Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.582
Peer-review started: September 15, 2022
First decision: November 26, 2022
Revised: December 3, 2022
Accepted: December 27, 2022
Article in press: December 27, 2022
Published online: January 28, 2023
Processing time: 127 Days and 2.5 Hours
Clostridioides difficile (C. difficile) is progressively colonizing humans and animals living with humans. During this process, hypervirulent strains and mutated toxin A and B of C. difficile (TcdA and TcdB) are originating and developing. While in healthy subjects colonization by C. difficile becomes a risk after the use of antibiotics that alter the microbiome, other categories of people are more susceptible to infection and at risk of relapse, such as those with inflammatory bowel disease (IBD). Recent in vitro studies suggest that this increased susceptibility could be due to the strong cytotoxic synergism between TcdB and proinflammatory cytokines the tumor necrosis factor-alpha and interferon-gamma (CKs). Therefore, in subjects with IBD the presence of an inflammatory state in the colon could be the driver that increases the susceptibility to C. difficile infection and its progression and relapses. TcdB is internalized in the cell via three receptors: chondroitin sulphate proteoglycan 4; poliovirus receptor-like 3; and Wnt receptor frizzled family. Chondroitin sulphate proteoglycan 4 and Wnt receptor frizzled family are involved in cell death by apoptosis or necrosis depending on the concentration of TcdB and cell types, while poliovirus receptor-like 3 induces only necrosis. It is possible that cytokines could also induce a greater expression of receptors for TcdB that are more involved in necrosis than in apoptosis. Therefore, in subjects with IBD there are the conditions: (1) For greater susceptibility to C. difficile infection, such as the inflammatory state, and abnormalities of the microbiome and of the immune system; (2) for the enhancement of the cytotoxic activity of TcdB +Cks; and (3) for a greater expression of TcdB receptors stimulated by cytokines that induce cell death by necrosis rather than apoptosis. The only therapeutic approach currently possible in IBD patients is monitoring of C. difficile colonization for interventions aimed at reducing tumor necrosis factor-alpha and interferon-gamma levels when the infection begins. The future perspective is to generate bacteriophages against C. difficile for targeted therapy.
Core Tip: Clostridioides difficile is an opportunistic pathogen that is progressively increasing worldwide. Patients with inflammatory bowel diseases are particularly susceptible due to altered immunological status and the therapies adopted that favor intestinal dysbiosis and colonization by Clostridioides difficile. Recent in vitro studies also suggest that the infection might be favored by the strong cytotoxic synergism between Clostridioides difficile toxin B and proinflammatory cytokines, thetumor necrosis factor-alpha and interferon-gamma. The therapeutic approaches are still limited, and those presently available rely on antibiotic therapy and fecal transplantation.
- Citation: Bassotti G, Fruganti A, Stracci F, Marconi P, Fettucciari K. Cytotoxic synergism of Clostridioides difficile toxin B with proinflammatory cytokines in subjects with inflammatory bowel diseases. World J Gastroenterol 2023; 29(4): 582-596
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.582
Clostridioides difficile (C. difficile)[1] is a gram-positive, anaerobic, and spore-forming bacterium[2-6], responsible for the most widespread healthcare-associated infection worldwide[7-11]. In the United States every year about 500000 individuals become infected, leading to approximately 29000 deaths, and in Europe there are 124000 cases of infected individuals per year with a mortality rate ranging from 3% to 30%[7-11]. C. difficile infection (CDI) accounts for more than 15%-25% of all opportunistic gastrointestinal infections[7-11]. The rate of C. difficile colonization is about 18%-90% of healthy infants in relation to infant age[12], and 4%-15% of healthy individuals[13]. Its transmission occurs by the fecal-oral route[6,14], mainly by spores. Hospitals and community healthcare settings are an important source of infection due to the presence of C. difficile-infected patients[15,16]. The latter create a microenvironment highly contaminated by C. difficile spores extremely resistant to common strong disinfectants and radiation[17-19].
The clinical manifestations of CDI vary from asymptomatic carriage or mild self-limiting diarrhea to pseudomembranous colitis, with complications including toxic megacolon, fulminant infection and death[2-5,10]. The disease strictly depends on C. difficile germination and the release of three toxins[2-5,10]. The Rho-glycosylating C. difficile toxins A (TcdA) and B (TcdB) are major toxins that are clearly responsible for diarrhea and colitis[2-5,10]. In addition, 5%-30% of clinical C. difficile strains produce a binary autoprotease domain-ribosylating toxin, called the C. difficile binary toxin (CDT), that modifies actin[20,21].
It has previously been highlighted how the continuous progressive spread of C. difficile in the anthropized environment and the ability to develop more virulent strains will allow it to colonize most of the human population in the near future[22,23]. Among the subjects who will be more progressively colonized/infected with the progressively expanding C. difficile are those with inflammatory bowel diseases (IBD)[24-28], a category which is increasing in number in both Western and Eastern countries[29,30,31].
The progressive C. difficile endemic spread and the growing number of IBD subjects[25,29,32,33] are already interacting, as evidenced by studies showing how the rate of CDI cases in patients with IBD has increased by approximately four times in recent years[25]. It is therefore important to understand the molecular and pathogenic events by which C. difficile colonizes and infects and how these can impact subjects with IBD characterized by a profound alteration of the microbiome, of the immune system, and of the inflammatory response.
CDI causes nosocomial/antibiotic-associated and community healthcare diarrhea with abdominal pain and cramps[6,10,32,33]. Colitis without pseudomembrane formation features watery diarrhea, trace blood in stool, nausea, abdominal pain, malaise, anorexia, low-grade fever, dehydration, pyrexia, and leucocytosis[6,10,32,33]. Clinical manifestations of pseudomembranous colitis consist of abdominal cramps, watery diarrhea with dehydration, hypoalbuminemia, and increased serum proteins, mucus, and inflammatory cells. Sometimes plaques (pseudomembranes) are found in the colorectal mucosa[6,10,32,33]. Fulminant colitis, observed in about 3% of CDI patients, induces serious complications such as perforation, prolonged ileus, megacolon, and death[6,10,32,33]. CDI may not be limited to the colon, and extra-colonic manifestations have been reported, including small bowel disease with the formation of pseudomembranes on the ileal mucosa, bacteremia, reactive arthritis, appendicitis, intra-abdominal abscesses, osteomyelitis, and empyema[34,35] In recent years, a significant rise in cases of fulminant colitis, which results in the development of symptoms, multiple organ failure, and increased mortality, has been associated with hypervirulent strains of C. difficile[6,10,32,33]. The disease strictly depends on C. difficile production and release of three toxins[2-5,10]. TcdA and TcdB are primarily responsible for clinical manifestations of disease[2-5,10]. However, 5%-30% of C. difficile clinical strains produce CDT, which contributes to disease by means of actin modification[20,21].
TcdA and TcdB are single chain proteins, with a molecular weight of 308 kDa for TcdA and 270 kDa for TcdB[2-5,32]. Tcds show 48% sequence identity and 66% sequence similarity, where the diversity of sequence is mainly limited to the C-terminal binding domain. TcdA and TcdB are constituted by four domains, each characterized by specific biological and functional properties: (1) A glucosyltransferase N-terminal domain (GTD); (2) An autoprotease domain; (3) A pore forming and translocation domain; and (4) A C-terminal binding repetitive oligopeptides domain (CROP)[2-5,32]. The CROP domain and other amino acids outside this domain allow the binding of Tcds to the cells for subsequent internalization[2-5]. Although TcdA and TcdB CROPs display the solenoid fold, they present distinct spatial and sequential arrangements of their repeat units. This agrees with findings that suggest that both TcdA and TcdB bind to different receptors[2-5,32]; therefore, TcdA and TcdB do not follow the rule of one toxin-one receptor[2-5,32,36].
Two different cell surface receptors have been proposed for TcdA: rabbit sucrase isomaltase and gp96[2-5,32,36]. Since many cells and tissues that are sensitive to TcdA do not express sucrase isomaltase and cells lacking gp96 are only partially resistant to TcdA intoxication, TcdA could bind to other receptor structures[2-5,32,36]. Three different receptors have been identified for TcdB[2-5,32,36]: Chondroitin sulphate proteoglycan 4 (CSPG4); poliovirus receptor-like 3 (PVRL3); and Wnt receptor frizzled family (FZD; FZD1, 2 and 7)[2-5,32,36]. TcdB binding to the CSPG4 receptor in HeLa and HT29 cells induces cell rounding and apoptosis at picomolar concentrations of TcdB but induces necrosis at higher concentrations of TcdB[32,36-38]. TcdB binding to the PVRL3 receptor induces necrosis at high doses (the nanomolar range) of TcdB[32,36-39]. TcdB binding to the FZD receptors induces cytopathic effects and apoptosis at picomolar concentrations of TcdB, indicating that FZD functions as an alternative receptor to CSPG4[36-40]. A further significant distinctiveness of TcdB is that it can bind to the membrane receptor with amino acid sequences that extend beyond the CROP sequences[36-40].
This description, in agreement with recent studies, indicates that TcdA and TcdB use more than one receptor for cell binding and uptake and highlights how the heterogeneity of the TcdB-bound receptors may impact on the diversity of the effects in relation to the receptor binding, concentrations of TcdB, and cell types[36-43]. It is possible that TcdB may utilize multiple receptors to broaden the selection of mammalian cells it can target[36-41]. Further, TcdB variants are highly diverse for their receptor preference, with relevant implications on colonic pathology[20,32,36,41].
The role played by CROP domains is also demonstrated by the fact that antibodies directed toward the CROP domains of TcdA and TcdB prevent uptake[2-5,36], and excess of the TcdA CROP domain compete with TcdA for cell binding[2-5,36]. However, TcdA and TcdB that lack CROP domains are still capable of internalization by the cells[2-5,36]. To understand some important aspects of the pathogenic strategy of C. difficile it is necessary to know what Tcd receptors are expressed on different cell types. Receptors for Tcds do not have well-defined molecular structures[20,32,36-40]; they are likely formed by a complex configuration of the polysaccharide chains that are recognized by the TcdA or TcdB binding domains with a lectin-like structure[42]. Furthermore, TcdA and TcdB have some important features of intrinsically disordered proteins[44] that allow Tcds to modify their conformation to adapt and more efficiently bind to the target structure of Tcd receptors[32,36-40]. The complex and intrinsically disordered structural features of the Tcds allow them to bind very different cell types[2-5,32], such as the cells of surface epithelium of the human colon[43], colonocytes[45], hepatic cells[46], nervous cells[47], enteric glial cells (EGCs)[48], and cardiac cells[49].
It has been hypothesized that when C. difficile spores germinate into their vegetative forms and replicate that their susceptibility to the cytotoxic activity of macrophages, polymorphonucleates, and lymphocytes will increase[32,50-52]. These immune cells induce and increase the inflammatory response[3,50-53] characterized by secretion of several proinflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α)[54-58]. C. difficile struggles against these immune cells by means of Tcds, capable of binding to receptors with different forms of carbohydrates that give rise to distinct structures. This result is obtained by the ability of TcdB to recognize three types of receptors[36-40] and by its intrinsically disordered structure[44]. Therefore, due to accidental molecular homology, Tcds could also be cytotoxic to other cell types not present in the infection site that express one or more of the Tcd receptors (e.g., endothelial, hepatic, nervous, EGCs, and cardiac cells)[32,46-49]. Furthermore, the binding capability of Tcds toward colonocytes deepens the tissue damage within and beyond the submucosa. The subsequent damage to the muscle and enteric nervous system cells creates conditions to expel via diarrhea the C. difficile vegetative forms that rapidly become spores and to start a new infection cycle. It is also possible that if the Tcd receptor domain mutates, the pathogenicity of C. difficile may become more severe.
Tcds, after binding to their cell membrane receptors, promote their uptake by endocytosis[2-5,32]. Tcds differ in uptake pathways. TcdB uptake is mediated by clathrin[2], while TcdA uptake is mediated by PACSIN2/syndapin-II[2]. In the endocytic vacuole the progressive pH decrease promotes a Tcd conformational change that leads to translocation across the endosomal membrane of the catalytic domain for cleavage through vacuole pore formation[2-5,32]. Then, Tcds undergo autoprocessing by the cysteine protease domain that follows the N-terminal GTD[2-5,32] in a host-factor dependent manner (e.g., inositolphosphates, mainly inositol hexakisphosphate)[2-5,32], releasing the GTD into the host cell cytosol[2-5,32].
The Tcds GTD, by the monoglucosylation of the catalytic site of Rho-GTPases, inhibits their activity[2-5,32]. The monoglucosylation of Rho-GTPases induces different effects in vitro and in vivo[2-5,32]. The effects mainly documented in vitro are: (1) Actin condensation, rearrangement of the cytoskeleton, and disruption of focal adhesions and tight junctions[2-5,32]. These effects induce cell rounding in cultured cells (cytopathic effect)[2-6,32,48]; (2) Arrest of the cell cycle by reduction of both the expression of cyclins and the activation of cyclin-dependent kinases that together mediate progression in the different cell cycle phases[2-4,32,48]; and (3) Cell death by apoptosis or necrosis (cytotoxic effect)[2-6,32,48]. Cell death occurs in a glucosylation-dependent/glucosylation-independent way, mainly by apoptosis with caspase-dependent or caspase-independent mechanisms[2-4,32,48]. Furthermore, the cytotoxic effects are dependent on the dose of Tcds, receptors involved, and cell types[2-6,32]. In fact, TcdB at high concentrations bind to CSPG4, FZDs, or PVRL3 and induce cell death by necrosis[36-39,59]. At low concentrations TcdB binds to CSPG4 or FZDs, and cell death occurs by apoptosis[36-39,48]. However, it must be considered that the effects of Tcds could depend on cell types, likely by selective expression of Tcd receptors, and by differential expression levels of Tcd receptors[2,3,6,32].
TcdA and TcdB, in vivo, disrupt epithelial tight junctions and induce cell death, provoking direct damage to the colonic epithelium[2-5,32]. In addition, Tcds induce an acute inflammatory response stimulating colonic epithelial cells to release proinflammatory cytokines and chemoattractants of neutrophils[2-5,32] that can induce tissue damage, modifying the barrier effect of the intestinal mucosa. A compromised intestinal barrier within the context of active inflammation subsequently leads to enhanced intestinal and vascular permeability[3,26,29,32,34]. Following the loss of a protective barrier, there is access of Tcds and/or bacteria into the lamina propria, which in turn increases intestinal inflammation[3,26,29,32,34]. TcdA primarily affects the intestinal epithelium, while TcdB has a broader cell tropism and is probably responsible for the major clinical effects of C. difficile due to its toxicity, which is approximately 1000 times higher than that of TcdA. Thus, TcdB represents the main virulence factor of CDI[2,3,26,29,34]. TcdB causes death in many different cell types[3,26,29,32,34] other than epithelial cells and colonic myofibroblasts, such as hepatocytes, cardiomyocytes, lung fibroblasts, immunocytes, enteric neurons, and EGCs[32,46-49].
Since immune cells that reach the replication area of the C. difficile vegetative form possess intrinsic motility, the molecular strategy to cause cytoskeleton alterations and cell cycle arrest is of crucial importance in order to decrease their functional ability to counteract CDI[2,3-5,6,32]. Tcds in these immune cells stimulate cytokine secretion, in particular proinflammatory cytokines such as IFN-γ and TNF-α[2,3,6,32,34] and anti-inflammatory cytokines such as IL-10[60]. Cytoskeletal disruption occurs in some cells after 30 min, representing the initial event that leads to cell rounding in most cell types in vitro[32,61] with detachment of cells. Tcds in vivo induces both retraction of colonocytes and basal membrane cells, favoring the additional in-depth penetration of C. difficile and promoting an extremely inflammatory environment that causes the expulsion of C. difficile in the external environment by diarrhea[32,62].
Various Tcd-infected cells, after cell-cycle arrest, die by apoptosis[2,3,5,6,32]. This could represent an important aspect of the molecular strategy of this pathogen to reduce the inflammatory response. Apoptosis is a form of cell death that occurs without inflammation and is primarily mediated by activation of the effector caspases-3 and -7, which can be triggered by a death receptor-dependent extrinsic or a mitochondria-dependent intrinsic pathway[63-65]. However, apoptosis can also be activated in a caspase-independent manner by the cleavage/activation of pro-apoptotic Bcl-2 family members and non-caspase proteases such as calpains and cathepsins[66-69]. Caspase-dependent TcdA- and TcdB-induced apoptosis has been extensively investigated[2,3,32,48,70], while there is only one study that found that TcdA can also induce caspase-independent apoptosis following cathepsin[71] and calpain activation[71].
Recently, Fettucciari et al[72] demonstrated that the mechanism by which TcdB induces apoptosis is much more complex than previously thought[48,70]. TcdB induced apoptosis in EGCs, a cell population of paramount importance for colonic pathophysiology, by three signaling pathways activated by calpains, caspases, and cathepsins, which are all involved in both induction and execution of apoptotic signaling[72]. Calpain activation by Ca2+ influx is the first pro-apoptotic event in TcdB-induced EGC apoptosis and causes caspase-3, caspase-7, and PARP activation. The latter is activated by caspases but also directly by calpains, which are responsible for the majority of apoptosis[72]. Caspase-3/caspase-7 and PARP activation is mediated also by activation of initiator caspase-8 by TcdB, and it contributes to one-third of apoptosis events[72]. Finally, cathepsin B contributes to triggering the pro-apoptotic signal and to one-third of apoptosis events by a caspase-independent manner. It appears to control the levels of caspase-3 and caspase-7 active fragments, highlighting the complex interaction between these cysteine protease families activated during TcdB-induced apoptosis[72].
Recently, we also demonstrated that pro-inflammatory cytokines, TNF-α plus IFN-γ (CKs) strongly increased apoptosis induced by TcdB, by an enhanced activation of the three pro-apoptotic pathways induced also by TcdB alone activated by calpains, caspases, and cathepsins, which are involved in both induction and execution of apoptotic signaling[72]. However, two important differences between TcdB- and TcdB + CKs-induced apoptosis are: (1) Apoptotic signaling activation by TcdB + CKs is enriched by TNF-α-induced NF-kB signaling, inhibition of JNK activation, and activation of AKT[72]; and (2) Apoptosis induced by TcdB + CKs increased strongly in the time course, with more than 60% of the cells undergoing apoptosis at 72 h, while apoptosis induced by TcdB increased slowly, with only about 18% of cells undergoing apoptosis at 72 h[72].
This capability of TcdB to trigger three different cell death pathways represents an extremely important C. difficile strategy[72] to overcome the possible intrinsic resistance of a cell type to one or two of the three apoptotic pathways. In fact, as reported above, Tcds are able of cause cell death in different non-immune[32,46-49] and immune cell types[2,3-32,73,74].
A further strategy adopted by C. difficile is its ability to induce different types of cell death that can lead to different consequences in the C. difficile pathogenesis[2-4,6,32]. In fact, TcdB causes cell death by both apoptosis and necrosis[2,3,6,32,75] that is dependent on the TcdB concentration and by the TcdB receptor expressed by the target cells[20,32,36-38]. TcdB at lower concentrations and binding to the CSPG4 or FZDs receptors[20,36-40] induces apoptosis in a glucosylation-dependent manner[2-6,32], while at higher concentrations (100 pM or above) causes cell death by necrosis[20,36-43], which does not require either the autoprocessing or glucosyltransferase activities of the toxin[2-6,75]. Necrosis is an early process occurring after 2-4 h of intoxication and is found in both cell culture and colon explant models[2,3,5,6,75]. Necrosis is characterized by quick ATP depletion, early loss of plasma membrane permeability and cellular leakage, and chromatin condensation without caspase-3 and caspase-7 activation[2,3,6,32,75].
TcdB causes necrosis through activation of a strong production of reactive oxygen species (ROS) by assembly of the NADPH oxidase complex on endosomes[2-5,75]. High levels of ROS trigger necrosis by DNA damage, lipid peroxidation, protein oxidation, and/or mitochondrial dysfunction[2,3,6,32,75]. It has been suggested that pore formation in the endocytic vacuoles is important for the glucosylation-independent necrotic cell death caused by TcdB. Indeed, a TcdB mutant, defective in pore formation, does not induce necrosis even at high nanomolar concentrations[2,4,6,75]. Unlike TcdB, TcdA does not trigger ROS production through the NADPH oxidase complex and causes glucosylation-dependent apoptosis at all concentrations tested[2-6]. The ability of TcdB, but not of TcdA, to cause necrosis may explain why a wild-type (TcdA+TcdB+) epidemic strain and an isogenic TcdA-TcdB+ mutant in animal infection models provoke considerably more damage to colon tissue than an isogenic TcdA+TcdB- mutant strain[2-6]. The glucosylation-independent mechanism of TcdB might play a similar role in the context of human disease; TcdB-induced necrosis likely contributes to the extensive gut damage observed in patients with severe forms of CDI[2-6].
TcdB induces an early cell death defined pyknotic cell death, characterized by chromatin condensation, cell cycle arrest, and ballooning of the nuclear envelope that is both glucosyltransferase domain-dependent and -independent[2,3,6,75] and occurs at concentrations 5000 times greater than necessary for Rho protein glucosylation and ROS production[2-5,75].
It has also been reported that TcdA and TcdB trigger pyrin inflammasome activation in an apoptosis-associated speck-like protein with a caspase recruitment domain-dependent manner, causing release of IL-1β[2-6]. In particular, TcdB-induced inflammasome activation triggers a type of cell death defined as “pyroptosis,” characterized by cell swelling and lysis with gross release of cellular content and inflammatory cytokines like IL-1β through pore formation in target cells caused by caspase-1-dependent activation of gasdermin D, which induces strong inflammation[2-6].
Most importantly, EGCs intoxicated with low doses of Tcds might revert to their normal functions after a brief cell cycle arrest[32,48,76], while EGCs that survive apoptotic activity of TcdB become senescent as a TcdB-mediated survival response to stressful stimulus[32,48,76]. This ability of cells surviving the cytotoxic activity of Tcds to become senescent may affect functionality of EGCs, intestinal neurons, and myocytes, contributing to altered bowel motility[32,77]. The acquisition of a senescence status by these cell types could have critical outcomes in the subsequent development of post-infectious irritable bowel syndrome and stimulation of preneoplastic cells[32,77].
Thus, we can postulate that when healing after an acute CDI patients can later have some important consequences such as a decrease in EGC number and impairment and alterations in EGC functionality[32]. After CDI, the structural and functional defects caused by the Tcds might be long-lasting in a significant percentage of patients and trigger low-grade inflammation and persistent dysmicrobism[25]. This implies that residual C. difficile bacteria that remain when healing after an acute CDI may benefit from this condition and provoke relapses. The latter might be due to cytotoxic synergism with inflammatory cytokines that can occur even after months without any evident cause[78]. Therefore, it is possible that C. difficile changes the large bowel environment to remain for a long time and favor easier relapses. This means a continual expansion of C. difficile carriers in the large bowel environment, with induction of an irritable bowel syndrome-like condition and with recurrences due to a latent or fluctuating inflammatory condition as in IBD subjects.
All this emphasizes the complex molecular strategy of C. difficile based on the cytotoxic synergism with some components of the inflammatory response as IFN-γ and TNF-α, which potentiate cytotoxicity of TcdB[48,72]. Therefore, it is conceivable that IFN-γ and TNF-α behave as drivers of the infection from the beginning of the infection, amplifying apoptosis induced by low doses of Tcds, and opening the way to infection progression[26,32,34,48,72].
Therefore, it is likely that antibiotic treatment, other than provoking dysmicrobism, by means of bacteriolytic activity builds an inflamed environment in the large bowel by release of bacterial factors from killed bacteria. Additionally, whatever inflammatory environment induced in the absence of antibiotic treatment could also help CDI in subjects with obesity or other pathologies accompanied by an inflammatory state[28].
An endemic spread of C. difficile in IBD patients is favored by:
(1) The extreme resistance of the spores to the external environment and the mechanism of spore germination[17-19,79].
C. difficile spores are extremely resistant to strong disinfectants and radiation[17-19]. The mechanism of germination is both complicated and distinctive compared to that of other microorganisms[79], due to the peculiar Tcd interactions with the host and to cellular microbial factors that facilitate the colonization and successive infection and relapses[79-83]. Moreover, C. difficile spores can also adhere to gut epithelial cells and penetrate them via a process of macropinocytosis-like endocytosis[84,85]. This macropinocytosis-like endocytosis is dependent on Fr-95B1 and Vn-avβ1 integrin and on the spore-surface collagen-like BclA3 exosporium protein[84,85]. In an in vivo model in mice, it has been shown that the entry of spores into intestinal epithelial cells in a dormant but reactivable state contributes to the recurrence of CDI[84,85].
(2) Progressive colonization of humans by the C. difficile spores depends on C. difficile to wait for the appropriate conditions that favor transition to the vegetative form capable of inducing infection and the clinical manifestations. Colonization is due to C. difficile transmission via the fecal-oral route, with the spores that traverse the acidic pH of the stomach to colonize the large bowel, where they remain inactive until appropriate conditions favor the passage to the vegetative form[83]. The conditions that favor intestinal germination of C. difficile spores are an increase of primary bile acids, butyrate, disaccharide, and trehalose and other substances produced by some bacterial species taxonomically identified in a perturbed microbiome that favors C. difficile overgrowth vs other pathogens and a reduction of secondary bile acids[86-91]. Of interest, these conditions have been described in patients with IBD[92,93]. In fact, C. difficile colonizes and infects the colon following antibiotic-immunosuppressant-induced gut dysbiosis[94-101]. The dysbiosis also depends on different factors such as age, type of drug used, administration of proton-pump inhibitors, types of foods, physical environment, the genetic and immune system of the individual, and concomitant pathologies (e.g., diabetes, obesity, autoimmune and allergic diseases, IBD)[94-101]. These predisposing factors have progressively broadened the range of subjects susceptible to colonization/infection by C. difficile[94-101]. In turn, C. difficile colonization causes gut flora perturbations that increase dysmicrobism and inflammation, promoting CDI and CDI relapses[94-101]. Moreover, changes in normal gut microflora after a first CDI could predispose individuals to recurrent CDIs, and the protracted antimicrobic therapy for C. difficile can cause, in a gut microbiota already modified, further and persistent dysbiosis and inflammation.
Although following primary CDI episodes the bacterial taxa restore with time, in some subjects some taxa may not recover fully and maintain a decreased resistance to colonization. This in turn promotes the subsequent growth of pathogens (including C. difficile), altering the composition of the gut microbiome. The frequent use of antibiotics to treat C. difficile increases the pool of antibiotic-resistant genes in the gut microbiome, thereby favoring recurrent CDIs[96-103]. Although antibiotic exposure, hospitalization, advanced age, and immunocompromised status increase the risk for disease, community-acquired infections in otherwise healthy young and adults not previously exposed to antibiotics are not infrequent[96-103].
The favorable conditions for C. difficile colonization are more widespread in “developed countries” due to the increase in antibiotic therapies[15] in all ages and changes in microbiota for various external factors[104]. Thus, a progressive increase of CDI and CDI-related deaths (at present in the range of 5%-30% with primary infection)[16] is foreseeable, with a more progressive rise of death rates following CDI relapses[105,106].
(3) Persistent dysmicrobism due to the use of antibiotics, immunosuppressive drugs, and most important a colonic environment characterized by waves of inflammatory response with a continuously active basal level. Persistent dysmicrobism enables the overgrowth of several intestinal pathogens, including C. difficile. Some particularities of C. difficile favor its growth in an altered environment characterized by low-grade inflammation[15,80-82,90,98-101,104], such as in a subject with IBD[24-31]. For primary CDI, the changes in gut microbial flora that favor overgrowth of C. difficile over other various intestinal pathogens (e.g., C. perfringens) are crucial, even though the role of the gut flora in regulating C. difficile is more complex than previously hypothesized. Indeed, in preventing C. difficile colonization, disease, and recurrence, the maintenance of enough density of the species creating an environment hostile to C. difficile expansion by means of both changes of biomass and composition rather than the simple reduction of some taxonomic groups plays a key role[9,90,94-101]. Furthermore, dysmicrobism depends primary on the factors and pathologies reported above (e.g., IBD)[9,90,94-101].
(4) Continued emergence of new C. difficile strains that are more hypervirulent or multidrug-resistant (e.g., ribotypes 015, 027, 078 or 176), many of which produce the binary toxin CDT[20,51,91,107,108].
(5) Production of variant Tcds by some C. difficile strains[51,80,91,99,102]. The expansion of CDI could be enhanced by C. difficile strains that produce Tcd variants[107-111]. In fact, many of these are hypervirulent and release the binary toxin CTD, which is linked with enhanced morbidity and mortality[107-111]. CTD stimulates the formation of long cellular filaments, which become anchor points for other C. difficile to epithelial cells, potentiating the infection[20,51,91,107,108]. The Tcd variants are also greatly different for enzymatic activity, immunogenicity, and their receptor preference, with important implications on colonic pathology[51,91,107,108].
(6) The complex equipment of surface antigens of C. difficile are flagella, fimbriae, pili, cell wall proteins, and biofilm, which act as colonization factors or mediate innate immune responses that can play a key role in the persistence of C. difficile[2,3,6,10,32] and induce proinflammatory cytokines such as TNF-α and IFN-γ[2-6].
TcdB is the most involved in CDI due to its presence in most toxic strains and its degree of pathogenicity is 1000 times greater than that of TcdA[2-6]. The functions of the TcdB receptors, CSPG4, PVRL3, and FZD, are summarized above. The variants of TcdB are further diversified for their receptor preference with relevant implications in the pathology of CDI[32,51,91,107,108] by the ability to bind extremely different cell types[2,3,6,32], including human colon epithelium cells[43], nerve cells[47], EGCs[48], neurons, liver cells, and heart cells[49].
What are the elements that can therefore impact subjects with IBD as a consequence of the heterogeneity of Tcds and of their receptors on the various cell types? The intestinal mucosa of IBD subjects is altered by inflammation resulting from the immune response and dysmicrobism. Although direct data on how this altered colonic environment may modify the expression of the various receptors for TcdB are not available, it is likely that this could occur, as suggested by in vitro data. Therefore, it is possible that conditions whereby a differential expression of TcdB receptors are expressed on colonic epithelial cells favoring primarily the necrotic effects of TcdB could arise, characterizing the trend of infection. Moreover, even the inflammatory immune cells that try to fight infections could be induced to express receptors that lead to their cell death by necrosis. Thus, in subjects with IBD an initial CDI could quickly become serious due to a strong inflammatory response that favors the expression of receptors for the TcdB that lead to death mainly by necrosis. This would profoundly change the environment already altered by necrosis to favor further relapses.
It is therefore possible that the progression and severity of CDI in subjects with IBD depends on an inflammatory environment inducing the prevalent expression of receptors that favor cell necrosis. Indeed, it has been demonstrated that expression of CSPG4 is increased by inflammatory conditions such as that induced by TNF-α and lipopolysaccharides[112,113].
CKs release potentiates apoptosis of EGCs treated with low doses of TcdB[48,72]. This phenomenon is relevant with profound implications in vivo[26,32,34,48,72], especially in subjects with IBD[29].
First, the enhancement of apoptosis induced by the synergism between TcdB +CKs also occurs when CKs are given to EGCs 18 h before TcdB[48]. Therefore, in an already inflamed environment, as soon as C. difficile begins to produce Tcds, cytotoxicity is immediately increased by the presence of pre-existing cytokines, paving the way for the progression of CDI.
Second, the cytotoxic synergism between TcdB and CKs is triggered even 3 d after infection with TcdB[48,72], implying that even if at the beginning of the infection there is no significant inflammatory response, the enhancement of cytotoxicity by cytokines can occur later.
Third, the cytotoxic synergism between TcdB + CKs at 24 h is mainly characterized by death by apoptosis[48,72], while cells surviving in the following days progressively die by apoptosis/necrosis[72]. This in contrast to the cells treated only with TcdB that die by apoptosis at 24 h, and in the following days there is only a slight increase in cell death by this mechanism[72].
Fourth, cell death induced by TcdB + CKs is characterized by the activation of three apoptotic pathways[72], with a primary role played by calpains and subsequently cathepsin B activation, which either directly or converging on the effector caspases (caspases-3 and -7) lead to cell death, bypassing any anti-apoptotic barrier in the first 24 h[72]. Thereafter, a process of amplification of the cell death process begins in the cells that have resisted apoptosis, with consequent death by apoptosis and necrosis[72]. It is therefore clear how the cytotoxic synergism between TcdB + CKs finds in subjects with IBD a particularly favorable environment that will favor CDI, characterized by a strong cell death response by necrosis, with an increase in mortality and major incidence of CDI relapses. These are due to the fact that, although partially restored with antibiotic therapy, the intestinal environment remains very susceptible to further CDI relapses for the characteristics of necrotic cell damage and the persistence of the inflammatory response.
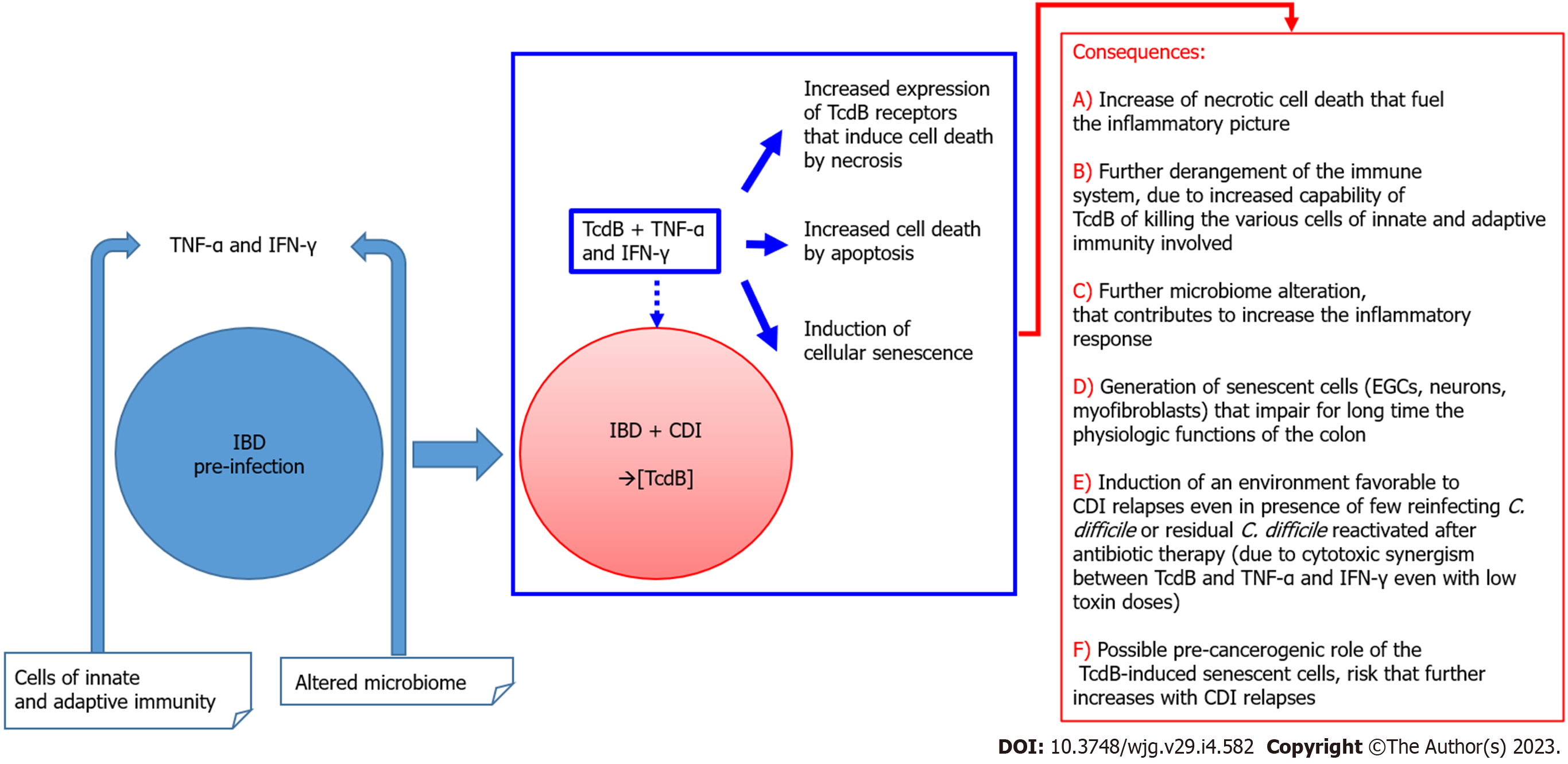
Therefore, in subjects with IBD a circuit of progressive cell damage can be activated, which feeds on itself based on the elements that characterize the first event of colonization/infection by C. difficile, i.e. the presence of an inflammatory state that enhances the cytotoxic synergism of TcdB + CKs, with induction of apoptosis and necrosis that in turn could lead to increased expression of receptors for TcdB. The latter, based on TcdB receptors involved and TcdB concentration, will promote cell death by apoptosis and/or necrosis. Cell death causes deeper tissue damage with a progressive increase in this loop that can only be stopped with antibiotic therapy, which adds an additional level of inflammatory response. Once the infection is resolved, the environment is even more susceptible to relapses.
It is clear that if CDI occurs in a subject with IBD the clinical picture can progress to an more acute form for the following reasons: (1) The expression of high levels of receptors for TcdB that induce cell death by necrosis; and (2) Higher levels of cytokines that enhance the development of receptors for TcdB, inducing mainly necrosis. The final result is enhancement of cell death by apoptosis/necrosis.
The above considerations could explain the differences in colonic environment during CDI in individuals with IBD and therefore the susceptibility to one or more relapses[29] (Figure 1).
Based on some peculiarities of the pathogenesis mechanisms of C. difficile in subjects with IBD we can try to adopt new strategies to counteract CDI in these subjects. However, we need to bear in mind that to date efficient strategies are strongly limited for the following reasons:
(1) It is not possible to block the endemic spread of C. difficile and to eradicate it in hospitals or nursing homes, which represent one of the most contaminated environments and the greatest cause of spread.
(2) Antibiotics or treatments preventing the development of conditions that favor relapses are not available.
(3) Fecal transplantation, although effective, is still a limited therapeutic.
(4) Immunotherapy with monoclonal antibodies to TcdA and TcdB has yielded very limited results.
(5) Vaccination against TcdA and TcdB did not yield significant clinical and eradication results for C. difficile.
Therefore, assuming that the spread of C. difficile cannot be stopped and that subjects with IBD are more at risk of contracting CDI, based on the most recent knowledge on the molecular pathogenesis of C. difficile, which methodology of interventions can we try to develop? Here we propose several approaches: (1) Monitor subjects with IBD for C. difficile with greater frequency to identify the onset of the active phase of the disease as early as possible; (2) In subjects with active IBD, the presence of colonization by C. difficile represents a serious risk and requires a strong reduction in the inflammatory response before the infection begins or spreads; (3) In subjects already colonized with C. difficile and quiescent IBD, continue microbiological monitoring by evaluating the extent of colonization over time. In cases of IBD exacerbation intervene to reduce TNF-α and IFN-γ levels using appropriate targeting drugs; (4) Due to the limited availability of specific antibiotics for CDI and the continuous emergence of antibiotic resistance, the research for alternative methods is under way. For instance, pangenomic analysis of this bacterium has revealed specific drug targets toward the core genome of C. difficile[114]. This, in the next future, will likely pave the road for more targeted therapeutic approaches; and (5) In high-risk subjects it would be important to develop selective prophylaxis and therapy based on highly specific bacteriophages for C. difficile. Indeed, this is becoming a hot topic due to the impending problem of multidrug-resistant bacteria. Since it is presently possible to analyze intestinal phages, this could represent a tool of paramount importance in the future development of phage therapy[115,116]. Of interest, the feasibility of combination phage therapy to treat infections associated with IBD was recently demonstrated[117].
The increasing worldwide spread of CDI represents a serious health problem, enforced by resistance of many bacterial strains to antibiotic therapy. This is particularly worrisome for some patient groups, such as elderly institutionalized subjects, immunocompromised subjects, and subjects with IBD[118]. The latter are particularly at risk due to the basal impaired immunological status and the frequent use of antibiotics and immunosuppressant agents for treatment. It is therefore of paramount importance to understand the mechanisms favoring CDI in these patients in order to develop more targeted and effective therapeutic strategies to limit/abolish relapses, morbidity, and mortality due to this infection. It is most important to have understood that C. difficile to efficient colonize humans, uses key inflammatory response elements such as proinflammatory cytokines, TNF-α and IFN-γ, that favor cell death by necrosis which increases inflammation and the expression of TcdB receptors that in turn promote further necrotic cell death. It is absolutely mandatory to find prophylactic and therapeutic methodologies to antagonize this synergism between C. difficile and the inflammatory response. This is important to protect patients with IBD and to prevent other at-risk populations.
| 1. | Oren A, Rupnik M. Clostridium difficile and Clostridioides difficile: Two validly published and correct names. Anaerobe. 2018;52:125-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Aktories K, Schwan C, Jank T. Clostridium difficile Toxin Biology. Annu Rev Microbiol. 2017;71:281-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 3. | Chandrasekaran R, Lacy DB. The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev. 2017;41:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 4. | Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2012;2:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Sun X, Savidge T, Feng H. The enterotoxicity of Clostridium difficile toxins. Toxins (Basel). 2010;2:1848-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 498] [Article Influence: 71.1] [Reference Citation Analysis (1)] |
| 7. | Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Balsells E, Shi T, Leese C, Lyell I, Burrows J, Wiuff C, Campbell H, Kyaw MH, Nair H. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health. 2019;9:010407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 9. | Furuya-Kanamori L, Marquess J, Yakob L, Riley TV, Paterson DL, Foster NF, Huber CA, Clements AC. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis. 2015;15:516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Goudarzi M, Seyedjavadi SS, Goudarzi H, Mehdizadeh Aghdam E, Nazeri S. Clostridium difficile Infection: Epidemiology, Pathogenesis, Risk Factors, and Therapeutic Options. Scientifica (Cairo). 2014;2014:916826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Acuña-Amador L, Quesada-Gómez C, Rodríguez C. Clostridioides difficile in Latin America: A comprehensive review of literature (1984-2021). Anaerobe. 2022;74:102547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Takakuwa H, Saikai T, Kobayashi K, Yamagishi T, Nakamura S. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J Med Microbiol. 2001;50:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Rousseau C, Levenez F, Fouqueray C, Doré J, Collignon A, Lepage P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol. 2011;49:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Daniels LM, Kufel WD. Clinical review of Clostridium difficile infection: an update on treatment and prevention. Expert Opin Pharmacother. 2018;19:1759-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Guh AY, Kutty PK. Clostridioides difficile Infection. Ann Intern Med. 2018;169:ITC49-ITC64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Wilcox MH, Fawley WN, Wigglesworth N, Parnell P, Verity P, Freeman J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect. 2003;54:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Boyce JM, Havill NL, Otter JA, McDonald LC, Adams NM, Cooper T, Thompson A, Wiggs L, Killgore G, Tauman A, Noble-Wang J. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol. 2008;29:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 19. | Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis. 2010;10:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Abeyawardhane DL, Godoy-Ruiz R, Adipietro KA, Varney KM, Rustandi RR, Pozharski E, Weber DJ. The Importance of Therapeutically Targeting the Binary Toxin from Clostridioides difficile. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, Wehland J, Aktories K. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Kachrimanidou M, Tzika E, Filioussis G. Clostridioides (Clostridium) Difficile in Food-Producing Animals, Horses and Household Pets: A Comprehensive Review. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Rodriguez Diaz C, Seyboldt C, Rupnik M. Non-human C. difficile Reservoirs and Sources: Animals, Food, Environment. Adv Exp Med Biol. 2018;1050:227-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Antonelli E, Baldoni M, Giovenali P, Villanacci V, Essatari M, Bassotti G. Intestinal superinfections in patients with inflammatory bowel diseases. J Crohns Colitis. 2012;6:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Bassotti G, Macchioni L, Corazzi L, Marconi P, Fettucciari K. Clostridium difficile-related postinfectious IBS: a case of enteroglial microbiological stalking and/or the solution of a conundrum? Cell Mol Life Sci. 2018;75:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Bassotti G, Marchegiani A, Marconi P, Fettucciari K. The cytotoxic synergy between Clostridioides difficile toxin B and proinflammatory cytokines: an unholy alliance favoring the onset of Clostridioides difficile infection and relapses. Microbiologyopen. 2020;9:e1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 27. | Bassotti G, Villanacci V, Nascimbeni R, Cadei M, Fisogni S, Antonelli E, Corazzi N, Salerni B. Enteric neuroglial apoptosis in inflammatory bowel diseases. J Crohns Colitis. 2009;3:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 28. | Arredondo-Hernandez R, Orduña-Estrada P, Lopez-Vidal Y, Ponce de Leon-Rosales S. Clostridium Difficile Infection: An Immunological Conundrum. Arch Med Res. 2018;49:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Bassotti G, Fruganti A, Maconi G, Marconi P, Fettucciari K. Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease May be Favoured by the Effects of Proinflammatory Cytokines on the Enteroglial Network. J Inflamm Res. 2021;14:7443-7453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Agrawal M, Christensen HS, Bøgsted M, Colombel JF, Jess T, Allin KH. The Rising Burden of Inflammatory Bowel Disease in Denmark Over Two Decades: A Nationwide Cohort Study. Gastroenterology. 2022;163:1547-1554.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 31. | Yang H, Zhou R, Bai X, Guo M, Ruan G, Wang L, Qian J. Trend and Geographic Variation in Incidence and Prevalence of Inflammatory Bowel Disease in Regions Across China: A Nationwide Employee Study Between 2013 and 2016. Front Med (Lausanne). 2022;9:900251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 32. | Fettucciari K, Marconi P, Marchegiani A, Fruganti A, Spaterna A, Bassotti G. Invisible steps for a global endemy: molecular strategies adopted by Clostridioides difficile. Therap Adv Gastroenterol. 2021;14:17562848211032797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Cho JM, Pardi DS, Khanna S. Update on Treatment of Clostridioides difficile Infection. Mayo Clin Proc. 2020;95:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Fettucciari K, Fruganti A, Marchegiani A, Brancorsini S, Marconi P, Bassotti G. Proinflammatory Cytokines: Possible Accomplices for the Systemic Effects of Clostridioides difficile Toxin B. J Inflamm Res. 2021;14:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Di Bella S, Ascenzi P, Siarakas S, Petrosillo N, di Masi A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 36. | Orrell KE, Zhang Z, Sugiman-Marangos SN, Melnyk RA. Clostridium difficile toxins A and B: Receptors, pores, and translocation into cells. Crit Rev Biochem Mol Biol. 2017;52:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Yuan P, Zhang H, Cai C, Zhu S, Zhou Y, Yang X, He R, Li C, Guo S, Li S, Huang T, Perez-Cordon G, Feng H, Wei W. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2015;25:157-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Henkel D, Tatge H, Schöttelndreier D, Tao L, Dong M, Gerhard R. Receptor Binding Domains of TcdB from Clostridioides difficile for Chondroitin Sulfate Proteoglycan-4 and Frizzled Proteins Are Functionally Independent and Additive. Toxins (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | LaFrance ME, Farrow MA, Chandrasekaran R, Sheng J, Rubin DH, Lacy DB. Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc Natl Acad Sci U S A. 2015;112:7073-7078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Tao L, Zhang J, Meraner P, Tovaglieri A, Wu X, Gerhard R, Zhang X, Stallcup WB, Miao J, He X, Hurdle JG, Breault DT, Brass AL, Dong M. Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature. 2016;538:350-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 41. | Pan Z, Zhang Y, Luo J, Li D, Zhou Y, He L, Yang Q, Dong M, Tao L. Functional analyses of epidemic Clostridioides difficile toxin B variants reveal their divergence in utilizing receptors and inducing pathology. PLoS Pathog. 2021;17:e1009197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Hartley-Tassell LE, Awad MM, Seib KL, Scarselli M, Savino S, Tiralongo J, Lyras D, Day CJ, Jennings MP. Lectin Activity of the TcdA and TcdB Toxins of Clostridium difficile. Infect Immun. 2019;87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Terada N, Ohno N, Murata S, Katoh R, Stallcup WB, Ohno S. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem Cell Biol. 2006;126:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | El Hadidy N, Uversky VN, Sun X. On the Potential Significance of the Intrinsically Disordered Regions in the Clostridiodes difficile Toxins A and B. Curr Protein Pept Sci. 2022;23:192-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 45. | Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT. Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology. 2005;129:1875-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Grossmann EM, Longo WE, Kaminski DL, Smith GS, Murphy CE, Durham RL, Shapiro MJ, Norman JG, Mazuski JE. Clostridium difficile toxin: cytoskeletal changes and lactate dehydrogenase release in hepatocytes. J Surg Res. 2000;88:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Zhang P, Hong J, Yoon IN, Kang JK, Hwang JS, Kim H. Clostridium difficile Toxin A Induces Reactive Oxygen Species Production and p38 MAPK Activation to Exert Cellular Toxicity in Neuronal Cells. J Microbiol Biotechnol. 2017;27:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Fettucciari K, Ponsini P, Gioè D, Macchioni L, Palumbo C, Antonelli E, Coaccioli S, Villanacci V, Corazzi L, Marconi P, Bassotti G. Enteric glial cells are susceptible to Clostridium difficile toxin B. Cell Mol Life Sci. 2017;74:1527-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Krijnen PA, Sipkens JA, Molling JW, Rauwerda JA, Stehouwer CD, Muller A, Paulus WJ, van Nieuw Amerongen GP, Hack CE, Verhoeven AJ, van Hinsbergh VW, Niessen HW. Inhibition of Rho-ROCK signaling induces apoptotic and non-apoptotic PS exposure in cardiomyocytes via inhibition of flippase. J Mol Cell Cardiol. 2010;49:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Paredes-Sabja D, Cofre-Araneda G, Brito-Silva C, Pizarro-Guajardo M, Sarker MR. Clostridium difficile spore-macrophage interactions: spore survival. PLoS One. 2012;7:e43635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Buckley AM, Spencer J, Candlish D, Irvine JJ, Douce GR. Infection of hamsters with the UK Clostridium difficile ribotype 027 outbreak strain R20291. J Med Microbiol. 2011;60:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Vargas E, Apewokin S, Madan R. Role of the leukocyte response in normal and immunocompromised host after Clostridium difficile infection. Anaerobe. 2017;45:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Péchiné S, Collignon A. Immune responses induced by Clostridium difficile. Anaerobe. 2016;41:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Flegel WA, Müller F, Däubener W, Fischer HG, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659-3666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Foschetti DA, Braga-Neto MB, Bolick D, Moore J, Alves LA, Martins CS, Bomfin LE, Santos A, Leitão R, Brito G, Warren CA. Clostridium difficile toxins or infection induce upregulation of adenosine receptors and IL-6 with early pro-inflammatory and late anti-inflammatory pattern. Braz J Med Biol Res. 2020;53:e9877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Hansen A, Alston L, Tulk SE, Schenck LP, Grassie ME, Alhassan BF, Veermalla AT, Al-Bashir S, Gendron FP, Altier C, MacDonald JA, Beck PL, Hirota SA. The P2Y6 receptor mediates Clostridium difficile toxin-induced CXCL8/IL-8 production and intestinal epithelial barrier dysfunction. PLoS One. 2013;8:e81491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | McDermott AJ, Falkowski NR, McDonald RA, Frank CR, Pandit CR, Young VB, Huffnagle GB. Role of interferon-γ and inflammatory monocytes in driving colonic inflammation during acute Clostridium difficile infection in mice. Immunology. 2017;150:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Wang Y, Wang S, Kelly CP, Feng H, Greenberg A, Sun X. TPL2 Is a Key Regulator of Intestinal Inflammation in Clostridium difficile Infection. Infect Immun. 2018;86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Farrow MA, Chumbler NM, Lapierre LA, Franklin JL, Rutherford SA, Goldenring JR, Lacy DB. Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc Natl Acad Sci U S A. 2013;110:18674-18679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Kim MN, Koh SJ, Kim JM, Im JP, Jung HC, Kim JS. Clostridium difficile infection aggravates colitis in interleukin 10-deficient mice. World J Gastroenterol. 2014;20:17084-17091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (3)] |
| 61. | Halabi-Cabezon I, Huelsenbeck J, May M, Ladwein M, Rottner K, Just I, Genth H. Prevention of the cytopathic effect induced by Clostridium difficile Toxin B by active Rac1. FEBS Lett. 2008;582:3751-3756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1112] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 63. | Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 470] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 64. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10243] [Cited by in RCA: 9948] [Article Influence: 523.6] [Reference Citation Analysis (0)] |
| 65. | Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1189] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 66. | Jäättelä M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 67. | Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jäättelä M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem. 2002;277:30738-30745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci. 2013;126:1905-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 70. | Macchioni L, Davidescu M, Fettucciari K, Petricciuolo M, Gatticchi L, Gioè D, Villanacci V, Bellini M, Marconi P, Roberti R, Bassotti G, Corazzi L. Enteric glial cells counteract Clostridium difficile Toxin B through a NADPH oxidase/ROS/JNK/caspase-3 axis, without involving mitochondrial pathways. Sci Rep. 2017;7:45569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Nottrott S, Schoentaube J, Genth H, Just I, Gerhard R. Clostridium difficile toxin A-induced apoptosis is p53-independent but depends on glucosylation of Rho GTPases. Apoptosis. 2007;12:1443-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Fettucciari K, Marguerie F, Fruganti A, Marchegiani A, Spaterna A, Brancorsini S, Marconi P, Bassotti G. Clostridioides difficile toxin B alone and with pro-inflammatory cytokines induces apoptosis in enteric glial cells by activating three different signalling pathways mediated by caspases, calpains and cathepsin B. Cell Mol Life Sci. 2022;79:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Solomon K, Webb J, Ali N, Robins RA, Mahida YR. Monocytes are highly sensitive to clostridium difficile toxin A-induced apoptotic and nonapoptotic cell death. Infect Immun. 2005;73:1625-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Mahida YR, Galvin A, Makh S, Hyde S, Sanfilippo L, Borriello SP, Sewell HF. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect Immun. 1998;66:5462-5469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Stieglitz F, Gerhard R, Hönig R, Giehl K, Pich A. TcdB of Clostridioides difficile Mediates RAS-Dependent Necrosis in Epithelial Cells. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Fettucciari K, Macchioni L, Davidescu M, Scarpelli P, Palumbo C, Corazzi L, Marchegiani A, Cerquetella M, Spaterna A, Marconi P, Bassotti G. Clostridium difficile toxin B induces senescence in enteric glial cells: A potential new mechanism of Clostridium difficile pathogenesis. Biochim Biophys Acta Mol Cell Res. 2018;1865:1945-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Schneider S, Wright CM, Heuckeroth RO. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu Rev Physiol. 2019;81:235-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Orenstein R, Patron RL, Seville MT. Why Does Clostridium difficile Infection Recur? J Am Osteopath Assoc. 2019;119:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Kochan TJ, Foley MH, Shoshiev MS, Somers MJ, Carlson PE, Hanna PC. Updates to Clostridium difficile Spore Germination. J Bacteriol. 2018;200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Edwards AN, Karim ST, Pascual RA, Jowhar LM, Anderson SE, McBride SM. Chemical and Stress Resistances of Clostridium difficile Spores and Vegetative Cells. Front Microbiol. 2016;7:1698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 81. | Larcombe S, Hutton ML, Lyras D. Involvement of Bacteria Other Than Clostridium difficile in Antibiotic-Associated Diarrhoea. Trends Microbiol. 2016;24:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 82. | Uzal FA, Navarro MA, Li J, Freedman JC, Shrestha A, McClane BA. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe. 2018;53:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 83. | Paredes-Sabja D, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol. 2014;22:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 317] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 84. | Shen A. Clostridioides difficile Spore Formation and Germination: New Insights and Opportunities for Intervention. Annu Rev Microbiol. 2020;74:545-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 85. | Castro-Córdova P, Mora-Uribe P, Reyes-Ramírez R, Cofré-Araneda G, Orozco-Aguilar J, Brito-Silva C, Mendoza-León MJ, Kuehne SA, Minton NP, Pizarro-Guajardo M, Paredes-Sabja D. Entry of spores into intestinal epithelial cells contributes to recurrence of Clostridioides difficile infection. Nat Commun. 2021;12:1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 86. | Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190:2505-2512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 568] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 87. | Eyre DW, Didelot X, Buckley AM, Freeman J, Moura IB, Crook DW, Peto TEA, Walker AS, Wilcox MH, Dingle KE. Clostridium difficile trehalose metabolism variants are common and not associated with adverse patient outcomes when variably present in the same lineage. EBioMedicine. 2019;43:347-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 406] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 89. | Mullish BH, Allegretti JR. The contribution of bile acid metabolism to the pathogenesis of Clostridioides difficile infection. Therap Adv Gastroenterol. 2021;14:17562848211017725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 90. | Sehgal K, Khanna S. Gut microbiome and Clostridioides difficile infection: a closer look at the microscopic interface. Therap Adv Gastroenterol. 2021;14:1756284821994736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 91. | Ofori E, Ramai D, Dhawan M, Mustafa F, Gasperino J, Reddy M. Community-acquired Clostridium difficile: epidemiology, ribotype, risk factors, hospital and intensive care unit outcomes, and current and emerging therapies. J Hosp Infect. 2018;99:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 92. | Yang ZH, Liu F, Zhu XR, Suo FY, Jia ZJ, Yao SK. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J Gastroenterol. 2021;27:3609-3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 93. | van der Lugt B, Vos MCP, Grootte Bromhaar M, Ijssennagger N, Vrieling F, Meijerink J, Steegenga WT. The effects of sulfated secondary bile acids on intestinal barrier function and immune response in an inflammatory in vitro human intestinal model. Heliyon. 2022;8:e08883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 860] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 95. | Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1031] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 96. | Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012;20:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 97. | Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Béliveau C, Oughton M, Brukner I, Dascal A. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (10)] |
| 98. | Bhattacharyya M, Debnath AK, Todi SK. Clostridium difficile and Antibiotic-associated Diarrhea. Indian J Crit Care Med. 2020;24:S162-S167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Anjuwon-Foster BR, Tamayo R. Phase variation of Clostridium difficile virulence factors. Gut Microbes. 2018;9:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 100. | Shin JH, Warren CA. Prevention and treatment of recurrent Clostridioides difficile infection. Curr Opin Infect Dis. 2019;32:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Pomares Bascuñana RÁ, Veses V, Sheth CC. Effectiveness of fecal microbiota transplant for the treatment of Clostridioides difficile diarrhea: a systematic review and meta-analysis. Lett Appl Microbiol. 2021;73:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Muñoz M, Restrepo-Montoya D, Kumar N, Iraola G, Camargo M, Díaz-Arévalo D, Roa-Molina NS, Tellez MA, Herrera G, Ríos-Chaparro DI, Birchenall C, Pinilla D, Pardo-Oviedo JM, Rodríguez-Leguizamón G, Josa DF, Lawley TD, Patarroyo MA, Ramírez JD. Integrated genomic epidemiology and phenotypic profiling of Clostridium difficile across intra-hospital and community populations in Colombia. Sci Rep. 2019;9:11293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Fu Y, Luo Y, Grinspan AM. Epidemiology of community-acquired and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2021;14:17562848211016248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 104. | Rodríguez C, Romero E, Garrido-Sanchez L, Alcaín-Martínez G, Andrade RJ, Taminiau B, Daube G, García-Fuentes E. MICROBIOTA INSIGHTS IN CLOSTRIDIUM DIFFICILE INFECTION AND INFLAMMATORY BOWEL DISEASE. Gut Microbes. 2020;12:1725220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 105. | Madoff SE, Urquiaga M, Alonso CD, Kelly CP. Prevention of recurrent Clostridioides difficile infection: A systematic review of randomized controlled trials. Anaerobe. 2020;61:102098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Olsen MA, Yan Y, Reske KA, Zilberberg MD, Dubberke ER. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect. 2015;21:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 107. | Cheng YW, Fischer M. Treatment of Severe and Fulminnant Clostridioides difficile Infection. Curr Treat Options Gastroenterol. 2019;17:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 108. | Badilla-Lobo A, Rodríguez C. Microbiological features, epidemiology, and clinical presentation of Clostridioides difficile strains from MLST Clade 2: A narrative review. Anaerobe. 2021;69:102355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Hunt JJ, Ballard JD. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol Mol Biol Rev. 2013;77:567-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 110. | Goorhuis A, Bakker D, Corver J, Debast SB, Harmanus C, Notermans DW, Bergwerff AA, Dekker FW, Kuijper EJ. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis. 2008;47:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 535] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 111. | Shen E, Zhu K, Li D, Pan Z, Luo Y, Bian Q, He L, Song X, Zhen Y, Jin D, Tao L. Subtyping analysis reveals new variants and accelerated evolution of Clostridioides difficile toxin B. Commun Biol. 2020;3:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 112. | Pellegatta S, Savoldo B, Di Ianni N, Corbetta C, Chen Y, Patané M, Sun C, Pollo B, Ferrone S, DiMeco F, Finocchiaro G, Dotti G. Constitutive and TNFα-inducible expression of chondroitin sulfate proteoglycan 4 in glioblastoma and neurospheres: Implications for CAR-T cell therapy. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 113. | Gao Q, Lu J, Huo Y, Baby N, Ling EA, Dheen ST. NG2, a member of chondroitin sulfate proteoglycans family mediates the inflammatory response of activated microglia. Neuroscience. 2010;165:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 114. | Golchha NC, Nighojkar A, Nighojkar S. Redefining genomic view of Clostridioides difficile through pangenome analysis and identification of drug targets from its core genome. Drug Target Insights. 2022;16:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 115. | Fujimoto K, Uematsu S. Phage therapy for Clostridioides difficile infection. Front Immunol. 2022;13:1057892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Federici S, Kviatcovsky D, Valdés-Mas R, Elinav E. Microbiome-phage interactions in inflammatory bowel disease. Clin Microbiol Infect. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 117. | Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K, Furuichi M, Oka A, Liu B, Fibelman M, Weiner IN, Khabra E, Cullin N, Ben-Yishai N, Inbar D, Ben-David H, Nicenboim J, Kowalsman N, Lieb W, Kario E, Cohen T, Geffen YF, Zelcbuch L, Cohen A, Rappo U, Gahali-Sass I, Golembo M, Lev V, Dori-Bachash M, Shapiro H, Moresi C, Cuevas-Sierra A, Mohapatra G, Kern L, Zheng D, Nobs SP, Suez J, Stettner N, Harmelin A, Zak N, Puttagunta S, Bassan M, Honda K, Sokol H, Bang C, Franke A, Schramm C, Maharshak N, Sartor RB, Sorek R, Elinav E. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185:2879-2898.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 365] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 118. | Lv T, Zheng L, Wu T, Shen P, Chen Y. Molecular characterization and antibiotic resistance of Clostridioides difficile in patients with inflammatory bowel disease from two hospitals in China. J Glob Antimicrob Resist. 2022;30:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Madian A, Egypt; Popovic DD, Serbia; Shen Z, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL