Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1588
Peer-review started: October 5, 2023
First decision: December 6, 2023
Revised: December 20, 2023
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 21, 2024
Processing time: 168 Days and 9.2 Hours
Acute liver failure (ALF) has a high mortality with widespread hepatocyte death involving ferroptosis and pyroptosis. The silent information regulator sirtuin 1 (SIRT1)-mediated deacetylation affects multiple biological processes, including cellular senescence, apoptosis, sugar and lipid metabolism, oxidative stress, and inflammation.
To investigate the association between ferroptosis and pyroptosis and the upstream regulatory mechanisms.
This study included 30 patients with ALF and 30 healthy individuals who underwent serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) testing. C57BL/6 mice were also intraperitoneally pretreated with SIRT1, p53, or glutathione peroxidase 4 (GPX4) inducers and inhibitors and injected with lipopolysaccharide (LPS)/D-galactosamine (D-GalN) to induce ALF. Gasdermin D (GSDMD)-/- mice were used as an experimental group. Histological changes in liver tissue were monitored by hematoxylin and eosin staining. ALT, AST, glutathione, reactive oxygen species, and iron levels were measured using commercial kits. Ferroptosis- and pyroptosis-related protein and mRNA expression was detected by western blot and quantitative real-time polymerase chain reaction. SIRT1, p53, and GSDMD were assessed by immunofluorescence analysis.
Serum AST and ALT levels were elevated in patients with ALF. SIRT1, solute carrier family 7a member 11 (SLC7A11), and GPX4 protein expression was decreased and acetylated p5, p53, GSDMD, and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein levels were elevated in human ALF liver tissue. In the p53 and ferroptosis inhibitor-treated and GSDMD-/- groups, serum interleukin (IL)-1β, tumour necrosis factor alpha, IL-6, IL-2 and C-C motif ligand 2 levels were decreased and hepatic impairment was mitigated. In mice with GSDMD knockout, p53 was reduced, GPX4 was increased, and ferroptotic events (depletion of SLC7A11, elevation of ACSL4, and iron accumulation) were detected. In vitro, knockdown of p53 and overexpression of GPX4 reduced AST and ALT levels, the cytostatic rate, and GSDMD expression, restoring SLC7A11 depletion. Moreover, SIRT1 agonist and overexpression of SIRT1 alleviated acute liver injury and decreased iron deposition compared with results in the model group, accompanied by reduced p53, GSDMD, and ACSL4, and increased SLC7A11 and GPX4. Inactivation of SIRT1 exacerbated ferroptotic and pyroptotic cell death and aggravated liver injury in LPS/D-GalN-induced in vitro and in vivo models.
SIRT1 activation attenuates LPS/D-GalN-induced ferroptosis and pyroptosis by inhibiting the p53/GPX4/GSDMD signaling pathway in ALF.
Core Tip: In this study, we investigated the involvement of ferroptosis and pyroptosis in acute liver failure (ALF) using lipopolysaccharide/D-galactosamine-induced ALF model mice. Our results showed that silent information regulator sirtuin 1 activation alleviated ALF through p53/glutathione peroxidase 4/gasdermin D, which mediated the ferroptosis and pyroptosis crosstalk. Our study established a link between ferroptosis and pyroptosis and the upstream regulatory mechanisms. These results may lead to the identification of potential therapeutic targets for ALF.
- Citation: Zhou XN, Zhang Q, Peng H, Qin YJ, Liu YH, Wang L, Cheng ML, Luo XH, Li H. Silent information regulator sirtuin 1 ameliorates acute liver failure via the p53/glutathione peroxidase 4/gasdermin D axis. World J Gastroenterol 2024; 30(11): 1588-1608
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1588.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1588
Acute liver failure (ALF) is a rare and severe consequence of abrupt hepatocyte injury, with a mortality rate of up to 30%[1]. ALF is caused by various factors, including drugs, toxins, virus, and metabolic diseases. The central event in ALF is the excessive and uncontrolled mass death of hepatocytes through necrosis and apoptosis, which can cause DNA damage and oxidation stress, accompanied by an inflammatory storm[2,3]. Accumulating evidence has suggested that ferroptosis and pyroptosis, which are novel programmed modes of cell death that are distinct from necrosis, apoptosis, and autophagy, play critical roles in ALF by each mediating different immunological effects and inflammatory responses[4-6].
Ferroptosis is characterized by intracellular iron overload, decreased glutathione peroxidase 4 (GPX4) activity, and accumulation of lipid reactive oxygen species (ROS)[7]. Solute carrier family 7a member 11 (SLC7A11), a component of the system XC− antiporter located in the cell membrane, is involved in glutathione (GSH) synthesis[8]. Jiang et al[9] showed that transcriptional suppression of SLC7A11 by p53 resulted in inhibited cystine uptake and sensitized cells to ferroptosis. p53 plays a critical role in the cellular response to various stresses, including DNA damage, hypoxia, nutrition starvation, and oncogene activation[10]. Additionally, p53 promotes ferroptosis by inhibiting cystine metabolism and ROS activity[9,11].
Pyroptosis is a form of lytic cell death induced by pathogen infection or an endogenous challenge[12]. When cells receive internal and external danger signals, pattern recognition receptors initiate various inflammasomes to activate caspase 1, leading to cleavage of the pyroptosis executor gasdermin D (GSDMD) into the active N terminal and inactive C terminal. The active N terminal fragment translocates from the cytosol into the medial cell membrane, forming a large number of sieve membrane pores, resulting in the release of cell contents, including interleukin (IL)-1β and IL-18, into the extracellular space. Recruitment of immune cells for targeted migration to the injury site mediates secondary immune damage[13]. Our previous study showed that GSDMD-mediated pyroptosis of hepatocytes played an important role in the pathogenesis of ALF[14]. Furthermore, GSDMD knockout reduced hepatocyte death and inflammatory responses, thus alleviating ALF. However, the relationship between ferroptosis and pyroptosis in ALF is unknown.
Silent information regulator sirtuin 1 (SIRT1), which is an NAD+-dependent protein deacetylase, regulates the acetylation of specific transcription factors and proteins, including p53. SIRT1 is involved in various functions, such as energy metabolism, stress responses, inflammation, and redox homeostasis[15,16]. Deacetylation of p53 by SIRT1 may act as a protective shield in sepsis-induced liver injury[17].
In the present study, we investigated the involvement of ferroptosis and pyroptosis in ALF using lipopolysaccharide (LPS)/D-galactosamine (D-GalN)-induced ALF model mice. Our study established an association between ferroptosis and pyroptosis and the upstream regulatory mechanisms. These results may lead to the identification of potential therapeutic targets for ALF.
Samples from 30 patients with ALF and 30 healthy individuals were collected from the Affiliated Hospital of Guizhou Medical University. ALF was diagnosed by following the definition in the 2017 EASL ALF Guidelines[18]. Whole livers of seven patients with ALF and donor trimmed portions from three liver transplant recipients were collected. This study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University (2018 Lun Audit No. 036) and complied with the ethical guidelines of the 1975 Declaration of Helsinki. The tissue donors and/or their families provided informed consent for the use of human residual material for research purposes.
Male C57BL/6 mice (8-10 wk, 22-26 g) were purchased from SPF Biotechnology Co. (Beijing, China). Male GSDMD systemic knockout (GSDMD-/-) mice (C57BL/6J strain, 6-8 wk) were a generous gift from Shao Feng’s Laboratory, Beijing Institute of Life Sciences, China. All animals were kept in the Laboratory Animal Centre of Guizhou Medical University (laboratory animal production license No. SYXK (Guizhou) 2023-0002, and laboratory animal use license No. SYXK (Guizhou) 2023-0002). The animal experimental methods designed in this study were reviewed and approved by the Animal Ethics Committee of Guizhou Medical University. The mice were maintained in a 12-h light-dark cycle environment and had free access to water and food. The mice acclimated to the environment for 1 wk before the experiments.
The mice were randomly divided into the following nine experimental groups (n = 10 per group): Vehicle (phosphate-buffered saline), resveratrol [a SIRT1 activator; MedChemExpress, New Jersey, United States; 30 mg/kg, intraperitoneal injection (i.p.) for 14 d], EX527 (a SIRT1 inhibitor; Sigma, St. Louis, Missouri, United States; 5 mg/kg, i.p. for 14 d), pifithrin-α (a p53 inhibitor; MedChemExpress; 2.2 mg/kg, i.p. for 3 d), nutlin-3α (a p53 inducer; MedChemExpress; 12 mg/kg, i.p. for 7 d), liproxstatin-1 (a ferroptosis inhibitor; MedChemExpress; 10 mg/kg, i.p. for 14 d), RAS-selective lethal 3 (RSL3) (a ferroptosis inducer; Sigma; 2 mg/kg, i.p. for 7 d), GSDMD-/-, and ALF model. The mice were pre-treated as described above before establishing the model.
To establish the ALF model, all groups except for the vehicle group, were injected with LPS (Sigma; 10 µg/kg, i.p.) and D-GalN (Sigma; 300 mg/kg, i.p.). After 48 h, survival was recorded and the surviving mice were sacrificed. Blood was collected from the eyeball vein and centrifuged at 3000 × g/min for 15 min. The serum was separated and stored at -80 °C. Liver tissues were harvested by portal vein perfusion. Some of the specimens were fixed using paraformaldehyde for 48 h, and a pathological examination was performed. The remaining tissues were quickly stored at -80 °C.
Liver tissues and cells were homogenized in an ice bath using the Iron Assay Kit (BC4355; Solarbio, Beijing, China). The samples were centrifuged at 4000 × g at 4 °C and the supernatants were removed. The supernatants were added to a 96-well plate, and the absorbance was measured at 520 nm with a microplate reader.
Liver tissues were fixed in 4% paraformaldehyde for 24 h and dehydrated with a concentration gradient of ethanol solutions, followed by paraffin embedding. Tissue sections were cut, de-waxed, rehydrated, and stained with hematoxylin and eosin (HE). The sections were subjected to microscope analysis.
Liver tissues were collected and routinely embedded in optimal cutting temperature compound. The sections were dewaxed three times for 30 min each and dehydrated three times for 30 min each, followed by three washes with phosphate-buffered saline. Liver sections were stained with antibodies against SIRT1 (ab110304; Abcam), p53 (80077-1-RR; Proteintech), and GSDMD (ab219800; Abcam) overnight at 4 °C. After extensive washing, the sections were incubated with the respective fluorescent secondary antibodies (ab150115 and ab150077; Abcam) for 2 h at room temperature.
SIRT1, p53, GPX4, and negative control (NC) short interfering (siRNAs) were obtained from GeneChem (China). HL7702 cells were transfected with siRNAs using Lipofectamine 3000 (Invitrogen, United States) by following the manufacturer’s instructions. After 6 h, the culture medium was replaced with fresh complete medium and the cells were cultured for 48 h.
The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were assessed using the ALT Kit (E-BC-K235-M; Elabscience, China) and AST Kit (E-BC-K236-M; Elabscience, China) by following the manufacturers’ instructions.
ROS activity in cells was measured with a fluorescent probe (DCFH-DA) (Beyotime, Shanghai, China). HL7702 cells in a six-well plate were treated with D-GalN/LPS (D-GalN 15 mmol/L + LPS 100 µg/mL) for 24 h, and DCFH-DA (2 µL/well) was added for 30 min at 37 °C. The cells were washed three times with serum-free medium. DCFH-DA was measured by flow cytometric analysis.
Liver tissue was lysed with Trizol (Omega, United States). Total RNA was extracted and tested for concentration and purity. The RNA was reverse transcribed using a reverse transcription kit (Takara, Japan) to synthesize cDNA. Expression levels of mRNA were evaluated using the SYBR Green detection system (Takara, Japan). All samples were measured in triplicate and gene expression was normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. The primer sequences were as follows: GAPDH, (forward) AGGTCGGTGTGAACGGATTTG and (reverse) GGGGTCGTTGATGGCAACA; p53, (forward) CCCCTGCATCTT TTGTCCCT and (reverse) AGCTGGCAGAATAGCTTATTGAG; GPX4, (forward) TGTGCATC CCGCGATGATT and (reverse) CCCTGTACTTATCCAGGCAGA; GSDMD, (forward) ATGCC ATCGGCCTTTGAGAAA and (reverse) AGGCTGTCCACCGGAATGA; SLC7A11, (forward) GGCACCGTCATCGGATCAG and (reverse) CTCCACAGGCAGACCAGAAAA; and acyl-CoA synthetase long-chain family member 4 (ACSL4), (forward) CCTGAGGGGCTTGAAATTCAC and (reverse) GTTTCGGTGTGAACGGATTTG.
Liver tissues and cells were rinsed twice with phosphate-buffered saline buffer and lysed on ice with RIPA lysis solution (Solarbio). The protein samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Millipore, United States). The membranes were incubated with mouse anti-Sirt1 (1:1000; Abcam; ab110304), mouse anti-p53 (1:1000; Abcam; ab26), rabbit anti-p53 (acetyl K382) (1:1000; Abcam; ab75754), mouse anti-GPX4 (1:2000; Proteintech; 67763-1-Ig), rabbit anti-GSDMD (1:1000; Abcam; ab129800), rabbit anti-SLC7A11 (1:2000; Origene; TA351958), and rabbit anti-ACSL4 (1:2000; Origene; TA349566) antibodies overnight at 4 °C. The membranes were incubated with secondary anti-mouse (1:5000; Proteintech; SA00001-1) or anti-rabbit horseradish peroxidase-conjugated antibodies (1:5000; Proteintech; SA00001-2) for 2 h at room temperature. The signals were visualized using enhanced chemiluminescence reagent (CLiNX; Chemiscop, Shanghai, China).
Statistical analyses were conducted using Prism 8.3.0 (GraphPad, United States). Values are expressed as the mean ± SD of at least three independent experiments. The normality and the homogeneity of variance were checked by the Shapiro-Wilk test before Student’s t-test and one-way analysis of variance. Student’s t-test was used to compare data between two groups, while one-way analysis of variance analysis was used for comparison among multiple groups. Statistical significance was considered if P value was < 0.05.
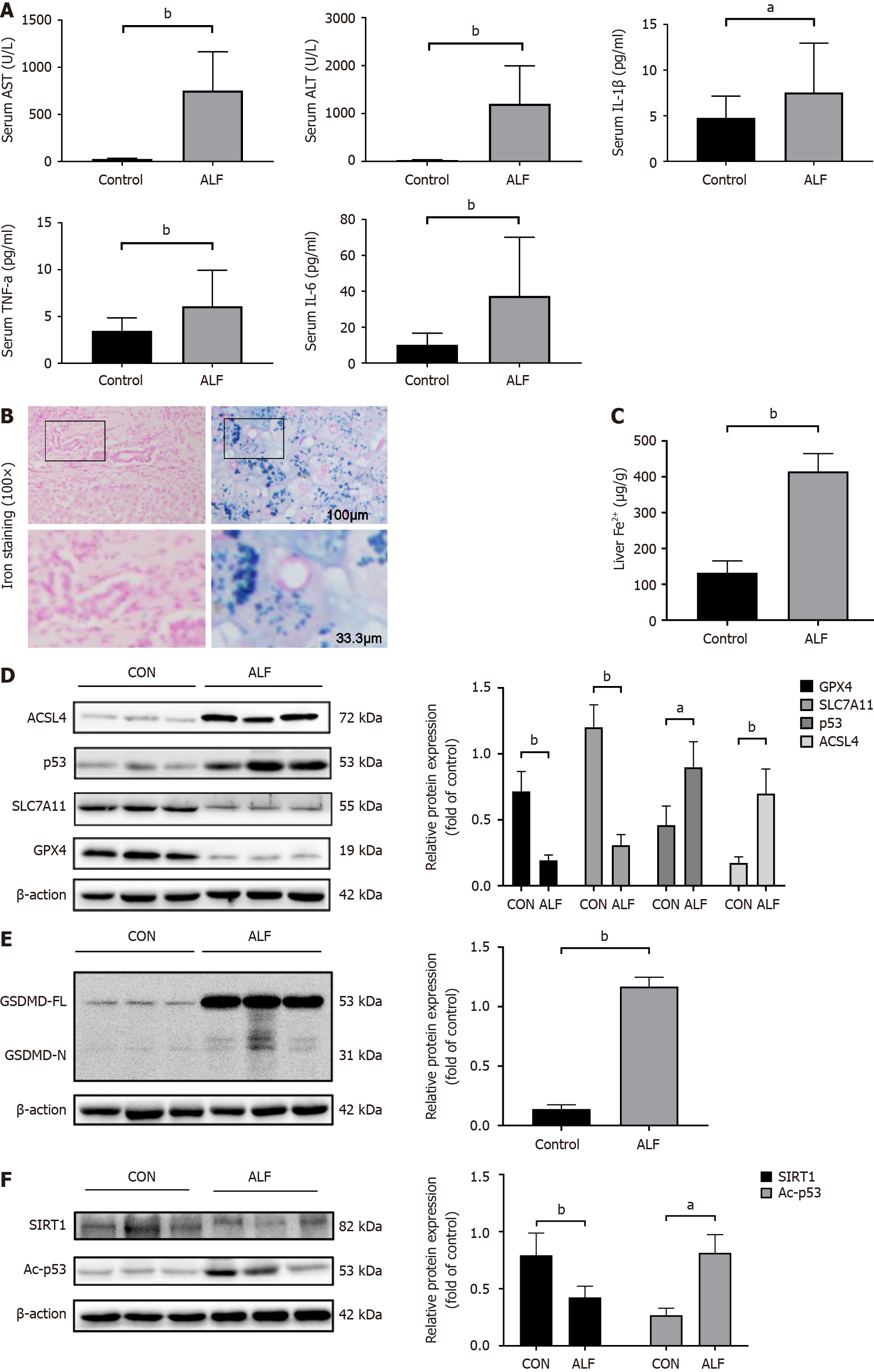
To examine the potential role of ferroptosis and pyroptosis in ALF, we first examined the inflammatory status, liver function biomarkers, and markers for ferroptosis and pyroptosis in the serum or liver tissue samples from patients with ALF. We found that AST, ALT, and inflammatory factor levels were increased in human ALF serum (Figure 1A). Iron staining showed iron accumulation in liver tissue in patients with ALF (Figure 1B and C). Additionally, the ferroptotic markers GPX4 and SLC7A11 showed decreased protein expression, whereas p53 and ACSL4 proteins, which are positive regulators of ferroptosis, were elevated in human ALF liver tissue (Figure 1D). In line with previous studies[14], GSDMD, which is a key regulatory factor of pyroptosis, was increased in human ALF tissue (Figure 1E). We also found that p53 and acetylated p53 (Ac-p53) were elevated and SIRT1 was decreased in human ALF tissue (Figure 1F).
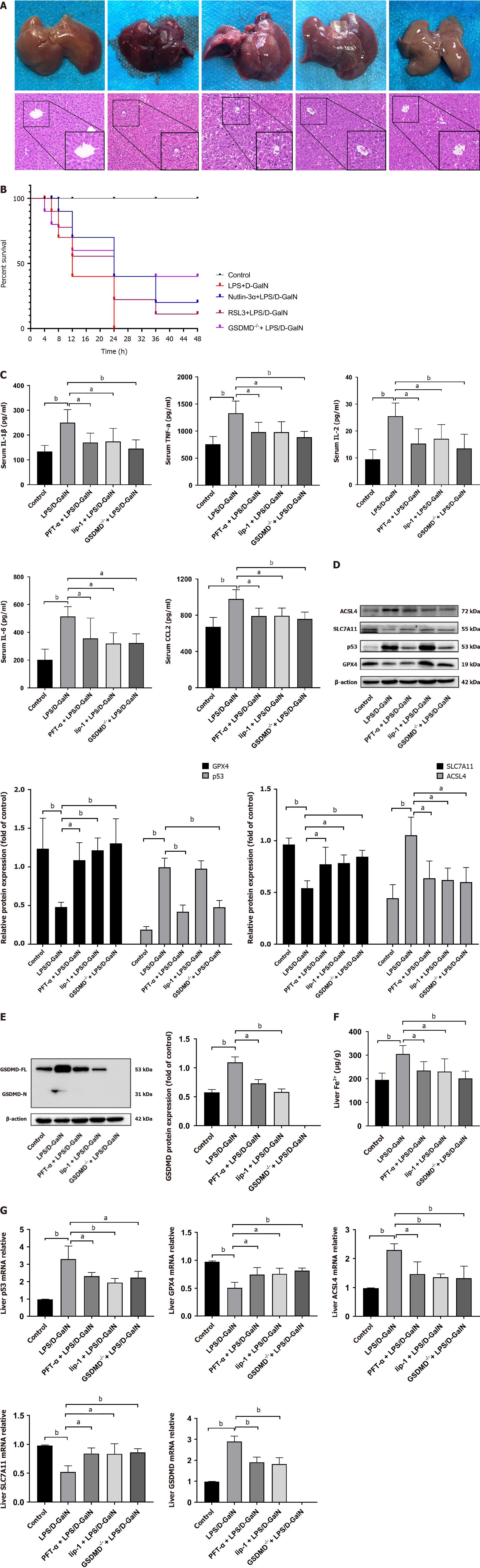
We observed an induction of ferroptosis and pyroptosis in human ALF liver tissues, accompanied by increased p53 protein expression. To further examine the mechanism of ferroptosis and pyroptosis in ALF, we inhibited ferroptosis and pyroptosis in mice with ALF induced by LPS/D-GalN. Untreated mice were first pretreated with pifithrin-α and liproxstatin-1, which inhibits GPX4 consumption[19,20]. GSDMD-/- mice were used as another experimental group. HE staining of liver sections showed that mice pre-treated with pifithrin-α or liproxstatin-1 and GSDMD-/- mice had less structural damage in liver tissue, and most liver lobules were structurally intact compared with those in the ALF model group. These mice also showed a prolonged survival compared with those in the ALF model group (Figure 2A and B). Furthermore, mice pre-treated with pifithrin-α or liproxstatin-1 and GSDMD-/- mice showed a reduction in elevated serum levels of IL-1β, tumour necrosis factor alpha (TNF-α), IL-6, IL-2, and C-C motif ligand 2 (CCL2), which are inflammatory factors associated with pyroptosis, compared with those in the ALF model group (Figure 2C). Western blot analysis and quantitative real-time polymerase chain reaction (qRT-PCR) showed that mice pre-treated with pifithrin-α and liproxstatin-1 had reduced protein and mRNA levels of GSDMD and ferroptotic markers (ACSL4 and p53) compared with those in the ALF model group. We also found that GSDMD-/- mice showed increased GPX4 and SLC7A11 and decreased p53 and ACSL4 expression, accompanied by a decrease in iron accumulation (Figure 2D-G). Notably, p53 protein levels were markedly lower in GSDMD-/- mice than in ALF mice, but they were not affected by liproxstatin-1. However, p53 mRNA levels in the liproxstatin-1 group were downregulated (Figure 2D). An immunofluorescence assay showed a decrease in GSDMD expression in the groups treated with pifithrin-α and liproxstatin-1 (Supplementary Figure 1A).
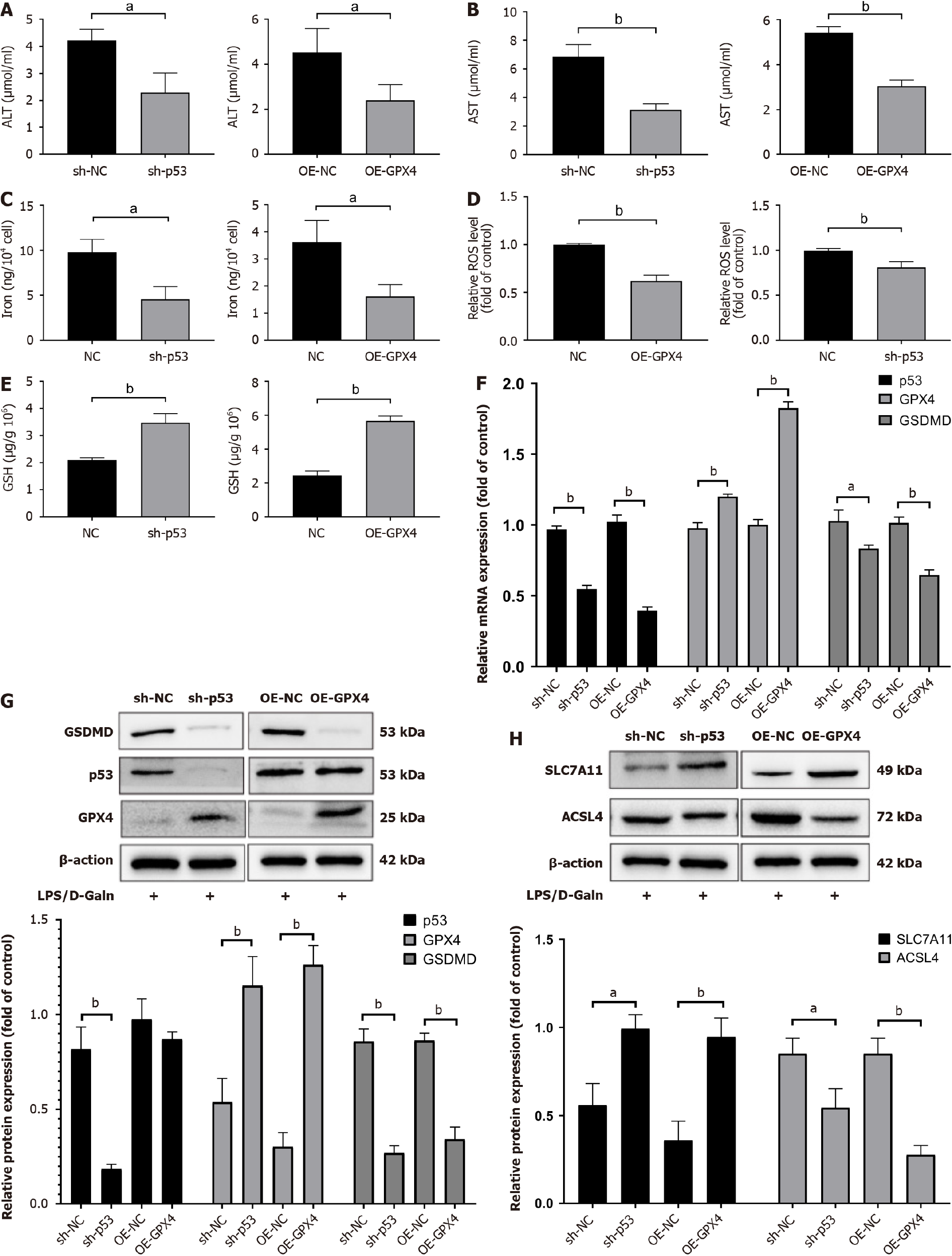
To gain insight into the potential mechanisms of ferroptosis and pyroptosis in ALF induced by LPS/D-GalN, HL7702 cells transfected with siRNA targeting p53 and plasmid overexpressing GPX4 were stimulated with LPS/D-GalN for 24 h. We then detected the cellular AST and ALT contents. We found that LPS/D-GalN significantly increased AST and ALT, while knocking down p53 and overexpressing GPX4 reduced AST and ALT (Figure 3A and B). We further examined iron, ROS, GSH, and lipid peroxidation products, and found that knocking down p53 and overexpressing GPX4 significantly reduced GSH, malondialdehyde, and ROS contents compared with those in the AFL model group (Figure 3C-E). Western blot analysis and qRT-PCR showed that when p53 was knocked down and GPX4 was overexpressed, SLC7A11 protein and mRNA expression levels were restored compared with control levels. This change occurred at the same time as a decrease in ACSL4 expression (Figure 3F-H). Notably, GSDMD expression was also reduced in the sh-p53 group compared with the sh-NC group. To evaluate the relationships between p53, GPX4, and GSDMD, we overexpressed GPX4 in HL7702 cells, and found that AST and ALT levels were increased (Supplementary Figure 1A). Furthermore, with GPX4 overexpression, iron, ROS, and GSH contents and ferroptotic events were higher than those in the control group (Supplementary Figure 2A). Western blot analysis and qRT-PCR showed that SLC7A11 protein and mRNA levels were downregulated, while p53, ACSL4, and GSDMD protein and mRNA levels were upregulated in the GPX4 knockdown group compared with the sh-NC group (Supplementary Figure 2B and C).
GPX4 and GSDMD play a prominent role in the occurrence of ferroptosis and pyroptosis. p53 promotes ferroptosis by inhibiting cystine metabolism and ROS activity. However, the relationships between p53, GPX4, and GSDMD are unknown. These results showed that the suppression of ferroptosis and pyroptosis attenuated LPS/D-GalN-induced hepatocyte injury by inhibiting the p53/GPX4/GSDMD signaling pathway and lipid peroxidation.
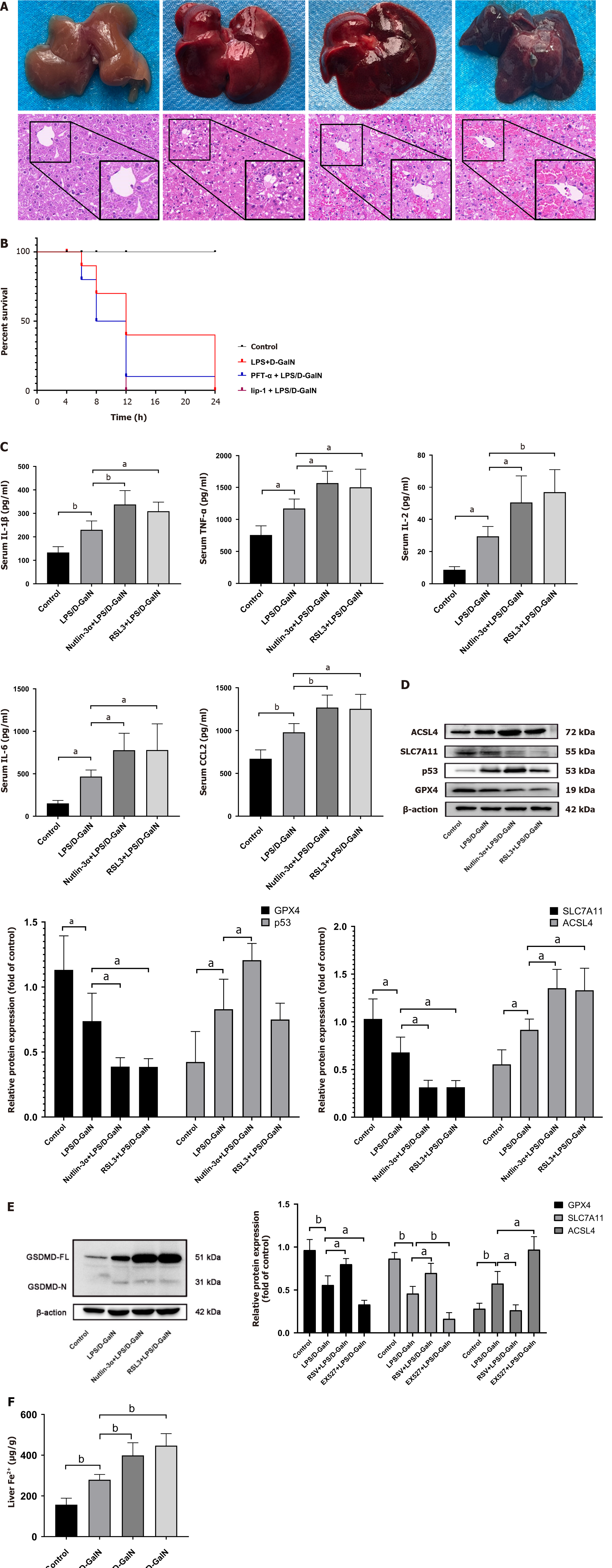
To further validate our hypothesis, we used nutlin-3α and RSL3 (ferroptosis inducer, which also causes GPX4 inactivation)[21,22] in ALF model mice. HE staining of liver tissue showed that the administration of nutlin-3α dramatically aggravated liver injury in ALF model mice to the same extent as that with RSL3 (Figure 4A). Furthermore, both treatments shortened the survival time of mice (Figure 4B). We also found that nutlin-3α and RSL3 worsened the inflammatory response in ALF model mice (Figure 4C). GPX4 and SLC7A11 were significantly decreased after nutlin-3α treatment, and ACSL4 protein levels were elevated (Figure 4D). Notably, p53 protein levels were upregulated slightly after RSL3 treatment, but this was not significant (Figure 4D). Western blot analysis showed that GSDMD and GSDMD-N protein levels were significantly higher in the nutlin-3α and RSL3 groups than in the ALF model group (Figure 4E), accompanied by increased iron deposition (Figure 4F). Immunofluorescence staining showed that nutlin-3α and RSL3 significantly exacerbated GSDMD expression in liver tissue, which suggested that inducing ferroptosis aggravated pyroptosis (Supplementary Figure 1B). Taken together, these data indicate that p53 upregulation may cause SLC7A11 and GPX4 downregulation, leading to ferroptosis, which in turn triggers pyroptosis to amplify the inflammatory response. This process may form a positive feedback loop, resulting in the deterioration of ALF.
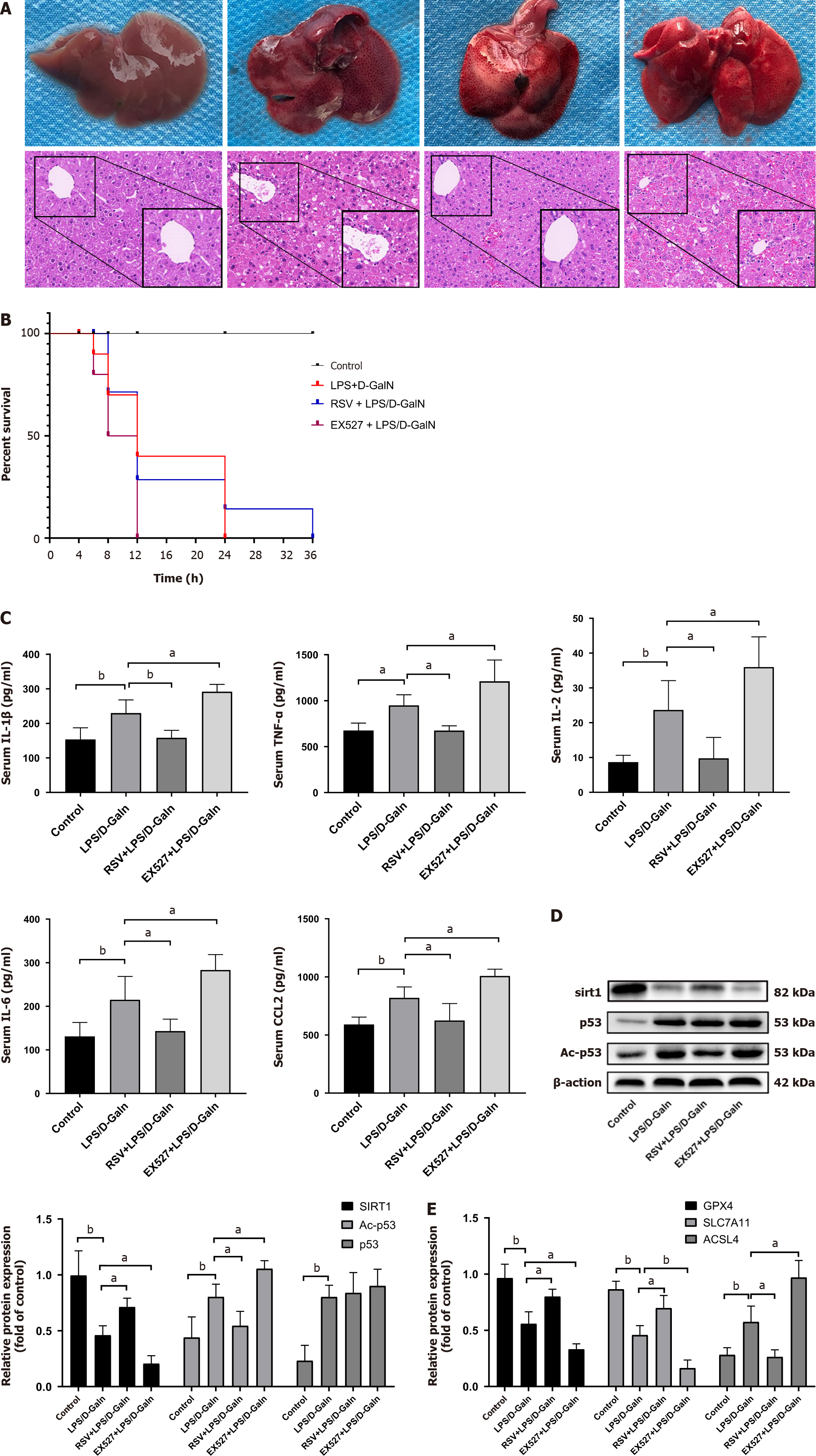
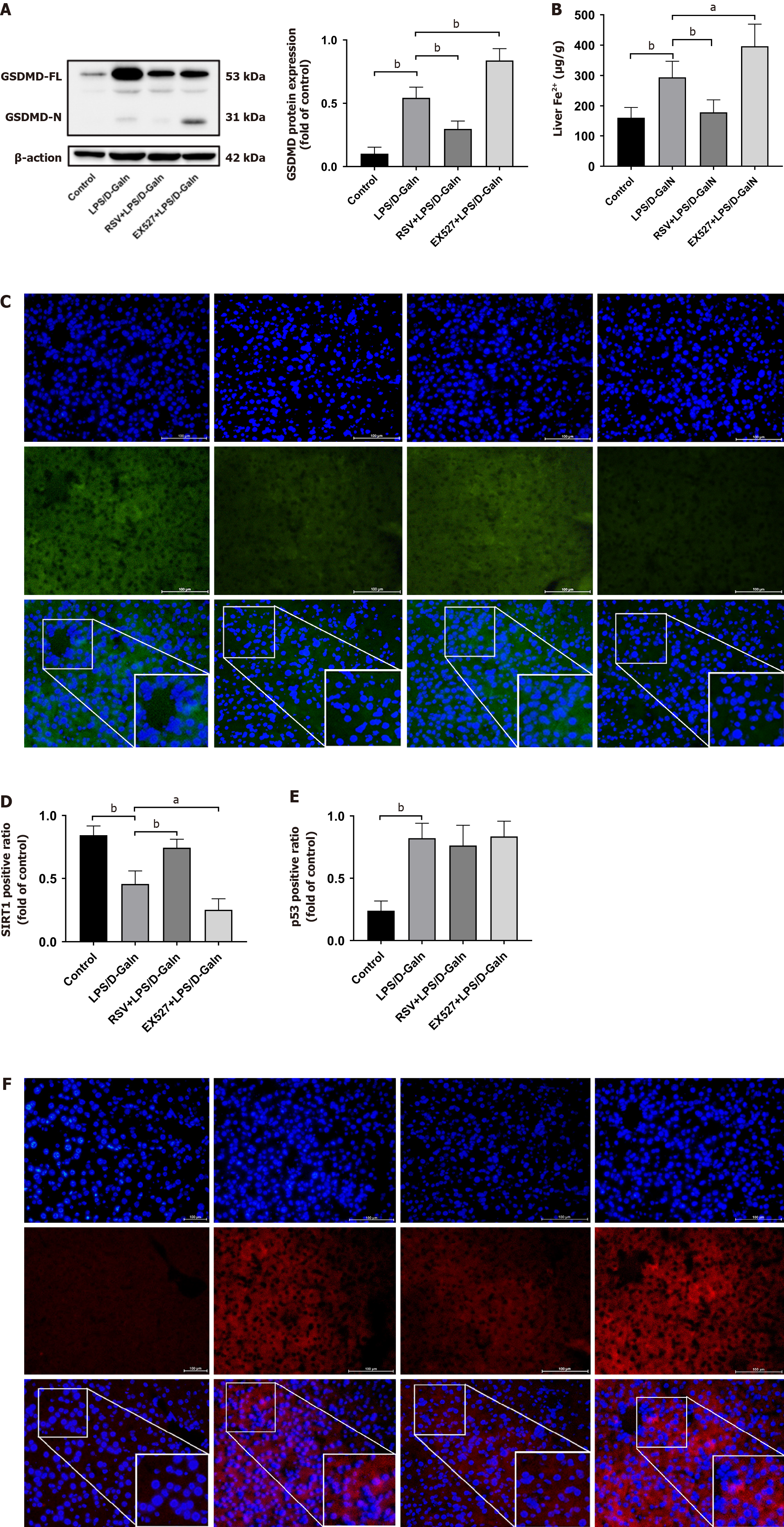
We found that SIRT1 levels were downregulated in human ALF, while Ac-p53 and p53 Levels were increased (Figure 1D and E). Previous studies have shown that SIRT1 inhibits p53 deacetylation. Our results confirmed that p53/GPX4/GSDMD mediated ferroptosis and pyroptosis. Therefore, we next pre-treated mice with EX527 and resveratrol. HE staining showed that the structure of the liver lobules was severely destroyed by the infiltration of inflammatory cells in the EX527 group compared with the ALF model group. In contrast, resveratrol treatment attenuated LPS/D-GalN-induced acute liver injury (Figure 5A) and prolonged the survival time of mice (Figure 5B). Multi-factor kits showed that EX527 exacerbated the inflammatory response (serum IL-1β, TNF-α, IL-6, IL-2, and CCL2 levels in mice), while resveratrol had the opposite effect (Figure 5C). Western blot analysis showed that, in liver tissue of resveratrol-treated mice with ALF, SIRT1 activation increased p53 acetylation, with no significant changes in p53 protein levels (Figure 5D). When SIRT1 was inhibited, GPX4 and SLC7A11 expression was decreased and ACSL4 expression was upregulated (Figure 5E). GSDMD and GSDMD-N expression was increased, especially GSDMD-N (Figure 6A), and iron accumulation was enhanced (Figure 6B). We found that resveratrol, as a SIRT1 inducer, increased SIRT1 expression. In contrast, EX527 decreased SIRT1 level (Figure 6C and D). We further performed immunofluorescence experiments, which showed no significant change in p53 protein expression in the resveratrol or EX527 treatment groups compared with the ALF model group (Figure 6E and F), which is consistent with our results mentioned above (Figure 5D). Overall, these data demonstrate that SIRT1 exerts protection against ALF by inhibiting p53 deacetylation, thereby inhibiting the GPX4/GSDMD signaling pathway.
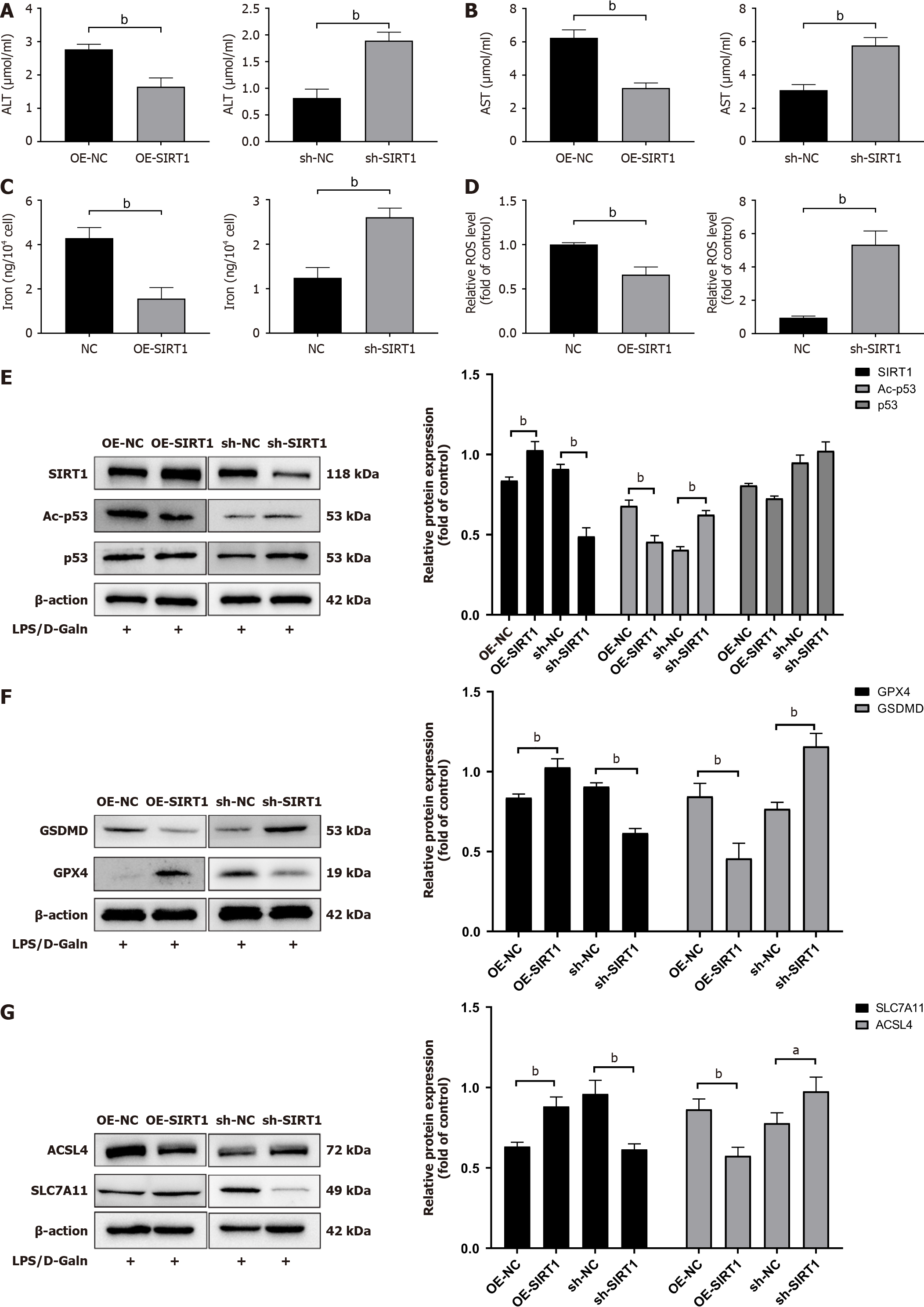
To further investigate the functional role of SIRT1 in hepatocyte ferroptosis and pyroptosis, we knocked down and overexpressed SIRT1 in HL7702 cells. ALT and AST levels were lower in the SIRT1 overexpression group and higher in the SIRT1 knockdown group than in the ALF model and knockdown groups (Figure 7A and B). Ferroptotic events (elevation of ROS activity and iron accumulation) were detected in the SIRT1 overexpression group (Figure 7C and D). Western blot analysis showed that Ac-p53 protein levels were significantly decreased in the SIRT1 overexpression group, while p53 did not change (Figure 7E). We next examined ferroptosis- and pyroptosis-related proteins (GPX4, SLC7A11, ACSL4, and GSDMD). We found that upregulation of SIRT1 increased GPX4 and SLC7A11 protein expression and decreased ACSL4 and GSDMD protein expression (Figure 7F and G). Furthermore, knockdown of SIRT1 inhibited the deacetylation and degradation of p53 and downregulated the levels of SLC7A11 and GPX4 (Figure 7F and G). These data show that SIRT1 overexpression alleviated ALF in HL7702 cells via a p53/GPX4/GSDMD-dependent mechanism.
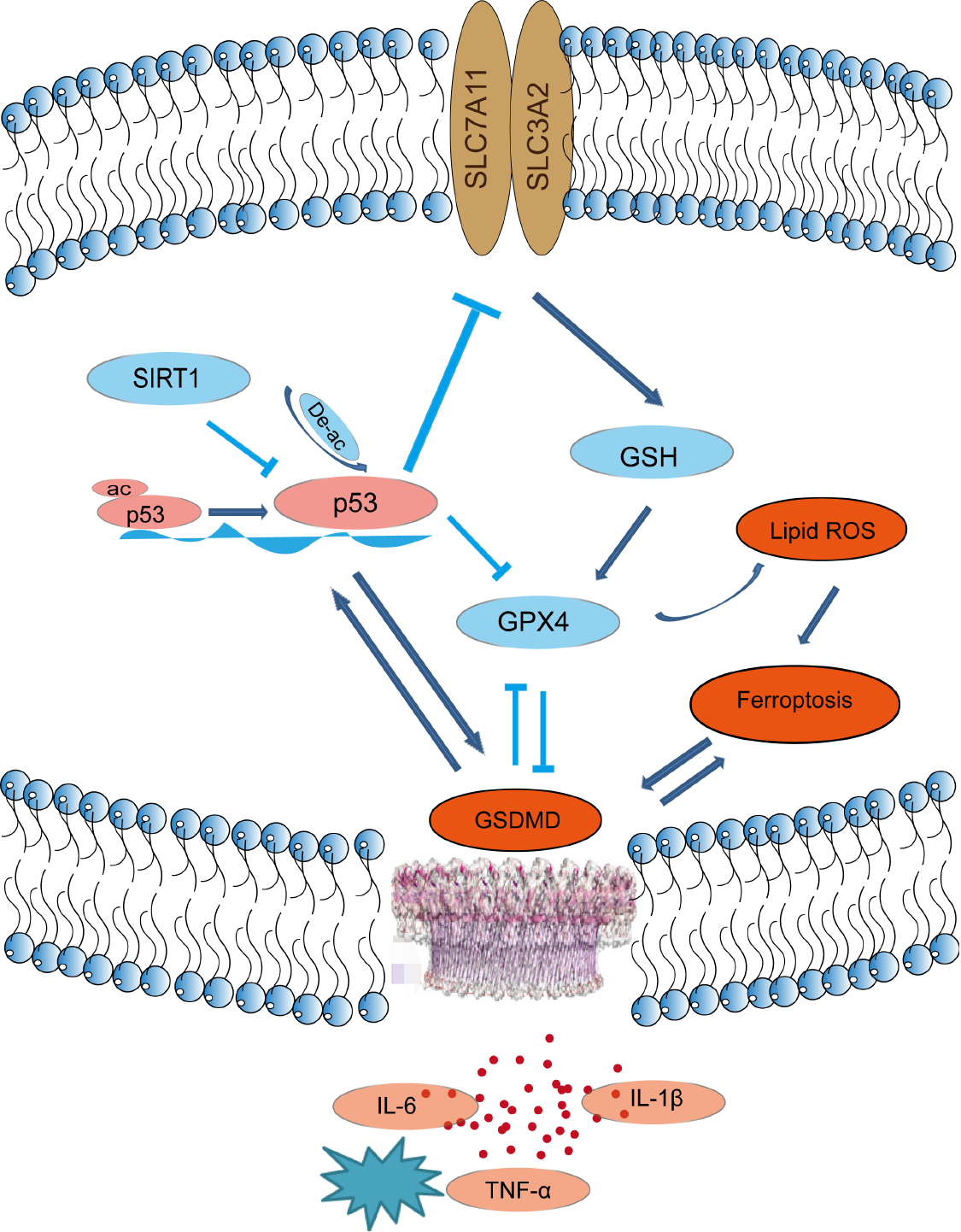
ALF is a life-threatening disease with high morbidity and mortality rates. The most effective treatment for ALF at present is liver transplantation[23]. However, liver transplants are not widely available because of the lack of donors and the high cost of medical care[18]. ALF is characterized by extensive hepatocellular cell death, leading to massive loss of parenchymal cells, with the release of cell contents, cell swelling, and inflammation. Emerging evidence has indicated that ferroptosis and pyroptosis, two newly identified forms of non-apoptotic cell death, play a crucial role in liver disease[24,25], but their precise role in ALF is unknown. In this study, we provided evidence that ferroptosis and pyroptosis occurred in ALF and established an association between them. We found that SIRT1 activation or inhibition of the p53/GPX4/GSDMD signaling pathway attenuated LPS/D-GalN-induced ferroptosis and pyroptosis in ALF (Figure 8).
In this study, we found that the activity of the enzymatic biomarkers ALT and AST was markedly increased in human ALF, accompanied by elevated inflammatory factors, such as TNF-α, IL-1β, and IL-6. Furthermore, we found that the ferroptosis-related antioxidant proteins GPX4 and SLC7A11 were reduced and iron deposition was aggravated. Additionally, GSDMD expression was increased.
The pathogenesis of ALF induced by LPS/D-GalN in mice is similar to that of fulminant hepatitis in humans[26,27]. Only some evidence has indicated that ferroptosis and pyroptosis occur in LPS/D-GalN-induced ALF, and inhibition of ferroptosis and pyroptosis attenuates ALF[28,29]. However, the association between ferroptosis and pyroptosis is unclear.
GPX4 and GSDMD play a prominent role in the occurrence of ferroptosis and pyroptosis. GSH biosynthesis and proper functioning of GPX4 are critical for inhibiting ferroptosis, and conditions that culminate in GPX4 inhibition/destabilization sensitize cells to ferroptosis or even trigger ferroptotic cell death[19,21,30]. GSDMD, a mediator of pyroptosis, is cleaved by inflammasome-activated caspase-1 and LPS-activated caspase-11/4/5 into active GSDMD-N and inactive GSDMD-C. Deletion of GSDMD completely abolishes cell pyroptosis[31,32]. An increasing number of studies have suggested that there is an interaction between ferroptosis and pyroptosis. The knockout of caspase-11, which is involved in the non-classical pathway of pyroptotic cell death, in myeloid cells conferred similar protection in septic myeloid cell–specific GPX4-knockout mice, which were susceptible to lethal infection[33]. The caspase-1-dependent NLRP3 inflammasome was also inhibited by GPX4, indicating that GPX4 has a broad role in inhibiting pyroptosis[33]. However, the association between ferroptosis and pyroptosis in ALF is unclear. The present study provided evidence that bridged the gap between ferroptosis and pyroptosis, and showed that blocking the p53/GPX4/GSDMD pathway regulated ferroptosis and pyroptosis in ALF. Inhibiting p53 and enhancing GPX4 in ALF model mice with drugs reduced AST and ALT levels and inflammatory reactions compared with the ALF model group, and ferroptotic events (depletion of GPX4, GSH, and SLC7A11, and iron accumulation) were reversed. Additionally, GSDMD-N protein levels were significantly decreased, consistent with the immunofluorescence results (Supplementary Figure 1). Notably, deletion of GSDMD decreased p53 expression and upregulated GPX4, which indicated crosstalk between ferroptosis and pyroptosis. The knockdown of GPX4 increased AST and ALT levels, accompanied by significantly increased ferroptotic markers and GSDMD (Supplementary Figure 2). Together, these results suggested that blocking the p53/GPX4/GSDMD signaling pathway alleviated ferroptosis and pyroptosis in ALF, and a positive feedback loop may exist.
In our study, we found that increased or decreased GPX4 mRNA or protein expression did not affect p53, but it acted indirectly by regulating GSDMD, suggesting that GPX4 is a downstream regulator of p53. Chen et al[34] reported that p53 levels were unaffected by the loss of ACSL4 and GPX4, and p53-driven ferroptosis was induced in a GPX4-independent manner. Nevertheless, enhancement of GPX4 reduced p53 transcription, which was inconsistent with the Western blot results. This difference between mRNA and protein levels suggests that post-transcriptional regulation, translational efficiency, and post-translational modifications alter protein levels. One possible explanation for this difference is that reduced translational efficiency may be compensated for by increased transcriptional activity[35]. However, the mechanism underlying the differences in transcription and translation of p53 is unclear.
SIRT1 has been widely reported to play a protective role in various biological processes including nutrient starvation, DNA repair, aging, oxidative stress, and the inflammatory response[36,37]. One study suggested that negative regulation of SIRT1 increased pyroptosis (GSDMD) and aggravated the acute hepatic pro-inflammatory reaction[38]. This finding is consistent with another study, which showed that SIRT1 was suppressed in APAP-induced hepatotoxicity[39]. Treatment with resveratrol, a small-molecule SIRT1 activator, shows a protective effect in mouse liver ischemia–reperfusion injury[40,41]. Similarly, in our study, we found that SIRT1 was decreased and p53 and Ac-p53 were increased in human ALF. SIRT1 is an NAD-dependent deacetylase that directly deacetylates p53 and mediates its function[37]. A study showed that SIRT1 overexpression abolished p53 acetylation levels and reduced the release of hepatic enzymes, hepatic oxidative, stress, and inflammation in non-alcoholic fatty liver disease[42]. Ma et al[43] demonstrated that SIRT1 inhibited ferroptosis-induced myocardial cell death through the p53/SLC7A11 axis in myocardial ischemia–reperfusion injury. Nevertheless, whether SIRT1 activation regulates p53 deacetylation to affect ferroptosis in ALF has not been shown. Our study showed that SIRT1 activation attenuated liver injury and the inflammatory response, accompanied by a reduction in ferroptosis and pyroptosis-related proteins in ALF. We demonstrated that SIRT1 activation inhibited p53/GPX4/GSDMD by inducing p53 acetylation, which attenuated LPS/D-GalN-induced ALF.
In conclusion, our study provides evidence that ferroptosis and pyroptosis are crucial modes of hepatocyte death in ALF, and the interactions between these modes of cell death advance the progression of ALF. SIRT1 plays an important role in ALF through p53/GPX4/GSDMD-mediated ferroptosis and pyroptosis. These results could provide new therapeutic targets to alleviate ALF. However, there are still many issues that need to be further investigated. First, p53 is a versatile protein with multiple roles in promoting physiological and pathological regulation. Although p53 can promote ferroptosis, it is also involved in several major functions, such as cell cycle arrest, DNA repair, angiogenesis, metastasis, and senescence. When p53 pro-death function is inhibited, its pro-survival function is concomitantly impaired. Therefore, how the unique p53-mediated pro-death function might be achieved is unclear. Second, our research was only limited to cellular and mouse models and has not been applied to clinical studies. Although we found decreased SIRT1 expression in human ALF liver tissue, whether an SIRT1 activator is effective for acute liver injury and failure in patients is unknown, and further safety and efficacy studies are required. Third, the mechanism of ALF may be associated with multiple modes of cell death, but our study was limited to ferroptosis and pyroptosis, and was not designed to examine other modes of death. The focus of our next study will be to further investigate the mechanism of ALF in depth.
Acute liver failure (ALF) has a high mortality with widespread hepatocyte death involving ferroptosis and pyroptosis, but their precise role in ALF is unknown. Silent information regulator sirtuin 1 (SIRT1)-mediated deacetylation influences multiple biological processes, including cellular senescence, apoptosis, sugar and lipid metabolism, oxidative stress, and inflammation. In this study, we examined the link between ferroptosis and pyroptosis and the upstream regulatory mechanisms.
The most effective treatment for ALF at present is liver transplantation. However, liver transplants are not widely available because of the lack of donors and the high cost of medical care. Therefore, therapies for ALF are imminent.
To explore the link between ferroptosis and pyroptosis and the upstream regulatory mechanisms.
Animal and cellular models of ALF were developed. The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were tested with automatic biochemistry instrument. Iron, reactive oxygen species, and glutathione levels were measured using commercial kits. SIRT1, p53, acetylated p53 (Ac-p53), glutathione peroxidase 4 (GPX4), gasdermin D (GSDMD), solute carrier family 7a member 11 (SLC7A11), and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expression levels were measured through Western blot analysis.
AST and ALT levels were elevated in the serum of ALF patients. SIRT1, SLC7A11, and GPX4 expressions were decreased and Ac-p53, p53, GSDMD, and ACSL4 levels were elevated in human ALF liver tissue. In p53 and ferroptosis inhibitor–treated and GSDMD-/- groups, serum interleukin (IL)-1β, tumour necrosis factor alpha, IL-6, IL-2, and C-C motif ligand 2 levels were decreased and hepatic impairment was mitigated. In mice with GSDMD knockout, p53 was reduced, GPX4 was increased, and ferroptotic events (depletion of SLC7A11, elevation of ACSL4, and iron accumulation) were detected.
SIRT1 activation attenuates lipopolysaccharide/D-galactosamine-induced ferroptosis and pyroptosis by inhibiting the p53/GPX4/GSDMD signaling pathway in ALF.
Our research is only limited to cellular and mouse models and has not been applied to clinical studies. Although we found decreased SIRT1 expression in human ALF liver tissue, whether SIRT1 activator is effective for acute liver injury and failure in patients is unknown, and further safety and efficacy studies are required.
We thank Ellen Knapp, PhD, for editing the English text of a draft of this manuscript.
| 1. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 631] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 2. | Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765-783.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 3. | Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 804] [Article Influence: 61.8] [Reference Citation Analysis (6)] |
| 4. | Green DR. The Coming Decade of Cell Death Research: Five Riddles. Cell. 2019;177:1094-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 5. | Mancardi D, Mezzanotte M, Arrigo E, Barinotti A, Roetto A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 6. | de Carvalho Ribeiro M, Szabo G. Role of the Inflammasome in Liver Disease. Annu Rev Pathol. 2022;17:345-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 7. | Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 2953] [Article Influence: 492.2] [Reference Citation Analysis (0)] |
| 8. | Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020;30:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 853] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 9. | Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 2644] [Article Influence: 240.4] [Reference Citation Analysis (0)] |
| 10. | Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1484] [Article Influence: 164.9] [Reference Citation Analysis (0)] |
| 11. | Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 543] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 12. | Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 834] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 13. | Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2501] [Cited by in RCA: 2480] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 14. | Li H, Zhao XK, Cheng YJ, Zhang Q, Wu J, Lu S, Zhang W, Liu Y, Zhou MY, Wang Y, Yang J, Cheng ML. Gasdermin D-mediated hepatocyte pyroptosis expands inflammatory responses that aggravate acute liver failure by upregulating monocyte chemotactic protein 1/CC chemokine receptor-2 to recruit macrophages. World J Gastroenterol. 2019;25:6527-6540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 15. | Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 16. | Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal. 2018;28:643-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 633] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 17. | Liu X, Yang X, Han L, Ye F, Liu M, Fan W, Zhang K, Kong Y, Zhang J, Shi L, Chen Y, Zhang X, Lin S. Pterostilbene alleviates polymicrobial sepsis-induced liver injury: Possible role of SIRT1 signaling. Int Immunopharmacol. 2017;49:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Flamm SL, Yang YX, Singh S, Falck-Ytter YT; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guidelines for the Diagnosis and Management of Acute Liver Failure. Gastroenterology. 2017;152:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 19. | Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 2877] [Article Influence: 239.8] [Reference Citation Analysis (0)] |
| 20. | Fan BY, Pang YL, Li WX, Zhao CX, Zhang Y, Wang X, Ning GZ, Kong XH, Liu C, Yao X, Feng SQ. Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen Res. 2021;16:561-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 21. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5756] [Article Influence: 479.7] [Reference Citation Analysis (1)] |
| 22. | Li S, He Y, Chen K, Sun J, Zhang L, Yu H, Li Q. RSL3 Drives Ferroptosis through NF-κB Pathway Activation and GPX4 Depletion in Glioblastoma. Oxid Med Cell Longev. 2021;2021:2915019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 23. | Rodgers SK, Horrow MM. Acute (fulminant) liver failure: a clinical and imaging review. Abdom Radiol (NY). 2021;46:3117-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 484] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 25. | Aizawa S, Brar G, Tsukamoto H. Cell Death and Liver Disease. Gut Liver. 2020;14:20-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Silverstein R. D-galactosamine lethality model: scope and limitations. J Endotoxin Res. 2004;10:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Wilhelm EA, Jesse CR, Roman SS, Nogueira CW, Savegnago L. Hepatoprotective effect of 3-alkynyl selenophene on acute liver injury induced by D-galactosamine and lipopolysaccharide. Exp Mol Pathol. 2009;87:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M, Lv J, Wang L, Gong Z. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020;53:e12829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 29. | Huang S, Wang Y, Xie S, Lai Y, Mo C, Zeng T, Kuang S, Deng G, Zhou C, Chen Y, Huang S, Gao L, Lv Z. Hepatic TGFβr1 Deficiency Attenuates Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure Through Inhibiting GSK3β-Nrf2-Mediated Hepatocyte Apoptosis and Ferroptosis. Cell Mol Gastroenterol Hepatol. 2022;13:1649-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 1147] [Article Influence: 163.9] [Reference Citation Analysis (1)] |
| 31. | Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2573] [Cited by in RCA: 5030] [Article Influence: 457.3] [Reference Citation Analysis (0)] |
| 32. | Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, Li Z, Wang Y, Zhao Q, Shao F, Ding J. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020;180:941-955.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 548] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 33. | Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe. 2018;24:97-108.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 494] [Article Influence: 61.8] [Reference Citation Analysis (1)] |
| 34. | Chen D, Chu B, Yang X, Liu Z, Jin Y, Kon N, Rabadan R, Jiang X, Stockwell BR, Gu W. iPLA2β-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat Commun. 2021;12:3644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 35. | Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet. 2009;18:2388-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, Tie J, Hu D. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol. 2022;13:831168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 362] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 37. | Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187:111215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 38. | Kadono K, Kageyama S, Nakamura K, Hirao H, Ito T, Kojima H, Dery KJ, Li X, Kupiec-Weglinski JW. Myeloid Ikaros-SIRT1 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed mouse and human liver. J Hepatol. 2022;76:896-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 39. | Wang C, Liu T, Tong Y, Cui R, Qu K, Liu C, Zhang J. Ulinastatin protects against acetaminophen-induced liver injury by alleviating ferroptosis via the SIRT1/NRF2/HO-1 pathway. Am J Transl Res. 2021;13:6031-6042. [PubMed] |
| 40. | Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa RA, Reed EF, Datta N, Zarrinpar A, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J Hepatol. 2017;67:1232-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 41. | Nakamura K, Kageyama S, Ke B, Fujii T, Sosa RA, Reed EF, Datta N, Zarrinpar A, Busuttil RW, Kupiec-Weglinski JW. Sirtuin 1 attenuates inflammation and hepatocellular damage in liver transplant ischemia/Reperfusion: From mouse to human. Liver Transpl. 2017;23:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Alshehri AS, El-Kott AF, El-Kenawy AE, Khalifa HS, AlRamlawy AM. Cadmium chloride induces non-alcoholic fatty liver disease in rats by stimulating miR-34a/SIRT1/FXR/p53 axis. Sci Total Environ. 2021;784:147182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Ma S, Sun L, Wu W, Wu J, Sun Z, Ren J. USP22 Protects Against Myocardial Ischemia-Reperfusion Injury via the SIRT1-p53/SLC7A11-Dependent Inhibition of Ferroptosis-Induced Cardiomyocyte Death. Front Physiol. 2020;11:551318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Motlagh B, Iran; Wang M, China; Xia B, China S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Yuan YY