Published online Dec 21, 2023. doi: 10.3748/wjg.v29.i47.6148
Peer-review started: September 6, 2023
First decision: November 1, 2023
Revised: November 4, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 21, 2023
Processing time: 103 Days and 18.1 Hours
Colorectal cancer (CRC) is a highly prevalent malignancy worldwide, and new therapeutic targets urgently need to be found to prolong patient survival. 5-methoxytryptophan (5-MTP) is a tryptophan metabolite found in animals and humans. However, the effects of 5-MTP on proliferation and apoptosis of CRC cells are currently unknown.
To investigate the effects of 5-MTP on the proliferation, migration, invasion, and apoptosis abilities of CRC cells. Additionally, we seek to explore whether 5-MTP has the potential to be utilized as a drug for the treatment of CRC.
In order to evaluate the effect of 5-MTP on CRC cells, a series of experiments were conducted for evaluation. Colony formation assay and Cell Counting Kit 8 assays were used to investigate the impact of 5-MTP on the proliferation of CRC cell lines. Cell cycle assays were employed to examine the effect of 5-MTP on cellular growth. In addition, we investigated the effects of 5-MTP on apoptosis and reactive oxygen species in HCT-116 cells. To obtain a deeper understanding of how 5-MTP affects CRC, we conducted a study to examine its influence on the PI3K/Akt signaling pathway in CRC cells.
This article showed that 5-MTP promoted apoptosis and cell cycle arrest and inhibited cell proliferation in CRC cells. In many articles, it has been reported that PI3K/Akt/FoxO3a signaling pathway is one of the most important signaling pathways involved in internal regulating cell proliferation and differentiation. Nevertheless, 5-MTP combined with PI3K/Akt/FoxO3a signaling pathway inhibitors significantly promoted apoptosis and cell cycle arrest and inhibited cell proliferation in CRC cells compared with 5-MTP alone in our study.
Therefore, there is strong evidence that 5-MTP can be used as an effective medicine for CRC treatment.
Core Tip: Colorectal cancer (CRC) is insensitive to radiotherapy and has poor therapeutic efficacy, and there is an urgent need to find new therapeutic targets to prolong patient survival. 5-methoxytryptophan (5-MTP) is a tryptophan metabolite present in both animals and humans. 5-MTP has a wide range of physiological functions such as stabilizing endothelial function, anti-inflammation, and antioxidant to prevent cellular damage. Our study found that 5-MTP combined with an inhibitor of the PI3K/Akt/FoxO3a signaling pathway significantly promoted apoptosis and cell cycle arrest and inhibited cell proliferation in CRC cells compared with 5-MTP alone.
- Citation: Zhao TL, Qi Y, Wang YF, Wang Y, Liang H, Pu YB. 5-methoxytryptophan induced apoptosis and PI3K/Akt/FoxO3a phosphorylation in colorectal cancer. World J Gastroenterol 2023; 29(47): 6148-6160
- URL: https://www.wjgnet.com/1007-9327/full/v29/i47/6148.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i47.6148
The incidence and mortality of colorectal cancer (CRC) have declined over the past 30 years[1]. However, CRC morbidity and mortality are rising among young adults[2,3]. Patients with advanced CRC have a poor prognosis. Pathologic classification is used to assess prognosis and inform the treatment of CRC[4]. Great efforts have been made to develop noninvasive biomarkers to detect early cancers and/or reflect individual cancer risk, which is critical for reducing CRC mortality[5,6]. However, little progress has been made in improving disease-free survival in CRC patients. Because the pathomechanism of CRC progression is not fully understood, more studies are needed to discover and develop effective medicines for CRC treatment.
Traditional Chinese medicine (TCM) has a long history of treating malignant tumors and is of great significance in reducing the recurrence and metastasis rate, reducing adverse reactions to chemotherapy, prolonging survival, and improving quality of life[7,8]. Also, some previous study demonstrate the significant change of tryptophan after TCM treatment in cancer patients[9,10]. However, the potential anti-cancer role of tryptophan-related metabolites is still yet to be elucidated. 5-methoxytryptophan (5-MTP) is a tryptophan metabolite found in animals and humans[11]. Tryptophan is first hydroxylated by tryptophan type 1 or type 2 hydroxylases (TPH-1, TPH-2) to generate 5-hydroxytryptophan, then methylase hydroxyindole-O-methyltransferase further methylates 5-hydroxytryptophan to 5-MTP[12]. 5-MTP has anti-inflammatory and anti-fibrotic effects and has become an indispensable therapeutic factor in diseases such as myocardial infarction and renal fibrosis[11,12]. However, there have not been any reports of CRC, so more specific roles need to be further teased out.
In addition, 5-MTP was demonstrated for the first time to inhibit proliferation, invasion, and migration at the CRC cell level; promote apoptosis, reactive oxygen species (ROS) levels, and cell cycle arrest; and combined with PI3K/Akt/p-FoxO3a signaling pathway to play a stronger therapeutic role. Therefore, 5-MTP may be used as an adjuvant new strategy for treating CRC.
The three major kinds of human colon cancer cell lines, including HCT-116, HCT15, and SW480, were purchased from American Type Culture Collection (https://www.atcc.org/) and cultured in McCoy’s 5A (Gibco, United States), RPMI-1640 (Gibco, United States) and DMEM medium (Gibco, United States), respectively, supplemented with 10% fetal bovine serum (Gibco, United States) and 1% penicillin/streptomycin (Sangon Biotech, China). The colon cancer cell lines were incubated in incubator (Thermo Scientific, United States) with 5% CO2 at 37 °C. We added 5-MTP (MedChemExpress, United States) in different concentrations during cell culture.
Cells were digested to make single-cell suspensions at logarithmic phase and uniformly seeded in 12-well plates at 1 × 105 cells per well. After 48 h, cells were collected and washed with 1 mL of precooled phosphate buffered saline (PBS). The cell pellet was resuspended with 1 mL of precooled 70% ethanol and fixed overnight at 4 °C. On the second day, 70% ethanol was discarded by centrifugation at 1000 × g for 5 min at 4 °C, washed with 1 mL of precooled PBS. Each sample was added with 500 μL propyl iodide (PI) staining solution, gently mixed, incubated at 37 °C in the dark for 30 min, and the cells were filtered with a 400-mesh cell strainer to detect the cell cycle of each group by flow cytometry.
Colon cancer cells in the logarithmic growth phase were seeded in 6-well plates, and 5-MTP-treated cells were added after the cells attached. Cells in each group were digested with ethylene diamine tetraacetic acid-free trypsin, washed with PBS, and collected at 1000 × g for 5 min. Binding buffer 100 μL was used to resuspend cells, and fluorescein isothiocyanate staining solution 5 μL and PI staining solution 10 μL were added. They were blown and mixed well by the pipette. The cells were allowed to stand at room temperature for 15 min. Before loading the machine, 400 μL of binding buffer solution was added to each tube and mixed well so that the final system was 500 μL. Apoptosis of cells in each group was detected by flow cytometry.
To test the ROS level in each group of cells, the colon cancer cells were inoculated into 6-well plates at logarithmic growth phase. The 2′,7′-dichlorofluorescin diacetate (Sangon Biotech, China) was added, incubated in a cell culture incubator at 37 °C for 20 minutes, and observed under a confocal microscope. Similarly, Hoechst 33342 Viable Cell Staining Solution (Sangon Biotech, China) was added and incubated in a 37 °C cell culture incubator for 10 min and observed under a confocal microscope.
Cells were treated with 5-MTP at various concentrations for 48 h at 12-well plate. Before incubation, 25 μL of 200X JC-10 concentrate (Sangon Biotech, China) was added to a 5 mL assay buffer to dilute JC-10. 500 μL of JC-1 solution was added to each well and incubated at 37 °C for 20 min. After incubated, washed twice with staining buffer, added 500 μL cell culture medium, and observed under an inverted fluorescence microscope.
CRC cell lines were treated with 5-MTP for 48 h at logarithmic growth phase before protein extraction. Then, cells were washed with precooled PBS, 500 μL of RIPA buffer (Beyotime, China) was added to each dish, scraped by cell scraper, and transferred to a new centrifuge tube, lysed on ice for 30 min and centrifuged (12000 × g, 15 min, centrifugation radius 30 cm) to collect the supernatant. After adjusting the protein concentration, 4 × loading buffer was added and placed in a metal bath for heating denaturation (95 °C, 10 min). Sodium-dodecyl sulfate gel electrophoresis electrophoresis was performed to separate proteins (80 V), transferred by wet membranes (300 mA, 90 min), and blocked with 5% skimmed milk overnight (4 °C). Primary antibodies were used to incubate overnight (4 °C), including anti-AKT (1:1000, Cell Signaling Technology, #4685), anti-p-AKT (1:1000, Cell Signaling Technology, #13038), anti-FoxO3a (1:1000, Cell Signaling Technology, #12829), anti-p-FoxO3a (1:1000, Cell Signaling Technology, #9466), anti-β-actin (1:1000, Cell Signaling Technology, #4970), anti-Bax (1:1000, Cell Signaling Technology, #41162), anti-Bcl2 (1:1000, Cell Signaling Technology, #15071), anti-PARP (1:1000, Cell Signaling Technology, #9532), anti-Caspase3 (1:1000, Cell Signaling Technology, #9662), anti-Bim (1:1000, Cell Signaling Technology, #2933), anti-P27 (1:1000, Cell Signaling Technology, #3688) and anti-Cyclin D1 (1:1000, Cell Signaling Technology, #55506). Membranes were washed, incubated with secondary antibodies (1:10000) for 1 h at room temperature, and finally developed with ECL (Jiapeng Biotech, China), cassette luminescence, and band data analysis was performed by ImageJ software.
Cells were seeded in six-well plates at 800 cells/well in cell suspension, and 5-MTP was added when the cells were completely attached and cultured in an incubator for about 10 d, and > 50 cells/colony were considered as one clone, stained with crystal violet, photographed and counted.
After adjusting the cell density of each group to 1 × 104 cells/mL, 200 μL of cell suspension was added to a 96-well plate and cultured at 37 °C for 0, 24, 48, and 72 h. Following this, 10 μL of Cell Counting Kit 8 (CCK-8) solution was added to each well. After incubation at 37 °C for 2 h, absorbance values were measured using a microplate reader (Flash Biotech, China) at 450 nm.
Invasion assays were performed at 37 °C using transwell chambers coated with matrigel. The cell density was adjusted to 4 × 105 cells/mL, 100 μL was added to the upper transwell chamber, and then 600 μL of medium containing 10% foetal bovine serum was added to the lower chamber. After incubation at 37 °C for 24 h, the cells on the lower surface of the membrane were fixed with 4% paraformaldehyde (Sangon Biotech, China) for 20 min and stained with 500 μL, 1% crystal violet (Sangon Biotech, China) for 30 min. Five fields were randomly selected to observe the number of cells in invasion using an inverted light microscope and photographed.
GraphPad Prism (v9.0.0) software was used for data processing. Measurement data were expressed as mean ± SD. An independent sample t-test was performed between two groups. A one-way analysis of variance was used to compare the means between multiple groups. P < 0.05 was considered with statistically significant.
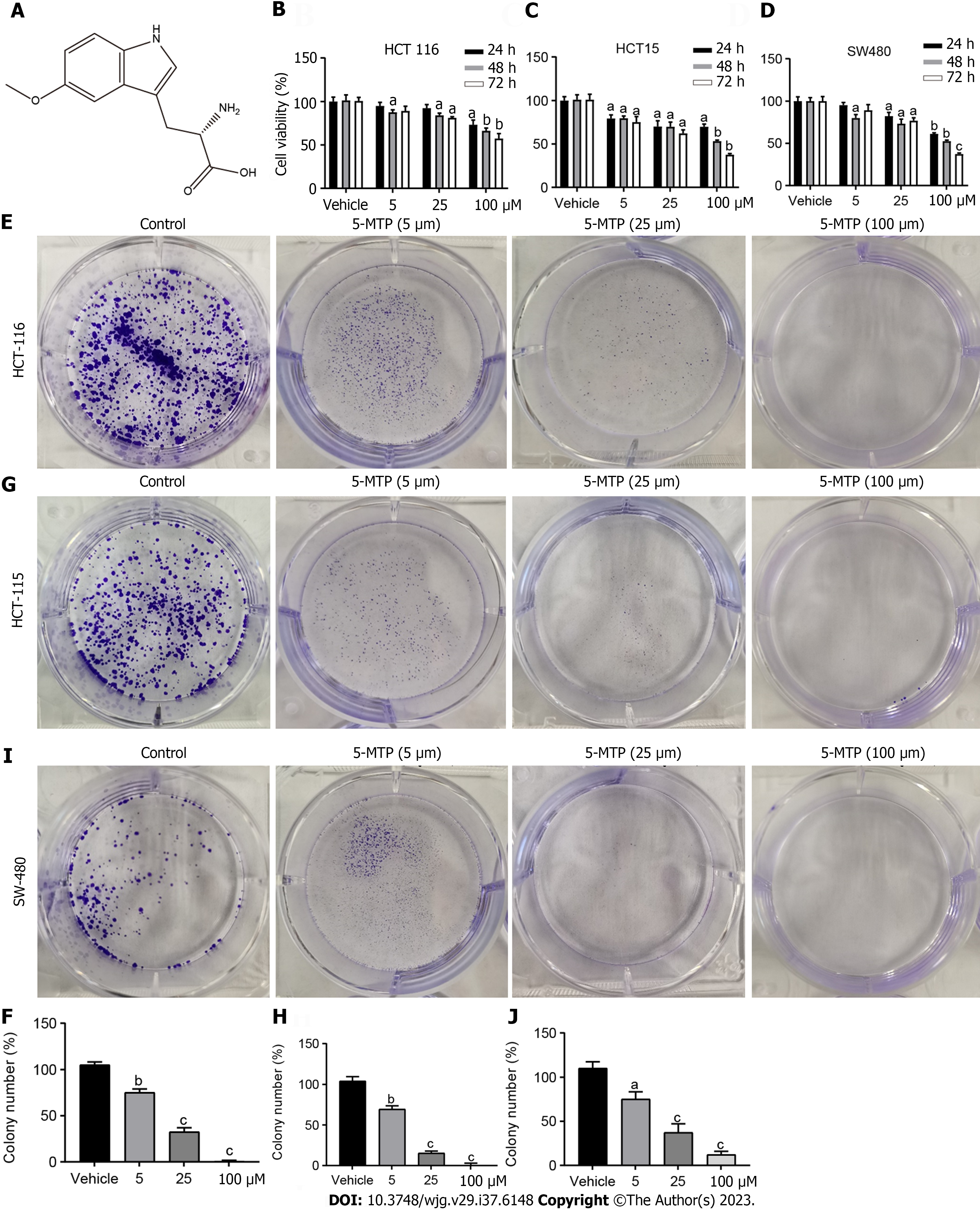
ATL was selected for further analysis, and its structure is shown in Figure 1A. To validate the role of 5-MTP in CRC, we selected HCT116, HCT15, and SW480 for subsequent experiments. In HCT116, HCT15, and SW480 cells, the results of CCK8 and colony formation assay showed that 5-MTP inhibited CRC cell proliferation gradually with increasing drug concentration (Figures 1B-J). These data suggested that 5-MTP inhibited human CRC cell proliferation.
Next, we examined the effects of 5-MTP on CRC cell apoptosis by measuring levels of JC-1 and ROS, among others. In HCT-116 cells, Hoechst staining showed that 5-MTP inhibited CRC cell activity progressively (Figures 2A and B). Flow cytometry results showed that 5-MTP significantly inhibited JC-1 levels and promoted the apoptosis rate and ROS levels (Figures 2C-H).
Flow cytometry results showed that the peak value became higher in the G2/M phase, that is, 5-MTP-induced HCT116 cell cycle arrest (Figures 3A and B). These results demonstrated that 5-MTP induced cell cycle arrest in HCT-116 cells.
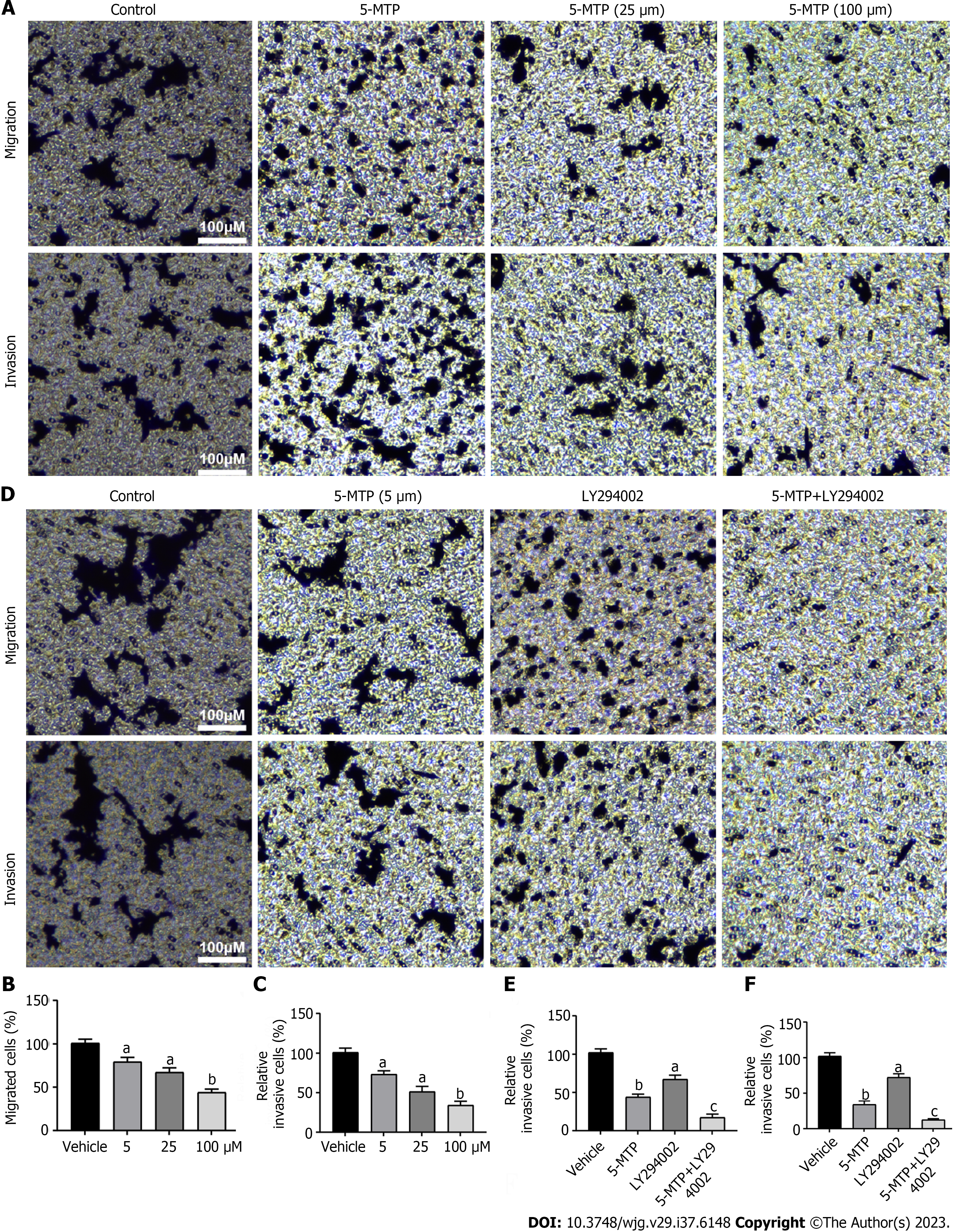
To further investigate the effect of 5-MTP on CRC cells, invasion and migration assays showed that 5-MTP inhibited CRC cell invasion and migration (Figures 4A-C). Ly294002 (PI3K) inhibitors have been shown to play a role in various tumors, including proliferation, metastasis, and apoptosis[13-15]. However, the effects of 5-MTP combined with ly294002 on invasion and migration in CRC cells are further enhanced is unknown. It was found that 5-MTP combined with ly294002 resulted in significantly less cell invasion and migration than 5-MTP (Figures 4D and F).
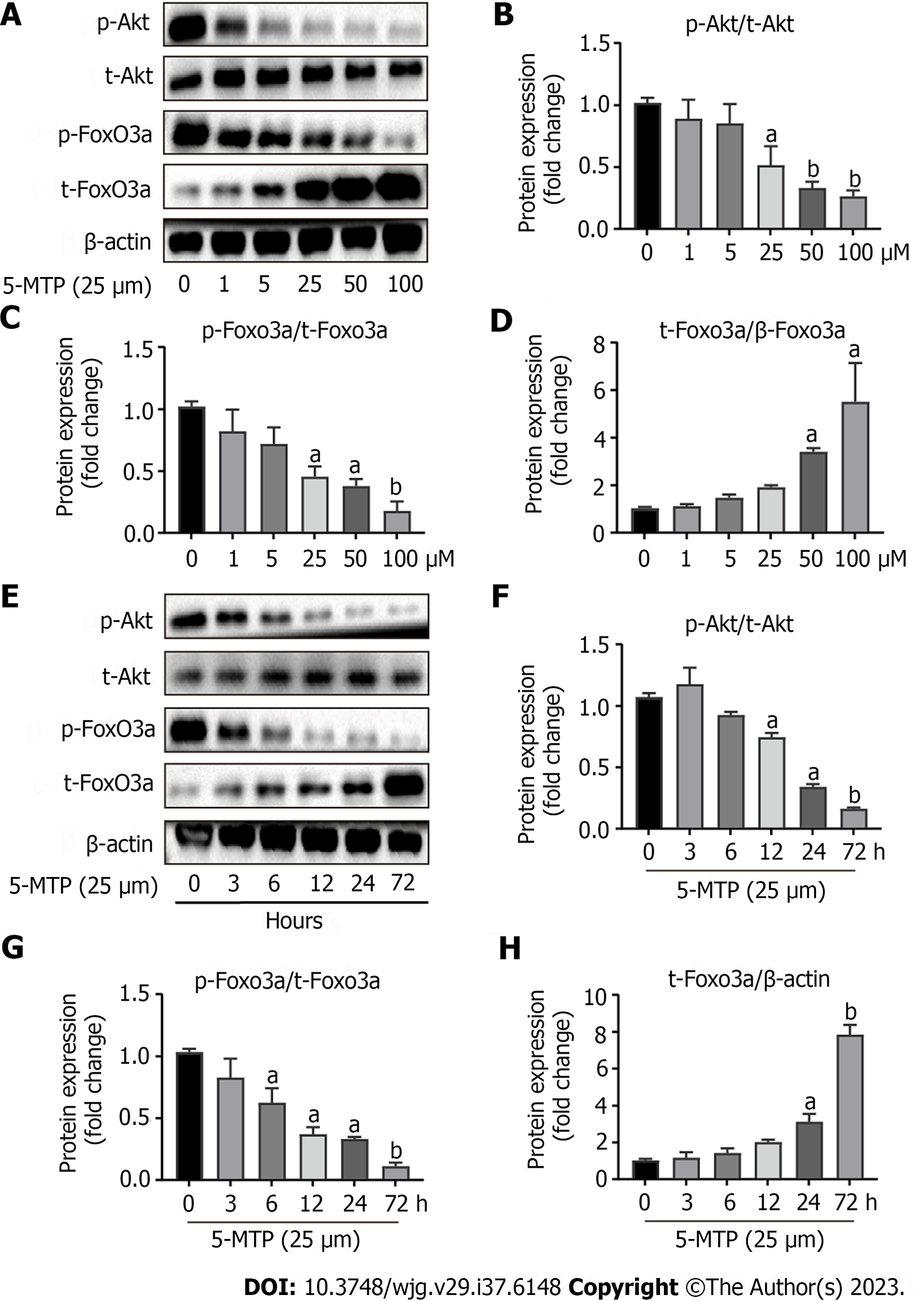
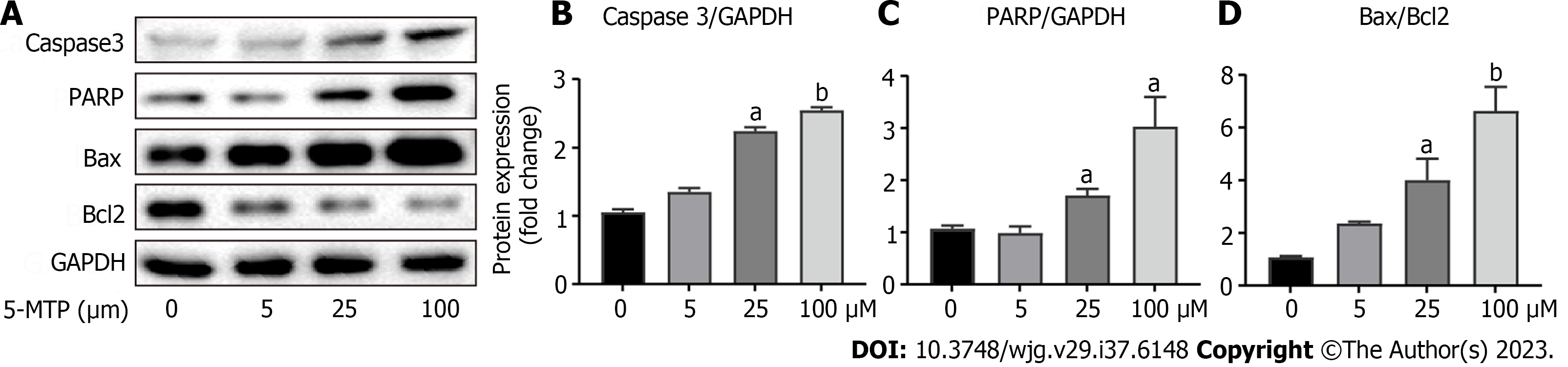
The PI3K/Akt/FoxO3a signaling pathwa plays an important role in various pathological processes in various tumors, including proliferation, metastasis, and apoptosis[16-18]. However, Figure 4 confirmed that 5-MTP combined with ly294002 inhibited CRC cell invasion and migration. The next experimental results showed that 5-MTP inhibited the relative protein levels of p-Akt/t-AKT and p-FoxO3a/t-FoxO3a with increasing drug concentration in HCT116 cells (Figures 5A-D). It was further found that the relative protein levels of p-Akt/t-AKT and p-FoxO3a/t-FoxO3a were also inhibited over time when the 5-MTP concentration was fixed, confirming the previous conclusions (Figures 5E-H). Meanwhile, western blot results showed that Caspase3, PARP, and BAX protein levels were increased, while Bcl2 protein levels were significantly decreased (Figures 6A-D). These results are the same as those in the Figure 1. Overall, our data indicated that 5-MTP induced dose-response and time course inhibition of PI3K/Akt/FoxO3a signaling pathway in HCT-116 cells.
To further verify that 5-MTP combined with PI3K/Akt/p-FoxO3a signaling pathway inhibitors played a better therapeutic effect, ly294002 (PI3K) inhibitor and AKT small interfering RNA (siRNA) were transfected into HCT116 cells. The results showed that the relative protein levels of p-Akt/AKT and p-FoxO3a/FoxO3a were significantly decreased in the 5-MTP combined with the ly294002 group compared with the 5-MTP group. At the same time, we also found that Cyclin D1 and P27 protein levels were significantly decreased while Bim was significantly increased in the 5-MTP combined ly294002 group compared with the 5-MTP group, suggesting that 5-MTP-induced cell cycle-related proteins are associated with the PI3K/Akt/p-FoxO3a signaling pathway (Figures 7A-D). It was further found that the 5-MTP combined with the AKT siRNA group came to the same conclusion as the 5-MTP group (Figures 7E-H).
CRC is a highly prevalent disease in countries around the world, and the incidence increases with age, accounting for nearly one-third of patients over 75 years of age[19,20]. The number of patients with early-onset CRC no more than 50 years of age also cannot be ignored[3,21]. The main treatments for CRC are surgery, radiotherapy, and chemotherapy[22]. However, 35% of patients are found to be in the advanced stage and lose the chance of radical surgery[23]. With the in-depth study of the formation, development, and treatment of CRC from the molecular and genetic levels, adjuvant therapy with TCM monomers optimizes the balance between tumor cell killing and non-targeted effects with the advantage of specific selection combined with oncogenic sites[24,25].
5-MTP, with a molecular formula of C12H14N2O3 and a molecular weight of 234.251, is a newly identified tryptophan metabolite produced by cells, such as fibroblasts, renal epithelial, smooth muscle and vascular endothelial cells[11,26,27]. Current studies have shown that 5-MTP has various physiological functions, such as stabilizing endothelial function, anti-inflammation, and anti-oxidation[11,28]. 5-MTP has been shown to be involved in regulating inflammatory responses and can maintain endothelial cell tight junctions to some extent[29]. Proinflammatory factors inhibit the expression of TPH-1, a key enzyme in 5-MTP synthesis in endothelial cells, reducing 5-MTP production, which leads to endothelial barrier disruption[30]. In addition, 5-MTP plays an important vaso-protective function by regulating vascular permeability, controlling systemic inflammation, and defending against systemic inflammation and multiple organ failure[31]. It has been found that 5-MTP may regulate cardiomyocyte growth-associated proteins, cytoskeleton, redox, and protein folding, thereby promoting wound healing, and preventing damaged cell death by maintaining redox balance and reducing endoplasmic reticulum stress[32].
PI3K/Akt signaling pathway is one of the most important signaling pathways involved in internal regulating cell proliferation and differentiation[33]. Akt is essential for the regulation of cell migration and growth[34]. P-Akt is the active form of Akt and is closely associated with CRC[35]. P-Akt plays an important role in CRC progression[36]. FOXO family members are major effector proteins of the PI3K/Akt signaling pathway[37]. The FOXO family is a subclass of the FOX family that contains four isoforms (FOXO1, FOXO3, FOXO4, and FOXO6), all of which structurally contain a Forkhead DNA-binding domain that binds to the same DAF-16-binding element binding site within the target gene promoter through a DNA domain, and thus regulates target gene expression[38]. FOXO3a has been found to be an important transcription factor for genes involved in cell cycle progression, apoptosis, metabolism, differentiation, and autophagy[39]. PI3K/Akt regulates the expression of a series of genes related to cell proliferation, apoptosis, and cycle arrest downstream of FOXO by working on phosphorylation sites on FOXO protein[40]. FOXO3A is involved in the biological effects of CRC, and this process may be related to cell proliferation and apoptosis[18]. For example, many FOXO factors are phosphorylated by Akt in the presence of growth factors, resulting in their translocation from the nucleus to the cytoplasm; PI3K/AKT pathway mediates hyperglycemia-induced apoptosis in ventricular myocytes of neonatal rats through translocation of FOXO3a to the nucleus[17,41].
In this study, the efficacy of 5-MTP in the treatment of CRC by in vitro cell experiments was investigated, and the effects of 5-MTP on CRC cells under different conditions were examined using CCK-8, colony formation, apoptosis rate detection, JC-1 level, ROS level and cell cycle detection. The results showed that 5-MTP promoted apoptosis and cell cycle arrest and inhibited cell proliferation in CRC cells. In subsequent experiments, the effect of 5-MTP combined with PI3K/Akt/p-FoxO3a signaling pathway inhibitors in the treatment of CRC was focused, confirming that combined PI3K/Akt/p-FoxO3a signaling pathway inhibitors played a more effective role in the treatment of CRC. There was a correlation between 5-MTP-induced cyclin-related proteins and PI3K/Akt/p-FoxO3a signaling pathway.
In summary, 5-MTP could inhibit proliferation and promote apoptosis of CRC cells, and combined ly294002 or AKT inhibitors played a more effective role in treating CRC. This study provided theoretical guidance for the clinical treatment of CRC with 5-MTP. However, there were some limitations, and in vivo experiments such as mice are still needed for further validation in the future.
Colorectal cancer (CRC) is a highly prevalent malignant tumor. Research is needed to find and develop effective drugs for the treatment of CRC. 5-methoxytryptophan (5-MTP) is a tryptophan metabolite found in animals and humans. The effect of 5-MTP on the proliferation and apoptosis of CRC cells is still unclear.
Our work explored the effects of 5-MTP on the proliferation, migration, invasion and apoptosis of CRC cells. We tried to explore the potential of 5-MTP as a drug for the treatment of CRC.
Here, we studied that 5-MTP combined with PI3K/Akt/FOXO3a signaling pathway inhibitor can significantly promote apoptosis and cell cycle arrest of CRC cells, and inhibit cell proliferation. 5-MTP can be used as an effective drug in the treatment of CRC.
In this study, a series of experiments were carried out. Colony forming assay and Cell Counting Kit were used to detect the effect of 5-MTP on the proliferation of CRC cell lines. The effect of 5-MTP on cell growth was detected by cell cycle analysis. In addition, the effects of 5-MTP on apoptosis and reactive oxygen species of HCT-116 cells were studied. In order to further explore the effect of 5-MTP on CRC, we studied the effect of 5-MTP on PI3K/Akt signaling pathway in CRC cells.
5-MTP can promote apoptosis and cell cycle arrest of CRC cells, and inhibit cell proliferation. However, compared with 5-MTP alone, 5-MTP combined with PI3K/Akt/FOXO3a signaling pathway inhibitors significantly promoted apoptosis and cell cycle arrest of CRC cells, and inhibited cell proliferation. It provides new insights into the mechanism of drug action.
5-MTP combined with PI3K/Akt/FOXO3a signaling pathway inhibitor significantly promoted apoptosis and cell cycle arrest of CRC cells, and inhibited cell proliferation.
This study confirmed that 5-MTP combined with PI3K/Akt/FOXO3a signaling pathway inhibitor could significantly promote the apoptosis and cell cycle arrest of CRC cells, and inhibit cell proliferation. These results provide a new direction for the drug treatment of CRC. However, the study of mechanism in this study is relatively limited. Therefore, further analysis, nude mouse experiments and more cell experiments are needed to explore its mechanism.
| 1. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1732] [Article Influence: 346.4] [Reference Citation Analysis (1)] |
| 2. | Kishore C, Bhadra P. Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur J Pharmacol. 2021;893:173819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 515] [Article Influence: 128.8] [Reference Citation Analysis (7)] |
| 4. | Luo XJ, Zhao Q, Liu J, Zheng JB, Qiu MZ, Ju HQ, Xu RH. Novel Genetic and Epigenetic Biomarkers of Prognostic and Predictive Significance in Stage II/III Colorectal Cancer. Mol Ther. 2021;29:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 5. | Eng C, Jácome AA, Agarwal R, Hayat MH, Byndloss MX, Holowatyj AN, Bailey C, Lieu CH. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 2022;23:e116-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 6. | Kasprzak A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 7. | Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer--From theory to practice. Biomed Pharmacother. 2021;137:111381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 8. | Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 9. | Hu XQ, Wei B, Song YN, Ji Q, Li Q, Luo YQ, Wang WH, Su SB. Plasma metabolic profiling on postoperative colorectal cancer patients with different traditional Chinese medicine syndromes. Complement Ther Med. 2018;36:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Cao B, Lin J, Wu Z, Liu H, Zhang D, Xu H, Xu R, Han L. Mechanisms exploration of Xiaojin Pills on lung cancer based on metabolomics and network pharmacology. J Pharm Pharmacol. 2021;73:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Wu KK, Kuo CC, Yet SF, Lee CM, Liou JY. 5-methoxytryptophan: an arsenal against vascular injury and inflammation. J Biomed Sci. 2020;27:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Chen DQ, Cao G, Chen H, Argyopoulos CP, Yu H, Su W, Chen L, Samuels DC, Zhuang S, Bayliss GP, Zhao S, Yu XY, Vaziri ND, Wang M, Liu D, Mao JR, Ma SX, Zhao J, Zhang Y, Shang YQ, Kang H, Ye F, Cheng XH, Li XR, Zhang L, Meng MX, Guo Y, Zhao YY. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat Commun. 2019;10:1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | Ji H, Ma J, Chen L, Chen T, Zhang S, Jia J, Yang X, Guo C, Xiao Z, Niu P. Pyrroloquinoline Quinine and LY294002 Changed Cell Cycle and Apoptosis by Regulating PI3K-AKT-GSK3β Pathway in SH-SY5Y Cells. Neurotox Res. 2020;38:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | A Z, J SW, A M, E L, I W, W R, J JG. LY294002 and sorafenib as inhibitors of intracellular survival pathways in the elimination of human glioma cells by programmed cell death. Cell Tissue Res. 2021;386:17-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 15. | Xing CG, Zhu BS, Liu HH, Lin F, Yao HH, Liang ZQ, Qin ZH. LY294002 induces p53-dependent apoptosis of SGC7901 gastric cancer cells. Acta Pharmacol Sin. 2008;29:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Lin Y, Wang L, Zhan H, Luo X, Zeng Y, Wu W, Zhang X, Wang F. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer's disease mice. Aging (Albany NY). 2020;12:20862-20879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 17. | Zhao C, Gu Y, Chen L, Su X. Upregulation of FoxO3a expression through PI3K/Akt pathway attenuates the progression of lupus nephritis in MRL/lpr mice. Int Immunopharmacol. 2020;89:107027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Abdullah ML, Al-Shabanah O, Hassan ZK, Hafez MM. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Slomski A. Evidence for a Colorectal Cancer Screening Benefit After Age 75 Years. JAMA. 2021;326:378. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA. 2018;319:2021-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 21. | Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 22. | Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 250] [Cited by in RCA: 528] [Article Influence: 58.7] [Reference Citation Analysis (12)] |
| 23. | Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 24. | Zhao H, He M, Zhang M, Sun Q, Zeng S, Chen L, Yang H, Liu M, Ren S, Meng X, Xu H. Colorectal Cancer, Gut Microbiota and Traditional Chinese Medicine: A Systematic Review. Am J Chin Med. 2021;49:805-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Li W, Li C, Zheng H, Chen G, Hua B. Therapeutic targets of Traditional Chinese Medicine for colorectal cancer. J Tradit Chin Med. 2016;36:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Cheng HH, Wang KH, Chu LY, Chang TC, Kuo CC, Wu KK. Quiescent and proliferative fibroblasts exhibit differential p300 HAT activation through control of 5-methoxytryptophan production. PLoS One. 2014;9:e88507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Wu KK. Control of Tissue Fibrosis by 5-Methoxytryptophan, an Innate Anti-Inflammatory Metabolite. Front Pharmacol. 2021;12:759199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Hsu WT, Tseng YH, Jui HY, Kuo CC, Wu KK, Lee CM. 5-Methoxytryptophan attenuates postinfarct cardiac injury by controlling oxidative stress and immune activation. J Mol Cell Cardiol. 2021;158:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Wang YF, Hsu YJ, Wu HF, Lee GL, Yang YS, Wu JY, Yet SF, Wu KK, Kuo CC. Endothelium-Derived 5-Methoxytryptophan Is a Circulating Anti-Inflammatory Molecule That Blocks Systemic Inflammation. Circ Res. 2016;119:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Cheng HH, Kuo CC, Yan JL, Chen HL, Lin WC, Wang KH, Tsai KK, Guvén H, Flaberg E, Szekely L, Klein G, Wu KK. Control of cyclooxygenase-2 expression and tumorigenesis by endogenous 5-methoxytryptophan. Proc Natl Acad Sci U S A. 2012;109:13231-13236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Chu LY, Wang YF, Cheng HH, Kuo CC, Wu KK. Endothelium-Derived 5-Methoxytryptophan Protects Endothelial Barrier Function by Blocking p38 MAPK Activation. PLoS One. 2016;11:e0152166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Chou HC, Chan HL. 5-Methoxytryptophan-dependent protection of cardiomyocytes from heart ischemia reperfusion injury. Arch Biochem Biophys. 2014;543:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 1193] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 34. | Ma L, Zhang R, Li D, Qiao T, Guo X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem Biol Interact. 2021;349:109659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Cui X, Feng J, Wu J, Zhang X, Ding M. Propofol postpones colorectal cancer development through circ_0026344/miR-645/Akt/mTOR signal pathway. Open Med (Wars). 2021;16:570-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Lan W, Zhao J, Chen W, Shang H, Peng J, Lin J. Anlotinib Overcomes Multiple Drug Resistant Colorectal Cancer Cells via Inactivating PI3K/AKT Pathway. Anticancer Agents Med Chem. 2021;21:1987-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Meng F, Zhang Z, Chen C, Liu Y, Yuan D, Hei Z, Luo G. PI3K/AKT activation attenuates acute kidney injury following liver transplantation by inducing FoxO3a nuclear export and deacetylation. Life Sci. 2021;272:119119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 38. | Calissi G, Lam EW, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. 2021;20:21-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 39. | Li J, Long H, Cong Y, Gao H, Lyu Q, Yu S, Kuang Y. Quercetin prevents primordial follicle loss via suppression of PI3K/Akt/Foxo3a pathway activation in cyclophosphamide-treated mice. Reprod Biol Endocrinol. 2021;19:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Liu L, Hu R, You H, Li J, Liu Y, Li Q, Wu X, Huang J, Cai X, Wang M, Wei L. Formononetin ameliorates muscle atrophy by regulating myostatin-mediated PI3K/Akt/FoxO3a pathway and satellite cell function in chronic kidney disease. J Cell Mol Med. 2021;25:1493-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, Yu W, Wang Y, Li P, Wang J. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan; Exbrayat JM, France S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX