Published online Feb 28, 2023. doi: 10.3748/wjg.v29.i8.1243
Peer-review started: September 21, 2022
First decision: January 3, 2023
Revised: January 6, 2023
Accepted: January 30, 2023
Article in press: January 30, 2023
Published online: February 28, 2023
Processing time: 160 Days and 5.1 Hours
Hepatocellular carcinoma (HCC) is the most frequent liver neoplasm, and its incidence rates are constantly increasing. Despite the availability of potentially curative treatments (liver transplantation, surgical resection, thermal ablation), long-term outcomes are affected by a high recurrence rate (up to 70% of cases 5 years after treatment). HCC recurrence within 2 years of treatment is defined as “early” and is generally caused by the occult intrahepatic spread of the primary neoplasm and related to the tumor burden. A recurrence that occurs after 2 years of treatment is defined as “late” and is related to de novo HCC, independent of the primary neoplasm. Early HCC recurrence has a significantly poorer prognosis and outcome than late recurrence. Different pathogenesis corresponds to different predictors of the risk of early or late recurrence. An adequate knowledge of predictive factors and recurrence risk stratification guides the therapeutic strategy and post-treatment surveillance. Patients at high risk of HCC recurrence should be referred to treatments with the lowest recurrence rate and when standardized to combined or adjuvant therapy regimens. This review aimed to expose the recurrence predictors and examine the differences between predictors of early and late recurrence.
Core Tip: Hepatocellular carcinoma is burdened by a high rate of both early and late recurrence. The knowledge of the predictive factors of recurrence and its risk stratification should allow optimization of the management of the patient with hepatocellular carcinoma.
- Citation: Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, Imbriani S, Claar E, Cozzolino D, Sasso FC, Marrone A, Adinolfi LE, Rinaldi L. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol 2023; 29(8): 1243-1260
- URL: https://www.wjgnet.com/1007-9327/full/v29/i8/1243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i8.1243
Liver cancers represent the fifth neoplasm by incidence and the fourth cause of cancer-related deaths worldwide, with a higher incidence in men than in women[1] and an epidemiological distribution that results from the variations of etiological factors for liver disease (with a current reduction in viral hepatitis and an increase in metabolic etiologies)[2,3]. Hepatocellular carcinoma (HCC) accounts for about 90% of liver cancer cases, with an increasing incidence of 75% from 1990 to 2015[1,4]. The onset of HCC generally follows chronic liver damage resulting in the development of fibrosis and especially liver cirrhosis, although cases of HCC in the absence of liver damage are possible and are generally related to aflatoxin exposure[5].
The main risk factors for HCC are represented by: liver cirrhosis and chronic hepatitis; chronic hepatitis B virus (HBV) infection (with or without hepatitis D virus)[6,7] or hepatitis C virus (HCV)[8,9]; chronic alcohol abuse; metabolic syndrome[10]; diabetes mellitus[11]; and obesity[12]. In particular, 1% to 8% of patients with liver cirrhosis develop HCC annually[13]. Low platelet count, severe portal hypertension, certain comorbidities (e.g., obesity, diabetes mellitus), cigarette smoking, concomitant alcohol consumption, older age and male sex are factors closely associated with the development of HCC in patients with liver cirrhosis[13-17].
The mortality rate of HCC patients, although improving, still appears to be extraordinarily high[18]. An early diagnosis and an appropriate therapeutic approach contribute to higher overall and disease-free survival rates. In particular, when diagnosed at an early stage, HCC can be effectively treated by liver transplantation (LT) or loco-regional techniques, including liver resection (LR) and radio-frequency ablation (RFA). Optimal treatment choice is based on both tumor burden and residual liver function[19-21]. HCC recurrence rates after LT accounts for about 13% of cases[22], and these rates appear significantly higher after loco-regional treatment, reaching 70% of cases at 5 years[4]. The recurrence of the neoplasm may reflect both the presence of intrahepatic metastases (“true” or “early” recurrence) and the development of de novo tumors (“late” recurrence). A 2-year cutoff is generally used to define the two entities[4].
There are numerous factors (related to the patient, the tumor and the type of treatment) able to predict the risk of recurrence after loco-regional treatment or LT. Although many predictors are common to early and late recurrence, other factors may be exclusive to one form due to the different pathogenesis. An adequate recurrence risk stratification would allow personalization of the therapeutic strategy for each patient, optimization of the surveillance program and ideally encourage the study, validation and use of adjuvant therapy in high-risk patients.
The aim of this review was to examine the predictors of HCC recurrence after LT and loco-regional treatment and discuss the differences between predictors of early and late recurrence.
In order to define the most appropriate therapeutic strategy able to maximize outcomes and reduce the risk of recurrence, HCC requires appropriate staging that considers cancer-related factors and residual liver function. The Barcelona Clinic Liver Cancer (BCLC) staging system is the most used to predict patient prognosis and establish treatment allocation[4]. It divides patients into 5 categories (0, A-D) based on tumor characteristics (uni- or multifocality, vascular invasion, size and extrahepatic spread) and liver function (assessed by Child Pugh score).
“Very early HCC stage” (BCLC 0) is defined as patients with preserved liver function and carcinoma in situ (single HCC lesion < 2 cm without vascular invasion), whereas “early stage” (BCLC A) is defined as patients with preserved liver function and a single lesion > 2 cm or 3 lesions with diameters < 3 cm. Patients at BCLC stage 0 and A are optimal candidates for a radical therapeutic strategy (LT, ablation, surgical resection). Patients in more advanced states (BCLC B-D) are candidates for palliative (chemoembolization, systemic therapy) or supportive treatment[4,21].
Due to the excellent long-term outcomes achievable, LT is considered the gold standard for the treatment of HCC[22,23]. It is indicated as the first-line therapy in patients with HCC who meet the Milan criteria (single tumor ≤ 5 cm or multiple tumors as ≤ 3 nodules size ≤ 3 cm) without vascular invasion and/or extrahepatic involvement[24]) but are not eligible for LR[4]. Recent evidence also suggests that patients beyond the Milan criteria could be reconsidered for LT after adequate downstaging of the tumor[25]. LR is the treatment of choice in HCC patients with or without cirrhosis with well-preserved liver function, solitary tumors and clinically mild portal hypertension (hepatic vein to portal system gradient ≤ 10 mmHg)[4,26], with the possibility (not standardized yet) to extend the indication to multicentric tumors or to tumors > 2 cm[4]. It is still one of the main local-regional curative-intent treatments, with a 5-year survival between 40% and 70%[27].
Thermal ablation with RFA or microwaves is the standard of care for HCC patients with BCLC 0 and A stage not suitable for surgery (LT or LR) or as an alternative to LR in very early HCC stage with favorable localization also in patients eligible for surgery[4]. Thermoablation is also indicated as a neoadjuvant therapy in patients who are candidates for LT (pre-transplant) in order to reduce the risk of recurrence. Selected patients with intermediate HCC-BCLC B (solitary > 3 cm or > 3 nodules < 3 cm or advanced liver failure not clinically decompensated) can be reasonably treated with RFA, even if medium- and long-term outcomes appear worse than patients with HCC BCLC 0 or A[28].
Transarterial chemoembolization (TACE) is the most widely used treatment for unresectable HCC and offers significant overall survival advantages over best supportive care[4], although it has no clear curative purposes and cannot be considered a radical approach. In particular, the survival rates after TACE are 70.3% at 1 year, 51.8% at 2 years, 40.4% at 3 years and 32.4% at 5 years[29]. The rationale of the technique is based on the intermediate HCC intense arterial neo-angiogenic activity and consists in the intra-arterial administration of cytotoxic agents (doxorubicin or platinum derivatives) followed by the embolization of peritumoral vessels inducing a cytotoxic and ischemic effect of the tumor mass. Combination treatments (e.g., TACE + RFA) and neoadjuvant or adjuvant systemic treatments are currently being studied.
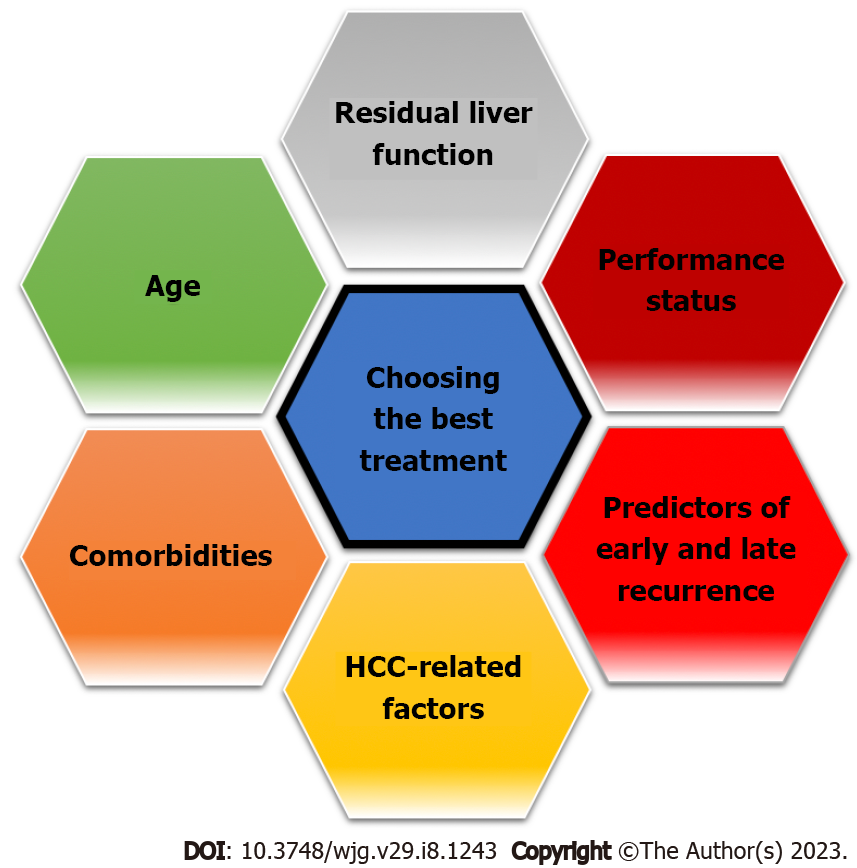
The recurrence of HCC represents a relevant clinical issue, affecting up to 70% of patients undergoing curative treatment[4]. Although many factors are involved in the decision-making process of the best possible treatment (Figure 1), the assessment of baseline risk recurrence significantly influences the choice of treatment type and its timing. Moreover, the treatment of a relapse is generally more complex than the treatment of the first lesion, due to the change in anatomical or functional liver conditions and a more advanced age. Patients at high risk of early recurrence are candidates for close surveillance and potentially adjuvant therapy, which has not yet been standardized[30,31].
The “true” recurrence (secondary to the presence of occult intrahepatic metastases) is generally “early” and accounts for more than 70% of tumor recurrence. It derives from an intrahepatic dissemination of the neoplasm through the portal circulation. A 2-year cutoff is used to distinguish it from late recurrence, generally resulting from de novo development of the neoplasm. However, this cutoff remains arbitrary and not universally accepted. Yamamoto et al[32], for example, identified 17 mo as the best cutoff to distinguish early and late recurrence on a cohort of 252 patients with recurrence of HCC after hepatectomy. Previously Hayashi et al[33] had hypothesized a 1 year cutoff instead. A further shorter observation period has recently been hypothesized by Xing et al[34] who identify 8 mo as the ideal threshold to define a recurrence of HCC as early or late. Although the temporal criterion is used to distinguish a pathogenesis related to the presence of occult intrahepatic metastases or to a de novo neoplasm, a precise differentiation would require the study of recurrence clonality by genetic/genomic analyzes[35].
The two types of recurrence are two distinct entities associated with different risk factors. Early recurrence is associated with tumor-related factors, whereas late recurrence is related to underlying liver disease. They have a substantially different clinical and biological profile and are burdened by different morbidity and mortality. In fact, an early recurrence represents a significant negative prognostic factor and has a far greater impact on overall survival than a late recurrence[32,36]. Xing et al[34] demonstrated that patients with early recurrence showed a median of survival free from cancer of 8.4 mo (7.5-10.0 mo) compared to a median of 21.3 mo (17.9-23.8 mo) for patients with late recurrence. In particular, an early recurrence, mainly determined by the aggressiveness of the primary tumor, is characterized by larger dimensions, higher rates of multifocality and intrahepatic spread, higher probability of vascular invasion and higher levels of alpha-fetoprotein (AFP) compared to a recurrence that develops later[36]. Conversely, late recurrence is generally related to etiology and cirrhosis, risk factors for hepatocarcinogenesis, and not to the primary tumor[37].
The factors affecting the possibility of early HCC relapse after LT or loco-regional treatment can be classified into three categories: those related to the tumor (size, number of nodules, differentiation, oncological markers); to the patient (e.g., age, comorbidity, liver function, possible viral load, presence and activity of hepatitis, presence and activity of liver cirrhosis); and treatment (type of treatment, margins, characteristics of resection) (Table 1)[38,39].
| Predictors related to HCC characteristics | Ref. | Predictors related to patient characteristics | Ref. | Predictors related to treatment | Ref. |
| Size | Liver cirrhosis | [37,41] | RFA (vs LR) | [41,88,89] | |
| > 2.6 cm | [42] | ||||
| > 3 cm | [36,41] | ||||
| Multifocality | [41] | Liver insufficiency (CPS, MELD, ALBI) | [37,56-58] | LR: | |
| Liver resection margins invasion | [44] | ||||
| Liver resection margins < 1 cm | [43,86] | ||||
| Non-anatomical resection1 | [45] | ||||
| Beyond Milan criteria | [43] | High total bilirubin | [42] | RFA: ablation margins < 1 cm | [91] |
| Microvascular invasion | [34,36,38,40,44,45] | Male sex | [37] | ||
| Lack of capsule integrity | [34,42] | High PLR | [49,59,60] | ||
| Poor histological differentiation | [36,43,47] | High NLR | [49,60] | ||
| High AFP | [36,41,50] | DAAs therapy | |||
| > 10 ng/mL | [48] | Predictor | [62,63] | ||
| > 32 ng/mL | [45] | Non-predictor | [64-76] | ||
| > 400 ng/mL | [40] | Protective | [77-83] | ||
| High PIVKA-II | [41,52,55] | High viral load in HBV infected patients | [109] | ||
| > 46.0 mAU/mL | [38] | ||||
| > 375.5 mAU/mL | [51] |
Several factors related to the characteristics of HCC are able to predict the risk of early recurrence (Table 1). This risk is closely related to the tumor burden and its aggressiveness, which increases the probability of occult intrahepatic spread and affects the radicality of the treatment[40].
Preoperative radiological imaging can already provide first indications about the risk of relapse. In this regard, the size of the tumor represents a relevant predictor of the risk of early recurrence. Jung et al[36] showed that a maximum diameter of HCC greater than 3 cm before a curative LR represented an independent risk factor for early recurrence (defined by the authors as occurring within 1 year of treatment). These results have recently been confirmed by Lee et al[41]. Zhu et al[42] identified a 4.77-fold higher risk of early recurrence after an LR for cancers with a maximum diameter greater than 2.6 cm compared to those with a smaller maximum diameter[42]. Similarly, the multifocality of HCC seems to be another significant risk factor for early recurrence[41]. Tumor size and number of lesions represents together the aforementioned Milan criteria. As expected, being beyond the Milan criteria represents an independent risk factor for early HCC recurrence[43].
At histological evaluation, the most predictive factors of the risk of early HCC recurrence are the presence of microvascular invasion, the integrity of the capsule and the degree of differentiation. In light of the fact that early recurrence is generally due to intrahepatic dissemination of the neoplasm through the portal circulation, it is not surprising that vascular invasion has been identified many times as an independent predictor of recurrence. If the involvement of the major vessels (e.g., portal vein, inferior vena cava) can be evaluated radiologically and often excludes the possibility of loco-regional treatment for radical purposes, microvascular invasion needs to be evaluated by histopathological analysis of the intraoperative sample. Microvascular invasion, although dependent upon the operator and influenced by possible sampling errors, represents an independent risk factor for early recurrence[34,44,45] affecting approximately one-third of HCC cases that underwent LR[37]. Since a qualitative and/or quantitative classification of microvascular invasion is not yet available, its only presence/absence is generally considered in the histopathological evaluation. However, in this regard, Roayaie et al[46] proposed a risk system based on histological features of microvascular invasion that includes invasion of a vessel with a muscular wall and invasion of vessels ≥ 1 cm away from the tumor capsule. According to the authors, this system stratifies patients into three distinct groups with significantly different risks of recurrence and death. In particular, patients with microvascular invasion and the aforementioned risk factors show outcomes (tumor recurrence rate, mortality rate) comparable to patients with macroscopic vascular invasion.
The integrity of the HCC capsule would instead represent a protective factor for the risk of recurrence[42]. The capsule would act as a barrier to the spread and metastasis of cancer cells. This function is lost in non-capsular HCC or those with a ruptured capsule[34,42]. Zhu et al[42] showed that non-capsular HCC was associated with a higher rate of poorer differentiation and vascular invasion than capsular HCC.
Histological differentiation is considered an independent risk factor for early HCC recurrence. It is classified according to the Edmonson-Steiner criteria: 1st and 2nd degree correspond to well differentiated neoplasms; and 3rd and 4th degree correspond to poorly differentiated neoplasms. In particular, grades 3 and 4 are related to a significantly increased risk of early recurrence[36,43]. In patients without microvascular invasion, the degree of differentiation seems to be the best histological predictor of the risk of early recurrence and overall survival[47].
In light of the growing evidence of the efficacy in predicting the risk of recurrence, histological evaluation will become crucial in choosing the optimal therapeutic strategy for each patient, allowing the implementation of the stratification of the basal recurrence risk with the postoperative one. Patients at high risk of recurrence should undergo an aggressive surveillance strategy and ideally adjuvant therapy.
Among the predictors of early recurrence, the markers expressed by the neoplasm are certainly the most studied. In particular, AFP, a glycoprotein physiologically produced by the liver and fetus, can increase in pathological conditions, such as liver cancer. In addition to the diagnostic phase, it plays an important role as a predictor and monitoring tool in the context of HCC recurrence. There is almost unanimous agreement on the predictive value of elevated AFP levels for the risk of early HCC recurrence after treatment[36,48,49], but there are no standardized cutoffs pre- and post-treatment.
Jung et al[36] showed that high AFP values both pre- and post-hepatectomy were associated with a higher risk of early recurrence (which the authors defined as < 1 year after treatment) but without a standardized threshold value. Using a retrospective data analysis, Kim et al[48] showed that preoperative AFP values > 10 ng/mL were a predisposing factor of disseminated HCC recurrence within 3 mo after hepatectomy for solitary HCC [odds ratio: 5.333; 95% confidence interval (CI): 1.095–25.985]. Fang et al[40] showed instead that preoperative AFP > 400 ng/mL correlated independently with the risk of early recurrence. Recently, AFP levels at 12 wk after achieving sustained virological response with direct antiviral agents (DAAs) treatment in chronic HCV patients have also been independently associated with a risk of HCC recurrence[50].
The protein induced by vitamin K absence or antagonist II (PIVKA-II) is an immature form of prothrombin, a cofactor of vitamin K that is synthesized by the liver when the latter is not produced or antagonized. Evaluation of PIVKA-II has been shown to be useful in diagnosing HCC and in stratifying the risk of recurrence[38,41,51,52]. In fact, it acts as a growth factor, promoting cell proliferation and tumor angiogenesis in HCC patients[52,53]. The evaluation of PIVKA-II appears to be complementary to AFP[54]. Low values of both AFP and PIVKA-II are associated with a better prognosis compared to elevated levels of AFP and/or PIVKA-II[55]. Serial measurements of both proteins allow a prompt diagnosis of early recurrence. In fact, pre- and post-treatment levels of PIVKA-II are good predictors of early HCC recurrence[38,41,51] since they are related to greater aggressiveness of the neoplasm, such as the presence of vascular invasion and intrahepatic metastasis of HCC cells[52].
Similarly to what is highlighted for AFP, there is no agreement on a PIVKA-II cutoff that is useful as a guide for assessing the risk of recurrence. In this regard, Wang et al[51] showed that in patients with a low risk of recurrence (tumor size < 5 cm, single tumor, absence of satellite lesions, absence of vascular invasions, high degree of histological differentiation, BCLC stage 0-A), a preoperative value of PIVKA-II > 375.5 mAU/mL was the strongest independent prognostic factor for the risk of early recurrence [hazard ratio (HR): 2877; 95%CI: 1524-5429]. Furthermore, patients who expressed high levels of PIVKA-II showed lower 1-year time-to-progression than patients with low levels (54.8% vs 20.2%, respectively). More recently, Hong et al[38] identified a cutoff of 46 mAU/mL for PIVKA-II for predicting an increased risk of early recurrence.
Predictors related to patient characteristics are associated with the risk of late recurrence. In fact, they represent the substrate for the risk of hepatocarcinogenesis and generally do not correlate with the aggressiveness of the primary tumor, which is strongly associated with early recurrence instead. However, some studies have shown a certain impact of the patient’s basal phenotype on HCC recurrence that occurs in the first 2 years after treatment (Table 1).
In this regard, the presence of liver cirrhosis and the degree of hepatic dysfunction are the factors that most increase the risk of early recurrence after curative treatment[37,41]. In the models used for risk stratification in patients undergoing hepatectomy, the degree of hepatic dysfunction is quantified by the Child Pugh score[56,57], the model for end-stage liver disease[41] or by the Albumin-Bilirubin score[37,58]. The latter in particular seems to have better discriminatory power in this setting since it helps to further stratify stage A of the Child-Pugh score, which includes almost all patients undergoing LR[58]. Total bilirubin levels, an expression of the degree of hepatic dysfunction, also correlate with the risk of early recurrence[42]. Furthermore, according to Chan et al[37] male patients undergoing hepatectomy show a higher risk of early recurrence than female patients.
The predictive value on outcomes (mortality and recurrence) of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in HCC patients has been extensively studied. These scores are an expression of the systemic inflammatory response, a known preoperative risk factor for HCC outcomes[49,59,60]. High PLR has been shown to predict lower overall survival (HR: 1.63, 95%CI: 1.34-1.98) and earlier HCC recurrence (HR: 1.52, 95%CI: 1.21-1.91)[59]. Similar results were also highlighted for NLR[49,60]. In addition, both the NLR and PLR were identified as independent risk factors for predicting overall survival and recurrence-free survival (RFS) in HCC patients[59,60].
In recent years, a heated scientific debate has been carried out on the hypothesis of an increased risk of HCC incidence and recurrence in chronic HCV patients treated with DAAs. Unlike interferon (IFN)-based therapeutic regimens, which have been proven to reduce the incidence and recurrence of HCC in cases of sustained virological response[61], some data indicated that after DAA therapy HCC risk may remain high[4,62]. In particular, the retrospective analysis of some patient cohorts revealed an unexpectedly high rate of early HCC recurrence in patients undergoing antiviral treatment with DAAs[62,63]. It has been hypothesized that the rapid fall in viral load induced by DAAs could lead to an altered immune surveillance, promoting the growth of already existing cancer clones[4]. However, the higher number of patients eligible for antiviral treatment with DAAs could influence the apparent raise in early recurrence rates. In fact, due to the manageability and safety profile of these therapeutic regimens, a larger number of patients with advanced liver disease at high risk of HCC (e.g., liver cirrhosis, advanced age, multiple morbidity) have undergone antiviral treatment than those who were candidates for IFN-based schemes. Subsequent prospective studies underlined that although the rates of early HCC recurrence remain high in patients who have obtained viral clearance by DAAs, these rates are comparable to those reported in the literature for patients not treated with these regimens[64-66].
Waziry et al[67], in a meta-analysis, concluded that there is no evidence that DAA therapy was associated with higher HCC recurrence and that the available data do not suggest differentiated surveillance pathways for HCC among patients receiving IFN-free therapeutic regimens or receiving DAA treatment. However, the heterogeneity of the studies included in this analysis limited the power of its results. Although there is no conclusive evidence at the moment in favor or against an increased risk of early HCC recurrence in patients undergoing treatment with DAAs, it is clear that the risk of early HCC recurrence in patients with HCV-related cirrhosis remains high despite viral eradication.
The latest guidelines of the European Association for the Study of the Liver (published in 2018) suggested a more intensive surveillance strategy (3-4 mo imaging intervals for the first 2 years that can be extended to 6-mo intervals thereafter) in this patient setting[4]. However, the updated literature available after the publication of these guidelines almost unanimously confirmed that DAA therapy was not associated with an increase in early HCC recurrence rates and that both recurrence rates and tumor patterns after initiation of antiviral therapy did not differ between patients who received IFN-based or IFN-free therapy[68-76]. On the contrary, there is now increasing evidence of a reduction in the risk of early (as well as late) HCC recurrence after viral eradication achieved by DAA[77-83].
The choice of treatment for HCC depends on the characteristics of the patient (in particular the residual liver function and the performance status) and the neoplasm characteristics (size, single or multifocal, vascular invasion, distant metastasis) as well as the indication and eligibility for LT.
In terms of recurrence risk, no conclusive data are available in the literature about which type of loco-regional treatment is more advantageous. In the absence of liver cirrhosis, LR probably offers the best prognostic prospects, with an early recurrence rate of approximately 10%[36]. Including patients with liver cirrhosis, the early recurrence rates of post-hepatectomy HCC reported in the literature range from 25% to 50%[34,84,85]. Metachronous HCC, new postoperative lesions and intrahepatic metastasis from primary HCC are in fact relatively common following hepatectomy[42]. In this regard, the resection margins after hepatectomy appear to be closely related to the risk of recurrence. In particular, neoplastic invasion of the resection margins represents a significant predictor of the risk of recurrence[44]. A resection margin < 1 cm also seems to be an independent risk factor for early HCC recurrence[43,86]. Some evidence also suggests that a non-anatomic hepatic resection (resection or enucleation without regard to sectoral structure) could lead to an increase in early recurrence rate[45]. In fact, an anatomic resection (resection of the entire liver parenchymal tissue supplied by the portal venous system draining the HCC tissue, independent of margin length) could hinder cell dissemination, reducing tumor cell flow throughout the portal circulation and consequently reduce the risk of intrahepatic metastasis and improve RFS. However, the absence of randomized clinical trials comparing anatomical and non-anatomical hepatic resection do not provide conclusive indications[87].
As previously mentioned, a maximum tumor diameter greater than 2.6 cm (according to Zhu et al[42]) or 3.0 cm (according to Jung et al[36]) before a curative LR is predictive of a high rate of early recurrence. The risk of early HCC recurrence with a diameter greater than 2.6 cm appears 4.77-fold higher than neoplasms with a diameter ≤ 2.6 cm[42]. Furthermore, high preoperative total bilirubin levels also correlate with an increased risk of recurrence[42]. In particular, this risk would be increased by 6% for every 1 mmol/L increase in preoperative bilirubin. Chan et al[37] in a large multicenter study provided pre- and post-hepatectomy risk stratification models (ERASL-pre and ERASL-post, respectively) with the ability to identify three early recurrence risk classes, with a 2-year malignancy free survival rate of 64.8%, 42.5% and 20.7% in low, intermediate and high risk patients, respectively. In these models male sex, large tumor size, multifocal tumor, high Albumin-Bilirubin score and high serum AFP represented the factors closely related to early recurrence after LR. Ideally, these models, once validated, would optimize the therapeutic strategy for patients with high baseline risk of early recurrence after hepa-tectomy (revaluating the possibility of LT or providing adjuvant therapy) and intensify the surveillance program.
Compared to LR, RFA treatment shows significantly higher recurrence rates[41,88,89]. In particular RFA results in shorter overall survival and time to recurrence (5-year overall survival of 71.1%, 5-year recurrence time of 63.8%) than LR (5-year overall survival of 61.1%, 5-year recurrence time of 71.7%)[88]. The HR for death (0.84, 95%CI: 0.74-0.95) and recurrence (0.74, 95%CI: 0.68-0.79) was significantly lower in patients undergoing LR than in those undergoing RFA. Recently, Lee et al[41], through a retrospective study comparing the two methods, showed that in patients with BCLC stage A HCC treatment with RFA was associated with a 1.8 times higher probability of HCC recurrence than hepatectomy (95%CI: 1541-2216). While confirming higher overall survival rates after LR compared to RFA, other data were unable to demonstrate significant differences in RFS between the two methods[90]. Finally, similar to what was shown for LR, an ablation margin ≥ 10 mm away from the tumor lesion guarantees better outcomes and a lower rate of recurrence compared to an ablation margin ≥ 5 mm and < 10 mm[91].
Conventionally, HCC recurrence is defined as late when it occurs 2 years after treatment of the primary tumor. Late recurrence is generally related to de novo development of the neoplasm and does not depend on the characteristics of the previous tumor. About 90% of late relapses consist in exclusively intrahepatic localization, whereas the remaining cases show both intrahepatic and extrahepatic localizations[92]. Therefore the risk factors are mainly related to underlying liver disease (Table 2).
| Predictors related to HCC characteristics | Ref. | Predictors related to patient characteristics | Ref. | Predictors related to treatment | Ref. |
| Size > 5 cm | [92] | Liver cirrhosis | [32,41,43,84,93-96] | RFA (vs LR) | [41,88,117-119] |
| Multifocality | [92,93] | Old age | [41,92] | ||
| Microvascular invasion | [92] | Male sex | [93] | ||
| AFP > 400 μg/L | [94] | LSM | [43,102] | ||
| Portal hypertension | |||||
| SSM (> 70 kPa) | [43] | ||||
| Splenic volume (> 165 mL) | [40] | ||||
| Platelet count | [43,103,104] | ||||
| Esophageal varices | [43,103] | ||||
| HBV infection | |||||
| High viral load | [105,108] | ||||
| Low viral load | [111] | ||||
| High HBsAg levels | [84,86,103,107] |
Among the predictive factors of late recurrence related to the basal characteristics of the patient, it is easily understood that the main predictive factor is the presence of liver cirrhosis, a fertile substrate for hepatocarcinogenesis[93-96]. In particular, the presence of liver cirrhosis increases the risk of late HCC recurrence by 3 or 4 times[41,93]. In fact, liver cirrhosis is a pre-malignant condition that due to an accelerated hepatocyte turnover induced by the chronic inflammatory state promotes the accumulation of gene aberrations and cell transformations. The high rate of gene errors and the subsequent uncontrolled cell proliferation therefore favor the development of HCC[97]. However, patients with liver cirrhosis undergoing curative treatment for HCC show a late recurrence rate significantly higher compared to de novo incidence rate in patients with no history of prior HCC[41]. This implies that the presence of liver cirrhosis is not the only factor that elicits the cancer risk in patients with late recurrence.
As highlighted by Lee et al[41], Xu et al[92] and Yang et al[93], the age and sex of the patient also contribute to the late recurrence risk stratification. Older and male patients show an increased risk of late HCC recurrence after curative treatment. The sex difference in the late recurrence rate could be explained by the potential protective role of estrogen on the development of HCC[98,99], resulting in more than 3 times higher probability of late recurrence in men compared to women[93].
The predictive role of liver stiffness (LSM) in the risk of HCC occurrence is well documented in the literature[100,101]. Since occurrence and late recurrence show similar pathogenesis (de novo hepatocarcinogenesis), it seems reasonable to believe that LSM can also represent a risk factor for late recurrence. In this regard, Jung et al[102] had already highlighted how the presence of liver cirrhosis assessed by LSM ≥ 13.5 kPa was associated with an increased overall risk of recurrence. More recently, Marasco et al[43] demonstrated that LSM but even more the splenic stiffness (surrogate for the degree of portal hypertension) were associated with an increased risk of late HCC recurrence. Late RFS was significantly different according to the splenic stiffness cutoff of 70 kPa.
These data find further evidence in the literature. Fang et al[40] in fact showed how the splenic volume (evaluated through automated volumetry software from preoperative computed tomography images) correlated independently with the probability of HCC recurrence 2 years after hepatic resection. In particular, for each 1 mL increase in splenic volume, the risk of late recurrence increased by 0.3%. In addition, patients with high splenic volume (> 165 mL) showed a lower 5-year RFS (5-year RFS 36%) than patients with low splenic volume (< 165 mL, 5-year RFS 71%). The correlation between stiffness/splenic volume and the risk of late HCC recurrence probably comes from the close association with the severity of liver disease and portal hypertension, both involved in hepatocarcinogenesis. The correlation that some studies showed between the risk of late recurrence and platelet counts or the presence of esophageal varices, which are also surrogates of the degree of portal hypertension and severity of liver damage[43,103,104], is therefore easily explained.
In patients with chronic HBV infection, the presence of viral replication increases the risk of late recurrence[105-108]. In particular, patients undergoing LR for HCC with high preoperative HBV-DNA levels show lower median overall survival and RFS compared to those with low viral load[106]. Patients with high viremia are characterized by higher Ishak inflammatory and fibrosis scores, favoring hepatocarcinogenesis[105]. The liver inflammatory activity induced by the high viral load may lead to necrosis and regeneration of hepatocytes, increasing the rate of gene errors and neoplastic transformation.
Recently, it was hypothesized that a high viral load (HBV-DNA > 104 copies/mL) was a risk factor also of early recurrence[109]. In fact, high preoperative levels of HBV-DNA increased the risk of microvascular invasion by about 40%[110]. In patients with high preoperative viral load, the initiation of antiviral treatment after surgical resection reduced both early and late HCC recurrence rates[106,109,110]. However, a similar effect on late recurrence rates was also found in patients with low preoperative viral loads[111]. Despite comparable efficacy in obtaining virological response, recent evidence suggested that tenofovir disoproxil fumarate (TDF) treatment was associated with a significantly lower risk of both HCC occurrence[112] and early and late recurrence[113] compared to treatment with entecavir (ETV). In particular, the HCC recurrence rate at 5 years from surgical resection was 33.6% in patients treated with TDF and 44.5% in those treated with ETV[113]. Unlike ETV, TDF induced the synthesis of high serum levels of IFN-lambda 3 (IFN-λ3)[114], which has been shown to exert a strong antitumor activity[115,116]. The antitumor effect of IFN-λ3 induced by TDF would therefore be additive to the capacity of breaking down viral replication and turn off necroinflammatory activity, which is common to both nucleoside (ETV) and nucleotide (TDF) analogs.
Increasing evidence is also available on the predictive role of hepatitis B surface antigen (HBsAg) levels on the rate of late HCC recurrence. In patients undergoing LR, preoperative HBsAg levels > 200 IU/mL were independent predictors of late recurrence (HR: 1778)[84,103]. If the role of the predictor is well defined, there is nevertheless a significant heterogeneity of the cutoffs used. Huang et al[86] in fact highlighted how in patients with low viral load the risk for HCC recurrence significantly increased with HBsAg levels ≥ 1000 IU/mL (5-year RFS rate of 46.1% in HBsAg ≥ 1000 IU/mL group vs 34.1% in HBsAg < 1000 IU/mL group). According to Sohn et al[107], HBsAg levels ≥ 4000 IU/mL were associated with late recurrence after curative resection in HBV-related HCC.
Several studies suggested that RFA treatment increases the risk of early and late recurrence (HR: 1872; 95%CI: 1290-2717) compared to LR[41,88,117-119]. Furthermore, the overall survival at 3 years and 5 years of patients undergoing LR appeared significantly higher than patients undergoing RFA[117,118]. In particular, the 5-year overall survival rates were 80% vs 66%, and 5-year RFS rates were 48% vs 18% for LR and RFA groups, respectively[118]. Because the choice of treatment is influenced by both tumor- and liver disease-related factors, some authors hypothesize that the correlation between RFA treatment and the risk of late recurrence could reflect both. In fact, patients undergoing RFA are generally older, have a greater number of comorbidities and a worse degree of liver function than patients undergoing LR. As noted above, these factors are closely related to the risk of both late and early recurrence. Therefore, the higher risk of late recurrence in patients who received RFA compared to those treated with resection could depend on more severe underlying liver disease.
Some evidence suggested (which was different from what was expected[95,96]) that factors related to tumor burden (size, multifocality) determined an increase in the risk of late and early recurrence[45,93,105]. Recently, Xu et al[92] confirmed in a multicenter retrospective analysis of patients who underwent curative LR for HCC that multifocality, dimensions greater than 5 cm and the presence of satellite nodules or vascular invasion (macroscopic or microscopic) represented independent risk factors of late recurrence. The presence of multifocal HCC at baseline increased the risk of a late recurrence after curative treatment by more than 3 times (HR: 3766, 95%CI: 2287–6201)[93]. In this regard, Cheng et al[94] also showed that pre-hepatectomy AFP levels > 400 ug/L were related to the risk of late (as well as early) recurrence. Given the dichotomy between early and late recurrence, these factors should correlate with an increased probability of occult intrahepatic dissemination (and therefore early recurrence) rather than an increased risk of hepatocarcinogenesis (late recurrence). These data suggest that the dichotomy between early and late recurrence is probably not so clear-cut, and pathogenetic mechanisms and clinical features may overlap. Therefore, the temporal criterion alone is not able to discriminate the pathogenesis and aggressiveness of a recurrence of HCC with certainty.
LT represents the most radical approach for HCC in patients with liver cirrhosis and is able to treat simultaneously the neoplasm and the underlying liver disease while minimizing the risk of both early and late recurrence when candidate selection is adequate[120]. In fact, a therapeutic strategy that provides for early listing for transplantation and a loco-regional bridging therapy in patients with HCC at high risk of recurrence has proven to be extremely valid and associated with excellent long-term outcomes[121]. The presence of occult extrahepatic dissemination and the persistence of the cause of liver damage (e.g., HBV infection or HBV/hepatitis D virus coinfection) account for the residual risk of early and late HCC recurrence, respectively. Overall, the estimated HCC recurrence rate in patients undergoing LT is between 12% and 20%[22,122,123].
The most important predictor of the risk of early post-transplant recurrence is certainly the tumor burden. Size and number of lesions closely correlate with this risk, although not in a linear way. In fact, in multifocal HCC, starting from three or more lesions, the increase in the risk of recurrence appears to be attenuated[124]. Conversely, this risk is proportional to the size of the tumor. In particular, the recurrence rate increases by 36% for each additional centimeter of HCC diameter[125]. The microvascular invasion is another determining factor in the risk of recurrence and HCC-related death[126,127]. Its presence increases the risk of recurrence by approximately 2.4 times and significantly reduces the 5-year rates of RFS (44% vs 64% in the absence of microvascular invasion)[126]. Similarly, the histological finding of poorly differentiated (grade 3 or 4) HCC determines an increased risk of recurrence (39.3% vs 13.0% for grade 1 and 2 tumors) and reduction of 5-year RFS (39.9% vs 57.7%)[127]. In this setting, the prognostic role of AFP is also relevant with an inverse relationship with the post-transplant survival rate[128]. An increase in AFP greater than 7.5 ng/mL per month is associated with the presence of microvascular invasion and is predictive of post-transplant recurrence[129].
Beyond the characteristics and aggressiveness of the tumor, the etiology of liver disease also affects the rate of recurrence, especially late recurrence. In particular, the highest recurrence rate was found in patients with chronic HBV infection (18%) compared to other etiologies (11%, 10% and 8% for HCV, alcoholic liver disease and nonalcoholic steatohepatitis, respectively). In this regard, preliminary evidence suggested that an increase in RFS for HCC patients following LT can be obtained by administering anti-HBV prophylaxis and/or anti-HBV immunoglobulins[130].
Finally, some comorbidities may also influence the risk of recurrence in HCC patients undergoing LT. In particular, obese patients show a significantly higher frequency of microvascular invasion and recurrence rates and lower RFS than normal weight patients[131,132]. This correlation could be explained by a more pronounced tumor neoangiogenesis in obese patients, secondary to the increased expression of vascular endothelial growth factor. Furthermore, in obese patients the reduction of adiponectin levels and the simultaneous increase of leptin induced a pro-oncogenic state and stimulated neoplastic proliferation[131,132].
The central theme in managing the risk of recurrence is the selection of patients eligible for LT. Currently, the strongest evidence identifies the Milan criteria as the best strategy to optimize the selection of LT candidates, stratifying the risk of early recurrence, strongly correlated to tumor size and number of focal lesions[22]. These criteria suggest the presence of single tumors ≤ 5 cm or multiple tumors ≤ 3 nodules sized ≤ 3 cm, without vascular invasion and/or extrahepatic involvement as boundaries for transplant eligibility[24]. The application of these criteria guarantees a post-transplant survival rate comparable to that of patients undergoing transplants for non-neoplastic causes[133]. On the other hand, patients undergoing organ transplantation beyond the Milan criteria show significantly higher HCC recurrence rates than patients within these criteria[22].
However, growing evidence suggests that these criteria, developed in 1996, may be excessively restrictive to date, leading to the exclusion of a subgroup of patients who could benefit from transplantation[120]. Yao et al[134] showed that expansion of the tumor size limits (solitary tumor ≤ 6.5 cm or ≤ 3 nodules with the largest lesion ≤ 4.5 cm and total diameter ≤ 8 cm-UCSF criteria) does not adversely impact survival post-transplant. Subsequently, the same authors validated these criteria, confirming a 5-year RFS rate of 81%[135]. The extension of the Milan criteria suggested by Yao et al[135] made the 5%-20% of previously excluded patients eligible for LT, guaranteeing comparable long-term survival rates. Also Mazzaferro et al[124] attempted to overcome the previous limits through the up-to-seven criteria: HCC with seven as the sum of the size of the largest tumor (in centimeters) and the number of tumors. They showed that in the absence of microvascular invasion patients who met these criteria demonstrated survival rates comparable to patients who met the original Milan criteria (5-year overall survival of 71.2%). Conversely, the presence of microvascular invasion doubled the likelihood of recurrence and significantly reduced the overall post-transplant survival rates in these patients (up-to-seven patients 5-year overall survival of 53.6% vs Milan criteria patients 5-year overall survival of 73.3%). Microvascular invasion was observed in 16.6% of patients who met the Milan criteria and in over half of patients beyond the Up-to-seven criteria[124].
Recently, Mazzaferro et al[136] attempted to identify factors associated with HCC-related deaths of patients who underwent LT and to provide a predictive model of survival. The number of lesions and their size, as well as the levels of AFP, were significantly associated with HCC-specific deaths. To ensure an HCC-specific post-transplant survival rate of at least 70%, the authors suggested that the sum of the number and size of tumors (in centimeters) should not exceed 7 when the level of AFP was < 200 ng/mL, should not exceed 5 when the level of AFP was 200-400 ng/mL and should not exceed 4 when the level of AFP was 400-1000 ng/mL. Several studies confirmed the predictive value of pretransplant AFP levels for HCC recurrence[22,137]. A similar role has also been demonstrated for PIVKA-II (5-fold increased risk for recurrence after transplantation)[138].
An approach independent of the size of the HCC and the number of lesions has been hypothesized through the “extended Toronto criteria”[137]. The eligibility for LT included tumors that do not have extrahepatic spread and/or macrovascular invasion and that have a low histological grade on preoperative biopsy and the patient enjoys a high performance status. The post-transplant survival rates in this case were shown to be independent of the patient’s status within or beyond the Milan criteria (5-year overall survival of 69% and 78% for patients who met or did not meet the Milan criteria, respectively, P = 0.3) and therefore independent of the size of HCC and the number of lesions[139].
Furthermore, growing evidence suggests that an effective and sustained tumor downstaging with locoregional, surgical or systemic therapies from beyond to within the Milan criteria favorably impact overall and tumor-free survival[25,140,141]. In particular, the success rate of downstaging reported in the literature was higher than 40%[142]. Patients eligible for LT who met the Milan criteria after HCC downstaging showed 5-year tumor event-free and overall survival of 76.8% and 77.5%, respectively, compared to 18.3% (P = 0.003) and 31.2% (P = 0.035) of patients who underwent standard of care (non-transplantation therapies)[25]. Regardless of the Milan criteria, in patients with HCC eligible for LT, a pretransplantation loco-regional therapeutic approach (neoadjuvant, thermal ablation) reduced the risk of recurrence[4].
HCC is currently a potentially curable disease. However, recurrence rates still appear to be extra-ordinarily high. Although several factors are involved, the identification of predictive factors for both early and late HCC recurrence could optimize treatment strategies (LT, LR, thermal ablation) and surveillance. High-risk patients should be referred to treatments with the lowest recurrence rate (e.g., LT[121]) and/or combination therapies, as well as intensive surveillance programs. The combination of multiple treatments or use of adjuvant or neoadjuvant therapeutic schemes (e.g., immunotherapy) could reduce the recurrence rate and improve overall survival[143]. Research should therefore aim to validate these combined therapeutic strategies, particularly in patients with strong pre- and postoperative predictors of early or late recurrence.
We want to thank the department staff who allow us to work at our best every day.
| 1. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 639] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 2. | Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 3. | Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. ; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6421] [Article Influence: 802.6] [Reference Citation Analysis (9)] |
| 5. | McCullough AK, Lloyd RS. Mechanisms underlying aflatoxin-associated mutagenesis - Implications in carcinogenesis. DNA Repair (Amst). 2019;77:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Jiang Y, Han Q, Zhao H, Zhang J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:435-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 7. | Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 8. | Khatun M, Ray R, Ray RB. Hepatitis C virus associated hepatocellular carcinoma. Adv Cancer Res. 2021;149:103-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | D'Ambrosio R, Lampertico P. Is it time to refine HCC surveillance strategies in HCV cured patients? Hepatology. 2022;76:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Banini BA, Sanyal AJ. NAFLD-related HCC. Adv Cancer Res. 2021;149:143-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 11. | Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 12. | Saitta C, Pollicino T, Raimondo G. Obesity and liver cancer. Ann Hepatol. 2019;18:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-945, 945.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 14. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 15. | Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15:1295-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 16. | Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20:12945-12955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Ajmera VH, Terrault NA, Harrison SA. Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? Hepatology. 2017;65:2090-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer. 2021;21:1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 395] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 20. | Vitale A, Saracino E, Boccagni P, Brolese A, D'Amico F, Gringeri E, Neri D, Srsen N, Valmasoni M, Zanus G, Carraro A, Violi P, Pauletto A, Bassi D, Polacco M, Burra P, Farinati F, Feltracco P, Romano A, D'Amico DF, Cillo U. Validation of the BCLC prognostic system in surgical hepatocellular cancer patients. Transplant Proc. 2009;41:1260-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3132] [Article Influence: 783.0] [Reference Citation Analysis (61)] |
| 22. | Tan DJH, Wong C, Ng CH, Poh CW, Jain SR, Huang DQ, Muthiah MD. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 23. | Santopaolo F, Lenci I, Milana M, Manzia TM, Baiocchi L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J Gastroenterol. 2019;25:2591-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (3)] |
| 24. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5394] [Article Influence: 179.8] [Reference Citation Analysis (7)] |
| 25. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 26. | Orcutt ST, Anaya DA. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control. 2018;25:1073274817744621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 27. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1127] [Article Influence: 93.9] [Reference Citation Analysis (1)] |
| 28. | Seror O, N'Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, Coderc E, Trinchet JC, Beaugrand M, Sellier N. Large (>or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology. 2008;248:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 553] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 30. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 833] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 31. | Wang H, Liu A, Bo W, Feng X, Hu Y, Tian L, Zhang H, Tang X. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis. 2016;48:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Hayashi M, Shimizu T, Hirokawa F, Inoue Y, Komeda K, Asakuma M, Miyamoto Y, Takeshita A, Shibayama Y, Tanigawa N. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am Surg. 2011;77:572-578. [PubMed] |
| 34. | Xing H, Zhang WG, Cescon M, Liang L, Li C, Wang MD, Wu H, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Schwartz M, Pawlik TM, Serenari M, Shen F, Wu MC, Yang T. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 2020;22:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 35. | Furuta M, Ueno M, Fujimoto A, Hayami S, Yasukawa S, Kojima F, Arihiro K, Kawakami Y, Wardell CP, Shiraishi Y, Tanaka H, Nakano K, Maejima K, Sasaki-Oku A, Tokunaga N, Boroevich KA, Abe T, Aikata H, Ohdan H, Gotoh K, Kubo M, Tsunoda T, Miyano S, Chayama K, Yamaue H, Nakagawa H. Whole genome sequencing discriminates hepatocellular carcinoma with intrahepatic metastasis from multi-centric tumors. J Hepatol. 2017;66:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Jung SM, Kim JM, Choi GS, Kwon CHD, Yi NJ, Lee KW, Suh KS, Joh JW. Characteristics of Early Recurrence After Curative Liver Resection for Solitary Hepatocellular Carcinoma. J Gastrointest Surg. 2019;23:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Mazzaferro V, García-Fiñana M, Kudo M, Kumada T, Roayaie S, Johnson PJ. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 38. | Hong YM, Cho M, Yoon KT, Chu CW, Yang KH, Park YM, Rhu JH. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol. 2017;39:1010428317720863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Fang T, Long G, Mi X, Su W, Mo L, Zhou L. Splenic Volume, an Easy-To-Use Predictor of HCC Late Recurrence for HCC Patients After Hepatectomy. Front Oncol. 2022;12:876668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 41. | Lee HA, Lee YS, Kim BK, Jung YK, Kim SU, Park JY, Kim JH, An H, Kim DY, Yim HJ, Ahn SH, Yeon JE, Byun KS, Han KH, Um SH, Seo YS. Change in the Recurrence Pattern and Predictors over Time after Complete Cure of Hepatocellular Carcinoma. Gut Liver. 2021;15:420-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Zhu Y, Gu L, Chen T, Zheng G, Ye C, Jia W. Factors influencing early recurrence of hepatocellular carcinoma after curative resection. J Int Med Res. 2020;48:300060520945552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, Cucchetti A, Cescon M, Festi D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 44. | Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, Taylor BR, Grant DR, Vollmer CM. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 45. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1263] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 46. | Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 562] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 47. | Zhou L, Rui JA, Zhou WX, Wang SB, Chen SG, Qu Q. Edmondson-Steiner grade: A crucial predictor of recurrence and survival in hepatocellular carcinoma without microvascular invasio. Pathol Res Pract. 2017;213:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 48. | Kim JM, Yi NJ, Kwon CHD, Lee KW, Suh KS, Joh JW. Early disseminated recurrence after liver resection in solitary hepatocellular carcinoma. Ann Surg Treat Res. 2018;94:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Tani J, Senoh T, Moriya A, Ogawa C, Deguchi A, Sakamoto T, Takuma K, Nakahara M, Oura K, Tadokoro T, Mimura S, Fujita K, Yoneyama H, Kobara H, Morishita A, Himoto T, Tsutsui A, Nagano T, Takaguchi K, Masaki T. Long-Term Outcomes and Evaluation of Hepatocellular Carcinoma Recurrence after Hepatitis C Virus Eradication by Direct-Acting Antiviral Treatment: All Kagawa Liver Disease Group (AKLDG) Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Wang BL, Tan QW, Gao XH, Wu J, Guo W. Elevated PIVKA-II is associated with early recurrence and poor prognosis in BCLC 0-A hepatocellular carcinomas. Asian Pac J Cancer Prev. 2014;15:6673-6678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin. Liver Int. 2011;31:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Matsubara M, Shiraha H, Kataoka J, Iwamuro M, Horiguchi S, Nishina S, Takaoka N, Uemura M, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yamamoto K. Des-γ-carboxyl prothrombin is associated with tumor angiogenesis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Kim DY, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, Lee KS, Chon CY, Han KH. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007;72 Suppl 1:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Kang SH, Kim DY, Jeon SM, Ahn SH, Park JY, Kim SU, Kim JK, Lee KS, Chon CY, Han KH. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur J Gastroenterol Hepatol. 2012;24:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 56. | Ang SF, Ng ES, Li H, Ong YH, Choo SP, Ngeow J, Toh HC, Lim KH, Yap HY, Tan CK, Ooi LL, Cheow PC, Chung AY, Chow PK, Foo KF, Tan MH. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS One. 2015;10:e0118658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 57. | Huang S, Huang GQ, Zhu GQ, Liu WY, You J, Shi KQ, Wang XB, Che HY, Chen GL, Fang JF, Zhou Y, Zhou MT, Chen YP, Braddock M, Zheng MH. Establishment and Validation of SSCLIP Scoring System to Estimate Survival in Hepatocellular Carcinoma Patients Who Received Curative Liver Resection. PLoS One. 2015;10:e0129000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2173] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 59. | Zheng J, Cai J, Li H, Zeng K, He L, Fu H, Zhang J, Chen L, Yao J, Zhang Y, Yang Y. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cell Physiol Biochem. 2017;44:967-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 60. | Zheng X, Ye B, Gou Y, Li Z, Chen C, Liao F, Liu X, Qin S. Neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers to predict relapse and survival in posthepatectomy HBV-related hepatocellular carcinoma: a meta-analysis and preliminary immune perspective. Transl Cancer Res. 2021;10:1261-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 61. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 62. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 713] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 63. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 818] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 64. | Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, Distefano M, Larocca L, Prestileo T, Tinè F, Bertino G, Giannitrapani L, Benanti F, Licata A, Scalisi I, Mazzola G, Cartabellotta F, Alessi N, Barbàra M, Russello M, Scifo G, Squadrito G, Raimondo G, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV). Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? Aliment Pharmacol Ther. 2017;46:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 65. | Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WTH, MacDonald DC, Agarwal K, Foster GR, Irving WL; HCV Research UK. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 66. | ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 67. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 68. | Nishibatake Kinoshita M, Minami T, Tateishi R, Wake T, Nakagomi R, Fujiwara N, Sato M, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Shiina S, Koike K. Impact of direct-acting antivirals on early recurrence of HCV-related HCC: Comparison with interferon-based therapy. J Hepatol. 2019;70:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 69. | Tajiri K, Ito H, Kawai K, Kashii Y, Hayashi Y, Murayama A, Minemura M, Takahara T, Shimizu Y, Yasuda I. Direct-acting antivirals for hepatitis C virus-infected patients with hepatocellular carcinoma. World J Hepatol. 2022;14:1190-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Sapena V, Enea M, Torres F, Celsa C, Rios J, Rizzo GEM, Nahon P, Mariño Z, Tateishi R, Minami T, Sangiovanni A, Forns X, Toyoda H, Brillanti S, Conti F, Degasperi E, Yu ML, Tsai PC, Jean K, El Kassas M, Shousha HI, Omar A, Zavaglia C, Nagata H, Nakagawa M, Asahina Y, Singal AG, Murphy C, Kohla M, Masetti C, Dufour JF, Merchante N, Cavalletto L, Chemello LL, Pol S, Crespo J, Calleja JL, Villani R, Serviddio G, Zanetto A, Shalaby S, Russo FP, Bielen R, Trevisani F, Cammà C, Bruix J, Cabibbo G, Reig M. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut. 2022;71:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 71. | Rutledge SM, Zheng H, Li DK, Chung RT. No evidence for higher rates of hepatocellular carcinoma after direct-acting antiviral treatment: a meta-analysis. Hepatoma Res. 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, Madonia S, Rossi M, Magro B, Rini F, Distefano M, Larocca L, Prestileo T, Malizia G, Bertino G, Benanti F, Licata A, Scalisi I, Mazzola G, Di Rosolini MA, Alaimo G, Averna A, Cartabellotta F, Alessi N, Guastella S, Russello M, Scifo G, Squadrito G, Raimondo G, Trevisani F, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia – HCV (RESIST-HCV) and Italian Liver Cancer (ITA. LI.CA.) Group. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 73. | Ismail MS, Mohamed I, Polychronopoulou E, Goss JA, Kuo YF, Kanwal F, Jalal PK. Outcomes in the Era of Interferon-Free Direct-Acting Antiviral Therapy After Liver Transplantation in Patients with Hepatitis C Virus and Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:701-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Vallet-Pichard A, Correas JM, Dorival C, Zoulim F, Tran A, Bourlière M, Calès P, Guyader D, Bronowicki JP, Larrey D, Hezode C, Loustaud-Ratti V, Gournay J, de Ledinghen V, Asselah T, Ganne N, Metivier S, Chazouillères O, Leroy V, Rosa I, Samuel D, Mathurin P, Cagnot C, Fontaine H, Carrat F, Pol S; AFEF ANRS study group. Absence of impact of direct acting antivirals for hepatitis C virus on recurrent hepatocellular carcinoma tumor growth in the AFEF/ANRS CO22 Hepather cohort. Clin Res Hepatol Gastroenterol. 2021;45:101459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Lin WC, Lin YS, Chang CW, Wang TE, Wang HY, Chen MJ. Impact of direct-acting antiviral therapy for hepatitis C-related hepatocellular carcinoma. PLoS One. 2020;15:e0233212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683-1692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 77. | Kuromatsu R, Ide T, Okamura S, Noda Y, Kamachi N, Nakano M, Shirono T, Shimose S, Iwamoto H, Kuwahara R, Arinaga-Hino T, Niizeki T, Zaizen Y, Takaki H, Shirachi M, Koga H, Torimura T. Hepatitis C Virus Elimination Using Direct Acting Antivirals after the Radical Cure of Hepatocellular Carcinoma Suppresses the Recurrence of the Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 78. | Lui FH, Moosvi Z, Patel A, Hussain S, Duong A, Duong J, Nguyen DL. Decreased risk of hepatocellular carcinoma recurrence with direct-acting antivirals compared with no treatment for hepatitis C: a meta-analysis. Ann Gastroenterol. 2020;33:293-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Chen YS, Huang KH, Wang PM, Chuang CH, Yong CC, Liu YW, Huang PY, Yao CC, Lin YP, Tsai MC. The Impact of Direct-Acting Antiviral Therapy on the Risk of Recurrence after Curative Resection in Patients with Hepatitis-C-Virus-Related Early Stage Hepatocellular Carcinoma. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 80. | Ikenaga H, Uchida-Kobayashi S, Tamori A, Odagiri N, Yoshida K, Kotani K, Motoyama H, Kozuka R, Kawamura E, Hagihara A, Fujii H, Enomoto M, Kawada N. Direct-acting antivirals reduce the risk of tumour progression of hepatocellular carcinoma after curative treatment. J Viral Hepat. 2022;29:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Ochi H, Hiraoka A, Hirooka M, Koizumi Y, Amano M, Azemoto N, Watanabe T, Yoshida O, Tokumoto Y, Mashiba T, Yokota T, Abe M, Michitaka K, Hiasa Y, Joko K. Direct-acting antivirals improve survival and recurrence rates after treatment of hepatocellular carcinoma within the Milan criteria. J Gastroenterol. 2021;56:90-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Ismail MS, Hassan M, Khaderi SA, Yousry WA, Kamal El-Din MM, Bahaa El-Din MM, El Sayed OA, Kaseb AO, Goss JA, Kanwal F, Jalal PK. Clinical efficacy of direct-acting antiviral therapy for recurrent hepatitis C virus infection after liver transplantation in patients with hepatocellular carcinoma. World J Hepatol. 2020;12:628-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Preda CM, Baicus C, Sandra I, Oproiu A, Manuc T, Constantinescu I, Gavrila D, Diculescu M, Dumitru R, Vasilescu C, Tieranu C, Istratescu D, Voiosu T, Manuc M. Recurrence rate of hepatocellular carcinoma in patients with treated hepatocellular carcinoma and hepatitis C virus-associated cirrhosis after ombitasvir/paritaprevir/ritonavir+dasabuvir+ribavirin therapy. United European Gastroenterol J. 2019;7:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Lee IC, Lei HJ, Chau GY, Yeh YC, Wu CJ, Su CW, Huo TI, Chao Y, Lin HC, Hou MC, Huang YH. Predictors of long-term recurrence and survival after resection of HBV-related hepatocellular carcinoma: the role of HBsAg. Am J Cancer Res. 2021;11:3711-3725. [PubMed] |
| 85. | Zheng J, Chou JF, Gönen M, Vachharajani N, Chapman WC, Majella Doyle MB, Turcotte S, Vandenbroucke-Menu F, Lapointe R, Buettner S, Groot Koerkamp B, Ijzermans JNM, Chan CY, Goh BKP, Teo JY, Kam JH, Jeyaraj PR, Cheow PC, Chung AYF, Chow PKH, Ooi LLPJ, Balachandran VP, Kingham TP, Allen PJ, D'Angelica MI, DeMatteo RP, Jarnagin WR, Lee SY. Prediction of Hepatocellular Carcinoma Recurrence Beyond Milan Criteria After Resection: Validation of a Clinical Risk Score in an International Cohort. Ann Surg. 2017;266:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 86. | Huang G, Lau WY, Zhou WP, Shen F, Pan ZY, Yuan SX, Wu MC. Prediction of Hepatocellular Carcinoma Recurrence in Patients With Low Hepatitis B Virus DNA Levels and High Preoperative Hepatitis B Surface Antigen Levels. JAMA Surg. 2014;149:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Kang KJ, Ahn KS. Anatomical resection of hepatocellular carcinoma: A critical review of the procedure and its benefits on survival. World J Gastroenterol. 2017;23:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 88. | Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, Matsuyama Y. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 89. | Gory I, Fink M, Bell S, Gow P, Nicoll A, Knight V, Dev A, Rode A, Bailey M, Cheung W, Kemp W, Roberts SK; Melbourne Liver Group. Radiofrequency ablation versus resection for the treatment of early stage hepatocellular carcinoma: a multicenter Australian study. Scand J Gastroenterol. 2015;50:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 90. | Li YC, Chen PH, Yeh JH, Hsiao P, Lo GH, Tan T, Cheng PN, Lin HY, Chen YS, Hsieh KC, Hsieh PM, Lin CW. Clinical outcomes of surgical resection versus radiofrequency ablation in very-early-stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. 2021;21:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Liao M, Zhong X, Zhang J, Liu Y, Zhu Z, Wu H, Zeng Y, Huang J. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: A prospective randomized trial. J Surg Oncol. 2017;115:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 93. | Yang Y, Chen Y, Ye F, Cao X, Xin Y, Wang Y, Lei Y, Li X, Feng D, Zhou X, Fan Q. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol. 2021;31:3053-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 94. | Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, Xia Y, Li J, Wang K, Yan Z, Wu D, Zhang B, Hüser N, Shen F. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 95. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 757] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 96. | Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500-507. [PubMed] |
| 97. | Nevola R, Rinaldi L, Giordano M, Marrone A, Adinolfi LE. Mechanisms and clinical behavior of hepatocellular carcinoma in HBV and HCV infection and alcoholic and non-alcoholic fatty liver disease. Hepatoma Res. 2018;4:55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Zheng B, Zhu YJ, Wang HY, Chen L. Gender disparity in hepatocellular carcinoma (HCC): multiple underlying mechanisms. Sci China Life Sci. 2017;60:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 99. | Hassan MM, Botrus G, Abdel-Wahab R, Wolff RA, Li D, Tweardy D, Phan AT, Hawk E, Javle M, Lee JS, Torres HA, Rashid A, Lenzi R, Hassabo HM, Abaza Y, Shalaby AS, Lacin S, Morris J, Patt YZ, Amos CI, Khaderi SA, Goss JA, Jalal PK, Kaseb AO. Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2017;15:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Rinaldi L, Guarino M, Perrella A, Pafundi PC, Valente G, Fontanella L, Nevola R, Guerrera B, Iuliano N, Imparato M, Trabucco A, Sasso FC, Morisco F, Ascione A, Piai G, Adinolfi LE. Role of Liver Stiffness Measurement in Predicting HCC Occurrence in Direct-Acting Antivirals Setting: A Real-Life Experience. Dig Dis Sci. 2019;64:3013-3019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Rosato V, Ascione A, Nevola R, Fracanzani AL, Piai G, Messina V, Claar E, Coppola C, Fontanella L, Lombardi R, Staiano L, Valente G, Fascione MC, Giorgione C, Mazzocca A, Galiero R, Perillo P, Marrone A, Sasso FC, Adinolfi LE, Rinaldi L. Factors affecting long-term changes of liver stiffness in direct-acting anti-hepatitis C virus therapy: A multicentre prospective study. J Viral Hepat. 2022;29:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Jung KS, Kim SU, Choi GH, Park JY, Park YN, Kim DY, Ahn SH, Chon CY, Kim KS, Choi EH, Choi JS, Han KH. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan®). Ann Surg Oncol. 2012;19:4278-4286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Wu CJ, Chau GY, Lee IC, Huo TI, Su CW, Hou MC, Huang YH. Early and late recurrence of surgically resected hepatitis B virus-related hepatocellular carcinoma on nucleos(t)ide analogues therapy. J Formos Med Assoc. 2021;120:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Pang Q, Qu K, Bi JB, Liu SS, Zhang JY, Song SD, Lin T, Xu XS, Wan Y, Tai MH, Liu HC, Dong YF, Liu C. Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: Systematic review and meta-analysis. World J Gastroenterol. 2015;21:7895-7906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 106. | Yang T, Lu JH, Zhai J, Lin C, Yang GS, Zhao RH, Shen F, Wu MC. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol. 2012;38:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Sohn W, Paik YH, Kim JM, Kwon CH, Joh JW, Cho JY, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21:2429-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 108. | Kim BK, Park JY, Kim DY, Kim JK, Kim KS, Choi JS, Moon BS, Han KH, Chon CY, Moon YM, Ahn SH. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Wang MD, Li C, Liang L, Xing H, Sun LY, Quan B, Wu H, Xu XF, Wu MC, Pawlik TM, Lau WY, Shen F, Yang T. Early and Late Recurrence of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Oncologist. 2020;25:e1541-e1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 110. | Li Z, Lei Z, Xia Y, Li J, Wang K, Zhang H, Wan X, Yang T, Zhou W, Wu M, Pawlik TM, Lau WY, Shen F. Association of Preoperative Antiviral Treatment With Incidences of Microvascular Invasion and Early Tumor Recurrence in Hepatitis B Virus-Related Hepatocellular Carcinoma. JAMA Surg. 2018;153:e182721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 111. | Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, Wang MC, Zhou WP. Antiviral Therapy Reduces Hepatocellular Carcinoma Recurrence in Patients With Low HBV-DNA Levels: A Randomized Controlled Trial. Ann Surg. 2018;268:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 112. | Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of Hepatocellular Carcinoma in Patients Treated With Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019;5:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 113. | Choi J, Jo C, Lim YS. Tenofovir Versus Entecavir on Recurrence of Hepatitis B Virus-Related Hepatocellular Carcinoma After Surgical Resection. Hepatology. 2021;73:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 114. | Murata K, Asano M, Matsumoto A, Sugiyama M, Nishida N, Tanaka E, Inoue T, Sakamoto M, Enomoto N, Shirasaki T, Honda M, Kaneko S, Gatanaga H, Oka S, Kawamura YI, Dohi T, Shuno Y, Yano H, Mizokami M. Induction of IFN-λ3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut. 2018;67:362-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 115. | Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686-7694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 116. | Yan Y, Wang L, He J, Liu P, Lv X, Zhang Y, Xu X, Zhang L. Synergy with interferon-lambda 3 and sorafenib suppresses hepatocellular carcinoma proliferation. Biomed Pharmacother. 2017;88:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Yin Z, Jin H, Ma T, Zhou Y, Yu M, Jian Z. A meta-analysis of long-term survival outcomes between surgical resection and radiofrequency ablation in patients with single hepatocellular carcinoma ≤ 2 cm (BCLC very early stage). Int J Surg. 2018;56:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann Surg. 2016;263:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 119. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 120. | Ju MR, Yopp AC. Evolving thresholds for liver transplantation in hepatocellular carcinoma: A Western experience. Ann Gastroenterol Surg. 2020;4:208-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Ferrer-Fàbrega J, Forner A, Liccioni A, Miquel R, Molina V, Navasa M, Fondevila C, García-Valdecasas JC, Bruix J, Fuster J. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. 2016;63:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 122. | Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 123. | Filgueira NA. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J Hepatol. 2019;11:261-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (3)] |
| 124. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1619] [Article Influence: 89.9] [Reference Citation Analysis (1)] |
| 125. | Welling TH, Eddinger K, Carrier K, Zhu D, Kleaveland T, Moore DE, Schaubel DE, Abt PL. Multicenter Study of Staging and Therapeutic Predictors of Hepatocellular Carcinoma Recurrence Following Transplantation. Liver Transpl. 2018;24:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 127. | Donat M, Alonso S, Pereira F, Ferrero E, Carrión L, Acin-Gándara D, Moreno E. Impact of Histological Factors of Hepatocellular Carcinoma on the Outcome of Liver Transplantation. Transplant Proc. 2016;48:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 129. | Giard JM, Mehta N, Dodge JL, Roberts JP, Yao FY. Alpha-Fetoprotein Slope >7.5 ng/mL per Month Predicts Microvascular Invasion and Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma. Transplantation. 2018;102:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Pazgan-Simon M, Simon KA, Jarowicz E, Rotter K, Szymanek-Pasternak A, Zuwała-Jagiełło J. Hepatitis B virus treatment in hepatocellular carcinoma patients prolongs survival and reduces the risk of cancer recurrence. Clin Exp Hepatol. 2018;4:210-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, Jacobson JS, Hershman DL, Verna EC, Zaretsky J, Halazun K, Dove L, Brown RS Jr, Neugut AI, Kato T, Remotti H, Coppleson YJ, Emond JC. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 132. | Mathur A, Franco ES, Leone JP, Osman-Mohamed H, Rojas H, Kemmer N, Neff GW, Rosemurgy AS, Alsina AE. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB (Oxford). 2013;15:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 133. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 134. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1718] [Article Influence: 68.7] [Reference Citation Analysis (1)] |
| 135. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 136. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 509] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 137. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD, Grant DR. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 138. | Lai Q, Iesari S, Levi Sandri GB, Lerut J. Des-gamma-carboxy prothrombin in hepatocellular cancer patients waiting for liver transplant: a systematic review and meta-analysis. Int J Biol Markers. 2017;32:e370-e374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 140. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK Jr, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 141. | Chapman WC, Garcia-Aroz S, Vachharajani N, Fowler K, Saad N, Lin Y, Wellen J, Tan B, Khan AS, Doyle MB. Liver Transplantation for Advanced Hepatocellular Carcinoma after Downstaging Without Up-Front Stage Restrictions. J Am Coll Surg. 2017;224:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 142. | Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21:1142-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 143. | Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ariyachet C, Thailand; Ke X, China; Pandit R, United States; Reddy NNR, India S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR