Published online Nov 14, 2023. doi: 10.3748/wjg.v29.i42.5768
Peer-review started: August 18, 2023
First decision: September 18, 2023
Revised: September 30, 2023
Accepted: October 29, 2023
Article in press: October 29, 2023
Published online: November 14, 2023
Processing time: 87 Days and 5 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) has been extensively used to treat portal hypertension-associated complications, including cirrhosis. The prediction of post-TIPS prognosis is important for cirrhotic patients, as more aggressive liver transplantation is needed when the post-TIPS prognosis is poor.
To construct a nutrition-based model that could predict the disease progression of cirrhotic patients after TIPS implantation in a sex-dependent manner.
This study retrospectively recruited cirrhotic patients undergoing TIPS implan
This study eventually included 186 cirrhotic patients receiving TIPS who were followed up for 30.5 ± 18.8 mo. For male patients, the 30-mo survival rate was significantly lower and the probability of progressive events was higher (3.257-fold) in the low-level SMI group than in the high-level SMI group. According to the multivariate Cox analysis of male patients, SMI < 32.8 was an independent risk factor for long-term adverse outcomes after TIPS implantation. A model was constructed, which involved creatinine, plasma ammonia, SMI, and acute-on-chronic liver failure and hepatic encephalopathy occurring within half a year after surgery. This model had an area under the receiver operating characteristic curve of 0.852, sensitivity of 0.926, and specificity of 0.652. According to the results of the DeLong test, this model outperformed other models (Child-Turcotte-Pugh, Model for End-Stage Liver Disease, and Freiburg index of post-TIPS survival) (P < 0.05).
SMI is strongly associated with poor long-term outcomes in male patients with cirrhosis who underwent TIPS implantation.
Core Tip: Our study highlights the strong association between the skeletal muscle index and long-term adverse outcomes in male patients with cirrhosis who received transjugular intrahepatic portosystemic shunt (TIPS) implantation, reflecting the necessity for adequate sex-stratified analysis. Additionally, nutritional indicator-based models hold tremendous promise for effectively predicting the prognosis of cirrhotic patients undergoing TIPS implantation and assisting clinicians in closely monitoring high-risk populations.
- Citation: Zhang Q, Long L, Zhu HL, Peng H, Luo XH, Zhu KS, Wang RP. Predicting disease progression in cirrhotic patients after transjugular intrahepatic portosystemic shunt implantation: A sex-stratified analysis. World J Gastroenterol 2023; 29(42): 5768-5780
- URL: https://www.wjgnet.com/1007-9327/full/v29/i42/5768.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i42.5768
Cirrhosis is frequently complicated by esophagogastric variceal bleeding and ascites, two decompensating events, which are closely associated with the presence of portal hypertension[1]. Transjugular intrahepatic portosystemic shunt (TIPS) has been extensively applied for the treatment of portal hypertension-related complications, which reduces the portal pressure gradient by creating a conduit between the portal and systemic circulations. At present, TIPS can be used for the treatment of cirrhosis complicated by refractory ascites or for secondary prevention of upper gastrointestinal hemorrhage in patients who cannot be treated by endoscopy[2-4].
Although TIPS implantation techniques have improved, there is still a subset of patients with a poor prognosis because such patients typically develop advanced cirrhosis with more than one complication when TIPS implantation is needed. Hence, it is warranted to screen patients with a potentially poor prognosis after TIPS for closer follow-up, as they may require more aggressive liver transplantation. Currently, the post-TIPS survival of patients is commonly predicted with models including Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD)[5,6]. In addition, recent studies have also elaborated that the post-TIPS survival of patients can be effectively predicted with the bilirubin-platelet and Freiburg index of post-TIPS survival (FIPS) models[7,8]. Nevertheless, the use of these models is limited by two factors. First, these models are constructed based on studies of Western patients who have different pathogeneses from Eastern patients. Consequently, the predictive value of these models for Eastern patients has yet to be verified. Second, these models focus on the short-term prognosis of patients after surgery and cannot assess the long-term survival of patients. Thus, a new alternative model is needed to accurately predict the long-term outcome of Asian patients with ascites- or variceal bleeding-induced end-stage liver disease who receive TIPS implantation.
In addition, the basic nutritional status of patients is also an important factor that affects the long-term survival of patients with end-stage liver disease[9,10]. Notably, measuring the skeletal muscle index (SMI) of the third lumbar spine (L3) by abdominal computed tomography (CT) is the most objective method for assessing nutritional status[11]. Intriguingly, prior studies demonstrated that SMI was more strongly associated with end-stage liver disease in men than in women[12,13]. Therefore, this study sought to establish a nutritional indicator-based model to predict the long-term survival of Asian patients with cirrhosis after TIPS implantation and stratify long-term survival by sex.
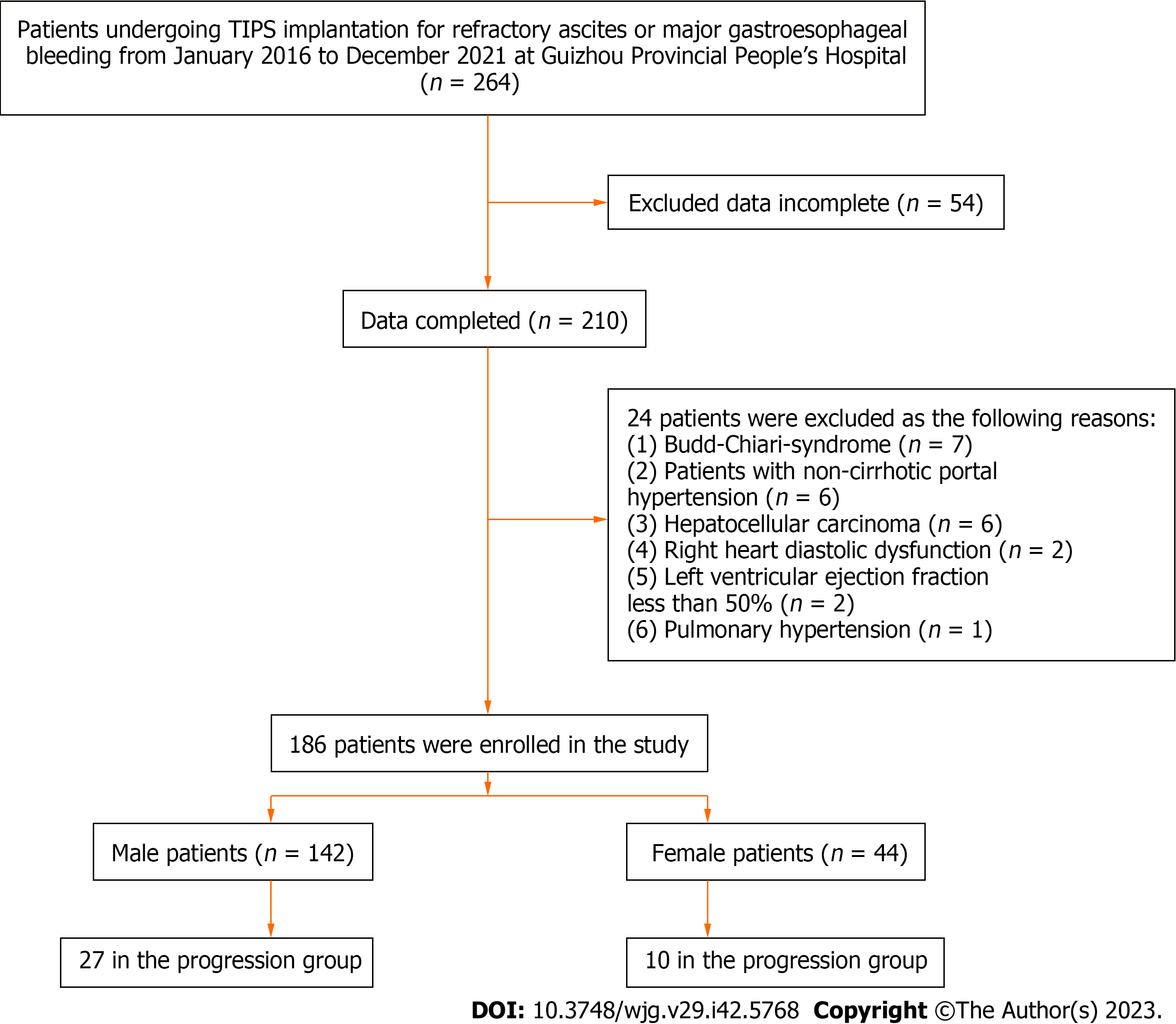
This retrospective study recruited 264 patients undergoing TIPS implantation for refractory ascites or major gastroesophageal bleeding at the Guizhou Provincial People’s Hospital from January 2016 to December 2021. Among these patients, we excluded those with incomplete data (n = 54), Budd-Chiari syndrome (n = 7), noncirrhotic portal hypertension (n = 6), hepatocellular carcinoma (n = 6), right heart diastolic dysfunction (the ratio of early-to-late diastolic flow velocity < 0.75) (n = 2), left ventricular ejection fraction of less than 50% evaluated by echocardiography within 1 mo before TIPS implantation (n = 2), or pulmonary hypertension (n = 1). Finally, 186 patients were included (Figure 1).
Refractory ascites referred to the failure to reduce ascites by sodium restriction and high-dose diuretics (spironolactone at a dose up to 400 mg/d and furosemide at a dose of 160 mg/d) or an untreatable condition due to complications[14]. Based on the Baveno VI criteria, major gastroesophageal bleeding was defined as a high risk of rebleeding after the first episode of gastroesophageal variceal bleeding was treated[15].
The indication for TIPS implantation was determined through the careful clinical evaluation of each patient by a panel of hepatologists and interventional specialists at our hospital. CT-guided puncture of the right internal jugular vein was used as peripheral vascular access for TIPS, and the patient underwent portal vein puncture guided by real-time CT. An expandable polytetrafluoroethylene-covered stent (10 mm; Viatorr TIPS endoprosthesis; Gore, Flagstaff, Arizona, USA) was initially expanded to 8 mm. The patency rate of TIPS in patients was monitored by Doppler ultrasound every 3-6 mo.
Demographic data and laboratory parameters of all cirrhotic patients were extracted from medical records. Laboratory parameters included blood biochemistry, routine blood tests, alpha-fetoprotein, and plasma ammonia. Prognostic scores (including MELD and CTP scores) were calculated with the latest test results before TIPS implantation. SMI at L3 was evaluated according to abdominal CT scans within one month before TIPS implantation. SMI was calculated according to our previous research[16]. The primary outcome was adverse progression within 30 mo after TIPS implantation, including liver transplantation or death from complications such as gastrointestinal bleeding, liver failure, and hepatic encephalopathy (HE).
This study was carried out in accordance with recommendations in the ethical guidelines of the latest version of the Declaration of Helsinki and those provided by the Guizhou Provincial People’s Hospital. The protocol was approved by the Ethics Committee of the Guizhou Provincial People’s Hospital. Individual consent was waived for this retrospective analysis.
Data were processed with SPSS 25.0 statistical analysis software (IBM Corp., Armonk, NY, United States) and R statistical analysis software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria). A difference significantly differed at P < 0.05 (two-sided). Continuous variables were compared by the Student's t test or the Mann-Whitney U test and are expressed as the mean ± standard error in the presence of a normal distribution or as the median in the absence of a normal distribution. Categorical data are presented as numbers (percentages) and were compared by the chi-square test. Spearman’s correlation coefficients (rs) were used for correlation analyses. Independent predictors of disease progression were evaluated with univariate and multivariate Cox proportional hazard models, followed by the assessment of model calibration by analyzing the C-index. The area under the receiver operating characteristic (ROC) curve (AUC) was compared by the DeLong test. Cumulative transplant-free survival rates were plotted as Kaplan-Meier curves and compared by the log-rank test.
This study ultimately included 186 cirrhotic patients receiving TIPS implantation who were followed up for 30.5 ± 18.8 mo on average. The main characteristics of the cohorts at baseline are presented in Table 1. These participants comprised 142 males (115 in the transplantation-free survival group and 27 in the progression group) and 44 females (34 in the transplantation-free survival group and 10 in the progression group), suggesting that the transplantation-free survival rate was 80.1% in total, 81.0% for male patients, and 77.3% for female patients. Subsequently, various baseline characteristics of the male and female patients were compared between the two groups. It was found that patients with poor outcomes were older with a higher neutrophil-to-lymphocyte ratio. For male patients, the SMI and albumin levels were lower but ammonia and the CTP scores were higher in the progression group than in the transplantation-free survival group. However, these trends were not obvious for female patients.
| Variable | All (n = 186) | Male (n = 142) | Female (n = 42) | ||||
| Transplantation-free survival (n = 115) | Progression (n = 27) | P value | Transplantation-free survival (n = 34) | Progression (n = 10) | P value | ||
| Age (yr) | 51 (44-57) | 48.0 (43.0-53.0) | 56.0 (45.0-63.0) | 0.017 | 55.0 (47.0-62.0) | 64.0 (62.0-66.0) | 0.001 |
| BMI (kg/m2) | 23.0 (21.4-24.0) | 23.0 (21.7-24.3) | 23.0 (20.8-23.0) | 0.098 | 22.1 (21.4-23.5) | 21.9 (19.4-23.7) | 0.73 |
| SMI (cm2/m2) | 38.9 (31.2-45.3) | 41.9 (36.6-48.1) | 37.6 (30.8-44.2) | 0.036 | 29.4 (25.4-33.4) | 28.68 (26.49-35.36) | 0.901 |
| SATI (cm2/m2) | 27.1 (14.1-45.6) | 24.8 (13.1-38.6) | 20.9 (10.4-36.5) | 0.252 | 43.79 (19.23-55.96) | 51.51 (32.58-81.8) | 0.128 |
| VATI (cm2/m2) | 37.6 (24.5-49.7) | 37.3 (22.7-53.1) | 39.0 (24.8-48.1) | 0.592 | 32.45 (24.58-41.92) | 39.90 (34.25-69.58) | 0.075 |
| MELD score | 9.0 (6.0-12.0) | 8.0 (5.0-11.0) | 11.0 (7.0-14.0) | 0.070 | 9.0 (6.0-12.0) | 10.0 (9.75-11.25) | 0.354 |
| CTP score | 7.0 (6.0-9.0) | 7.0 (6.0-9.0) | 9.0 (7.0-9.0) | 0.014 | 7.5 (6.0-8.0) | 7.0 (6.0-8.0) | 0.572 |
| CTP classification | 0.148 | 0.513 | |||||
| A (%) | 55 (29.6) | 38 (33.0) | 4 (14.8) | 10 (20.4) | 3 (30.0) | ||
| B (%) | 113 (60.8) | 67 (58.3) | 19 (70.4) | 20 (58.8) | 7 (70.0) | ||
| C (%) | 18 (9.7) | 10 (8.7) | 4 (14.8) | 4 (11.8) | 0 (0) | ||
| Etiology (%) | 0.051 | 0.09 | |||||
| HBV/HCV | 104 (55.9) | 69 (60.0) | 12 (44.4) | 20 (58.8) | 3 (30.0) | ||
| Alcohol | 51 (27.4) | 40 (34.8) | 10 (37.0) | 1 (2.9) | 0 (0) | ||
| Others | 31 (16.7) | 6 (5.2) | 5 (18.6) | 13 (38.2) | 7 (70.0) | ||
| Ascites (%) | 0.43 | 0.993 | |||||
| None | 48 (25.8) | 28 (24.3) | 4 (14.8) | 12 (35.3) | 4 (40.0) | ||
| Mild | 51 (27.4) | 35 (30.4) | 7 (25.9) | 7 (20.6) | 2 (20.0) | ||
| Moderate | 45 (24.2) | 29 (25.2) | 7 (25.9) | 7 (20.6) | 2 (20.0) | ||
| Massive | 42 (22.6) | 23 (20.0) | 9 (33.3) | 8 (23.5) | 2 (20.0) | ||
| Portal vein thrombosis (%) | 37 (19.9) | 23 (20.0) | 6 (22.2) | 0.797 | 8 (23.5) | 0 (0) | |
| Portal vein pressure (mm Hg) | 32.0 (28.0-36.8) | 32.3 (28.4-36.9) | 33.8 (30.1-38.4) | 0.141 | 30.5 (24.4-33.1) | 30.5 (27.0-32.3) | 0.553 |
| Albumin (g/L) | 30.45 (26.80-33.90) | 30.85 (26.95-33.93) | 28.60 (24.90-30.80) | 0.041 | 31.05 (27.33-34.68) | 29.90 (23.43-33.45) | 0.464 |
| International normalized ratio | 1.28 (1.16-1.38) | 1.29 (1.18-1.37) | 1.34 (1.15-1.50) | 0.295 | 1.23 (1.07-1.37) | 1.18 (1.13-1.38) | 0.61 |
| Total bilirubin (mol/L) | 22.60 (14.90-31.65) | 20.7 (13.6-29.9) | 28.3 (12.6-43.6) | 0.236 | 24.15 (17.88-37.18) | 28.35 (22.20-32.68) | 0.649 |
| Creatinine (mol/L) | 70.5 (59.0-82.3) | 72.55 (62.38-83.18) | 79.60 (64.80-114.10) | 0.069 | 57.05 (49.75-65.88) | 58.50 (51.50-80.00) | 0.499 |
| Sodium (mmol/l) | 138.45 (136.0-140.8) | 138.25 (136.00-140.48) | 138.20 (135.00-141.00) | 0.985 | 139.00 (135.75-140.85) | 139.00 (133.90-140.80) | 0.585 |
| Alanine aminotransferase (U/L) | 21.0 (15.0-32.3) | 21.0 (15.0-30.0) | 24.0 (14.0-45.0) | 0.274 | 19.5 (15.8-32.8) | 28.4 (22.2-32.7) | 0.989 |
| Plasma ammonia (mol/L) | 47.8 (34.7-54.6) | 44.84 (33.80-49.20) | 50.56 (44.40-69.07) | 0.005 | 47.80 (34.46-54.84) | 48.18 (46.20-62.12) | 0.354 |
| White blood cells (× 109/L) | 3.76 (2.60-5.66) | 3.71 (2.60-5.50) | 4.78 (3.20-8.44) | 0.108 | 3.32 (2.17-5.55) | 3.79 (3.07-6.22) | 0.312 |
| Platelets (× 109/L) | 64.5 (46.0-91.3) | 65.0 (47.0-92.0) | 70.0 (49.0-92.0) | 0.888 | 66.0 (46.5-83.5) | 51.5 (39.0-80.8) | 0.354 |
| Neutrophils (× 109/L) | 2.60 (1.58-4.23) | 2.49 (1.61-3.99) | 3.93 (2.12-6.79) | 0.048 | 2.11 (1.34-4.00) | 2.66 (2.28-5.03) | 0.273 |
| Lymphocytes (× 109/L) | 0.67 (0.49-0.97) | 0.68 (0.49-0.96) | 0.65 (0.46-1.08) | 0.944 | 0.64 (0.54-1.09) | 0.52 (0.40-0.78) | 0.108 |
| NLR | 3.45 (2.52-5.79) | 3.38 (2.41-5.64) | 5.22 (3.16-7.28) | 0.046 | 2.70 (1.99-4.50) | 6.38 (3.97-8.59) | 0.001 |
| PLR | 94.25 (67.80-133.51) | 95.39 (68.91-133.33) | 86.36 (64.12-153.33) | 0.670 | 100.00 (67.18-127.60) | 101.80 (78.06-149.53) | 0.591 |
| Alpha-fetoprotein, mmol/L | 4.10 (2.19-5.36) | 4.09 (2.10-5.36) | 3.92 (2.40-5.36) | 0.796 | 3.48 (2.07-5.36) | 5.36 (5.36-5.53) | 0.016 |
To further identify the impact of SMI on the outcomes of patients, we used SMI to plot ROC curves and selected the optimal cutoff value (SMI = 32.8) based on the Youden index. Based on the cutoff value, patients were arranged into low-level SMI and high-level SMI groups, followed by the comparison of the long-term cumulative survival rates between the two groups. For male patients, the 30-mo survival rate was significantly lower in the low-level SMI group (58.0%) than in the high-level SMI group (86.6%) (P < 0.001), accompanied by a higher probability (3.257-fold) of progressive events (liver transplantation or death), as shown in the Cox proportional hazard regression analysis. Conversely, no significant difference was observed in the long-term cumulative survival rate of female patients between the two groups (Figure 2).
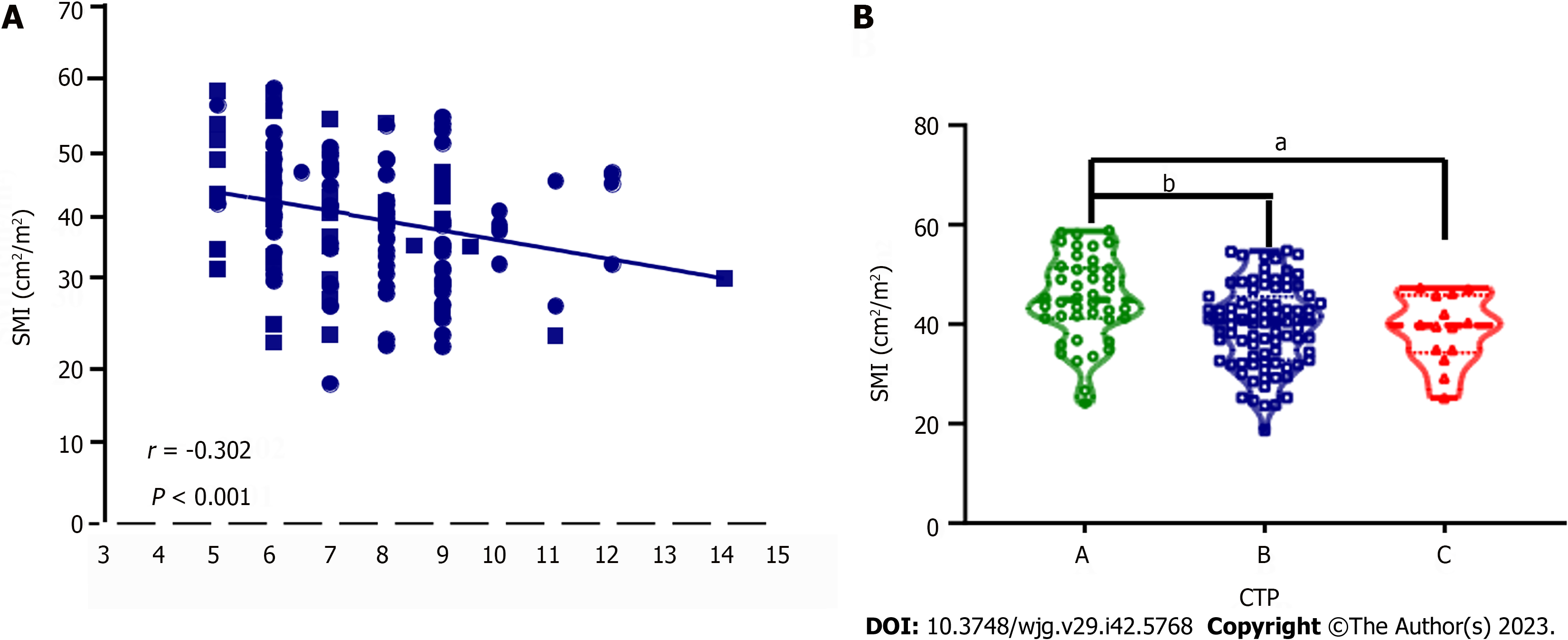
In the entire cohort, significantly different indicators in the univariate analysis and body mass index were combined for further multivariate Cox proportional hazard regression analyses. The results revealed that etiology, creatinine, plasma ammonia, alpha-fetoprotein, SMI < 32.8, and HE occurring within half a year after surgery were independent risk factors for 30-mo adverse outcomes in cirrhotic patients undergoing TIPS. In male patients, SMI < 32.8 was also found to be an independent risk factor for long-term adverse outcomes after TIPS but not in female patients (Table 2). Further analyses exhibited a negative correlation between SMI and CTP scores (r = -0.302, P < 0.001), as confirmed by the lower SMI in patients with CTP grades B and C than in the patients with CTP grade A (P < 0.05) (Figure 3).
| Death or liver transplantation | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| All (n = 186) | ||||
| Age (yr) | 1.073 (1.039-1.108) | < 0.001 | ||
| BMI | 0.914 (0.814-1.026) | 0.128 | ||
| CTP score | 1.212 (1.012-1.451) | 0.037 | ||
| Etiology (%) | ||||
| HBV/HCV | Reference | Reference | ||
| Alcohol | 1.421 (0.638-3.165) | 0.39 | 1.205 (0.503-2.888) | 0.675 |
| Others | 2.862 (1.337-6.125) | 0.007 | 3.807 (1.680-8.623) | 0.001 |
| Creatinine (mol/L) | 1.008 (1.005-1.012) | < 0.001 | 1.006 (1.002-1.011) | 0.005 |
| Plasma ammonia (mol/L) | 1.027 (1.013-1.041) | < 0.001 | 1.025 (1.009-1.041) | 0.003 |
| Alpha-fetoprotein (mmol/L) | 1.030 (1.017-1.044) | < 0.001 | 1.021 (1.005-1.036) | 0.009 |
| NLR | 1.088 (1.036-1.142) | 0.001 | ||
| SMI < 32.8 | 2.250 (1.179-4.291) | 0.014 | 2.192 (1.008-4.769) | 0.048 |
| Postoperative ACLF | 2.927 (1.218-7.037) | 0.016 | ||
| Postoperative HE | 10.115 (4.851-21.091) | < 0.001 | 8.721 (4.065-18.710) | < 0.001 |
| Male (n = 142) | ||||
| Age (yr) | 1.062 (1.024-1.100) | 0.001 | ||
| BMI | 0.897 (0.781-1.030) | 0.897 | ||
| CTP score | 1.294 (1.069-1.566) | 0.008 | ||
| MELD score | 1.122 (1.034-1.217) | 0.006 | ||
| Etiology (%) | ||||
| HBV/HCV | Reference | |||
| Alcohol | 1.453 (0.627-3.367) | 0.384 | ||
| Others | 3.396 (1.193-9.670) | 0.022 | ||
| Creatinine (mol/L) | 1.009 (1.005-1.013) | < 0.001 | 1.008 (1.004-1.013) | 0.001 |
| Plasma ammonia (mol/L) | 1.028 (1.012-1.044) | < 0.001 | 1.028 (1.011-1.046) | 0.001 |
| Alpha-fetoprotein (mmol/L) | 1.032 (1.015-1.049) | < 0.001 | ||
| NLR | 1.080 (1.018-1.146) | 0.011 | ||
| SMI < 32.8 | 3.275 (1.516-7.073) | 0.003 | 3.277 (1.306-8.226) | 0.011 |
| Postoperative ACLF | 10.066 (3.249-31.187) | < 0.001 | 5.621 (1.685-18.752) | 0.005 |
| Postoperative HE | 6.697 (3.015-14.876) | < 0.001 | 5.110 (2.153-12.126) | < 0.001 |
To further analyze the predictive value of SMI for the long-term prognosis of male patients, we used 5-fold cross-validation to classify male patients into five folds, among which four folds were used as the training set and a fold as the validation set. As demonstrated in Table 3, SMI was included as a predictor in all five models. The results showed that the other models had more stable prediction performance in both the training and validation sets (the AUC values were all > 0.8), except for model 3, which had slightly worse prediction performance. To perform a comprehensive assessment of the entire male data set, we reconducted the multivariate Cox proportional hazard regression analysis of the characteristics screened for each submodel in the 5-fold cross-validation across the entire male data set. Eventually, a model was constructed by including creatinine, plasma ammonia, SMI, and acute-on-chronic liver failure (ACLF) and HE occurring within half a year after surgery, which had an AUC of 0.852 [95% confidence interval (CI): 0.782-0.923], sensitivity of 0.926, and specificity of 0.652.
| Model | Variable | Training set AUC (95%CI) | Sensitivity | Specificity | Test set AUC (95%CI) | Sensitivity | Specificity |
| Model 1 | Creatinine, plasma ammonia, SMI, ACLF, HE | 0.842 (0.763-0.921) | 0.957 | 0.652 | 0.875 (0.672-1.000) | 0.75 | 0.962 |
| Model 2 | Creatinine, plasma ammonia, SMI, ACLF, HE | 0.864 (0.785-0.942) | 0.864 | 0.707 | 0.809 (0.631-0.986) | 1.000 | 0.609 |
| Model 3 | Creatinine, SMI, HE | 0.804 (0.689-0.918) | 0.857 | 0.674 | 0.768 (0.581-0.955) | 0.833 | 0.696 |
| Model 4 | Creatinine, plasma ammonia, SMI, ACLF, HE | 0.842 (0.761-0.923) | 0.783 | 0.813 | 0.948 (0.844-1.00) | 1.000 | 0.792 |
| Model 5 | Creatinine, plasma ammonia, SMI, HE | 0.840 (0.760-0.921) | 0.957 | 0.636 | 0.823 (0.659-0.987) | 1.000 | 0.667 |
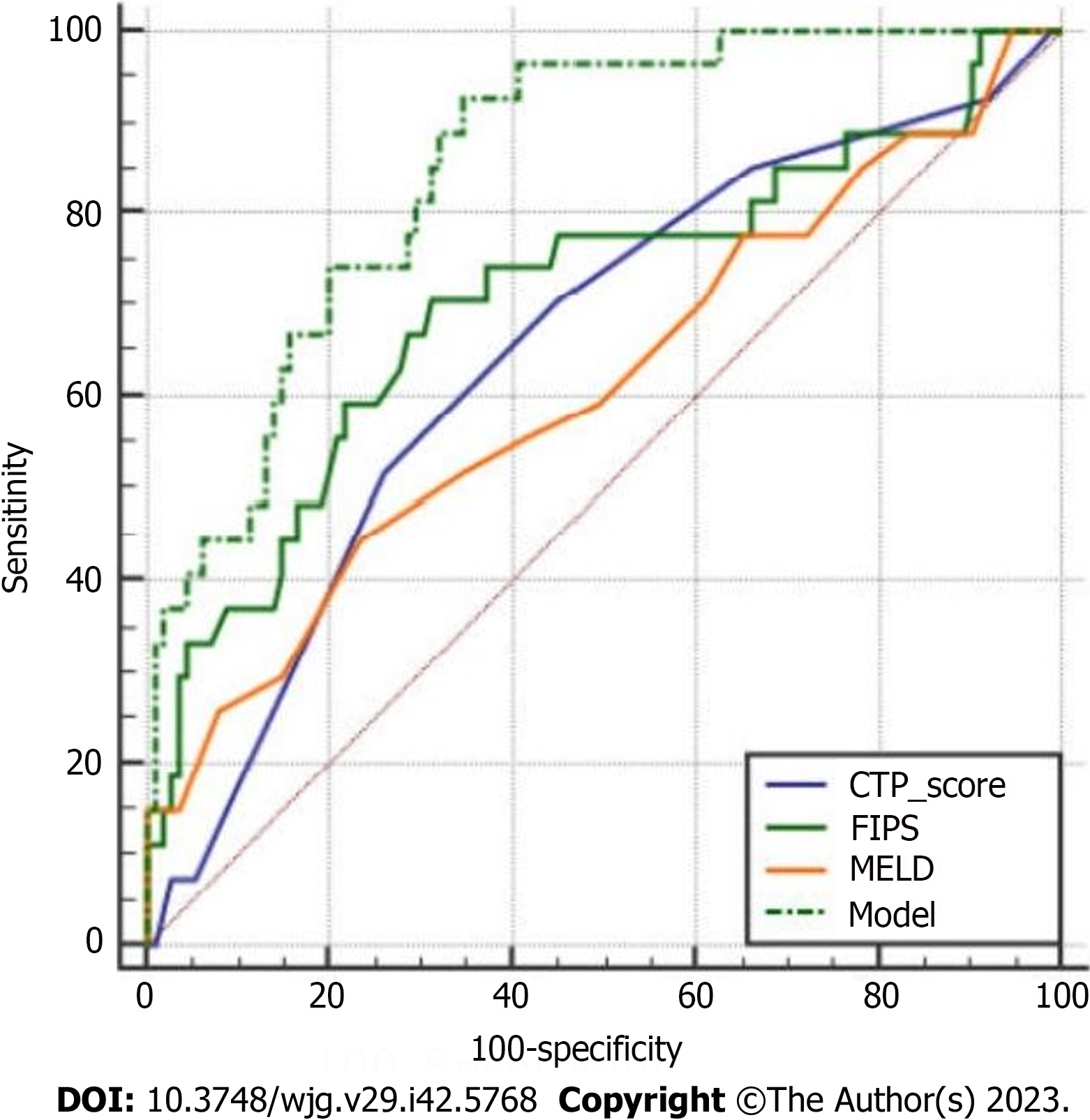
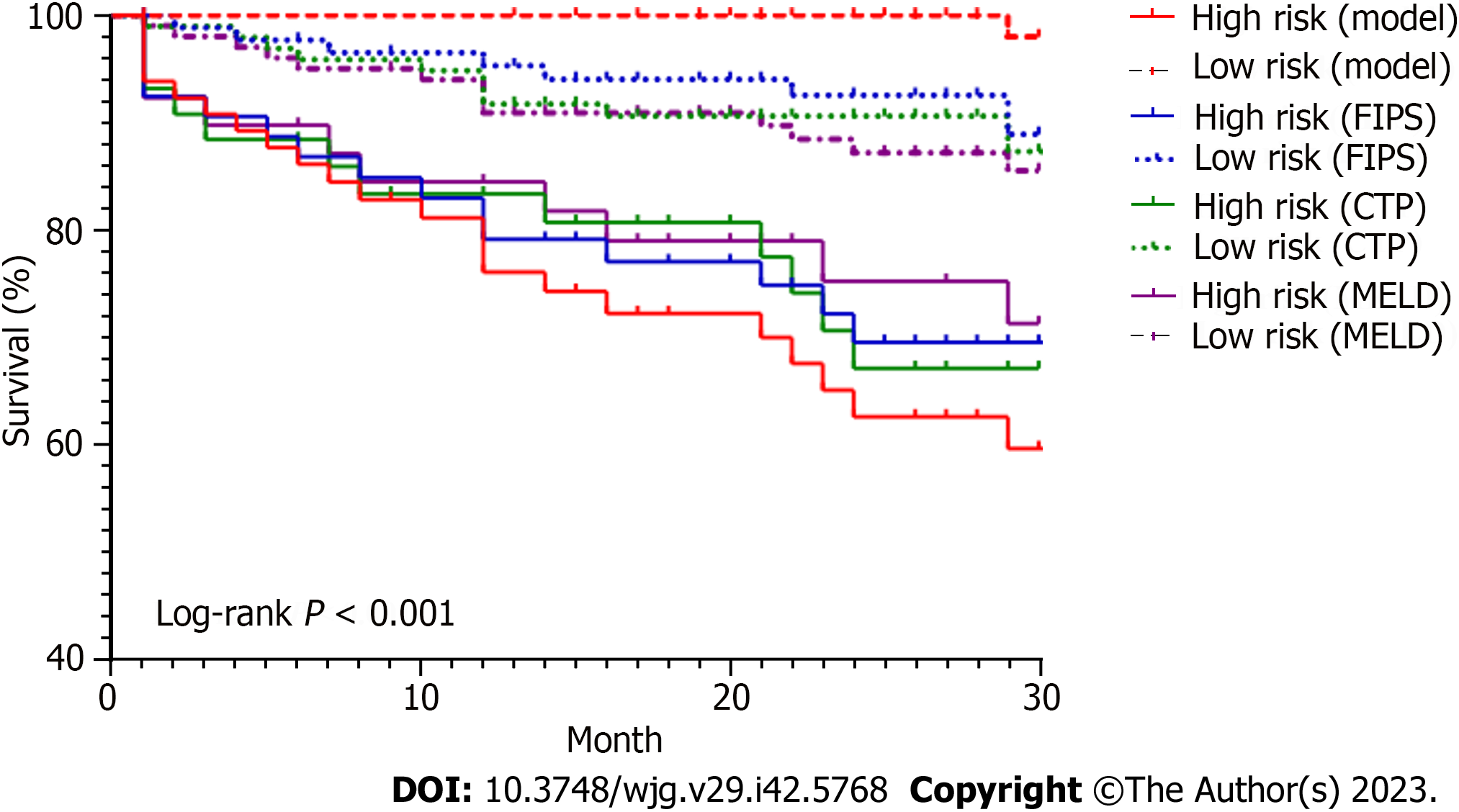
Finally, our model was compared with other models, including MELD, CTP, and FIPS, in terms of the C-index, AUC, and survival rate. The C-index and AUC of our model for 30-mo survival were 0.890 and 0.852 (95%CI = 0.782-0.923), respectively, significantly higher than those of MELD [C-index = 0.603, AUC = 0.612 (P < 0.001)], CTP [C-index = 0.644, AUC = 0.651 (P = 0.003)], and FIPS [C-index = 0.737, AUC = 0.709 (P = 0.015)] (Figure 4). Moreover, the 30-mo cumulative survival rates were compared between the low- and high-risk groups in the different models. For the high-risk group, the survival rate was extremely low, at 59.5%, according to our model, 67.0% according to CTP, 71.2% according to MELD, and 69.5% according to FIPS. Similarly, the median survival was 22.9 mo based on our model (95%CI: 20.3-25.5), 24.1 mo based on CTP (95%CI: 21.1-27.2), 24.8 mo based on MELD (95%CI: 21.6-27.9), and 24.0 mo based on FIPS (95%CI: 21.3-26.8). The survival curves were significantly different among the groups (P < 0.001) (Figure 5).
Our data demonstrated that among 186 cirrhotic patients receiving TIPS implantation, approximately 20% experienced liver transplantation or death within 30 mo after implantation, largely consistent with some previous studies[17,18]. Interestingly, our results also exhibited a significant association between SMI and long-term poor prognoses only in male patients with cirrhosis. Subsequent multivariate regression analyses elucidated that a low level of SMI in males was an independent risk factor for long-term adverse outcomes in cirrhotic patients receiving TIPS. Under the low level of SMI, the 30-mo postoperative adverse outcome rate was 42.0% in male patients and 22.6% in female patients. These findings essentially reveal the differential impacts of SMI on the long-term prognosis of patients undergoing TIPS implantation in the context of sex differences. Therefore, it is necessary to raise awareness of sex-specific therapeutic interventions in clinical practice.
A low level of SMI has been reported to share an association with various adverse outcomes in extrahepatic and intrahepatic diseases[19-21]. Meanwhile, some studies have also described the relationship between SMI and TIPS implantation in cirrhotic patients under a wide spectrum of pathological conditions and the possible related pathogenesis. For instance, in a recent study involving 224 patients undergoing TIPS, SMI was measured preoperatively and at 2, 5, and 12 mo postoperatively. The results showed that sarcopenia was associated with an increased risk of post-TIPS mortality and that the reversal of sarcopenia after TIPS may reduce the risk of mortality[22]. Other studies have also displayed an association of reduced skeletal muscle with HE, ACLF, and increased mortality after TIPS implantation[23-25]. Of note, a prior study was conducted in 2019 to investigate sarcopenia and frailty in patients with cirrhosis, which emphasized the importance of sex-stratified analyses[12]. This study revealed that two-thirds of male patients with frailty showed signs of sarcopenia, while only a quarter of female patients with frailty exhibited signs of sarcopenia, indicating that frailty-inducing factors might vary by sex. Another study in 2021 also demonstrated that SMI level reduction was associated with multidimensional frailty in male patients with cirrhosis[13]. Accordingly, sex-specific therapeutic strategies are needed to reduce frailty in this population. In this context, our follow-up cohort study was performed for in-depth analysis. Interestingly, our study illustrated SMI as an independent risk factor for long-term adverse outcomes in male patients with cirrhosis following TIPS implantation. Furthermore, the newly constructed prediction model based on SMI had a higher prediction efficiency than previous models. We believe that this finding is plausible and informative since a growing body of research has demonstrated a strong correlation of nutritional status with disease progression and outcomes in cirrhotic patients.
In addition, our findings also suggested sex differences in the relationship between a low level of SMI and long-term prognosis after TIPS implantation, concordant with findings in other phenotypic studies of chronic liver disease[12,13]. This difference can be explained by two facts. First, cirrhotic patients usually have hormonal changes, such as a substan
Subsequently, we noted that the poor prognosis of cirrhotic patients undergoing TIPS implantation was also closely related to elevated plasma ammonia levels. Reportedly, hyperammonaemia is not only a primary cause of HE but is also associated with damage to multiple systems, causing numerous complications of decompensated cirrhosis, including bacterial infection, variceal bleeding, and ascites[28]. For instance, plasma ammonia can promote the progression of liver injury, liver fibrosis, and portal hypertension by activating hepatic stellate cells through direct induction of hepatocyte death[29,30]. In addition, hyperammonaemia exhibits an association with innate immune dysfunction in patients with cirrhosis[31]. More importantly, progressive skeletal muscle loss leads to the deficiency of compensatory pathways for extrahepatic ammonia detoxification via glutamine synthetase, and ammonia accumulation has been documented to contribute to skeletal muscle loss through a variety of mechanisms[32]. Of note, previous studies have also confirmed that sarcopenia is associated with the increased mortality of patients with cirrhosis and is attributable to hyperammonemia[33]. Furthermore, hyperammonaemia in patients with cirrhosis may be the result of liver dysfunction, portosystemic shunts, skeletal muscle loss (reduced extrahepatic ammonia clearance), renal dysfunction, and intestinal dysbacteriosis[34]. Hence, the occurrence of hyperammonaemia reflects a complex and multiorgan interaction in cirrhosis that involves the dysfunction of the liver, kidneys, nerves, immunity, and skeletal muscle, and it is a critical biomarker for predicting the prognosis of patients with cirrhosis.
In addition to SMI levels and hyperammonaemia, creatinine levels also exert effects on the prognosis of cirrhosis. It has been reported that some patients with cirrhosis may also develop hepatorenal syndrome. Prior studies have indicated that renal dysfunction can be considered the result of hemodynamic damage associated with the severity of liver diseases, illustrating that renal dysfunction may also affect the long-term prognosis of patients receiving TIPS implantation[8]. Importantly, the postoperative level of creatinine, a fairly reliable index of renal function, can reflect renal function. ACLF and HE are also two frequent complications of TIPS implantation, which can result in the deterioration of liver function and are tightly related to poor prognoses in patients. In summary, the long-term survival of patients after TIPS implantation can be more accurately predicted by prognostic models constructed based on SMI, creatinine, and plasma ammonia levels, as well as the short-term postoperative complications of ACLF and HE in patients.
Our study has some limitations. First, because of the nature of observational studies, we only observed an association between SMI level reduction and long-term prognosis in male patients with cirrhosis after TIPS implantation. Nevertheless, it is not clear whether changes in muscle mass are a cause of disease progression, an aggravating factor of persistent pathological progression, or an incidental phenomenon reflecting a general adverse condition in patients with cirrhosis. Second, our study did not analyze and assess changes in the sex hormone levels of each patient in the cohort. Therefore, the different potential roles of this factor in muscle formation between men and women is only speculative. Third, our model lacked external validation in similar populations in other centers. Accordingly, further multicenter studies are warranted to validate our findings.
Our study highlights the strong correlation between SMI and long-term adverse outcomes in male patients with cirrhosis after TIPS implantation, favoring adequate sex-stratified analyses. Moreover, models constructed based on nutritional indicators hold promise for the effective prediction of prognosis in cirrhotic patients receiving TIPS implantation, assisting clinicians in closely monitoring the dynamics of high-risk populations.
Transjugular intrahepatic portosystemic shunt (TIPS) has been extensively applied for the treatment of portal hypertension-related complications. However, there is still a subset of patients with a poor prognosis because such patients typically develop advanced cirrhosis with more than one complication when TIPS implantation is needed. Hence, it is warranted to screen patients with a potentially poor prognosis after TIPS for closer follow-up, as they may require more aggressive liver transplantation.
Currently, the post-TIPS survival of patients is commonly predicted by models including Child-Turcotte-Pugh and Model for End-Stage Liver Disease. Nevertheless, the use of these models is limited by two factors. First, these models are constructed based on studies of Western patients who have different pathogeneses from Eastern patients. Second, these models focus on the short-term prognosis of patients after surgery and cannot assess the long-term survival of patients. Thus, a new alternative model is needed to accurately predict the long-term outcome of Asian patients with ascites- or variceal bleeding-induced end-stage liver disease who receive TIPS implantation.
This study aimed to construct a nutrition-based model that could predict the disease progression of cirrhotic patients after TIPS implantation in a sex-dependent manner.
According to the inclusion and exclusion criteria, 186 patients were eventually enrolled in this retrospective study. Skeletal muscle index (SMI) at the third lumbar spine was evaluated according to abdominal computed tomography scans within one month before TIPS implantation. A difference significantly differed at P < 0.05. Continuous variables were compared by the Student's t test or the Mann-Whitney U test. Categorical data are presented as numbers (percentages) and were compared by the chi-square test. Spearman’s correlation coefficients (rs) were used for correlation analyses. Independent predictors of disease progression were evaluated with univariate and multivariate Cox proportional hazard models, followed by the assessment of model calibration by analyzing the C-index. The area under the receiver operating characteristic curve was compared by the DeLong test. Cumulative transplant-free survival rates were plotted as Kaplan-Meier curves and compared by the log-rank test.
Our data demonstrated that among 186 cirrhotic patients receiving TIPS implantation, approximately 20% experienced liver transplantation or death within 30 mo after implantation. Interestingly, our results also exhibited a significant association between SMI and long-term poor prognoses only in male patients with cirrhosis. Subsequent multivariate regression analyses elucidated that a low level of SMI in males was an independent risk factor for long-term adverse outcomes in cirrhotic patients receiving TIPS. Under the low level of SMI, the 30-mo postoperative adverse outcome rate was 42.0% in male patients and 22.6% in female patients. These findings essentially reveal the differential impacts of SMI on the long-term prognosis of patients undergoing TIPS implantation in the context of sex differences. Further multicenter studies are warranted to validate our findings.
Our study highlights the strong correlation between SMI and long-term adverse outcomes in male patients with cirrhosis after TIPS implantation, favoring adequate sex-stratified analyses.
Models constructed based on nutritional indicators hold promise for the effective prediction of prognosis in cirrhotic patients receiving TIPS implantation, assisting clinicians in closely monitoring the dynamics of high-risk populations. Therefore, it is necessary to raise awareness of sex-specific therapeutic interventions in clinical practice.
We would like to acknowledge the reviewers for their helpful comments on this paper.
| 1. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2211] [Article Influence: 110.6] [Reference Citation Analysis (3)] |
| 2. | Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, Garcia-Tsao G, Lee SS. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, Bai W, Guo W, Yu T, Yuan X, Zhang H, Xie H, Yao L, Wang J, Li T, Wang Q, Chen H, Wang E, Xia D, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Cai H, Xia J, Yin Z, Wu K, Fan D, Han G; AVB-TIPS Study Group. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (1)] |
| 4. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 864] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 5. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2104] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 6. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3775] [Article Influence: 151.0] [Reference Citation Analysis (2)] |
| 7. | Bureau C, Métivier S, D'Amico M, Péron JM, Otal P, Pagan JC, Chabbert V, Chagneau-Derrode C, Procopet B, Rousseau H, Bosch J, Vinel JP. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPS. J Hepatol. 2011;54:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Bettinger D, Sturm L, Pfaff L, Hahn F, Kloeckner R, Volkwein L, Praktiknjo M, Lv Y, Han G, Huber JP, Boettler T, Reincke M, Klinger C, Caca K, Heinzow H, Seifert LL, Weiss KH, Rupp C, Piecha F, Kluwe J, Zipprich A, Luxenburger H, Neumann-Haefelin C, Schmidt A, Jansen C, Meyer C, Uschner FE, Brol MJ, Trebicka J, Rössle M, Thimme R, Schultheiss M. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74:1362-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, Engelmann C, Zhang P, Jeong JY, van Vugt JLA, Xiao H, Deng H, Gao X, Ye Q, Zhang J, Yang L, Cai Y, Liu N, Li Z, Han T, Kaido T, Sohn JH, Strassburg C, Berg T, Trebicka J, Hsu YC, IJzermans JNM, Wang J, Su GL, Ji F, Nguyen MH. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;76:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (2)] |
| 10. | Montagnese S, Russo FP, Amodio P, Burra P, Gasbarrini A, Loguercio C, Marchesini G, Merli M, Ponziani FR, Riggio O, Scarpignato C. Hepatic encephalopathy 2018: A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Dig Liver Dis. 2019;51:190-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 473] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 12. | Fozouni L, Wang CW, Lai JC. Sex Differences in the Association Between Frailty and Sarcopenia in Patients With Cirrhosis. Clin Transl Gastroenterol. 2019;10:e00102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Feng H, Wang X, Mao L, Yu Z, Cui B, Lin L, Hui Y, Zhao X, Xu X, Fan X, Wang B, Yu Q, Jiang K, Sun C. Relationship between sarcopenia/myosteatosis and frailty in hospitalized patients with cirrhosis: a sex-stratified analysis. Ther Adv Chronic Dis. 2021;12:20406223211026996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1022] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 15. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2352] [Article Influence: 213.8] [Reference Citation Analysis (4)] |
| 16. | Peng H, Zhang Q, Luo L, Lei S, Xiong T, Long L, Xiong Y, Zhang L, Zheng J, Luo X. A prognostic model of acute-on-chronic liver failure based on sarcopenia. Hepatol Int. 2022;16:964-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 17. | Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 18. | Li J, Tang S, Zhao J, Zhang C, Jiang Z, Xue H, Sun J, Zhu X, Ren W, Wang Q, Wang E, Lv Y, Guo S, Wang Z, Yang Q, Niu J, Yin Z, Xia J, Fan D, Han G. Long-term survival prediction for transjugular intrahepatic portosystemic shunt in severe cirrhotic ascites: assessment of ten prognostic models. Eur J Gastroenterol Hepatol. 2021;33:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Wijarnpreecha K, Werlang M, Panjawatanan P, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Ungprasert P. Association between sarcopenia and hepatic encephalopathy: A systematic review and meta-analysis. Ann Hepatol. 2020;19:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, Montano-Loza AJ. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 21. | Papadopoulou SK. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 342] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 22. | Liu J, Ma J, Yang C, Chen M, Shi Q, Zhou C, Huang S, Chen Y, Wang Y, Li T, Xiong B. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology. 2022;303:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, Riggio O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin Gastroenterol Hepatol. 2017;15:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 24. | Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, Pohlmann A, Lattanzi B, Krabbe VK, Strassburg CP, Arroyo V, Merli M, Meyer C, Trebicka J. Sarcopenia Is Associated With Development of Acute-on-Chronic Liver Failure in Decompensated Liver Cirrhosis Receiving Transjugular Intrahepatic Portosystemic Shunt. Clin Transl Gastroenterol. 2019;10:e00025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Yang C, Zhu X, Liu J, Shi Q, Du H, Chen Y, Huang S, Zhou C, Wang Y, Li T, Bai Y, Xiong B. Development and Validation of Prognostic Models to Estimate the Risk of Overt Hepatic Encephalopathy After TIPS Creation: A Multicenter Study. Clin Transl Gastroenterol. 2022;13:e00461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 27. | Kahl KG, Utanir F, Schweiger U, Krüger TH, Frieling H, Bleich S, Gutberlet M, Hartung D. Reduced muscle mass in middle-aged depressed patients is associated with male gender and chronicity. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Tranah TH, Ballester MP, Carbonell-Asins JA, Ampuero J, Alexandrino G, Caracostea A, Sánchez-Torrijos Y, Thomsen KL, Kerbert AJC, Capilla-Lozano M, Romero-Gómez M, Escudero-García D, Montoliu C, Jalan R, Shawcross DL. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis. J Hepatol. 2022;77:1554-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 29. | Jia B, Yu ZJ, Duan ZF, Lü XQ, Li JJ, Liu XR, Sun R, Gao XJ, Wang YF, Yan JY, Kan QC. Hyperammonaemia induces hepatic injury with alteration of gene expression profiles. Liver Int. 2014;34:748-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Jalan R, De Chiara F, Balasubramaniyan V, Andreola F, Khetan V, Malago M, Pinzani M, Mookerjee RP, Rombouts K. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension. J Hepatol. 2016;64:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, Pitsillides AA, Jalan R. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology. 2008;48:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 34. | Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015;90:646-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Kumar R, India S-Editor: Lin C L-Editor: Wang TQ P-Editor: Cai YX