Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1329
Peer-review started: December 5, 2023
First decision: January 4, 2024
Revised: January 15, 2024
Accepted: February 25, 2024
Article in press: February 25, 2024
Published online: March 14, 2024
Processing time: 100 Days and 3.8 Hours
Postoperative pancreatic fistula (POPF) is a frequent complication after pancreatectomy, leading to increased morbidity and mortality. Optimizing prediction models for POPF has emerged as a critical focus in surgical research. Although over sixty models following pancreaticoduodenectomy, predominantly reliant on a variety of clinical, surgical, and radiological parameters, have been documented, their predictive accuracy remains suboptimal in external validation and across diverse populations. As models after distal pancreatectomy continue to be pro
Core Tip: Postoperative pancreatic fistula (POPF) is a common complication following pancreatectomy, associated with increased morbidity and mortality. Optimizing prediction models for POPF is a critical focus in surgical research. Although over sixty models following pancreaticoduodenectomy have been documented, their predictive accuracy remains suboptimal across diverse populations. The validation of models after distal pancreatectomy is anticipated, while POPF prediction after central pancreatectomy requires further development and validation. Machine learning and big data analytics offer promising prospects for enhancing prediction model accuracy. Personalized prediction models and novel imaging technologies, such as AI-based radiomics, may further refine predictive models.
- Citation: Yang F, Windsor JA, Fu DL. Optimizing prediction models for pancreatic fistula after pancreatectomy: Current status and future perspectives. World J Gastroenterol 2024; 30(10): 1329-1345
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1329.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1329
With the ongoing development of surgical techniques and technologies, the outcomes of pancreatectomy has significantly improved. Although the mortality rate after pancreatectomy has decreased to less than 5%, the occurrence of morbidity remains high, ranging from 15% to 65%[1,2]. One frequent complication that arises after pancreatectomy is postoperative pancreatic fistula (POPF), which varies in incidence depending on many factors including the definition of POPF and type of pancreatic anastomosis employed. The rate of POPF has not shown significant changes over time. Recent reports indicate that the incidence of POPF after pancreaticoduodenectomy (PD) is about 15%-20%[3], and after distal pancreatectomy (DP) 20%-30%[4]. Central pancreatectomy (CP) has the highest incidence of POPF, exceeding 30%[5]. The consequences of POPF include secondary complications of intra-abdominal abscess, sepsis, and life-threatening massive hemorrhage, which combine to further extend hospital stay and increase healthcare costs.
While accurate prediction of patients at high risk of POPF is a high priority, it remains a challenge. Predictive models serve as useful tools for risk stratification and resource allocation, with a focus on patients who stand to benefit the most. By efficiently identifying patients at a higher risk of POPF, these models allow healthcare providers to tailor their management approach based on an individual patient's risk profile. With the ability to pinpoint high-risk patients, predictive models empower providers to proactively implement preventive strategies, including appropriate anastomotic technique, octreotide administration, prophylactic drains, and Wirsung's duct stenting, while also initiating closer postoperative monitoring. Furthermore, predictive models offer valuable information for shared decision-making between healthcare providers and patients. This ensures that patients are well-informed about their risk of developing POPF, along with the potential benefits and risks associated with various prevention and management strategies. As a result, patients can actively participate in decisions regarding their treatment and care. These models utilize a range of risk factors, including clinical parameters, to determine the likelihood of POPF in individual patients, thereby improving surgical outcomes and reducing healthcare burden. Future iterations of these models hold the potential to further enhance their accuracy and effectiveness by incorporating valid risk factors and improving predictive algorithms. The aim of this paper is to provide a reference for surgeons to select suitable models in their clinical practice, and to propose strategies for optimizing these models.
A comprehensive literature search was conducted in the PubMed database to identify relevant studies on prediction models for POPF after pancreatectomy. The search strategy included the terms "pancreatic fistula" AND "predictive model" or "score" AND "pancreaticoduodenectomy " or "pancreatic resection". Only studies published in English between January 2005 and October 2023 were included in the screening process.
The definition (drain fluid amylase level from postoperative day 3 exceeds 3 times the serum amylase activity) and grading system of POPF was first published in 2005[6] and later revised by the International Study Group of Pancreatic Surgery (ISGPS) in 2016[7]. This system is now widely accepted and utilized, grading POPF on the basis of its severity. Grade A refers to a ‘biochemical leak’ that is characterized by an elevated drain fluid amylase level. However, it does not result in adverse clinical consequences and is no longer considered a true POPF. Grade B affects postoperative recovery and requires intervention, although it does not lead to severe consequences. This grade is clinically relevant as it can interfere with the management and impact clinical outcome. Within Grade B, there are three subtypes: B1, B2, and B3, each increasing in severity[8]. B1 is the least prevalent subtype and is characterized by persistent abdominal drainage for more than three weeks. Although it does not require specific treatment, it still requires monitoring. B2 is the most common subtype and necessitates medical therapy, including antibiotics, enteral or parenteral nutrition, somatostatin and analogues, and transfusions, regardless of the need for extended catheter drainage. B3 is the most severe subtype, which demands interventional procedures under general anesthesia. Grade C is the most severe form of POPF and is associated with significant clinical implications, including organ failure and death. This grade requires immediate attention and intervention. Clinically relevant POPF (CR-POPF, B+C grades only) is accompanied by clinically relevant developments or conditions directly related to the POPF. By using this grading system, healthcare professionals can effectively grade and manage POPF based on its severity, helping to ensure appropriate treatment.
In recent study, it has been observed that patients who experienced postoperative pancreatitis (POAP) had an increased likelihood of developing CR-POPF[9]. Although the exact mechanism by which POAP leads to CR-POPF formation is yet to be determined, the association between them suggests a potential link. Postoperative hyperamylasemia, which is considered a biochemical marker of pancreatic tissue irritation, can be likened to a biochemical leak. Its significance in terms of clinical outcomes is not well understood. Additional research is required to clarify the clinical implications of postoperative hyperamylasemia and its relationship with the development of POPF[10].
Numerous risk factors have been identified in association with POPF (Table 1), leading to the development of several prediction models based on these factors. The risk factors can be described in three groups: preoperative, intraoperative, and postoperative factors[11]. It’s important to note that the risk factors of POPF may vary depending on the type of pancreatic resection being performed[12].
| Stage | Factors |
| Preoperative | (1) Sex, (2) age, (3) BMI, (4) weight, (5) weight loss, (6) smoking history, (7) hypertension, (8) diabetes mellitus, (9) history of acute pancreatitis, (10) history of abdominal surgery, (11) chronic steroid use, (12) ASA score, (13) preoperative biliary drainage, (14) preoperative chemotherapy, (15) albumin, (16) bilirubin, (17) alanine transaminase, (18) creatine, (19) tumor site, (20) MPD diameter, (21) MPD index1, (22) pancreatic thickness, (23) pancreatic density, (24) pancreatic texture, (25) relation with PV on CT, (26) pancreatic density index, (27) intra-abdominal fat thickness, (28) visceral adipose tissue, (29) total adipose tissue, (30) sarcopenic obesity, (31) L3 subcutaneous fat area, (32) pancreatic remnant volume, (33) stump area, (34) fat score, (35) atrophy score, (36) A/L ratio, (37) subcutaneous fat index, (38) radiomics score, (39) combined radiomics score, (40) liver density, (41) muscle attenuation, (42) PS SIratio, (43) PM SIratio, (44) fat mass at BIVA, (45) SWV value of pancreas, (46) MIPD experience, (47) preoperative diagnosis |
| Intraoperative | (A) MPD diameter, (B) pancreatic texture, (C) operating time, (D) estimated blood loss, (E) transfusion, (F) intraoperative colloid infusion, (G) surgical approach, (H) minimally invasive approach, (I) open conversion, (J) pancreatic anastomosis, (K) gastrojejunostomy, (L) extended lymphadenectomy, (M) venous resection, (N) nasojejunal feeding tube |
| Postoperative | (a) Postoperative DFA, (b) change of postoperative DFA, (c) WBC on POD1, (d) change of postoperative WBC, (e) neutrophil on POD3, (f) postoperative CRP, (g) temperature on POD3, (h) postoperative albumin, (i) albumin difference2, (j) postoperative CRP/albumin, (k) serum creatinine on POD1, (l) hyperamylasemia on POD1-2, (m) serum lipase on POD1, (n) DFL on POD1, (o) pathology, (p) PV invasion, (q) pancreatic fibrosis, (r) pancreatic steatosis, (s) deep surgical site infection, (t) DGE |
Preoperative risk factors for POPF in patients undergoing PD include demographic characteristics such as gender, age, and body mass index (BMI)[13-15]. Comorbidities such as diabetes and pancreatitis, as well as imaging findings including pancreatic density, main pancreatic duct (MPD) diameter, visceral adipose tissue and radiomics score, are also risk factors[13,15-19]. Furthermore, biochemical markers like preoperative bilirubin and albumin levels, as well as preoperative biliary drainage and neoadjuvant chemotherapy, also contribute to the risk.
Intraoperative risk factors for POPF include pancreas-specific characteristics, such as soft pancreas and small MPD diameter. The surgical approach utilized (open, laparoscopic, and robotic) and type of anastomosis are also important. Other intraoperative risk factors include extended operating time, massive blood loss, combined venous resection, and extended lymphadenectomy[20-23].
Postoperative risk factors for POPF include high drain amylase levels, hyperamylasemia, hyperlipasemia, hypoalbuminemia, elevated C-reactive protein (CRP) level, and increased neutrophil count. Delayed gastric emptying is also a risk factor[24]. The pathology report may describe risk factors for POPF such as pancreatosteatosis and the absence of pancreatic fibrosis. Many prediction models for POPF after PD have been developed, and the reported predictors for these models are detailed in Table 1.
Numerous studies have examined the risk factors associated with POPF following DP. However, compared with PD, there are fewer reported risk factors. These predictors can also be grouped as preoperative, intraoperative, and postoperative factors.
Preoperative risk factors for DP include young age, high BMI, the presence of preoperative comorbidities such as diabetes and coronary artery disease, hypoalbuminemia and certain pancreas-specific characteristics[25-27]. For instance, large MPD diameter and thick pancreas have been identified as potential risk factors.
Intraoperative risk factors include extended operating time, massive blood loss, soft pancreas, transection at pancreatic neck, and vascular resection.
Postoperative risk factors include surgical drain characteristics such as high amylase levels, elevated CRP, and the presence of high-risk pathology. Interestingly, there is a reversed predictive effect of MPD diameter between DP and PD. While a wider diameter is considered a risk factor for DP, it is reported as having a protective effect in PD[28]. Several models for predicting POPF following DP have been developed, with their reported predictors summarized in Table 2. Further research is needed to expand the understanding risk factors for POPF after DP, as well as to identify additional indicators that may contribute to more accurate prediction models. By considering a broader range of factors and conducting larger-scale studies, researchers can gain a more comprehensive understanding of POPF risk and develop effective strategies for its prevention and management.
| Preoperative | Intraoperative | Postoperative |
| (1) Age | (A) Epidural use | (a) CRP on POD1 |
| (2) BMI | (B) Operating time | (b) DFA on POD1 |
| (3) Diabetes mellitus | (C) Estimated blood loss | (c) DFA on POD3 |
| (4) Coronary artery disease | (D) Transfusion | (d) Change of postoperative DFA |
| (5) ASA score | (E) Pancreatic texture | (e) Pathology |
| (6) Albumin | (F) Transection site | |
| (7) MPD diameter | (G) Splenectomy | |
| (8) Pancreatic thickness | (H) Vascular resection | |
| (9) Pancreatic neck major diameter | ||
| (10) Pancreatic neck minor diameter | ||
| (11) Predicted pancreatic neck area |
Only a limited number of risk factors for POPF after CP have been reported, and of potential value for establishing prediction models. These risk factors include sex, BMI, diabetes, MPD diameter, pancreatic thickness and texture, operating time, transection site, technique of pancreatic anastomosis, and pathology (Table 3). As research in this field progresses, it is expected that additional risk factors will be identified to enhance our understanding of POPF risk after CP.
| Preoperative | Intraoperative | Postoperative |
| (1) Sex | (A) Operating time | (a) Pathology |
| (2) BMI | (B) Pancreatic texture | |
| (3) Diabetes mellitus | (C) Transection site | |
| (4) Cephalic MPD diameter | (D) Pancreatic anastomosis | |
| (5) Distal MPD diameter | ||
| (6) Pancreatic thickness |
A range of statistical methods are used to develop POPF prediction models. These models can take the form of scores, calculation formulas, or nomograms, providing clinicians with a tool to assess individual patient risk. It is important to note that certain risk factor can be evaluated at different stages of the patient’s journey. For example, the MPD diameter can be measured preoperatively using enhanced CT/MRI scans or during the surgical procedure itself. Although both measurements may introduce some degree of error, MPD diameter measured through preoperative imaging is generally considered accurate[29]. Similarly, while pathology is typically assessed postoperative, a preoperative diagnosis by radiological imaging or biopsy serves as a reliable proxy. Furthermore, advancements in imaging technology have enabled evaluation of pancreatic texture not only during surgery but also with preoperative imaging by CT/MRI scans, and elastography[30]. This expanded imaging capability provides additional insights into the identification of risk factors such as pancreatic fibrosis or inflammation.
In the past decade, over sixty prediction models for POPF after PD have demonstrated potential value in clinical practice. Among these models, one of the earliest reported prediction scores, originating from a single-center prospective study conducted in 2010, categorized patients into four subgroups based on the presence of three risk factors: BMI, pancreatic steatosis and fibrosis[31]. This model shed light on the impact of pancreatic fat infiltration and fibrosis on the potential for POPF and showed high accuracy in predicting grade B and C POPF. However, the reliance on histological analysis for determining the scores of pancreatic steatosis and fibrosis is only available after surgery and cannot be used for surgical strategies, limiting the applicability of this model for instituting steps to prevent or reduce the risk of POPF. Subsequently, Wellner et al[13] and Yamamoto et al[14] proposed models that utilized preoperative indicators to predict the occurrence of POPF. However, these models did not gain widespread acceptance possibly due to the challenges associated with evaluating certain variables, including the MPD index and the distance from the portal vein. Fur
Despite the limitations of early prediction models, continued research efforts have led to more models. These new models take into consideration a broader range of variables and aim to improve accuracy and clinical applicability. In 2013, a prospective study introduced the fistula risk score (FRS) to predict the risk of POPF[34]. The FRS is based on four variables: pancreatic texture, MPD diameter, intraoperative blood loss, and pathology. This scoring system was developed to address the limitations of preoperative assessments and has been validated by several studies, demonstrating its acceptable predictive performance with a c-statistic of over 0.7[35,36]. One of the areas of debated with this model is the relationship between blood loss and the occurrence of POPF[37]. It has been observed that minimally invasive surgery, which results in lower blood loss compared to open surgery, is not consistently associated with a reduced incidence of POPF. In light of this, Mungroop et al[37] proposed an alternative FRS (a-FRS), which removes the variables of intraoperative blood loss and pathological diagnosis. Instead, it includes the BMI as an additional variable. Subsequently, an updated alternative FRS (ua-FRS) was introduced, which incorporates the gender variable specifically for patients undergoing minimally invasive PD (MIPD)[38]. These modified scoring systems have shown improved convenience and enhanced predictive performance compared to the original FRS in subsequent external validations[39].
Preoperative prediction models may have the potential to help in enabling preventive measures and guiding surgical decision-making compared to intraoperative and postoperative prediction models. One such preoperative predictive score was developed by Roberts et al[15], utilizing only BMI and MPD diameter, and it showed a significant increase with increasing severity of POPF (P < 0.001) in a subsequent multicenter study[40]. Building on this, Perri et al[41] established a more simplified risk-tree using the same two parameters. This risk-tree effectively categorized patients into three distinct risk groups with significantly different rates of POPF. However, it is worth noting that the area under the curve (AUC) for this risk-tree in the validation cohort was 0.65, indicating only moderate predictive accuracy. This would mean that 35% of patients are misclassified, which is not sufficiently accurate for application to individual patients.
With the advancements in medical imaging technology, imaging parameters have gained prominence in POPF prediction, and many preoperative prediction models now rely on these parameters (Table 4)[13-15,17,19,41-53]. However, certain imaging parameters require external software for preoperative evaluation, which poses challenges in terms of accessibility, standardization, and compatibility with different imaging systems, as well as external validation for these models. Additionally, the past three years have witnessed the development of over 10 POPF prediction models based on machine learning algorithms (Table 5)[22,48,54-64]. While these models are often considered superior to traditional regression models, it is important to highlight that a recent study revealed machine learning did not outperform logistic regression in predicting POPF after PD[22]. Furthermore, the predictive models developed using nationwide population data exhibited lower AUC values compared to models developed in single- and multicenter studies[22,60,62,63]. This discrepancy implies that the generalisability of the latter two models may be compromised in terms of their predictive value.
| Ref. | Year | Country | Center | Study period | Design cohort | CR-POPF (%) | Variables5 | C-index/AUC (95%CI) | Validation |
| Wellner et al[13] | 2010 | Germany | Single | 2006-2008 | 62 | 30.64 | (2)(5)(6)(9)(47) | Internal | |
| Yamamoto et al[14] | 2011 | Japan | Single | 2004-2007 | 279 | 36.9 | (1)(21)(25)(27)(47) | 0.808 (0.757-0.860) | Internal |
| Roberts et al[15] | 2014 | United Kingdom | Single1 | 2007-2012 | 217 | 22.14 | (3)(20) | 0.832 (0.768-0.897) | Internal |
| Casadei et al[42] | 2015 | Italy | Single | 2008-2012 | 2082 | 20.2 | (3)(20)(47) | ||
| Zhang et al[43] | 2018 | China | Single | 80 | 42.54 | (38) | 0.825 (0.736-0.913) | Internal | |
| Shi et al[44] | 2020 | China | Multi | 2009-2019 | 718 | 15.6 | (20)(32)(33)(34)(35) | 0.729 (0.678-0.775) | External |
| Yu et al[17] | 2021 | China | Single | 2016-2018 | 124 | 25.8 | (21)(23) | 0.775 (0.687-0.862) | Internal |
| Lin et al[19] | 2021 | China | Single | 2013-2019 | 175 | 21.1 | (38) | 0.801 (0.719-0.884) | Internal |
| (39) | 0.871 (0.816-0.926) | ||||||||
| Tang et al[45] | 2021 | China | Single | 2013-2019 | 239 | 19.7 | (3)(20)(36) | 0.823 (0.769-0.877) | |
| Lapshyn et al[46] | 2021 | Germany | Single1 | 2012-2018 | 120 | 193 | (1)(20)(22) | 0.808 (0.726-0.874) | Internal |
| Perri et al[41] | 2021 | Italy | Multi | 2017-2019 | 566 | 20 | (3)(20) | 0.70 (0.63-0.77) | External |
| Savin et al[47] | 2021 | Romania | Single | 2015-2020 | 78 | 28.2 | (20)(23)(32) | 0.846 (0.694-0.941) | |
| (20)(32)(40) | 0.774 (0.599-0.850) | ||||||||
| Skawran et al[48] | 2021 | Switzerland | Single | 2008-2018 | 62 | 27.4 | (43) | 0.75 (0.63-0.84) | |
| Box et al[49] | 2021 | United States | Single | 2013-2018 | 220 | 15.94 | (3)(20)(37) | 0.822 | |
| (3)(20)(26) | 0.757 | ||||||||
| (3)(20)(26)(37) | 0.844 | ||||||||
| Kolbinger et al[50] | 2022 | Germany | Single | 2012-2021 | 195 | 28.7 | (20)(24)(47) | 0.82 | Internal |
| (20)(24)(32)(47) | 0.83 | ||||||||
| Maqueda González et al[51] | 2022 | Spain | Single | 2010-2019 | 103 | 30.1 | (20)(29) | 0.78 (0.68-0.87) | |
| Zou et al[52] | 2023 | China | Single | 2015-2021 | 125 | 17.6 | (20)(28)(42) | 0.903 | Internal |
| Tian et al[53] | 2023 | China | Single1 | 2020-2021 | 1432 | 36 | (20)(45) | 0.866 | Internal |
| Ref. | Year | Country | Center | Study period | Design cohort | CR-POPF (%) | C-index/AUC (95%CI) | Validation |
| Mu et al[54] | 2020 | China | Multi | 2006-2019 | 359 | 15.6 | 0.85 (0.80-0.90) | Internal-external |
| Han et al[55] | 2020 | Korea | Single | 2007-2016 | 1769 | 12.5 | 0.74 | |
| Skawran et al[48] | 2021 | Switzerland | Single | 2008-2018 | 62 | 27.4 | 0.82 (0.74-0.89), 0.74 (0.63-0.89), 0.90 (0.84-0.95) | Internal |
| Giovinazzo et al[56] | 2021 | Multinational | Multi | 1638 | 27 | 0.962 (0.940-0.984) | ||
| Shen et al[57] | 2022 | China | Single | 2010-2021 | 2421 | 17.5 | 0.79-0.81 | Internal |
| Long et al[58] | 2022 | China | Multi | 2012-2021 | 618 | 18.1 | 0.897 (0.370-1.424) | Internal |
| Capretti et al[59] | 2022 | Italy | Single1 | 2011-2019 | 100 | 20 | 0.807, 0.749 | Internal |
| Chen et al[60] | 2022 | United States | Nationwide | 2014-2019 | 13940 | 14.4 | 0.746 (0.733-0.760) | Internal-external |
| Zheng et al[61] | 2023 | China | Single | 2013-2021 | 2572 | 21.8 | 0.977 | Internal |
| Ingwersen et al[22] | 2023 | Netherlands | Nationwide | 2014-2020 | 4912 | 16.3 | 0.74 (0.73-0.74) | |
| Verma et al[62] | 2023 | United States | Nationwide | 2014-2018 | 8597 | 11 | 0.74 (0.72-0.76) | Internal-external |
| Ashraf Ganjouei et al[63] | 2023 | United States | Nationwide | 2014-2019 | 8666 | 13 | 0.67-0.72 | Internal |
| Ingwersen et al[64] | 2023 | Multinational | Multi | 2013-2018 | 118 | 42.4 | 0.9 (0.71-0.99), 0.86, 0.81, 0.8 | Internal-external |
The simplest intraoperative prediction model for POPF is known as the ISGPS risk classification. This classification categorizes patients into four risk groups based on intraoperative measurements of MPD diameter and pancreatic texture[65]. Interestingly, a nationwide validation study of this classification revealed no significant difference between the two intermediate risk categories, leading to the proposal of a simplified three-tier system[66]. The current literature indicates that a-FRS[37] and ua-FRS[38] have been validated by numerous external studies with acceptable accuracy and are two recommended models. However, the surgeon's determination of pancreatic texture by intraoperative palpation is subjective and prone to bias. Specific details regarding more intraoperative POPF prediction model are shown in Table 6[16,18,21,22,37,38,50,61,65,67-71].
| Ref. | Year | Country | Center | Study period | Design cohort | CR-POPF (%) | Variables5 | C-index/AUC (95%CI) | Validation |
| Kim et al[67] | 2013 | Korea | Single | 2003-2008 | 100 | 414 | (A)(B)(M) | 0.728 (0.630-0.812) | Internal |
| Chen et al[68] | 2015 | China | Single | 2008-2013 | 921 | 9.7 | (3)(A)(B)(D)(E) | 0.812 (0.766-0.858) | |
| Kantor et al[69] | 2017 | United States | Nationwide | 2011-2012 | 1731 | 18.3 | (1)(3)(16)(A)(B) | 0.70 (0.65-0.74) | Internal-external |
| Li et al[70] | 2019 | China | Single | 2011-2014 | 189 | 20.1 | (15)(A)(B)(D) | 0.821 (0.736-0.905) | Internal |
| Mungroop et al[37] | 2019 | Multinational | Multi | 2007-2016 | 1924 | 124 | (3)(20)(B) | 0.75 (0.71-0.78) | Internal-external |
| Angrisani et al[21] | 2020 | Italy | Multi1 | 2016-2018 | 148 | 19.6 | (44)(A)(B)(D) | 0.774 (0.683-0.866) | |
| (44)(A)(B) | 0.784 (0.680-0.888) | ||||||||
| Zhang et al[16] | 2021 | China | Single | 2012-2020 | 232 | 7.8 | (7)(8)(10)(B)(K) | 0.916 | |
| Mungroop et al[38] | 2021 | Multinational | Multi | 2007-2017 | 9522 | 21 | (1)(3)(20)(B) | 0.75 (0.71-0.79) | External |
| Kolbinger et al[50] | 2022 | Germany | Single | 2012-2021 | 195 | 28.7 | (47)(A)(B) | 0.82 | Internal |
| Lucassen et al[18] | 2022 | Netherlands | Single | 2009-2018 | 329 | 16.7 | (20)(41)(B) | 0.73 (0.68-0.79) | |
| (20)(28)(B) | 0.81 (0.75-0.86) | ||||||||
| (20)(28)(41)(B) | 0.81 (0.75-0.86) | ||||||||
| Zheng et al[61] | 2023 | China | Single | 2013-2021 | 257 | 21.8 | (3)(20)(B) | 0.743 | Internal |
| Hayashi et al[71] | 2023 | Japan | Single | 2010-2021 | 169 | 22.5 | (30)(31)(B) | 0.832 | |
| Ingwersen et al[22] | 2023 | Netherlands | Nationwide | 2014-2020 | 4912 | 16.3 | (1)(3)(5)(13)(16)(19)(A)(B)(G)(J)(M)(N) | 0.73 | |
| Schuh et al[65] | 2023 | Multinational | Multi | 2004-2019 | 55333 | 15.7 | (A)(B) | External |
Recent studies have made significant advances in identifying early postoperative variables that are closely associated with POPF, including high drain fluid amylase (DFA), hyperamylasemia, and high-risk pathology, among others. These variables, combined with postoperative clinical data, biochemical indicators, and histopathological analysis, contribute to the development of dynamic POPF prediction models (Table 7)[20,23,24,31,34,72-88]. One particularly intriguing model is the "90-1000" score, which demonstrates superior performance in predicting POPF after PD compared to intraoperative pancreatic parenchymal features[79]. This model relies on the measurement of DFA and serum CRP levels on the first postoperative day. Its simplicity makes it particularly suitable for clinical practice; however, further validation is needed to establish its reliability, accuracy and applicability.
| Ref. | Year | Country | Center | Study period | Design cohort | CR-POPF (%) | Variables6 | C-index/AUC (95%CI) | Validation |
| Gaujoux et al[31] | 2010 | France | Single1 | 2004-2005 | 100 | 24 | (3)(q)(r) | 0.82 | |
| Callery et al[34] | 2013 | United States | Single1 | 2002-2007 | 233 | 13 | (A)(B)(D)(o) | 0.942 | Internal |
| Xia et al[23] | 2018 | China | Single | 2009-2017 | 225 | 17.8 | (A)(B)(L)(h) | 0.813 (0.737-0.889) | Internal |
| Xingjun et al[72] | 2019 | China | Multi | 2014-2017 | 457 | 12.65 | (A)(q)(r) | 0.868 | External |
| You et al[73] | 2019 | Korea | Single | 2007-2016 | 1771 | 12.5 | (1)(3)(12)(15)(A)(o) | 0.709 | Internal |
| Guo et al[74] | 2020 | China | Single | 2012-2016 | 220 | 22.7 | (A)(B)(o)(p) | 0.793 (0.731-0.855) | Internal |
| Li et al[75] | 2021 | China | Single | 2018-2020 | 176 | 21.1 | (a)(e)(f)(g)(k) | 0.814 (0.736-0.892) | |
| Shen et al[76] | 2021 | China | Single | 2016-2020 | 302 | 16.6 | (3)(B)(a)(i) | 0.87 (0.81-0.94) | Internal |
| Liu et al[77] | 2021 | China | Single | 2016-2019 | 2514 | 7.6 | (15)(18)(a)(j) | 0.866 (0.737-0.996) | |
| (15)(18)(a)(j) | 0.896 (0.814-0.978) | ||||||||
| (15)(18)(a)(j) | 0.888 (0.806-0.971) | ||||||||
| Huang et al[78] | 2021 | China | Multi | 2010-2018 | 762 | 11.4 | (3)(A)(a) | 0.934 (0.914-0.950) | External |
| Guilbaud et al[79] | 2021 | France | Multi1 | 2017-2019 | 1823 | 21.2 | (a)(f) | 0.834 (0.769-0.900) | |
| Honselmann et al[20] | 2021 | Germany | Single | 2012-2017 | 182 | 16 | (12)(A)(C)(c)(m) | 0.903 | Internal |
| (12)(B)(c)(d)(m) | 0.891 | ||||||||
| Suzuki et al[80] | 2021 | Japan | Single | 2007-2012 | 349 | 17.5 | (20)(B)(b)(n) | ||
| Al Abbas et al[81] | 2021 | United Sates | Nationwide | 2014-2016 | 9867 | 13.9 | (1)(2)(3)(7)(8)(A)(B)(o) | 0.70 (0.69-0.71) | Internal |
| Yin et al[82] | 2022 | China | Single | 2012-2016 | 662 | 16.3 | (17)(A)(F)(M)(o) | 0.667 | Internal |
| (A)(F)(a)(e) | 0.809 | ||||||||
| Gu et al[24] | 2023 | China | Nationwide | 2014-2017 | 36092 | 16.7 | (4)(20)(B)(o)(s)(t) | 0.855 (0.702-0.853) | External |
| Bannone et al[83] | 2023 | Italy | Single1 | 2016-2021 | 905 | 20.2 | (A)(B)(D)(a)(o) | 0.85 (0.82-0.87) | |
| (A)(B)(D)(a)(l)(o) | 0.87 (0.84-0.89) | ||||||||
| (A)(B)(D)(a)(f)(l)(o) | 0.90 (0.87-0.91) | ||||||||
| Choi et al[84] | 2023 | Korea | Multi | 2012-2020 | 4294 | 12.4 | (12)(20)(46)(B)(E)(H)(I)(o) | 0.739 (0.668-0.800) | Internal |
| van Dongen et al[85] | 2023 | Netherlands | Nationwide | 2014-2018 | 3271 | 14.6 | (1)(3)(8)(20)(o) | 0.73 | External |
| Raza et al[86] | 2023 | United Kingdom | Multi | 2009-2019 | 187 | 12.8 | (1)(a)(f)(h) | 0.78 | External |
| Mohamed et al[87] | 2023 | United Sates | Nationwide | 2015-2018 | 5975 | 17 | (1)(3)(14)(A)(B)(o) | 0.72 (0.704-0.737) | |
| Ahmad et al[88] | 2023 | United States | Nationwide | 2014-2017 | 2417 | 12.6 | (3)(11)(B)(C)(a) | 0.720 (0.687-0.752) | Internal |
| (3)(11)(B)(C)(a)(b) | 0.758 (0.726-0.789) |
Many postoperative prediction models incorporate a combination of preoperative and intraoperative parameters. This approach holds the potential to enhance the clinical risk stratification of POPF and may offer a window of opportunity for pre-emptive interventions before the actual occurrence of POPF.
Compared to PD, there have been fewer studies of prediction models for POPF after DP. Efforts in developing reliable models after DP are relatively limited. Although DP involves fewer anastomoses, it is appears to be associated with a higher incidence of POPF[2]. A retrospective study conducted on 2026 patients from 10 institutions identified several risk factors for CR-POPF after DP[25]. These risk factors included age below 60 years, obesity, low levels of albumin, absence of epidural use, high-risk pathology such as neuroendocrine and benign tumors, combined splenectomy, and vascular resection. However, the model constructed using these factors exhibited relatively poor accuracy in predicting POPF, with a c-statistic of 0.654 [95% confidence interval (CI): 0.620-0.688].
Recently, De Pastena et al[27] developed two DP fistula risk score (D-FRS) models. The preoperative model included two factors (pancreatic thickness and MPD diameter) and showed good predictive performance with an AUC of 0.83 (95%CI: 0.78-0.88) and 0.73 (95%CI: 0.70-0.76) for internal and external validation, respectively. In addition to pancreatic thickness and MPD diameter, the intraoperative D-FRS model included BMI, pancreatic texture, and operating time as factors and this achieved an AUC of 0.80 (95%CI: 0.74-0.85) without external validation. The DISPAIR model, developed by Bonsdorff et al[89] in the same year, incorporated three parameters: transection at pancreatic neck, pancreatic thickness, and diabetes. The model's internal and external validation resulted in notable AUC values of 0.904 (95%CI: 0.855-0.949) and 0.798 (95%CI: 0.748-0.848), respectively. While these models offer valuable insights, it is vital to consider their limitations, one of which is the need for external validation across diverse populations. To address this, Xu et al[28] conducted a validation study on the D-FRS and DISPAIR models using 653 Chinese patients who underwent DP. The study demonstrated acceptable discrimination for both models, with no significant differences between them. The AUC values were as follows: preoperative D-FRS 0.723 (95%CI: 0.687-0.757), intraoperative D-FRS 0.737 (95%CI: 0.701-0.770), and DISPAIR model 0.721 (95%CI: 0.685-0.755). The preoperative D-FRS is the most recommended model due to its practicality and ease of use, as it relies on easily measurable radiographic images to assess pancreatic thickness and MPD diameter for preoperative risk stratification. Despite these positive outcomes, there is still room for improvement in the performance of the predictive models. Standardizing classification thresholds may help to enhance their accuracy. There has been the emergence of additional prediction models for POPF after DP, and these are summarized in Table 8[26,79,90-92].
| Ref. | Year | Country | Center | Design cohort | Study period | CR-POPF (%) | Variables3 | AUC (95%CI) | Validation |
| Ecker et al[25] | 2019 | Multinational | Multi | 2026 | 2001-2016 | 15.1 | (1)(2)(6)(A)(G)(H)(e) | 0.654 (0.620-0.688) | |
| Guilbaud et al[79] | 2021 | France | Multi1 | 922 | 2017-2019 | 21.2 | (a)(b) | 0.762 (0.640-0.885) | |
| Rollin et al[90] | 2022 | France | Single | 103 | 2015-2019 | 32 | (2)(8)(B)(c) | 0.83 (0.75-0.92) | |
| Nassour et al[91] | 2022 | USA | Nationwide | 692 | 2014-2018 | 15.9 | (1)(B)(D)(b) | 0.731 (0.685-0.796) | Internal |
| (1)(B)(b)(d) | 0.791 (0.742-0.836) | ||||||||
| Bonsdorff et al[89] | 2022 | Multinational | Multi | 266 | 2013-2021 | 19.5 | (3)(8)(F) | 0.904 (0.855-0.949) | Internal-external |
| He et al[92] | 2023 | China | Single | 115 | 2005-2020 | 33 | (2)(6)(8)(E) | 0.842 (0.762-0.921) | |
| Pecorelli et al[26] | 2023 | Italy | Single | 220 | 2016-2019 | 33.6 | (2)(3)(4)(5) | 0.651 (0.58-0.73) | Internal |
| (2)(9)(10)(C) | 0.725 (0.66-0.79) | ||||||||
| (5)(11)(C) | 0.733 (0.64-0.80) | ||||||||
| De Pastena et al[27] | 2023 | Multinational | Multi1 | 339 | 2014-2016 | 23 | (7)(8) | 0.731 (0.70-0.76) | Internal-external |
| (2)(7)(8)(B)(E) | 0.851 (0.80-0.90) | Internal |
There is a trend toward offering CP more frequently in clinical practice because it allows for preservation of more pancreatic endocrine and exocrine function by resecting less normal pancreatic tissue. However, it appears to have a higher risk of POPF compared with PD and DP because of the presence of two pancreatic stumps[93]. While previous studies have primarily focused on the safety of CP and risk factors for POPF, the exploration of prediction models for POPF after CP has been relatively recent (Table 9). In a study by Ouyang et al[94], involving 194 CP patients, independent risk factors for POPF were identified as obesity and pancreatic anastomosis technique. They constructed a nomogram based on these variables, which demonstrated a modest AUC of 0.678. However, this study overlooked certain pancreas-specific features such as pancreatic texture, MPD diameters on both sides of the transection, and pancreatic thickness, despite its large sample size.
| Ref. | Year | Design cohort | Study period | CR-POPF (%) | Variables1 | AUC (95%CI) |
| Ouyang et al[94] | 2022 | 194 | 2009-2020 | 45.9 | (2)(D) | 0.678 |
| Yang et al[95] | 2023 | 115 | 2010-2022 | 30.4 | (2)(4)(5)(6) | 0.832 (0.751-0.895) |
| (2)(4)(5)(6)(B) | 0.827 (0.745-0.891) | |||||
| (1)(2)(4)(5)(6)(B) | 0.828 (0.746-0.892) | |||||
| (1)(2)(3)(4)(5)(6)(a) | 0.826 (0.744-0.890) | |||||
| (2)(4)(5)(6)(A)(B) | 0.845 (0.766-0.906) | |||||
| (2)(4)(5)(6)(A)(B) | 0.847 (0.768-0.907) | |||||
| (1)(2)(4)(5)(6)(A)(B) | 0.823 (0.741-0.888) | |||||
| (1)(2)(3)(4)(5)(6)(A)(B)(a) | 0.840 (0.760-0.902) | |||||
| (2)(3)(5)(6)(C) | 0.758 (0.669-0.833) | |||||
| (2)(3)(5)(6)(B)(C) | 0.748 (0.659-0.824) | |||||
| (1)(2)(3)(5)(6)(B)(C) | 0.784 (0.698-0.855) | |||||
| (1)(2)(3)(5)(6)(C)(a) | 0.750 (0.661-0.826) |
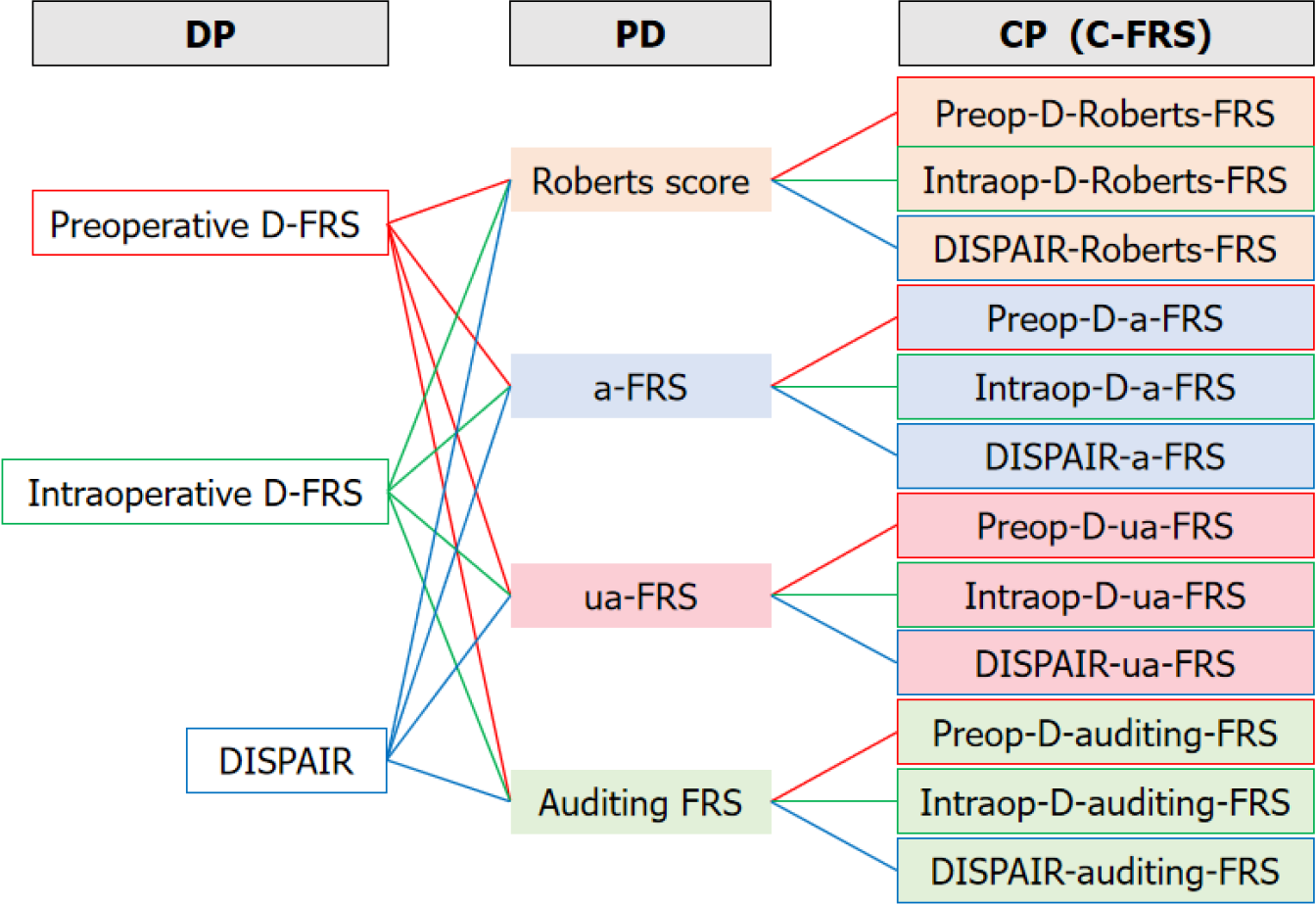
In light of these omissions, Yang et al[95] conducted a study that identified additional risk factors for POPF after CP. They found that BMI, pancreatic thickness, and MPD diameters at both ends of the lesion were independent predictors. Building upon the probability (P) of the union of two events [formula: P(PD∪DP) = P(PD) + P(DP) - P(PD∩DP)], they innovatively combined the existing FRS for PD and DP to develop specific FRS for CP (Figure 1). Consequently, they obtained a total of 12 central FRS (C-FRS) models. The predictive performance of these C-FRS models was generally acceptable, with AUC values ranging from 0.748 to 0.847. Particularly, the Preop-D-Roberts-FRS model emerged as a preoperative prediction model composed of four parameters: BMI, MPD diameters at both ends of the lesion, and pancreatic thickness. This model exhibited an AUC of 0.832 (95%CI, 0.751-0.895). Using this model, patients were categorized into three risk groups: low risk (< 25%), intermediate risk (25%-45%), and high risk (> 45%). The corresponding incidence of POPF in these risk groups was 0%, 30%, and 66.7%, respectively. Due to its ease of use and accurate preoperative prediction, the Preop-D-Roberts-FRS is recommended for clinical practice.
It is worth noting, however, that despite the promising predictive efficacy of these models, they were both derived from single-center retrospective studies in China and lacked valid external validation. Therefore, further prospective studies involving multiple centers and diverse populations are required for external validation and generalizability of the C-FRS models.
Due to the influence of multiple risk factors (pre, intra and postoperative) and the inherent complexity of pancreatectomy, there is still room to improve the accuracy of predicting POPF. It is unlikely that a single model will be possible for all circumstances. The pathophysiological mechanisms relevant to POPF are not fully understood, and with time other factors might be identified. Moreover, the lack of consensus on diagnostic thresholds, judging criteria, non-blinded assessment of predictors, different statistical methods and potential interactions among various risk factors contribute to inferior performance of prediction models. A comprehensive review of 52 prediction models revealed that the average adherence rate to the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines for POPF prediction models after PD was 65%[33]. Only 13 models surpassed this average TRIPOD adherence rate, indicating the importance of improving reporting standards and ensuring transparency in model development and evaluation.
Despite the development of many POPF prediction models, including some that are based on multicenter or nationwide cohort studies, over 80% of them lack external validation or demonstrate modest performance during subsequent validation[96], with AUCs ranging from 0.62 to 0.70[97]. One of the main reasons is their reliance on retrospective data, which may not encompass all the relevant factors contributing to POPF. Additionally, different models may incorporate varying variables and scoring systems, creating challenges when it comes to comparing and validating their performance. The lack of standardized and objective variables and scoring systems further hampers the universal applicability and reliability of these models. Another significant factor that has been overlooked in most models is the impact of individual surgeon experience and skill on the occurrence of POPF[98]. The surgical technique employed, decision-making process during operation, and proficiency of the surgeon can all have a substantial influence on the development of postoperative complications, including POPF. Ignoring these important aspects in the prediction models may contribute to their modest performance and decrease the translation of POPF prediction models into clinical practice.
Notably, while existing POPF prediction models show good performance in sample populations, their ability to predict and generalize may be limited when applied to ethnically diverse populations. Blunck et al[99] conducted an external validation study and found that although some models performed well for the overall population, their predictive value was limited for Black patients. Kang et al[100] validated three prediction models in a Korean population, yielding AUCs of 0.61-0.64, which were significantly lower than the original reports[15,34,37]. It is important to recognize that models developed in Western countries may not be directly applicable to Asian populations. In recent years, numerous prediction models have been developed in Asian countries such as China, Japan, and Korea. However, most of these models are from single-center retrospective studies and lack external validation. Consequently, there is a pressing need for large-scale prospective studies that integrate various factors to establish prediction models specifically suitable for respective populations.
The risk of POPF following pancreatectomy remains high, highlighting the need for a thorough understanding of pathophysiology and risk factors in order to reduce the risk where possible and improve surgical outcomes. Continuous improvement and refinement of POPF prediction models is necessary for better clinical utility. This iterative process allows for development of personalized treatment strategies to optimize patient outcomes. To overcome the limitations and challenges faced by current models, future efforts should consider collecting comprehensive and standardized data. Ongoing research is directed towards developing robust models that account for the multifactorial nature of POPF. By predicting the risk of POPF based on preoperative factors, clinicians will be able to adequately prepare patients before surgery, choose appropriate surgical procedure, and make timely decisions regarding whether the patient should be transferred to a specialized surgical center for further treatment. In addition to static variables, efforts should also be focused on developing models that incorporate dynamic variables. Intraoperative findings and early postoperative markers, which can provide valuable real-time information, could be integrated into the prediction models. By including these factors, the models can better adapt to individual patient characteristics and enhance their predictive power. The dynamic monitoring models can guide surgeons in determining the best course of postoperative treatment for patients affected by POPF.
Prospective studies involving large cohorts and multiple centers are of utmost importance to establish reliable prediction models. Advancements in imaging techniques hold great promise in refining prediction models. High-resolution imaging modalities can provide detailed information about pancreatic and abdominal features and help identify important predictive factors. The integration of machine learning algorithms and artificial intelligence systems are likely to enhance the predictive capabilities of these models. By continuously learning from real-time data and adapting to new information, it is anticipated these systems will provide more accurate predictions of POPF. Nevertheless, it is crucial to validate the developed models externally to ensure their generalizability across different clinical settings and patient populations.
| 1. | Yang F, Jin C, Hao S, Fu D. Drain Contamination after Distal Pancreatectomy: Incidence, Risk Factors, and Association with Postoperative Pancreatic Fistula. J Gastrointest Surg. 2019;23:2449-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 2. | McMillan MT, Christein JD, Callery MP, Behrman SW, Drebin JA, Hollis RH, Kent TS, Miller BC, Sprys MH, Watkins AA, Strasberg SM, Vollmer CM Jr. Comparing the burden of pancreatic fistulas after pancreatoduodenectomy and distal pancreatectomy. Surgery. 2016;159:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Yang F, Jin C, Li J, Di Y, Zhang J, Fu D. Clinical significance of drain fluid culture after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Chong E, Ratnayake B, Lee S, French JJ, Wilson C, Roberts KJ, Loveday BPT, Manas D, Windsor J, White S, Pandanaboyana S. Systematic review and meta-analysis of risk factors of postoperative pancreatic fistula after distal pancreatectomy in the era of 2016 International Study Group pancreatic fistula definition. HPB (Oxford). 2021;23:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, Bortolasi L, Guglielmi A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3557] [Article Influence: 169.4] [Reference Citation Analysis (35)] |
| 7. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 3225] [Article Influence: 358.3] [Reference Citation Analysis (35)] |
| 8. | Maggino L, Malleo G, Bassi C, Allegrini V, McMillan MT, Borin A, Chen B, Drebin JA, Ecker BL, Fraker DL, Lee MK, Paiella S, Roses RE, Salvia R, Vollmer CM Jr. Decoding Grade B Pancreatic Fistula: A Clinical and Economical Analysis and Subclassification Proposal. Ann Surg. 2019;269:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Bonsdorff A, Helanterä I, Tarvainen T, Sirén J, Kokkola A, Sallinen V. Prediction and consequences of postoperative pancreatitis after pancreaticoduodenectomy. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Chui JN, Yang AJ, Nahm CB, Connor S, Gill AJ, Samra JS, Mittal A. Clinical validation of the international study group of pancreatic surgery (ISGPS) definition for post-pancreatectomy acute pancreatitis. HPB (Oxford). 2023;25:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Bonsdorff A, Sallinen V. Prediction of postoperative pancreatic fistula and pancreatitis after pancreatoduodenectomy or distal pancreatectomy: A review. Scand J Surg. 2023;112:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 12. | Yang F, Jin C, Fu D. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;371:875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Wellner UF, Kayser G, Lapshyn H, Sick O, Makowiec F, Höppner J, Hopt UT, Keck T. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB (Oxford). 2010;12:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2011;35:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Roberts KJ, Hodson J, Mehrzad H, Marudanayagam R, Sutcliffe RP, Muiesan P, Isaac J, Bramhall SR, Mirza DF. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB (Oxford). 2014;16:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Zhang JY, Huang J, Zhao SY, Liu X, Xiong ZC, Yang ZY. Risk Factors and a New Prediction Model for Pancreatic Fistula After Pancreaticoduodenectomy. Risk Manag Healthc Policy. 2021;14:1897-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Yu J, Ren CY, Wang J, Cui W, Zhang JJ, Wang YJ. Establishment of risk prediction model of postoperative pancreatic fistula after pancreatoduodenectomy: 2016 edition of definition and grading system of pancreatic fistula: a single center experience with 223 cases. World J Surg Oncol. 2021;19:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Lucassen CJ, Groen JV, Aziz MH, Bastiaannet E, Bonsing BA, Leistra E, Shahbazi Feshtali S, Vahrmeijer AL, Droop A, Mieog JSD. Visceral adipose tissue is a better predictor than BMI in the alternative Fistula Risk Score in patients undergoing pancreatoduodenectomy. HPB (Oxford). 2022;24:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Lin Z, Tang B, Cai J, Wang X, Li C, Tian X, Yang Y. Preoperative prediction of clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy. Eur J Radiol. 2021;139:109693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Honselmann KC, Antoine C, Frohneberg L, Deichmann S, Bolm L, Braun R, Lapshyn H, Petrova E, Keck T, Wellner U, Bausch D. A simple nomogram for early postoperative risk prediction of clinically relevant pancreatic fistula after pancreatoduodenectomy. Langenbecks Arch Surg. 2021;406:2343-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Angrisani M, Sandini M, Cereda M, Paiella S, Capretti G, Nappo G, Roccamatisi L, Casciani F, Caccialanza R, Bassi C, Zerbi A, Gianotti L. Preoperative adiposity at bioimpedance vector analysis improves the ability of Fistula Risk Score (FRS) in predicting pancreatic fistula after pancreatoduodenectomy. Pancreatology. 2020;20:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Ingwersen EW, Stam WT, Meijs BJV, Roor J, Besselink MG, Groot Koerkamp B, de Hingh IHJT, van Santvoort HC, Stommel MWJ, Daams F; Dutch Pancreatic Cancer Group. Machine learning versus logistic regression for the prediction of complications after pancreatoduodenectomy. Surgery. 2023;174:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Xia W, Zhou Y, Lin Y, Yu M, Yin Z, Lu X, Hou B, Jian Z. A Predictive Risk Scoring System for Clinically Relevant Pancreatic Fistula After Pancreaticoduodenectomy. Med Sci Monit. 2018;24:5719-5728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Gu Z, Du Y, Wang P, Zheng X, He J, Wang C, Zhang J. Development and validation of a novel nomogram to predict postoperative pancreatic fistula after pancreatoduodenectomy using lasso-logistic regression: an international multi-institutional observational study. Int J Surg. 2023;109:4027-4040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Ecker BL, McMillan MT, Allegrini V, Bassi C, Beane JD, Beckman RM, Behrman SW, Dickson EJ, Callery MP, Christein JD, Drebin JA, Hollis RH, House MG, Jamieson NB, Javed AA, Kent TS, Kluger MD, Kowalsky SJ, Maggino L, Malleo G, Valero V 3rd, Velu LKP, Watkins AA, Wolfgang CL, Zureikat AH, Vollmer CM Jr. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 Resections From the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2019;269:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 26. | Pecorelli N, Palumbo D, Guarneri G, Gritti C, Prato F, Schiavo Lena M, Vallorani A, Partelli S, Crippa S, Doglioni C, De Cobelli F, Falconi M. Preoperative CT image analysis to improve risk stratification for clinically relevant pancreatic fistula after distal pancreatectomy. Br J Surg. 2023;110:891-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | De Pastena M, van Bodegraven EA, Mungroop TH, Vissers FL, Jones LR, Marchegiani G, Balduzzi A, Klompmaker S, Paiella S, Tavakoli Rad S, Groot Koerkamp B, van Eijck C, Busch OR, de Hingh I, Luyer M, Barnhill C, Seykora T, Maxwell T T, de Rooij T, Tuveri M, Malleo G, Esposito A, Landoni L, Casetti L, Alseidi A, Salvia R, Steyerberg EW, Abu Hilal M, Vollmer CM, Besselink MG, Bassi C. Distal Pancreatectomy Fistula Risk Score (D-FRS): Development and International Validation. Ann Surg. 2023;277:e1099-e1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 28. | Xu Y, Jin C, Fu D, Yang F. External validation of fistula risk scores for postoperative pancreatic fistula after distal pancreatectomy. Surgery. 2023;174:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Roberts KJ, Storey R, Hodson J, Smith AM, Morris-Stiff G. Pre-operative prediction of pancreatic fistula: is it possible? Pancreatology. 2013;13:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kalayarasan R, Himaja M, Ramesh A, Kokila K. Radiological parameters to predict pancreatic texture: Current evidence and future perspectives. World J Radiol. 2023;15:170-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 32. | Adamu M, Plodeck V, Adam C, Roehnert A, Welsch T, Weitz J, Distler M. Predicting postoperative pancreatic fistula in pancreatic head resections: which score fits all? Langenbecks Arch Surg. 2022;407:175-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Alhulaili ZM, Linnemann RJ, Dascau L, Pleijhuis RG, Klaase JM. A Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis analysis to evaluate the quality of reporting of postoperative pancreatic fistula prediction models after pancreatoduodenectomy: A systematic review. Surgery. 2023;174:684-691. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 981] [Article Influence: 70.1] [Reference Citation Analysis (2)] |
| 35. | Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, Vollmer CM Jr. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014;18:172-79; discussion 179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Shubert CR, Wagie AE, Farnell MB, Nagorney DM, Que FG, Reid Lombardo KM, Truty MJ, Smoot RL, Kendrick ML. Clinical Risk Score to Predict Pancreatic Fistula after Pancreatoduodenectomy: Independent External Validation for Open and Laparoscopic Approaches. J Am Coll Surg. 2015;221:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, de Pastena M, Klompmaker S, Marchegiani G, Ecker BL, van Dieren S, Bonsing B, Busch OR, van Dam RM, Erdmann J, van Eijck CH, Gerhards MF, van Goor H, van der Harst E, de Hingh IH, de Jong KP, Kazemier G, Luyer M, Shamali A, Barbaro S, Armstrong T, Takhar A, Hamady Z, Klaase J, Lips DJ, Molenaar IQ, Nieuwenhuijs VB, Rupert C, van Santvoort HC, Scheepers JJ, van der Schelling GP, Bassi C, Vollmer CM, Steyerberg EW, Abu Hilal M, Groot Koerkamp B, Besselink MG; Dutch Pancreatic Cancer Group. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and International External Validation. Ann Surg. 2019;269:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 308] [Article Influence: 44.0] [Reference Citation Analysis (1)] |
| 38. | Mungroop TH, Klompmaker S, Wellner UF, Steyerberg EW, Coratti A, D'Hondt M, de Pastena M, Dokmak S, Khatkov I, Saint-Marc O, Wittel U, Abu Hilal M, Fuks D, Poves I, Keck T, Boggi U, Besselink MG; European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS). Updated Alternative Fistula Risk Score (ua-FRS) to Include Minimally Invasive Pancreatoduodenectomy: Pan-European Validation. Ann Surg. 2021;273:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 39. | Shinde RS, Acharya R, Chaudhari VA, Bhandare MS, Mungroop TH, Klompmaker S, Besselink MG, Shrikhande SV. External validation and comparison of the original, alternative and updated-alternative fistula risk scores for the prediction of postoperative pancreatic fistula after pancreatoduodenectomy. Pancreatology. 2020;20:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Roberts KJ, Sutcliffe RP, Marudanayagam R, Hodson J, Isaac J, Muiesan P, Navarro A, Patel K, Jah A, Napetti S, Adair A, Lazaridis S, Prachalias A, Shingler G, Al-Sarireh B, Storey R, Smith AM, Shah N, Fusai G, Ahmed J, Abu Hilal M, Mirza DF. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy: A UK Multicenter Study. Ann Surg. 2015;261:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 41. | Perri G, Marchegiani G, Partelli S, Crippa S, Bianchi B, Cinelli L, Esposito A, Pecorelli N, Falconi M, Bassi C, Salvia R. Preoperative risk stratification of postoperative pancreatic fistula: A risk-tree predictive model for pancreatoduodenectomy. Surgery. 2021;170:1596-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Casadei R, Ricci C, Taffurelli G, D'Ambra M, Pacilio CA, Ingaldi C, Minni F. Are there preoperative factors related to a "soft pancreas" and are they predictive of pancreatic fistulas after pancreatic resection? Surg Today. 2015;45:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Zhang W, Cai W, He B, Xiang N, Fang C, Jia F. A radiomics-based formula for the preoperative prediction of postoperative pancreatic fistula in patients with pancreaticoduodenectomy. Cancer Manag Res. 2018;10:6469-6478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Shi Y, Gao F, Qi Y, Lu H, Ai F, Hou Y, Liu C, Xu Y, Zhang X, Cai X. Computed tomography-adjusted fistula risk score for predicting clinically relevant postoperative pancreatic fistula after pancreatoduodenectomy: Training and external validation of model upgrade. EBioMedicine. 2020;62:103096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Tang B, Lin Z, Ma Y, Zhang A, Liu W, Zhang J, Wang X, Tian X, Yang Y. A modified alternative fistula risk score (a-FRS) obtained from the computed tomography enhancement pattern of the pancreatic parenchyma predicts pancreatic fistula after pancreatoduodenectomy. HPB (Oxford). 2021;23:1759-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Lapshyn H, Petruch N, Thomaschewski M, Sondermann S, May K, Frohneberg L, Petrova E, Zemskov S, Honselmann KC, Braun R, Keck T, Wellner UF, Bolm L. A simple preoperative stratification tool predicting the risk of postoperative pancreatic fistula after pancreatoduodenectomy. Pancreatology. 2021;21:957-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Savin ML, Mihai F, Gheorghe L, Lupascu Ursulescu C, Negru D, Trofin AM, Zabara M, Nutu V, Cadar R, Blaj M, Lovin O, Crumpei F, Lupascu C. Proposal of a Preoperative CT-Based Score to Predict the Risk of Clinically Relevant Pancreatic Fistula after Cephalic Pancreatoduodenectomy. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 48. | Skawran SM, Kambakamba P, Baessler B, von Spiczak J, Kupka M, Müller PC, Moeckli B, Linecker M, Petrowsky H, Reiner CS. Can magnetic resonance imaging radiomics of the pancreas predict postoperative pancreatic fistula? Eur J Radiol. 2021;140:109733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Box EW, Deng L, Morgan DE, Xie R, Kirklin JK, Wang TN, Heslin MJ, Reddy S, Vickers S, Dudeia V, Rose JB. Preoperative anthropomorphic radiographic measurements can predict postoperative pancreatic fistula formation following pancreatoduodenectomy. Am J Surg. 2021;222:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Kolbinger FR, Lambrecht J, Leger S, Ittermann T, Speidel S, Weitz J, Hoffmann RT, Distler M, Kühn JP. The image-based preoperative fistula risk score (preFRS) predicts postoperative pancreatic fistula in patients undergoing pancreatic head resection. Sci Rep. 2022;12:4064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Maqueda González R, Di Martino M, Galán González I, Rodríguez Carnero P, Martín-Pérez E. Development of a prediction model of pancreatic fistula after duodenopancreatectomy and soft pancreas by assessing the preoperative image. Langenbecks Arch Surg. 2022;407:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 52. | Zou J, Xue X, Qin L. Development of a Nomogram to Predict Clinically Relevant Postoperative Pancreatic Fistula After Pancreaticoduodenectomy on the Basis of Visceral Fat Area and Magnetic Resonance Imaging. Ann Surg Oncol. 2023;30:7712-7719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Tian XF, Zhang L, Lou WH, Qiu YJ, Zuo D, Wang WP, Dong Y. Application of ultrasound shear wave elastography in pre-operative and quantitative prediction of clinically relevant post-operative pancreatic fistula after pancreatectomy: a prospective study for the investigation of risk evaluation model. Eur Radiol. 2023;33:7866-7876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Mu W, Liu C, Gao F, Qi Y, Lu H, Liu Z, Zhang X, Cai X, Ji RY, Hou Y, Tian J, Shi Y. Prediction of clinically relevant Pancreatico-enteric Anastomotic Fistulas after Pancreatoduodenectomy using deep learning of Preoperative Computed Tomography. Theranostics. 2020;10:9779-9788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Han IW, Cho K, Ryu Y, Shin SH, Heo JS, Choi DW, Chung MJ, Kwon OC, Cho BH. Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence. World J Gastroenterol. 2020;26:4453-4464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Giovinazzo F, Linneman R, Riva GVD, Greener D, Morano C, Patijn GA, Besselink MGH, Nieuwenhuijs VB, Abu Hilal M; Artificial Intelligence Pancreatic Fistula Group, de Hingh IH, Kazemier G, Festen S, de Jong KP, van Eijck CHJ, Scheepers JJG, van der Kolk M, den Dulk M, Bosscha K, Boerma D, van der Harst E, Armstrong T, Takhar A, Hamady Z. Clinical relevant pancreatic fistula after pancreatoduodenectomy: when negative amylase levels tell the truth. Updates Surg. 2021;73:1391-1397. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 57. | Shen Z, Chen H, Wang W, Xu W, Zhou Y, Weng Y, Xu Z, Deng X, Peng C, Lu X, Shen B. Machine learning algorithms as early diagnostic tools for pancreatic fistula following pancreaticoduodenectomy and guide drain removal: A retrospective cohort study. Int J Surg. 2022;102:106638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Long ZD, Lu C, Xia XG, Chen B, Xing ZX, Bie L, Zhou P, Ma ZL, Wang R. Personal predictive model based on systemic inflammation markers for estimation of postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastrointest Surg. 2022;14:963-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Capretti G, Bonifacio C, De Palma C, Nebbia M, Giannitto C, Cancian P, Laino ME, Balzarini L, Papanikolaou N, Savevski V, Zerbi A. A machine learning risk model based on preoperative computed tomography scan to predict postoperative outcomes after pancreatoduodenectomy. Updates Surg. 2022;74:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Chen KA, Berginski ME, Desai CS, Guillem JG, Stem J, Gomez SM, Kapadia MR. Differential Performance of Machine Learning Models in Prediction of Procedure-Specific Outcomes. J Gastrointest Surg. 2022;26:1732-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 61. | Zheng J, Lv X, Jiang L, Liu H, Zhao X. Development of a Pancreatic Fistula Prediction Model After Pancreaticoduodenectomy Based on a Decision Tree and Random Forest Algorithm. Am Surg. 2023;31348231158692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Verma A, Balian J, Hadaya J, Premji A, Shimizu T, Donahue T, Benharash P. Machine Learning Based Prediction of Postoperative Pancreatic Fistula Following Pancreaticoduodenectomy. Ann Surg. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Ashraf Ganjouei A, Romero-Hernandez F, Wang JJ, Casey M, Frye W, Hoffman D, Hirose K, Nakakura E, Corvera C, Maker AV, Kirkwood KS, Alseidi A, Adam MA. A Machine Learning Approach to Predict Postoperative Pancreatic Fistula After Pancreaticoduodenectomy Using Only Preoperatively Known Data. Ann Surg Oncol. 2023;30:7738-7747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 64. | Ingwersen EW, Bereska JI, Balduzzi A, Janssen BV, Besselink MG, Kazemier G, Marchegiani G, Malleo G, Marquering HA, Nio CY, de Robertis R, Salvia R, Steyerberg EW, Stoker J, Struik F, Verpalen IM, Daams F; Pancreatobiliary and Hepatic Artificial Intelligence Research (PHAIR) consortium. Radiomics preoperative-Fistula Risk Score (RAD-FRS) for pancreatoduodenectomy: development and external validation. BJS Open. 2023;7:zrad100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 65. | Schuh F, Mihaljevic AL, Probst P, Trudeau MT, Müller PC, Marchegiani G, Besselink MG, Uzunoglu F, Izbicki JR, Falconi M, Castillo CF, Adham M, Z'graggen K, Friess H, Werner J, Weitz J, Strobel O, Hackert T, Radenkovic D, Kelemen D, Wolfgang C, Miao YI, Shrikhande SV, Lillemoe KD, Dervenis C, Bassi C, Neoptolemos JP, Diener MK, Vollmer CM Jr, Büchler MW. A Simple Classification of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula: A classification of the International Study Group of Pancreatic Surgery. Ann Surg. 2023;277:e597-e608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 66. | Suurmeijer JA, Emmen AM, Bonsing BA, Busch OR, Daams F, van Eijck CH, van Dieren S, de Hingh IH, Mackay TM, Mieog JS, Molenaar IQ, Stommel MW, de Meijer VE, van Santvoort HC, Groot Koerkamp B, Besselink MG; Dutch Pancreatic Cancer Group. Nationwide validation of the ISGPS risk classification for postoperative pancreatic fistula after pancreatoduodenectomy: "Less is more". Surgery. 2023;173:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 67. | Kim JY, Park JS, Kim JK, Yoon DS. A model for predicting pancreatic leakage after pancreaticoduodenectomy based on the international study group of pancreatic surgery classification. Korean J Hepatobiliary Pancreat Surg. 2013;17:166-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Chen JY, Feng J, Wang XQ, Cai SW, Dong JH, Chen YL. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2015;21:5926-5933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Kantor O, Talamonti MS, Pitt HA, Vollmer CM, Riall TS, Hall BL, Wang CH, Baker MS. Using the NSQIP Pancreatic Demonstration Project to Derive a Modified Fistula Risk Score for Preoperative Risk Stratification in Patients Undergoing Pancreaticoduodenectomy. J Am Coll Surg. 2017;224:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 70. | Li Y, Zhou F, Zhu DM, Zhang ZX, Yang J, Yao J, Wei YJ, Xu YL, Li DC, Zhou J. Novel risk scoring system for prediction of pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2019;25:2650-2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, Hosoda K, Sakai H, Ikehara T, Soejima Y. A new fistula risk score using sarcopenic obesity and subcutaneous fat area for predicting postoperative pancreatic fistula after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2023;30:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 72. | Xingjun G, Feng Z, Meiwen Y, Jianxin J, Zheng H, Jun G, Tao H, Rui Z, Leida Z, Min W, Renyi Q; FACS. A score model based on pancreatic steatosis and fibrosis and pancreatic duct diameter to predict postoperative pancreatic fistula after Pancreatoduodenectomy. BMC Surg. 2019;19:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | You Y, Han IW, Choi DW, Heo JS, Ryu Y, Park DJ, Choi SH, Han S. Nomogram for predicting postoperative pancreatic fistula. HPB (Oxford). 2019;21:1436-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Guo CX, Shen YN, Zhang Q, Zhang XZ, Wang JL, Gao SL, Lou JY, Que RS, Ma T, Liang TB, Bai XL. Prediction of postoperative pancreatic fistula using a nomogram based on the updated definition. Ann Surg Treat Res. 2020;98:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Li B, Pu N, Chen Q, Mei Y, Wang D, Jin D, Wu W, Zhang L, Lou W. Comprehensive Diagnostic Nomogram for Predicting Clinically Relevant Postoperative Pancreatic Fistula After Pancreatoduodenectomy. Front Oncol. 2021;11:717087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Shen J, Guo F, Sun Y, Zhao J, Hu J, Ke Z, Zhang Y, Jin X, Wu H. Predictive nomogram for postoperative pancreatic fistula following pancreaticoduodenectomy: a retrospective study. BMC Cancer. 2021;21:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Liu R, Cai Y, Cai H, Lan Y, Meng L, Li Y, Peng B. Dynamic prediction for clinically relevant pancreatic fistula: a novel prediction model for laparoscopic pancreaticoduodenectomy. BMC Surg. 2021;21:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Huang XT, Huang CS, Liu C, Chen W, Cai JP, Cheng H, Jiang XX, Liang LJ, Yu XJ, Yin XY. Development and Validation of a New Nomogram for Predicting Clinically Relevant Postoperative Pancreatic Fistula After Pancreatoduodenectomy. World J Surg. 2021;45:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Guilbaud T, Garnier J, Girard E, Ewald J, Risse O, Moutardier V, Chirica M, Birnbaum DJ, Turrini O. Postoperative day 1 combination of serum C-reactive protein and drain amylase values predicts risks of clinically relevant pancreatic fistula. The "90-1000" score. Surgery. 2021;170:1508-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Suzuki S, Shimoda M, Shimazaki J, Oshiro Y, Nishida K, Shiihara M, Izumo W, Yamamoto M. Drain Lipase Levels and Decreased Rate of Drain Amylase Levels as Independent Predictors of Pancreatic Fistula with Nomogram After Pancreaticoduodenectomy. World J Surg. 2021;45:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Al Abbas AI, Borrebach JD, Pitt HA, Bellon J, Hogg ME, Zeh HJ 3rd, Zureikat AH. Development of a Novel Pancreatoduodenectomy-Specific Risk Calculator: an Analysis of 10,000 Patients. J Gastrointest Surg. 2021;25:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 82. | Yin J, Zhu Q, Zhang K, Gao W, Wu J, Lu Z, Jiang K, Miao Y. Development and validation of risk prediction nomogram for pancreatic fistula and risk-stratified strategy for drainage management after pancreaticoduodenectomy. Gland Surg. 2022;11:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 83. | Bannone E, Marchegiani G, Vollmer C, Perri G, Procida G, Corvino G, Peressotti S, Vacca PG, Salvia R, Bassi C. Postoperative Serum Hyperamylasemia Adds Sequential Value to the Fistula Risk Score in Predicting Pancreatic Fistula after Pancreatoduodenectomy. Ann Surg. 2023;278:e293-e301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 84. | Choi M, Lee JH, Roh YH, Kim H, Jang JY, Choi SH, Kang CM. Multidimensional Nomogram to Predict Postoperative Pancreatic Fistula after Minimally Invasive Pancreaticoduodenectomy. Ann Surg Oncol. 2023;30:5083-5090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | van Dongen JC, van Dam JL, Besselink MG, Bonsing BA, Bosscha K, Busch OR, van Dam RM, Festen S, van der Harst E, de Hingh IH, Kazemier G, Liem MSL, de Meijer VE, Mieog JSD, Molenaar IQ, Patijn GA, van Santvoort HC, Wijsman JH, Stommel MWJ, Wit F, De Wilde RF, van Eijck CHJ, Groot Koerkamp B; Dutch Pancreatic Cancer Group. Fistula Risk Score for Auditing Pancreatoduodenectomy: The Auditing-FRS. Ann Surg. 2023;278:e272-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Raza SS, Nutu A, Powell-Brett S, Marchetti A, Perri G, Carvalheiro Boteon A, Hodson J, Chatzizacharias N, Dasari BV, Isaac J, Abradelo M, Marudanayagam R, Mirza DF, Roberts JK, Marchegiani G, Salvia R, Sutcliffe RP. Early postoperative risk stratification in patients with pancreatic fistula after pancreaticoduodenectomy. Surgery. 2023;173:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Mohamed A, Nicolais L, Fitzgerald TL. Revisiting the Pancreatic Fistula Risk Score: Clinical Nomogram Accurately Assesses Risk. Am Surg. 2023;89:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 88. | Ahmad SB, Hodges JC, Nassour I, Casciani F, Lee KK, Paniccia A, Vollmer CM Jr, Zureikat AH. The risk of clinically-relevant pancreatic fistula after pancreaticoduodenectomy is better predicted by a postoperative trend in drain fluid amylase compared to day 1 values in isolation. Surgery. 2023;174:916-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Bonsdorff A, Ghorbani P, Helanterä I, Tarvainen T, Kontio T, Belfrage H, Sirén J, Kokkola A, Sparrelid E, Sallinen V. Development and external validation of DISPAIR fistula risk score for clinically relevant postoperative pancreatic fistula risk after distal pancreatectomy. Br J Surg. 2022;109:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 90. | Rollin N, Cassese G, Pineton DE Chambrun G, Serrand C, Navarro F, Blanc P, Panaro F, Valats JC. An easy-to-use score to predict clinically relevant postoperative pancreatic fistula after distal pancreatectomy. Minerva Surg. 2022;77:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Nassour I, AlMasri S, Hodges JC, Hughes SJ, Zureikat A, Paniccia A. Novel Calculator to Estimate the Risk of Clinically Relevant Postoperative Pancreatic Fistula Following Distal Pancreatectomy. J Gastrointest Surg. 2022;26:1436-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | He C, Zhang Y, Li L, Zhao M, Wang C, Tang Y. Risk factor analysis and prediction of postoperative clinically relevant pancreatic fistula after distal pancreatectomy. BMC Surg. 2023;23:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 93. | Yang F, Jin C, Di Y, He H, Hao S, Yao L, Li J, Fu D. Central pancreatectomy with external drainage of monolayer pancreaticojejunostomy for prevention of postoperative pancreatic fistula: A retrospective cohort study. Int J Surg. 2018;51:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Ouyang L, Liu RD, Ren YW, Nie G, He TL, Li G, Zhou YQ, Huang ZP, Zhang YJ, Hu XG, Jin G. Nomogram predicts CR-POPF in open central pancreatectomy patients with benign or low-grade malignant pancreatic neoplasms. Front Oncol. 2022;12:1030080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Yang F, Xu Y, Jin C, Windsor JA, Fu D. Predicting pancreatic fistula after central pancreatectomy using current fistula risk scores for pancreaticoduodenectomy and distal pancreatectomy. Pancreatology. 2023;23:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | PARANOIA Study Group; Writing committee, Pande R, Halle-Smith JM, Phelan L, Thorne T, Panikkar M, Hodson J, Roberts KJ; Steering committee, Arshad A, Connor S, Conlon KC, Dickson EJ, Giovinazzo F, Harrison E, de Liguori Carino N, Hore T, Knight SR, Loveday B, Magill L, Mirza D, Pandanaboyana S, Perry RJ, Pinkney T, Siriwardena AK, Satoi S, Skipworth J, Stättner S, Sutcliffe RP, Tingstedt B. External validation of postoperative pancreatic fistula prediction scores in pancreatoduodenectomy: a systematic review and meta-analysis. HPB (Oxford). 2022;24:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 97. | Schouten TJ, Henry AC, Smits FJ, Besselink MG, Bonsing BA, Bosscha K, Busch OR, van Dam RM, van Eijck CH, Festen S, Groot Koerkamp B, van der Harst E, de Hingh IHJT, Kazemier G, Liem MSL, de Meijer VE, Patijn GA, Roos D, Schreinemakers JMJ, Stommel MWJ, Wit F, Daamen LA, Molenaar IQ, van Santvoort HC; Dutch Pancreatic Cancer Group. Risk Models for Developing Pancreatic Fistula After Pancreatoduodenectomy: Validation in a Nationwide Prospective Cohort. Ann Surg. 2023;278:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 98. | Casciani F, Trudeau MT, Asbun HJ, Ball CG, Bassi C, Behrman SW, Berger AC, Bloomston MP, Callery MP, Christein JD, Falconi M, Fernandez-Del Castillo C, Dillhoff ME, Dickson EJ, Dixon E, Fisher WE, House MG, Hughes SJ, Kent TS, Malleo G, Partelli S, Salem RR, Stauffer JA, Wolfgang CL, Zureikat AH, Vollmer CM Jr; Pancreas Fistula Study Group. Surgeon experience contributes to improved outcomes in pancreatoduodenectomies at high risk for fistula development. Surgery. 2021;169:708-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 99. | Blunck CK, Vickers SM, Wang TN, Dudeja V, Reddy S, Rose JB. External validation of four Pancreatic Fistula Risk Score models in the Deep South US: Do racial disparities affect pancreatic fistula prediction? Am J Surg. 2022;224:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 100. | Kang JS, Park T, Han Y, Lee S, Kim JR, Kim H, Kwon W, Kim SW, Heo JS, Choi SH, Choi DW, Kim SC, Hong TH, Yoon DS, Park JS, Park SJ, Han SS, Choi SB, Kim JS, Lim CS, Jang JY. Clinical validation of scoring systems of postoperative pancreatic fistula after pancreatoduodenectomy: applicability to Eastern cohorts? Hepatobiliary Surg Nutr. 2019;8:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kelemen D, Hungary S-Editor: Yan JP L-Editor: A P-Editor: Zhao YQ