Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.208
Revised: April 14, 1997
Accepted: May 6, 1997
Published online: December 15, 1997
AIM: To study the clinical significance of detecting the expression of CD44v mRNA in the blood of patients with hepatocellular carcinoma (HCC).
METHODS: The expression of CD44v mRNA was detected in blood with RT and diploid PCR, and the clinical significance was discussed based on the result of pathological examination and follow-up.
RESULTS: CD44v mRNA was detected in the blood of 10/15 patients, giving a positive rate of 66.67%. In the 13 patients who showed response in the follow up period, the CD44v mRNA expression was positive in 9 and negative in 4. Recurrence rate was higher in the patients with positive CD44v mRNA expression than in those with negative CD44v mRNA expression, and the clinical pathological indices were also higher in the former than in the latter.
CONCLUSION: Detection of the expression level of CD44v mRNA in blood of the patients with HCC can be used as an adjuvant means for differential diagnosis, prediction and monitoring of HCC recurrence.
- Citation: Liu PF, Wu MC, Cheng H, Qian GX, Fu JL. Clinical significance of CD44v mRNA detection by PCR in peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol 1997; 3(4): 208-209
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.208
CD44 is an adhesive molecule located on the cell surface, where it participates in cell-cell and cell-matrix interactions[1,2]. “Standard” CD44 can be modified by the insertion of transcripts from at least five extra exons[3], after which splice variants of CD44 (CD44v) can be produced. A single cell may contain two or more variant mRNAs from the same gene simultaneously. CD44v may play an important role in tumor growth and metastasis. The human homologue of rat metastasis-associated CD44v has been isolated from the cell lines of human non-small cell lung carcinoma, colon carcinoma, and breast carcinoma[4]. Furthermore, CD44v expression has been detected in fresh human tumor tissues of the breast, colon[7], and gastrointestinal tract[8], as well as in melanoma[9] and metastases in brain[10].
Gene expression of a single tumor cell in 107 white blood cells can be detected by sensitive RT-PCR[7]. Thus, it is possible to detect the gene expression of a free tumor cell in the blood of patients with malignant diseases. In the study described herein, we explored an auxiliary method for the diagnosis and monitoring of recurrence of malignant tumors by detecting CC44v mRNA expression in the peripheral blood of patients with hepatocellular carcinoma (HCC).
On a Ficoll-Hypaque gradient, free tumor cells were collected from blood samples obtained from the peripheral vein of 15 patients with HCC prior to surgical intervention. Meanwhile, tumor tissue samples were obtained from the resected HCC specimens and control blood samples were obtained from 16 patients with benign diseases (including chronic cholecystitis, chronic abscess of liver, chronic hepatitis B, cirrhosis, inflammatory pseudotumor of liver, and cavernous hemangioma of liver). All samples were stored at 196 °C and kept frozen until use.
RNA was extracted from the free tumor cells and the tumor tissue samples according to the method of Chomzynski and Sacchi[11]. RT was carried out on the total RNA sample using an AMV reverse transcriptase kit (Promega Co., USA). First-strand cDNA was synthesized at 42 °C for 60 min in 20 μL reaction solution. The first round of PCR was carried out with the synthesized cDNA in 50 μL reaction solution (upstream primer: 5’-GACAGACACCTCAGTTTTTCTGGA-3’; downstream primer: 5’- TTCCTTCGTGTGTGGGTAATGAGA-3’) for 30 cycles. The conditions of PCR were 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1.5 min. The second round of PCR was carried out under the same conditions as the first round (upstream primer: 5’-GACAGACACCTCAGTTTTTCTGGA-3’; downstream primer: 5’-TTCCTTCGTGTGTGGGTAATGAGA-3’) fro 30 cycles. PCR products (20 μL) were separated on an ethidium bromide-stained 1.5% agarose gel and analyzed.
All tumor samples were observed under microscopy to determine whether the tumor capsule was intact or deficient, whether it had been penetrated by tumor cells, whether tumor cells had migrated into the portal vein of normal liver tissues, and whether daughter tumor nodules appeared in normal liver tissues near the main mass. Follow-up after tumor resection was carried out for 20-22 mo and was conducted by questionnaire.
Statistical analysis was carried out using the chi-square test.
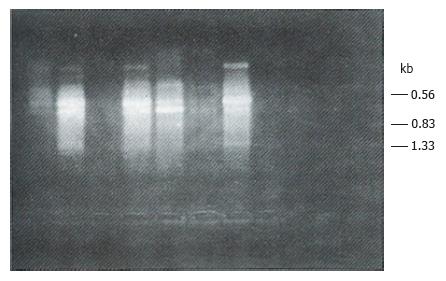
All the tumor tissue samples and 10 of the 15 blood samples obtained from the patients with HCC expressed CD44v mRNA. No CD44v mRNA expression was detected in the control blood samples (Tables 1 and 2, Figure 1).
| CD44vmRNA in blood | Detected, n | Positive AFP | Followed up, n | Responders, n | Recurrence, n | Recurrence rate, % | Survivors, n | Survival rate, % |
| + | 10 | 8 | 10 | 9 | 9 | 100.00 | 1 | 11.11 |
| - | 5 | 3 | 5 | 4 | 1 | 25.00 | 3 | 75.00 |
| Total | 15 | 11 | 15 | 13 | 10 | 76.92 | 4 | 30.77 |
| CD44v MRNA in blood | Followed up, n | Responders, n | DTC | PTC | TEPV | DTN |
| + | 10 | 9 | 8 | 2 | 9 | 7 |
| - | 5 | 4 | 1 | 1 | 2 | 1 |
| Total | 15 | 13 | 9 | 3 | 11 | 8 |
In the patients with positive CD44v mRNA expression in blood, the recurrence rate was higher and the survival rate was lower than in the patients with negative CD44v mRNA expression (χ2 = 8.775, P < 0.005 and χ2 = 5.7778, P < 0.025).
Free tumor cells can appear in the peripheral vein of patients with malignant tumors of the digestive system[12]. Tumor cells can enter into the vein through the short path involving the microartery vein and elastic blood capillaries[13]. Our study has shown that liver cancer cells may enter into the peripheral vein via 1) collateral circulation of the portal caval vein, or 2) liver vein → inferior caval vein → capillary of lung → artery → blood capillary of tissue → peripheral vein of body.
It is possible to detect the CD44v mRNA expression of free tumor cells in blood by RT-PCR. This method may be an auxiliary indicator for diagnosis of malignant tumors. CD44v can confer metastatic potential on tumor cells, which was supported by the findings of our study[14]. In this study, the metastatic data were more robust in patients with positive CD44v mRNA expression in blood than in patients with negative CD44v mRNA expression; additionally, the recurrence rate was higher and the survival rate was lower in the former than in the latter. Thus, CD44v expression in blood may indicate metastatic potential of cancer and become an auxiliary means for monitoring recurrence.
Although detection of alpha-fetoprotein (AFP) is considered the most effective method for HCC diagnosis, there are still about 10% to 30% of patients with HCC who present with negativity for AFP. In the current study, we found that the CD44v mRNA expression in blood of patients with HCC was not related to AFP, suggesting that this method can be a complementary indicator for HCC diagnosis.
However, CD44v mRNA expression in blood cannot be a specific diagnostic index for primary hepatic carcinoma because the specificity of CD44v in different tumors was not clear. Yet, it may be an auxiliary method for differential diagnosis of malignant and benign diseases (i.e., HCC and non-typical abscess of liver, inflammatory pseudotumor of liver, etc.).
The reasons for CD44v mRNA expression being positive in only 10 of 15 blood samples of HCC in the current study, but being positive in all of the 15 tumor tissue samples, may be as follows: (1) none of tumor cells were collected from blood or there may have been no free tumor cells in the blood; (2) the experimental method used was not optimal or sufficient; and (3) the expression level of CD44v mRNA in tumors was low. Therefore, the detection method for CD44v mRNA expression in blood should be further improved.
| 1. | Haynes BF, Telen MJ, Hale LP, Denning SM. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989;10:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 415] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991;3:347-350. [PubMed] |
| 3. | Hofmann M, Rudy W, Zöller M, Tölg C, Ponta H, Herrlich P, Günthert U. CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res. 1991;51:5292-5297. [PubMed] |
| 4. | Smith CW, Patton JG, Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 553] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1240] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 6. | Reber S, Matzku S, Günthert U, Ponta H, Herrlich P, Zöller M. Retardation of metastatic tumor growth after immunization with metastasis-specific monoclonal antibodies. Int J Cancer. 1990;46:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Matsumura Y, Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992;340:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 340] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Guo YJ, Liu G, Wang X, Jin D, Wu M, Ma J, Sy MS. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994;54:422-426. [PubMed] |
| 9. | Birch M, Mitchell S, Hart IR. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991;51:6660-6667. [PubMed] |
| 10. | Li H, Hamou MF, de Tribolet N, Jaufeerally R, Hofmann M, Diserens AC, Van Meir EG. Variant CD44 adhesion molecules are expressed in human brain metastases but not in glioblastomas. Cancer Res. 1993;53:5345-5349. [PubMed] |
| 11. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3020] [Cited by in RCA: 13201] [Article Influence: 338.5] [Reference Citation Analysis (0)] |
| 12. | Cole WH, McDonald G, Roberts SS. Dissemination of cancer, prevention and therapy. New York: Appleton-Century-Crofts, Inc 1961; 155. |
| 13. | Cole WH, McDonald G, Roberts SS. Dissemination of cancer, prevention and therapy. New York: Appleton-Century-Crofts, Inc 1961; 137. |
| 14. | Liu PF, Wu MC, Cheng H, Qian GX, Fu JL. Expression of splice variants of CD44 and significance in human liver carcinoma. . Jiefangjun Yixue Zazhi. 1996;21:189-190. |
Original title:
S- Editor: A L- Editor: Filipodia E- Editor: Li RF