Published online Mar 7, 2023. doi: 10.3748/wjg.v29.i9.1492
Peer-review started: November 17, 2022
First decision: January 23, 2023
Revised: February 7, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 7, 2023
Processing time: 110 Days and 16 Hours
Since its complete roll-out in 2009, the French colorectal cancer screening program (CRCSP) experienced 3 major constraints [use of a less efficient Guaiac-test (gFOBT), stopping the supply of Fecal-Immunochemical-Test kits (FIT), and suspension of the program due to the coronavirus disease 2019 (COVID-19)] affecting its effectiveness.
To describe the impact of the constraints in terms of changes in the quality of screening-colonoscopy (Quali-Colo).
This retrospective cohort study included screening-colonoscopies performed by gastroenterologists between Jan-2010 and Dec-2020 in people aged 50-74 living in Ile-de-France (France). The changes in Quali-colo (Proportion of colonoscopies performed beyond 7 mo (Colo_7 mo), Frequency of serious adverse events (SAE) and Colonoscopy detection rate) were described in a cohort of Gastroenterologists who performed at least one colonoscopy over each of the four periods defined according to the chronology of the constraints [gFOBT: Normal progress of the CRCSP using gFOBT (2010-2014); FIT: Normal progress of the CRCSP using FIT (2015-2018); STOP-FIT: Year (2019) during which the CRCSP experienced the cessation of the supply of test kits; COVID: Program suspension due to the COVID-19 health crisis (2020)]. The link between each dependent variable (Colo_7 mo; SAE occurrence, neoplasm detection rate) and the predictive factors was analyzed in a two-level multivariate hierarchical model.
The 533 gastroenterologists (cohort) achieved 21509 screening colonoscopies over gFOBT period, 38352 over FIT, 7342 over STOP-FIT and 7995 over COVID period. The frequency of SAE did not change between periods (gFOBT: 0.3%; FIT: 0.3%; STOP-FIT: 0.3%; and COVID: 0.2%; P = 0.10). The risk of Colo_7 mo doubled between FIT [adjusted odds ratio (aOR): 1.2 (1.1; 1.2)] and STOP-FIT [aOR: 2.4 (2.1; 2.6)]; then, decreased by 40% between STOP-FIT and COVID [aOR: 2.0 (1.8; 2.2)]. Regardless of the period, this Colo_7 mo’s risk was twice as high for screening colonoscopy performed in a public hospital [aOR: 2.1 (1.3; 3.6)] compared to screening-colonoscopy performed in a private clinic. The neoplasm detection, which increased by 60% between gFOBT and FIT [aOR: 1.6 (1.5; 1.7)], decreased by 40% between FIT and COVID [aOR: 1.1 (1.0; 1.3)].
The constraints likely affected the time-to-colonoscopy as well as the colonoscopy detection rate without impacting the SAE’s occurrence, highlighting the need for a respectable reference time-to-colonoscopy in CRCSP.
Core Tip: The study showed that the detection rate of colonoscopy dropped significantly in France during the years 2019 and 2020, probably due to the coronavirus disease health crisis. The risk of a long delay (> 7 mo) in performing the colonoscopy was twice as high in a public hospital compared to colonoscopies performed in a private endoscopy practice. The constraints likely affected the time to colonoscopy as well as the colonoscopy detection rate without impacting the occurrence of serious adverse events.
- Citation: Koïvogui A, Vincelet C, Abihsera G, Ait-Hadad H, Delattre H, Le Trung T, Bernoux A, Carroll R, Nicolet J. Supply and quality of colonoscopy according to the characteristics of gastroenterologists in the French population-based colorectal-cancer screening program. World J Gastroenterol 2023; 29(9): 1492-1508
- URL: https://www.wjgnet.com/1007-9327/full/v29/i9/1492.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i9.1492
The impact of the Screening program on controlling colorectal cancer (CRC) morbidity and mortality has been widely proved[1-4]. But since its complete roll-out in France in 2009, the population-based colorectal cancer screening program (CRCSP) has continued to face constraints affecting its effectiveness. Despite the existence of the fecal immunochemical test (FIT) in certain European programs (i.e., Italy, Czech Republic) when the program roll-out was completed in France[5], the health authority chose the Guaiac Hemoccult II test® (gFOBT). It later turned out that gFOBT only identified 50% of colorectal cancer (CRC) lesions and a third of adenomas[6], which led some GPs to be wary of it, at the risk of seeing some of their patients fall through the cracks[6,7].
To consider this first constraint induced using a low sensitivity/specificity screening test, the health authority decided to replace gFOBT in 2015, with the FIT (Threshold set at 150 ng hemoglobin/mL of stool, “Institut National du Cancer”, www.e-cancer.fr). While admitting an improvement in participation with FIT compared to gFOBT, most studies published in France have confirmed the high sensitivity (detection of advanced adenomas and CRC) of FIT and its better acceptability by the population and GPs[8-12]. This performance of the FIT inevitably leads to an increase in colonoscopy requests in the screened population and subsequently to an extension of the time to colonoscopy after a positive FIT result[13]. However, these analyses of the time to colonoscopy only considered the characteristics of the target population without any adjustment to the characteristics of the colonoscopy supply.
On April 25, 2018, the Paris Administrative Court cancelled, during an appeal session, the contract concluded in 2014 between the Health Insurance Agency and the Cerba-Daklapack® consortium (www.slbc.fr). This contract, which related to the supply of screening test kits and the laboratory analysis of the tests carried out, had thus been cancelled only three years after the introduction of the FIT in CRCSP. This legal and administrative confusion led to a market shutdown between March and September 2019. In the Ile-de-France (IDF) region, this shutdown led to a drastic decrease in the number of tests carried out in 2019, compared to forecasts (annual activity report 2019).
Only a few months after the resumption of the test kits’ market, the World Health Organization (WHO) announced the pandemic of COVID-19[14]. This pandemic constraint required a relocation of health care resources to control this global health crisis. Screening programs, in particular the CRCSP, were suspended in many countries. The aim of this study was to describe the impact of the constraints listed above in terms of changes to the quality of screening colonoscopies (Quali-colo) in a cohort of gastroenterologists (GEs) practicing in IDF.
This retrospective cohort study included all screening colonoscopies, performed between 01/01/2010 and 31/12/2020 by GEs in the IDF region and collected by the eight sites (Paris, Seine-et-Marne, Yvelines, Essonne, Hauts-de-Seine, Seine-Saint-Denis, Val-de-Marne and Val-d’Oise) of the IDF CRCSP Coordination Centre (CRCDC-IDF). These screening colonoscopies were performed following a positive screening test in people aged 50-74, living in IDF, France.
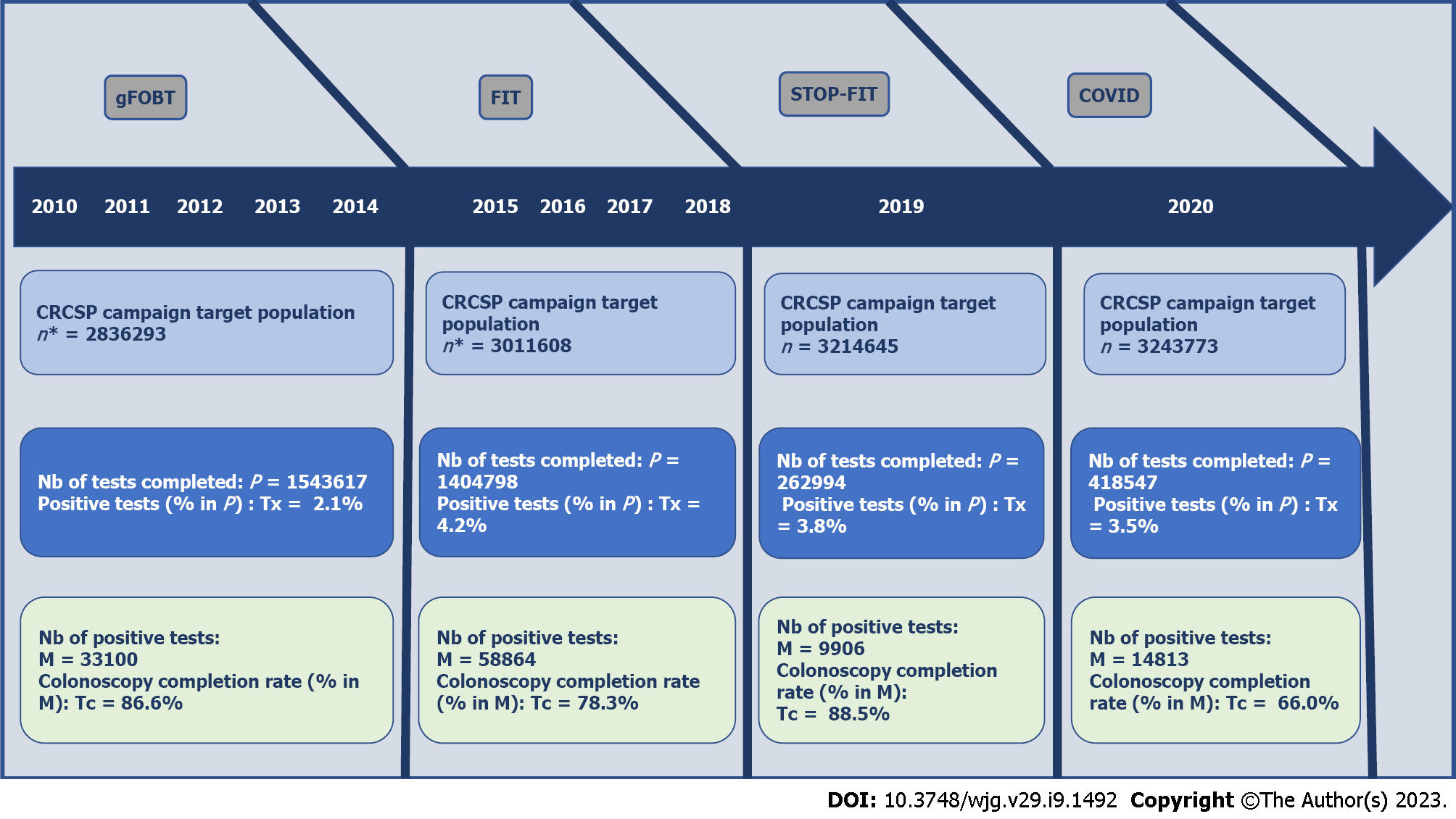
Considering the chronology of the constraints in the CRCSP, four periods for carrying out the colonoscopy were distinguished (Figure 1). The first period (gFOBT) corresponded to the five years (2010-2014) of normal progress of the CRCSP using gFOBT. The second period (FIT) corresponded to the four years (2015-2018) of normal progress of the CRCSP using FIT. The third (FIT-STOP) corresponded to the year (2019) during which the CRCSP experienced the cessation of the supply of test kits and the fourth (COVID) corresponded to the program suspension due to the COVID-19 health crisis (2020).
The supply of screening colonoscopy was described by the number and type of practice of GEs practicing in IDF and having performed a screening colonoscopy in a person living in IDF. The Quali-colo was described in terms of time to colonoscopy, yield of colonoscopy and frequency of undesirable events (incidents/accidents, incomplete colonoscopy, refusal of 2nd colonoscopy).
Descriptive and evolutive analyses (supply and Quali-colo) were carried out between the periods (gFOBT, FIT, FIT-STOP, and COVID). These changes were first described according to the characteristics of the GEs who performed the screening colonoscopies. Secondly, the impact of constraints was described in terms of changes in Quali-colo indicators between the four periods, in a cohort of GEs (Cohort-GE) who performed at least one colonoscopy in each of the four periods.
The National Council of the Order of Physicians (Research and Statistics Study Department) provided the medical demographic data. Screening data were extracted from CRCDC-IDF departmental databases. Over the study period, the CRCSP campaigns were organized following the CRCSP specifications[15,16]. As a preliminary to each campaign in each study department, an update of the files of eligible people was made after the transmission of individual data by the partners (Health Insurance plans, Medical Information Services of hospitals, Pathologists, GEs, Surgeons, GPs, patients). Anyone who had a screening test did not need a screening colonoscopy if the test result was negative. In case of a positive test result, the person was subsequently invited five years after a normal colonoscopy or excluded from the CRCSP after a positive colonoscopy result (polyp or CRC).
The screening colonoscopy (complete or incomplete) was considered completed only if the result was provided with or without a completion date. When the completion date was provided, the time to screening colonoscopy was expressed as the number of months between the date of completion of the screening test and the date of completion of the colonoscopy. In the cases where several colonoscopies were carried out to investigate the same positive test, the time to screening colonoscopy was that related to the first colonoscopy. The proportion of screening colonoscopies with an abnormally long time to access colonoscopy (Long-delay-colo) was estimated by the frequency of colonoscopies performed beyond a 7-mo delay among the screening colonoscopies for which the completion date was provided. This delay threshold considers the fact that the risk of colorectal cancer is increased by about 40% for any colonoscopy performed after a waiting period of 7-12 mo[17].
The screening colonoscopy was complete when the colon was examined until crossing the Bauhin valve. The reasons for an incomplete colonoscopy were: Insufficient preparation, Anatomical (dolichocolon, Presence of an obstructive lesion requiring a second colonoscopy or surgery). The accidents related to screening colonoscopy were: exterior hemorrhage with or without transfusion, perforation, death. Incidents related to anesthesia or general condition (cardiorespiratory disorders) were distinguished from those related to endoscopy (i.e., difficulty crossing a cul-de-sac, placement of clips to stop bleeding after a polypectomy). The proportion of serious adverse events (SAEs) was estimated by the frequency of screening colonoscopies during which an incident/accident was notified.
The screening colonoscopy was classified as positive when a neoplasm (Polyp/adenoma/CRC) was discovered, negative if not. The screening colonoscopy detection rate (yield of colonoscopy) was estimated by the proportion of positive colonoscopies among the screening colonoscopies performed. The CRC and polyps/adenomas diagnoses were those coded C18-C20 and D12 according to the 10th version of the WHO International Classification of Diseases (ICD10)[18]. The CRC was considered “seen at colonoscopy” when an ulcerative-budding/ulcerative-necrotizing lesion was described by the GE. The high-risk polyps were adenomatous or scalloped polyps with a diameter of ≥ 10 mm (except hyperplastic polyps), high-grade dysplasia adenomas, villous or tubulo-villous adenomas. The TNM classification[19] has been used to define CRC severity. Any CRC ≥ T3 (subserous invaded) or ≥ N1 (at least one regional node invaded) or M1 (with metastasis) was considered severe CRC.
For each GE practicing in IDF region, having performed at least one screening colonoscopy, the factors studied were: (1) The existence of a gastroenterology consultation carried out before the screening colonoscopy completion date; (2) the annual number of screening colonoscopies performed (1, 2-30, 31-100, and > 100 colonoscopies); (3) the place of performance of the screening colonoscopy (1-Private clinic in the IDF; 2-Private hospitals in the IDF; 3-Public hospital in the IDF including: The Public Assistance of Paris hospitals -APHP-, Other public hospitals in the IDF including army hospitals and municipal health centers). The colonoscopies performed by GEs practicing in ≥ 2 locations, the locations of which had not been specified (n = 2), were attributed to the locations most frequented by these GEs over the period. Similarly, Colo for which the location was specified but for which the GEs were not specified (n = 6), were attributed to the GEs who performed the greatest number of colonoscopies on the location and over the period. Colo performed in a country other than France were classified as “Place Unspecified”. Colonoscopies performed in another region of France were classified “Outside-IDF”; (4) the annual number of colonoscopy locations (1 Location, ≥ 2 Locations); (5) the density of GEs in the municipality where the GE performed the screening colonoscopy. The density (D) of GEs was estimated as number of GEs/100000 inhabitants. Each colonoscopy year, with reference to a regional average density (M) and standard deviation (SD). Low density of GE was: D < M-SD, average-density of GE was: D in M ± SD, high density of GE was: D > M + SD; (6) the seniority of the GE (for any year “A”, the GE having no screening colonoscopy in the years prior to “A” was considered a new GE); (7) the residence of the CRCSP target patient treated by the GE (1-the Colonoscopy’s supply municipality, 2-other municipality in the Colonoscopy’s supply department, 3-other IDF departments). As a reminder, in 2018, The National Institute of Statistics and Economic Studies (INSEE) counted 1267 municipalities in IDF in addition to the city of Paris; and (8) the age of the CRCSP target patient treated by the GE (50-54, 55-59, 60-64, 65-69, and ≥ 70 years).
The proportions (Colo performed within one month or after a waiting delay > 7 mo, incomplete and redone Colo, incidents/accidents, positive Colo, high_risk_polyp, CRC seen at Colo, CRC with provided status, severe CRC) were described and compared between periods (gFOBT, FIT, FIT-STOP, and COVID) by the Pearson’ Chi-2 test. In the strata defined according to the characteristics of the cohort-GE, the time to perform the screening colonoscopy (in months) was analyzed in terms of average and confidence interval (CI) then, an analysis of variance (ANOVA on repeated measures) was used to compare the average delays between periods (gFOBT, FIT, FIT-STOP vs COVID). In the strata defined according to the characteristics of the cohort-GE, the proportions (colonoscopies performed after > 7 mo delay, proportion of SAEs, yield of screening colonoscopy) were compared between periods (gFOBT, FIT, FIT-STOP vs COVID) by Cochran’s Q test.
The link between each dependent variable (binary variables 0/1: Long-delay-colo; SAEs, Yield of screening colonoscopy) and the predictive factors (annual number of screening colonoscopies performed, Place of performance of the screening colonoscopy, Annual number of colonoscopy locations, Density of GE, Residence of the patient, Age of the patient) was analyzed in a multivariate and two level (colonoscopy and GE) hierarchical regression model. The generalized linear model (family: Bernoulli, link: Logit) with mixed effect was preferred. This multivariate analysis was performed using a model with all covariates regardless of their relationship in univariate analysis. In addition, a strong correlation existed between several covariates (i.e., annual number of screening colonoscopies and Place of performance, Annual number of screening colonoscopies and Municipal density of GEs, Annual number of screening colonoscopies and Period), the model was extended to these terms of interaction between covariates. Only the significant interaction terms (P < 0.05 in univariate analysis) were kept in the final model evaluated by the likelihood ratio test. A biomedical statistician performed the statistical review. All the analyses were carried out at the 5% threshold with version 13 of the STATA software (College Station, TX, United States).
Before analysis, all data were anonymized. The screening database had a favourable opinion from the institution that oversees the ethics of data collection (“Commission nationale de l’informatique et des
Out of a total of 1267 municipalities listed in the IDF region, only 155 municipalities had at least one GE in 2010. This number of municipalities having at least one GE falling from 155 in 2010 to 142 in 2020. In the municipalities having at least one GE, the average annual density of GEs fluctuated between a minimum of 6.3 (in 2014) and a maximum of 6.5 GE/100000 inhabitants over the study period (Table 1).
| Year of colonoscopy | Nb of GE in IDF1 | Number of gastroenterologists who performed a screening colonoscopy2 | |||||||||||||
| Number of GE by seniority | Number of GE by density of GE in the municipality of practice of the GE | Number of GE by place of performance of the colonoscopy | Number of GE by annual number (A) of colonoscopies performed | Total (n) of GE in IDF (% GE ≥ 2 location) | |||||||||||
| Nb of GE (density)3 | Nb of municipalities with GE | Senior | New (% in n) | Low | Average | High | Private clinic | Private Hop. | Public Hop. | A = 1 | A = 2-30 | A = 30-100 | A > 100 | ||
| 2010 | 761 (6.5) | 155 | 627 | - | 134 | 85 | 493 | 415 | 114 | 214 | 119 | 473 | 35 | - | 627 (20.6) |
| 2011 | 756 (6.4) | 156 | 534 | 77 (12.6) | 140 | 71 | 474 | 408 | 117 | 201 | 106 | 465 | 40 | - | 611 (17.2) |
| 2012 | 759 (6.4) | 155 | 538 | 57 (9.6) | 116 | 79 | 473 | 383 | 112 | 206 | 117 | 454 | 24 | - | 595 (16.8) |
| 2013 | 761 (6.4) | 154 | 539 | 30 (5.3) | 98 | 92 | 442 | 378 | 115 | 181 | 107 | 448 | 14 | - | 569 (16.5) |
| 2014 | 757 (6.3) | 155 | 522 | 63 (10.8) | 129 | 75 | 451 | 384 | 106 | 193 | 123 | 448 | 14 | - | 585 (17.4) |
| 2015 | 776 (6.4) | 154 | 526 | 44 (7.7) | 117 | 53 | 449 | 379 | 103 | 178 | 140 | 419 | 11 | - | 570 (13.9) |
| 2016 | 784 (6.5) | 154 | 629 | 98 (13.5) | 128 | 65 | 628 | 432 | 143 | 312 | 97 | 447 | 175 | 8 | 727 (18.8) |
| 2017 | 793 (6.5) | 152 | 642 | 72 (10.1) | 142 | 56 | 603 | 418 | 142 | 312 | 93 | 486 | 134 | 1 | 714 (19.9) |
| 2018 | 799 (6.5) | 149 | 665 | 64 (8.8) | 141 | 51 | 626 | 424 | 151 | 312 | 100 | 488 | 139 | 2 | 729 (20.7) |
| 2019 | 798 (6.5) | 147 | 643 | 32 (4.7) | 123 | 63 | 574 | 388 | 152 | 287 | 92 | 512 | 71 | - | 675 (21.8) |
| 2020 | 802 (6.5) | 142 | 619 | 76 (10.9) | 147 | 50 | 582 | 412 | 162 | 265 | 124 | 475 | 96 | - | 695 (19.7) |
The gap between the number of GEs registered in the medical demographic database and the number of GEs having performed at least one screening colonoscopy, increased from 134 in 2010 (761 registered vs 627 having performed ≥ 1 screening colonoscopy), to 206 in 2015 (776 vs 570) before being reduced to 123 in 2019 (798 vs 675). The proportion of GEs performing screening colonoscopies at two or more locations varied from 20.6% in 2010 to 13.9% in 2015, then 21.8% in 2019. The proportion of new GEs decreased from 12.6% in 2011 to 7.7% in 2015, then increased to 13.5% in 2016 and further decreased to 4.7% in 2019. In 2016, a total of 727 GEs performed at least one colonoscopy. Among them, 97 GE performed only one screening colonoscopy and 8 GEs exceeded an annual number of 100 screening colonoscopies (Table 1).
In 2011, out of a total of 6428 colonoscopies performed in IDF, the proportion of colonoscopies performed by new GEs was 2.0%, the proportion of colonoscopies performed in a municipality with a high density of GEs was 62.2%, the proportion of colonoscopies performed in a public hospital was 12.5%. In 2016, 1041 screening colonoscopies were performed by the GEs having an annual volume of > 100 screening colonoscopies and 9148 (58.9%) screening colonoscopies were performed by the GEs having an annual volume of 30-100 screening colonoscopies. Compared to 2010 (1.7%), the proportion of screening colonoscopies performed outside the IDF region was significantly higher in 2020 (2.5%; P < 0.0001). Similarly, compared to 2019 (16.8%), the proportion of screening colonoscopies performed in public hospitals decreased significantly in 2020 (13.0%, P < 0.0001) (Table 2).
| Year of colonoscopy | Number of colonoscopies performed according to GE characteristics | ||||||||||||||
| Number of colonoscopies by seniority of GE | Number of colonoscopies by density of GE in the municipality of practice of the GE1 | Number of colonoscopies by place of performance of the colonoscopy | Number of colonoscopies by GE’s annual number (A) of colonoscopies performed | Total | |||||||||||
| Senior | New | Low | Average | High | Clinic | Private Hop. | Public Hop. | A = 1 | A = 2-30 | A = 31-100 | A > 100 | Nb (n) of Colo performed in IDF (average Nb of Colo by GE) | Nb of Colo with place specified (% outside IDF) | Nb of Colo (% Place unspecified) | |
| 2010 | 6059 | - | 1535 | 900 (14.9) | 3624 (59.8) | 4507 | 830 (13.7) | 722 (11.9) | 119 | 4493 (74.2) | 1447 (23.9) | - | 6059 (11) | 6161 (1.7) | 6441 (4.4) |
| 2011 | 6300 | 128 (2.0) | 1684 | 712 (11.1) | 4032 (62.7) | 4677 | 946 (14.7) | 805 (12.5) | 106 | 4578 (71.2) | 1744 (27.1) | - | 6428 (12) | 6543 (1.8) | 6928 (5.6) |
| 2012 | 5355 | 76 (1.4) | 1186 | 766 (14.1) | 3479 (64.1) | 3818 | 830 (15.3) | 783 (14.4) | 117 | 4284 (78.9) | 1030 (19.0) | - | 5431 (11) | 5533 (1.8) | 5852 (5.5) |
| 2013 | 4309 | 47 (1.1) | 1045 | 737 (16.9) | 2574 (59.1) | 3156 | 660 (15.2) | 540 (12.4) | 107 | 3725 (85.5) | 524 (12.0) | - | 4356 (9) | 4409 (1.2) | 4712 (6.4) |
| 2014 | 4320 | 132 (3.0) | 1104 | 611 (13.7) | 2737 (61.5) | 3199 | 650 (14.6) | 603 (13.5) | 123 | 3718(83.5) | 611 (13.7) | - | 4452 (9) | 4515 (1.4) | 4746 (4.9) |
| 2015 | 3712 | 63 (1.7) | 879 | 446 (11.8) | 2450 (64.9) | 2692 | 604 (16.0) | 479 (12.7) | 140 | 3198 (84.7) | 437 (11.6) | - | 3775 (8) | 3818 (1.1) | 4034 (5.4) |
| 2016 | 15196 | 333 (2.1) | 3406 | 1862 (12.0) | 10261 (66.1) | 10886 | 2527 (16.3) | 2116 (13.6) | 97 | 5243 (33.8) | 9148 (58.9) | 1041 | 15529 (25) | 15811 (1.8) | 16651 (5.0) |
| 2017 | 11519 | 192 (1.6) | 2876 | 1262 (10.8) | 7573 (64.7) | 7919 | 1937 (16.5) | 1855 (15.8) | 93 | 5370 (45.9) | 6137(52.4) | 111 | 11711 (18) | 11920 (1.8) | 12345 (3.4) |
| 2018 | 12181 | 164 (1.3) | 2758 | 1190 (9.6) | 8397 (68.0) | 8233 | 2300 (18.6) | 1812 (14.7) | 100 | 5331 (43.2) | 6684 (54.1) | 230 | 12345 (19) | 12602 (2.0) | 13057 (3.5) |
| 2019 | 8189 | 98 (1.2) | 1582 | 932 (11.3) | 5773 (69.7) | 5365 | 1532 (18.5) | 1390 (16.8) | 92 | 5261 (63.5) | 2934 (35.4) | - | 8287 (13) | 8487 (2.4) | 8767 (3.2) |
| 2020 | 9103 | 158 (1.7) | 2088 | 755 (8.2) | 6418 (69.3) | 6654 | 1900 (20.5) | 1199 (13.0) | 124 | 5049 (54.5) | 4088 (44.1) | - | 9261 (15) | 9501 (2.5) | 9793 (3.0) |
Overall, the time to screening colonoscopy was significantly longer over STOP-FIT (gFOBT: 2.6 ± 2.9 vs FIT: 3.0 ± 3.0; STOP-FIT: 3.9 ± 3.9, COVID: 3.5 ± 3.9, P < 0.0001). Over the gFOBT period, 3.1% of the 28679 colonoscopies performed were incomplete (20.7% were redone) for reasons: Anatomical (60.6%), insufficient preparation (16.1%). The proportion of incomplete and redone colonoscopies was significantly higher over FIT (P < 0.001). Although one case of death was reported during the gFOBT period, the proportion of adverse events was not significantly related to the period (0.05). The proportion of cancers seen at colonoscopy was lower over FIT (gFOBT: 61.4%, vs FIT: 55.2% or STOP-FIT: 57.5% or COVID: 56.1%; P < 0.0001) (Table 3).
| Quality indicator | Period | ||||
| gFOBT | FIT | STOP-FIT | COVID | P value1 | |
| Total number (n) of colonoscopies | 28679 | 46087 | 8767 | 9783 | |
| Existence of a GE consultation before colonoscopy | |||||
| Nb (A) colonoscopies with date of consultation | 5267 (18.4) | 1517 (3.3) | 406 (4.6) | 198 (2.0) | < 10-3 |
| Date of consultation ≠ Date of colonoscopy | |||||
| Nb colonoscopies of which date of consultation ≠ colon date (% in A) | 298.4 (56.7) | 883 (58.2) | 402 (99.0) | 191 (96.5) | < 10-3 |
| Time to colonoscopy | |||||
| Average (in mean ± SD) | 2.6 ± 2.9 | 3.0 ± 3.0 | 3.9 ± 3.9 | 3.5 ± 2.9 | < 10-3* |
| Number of colonoscopies performed within one month | 4957 (17.3) | 4572 (9.9) | 458 (5.2) | 726 (7.4) | < 10-3 |
| Number of colonoscopies performed beyond 7 mo | 1520 (5.3) | 2949 (6.4) | 1034 (11.8) | 933 (9.5) | < 10-3 |
| Complete colonoscopy | < 10-3 | ||||
| Nb colonoscopies without information on performance | 1263 (4.4) | 2360 (5.1) | 410 (4.7) | 432 (4.4) | |
| Number of complete colonoscopies | 26530 (92.5) | 41695(90.5) | 8004 (91.3) | 8981 (91.8) | |
| Nb (B) of incomplete colonoscopies | 886 (3.1) | 2032 (4.4) | 357 (4.1) | 376 (3.8) | |
| Reasons for incomplete colonoscopies | < 10-3 | ||||
| Unspecified: n (% in B) | 206 (23.3) | 617 (30.4) | 109 (30.5) | 114 (30.3) | |
| Anatomical reason/Obstruction by lesion: n (% in B) | 537 (60.6) | 845 (41.6) | 150 (42.0) | 161 (42.8) | |
| Insufficient preparation: n (% in B) | 143 (16.1) | 570 (28.1) | 98 (27.5) | 101 (26.9) | |
| Redone incomplete colonoscopy | |||||
| Number of redone colonoscopies (% B) | 183 (20.7) | 960 (47.2) | 158 (44.3) | 163 (43.3) | < 10-3 |
| Frequency of incidents | 0.14 | ||||
| No incidents reported: n | 28873 (99.6) | 45947 (99.7) | 8740 (99.7) | 9763 (99.8) | |
| Related to anaesthesia/general condition: n | 18 (0.06) | 24 (0.05) | 3 (0.03) | 2 (0.02) | |
| Related to endoscopy: n | 88 (0.3) | 116 (0.3) | 22 (0.3) | 18 (0.2) | |
| Frequency of accidents | 0.17 | ||||
| No accidents reported: n | 28589 (99.7) | 45970 (99.8) | 8749 (99.8) | 9763 (99.8) | |
| Suspected complication: n | 24 (0.08) | 23 (0.05) | 5 (0.04) | 3 (0.03) | |
| Exterior bleeding: n | 57 (0.2) | 66 (0.1) | 14 (0.2) | 12 (0.1) | |
| Perforation: n | 8 (0.03) | 28 (0.06) | 3 (0.03) | 4 (0.04) | |
| Deaths: n | 1 (0.0) | 0 | 0 | 0 | |
| Colonoscopies results | |||||
| Detection rate: Nb of lesions | 14857 (51.8) | 29843 (64.8) | 5565 (63.5) | 5967 (60.1) | < 10-3 |
| Nb Polyps (% HRP) | 12947 (44.2) | 26624 (56.4) | 5040 (53.3) | 5425 (51.8) | < 10-3 |
| Nb of CRC (% CRC seen at colonoscopy) | 1910 (61.4) | 3219 (55.2) | 525 (57.3) | 542 (56.1) | < 10-3 |
| % CRC with severity stage specified among Nb CRC2 | 90.3 | 80.5 | 74.3 | 72.3 | < 10-3 |
| Nb CRC with severity stage specified (% severe CRC) 2 | 1724 (50.7) | 2592 (40.9) | 390 (39.5) | 392 (39.5) | < 10-3 |
The cohort of 533 GE achieved 21509 Screening colonoscopies over the gFOBT period, 38352 over FIT, 7342 over STOP-FIT and 7995 over the COVID period. In this cohort, the difference in time (months) to screening colonoscopy between periods was globally significant [gFOBT: 2.6 (2.5; 2.6) vs FIT: 3.0 (2.9; 3.0); STOP-FIT: 3.9 (3.8; 4.0) and COVID: 3.5 (3.4; 3.6); P < 0.0001]. The average time to colonoscopy was longer in public hospitals compared to clinics or private hospital, regardless of the period. This average time was paradoxically shorter over the COVID period compared to the STOP-FIT period, regardless of the type of establishment [in STOP-FIT clinic: 3.7 (3.6; 3.7) vs COVID: 3.4 (3.3-3.5) in public hospitals STOP-FIT: 5.1 (4.7-5.9) vs COVID: 4.2 (3.8; 4.7)]. The average time to colonoscopy was significantly lower among GEs practicing in low-density areas of GEs compared to those practicing in high-density areas of GEs, over the gFOBT and FIT periods, conversely, depending on the density area the confidence intervals were not significant over the STOP-FIT and COVID periods (Table 4).
| Characteristics of the cohort of gastroenterologists | Nb of GE | Average time (in months) to colonoscopy, by period | ||||||||
| gFOBT | FIT | STOP-FIT | COVID | P1 | ||||||
| Nb of Colo | Average, 95%CI | Nb of Colo | Average, 95%CI | Nb of Colo | Average, 95%CI | Nb of Colo | Average, 95%CI | |||
| Overall | 533 | 21509 | 2.6 [2.5; 2.6] | 38352 | 3.0 [2.9; 3.0] | 7342 | 3.9 [3.8; 4.0] | 7995 | 3.5 [3.4; 3.6] | < 10-3 |
| Annual Nb of Colo | ||||||||||
| 1 | 2012 | 304 | 3.1 [2.8; 3.5] | 150 | 3.3 [2.7; 3.8] | 38 | 4.3 [3.1; 4.8] | 51 | 4.0 [3.5; 4.6] | 0.08 |
| 2-30 | 4812 | 16819 | 2.6 [2.5; 2.6] | 15970 | 3.0 [3.0; 3.1] | 4887 | 3.9 [3.8; 4.0] | 4211 | 3.5 [3.4; 3.6] | < 10-3 |
| 31-100 | 442 | 4386 | 2.4 [2.3; 2.5] | 21137 | 3.0 [2.9; 3.0] | 2817 | 3.8 [3.6; 3.9] | 3733 | 3.5 [3.4; 3.6] | < 10-3 |
| > 100 | 0 | 0 | 1095 | 2.5 [2.3; 2.6] | 0 | 0 | ||||
| Place of S-colo performance | ||||||||||
| Clinic | 3552 | 15745 | 2.4 [2.4; 2.5] | 27003 | 2.9 [2.8; 2.9] | 5039 | 3.7 [3.6; 3.7] | 5560 | 3.4 [3.3; 3.5] | < 10-3 |
| Private hospital | 1252 | 3041 | 2.5 [2.4; 2.6] | 6500 | 2.9 [2.8; 3.0] | 1359 | 3.6 [3.5; 3.7] | 1621 | 3.4 [3.3; 3.5] | < 10-3 |
| Public hospital | 2352 | 2723 | 3.3 [3.2; 3.4] | 4849 | 3.8 [3.7; 3.9] | 940 | 5.1 [4.7; 5.9] | 795 | 4.2 [3.8; 4.7] | < 10-3 |
| Average density of GE (GE/100000iHbts) | ||||||||||
| Low | 1272 | 4643 | 2.4 [2.3; 2.5] | 8419 | 2.9 [2.8; 2.9] | 1519 | 3.9 [3.8; 4.1] | 1800 | 3.4 [3.3; 3.5] | < 10-3 |
| Average | 1082 | 3245 | 2.5 [2.4; 2.5] | 4314 | 2.9 [2.8; 2.9] | 810 | 4.1 [3.8; 4.4] | 781 | 3.6 [3.4; 3.9] | < 10-3 |
| High | 4672 | 13621 | 2.6 [2.6; 2.7] | 25619 | 3.0 [3.0; 3.1] | 5009 | 3.8 [3.7; 3.9] | 5395 | 3.5 [3.4; 3.6] | < 10-3 |
| Annual Nb of Colo locations | ||||||||||
| 1 location | 4832 | 14437 | 2.6 [2.6; 2.7] | 24851 | 3.0 [3.0; 3.1] | 4763 | 3.8 [3.7; 3.9] | 5160 | 3.5 [3.4; 3.6] | < 10-3 |
| ≥ 2 locations | 1532 | 7072 | 2.4 [2.4; 2.5] | 13501 | 2.9 [2.9; 3.0] | 2575 | 4.0 [3.9; 4.1] | 2816 | 3.5 [3.4; 3.7] | < 10-3 |
| Residence of the patient | ||||||||||
| Colonoscopy’s supply municipality | 3382 | 4947 | 2.5 [2.4; 2.5] | 7775 | 2.9 [2.9; 3.0] | 1502 | 3.9 [3.7; 4.1] | 1530 | 3.5 [3.3; 3.6] | < 10-3 |
| Other municipality in Colonoscopy’s supply department | 4802 | 13259 | 2.5 [2.5; 2.6] | 23754 | 3.0 [3.0; 3.1] | 4401 | 3.9 [3.8; 4.0] | 4982 | 3.5 [3.4; 3.6] | < 10-3 |
| Other departments in IDF | 4192 | 3303 | 2.7 [2.6; 2.8] | 6823 | 2.9[2.9; 3.0] | 1435 | 3.8 [3.6; 4.0] | 1464 | 3.6 [3.5; 3.8] | < 10-3 |
| Age (in yr) of the patients | ||||||||||
| 50-54 | 4852 | 4995 | 2.7 [2.6; 2.8] | 8018 | 3.1 [3.0; 3.2] | 1695 | 4.1 [3.9; 4.2] | 1616 | 3.8 [3.7; 4.0] | < 10-3 |
| 55-59 | 4522 | 4669 | 2.6 [2.5; 2.7] | 7355 | 3.1 [3.0; 3.1] | 1446 | 3.9 [3.7; 4.1] | 1560 | 3.6 [3.4; 3.7] | < 10-3 |
| 60-64 | 4662 | 4889 | 2.5 [2.4; 2.6] | 7851 | 3.0 [2.9; 3.0] | 1478 | 3.7 [3.5; 3.9] | 1531 | 3.5 [3.4; 3.7] | < 10-3 |
| 65-69 | 4642 | 3766 | 2.5 [2.4; 2.5] | 8511 | 2.9 [2.8; 2.9] | 1403 | 3.8 [3.6; 4.0] | 1590 | 3.3 [3.1; 3.5] | < 10-3 |
| ≥ 70 | 4312 | 3190 | 2.4 [2.3; 2.5] | 6617 | 2.9 [2.8; 3.0] | 1316 | 3.7 [3.5; 3.8] | 1679 | 3.4 [3.3; 3.6] | < 10-3 |
Regardless of the GE’s characteristics, the proportion of screening colonoscopy performed in > 7 mo delay was significantly higher over STOP-FIT (P < 0.001). The proportion of colonoscopies performed in > 7 mo delay was higher in public hospitals compared to clinics and private hospitals, regardless of the period (P < 0.001 in each period). This proportion of colonoscopies performed in > 7 mo delay decreased during the COVID period compared to the STOP-FIT period, regardless of the place of colonoscopy P < 0.001 for each place). The proportion of colonoscopies performed in > 7 mo delay was higher in the 50-54 age group, regardless of the period P < 0.001 in each period) (Table 5).
| Characteristics of the cohort of gastroenterologists | Proportion of colonoscopies performed beyond 7 mo by period | Proportion of serious adverse events by period | ||||||||
| gFOBT | FIT | STOP-FIT | COVID | P1 | gFOBT | FIT | STOP-FIT | COVID | P1 | |
| Nb of Colo (% > 7 mo) | Nb of Colo (% > 7 mo) | Nb of Colo (% > 7mo) | Nb of Colo (% > 7 mo) | %EI | %EI | %EI | ||||
| Overall | 21509 (5.3) | 38352 (6.2) | 7342 (11.3) | 7995 (9.2) | < 10-3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.10 |
| Annual Nb of Colo | ||||||||||
| 1 | 304 (10.1) | 150 (11.8) | 38 (18.4) | 51 (12.0) | < 10-3 | 0.3 | 0 | 0 | 0 | 0.67 |
| 2-30 | 16819 (5.5) | 15970 (6.9) | 4887 (11.8) | 4211 (9.4) | < 10-3 | 0.3 | 0.4 | 0.3 | 0.1 | 0.09 |
| 31-100 | 4386 (4.1) | 21137 (5.7) | 2817 (10.6) | 3733 (9.0) | < 10-3 | 0.4 | 0.2 | 0.3 | 0.2 | 0.25 |
| > 100 | 1095 (3.7) | 0.2 | ||||||||
| Place of Colo performance | ||||||||||
| Clinic | 15745 (4.8) | 27003 (5.4) | 5039 (10.4) | 5560 (8.3) | < 10-3 | 0.3 | 0.3 | 0.3 | 0.1 | 0.16 |
| Private hospital | 3041 (5.1) | 6500 (5.9) | 1359 (9.6) | 1621 (9.5) | < 10-3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.71 |
| Public hospital | 2723 (8.5) | 4849 (10.6) | 940 (18.8) | 795 (15.1) | < 10-3 | 0.6 | 0.6 | 0.2 | 0.4 | 0.48 |
| Average density of GE (GE/100000 iHbts) | ||||||||||
| Low | 4643 (5.1) | 8419 (5.8) | 1519 (11.5) | 1800 (9.1) | < 10-3 | 0.3 | 0.2 | 0.3 | 0.1 | 0.59 |
| Average | 3245 (4.9) | 4314 (6.5) | 810 (11.0) | 781 (9.9) | < 10-3 | 0.2 | 0.3 | 0.1 | 0.5 | 0.48 |
| High | 13621 (5.5) | 25619 (6.2) | 5009 (11.3) | 5395 (9.2) | < 10-3 | 0.4 | 0.3 | 0.3 | 0.1 | 0.04 |
| Annual Nb of Colo locations | ||||||||||
| 1 location | 14437 (5.6) | 24851 (6.3) | 4763 (10.6) | 5160 (9.5) | < 10-3 | 0.4 | 0.3 | 0.3 | 0.2 | 0.17 |
| ≥ 2 location | 7072 (4.8) | 13501 (6.0) | 2575 (12.7) | 2816 (8.7) | < 10-3 | 0.2 | 0.2 | 0.2 | 0.1 | 0.80 |
| Residence of the patient | ||||||||||
| Colonoscopy’s supply municipality | 4947 (4.9) | 7775 (5.9) | 1502 (11.6) | 1530 (8.0) | < 10-3 | 0.2 | 0.3 | 0.3 | 0.0 | 0.18 |
| Other municipality in Colonoscopy’s supply department | 13259 (5.1) | 23754 (6.0) | 4401 (11.0) | 4982 (9.6) | < 10-3 | 0.4 | 0.3 | 0.2 | 0.2 | 0.02 |
| Other departments in IDF | 3303 (6.6) | 6823 (6.9) | 1435 (12.1) | 1464 (9.2) | < 10-3 | 0.3 | 0.4 | 0.4 | 0.3 | 0.80 |
| Age (in yr) of the patients | ||||||||||
| 50-54 | 4995 (6.7) | 8018 (7.0) | 1695 (12.2) | 1616 (11.0) | < 10-3 | 0.2 | 0.2 | 0.1 | 0.1 | 0.43 |
| 55-59 | 4669 (5.7) | 7355 (6.6) | 1446 (11.8) | 1560 (9.9) | < 10-3 | 0.3 | 0.3 | 0.4 | 0.2 | 0.79 |
| 60-64 | 4889 (4.7) | 7851 (5.9) | 1478 (10.3) | 1531 (8.9) | < 10-3 | 0.4 | 0.3 | 0.5 | 0.3 | 0.76 |
| 65-69 | 3766 (4.4) | 8511 (5.5) | 1403 (11.7) | 1590 (8.2) | < 10-3 | 0.4 | 0.3 | 0.3 | 0.1 | 0.20 |
| ≥ 70 | 3190 (4.6) | 6617 (5.7) | 1316 (10.6) | 1679 (8.2) | < 10-3 | 0.5 | 0.4 | 0.2 | 0.2 | 0.49 |
Whatever the characteristics of the Cohort-GE, the decline in colonoscopy detection rate was significant between the FIT and COVID period (Table 6). The risk of having a long delay to colonoscopy was twice as high for screening-colonoscopy performed in a public hospital [adjusted odds ratio (aOR): 2.1 (1.3; 3.6)] compared to screening colonoscopy performed in a private IDF clinic. Except for the patient’s age, the risk of adverse events was not related to any other predictive factor. Compared to patients aged 50-54, patients aged 70 had a 70% increased risk of neoplasm detection. The risk of neoplasm detection decreased by about 40% between the periods FIT [aOR: 1.6 (1.5; 1.7)] and COVID [aOR: 1.1 (1.0; 1.3)] (Table 7).
| Characteristics of the cohort of gastroenterologists | Neoplasm detection rate at colonoscopy by period | ||||
| gFOBT | FIT-1 | STOP-FIT | COVID | P1 | |
| Nb of Colo (% Colo+) | Nb of Colo (% Colo+) | Nb of Colo (% Colo+) | Nb of Colo (% Colo+) | ||
| Overall | 21509 (52.3) | 38352 (65.0) | 7342 (63.3) | 7995 (60.1) | < 10-3 |
| Annual Nb of Colo | |||||
| 1 | 304 (50.7) | 150 (62.0) | 38 (71.1) | 51 (51.0) | 0.02 |
| 2-30 | 16819 (53.6) | 15970 (64.5) | 4887 (63.7) | 4211 (59.6) | < 10-3 |
| 31-100 | 4386 (47.6) | 21137 (65.8) | 2817 (62.5) | 3733 (61.8) | < 10-3 |
| > 100 | 1095 (58.6) | 0 | 0 | ||
| Place of S-colo performance | |||||
| Clinic | 15745 (52.4) | 27 003 (64.9) | 5039 (62.8) | 5560 (60.1) | < 10-3 |
| Private hospital | 3041 (54.3) | 6500 (65.0) | 1359 (64.8) | 1621 (62.7) | < 10-3 |
| Public hospital | 2723 (49.8) | 4849 (65.6) | 940 (63.7) | 795 (59.5) | < 10-3 |
| Average Density of GE (GE/100000iHbts) | |||||
| Low | 4643 (53.0) | 8419 (64.5) | 1519 (61.7) | 1800 (58.4) | < 10-3 |
| Average | 3245 (53.1) | 4314 (64.1) | 810 (65.2) | 781 (61.1) | < 10-3 |
| High | 13621 (51.9) | 25619 (65.4) | 5009 (63.4) | 5395 (61.2) | < 10-3 |
| Annual Nb of S-colo locations | |||||
| 1 location | 14437 (52.8) | 24851 (64.8) | 4763 (63.0) | 5160 (60.8) | < 10-3 |
| ≥ 2 locations | 7072 (51.3) | 13501 (65.6) | 2575 (63.8) | 2816 (60.2) | < 10-3 |
| Residence of the patient | |||||
| Colonoscopy’s supply municipality | 4947 (53.4) | 7775 (65.3) | 1502 (61.1) | 1530 (59.5) | < 10-3 |
| Other municipality in Colonoscopy’s supply department | 13259 (51.7) | 23754 (65.0) | 4401 (64.2) | 4982 (61.2) | < 10-3 |
| Other departments in IDF | 3303 (53.1) | 6823 (64.9) | 1435 (62.8) | 1464 (59.6) | < 10-3 |
| Age (in yrs) of the patients | |||||
| 50-54 | 4995 (44.9) | 8018 (56.5) | 1695 (55.2) | 1616 (52.9) | < 10-3 |
| 55-59 | 4669 (50.8) | 7355 (63.1) | 1446 (61.3) | 1560 (58.9) | < 10-3 |
| 60-64 | 4889 (54.2) | 7851 (67.9) | 1478 (65.5) | 1531 (63.1) | < 10-3 |
| 65-69 | 3766 (57.1) | 8511 (68.9) | 1403 (68.3) | 1590 (65.3) | < 10-3 |
| ≥ 70 | 3190 (57.5) | 6617 (69.2) | 1316 (67.9) | 1679 (62.8) | < 10-3 |
| Characteristics of the cohort of gastroenterologists | Colo performed beyond a 7-mo risk analysis | Serious adverse events risk analysis | Neoplasms risk analysis | |||
| ORa, 95%CI | P1 | ORa, 95%CI | P1 | ORa, 95%CI | P1 | |
| Annual Nb of Colo (Ref: 1 Colo) | ||||||
| 2-30 | 0.7 [0.6; 1.0] | 0.002 | 2.5 [0.3; 18.2] | 0.37 | 0.9 [0.7; 1.1] | 0.41 |
| > 30 | 0.7 [0.3; 0.9] | 0.008 | 2.9 [0.9; 23.0] | 0.05 | 0.8 [0.6; 1.1] | 0.32 |
| Place of S-colo performance (Ref: Clinic) | ||||||
| Private hospital | 1.2 [0.9; 1.6] | 0.18 | 0.7 [0.3; 1.8] | 0.47 | 1.1 [0.9; 1.3] | 0.41 |
| Public hospital | 2.1 [1.3; 3.6] | 0.001 | 1.6 [0.3; 8.7] | 0.60 | 1.1 [0.8; 1.4] | 0.20 |
| Density of GE (Ref: Low) | ||||||
| Average | 0.9 [0.8; 1.0] | 0.05 | 1.2 [0.6; 2.2] | 0.59 | 1.0 [0.9; 1.1] | 0.76 |
| High | 1.0 [1.0; 1.2] | 0.28 | 1.2 [0.6; 2.3] | 0.65 | 0.9 [0.8; 1.0] | 0.04 |
| Annual Nb of S-colo locations (Ref: 1 location) | ||||||
| ≥ 2 locations | 1.1 [0.8; 1.5] | 0.11 | 1.6 [0.5; 4;4] | 0.41 | 1.0 [0.8; 1.3] | 0.84 |
| Residence of the patient (Ref: Colonoscopy’s supply municipality) | ||||||
| Other municipality in Colonoscopy’s supply department | 1.0 [0.9; 1.0] | 0.30 | 1.0 [0.5; 2.1] | 0.97 | 1.0 [0.9; 1.0] | 0.31 |
| Other departments in IDF | 1.2 [1.1; 1.3] | < 10-3 | 1.2 [0.3; 5.2] | 0.81 | 0.9 [0.8; 1.0] | 0.13 |
| Age (yrs) of the patients (Ref: 50-54 yr) | ||||||
| 55-59 | 0.9 [0.8; 1.0] | 0.03 | 1.6 [1.0; 2.6] | 0.04 | 1.3 [1.2; 1.4] | < 10-3 |
| 60-64 | 0.8 [0.7; 0.9] | 0.001 | 2.0 [1.2; 3.1] | 0.006 | 1.6 [1.5; 1.6] | < 10-3 |
| 65-69 | 0.7 [0.7; 0.8] | < 10-3 | 1.9 [1.2; 3.0] | 0.01 | 1.7 [1.6; 1.8] | < 10-3 |
| ≥ 70 | 0.7 [0.6; 0.8] | 0.003 | 2.1 [1.3; 3.4] | 0.002 | 1.7 [1.6; 1.8] | < 10-3 |
| Period (Ref.: gFOBT) | ||||||
| FIT | 1.2 [1.1; 1.2] | < 10-3 | 0.8 [0.4; 1.5] | 0.11 | 1.6 [1.5; 1.7] | < 10-3 |
| STOP-FIT | 2.4 [2.1; 2.6] | < 10-3 | 0.8 [0.5; 1.3] | 0.27 | 1.3 [1.1; 1.5] | < 10-3 |
| COVID | 2.0 [1.8; 2.2] | < 10-3 | 0.5 [0.3; 0.9] | 0.02 | 1.1 [1.0; 1.3] | 0.08 |
The European guide for quality assurance of colorectal cancer screening recommends performing a colonoscopy within 31 d following a positive test result[21]. In our Cohort-GE, if the increase in the time to screening colonoscopy between the first and the second period was attributable to the introduction of FIT, its increase after the second period was attributable to the malfunction of the program due to the slowdown of the kit market and the COVID-19 health crisis. There is certainly no relationship between the kit market and the colonoscopy offer, but the unexplained increase in the time to perform colonoscopy during a year that saw a market slowdown can be explained factually by this market crisis. The hypothesis would be that general practitioners reacted to the market crisis by relaxing the program, in particular the follow-up of people who had a positive test. Indeed, in France, in addition to the distribution of the test kit, the training doctors are real facilitators of access to colonoscopy (helping the patient to make an appointment with a gastroenterologist, motivating the patient to have the colonoscopy). This hypothesis is confirmed by the slight decrease in the time to colonoscopy in 2020 compared to 2019, despite the COVID-19 health crisis. The year 2020 was moreover affected by this kit market crisis than by the COVID-19 health crisis. Indeed, after the resumption of the kit market in September 2019, several people who had a positive test during the last quarter of 2019 were inevitably the first to be affected by colonoscopy postponements at the start of the first confinement in March 2020. However, the improvement in the time to colonoscopy during the pandemic (compared to the STOP-FIT period) could also be linked to the fact that people have refocused their concerns on their health. Regardless of the characteristics of the Cohort-GE, the screening colonoscopy detection rate dropped significantly between the STOP-FIT and COVID periods, while the proportions of SAEs stayed unchanged.
The long delay to access colonoscopy observed on the gFOBT and FIT periods converges with the results of another French study[22], although it is clearly higher than those observed elsewhere[23,24]. The definition of a reference delay and the obligation of compliance with it by all GEs taking part in CRCSP would effectively reduce the delay in France. This reframing is necessary, especially since the number of GEs is large, but with an increased disparity in terms of the number of screening colonoscopies performed by GEs.
Despite this longer waiting time to colonoscopy, the proportion of colonoscopies during which a SAE was reported did not change between periods. Although high, the frequency of perforations remains lower than that (1.1%) found in Alsace[25]. In the program, there was no nationally standardized forms for collecting screening colonoscopy data. Information concerning the date of consultation before the colonoscopy, or the progress of the examination can sometimes be missed or be considered irrelevant during this collection. Therefore, the low frequency of SAEs reported in this study could be the consequence of under-reporting.
The high proportion of incomplete colonoscopies due to insufficient preparation should alert to the need to set up a specific preparation protocol for screening colonoscopy. To date, it is impossible to evaluate with relevance the preparation of a colonoscopy in outpatients, who are not hospitalized at the time of the preparation. Similarly, there is no standard preparation scheme imposed in the French screening program, each GE proposing the method of his choice to the patient. However, although a non-superiority of a preparation scheme (Enema vs Oral preparation) was argued[21], studies admitted that a short time (1-6 h vs > 8 h) between the colic preparation and colonoscopy is associated with a better quality of colonic preparation[26].
Compared to gFOBT, the high proportion of 2nd colonoscopies over the FIT period would confirm the literature on the performance of FIT in screening for precancerous lesions[27], which most often only require endoscopic resection. However, in addition to a high proportion of obstructive lesions, the proportion of severe cancers was significantly higher over the gFOBT period.
Several study results converge on a link between the long delay in access to colonoscopy and the CRC risk. Forbes et al[28] propose that wherever possible, colonoscopy should not be delayed beyond 6 mo of positive fecal testing as an aspirational target (with 9 mo as an upper limit). In the Kaiser Permanente (California) health plan members, the risk of CRC was increased by about 40% for any colonoscopy performed after a waiting period of 7-12 mo[17]. A recent meta-analysis shows that the risk of colorectal cancer is increased by 42%, and that the risk of cancer at an advanced stage was multiplied by 2 or even more, when colonoscopy was performed more than 6 mo after a positive test[29]. In this study, the time to access colonoscopy as well as its lengthening, induced first by the change of the test and then by the health crisis, had no impact in terms of the CRC severity, probably because of the discriminatory approach prioritizing patients with already existing symptoms. As a reminder, the French Society of Digestive Endoscopy had made, in mid-April 2020, the specific recommendation to postpone by 6 wk any colonoscopy following a positive screening test result, if there was no clinical nor biological sign of CCR[30]. In addition, since FIT was introduced in 2015 in a population screened biannually with gFOBT, the severe CRC screened by FIT are likely to be those not detected at an early stage by gFOBT. This hypothesis is confirmed by the drop in the colonoscopy detection rate and by the proportion of severe CRC over STOP-FIT and COVID periods.
To celebrate the tenth anniversary of the first atlas of medical demography, the National Council of the Order of Physicians focused on the gradual transfer from liberal activity to salaried activity. The focus also mentioned the widening of territorial inequalities to the detriment of regions and departments already in difficulty in terms of medical density[31]. Although the number of GEs is unevenly distributed over the 1268 IDF municipalities, the density of GEs in the IDF region was well above the range (4.2 to 4.9) of the national average observed in 2017[31].
Each GE participating in a CRCSP must perform at least 300 colonoscopies per year[21]. Despite the superiority of the regional offer compared to the national average, the annual number of colonoscopies per GE stays very disparate and below 300, especially for GEs in public hospitals. The main limitation of this study is the fact that it only gives an opinion on screening colonoscopies. Indeed, screening colonoscopies only represented 5.5% of all colonoscopies performed in France in 2012 (gFOBT-period) and about 10% in 2016 (FIT-period)[32]. Since the patient base of a GE is not limited to the population of the region of practice, several GEs in the IDF region could reach or exceed this recommended annual number, in particular GEs practicing in a private clinic. The other limit of the study would come from the fact that the measurements of the indicators cannot be generalized over the whole of France. Indeed, the density of gastroenterologists and the types of practice (clinical hospital, etc.) may vary from one municipality (or department or region) to another. Only access to databases for the reimbursement of colonoscopy procedures could allow the exhaustive evaluation of such a quality indicator.
Although GEs are unevenly distributed over the municipalities of the IDF region, the supply of colonoscopies has remained almost constant between 2010 and 2020. The increase in colonoscopy requests induced by the change of the test kit has led to an increase in the average annual number of colonoscopies performed by GEs at the start of the FIT period. This very disparate annual average number between GEs fell over the STOP-FIT and COVID periods, due to the decrease in demand induced by the shutdown of the test kit market and the COVID-19 health crisis. The definition of a reference time and the obligation to respect it by all GEs would effectively reduce the time to access screening colonoscopy in France. The increase in the time to colonoscopy between the first and the second period was attributable to the introduction of the FIT, its increase after the second period was probably attributable to the malfunction of the program due to the slowdown of the kit market and the COVID-19 health crisis. Regardless of the characteristics of the GEs, the colonoscopy detection rate dropped significantly between the STOP-FIT and COVID periods, while the proportions of SAEs remained unchanged. However, the time to colonoscopy as well as its lengthening induced by the constraints had no impact in terms of CRC severity, probably because of a discriminatory approach prioritizing patients with existing symptoms.
The impact of the Screening program on controlling the colorectal cancer (CRC) morbidity and mortality has been proved. But since its complete roll-out in 2009, the French population-based colorectal cancer screening program (CRCSP) experienced 3 major constraints [use of a less efficient Guaiac-test (gFOBT), Stopping the supply of Faecal-Immunochemical-Test kits (FIT), Suspension of the program due to the coronavirus disease 2019 (COVID-19)] affecting its effectiveness.
At this time when all the spotlights are focused on the impact of the health crisis linked to COVID-19, our motivation was to warn of the continued deterioration in the quality of screening colonoscopies in France.
To describe the impact of the constraints in terms of changes to the quality of screening colonoscopies.
This retrospective cohort study included screening colonoscopies performed by the gastroenterologists between January 2010 and December 2020 in people aged 50-74 Living in Ile-de-France (France). The changes to the quality of screening colonoscopy (proportion of colonoscopies performed beyond 7 mo, Frequency of serious adverse events and the colonoscopy detection rate) were described in a cohort of Gastroenterologists who performed at least one colonoscopy over each of the four periods defined according to the chronology of the constraints [gFOBT: Normal progress of the CRCSP using gFOBT (2010-2014); FIT: Normal progress of the CRCSP using FIT(2015-2018); STOP-FIT: Year (2019) during which the CRCSP experienced the cessation of the supply of test kits; COVID: program suspension due to the COVID-19 health crisis (2020)]. The link between each dependent variable (Colo_7 mo; SAE Occurrence, Neoplasm detection rate) and the predictive factors was analyzed in a two-level multivariate hierarchical model.
The retrospective cohort was made up of 533 gastroenterologists. These 533 gastroenterologists achieved 21509 screening colonoscopies over the gFOBT period, 38,352 over FIT, 7342 over STOP-FIT and 7995 over the COVID period. The frequency of serious adverse events did not change between periods (gFOBT: 0.3%; FIT: 0.3%; STOP-FIT: 0.3%, and COVID: 0.2%; P = 0.10). The risk of colonoscopies performed beyond 7 mo doubled between FIT [adjusted-odds-ratio (aOR): 1.2 (1.1; 1.2)] and STOP-FIT [aOR: 2.4 (2.1; 2.6)], then decreased by 40% between STOP-FIT and COVID [aOR: 2.0 (1.8; 2.2)]. Regardless of the period, this Colo_7 mo’s risk was twice as high for screening colonoscopy performed in a public hospital [aOR: 2.1 (1.3; 3.6)] compared to screening-colonoscopy performed in a private clinic. The neoplasm detection, which increased by 60% between gFOBT and FIT [aOR: 1.6 (1.5; 1.7)], decreased by 40% between FIT and COVID [aOR: 1.1 (1.0; 1.3)].
The study showed that the constraints likely affected the time-to-colonoscopy as well as the colonoscopy detection rate without impacting the occurrence of the serious adverse events, highlighting the need for a respectable reference time-to-colonoscopy in CRCSP.
At the end of this study, we initially aim to develop, evaluate, and validate a standard form for collecting data from screening colonoscopies in France. In a second step, we will evaluate the impact of the patient’s motivation by the attending physician on the time taken to perform the colonoscopy.
The authors would like to thank the staff of the CRCDC-IDF. The authors would like to thank Stéphanie RASSE (National Council of the Order of Physicians, Research and Statistics Study Department) who facilitated access to medical demographic data. The authors are grateful to all contributors who participated in the final revisions.
| 1. | Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 1840] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 3. | Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004;39:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Faivre J, Lepage C, Viguier J. [Colorectal cancer: from diagnosis to screening]. Gastroenterol Clin Biol. 2009;33:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Cardoso R, Guo F, Heisser T, Hoffmeister M, Brenner H. Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Guignot C. Cancer colorectal. Vers un dépistage mieux accepté. Science et Santé. [cited 20 January 2023]. Available from: https://www.ipubli.inserm.fr/bitstream/handle/10608/9439/2015_26_36.pdf?sequence=1. |
| 7. | Le Pimpec F, Moutel G, Piette C, Lièvre A, Bretagne JF. Fecal immunological blood test is more appealing than the guaiac-based test for colorectal cancer screening. Dig Liver Dis. 2017;49:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Koïvogui A, Mab GL, Benamouzig R. Detection of Colorectal Neoplasia in a Cohort Before and After the Change of Fecal Occult Blood Test in a French Colorectal Cancer Screening Program. Am J Gastroenterol. 2018;113:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Vitellius C, Laly M, Banaszuk AS, Deherce I, Cornet N, Bertrais S, Saulnier P, Caroli-Bosc FX. Contribution of the OC Sensor(®) immunoassay in comparison to the Hemoccult II(®) guaiac-test in organized colorectal cancer screening. Eur J Epidemiol. 2019;34:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Senore C, Basu P, Anttila A, Ponti A, Tomatis M, Vale DB, Ronco G, Soerjomataram I, Primic-Žakelj M, Riggi E, Dillner J, Elfström MK, Lönnberg S, Sankaranarayanan R, Segnan N. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut. 2019;68:1232-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Bretagne JF, Piette C, Cosson M, Durand G, Lièvre A. Switching from guaiac to immunochemical faecal occult blood test increases participation and diagnostic yield of colorectal cancer screening. Dig Liver Dis. 2019;51:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Bretagne JF, Carlo A, Piette C, Rousseau C, Cosson M, Lièvre A. Significant decrease in interval colorectal cancer incidence after implementing immunochemical testing in a multiple-round guaiac-based screening programme. Br J Cancer. 2021;125:1494-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Kaufmanis A, Vincelet C, Koivogui A, Ait Hadad H, Bercier S, Brixi Z, Delattre-Massy H, Deyra J, Le Mab G, Le Trung T, Liautaud A. Dépistage organisé du cancer colorectal en Ile-de-France: état des lieux du délai de réalisation de la coloscopie avant et après l’introduction du test immunologique. Journées Francophones d’Hépato-Gastroentérolgie et d’Oncologie Digestive (JFHOD) de la Société Nationale Française de Gastro-Entérologie (SNFGE). March 22, 2018. [cited 20 January 2023]. Available from: https://www.snfge.org/content/depistage-organise-du-cancer-colorectal-en-ile-de-france-etat-des-lieux-du-delai-de. |
| 14. | Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 Pandemic on Colorectal Cancer Screening: a Systematic Review. J Gastrointest Cancer. 2022;53:730-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Goulard H, Boussac-Zarebska M, Bloch J. Epidemiological assessment of the pilot programme for organized colorectal cancer screening. 2009. [cited 20 January 2023]. Available from: https://www.semanticscholar.org/paper/Epidemiological-assessment-of-the-pilot-programme-Goulard-Boussac-Zarebska/6ba13198a01d64068277b9249d6ef9d42d417d7f. |
| 16. | Leuraud K, Jezewski-Serra D, Viguier J, Salines E. Colorectal cancer screening by guaiac faecal occult blood test in France: Evaluation of the programme two years after launching. Cancer Epidemiol. 2013;37:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Schottinger JE, Marks AR, Zhao WK, Ghai NR, Lee AT, Contreras R, Quesenberry CP, Fireman BH, Levin TR. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA. 2017;317:1631-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 18. | World Health Oorganization. ICD-10 Version: 2008. 10th International Classification of Diseases. [cited 20 January 2023]. Available from: https://icd.who.int/browse10/2008/fr. |
| 19. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017. |
| 20. | Journal Officiel de la République Française. Délibération n 2017-215 du 13 juillet 2017 portant adoption d’une norme destinée à simplifier l’obligation de déclaration des traitements de données à caractère personnel ayant pour finalité le dépistage organisé du cancer du sein, du cancer colorectal et du cancer du col de l’utérus mis en œuvre par les structures de gestion conventionnées, et abrogeant la délibération n 2015-175 du 11 juin 2015 (décision d’autorisation unique n AU-043) (NS-059). [cited 20 January 2023]. Available from: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000035484848&categorieLien=id. |
| 21. | Valori R, Rey JF, Atkin WS, Bretthauer M, Senore C, Hoff G, Kuipers EJ, Altenhofen L, Lambert R, Minoli G; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012;44 Suppl 3:SE88-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Cariou M, El Fettouhi A, Kermarrec T, Bommelaere F, Foll Y, Nousbaum JB, Robaszkiewicz M, Quénéhervé L. Comparative evaluation of two colorectal cancer screening campaigns using different faecal occult blood tests in a French area. Cancer Epidemiol. 2020;69:101839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Beshara A, Ahoroni M, Comanester D, Vilkin A, Boltin D, Dotan I, Niv Y, Cohen AD, Levi Z. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int J Cancer. 2020;146:1532-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Demb J, Liu L, Bustamante R, Dominitz JA, Earles A, Shah SC, Gawron AJ, Martinez ME, Gupta S. COVID-19 Pandemic Had Minimal Impact on Colonoscopy Completion After Colorectal Cancer Red Flag Sign or Symptoms in US Veterans. Dig Dis Sci. 2022;1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Denis B, Gendre I, Weber S, Perrin P. Adverse events of colonoscopy in a colorectal cancer screening program with fecal immunochemical testing: a population-based observational study. Endosc Int Open. 2021;9:E224-E232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Colonoscopy Outcomes by Duration of NPO Status Prior to Colonoscopy with Moderate or Deep Sedation [Internet]. Washington (DC): Department of Veterans Affairs (US); 2015 Jan- . [PubMed] |
| 27. | van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, Verbeek AL, Jansen JB, Dekker E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 28. | Forbes N, Hilsden RJ, Martel M, Ruan Y, Dube C, Rostom A, Shorr R, Menard C, Brenner DR, Barkun AN, Heitman SJ. Association Between Time to Colonoscopy After Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review. Clin Gastroenterol Hepatol. 2021;19:1344-1354.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Mutneja HR, Bhurwal A, Arora S, Vohra I, Attar BM. A delay in colonoscopy after positive fecal tests leads to higher incidence of colorectal cancer: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Société Française d’Endoscopie Digestive. Epidémie de COVID-19-Recommandations en endoscopie digestive 11 mars 2020. Société Française d’Endoscopie Digestive (SFED). Mar 11, 2020. [cited 20 January 2023]. Available from: https://www.sfed.org/sites/www.sfed.org/files/2021-10/covid19endo_reco.pdf. |
| 31. | Conseil National de l’Ordre des Médecins. Atlas de la démographie médicale en france 2017. Profils comparés : 2007/2017-les territoires au cœur de la réflexion. Paris, France: Conseil National de l’Ordre des Médecins (CNOM). 2018. [cited 20 January 2023]. Available from: https://www.conseil-national.medecin.fr/sites/default/files/external-package/analyse_etude/1sogkeq/atlas_de_la_demographie_medicale_2017.pdf. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bustamante-Balen M, Spain; Gencdal G, Turkey; Muguruma N, Japan; Teramoto-Matsubara OT, Mexico S-Editor: Chen YL L-Editor: A P-Editor: Chen YL