Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1450
Peer-review started: December 27, 2023
First decision: January 5, 2024
Revised: January 13, 2024
Accepted: January 31, 2024
Article in press: January 31, 2024
Published online: March 14, 2024
Processing time: 78 Days and 0.9 Hours
Direct-acting antiviral agents (DAAs) are highly effective treatment for chronic hepatitis C (CHC) with a significant rate of sustained virologic response (SVR). The achievement of SVR is crucial to prevent additional liver damage and slow down fibrosis progression. The assessment of fibrosis degree can be performed with transient elastography, magnetic resonance elastography or shear-wave elastography (SWE). Liver elastography could function as a predictor for hepatocellular carcinoma (HCC) in CHC patients treated with DAAs.
To explore the predictive value of SWE for HCC development after complete clearance of hepatitis C virus (HCV).
A comprehensive literature search of clinical studies was performed to identify the ability of SWE to predict HCC occurrence after HCV clearance. In accordance with the study protocol, a qualitative and quantitative analysis of the evidence was planned.
At baseline and after 12 wk of follow-up, a trend was shown towards greater liver stiffness (LS) in those who go on to develop HCC compared to those who do not [baseline LS standardized mean difference (SMD): 1.15, 95% confidence interval (95%CI): 020-2.50; LS SMD after 12 wk: 0.83, 95%CI: 0.33-1.98]. The absence of a statistically significant difference between the mean LS in those who developed HCC or not may be related to the inability to correct for confounding factors and the absence of raw source data. There was a statistically significant LS SMD at 24 wk of follow-up between patients who developed HCC vs not (0.64; 95%CI: 0.04-1.24).
SWE could be a promising tool for prediction of HCC occurrence in patients treated with DAAs. Further studies with larger cohorts and standardized timing of elastographic evaluation are needed to confirm these data.
Core Tip: The role of shear wave-elastography (SWE) is still unclear in predicting hepatocellular carcinoma (HCC) after hepatitis C virus eradication. This is the first systematic review and meta-analysis that focuses on SWE as a predictor of HCC in sustained virologic response patients.
- Citation: Esposto G, Santini P, Galasso L, Mignini I, Ainora ME, Gasbarrini A, Zocco MA. Shear-wave elastography to predict hepatocellular carcinoma after hepatitis C virus eradication: A systematic review and meta-analysis. World J Gastroenterol 2024; 30(10): 1450-1460
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1450
The hepatitis C virus (HCV) is an RNA virus that predominantly affects the liver, leading to both acute and chronic hepatitis. Its prolonged presence often leads to progressive liver damage, which can manifest as cirrhosis, decompensated liver disease, and hepatocellular carcinoma (HCC). In 2016, the World Health Organization aimed to eliminate HCV infection as a significant public health threat by 2030[1]. Despite notable advancements, an estimated 57 million individuals were still grappling with HCV infection in 2020, with an annual toll of 300000 HCV-related deaths[2].
Direct-acting antiviral agents (DAAs) have emerged as highly effective treatments for chronic hepatitis C (CHC)[3,4]. Numerous studies have highlighted the exceptional tolerability of these drugs, even among the most vulnerable patients, including those undergoing dialysis[5], awaiting liver transplantation[6], or affected by cardiac pathologies[7]. This tolerability has led to a significant increase in the number of treated patients achieving a sustained virologic response (SVR), which exceeds 90%[8] and represents a crucial benchmark for success in hepatitis C treatment. In fact, the primary objective of achieving SVR is the prevention of additional liver damage or complications associated with CHC infection.
However, despite positive data regarding DAA treatment response and improved outcomes, several cases of pharmacological resistance have been described[9], making HCV infection increasingly challenging and difficult to manage. Furthermore, emerging evidence suggests a persistent risk of HCC in CHC patients post-DAAs therapy, even after achieving SVR[10]. Therefore, in the effort to eradicate HCV and subsequently minimize its associated complications, there are countless studies focused on identifying high-risk patient profiles prone to post-SVR complications[11,12].
A recent study by Zou et al[13] demonstrated the ability to identify individuals who achieved SVR but remain susceptible to HCC. They developed a risk prediction model that includes liver function laboratory indices, which proved to be a pivotal step toward identification of patients at higher risk.
In addition, DAA therapy exerts an impact at the organic level, improving the degree of liver fibrosis and reducing key clinical scores such as Model for End-Stage Liver Disease, Child-Pugh-Turcotte (CPT) and Fibrosis-4 score[14,15].
For assessing the degree of hepatic fibrosis, liver stiffness (LS) measurement techniques have been implemented alongside clinical scores. Ultrasound (US) elastography[16] or magnetic resonance elastography (MRE)[17] are dependable alternatives to liver biopsy for evaluating fibrosis levels. These validated non-invasive methods play a crucial role in monitoring fibrosis regression and forecasting the risk of complications following DAA therapy[18].
In this context, various studies have aimed to understand how liver elastography could function as a predictor for HCC in CHC patients treated with DAAs, highlighting its role in assessing the risk of HCC[19-22]. These studies mainly employed Fibroscan®, and a recent meta-analysis[23] confirmed the capability of LS in predicting HCC onset in DAA-treated CHC patients.
Alongside Fibroscan® and MRE, shear-wave elastography (SWE) represents another novel technique used for evaluation of liver fibrosis. SWE holds several advantages over Fibroscan® as it allows the operator to vary depth and to select areas of liver parenchyma without vessels or structures that could interfere with the measurement. Additionally, while Fibroscan® requires dedicated machinery solely for measurements without offering a panoramic view of the liver, SWE is performed using the same US device used for US B-mode imaging, providing a higher quality B-mode image compared to that obtained with Fibroscan®[24]. This could also influence operator variability, making SWE less exposed to it.
To the best of our knowledge, this represents the first systematic review and meta-analysis examining how SWE could serve as a predictor of HCC post-SVR.
A systematic literature review was conducted to answer the following research question: “Can liver Shear Wave Elastography be used to predict risk of developing HCC after HCV eradication?”
The study was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses[25,26] and synthetized with meta-analysis. The protocol for this systematic review was written and submitted to the International Register of Systematic Reviews (PROSPERO, ID: CRD42023491042) prior to the completion of the literature search.
The search was performed in the following electronic bibliographic databases: Medline (via PubMed), Embase (via Ovid), and Web of Science. To guarantee adequate sensitivity, the search strategy included terms strictly related to the diagnostic technique evaluated and the target population of patients affected by HCV who had undergone DAAs therapy and obtained SVR. Therefore, the three domains were combined regarding SVR, HCV and elasticity. The search string for each database can be found in Supplementary Table 1. The search was complemented by manual review of retrieved articles’ references and of prior systematic reviews on this topic.
According to the prognostic study design, studies were considered eligible if they met the following criteria: (1) Prospective and retrospective cohort studies; (2) written in English; (3) describing patients with HCV treated with DAAs who reached SVR; and (4) being evaluated with SWE after SVR. Moreover, meeting abstracts and oral or poster communications at scientific congresses were excluded. The results of the literature search were combined using EndNote™. Then, individual records were manually screened with title and abstract analysis by two independent reviewers (Esposto G and Galasso L). Any disagreement was settled by discussion. Records selected by manual screening were eligible for full-text analysis. Study selection, full-text analysis, and data extraction were performed by two reviewers (Esposto G and Galasso L). In the case of multiple records on a single study, the most recent published paper in which the outcomes of the review were reported in the most exhaustive and complete way was included.
The following data were collected: Author, location, publication year, study design, dates of study, origin of the study population, type of DAAs treatment, total number of patients, Fibrosis index (FI) measured via ultrasound SWE, and confounding factors reported in each study. In the case of ambiguity, the study investigators were contacted to provide clarification. Missing data were requested from study authors. In accordance with the study protocol, a qualitative analysis of the evidence was planned.
LS measured via SWE was reported as FI, i.e. the Young’s modulus expressed in kPa. Where the original article expressed LS in terms of shear-wave velocity (Vs) expressed in m/s, a conversion formula was used. In fact, assuming an isotropic tissue density of 1 g/mL, shear-wave velocity can be converted into FI according to the following equation: 3 × Vs (m/s)2 = FI (kPa)[27,28].
The quantitative synthesis of the data was performed through analysis of the Hedges’ g standardized difference of the LS means in those who received a diagnosis of HCC vs those who did not develop this complication. Whenever the original work did not report the results in terms of mean and standard deviation, the relative median and interquartile range were converted according to the indications of the Cochrane Library[29]. Hence, standardized mean differences (SMDs) were meta-analyzed using a Random Effect model due the very high heterogeneity documented.
It was not possible to perform a quantitative synthesis of effect measures [i.e. hazard ratio (HR) or odds ratio] due to the absence of a predefined LS cut-off and the consequent non-comparability of the evidence reported by the individual studies.
Heterogeneity was assessed by τ2 and I2 statistics. The first accounts for the between-studies variance, while the latter represents the proportion of total variation across studies due to heterogeneity rather than chance.
Risk of bias of eligible studies was assessed with the Quality Assessment of Prognostic Accuracy Studies (QUAPAS) tool[30]. This assessment was performed by two independent authors (Santini P and Esposto G), and any disagreement between the two reviewers was settled by discussion with involvement of a third review author if necessary.
The number of studies was insufficient to allow a graphical assessment of publication bias by funnel plot or statistical assessment by Egger’s test. However, all of the identified studies showed a statistically different LS by univariate or multivariate analysis between patients who developed HCC and those who did not. Consequently, it cannot be excluded that this could reflect the presence of a certain degree of unmeasurable publication bias.
The primary outcome of this systematic review and meta-analysis is the development of HCC in HCV patients treated with DAAs who reached SVR. HCC was defined as an established radiological diagnosis with contrast-enhanced abdominal ultrasound, magnetic resonance imaging, or CT.
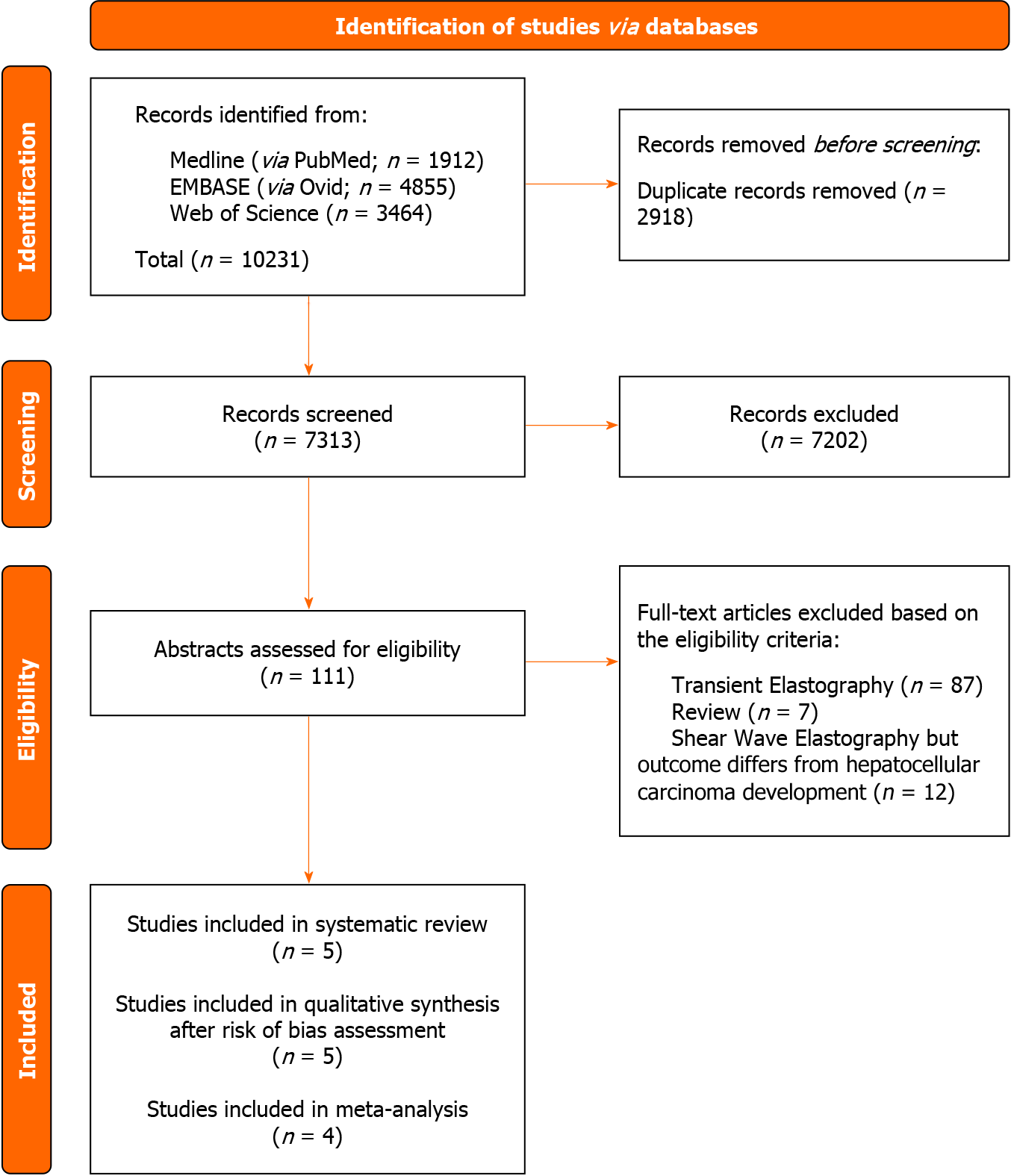
Screening of three biomedical databases was conducted using the prespecified search methods on 21 November 2023, and a total of 10231 studies were found (Medline via PubMed: 1912; Embase: 4855; Web of Science: 3464). After duplicates were removed, 7313 records were screened for eligibility based on title and abstract. A total of 111 papers were considered eligible for full-text analysis. Exclusion occurred for 99 experimental studies: 87 studies evaluated the use of transient elastography, while 12 studies did not evaluate HCC development as an outcome. Moreover, we excluded seven previously published reviews according to the aforementioned inclusion criteria. Finally, five studies matched the pre-established eligibility requirements for the current systematic review and meta-analysis.
After a comprehensive risk-of-bias assessment, one original paper was excluded from quantitative synthesis due to an estimated high concern about applicability related to the systematic review and meta-analysis research question.
Figure 1 shows the PRISMA selection flow diagram that describes the study-selection process in detail.
Using the QUAPAS tool for QUAPAS[30], a structured examination of the risk of bias was conducted to assess the internal and external validity of each included study (Tables 1 and 2). It is important to emphasize that the risk-of-bias assessment does not judge the overall scientific quality of each included study; rather, it assesses each study in relation to the research questions of the current systematic review and meta-analysis.
Three studies were categorized as having an unknown risk of bias in the “Participants” domain because the enrollment strategy was not provided. Selection bias may have occurred because patients in these retrospective analyses are not specified to be consecutive or not[31-33]. In the same domain, one study was found to be at high risk of bias due to the exclusion of study subjects who had CPT stage C liver cirrhosis. The exclusion of these patients determines a selection of those who present lower HCC risk[34]. The “Index test” domain was judged to be at low risk of bias for all studies, although the question about the use of a prespecified threshold was ignored due to the absence of a validated LS cutoff predictive for the development of HCC. All studies were assessed as being at unclear risk of bias regarding the “Outcome” domain. In fact, no study has specified whether the subsequent screening for HCC was performed blind to the elastographic measurement of LS. One study was at high risk of bias in the “Flow and timing” and “Analysis” domains due to the lack of reporting of patients lost to follow-up and the lack of description of the statistical management of these subjects and censoring events[31].
The evaluation of concerns about applicability was found to be at high risk in the “Participants” domain in one study[31]. Hamada and colleagues described patients treated with both DAAs and interferon[31]. The impossibility of performing a sub-analysis of those who received therapy with DAAs determines an assessment of non-applicability of the results of this study to the research question of the present systematic review and meta-analysis. Consequently, this study was excluded from quantitative synthesis. A visual summary of distribution of Risk of Bias and Applicability Concerns across QUAPAS domains can be found in Supplementary Figures 1 and 2.
The five studies considered eligible for qualitative synthesis had different study designs, inclusion and exclusion criteria, different baseline SWE evaluation and timing of elastographic evaluation after SVR (Table 3). A summary of the baseline characteristics of patients included in each study is available in Table 4. The number of study participants run from 196 to 525, for a total of 1458 patients. All of the included studies used SWE in the evaluation of LS. All five studies performed the first assessment of LS before treatment initiation[31-35], while only two evaluated LS at the End of Treatment (EOT)[32,33]. The successive SWE evaluations were conducted at 12 wk after EOT in three out of five studies[32,33,35] and at 24 wk after EOT in four out of five studies[31-34].
| Ref. | Country | Study design | Patients, n | SWE evaluation | Follow-up time |
| Hamada et al[31], 2018 | Japan | Retrospective | 196 | 24 wk | 26 (5-109) mo |
| Gyotoku et al[32], 2022 | Japan | Retrospective | 229 | EOT, 12 and 24 wk | 32.6 ± 19.5 mo |
| Kumada et al[35], 2022 | Japan | Prospective | 525 | 12 wk | 5.0 (4.0-5.4) yr |
| Masaoka et al[33], 2023 | Japan | Retrospective | 279 | EOT, 12 and 24 wk | 33.8 (6-85) mo |
| Nicoletti et al[34], 2023 | Italy | Prospective | 229 | 24 wk | 3.25 (0.5-4.7) yr |
| Ref. | Age in yr | Sex as male/female | Fib4 score | HCC, n | Time to HCC in mo |
| Hamada et al[31], 2018 | 62 (29-89) | 89/107 | 2.56 (0.39-12.13) | 8 | 28 (6-46) |
| Gyotoku et al[32], 2022 | 66 (21-84) | 93/136 | 3.49 (0.30-19.10) | 8 | 21.3 |
| Kumada et al[35], 2022 | 72 (65-79) | 227/298 | 2.73 (1.90-4.23) | 21 | Not reported |
| Masaoka et al[33], 2023 | 66 (21-86) | 118/161 | 3.41 (0.23-22.00) | 12 | 33.8 (6-85) |
| Nicoletti et al[34], 2023 | 67 ± 11 | 136/93 | 5.77 ± 5.40 | 30 | 24 ± 14.5 |
Hamada et al[31] included 196 patients treated with DAAs (n = 107) or interferon (n = 89). SVR was defined as negative search of HCV RNA at 24 wk after EOT. SWE was conducted with an Aixplorer® ultrasound system (SuperSonic Imagine S.A., Aix-en-Provence, France) to measure LS (kPa). The authors performed SWE before treatment initiation and at SVR 24 wk (SVR24). Median follow-up time after SVR was 26 [interquartile range (IQR): 5-109] mo, during which patients underwent regular liver screening for HCC every 3-4 mo. Eight patients developed HCC, and the median time to develop HCC from SVR was 28 (IQR: 6-46) mo. SWE results were 8.3 kPa (IQR: 3.4-36.2 kPa) at baseline and 5.9 kPa (IQR 2.7-31.3 kPa) at SVR24. Several factors were associated with HCC development, including SWE at SVR24. Multivariate analysis revealed that this association was independent and that the cut-off, determined by a receiver operator characteristics (ROC) analysis, was ≥ 11 kPa [relative risk= 28.71, HR: 28.71, 95% confidence interval (95%CI): 2.58-320.03; P = 0.006]. Patients with SWE values below this level had substantially lower risk of developing HCC during follow-up time.
Gyotoku et al[32] included 229 patients treated with DAAs. SVR was defined as negative search of HCV RNA at 12 wk after EOT. SWE was conducted with a LOGIQ E9 ultrasound system (GE Healthcare, Milwaukee, WI, United States) to measure shear wave velocity (Vs, m/s). All patients underwent SWE measurement before initiating treatment and later at EOT, 12 and 24 wk after EOT. During follow-up time, abdominal ultrasound was performed every 6 mo to screen for potential HCC. The mean observation time was 32.6 ± 19.5 mo, and HCC was diagnosed in eight patients with a mean time from EOT to HCC development of 21.3 mo. Patient characteristics were then grouped into those who developed HCC and those who did not. Vs values decreased during follow-up and were statistically higher in the HCC group than those in the non-HCC group at various stages of evaluation. At baseline, Vs was 1.86 ± 0.20 m/s in the HCC group compared to 1.58 ± 0.26 m/s in the non-HCC group (P = 0.004). Vs at EOT was 1.58 ± 0.26 m/s in the non-HCC group compared to 1.75 ± 0.22 m/s in the HCC group (P = 0.004). Vs at 12 wk follow-up was 1.49 ± 0.25 m/s compared to 1.66 ± 0.15 m/s in thee HCC group (P = 0.018). At 24 wk follow-up, Vs was 1.44 ± 0.24 m/s in the non-HCC group compared to 1.69 ± 0.121 m/s of HCC group (P = 0.031). ROC curve analysis revealed an area under the curve of 0.80 at baseline, 0.75 at EOT, 0.72 at 12 wk follow-up and 0.86 at 24 wk follow-up. A cut-off value with corresponding HR was not calculated.
In the study by Kumada et al[35], 525 patients treated with DAAs were enrolled. SVR was defined as undetectable HCV RNA at 12 wk after EOT. SWE was conducted with a LOGIQ S8 or E9 US system (GE Healthcare, Milwaukee, WI, United States) to measure LS (kPa). All patients underwent baseline measurement before initiating treatment and 12 wk after EOT. Patients were monitored for HCC every 6 mo for a median follow-up time of 5.01 (IQR: 3.97-5.41) years. In this period, HCC was diagnosed in 21 patients with a median time to HCC of 3.70 (IQR: 2.44-4.50) years. Median FI at baseline was 7.8 (IQR: 6.1-10.2) kPa in non-HCC group vs 15.7 (IQR: 11.7-19.1) kPa in HCC patients (P < 0.001). Median SWE at SVR12 was 6.8 (IQR: 5.6-8.6) kPa in non-HCC group vs 11.6 (IQR: 8.0-15.6) kPa in HCC patients (P < 0.001). ROC analysis identified a value of 11.7 kPa at baseline as the cut-off above which HCC risk increases (incidence of 3.3% and 8.9% at 2.5 years and 5 years vs 0.0% and 0.9% if FI < 11.7 kPa; P = 0.001).
Masaoka et al[33] included 279 patients treated with DAAs. SVR was defined as the absence of HCV RNA at 12 wk after EOT. SWE was conducted with a LOGIQ E9 ultrasound system (GE Healthcare, Milwaukee, WI, United States) to measure Vs (m/s). All patients underwent SWE measurement before initiating treatment and later at EOT, 12 and 24 wk after EOT. Abdominal ultrasound was performed every 6 mo after EOT for surveillance of HCC. The median observation time was 33.8 (IQR: 6-85) mo, and HCC was diagnosed in 12 patients. Based on age-male-albumin-bilirubin-platelets (aMAP) score, patients were divided into low, medium, and high-risk groups. Those in the medium and high-risk groups (aMAP scores ≥ 50, number of patients = 237) at 12 wk follow-up were further divided into HCC and non-HCC groups. In the non-HCC group, the median Vs was 1.45 (IQR: 0.95-2.14) m/s while it was 1.69 (IQR: 1.45-2.31) m/s (P = 0.0011) in the HCC group. Multiple regression analysis at 12 wk follow-up revealed a statistical difference in Vs (P = 0.030). Through ROC curve, the authors derived a cut-off value for Vs of 1.53 m/s for HCC development.
Nicoletti et al[34] enrolled 229 patients treated with DAAs. SVR was defined as negativity of HCV RNA at 12 wk after EOT. SWE was conducted with an Aixplorer® US system (Supersonic Imagine S.A., Aix-en-Provence, France) and LS was measured in kPa. Patients were evaluated within 3 mo before initiating therapy (baseline) and after 24 (T1) and 48 (T2) wk from EOT. Patients were then followed up for a median time of 3.25 years (IQR: 0.50-4.70) and HCC was diagnosed in 30 out of 229 patients, with a mean time to HCC of 24 ± 14.5 mo. LS decreased over time after treatment (EOT: 18.1 ± 6.6 kPa, T1: 13.6 ± 6.1 kPa, T2: 12.5 ± 6.1 kPa (P < 0.001) and was inversely correlated to HCC development. ROC curve analysis identified a decrease of at least 20% at 1-year follow-up as the optimal cut-off for risk of HCC (AUC: 0.690, sensitivity: 74%, specificity: 65%). Multivariate analysis confirmed that 1-year delta LS < 20% (HR: 2.98; 95%CI: 1.01-8.11; P = 0.03) was independently associated with HCC development. Hence, patients with a difference in LS less than 20% had higher risk for HCC at 1-year follow-up. This cut-off maintained its predictive capacity at 24, 48, 36 and 60 mo.
Table 5 shows the predictive LS threshold for developing HCC identified by each study and the related HR, when calculated.
| Ref. | LS threshold in kPa | SWE timing | aHR (95%CI) | P value | AUC |
| Hamada et al[31], 2018 | ≥ 11.0 | 24 wk after EOT | 28.71 (2.58-320.03) | 0.006 | 0.93 |
| Gyotoku et al[32], 2022 | Not reported | 24 wk after EOT | Not reported | Not reported | 0.86 |
| Kumada et al[35], 2022 | 11.7 | Baseline | 28.08 (5.53-132.60) | < 0.001 | 0.93 |
| Masaoka et al[33], 2023 | 7.0 | 12 wk after EOT | Not reported | Not reported | Not reported |
| Nicoletti et al[34], 2023 | ΔLS < 20% | Baseline and 48 wk after EOT | 2.98 (1.01-8.11) | 0.03 | 0.69 |
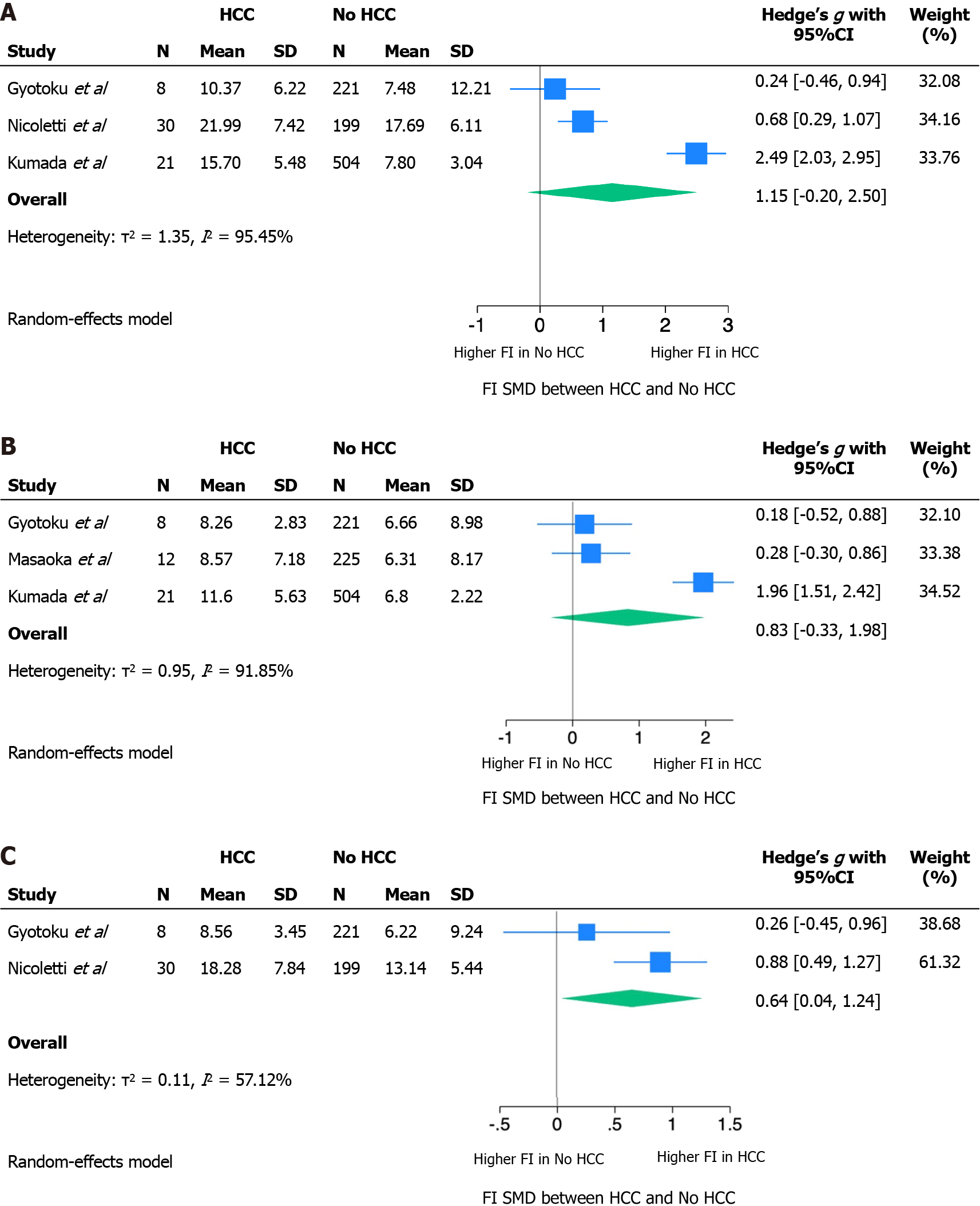
The absence of a validated LS threshold predictive for HCC has determined a non-comparability of the effect measures reported by individual studies. Our quantitative synthesis then focused on the distribution of mean LS among those who did or did not develop HCC during follow-up. The SMD, calculated as Hedges’ g SMD, at baseline and at 12 and 24 wk of follow-up is reported in Figure 2. Because of very high heterogeneity, SMDs were meta-analyzed using a Random Effect model.
At baseline and after 12 wk, standardizing the difference between LS means by the standard deviation of the measurement itself resulted in an SMD that was not statistically significant. The absence of a statistically significant difference between the mean LS in those who developed HCC or not may be related to an inability to correct for confounding factors in the absence of raw source data. Nonetheless, at these follow-up times, a trend was shown towards greater LS in those who will develop HCC compared to those who will not (baseline LS SMD: 1.15, 95%CI: 020-2.50; LS SMD after 12 wk: 0.83, 95%CI: 0.33-1.98). The LS measured at 24 wk showed a statistically significant SMD of 0.64 (95%CI: 0.04-1.24).
The measurement of LS in the two groups was burdened by very high heterogeneity at each of the follow-up times, as shown by the τ2 and I2 statistics, with a trend towards lower heterogeneity at 24 wk of follow-up.
In this meta-analysis, we included four out of five studies for a total of 1261 patients. The exclusion of Hamada et al[31] in 2018 from the quantitative synthesis is due to the heterogeneous population that included both patients treated with DAAs and interferon without the possibility to separate the data by treatment. The quantitative analysis of the remaining four studies showed a positive trend between higher LS values, measured in kPa, and risk of developing HCC, although statistical significance was not reached at EOT and 12 wk of follow-up, while LS at 24 wk showed a statically significant association with HCC development. The lack of statistical significance at EOT and 12 wk could be due to the small number of patients included and to the great heterogeneity among the studies, mainly in terms of included patients and timing of elastographic evaluation. However, it is possible that patients with higher LS at 24 wk after EOT have a percentage of liver fibrosis that cannot regress with antiviral treatment and therefore are at higher risk of HCC.
Concerning the heterogeneity, only two out of four studies measured SWE at EOT[32,33], while the other two studies[34,35] considered the baseline values as those measured before starting antiviral treatment. SVR evaluation, intended as SWE measure at 12 wk after EOT, was performed in three out of four studies[32,33,35]. Three studies explored SWE values at 24 wk after EOT[32-34], but only two of them reported complete data for HCC and non-HCC groups[32,34]. The absence of complete data for Gyotoku et al[32] and Masaoka et al[33] could have influenced the quantitative analysis and the capability to reach statistical significance.
In terms of patient inclusion, Nicoletti et al[34] excluded patients with CPT stage C cirrhosis, possibly leading to an underestimation of HCC occurrence. These exclusion criteria are explained by the nature of the study that aimed to identify non-invasive parameters that could predict liver-related complications; hence patients with decompensated cirrhosis had to be excluded from the study population.
The major difference between mean LS from HCC and non-HCC groups was seen in the study of Kumada et al[35]. Apart from the higher number of subjects in the study, this could be explained by the design of the study itself. The authors followed up with patients with both SWE and MRE. Therefore, this could have led to an increased rate of detection of low-grade HCC.
These data are consistent with previous studies that focused mainly on TE. A recent meta-analysis by You et al[23] on a total of 3398 patients subjected to TE concluded that the pooled HR for HCC development between positive and negative LS results was 3.45 (95%CI: 1.63-7.19). This implies that a high LS is associated with higher HCC occurrence rate, as we found in our meta-analysis of SWE.
Our study has several limitations, starting from the small number of included studies and patients. These studies were heterogeneous in many aspects, including population, SVR definition, LS unit measure (m/s and kPa) and timing of LS measurement. Due to the absence of a validated LS threshold for HCC prediction, we could not compare the effect measures reported by individual studies nor derive a pooled HR for the intended outcome. Hence, we focused the quantitative synthesis on the distribution of mean LS among those who developed HCC and those who did not. Moreover, the comparison of LS between the two sets of patients was burdened by a significant heterogeneity at each of the follow-up times, as shown by the τ2 and I2 statistics, with a trend towards lower heterogeneity at 24 wk after EOT.
Despite the described limitations, SWE seems to be a promising tool for predicting HCC occurrence. To explore the presence of a statistically significant relationship between SWE measured LS and HCC occurrence, future studies need to be designed in a more homogenous way in order to be easily compared. SWE evaluation should be conducted prior to initiating treatment, at EOT, 12 and 24 wk after EOT, to derive the optimal follow-up point in which SWE has the higher predictive capability for HCC occurrence.
This meta-analysis highlights the potential value of SWE in routine US follow-up of SVR patients, for early identification of those at higher risk of developing HCC. SWE acquisition can be performed with the same ultrasound machine used for B-mode evaluation and it takes only a few minutes more than standard US. LS measure with Fibroscan® has already shown its value in routine follow-up of CHC patients. Because SWE could soon replace TE, further studies with larger cohorts and standardized timeline are needed to confirm its value in prediction of HCC after clearance of HCV.
Direct-acting antiviral agents (DAAs) modified the natural history of chronic hepatitis C. However, despite the advancements in sustained virologic response (SVR), some patients remain at risk of developing hepatocellular carcinoma (HCC). In this context, liver stiffness (LS) measurement could serve as a predictor of HCC, allowing for the identification of patients at higher risk.
Identification of SVR patients at higher risk of HCC development could enhance follow-up timing and improve early diagnosis and overall prognosis.
The aim of this meta-analysis is to investigate shear-wave elastography (SWE) as a predictor of HCC occurrence after hepatitis C virus (HCV) clearance with DAAs.
We conducted a systematic literature review and planned a qualitative and quantitative synthesis of the evidence through analysis of the Hedges’ g standardized difference of the LS means in those who developed HCC and those who did not. The absence of a predefined LS cut-off prevented us from deriving the effect measures (i.e. hazard ratio or odds ratio).
LS at baseline and 12 wk follow-up showed a trend towards greater values in those who will develop HCC compared to those who will not [baseline LS standardized mean difference (SMD): 1.15, 95% confidence interval (95%CI): 020-2.50; LS SMD after 12 wk: 0.83, 95%CI: 0.33-1.98]. The standardized mean difference between LS in the two groups at 24 wk follow-up was statistically significant (0.64; 95%CI: 0.04-1.24).
This study explored the ability of SWE to predict HCC after HCV eradication.
LS, measured by SWE, looks a promising predictor of HCC occurrence in SVR patients. These results need to be further confirmed by larger cohorts.
| 1. | Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, Séguy N, Rewari BB, Chan PL, Le LV, Doherty M, Luhmann N, Easterbrook P, Dirac M, de Martel C, Nayagam S, Hallett TB, Vickerman P, Razavi H, Lesi O, Low-Beer D. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 2. | Martinello M, Solomon SS, Terrault NA, Dore GJ. Hepatitis C. Lancet. 2023;402:1085-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 117] [Reference Citation Analysis (0)] |
| 3. | Fehily SR, Papaluca T, Thompson AJ. Long-Term Impact of Direct-Acting Antiviral Agent Therapy in HCV Cirrhosis: Critical Review. Semin Liver Dis. 2019;39:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Pugliese N, Polverini D, Arcari I, De Nicola S, Colapietro F, Masetti C, Ormas M, Ceriani R, Lleo A, Aghemo A. Hepatitis C Virus Infection in the Elderly in the Era of Direct-Acting Antivirals: Evidence from Clinical Trials and Real Life. Trop Med Infect Dis. 2023;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Toyoda H, Kikuchi K. Management of dialysis patients with hepatitis C virus in the era of direct-acting antiviral therapy. Ther Apher Dial. 2023;27:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Pacheco LS, Ventura PE, Kist R, Garcia VD, Meinerz G, Tovo CV, Cantisani GPC, Zanotelli ML, Mucenic M, Keitel E. Real-world effectiveness and safety of direct-acting antivirals for the treatment of hepatitis C virus in kidney and liver transplant recipients: experience of a large transplant center in Brazil. Rev Inst Med Trop Sao Paulo. 2023;65:e59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Roguljic H, Nincevic V, Bojanic K, Kuna L, Smolic R, Vcev A, Primorac D, Vceva A, Wu GY, Smolic M. Impact of DAA Treatment on Cardiovascular Disease Risk in Chronic HCV Infection: An Update. Front Pharmacol. 2021;12:678546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Zarębska-Michaluk D, Brzdęk M, Jaroszewicz J, Tudrujek-Zdunek M, Lorenc B, Klapaczyński J, Mazur W, Kazek A, Sitko M, Berak H, Janocha-Litwin J, Dybowska D, Supronowicz Ł, Krygier R, Citko J, Piekarska A, Flisiak R. Best therapy for the easiest to treat hepatitis C virus genotype 1b-infected patients. World J Gastroenterol. 2022;28:6380-6396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Izhari MA. Molecular Mechanisms of Resistance to Direct-Acting Antiviral (DAA) Drugs for the Treatment of Hepatitis C Virus Infections. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 10. | Hsu CC, Gopalakrishna H, Mironova M, Lee MH, Chen CJ, Yang HI, Wiese M, Chang KM, Wright EC, Abijo T, Feld JJ, Kaplan DE. Risk of Hepatocellular Carcinoma After Spontaneous Clearance of Hepatitis C Virus and in Noncirrhosis Chronic Hepatitis C Patients With Sustained Virological Response: A Systematic Review. Clin Infect Dis. 2023;77:S245-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Toyoda H, Kanneganti M, Melendez-Torres J, Parikh ND, Jalal PK, Piñero F, Mendizabal M, Ridruejo E, Cheinquer H, Casadei-Gardini A, Weinmann A, Peck-Radosavljevic M, Dufour JF, Radu P, Shiha G, Soliman R, Sarin SK, Kumar M, Wang JH, Tangkijvanich P, Sukeepaisarnjaroen W, Atsukawa M, Uojima H, Nozaki A, Nakamuta M, Takaguchi K, Hiraoka A, Abe H, Matsuura K, Watanabe T, Shimada N, Tsuji K, Ishikawa T, Mikami S, Itobayashi E, Singal AG, Johnson PJ. Regional Differences in Clinical Presentation and Prognosis of Patients With Post-Sustained Virologic Response Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2024;22:72-80.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Fraile-López M, Alvarez-Navascués C, González-Diéguez ML, Cadahía V, Chiminazzo V, Castaño A, Varela M, Rodríguez M. Predictive models for hepatocellular carcinoma development after sustained virological response in advanced hepatitis C. Gastroenterol Hepatol. 2023;46:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Zou Y, Yue M, Jia L, Wang Y, Chen H, Zhang A, Xia X, Liu W, Yu R, Yang S, Huang P. Accurate prediction of HCC risk after SVR in patients with hepatitis C cirrhosis based on longitudinal data. BMC Cancer. 2023;23:1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | El-Sherif O, Jiang ZG, Tapper EB, Huang KC, Zhong A, Osinusi A, Charlton M, Manns M, Afdhal NH, Mukamal K, McHutchison J, Brainard DM, Terrault N, Curry MP. Baseline Factors Associated With Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology. 2018;154:2111-2121.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Thi Thu PN, Hoang Van D, Ngo Thi Quynh M, Tran Thi N, Pham Minh K, Pham Van L. Metabolic, renal, and hematological changes in chronic hepatitis C patients achieving rapid virologic response after 12 weeks of direct-acting antiviral treatment: A prospective cohort study. PLoS One. 2023;18:e0290235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Li Q, Chen T, Shi N, Ye W, Yuan M, Shi Y. Quantitative evaluation of hepatic fibrosis by fibro Scan and Gd-EOB-DTPA-enhanced T1 mapping magnetic resonance imaging in chronic hepatitis B. Abdom Radiol (NY). 2022;47:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Kumada T, Toyoda H, Yasuda S, Sone Y, Ogawa S, Takeshima K, Tada T, Ito T, Sumida Y, Tanaka J. Prediction of Hepatocellular Carcinoma by Liver Stiffness Measurements Using Magnetic Resonance Elastography After Eradicating Hepatitis C Virus. Clin Transl Gastroenterol. 2021;12:e00337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Cerrito L, Ainora ME, Nicoletti A, Garcovich M, Riccardi L, Pompili M, Gasbarrini A, Zocco MA. Elastography as a predictor of liver cirrhosis complications after hepatitis C virus eradication in the era of direct-acting antivirals. World J Hepatol. 2021;13:1663-1676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Nakai M, Yamamoto Y, Baba M, Suda G, Kubo A, Tokuchi Y, Kitagataya T, Yamada R, Shigesawa T, Suzuki K, Nakamura A, Sho T, Morikawa K, Ogawa K, Furuya K, Sakamoto N. Prediction of hepatocellular carcinoma using age and liver stiffness on transient elastography after hepatitis C virus eradication. Sci Rep. 2022;12:1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | John BV, Dang Y, Kaplan DE, Jou JH, Taddei TH, Spector SA, Martin P, Bastaich DR, Chao HH, Dahman B. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Rodprasert N, Hongboontry T, Cherdchoochart C, Chaiteerakij R. Association between Liver Stiffness and Liver-Related Events in HCV-Infected Patients after Successful Treatment with Direct-Acting Antivirals. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Cossiga V, La Civita E, Bruzzese D, Guarino M, Fiorentino A, Sorrentino R, Pontillo G, Vallefuoco L, Brusa S, Montella E, Terracciano D, Morisco F, Portella G. Enhanced liver fibrosis score as a noninvasive biomarker in hepatitis C virus patients after direct-acting antiviral agents. Front Pharmacol. 2022;13:891398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | You MW, Kim KW, Shim JJ, Pyo J. Impact of liver-stiffness measurement on hepatocellular carcinoma development in chronic hepatitis C patients treated with direct-acting antivirals: A systematic review and time-to-event meta-analysis. J Gastroenterol Hepatol. 2021;36:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Osman AM, El Shimy A, Abd El Aziz MM. 2D shear wave elastography (SWE) performance versus vibration-controlled transient elastography (VCTE/fibroscan) in the assessment of liver stiffness in chronic hepatitis. Insights Imaging. 2020;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7062] [Cited by in RCA: 5642] [Article Influence: 1128.4] [Reference Citation Analysis (33)] |
| 26. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 2332] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 27. | Palmeri ML, Nightingale KR. Acoustic radiation force-based elasticity imaging methods. Interface Focus. 2011;1:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255-268. [PubMed] |
| 29. | Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Hoboken: Wiley, 2023. [DOI] [Full Text] |
| 30. | Lee J, Mulder F, Leeflang M, Wolff R, Whiting P, Bossuyt PM. QUAPAS: An Adaptation of the QUADAS-2 Tool to Assess Prognostic Accuracy Studies. Ann Intern Med. 2022;175:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Hamada K, Saitoh S, Nishino N, Fukushima D, Horikawa Y, Nishida S, Honda M. Shear wave elastography predicts hepatocellular carcinoma risk in hepatitis C patients after sustained virological response. PLoS One. 2018;13:e0195173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Gyotoku Y, Shirahashi R, Suda T, Tamano M. Role of liver stiffness measurements in patients who develop hepatocellular carcinoma after clearance of the hepatitis C virus. J Med Ultrason (2001). 2022;49:253-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Masaoka R, Gyotoku Y, Shirahashi R, Suda T, Tamano M. Combining the age-male-albumin-bilirubin-platelets score and shear wave elastography stratifies carcinogenic risk in hepatitis C patients after viral clearance. World J Clin Cases. 2023;11:5204-5214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Nicoletti A, Ainora ME, Cintoni M, Garcovich M, Funaro B, Pecere S, De Siena M, Santopaolo F, Ponziani FR, Riccardi L, Grieco A, Pompili M, Gasbarrini A, Zocco MA. Dynamics of liver stiffness predicts complications in patients with HCV related cirrhosis treated with direct-acting antivirals. Dig Liver Dis. 2023;55:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Kumada T, Toyoda H, Yasuda S, Ogawa S, Gotoh T, Tada T, Ito T, Sumida Y, Tanaka J. Combined ultrasound and magnetic resonance elastography predict hepatocellular carcinoma after hepatitis C virus eradication. Hepatol Res. 2022;52:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang ZX, China S-Editor: Lin C L-Editor: Filipodia P-Editor: Yuan YY