpoint inhibitors, the article underscores potential breakthroughs in treating microsatellite stable CRC-the predominant cases-providing insights into bettering prognosis and trial outcomes in CRC treatment.
- Citation: Sharma S, Singh N, Turk AA, Wan I, Guttikonda A, Dong JL, Zhang X, Opyrchal M. Molecular insights into clinical trials for immune checkpoint inhibitors in colorectal cancer: Unravelling challenges and future directions. World J Gastroenterol 2024; 30(13): 1815-1835
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1815
Colorectal cancer (CRC) is a prevalent malignancy recognized worldwide for its intricate pathogenesis, diverse etiologies, and clinical outcomes[1,2]. Approximately 147950 new cases are expected to be diagnosed in 2023, along with an estimated number of 53200 deaths due to CRC[3]. Moreover, the incidence of early onset CRC is increasing as well[4]. CRC arises from the malignant transformation of epithelial cells lining the colon or rectum. The development of CRC is influenced by a multitude of risk factors, including advanced age, dietary choices, obesity, and inflammatory bowel disease[5-7]. The molecular pathogenesis of CRC is complex, with genetic and epigenetic alterations that drive tumorigenesis and contribute to disease progression[8-12]. These alterations intricately disrupt essential signaling pathways, such as WNT/β-catenin pathway, KRAS/BRAF/MEK/ERK pathway, and PI3K/AKT/mTOR pathway governing critical cellular processes, including cell proliferation, differentiation, and survival[8,9,13-15].
Currently, several approaches are employed for CRC treatment, including surgical procedures, chemotherapy, radiation therapy, targeted therapy, and immunotherapy[16]. However, following preliminary diagnosis, the 5-year survival rate of CRC patients is 65.0%, which significantly decreases to approximately 13% for metastatic CRC (mCRC)[17]. Treatment of advanced or mCRC is hindered by several challenges. Treatment options are particularly limited for patients who have exhausted multiple lines of treatment. Additionally, CRC tumors can develop resistance to chemotherapy, diminishing treatment efficacy over time[18,19]. The toxicity associated with chemotherapy and targeted therapies further complicates treatment and affects patients' quality of life. mCRC also has poor prognosis. Tumor heterogeneity adds another layer of complexity, contributing to treatment resistance and variability in patient responses[20,21]. To this end, immunotherapy targeting immune checkpoints such as PD-1/PD-L1 axis and CTLA-4 shows promise in treating advanced CRC, particularly in CRC tumors with microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR)[22]. Combination therapies, involving immunotherapy with chemotherapy, targeted therapies, and other immunomodulators, further offer the potential of synergistic effects and enhanced treatment efficacy[23,24]. Ongoing research efforts on predictive biomarkers, such as tumor mutational burden (TMB) and immune cell infiltration patterns, aim to identify patients most likely to benefit from immunotherapy[25,26]. Hence, immunotherapy holds promise as a transformative approach for the management of advanced or mCRC with durable responses and improved patient outcomes.
Immunotherapy using immune checkpoint inhibitions (ICIs) has recently emerged as a promising therapeutic approach for various cancers, including CRC. Understanding the immune infiltration patterns in CRC patients with microsatellite-stable (MSS) vs microsatellite-instability (MSI) phenotype is crucial for developing immunotherapeutic strategies. While MSI-H tumors may benefit from immunotherapy due to their higher immune infiltration and mutational load, MSS tumors may still require alternative or combination approaches to enhance the antitumor immune response and improve treatment outcomes[27,28]. To this end, ongoing research efforts aim to unravel the complexities of immune infiltration in different CRC subtypes to guide the development of more effective and personalized therapeutic interventions. Initial studies conducted between 2010 and 2013 showed limited clinical activity of ICIs in patients who were not selected based on specific biomarkers or treatment history[29-33]. Eventually, several promising findings have led to the approval of ICIs for MSI-H or dMMR CRC. Nonetheless, a low response to immunotherapy remains a significant challenge in the treatment of MSS or proficient MMR (pMMR) CRC, highlighting the need for further research to enhance effectiveness and identify biomarkers to improve treatment outcomes[34,35]. Newer immunotherapeutic approaches have been investigated for CRC treatment, including cancer vaccines, adoptive T-cell therapy, and oncolytic viruses[36,37]. These approaches aim to stimulate the immune response against cancer cells by various means, including inducing antigen-specific T cell responses, genetically modifying T-cells to recognize and attack cancer cells, and using viruses to selectively target and destroy cancer cells[38,39]. A multitude of clinical trials, spanning both ongoing and concluding studies, have been conducted to explore the efficacy and safety of diverse drugs and combination therapies for CRC treatment. This review provides insights into the current landscape, challenges, and potential advancements in this field. CRC clinical trials involving ICIs and their mechanistic actions are outlined, treatment strategies and the future trajectory of CRC therapeutics are discussed.
Molecular characterization of CRC has identified two major subtypes, MSS and MSI CRC that account for approximately 85% and 15% of all CRC cases, respectively[40-42]. Clinical and pathological features of MSS CRC differ from those of MSI CRC[2]. Specifically, MSS CRC is typically associated with older age, male sex, and distal colon location, whereas MSI CRC is associated with younger age, female sex, proximal colon location, and better prognosis[41]. Furthermore, the MMR status and CRC are intricately linked due to their role in maintaining genome integrity and preventing the accumulation of mutations that can lead to cancer[43,44]. MSI-H CRC tends to have a dMMR status, a higher mutational load, and a distinct molecular profile compared to MSS CRC, which has a pMMR[43-45]. In particular, MSI and MMR status are predictive biomarkers for response to ICIs therapy[34,42]. The consensus molecular subtype (CMS) classification system divides CRC into four distinct subtypes based on gene expression profiles: CMS1 (immune), CMS2 (canonical), CMS3 (metabolic), and CMS4 (mesenchymal)[46]. Each CMS subtype has a distinct molecular signature and clinical phenotype. The system thus offers a clear biological understanding and serves as a foundation for future clinical stratification and targeted interventions based on specific subtypes.
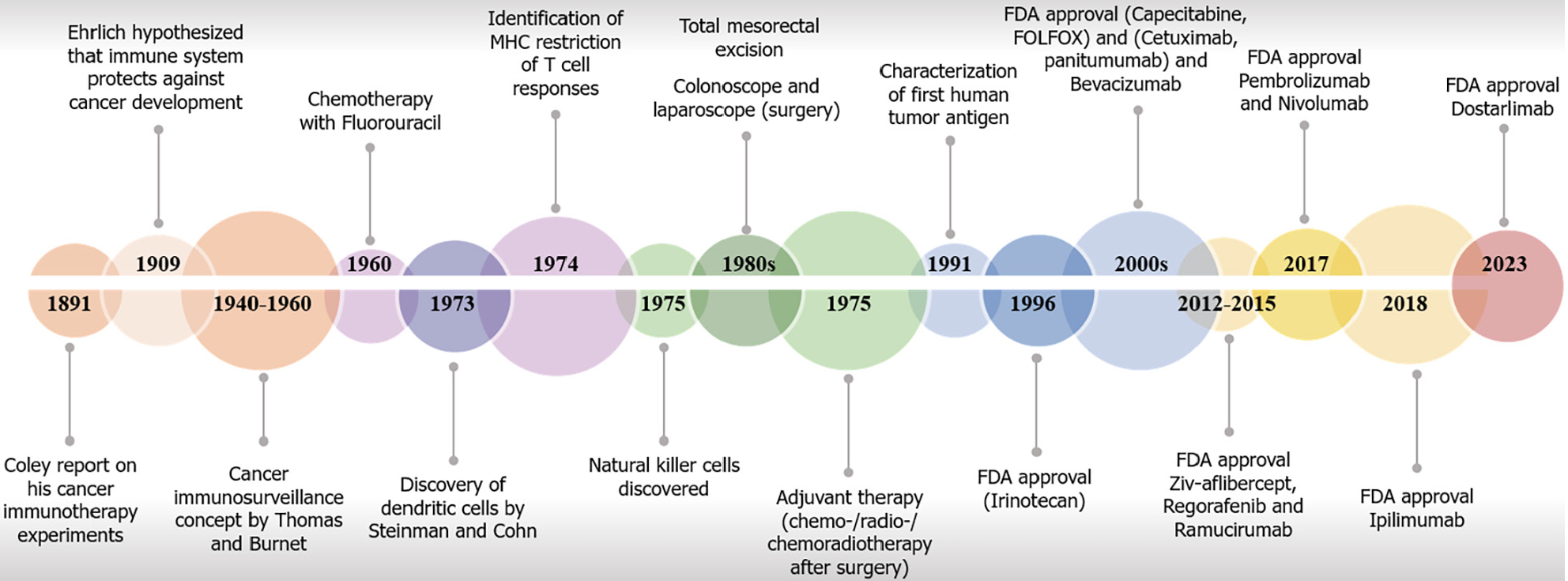
Over the years, substantial progress has been achieved in the development of CRC therapeutics, resulting in enhanced survival rates attributed to advancements in primary and adjuvant treatment modalities[47]. Notably, the inclusion of chemotherapy as a neoadjuvant or adjuvant intervention has emerged as a strategic approach aimed at mitigating the tumor burden, reduction, and stabilization[48-51]. Chemotherapy maintains its pivotal status in current treatment strategies. However, the utility of chemotherapy is curtailed by a restricted therapeutic range, significant adverse reactions, and the frequent occurrence of acquired resistance[52]. Several chemotherapy agents, radiotherapies, and other physical forces also induce destruction of cells and tissues, leading to death of immune cells and subsequently enhancing therapeutic outcomes[53-56]. Immunotherapy with checkpoint inhibitors has provided a significant improvement in cancer treatment, demonstrating high efficacy and manageable side effects in various tumor types[57-62]. However, the success of immunotherapy in CRC patients remains limited, with only a small subset of cases characterized by MSI-H or dMMR benefiting from treatment[63,64]. Thus, despite over 50 decades of research on immunotherapy for CRC treatment and major advancements, significant challenges remain in the diagnosis and management of CRC, particularly in the context of advanced or metastatic disease[27] (Figure 1).
Immuno-oncology is an emerging field of cancer treatment that involves harnessing the patient’s immune system to recognize and eliminate cancer cells[64]. Immunotherapy can potentially improve treatment outcomes for patients with a wide range of malignancies[38]. Immunotherapy is often considered more beneficial than chemotherapy due to its ability to induce durable immune responses. Unlike chemotherapy, which primarily induces short-term cytotoxic effects on cancer cells and eliminates immunosuppressive cells[65-67], immunotherapy activates the immune system, particularly cytotoxic T-cells, to recognize and target cancer cells[38]. Consequently, immunotherapy offers the potential for sustained protection against cancer by maintaining an immunological "memory" that can detect and eliminate cancer cells in case of re-encounter[68,69]. Immunotherapy for cancer has thus brought about revolutionary transformations in the field of oncology, extending the survival of individuals diagnosed with aggressive life-threatening malignancies[57-61]. CRC patients with MSI-H or dMMR status show higher mutation rates, more neoantigens, and increased tumor-infiltrating lymphocytes (TILs), particularly cytotoxic T-cells[70], fostering a robust antitumor immune response. In contrast, MSS tumors have an immunosuppressive microenvironment with regulatory T-cells (Tregs) and other immunosuppressive cell types that hinder effector T cell activity[71,72]. Eventually, the prognostic value of the immunoscore was initially established in individuals with colon cancer, showing its ability to assess prognosis based on factors such as the density, type, and localization of infiltrating immune cells[73].
ICIs are drugs that blocks certain key proteins on the surface of immune cells, particularly T-cells, and cancer cells, and release the brakes on the immune response. The development of ICIs has been a breakthrough in the field of cancer immunotherapy[36,69,74-76]. These proteins, known as immune checkpoints, play crucial roles in regulating the immune response. By blocking these checkpoints, ICIs enhance the ability of the immune system to recognize and attack cancer cells, thereby boosting the body's natural anti-cancer response[38]. ICIs have become a cornerstone of cancer therapy, with a wide range of approved agents available for multiple malignancies, leading to increased utilization in various treatment settings, including (neo)adjuvant and maintenance therapy. Thus, ICIs are accessible to nearly half of metastatic cancer patients in economically developed countries[77-79].
A solid understanding of the molecular drivers of CRC and identification of biomarkers of treatment response are essential for improving immunotherapy outcomes in patients with this disease[28,73,80-85]. A key element is the high TMB caused by genetic or sporadic mutations in MMR genes (such as MLH1, MSH2, MSH6, and PMS2), resulting in a deficiency of MMR proteins. This leads to an accumulation of genetic mutations in microsatellites[15,41], as observed in MSI-H or dMMR CRC tumors, compared to MSI-low (MSI-L) or pMMR. Consequently, enhanced immunogenicity is observed in such CRC cases, characterized by a higher count of neoantigens and substantial immune cell infiltration, including high numbers of tumor-infiltrating immune cells, such as CD8+ and CD4+ T-cells and macrophages[40,73,86-90]. Additionally, these tumors exhibit a microenvironment enriched with type I interferons, which distinguishes them from other CRC subtypes[87]. This immune-rich trait has been linked to improved rates of response to ICIs that block the PD-1/PD-L1 axis and T-cell activation[40,91]. In contrast, CRC tumors exhibiting pMMR along with MSS exhibit a low burden of mutations and low infiltration of CD4 and CD8 immune cells, resulting in evasion of the immune response.
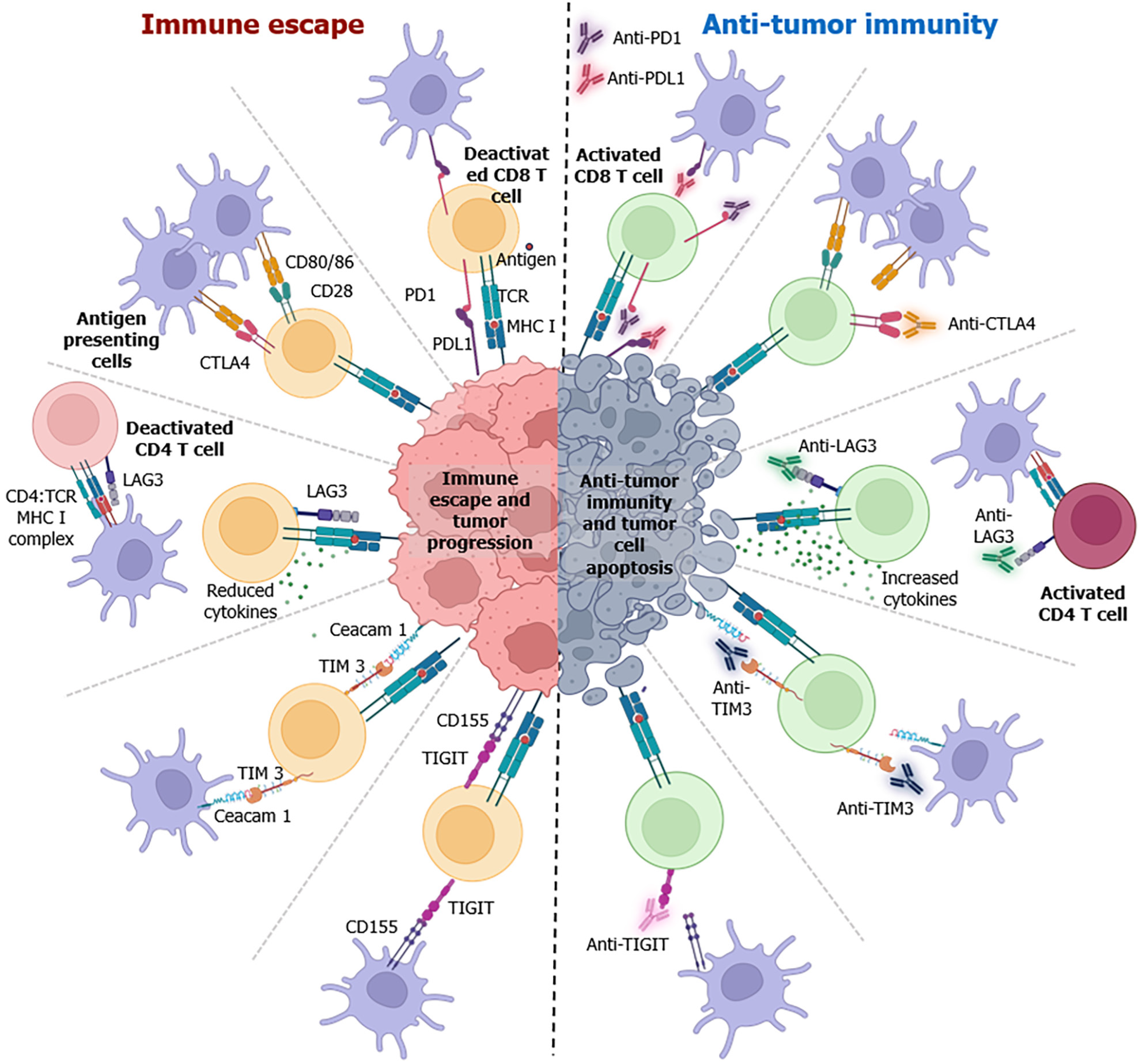
ICIs enhance the recognition and elimination of cancer cells by activating the immune system, resulting in a more potent and sustained anticancer immune response. Understanding the mechanisms of synchronization with the disease pathophysiology is crucial for optimizing the therapeutic potential of ICIs and improving patient outcomes. The main mechanisms of action of ICIs include blockade of the PD-1/PD-L1 pathway and CTLA-4, which regulates T-cell activity and is often upregulated in tumors to evade the immune system (Figure 2)[38]. Indeed, PD-1 and CTLA-4 serve as negative regulators of T-cell activation and exert their biological effects at specific anatomical locations and at various points throughout the lifespan of T-cells[92]. The varied and late-onset autoimmune manifestations observed in Pdcd1–/– mice differ significantly from those in Ctla4-/- animals, highlighting that the PD1 axis governs T-cell biology in a distinct manner compared to CTLA4[92,93].
The PD-1/PD-L1 axis plays a role in autoimmunity by negatively regulating T-cell activation[94]. Functional loss of PD1 protein results in splenomegaly in mice models[94]. Additionally, mouse models lacking the PD-1 gene exhibit cardiac inflammation, leading to dilated cardiomyopathy and accelerated type 1 diabetes mellitus[95,96]. The PD1 axis is crucial for regulating differentiated effectors in T-cells[93,97,98]. Upon binding to PD-L1, PD1 exerts inhibitory intracellular signaling, leading to T-cell exhaustion, and eventually suppressing the immune response[99-101]. In addition to its role in regulating conventional T-cells, PD-L1 on antigen presenting cells (APCs) also plays a role in controlling Treg differentiation and immunosuppressive activity[102]. Tumor cells upregulate PD-L1 surface expression to take advantage of the PD-1/PD-L1 axis and escape immune response.
Anti-PD-1 antibodies such as pembrolizumab and nivolumab are ICIs used in cancer immunotherapy. Anti-PD-1 antibodies not only enhance the activity of cytotoxic T-cells but also affect the overall tumor microenvironment (TME) as well. These antibodies can alter the balance of immune cell populations by reducing the number of immunosuppressive cells such as Tregs and myeloid-derived suppressor cells[103]. This shift contributes to a more favorable immunological milieu for anti-tumor responses[104]. Anti-PD-1 antibodies promote increased production of pro-inflammatory cytokines, such as IFN-γ. These cytokines play key roles in amplifying the anti-tumor immune response by activating other immune cells and enhancing the recognition and elimination of cancer cells. In contrast, anti-PD-L1 antibodies target PD-L1 ligands on cancer cells. By blocking the interaction between PD-L1 and PD-1 on T-cells, these antibodies disrupt a key immune evasion mechanism employed by cancer cells. Anti-PD-1/PD-L1 antibodies help overcome adaptive immune resistance by enabling T-cells to recognize and target cancer cells more effectively. This leads to continuous adaptation of the immune response against evolving tumor cells. Immunotherapeutic responses are often associated with the expression of specific immunological biomarkers[105-107]. For anti-PD-1/PD-L1 therapy, the expression of PD-L1 on tumor cells is a commonly used biomarker. Tumors with high PD-L1 expression may have a higher likelihood of responding to anti-PD-1 antibodies. The presence of TILs is also considered a positive prognostic indicator of immunotherapy response.
CTLA-4, a vital immune checkpoint, exhibits low basal levels in conventional T-cell. However, its expression is significantly induced after antigen activation. Activated T-cells expressing CTLA4 impede the interaction between B7-1 and B7-2 molecules on APCs and CD28, and thereby induce anergy and reduce T-cell activation[108-110]. TCR signaling studies affirm CTLA4's role in inhibiting T-cell activation and proliferation[111-113]. Ctla4-knockout mice were found to develop T-cell mediated autoimmune disease, which is mitigated by treatment with the CTLA4: Fc fusion protein (CTLA4Ig)[114-116]. Notably, CD4+ CD25+ Tregs, which are known for their immunosuppressive function, constitutively express CTLA4 and are necessary for the release of anti-inflammatory cytokines from Tregs[117,118]. These findings confirm that CTLA4 is a T-cell activation inhibitor with potential as a therapeutic agent against cancer[119]. Pre-clinical studies using anti-CTLA-4 antibodies aimed to prevent inhibitory signals, allowing for a more effective CD28 interaction with B7[120]. However, the results were found to depend on tissue specificity and tumor size[119,121]. Additionally, blocking CTLA4 enhances T-cell responses to tumor-associated neoantigens, and a high neoantigen burden predicts a positive response to anti-CTLA4 therapy.
Blocking CTLA-4 with anti-CTLA-4 antibodies promotes a sustained and enhanced T-cell activation. CD28 is a co-stimulator of T-cell activation that benefits from the increased availability of anti-CTLA-4 antibodies and facilitates enhanced binding to B7. This then amplifies co-stimulatory signals, promoting T-cell proliferation and function[120]. These antibodies also modify the TME by decreasing immunosuppressive cells, such as Tregs, creating a more favorable setting for anti-tumor immune responses[122,123]. Additionally, anti-CTLA-4 antibodies induce antibody-dependent cell-mediated cytotoxicity, with immune cells, particularly natural killer cells, recognizing and eliminating target cells marked with therapeutic antibodies[124]. Often used in combination with other ICIs, such as anti-PD-1 or anti-PD-L1 antibodies, this approach targets multiple checkpoints simultaneously, enhancing the overall anti-tumor immune response. Anti-CTLA-4 therapy induces systemic immune activation, affecting not only the tumor site but also distant metastases, contributing to the potential for durable responses and efficacy.
ICIs can also enhance antigen presentation by dendritic cells (DCs), and thus facilitate the priming of T-cells to initiate a robust and targeted immune response against cancer[125,126]. Activated DCs present tumor antigens to T-cells effectively to promote T-cell activation and proliferation. Adaptive immune resistance involves a dynamic interplay between the immune system and cancer cells. Successful ICIs therapy is associated with memory T-cell generation. Memory T-cells contribute to long-term immune memory by enabling the immune system to respond rapidly to cancer cell recurrence. Understanding these immunological nuances provides insights into how ICIs contribute to unleashing and potentiating the ability of the immune system to recognize and eliminate cancer cells. Despite its success in MSI-H CRC, the clinical efficacy of immunotherapy in MSS CRC remains very limited[28]. Recent studies have found that characteristics such as high levels of TILs and expression of immune checkpoint molecules such as PD-L1 may help identify patients with MSS CRC who are more likely to benefit from ICI treatment.
The emergence of checkpoint inhibitors has brought a remarkable shift in our approach to treatment of cancers, including CRC. Notably, these inhibitors have exhibited encouraging treatment outcomes in specific subsets of patients, such as those with MSI-H or dMMR tumors, which are characterized by augmented levels of TILs and heightened susceptibility to immune checkpoint blockade. This section focuses on noteworthy clinical studies on application, efficacy, and potential benefits of ICIs in CRC. In 2014, the Food and Drug Administration (FDA) approved Pembrolizumab, a PD-1 immune checkpoint inhibitor for melanoma treatment[127]. Tumor cells evade the immune system through the PD-1 pathway where PDL1 and PDL2 Ligands on tumor cells binds to the PD-1 receptors on T cells to inactivate T cells. Pembrolizumab binds to these PD-1 receptor and blocks their interaction with PDL1 and PDL2, thereby restoring the immune response[128]. Subsequently, in 2020, the FDA approved this drug for patients with unresectable or metastatic MSI-H or dMMR CRC, based on results from key clinical trials. Phase 2 open-label, multicenter trial (NCT01876511) was conducted to evaluate the safety, efficacy, and tolerability of pembrolizumab in MSI-H-positive patients[129]. Trials have shown no dose-limiting toxicities associated with pembrolizumab, with a promising disease control rate of 80% in patients with MSI-positive CRC, suggesting the potential of Pembrolizumab in CRC treatment. Subsequently, another trial (NCT02460198) postulated the efficacy of pembrolizumab in patients with unresectable tumors who underwent standard chemotherapy[130,131]. The results showed a promising overall progression response rate of 32 to 34 months in both cohorts. This study demonstrated the potential of pembrolizumab as an effective treatment option for patients with dMMR and MSI-H mCRC.
Chemotherapy has been used over the years for the treatment of patients with CRC to shrink tumor volume[132]. In 2015, a phase 3 clinical trial (NCT02563002) was conducted to test pembrolizumab as a first-line treatment in comparison with standard chemotherapy treatment in mCRC patients with MSI-H or dMMR tumors[133,134]. The results showed a significant improvement in the progression-free survival (PFS) rate of 16.5 months in comparison to the standard chemotherapy group at 8.2 months. The trial demonstrated pembrolizumab monotherapy to be superior to standard chemotherapy in terms of PFS and overall response rate (ORR) for patients with MSI-H or dMMR mCRC as a standard care option. The potential efficacy of pembrolizumab in these patients was demonstrated by increased production of neoantigens resulting from an elevated mutational burden. This, in turn, leads to a heightened recognition of tumor cells by cytotoxic T cells, which are primed by blocking the interaction between PD-1 and PD-L1. These results thus led to a paradigm shift in the treatment of this patient population.
The evolution of pembrolizumab has led to the development of more PD-1-targeting drugs whose efficacy and safety profiles were assessed. Nivolumab, another PD-1 monoclonal antibody, was approved in 2017 for use in mCRC treatment[28]. Both drugs exhibited similar modes of action in blocking PD1 and inducing increased CTLs cytotoxicity. However, these two antibodies also exhibited significant structural differences in their binding to PD-1[135]. The epitope region of pembrolizumab displayed a considerably larger overlap with the PD-L1 binding site compared to that of nivolumab. Notably, the binding sites of pembrolizumab and nivolumab on PD-1 showed almost no convergence[135]. A study published in 2017 compared the effectiveness of drugs with comparable effectiveness, which may potentially be interchangeable. The effectiveness of nivolumab has been studied in NCT02060188 MSI-H or dMMR mCRC patients with or without the CTLA-4 inhibitor drug Ipilimumab[136-138]. The treatment showed promising results, with a disease control rate of 80% with nivolumab alone. Combination treatment with a CTLA-4 inhibitor was effective in 51 of the 74 patients who achieved disease control for a minimum of 12 wk. However, further studies are still needed to determine the optimal treatment duration for pembrolizumab and to identify predictive biomarkers of response to immunotherapy in this population. Overall, Nivolumab plus ipilimumab combination therapy is a promising treatment with a better disease control rate and objective response.
Atezolizumab, a monoclonal antibody targeting PD-L1, was approved by the FDA in 2016 for the treatment of non-small cell lung cancer tumors[139]. The mechanism of action of Atezolizumab differs from those of pembrolizumab and nivolumab. Instead of binding to PD-1, atezolizumab binds to PD-L1 on tumor cells, and thereby provides a mode of action similar to that of the PD-1 antibody. A clinical trial (NCT02788279) was conducted to evaluate the efficacy of this drug alone and in combination with a MEK inhibitor, cobimetinib, compared to regorafenib (a multi-kinase inhibitor)[140]. This combination is used as a therapeutic alternative to a MEK inhibitor that increases T-cell proliferation and CD8+ T cell infiltration, and PD-1 treatment to upregulate the PD-1/PD-L1 interaction, thereby downregulating the immunosuppressive TME. The results of the trial showed an improved PFS rate 1.91 of 2 months after cobimetinib treatment. In conclusion, combination of atezolizumab and cobimetinib improved PFS and ORR compared to regorafenib as a second-line treatment for patients with mCRC, yet did not significantly improve OS. These results underscore the potential of combination therapy and suggest improved investigations of combined immunotherapy drugs as promising targeted treatment approaches for mCRC. Combination therapy with MEK inhibition and PD-L1 blockade led to impressive long-term survival rates. MEK inhibitors act during the post-naïve stage of T-cell differentiation. MEK inhibition counteracts the expression of Nur77, which is associated with exhaustive T cell death induced by antigen-specific CD8+ T cells, thereby rescuing T cell exhaustion[16].
Another PD-1 monoclonal antibody, dostarlimab, was approved in 2021 and tested in clinical trials (NCT04165772) in patients with MSI-H or dMMR CRC[141]. A high-resolution structure revealed that Dostarlimab binds to the flexible loops of PD-1, including the BC, C’D, and FG loops, in contrast to the binding modes of Pembrolizumab or Nivolumab[94]. The initial findings of the trials were published for 12 patients. Accordingly, all the patients (100%; 95%CI: 74-100) showed a complete clinical response[142]. The response was confirmed using magnetic resonance imaging, which showed no evidence of tumors. At that time, none of the patients had received chemoradiotherapy or undergone surgery. No cases of progression or recurrence were observed during follow-up (6–25 months). The study listed no grade 3 or higher AEs during the trial period. This study thus demonstrated the high sensitivity of dMMR locally advanced rectal cancer to single-agent PD-1 blockade. Despite these promising results, a longer follow-up period and a larger sample size are still needed to assess the duration of the response.
Following the improved success of ICIs in CRC patients with MSI-H or dMMR tumors, researchers have investigated their efficacy in MSS or pMMR CRC patients as well. These data suggested that more than 85% of CRC patients with MSS tumors show resistance to ICIs therapy. A clinical trial (NCT01876511) illustrating the potential of pembrolizumab in MSI-H or dMMR CRC patients has shown no measurable responses in any of the 18 patients with pMMR CRC, as defined by the RECIST criteria[129]. To overcome these limitations, combination treatments have been used to improve the drug responses. A clinical trial was conducted using PD-L1 with a multi kinase inhibitor in patients with MSS or pMMR CRC. Regorafenib targets stromal and oncogenic receptor tyrosine kinases, and shows anti-angiogenic activity due to its dual-target VEGFR2-TIE2 tyrosine kinase inhibition[143]. The NCT04126733 trial conducted between 2019 and 2022 included 94 CRC patients with MSS or pMMR[144]. The results showed an ORR of 7% and overall survival (OS) rate of 11.9%. The relatively reduced success observed in patients with MSS or pMMR CRC indicates the need for further advancement of drug efficacy to provide better outcomes in MSS tumors.
In 2016, a combination trial (NCT01988896) was performed in CRC patients with BRAF/KRAS mutations using cobimetinib and atezolizumab to study OS and PFS in MSS or pMMR CRCs patients[145]. These mutations are rarely identified but are more frequently found in patients with MSS CRC. BRAF and KRAS mutations are mutually exclusive, and BRAF-mt induces aberrant and inappropriate activation of the MAPK/ERK pathway, making it a good candidate for combination therapy with ICIs and kinases or MEK inhibitors[146]. As expected, the combination provided relatively better outcomes in patients with BRAF/KRAS mutations, with an OS rate of 43%. These findings provide compelling evidence that MAPK pathway blockade therapy combined with ICIs is promising for improving treatment efficacy in mCRC patients with MSS/pMMR BRAF mutations.
Recent developments have improved targeted immunotherapies using engineered ICIs to increase the success rate in patients with MSS or pMMR CRC[147]. A phase 1 clinical trial was conducted to categorize the adverse effects and dose-limiting toxicity of botensilimab, an Fc-engineered anti-CTLA-4 monoclonal antibody, in patients with MSS CRC. This Fc engineering promotes intratumoral Treg depletion and reduces complement fixation. This modification provides optimized T-cell priming, activation, and memory formation by strengthening antigen-presenting cell/T-cell co-engagement. The trial showed an ORR of 22% (95%CI: 12-35) and a disease control rate of 73% (95%CI: 42-75) for patients with non-hepatic disease in refractory CRC. The trial showed the efficacy of the anti-tumor activity in MSS CRC patients with active liver metastatic disease, and a phase 2 trial (NCT05608044) for MSS CRC has begun to study its potential in controlling tumor progression.
The success of ICIs in patients with MSI-H CRC has been hindered by their reduced potential in MSS CRC. The results from clinical trials in patients with MSS CRC undergoing immune checkpoint blockade immunotherapy suggest the need for the development of new pre-clinical mouse models to replicate the microenvironment of human CRC, and potentiate new targeted therapies to improve patient survival.
Although ICI therapy holds benefits, patients also often experience autoimmune-like effects known as immune-related adverse events (irAEs). This is likely the result of generalized, non-antigen-specific activation of the immune system following a checkpoint blockade. Inhibition of a naturally occurring central immune checkpoint releases potent immune effector mechanisms that may not adhere to the usual boundaries of immune tolerance towards self-tissues[148]. Human loss-of-function mutations in CTLA4 and its regulatory partner, LPS Responsive Beige-Like Anchor Protein, mimic the immune-related side effects of anti-CTLA4 therapy[149,150]. irAEs have been reported in 15%–90% of patients[57], with severe events requiring intervention being observed in 30% and 15% of patients treated with CTLA4 and PD1 axis inhibitors, respectively[151]. This immune checkpoint inhibitor leads to toxicity in naïve T cells and accumulation of memory T cells in peripheral organs[152,153]. Compared with PD-1, CTLA4 therapy possess with severe autoimmune complications, as seen in pre-clinical and clinical trials[154].
Colitis is a frequent complication observed in ICIs therapy[155]. Anti-CTLA4 treatment resulted in a potentially higher colitis rate, ranging from 5.7% to 22% of patients, compared to 0.7% to 1.6% with anti–PD-1 agents[156]. The development of ICI-mediated colitis and diarrhea (IMC) may involve cytotoxic CD8+ T cells. Recent analysis of single-cell RNA sequences from patients with IMC revealed an expansion of tissue-resident memory CD8+ T cells into inflammatory populations within the colon tissue, suggesting that the activation or alteration of CD8+ T cell populations could be a potential mechanism for colitis induced by ICIs[157]. Therefore, with the potential use of immune checkpoint blockade, the current research should aim to identify potential predictive markers for organ-specific toxicities caused by immunotherapy.
Patients comprising MSI-H or dMMR tumors have a significantly high overall mutation burden, with approximately 12 mutations per million DNA bases. In contrast, pMMR/MSI-L tumors have a relatively reduced tumor burden, with fewer than eight mutations per million DNA bases[158]. This phenomenon is primarily attributed to somatic defects in the function of MMR genes, with the most prevalent mechanism being hypermethylation of the MLH1 promoter, which serves as a prognostic marker. In MSI, frameshift mutations in protein-coding sequences can create diverse peptides that serve as potential neoepitopes, which are recognized as foreign by the immune system. Mutant peptides form complexes with major histocompatibility complex class I molecules and act as foreign neoantigens that initiate immune cell priming and infiltration. Within the TME, tumor-associated macrophages play a crucial role in influencing tumor growth and progression. Recent study has demonstrated a frameshift mutation in the TGFβRII producing an immunogenic peptide called p538[159]. This peptide is present in over 90% of tumors with dMMR, indicating its broad relevance in the field. These tumors exhibit robust infiltration by immune cells, particularly CD8+ TILs, Th1 CD4+ TILs, and macrophages[73]. Furthermore, the microenvironment of these tumors is notably enriched in type I interferons, which distinguishes them from other CRCs types.
Approximately 15% of all CRCs exhibit MSI-H or dMMR characteristics[160]. Patients with MSI-H or dMMR tumors before ICIs therapy continue to have a poor prognosis. Cancers show significantly upregulated expression of PD1, PDL1, and CTLA4, rendering them potentially susceptible to ICIs[87]. In contrast, MSS or pMMR tumors lacking neoantigens are characterized by reduced T cell infiltration and elevated levels of immunosuppressive ligands. These characteristics offer insights into the disagreement between MSI-H or dMMR and MSS or pMMR CRCs in ICIs responses and could potentially serve as prognostic biomarkers for patient selection. As shown in previously described clinical trials, immunotherapy as a neoadjuvant approach has not shown any clinical benefit in patients with MSS or pMMR CRC, including individuals with mCRC.
Contrastingly, the MSS CRC, majorly referred to as an "immune cold" cancer type, are predisposed by various molecular factors contributing to the underlying resistance to immunotherapy[161]. MSS-CRC is characterized by larger chromosomal aberrations that mark the phenotype of MSS-CRC, resulting in a lower tumor mutation burden and reduced neoantigen configuration. This framework partially elucidates the disparate clinical responses to ICIs observed in these CRC subgroups. The MSS CRC TME hosts more tumor-associated macrophages, which have been associated with poor prognosis in most studies. Notably, a pioneer study identified that increased β-catenin activation (a downstream effect of APC mutation) resulted in reduced infiltration of CD8+ and CD103+ DCs, orchestrating an immune suppressive environment via T-cell exclusion[16]. The APC protein is mutated in more than 70% and 20% of MSS and MSI-H CRCs, respectively, driving the distinct oncogenesis mechanisms and subsequent “immune hot” and “immune cold” TME[162]. These differences are reflected in clinical trials with ICIs, where MSS tumors have very low response rates compared to MSI tumors (Table 1).
| Trial identified (number of patients) | Treatment groups | Patients enrolled | Primary and secondary outcomes |
| Checkpoint inhibitor: Pembrolizumab | |||
| NCT01876511 (n = 113) | Pembrolizumab | Cohort A: MSI positive (pMMR) CRC | Cohort A: ORR: 54.0% (95%CI: 37.0–69.0); PFS: 70% (95%CI: 57–86); OS: NA (95%CI: 151.86-NA) |
| Cohort B: MSI negative (dMMR) CRC | Cohort B: ORR: 0% (95%CI: 0.0–14.0); PFS: 16% (95%CI: 6–41); OS: 36.71 (95%CI: 21.29-69.43) | ||
| NCT02460198 (n = 124) | Pembrolizumab | mCRC with dMMR or MSI–H status Cohort A: Participants must have received prior treatment with standard therapies | Cohort A: ORR: 32.8 (95%CI: 21.3 to 46.0); PFS: 2.3 (95%CI: 2.1–8.1); OS: 31.4 (95%CI: 21.4–58) |
| Cohort B: Participants must have undergone at least one line of systemic standard of care therapy | Cohort B: ORR: 34.9 (95%CI: 23.3–48.0); PFS: 4.1 (95%CI: 2.1–18.9); OS: 47 (19.2–NA) | ||
| NCT02563002 (n = 307) | Arm A: Pembrolizumab | mCRC with high MSI–H or dMMR | Arm A: ORR: 45.1% (95%CI: 37.1–53.3); PFS: 16.5 (95%CI: 5.4–38.1); OS: NA (95%CI: 49.2–NA) |
| Arm B: mFOLFOX6/FOLFIRI/Bevacizumab/Cetuximab/Pembrolizumab | Arm B: ORR: 33.1% (95%CI: 25.8–41.1); PFS: 8.2 (95%CI: 6.1–10.2); OS: 27.6 (95%CI: 27.6–NA) | ||
| Checkpoint inhibitor: Nivolumab + Regorafenib | |||
| NCT04126733 (n = 94) | Regorafenib and Nivolumab | Patients with pMMR or MSS CRC | ORR: 7% (95%CI: 2.4–15.9); PFS: 1.8 (95%CI: 1.8–2.4); OS: 11.9 (95%CI: 7.0 to NA) |
| Checkpoint inhibitor: Nivolumab + Ipilimumab | |||
| NCT02060188 (n = 119) | Arm A: Nivolumab | MSI–H or dMMR mCRC | Arm A: No results posted |
| Arm B: Nivolumab + Ipilimumab | Arm B: ORR: 55% (95%CI: 45.2%–63.8%); PFS: 71% (95%CI: 61.4 to 78.7); OS: 85% (95%CI: 77.0 to 90.2) | ||
| Arm C: Cobimetinib + Nivolumab + Ipilimumab | Arm C: No results posted | ||
| Arm D: Nivolumab + Daratumumab | Arm D: No results posted | ||
| Arm E: Nivolumab + BMS–986016 | Arm E: No results posted | ||
| Checkpoint inhibitor: Atezolizumab | |||
| NCT02788279 (n = 363) | Arm A: Atezolizumab | Patients with mCRC (MSI or MSS status unknown) | Arm A: PFS: 1.94 (95%CI: 1.91 to 2.10); OS: 7.10 (95%CI: 6.05–10.05) |
| Arm B: Cobimetinib + Atezolizumab | Arm B: PFS: 1.91 (95%CI: 1.87 to 1.97); OS: 8.87 (95%CI: 7.00–10.61) | ||
| Arm C: Regorafenib | Arm C: PFS: 2 (95%CI: 1.87–3.61); OS: 8.51 (95%CI: 6.41–10.71) | ||
| NCT01988896 (n = 84) | Atezolizumab + Cobimetinib | Patients having BRAF/KRAS mutation in mCRC | ORR: 8% (7/84) (6 patients: MSS, 1 patient: MSI) |
| NCT01633970 (n = 10) | Arm A: Atezolizumab + Bevacizumab | No results posted | No results posted |
| Arm B: Atezolizumab + Bevacizumab + FOLFOX | |||
| Arm C: Atezolizumab + Carboplatin + Paclitaxel | |||
| Arm D: Atezolizumab + Carboplatin + Pemetrexed | |||
| Arm E: Atezolizumab + Carboplatin + Nab–paclitaxel | |||
| Arm F: Atezolizumab + Nab–paclitaxel | |||
| Checkpoint inhibitor: Durvalumab + Tremelimumab | |||
| NCT03122509 (n = 25) | Arm A: Durvalumab + Tremelimumab + Radiotherapy | Metastatic Colorectal Cancer (MSI or MSS status unknown) | Arm A: ORR: 8%; Stable response: 12%; Progressive disease: 76% |
| Arm B: Durvalumab + Tremelimumab + Ablation | Arm B: No participants enrolled | ||
| Checkpoint inhibitor: Dostarlimab | |||
| NCT04165772 (n = 200) | Arm A: Dostarlimab | Patients with dMMR rectal adenocarcinoma | Arm A: Complete response: 12 patients (100%; 95%CI: 74–100) |
| Arm B: Dostarlimab + Capecitabine or 5–FU + IMRT | Arm B: No participants enrolled | ||
Mouse- and cell-based models have been used for decades to investigate the molecular origins of CRC, and more recently, to identify drug and immune responses in specific CRC types (Figure 1). These efforts have yielded tremendous improvement in our basic understanding of the disease and TME. However, despite recent approvals, the majority of patients continue to have limited immunotherapy options. A recurring challenge highlighted in the literature is the absence of a mouse model that precisely replicates the progression of human CRC from adenoma and adenocarcinoma to metastasis, including changes in the microenvironment. Initial mouse models lacked significant penetrance of the metastatic phenotype, often forming tumors in the small intestine rather than in the colon, unless induced by laparoscopy[163,164]. In 2013, the National Institute of Health formally concluded the Mouse Models in Human Cancer Consortium, leading researchers to explore alternative models, such as patient-derived xenografts and patient-derived organoids, to study the disease. The significance of the TME in metastasis remains a focus of current research, and the potential of checkpoint inhibitors and other immunological and inhibitor therapies are being explored. However, an ideal model still does not exist, highlighting the importance of an immunocompetent autochthonous model. Notably, single-cell RNA sequencing of mouse tumors to understand the mechanisms underlying immune-modulating therapies could help draw more impactful conclusions[165]. Despite these limitations, the diversity of the methods employed by researchers with mice, the adaptability of the system, and the deductive formation of CRC images from diverse models remain impressive.
Various transplantation techniques have been introduced over the years to replicate complex TME in mouse models. The subcutaneous injection model has been widely used[166], yet it has limitations, such as creating an ectopic environment and lacking accuracy in mimicking the metastatic spread of cancer[167]. The orthotopic transplantation model has emerged as a promising alternative to address these issues. This involves a precise injection into the intestinal region, such as the cecal wall, colon, or rectum. Among these, the orthotopic CRC model using cecal wall injections has been widely adopted. However, it is essential to recognize that even this model has constraints as it does not faithfully replicate the anatomical location where tumors typically occur in humans and exhibits a microenvironment distinct from the rest of the colon[168].
Genetically engineered mouse models (GEMMs) are based on genetic engineering techniques, particularly the Cre recombinase lox-P system, to simulate tumorigenesis by modifying the structure of the genome[169]. GEMMs, including those incorporating mutations in genes, such as APC, KRAS, p53, and MSH2, provide valuable insights into the molecular mechanisms underlying CRC and play a pivotal role in the study of CRC development and therapeutic strategies. APC mutations activate Wnt/b-catenin, causing increased b-catenin levels and tumor development[170,171], restricting tumor growth within the intestines, and mimicking human CRC. In addition, compared with previous single-mutation GEMMs for CRC, transgenic mice established via combined APC/KRAS mutations have been shown to represent CRC initiation, progression, and metastasis more accurately into nearby tissues[163,172]. Advantages include specificity in mirroring human CRC growth, representation of TMEs, and the ability to visualize the CRC through colonoscopy. Immunotherapeutic studies using transgenic mice have revealed promising avenues for targeted treatments. However, limitations such as extended metastatic development time and limited colon-specific models warrant further refinement for comprehensive CRC research[173]. As a result, transgenic mice are often ineffective in representing the later stages of tumor development owing to highly variable metastasis[166]. Additionally, there are few current transgenic mouse models that lead to specific CRC development in the colon, as the majority of pre-existing models lead to CRC development within the small intestine or other nearby tissues, contributing to the development of familial cancers rather than specific GEMM mutation-derived CRC[174].
The observation that tumors in mice have a narrower phenotype than human tumors suggests that the mice themselves need to be subtyped before drawing comparisons with human subtypes. This recognition is crucial, especially considering that murine backgrounds are often congenic (with the same genetic makeup) and artificially altered for experimental purposes. In summary, the critical importance of selecting appropriate preclinical models in CRC research requires better understanding. Mouse models are indispensable tools for discovering effective therapeutic interventions. The evaluation of various transplantation methods, with particular emphasis on the orthotopic CRC model via cecal wall injection, provides a nuanced understanding of their utility while acknowledging the inherent limitations associated with this approach. This recognition is vital for refining experimental design and interpretation to better translate findings from preclinical studies to human clinical scenarios.
Overall, immunotherapy, particularly with the use of ICIs like pembrolizumab and nivolumab has demonstrated significant clinical benefit in MSI-H CRC, while its efficacy in MSS CRC remains limited[91,130,133,138]. However, even in MSI-H tumors, the upregulation of immune checkpoint proteins, the presence of other immunosuppressive mechanisms within the TME, and the heterogeneity of MSI-H CRC tumors within the primary tumor and across metastatic sites can contribute to varied responses to immunotherapy. Understanding these factors and further research on the mechanisms of immune resistance in patients with MSI-H CRC are essential to improve the outcomes of immunotherapy in this patient population.
In the field of ICI therapy for CRC, various research avenues to enhance treatment efficacy and broaden its scope of application are being actively pursued. Therefore, there is a need to identify response biomarkers and devise novel treatment approaches to address these challenges. One area of focus is the investigation of combination therapies in which ICIs are used in conjunction with chemotherapy, targeted therapies, and other immunotherapies. Similarly, combining immunotherapy with targeted therapy directed against specific signaling pathways, such as the MAPK pathway, may also improve treatment outcomes[136-138,140,175]. Here, the goal is to enhance the response rates and improve patient survival by leveraging the synergistic effects of different treatment modalities. Another important research avenue is the discovery of reliable biomarkers that can accurately predict patient response to ICIs. Although PD-L1 expression is currently used as a biomarker for some cancer types[176,177], its predictive value for CRC is limited[129,178]. For example, tumors with elevated PD-L1 expression may be more responsive to anti-PD-1/PD-L1 therapy, whereas tumors with low PD-L1 expression may require combination therapy to achieve a response. Similarly, tumors with specific genetic mutations such as BRAF V600E may require targeted therapy in addition to immunotherapy to achieve a response. Mutations in genes such as JAK1, JAK2, and B2M may contribute to treatment resistance[179-181]. Truncating mutations in B2M affect antigen presentation, and can lead to pembrolizumab resistance. Evidence indicates that a high somatic mutational load and neoantigen density are associated with an improved response to immune checkpoint blockade in various cancers. This is attributed to the increased presence of mutation-associated neoantigens, which contribute to greater T cell diversity[182]. Moreover, there are also ongoing research efforts on the development of novel immunotherapeutic agents such as bispecific antibodies, chimeric antigen receptor (CAR) T-cells, and vaccines, which may provide new treatment options for CRC patients[183-188]. Efforts are underway to identify additional biomarkers that assist patient selection and treatment decisions. These targeted therapies, when combined with combination therapies, have shown considerable potential in enhancing treatment efficacy and overcoming drug resistance. By simultaneously targeting different pathways implicated in CRC progression, these approaches aim to maximize therapeutic benefits while minimizing adverse effects. Future research should focus on identifying optimal drug combinations, elucidating synergistic interactions, and refining treatment regimens to improve patient response.
A recent study revealed that immune cells form multicellular hubs in CRC samples that are spatially organized and functionally distinct from the surrounding immune cells[85]. The findings indicated that these immune hubs are composed of different cell types, including T-cells, B-cells, and myeloid cells, and are enriched in specific functional pathways related to the immune response and cell-cell communication. Researchers have also observed that the distribution and composition of immune hubs vary between patients and may be influenced by factors such as tumor stage and treatment history. Furthermore, the findings also demonstrated that the presence of immune hubs was associated with better clinical outcomes in CRC patients, suggesting a crucial role in the immune response to cancer. The authors proposed that targeting immune hubs could be a promising strategy for enhancing the efficacy of immunotherapy in CRC[85].
Additionally, there is a growing interest in exploring the use of ICIs in the early stages of CRC, such as adjuvant therapy following surgery. Early detection and intervention are pivotal for improving CRC outcomes. Emerging technologies, such as liquid biopsies and advanced imaging modalities, hold promise for the detection of CRC at earlier stages when treatment options are more effective[189-191]. Additionally, minimally invasive surgical techniques and organ-preserving approaches offer less invasive alternatives for managing early stage CRC, reducing morbidity, and improving the quality of life of patients[192].
Current research focuses on understanding the intricate mechanisms underlying drug resistance, including genetic mutations, TME interactions, and adaptive signaling pathways. Strategies for overcoming resistance include developing combination therapies that target multiple pathways, repurposing existing drugs, and developing novel agents to evade resistance mechanisms. Precision medicine approaches such as tumor molecular profiling and real-time monitoring facilitate the early detection of resistance mechanisms, allowing prompt adjustments to treatment strategies[193,194]. Furthermore, biomarker research in CRC is rapidly evolving with the aim of identifying molecular signatures crucial for diagnosis, prognosis, and treatment decisions. Biomarkers, such as mutations in genes such as KRAS and BRAF, not only influence tumor behavior, but also affect responses to targeted therapies, notably anti-EGFR antibodies[24]. Additionally, MSI status serves not only as a guide for immunotherapy but also as a valuable prognostic indicator as well. Liquid biopsies offer a noninvasive method to analyze circulating tumor DNA and to monitor disease progression and treatment responses[189]. Epigenetic alterations, such as DNA methylation patterns and microRNA expression profiles, are promising diagnostic and prognostic markers[24,195]. Traditional biomarkers, such as carcinoembryonic antigen, provide insights into tumor burden and treatment response, while gene expression signatures, such as Oncotype DX and ColoPrint, offer predictive value for treatment outcomes and recurrence risk assessment[196]. Integrating these diverse biomarkers into clinical practice can help personalize treatment strategies, optimize patient management, and ultimately enhance the survival outcomes for CRC patients. However, drug resistance remains a significant challenge, com
Personalized medicine has revolutionized CRC treatment by tailoring interventions to individual patient characteristics, including genetic and molecular factors. Over the years, personalized medicine has gained traction and treatment approaches have been tailored based on individual patient characteristics. This involves integrating tumor genetic profiling, immune profiling, and other personalized medicine strategies to identify the most effective treatment options for each patient[194,210,211]. Comprehensive genomic profiling and clinical data integration enable the identification of actionable targets and personalized treatment regimens. Artificial intelligence enhances data interpretation and improves the accuracy of treatment response prediction. Liquid biopsies provide a noninvasive method for monitoring disease progression and identifying therapeutic targets. Personalized medicine integrates liquid biopsy-based monitoring into treatment management, allowing for real-time therapy adjustments. This approach promises to optimize treatment strategies and improve the clinical outcomes for CRC patients with CRC. Advancements in biomarker research coupled with efforts to overcome drug resistance and implement personalized medicine offer a multifaceted approach to CRC management that holds great promise for enhancing patient care and outcomes in the future.
Moreover, additional novel strategies for CRC treatment such as mRNA vaccines, TILs therapy, CAR-T therapy, oncolytic virus therapy, bispecific T-cell engagers, and combination strategies aim to improve treatment outcomes by specifically considering metastatic location and TME regulation. Novel agents and therapeutic strategies are being developed to expand the range of options available for immune modulation of this disease. Accordingly, in addition to the development of new biomarkers and therapeutic strategies, there is also a need for better pre-clinical mouse models that can potentially or closely replicate the human CRC microenvironment, thus providing a better opportunity to unmask novel approaches for treatment. As the field of immunotherapy evolves, these directions hold great promise for advancing immune checkpoint inhibitor therapy and other immunotherapeutic approaches for CRC, ultimately resulting in improved patient outcomes.
Immunotherapy has significantly reshaped the CRC treatment landscape, particularly for patients with MSI-H or dMMR tumors. Key accomplishments include the FDA approval of PD-1 inhibitors, such as pembrolizumab and nivolumab, for these patient subsets. Pembrolizumab has demonstrated promising outcomes both as a monotherapy and in combination with chemotherapy, surpassing standard treatments in terms of PFS and ORR[133,134]. Furthermore, combination therapies have shown promise, such as the use of nivolumab with ipilimumab (a CTLA-4 inhibitor), which has demonstrated improved disease control rates[136-138]. Additionally, atezolizumab in combination with Cobimetinib has shown enhanced PFS rates in second-line treatments, although further studies are needed to establish its effects on OS[145]. A recent study established Dostarlimab as a drug with 100% effectiveness against MSI-H or dMMR CRC tumors. Despite the preliminary success of immuno-oncology, challenges persist for CRC treatment, particularly those pertaining to the extension of immunotherapeutic benefits to MSS or pMMR tumors, which commonly exhibit resistance to ICIs. In this regard, irAEs associated with ICIs should be managed effectively, which requires identification of predictive biomarkers and the development of mitigation strategies. Combination therapies, as exemplified by the synergistic effects observed with nivolumab and ipilimumab, require further investigation to optimize their performance and to identify their underlying mechanisms. Exploring novel therapeutic targets beyond immune checkpoint blockade, including targeted therapies and engineered immunotherapies, holds promise for overcoming resistance mechanisms. Addressing these challenges requires interdisciplinary collaboration, ongoing preclinical and clinical research, and rigorous validation through well-controlled trials. By overcoming these obstacles, advancements in CRC treatment can be realized, leading to improved clinical outcomes and enhanced quality of life in affected patients. In conclusion, immunotherapy has revolutionized CRC treatment, resulting in improved outcomes and survival rates in MSI-H or dMMR patients. However, challenges persist in extending these benefits to patients with MSS or pMMR and in the management of irAEs. Future research should focus on optimizing combination therapies, identifying predictive biomarkers, and mitigating treatment-related toxicities to realize the full potential of immunotherapy in CRC management.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68447] [Article Influence: 13689.4] [Reference Citation Analysis (201)] |
| 2. | Li K, Luo H, Huang L, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 2020;20:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 349] [Article Influence: 58.2] [Reference Citation Analysis (1)] |
| 3. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1831] [Reference Citation Analysis (5)] |
| 4. | Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med. 2022;386:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 346] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 5. | Ewing I, Hurley JJ, Josephides E, Millar A. The molecular genetics of colorectal cancer. Frontline Gastroenterol. 2014;5:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 6. | Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1040] [Article Influence: 130.0] [Reference Citation Analysis (3)] |
| 7. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3398] [Article Influence: 485.4] [Reference Citation Analysis (4)] |
| 8. | Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, Bacher J, Bigley C, Nelsen L, Goodfellow PJ, Goldberg RM, Paskett E, Shields PG, Freudenheim JL, Stanich PP, Lattimer I, Arnold M, Liyanarachchi S, Kalady M, Heald B, Greenwood C, Paquette I, Prues M, Draper DJ, Lindeman C, Kuebler JP, Reynolds K, Brell JM, Shaper AA, Mahesh S, Buie N, Weeman K, Shine K, Haut M, Edwards J, Bastola S, Wickham K, Khanduja KS, Zacks R, Pritchard CC, Shirts BH, Jacobson A, Allen B, de la Chapelle A, Hampel H; Ohio Colorectal Cancer Prevention Initiative Study Group. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 545] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 9. | Barnetson RA, Tenesa A, Farrington SM, Nicholl ID, Cetnarskyj R, Porteous ME, Campbell H, Dunlop MG. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354:2751-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 347] [Article Influence: 17.4] [Reference Citation Analysis (2)] |
| 10. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, Lockman J, LaJeunesse J, Comeras I, de la Chapelle A. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 669] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 11. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1035] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Yurgelun MB, Allen B, Kaldate RR, Bowles KR, Judkins T, Kaushik P, Roa BB, Wenstrup RJ, Hartman AR, Syngal S. Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients With Suspected Lynch Syndrome. Gastroenterology. 2015;149:604-13.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 13. | Sinicrope FA. Lynch Syndrome-Associated Colorectal Cancer. N Engl J Med. 2018;379:764-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 14. | Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 15. | Arends MJ. Pathways of colorectal carcinogenesis. Appl Immunohistochem Mol Morphol. 2013;21:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 16. | Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 448] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (5)] |
| 17. | National Cancer Institute. All Cancer Sites Combined Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2020. [cited 23 August 2023]. Available from: https://seer.cancer.gov/statistics-network/explorer/application.html. |
| 18. | Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 1161] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 19. | Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, Danenberg PV. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322-1327. [PubMed] |
| 20. | Källberg J, Harrison A, March V, Bērziņa S, Nemazanyy I, Kepp O, Kroemer G, Mouillet-Richard S, Laurent-Puig P, Taly V, Xiao W. Intratumor heterogeneity and cell secretome promote chemotherapy resistance and progression of colorectal cancer. Cell Death Dis. 2023;14:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (2)] |
| 21. | Molinari C, Marisi G, Passardi A, Matteucci L, De Maio G, Ulivi P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (2)] |
| 22. | Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol. 2020;13:1756284820917527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 23. | Péraudeau E, Renoux B, Emambux S, Poinot P, Châtre R, Thoreau F, Riss Yaw B, Tougeron D, Clarhaut J, Papot S. Combination of Targeted Therapies for Colorectal Cancer Treatment. Mol Pharm. 2023;20:4537-4545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 24. | Alese OB, Wu C, Chapin WJ, Ulanja MB, Zheng-Lin B, Amankwah M, Eads J. Update on Emerging Therapies for Advanced Colorectal Cancer. Am Soc Clin Oncol Educ Book. 2023;43:e389574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 25. | Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D'Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2239] [Cited by in RCA: 2979] [Article Influence: 425.6] [Reference Citation Analysis (1)] |
| 26. | Zheng M. Tumor mutation burden for predicting immune checkpoint blockade response: the more, the better. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 27. | Ganesh K. Optimizing immunotherapy for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2022;19:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 28. | Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1362] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 29. | Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2447] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 30. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6405] [Article Influence: 457.5] [Reference Citation Analysis (0)] |
| 31. | Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, Saltz LB. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485-3490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 32. | Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 438] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 33. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 10130] [Article Influence: 723.6] [Reference Citation Analysis (5)] |
| 34. | Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY, Riches JC, Tuvy Y, Kundra R, Bouvier N, Vakiani E, Gao J, Heins ZJ, Gross BE, Kelsen DP, Zhang L, Strong VE, Schattner M, Gerdes H, Coit DG, Bains M, Stadler ZK, Rusch VW, Jones DR, Molena D, Shia J, Robson ME, Capanu M, Middha S, Zehir A, Hyman DM, Scaltriti M, Ladanyi M, Rosen N, Ilson DH, Berger MF, Tang L, Taylor BS, Solit DB, Schultz N. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov. 2018;8:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 35. | Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 36. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4973] [Article Influence: 621.6] [Reference Citation Analysis (0)] |
| 37. | Galluzzi L, Chan TA, Kroemer G, Wolchok JD, López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 317] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 38. | Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2409] [Cited by in RCA: 2928] [Article Influence: 488.0] [Reference Citation Analysis (0)] |
| 39. | Sumransub N, Vantanasiri K, Prakash A, Lou E. Advances and new frontiers for immunotherapy in colorectal cancer: Setting the stage for neoadjuvant success? Mol Ther Oncolytics. 2021;22:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (6)] |
| 40. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 5167] [Article Influence: 574.1] [Reference Citation Analysis (0)] |
| 41. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1613] [Article Influence: 100.8] [Reference Citation Analysis (2)] |
| 42. | Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 770] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 43. | Jin Z, Sanhueza CT, Johnson B, Nagorney DM, Larson DW, Mara KC, Harmsen WC, Smyrk TC, Grothey A, Hubbard JM. Outcome of Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Mayo Clinic Experience. Oncologist. 2018;23:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Li SKH, Martin A. Mismatch Repair and Colon Cancer: Mechanisms and Therapies Explored. Trends Mol Med. 2016;22:274-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 45. | Battaglin F, Naseem M, Lenz HJ, Salem ME. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol. 2018;16:735-745. [PubMed] |
| 46. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3806] [Article Influence: 346.0] [Reference Citation Analysis (8)] |
| 47. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1185] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 48. | Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D; ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64-vi72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 49. | Van Cutsem E, Cervantes A, Nordlinger B, Arnold D; ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1-iii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 820] [Article Influence: 68.3] [Reference Citation Analysis (1)] |
| 50. | Brown KGM, Solomon MJ, Mahon K, O'Shannassy S. Management of colorectal cancer. BMJ. 2019;366:l4561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1073] [Article Influence: 214.6] [Reference Citation Analysis (16)] |
| 52. | Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol. 2019;54:407-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 623] [Reference Citation Analysis (2)] |
| 53. | Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2191] [Article Influence: 219.1] [Reference Citation Analysis (0)] |
| 54. | Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21:120-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 55. | Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 729] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 56. | Zhai J, Gu X, Liu Y, Hu Y, Jiang Y, Zhang Z. Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: An update review. Front Pharmacol. 2023;14:1152934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 80] [Reference Citation Analysis (1)] |
| 57. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 12016] [Article Influence: 751.0] [Reference Citation Analysis (0)] |
| 58. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4701] [Article Influence: 427.4] [Reference Citation Analysis (1)] |
| 59. | Ribas A. Releasing the Brakes on Cancer Immunotherapy. N Engl J Med. 2015;373:1490-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 60. | Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA; IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379:2108-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 3171] [Article Influence: 396.4] [Reference Citation Analysis (4)] |
| 61. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 5061] [Article Influence: 632.6] [Reference Citation Analysis (6)] |
| 62. | Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;379:e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 63. | Franke AJ, Skelton WP, Starr JS, Parekh H, Lee JJ, Overman MJ, Allegra C, George TJ. Immunotherapy for Colorectal Cancer: A Review of Current and Novel Therapeutic Approaches. J Natl Cancer Inst. 2019;111:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 64. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3724] [Article Influence: 338.5] [Reference Citation Analysis (0)] |
| 65. | Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713-6721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 839] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 66. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 991] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 67. | Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 68. | Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099-8103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 307] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 69. | Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2903] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 70. | Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, Wu C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121:809-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 71. | Aristin Revilla S, Kranenburg O, Coffer PJ. Colorectal Cancer-Infiltrating Regulatory T Cells: Functional Heterogeneity, Metabolic Adaptation, and Therapeutic Targeting. Front Immunol. 2022;13:903564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 72. | Olguín JE, Medina-Andrade I, Rodríguez T, Rodríguez-Sosa M, Terrazas LI. Relevance of Regulatory T Cells during Colorectal Cancer Development. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 73. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 5009] [Article Influence: 250.5] [Reference Citation Analysis (19)] |
| 74. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3572] [Cited by in RCA: 4165] [Article Influence: 160.2] [Reference Citation Analysis (1)] |
| 75. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1823] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 76. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10704] [Article Influence: 764.6] [Reference Citation Analysis (34)] |
| 77. | Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. J Leukoc Biol. 2016;100:275-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 78. | Haslam A, Gill J, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw Open. 2020;3:e200423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 79. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Drilon A, Wolchok JD, Carvajal RD, McHenry MB, Hosein F, Harbison CT, Grosso JF, Sznol M. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019;5:1411-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 457] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 80. | van der Stok EP, Spaander MCW, Grünhagen DJ, Verhoef C, Kuipers EJ. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol. 2017;14:297-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 81. | Sánchez-Gundín J, Fernández-Carballido AM, Martínez-Valdivieso L, Barreda-Hernández D, Torres-Suárez AI. New Trends in the Therapeutic Approach to Metastatic Colorectal Cancer. Int J Med Sci. 2018;15:659-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 82. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1361] [Article Influence: 170.1] [Reference Citation Analysis (1)] |
| 83. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1763] [Article Influence: 251.9] [Reference Citation Analysis (2)] |
| 84. | Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 745] [Article Influence: 93.1] [Reference Citation Analysis (1)] |
| 85. | Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, Bejnood A, Dionne D, Ge WH, Xu KH, Chao SX, Zollinger DR, Lieb DJ, Reeves JW, Fuhrman CA, Hoang ML, Delorey T, Nguyen LT, Waldman J, Klapholz M, Wakiro I, Cohen O, Albers J, Smillie CS, Cuoco MS, Wu J, Su MJ, Yeung J, Vijaykumar B, Magnuson AM, Asinovski N, Moll T, Goder-Reiser MN, Applebaum AS, Brais LK, DelloStritto LK, Denning SL, Phillips ST, Hill EK, Meehan JK, Frederick DT, Sharova T, Kanodia A, Todres EZ, Jané-Valbuena J, Biton M, Izar B, Lambden CD, Clancy TE, Bleday R, Melnitchouk N, Irani J, Kunitake H, Berger DL, Srivastava A, Hornick JL, Ogino S, Rotem A, Vigneau S, Johnson BE, Corcoran RB, Sharpe AH, Kuchroo VK, Ng K, Giannakis M, Nieman LT, Boland GM, Aguirre AJ, Anderson AC, Rozenblatt-Rosen O, Regev A, Hacohen N. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184:4734-4752.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 548] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 86. | Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, Sukawa Y, Stewart C, Rosenberg M, Mima K, Inamura K, Nosho K, Nowak JA, Lawrence MS, Giovannucci EL, Chan AT, Ng K, Meyerhardt JA, Van Allen EM, Getz G, Gabriel SB, Lander ES, Wu CJ, Fuchs CS, Ogino S, Garraway LA. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 87. | Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1201] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 88. | Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 382] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 89. | Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med. 2007;5:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 90. | Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417-2422. [PubMed] |
| 91. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 2041] [Article Influence: 340.2] [Reference Citation Analysis (0)] |
| 92. | Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 787] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 93. | Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1529] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 94. | Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1665] [Article Influence: 185.0] [Reference Citation Analysis (0)] |
| 95. | Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1426] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 96. | Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823-11828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 97. | Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 1058] [Article Influence: 151.1] [Reference Citation Analysis (5)] |
| 98. | Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 388] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 99. | Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2815] [Cited by in RCA: 3235] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 100. | Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 621] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 101. | Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, Allison JP. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 2017;170:1120-1133.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 1026] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 102. | Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015-3029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1346] [Cited by in RCA: 1664] [Article Influence: 97.9] [Reference Citation Analysis (1)] |
| 103. | Hou A, Hou K, Huang Q, Lei Y, Chen W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front Immunol. 2020;11:783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 104. | Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 105. | Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360-3365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1208] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 106. | Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 107. | Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381-3385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 693] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 108. | Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1337] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 109. | Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 744] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 110. | Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 111. | Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1519] [Cited by in RCA: 1677] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 112. | Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 715] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 113. | Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1559] [Cited by in RCA: 1665] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 114. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2111] [Cited by in RCA: 2149] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 115. | Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). J Exp Med. 1999;189:435-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 116. | Tivol EA, Boyd SD, McKeon S, Borriello F, Nickerson P, Strom TB, Sharpe AH. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J Immunol. 1997;158:5091-5094. [PubMed] |
| 117. | Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155-5163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 118. | Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1642] [Cited by in RCA: 1719] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 119. | Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PubMed] |
| 120. | Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 121. | Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036-4041. [PubMed] |
| 122. | Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 751] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 123. | Sharma N, Vacher J, Allison JP. TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion. Proc Natl Acad Sci U S A. 2019;116:10453-10462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 124. | Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RL. CTLA-4⁺ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75:2200-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 125. | Hou TZ, Qureshi OS, Wang CJ, Baker J, Young SP, Walker LS, Sansom DM. A transendocytosis model of CTLA-4 function predicts its suppressive behavior on regulatory T cells. J Immunol. 2015;194:2148-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (2)] |
| 126. | Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1406] [Cited by in RCA: 1384] [Article Influence: 92.3] [Reference Citation Analysis (3)] |
| 127. | Raedler LA. Keytruda (Pembrolizumab): First PD-1 Inhibitor Approved for Previously Treated Unresectable or Metastatic Melanoma. Am Health Drug Benefits. 2015;8:96-100. [PubMed] |
| 128. | Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021;12:2735-2746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 129. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7483] [Article Influence: 680.3] [Reference Citation Analysis (2)] |
| 130. | Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Elez E, Al-Batran SE, Boland PM, Crocenzi T, Atreya CE, Cui Y, Dai T, Marinello P, Diaz LA Jr, André T. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 746] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 131. | van Vugt MJH, Stone JA, De Greef RHJMM, Snyder ES, Lipka L, Turner DC, Chain A, Lala M, Li M, Robey SH, Kondic AG, De Alwis D, Mayawala K, Jain L, Freshwater T. Immunogenicity of pembrolizumab in patients with advanced tumors. J Immunother Cancer. 2019;7:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 132. | Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8:57-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 397] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 133. | Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T; KEYNOTE-177 Investigators. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 601] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 134. | Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, Sevilla I, De La Fouchardiere C, Rivera F, Elez E, Diaz LA Jr, Yoshino T, Van Cutsem E, Yang P, Farooqui M, Le DT. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:665-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 135. | Fessas P, Lee H, Ikemizu S, Janowitz T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44:136-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 136. | Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1537] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 137. | Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 476] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 138. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2179] [Article Influence: 242.1] [Reference Citation Analysis (9)] |
| 139. | Shah NJ, Kelly WJ, Liu SV, Choquette K, Spira A. Product review on the Anti-PD-L1 antibody atezolizumab. Hum Vaccin Immunother. 2018;14:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 140. | Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, Sobrero A, Yan Y, Chang I, Uyei A, Roberts L, Ciardiello F; IMblaze370 Investigators. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 141. | André T, Berton D, Curigliano G, Sabatier R, Tinker AV, Oaknin A, Ellard S, de Braud F, Arkenau HT, Trigo J, Gravina A, Kristeleit R, Moreno V, Abdeddaim C, Vano YA, Samouëlian V, Miller R, Boni V, Torres AA, Gilbert L, Brown J, Dewal N, Dabrowski C, Antony G, Zografos E, Veneris J, Banerjee S. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients With Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw Open. 2023;6:e2341165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 142. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1068] [Article Influence: 267.0] [Reference Citation Analysis (0)] |
| 143. | Bajbouj K, Qaisar R, Alshura MA, Ibrahim Z, Alebaji MB, Al Ani AW, Janajrah HM, Bilalaga MM, Omara AI, Abou Assaleh RS, Saber-Ayad MM, Elmoselhi AB. Synergistic Anti-Angiogenic Effect of Combined VEGFR Kinase Inhibitors, Lenvatinib, and Regorafenib: A Therapeutic Potential for Breast Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 144. | Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Tam R, D'Adamo D, Sharma N, Brennan BJ, Wang YA, Coppieters S, Zebger-Gong H, Weispfenning A, Seidel H, Ploeger BA, Mueller U, Oliveira CSV, Paulson AS. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023;58:101917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 145. | Bendell J, Ciardiello F, Tabernero J, Tebbutt N, Eng C, Bartolomeo MD, Falcone A, Fakih M, Kozloff M, Segal N, Sobrero A, Shi Y, Roberts L, Yan Y, Chang I, Uyei A, Kim T. Efficacy and safety results from IMblaze370, a randomised Phase III study comparing atezolizumab+cobimetinib and atezolizumab monotherapy vs regorafenib in chemotherapy-refractory metastatic colorectal cancer. Ann Oncol. 2018;29:v123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Khaddour K, Maahs L, Avila-Rodriguez AM, Maamar Y, Samaan S, Ansstas G. Melanoma Targeted Therapies beyond BRAF-Mutant Melanoma: Potential Druggable Mutations and Novel Treatment Approaches. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 147. | El-Khoueiry AB, Fakih M, Gordon MS, Tsimberidou AM, Bullock AJ, Wilky BA, Trent JC, Margolin KA, Mahadevan D, Balmanoukian AS, Sanborn RE, Schwartz GK, Bockorny B, Moser JC, Grossman JE, Feliu WIO, Rosenthal K, O'Day S, Lenz HJ, and Schlechter BL. Results from a phase 1a/1b study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC). J Clin Oncol. 2023;41 :Suppl 4: LBA8-LBA. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 148. | Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J Exp Med. 2019;216:1244-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 149. | Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, Zhang Y, Liu Z, Fritz JM, Marsh R, Husami A, Kissell D, Nortman S, Chaturvedi V, Haines H, Young LR, Mo J, Filipovich AH, Bleesing JJ, Mustillo P, Stephens M, Rueda CM, Chougnet CA, Hoebe K, McElwee J, Hughes JD, Karakoc-Aydiner E, Matthews HF, Price S, Su HC, Rao VK, Lenardo MJ, Jordan MB. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 503] [Article Influence: 45.7] [Reference Citation Analysis (2)] |
| 150. | Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, Schickel JN, Tran DQ, Stoddard J, Zhang Y, Frucht DM, Dumitriu B, Scheinberg P, Folio LR, Frein CA, Price S, Koh C, Heller T, Seroogy CM, Huttenlocher A, Rao VK, Su HC, Kleiner D, Notarangelo LD, Rampertaap Y, Olivier KN, McElwee J, Hughes J, Pittaluga S, Oliveira JB, Meffre E, Fleisher TA, Holland SM, Lenardo MJ, Tangye SG, Uzel G. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 696] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 151. | Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1626] [Article Influence: 162.6] [Reference Citation Analysis (0)] |
| 152. | Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol. 2017;8:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 443] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 153. | Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, Kern JA, Lacouture ME. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 154. | Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol. 2017;8:730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (1)] |
| 155. | Weingarden AR, Rubin SJS, Gubatan J. Immune checkpoint inhibitor-mediated colitis in gastrointestinal malignancies and inflammatory bowel disease. World J Gastrointest Oncol. 2021;13:772-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 156. | Hashash JG, Francis FF, Farraye FA. Diagnosis and Management of Immune Checkpoint Inhibitor Colitis. Gastroenterol Hepatol (NY). 2021;17:358-366. [PubMed] |
| 157. | Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, Bowling P, Hodi FS, Rahma O, Sullivan RJ, Boland GM, Nowak JA, Dougan SK, Dougan M, Yuan GC, Wucherpfennig KW. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell. 2020;182:655-671.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 158. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6848] [Article Influence: 489.1] [Reference Citation Analysis (10)] |
| 159. | Saeterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A, Gaudernack G. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:13255-13260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 160. | Mulet-Margalef N, Linares J, Badia-Ramentol J, Jimeno M, Sanz Monte C, Manzano Mozo JL, Calon A. Challenges and Therapeutic Opportunities in the dMMR/MSI-H Colorectal Cancer Landscape. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (1)] |
| 161. | Wu Y, Zhuang J, Qu Z, Yang X, Han S. Advances in immunotyping of colorectal cancer. Front Immunol. 2023;14:1259461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (3)] |
| 162. | Sahin IH, Ciombor KK, Diaz LA, Yu J, Kim R. Immunotherapy for Microsatellite Stable Colorectal Cancers: Challenges and Novel Therapeutic Avenues. Am Soc Clin Oncol Educ Book. 2022;42:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 163. | Hung KE, Maricevich MA, Richard LG, Chen WY, Richardson MP, Kunin A, Bronson RT, Mahmood U, Kucherlapati R. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci U S A. 2010;107:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 164. | Roper J, Tammela T, Akkad A, Almeqdadi M, Santos SB, Jacks T, Yilmaz ÖH. Colonoscopy-based colorectal cancer modeling in mice with CRISPR-Cas9 genome editing and organoid transplantation. Nat Protoc. 2018;13:217-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 165. | Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, Nguyen JM, Curiel TJ, Cadungog MG, Singhal S, Eruslanov EB, Zhang P, Tchou J, Zhang R, Conejo-Garcia JR. Tumor Cell-Independent Estrogen Signaling Drives Disease Progression through Mobilization of Myeloid-Derived Suppressor Cells. Cancer Discov. 2017;7:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 166. | Oliveira RC, Abrantes AM, Tralhão JG, Botelho MF. The role of mouse models in colorectal cancer research-The need and the importance of the orthotopic models. Animal Model Exp Med. 2020;3:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 167. | Bürtin F, Mullins CS, Linnebacher M. Mouse models of colorectal cancer: Past, present and future perspectives. World J Gastroenterol. 2020;26:1394-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (2)] |
| 168. | Tseng W, Leong X, Engleman E. Orthotopic mouse model of colorectal cancer. J Vis Exp. 2007;484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 169. | Kim H, Kim M, Im SK, Fang S. Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab Anim Res. 2018;34:147-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 170. | Jiang L, Hermeking H. miR-34a and miR-34b/c Suppress Intestinal Tumorigenesis. Cancer Res. 2017;77:2746-2758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 171. | Parker TW, Neufeld KL. APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes. Sci Rep. 2020;10:2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 172. | Tetteh PW, Kretzschmar K, Begthel H, van den Born M, Korving J, Morsink F, Farin H, van Es JH, Offerhaus GJ, Clevers H. Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research. Proc Natl Acad Sci U S A. 2016;113:11859-11864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 173. | Li C, Lau HC, Zhang X, Yu J. Mouse Models for Application in Colorectal Cancer: Understanding the Pathogenesis and Relevance to the Human Condition. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 174. | Xue Y, Johnson R, Desmet M, Snyder PW, Fleet JC. Generation of a transgenic mouse for colorectal cancer research with intestinal cre expression limited to the large intestine. Mol Cancer Res. 2010;8:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 175. | Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, Canon JL, Guan X, Demonty G, Schwartzberg LS. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis. 2017;32:1179-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 176. | Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4993] [Article Influence: 453.9] [Reference Citation Analysis (0)] |
| 177. | Hersom M, Jørgensen JT. Companion and Complementary Diagnostics-Focus on PD-L1 Expression Assays for PD-1/PD-L1 Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Ther Drug Monit. 2018;40:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 178. | André T, Overman M, Lonardi S, Aglietta M, McDermott R, Wong KYM, Morse M, Hendlisz A, Moss RA, Ledeine JM, Tang H, Cao ZA, Kopetz S. Analysis of tumor PD-L1 expression and biomarkers in relation to clinical activity in patients (pts) with deficient DNA mismatch repair (dMMR)/high microsatellite instability (MSI-H) metastatic colorectal cancer (mCRC) treated with nivolumab (NIVO) + ipilimumab (IPI): CheckMate 142. Ann Oncol. 2017;28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 179. | Koelzer VH, Baker K, Kassahn D, Baumhoer D, Zlobec I. Prognostic impact of β-2-microglobulin expression in colorectal cancers stratified by mismatch repair status. J Clin Pathol. 2012;65:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 180. | Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, Palaskas N, Rodriguez GA, Parisi G, Azhdam A, Chmielowski B, Cherry G, Seja E, Berent-Maoz B, Shintaku IP, Le DT, Pardoll DM, Diaz LA Jr, Tumeh PC, Graeber TG, Lo RS, Comin-Anduix B, Ribas A. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017;7:188-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 1044] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 181. | Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2518] [Article Influence: 251.8] [Reference Citation Analysis (0)] |
| 182. | Li B, Li T, Pignon JC, Wang B, Wang J, Shukla SA, Dou R, Chen Q, Hodi FS, Choueiri TK, Wu C, Hacohen N, Signoretti S, Liu JS, Liu XS. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet. 2016;48:725-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 183. | Aurisicchio L, Fridman A, Mauro D, Sheloditna R, Chiappori A, Bagchi A, Ciliberto G. Safety, tolerability and immunogenicity of V934/V935 hTERT vaccination in cancer patients with selected solid tumors: a phase I study. J Transl Med. 2020;18:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 184. | Bartnik A, Nirmal AJ, Yang SY. Peptide Vaccine Therapy in Colorectal Cancer. Vaccines (Basel). 2012;1:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 185. | Heery CR, Singh BH, Rauckhorst M, Marté JL, Donahue RN, Grenga I, Rodell TC, Dahut W, Arlen PM, Madan RA, Schlom J, Gulley JL. Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol Res. 2015;3:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 186. | Li M, Yuan YH, Han Y, Liu YX, Yan L, Wang Y, Gu J. Expression profile of cancer-testis genes in 121 human colorectal cancer tissue and adjacent normal tissue. Clin Cancer Res. 2005;11:1809-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 187. | Liu C, Xie Y, Sun B, Geng F, Zhang F, Guo Q, Wu H, Yu B, Wu J, Yu X, Kong W, Zhang H. MUC1- and Survivin-based DNA Vaccine Combining Immunoadjuvants CpG and interleukin-2 in a Bicistronic Expression Plasmid Generates Specific Immune Responses and Antitumour Effects in a Murine Colorectal Carcinoma Model. Scand J Immunol. 2018;87:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 188. | Snook AE, Baybutt TR, Xiang B, Abraham TS, Flickinger JC Jr, Hyslop T, Zhan T, Kraft WK, Sato T, Waldman SA. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J Immunother Cancer. 2019;7:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 189. | Connal S, Cameron JM, Sala A, Brennan PM, Palmer DS, Palmer JD, Perlow H, Baker MJ. Liquid biopsies: the future of cancer early detection. J Transl Med. 2023;21:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 190. | Fukumoto M, Kurohara A, Akagi N, Yoshida D, Yoshida S. Ga-67 visualization of the coexistence of two mucosa-associated lymphoid tissue (MALT) lymphomas in the thyroid and stomach. Clin Nucl Med. 1998;23:484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 191. | Tang Q, Wang J, Frank A, Lin J, Li Z, Chen CW, Jin L, Wu T, Greenwald BD, Mashimo H, Chen Y. Depth-resolved imaging of colon tumor using optical coherence tomography and fluorescence laminar optical tomography. Biomed Opt Express. 2016;7:5218-5232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 192. | Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Zhao WK, Marks AR, Schottinger JE, Ghai NR, Lee AT, Contreras R, Klabunde CN, Quesenberry CP, Levin TR, Mysliwiec PA. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016;164:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 193. | Louie BH, Kato S, Kim KH, Lim HJ, Lee S, Okamura R, Fanta PT, Kurzrock R. Precision medicine-based therapies in advanced colorectal cancer: The University of California San Diego Molecular Tumor Board experience. Mol Oncol. 2022;16:2575-2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 194. | Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 590] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 195. | Jung G, Hernández-Illán E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17:111-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 562] [Article Influence: 93.7] [Reference Citation Analysis (4)] |
| 196. | Gao S, Tibiche C, Zou J, Zaman N, Trifiro M, O'Connor-McCourt M, Wang E. Identification and Construction of Combinatory Cancer Hallmark-Based Gene Signature Sets to Predict Recurrence and Chemotherapy Benefit in Stage II Colorectal Cancer. JAMA Oncol. 2016;2:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 197. | Azwar S, Seow HF, Abdullah M, Faisal Jabar M, Mohtarrudin N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 198. | Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020;111:3142-3154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 199. | Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 200. | Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 201. | Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1692] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 202. | Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 1330] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 203. | Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM, Sarantopoulos J, Bedard PL, Lin CC, Hodi FS, Wilgenhof S, Santoro A, Sabatos-Peyton CA, Longmire TA, Xyrafas A, Sun H, Gutzwiller S, Manenti L, Naing A. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin Cancer Res. 2021;27:3620-3629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 204. | Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA, Moudgil T, Miller W, Haley D, Coffey T, Fisher B, Delanty-Miller L, Rymarchyk N, Kelly T, Crocenzi T, Bernstein E, Sanborn R, Urba WJ, Weinberg AD. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189-7198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 205. | Harding JJ, Moreno V, Bang YJ, Hong MH, Patnaik A, Trigo J, Szpurka AM, Yamamoto N, Doi T, Fu S, Calderon B, Velez de Mendizabal N, Calvo E, Yu D, Gandhi L, Liu ZT, Galvao VR, Leow CC, de Miguel MJ. Blocking TIM-3 in Treatment-refractory Advanced Solid Tumors: A Phase Ia/b Study of LY3321367 with or without an Anti-PD-L1 Antibody. Clin Cancer Res. 2021;27:2168-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 206. | Mettu NB, Ulahannan SV, Bendell JC, Garrido-Laguna I, Strickler JH, Moore KN, Stagg R, Kapoun AM, Faoro L, Sharma S. A Phase 1a/b Open-Label, Dose-Escalation Study of Etigilimab Alone or in Combination with Nivolumab in Patients with Locally Advanced or Metastatic Solid Tumors. Clin Cancer Res. 2022;28:882-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 207. | Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 768] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 208. | Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 209. | Ward-Kavanagh LK, Lin WW, Šedý JR, Ware CF. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity. 2016;44:1005-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 210. | Lyons YA, Wu SY, Overwijk WW, Baggerly KA, Sood AK. Immune cell profiling in cancer: molecular approaches to cell-specific identification. NPJ Precis Oncol. 2017;1:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 211. | Mukherjee S. Genomics-Guided Immunotherapy for Precision Medicine in Cancer. Cancer Biother Radiopharm. 2019;34:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for Cancer Research, 1215631.
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu HT, China S-Editor: Lin C L-Editor: A P-Editor: Yu HG