Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1636
Peer-review started: January 19, 2024
First decision: January 31, 2024
Revised: February 2, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: March 21, 2024
Processing time: 62 Days and 6.3 Hours
Metastatic cardiac tumors are known to occur more frequently than primary cardiac tumors, however, they often remain asymptomatic and are commonly dis
The case of a 60-year-old man who complained of dysphagia is presented. Upper gastrointestinal endoscopy showed a submucosal tumor-like elevated lesion in the esophagus causing stenosis. Contrast-enhanced computed tomography showed left atrial compression due to the esophageal tumor, multiple liver and lung metastases, and a left pleural effusion. Pathological examination of a biopsy speci
To the best of our knowledge, there have been no prior reports of cardiac metastasis of esophageal SCC. This case highlights our experience with a patient with esophageal SCC who progressed rapidly and died from the disease, with the autopsy examination showing cardiac metastasis.
Core Tip: Spindle cell carcinoma (SCC) is rare, accounting for about 0.1%-1.5% of esophageal cancers. Tumor cells show sarcomatoid differentiation with spindle cells and are accompanied by components of conventional carcinoma including squamous cell carcinoma in some cases. Many of cardiac metastatic tumors are asymptomatic and are typically discovered through pathological autopsy, with metastatic cardiac tumors being identified in 2% to 18% of cancer patients undergoing autopsy. There has been no report of cardiac metastasis in esophageal SCC.
- Citation: Shibata Y, Ohmura H, Komatsu K, Sagara K, Matsuyama A, Nakano R, Baba E. Myocardial metastasis from ZEB1- and TWIST-positive spindle cell carcinoma of the esophagus: A case report. World J Gastroenterol 2024; 30(11): 1636-1643
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1636
Cardiac tumors are classified into primary tumors, originating within the heart, and metastatic tumors from other organs. Primary cardiac tumors include benign tumors, represented by myxoma, and malignant tumors, including sarcomas and lymphomas. Primary cardiac tumors are extremely rare, with prevalence rates ranging from 0.001% to 0.3% in autopsy studies. Furthermore, the proportion of malignant tumors among primary cardiac tumors is approximately 10%. On the other hand, metastatic cardiac tumors are much more frequent than primary cardiac tumors, with an estimated occurrence rate that is approximately 20 to 40 times higher. Many of these cases remain asymptomatic and are typically discovered on autopsy, with metastatic cardiac tumors being identified in 2% to 18% of cancer patients undergoing autopsy examination[1-3]. Excluding direct infiltration from adjacent organs, the mechanisms of cardiac metastasis are considered to include lymphatic or hematogenous metastasis, as well as composite types. In cases with cardiac metastasis, 69.4% had pericardial metastasis, 34.2% had epicardial metastasis, 31.8% had myocardial metastasis, and endocardial metastasis was uncommon, at 5%. Malignant tumors reported to have a relatively high frequency of cardiac metastasis include malignant pleural mesothelioma, melanoma, lung cancer, and breast cancer, however, reports of cardiac metastasis in esophageal cancer are rare[2]. Spindle cell carcinoma (SCC) is rare, accounting for about 0.1%-1.5% of esophageal cancers. Tumor cells show sarcomatoid differentiation with spindle cells and are accompanied by components of conventional carcinoma, including squamous cell carcinoma in some cases. There are reports of a high frequency of lymphatic metastasis and of a higher frequency of hematogenous metastasis[4].
The efficacy of 5-fluorouracil + cisplatin + anti-programmed cell death 1 antibody (nivolumab or pembrolizumab) and anti-programmed cell death 1 antibody + anti- cytotoxic T lymphocyte-associated antigen-4 antibody (nivolumab + ipilimumab) as first-line treatment has been demonstrated for metastatic/recurrent esophageal squamous cell carcinoma and adenocarcinoma, and they are standard treatments[5,6]. However, a standard treatment for advanced SCC has not been established. There have been no previous reports of myocardial metastasis from esophageal SCC. A case of esophageal SCC with rapid disease progression leading to death that showed myocardial metastasis on autopsy is presented.
A 60-year-old man was admitted to our hospital with complaints of difficulty in swallowing and left chest pain.
The patient had a 6-month history of persistent, worsening difficulty in swallowing since December 2023.
The patient had no significant history.
Mother of the patient had the history of uterine cancer.
The patient showed a poor general condition, with Eastern Comprehensive Oncology Group Performance Status (ECOG PS 3).
Blood tests showed preserved bone marrow, liver, and kidney functions, without coagulation abnormalities. Serum levels of tumor markers (carcinoembryonic antigen, SCC) were not increased.
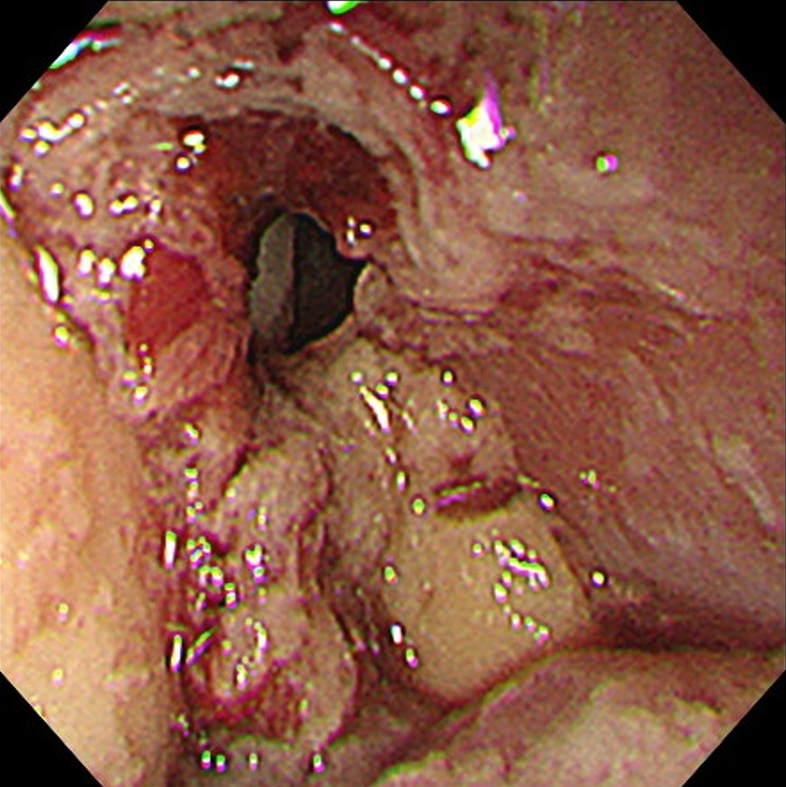
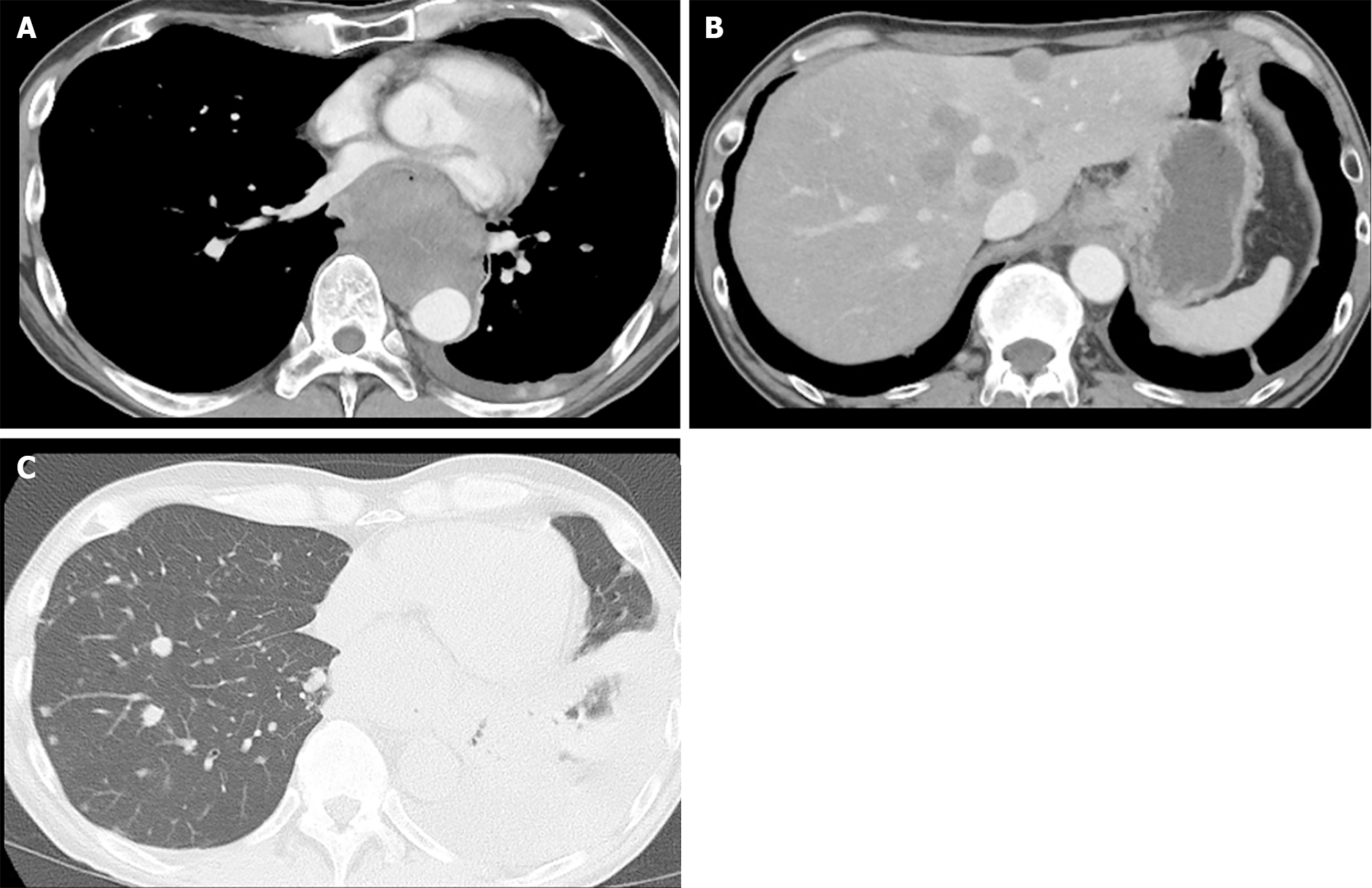
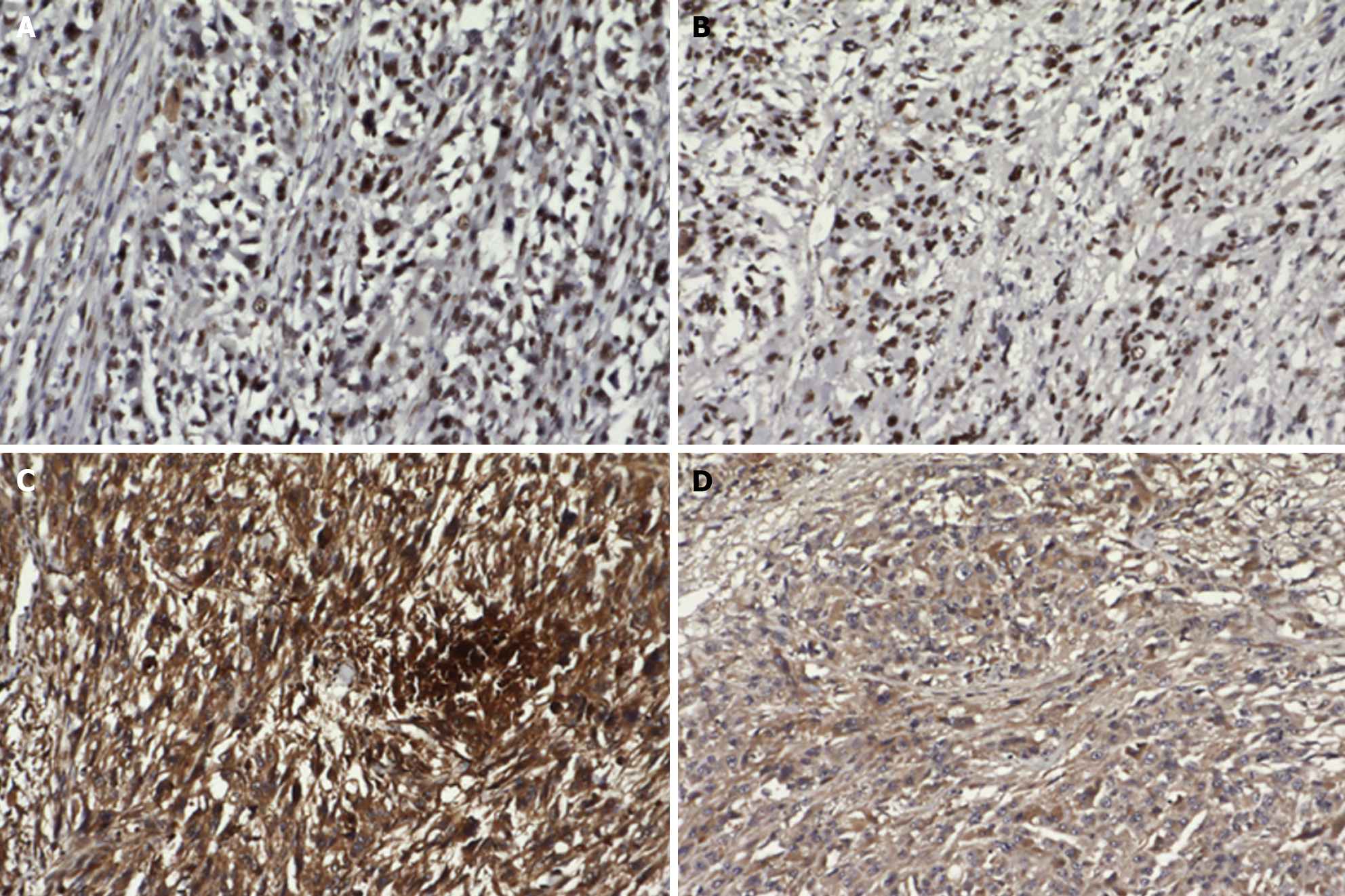
Upper gastrointestinal endoscopy showed a submucosal tumor-like protruding lesion, occupying nearly the entire lumen from the mid to lower thoracic esophagus, causing stenosis (Figure 1). Contrast-enhanced computed tomography (CT) showed esophageal tumor invasion into the left atrium, multiple liver and lung metastases, and a left pleural effusion (Figure 2). The biopsy specimen of the esophageal tumor showed spindle cells, positive for the mesenchymal marker vimentin and negative for epithelial markers including AE1/AE3, CAM5.2, p40, and cytokeratin 7, leading to a suspicion of esophageal sarcoma, and the patient was referred to our hospital for treatment. Cardiac ultrasonography showed a tumorous lesion on the posterior side of the left atrium, however, no invasion into the heart or intramyocardial tumor was observed. Chest X-ray and ultrasonography showed a pleural effusion, and thoracentesis was performed to alleviate symptoms and make a diagnosis, draining 1000 mL of slightly turbid, bloody pleural fluid. However, chest X-ray the next day showed re-accumulation of pleural fluid to the same degree as before drainage. Upper gastrointestinal endoscopy allowed passage of a slim scope, and biopsy of the primary lesion was performed. Histopathologically, atypical spindle cells and polymorphic cells, however, no epithelial components, were observed and immunohistological staining was negative for AE1/AE3, CAM5.2, cytokeratin 5/6, and p63, similar to the previous pathological report; thus, an epithelial malignant tumor could not be confirmed. The programmed death-ligand 1 Combined Positive Score (CPS) was ≥ 10. Pleural fluid cytology showed malignant cells, and cell block immunostaining showed similar findings to those of the primary lesion. Cancer stem cell markers including ZEB1 and TWIST were positive in both the primary and metastatic cardiac lesions (Figure 3).
Histopathologically, advanced esophageal squamous cell carcinoma or non-small round cell sarcoma was considered.
Disease progression was aggressive; thus, systemic chemotherapy for advanced esophageal squamous cell carcinoma or non-small round cell sarcoma was considered, however, it was deemed infeasible due to the patient’s poor general condition (ECOG PS 3). Expecting efficacy of nivolumab monotherapy given the high CPS, administration of nivolumab was started in January 2023.
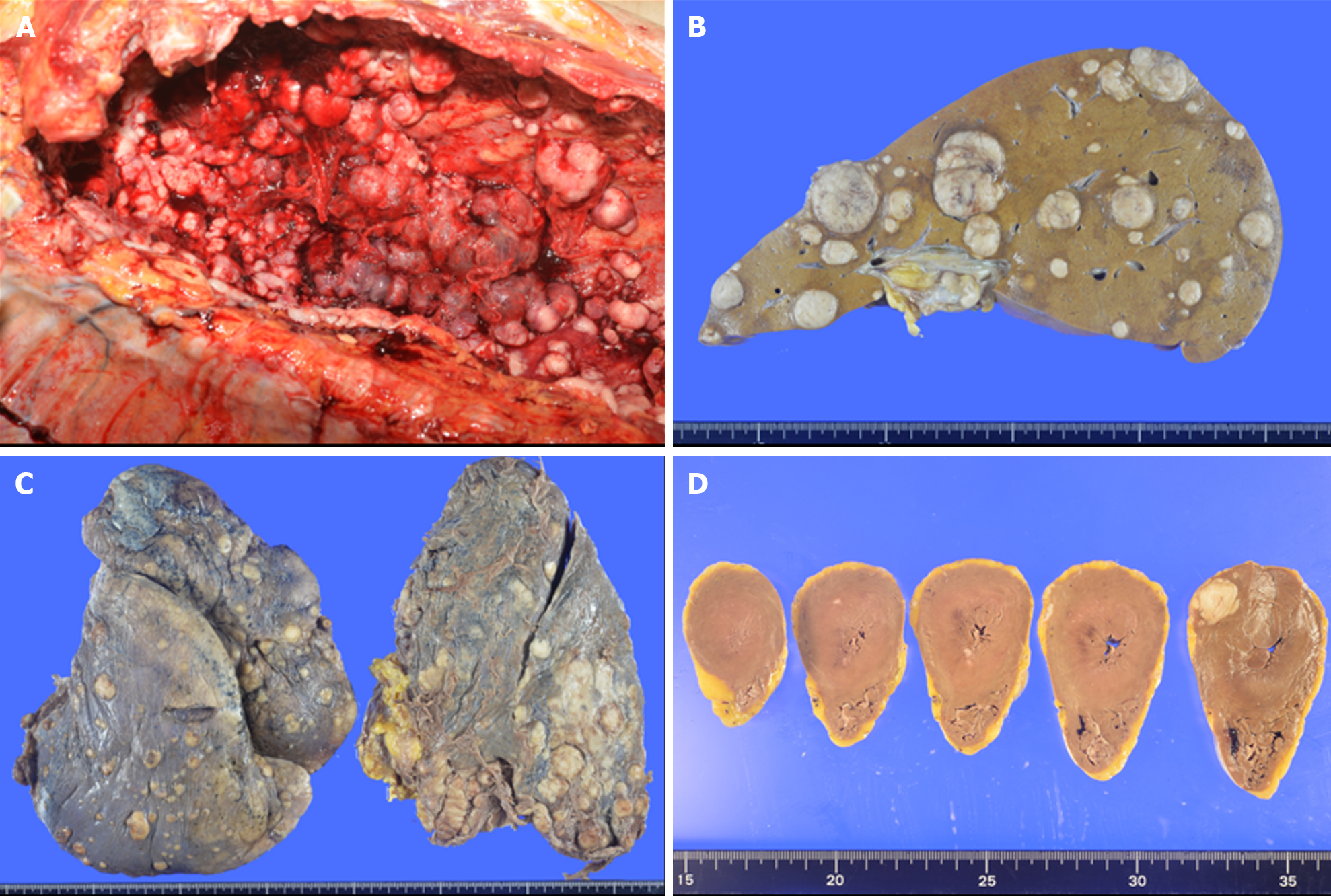
However, on the day of administration of nivolumab, the patient lost consciousness, developed lower jaw breathing, and then developed respiratory and cardiac arrest resulting in death. Pathological autopsy examination was performed with the prior written, informed consent from the patient and family to investigate the cause of death. In the pathological autopsy examination, A whitish, protruding lesion was observed from the mid to lower esophagus, mainly on the lateral to posterior walls, bulging into the lumen with severe stenosis of the esophageal lumen. Histologically, atypical epithelioid cells were densely proliferating with poor cohesion admixed with spindle cells and pleomorphic cells. Numerous mitotic figures were observed. The presence of an area considered to be carcinoma in situ at the border between tumor and non-tumor areas in the anterior and lateral esophageal walls, and partial positivity of the atypical cells for AE1/AE3 and cytokeratin 5/6 on immunohistochemistry, along with negative smooth muscle actin, desmin, c-kit, and no other distinct differentiation, led to a diagnosis of spindle cell squamous cell carcinoma arising from esophageal squamous cell carcinoma. The mediastinal lymph nodes were fused into a single mass by tumor metastases, with indistinct original nodal structures. Widespread tumor metastasis was observed in both lungs and the liver, accompanied by marked vascular invasion. In addition to a 15 mm × 12 mm nodular lesion in the left ventricular lateral wall, microscopic metastases were also observed in the left ventricular lateral and posterior walls and interventricular septum. Histologically, pleomorphic to spindle-shaped atypical cells with hyperchromatic nuclei, distinct nucleoli, and eosinophilic cytoplasm showed poorly cohesive proliferation, often admixed with polymorphic cells. There was no major histological difference between the primary and metastatic lesions. Numerous disseminated nodules were also observed in the left pleural cavity, with accumulation of a bloody pleural effusion. Death was ascribed to multi-organ tumor metastases, with respiratory failure due to pleural effusion also potentially contributing as a cause of death (Figure 4).
Cardiac metastasis occurs more often than primary cardiac tumors. In an autopsy study of 18751 cases, malignant tumors were identified in 7289 cases, of which 622 (9.1%) had cardiac metastases[2]. The most common metastases to the pericardium are from mesothelioma, lung cancer, uterine cancer, gastric cancer, and prostate cancer. Metastases to the epicardium are most common from melanoma, squamous cell carcinoma, and adenocarcinoma of the lung. Myocardial metastases occur most often from melanoma and hematological malignancies, whereas endocardial metastases are most common from melanoma, renal cell carcinoma, and hepatocellular carcinoma. In a study of 111 autopsies of esophageal cancer patients, epicardial metastases were found in 13%, and no myocardial metastases or endocardial metastases were identified[7]. Besides direct invasion, the mechanisms of cardiac metastasis are thought to include lymphatic spread, hematogenous spread, and their combination. Pericardial metastases, excluding direct invasion from intrathoracic or mediastinal tumors, are considered to arise from retrograde lymphatic spread from the trachea and mediastinal lymph nodes. Myocardial metastases and epicardial metastases may result from lymphatic spread and seeding from pericardial metastases. Hematogenous spread via the coronary arteries also contributes to myocardial metastases. In the present case, direct invasion and pericardial metastases were absent, and myocardial metastasis due to hematogenous spread was presumed.
SCC often shows positivity for vimentin and p53 on immunohistochemistry, whereas the epithelial component is frequently positive for pan cytokeratin (AE1/AE3). Li et al[8] reported that, in 23.2% of cases of primary esophageal SCC, both the spindle cells and the epithelial cells were positive for AE1/AE3, and in 8.5% of cases, both components were also positive for vimentin. In the present case, AE1/AE3 expression was limited to only a small portion, with loss of epithelial characteristics in the majority, suggesting difficulty in making a definitive diagnosis on biopsy. ZEB1 and TWIST positivity in primary esophageal SCC has been reported, implicating the epithelial-mesenchymal transition in its pathogenesis. Both the primary and metastatic cardiac tumors in the present case were positive for ZEB1 and TWIST[9]. To the best of our knowledge, this is the first analysis of these molecules in the rare occurrence of cardiac metastasis from primary esophageal SCC.
A previous report showed that primary esophageal SCC had a better prognosis than squamous cell carcinoma[10]. SCC often forms intraluminal protruding lesions, leading to early symptoms of dysphagia, which may account for its detection at an early stage. It is usually diagnosed as early cancer, with a lower frequency of lymph node metastasis. Iyomasa et al[11] compared 20 cases of carcinosarcoma to 773 cases of squamous cell carcinoma, reporting 3-year survival rates of 62.8% vs 28.1%, however, similar 5-year survival rates of 26.7% vs 22.4%, respectively[11]. Most cases are diagnosed and surgically resected before developing distant metastases, and surgery remains the only curative treatment of SCC. Other therapies including radiation and chemotherapy have been reported, however, the roles of radiation therapy and chemotherapy are unclear because of insufficient evidence. Schizas et al[12] reported that perioperative chemotherapy did not improve survival. Iwaya et al[13] described failure to control tumor progression with radiation therapy. In contrast, Sanada et al[14] reported tumor shrinkage with chemoradiation. Yamauchi et al[15] observed tumor reduction with chemoradiotherapy followed by rapid regrowth, speculating that the squamous component responded to the therapy, however, the sarcomatous component did not[12-15]. In the present case, biopsy pathology and the cell block of pleural fluid showed no epithelial component, suggesting sarcoma, and carcinosarcoma could not be ruled out. Standard therapy for sarcoma with doxorubicin alone or doxorubicin + ifosfamide could not be administered due to the patient’s poor general condition. A high CPS has been associated with response to immune checkpoint inhibitors (ICIs) in several cancers[16-18], and the CPS was greater than 10 in the present case, and a response to ICI therapy was expected. However, the disease progressed rapidly, and the patient died before a response to ICI therapy could be obtained. Although death on the day of ICI administration raises the possibility of a drug-related event, the patient’s vital signs were normal at the time of administration, without signs of allergy or anaphylaxis. Autopsy findings of rapid tumor progression and widespread dissemination support cancer death as the cause. Cardiac metastases are often asymptomatic and found incidentally on postmortem examination. Fluorodeoxyglucose positron emission tomography/CT has also shown asymptomatic myocardial metastases prior to death[19]. When symptomatic, widespread metastases to other sites are usually present, and death more commonly results from metastases elsewhere rather than the cardiac metastasis. However, pericardial metastases can cause cardiac tamponade requiring emergency drainage[20]. Myocardial metastases may cause symptoms mimicking myocardial infarction[21]. Endocardial metastasis causing an intracavitary cardiac mass from esophageal cancer has been resected to prevent sudden death and potentially prolong survival[22,23]. In the present case, the patient died before response assessment, and myocardial metastasis was found at autopsy, however, it did not appear to cause symptoms or affect prognosis.
Myocardial metastasis from esophageal cancer is rare, and among esophageal cancers, SCC is uncommon, so its metastatic patterns are unclear with no prior reports of myocardial metastasis. This report presents the first case of myocardial metastasis from ZEB1- and TWIST-positive esophageal SCC and characterizes its immunohistochemical profile.
The authors are grateful to the patient for granting us permission for this case report.
| 1. | Glancy DL, Roberts WC. The heart in malignant melanoma. A study of 70 autopsy cases. Am J Cardiol. 1968;21:555-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 150] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, Barbato L, Verzini A, Fortunato G, di Summa M. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999;68:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 231] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 322] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Raza MA, Mazzara PF. Sarcomatoid carcinoma of esophagus. Arch Pathol Lab Med. 2011;135:945-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 1085] [Article Influence: 217.0] [Reference Citation Analysis (0)] |
| 6. | Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 795] [Article Influence: 198.8] [Reference Citation Analysis (2)] |
| 7. | Mandard AM, Chasle J, Marnay J, Villedieu B, Bianco C, Roussel A, Elie H, Vernhes JC. Autopsy findings in 111 cases of esophageal cancer. Cancer. 1981;48:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Li XM, Song X, Zhao XK, Hu SJ, Cheng R, Lv S, Du DF, Zhang XY, Lu JL, Ku JW, Zhang DY, Zhang Y, Fan ZM, Wang LD. The alterations of cytokeratin and vimentin protein expressions in primary esophageal spindle cell carcinoma. BMC Cancer. 2018;18:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Nakazawa T, Nobusawa S, Ikota H, Kuwano H, Takeyoshi I, Yokoo H. Wide expression of ZEB1 in sarcomatous component of spindle cell carcinoma of the esophagus. Pathol Int. 2015;65:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | McCort JJ. Esophageal carcinosarcoma and pesudosarcoma. Radiology. 1972;102:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Iyomasa S, Kato H, Tachimori Y, Watanabe H, Yamaguchi H, Itabashi M. Carcinosarcoma of the esophagus: a twenty-case study. Jpn J Clin Oncol. 1990;20:99-106. [PubMed] |
| 12. | Schizas D, Mastoraki A, Bagias G, Ioannidi M, Kanavidis P, Moris D, Tsilimigras DI, Spartalis E, Arkadopoulos N, Liakakos T. Carcinosarcomas of the esophagus: systematic review of a rare nosologic entity. J BUON. 2018;23:1432-1438. [PubMed] |
| 13. | Iwaya T, Maesawa C, Uesugi N, Kimura T, Ogasawara S, Ikeda K, Kimura Y, Mitomo S, Ishida K, Sato N, Saito K, Masuda T. True carcinosarcoma of the esophagus. Dis Esophagus. 2006;19:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sanada Y, Hihara J, Yoshida K, Yamaguchi Y. Esophageal carcinosarcoma with intramural metastasis. Dis Esophagus. 2006;19:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Yamauchi T, Taniyama Y, Fujishima F, Sasano H, Unno M, Kamei T. Rapidly growing carcinosarcoma of the esophagus following definitive chemoradiotherapy: A case report and the literature review. Int J Surg Case Rep. 2022;94:107116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Ulas EB, Hashemi SMS, Houda I, Kaynak A, Veltman JD, Fransen MF, Radonic T, Bahce I. Predictive Value of Combined Positive Score and Tumor Proportion Score for Immunotherapy Response in Advanced NSCLC. JTO Clin Res Rep. 2023;4:100532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 17. | Shin M, Ahn S, Jung J, Hyung S, Kim KM, Kim ST, Kang WK, Lee J. Impact of programmed death-ligand 1 (PD-L1) positivity on clinical and molecular features of patients with metastatic gastric cancer. Cancer Med. 2023;12:18633-18642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2299] [Article Influence: 328.4] [Reference Citation Analysis (0)] |
| 19. | Puranik AD, Purandare NC, Sawant S, Agrawal A, Shah S, Jatale P, Rangarajan V. Asymptomatic myocardial metastasis from cancers of upper aero-digestive tract detected on FDG PET/CT: a series of 4 cases. Cancer Imaging. 2014;14:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Kishino T, Kumamoto K, Matsukawa H, Kondo A, Ando Y, Uemura J, Suto H, Asano E, Oshima M, Chiba Y, Ueno M, Nakagawa S, Kitamoto S, Yamashita Y, Horii T, Okano K. Constrictive pericarditis caused by pericardial metastasis from esophageal squamous cell carcinoma: a case report. Int Cancer Conf J. 2022;11:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Oliveira SM, Gonçalves A, Cruz C, Almeida J, Madureira AJ, Amendoeira I, Maciel MJ. Cardiac metastasis from epidermoid esophageal cancer mimicking anterior myocardial infarction. Rev Port Cardiol. 2012;31:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Yamazaki I, Yanagi H, Goda M, Suzuki S, Masuda M. Isolated intracavitary metastatic esophageal cancer of the right atrium and right ventricle. Jpn J Cardiovasc Surg. 2011;40:310-313. [DOI] [Full Text] |
| 23. | Kiryu K, Kadohama T, Yamaura G, Chida Y, Tanaka F, Takagi D, Itagaki Y, Yamamoto H. Tumorectomy to avoid sudden death by pulmonary embolism in a patient with a cardiac tumor originating from the esophagus. Jpn J Cardiovasc Surg. 2019;48:170-172. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang LQ, China S-Editor: Li L L-Editor: A P-Editor: Cai YX