Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1556
Peer-review started: December 17, 2023
First decision: December 25, 2023
Revised: January 8, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: March 21, 2024
Processing time: 95 Days and 2 Hours
Hepatitis B cirrhosis (HBC) is a chronic disease characterized by irreversible diffuse liver damage and aggravated by intestinal microbial imbalance and metabolic dysfunction. Although the relationship between certain single probiotics and HBC has been explored, the impact of the complex ready-to-eat Lactobacillus paracasei N1115 (LP N1115) supplement on patients with HBC has not been determined.
To compare the changes in the microbiota, inflammatory factor levels, and liver function before and after probiotic treatment in HBC patients.
This study included 160 HBC patients diagnosed at the General Hospital of Ningxia Medical University between October 2018 and December 2020. Patients were randomly divided into an intervention group that received LP N1115 supplementation and routine treatment and a control group that received routine treatment only. Fecal samples were collected at the onset and conclusion of the 12-wk intervention period. The structure of the intestinal microbiota and the levels of serological indicators, such as liver function and inflammatory factors, were assessed.
Following LP N1115 intervention, the intestinal microbial diversity significantly increased in the intervention group (P < 0.05), and the structure of the intestinal microbiota was characterized by an increase in the proportions of probiotic microbes and a reduction in harmful bacteria. Additionally, the intervention group demonstrated notable improvements in liver function indices and significantly lower levels of inflammatory factors (P < 0.05).
LP N1115 is a promising treatment for ameliorating intestinal microbial imbalance in HBC patients by modulating the structure of the intestinal microbiota, improving liver function, and reducing inflammatory factor levels.
Core Tip: Intestinal microbial imbalance and metabolic dysfunction may accelerate the process of liver cirrhosis. We explored the role of probiotic intervention in patients with hepatitis B cirrhosis in this study. After an intervention with the N1115 ready-to-eat Lactobacillus supplement, we found the following significant changes: an increase in gut microbial diversity, structural changes in the microbiota favoring the growth of probiotic microbes, improvements in liver function, and decreases in inflammatory factor levels. We conclude that supplementation with the N1115 ready-to-eat Lactobacillus product may be a beneficial intervention in patients with cirrhosis.
- Citation: Hu YC, Ding XC, Liu HJ, Ma WL, Feng XY, Ma LN. Effects of Lactobacillus paracasei N1115 on gut microbial imbalance and liver function in patients with hepatitis B-related cirrhosis. World J Gastroenterol 2024; 30(11): 1556-1571
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1556.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1556
Liver cirrhosis (LC) is a severe chronic liver disease characterized by extensive hepatocyte degeneration, fibrosis, and nodular regeneration caused by various factors[1]. In China, 85% of cirrhosis and hepatocellular carcinoma (HCC) cases are attributed to hepatitis B virus (HBV) infection[2]. Approximately 257 million people are infected with HBV annually worldwide, leading to approximately 887000 deaths annually, approximately 30% of which result from LC[2]. Decompensated hepatitis B cirrhosis (HBC) often leads to multiple-organ dysfunction, such as gastrointestinal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis (SBP), primary liver cancer (PLC), and hepatorenal syndrome, often necessitating liver transplantation for survival[3].
Patients with LC often experience varying degrees of intestinal flora imbalance. The intestinal microbiota not only directly affects intestinal health but also influences liver metabolism and immunity. The gut microbiota plays a critical role in the development of various chronic liver diseases, including nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), LC, and PLC. Alterations in the gut microbiota can lead to immune dysregulation, intestinal barrier dysfunction, and systemic inflammation through the gut-liver axis, thus promoting disease progression. The gut microbiota is closely associated with LC complications and liver disease[4-11]. Given the growing understanding of intestinal flora imbalance in patients with LC, modulating the gut microbiota has emerged as a new therapeutic approach for the treatment of LC. Probiotics, which promote the growth of beneficial bacteria and reduce the growth of harmful bacteria, have particularly gained attention for their therapeutic effects on various liver diseases[12,13]. For instance, supplementation with Lactobacillus casei (L. casei) can improve lipid metabolism and regulate intestinal flora disorders in patients with alcoholic liver injury[14]. However, L. rhamnosus and its culture medium can ameliorate alcohol-induced liver function damage and steatosis[15]. Supplementation with Lactobacillus, Bifidobacterium, Escherichia coli, Clostridium butyricum, Streptococcus salivarius, and VSL#3 strains has shown promise in improving hepatic encephalopathy[16,17]. Although numerous studies have confirmed the effectiveness of probiotics in the treatment of liver disease in animal models and hospitalized patients, controversies remain, and limited options are available for probiotic selection in patients with hepatitis B virus-induced LC (HBC), necessitating further investigation of their efficacy[18].
N1115 is a ready-to-eat supplement containing fructooligosaccharides (FOS), lactitol, Lactobacillus paracasei N1115 (LP N1115), L. plantarum, Bifidobacterium bifidobacterium, and L. acidophilus, with LP N1115 accounting for > 80% of its composition. LP N1115 is isolated from traditional fermented dairy products and has probiotic effects, such as acid resistance, bile salt tolerance, and promotion of intestinal cell growth[19]. In combination with FOS, LP N1115 promotes recovery of the p38 mitogen-activated protein kinase pathway, enhances the expression of cohesin-1, improves intestinal barrier function, and preserves histological integrity[20]. The combinedapplication of LP N1115 and FOS reduces endotoxin levels, inhibits the activation of the lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4) signaling pathway, decreases the release of inflammatory factors, and alleviates NAFLD-related insulin resistance in mice[21]. Stadlbauer et al demonstrated that L. casei preparations regulated the expression of TLR4, promoted interleukin (IL)-10 secretion, and restored neutrophil phagocytic ability in patients with ALD[22]. However, it remains unclear whether the complex ready-to-eat LP N1115 supplement can modulate the prognosis of patients with HBC by regulating the gut microbiota.
Based on the aforementioned background, this study recruited 160 HBC patients and evaluated them by comparing the changes in microbiota before and after probiotic treatment as well as the changes in the levels of inflammatory factors and liver function to assess whether LP N1115 had beneficial effects on the gut microbiota in HBC patients.
A total of 160 patients diagnosed with HBC and treated at the General Hospital of Ningxia Medical University between October 2018 and December 2020 were enrolled in this study. Patients were randomly divided into two groups. The intervention group consisted of 86 patients who received LP N1115 supplementation in addition to general treatment for 3 months. Stool samples were collected at baseline and at the end of the intervention (54 patients did not provide stool samples after the intervention owing to the use of antibiotics or proton pump inhibitors during LP N1115 treatment). The nonintervention group consisted of 74 patients who received general treatment only. The diagnosis of HBC was based on the guidelines for the prevention and treatment of chronic hepatitis B (2019 edition) issued by the Chinese Society of Hepatology and Chinese Society of Infectious Diseases[23]. The exclusion criteria were as follows: (1) Patients with alcoholic, autoimmune, or fatty liver disease, with acquired immunodeficiency, or with other viral liver diseases or other liver diseases; (2) patients with hypertension, diabetes, obesity, metabolic syndrome, inflammatory bowel disease, autoimmune disease (such as rheumatoid arthritis or multiple sclerosis) or various tumors; (3) patients who had not used antibiotics, microecological agents, or proton pump inhibitors within 2 wk before enrollment; and (4) pregnant and lactating women. This study was approved by the Medical Ethics Committee of the General Hospital of Ningxia Medical University (approval number: 2016-252), and all participants provided informed consent.
The Laboratory Department of the General Hospital of Ningxia Medical University conducted blood tests for high-sensitivity C-reactive protein (CRP), endotoxin, total bilirubin (TBIL), creatinine (Cr), albumin (Alb), prothrombin time (PT), and the prothrombin international standard ratio (INR). Abdominal color Doppler ultrasound was performed in the Department of Ultrasound at Ningxia Medical University.
The Child-Turcotte-Pugh (CTP)[24] scoring system was used as a clinical classification standard to quantitatively assess liver reserve function in patients with LC.
The Model for End-Stage Liver Disease (MELD) score was used to assess disease severity. The formula was as follows: r = 0.378 Ln [bilirubin (mg/dL)] + 1.12 Ln (INR) + 0.95 Ln [Cr (mg/dL)] + 0.64 (cause: Biliary or alcoholic 0, others 1). A higher R value indicated a greater risk and lower survival rate.
The prognostic index (PI)[25], which is based on serum CRP levels and white blood cell (WBC) counts, primarily reflects acute inflammation in the body. A CRP level of ≤ 10 mg/L and WBC count of ≤ 11 × 109/L score 0 points; CRP ≤ 10 mg/L and WBC count > 11 × 109/L score 1 point; CRP > 10 mg/L and WBC count ≤ 11 × 109/L score 1 point; CRP > 10 mg/L and WBC count > 11 × 109/L score 1 point; and CRP > 10 mg/L and WBC count > 11 × 109/L score 2 points.
The probiotic used in this study was LP N1115, which was produced by Shijiazhuang Junlebao Dairy Co., Ltd. (Shijiazhuang City, China) and contains fructooligosaccharides, the food additive lactitol, LP N1115, L. plantarum, Bifidobacterium bifidum, and L. acidophilus. The dosage of active Lactobacillus added was ≥ 5 × 1010 CFU per bag, with each bag weighing 2 g. The probiotic was administered by mixing an appropriate amount of warm water or milk with the recommended dosage of one bag twice a day. The storage instructions recommend keeping the probiotics in a cool, dry, or refrigerated place.
Fresh fecal samples were collected from the participants and promptly placed in liquid nitrogen tanks for preservation during transportation to the laboratory. The collection process involved weighing the fecal samples and repackaging them in sterile centrifuge tubes. Three samples were collected from each participant. The subpacked specimens were rapidly transferred to a low-temperature refrigerator at -80 °C for frozen storage. The entire collection and repackaging process was completed within 30 min.
All the samples were subjected to the same procedures for DNA extraction and polymerase chain reaction (PCR) amplification by the same laboratory staff. The samples were suspended in 790 μL of sterile lysis buffer [4 M guanidine thiocyanate, 10% N-lauroyl sarcosine, and 5% N-lauroyl sarcosine in 0.1 M phosphate buffer (pH 8.0)] in a 2 mL screw-cap tube containing 1 g of glass beads (0.1 mm; BioSpec Products, Inc., United States). This mixture was vortexed vigorously and thenincubated at 70 °C for 1 h. After incubation, the mixture was subjected to bead beating for 10 min at maximum speed. Bacterial DNA was extracted using an E.Z.N.A.® Stool DNA Kit (Omega Biotek, Inc., GA) following the manufacturer’s instructions, which excluded lysis steps; the extracted DNA was stored at -20 °C for further analysis.
The extracted DNA from each sample was used as the template for amplifying the V3-V4 region of the 16S rRNA gene. The primers F1 and R2 (5’-CCTTCGGGNGGCWGCAG-3’ and 5’-GACTACHVGGGTATCTAATCC-3’) corresponding to positions 341 to 805 of the Escherichia coli 16S rRNA gene were used to amplify the V3-V4 region of each fecal sample by PCR. PCRs were run on an EasyCycler 96 PCR system (Analytik Jena Corp., AG). The products from different samples were indexed and mixed at equal ratios for sequencing using the MiSeq platform (Illumina, Inc., United States) according to the manufacturer’s instructions.
Clean data were extracted from the raw data using USEARCH software (version 11.0.667). The quality-filtered sequences were clustered into unique sequences and sorted in order of decreasing abundance to identify representative sequences using UPARSE according to the UPARSE operational taxonomic unit (OTU) analysis pipeline, and singletons were omitted from this step. OTUs were classified based on 97% similarity after chimeric sequences were removed using UPARSE (version 7.1; http://drive5.com/uparse/) and annotated using the SILVA reference database (SSU138) in qiime2-2020.11. Taxonomic analysis was performed on the representative sequences of the OTUs, and the community composition at each taxonomic level (domain, kingdom, phylum, class, order, family, genus, and species) was determined for each sample.
The Mann-Whitney U test (Wilcoxon rank-sum test) was used to compare the levels of CRP, endotoxin, and CTP between the two groups. The MELD score and PI were compared before and after treatment. P < 0.05 indicated statistical significance. The chi-square test was used to compare the incidence of ascites.
Bacterial α diversity was assessed using the Shannon, Simpson, Chao1, and The ACE estimator (ACE) indices. The Wilcoxon rank-sum test was also used to assess the α diversity among the different groups. The nonparametric factorial Kruskal-Wallis rank-sum test was used to detect differences in microbial abundances. Linear discriminant analysis (LDA) effect size (LEfSe) was used to detect taxa with differential abundance among the groups (LEfSe1.1, https://github.com/SegataLab/Lefse).
The baseline characteristics of the participants, including their clinical and biochemical characteristics, are summarized in Table 1. Before the intervention, no significant differences in age, sex, TBIL level, Cr level, PT time, WBC count, or platelet count were observed between the intervention and nonintervention groups. There were significant alterations observed in the levels of Alb, Cr, PT, INR, and CRP before and after treatment in the intervention group. Conversely, no notable changes were found in clinical parameters before or after treatment in the nonintervention group (Table 2).
| Characteristic | Treat (n = 86) | Untreat (n = 74) | P value |
| Age (yr), median (IQR) | 58 (51.25, 64.75) | 60 (51, 70) | 0.219 |
| Sex, n (%) | 0.176 | ||
| Male | 68 (79.07) | 53 (71.62) | |
| Female | 18 (20.93) | 21 (28.38) | |
| TBIL (3.0-22.0 μmol/L) | 29.26 (21.025, 43.095) | 33.875 (26.168, 53.657) | 0.117 |
| Alb (35.0-50.0 g/L) | 32.75 (25, 39.6) | 30.745 (26.575, 34.825) | 0.341 |
| Cr (58-110 μmol/L) | 64.5 (54.45, 75.325) | 60.55 (51.35, 76.925) | 0.498 |
| WBC (3.5-9.5 × 109/L) | 3.89 (2.728, 5.578) | 3.88 (2.9075, 5.06) | 0.855 |
| PLT (125.0-350.0 × 1012/L) | 78 (43.25, 110.25) | 69 (48.25, 111.5) | 0.943 |
| PT (9.4-12.5 s) | 16.15 (14.6, 18.125) | 16.25 (14.625, 18.275) | 0.986 |
| INR (0.85-1.14) | 1.345 (1.216, 1.518) | 1.36 (1.193, 1.505) | 0.907 |
| CRP (< 10) | 4.75 (1.74, 12.8) | 4.995 (1.72, 9.403) | 0.836 |
| Characteristics | Treat | P value | Untreat | P value | ||
| Before | After | Before | After | |||
| TBIL (μmol/L), median (IQR) | 29.26 (21.025, 43.095) | 27.515 (18.237, 44.22) | 0.707 | 33.875 (26.168, 53.657) | 33.3 (20.8, 57.315) | 0.637 |
| Alb (g/L), median (IQR) | 32.75 (25, 39.6) | 37.85 (31.635, 43.773) | 0.007 | 30.745 (26.575, 34.825) | 29.9 (26.775, 38.025) | 0.625 |
| Cr (μmol/L), median (IQR) | 64.5 (54.45, 75.325) | 0.85 (48.425, 69.325) | 0.03 | 60.55 (51.35, 76.925) | 61.9 (51.4, 82.125) | 0.689 |
| WBC (109/L), median (IQR) | 3.89 (2.7275, 5.5775) | 3.505 (2.383, 5.155) | 0.321 | 3.88 (2.9075, 5.06) | 4.115 (3, 5.493) | 0.439 |
| PLT (1012/L), median (IQR) | 78 (43.25, 110.25) | 71.5 (50.25, 123.25) | 0.760 | 69 (48.25, 111.5) | 69 (48.25, 103.75) | 0.833 |
| PT (s), median (IQR) | 16.15 (14.6, 18.125) | 15.05 (13.35, 16.6) | 0.003 | 16.25 (14.625, 18.275) | 15.95 (14, 17.75) | 0.342 |
| INR, median (IQR) | 1.345 (1.216, 1.518) | 1.29 (1.123, 1.408) | 0.018 | 1.36 (1.193, 1.505) | 1.315 (1.185, 1.448) | 0.526 |
| CRP, median (IQR) | 4.75 (1.74, 12.8) | 1.845 (0.775, 4.343) | 0.005 | 4.995 (1.72, 9.403) | 1.985 (2.035, 1.015) | 0.822 |
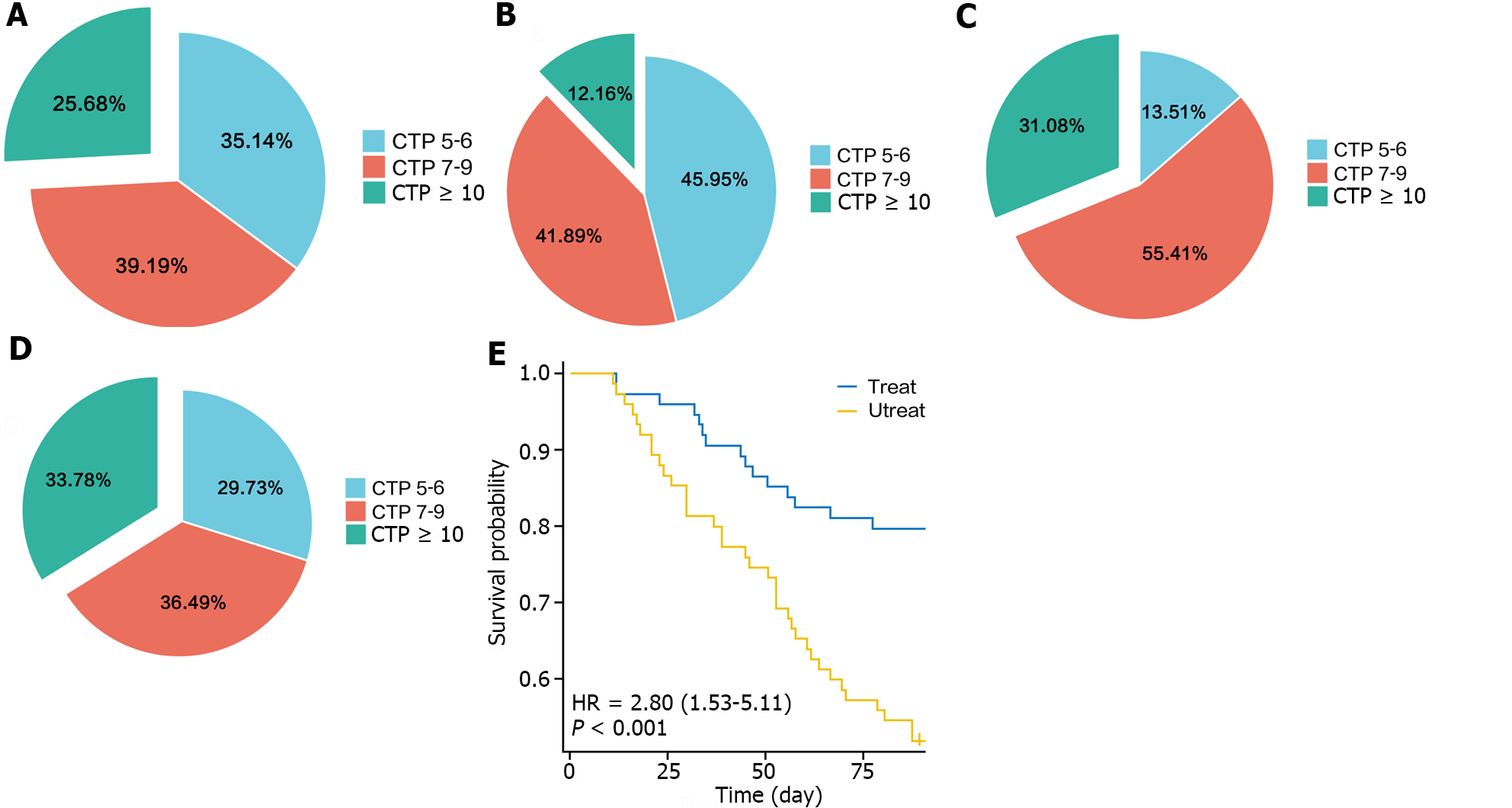
The CTP and MELD scores were used to assess liver function before and after treatment in the intervention and nonintervention groups, respectively. The results showed that after treatment, the CTP score of the intervention group was better than that before treatment, with a decrease in the proportion of patients with CTP grade C disease from 25.68% to 12.16%. In contrast, the proportion of patients with CTP grade C disease in the nonintervention group increased from 31.08% to 33.78% (Figure 1A-D). Survival curves revealed that the probability of recurrence of ascites in the intervention group was significantly lower than that in the nonintervention group (Figure 1E).
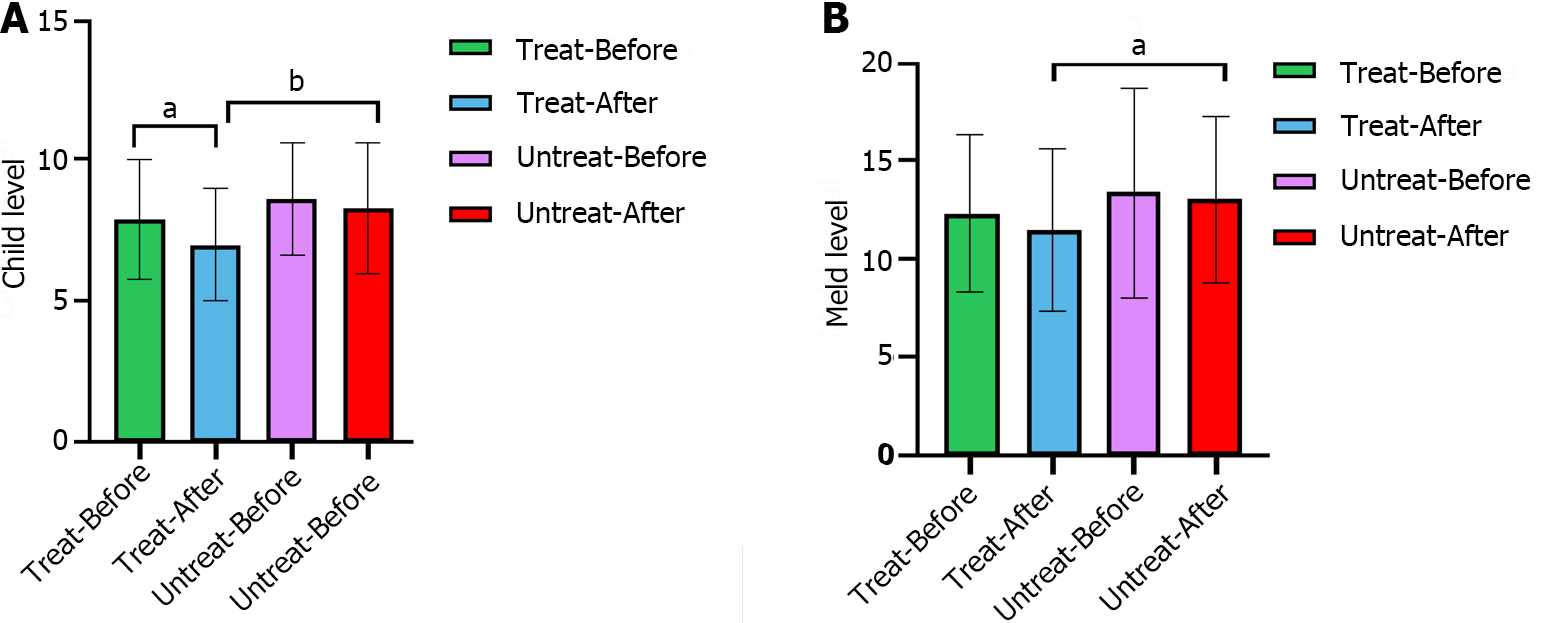
The CTP grade in the intervention group significantly decreased after treatment (P = 0.007), whereas that in the nonintervention group did not significantly change (P = 0.489). The MELD score, which reflects the severity of end-stage liver disease, was not significantly different between the intervention and nonintervention groups before treatment, whereas after treatment, the MELD score of the intervention group was significantly lower than that of the nonintervention group (P = 0.017), indicating that the severity of liver disease was lower in the intervention group than in the nonintervention group (Figure 2).
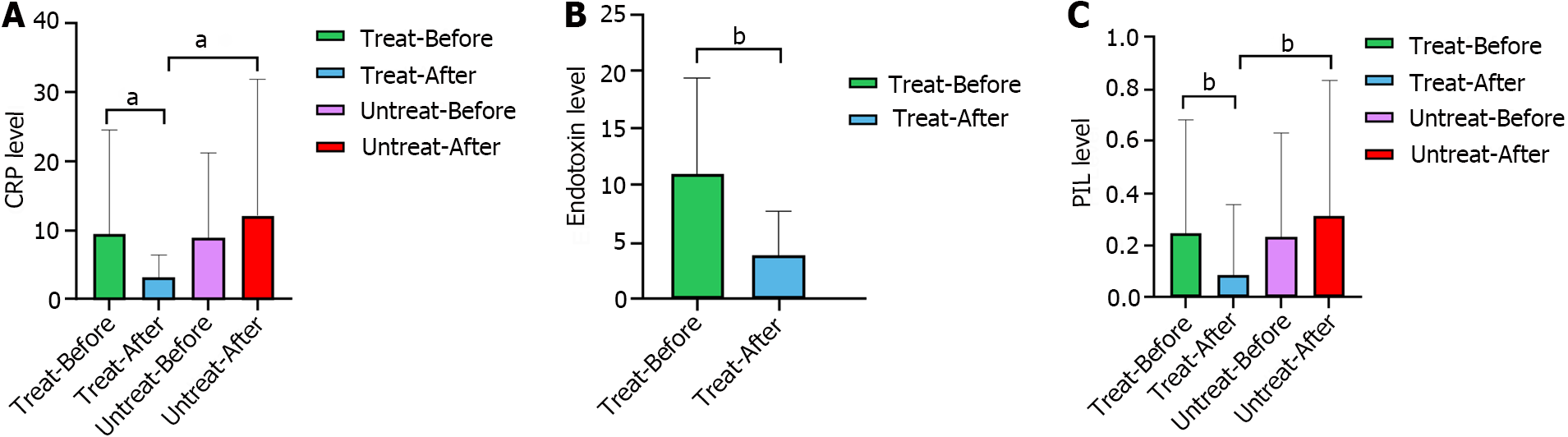
The results showed no significant differences in CRP levels or PI between the intervention and nonintervention groups at baseline, whereas after the intervention, there were significant differences (P < 0.001 and P = 0.001, respectively). Compared with those in the pretreatment group, the CRP level and PI in the intervention group decreased significantly after treatment (P < 0.001 and P = 0.006, respectively), whereas there was no significant reduction in the nonintervention group after treatment (P = 0.823 and P = 0.306, respectively). The endotoxin levels were significantly lower after treatment in the intervention group (P = 0.007) (Figure 3).
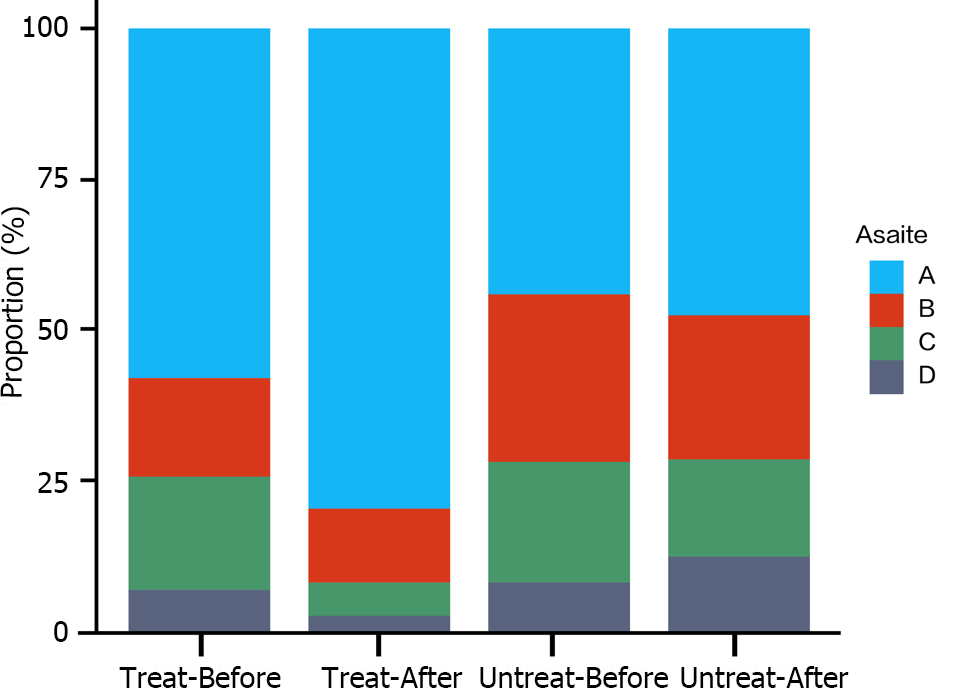
There was no significant difference in the incidence of ascites between the intervention and nonintervention groups before treatment (P = 1.0). After treatment with LP N1115, the incidence of ascites in the intervention group was significantly lower than that before treatment, whereas there was no significant change in the nonintervention group. The incidence of massive ascites decreased from 6.76% to 2.7%in the intervention group, whereas it increased from 8.11% to 12.16% in the nonintervention group. The incidence of ascites after treatment was significantly lower in the intervention group than in the nonintervention group (P = 0.001) (Figure 4).
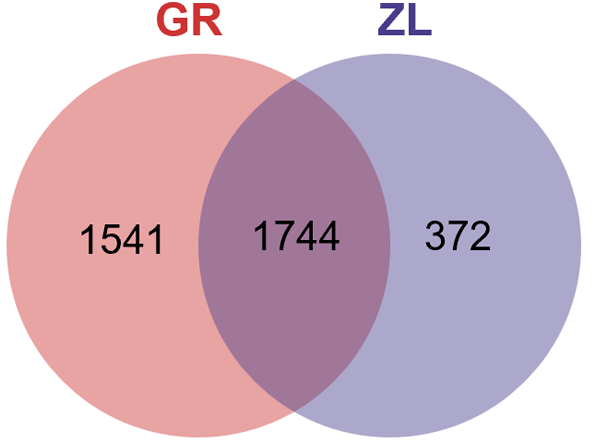
Venn diagram of the OTU distribution: The microbiota in a total of 118 fecal samples collected from the intervention group were tested, including 86 samples collected before the intervention and 32 samples after the intervention. A Venn diagram showed that there were 1744 OTUs shared in the samples before and after treatment, while 1541 OTUs were specifically detected before treatment, and 372 OTUs were specifically detected after treatment (Figure 5).
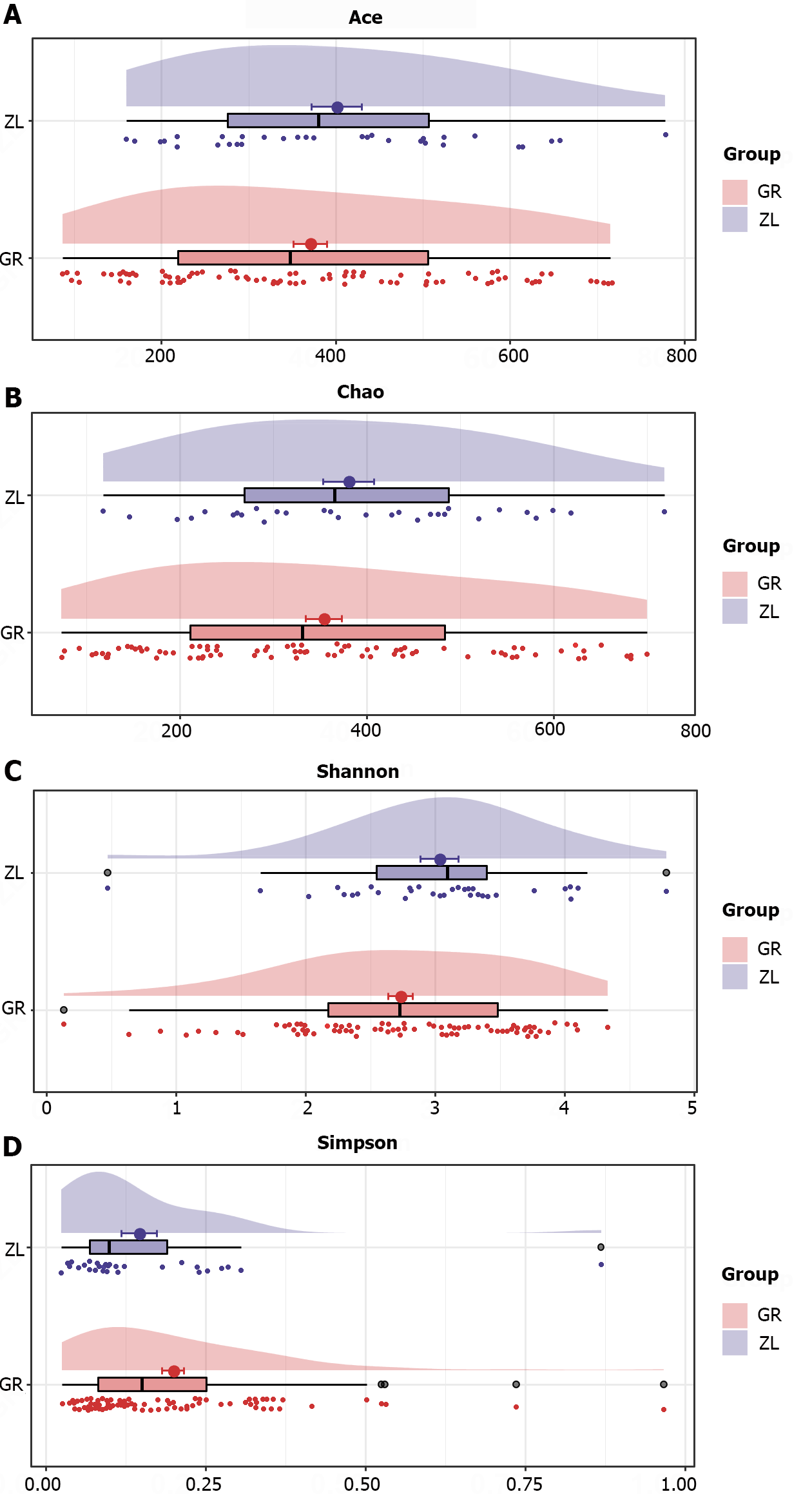
Alpha diversity analysis: Alpha diversity was used to assess the richness (Chao1 and ACE) and diversity (Shannon and Simpson indices) of the gut microbiota. Alpha-diversity analysis of the intervention group revealed changes in the bacterial richness and diversity before and after treatment. The richness of the intestinal flora tended to increase after the intervention, but the difference was not significant (P > 0.05). The Simpson index was significantly lower after the intervention than before the intervention (P < 0.05), indicating that the diversity of the intestinal flora tended to increase after intervention with probiotics (Figure 6).
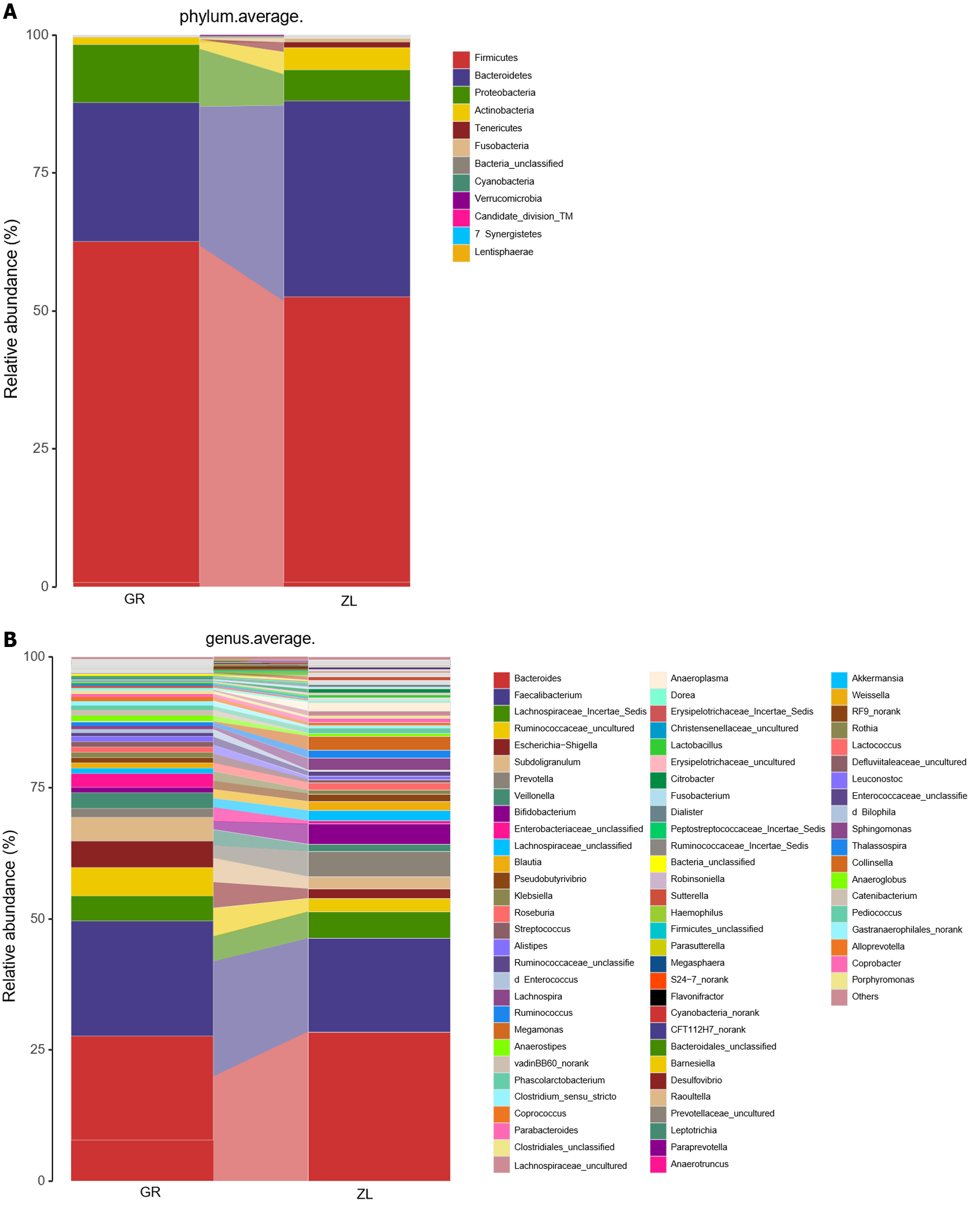
Composition of the intestinal flora before and after probiotic treatment: At the phylum level, Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Fusobacteria were the main phyla identified both before and after treatment. However, the proportion of each phylum changed after the treatment: the proportion of Bacteroidetes (25.2%-35.3%) increased significantly, and those of Firmicutes (61.8%-51.7%) and Proteobacteria (10.5%-5.6%) decreased significantly (Table 3 and Figure 7).
| Phylum of bacteria (%) | GR | ZL | P value |
| Firmicutes | 61.8 | 51.7 | 0.046 |
| Bacteroidetes | 25.2 | 35.5 | 0.008 |
| Proteobacteria | 10.5 | 5.6 | 0.468 |
| Actinobacteria | 1.7 | 4 | 0.057 |
| Fusobacteria | 0.3 | 0.8 | 0.494 |
| Bacteroides | 19.8 | 28.4 | 0.014 |
| Faecalibacterium | 22 | 17.8 | 0.803 |
| Lachnospiraceae_Incertae_Sedis | 4.8 | 5 | 0.001 |
| Ruminococcaceae_uncultured | 5.4 | 2.6 | 0.027 |
| Escherichia-Shigella | 5 | 1.8 | 0.348 |
| Subdoligranulum | 4.5 | 2.3 | 0.561 |
| Prevotella | 2.4 | 4.9 | 0.211 |
| Veillonella | 3 | 1.3 | 0.315 |
| Bifidobacterium | 1.6 | 3.9 | 0.039 |
| Enterobacteriaceae_unclassified | 2.6 | 0.7 | 0.629 |
| Anaerostipes | 1 | 0.6 | 0.007 |
The main genera identified before and after treatment were Bacteroides, Faecalibacterium, Lachnospiraceae incertae sedis, and Ruminococcaceae_unclassified. However, after treatment, the proportions of the potentially pathogenic bacteria Enterobacteriaceae_unclassified (2.6%-0.7%), Escherichia, Shigella (5%-1.8%), and Streptococcus (1.8%-0.6%) decreased in abundance. In contrast, the proportions of Bacteroides (19.8%-28.4%), Bifidobacterium (1.6%-4%), Ruminococcus (0.7%-1.5%), Prevotella (2.4%-4.9%), and Lachnospiraceae incertae sedis (4.8%-5.0%) increased (Table 3 and Figure 8).
Comparison of the abundance of flora before and after treatment: LEfSe analysis was used to identify the key phylotypes with significant differences in abundance before and after treatment. LEfSe analysis revealed that, compared to those before treatment, the proportions of Firmicutes (LDA = 4.74, P = 0.05), Clostridium (LDA = 3.8, P = 0.03), Pseudobutyrivibrio (LDA = 3.5, P = 0.03), and Anaerostipes (LDA = 3.46, P = 0.01) significantly decreased after treatment. In contrast, the proportions of Bacteroidetes (LDA = 4.66, P = 0.01), Bifidobacterium (LDA = 4.47, P = 0.04), Veillonellaceae (LDA = 4.0, P = 0.01), Lachnospiraceae (LDA = 3.95, P = 0.001) and Eggerthella (LDA = 3.47, P = 0.01) significantly increased after treatment (Figure 8).
Correlation analysis between differential flora and CTP classification: To further understand the relationship between the differential bacteria and the patient's CTP classification of liver function, we performed a correlation analysis between the differential flora and the CTP classification. There was no correlation between the occurrence of Veillonella, Streptococcus, or Lachnospiraceae incertae sedis and the CTP classification before treatment.However, after treatment, there was a positive correlation (Table 4).
| GR | ZL | |||
| r | P value | r | P value | |
| Bacteroides | -0.436 | 0.013 | -0.004 | 0.981 |
| Lachnospiraceae_Incertae_Sedis | 0.312 | 0.082 | 0.353 | 0.048 |
| Veillonella | 0.272 | 0.132 | 0.449 | 0.01 |
| Streptococcus | 0.143 | 0.433 | 0.354 | 0.047 |
| Escherichia-Shigella | 0.275 | 0.128 | 0.016 | 0.929 |
| Prevotella | 0.22 | 0.226 | 0.069 | 0.706 |
| Enterobacteriaceae_unclassified | 0.366 | 0.04 | 0.198 | 0.277 |
| Firmicutes/Bacteroidetes | 0.299 | 0.097 | 0.253 | 0.126 |
Intestinal flora imbalance in patients with decompensated LC has garnered significant attention[8]. Studies have indicated that the intestinal microbiota may contribute to the progression of HBV-related chronic liver disease to severe liver failure by promoting the accumulation of inflammatory factors and pathogenic metabolites[26]. An imbalanced intestinal ecology in patients with LC can lead to impaired intestinal barrier function and microbial translocation, which in turn contributes to the development of SBP[27]. Moreover, intestinal flora imbalance after disruption of intestinal barrier function results in increased levels of Klebsiella and Proteus spp., which, through the production of ammonia and endotoxins, directly causes hepatic encephalopathy owing to an increase in blood ammonia levels[28]. Patients with HCC often exhibit elevated levels of gram-negative bacteria commonly found in the gut microbiome, such as Escherichia coli, and decreased levels of beneficial bacteria, including Lactobacillus, Bifidobacterium, and Enterococcus. This imbalance is primarily caused by increased intestinal permeability, leading to bacterial translocation and endotoxin accumulation, which in turn results in intestinal bacterial overgrowth and alterations in the composition of the intestinal microbiota[29].
A previous study involving patients with HBC demonstrated no significant differences in alpha diversity among patients with HBV-related chronic diseases, HBC, or HCC[30]. However, in the present study, the results indicated an increasing trend in gut microbiota diversity after treatment.
L. paracasei ameliorated diarrhea by inhibiting activation of the NF-κB-MLCK pathway and increasing the abundance of gut microbiota that produce short-chain fatty acids (SCFA)[31]. Lp N1115 was able to enhance the contentof Lactobacillus and maintain fecal pH levels. Its beneficial effects on gut development were more obvious in 6-12-month-old infants[32]. These findings suggest that intervention with the N1115 ready-to-eat Lactobacillus supplement may have a modulatory effect on gut microbiota dysbiosis in patients with LC. The increased diversity of the gut microbiota after treatment may help restore a healthy gut state. However, further studies are required to validate and explore the effectiveness and mechanisms of this intervention.
Although numerous studies have confirmed the efficacy of probiotics in animal models and in the treatment of hospitalized patients with liver disease, several controversies remain. The choice between single or combined probiotics as well as the efficacy of different bacterial combinations in different probiotics for chronic liver disease require further investigation. Interestingly, we found a close relationship among dysbiosis, inflammatory markers, and liver function. The partial recovery of inflammatory marker levels and liver function in patients after LP N1115 intervention was even more surprising. Our study demonstrated a significant decrease in CRP levels and a lower CRP level in the intervention group than in the nonintervention group after treatment. Moreover, endotoxin synthesis decreased in the intervention group. The PI, which reflects acute inflammation in the body, significantly decreased in the intervention group after treatment and was significantly lower than that in the nonintervention group, suggesting improved acute inflammation in the intervention group after treatment, whereas the nonintervention group receiving conventional treatment exhibited no significant changes. Patients with HBC often experience bacterial translocation and increased endotoxin levels, leading to changes in intestinal permeability and increased activation of the LPS/TLR4 signaling pathway in the liver[4,33]. TLR4 activation triggers the production of proinflammatory, antiviral, and antibacterial cytokines. The levels of IL-10 and tumor necrosis factor-α are significantly greater in HBC patients than in control individuals, and increased endotoxin expression promotes factor synthesis through signaling pathways[19,34,35]. L. casei preparations can regulate TLR4 expression and IL-10 secretion. Therefore, we propose that LP N1115inhibits the activation of the LPS/TLR4 signaling pathway, reduces the release of inflammatory factors, and effectively alleviates the inflammatory state of patients when combined with conventional treatment[36].
However, whether there is a correlation between intestinal flora imbalance and liver function needs to be further explored. The main pathophysiological changes in patients with LC are liver function impairment and portal hypertension, which are crucial factors to consider when evaluating disease conditions and prognosis. Widely used evaluation systems, such as the CTP classification and MELD score, are commonly used both domestically and internationally. An imbalance in the gut microbiota can trigger an inflammatory response and exacerbate liver fibrosis by activating TLRs, thereby promoting the progression of cirrhosis to decompensation and liver failure[27]. Probiotics have been found to reduce liver function damage and improve the levels of markers, such as alanine aminotransferase, aspartate aminotransferase, cholesterol, low-density lipoprotein, and triglycerides, and waist circumference in children with NAFLD[37]. One study showed that Bullavirinae, Felixounavirus, Streptococcus, Escherichia and Pseudomonas phages were positively linked with the MELD, whereas Faecalibacterium phages were negatively linked with the MELD[38]. In this study, we aimed to further understand the effects of LP N1115, a ready-to-eat Lactobacillus supplement, on liver function. We compared the CTP and MELD scores between the intervention and nonintervention groups before and after the intervention. The results showed a significant improvement in the CTP classification score and a decrease in the MELD score after the intervention. We further validated liver function by assessing the incidence of ascites. The incidence of ascites in patients treated with LP N1115 was significantly lower, including a lower incidence of massive ascites, than that in patients who did not receive the N1115 intervention and who only received conventional treatment during the same period. Therefore, LP N1115 is highly important for improving liver function and liver reserve function in patients.
In patients with HBC, an increase in the proportion of Enterobacteriaceae, Fusobacteriaceae, and alkali-producing bacteria in the gut was observed, accompanied by a decrease in the abundance of Ruminococcaceae and Pilospirillaceae. Many bacteria from the Ruminococcaceae and Pilospirillaceae families possess bile acid hydrolases that are closely associated with the production of secondary bile acids[27]. Bile acids can disrupt the gut barrier and immune function, affecting the regulation of the intestinal flora structure. At the phylum level, the dominant species remained consistent before and after treatment, although the proportion of each phylum changed. After treatment, the abundance of some potentially pathogenic bacteria decreased, whereas that of beneficial bacteria increased. LEfSe analysis revealed significant differences in the abundance of Bacteroides, Bifidobacterium, Lachnospirillaceae, and Eggerthella after treatment. These results are consistent with the relative abundance map of the dominant species, suggesting that LP N1115 effectively regulates the homeostasis of the intestinal flora. Surprisingly, we found a significant difference in Lachnospiraceae incertae sedis levels after N1115 intervention, which was positively correlated with the CTP classification score. Previous studies by Bajaj et al[8] indicated that beneficial bacteria, such as Spirillaceae and Verrucomicrobacteriaceae, decrease in abundance in the intestines of patients with LC, whereas harmful bacteria, such as Enterobacteriaceae and Bacteroidaceae, increase in abundance. Triclospira, a member of Lachnospiraceae incertae sedis, produces anti-inflammatory SCFA that help maintain water and electrolyte balance, improve intestinal mucosa function and morphology, inhibit tumor cell proliferation, induce macrophage apoptosis, inhibit histone deacetylation, promote the migration of regulatory T cells, and induce the production of anti-inflammatory IgA by mucosal B cells[39,40]. Lachnospiraceae incertae sedis possesses bile salt hydrolase, which is involved in secondary bile acid production. Bile acids can directly damage bacterial outer membranesand exert bactericidal effects. These bacteria can also generate nitric oxide and IL-18 through the "bile acid-G protein-coupled receptor (targeting of bile acid receptor 5) TGR5-farnesoid X receptor-camp" pathway, thereby influencing the intestinal flora via the immune system, which plays a crucial role in maintaining the balance of the intestinal flora[39,41]. Additionally, the liver secretes bile acids into the intestine through the biliary tract, which can affect the composition and abundance of the intestinal flora. LC is often accompanied by bile acid excretion disorders. Patients with decompensated cirrhosis may develop portal hypertension, leading to intestinal mucosal congestion, edema, impaired small intestinal motility, and intestinal content retention. Complications such as esophageal and gastric fundus variceal bleeding are accompanied by intestinal mucosal ischemia/reperfusion injury, leading to bacterial overgrowth and translocation to the small intestine[42]. Qin et al[42] reported that the intestinal flora of patients with LC contained a high proportion of oral bacteria, such as Streptococcus and Veillonella, indicating that the oral microbial flora invades the gut and contributes to LC deterioration. Reduced gastric acid secretion and altered bile acid secretion in patients with LC may facilitate the translocation of oral bacteria to the gut. Similarly, we observed an increased proportion of Streptococcus and Veillonella before the intervention, although this difference was not correlated with the CTP classification score. However, after the intervention, the proportion of patients who died decreased and was positively correlated with the CTP classification score. Therefore, we believe that LP N1115 may influence the level of intestinal bile acid by regulating the level of Lachnospiraceae incertae sedis, reducing the translocation of Streptococcus and Veillonella to the intestine, and assisting conventional treatment to improve inflammation levels and liver function in patients with hepatobiliary diseases, thereby significantly affecting patient prognosis.
Wang et al[43] discovered that patients with hepatitis B-decompensatedcirrhosis exhibited a significant reduction in the abundance of Firmicutes, Trichospirillum, Dorea, and Dialister. Conversely, there was a significant increase in the abundance of Streptococcus, Fusobacterium, Veillonella, and Haemophilus spp. In our analysis of patients with HBC at the phylum level, we observed a relatively high proportion of Firmicutes before the intervention, which decreased after the intervention, demonstrating slight differences from previous studies. We think that a phylum-based analysis is generally not applicable to patients with cirrhosis because of the inclusion of pathogenic groups, such as staphylococci and Enterococcaceae, within Firmicutes, which are more abundant in severely ill individuals. Following the intervention with LP N1115, the proportions of cocci and Enterococcaceae within the phylum Firmicutes decreased.
This study is limited by its small sample size, single- center analysis, absence of an intervention treatment in the control group and no additional stratification. Therefore, whether the effects of the N1115 ready-to-eat Lactobacillus supplement on the intestinal flora are limited by differences in region and dietary habits is unknown; additionally, whether the changes in the intestinal flora in patients with different complications are consistent needs to be further explored. Our future work will involve designing larger multicenter trials.
In conclusion, we propose that LP N1115 modulates intestinal bile acid levels by regulating the abundance of Lachnospiraceae incertae sedis, consequently reducing the translocation of Streptococcus and Veillonella to the intestine. This intervention can effectively complement conventional treatments, leading to improvements in inflammation and liver function in HBC patients. As a potential therapeutic target, it is crucial to consider the timing, duration, and dosage of this intervention. Therefore, conductinglarge-scale, multicenter, randomized, placebo-controlled trials investigating the microbiome and metabolomics of LP N1115-treated patients with HBC in the future would provide valuable insights.
Hepatitis B cirrhosis (HBC) is a prevalent chronic disease associated with significant morbidity and mortality. Numerous studies have consistently demonstrated the occurrence of intestinal flora dysbiosis in patients diagnosed with HBC. Alterations in the composition of the intestinal flora can disrupt immune regulation, impair intestinal barrier function, and induce systemic inflammatory changes via the gut-liver axis, thereby hastening the progression of cirrhosis. Interventions utilizing microecological preparations hold immense significance in enhancing prognosis.
Although numerous individual probiotics have been documented in relation to HBC, the impact of the N1115 compound ready-to-eat Lactobacillus supplement in patients with HBC remains uncertain.
The primary objective was to assess the impact of the N1115 ready-to-consume lactic acid bacterial supplement on hepatic function, inflammation, and ascites in patients with HBC. This study aimed to investigate the therapeutic potential of the intestinal microecology in managing HBC. HBC patients were administered the N1115 ready-to-eat lactic acid bacterial supplement for 3 months, which resulted in a significant increase in intestinal microbial diversity and notable alterations in the composition of the intestinal microbiota. There was a remarkable improvement in liver function parameters and a decrease in the levels of inflammatory markers among the patients. This investigation offers novel insights into optimizing interventions targeting the intestinal microflora in individuals with HBC.
This study included 160 HBC patients who were diagnosed at the General Hospital of Ningxia Medical University between October 2018 and December 2020. Patients were randomly divided into an intervention group that received Lactobacillus paracasei N1115 (LP N1115) supplementation along with routine treatment and a control group that received routine treatment only. Fecal samples were collected at the onset and conclusion of the 12-wk intervention period. The structure of the intestinal microbiota and the levels of serological indicators, such as liver function and inflammatory factors, were assessed.
The patients were assessed after 3 months of treatment with the N1115 ready-to-eat Lactobacillus supplement: (1) There were significant changes in the levels of albumin, creatinine, prothrombin time, international standard ratio, and C-reactive protein and in the Child-Turcotte-Pugh and Model for End-Stage Liver Disease scores before and after treatment in the intervention group; (2) the probability of recurrence of ascites in the intervention group was significantly lower than that in the nonintervention group; and (3) the diversity of the intestinal flora tended to increase after intervention with probiotics. At the phylum level, the proportion of Bacteroidetes increased significantly, and those of Firmicutes and Proteobacteria decreased significantly. At the genus level, the proportions of the potentially pathogenic bacteria Enterobacteriaceae_unclassified, Escherichia, Shigella, and Streptococcus decreased. In contrast, the proportions of Bacteroides, Bifidobacterium, Ruminococcus, Prevotella, and Lachnospiraceae incertae sedis increased.
LP N1115 supplementation is promising for ameliorating intestinal microbial imbalance in patients with HBC by modulating the structure of the intestinal microbiota, improving liver function, and reducing inflammatory factor levels.
Future studies should prioritize investigating the structural and metabolic alterations in the composition of intestinal biota among patients with various complications of HBC, along with elucidating their underlying mechanisms. To address these inquiries, our research group intends to conduct large-scale, long-term clinical trials.
We thank all the patients who participated in this study and the physicians in the Department of Infectious Diseases, General Hospital, Ningxia Medical University. We also thank Shanghai Mobio Biomedical Technology Co. for their technical support.
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 1060] [Article Influence: 212.0] [Reference Citation Analysis (2)] |
| 2. | Yang F, Ma L, Yang Y, Liu W, Zhao J, Chen X, Wang M, Zhang H, Cheng S, Shen F, Wang H, Zhou W, Cao G. Contribution of Hepatitis B Virus Infection to the Aggressiveness of Primary Liver Cancer: A Clinical Epidemiological Study in Eastern China. Front Oncol. 2019;9:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Lee NY, Yoon SJ, Han DH, Gupta H, Youn GS, Shin MJ, Ham YL, Kwak MJ, Kim BY, Yu JS, Lee DY, Park TS, Park SH, Kim BK, Joung HC, Choi IS, Hong JT, Kim DJ, Han SH, Suk KT. Lactobacillus and Pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes. 2020;11:882-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Duseja A, Acharya SK, Mehta M, Chhabra S; Shalimar, Rana S, Das A, Dattagupta S, Dhiman RK, Chawla YK. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019;6:e000315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS. Altered Microbiota in Cirrhosis and Its Relationship to the Development of Infection. Clin Liver Dis (Hoboken). 2019;14:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 823] [Article Influence: 54.9] [Reference Citation Analysis (3)] |
| 8. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (1)] |
| 9. | Acharya C, Bajaj JS. Altered Microbiome in Patients With Cirrhosis and Complications. Clin Gastroenterol Hepatol. 2019;17:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. 2021;27:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 12. | Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol. 2012;2:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 13. | Shin D, Chang SY, Bogere P, Won K, Choi JY, Choi YJ, Lee HK, Hur J, Park BY, Kim Y, Heo J. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS One. 2019;14:e0220843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Ren T, Huang C, Cheng M. Dietary blueberry and bifidobacteria attenuate nonalcoholic fatty liver disease in rats by affecting SIRT1-mediated signaling pathway. Oxid Med Cell Longev. 2014;2014:469059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Wang C, Zhao H, Zhao C, Chen Y, Wang Y, McClain C, Feng W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J Nutr Biochem. 2015;26:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Holte K, Krag A, Gluud LL. Systematic review and meta-analysis of randomized trials on probiotics for hepatic encephalopathy. Hepatol Res. 2012;42:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, Chen XX, Xiao CX, Yu XF, Xu HZ, Xu LZ, Tang YN, Zhou F, Wang XL, Chen MY, Chen LG, Hong MZ, Ren JL, Pan JS. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Acharya C, Bajaj JS. Gut Microbiota and Complications of Liver Disease. Gastroenterol Clin North Am. 2017;46:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Yao F, Jia R, Huang H, Yu Y, Mei L, Bai L, Ding Y, Zheng P. Effect of Lactobacillus paracasei N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch Med Sci. 2019;15:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Yao FF, Zheng PY, Huang H, Bai LM, Ding YR, Mei L, Liu SM. [Effects of Lactobacillus paracasei N1115 combined with fructooligosaccharides on non-alcoholic fatty liver disease induced by high-fat diet in mice]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 123] [Reference Citation Analysis (1)] |
| 24. | Zuberi BF, Rasheed T, Ali FS, Bader N, Sadaf R. Assessment of frailty in cirrhosis using bedside measures and its correlation with Child-Turcotte-Pugh, MELD & MELD-Na Scores. Pak J Med Sci. 2022;38:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Yang KL, Chen JB, Zhu YQ, Zhou ZG, Chen MS. Inflammation Prognostic Score and its Correlation with the Prognosis of Radiofrequency Ablation in Liver Cancer. Guangdong Yixue. 2018;33:2294-2299. [DOI] [Full Text] |
| 26. | Wang WW, Zhang Y, Huang XB, You N, Zheng L, Li J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J Gastroenterol. 2017;23:6983-6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Oikonomou T, Papatheodoridis GV, Samarkos M, Goulis I, Cholongitas E. Clinical impact of microbiome in patients with decompensated cirrhosis. World J Gastroenterol. 2018;24:3813-3820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 30. | Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen J, Yang B, Ou Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Ren S, Wang C, Chen A, Lv W, Gao R. The Probiotic Lactobacillus paracasei Ameliorates Diarrhea Cause by Escherichia coli O(8) via Gut Microbiota Modulation(1). Front Nutr. 2022;9:878808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Li P, Ren Z, Zhou J, Zhao A, Wang S, Xun Y, Jiang H, Wang P, Yuan Q, Zhang Y. Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Fukui H. Gut Microbiome-based Therapeutics in Liver Cirrhosis: Basic Consideration for the Next Step. J Clin Transl Hepatol. 2017;5:249-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Moratalla A, Gómez-Hurtado I, Moya-Pérez Á, Zapater P, Peiró G, González-Navajas JM, Gómez Del Pulgar EM, Such J, Sanz Y, Francés R. Bifidobacterium pseudocatenulatum CECT7765 promotes a TLR2-dependent anti-inflammatory response in intestinal lymphocytes from mice with cirrhosis. Eur J Nutr. 2016;55:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (6)] |
| 36. | Bajaj JS, Sikaroodi M, Shamsaddini A, Henseler Z, Santiago-Rodriguez T, Acharya C, Fagan A, Hylemon PB, Fuchs M, Gavis E, Ward T, Knights D, Gillevet PM. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut. 2021;70:1162-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1456] [Article Influence: 242.7] [Reference Citation Analysis (1)] |
| 38. | Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 39. | Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 2021] [Article Influence: 202.1] [Reference Citation Analysis (0)] |
| 41. | Quan M, Xing HC. Intestinal flora research progress associated with chronic liver disease. Zhonguo Ganzangbing Zazhi (Dianziban). 11:26-30. |
| 42. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1614] [Article Influence: 134.5] [Reference Citation Analysis (43)] |
| 43. | Wang YX, Li W, Cheng DY, Liu SA, Zhang S, Han M, Zhu M, Sun J, Xing HC. Characteristics of intestinal flora of patients with hepatitis B related decompensated cirrhosis. Zhonghua Shiyan He Linchuang Ganranbing Zazhi (Dianziban). 2019;13:110-116. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wierzbicka A, Poland S-Editor: Qu XL L-Editor: A P-Editor: Cai YX