Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1295
Peer-review started: November 17, 2023
First decision: December 14, 2023
Revised: December 25, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 14, 2024
Processing time: 117 Days and 23.5 Hours
Hepatitis B virus (HBV) reactivation is a clinically significant challenge in disease management. This review explores the immunological mechanisms underlying HBV reactivation, emphasizing disease progression and management. It delves into host immune responses and reactivation’s delicate balance, spanning innate and adaptive immunity. Viral factors’ disruption of this balance, as are interac
Core Tip: Hepatitis B virus (HBV) reactivation poses a substantial clinical challenge, demanding a nuanced understanding of immunological mechanisms for effective management. This comprehensive review navigates the intricate landscape of HBV reactivation, spotlighting the delicate balance between host immune responses and viral factors. Emphasis is placed on the roles of T cells, natural killer cells, and antigen-presenting cells in disease progression, alongside the repercussions on severity, hepatic flares, liver fibrosis, and hepatocellular carcinoma. Critical analysis of management strategies, spanning anti-viral and immunomodulatory approaches, informs evidence-based practices. Prophylactic anti-viral therapy’s role during immunosuppression and the potential of innovative immunotherapies are explored, contributing significantly to informed disease management and improved patient outcomes.
- Citation: Ma H, Yan QZ, Ma JR, Li DF, Yang JL. Overview of the immunological mechanisms in hepatitis B virus reactivation: Implications for disease progression and management strategies. World J Gastroenterol 2024; 30(10): 1295-1312
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1295.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1295
Hepatitis B virus (HBV) infection is a significant global health challenge, affecting two billion individuals worldwide. It is a major cause of chronic liver diseases, including Cirrhosis and hepatocellular carcinoma, and 820000 individuals succumbed to diseases associated with HBV in 2019. In 2016, it was estimated that over 86 million individuals in China were afflicted with chronic HBV infection, accounting for approximately 6.1% of the total population[1]. HBV is primarily transmitted through contact with infected blood bodily fluids or from mother to child during childbirth. The infection can lead to a broad spectrum of outcomes, ranging from asymptomatic carrier states to acute hepatitis, chronic hepatitis, and even death in severe cases[2]. Chronic HBV infection poses a particularly concerning scenario, as it can lead to long-term complications such as primarily targeting the liver, leading to inflammation, liver fibrosis, cirrhosis, and an increased risk of liver cancer[3]. Developing effective prevention strategies, including vaccination and anti-viral treatments, has significantly contributed to reducing the burden of HBV infection, although challenges remain, especially in regions with high prevalence rates. While advancements in anti-viral therapies have improved outcomes for many patients, the virus can persist in a latent state within the body, posing the risk of reactivation[4,5].
HBV reactivation is characterized by the sudden reappearance or upsurge of HBV DNA in the bloodstream of individuals who had previously had inactive or resolved HBV infection. The reactivation, also known as flare or exacerbation, of hepatitis B is distinguished by a sudden increase in serum alanine aminotransferase (ALT) levels. Typically, the term “it” denotes a sudden elevation in serum ALT levels that surpasses 5-10 times the upper limit of normal or exceeds 3 times the initial baseline level. Mutations in the HBV genome, immunosuppressive therapy, and viral or drug-induced injury are common reactivation causes. The leading factor contributing to acute liver injury in individuals with chronic hepatitis B (CHB) in Eastern areas has been identified. It has been predicted that around 250 million individuals are affected by CHB[6-8]. The leading cause of HBV reactivation is an imbalance between the host’s immune response and virus replication. This phenomenon is of particular concern in individuals undergoing immunosuppressive therapies, such as chemotherapy or transplantation, chronic inflammatory diseases, and those with compromised immune systems[9,10].
According to prior research, HBV reactivation after chemotherapy has been shown in multiple studies, with a median of 4 months (range, 1-9 months) separating the start of reactivation from the end of chemotherapy. In patients with chronic HBV who have positive serum hepatitis B surface antigen (HBsAg), the rate of HBV reactivation ranges from 24-88%, while in those with positive HBcAb, it ranges from 3%-22%. There is a 23%-71% mortality rate in cases of HBV reactivation[11,12]. The rate of HBV reactivation in cancer patients with a history of HBV infection following chemotherapy or immunosuppressive medication was found to be 25%, ranging from 4% to 68%. Around 65% of these individuals experienced disease progression, potentially leading to hepatic failure, necessitating either liver transplantation or death[13]. A new research study conducted in Egypt investigated the occurrence of HBV reactivation in patients who were positive for HBsAg and undergoing treatment with direct-acting anti-virals for the hepatitis C virus. The study revealed that 28.6% of the patients experienced HBV reactivation, although only 10.0% exhibited liver hepatitis[14,15].
Therefore, understanding the immunological mechanisms underlying HBV reactivation is crucial for developing effective management strategies to mitigate its potential impact on disease progression and patient outcomes. The immune system plays a central role in controlling HBV infection and contributing to the potential for reactivation[16]. Dissecting these mechanisms provides insights into the delicate balance between viral suppression and immune responses, which, when disrupted, can lead to HBV reactivation and its associated complications. By unraveling the intricate interplay between viral factors, immune cells, and signaling pathways, researchers and clinicians understand how reactivation occurs and its implications for disease advancement[17]. Furthermore, insights into the immunological underpinnings of HBV reactivation offer opportunities to develop targeted and personalized management strategies. Leveraging this understanding, healthcare professionals can tailor therapeutic interventions to bolster the immune response and prevent reactivation in vulnerable populations[18]. This knowledge can guide the design of prophylactic anti-viral therapies for individuals undergoing immunosuppressive treatments, reducing the risk of HBV reactivation and its potential impact on liver function. Additionally, insights into immunomodulatory mechanisms can inform the exploration of novel therapeutic approaches that restore immune control over HBV, potentially leading to innovative immunotherapies[19]. Thus, comprehending the immunological intricacies of HBV reactivation not only enhances our understanding of disease progression but also empowers the medical community to devise more effective and targeted strategies for its management[20,21].
This review explores the intricate immunological mechanisms underlying HBV reactivation and its profound implications for disease progression and management. With a primary focus on immunological aspects, the review delves into the dynamic interactions between host immune responses and HBV reactivation, shedding light on the intricate processes that govern this phenomenon. By dissecting the roles of various immune cells, cytokine networks, and signaling pathways, the review seeks to elucidate the underlying mechanisms contributing to HBV reactivation, providing a foun
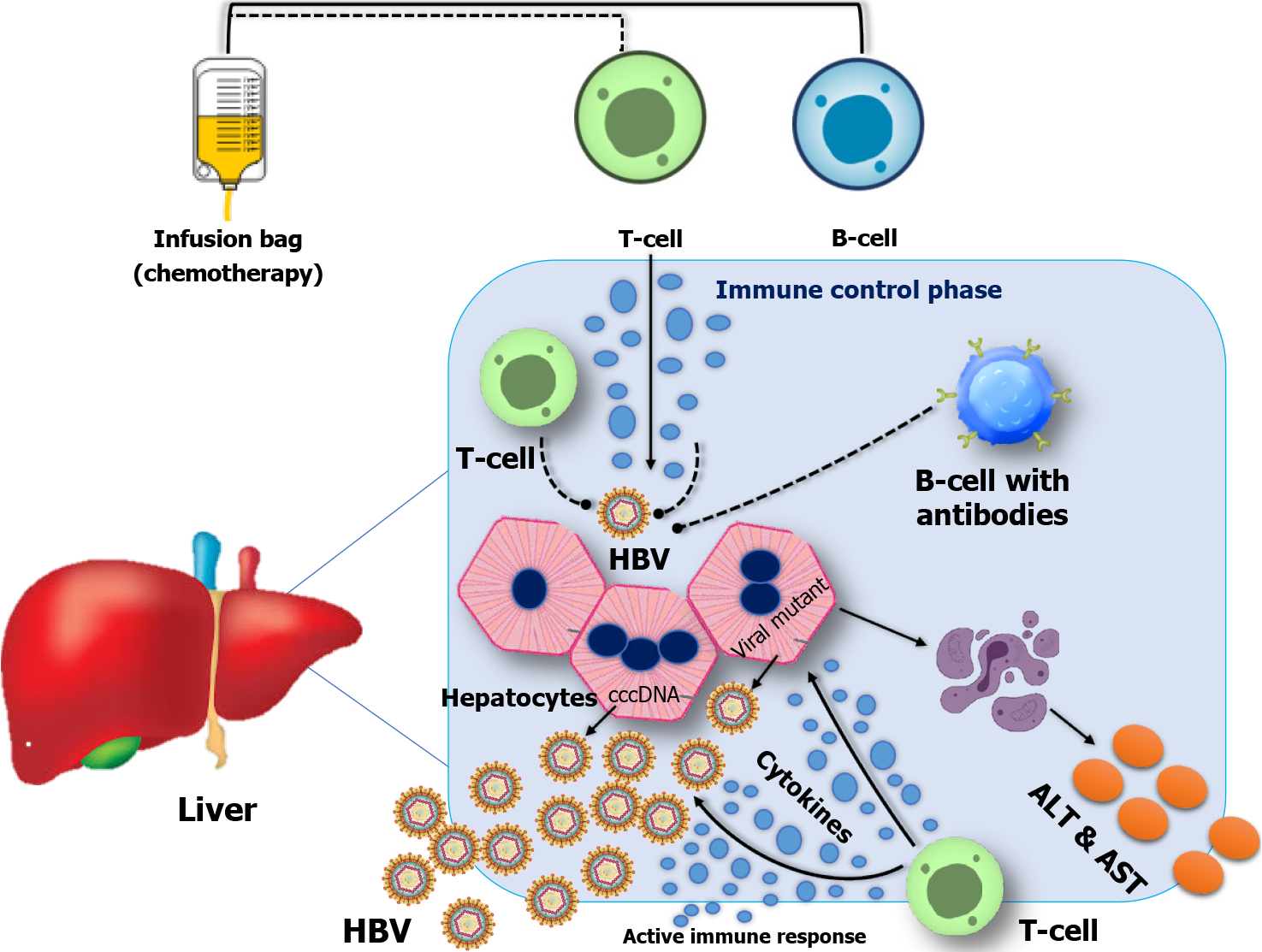
HBV reactivation is characterized by the sudden return or increase in the concentration of HBV DNA in the serum of individuals who have previously experienced resolved or dormant chronic HBV infection. The administration of either anti-cancer drugs, immunosuppressive medicines, or biological therapy can initiate this occurrence. CD8+ T cell exhaustion resulting from the overexpression of PD-1 is observed in persistent viral infections, such as chronic hepatitis B[22,23] (Figure 1).
The innate immune responses serve as the initial barrier of immunological protection against viral, bacterial, and tumorous pathogens. Soluble factors such as complement components, chemokines, and cytokines constitute integral components of the innate immune system. Granulocytes, dendritic cells (DCs), macrophages, mast cells, and natural killer (NK) cells play crucial roles as effector cells in various biological processes. The initiation of an effective innate immune response typically occurs when pathogen-associated molecular pattern (PAMP) molecules interact with pattern recognition receptors (PRRs)[24,25]. This interaction triggers the production of chemokines and pro-inflammatory cytokines and the activation of innate immune cells. Consequently, this immune response eliminates viral pathogens[26]. Immune system dysfunction plays a pivotal role in HBV reactivation, with impaired host immune responses against HBV-infected cells as a central mechanism.
Interferons type I and HBV reactivation: Interferons type I (IFN-1) plays a crucial role in orchestrating the immune response during the reactivation of HBV. When viral components are recognized by PRRs such as Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors, and melanoma differentiation-associated protein 5, in liver cells known as hepatocytes and hepatic DCs, it leads to a reduced response to PAMPs and a compromised production of IFN-I, which include IFN-alpha and IFN-beta[27,28]. According to a report by Faure-Dupuy and Baumert[29], it has been found that HBV infection leads to an increase in the expression of microRNA-146a (miR-146a) in liver cells. This increase in miR-146a subsequently inhibits the expression of RIG-I-like receptors. According to Faure-Dupuy and Baumert[29], IFN-I production is suppressed.
Furthermore, Wang et al[30] revealed that HBsAg, hepatitis B e antigen (HBeAg), hepatitis B x, and HBV virions possess the capability to impede the synthesis of IFN-β, hence reducing mitochondrial anti-viral signaling (MAVS) and disrupt the link between MAVS and RIG-I. The study conducted by Yang et al[31] demonstrates that IFN-I can directly reduce HBV infection by activating IFN-stimulated genes upon binding to the IFN receptor. This activation subsequently impedes viral replication. Nevertheless, HBV can substantially impair the signal transduction triggered by IFN-I and attenuate the immunological responses facilitated by IFN-I[31].
Moreover, the work conducted by He et al[32] demonstrates that the regulatory effects of IFN-α on HBV covalently closed circular DNA (cccDNA) can be linked to its capacity to disrupt the methylation and succinylation of histone H3 lysine residues, which is mediated by the general control non-depressible (GCN5) enzyme. As mentioned above, the disruption finally results in eradicating HBV cccDNA. The effect of IFN-α on the regulation of HBV cccDNA can be attributed to its ability to disrupt the methylation succinylation process of histone H3 Lysine, which is facilitated by GCN5[32]. According to Wei et al[33], the researchers have noticed that MX dynamin-like GTPase 2 exhibits an inhibitory effect on converting relaxed circular DNA into cccDNA of the HBV. This inhibitory effect indirectly leads to a decrease in the quantity of cccDNA. Bratulic et al[34] demonstrated that IFN-α can induce the synthesis of soluble constituents that can successfully rival HBV in their affinity for heparin glycosaminoglycans, hence hindering the HBV infection process. This finally results in the augmentation of adaptive immune responses. However, chronic HBV infection can result in the impairment of IFN-I signaling. This impairment allows the virus to evade the host’s immune defenses and contributes to reactivation.
DCs and HBV reactivation: DCs play a pivotal role in shaping the immune response during HBV reactivation by bridging the gap between innate and adaptive immunity[35]. Previous studies conducted by Soto et al[36] have shown compelling evidence suggesting that persons diagnosed with CHB demonstrate a significant reduction in the quantity of peripheral blood DCs in comparison to individuals without the condition. A decline follows the decrease in DCs’ functional capacity, directly leading to the impairment of HBV-specific T-cell activity. As professional antigen-presenting cells, DCs are essential for initiating and directing immune responses upon encountering viral antigens[37,38]. During HBV reactivation, infected hepatocytes release viral antigens captured by DCs, which then migrate to secondary lymphoid tissues to present these antigens to T cells. Feola et al[39] revealed that DCs activate CD8+ cytotoxic T lymphocytes (CTLs) by presenting HBV-derived peptides in the context of primary histocompatibility complex class I (MHC-I) molecules. This primes CTLs to recognize and eliminate HBV-infected cells, contributing to viral control.
However, DCs can exhibit functional impairment in chronic HBV infection, including reduced antigen presentation capacity and altered cytokine production (Table 1). These deficits can hinder the activation of effective anti-viral T-cell responses, potentially leading to viral persistence and reactivation[40]. Tang et al[41] conducted an in vitro investigation wherein DCs obtained from healthy individuals were cultivated with HBV DNA. The study revealed decreased functionality of DCs when exposed to HBV DNA. However, the addition of lamivudine resulted in a reduction of HBV DNA levels and a subsequent recovery of DC function. These findings show a direct impact of HBV on the functionality of DCs[41].
| Immune cells | Mechanism of impairment | Outcomes | Ref. |
| Innate immune cell responses | |||
| Natural killer cells | Downregulation of activating receptors (NKp30, NKp46, and CD56dim), inhibitory cytokine production (IFN- and TNF-) | Reduced viral clearance, increased reactivation risk | [62,64] |
| Dendritic cells | Reduced antigen presentation (CD8+ CTLs), impaired cytokine (IL-12 and IL-18) production | Impaired antiviral response, increased viral persistence | [40,65] |
| Macrophages | Dysregulated cytokine secretion (IL-1β, IL-6, and TNF-α) | Altered immune balance, increased inflammation | [49] |
| Neutrophils | Impaired chemotaxis, reduced phagocytosis | Ineffective pathogen clearance, prolonged viremia | [55] |
| Adaptive immune cell responses | |||
| CD8+ T cells | Exhaustion (CD8+ T cells), reduced cytotoxic activity | Inadequate viral control, viral persistence | [66] |
| CD4+ T cells | Decreased help for B and CD8+ T cells | Impaired adaptive immune response | [67] |
| B cells | Altered antibody production | Reduced neutralizing antibodies, prolonged viremia | [68] |
| Regulatory T cells | Dysfunction, reduced suppression | Dysregulated immune response, increased inflammation | [69] |
Furthermore, the role of DCs in HBV reactivation extends beyond antigen presentation. DCs secrete cytokines and chemokines that modulate the immune response’s direction and magnitude. For instance, DCs release interleukin-12 (IL-12) and IL-18, promoting the differentiation of T helper 1 (Th1) cells that enhance anti-viral immune responses[42,43]. However, the immunosuppressive cytokine IL-10 produced by DCs can inhibit immune activation and lead to immune tolerance, facilitating viral persistence. Additionally, DCs can interact with other immune cells, such as NK cells and regulatory T cells (Tregs), influencing their activity and contributing to the delicate balance between immune control and tolerance[44,45]. Further research is necessary to investigate the mechanisms underlying DC impairment resulting from HBV reactivation.
Reactivation of HBV in macrophages and monocytes: Macrophages and monocytes, key innate immune system components, play intricate and interrelated roles in HBV reactivation. These versatile phagocytic cells are pivotal in recognizing, engulfing, and eliminating viral particles and infected cells. Monocytes, circulating precursors of macrophages, are recruited to sites of infection, where they differentiate into tissue-resident macrophages specialized in responding to viral threats[46]. Upon encountering HBV antigens, monocytes and macrophages initiate a cascade of immune responses. Macrophages release pro-inflammatory cytokines, such as IL-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α), creating an inflammatory microenvironment that attracts and activates other immune cells[47,48] (Table 1). Macrophages play a significant role in antigen presentation, wherein they present viral peptides to adaptive immune cells, specifically CD4+ and CD8+ T cells, to elicit targeted immune responses[49].
Macrophages help to contain viral replication and reduce viral load by phagocytosing infected hepatocytes. Monocytes and macrophages are also involved in the phagocytosis and clearance of viral particles and infected hepatocytes, contributing to viral containment. By phagocytosing infected hepatocytes, macrophages help to contain viral replication and reduce viral load[50,51]. The dynamic interplay between macrophages and monocytes in HBV reactivation extends beyond their direct anti-viral functions. HBV has evolved strategies to modulate the polarization and activity of these immune cells. While macrophages exhibit plasticity between M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes, chronic HBV infection may promote an immunosuppressive M2-like phenotype, which could contribute to impaired viral clearance and immune evasion[52]. Monocytes and macrophages are also key players in initiating and maintaining inflammation-induced tissue damage. Their interactions with hepatic stellate cells (HSCs) and other liver-resident cells can contribute to fibrosis, a hallmark of chronic HBV infection[53,54].
Neutrophils and HBV reactivation: Neutrophils, prominent members of the innate immune system, play a complex and multifaceted role in HBV reactivation. These rapid-response immune cells are attracted to sites of infection in response to chemotactic signals and are involved in both antimicrobial and inflammatory functions (Table 1). Neutrophils release antimicrobial proteins and reactive oxygen species, killing viral particles and infected hepatocytes[55]. However, excessive neutrophil activation can lead to tissue damage and inflammation, potentially exacerbating liver injury. Neutrophils also contribute to immune surveillance by forming neutrophil extracellular traps (NETs), web-like structures composed of DNA and antimicrobial proteins, which can capture and neutralize pathogens, including HBV[56]. For example, Maronek and Gardlik[57] explained that patients diagnosed with liver cirrhosis demonstrate a reduced capacity of neutrophils to discharge NETs. This impairment concomitates a decline in CD69 and CD80 expression.
Moreover, the study conducted by Sarkar et al[58] showed that antigens linked to HBV, namely HBeAg and hepatitis B core antigen (HBcAg), exhibit the ability to diminish the release of NETs through the inhibition of p38 mitogen-activated protein kinase (MAPK) and ERK activation, as well as autophagy. Utilizing this mechanism facilitates the evasion of the immune response by the HBV, therefore enhancing its reproduction and ensuring its prolonged survival[58]. The intricate balance between neutrophils’ beneficial anti-viral effects and their potential to induce tissue damage underscores their role in the delicate immune response during HBV reactivation, highlighting the need for a comprehensive understanding to inform potential therapeutic strategies that harness their anti-viral potential while minimizing detrimental effects.
NK cells and HBV reactivation: Impairment of NK cells has been recognized as a significant factor in the reactivation of HBV infection. NK cells play a crucial role in the body’s defense against viral infections and tumors, primarily by identifying and eliminating infected or malignant cells. However, during HBV reactivation, the activity and function of NK cells can be compromised, leading to inadequate immune responses and allowing the virus to replicate and increase. This impairment may result from various factors, including HBV-induced changes in the expression of activating receptors on NK cells and the production of inhibitory cytokines that dampen NK cell function[59,60]. Poor prognosis and survival in individuals with liver cancer have been associated with the persistence of CHB infection and the development of hepatocellular carcinoma (HCC). As Chu et al[61] reported, hepatic NK cell activity is reduced, and NK cell receptors are expressed abnormally. According to the findings of Zhang et al[62], the levels of activating receptors such as NKp30, NKp46, and NK group 2 member D, as well as cytokines such as IFN- and TNF-, are significantly decreased in those who have been diagnosed with chronic hepatitis B (Table 1)[63-69]. These receptors include NKG2A, IL-10, T cell immunoglobulin, and mucin domain-containing protein 3 (Tim-3)[62,63].
Furthermore, in the context of CHB infection, Marotel et al[70] observed a correlation between the poor functionality of NK cells and the reduced expression of CD122. CD122 is the shared β chain of the IL-2 receptor found on CD56dim NK cells. You et al[71] explained. The precise effects of circulating antigens associated with HBV, such as Hepatitis B surface antigen (HBsAg) and HBeAg, on suppressing NK cells remain uncertain. Researchers have observed the limitation of NK cell cytotoxicity and cytokine production by HBsAg and HBeAg. This limitation occurs through interference with the activation of STAT1, nuclear factor-kappa B (NF-κB), and p38 MAPK[71]. Cao et al[72] showed that the reduction in STAT3 expression induced by HBsAg is associated with degranulation and cytokine production in people diagnosed with HBeAg-negative chronic hepatitis B. Monocytes treated with HBsAg can transform NK cells into regulatory NK cells that produce IL-10. This transformation is facilitated by signals from PD-L1 and MHC class I and E, and it plays a role in the persistence of chronic hepatitis B infection[72]. The study by Kar et al[73] revealed that exosomes derived from patients with CHB have a role in the transportation of HBV nucleic acids to NK cells. This process suppresses NK cell activity during HBV infection, achieved through inhibiting several signaling pathways, including RIG-I, NF-κB, and p38 MAPK (Figure 1).
T-lymphocytes and HBV reactivation: T-lymphocytes (T cells), central players in adaptive immunity, profoundly influence the dynamics of HBV reactivation through their multifaceted roles in viral clearance and immune regulation. HBV-infected hepatocytes are easily identifiable and eliminated by CD8+ CTLs. CTLs directly induce apoptosis in infected cells by recognizing viral peptides displayed on MHC-I molecules[74,75]. During acute HBV infection and reactivation, robust CTL responses are associated with viral control and recovery. However, chronic HBV infection can lead to T-cell exhaustion and functional impairment (Table 1), allowing the virus to persist[76]. According to Jin and Bi[66], a microarray study shows that HBV significantly increases the expression of Bcl-2-like protein 11 in HBV-specific CD8+ T cells, pointing to a critical mechanism for CD8+ T cell depletion during CHB infection. Inhibitory receptors such as PD-1, CTLA-4, CD244 (2B4), Tim-3, and lymphocyte activation gene 3 are present on exhausted HBV-specific CD8+ T cells, and these receptors closely resemble the transcriptional patterns of CD8+ T cells[66,77].
Furthermore, Tregs, a subset of CD4+ T cells, play a role in maintaining immune tolerance and preventing excessive inflammation. While their role is critical for immune homeostasis, the expansion of Tregs during chronic HBV infection can hinder effective anti-viral immune responses and contribute to viral persistence (Table 1). CD4+ T helper (Th) cells also coordinate immune responses[37,38,78]. Previous research shows that HBV-related antigens, namely HBcAg and HBsAg, can increase CD4+ T cell production of inhibitory molecules. Chuang et al[67] found that HBcAg enhanced PD-1 expression on CD4+ T cells, disrupting their function via JNK, ERK, and PI3K/AKT signaling pathways[79]. Moreover, the expression of human protein inhibitors of activated STAT1 (dependent on ERK and p38 MAPK signaling pathways) increased in CHB patients, making standard therapies ineffectual. CD4+ T cells develop into Foxp3+ Treg cells, which release inhibitory cytokines IL-10 and TGF-β, leading to a decline in HBV-specific CD8+ T cells[80]. According to Churiso et al[69], CD4+ T cells directly influence HBV clearance by regulating CD8+ T cells. IFN- is secreted by Th1 cells to activate macrophages and CTLs, boosting anti-viral activity. Th2 and Th17 cells may promote inflammation (Table 1), contributing to liver damage. Furthermore, the balance between different subsets of T cells shapes the immune response during HBV reactivation.
B-lymphocytes and HBV reactivation: B-lymphocytes (B cells), prominent components of the adaptive immune system, contribute to the complex immunological landscape of HBV reactivation through their roles in antibody production, immune regulation, and memory formation. Upon encountering viral antigens, B cells undergo activation, leading to the differentiation of plasma cells that secrete antibodies specific to HBV components. These antibodies, including anti-HBs and anti-HBc, can neutralize viral particles and contribute to viral clearance[81]. A prior study indicated a decrease in HBsAg-specific B cells in CHB patients. CHB patients also had deficient anti-HB production (Table 1). It was found that HBsAg-specific B cells in CHB patients had a CD21-CD27-atypical memory B cell (atMBC) phenotype with high levels of inhibitory receptors like PD-1, BTLA, and CD22[82,83]. AtMBCs in CHB patients have decreased survival, proliferation, and cytokine production and cannot develop into antibody-producing plasma cells, resulting in reduced humoral immune responses. Vanwolleghem et al[68] discovered that HBcAg binding to B cells leads to increased expression of inhibitory receptors FcRL4 and FcRL5, dysfunctional phenotypes, and suppressed B cell proliferation (Table 1) and activation via B cell receptor and TLR signaling[68,84].
Furthermore, Ma et al[85] revealed that B cells also play a role in immune regulation and memory formation during HBV reactivation. Regulatory B cells (Bregs) have immunosuppressive functions and can modulate immune responses to prevent excessive inflammation. Bregs produce anti-inflammatory cytokines and interact with Tregs, influencing the balance between pro-inflammatory and anti-inflammatory immune pathways. Likewise, HBeAg can stimulate the activation of B cells by promoting the production of B-cell activating factors through the secretion of IL-6 and IFN-γ. It is indicated that IL-6, in turn, can play a role in fighting against HBV by inducing the decay of cccDNA, reducing HBV transcription, and downregulating the NTPC receptor[86].
Moreover, B cells contribute to the formation of memory responses. Lam et al[87] reported that memory B cells generated during acute HBV infection could provide rapid and robust antibody responses upon re-exposure to the virus, contributing to subsequent immune control. However, chronic HBV infection can lead to B cell dysfunction, impaired antibody responses, and immune tolerance.
The escalation of hepatic inflammation is a crucial factor that highlights the possibility of severe consequences, such as fulminant hepatitis (FH). The reactivation of HBV elicits a renewed phase of viral replication and the subsequent release of viral antigens, hence inducing an intensified immune response. The activation of the immune system leads to the migration of immune cells, including macrophages, neutrophils, and T cells, into the liver[88,89]. According to Shi et al[90], it was found that liver injury occurring during a spontaneous exacerbation is likely influenced by an increased population of T cells that exhibit reactivity towards HBeAg and HBcAg, which demonstrate cross-reactivity at the T-cell level. These cells then release a wide range of pro-inflammatory cytokines and chemokines. Kawagishi et al[91] reported study showed elevated levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6.
Furthermore, Liu et al[93] have shown that chemokines such as CCL2 (MCP-1), CXCL8 (IL-8), and CXCL10 (IP-10) are consistently found in liver inflammation associated with HBV reactivation. These chemokines play crucial roles in attracting immune cells to the liver parenchyma. The resultant inflammatory milieu exacerbates hepatocellular damage and liver inflammation, leading to potentially severe clinical outcomes[92,93].
Moreover, the heightened liver inflammation that occurs after the reactivation of the HBV is of concern due to its correlation with FH. FH is a term used to describe the development of hepatic encephalopathy, which characterizes a severe clinical manifestation of hepatitis, including abrupt onset, rapid progression, complex clinical presentations, and unfavorable prognostic outcomes. It may cause 5%-18% of FH in Europe, 13%-15% in Bangladesh and India, and 22% in Sudan. HBV accounts for approximately 7% of United States FH cases[92]. The study by Kayesh et al[94] sheds light on the alarming phenomenon of FH resulting from HBV reactivation. Their research delves into the mechanisms underlying this severe condition, emphasizing the critical role of immune responses in HBV reactivation-related liver damage. Through a comprehensive analysis of clinical cases and molecular studies, Lam et al[87] elucidate the intricate interplay between viral factors, host immune responses, and the hepatic microenvironment, contributing to the development of FH. Their findings underscore the urgent need for vigilant monitoring and proactive management strategies in patients at risk of HBV reactivation, particularly those undergoing immunosuppressive treatments or chemotherapy[95]. The early onset of acute liver failure is attributed to the destructive effects of HBV reactivation-induced immunological responses, which are accompanied by the substantial release of inflammatory mediators. This situation can result in hepatic encephalopathy, coagulopathy, and multi-organ failure[96].
Liver fibrosis and Cirrhosis are complex clinical phenomena that the reactivation of HBV can further aggravate. The reactivation of HBV initiates a cascade of immunological reactions that facilitate the attraction and stimulation of various immune cells, such as macrophages, T cells, and neutrophils, within the milieu of the liver[49,97]. According to Lee et al[98], these immune cells release cytokines, chemokines, and profibrotic mediators that cause HSCs to change phenotypically into myofibroblast-like cells. The excessive synthesis and accumulation of extracellular matrix components by the activated HSCs contribute to fibrotic scarring. The chronic activation of the immune system resulting from HBV reactivation leads to the continuous presence of immune cells and the ongoing production of inflammatory mediators, which produce an environment favorable for the sustained development of fibrosis[46,47,99].
Moreover, in their study, Jagdish et al[100] discussed the complex immunological mechanisms and intricate feedback loops that contribute to the pathophysiological processes of liver fibrosis and Cirrhosis in the context of HBV reactivation, according to examination by Peiseler et al[101], immune cell activation and the subsequent release of cytokines not only promote fibrogenesis but also maintain a state of chronic inflammation. A self-sustaining cascade of inflammation and fibrosis starts due to the persistent immunological responses, which trigger the release of additional pro-inflammatory cytokines and chemokines. Furthermore, as understood by Gherlan et al[102], the presence of immune-suppressive components, such as Tregs, can reduce the efficiency of anti-viral immune responses and promote the growth of fibrosis by creating an immunologically tolerable environment. In people with HBV reactivation, the progression of liver fibrosis and cirrhosis is caused by a complex dynamic involving the ongoing interaction of immune activation, fibrogenesis, and immune suppression[102,103]. Comprehending the complex immunological mechanisms involved is of utmost importance to facilitate the formulation of precise therapy strategies that might effectively disrupt these processes and impede the progression of liver fibrosis and Cirrhosis in the context of HBV reactivation[104].
The pathogenesis of HCC is closely linked to the fundamental involvement of chronic inflammation in oncogenesis. Chronic HBV infection represents a significant risk factor for HCC, a liver cancer. The risk is further exacerbated by HBV reactivation, which sustains a continuous cycle of persistent inflammation, contributing to the development of HCC. In chronic carriers with HCC receiving chemotherapy, reported rates of HBV reactivation range from 4% to 67%[105]. According to a recent study, the administration of anti-cancer therapy for HCC has been associated with HBV’s reactivation. In a study by Midorikawa et al[106], 1609 patients who underwent hepatectomy were examined. This study revealed a significant independent association between HBV reactivation and reduced overall and recurrence-free survival. Moreover, Shiri et al[107] recommend delaying the planned therapy for HCC until the impaired liver function has been restored in cases of reactivation. Two prospective studies have shown that the reactivation of HCC has resulted in delayed or prematurely terminated treatment regimens.
During the process of HBV reactivation, there has been a significant rise in viral replication, leading to the release of viral antigens. This, in turn, triggers robust immunological responses. However, the continuous activation of the immune system can lead to the release of pro-inflammatory cytokines and chemokines[108]. This creates an environment that is conducive to DNA damage and the transformation of cells. According to Feitelson et al[109], prolonged exposure to viral antigens and persistent immune responses create an environment that promotes genetic mutations and epigenetic alterations in hepatocytes. This makes the hepatocytes more vulnerable to malignant transformation.
Furthermore, the significance of chronic inflammation in HCC linked with HBV reactivation is further emphasized by activating pivotal signaling pathways. The study conducted by Sivasudhan et al[110] demonstrated that the activation of the NF-κB and MAPKs signaling pathways, frequently observed in chronic inflammation cases, exert a substantial influence on the progression of HCC. The pathways mentioned above influence cell survival, proliferation, and the circumvention of apoptosis, all of which are vital facets of tumor progression. The enduring immunological responses and inflammatory mediators can promote oxidative stress and DNA damage, intensifying carcinogenic potential[111,112]. In addition, Chekol et al[113] have highlighted that the inflammatory response can lead to the production of immunomodulatory substances, including Tregs and anti-inflammatory cytokines. These substances may hinder immune surveillance and promote immunological tolerance. This allows modified hepatocytes to avoid immune detection and subsequent immune response.
The primary objective of existing therapeutic interventions for HBV reactivation is to inhibit viral replication and reinstate immunological regulation. Nucleoside and nucleotide analogs (NAs) are fundamental in treating HBV. The medications mentioned, namely lamivudine, entecavir, tenofovir, and adefovir, act as competitive inhibitors of HBV reverse transcriptase, thereby interfering with the synthesis of viral DNA[114]. NAs demonstrate significant anti-viral properties, resulting in the long-term inhibition of viral activity and decreased HBV DNA levels. The decrease in viral load mitigates hepatic inflammation and contributes to preventing HBV reactivation relapse. It is of utmost significance that the implementation of efficient anti-viral medication has the potential to impede the advancement of liver fibrosis and Cirrhosis, offering a pivotal means of managing individuals who are susceptible to severe liver disease resulting from HBV reactivation[115,116]. In addition to their anti-viral properties, the immunomodulatory capacities of NAs, as revealed by Zheng et al[25], are involved in regulating immunological reactions during the reactivation of HBV. Nucleic acid-based therapies have been observed to lower viral load, reducing viral antigen exposure effectively. Consequently, this reduction in viral antigen exposure leads to a subsequent decrease in immune activation triggered by antigens. Therefore, this mitigates the inflammation commonly associated with the reactivation of HBV[117].
Furthermore, nanoparticles (NAs) can augment the functionality of several immune cells, including NK cells, T cells, and DCs, hence facilitating the development of anti-viral immune responses. The simultaneous effect of NAs encompasses inhibiting viral replication and promoting immunological homeostasis restoration[118]. Nevertheless, it is crucial to acknowledge that although NAs exhibit significant efficacy, they generally do not result in a comprehensive eradication of the viral infection. Sustained viral suppression and relapse prevention often need the ongoing administration of these medications over an extended period[119].
Entecavir: Using Entecavir, an NA, has become a key strategy in managing HBV reactivation. The anti-viral actions of this substance are exerted through the inhibition of HBV DNA polymerase, resulting in the efficient suppression of viral replication (Table 2). Entecavir, a potent and specific inhibitor, effectively decreased the amounts of HBV DNA, resulting in enhanced liver function and reduced hepatic inflammation related to HBV reactivation[120]. This treatment option’s high genetic barrier to resistance makes it an appealing selection for extended therapeutic interventions, especially in patients susceptible to recurring HBV reactivation[121]. Moreover, the anti-viral effectiveness of Entecavir has a signi
| Therapy | Model | Mechanism | Efficacy | Success rate, % | Resistance | Ref. |
| Anti-viral therapy | ||||||
| Nucleos(t)ide | Lamivudine | Inhibits viral DNA synthesis | High | 80% | Low | [30] |
| Entecavir | Potent viral DNA polymerase | High | 90% | Rare | [120] | |
| Adefovir | Inhibits viral DNA polymerase | Moderate | 70%-80% | Occasional | [128] | |
| Tenofovir | Inhibits viral DNA synthesis | High | 90% | Rare | [124] | |
| Monoclonal antibodies | Anti-HBV antibodies | Viral neutralization | Moderate | 70% | Occasional | [137] |
| Combination therapy | Tenofovir + emtricitabine | Inhibits viral DNA synthesis | High | 95% | Low | [138] |
| Immune-modulating therapy | ||||||
| Toll-like receptor agonists | Immune activation | Moderate | 70% | Variable | [139] | |
| Interferon | Antiviral and immune activation | High | 80% | Occasional | [140] | |
| Personalized treatment approaches | ||||||
| Tailored | Targeted antiviral therapy based on genomic profile | Variable | 75%-90% | Variable | [141] | |
| Treatment | ||||||
| Combination therapy | Nucleos(t)ide + immune-modulating therapy | Antiviral + immunomodulation | High | 90%-95% | Low | [142] |
| Monoclonal antibodies | Individualized treatment | Targeted viral neutralization based on antibody profiling | Varies | 60%-80% | Occasional | [143] |
Tenofovir: Tenofovir, an NA, has been identified as a fundamental intervention in managing HBV reactivation. The strong inhibitory activity of this compound on the DNA polymerase of the HBV efficiently hampers the reproduction of the virus (Table 2), resulting in a quick decrease in viral load and relief from liver inflammation associated with the infection[124]. According to Mizushima et al[125], the efficacy of tenofovir in individuals with HBV reactivation, regardless of their prior treatment history, can be due to its extensive anti-viral activity and strong resistance barrier. In addition, a study by Hsu et al[126] has shown that using tenofovir can effectively reverse liver fibrosis and cirrhosis, leading to persistent viral suppression. This highlights the importance of tenofovir in preventing the development of severe liver diseases. The availability of both oral and injectable forms of medication allows for greater flexibility in tailoring treatment to meet each patient’s unique preferences and needs[126].
Nevertheless, it is crucial to consider the potential renal and bone health consequences that may arise from using tenofovir. In a study, Fu et al[127] proposed that tenofovir possesses strong anti-viral properties and beneficial resistance characteristics, making it an essential component in treating HBV reactivation. This highlights the significance of tailoring treatment approaches to individual patients to maximize outcomes’ effectiveness.
Adefovir: The potential use of adefovir, an NA, as a therapeutic intervention for the reactivation of HBV has been investigated, particularly in situations where alternative treatment options may be impractical or insufficient. The mechanism of action involves the inhibition of HBV DNA polymerase (Table 2), resulting in decreased viral replication and subsequent reduction in viral load[128]. The anti-viral activity of adefovir has demonstrated effectiveness in suppressing HBV reactivation and enhancing liver function. Nevertheless, this treatment has been linked to an elevated susceptibility to resistance in contrast to more contemporary anti-viral medications such as entecavir and tenofovir. The aforementioned highlights the significance of meticulous patient selection, consistent monitoring, and the potential utilization of combination therapy to mitigate resistance development[129]. With more advanced anti-viral drugs emerging, adefovir’s potential utility in managing HBV reactivation may be restricted to particular situations, underscoring the importance of tailored treatment strategies to get the best possible results[130].
Lamivudine: Lamivudine’s early chain termination-induced HBV replication reduction was discovered in 1995. The medication successfully treated HBV reactivation in a non-Hodgkin’s lymphoma patient in 1998. Lamivudine reduces HBV replication within days to weeks of starting treatment, with moderate side effects (Table 2). The conventional treatment for HBV replication is extensively used due to its efficacy, few side effects, high tolerance, and once-daily dosing. While most patients responded well to lamivudine, the treated group had mortality rates of 18% to 40%[131,132]. The study found that non-responders had decompensated liver disease before therapy. The effectiveness of Lamivudine may be diminished in severe hepatic damage. Thus, HBV reactivation, indicated by higher HBV-DNA levels, should be treated immediately. The therapy duration is unclear. Anti-viral drugs reduce reactivation rates. However, a study found a 24% reactivation rate three months after lamivudine cessation[133].
After immunosuppressive therapy, six months of treatment is advised. However, some authors recommend a year-long treatment to prevent HBV reactivation. Drug-resistant mutant strains of HBV constitute a significant concern with extended treatment. Viral resistance is the re-emergence of serum HBV DNA after viremia clearance, even with anti-viral therapy. The incidence of lamivudine-resistant strains with tyrosine-methionine-aspartate (YMDD) mutations increases with treatment duration[134]. These symptoms usually appear after six months of treatment. The prevalence of these symptoms is 15% in the first year, 38% in the second, 56% in the third, and 65% in the fifth year of treatment. While multiple studies have shown that the YMDD mutant virus does not affect clinical outcomes, one found a greater rate of hepatitis flares and other severe adverse effects in the fifth and sixth years of treatment. Mutations that confer lamivudine resistance caused these outcomes[135,136]. The influence on chronic HBV management is apparent; however, the effects on HBV reactivation therapy are unclear (Table 2)[137-143].
The potential efficacy of immune-modulating medications, such as interferon-based therapy, in managing HBV reactivation is encouraging. These therapies can enhance immune surveillance and facilitate viral clearance. Interferons are a class of cytokines that elicit anti-viral responses, augmenting the immune system’s capacity to identify and counteract viral infections[140,144]. In the setting of HBV reactivation, therapies based on interferon can elicit immune responses that are both innate and adaptive. The activation of NK cells, DCs, and macrophages is observed, enhancing their ability to identify and eliminate cells infected with HBV[12,145]. In addition, interferons can augment the antigen presentation capability of DCs, promoting T-cell solid responses that specifically target infected hepatocytes. By coordinating a diverse immune response, therapies based on interferon can effectively suppress viral replication (Table 2), impede the advancement of HBV reactivation, and potentially facilitate the resolution of viral infection[146].
Recently, a growing interest has been in utilizing TLR agonists as vaccine adjuvants or immune modulators. This interest stems from their capacity to stimulate the production of IFN, pro-inflammatory cytokines, and chemokines, which can potentially elicit anti-HBV effects. In PHH, TLR1/2 and TLR3 agonists decrease HBV replication (Table 2). Another study found that oral TLR7 agonist GS-9620 (vesatolimod) and nucleos(t)ide analogs increased T cell and NK cell responses and reduced NK cell suppression of T cells in chronically infected patients[139,147].
The significance of tailored treatment strategies for persons encountering HBV reactivation cannot be overemphasized, given that the efficacy of therapies can differ considerably depending on patient-specific variables. Individuals’ immunological profiles are paramount in assessing and predicting treatment outcomes. Certain patients may exhibit strong immune responses that can be effectively utilized to manage the reactivation of HBV. In contrast, others may necessitate more intensive immune modulation to get the desired effects[148]. Genetic variables additionally influence treatment variability. The presence of genetic differences has the potential to impact drug metabolism, immunological responses, and the likelihood of experiencing adverse effects. As a result, it is crucial to customize treatment approaches to optimize outcomes[149,150].
Moreover, the presence of many genotypes of HBV introduces an additional level of intricacy. Various genotypes display varied levels of virulence and may demonstrate distinct responses to anti-viral or immune-based treatments. Therefore, it is imperative to include the HBV genotype when designing personalized treatment plans to maximize interventions for the individual viral strain[151].
Precision medicine and biomarker research have witnessed significant progress, presenting encouraging prospects for customizing treatment based on specific patient characteristics. Biomarkers, including viral load, liver function tests, and specific immune cell subsets, can offer valuable insights into the patient’s response to therapy and facilitate informed decisions regarding treatment modifications[152]. Genetic testing can detect genetic variants that could influence the results of treatment or the metabolism of drugs, thereby facilitating the selection of the most suitable therapies[153]. Furthermore, viral genotyping might provide valuable insights in selecting appropriate anti-viral medicines and forecasting their effectiveness against certain strains of HBV. Incorporating these individualized characteristics into treatment determinations can optimize treatment results, mitigate unfavorable consequences, and increase patients’ overall quality of life[110,154]. The progress of personalized medicine can significantly impact the management of HBV reactivation by introducing patient-specific treatment approaches. This advancement can substantially improve clinical outcomes and enhance treatments.
Despite considerable progress in elucidating the complex immunological mechanisms behind the reactivation of HBV, specific knowledge gaps hinder a thorough comprehension of its pathophysiology. A significant deficiency exists in the exact coordination of immune responses during the reactivation of HBV and its subsequent implications for the course of the disease. The involvement of immune cells, including T cells, B cells, and innate immune components, has been widely recognized. However, there is ongoing research to determine the precise sequence of events, factors that influence immunological dominance, and the interactions that occur within the intricate hepatic milieu[155]. Furthermore, there is a need for more significant investigation into the mechanisms that govern the shift from regulated viral replication to reactivation and the subsequent effects on immune responses. Examining alternative avenues is necessary to identify specific immunological checkpoints or regulatory pathways that can be altered for therapeutic benefit. Bridging these information gaps is essential in developing precise therapies that aim to avoid the reactivation of HBV and minimize its potentially severe consequences[156].
Moreover, the impact of genetic and epigenetic variables on immune responses and disease course in HBV reactivation has not been thoroughly investigated. Genetic variants among individuals may influence the characteristics and efficacy of immune responses, offering a plausible explanation for the observed variability in patient outcomes. The influence of epigenetic changes, including DNA methylation and histone acetylation, on immune cell activity and their potential impact on the progression of HBV reactivation is a subject of interest[157]. Furthermore, the influence of comorbidities, such as obesity, diabetes, or co-infections, on immune responses during HBV reactivation has yet to be well investigated. The complete understanding of how these parameters intersect with immune systems can enhance our understanding of the illness spectrum and inform the development of customized treatment methods. The imperative to improve our comprehension of HBV reactivation and its related difficulties becomes increasingly significant as research progresses and novel technologies emerge[158,159].
Future research efforts in HBV reactivation should prioritize numerous prospective avenues to enhance our comprehension and therapeutic approaches. One potential approach involves investigating innovative immune-based treatments that use the complex interaction between immune cells and viral elements in the context of HBV reactivation. The exploration of immune checkpoint inhibitors, adoptive T-cell treatments, and customized immune cells designed to target HBV-infected hepatocytes specifically provide novel strategies for augmenting immune responses and achieving long-term viral suppression[160]. Furthermore, exploring the potential of tailored immunotherapies that leverage patient-specific immune profiles has significant opportunities for enhancing treatment outcomes. To effectively advance the development of innovative therapeutic strategies, it is imperative to conduct comprehensive studies investigating the dynamics of immune cell populations, cytokine profiles, and immunological checkpoint expression during HBV reactivation[161].
Another field of prospective investigation pertains to elucidating the complex intercommunication between the gastrointestinal tract and liver, commonly called the gut-liver axis, within the framework of HBV reactivation. Recent research indicates that increasing evidence supports the notion that the gut microbiota and their metabolic byproducts significantly impact liver immunity and inflammation. Examining the impact of the gut-liver axis on immune responses during HBV reactivation holds promise for shedding fresh light on the etiology of the illness and identifying possible targets for therapeutic intervention[162]. Moreover, gaining insight into the impact of changes in the composition and functioning of gut microbiota on the immunological dysregulation found in HBV reactivation provides opportunities for novel therapies, such as manipulating the gut microbiome to bolster anti-viral immune responses. Adopting a multidisciplinary approach can illuminate aspects of HBV reactivation that have not been thoroughly investigated before and may present innovative therapeutic approaches[163].
Likewise, it is necessary to thoroughly analyze the effects of HBV reactivation on the overall immune system. Although the liver is known to be a primary site for HBV infection and reactivation, there is a lack of comprehensive research on its impact on immune cell distribution, functioning, and memory responses throughout the body[164]. Examining the effects of HBV reactivation on the immunological landscape outside of the liver may yield valuable insights into immune aging and immune exhaustion and potentially inform the formulation of preventive measures against immunosuppression in several scenarios[165]. By incorporating state-of-the-art methodologies like single-cell RNA sequencing and advanced imaging modalities into these inquiries, it is possible to reveal novel understandings regarding the broader consequences of HBV reactivation and establish a foundation for comprehensive treatment interventions[166].
In conclusion, this in-depth review article has illuminated the complex immunological mechanisms behind HBV reactivation and their consequences for the disease and treatment approaches. The immunological components examined highlight the complexity of HBV reactivation, particularly the interaction between viral and host immune responses. These mechanisms underscore the need for close monitoring in high-risk populations by contributing to various clinical presentations, from asymptomatic instances to severe liver damage. Furthermore, understanding the immunopathogenesis of HBV reactivation points to effective treatment approaches. Anti-viral treatments that target particular immunological pathways and novel immunomodulatory drugs that may lessen the severity of reactivation and enhance patient outcomes are under development. The information compiled in this review article offers a vital basis for directing clinical practice, improving our comprehension of HBV reactivation dynamics, and encouraging the creation of more efficient management strategies in an era characterized by the development of immunotherapies.
Moreover, the consequences of this review go beyond the field of medicine. They emphasize the significance of treating HBV reactivation holistically, combining immunomodulation techniques with anti-viral treatments. Furthermore, they emphasize the necessity of continued research endeavors to unearth additional complexities in the immunological pathways underlying HBV reactivation. We will be better able to anticipate and stop reactivation occurrences due to this knowledge, which will also help us comprehend the whole picture of viral-host interactions in chronic HBV infection. Conclusively, this review’s synthesis of immunological insights and their clinical implications is an essential tool for healthcare professionals, researchers, and clinicians. It will help those at risk of HBV reactivation receive better care and achieve better results.
| 1. | Zheng P, Dou Y, Wang Q. Immune response and treatment targets of chronic hepatitis B virus infection: innate and adaptive immunity. Front Cell Infect Microbiol. 2023;13:1206720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Aliu TB, Majiyebo AJ, Tsado AN, Ibrahim HA, Berinyuy EB. Biology and molecular pathogenesis of hepatitis B virus infection. Biomed Natu and App Sci. 2022;2:28-36. [DOI] [Full Text] |
| 3. | Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 363] [Reference Citation Analysis (1)] |
| 4. | Yuan C, Peng J, Xia R, He J, Qiu T, Yao Y. Reactivation of Occult Hepatitis B Virus Infection During Long-Term Entecavir Antiviral Therapy. Front Microbiol. 2022;13:865124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Bhat SA, Hasan SK, Parray ZA, Siddiqui ZI, Ansari S, Anwer A, Khan S, Amir F, Mehmankhah M, Islam A, Minuchehr Z, Kazim SN. Potential antiviral activities of chrysin against hepatitis B virus. Gut Pathog. 2023;15:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | de Almeida Pondé RA. Detection of the serological markers hepatitis B virus surface antigen (HBsAg) and hepatitis B core IgM antibody (anti-HBcIgM) in the diagnosis of acute hepatitis B virus infection after recent exposure. Microbiol Immunol. 2022;66:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Rosenberg M, Poluch M, Thomas C, Sindaco P, Khoo A, Porcu P. Hepatitis B Virus and B-cell lymphoma: evidence, unmet need, clinical impact, and opportunities. Front Oncol. 2023;13:1275800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 8. | Benjamin I, Louis H, Udoikono AD, Agwamba EC, Unimuke TO, Ahuekwe EF. Hydrazineylidene‐3‐oxopropanal derivatives as antiviral agents for treatment of HBV and HCV: experimental, DFT, and molecular docking studies. Vietnam J Chem. 2023;61:109-125. [DOI] [Full Text] |
| 9. | Chang Y, Jeong SW, Jang JY. Hepatitis B Virus Reactivation Associated With Therapeutic Interventions. Front Med (Lausanne). 2021;8:770124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 10. | Mak JWY, Law AWH, Law KWT, Ho R, Cheung CKM, Law MF. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies in the targeted therapy era. World J Gastroenterol. 2023;29:4942-4961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (1)] |
| 11. | Papatheodoridis GV, Lekakis V, Voulgaris T, Lampertico P, Berg T, Chan HLY, Kao JH, Terrault N, Lok AS, Reddy KR. Hepatitis B virus reactivation associated with new classes of immunosuppressants and immunomodulators: A systematic review, meta-analysis, and expert opinion. J Hepatol. 2022;77:1670-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Dusheiko G, Agarwal K, Maini MK. New Approaches to Chronic Hepatitis B. N Engl J Med. 2023;388:55-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 13. | Zhu Y, Li H, Wang X, Zheng X, Huang Y, Chen J, Meng Z, Gao Y, Qian Z, Liu F, Lu X, Shi Y, Shang J, Yan H, Zheng Y, Qiao L, Zhang Y, Xiang X, Dan Y, Sun S, Hou Y, Zhang Q, Xiong Y, Li S, Huang Z, Li B, Jiang X, Luo S, Chen Y, Gao N, Liu C, Ji L, Yuan W, Li J, Li T, Zheng R, Zhou X, Ren H, Zhou Y, Xu B, Yu R, Tan W, Deng G. Hepatitis B Virus Reactivation Increased the Risk of Developing Hepatic Failure and Mortality in Cirrhosis With Acute Exacerbation. Front Microbiol. 2022;13:910549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Hassnine AA, Saber MA, Fouad YM, Sarhan H, Elsayed MM, Zaki ZM, Abdelraheem EM, Abdelhalim SM, Elsayed AM. Clinical study on the efficacy of hepatitis B vaccination in hepatitis C virus related chronic liver diseases in Egypt. Virus Res. 2023;323:198953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhou MJ, Zhang C, Fu YJ, Wang H, Ji Y, Huang X, Li L, Wang Y, Qing S, Shi Y, Shen L, Wang YY, Li XY, Li YY, Chen SY, Zhen C, Xu R, Shi M, Wang FS, Cheng Y. Cured HCV patients with suboptimal hepatitis B vaccine response exhibit high self-reactive immune signatures. Hepatol Commun. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Liu C, Shih YF, Liu CJ. Immunopathogenesis of Acute Flare of Chronic Hepatitis B: With Emphasis on the Role of Cytokines and Chemokines. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Cacoub P, Asselah T. Hepatitis B Virus Infection and Extra-Hepatic Manifestations: A Systemic Disease. Am J Gastroenterol. 2022;117:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Scott D, Singer DS. Harnessing the Power of Discovery. Cancer Discov. 2023;13:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Kim SW, Yoon JS, Lee M, Cho Y. Toward a complete cure for chronic hepatitis B: Novel therapeutic targets for hepatitis B virus. Clin Mol Hepatol. 2022;28:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Gramantieri L, Fornari F, Giovannini C, Trerè D. MicroRNAs at the Crossroad between Immunoediting and Oncogenic Drivers in Hepatocellular Carcinoma. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Su Y, Lu Y, An H, Liu J, Ye F, Shen J, Ni Z, Huang B, Lin J. MicroRNA-204-5p Inhibits Hepatocellular Carcinoma by Targeting the Regulator of G Protein Signaling 20. ACS Pharmacol Transl Sci. 2023;6:1817-1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Muto S, Matsubara T, Inoue T, Kitamura H, Yamamoto K, Ishii T, Yazawa M, Yamamoto R, Okada N, Mori K, Yamada H, Kuwabara T, Yonezawa A, Fujimaru T, Kawano H, Yokoi H, Doi K, Hoshino J, Yanagita M. Chapter 1: Evaluation of kidney function in patients undergoing anticancer drug therapy, from clinical practice guidelines for the management of kidney injury during anticancer drug therapy 2022. Int J Clin Oncol. 2023;28:1259-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Ando Y, Nishiyama H, Shimodaira H, Takano N, Sakaida E, Matsumoto K, Nakanishi K, Sakai H, Tsukamoto S, Komine K, Yasuda Y, Kato T, Fujiwara Y, Koyama T, Kitamura H, Kuwabara T, Yonezawa A, Okumura Y, Yakushijin K, Nozawa K, Goto H, Matsubara T, Hoshino J, Yanagita M; Committee of Clinical Practice Guidelines for the Management of Kidney Disease During Anticancer Drug Therapy 2022. Chapter 3: Management of kidney injury caused by cancer drug therapy, from clinical practice guidelines for the management of kidney injury during anticancer drug therapy 2022. Int J Clin Oncol. 2023;28:1315-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Zhao HJ, Hu YF, Han QJ, Zhang J. Innate and adaptive immune escape mechanisms of hepatitis B virus. World J Gastroenterol. 2022;28:881-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 25. | Zheng JR, Wang ZL, Feng B. Hepatitis B functional cure and immune response. Front Immunol. 2022;13:1075916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Santos Apolonio J, Lima de Souza Gonçalves V, Cordeiro Santos ML, Silva Luz M, Silva Souza JV, Rocha Pinheiro SL, de Souza WR, Sande Loureiro M, de Melo FF. Oncolytic virus therapy in cancer: A current review. World J Virol. 2021;10:229-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (20)] |
| 27. | Maqsood Q, Sumrin A, Iqbal M, Younas S, Hussain N, Mahnoor M, Wajid A. Hepatitis C virus/Hepatitis B virus coinfection: Current prospectives. Antivir Ther. 2023;28:13596535231189643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Medina C, García AH, Crespo FI, Toro FI, Mayora SJ, De Sanctis JB. A Synopsis of Hepatitis C Virus Treatments and Future Perspectives. Curr Issues Mol Biol. 2023;45:8255-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Faure-Dupuy S, Baumert TF. Targeting immuno-metabolism and anti-viral immune responses in chronic hepatitis B. Hepatol Int. 2023;17:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Wang L, Sun Y, Song X, Wang Z, Zhang Y, Zhao Y, Peng X, Zhang X, Li C, Gao C, Li N, Gao L, Liang X, Wu Z, Ma C. Hepatitis B virus evades immune recognition via RNA adenosine deaminase ADAR1-mediated viral RNA editing in hepatocytes. Cell Mol Immunol. 2021;18:1871-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Yang Z, Sun B, Xiang J, Wu H, Kan S, Hao M, Chang L, Liu H, Wang D, Liu W. Role of epigenetic modification in interferon treatment of hepatitis B virus infection. Front Immunol. 2022;13:1018053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 32. | He P, Zhang P, Fang Y, Han N, Yang W, Xia Z, Zhu Y, Zhang Z, Shen J. The role of HBV cccDNA in occult hepatitis B virus infection. Mol Cell Biochem. 2023;478:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Wei L, Cafiero TR, Tseng A, Gertje HP, Berneshawi A, Crossland NA, Ploss A. Conversion of hepatitis B virus relaxed circular to covalently closed circular DNA is supported in murine cells. JHEP Rep. 2022;4:100534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Bratulic S, Limeta A, Dabestani S, Birgisson H, Enblad G, Stålberg K, Hesselager G, Häggman M, Höglund M, Simonson OE, Stålberg P, Lindman H, Bång-Rudenstam A, Ekstrand M, Kumar G, Cavarretta I, Alfano M, Pellegrino F, Mandel-Clausen T, Salanti A, Maccari F, Galeotti F, Volpi N, Daugaard M, Belting M, Lundstam S, Stierner U, Nyman J, Bergman B, Edqvist PH, Levin M, Salonia A, Kjölhede H, Jonasch E, Nielsen J, Gatto F. Noninvasive detection of any-stage cancer using free glycosaminoglycans. Proc Natl Acad Sci U S A. 2022;119:e2115328119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, Tabachnikova A, Greene K, Tabacof L, Malik AA, Silva Monteiro V, Silva J, Kamath K, Zhang M, Dhal A, Ott IM, Valle G, Peña-Hernández M, Mao T, Bhattacharjee B, Takahashi T, Lucas C, Song E, McCarthy D, Breyman E, Tosto-Mancuso J, Dai Y, Perotti E, Akduman K, Tzeng TJ, Xu L, Geraghty AC, Monje M, Yildirim I, Shon J, Medzhitov R, Lutchmansingh D, Possick JD, Kaminski N, Omer SB, Krumholz HM, Guan L, Dela Cruz CS, van Dijk D, Ring AM, Putrino D, Iwasaki A. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 369] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 36. | Soto JA, Gálvez NMS, Andrade CA, Pacheco GA, Bohmwald K, Berrios RV, Bueno SM, Kalergis AM. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front Immunol. 2020;11:1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Fisicaro P, Barili V, Rossi M, Montali I, Vecchi A, Acerbi G, Laccabue D, Zecca A, Penna A, Missale G, Ferrari C, Boni C. Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches. Front Immunol. 2020;11:849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 38. | Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 331] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 39. | Feola S, Chiaro J, Cerullo V. Integrating immunopeptidome analysis for the design and development of cancer vaccines. Semin Immunol. 2023;67:101750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 40. | De Pasquale C, Campana S, Barberi C, Sidoti Migliore G, Oliveri D, Lanza M, Musolino C, Raimondo G, Ferrone S, Pollicino T, Ferlazzo G. Human Hepatitis B Virus Negatively Impacts the Protective Immune Crosstalk Between Natural Killer and Dendritic Cells. Hepatology. 2021;74:550-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Tang L, Kottilil S, Wilson E. Strategies to eliminate HBV infection: an update. Futr Virol. 2020;15:35-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Ciardi MR, Iannetta M, Zingaropoli MA, Salpini R, Aragri M, Annecca R, Pontecorvo S, Altieri M, Russo G, Svicher V, Mastroianni CM, Vullo V. Reactivation of Hepatitis B Virus With Immune-Escape Mutations After Ocrelizumab Treatment for Multiple Sclerosis. Open Forum Infect Dis. 2019;6:ofy356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Korsukewitz C, Reddel SW, Bar-Or A, Wiendl H. Neurological immunotherapy in the era of COVID-19 - looking for consensus in the literature. Nat Rev Neurol. 2020;16:493-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Yaseen MM, Abuharfeil NM, Darmani H, Daoud A. Mechanisms of immune suppression by myeloid-derived suppressor cells: the role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020;10:200111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 45. | Ding L, Wang N, Wang Q, Fan X, Xin Y, Wang S. Midkine inhibition enhances anti-PD-1 immunotherapy in sorafenib-treated hepatocellular carcinoma via preventing immunosuppressive MDSCs infiltration. Cell Death Discov. 2023;9:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 46. | Khanam A, Chua JV, Kottilil S. Immunopathology of Chronic Hepatitis B Infection: Role of Innate and Adaptive Immune Response in Disease Progression. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 47. | Raje N, Anderson K, Einsele H, Efebera Y, Gay F, Hammond SP, Lesokhin AM, Lonial S, Ludwig H, Moreau P, Patel K, Ramasamy K, Mateos MV. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: consensus recommendations from an expert panel. Blood Cancer J. 2023;13:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 48. | Jiang P, Jia H, Qian X, Tang T, Han Y, Zhang Z, Jiang L, Yu Z, Zheng L, Yu G, Cai H, Zhang S, Zhang X, Gu J, Ye C, Yang L, Lu Y, Liu H, Lu X, Jin C, Ren Y, Lu M, Xu L, Yu J, Jin X, Yang Y, Qian P. Single-cell RNA sequencing reveals the immunoregulatory roles of PegIFN-α in patients with chronic hepatitis B. Hepatology. 2024;79:167-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 49. | Binatti E, Gerussi A, Barisani D, Invernizzi P. The Role of Macrophages in Liver Fibrosis: New Therapeutic Opportunities. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 50. | Tsounis EP, Tourkochristou E, Mouzaki A, Triantos C. Toward a new era of hepatitis B virus therapeutics: The pursuit of a functional cure. World J Gastroenterol. 2021;27:2727-2757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (3)] |
| 51. | Bassit L, Amblard F, Patel D, Biteau N, Chen Z, Kasthuri M, Zhou S, Schinazi RF. The premise of capsid assembly modulators towards eliminating HBV persistence. Expert Opin Drug Discov. 2023;18:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Bao Z, Chen X, Li Y, Jiang W, Pan D, Ma L, Wu Y, Chen Y, Chen C, Wang L, Zhao S, Wang T, Lu WY, Ma C, Wang S. The hepatic GABAergic system promotes liver macrophage M2 polarization and mediates HBV replication in mice. Antiviral Res. 2023;217:105680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 53. | Koda Y, Teratani T, Chu PS, Hagihara Y, Mikami Y, Harada Y, Tsujikawa H, Miyamoto K, Suzuki T, Taniki N, Sujino T, Sakamoto M, Kanai T, Nakamoto N. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat Commun. 2021;12:4474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 54. | Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 459] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 55. | Liu K, Wang FS, Xu R. Neutrophils in liver diseases: pathogenesis and therapeutic targets. Cell Mol Immunol. 2021;18:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 56. | Sadeghi M, Dehnavi S, Jamialahmadi T, Johnston TP, Sahebkar A. Neutrophil extracellular trap: A key player in the pathogenesis of autoimmune diseases. Int Immunopharmacol. 2023;116:109843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 57. | Maronek M, Gardlik R. The Citrullination-Neutrophil Extracellular Trap Axis in Chronic Diseases. J Innate Immun. 2022;14:393-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Sarkar T, Sharma K, Ramakrishnan A. A comprehensive review of factors that enhance the readiness level of the immune system and also those that impair immunity. 2022. [cited 22 December 2023]. Available from: https://www.researchgate.net/publication/361424318_A_comprehensive_review_of_factors_that_enhance_the_readiness_level_of_the_immune_system_and_also_those_that_impair_immunity. |
| 59. | Björkström NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22:112-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 331] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 60. | Li H, Huang QZ, Zhang H, Liu ZX, Chen XH, Ye LL, Luo Y. The land-scape of immune response to monkeypox virus. EBioMedicine. 2023;87:104424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 89] [Reference Citation Analysis (0)] |
| 61. | Chu J, Gao F, Yan M, Zhao S, Yan Z, Shi B, Liu Y. Natural killer cells: a promising immunotherapy for cancer. J Transl Med. 2022;20:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 62. | Zhang C, Wang XM, Li SR, Twelkmeyer T, Wang WH, Zhang SY, Wang SF, Chen JZ, Jin X, Wu YZ, Chen XW, Wang SD, Niu JQ, Chen HR, Tang H. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat Commun. 2019;10:1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 63. | Gong L, Kwong DL, Dai W, Wu P, Li S, Yan Q, Zhang Y, Zhang B, Fang X, Liu L, Luo M, Liu B, Chow LK, Chen Q, Huang J, Lee VH, Lam KO, Lo AW, Chen Z, Wang Y, Lee AW, Guan XY. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat Commun. 2021;12:1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 64. | Lee J, Park SS, Kim TY, Lee DG, Kim DW. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 65. | Li M, Gao Y, Yang L, Lin Y, Deng W, Jiang T, Bi X, Lu Y, Zhang L, Shen G, Liu R, Wu S, Chang M, Xu M, Hu L, Song R, Jiang Y, Yi W, Xie Y. Dynamic changes of cytokine profiles and virological markers during 48 weeks of entecavir treatment for HBeAg-positive chronic hepatitis B. Front Immunol. 2022;13:1024333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Jin X, Bi J. Prospects for NK-based immunotherapy of chronic HBV infection. Front Immunol. 2022;13:1084109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 67. | Chuang YC, Tsai KN, Ou JJ. Pathogenicity and virulence of Hepatitis B virus. Virulence. 2022;13:258-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 68. | Vanwolleghem T, Adomati T, Van Hees S, Janssen HLA. Humoral immunity in hepatitis B virus infection: Rehabilitating the B in HBV. JHEP Rep. 2022;4:100398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Churiso G, Husen G, Bulbula D, Abebe L. Immunity Cell Responses to RSV and the Role of Antiviral Inhibitors: A Systematic Review. Infect Drug Resist. 2022;15:7413-7430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 70. | Marotel M, Villard M, Drouillard A, Tout I, Besson L, Allatif O, Pujol M, Rocca Y, Ainouze M, Roblot G, Viel S, Gomez M, Loustaud V, Alain S, Durantel D, Walzer T, Hasan U, Marçais A. Peripheral natural killer cells in chronic hepatitis B patients display multiple molecular features of T cell exhaustion. Elife. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | You H, Qin S, Zhang F, Hu W, Li X, Liu D, Kong F, Pan X, Zheng K, Tang R. Regulation of Pattern-Recognition Receptor Signaling by HBX During Hepatitis B Virus Infection. Front Immunol. 2022;13:829923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 72. | Cao W, Lu H, Zhang L, Wang S, Deng W, Jiang T, Lin Y, Yang L, Bi X, Lu Y, Shen G, Liu R, Chang M, Wu S, Gao Y, Hao H, Xu M, Chen X, Hu L, Xie Y, Li M. Functional molecular expression of nature killer cells correlated to HBsAg clearance in HBeAg-positive chronic hepatitis B patients during PEG-IFN α-2a therapy. Front Immunol. 2022;13:1067362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Kar A, Samanta A, Mukherjee S, Barik S, Biswas A. The HBV web: An insight into molecular interactomes between the hepatitis B virus and its host en route to hepatocellular carcinoma. J Med Virol. 2023;95:e28436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 74. | Ganesan M, Mathews S, Makarov E, Petrosyan A, Kharbanda KK, Kidambi S, Poluektova LY, Casey CA, Osna NA. Acetaldehyde suppresses HBV-MHC class I complex presentation on hepatocytes via induction of ER stress and Golgi fragmentation. Am J Physiol Gastrointest Liver Physiol. 2020;319:G432-G442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Bybee G, Moeun Y, Wang W, Kharbanda KK, Poluektova LY, Kidambi S, Osna NA, Ganesan M. Increased liver stiffness promotes hepatitis B progression by impairing innate immunity in CCl4-induced fibrotic HBV(+) transgenic mice. Front Immunol. 2023;14:1166171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol. 2019;37:457-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1432] [Article Influence: 204.6] [Reference Citation Analysis (10)] |
| 77. | Zhang YX, Ou MY, Yang ZH, Sun Y, Li QF, Zhou SB. Adipose tissue aging is regulated by an altered immune system. Front Immunol. 2023;14:1125395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 78. | Kramvis A, Chang KM, Dandri M, Farci P, Glebe D, Hu J, Janssen HLA, Lau DTY, Penicaud C, Pollicino T, Testoni B, Van Bömmel F, Andrisani O, Beumont-Mauviel M, Block TM, Chan HLY, Cloherty GA, Delaney WE, Geretti AM, Gehring A, Jackson K, Lenz O, Maini MK, Miller V, Protzer U, Yang JC, Yuen MF, Zoulim F, Revill PA. A roadmap for serum biomarkers for hepatitis B virus: current status and future outlook. Nat Rev Gastroenterol Hepatol. 2022;19:727-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 79. | Prange R. Hepatitis B virus movement through the hepatocyte: An update. Biol Cell. 2022;114:325-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 80. | Wan Z, Zhou Z, Liu Y, Lai Y, Luo Y, Peng X, Zou W. Regulatory T cells and T helper 17 cells in viral infection. Scand J Immunol. 2020;91:e12873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 81. | Mancuso S, Mattana M, Carlisi M, Santoro M, Siragusa S. Effects of B-Cell Lymphoma on the Immune System and Immune Recovery after Treatment: The Paradigm of Targeted Therapy. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 82. | Wu W, Sun S, Wang Y, Zhao R, Ren H, Li Z, Zhao H, Zhang Y, Sheng J, Chen Z, Shi Y. Circulating Neutrophil Dysfunction in HBV-Related Acute-on-Chronic Liver Failure. Front Immunol. 2021;12:620365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | You H, Zhang N, Yu T, Ma L, Li Q, Wang X, Yuan D, Kong D, Liu X, Hu W, Liu D, Kong F, Zheng K, Tang R. Hepatitis B virus X protein promotes MAN1B1 expression by enhancing stability of GRP78 via TRIM25 to facilitate hepatocarcinogenesis. Br J Cancer. 2023;128:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Suresh M, Menne S. Recent Drug Development in the Woodchuck Model of Chronic Hepatitis B. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 85. | Ma L, Sun X, Kong X, Gao Y. B cell dysfunction in chronic hepatitis B virus infection. Liver Res. 2021;5:11-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Mori T, Yoshio S, Yoshikawa S, Tsustui Y, Sakata T, Yoshida Y, Sakamoto Y, Kawai H, Osawa Y, Yamazoe T, Aoki Y, Fletcher SP, Kanto T. Toll-like receptor 7 agonist, GS-986, is an immune-stimulant inducing follicular helper T cells and expanding HBs antigen-specific B cells in vitro. Liver Int. 2023;43:1213-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Lam JH, Smith FL, Baumgarth N. B Cell Activation and Response Regulation During Viral Infections. Viral Immunol. 2020;33:294-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 88. | Meng Z, Chen Y, Lu M. Advances in Targeting the Innate and Adaptive Immune Systems to Cure Chronic Hepatitis B Virus Infection. Front Immunol. 2019;10:3127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 89. | Zhao H, Yu Y, Wang Y, Zhao L, Yang A, Hu Y, Pan Z, Wang Z, Yang J, Han Q, Tian Z, Zhang J. Cholesterol accumulation on dendritic cells reverses chronic hepatitis B virus infection-induced dysfunction. Cell Mol Immunol. 2022;19:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Shi Y, Wang Z, Ge S, Xia N, Yuan Q. Hepatitis B Core Antibody Level: A Surrogate Marker for Host Antiviral Immunity in Chronic Hepatitis B Virus Infections. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Kawagishi N, Suda G, Sakamori R, Matsui T, Onozawa M, Yang Z, Yoshida S, Ohara M, Kimura M, Kubo A, Maehara O, Fu Q, Hosoda S, Tokuchi Y, Suzuki K, Nakai M, Sho T, Morikawa K, Natsuizaka M, Ogawa K, Sakai H, Ohnishi S, Baba M, Takehara T, Sakamoto N. Serum IL-1β predicts de novo hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C, not during anti-cancer/immunosuppressive therapy. Sci Rep. 2022;12:16800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Al-Shimari FH, Rencken CA, Kirkwood CD, Kumar R, Vannice KS, Stewart BT. Systematic review of global hepatitis E outbreaks to inform response and coordination initiatives. BMC Public Health. 2023;23:1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 93. | Liu J, Lo CM, Man K. Role of intrahepatic regional immunity in post-transplant cancer recurrence. Engineering. 2022;10:57-64. [DOI] [Full Text] |
| 94. | Kayesh MEH, Hashem MA, Kohara M, Tsukiyama-Kohara K. In vivo Delivery Tools for Clustered Regularly Interspaced Short Palindromic Repeat/Associated Protein 9-Mediated Inhibition of Hepatitis B Virus Infection: An Update. Front Microbiol. 2022;13:953218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 95. | Datfar T, Doulberis M, Papaefthymiou A, Hines IN, Manzini G. Viral Hepatitis and Hepatocellular Carcinoma: State of the Art. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Hernandez N, Bessone F. Hepatotoxicity Induced by Biological Agents: Clinical Features and Current Controversies. J Clin Transl Hepatol. 2022;10:486-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Antar SA, Ashour NA, Marawan ME, Al-Karmalawy AA. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 212] [Reference Citation Analysis (0)] |
| 98. | Lee C, Kim M, Han J, Yoon M, Jung Y. Mesenchymal Stem Cells Influence Activation of Hepatic Stellate Cells, and Constitute a Promising Therapy for Liver Fibrosis. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Han F, Cao D, Zhu X, Shen L, Wu J, Chen Y, Xu Y, Xu L, Cheng X, Zhang Y. Construction and validation of a prognostic model for hepatocellular carcinoma: Inflammatory ferroptosis and mitochondrial metabolism indicate a poor prognosis. Front Oncol. 2022;12:972434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 100. | Jagdish RK, Roy A, Kumar K, Premkumar M, Sharma M, Rao PN, Reddy DN, Kulkarni AV. Pathophysiology and management of liver cirrhosis: from portal hypertension to acute-on-chronic liver failure. Front Med (Lausanne). 2023;10:1060073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 101. | Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 375] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 102. | Gherlan GS. Occult hepatitis B - the result of the host immune response interaction with different genomic expressions of the virus. World J Clin Cases. 2022;10:5518-5530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Zaltron S, Cambianica A, Di Gregorio M, Colangelo C, Storti S, Tiecco G, Castelli F, Quiros-Roldan E. Case report: An occult hepatitis B virus infection reactivation in an HIV/HCV coinfected patient during an immune reconstitution inflammatory syndrome. Front Cell Infect Microbiol. 2023;13:1143346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 104. | Iacob DG, Luminos M, Benea OE, Tudor AM, Olariu CM, Iacob SA, Ruta S. Liver fibrosis progression in a cohort of young HIV and HIV/ HBV co-infected patients: A longitudinal study using non-invasive APRI and Fib-4 scores. Front Med (Lausanne). 2022;9:888050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 105. | Zhou Q, Zhang Q, Wang K, Huang T, Deng S, Wang Y, Cheng C. Anti-rheumatic drug-induced hepatitis B virus reactivation and preventive strategies for hepatocellular carcinoma. Pharmacol Res. 2022;178:106181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Midorikawa Y, Takayama T, Moriguchi M, Yagi R, Yamagishi S, Nakayama H, Aramaki O, Yamazaki S, Tsuji S, Higaki T. Liver Resection Versus Embolization for Recurrent Hepatocellular Carcinoma. World J Surg. 2020;44:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Shiri Aghbash P, Ebrahimzadeh Leylabadlo H, Fathi H, Bahmani M, Chegini R, Bannazadeh Baghi H. Hepatic Disorders and COVID-19: From Pathophysiology to Treatment Strategy. Can J Gastroenterol Hepatol. 2022;2022:4291758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Zaki MYW, Fathi AM, Samir S, Eldafashi N, William KY, Nazmy MH, Fathy M, Gill US, Shetty S. Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 109. | Feitelson MA, Arzumanyan A, Spector I, Medhat A. Hepatitis B x (HBx) as a Component of a Functional Cure for Chronic Hepatitis B. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 110. | Sivasudhan E, Blake N, Lu Z, Meng J, Rong R. Hepatitis B Viral Protein HBx and the Molecular Mechanisms Modulating the Hallmarks of Hepatocellular Carcinoma: A Comprehensive Review. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 111. | Du G, Yang R, Qiu J, Xia J. Multifaceted Influence of Histone Deacetylases on DNA Damage Repair: Implications for Hepatocellular Carcinoma. J Clin Transl Hepatol. 2023;11:231-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 112. | MacDonald CA, Qian H, Pundir P, Kulka M. Sodium butyrate supresses malignant human mast cell proliferation, downregulates expression of KIT and promotes differentiation. Front Allergy. 2023;4:1109717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 113. | Chekol Abebe E, Asmamaw Dejenie T, Mengie Ayele T, Dagnew Baye N, Agegnehu Teshome A, Tilahun Muche Z. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J Inflamm Res. 2021;14:75-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 114. | Ogunnaike M, Das S, Raut SS, Sultana A, Nayan MU, Ganesan M, Edagwa BJ, Osna NA, Poluektova LY. Chronic Hepatitis B Infection: New Approaches towards Cure. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 115. | Umemura M, Ogawa K, Morikawa K, Kubo A, Tokuchi Y, Yamada R, Kitagataya T, Shigesawa T, Shimazaki T, Kimura M, Suzuki K, Nakamura A, Ohara M, Kawagishi N, Izumi T, Nakai M, Sho T, Suda G, Natsuizaka M, Ono K, Murata K, Sugiyama M, Mizokami M, Sakamoto N. Effects of nucleos(t)ide analogs on hepatitis B surface antigen reduction with interferon-lambda 3 induction in chronic hepatitis B patients. Hepatol Res. 2022;52:586-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Yip TCF, Lai JCT, Liang LY, Hui VWK, Wong VWS, Wong GLH. Risk of HCC in Patients with HBV, Role of Antiviral Treatment. Curr Hepatology Rep. 2022;21:76-86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Osmani Z, Boonstra A. Recent Insights into the Role of B Cells in Chronic Hepatitis B and C Infections. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 118. | Pishavar E, Oroojalian F, Salmasi Z, Hashemi E, Hashemi M. Recent advances of dendrimer in targeted delivery of drugs and genes to stem cells as cellular vehicles. Biotechnol Prog. 2021;37:e3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Hui RWH, Mak LY, Cheung KS, Fung J, Seto WK, Yuen MF. Novel Combination Strategies With Investigational Agents for Functional Cure of Chronic Hepatitis B Infection. Curr Hepatology Rep. 2022;21:59-67. [DOI] [Full Text] |
| 120. | Degasperi E, Anolli MP, Lampertico P. Towards a Functional Cure for Hepatitis B Virus: A 2022 Update on New Antiviral Strategies. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 121. | Zi J, Gao X, Du J, Xu H, Niu J, Chi X. Multiple Regions Drive Hepatitis Delta Virus Proliferation and Are Therapeutic Targets. Front Microbiol. 2022;13:838382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 122. | Oh H, Lee HY, Kim J, Kim YJ. Systematic Review with Meta-Analysis: Comparison of the Risk of Hepatocellular Carcinoma in Antiviral-Naive Chronic Hepatitis B Patients Treated with Entecavir versus Tenofovir: The Devil in the Detail. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Shahini E, Donghia R, Facciorusso A. The power of prevention: how tenofovir and entecavir are changing the game in hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2023;12:936-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 124. | Suda G, Baba M, Yamamoto Y, Sho T, Ogawa K, Kimura M, Hosoda S, Yoshida S, Kubo A, Fu Q, Yang Z, Tokuchi Y, Kitagataya T, Maehara O, Ohnishi S, Yamada R, Ohara M, Kawagishi N, Natsuizaka M, Nakai M, Morikawa K, Furuya K, Suzuki K, Izumi T, Meguro T, Terashita K, Ito J, Kobayashi T, Tsunematsu I, Sakamoto N. Prophylactic tenofovir alafenamide for hepatitis B virus reactivation and reactivation-related hepatitis. J Med Virol. 2023;95:e28452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 125. | Mizushima D, Takano M, Aoki T, Ando N, Uemura H, Yanagawa Y, Watanabe K, Gatanaga H, Kikuchi Y, Oka S. Effect of tenofovir-based HIV pre-exposure prophylaxis against HBV infection in men who have sex with men. Hepatology. 2023;77:2084-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 126. | Hsu CW, Chen SC, Wang PN, Wang HM, Chen YC, Yeh CT. Preventing viral relapse with prophylactic tenofovir in hepatitis B carriers receiving chemotherapy: A phase IV randomized study in Taiwan. 2023. [cited 22 December 2023]. Available from: https://www.researchsquare.com/article/rs-3013457/v1. |
| 127. | Fu S, Zhang Q, Jing R, Zu C, Ni F, Lv Y, Cui J, Zheng H, Zhang Y, Zhang M, Wei G, Cen Z, Chang AH, Hu Y, Huang H. HBV reactivation in patients with chronic or resolved HBV infection following BCMA-targeted CAR-T cell therapy. Bone Marrow Transplant. 2023;58:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 128. | Ding ZN, Meng GX, Xue JS, Yan LJ, Liu H, Yan YC, Chen ZQ, Hong JG, Wang DX, Dong ZR, Li T. Hepatitis B virus reactivation in patients undergoing immune checkpoint inhibition: systematic review with meta-analysis. J Cancer Res Clin Oncol. 2023;149:1993-2008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Di Stefano M, Faleo G, Leitner T, Zheng W, Zhang Y, Hassan A, Alwazzeh MJ, Fiore JR, Ismail M, Santantonio TA. Molecular and Genetic Characterization of Hepatitis B Virus (HBV) among Saudi Chronically HBV-Infected Individuals. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 130. | Deng M, Tong M, Fu F, Wei D. Comparative untargeted metabolomics analysis of serum metabolic alterations in patients infected with hepatitis B virus genotypes B and C. Arab J Chem. 2023;16:105155. [DOI] [Full Text] |
| 131. | Wang X, Liu X, Wang P, Yu L, Yan F, Yan H, Zhou D, Yang Z. Antiviral Therapy Reduces Mortality in Hepatocellular Carcinoma Patients with Low-Level Hepatitis B Viremia. J Hepatocell Carcinoma. 2021;8:1253-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 132. | Block TM, Chang KM, Guo JT. Prospects for the Global Elimination of Hepatitis B. Annu Rev Virol. 2021;8:437-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 133. | Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019;13:227-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 134. | Abdelaal MA. Lamivudine: An antiviral drug with high risk factor for selection of resistance in HBV patients. Rec of Pharm and Bio Sci. 2021;5:81-84. [DOI] [Full Text] |
| 135. | Mokaya J, McNaughton AL, Bester PA, Goedhals D, Barnes E, Marsden BD, Matthews PC. Hepatitis B virus resistance to tenofovir: fact or fiction? A systematic literature review and structural analysis of drug resistance mechanisms. Wellcome Open Res. 2020;5:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 136. | Pley C, Lourenço J, McNaughton AL, Matthews PC. Spacer Domain in Hepatitis B Virus Polymerase: Plugging a Hole or Performing a Role? J Virol. 2022;96:e0005122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 137. | Su YT, Chang ML, Chien RN, Liaw YF. Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Mican R, Busca Arenzana C, Vasquez J, Daroca G, Perez-Valero I, Martin-Carbonero L. Hepatitis B reactivation after tenofovir withdrawal in an HIV-infected patient with history of cured hepatitis B virus infection and poor immunological status. AIDS. 2021;35:1707-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | Kayesh MEH, Kohara M, Tsukiyama-Kohara K. Toll-Like Receptor Response to Hepatitis B Virus Infection and Potential of TLR Agonists as Immunomodulators for Treating Chronic Hepatitis B: An Overview. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 140. | Shen J, Wang X, Wang N, Wen S, Yang G, Li L, Fu J, Pan X. HBV reactivation and its effect on survival in HBV-related hepatocarcinoma patients undergoing transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors. Front Cell Infect Microbiol. 2023;13:1179689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 141. | Watanabe T, Inoue T, Tanaka Y. Hepatitis B Core-Related Antigen and New Therapies for Hepatitis B. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 142. | Xu H, Locarnini S, Wong D, Hammond R, Colledge D, Soppe S, Huynh T, Shaw T, Thompson AJ, Revill PA, Hogarth PM, Wines BD, Walsh R, Warner N. Role of anti-HBs in functional cure of HBeAg+ chronic hepatitis B patients infected with HBV genotype A. J Hepatol. 2022;76:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Huang SC, Yang HC, Kao JH. Hepatitis B reactivation: diagnosis and management. Expert Rev Gastroenterol Hepatol. 2020;14:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 144. | Herr KJ, Shen SP, Liu Y, Yang CC, Tang CH. The growing burden of generalized myasthenia gravis: a population-based retrospective cohort study in Taiwan. Front Neurol. 2023;14:1203679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 145. | Feld JJ, Lok AS, Zoulim F. New Perspectives on Development of Curative Strategies for Chronic Hepatitis B. Clin Gastroenterol Hepatol. 2023;21:2040-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 146. | Buschow SI, Jansen DTSL. CD4(+) T Cells in Chronic Hepatitis B and T Cell-Directed Immunotherapy. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 147. | Hui RW, Mak LY, Seto WK, Yuen MF. RNA interference as a novel treatment strategy for chronic hepatitis B infection. Clin Mol Hepatol. 2022;28:408-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 148. | Colombatto P, Coco B, Bonino F, Brunetto MR. Management and Treatment of Patients with Chronic Hepatitis B: Towards Personalized Medicine. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Hudu SA, Jimoh AO, Ibrahim KG, Alshrari AS. Hepatitis B Therapeutic Vaccine: A Patent Review. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 150. | Ruzzi F, Semprini MS, Scalambra L, Palladini A, Angelicola S, Cappello C, Pittino OM, Nanni P, Lollini PL. Virus-like Particle (VLP) Vaccines for Cancer Immunotherapy. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 151. | Chan SL, Wong N, Lam WKJ, Kuang M. Personalized treatment for hepatocellular carcinoma: Current status and future perspectives. J Gastroenterol Hepatol. 2022;37:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Salpini R, D'Anna S, Benedetti L, Piermatteo L, Gill U, Svicher V, Kennedy PTF. Hepatitis B virus DNA integration as a novel biomarker of hepatitis B virus-mediated pathogenetic properties and a barrier to the current strategies for hepatitis B virus cure. Front Microbiol. 2022;13:972687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 153. | Shang D, Wang P, Tang W, Mo R, Lai R, Lu J, Li Z, Wang X, Cai W, Wang H, Zhao G, Xie Q, Xiang X. Genetic Variations of ALDH (rs671) Are Associated With the Persistence of HBV Infection Among the Chinese Han Population. Front Med (Lausanne). 2022;9:811639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 154. | Rajendren S, Karijolich J. The impact of RNA modifications on the biology of DNA virus infection. Eur J Cell Biol. 2022;101:151239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 155. | Salama II, Sami SM, Salama SI, Abdel-Latif GA, Shaaban FA, Fouad WA, Abdelmohsen AM, Raslan HM. Current and novel modalities for management of chronic hepatitis B infection. World J Hepatol. 2023;15:585-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 156. | Manea M, Apostol D, Constantinescu I. The Connection between MiR-122 and Lymphocytes in Patients Receiving Treatment for Chronic Hepatitis B Virus Infection. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 157. | Viswanathan U, Mani N, Hu Z, Ban H, Du Y, Hu J, Chang J, Guo JT. Targeting the multifunctional HBV core protein as a potential cure for chronic hepatitis B. Antiviral Res. 2020;182:104917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 158. | Stroffolini T, Ciancio A, Furlan C, Vinci M, Niro GA, Russello M, Colloredo G, Morisco F, Coppola N, Babudieri S, Ferrigno L, Sagnelli C, Sagnelli E; Collaborating group. Chronic hepatitis B virus infection in Italy during the twenty-first century: an updated survey in 2019. Eur J Clin Microbiol Infect Dis. 2021;40:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 159. | Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 160. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 161. | Gehring AJ, Mendez P, Richter K, Ertl H, Donaldson EF, Mishra P, Maini M, Boonstra A, Lauer G, de Creus A, Whitaker K, Martinez SF, Weber J, Gainor E, Miller V. Immunological biomarker discovery in cure regimens for chronic hepatitis B virus infection. J Hepatol. 2022;77:525-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 162. | Gallage S, García-Beccaria M, Szydlowska M, Rahbari M, Mohr R, Tacke F, Heikenwalder M. The therapeutic landscape of hepatocellular carcinoma. Med. 2021;2:505-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 163. | Yang M, Yang Y, He Q, Zhu P, Liu M, Xu J, Zhao M. Intestinal Microbiota-A Promising Target for Antiviral Therapy? Front Immunol. 2021;12:676232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 164. | Wirusanti NI, Baldridge MT, Harris VC. Microbiota regulation of viral infections through interferon signaling. Trends Microbiol. 2022;30:778-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 165. | Milardi G, Lleo A. Tumor-Infiltrating B Lymphocytes: Promising Immunotherapeutic Targets for Primary Liver Cancer Treatment. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 166. | Wen L, Li G, Huang T, Geng W, Pei H, Yang J, Zhu M, Zhang P, Hou R, Tian G, Su W, Chen J, Zhang D, Zhu P, Zhang W, Zhang X, Zhang N, Zhao Y, Cao X, Peng G, Ren X, Jiang N, Tian C, Chen ZJ. Single-cell technologies: From research to application. Innovation (Camb). 2022;3:100342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotlyarov S, Russia S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ