Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1280
Peer-review started: December 23, 2023
First decision: January 13, 2024
Revised: January 22, 2024
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: March 14, 2024
Processing time: 82 Days and 14.5 Hours
Yu et al’s study in the World Journal of Gastroenterology (2023) introduced a novel regimen of Vonoprazan-amoxicillin dual therapy combined with Saccharomyces boulardii (S. boulardii) for the rescue therapy against Helicobacter pylori (H. pylori), a pathogen responsible for peptic ulcers and gastric cancer. Vonoprazan is a potassium-competitive acid blocker renowned for its rapid and long-lasting acid suppression, which is minimally affected by mealtime. Compared to proton pump inhibitors, which bind irreversibly to cysteine residues in the H+/K+-ATPase pump, Vonoprazan competes with the K+ ions, prevents the ions from binding to the pump and blocks acid secretion. Concerns with increasing antibiotic resistance, effects on the gut microbiota, patient compliance, and side effects have led to the advent of a dual regimen for H. pylori. Previous studies suggested that S. boulardii plays a role in stabilizing the gut barrier which improves H. pylori eradication rate. With an acceptable safety profile, the dual-adjunct regimen was effective regardless of prior treatment failure and antibiotic resistance profile, thereby strengthening the applicability in clinical settings. Nonetheless, S. boulardii comes in various formulations and dosages, warranting further exploration into the optimal dosage for supplementation in rescue therapy. Additionally, larger, randomized, double-blinded controlled trials are warranted to confirm these promising results.
Core Tip: Vonoprazan-amoxicillin dual therapy with Saccharomyces boulardii (S. boulardii, VAS regimen) emerges as a novel rescue therapy for eradicating Helicobacter pylori (H. pylori). Vonoprazan, a potassium-competitive acid blocker, exhibits superior acid suppression compared to proton pump inhibitors. Notably, dual therapy minimizes the use of an additional antibiotic while maintaining efficacy comparable to traditional triple therapy. This paper highlights the role of S. boulardii, a probiotic, in enhancing the efficacy of Vonoprazan dual therapy by restoring gut microbiota balance, directly affecting H. pylori, and regulating immunomodulation. The VAS regimen emerges as a promising treatment alternative, demonstrating remarkable eradication of H. pylori, even in triple-resistant strains.
- Citation: Dirjayanto VJ, Audrey J, Simadibrata DM. Vonoprazan-amoxicillin dual regimen with Saccharomyces boulardii as a rescue therapy for Helicobacter pylori: Current perspectives and implications. World J Gastroenterol 2024; 30(10): 1280-1286
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1280.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1280
Yu et al’s study introduced a novel regimen involving vonoprazan-amoxicillin dual therapy with Saccharomyces boulardii
Dual therapy against H. pylori is a relatively new treatment regimen approach. Previously, the standard triple therapy containing PPIs, Clarithromycin, and Amoxicillin or Metronidazole was the mainstay treatment for H. pylori eradication. However, antibiotic resistance has caused the eradication rate to diminish (< 80%)[6]. In fact, resistance to clarithromycin has continuously increased, with reports in Japan describing an increase from 7% to 28.5% over the course of 14 years[7] and in Australia suggesting an increase of 3.7% annually over the past 20 years[8]. In the United States, eradication rates of triple therapy with PPI have declined to less than 80%, attributable to both antibiotic resistance and failure to maintain the intragastric pH required for effective antimicrobial activity[9].
Yu et al’s prospective single-arm trial in the World Journal of Gastroenterology introduced a novel regimen involving vonoprazan-amoxicillin dual therapy with the addition of S. boulardii (VAS regimen) for the rescue therapy against H. pylori in patients with a history of treatment failure[1]. In this study, the resistance of H. pylori to clarithromycin, metronidazole, and levofloxacin was 91.3%, 100%, and 60.9%, respectively. Overall, the eradication rate of H. pylori was 92.6% (63/68). Interestingly, out of the patients with triple-resistant H. pylori (60.9%; n = 14/23), a 92.9% eradication rate was achieved with the vonoprazan-based rescue therapy. This suggested that such a regimen was effective and safe regardless of antibiotic resistance.
Previous studies investigating treatment-naïve patients showed promising findings for Vonoprazan dual therapy. In Chey et al’s phase 3 randomized controlled trial (RCT), the reported eradication rate for clarithromycin-resistant H. pylori with Vonoprazan-Amoxicillin dual therapy was 69.6%, while eradication rates were 65.8% with vonoprazan-amoxicillin-clarithromycin triple therapy and 31.9% with Lansoprazole triple therapy[9]. Despite not reaching values above 90%, the eradication rate was numerically higher in the dual therapy, suggesting that adding Clarithromycin may be unnecessary in treating clarithromycin-resistant strains. Zuberi et al’s study reported that the vonoprazan-based regimen was superior to the PPI triple therapy regimen in eradicating H. pylori (93.5% vs 83.9%)[10]. Similarly, Liu et al’s network meta-analysis suggested that vonoprazan-based therapies were significantly more effective in eradicating H. pylori than PPI triple therapy, with the best safety profile shown by the vonoprazan dual therapy[11]. Therefore, vonoprazan dual therapy presents as a lower-cost, simple, yet effective treatment option for H. pylori eradication.
As for patients with a history of two treatment failures, vonoprazan-based triple therapy yielded a significantly higher success rate in comparison to esomeprazole-based therapy[12]. Similarly, for third-line therapy, the eradication rate with the vonoprazan-amoxicillin-sitafloxacin regimen was higher than the PPI-based regimen (75.8% vs 53.3%), despite no significant difference in the intention-to-treat analyses (P = 0.071)[13]. The duration of therapy might explain the insignificant difference in eradication rates since Sue et al[13] had prescribed the treatment regimens for only seven days, whereas Yu et al[1] provided the regimen for 14 d. Therefore, although no study has investigated the effect of therapy duration on eradication rates, available data suggest that long-term regimens may be more effective.
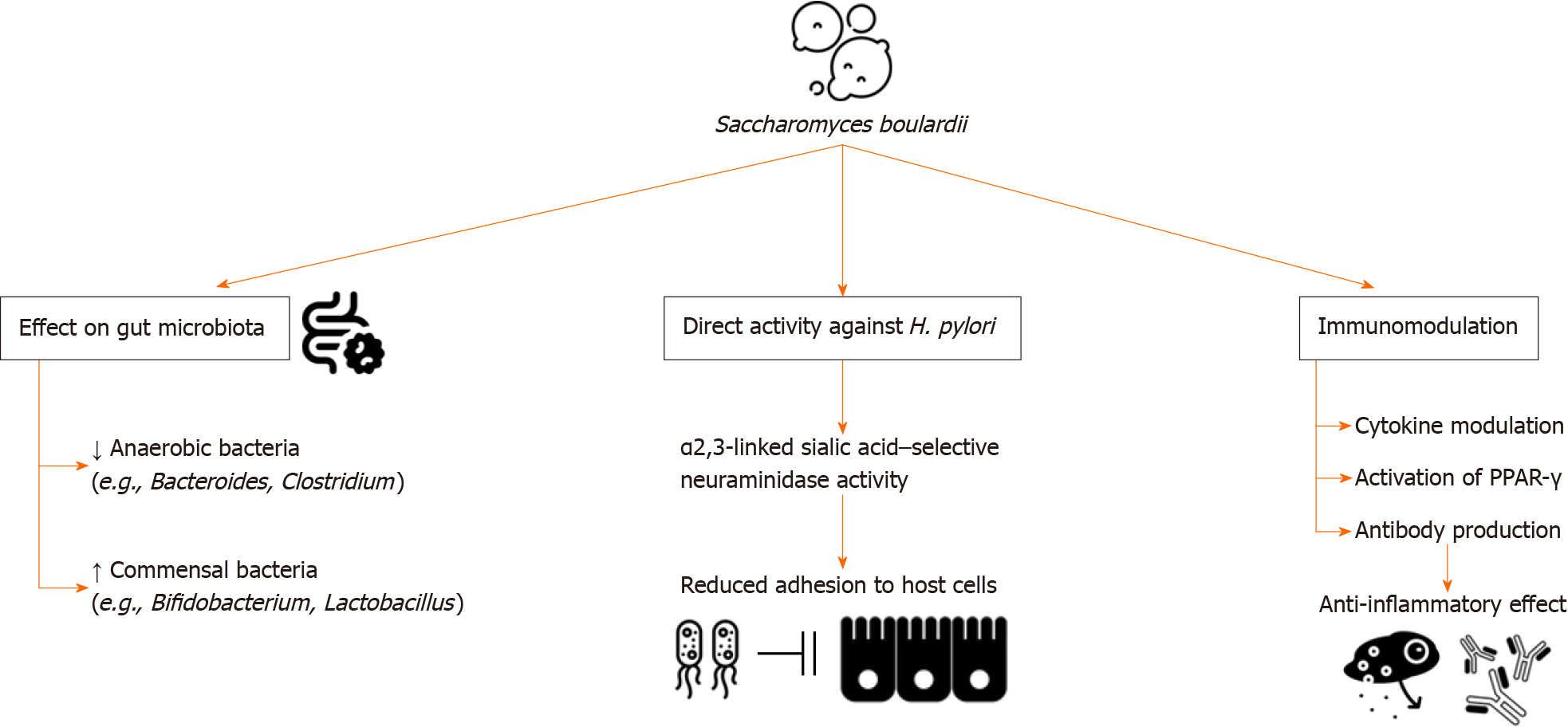
The use of antibiotics in treatment regimens for eradicating H. pylori has been shown to disrupt the balance of gut microbiota and may elicit adverse events. Recent studies have shown that probiotics, including S. boulardii, may contribute to mitigating these effects by: (1) Maintaining the balance and integrity of the normal gut microbiota; (2) direct activity against the pathogenesis of H. pylori; and (3) immunomodulation (Figure 1)[14]. In the context of therapy, Keikha and Kamali[14] investigated the addition of S. boulardii to the standard triple regimen, and the addition of S. boulardii was shown to be associated with a lower proportion of anaerobic bacteria, including Bacteroides and Clostridium, as well as higher proportion of commensal bacteria including Bifidobacterium and Lactobacillus[14]. Sakarya and Gunay[15] in vivo study proved that S. boulardii may also directly contribute to the eradication of H. pylori via the α2,3-linked sialic acid–selective neuraminidase activity, leading to reduced adhesion to host cells. Additionally, S. boulardii may produce proteins that modulate cytokines, contribute to the activation of peroxisome proliferator-activated receptor-gamma, and trigger antibody production, leading to an anti-inflammatory effect that sustains the gastrointestinal system[16].
Yu et al[1] were the first to investigate the combination of a vonoprazan dual regimen with S. boulardii as an adjunct therapy, providing new insights into its efficacy in rescue therapy. This data builds on previous studies investigating the addition of S. boulardii for standard triple or quadruple therapy. Qu et al[17] provided a two-stage intervention for rescue therapy, during which the patients were administered S. boulardii as monotherapy for two weeks, followed by bismuth quadruple therapy if required. The eradication rates in patients receiving S. boulardii were reported to be higher than those who did not receive S. boulardii, thus supporting its effectiveness. However, several other studies, including Zojaji et al[18], did not show any significant improvement in eradication with the addition of S. boulardii; however, it was noted that the side effects, including nausea, bloating, and diarrhea, were lower. In addition, a meta-analysis by Liu et al[11] investigating S. boulardii in addition to standard triple therapy suggested that it yielded beneficial outcomes in eradication, the occurrence of adverse effects, and symptom reduction. Another meta-analysis of 18 RCTs by Zhou et al[19] demonstrated a slight pooled improvement in the eradication rate by 9% while decreasing the adverse effects by half. Therefore, while the effectiveness of adding S. boulardii for eradicating H. pylori might be modest, the reduction of adverse effects makes it worthwhile for this probiotic to be added to the regimen.
Demographic factors such as age, gender, smoking history, and alcohol consumption were not associated with the VAS treatment regimen failure in Yu et al[1] study. Several socio-demographic characteristics, such as gender and areas of residence, have been significantly associated with H. pylori eradication failure[20] despite inconsistent results observed across studies[21,22]. This suggests that the impact of socioeconomic and demographic factors on treatment outcomes may vary across different patient populations. Such variations may stem from differences in antibiotic usage patterns, antimicrobial resistance, and medication adherence[23-25].
It is interesting to note that anxiety was identified as a risk factor for treatment failure with the VAS treatment regimen. This finding aligns with previous research linking psychological factors to dyspeptic symptoms. For instance, patients with disorders of the gut-brain interaction were noted to have higher rates of anxiety and depression[26]. This relationship may be correlated to the intricate brain-gut axis, a circuit linking the central, peripheral, and autonomic nervous systems with gastrointestinal functions. Gut microorganisms, including H. pylori infection, were hypothesized to interact with this axis, as evident by the observation that stress and emotional disorders negatively impact intestinal flora and digestive function[27]. Further investigations suggest that this relationship may be bidirectional. H. pylori infection was associated with altered eating behavior, anxiety and depression-like behaviors, cognitive dysfunction, and lower pain thresholds[27-29]. On the other hand, a study in mice models by Guo et al[30] demonstrated that the induction of psychological stress significantly increased H. pylori colonization and was associated with more extensive gastric mucosal injury. The underlying mechanisms of altered brain-gut axis potentially involve direct neurotoxic effects, activation of proinflammatory responses, and micronutrient deficiencies, areas which are still highly subject to research[27]. The complex interplay between psychological disorders and H. pylori infection underscores the importance of psychological assessments and interventions, such as cognitive behavioral therapy or counseling sessions, to enhance treatment success in H. pylori infections[31,32].
Notably, Yu et al[1] also showed that the number of previous treatment failures was not associated with treatment failure in this VAS regimen. Eradication rates were consistently high, irrespective of resistance to clarithromycin and levofloxacin. This is in contrast to a prior study that identified any prior exposure to antibiotics as a risk factor for treatment failure with a clarithromycin-containing triple therapy regimen[33]. Clarithromycin resistance was shown to be associated with Metronidazole resistance[34], leading to the prevalence of double-resistant strains, particularly in individuals who had previously failed two eradication treatments[35-38]. Furthermore, sufficient acid inhibition is required for successful H. pylori eradication, as it influences the stability and bioavailability of some antibiotics, including amoxicillin. Eradication failure was often observed in patients who are extensive CYP2C19 metabolizers of PPI, as they exhibit rapid PPI inactivation and insufficient acid suppression[39,40]. Vonoprazan exhibits stronger and longer acid suppression than PPI[41], which may explain the significant superiority of a vonoprazan-based regimen over PPI-based therapy regarding H. pylori eradication success[42]. This suggests the potential use of the VAS regimen as a rescue therapy for H. pylori infections resistant to other essential antibiotics, particularly in the context of increasing global antimicrobial resistance.
The impact of adverse events on therapy discontinuation and treatment adherence is a critical aspect of any treatment regimen. In Yu et al’s study[1], the VAS regimen exhibited a low rate of adverse events, all of which were reported as mild or moderate. The safety profile of vonoprazan, as reported in numerous clinical studies, consistently demonstrates its superiority or, at the very least, equivalence to that of PPIs. A meta-analysis of RCTs demonstrated a significantly lower rate of adverse events with vonoprazan-based triple therapy (32.7%) compared to PPI-based triple therapy (40.5%) while maintaining a higher efficacy in terms of H. pylori eradication rate[43].
Commonly reported adverse events include diarrhea, dysgeusia, loose stool, and skin eruption[44]. While Suzuki et al[45] noted a slightly higher incidence of skin rash in vonoprazan-based therapy, it is noteworthy that the vonoprazan-based regimen was generally well-tolerated, and no instances of therapy discontinuation occurred due to the adverse events.
The superiority of the vonoprazan-based regimen in terms of both efficacy and safety highlights its potential as an excellent alternative for H. pylori treatment and positions it as an effective option for rescue therapy. Notably, the vonoprazan-amoxicillin dual therapy has exhibited acceptable efficacy in H. pylori eradication, comparable to the outcomes of vonoprazan-based triple therapy. Given the increasing rates of clarithromycin resistance in various geographical regions, adding clarithromycin to vonoprazan and amoxicillin may only offer a marginal benefit. The dual regimen minimizes the use of an unnecessary additional antibiotic while maintaining efficacy similar to that of triple therapy, a crucial consideration amid the current surge in antibiotic resistance[46]. Additionally, the supplementation of S. boulardii as an adjunct therapy to vonoprazan-based regimens has shown positive effects on H. pylori eradication and reduced adverse events, possibly attributed to the maintenance of normal gut microbiota.
However, it is essential to acknowledge certain limitations in this study. While this study supported the efficacy of a dual vonoprazan-based regimen with the addition of S. boulardii for rescue therapy, it should be noted that the number of study participants is considered small. Additionally, the generalizability of the reduction of adverse events seen with the addition of S. boulardii might be limited since different populations possess different gut microbiota, which is affected by geography and dietary habits[47]. Given the various formulations and dosages available, further exploration is needed to determine the optimal dosing of S. boulardii supplementation in such rescue therapy. Lastly, as this study employed a single-arm design, direct comparisons of the VAS regimen to currently recommended regimens are lacking. Therefore, randomized double-blinded controlled trials with large sample sizes are required to validate these promising results.
| 1. | Yu J, Lv YM, Yang P, Jiang YZ, Qin XR, Wang XY. Safety and effectiveness of vonoprazan-based rescue therapy for Helicobacter pylori infection. World J Gastroenterol. 2023;29:3133-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Garnock-Jones KP. Vonoprazan: first global approval. Drugs. 2015;75:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Laine L, Sharma P, Mulford DJ, Hunt B, Leifke E, Smith N, Howden CW. Pharmacodynamics and Pharmacokinetics of the Potassium-Competitive Acid Blocker Vonoprazan and the Proton Pump Inhibitor Lansoprazole in US Subjects. Am J Gastroenterol. 2022;117:1158-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Luo HJ, Deng WQ, Zou K. Protonated form: the potent form of potassium-competitive acid blockers. PLoS One. 2014;9:e97688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Therap Adv Gastroenterol. 2018;11:1756283X17745776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Cho JH, Jin SY. Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea. World J Clin Cases. 2022;10:6349-6359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (9)] |
| 7. | Okamura T, Suga T, Nagaya T, Arakura N, Matsumoto T, Nakayama Y, Tanaka E. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter. 2014;19:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Schubert JP, Warner MS, Rayner CK, Roberts-Thomson IC, Mangoni AA, Costello S, Bryant RV. Increasing Helicobacter pylori clarithromycin resistance in Australia over 20 years. Intern Med J. 2022;52:1554-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology. 2022;163:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (2)] |
| 10. | Zuberi BF, Ali FS, Rasheed T, Bader N, Hussain SM, Saleem A. Comparison of Vonoprazan and Amoxicillin Dual Therapy with Standard Triple Therapy with Proton Pump Inhibitor for Helicobacter Pylori eradication: A Randomized Control Trial. Pak J Med Sci. 2022;38:965-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Liu L, Li F, Shi H, Nahata MC. The Efficacy and Safety of Vonoprazan and Amoxicillin Dual Therapy for Helicobacter pylori Infection: A Systematic Review and Network Meta-Analysis. Antibiotics (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Saito Y, Konno K, Sato M, Nakano M, Kato Y, Saito H, Serizawa H. Vonoprazan-Based Third-Line Therapy Has a Higher Eradication Rate against Sitafloxacin-Resistant Helicobacter pylori. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Sue S, Shibata W, Sasaki T, Kaneko H, Irie K, Kondo M, Maeda S. Randomized trial of vonoprazan-based vs proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol. 2019;34:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Keikha M, Kamali H. The impact of Saccharomyces boulardii adjuvant supplementation on alternation of gut microbiota after H. pylori eradication; a metagenomics analysis. Gene Rep. 2022;26:101499. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Sakarya S, Gunay N. Saccharomyces boulardii expresses neuraminidase activity selective for α2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS. 2014;122:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther. 2009;30:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Qu P, Liu X, Xia X, Xie X, Luo J, Cheng S, Chi J, Liu P, Li H, Zhao W, Yang H, Xu C. Saccharomyces boulardii Allows Partial Patients to Avoid Reusing Bismuth Quadruple for Helicobacter pylori Rescue Therapy: A Single-Center Randomized Controlled Study. Front Cell Infect Microbiol. 2022;12:903002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Zojaji H, Ghobakhlou M, Rajabalinia H, Ataei E, Jahani Sherafat S, Moghimi-Dehkordi B, Bahreiny R. The efficacy and safety of adding the probiotic Saccharomyces boulardiito standard triple therapy for eradication of H.pylori: a randomized controlled trial. Gastroenterol Hepatol Bed Bench. 2013;6:S99-S104. [PubMed] |
| 19. | Zhou BG, Chen LX, Li B, Wan LY, Ai YW. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019;24:e12651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Peña-Galo E, Gotor J, Harb Y, Alonso M, Alcedo J. Socioeconomic and demographic factors associated with failure in Helicobacter pylori eradication using the standard triple therapy. Gastroenterol Hepatol Bed Bench. 2021;14:53-58. [PubMed] |
| 21. | Smith S, Jolaiya T, Fowora M, Palamides P, Ngoka F, Bamidele M, Lesi O, Onyekwere C, Ugiagbe R, Agbo I, Ndububa D, Adekanle O, Adedeji A, Adeleye I, Harrison U. Clinical and Socio- Demographic Risk Factors for Acquisition of Helicobacter pylori Infection in Nigeria. Asian Pac J Cancer Prev. 2018;19:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Gebeyehu E, Nigatu D, Engidawork E. Helicobacter pylori eradication rate of standard triple therapy and factors affecting eradication rate at Bahir Dar city administration, Northwest Ethiopia: A prospective follow up study. PLoS One. 2019;14:e0217645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Nayar DS. Current eradication rate of Helicobacter pylori with clarithromycin-based triple therapy in a gastroenterology practice in the New York metropolitan area. Infect Drug Resist. 2018;11:205-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Lim SG, Park RW, Shin SJ, Yoon D, Kang JK, Hwang JC, Kim SS, Kim JH, Lee KM. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig Liver Dis. 2016;48:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Lefebvre M, Chang HJ, Morse A, van Zanten SV, Goodman KJ; CANHelp Working Group. Adherence and barriers to H. pylori treatment in Arctic Canada. Int J Circumpolar Health. 2013;72:22791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Xiong RG, Li J, Cheng J, Zhou DD, Wu SX, Huang SY, Saimaiti A, Yang ZJ, Gan RY, Li HB. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 27. | Budzyński J, Kłopocka M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J Gastroenterol. 2014;20:5212-5225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 28. | Bercik P, Verdú EF, Foster JA, Lu J, Scharringa A, Kean I, Wang L, Blennerhassett P, Collins SM. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009;296:R587-R594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 29. | Gorlé N, Bauwens E, Haesebrouck F, Smet A, Vandenbroucke RE. Helicobacter and the Potential Role in Neurological Disorders: There Is More Than Helicobacter pylori. Front Immunol. 2020;11:584165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Guo G, Jia KR, Shi Y, Liu XF, Liu KY, Qi W, Guo Y, Zhang WJ, Wang T, Xiao B, Zou QM. Psychological stress enhances the colonization of the stomach by Helicobacter pylori in the BALB/c mouse. Stress. 2009;12:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kabeer KK, Ananthakrishnan N, Anand C, Balasundaram S. Prevalence of Helicobacter Pylori Infection and Stress, Anxiety or Depression in Functional Dyspepsia and Outcome after Appropriate Intervention. J Clin Diagn Res. 2017;11:VC11-VC15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Haag S, Senf W, Tagay S, Langkafel M, Braun-Lang U, Pietsch A, Heuft G, Talley NJ, Holtmann G. Is there a benefit from intensified medical and psychological interventions in patients with functional dyspepsia not responding to conventional therapy? Aliment Pharmacol Ther. 2007;25:973-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Guo CG, Jiang F, Cheung KS, Li B, Ooi PH, Leung WK. Timing of prior exposure to antibiotics and failure of Helicobacter pylori eradication: a population-based study. J Antimicrob Chemother. 2022;77:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Heep M, Kist M, Strobel S, Beck D, Lehn N. Secondary resistance among 554 isolates of Helicobacter pylori after failure of therapy. Eur J Clin Microbiol Infect Dis. 2000;19:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 691] [Article Influence: 31.4] [Reference Citation Analysis (2)] |
| 36. | Gisbert JP. "Rescue" regimens after Helicobacter pylori treatment failure. World J Gastroenterol. 2008;14:5385-5402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 37. | Cammarota G, Martino A, Pirozzi G, Cianci R, Branca G, Nista EC, Cazzato A, Cannizzaro O, Miele L, Grieco A, Gasbarrini A, Gasbarrini G. High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;19:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 38. | Hwang JY, Kim C, Kwon YH, Lee JE, Jeon SW, Nam SY, Seo AN, Han MH, Park JH. Dual Clarithromycin and Metronidazole Resistance Is the Main Cause of Failure in Ultimate Helicobacter pylori Eradication. Dig Dis. 2021;39:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Kuo CH, Lu CY, Shih HY, Liu CJ, Wu MC, Hu HM, Hsu WH, Yu FJ, Wu DC, Kuo FC. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014;20:16029-16036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (2)] |
| 40. | Zhao X, Zhang Z, Lu F, Xiong M, Jiang L, Tang K, Fu M, Wu Y, He B. Effects of CYP2C19 genetic polymorphisms on the cure rates of H. pylori in patients treated with the proton pump inhibitors: An updated meta-analysis. Front Pharmacol. 2022;13:938419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 41. | Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, Miyajima H, Furuta T. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 42. | Shinozaki S, Kobayashi Y, Osawa H, Sakamoto H, Hayashi Y, Lefor AK, Yamamoto H. Effectiveness and Safety of Vonoprazan versus Proton Pump Inhibitors for Second-Line Helicobacter pylori Eradication Therapy: Systematic Review and Meta-Analysis. Digestion. 2021;102:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | Lyu QJ, Pu QH, Zhong XF, Zhang J. Efficacy and Safety of Vonoprazan-Based versus Proton Pump Inhibitor-Based Triple Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Randomized Clinical Trials. Biomed Res Int. 2019;2019:9781212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Maruyama M, Tanaka N, Kubota D, Miyajima M, Kimura T, Tokutake K, Imai R, Fujisawa T, Mori H, Matsuda Y, Wada S, Horiuchi A, Kiyosawa K. Vonoprazan-Based Regimen Is More Useful than PPI-Based One as a First-Line Helicobacter pylori Eradication: A Randomized Controlled Trial. Can J Gastroenterol Hepatol. 2017;2017:4385161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 45. | Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The Efficacy and Tolerability of a Triple Therapy Containing a Potassium-Competitive Acid Blocker Compared With a 7-Day PPI-Based Low-Dose Clarithromycin Triple Therapy. Am J Gastroenterol. 2016;111:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Zhang WL, Lin BS, Li YY, Ding YM, Han ZX, Ji R. Efficacy and Safety of Vonoprazan and Amoxicillin Dual Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis. Digestion. 2023;104:249-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Senghor B, Sokhna C, Ruimy R, Lagier JC. Gut microbiota diversity according to dietary habits and geographical provenance. Human Microbiome J. 2018;7–8:1-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oka A, Japan S-Editor: Lin C L-Editor: A P-Editor: Zhao YQ